Introduction
Erythropoietin (Epo), a 34-kDa glycoprotein hormone,
was originally characterized as the principal regulator of
hematopoiesis due to its ability to inhibit apoptosis and stimulate
the proliferation and differentiation of erythroid precursor cells
(1,2). Epo was recognized as an engine for
blood generation in various hematopoietic and non-hematopoietic
mammalian tissues, including the central and peripheral nerve
systems. Various experimental studies have shown that Epo exerts a
marked neuroprotective effect in vivo and in vitro in
nervous system disorders of the brain (3,4),
spinal cord (5,6) and retina (7-9),
among others.
Epo is initially produced in the liver and
translocated to the kidney (10,11). Epo circulates in the blood stream
and binds to its obligatory receptor, the Epo receptor (Epo-R),
which is expressed in progenitor hematopoietic and
non-hematopoietic cells, including endothelial cells, skeletal
muscle cells and neural cells (12-14). Epo can exert an effect only by
binding to the Epo-R homodimer, which alters the conformation of
Epo-R, activates the phosphorylation of tyrosine-protein kinase
JAK2 and Epo-R, and finally results in signal transduction
involving signal transducer and activator of transcription 5A,
phosphoinositide 3-kinase, mitogen-activated protein kinase and
other signaling molecules (15).
In the meantime, the binding of Epo and Epo-R may have an indirect
influence on neuronal survival by modulating the pro-inflammatory
environment and decreasing the subsequent neural apoptosis
(5,16). Such findings from brain and spinal
cord studies indicate that Epo and Epo-R are essential for
neuroprotection. In addition, a previous study revealed that
recombinant Epo most likely has otoprotective effects in newborn
hypoxic-ischemic encephalopathy-induced cellular pathology and that
it attenuates hearing loss (17).
Sound stimulation of the auditory hair cells (HCs)
is transduced into electrical signals that are transmitted to the
brain via the spiral ganglion neurons (SGNs). The cochlea in the
inner ear transduces complex sound waves into electrical neural
activity in the auditory nerve. The SGNs, the inner and outer HCs,
and the stria vascularis (SV) are crucial elementary structures of
the cochlea in the inner ear (18). Depolarization of the SGNs is
initiated by the inner HCs once sound stimulation has activated the
complicated mechanism of auditory transduction (19). Degeneration and apoptosis of the
SGNs leads to sensorineural hearing loss (SNHL) after deafening,
which is associated with damage to the inner ear. Certain exogenous
neurotrophins or neuroprotectants have been shown to be effective
in preventing the degeneration or death of SGN following SNHL
(20,21). Certain studies have shown that
using recombinant adenovirus vector-mediated gene therapy to
provide certain neuroprotectants can enhance the survival rate or
the regenerative sprouting of SGNs (22,23).
The potential neuroprotective effect of Epo in the
inner ear depends on whether Epo and Epo-R are expressed in SGNs
from the inner ear. However, the exact expression patterns of Epo
and Epo-R in the rat inner ear and their role in hearing in general
remain poorly understood. Additionally, there is a requirement for
an effective interventional approach for enhancing Epo expression
in the SGNs. The present study was designed to investigate whether
Epo and Epo-R are expressed in the SGN of the rat inner ear. An Epo
adenovirus vector (Ad-Epo) was constructed to investigate the
potential neuroprotective effect of Epo on the SGNs in the inner
ear, with the aim of providing a basis for the prevention or
alleviation of SNHL.
Materials and methods
Dissociation and preparation of cochlear
specimens
A total of 30 healthy neonatal (2-3 days postnatal)
Sprague-Dawley rats of a clean grade (weighing 6-8 g) were provided
by the Animal Medical Center of the Third Military Medical
University (Chongqing, China). Rats were maintained under standard
laboratory conditions, at 18-27°C and 40-70% humidity under a 12-h
light/dark cycle, with free access to clean water and food. The
experimental protocol was approved and supervised by the Laboratory
Animal Welfare and Ethics Committee of the Third Military Medical
University (production license no. SCXK-PLA-20070015; occupancy
permit no. SYXK-PLA-20070035). In this study, the guidelines for
ensuring the welfare of laboratory animals were followed, and the
welfare of the rodents was optimized by improving their living
environments. The animals were maintained under standard laboratory
conditions and were provided with clean water and food. To relieve
their pain and fear, all rats were sacrificed by rapid
decapitation.
Tissue preparation for
immunohistochemistry
Dissection of the cochleae was performed as
previously described (24,25).
In total, 60 ears from 30 rats were used: 5 were sectioned for
staining, 15 were used for the culture and the other 10 were used
for the apoptosis test. Both ears from each rat were used.
The temporal bones were dissected to remove the
cochleae, and the capsules were fractured to reveal the membranous
cochlear duct and the bony modiolus. The modiolus with the encased
acoustic ganglion neurons and the attached cochlear duct was
dissected and transferred to a different Petri dish, where was
opened in 37°C Dulbecco's phosphate-buffered saline (PBS). The
middle ear was then opened, and the cochlea was cautiously perfused
with 1.5% paraformaldehyde. The fixative was slowly injected into
the tympanic scale of the basal turn through the round-window
plasma membrane. A small opening was made at the cochlear apex to
allow cochlear perfusion. The specimens were submersed in the same
fixative at 4°C for at least 1 week to ensure complete fixation of
the osseous tissue components. The cochlea was split into two by a
longitudinal midmodiolar transaction and was subsequently embedded
in paraffin. To optimize the immunostaining procedure, the
concentration of the primary antibody was titrated, and different
antigen-retrieval procedures, including microwave oven treatment
using different buffers, and secondary visualization methods were
investigated. Animals were anaesthetized with 100 mg/kg sodium
pentobarbital and then perfused with 4% paraformaldehyde. The brain
was dissected, removed and post-fixed overnight in the same
fixative, and then transferred to 30% sucrose at 4°C until sinking
to the bottom. The brain was sectioned in a coronal plane (5-10
μm) on a paraffin machine. The brain tissues staining method
is the same as that of inner ear.
Sections of 5-10 μm in thickness were cut
from the embedded tissue and dried at 60°C to melt the paraffin.
De-paraffinization of the tissues was performed using xylene, and
rehydration was achieved using decreasing concentrations of ethanol
to water. All blocking and incubations were at room temperature. A
10-min Tris-buffered saline rinse was performed, and the endogenous
peroxidase activity was blocked by incubation in 3%
h2O2 for 5 min followed by a 30-min
incubation with the primary antibodies: Goat anti-Epo antibody
(N-19; 1:100; cat. no. sc-1310; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and rabbit anti-Epo-R antibody (1:50; cat. no.
bs-1424R; Bioss, Inc., Woburn, MA, USA). Sections were subsequently
incubated with anti-goat (1:150; cat. no. SP9000) or anti-rabbit
(1:150; cat. no. SP9001) secondary antibodies from
immunohistochemistry staining kits purchased from Zhongshan Golden
Bridge Biotechnology Co., Ltd. (Beijing, China). The tissue was
rinsed in running tap water for 5 min. Negative control staining
was performed using hematoxylin and eosin (H&E) for 45 sec,
followed by repeated rinses using running tap water.
Culture of SGNs
The majority of the protocols followed previously
described methods (25). The
cochleae were removed from the capsules, and the spiral ligament
and SV were removed carefully. The spiral ganglion and modiolus
were separated from the other peripheral tissue by a cut at the
border between the spiral ganglion and the limbus. Once the spiral
ganglia had been isolated, enzymatic dissociation was performed for
20 min in hanks' balanced salt solution containing 0.1% trypsin and
0.01% DNase I (Roche Diagnostics GmbH, Mannheim, Germany) and was
stopped using fetal calf serum (FCS) (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The spiral ganglia were washed
using culture medium prior to being mechanically dissociated, and
this process was followed by trituration, as previously described
(26). The culture medium was
Dulbecco's modified Eagle's medium supplemented with
4-(2-hydroxyethyl)-1-piperazineethane-sulfonic acid (both
Invitrogen; Thermo Fisher Scientific, Inc.), glucose, penicillin
and other components, including hanks' solution (Hyclone, Logan,
UT, USA), trypsin and collagenase I (both Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany), and FCS (Invitrogen; Thermo Fisher
Scientific, Inc.). The SGNs were seeded in the laminin-coated wells
of a 96-well culture plate at 1×105/ml.
Immunohistochemical analysis
The anti-neurofilament antibody (1:50; cat. no.
ab129349) was obtained from Abcam (Cambridge, UK). The primary
antibodies, goat anti-Epo and rabbit anti-Epo-R were the same as
those mentioned in the tissue preparation section. The dissected
cochlea specimens were rinsed using PBS prior to incubation with a
secondary antibody at a 1:2,000 dilution in 1.5% normal horse serum
for 30 min at room temperature. The secondary antibodies were the
same as those mentioned in the tissue preparation section. All the
specimens were immunostained with an anti-neurofilament antibody to
aid the visualization and identification of the SGNs and their
processes. For primary staining, the cultures were incubated with
the neurofilament antibody for 1 h at 37°C. When the presence of
SGNs was confirmed, the anti-Epo and the rabbit anti-Epo-R
antibodies were used in the subsequent protocol. The cells were
rinsed using PBS prior to incubation with a secondary biotinylated
antibody at a 1:2,000 dilution in 1.5% normal horse serum for 30
min at room temperature. Subsequent to washing with PBS, the cells
were treated with the ABC complex solution (Vector Laboratories,
Inc., Burlingame, CA, USA) according to the manufacturer's
protocols. The staining was visualized using diaminobenzidine for 5
min at room temperature under a BhF342 fluorescence microscope
(Olympus Inc., Tokyo, Japan). Controls were performed by
eliminating the respective antibodies from the protocol, and no
deposition of immunore-active products occurred.
Dual immunofluorescence staining
The sections were fixed for 45 min at room
temperature using 4% paraformaldehyde in PBS (24). The aforementioned Epo and Epo-R
antibodies were used, and the secondary antibody for fluorescence
detection was a mouse monoclonal anti-red fluorescent
protein-tagged antibody (1:50; cat. no. CW0254; CWBio, Beijing,
China). The sections were stained at room temperature with DAPI
(Sigma-Aldrich; Merck KGaA) as a nuclear counterstain. The
specimens were then rinsed for 3-10 min using PBS. Next, the
specimens were incubated overnight at 4°C with primary antibodies
diluted in blocking solution followed by incubation with
species-specific secondary antibodies conjugated to fluorescein
isothiocyanate or phycoerythrin. The samples were examined using a
Leica model TCS SP5 confocal laser-scanning microscope (Leica
Biosystems, Wetzlar, Germany). LAS-AF-Lite software (version
2.4.1_6384; Flexera Software, Inc., Maidenhead, UK) was used for
image capture and deconvolution analysis.
Construction and transfection of
Ad-Epo
Ad-Epo was prepared as previously described
(27-29). The virus was grown to high titers
in cells of the E1-containing human kidney 293 cell line. The
buffer was replaced with 10 mM Tris (ph 7.5) and 1 mM MgCl. The
virus was sterilized by passage through a sterile 0.2-mm filter and
frozen until use. The cultured SGNs were transfected with the
recombinant adenovirus Ad-Epo. The SGNs were then seeded into
six-well plates at 1×105/ml and cultured for 48 h at
37°C. Subsequently, the serum-free medium DMEM/F12 (Hyclone) was
changed and SGNs were harvested at the indicated time-points (at 24
or 48 h after Ad-Epo transfection respectively). SGNs grown in
serum-free medium formed the negative control group, neurons that
were infected with the green fluorescent protein expression vector
(Ad-GFP) formed the vector control group, and neurons infected with
Ad-Epo formed the Ad-Epo group.
Apoptosis of SGNs
SGNs were cultured under different conditions,
collected and fixed using cold 80% ethanol at 4°C overnight. The
SGNs were then resuspended in PBS for 15 min at room temperature,
1.0 ml of propidium iodide (PI) containing RNase A (10 mg/ml) was
added and the SGNs were incubated at 37°C for 30 min in the dark.
The apoptosis rate was determined at days 4 and 7 in the negative
control group, at day 7 in the vector control group and at day 7 in
the Ad-Epo group. The apoptotic cells, which were stained with PI,
were detected via flow cytometry using a BD Accuri C6 flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA) at an
excitation wavelength of 488 nm.
Western blotting
At 48 h post-Ad-Epo infection, total protein from
the cultured SGNs was extracted using a Protein Extraction kit
(P0027; Beyotime Biotech, Beijing, China) and its concentration was
determined using a BCA Assay kit (P0010; Beyotime Biotech). Samples
(35 μg) were loaded into 3-8% SDS-PAGE. Immunolabeling of
the polyvinylidene difluoride membranes was performed as previously
described (30). The membrane was
blocked with 5% non-fat milk-TBST overnight at 4°C. The membrane
was then washed with TBST and incubated with antibody recognizing
Epo as aforementioned overnight at 4°C, followed by 3 washes with
TBST and incubation with peroxidase-conjugated goat anti-mouse IgG
(1:1,000; Zhongshan Biotech, Bejing, China) or goat anti-rabbit IgG
secondary antibodies (1:1,000, Zhongshan Biotech) for 1.5 h at room
temperature. Monoclonal anti-β-actin antibody (1:1,000; AA128;
Beyotime Biotech) was used for the reference protein. ECL Plus kit
(Pierce Inc., Appleton, WI, USA) was used for the detection of blot
visualization and analyzed by Image Pro Plus software 5.0 (Media
Cybernetics, Rockville, USA). The expression of SGNs was also
labeled by GFP and observed prior to and following Ad-Epo
infection.
Statistical analysis
Data analysis was performed using SPSS 13.0 software
(SPSS, Inc., Chicago, IL, USA). The results of the statistical
analysis are expressed as the mean ± standard deviation. Apoptosis
rate and Epo protein level data were analyzed using one-way
analysis of variance and P<0.05 was considered to indicate a
statistically significant difference.
Results
Immunohistochemical detection of Epo and
Epo-R expression in the inner ear
The positive control for Epo immunohisto-logical
staining was the brain (Fig. 1A),
whereas the negative controls for Epo and Epo-R immunostaining were
inner ear sections stained only with H&E (Fig. 1B). Epo labeling was observed in
the majority of the neurons of the inner ear as coarse granulated
staining on the plasma membrane, in the cytoplasm surrounding the
nuclei, which were stained blue (Fig.
1C), in the organ of Corti, including the HCs, and in the SV
(Fig. 1D). Epo-R labeling was
observed as clear granulated staining in the plasma membrane and
cytoplasm of SGNs in the inner ear (Fig. 1E), in the phalangeal cells, in the
organ of Corti (Fig. 1E and F),
in the HCs and in the SV (Fig.
1F).
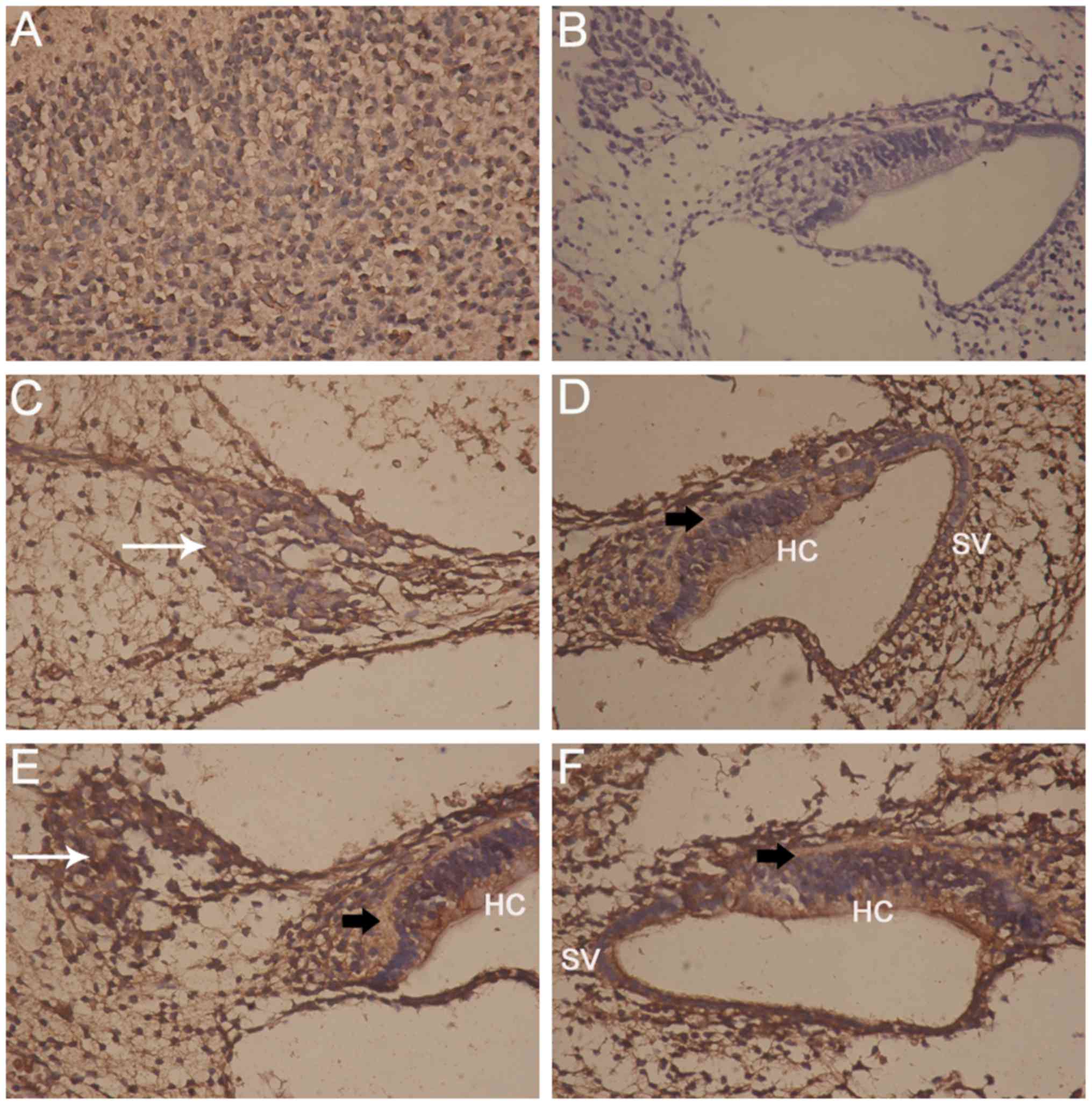 | Figure 1Micrographs showing Epo and Epo-R,
and control staining. (A) Positive control staining of the brain
(IHC; magnification, ×400); (B) negative control staining of the
inner ear (hematoxylin and eosin staining; magnification, ×100);
(C) Epo is localized in rat SGNs (white arrow; magnification,
×400); (D) Epo is localized in the organ of Corti (black arrow),
the HCs and the SV (magnification, ×400); (E) Epo-R staining of
SGNs (white arrow). One region of the organ of Corti (black arrow)
is displayed, showing staining in the HCs (magnification, ×400);
(F) Epo-R staining of the entire organ of Corti (black arrow),
showing Epo-R in the HCs and the SV (magnification, ×400). Epo,
erythropoietin; Epo-R, Epo receptor; IHC, immunohistochemical;
SGNs, spiral ganglion neurons; HC, hair cell; SV, stria
vascularis. |
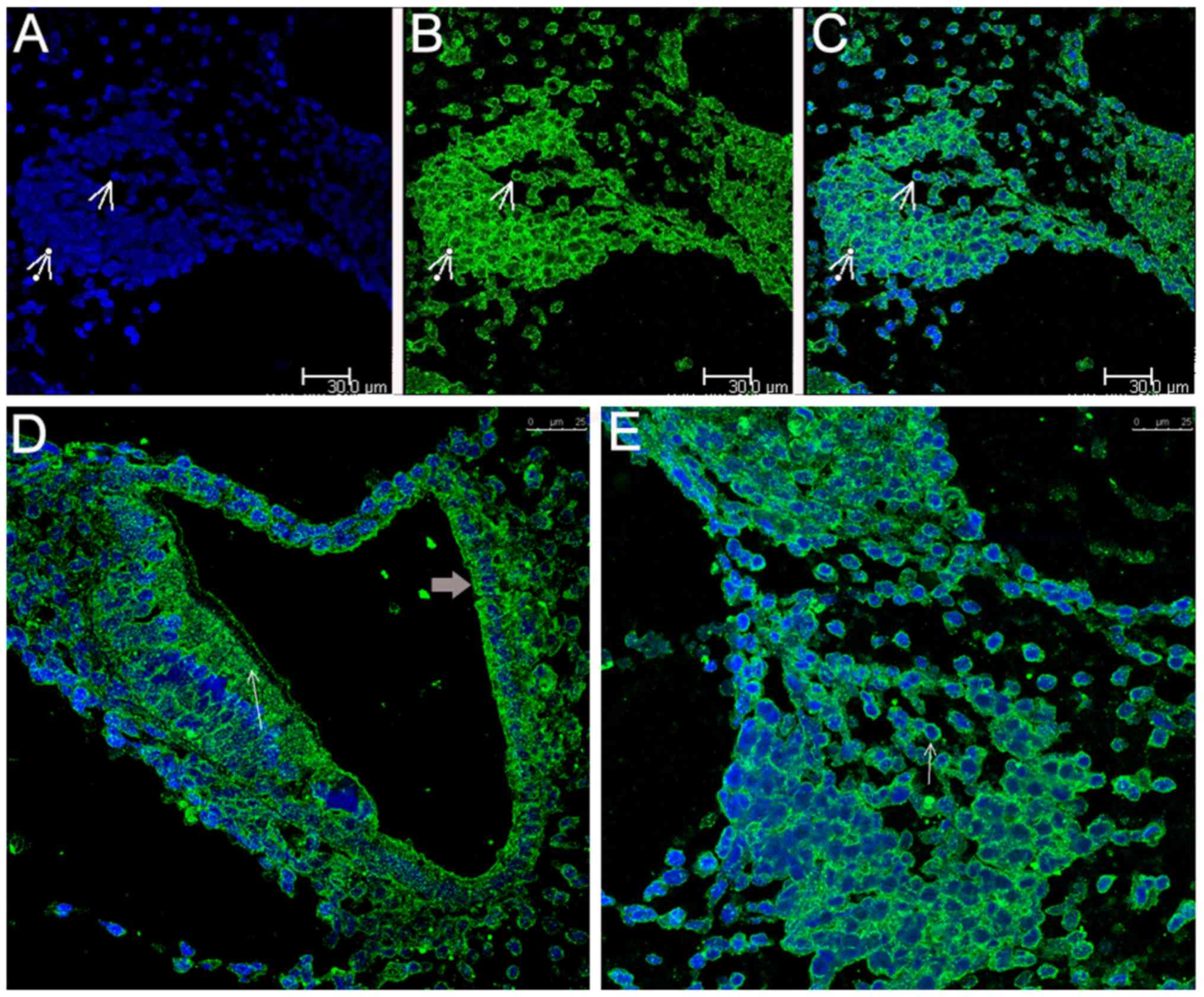
Scanning confocal fluorescence
microscopy
Following immunohistochemical detection of Epo,
inner ear specimens were stained for Epo using immunofluorescence
and were observed under a confocal laser-scanning fluorescence
microscope, which clearly revealed the blue DAPI-stained nuclei of
numerous SGNs (Fig. 2A). Positive
labeling with anti-Epo appeared as fluorescence in the plasma
membrane and in the cytoplasm surrounding the nucleus of the SGNs
(Fig. 2B). The merged images
display the localization of Epo in the SGNs in detail and clearly
show that Epo was present on the plasma membrane and in the
cytoplasm surrounding the nucleus of the SGNs (Fig. 2C). Additionally, Epo expression
was also detected in the organ of Corti, including the phalangeal
cells and the HCs, and in the SV (Fig. 2D). More details of Epo
presentation in the SGNs can be seen in Fig. 2E.
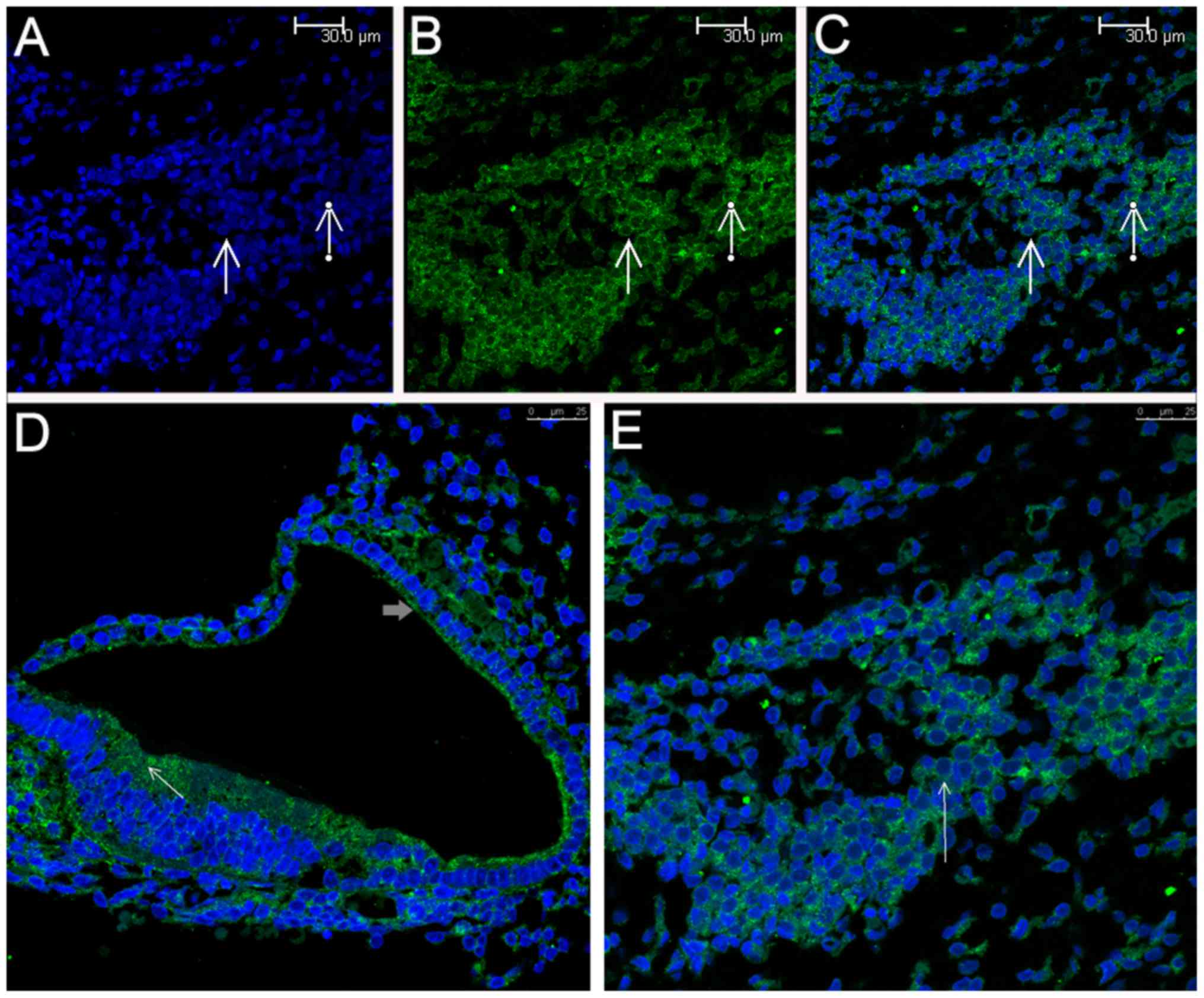
Simila rly, Epo-R was also detected in the SGNs
(Fig. 3B and C), which was in
agreement with the results of a previous study (31), and in the organ of Corti (e.g., in
HCs) and the SV (Fig. 3D). More
details of Epo-R presentation in the SGNs can be seen in Fig. 3E. Based on a coarse comparison of
the levels of Epo and Epo-R staining, it appeared that Epo was more
highly expressed in the SGNs than Epo-R.
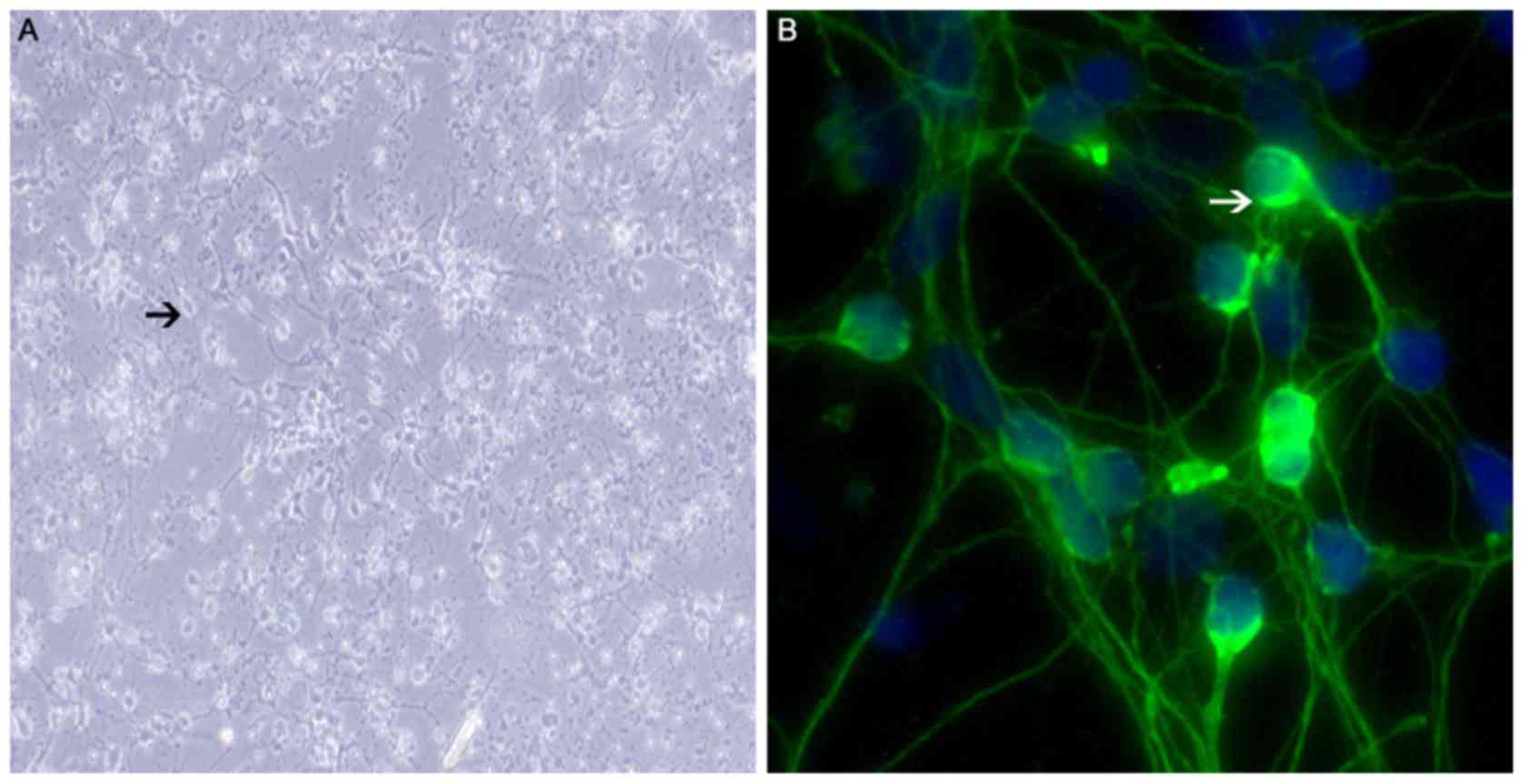
Identification of SGNs
SGNs were seeded into six-well plates and cultured.
The cultured SGNs, which exhibited typical bipolar shapes (Fig. 4A and B), were stained with an
anti-neurofilament protein antibody for identification. The cells
displayed green fluorescence in the plasma membranes, but not the
nuclei, confirming their identity as neurons (Fig. 4B).
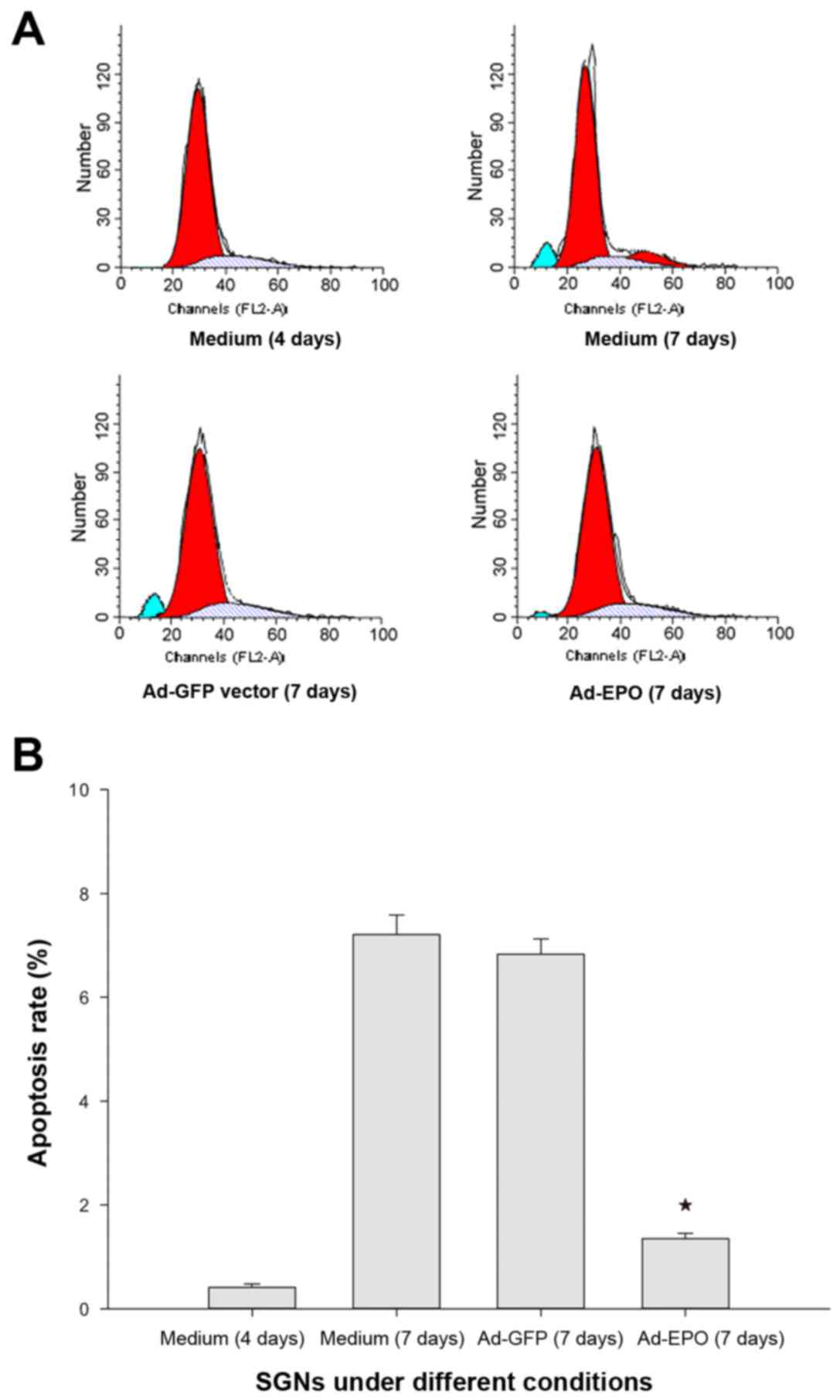
Apoptosis of cultured SGNs
Detection of apoptosis among the SGNs was based on
staining of the cells with PI, which can enter dead cells via
permeable plasma membranes and intercalate into the DNA (Fig. 5A). Apoptotic cells were seldom
observed at day 4 of culture of the SGNs in the negative control
group. At day 7, marked apoptotic cells were detected in the
negative control group and the vector control group. The rate of
apoptosis observed in the Ad-Epo group was significantly lower than
that observed in the negative control group or in the vector
control group at day 7 (P<0.05) (Fig. 5B).
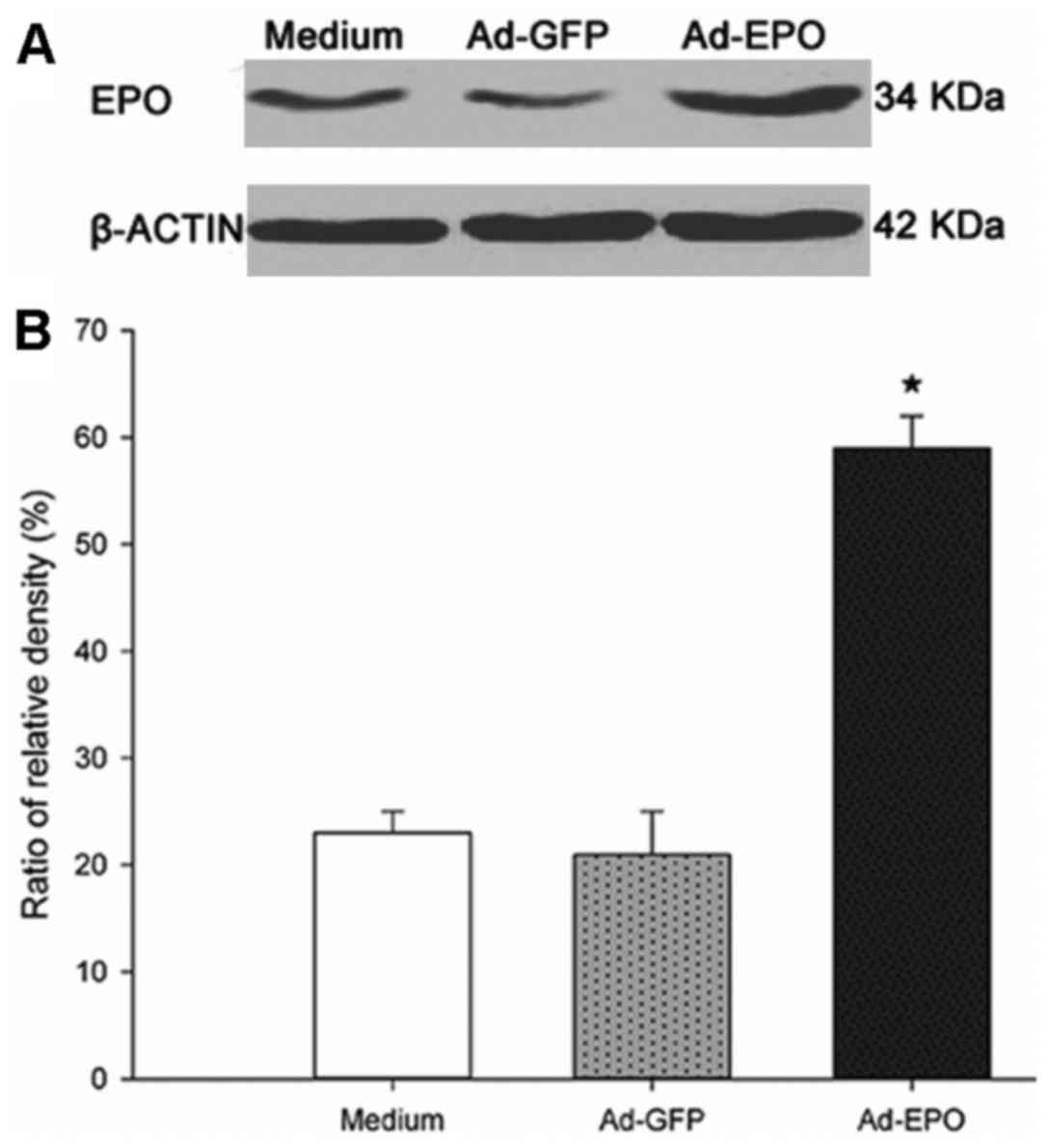
Epo expression following Ad-Epo
infection
The virus titer determined in the plaque assay using
293 cells was 1.17×1011 IU/ml. The level of Epo protein
in the SGNs was determined by western blotting 48 h after the SGNs
were infected with the adenovirus. Epo protein expression was
detected in the SGNs in the negative control group, the vector
control group and the Ad-Epo group (Fig. 6A). Ad-Epo was capable of infecting
SGNs, and Epo expression was upregulated in the SGNs in the Ad-Epo
group compared with the levels in the other two groups (P<0.05)
(Fig. 6B). Expression of Epo
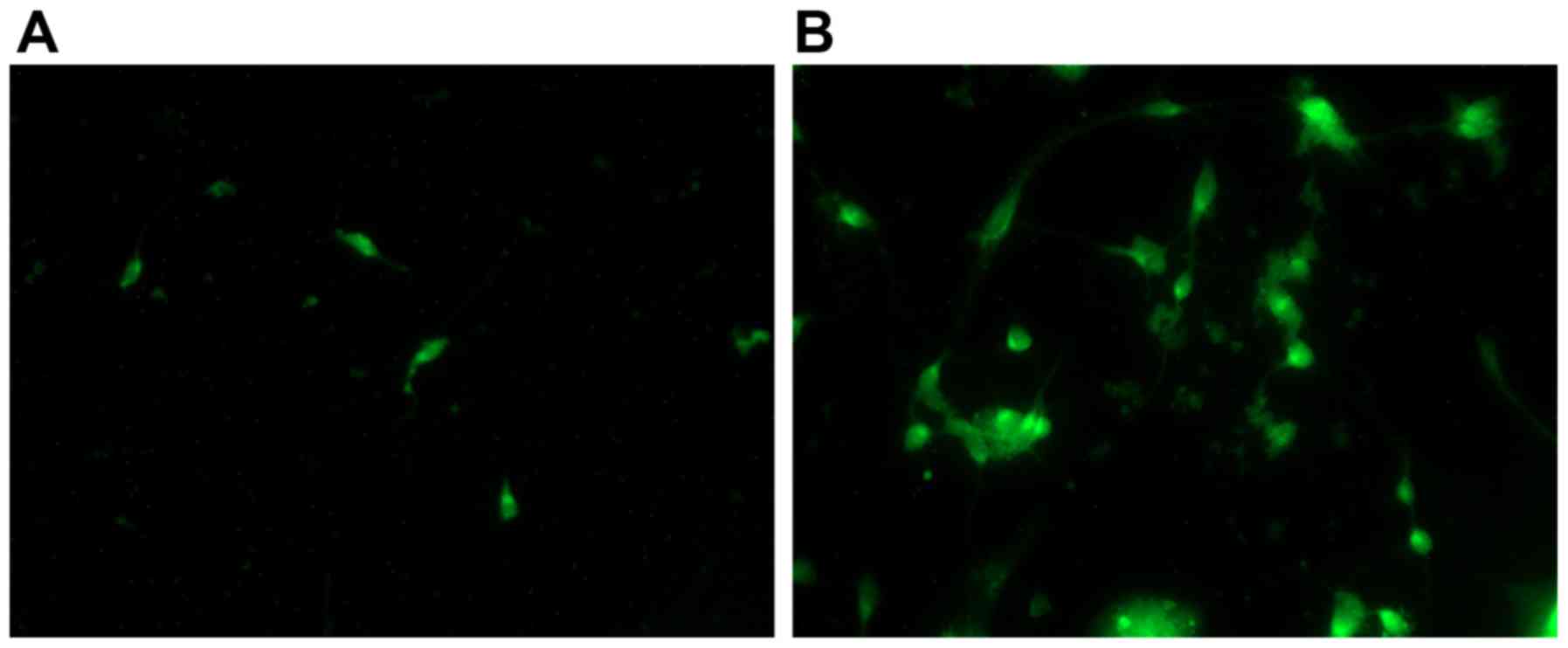
labeled by GFP prior to and following Ad-Epo infection demonstrated
increased Epo expression following infection. The staining in the
micrographic views was lower in the non-infected SGNs (Fig. 7A), but higher in the SGNs infected
by Ad-EPO (Fig. 7B).
Discussion
SNHL is a common and serious health problem
(32,33) that largely results from various
types of injuries to the HCs, SGNs or other structures of the inner
ear (34,35). Serious and long-term SNHL is
always incurable and irreversible; thus, potential methods for
preventing damage to SGNs and other inner ear components, or
possibly restoring their normal function, have attracted much
attention (36-39). To the best of our knowledge, the
present study is the first to examine the expression of Epo and
Epo-R in the inner ear of neonatal rats and in isolated primary
cultured SGNs, and to examine the effects of adenoviral
transfection of isolated primary cultured SGNs.
Epo is the primary stimulator of red blood cell
formation (11) and is a
pleiotropic cytokine with neuroprotective effects that have been
largely investigated in the central nervous system, particularly in
the brain (40). Astrocytes have
been shown to be responsible for the production of brain Epo
(41), and Epo-R (42) is abundantly expressed in the
brains of rodents (2,4,24,43,44). Epo is regarded as an important
neuro-protective candidate for treating trauma, stroke,
inflammation and other impairments (3,16,26,45). The role of Epo and Epo-R in the
SGNs and the inner ear remains unclear. It has been found that Epo
and Epo-R are expressed in the inner ear of guinea pigs (24), and that Epo-R is expressed in the
cultured rat SGNs (31). Other
evidence for the potential protective effect of Epo is that it
prevents amino glycoside-induced (46) and ischemia-induced HC death
(47). The neuroprotective effect
of Epo may involve at least two mechanisms, including a decrease in
the HC death rate and an increase in the expression of angiogenic
genes, such as vascular endothelial growth factor and C-X-C
chemokine receptor type 4 (48).
Epo has also been found to protect against gentamicin-induced
auditory HC damage in vitro (49). Previous findings indicated that
brain- derived neurotrophic factor and Epo may promote neuronal
survival in the inner ear in vitro (50). Additionally, Epo was found to act
as an otoprotectant in a DFNB12 mouse model with progressive
hearing loss (51).
In the present study, it was demonstrated that Epo
and Epo-R are expressed in SGNs and in other structures of the
inner ear of normal neonatal rats. The observed wide distribution
of Epo and Epo-R in the SGNs, the organ of Corti and the SV in the
cytoplasm surrounding the nuclei is consistent with their
localization in neurons (24,43). Considering the confirmed
neuroprotective effect of Epo in the brain and its localization in
the inner ear, it was concluded that Epo and Epo-R may be important
neuroprotectants of SGNs in the inner ear.
However, contrary to our hypothesis, in a previous
study, it was observed that Epo induced neurite outgrowth rather
than increased survival of the SGNs. Epo was proposed as a possible
candidate that could be used to enhance and modulate the
regenerative effects of known neurotrophic factors on SGNs
(31). However, Frederiksen et
al (52) reported a result
that contradicted the beneficial effects of Epo that had been
reported by the vast majority of researchers. In this study, Epo
was found to augment noise-induced hearing loss by altering the
dynamics of blood flow to the cochlear vascular bed through
potentially inducing vasoconstriction; the pathophysiological
alterations also included reduced cochlear blood flow, such as
localized periods of stasis, alterations in vascular permeability
and local ischemia, which may result in temporary or even permanent
deafness. The discrepancies between the results of this study and
other earlier results may be associated with the aforementioned
unexpected finding from the present study that Epo and Epo-R are
expressed in the SV. Further study is therefore required.
When examining the potential neuroprotective or
cytoprotective roles of Epo and Epo-R in the inner ear, the
blood-labyrinth barrier must inevitably be taken into consideration
(36,46,53). The blood-perilymph barrier may
preclude the passage of circulating Epo to the inner ear, although
a local paracrine system may exist. Thus, the systemic
administration of Epo, even at high doses, may not sufficiently
increase the endocochlear concentration of Epo (24). In vitro investigations are
therefore necessary. Cultures of dissociated SGNs may provide an
experimental environment in which the molecular mechanism
underlying the effects of Epo on SGNs may be examined. Differences
in the culture preparations and conditions used in different
studies are likely to underlie differences in the results. For
example, the majority of neurons in adult spiral ganglia in
vivo are bipolar. However, spiral ganglion cultures often
exhibit a mix of neuronal morphologies, including bipolar,
monopolar or even a lack of neurite growth. The goal of neuron
culture is to induce these cells to regrow their neurites in an
observable and quantifiable way (54). Thus, although the majority of
neurons are bipolar in vivo, neurons with different
morphologies may still be found in vitro. The dissociation,
culture and detection of SGNs, and the consequent experiments
involve delicate procedures that must be performed carefully and
patiently.
Adenovirus vectors have been widely used in a number
of fields (55,56). In the present study, an Ad-Epo was
successfully constructed and transfected into the cultured SGNs.
The results showed that Epo protein expression was markedly
upregulated, indicating that Ad-Epo is a powerful tool for
examining how Epo affects SGNs. At day 7 of culture, the rate of
apoptosis was significantly higher in the vector control group
compared with that in the Ad-Epo group, indicating that Epo may
facilitate the survival of the cultured unimpaired SGNs and that
apoptosis could be reduced using adenovirus transfection and other
molecular biological techniques.
This study presented a thorough and precise
description of Epo and Epo-R localization in the inner ear of
neonatal rats using morphological and biomolecular methods. The
expression pattern of Epo and Epo-R in normal neonatal rats was
visualized using immunohistochemistry and fluorescence staining,
and Epo protein expression was also detected using western
blotting. Additionally, it was found that Epo had a potential
neuroprotective effect on normal cultured SGNs, as the rate of
apoptosis was decreased by adenovirus vector-mediated upregulation
of Epo expression. The results of this study may provide an
experimental basis for a possible neuroprotective role of Epo based
on down-regulation of the apoptosis rate in the mixed spiral
ganglion cultures. Furthermore, the results of this study may add a
novel dimension to our understanding of the complex picture of the
neuroprotective effect of this clinically familiar drug and its
possible mechanism of action in the inner ear.
Despite these findings, the following issues remain
unclear: i) The exact pattern of Epo and Epo-R expression in the
inner ears of neonatal rats; ii) the neuroprotective potential of
Epo for SGNs in the inner ear; iii) the other cellular activities
that are regulated by Epo; and iv) the mechanism by which Epo
exerts its modulatory effects. Thus, further study is required to
clarify these issues and to elucidate the protective role of Epo
and its underlying mechanism. In the future, other intervention
factors may be employed to cause damage to SGNs, or in vivo
experiments may be performed with hearing-impaired individuals.
Glossary
Abbreviations
Abbreviations:
|
Epo
|
erythropoietin
|
|
Epo-R
|
erythropoietin receptor
|
|
SGNs
|
spiral ganglion neurons
|
|
Ad-Epo
|
erythropoietin adenovirus vector
|
|
SNHL
|
sensorineural hearing loss
|
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81100720).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Bartesaghi S, Marinovich M, Corsini E,
Galli CL and Viviani B: Erythropoietin: a novel neuroprotective
cytokine. Neurotoxicology. 26:923–928. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rankin EB, Wu C, Khatri R, Wilson TL,
Andersen R, Araldi E, Rankin AL, Yuan J, Kuo CJ, Schipani E, et al:
The HIF signaling pathway in osteoblasts directly modulates
erythropoiesis through the production of EPO. Cell. 149:63–74.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tugyan K, Ozbal S, Cilaker S, Kiray M,
Pekcetin C, Ergur BU and Kumral A: Neuroprotective effect of
erythropoietin on nandrolone decanoate-induced brain injury in
rats. Neurosci Lett. 533:28–33. 2013. View Article : Google Scholar
|
|
4
|
Traudt CM and Juul SE: Erythropoietin as a
neuroprotectant for neonatal brain injury: animal models. Methods
Mol Biol. 982:113–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kwon BK, Okon E, Hillyer J, Mann C,
Baptiste D, Weaver LC, Fehlings MG and Tetzlaff W: A systematic
review of non-invasive pharmacologic neuroprotective treatments for
acute spinal cord injury. J Neurotrauma. 28:1545–1588. 2011.
View Article : Google Scholar :
|
|
6
|
Wang Y, Yao M, Zhou C, Dong D, Jiang Y,
Wei G and Cui X: Erythropoietin promotes spinal cord-derived neural
progenitor cell proliferation by regulating cell cycle.
Neuroscience. 167:750–757. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Colella P and Auricchio A: Photoreceptor
degeneration in mice: adeno-associated viral vector-mediated
delivery of erythropoietin. Methods Mol Biol. 982:237–263. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang ZY, Yeh MK, Chiang CH, Chen YH and
Lu DW: Erythropoietin protects adult retinal ganglion cells against
NMDA-, trophic factor withdrawal-, and TNF-α-induced damage. PLoS
One. 8:e552912013. View Article : Google Scholar
|
|
9
|
Yoshida S, Nakama T, Ishikawa K, Arima M,
Tachibana T, Nakao S, Sassa Y, Yasuda M, Enaida H, Oshima Y, et al:
Antiangiogenic shift in vitreous after vitrectomy in patients with
proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci.
53:6997–7003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jacobson LO, Goldwasser E, Fried W and
Plzak LF: Studies on erythropoiesis. VII. The role of the kidney in
the production of erythropoietin. Trans Assoc Am Physicians.
70:305–317. 1957.PubMed/NCBI
|
|
11
|
Jelkmann W: Erythropoietin: structure,
control of production, and function. Physiol Rev. 72:449–489. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lundby C, Hellsten Y, Jensen MB, Munch AS
and Pilegaard H: Erythropoietin receptor in human skeletal muscle
and the effects of acute and long-term injections with recombinant
human erythropoietin on the skeletal muscle. J Appl Physiol (1985).
104:1154–1160. 2008. View Article : Google Scholar
|
|
13
|
Anagnostou A, Liu Z, Steiner M, Chin K,
Lee ES, Kessimian N and Noguchi CT: Erythropoietin receptor mRNA
expression in human endothelial cells. Proc Natl Acad Sci USA.
91:3974–3978. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shingo T, Sorokan ST, Shimazaki T and
Weiss S: Erythropoietin regulates the in vitro and in vivo
production of neuronal progenitors by mammalian forebrain neural
stem cells. J Neurosci. 21:9733–9743. 2001.PubMed/NCBI
|
|
15
|
Zhao W, Kitidis C, Fleming MD, Lodish HF
and Ghaffari S: Erythropoietin stimulates phosphorylation and
activation of GATA-1 via the PI3-kinase/AKT signaling pathway.
Blood. 107:907–915. 2006. View Article : Google Scholar
|
|
16
|
Wenker SD, Chamorro ME, Vittori DC and
Nesse AB: Protective action of erythropoietin on neuronal damage
induced by activated microglia. FEBS J. 280:1630–1642. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Olgun Y, Kırkım G, Kolatan E, Kıray M,
Bağrıyanık A, Şerbetçioğlu B, Yılmaz O, Gökmen N, Ellidokuz H,
Kumral A, et al: Otoprotective effect of recombinant
erythro-poietin in a model of newborn hypoxic-ischemic
encephalopathy. Int J Pediatr Otorhinolaryngol. 77:739–746. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stephenson L: Structure and innervation of
the cochlea and organ of corti. J Vis Commun Med. 35:1592012.
View Article : Google Scholar
|
|
19
|
Raphael Y and Altschuler RA: Structure and
innervation of the cochlea. Brain Res Bull. 60:397–422. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vandenbosch R, Chocholova E, Robe PA, Wang
Y, Lambert C, Moonen G, Lallemend F, Malgrange B and Hadjab S: A
role for the canonical nuclear factor-κB pathway in coupling
neurotrophin-induced differential survival of developing spiral
ganglion neurons. Front Cell Neurosci. 7:2422013. View Article : Google Scholar
|
|
21
|
Shepherd RK, Coco A, Epp SB and Crook JM:
Chronic depolarization enhances the trophic effects of
brain-derived neurotrophic factor in rescuing auditory neurons
following a sensorineural hearing loss. J Comp Neurol. 486:145–158.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakaizumi T, Kawamoto K, Minoda R and
Raphael Y: Adenovirus-mediated expression of brain-derived
neurotrophic factor protects spiral ganglion neurons from ototoxic
damage. Audiol Neurootol. 9:135–143. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fukui H, Wong HT, Beyer LA, Case BG,
Swiderski DL, Di Polo A, Ryan AF and Raphael Y: BDNF gene therapy
induces auditory nerve survival and fiber sprouting in deaf Pou4f3
mutant mice. Sci Rep. 2:8382012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cayé-Thomasen P, Wagner N, Lidegaard
Frederiksen B, Asal K and Thomsen J: Erythropoietin and
erythropoietin receptor expression in the guinea pig inner ear.
Hear Res. 203:21–27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lefebvre PP, Van de Water TR, Weber T,
Rogister B and Moonen G: Growth factor interactions in cultures of
dissociated adult acoustic ganglia: neuronotrophic effects. Brain
Res. 567:306–312. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wefstaedt P, Scheper V, Lenarz T and
Stöver T: Brain-derived neurotrophic factor/glial cell line-derived
neurotrophic factor survival effects on auditory neurons are not
limited by dexamethasone. Neuroreport. 16:2011–2014. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu D, Hu L, Zhang Z, Li QY and Wang G:
Construction of human BMP2-IRES-hIF1αmu adenovirus
expression vector and its expression in mesenchymal stem cells. Mol
Med Rep. 7:659–663. 2013. View Article : Google Scholar
|
|
28
|
Shen CF and Kamen A: Hyperosmotic pressure
on HEK 293 cells during the growth phase, but not the production
phase, improves adenovirus production. J Biotechnol. 157:228–236.
2012. View Article : Google Scholar
|
|
29
|
Graham FL and Prevec L: Manipulation of
adenovirus vectors. Methods Mol Biol. 7:109–128. 1991.PubMed/NCBI
|
|
30
|
Struglics A, Larsson S, Pratta MA, Kumar
S, Lark MW and Lohmander LS: Human osteoarthritis synovial fluid
and joint cartilage contain both aggrecanase- and matrix
metalloproteinase-generated aggrecan fragments. Osteoarthritis
Cartilage. 14:101–113. 2006. View Article : Google Scholar
|
|
31
|
Berkingali N, Warnecke A, Gomes P, Paasche
G, Tack J, Lenarz T and Stöver T: Neurite outgrowth on cultured
spiral ganglion neurons induced by erythropoietin. Hear Res.
243:121–126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stachler RJ, Chandrasekhar SS, Archer SM,
Rosenfeld RM, Schwartz SR, Barrs DM, Brown SR, Fife TD, Ford P,
Ganiats TG, et al American Academy of Otolaryngology-Head and Neck
Surgery: Clinical practice guideline: sudden hearing loss.
Otolaryngol head Neck Surg. 146(Suppl 3): S1–S35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mattox DE and Simmons FB: Natural history
of sudden sensori-neural hearing loss. Ann Otol Rhinol Laryngol.
86:463–480. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hellier WP, Wagstaff SA, O'Leary SJ and
Shepherd RK: Functional and morphological response of the stria
vascularis following a sensorineural hearing loss. Hear Res.
172:127–136. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feng H, Yin SH, Tang AZ and Tan SH:
Salicylate initiates apoptosis in the spiral ganglion neuron of
guinea pig cochlea by activating caspase-3. Neurochem Res.
36:1108–1115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Choi MY, Yeo SW and Park Kh: Hearing
restoration in a deaf animal model with intravenous transplantation
of mesenchymal stem cells derived from human umbilical cord blood.
Biochem Biophys Res Commun. 427:629–636. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Needham K, Nayagam BA, Minter RL and
O'Leary SJ: Combined application of brain-derived neurotrophic
factor and neurotrophin-3 and its impact on spiral ganglion neuron
firing properties and hyperpolarization-activated currents. Hear
Res. 291:1–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shibata SB, Budenz CL, Bowling SA, Pfingst
BE and Raphael Y: Nerve maintenance and regeneration in the damaged
cochlea. Hear Res. 281:56–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Conde de Felipe MM, Feijoo Redondo A,
García-Sancho J, Schimmang T and Durán Alonso MB: Cell- and gene-
therapy approaches to inner ear repair. Histol Histopathol.
26:923–940. 2011.PubMed/NCBI
|
|
40
|
Tan CC, Eckardt KU, Firth JD and Ratcliffe
PJ: Feedback modulation of renal and hepatic erythropoietin mRNA in
response to graded anemia and hypoxia. Am J Physiol. 263:F474–F481.
1992.PubMed/NCBI
|
|
41
|
Masuda S, Okano M, Yamagishi K, Nagao M,
Ueda M and Sasaki R: A novel site of erythropoietin production.
Oxygen-dependent production in cultured rat astrocytes. J Biol
Chem. 269:19488–19493. 1994.PubMed/NCBI
|
|
42
|
Liu C, Shen K, Liu Z and Noguchi CT:
Regulated human eryth-ropoietin receptor expression in mouse brain.
J Biol Chem. 272:32395–32400. 1997. View Article : Google Scholar
|
|
43
|
Sasaki R, Masuda S and Nagao M:
Erythropoietin: multiple physiological functions and regulation of
biosynthesis. Biosci Biotechnol Biochem. 64:1775–1793. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rahimi Nedjat M, Wähmann M, Bächli H,
Güresir E, Vatter H, Raabe A, Heimann A, Kempski O and Alessandri
B: Erythropoietin neuroprotection is enhanced by direct cortical
application following subdural blood evacuation in a rat model of
acute subdural hematoma. Neuroscience. 238:125–134. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Undén J, Sjölund C, Länsberg JK, Wieloch
T, Ruscher K and Romner B: Post-ischemic continuous infusion of
erythropoeitin enhances recovery of lost memory function after
global cerebral ischemia in the rat. BMC Neurosci. 14:272013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Monge A, Nagy I, Bonabi S, Schmid S,
Gassmann M and Bodmer D: The effect of erythropoietin on
gentamicin-induced auditory hair cell loss. Laryngoscope.
116:312–316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Andreeva N, Nyamaa A, Haupt H, Gross J and
Mazurek B: Recombinant human erythropoietin prevents
ischemia-induced apoptosis and necrosis in explant cultures of the
rat organ of Corti. Neurosci Lett. 396:86–90. 2006. View Article : Google Scholar
|
|
48
|
Gross J, Moller R, Amarjargal N, Machulik
A, Fuchs J, Ungethüm U, Kuban RJ, Henke W, Haupt H and Mazurek B:
Expression of erythropoietin and angiogenic growth factors
following inner ear injury of newborn rats. Prague Med Rep.
110:310–331. 2009.
|
|
49
|
Monge Naldi A, Gassmann M and Bodmer D:
Erythropoietin but not VEGF has a protective effect on auditory
hair cells in the inner ear. Cell Mol Life Sci. 66:3595–3599. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kaiser O, Paasche G, Stöver T, Ernst S,
Lenarz T, Kral A and Warnecke A: TGF-beta superfamily member
activin A acts with BDNF and erythropoietin to improve survival of
spiral ganglion neurons in vitro. Neuropharmacology. 75:416–425.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Han F, Yu H, Zheng T, Ma X, Zhao X, Li P,
Le L, Su Y and Zheng QY: Otoprotective effects of erythropoietin on
Cdh23erl/erl mice. Neuroscience. 237:1–6. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Frederiksen BL, Cayé-Thomasen P, Lund SP,
Wagner N, Asal K, Olsen NV and Thomsen J: Does erythropoietin
augment noise induced hearing loss. Hear Res. 223:129–137. 2007.
View Article : Google Scholar
|
|
53
|
Buemi M, Cavallaro E, Floccari F, Sturiale
A, Aloisi C, Trimarchi M, Corica F and Frisina N: The pleiotropic
effects of erythropoietin in the central nervous system. J
Neuropathol Exp Neurol. 62:228–236. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Whitlon DS, Grover M, Tristano J, Williams
T and Coulson MT: Culture conditions determine the prevalence of
bipolar and monopolar neurons in cultures of dissociated spiral
ganglion. Neuroscience. 146:833–840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yilmaz M, Chemaly RF, Han XY, Thall PF,
Fox PS, Tarrand JJ, De Lima MJ, Hosing CM, Popat UR, Shpall E, et
al: Adenoviral infections in adult allogeneic hematopoietic SCT
recipients: a single center experience. Bone Marrow Transplant.
48:1218–1223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhao B, Li X, Dai X and Gong N:
Adenovirus-mediated anti-sense extracellular signal-regulated
kinase 2 gene therapy inhibits activation of vascular smooth muscle
cells and angio-genesis, and ameliorates transplant
arteriosclerosis. Transplant Proc. 45:639–642. 2013. View Article : Google Scholar : PubMed/NCBI
|