Introduction
Hepatocellular carcinoma (HCC) ranks as the sixth
most common type of malignancy in the world and is the second
leading cause of cancer-associated mortality (1). The development and progression of
HCC is affected by various factors. Hepatitis virus infection
(hepatitis B or C viruses), alcohol-related liver cirrhosis, and
non-alcoholic steatohepatitis are the major risk factors for
developing HCC (2). Surgery is
the most effective treatment for HCC, although the curative
resection ratio is low and the rate of recurrent metastasis remains
high. Other treatment strategies depend on the tumor stage, and
suitable candidates include radiofrequency ablation, transarterial
chemoembolization and target therapy (3).
Molecule-targeted therapy, which aims to investigate
carcinogenic mechanisms and molecular biology provides a novel
approach in HCC treatment, particularly for patients with advanced
HCC. Sorafenib (SOR), an oral multikinase inhibitor, was the first
approved by the Food and Drug Administration for the treatment of
'unresectable' HCC, and has been considered as a novel
molecular-targeted therapy for HCC as it prolongs survival rates by
2-3 months in cases of advanced and inoperable HCC (4,5).
SOR can inhibit tumor angiogenesis and growth by suppressing the
RAS/RAF/mitogen-activated protein kinase (MAPK) signaling pathway
and other extracellular receptor tyrosine kinases, including
vascular endothelial growth factor receptor (6). However, clinical results have been
disappointing; they have demonstrated that the prognosis of
patients with HCC treated with SOR remained less than satisfactory
(7). Therefore, it is urgent and
necessary to identify the molecular mechanisms underlying
hepatocarcinogenesis, and investigate novel therapeutic approaches
to combat HCC.
Clusterin (CLU), a conserved heterodimeric
disulfide- linked glycoprotein, is widely distributed in tissues
and body fluids, and is crucial in tissue remodeling, reproduction,
lipid transport, complement regulation and apoptosis (8-10).
CLU appears to have two main isoforms, which result from
alternative splicing. The secreted form of the CLU protein (sCLU)
starts as a precursor peptide of ~60 kDa, which represents the
predominant translation product of the CLU gene. Nuclear clusterin
is translated from an alternatively spliced CLU transcript, which
bypasses the endoplasmic reticulum signal peptide (11,12). sCLU has been recognized as an
important contributor to chemoresistance against anticancer agents
(13,14). Our previous studies also
demonstrated that sCLU contributes to oxaliplatin-resistance by
activating the Akt pathway in HCC (15) and that downregulating sCLU enhance
the sensitivity of HCC cells to gemcitabine via activating the
intrinsic apoptotic pathway (16). However, the effect of sCLU on SOR
in the treatment of HCC remains to be fully elucidated. Therefore,
the present study tested the hypothesis that inhibiting sCLU
enhances the sensitivity of HCC cells to SOR, and aimed to
investigate the role and potential downstream pathways of sCLU in
SOR-induced cytotoxicity in human HCC cells.
Materials and methods
Cell culture
The human HepG2, Bel-7402 and Bel-7404 HCC cell
lines were obtained from the American Type Culture Collection
(Rockville, MD, USA). The SMMC-7721 and Huh-7 cell lines were
purchased from the Type Culture Collection Cell Bank, Chinese
Academy of Science (Shanghai, China). The cells were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA)/Dulbecco's modified Eagle's medium (DMEM; HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) containing 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 μg/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in 95% air and 5% CO2.
Reagents and antibodies
SOR tosylate was purchased from Bayer AG (Berlin,
Germany). The SOR was dissolved in 100% dimethyl sulfoxide (DMSO)
to produce a stock solution and was diluted with RPMI-1640/DMEM to
the desired concentrations with a final DMSO concentration of 0.1%
for the in vitro assays. PD98059, a specific inhibitor of
ERK kinase, was obtained from Sigma-Aldrich; Merck Millipore
(Darmstadt, Germany). Primary antibodies targeting B-cell lymphoma
2 (Bcl-2; cat. no. 2872) and Bcl-2-associated X protein (Bax; cat.
no. 2772) were obtained from Cell Signaling Technology, Inc.
(Danvers, MA, USA) and anti-GAPDH antibody (cat. no. ab37168) was
obtained from Abcam (Cambridge, UK). Antibodies targeting sCLU
(cat. no. sc-5289) and anti-ERK1/2 (Phospho-Thr202/Tyr204) (cat.
no. sc-136521) antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA).
Cell viability, apoptosis and western
blot analysis
These methods were as previously described (17,18). Briefly, cell viability assays were
performed using the Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan). Cells (5×103/well)
were seeded onto 96-well plates and incubated in DMEM (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and
100 μg/ml streptomycin (Gibco; Thermo Fisher Scientific,
Inc.) at 37°C for 24 h. Following adaptation, cells were treated
with different concentrations of SOR (0, 2.5, 5, 7.5, 10 and 12.5
μM) for 48 h. Finally, cells were incubated with DMEM
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 μg/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) containing 10 μl CCK-8 solution at 37°C
for 4 h, and the optical density (OD) was measured at 450 nm using
a Spectra Max 190 (Molecular Devices, LLC, Sunnyvale, CA, USA). For
the apoptotic assay, the cells were incubated with 5 μl of
Annexin V and 5 μl of propidium iodide for 15 min at room
temperature in the dark, according to the manufacturer's protocol
(BD Biosciences, San Jose, CA, USA), and were then subjected to
flow cytometry to measure the apoptotic rate (%). For the western
blot analysis, cells were treated with either SOR (0, 2.5, 5, 7.5
and 10 μM) alone, PD98059 (5 μM) alone, or in
combination (10 μM SOR and 5 μM PD98059) for 3-24 h,
and then lysed in cold radioimmunoprecipitation assay lysis buffer
with 1 nM phenylmethylsufonyl fluoride, followed by centrifugation
at 12,000 × g for 10 min at 4°C. The protein concentrations in cell
extracts were determined using a bicinchoninic acid assay (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Total proteins (20-40
μg) were separated using SDS-PAGE (10-12% gel) and
electrotransferred to poly-vinylidene fluoride membranes (EMD
Millipore, Billerica, MA, USA). Membranes were blocked using 5%
skimmed milk in Tris-buffered saline (T5912, pH 7.4; Sigma-Aldrich;
Merck Millipore) containing 0.05% Tween 20 (P1379; Sigma-Aldrich;
Merck Millipore) and were incubated with primary antibodies
overnight at 4°C (1:1,000 for sCLU, Bcl-2, Bax and pERK1/2).
Membranes were then incubated with horseradish
peroxidase-conjugated secondary antibodies (cat. no. 7074/7076;
1:2,000; Cell Signaling Technology, Inc.) for 1 h at room
temperature followed by visualization with enhanced
chemiluminescence immunoblotting detection reagents (Merck
Millipore). The levels of protein band intensities were quantified
using ImageJ software (version 1.6.0_20; National Institutes of
Health, Bethesda, MD, USA).
Knockdown of sCLU using short hairpin
(sh)RNA
Four pMAGic7.1-based shRNA vectors (CLU1, CLU2, CLU3
and CLU4), a scrambled shRNA vector (Table I) and a full-length human CLU cDNA
(NM_203339; GenBank, National Institutes of Health) were provided
by Shanghai Sunbio Biotechnology Co., Ltd. (Shanghai, China)
(15). Transfection of cells was
performed using GenJet DNA in vitro transfection reagent
(SignaGen, Rockville, MD, USA).
 | Table ISequences of oligonucleotides used as
CLU RNAi primers. |
Table I
Sequences of oligonucleotides used as
CLU RNAi primers.
| Construct | Position | Sequence | Target
sequence |
|---|
| CLU1 | Upstream |
5′-CCGGGCTCCAGGAAATGTCCAATttcaagagaATTGGACATTTCCTGGAGCTTTTTTg-3′ |
GCTCCAGGAAATGTCCAAT |
| Downstream |
5′-AATTCAAAAAAGCTCCAGGAAATGTCCAATtctcttgaaATTGGACATTTCCTGGAGC-3′ |
| CLU2 | Upstream |
5′-CCGGGGTTGACCAGGAAATACAAttcaagagaTTGTATTTCCTGGTCAACCTTTTTTg-3′ |
GGTTGACCAGGAAATACAA |
| Downstream |
5′-AATTCAAAAAAGGTTGACCAGGAAATACAAtctcttgaaTTGTATTTCCTGGTCAACC-3′ |
| CLU3 | Upstream |
5′-CCGGGGGATATGATGACAAGGTTctcaagagaAACCTTGTCATCATATCCC
TTTTTTg-3′ |
GGGATATGATGACAAGGTT |
| Downstream |
5′-AATTCAAAAAAGGGATATGATGACAAGGTTtctcttgagAACCTTGTCATCATATCCC-3′ |
| CLU4 | Upstream |
5′-CCGGCAGGGAAGTAAGTACGTCAATctcgagATTGACGTACTTACTTCCCTGTTTTTTg-3′ | CAGGGAAGT
AAGTACGTCAAT |
| Downstream |
5′-AATTCAAAAAACAGGGAAGTAAGTACGTCAATctcgagATTGACGTACTTACTTCCCTG-3′ |
| Scramble | Upstream |
5′-CCGGTTCTCCGAACGTGTCACGTttcaagagaTTCTCCGAACGTGTCACGTTTTTTTg-3′ |
TTCTCCGAACGTGTCACGT |
| Downstream |
5′-AATTCAAAAAATTCTCCGAACGTGTCACGTtctcttgaaTTCTCCGAACGTGTCACGT-3′ |
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed using one-way analysis of variance followed by
Dunnett's test using SPSS software (version 17.0; SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
HCC cells express sCLU and respond to SOR
treatment
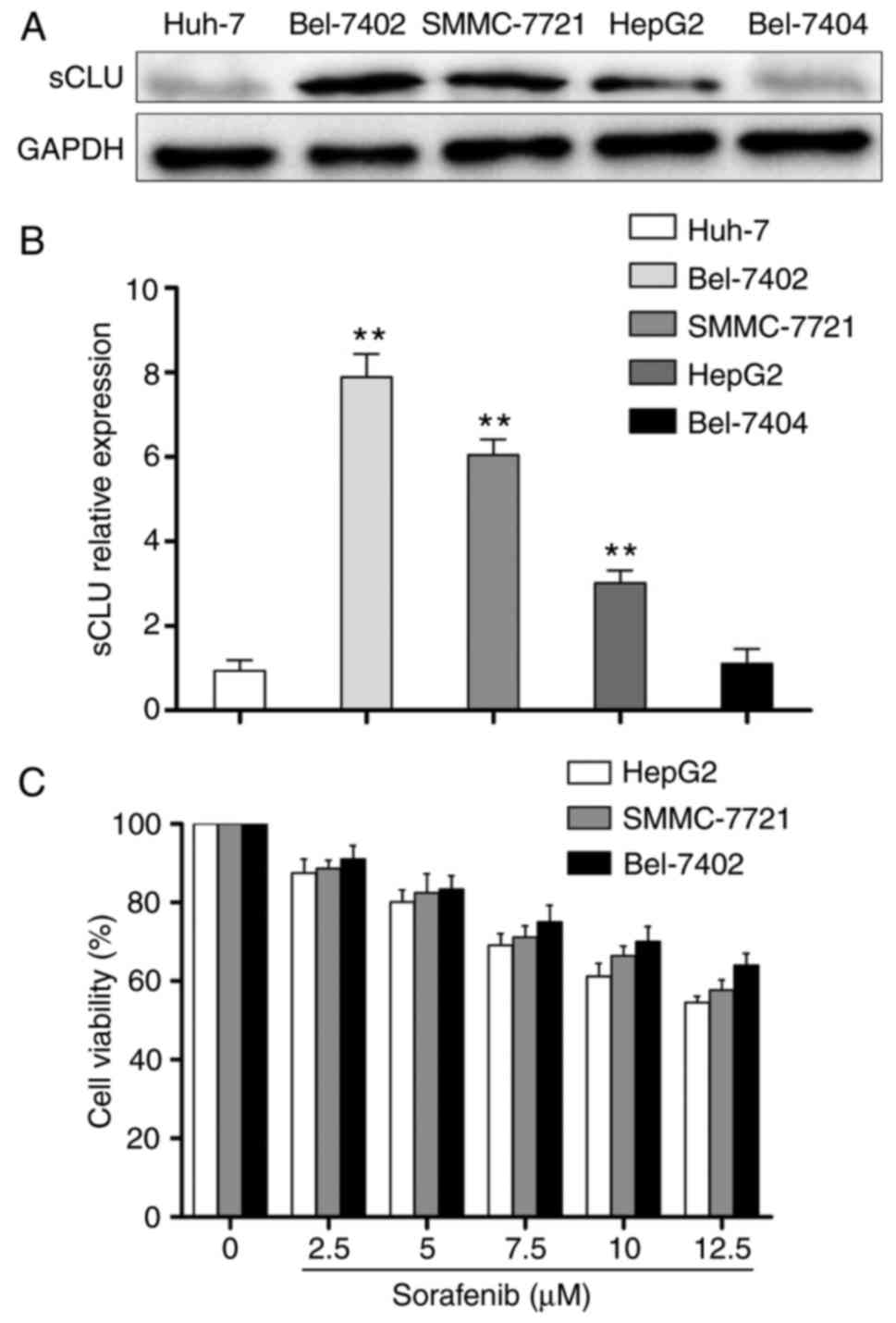
The present study first examined the protein
expression of sCLU using western blot analysis. As shown in
Fig. 1A and B, the HCC cell lines
expressed different protein levels of sCLU, and the Bel-7402,
SMMC-7721 and HepG2 cells exhibited high expression levels of sCLU.
However, the Huh-7 and Bel-7404 cells exhibited weak expression of
sCLU. Therefore, the Bel-7402, SMMC-7721 and HepG2 cells were
selected for examining the inhibitory effects of SOR on the
viability of cells. The cells were treated with different
concentrations of SOR (0-12.5 μM) for 48 h, and the
inhibition of cell proliferation rates was assessed using a CCK-8
assay. The results showed that all three HCC cell lines exhibited
resistance to SOR treatment at different levels, particularly in
the Bel-7402 cells (Fig. 1C).
These results suggested that intrinsic level of sCLU in HCC cells
was correlated with sensitivity to SOR.
SOR treatment upregulates sCLU in HCC
cells in vitro
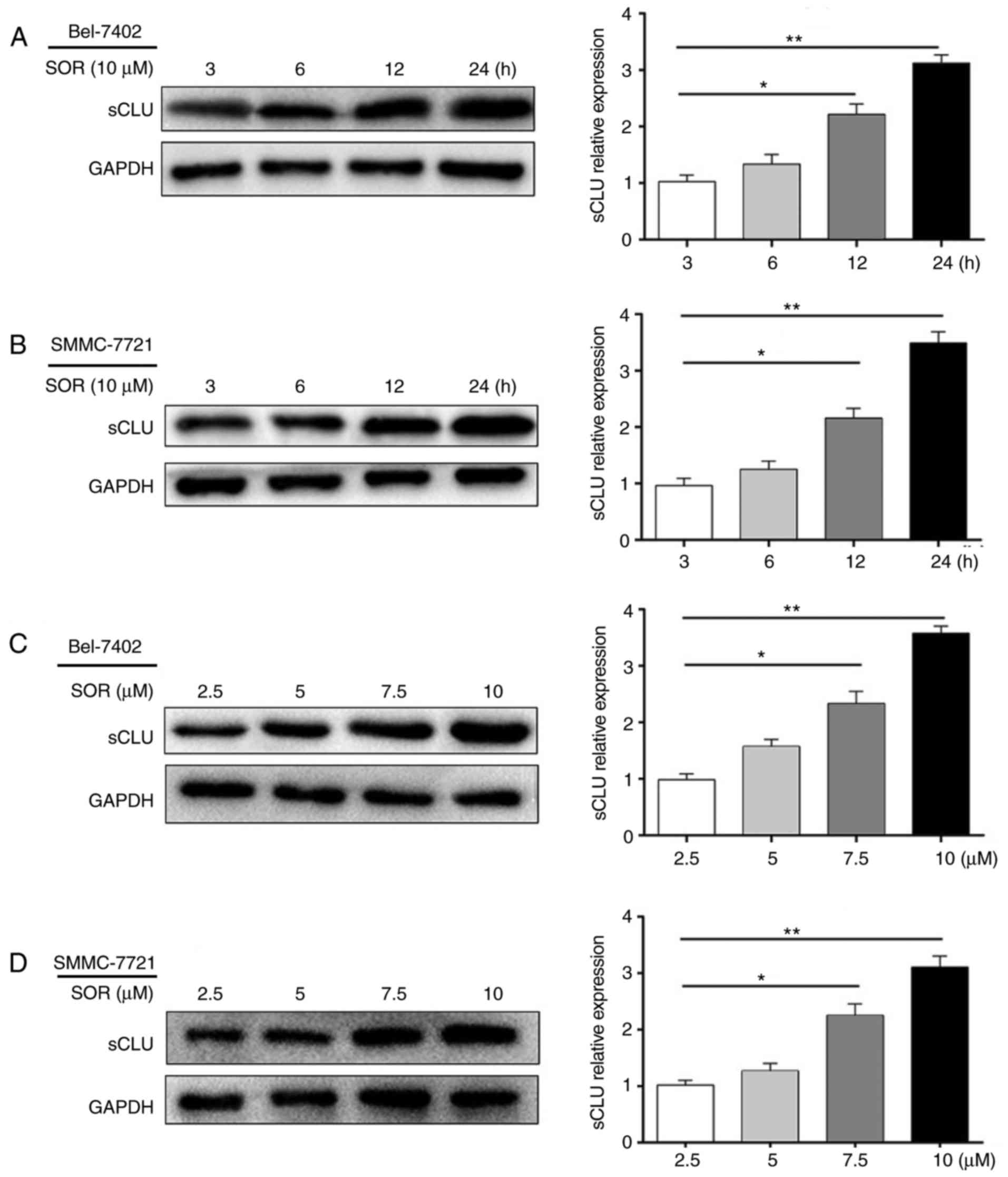
To examine whether the upregulation observed in the
expression of sCLU is associated with SOR-induced resistance,
Bel-7402 and SMMC-7721 cells were exposed to 10 μM of SOR
for 3-24 h, or with concentrations of 2.5-10 μM for 24 h. As
shown in Fig. 2A-D, SOR
significantly increased the expression of sCLU in a time- and
concentration-dependent manner. These data supported the hypothesis
that the expression of sCLU may be altered in response to SOR
treatment.
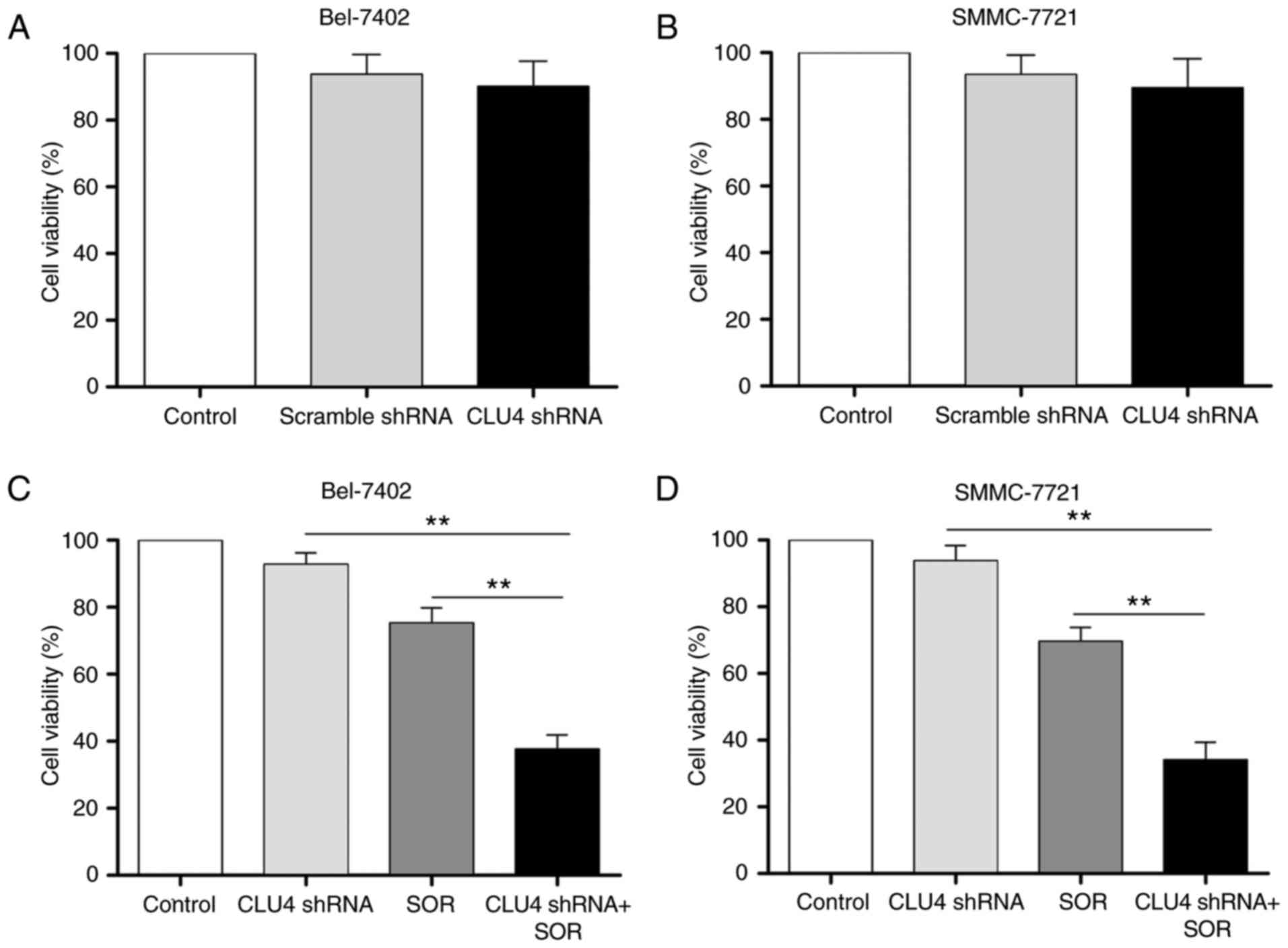
Silencing of sCLU enhances SOR-induced
cytotoxicity in HCC cells in vitro
Chemoresistance is one of the main factors limiting
effective cancer therapy. In the present study, to investigate
whether the suppression of sCLU enhanced SOR-induced cytotoxicity,
the cell viability of HCC cells transfected with CLU shRNA was
determined. In our previous study (15), four pMAGic7.1-based shRNA vectors
(CLU1, CLU2, CLU3 and CLU4) were designed to knock down the
expression of sCLU in HCC cell lines, and the results showed that
CLU4 shRNA exhibited the most marked gene-silencing ability.
Therefore, CLU4 shRNA was used in the present study. As shown in
Fig. 3A and B, Bel-7402 and
SMMC-7721 cell viabilities were only marginally altered by
sCLU-knockdown. However, as shown in Fig. 3C and D, transfection with CLU4
shRNA notably reduced the viability of the Bel-7402 and SMMC-7721
cells treated with SOR. The SOR-induced expression of sCLU
suggested the potential association between the expression of sCLU
and apoptosis in Bel-7402 and SMMC-7721 cells. To clarify this, the
role of sCLU in SOR-induced apoptosis of HCC cells was we examined.
Transfection of the cells with CLU4 shRNA, which reduced the extent
of sCLU, notably enhanced increase of cell apoptosis (Fig. 4A) and expression of Bax, and
downregulated the expression of Bcl-2 (Fig. 4B and C) in the SOR (10
μM)-treated Bel-7402 and SMMC-7721 cells. These data
suggested that the expression of sCLU may be correlated to the
chemoresistance of human HCC cells to SOR.
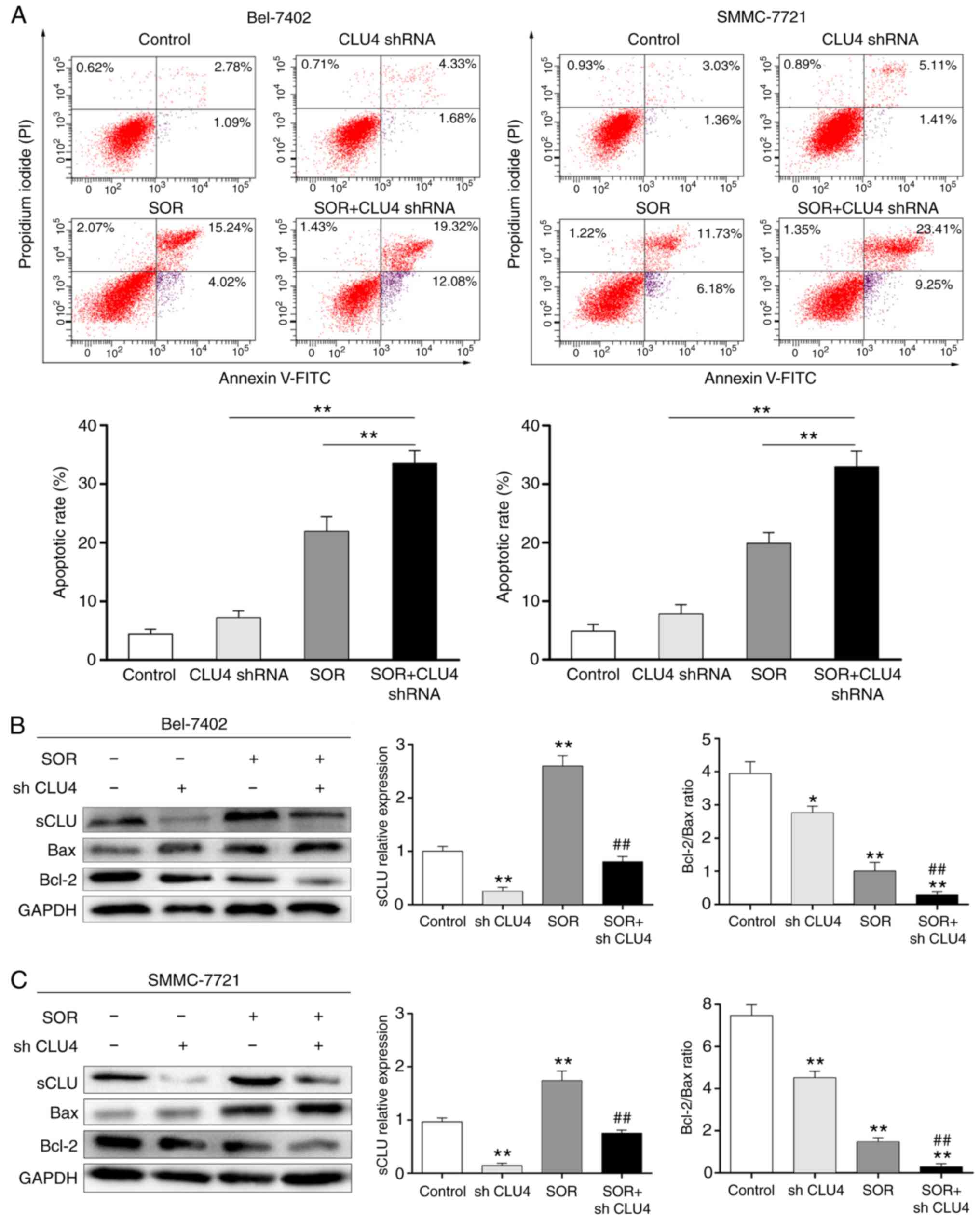 | Figure 4Combination effects of SOR and
sCLU-knockdown on the apoptosis of hepatocellular carcinoma cells
in vitro. (A) Bel-7402 and SMMC-7721 cells only (control),
and the cells transfected with the CLU4 shRNA vector were incubated
in the absence or presence of SOR (10 μM) for 24 h, and then
cell apoptosis was determined using flow cytometry with Annexin
V-FITC and propidium iodide. Quantitative analysis of the total
apoptosis (early and late) population is presented. Data are
presented as the mean ± standard deviation of three independent
experiments. **P<0.01. (B) Bel-7402 and (C) SMMC-7721
cells alone or the cells transfected with CLU4 shRNA vectors were
incubated in the absence or presence of 10 μM SOR for 24 h.
Cell lysates were harvested and analyzed using western blot
analysis with specific antibodies against sCLU, Bax and Bcl-2.
Levels of GAPDH served as a loading control. *P<0.05
and **P<0.01, vs. control; ##P<0.01,
vs. SOR. The data shown are representative of three independent
experiments. sCLU, secretory clusterin; SOR, sorafenib; shRNA,
short hairpin RNA; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated
X factor. |
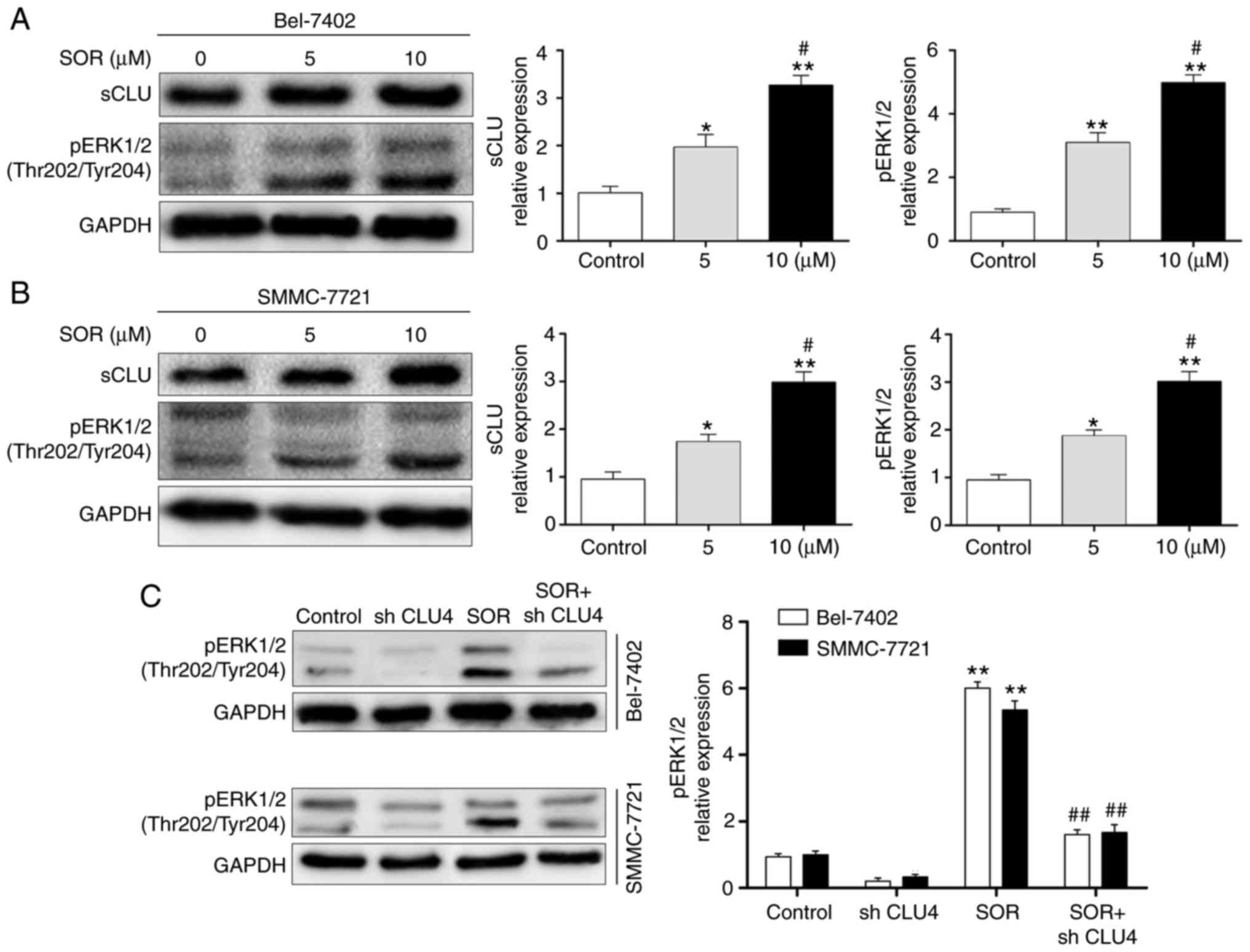
SOR treatment induces the sCLU-dependent
upregulation of pERK1/2 in human HCC cells in vitro
The activation of ERK1/2 leads to the
phosphorylation of several intracellular proteins, and is crucial
in regulating cell growth, proliferation, invasion/migration,
survival and motility (19-22). In the present study, the Bel-7402
and SMMC-7721 cells were treated with different concentrations of
SOR (0 and 10 μM) for 24 h. The protein expression levels
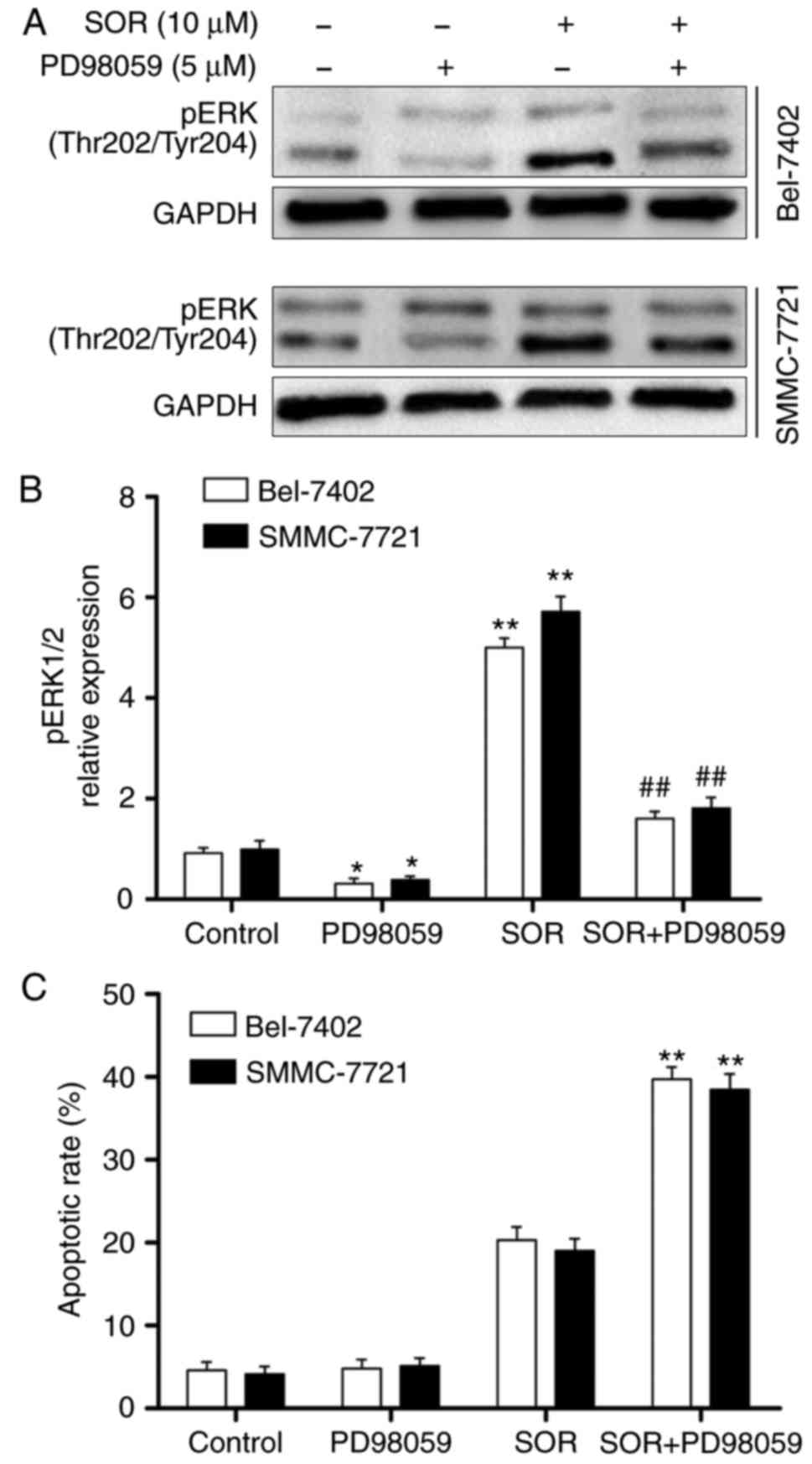
were evaluated using western blot analysis. As shown in Fig. 5A and B, SOR significantly enhanced
the expression of sCLU and pERK1/2 in a concentration-dependent
manner. To investigate whether the protein expression of pERK1/2
was sCLU-dependent, the Bel-7402 and SMMC-7721 cells were
transfected with CLU4 shRNA and then treated with SOR. As
demonstrated in Fig. 5C, the
combination treatment of SOR and CLU4 shRNA significantly decreased
the activation of pERK1/2 in the two cell lines. These data
suggested that SOR induced the sCLU-dependent activation of pERK1/2
in HCC cells.
Inactivated ERK1/2 sensitizes HCC cells
to SOR treatment
The Bel-7402 and SMMC-7721 cells were treated with
PD98059, an ERK-specific inhibitor, and then treated with different
concentrations of SOR (0 and 10 μM) for 24 h. As shown in
Fig. 6A and B, co-administration
with PD98059 markedly abrogated the expression of pERK1/2 in the
SOR-treated Bel-7402 and SMMC-7721 cells. To further examine
whether the ERK1/2 signaling pathway protects HCC cells from
SOR-induced cell death, PD98059 was used to inhibit pERK1/2. As
expected, the Bel-7402 and SMMC-7721 cells were significantly more
sensitive to SOR-induced apoptosis, compared with the cells treated
with SOR in the absence of PD98059 (Fig. 6C). These results revealed that the
inactivation of ERK1/2 is important in the potentiation of SOR
lethality.
Discussion
Systemic chemotherapy is the only method capable of
improving survival rates in patients with advanced HCC. However,
the treatment of HCC using cytotoxic chemotherapy has reached a
therapeutic bottleneck. Therefore, novel treatment modalities to
treat HCC are urgently required. SOR, a multikinase inhibitor of
Raf/MAPK kinase/ERK signaling and the receptor tyrosine kinase, has
been recognized as the standard therapeutic strategy for patients
with advanced HCC. However, the detailed mechanism of tumor
lethality regulated by SOR and the precise mechanism of drug
resistance have not been fully elucidated. In the present study,
the results showed that SOR was capable of upregulating the
expression of sCLU in human HCC cells. sCLU-knockdown using shRNA
(CLU4 shRNA) or the ERK-inhibitor (PD98059) significantly
potentiated the SOR-induced HCC cell apoptosis.
sCLU, also known as testosterone-repressed prostate
message-2, apolipoprotein J, or sulphated glycoprotein-2, is an
ATP-independent and cytoprotective chaperone, which consists of two
chains of ~40 kDa linked by five disulfide bonds (23,24). sCLU is key in stabilizing the
conformations of proteins during cellular stress, suppressing
protein aggregation and precipitation, and protecting cells from
numerous therapeutic factors (25-27). Accumulating evidence has also
revealed that sCLU exerts inhibitory effects against cytotoxic
chemotherapy in several types of cancer (28-31). The constitutive overexpression of
sCLU has been recognized to confer drug-resistance in cancer
therapy, whereas the downregulation of sCLU by RNA interference or
chemical inhibition can enhance chemosensitivity (13,32-35). Our previous studies demonstrated
that targeting sCLU enhances oxaliplatin or gemcitabine lethality
in HCC via activating the Akt pathway or the intrinsic apoptotic
pathway. However, the overexpression of sCLU significantly
abrogates the inhibition of cell growth and the induction of cell
apoptosis by oxaliplatin (15,16). Chemoresistance remains one of the
major factors leading to poor survival rates in patients with HCC.
Identifying mechanisms of chemoresistance are important in the
treatment of HCC. For the present study, it was hypothesized that
sCLU is involved in the resistance of HCC to SOR. The results
demonstrated that the treatment of Bel-7402 and SMMC-7721 cells
with SOR induced the upregulation of sCLU. sCLU-knockdown using
shRNA (CLU4 shRNA) significantly potentiated the cytotoxic and
apoptotic effect of SOR in HCC cells. Taken together, thee results
revealed the importance of sCLU in protecting cancer cells from
SOR-induced apoptosis. Targeting sCLU in the two HCC cell lines
examined distinctly increased their sensitivity to SOR.
The activation of ERK1/2 is important in regulating
various cellular functions, ranging from proliferation to
differentiation (36,37). Several studies have reported that
ERKs can also be activated in response to chemotherapeutic drugs
(38,39). In the present study, the data
demonstrated that pERK1/2 was crucial in the resistance of Bel-7402
and SMMC-7721 cells to SOR. In addition, it was found that the
inhibition of ERK1/2 by the ERK inhibitor (PD98059) significantly
potentiated the chemotherapeutic potential of SOR in HCC cells
in vitro. Several studies have demonstrated that sCLU is
important in regulating the ERK1/2 signaling pathway (40,41). Therefore, the present study
investigated whether the downregulation of sCLU sensitizes Bel-7402
and SMMC-7721 cells to SOR chemotherapy via the ERK1/2 signaling
pathway. The data revealed that the knockdown of sCLU by CLU4 shRNA
sensitized HCC cells to SOR treatment through inhibiting the
activation of pERK1/2. These results suggested that the
downregulation of sCLU sensitized HCC cell lines to SOR via the
pERK1/2-dependent signaling pathway.
In conclusion, SOR may affect the behavior of HCC
cell lines through the upregulation of sCLU, which may be important
in the effect of SOR. The downregulation of sCLU or exposure to the
ERK inhibitor (PD98059) further potentiated SOR-induced HCC cell
apoptosis. These findings indicate a potent strategy for the
treatment against HCC.
Acknowledgments
The authors would like to thank Dr Edward C. Mignot,
Shandong University (Shandong, China) for his linguistic
advice.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Czaja AJ: Current management strategies
for hepatocellular carcinoma. Minerva Gastroenterol Dietol.
59:143–159. 2013.PubMed/NCBI
|
|
4
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar
|
|
6
|
Wilhelm SM, Carter C, Tang L, Wilkie D,
McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al:
BAY 43-9006 exhibits broad spectrum oral antitumor activity and
targets the RAF/MEK/ERK pathway and receptor tyrosine kinases
involved in tumor progression and angiogenesis. Cancer Res.
64:7099–7109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ogasawara S, Chiba T, Ooka Y, Kanogawa N,
Motoyama T, Suzuki E, Tawada A, Kanai F, Yoshikawa M and Yokosuka
O: Efficacy of sorafenib in intermediate-stage hepatocellular
carcinoma patients refractory to transarterial chemoembolization.
Oncology. 87:330–341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamamoto Y, Lin PJ, Beraldi E, Zhang F,
Kawai Y, Leong J, Katsumi H, Fazli L, Fraser R, Cullis PR and
Gleave M: siRNA lipid nanoparticle potently silence clusterin and
delay progression when combined with androgen receptor cotargeting
in enzalutamide resistant prostate cancer. Clin Cancer Res.
21:4845–4855. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rubenstein M, Tsui P and Guinan P:
Treatment of prostate and breast tumors employing mono- and
bi-specific antisense oligonucleotides targeting apoptosis
inhibitory proteins clusterin and bcl-2. Med Oncol. 27:592–599.
2010. View Article : Google Scholar
|
|
10
|
Rull A, Martinez-Bujidos M, Perez-Cuellar
M, Pérez A, Ordóñez-Llanos J and Sánchez-Quesada JL: Increased
concentration of clusterin/apolipoprotein J (apoJ) in hyperlipemic
serum is paradoxically associated with decreased apoJ content in
lipoproteins. Atherosclerosis. 241:463–470. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Trougakos IP, Djeu JY, Gonos ES and
Boothman DA: Advances and challenges in basic and translational
research on clusterin. Cancer Res. 69:403–406. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Trougakos IP, Lourda M, Antonelou MH,
Kletsas D, Gorgoulis VG, Papassideri IS, Zou Y, Margaritis LH,
Boothman DA and Gonos ES: Intracellular clusterin inhibits
mitochondrial apoptosis by suppressing p53-activating stress
signals and stabilizing the cytosolic Ku70-Bax protein complex.
Clin Cancer Res. 15:48–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang B, Zhang K, Liu Z, Hao F, Wang M, Li
X, Yin Z and Liang H: Secreted clusterin gene silencing enhances
chemosensitivity of a549 cells to cisplatin through AKT and ERK1/2
pathways in vitro. Cell Physiol Biochem. 33:1162–1175. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koltai T: Clusterin: A key player in
cancer chemoresistance and its inhibition. Onco Targets Ther.
7:447–456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiu P, Dong X, Dong X, Xu Z, Zhu H, Liu F,
Wei Z, Zhai B, Kanwar JR, Jiang H, et al: Secretory clusterin
contributes to oxaliplatin resistance by activating Akt pathway in
hepatocellular carcinoma. Cancer Sci. 104:375–382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiu P, Xu Z, Liu F, Li Z, Li T, Zou F, Sun
X and Li J: Down-regulating sCLU enhances the sensitivity of
hepatocellular carcinoma cells to gemcitabine by activating the
intrinsic apoptosis pathway. Dig Dis Sci. 59:1798–1809. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong J, Xiu P, Dong X, Wang F, Wei H,
Wang X, Xu Z, Liu F, Li T, Wang Y and Li J: Meloxicam combined with
sorafenib synergistically inhibits tumor growth of human
hepatocellular carcinoma cells via ER stress-related apoptosis.
Oncol Rep. 34:2142–2150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dong X, Li R, Xiu P, Dong X, Xu Z, Zhai B,
Liu F, Jiang H, Sun X, Li J and Qiao H: Meloxicam executes its
antitumor effects against hepatocellular carcinoma in
COX-2-dependent and -independent pathways. PLoS One. 9:e928642014.
View Article : Google Scholar
|
|
19
|
Zhen Y, Zhang W, Liu C, He J, Lu Y, Guo R,
Feng J, Zhang Y and Chen J: Exogenous hydrogen sulfide promotes C6
glioma cell growth through activation of the p38 MAPK/ERK1/2-COX-2
pathways. Oncol Rep. 34:2413–2422. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li W, Liu Z, Zhao C and Zhai L: Binding of
MMP-9-degraded fibronectin to β6 integrin promotes invasion via the
FAK-Src-related Erk1/2 and PI3K/Akt/Smad-1/5/8 pathways in breast
cancer. Oncol Rep. 34:1345–1352. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng X, Zhou Y, Tian H, Yang G, Li C, Geng
Y, Wu S and Wu W: Sulforaphane inhibits invasion by phosphorylating
ERK1/2 to regulate E-cadherin and CD44v6 in human prostate cancer
DU145 cells. Oncol Rep. 34:1565–1572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng X, Li F, Wang P, Jia S, Sun L and Huo
H: Apelin-13 induces MCF-7 cell proliferation and invasion via
phosphorylation of ERK1/2. Int J Mol Med. 36:733–738. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shannan B, Seifert M, Leskov K, Willis J,
Boothman D, Tilgen W and Reichrath J: Challenge and promise: Roles
for clusterin in pathogenesis, progression and therapy of cancer.
Cell Death Differ. 13:12–19. 2006. View Article : Google Scholar
|
|
24
|
Sowery RD, Hadaschik BA, So AI, Zoubeidi
A, Fazli L, Hurtado-Coll A and Gleave ME: Clusterin knockdown using
the antisense oligonucleotide OGX-011 re-sensitizes
docetaxel-refractory prostate cancer PC-3 cells to chemotherapy.
BJU Int. 102:389–397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Muhammad LA and Saad F: The role of
clusterin in prostate cancer: Treatment resistance and potential as
a therapeutic target. Expert Rev Anticancer Ther. 15:1049–1061.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song HB, Jun HO and Kim JH, Yu YS, Kim KW,
Min BH and Kim JH: Anti-apoptotic effect of clusterin on
cisplatin-induced cell death of retinoblastoma cells. Oncol Rep.
30:2713–2718. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang C, Jin G, Jin H, Wang N, Luo Q, Zhang
Y, Gao D, Jiang K, Gu D, Shen Q, et al: Clusterin facilitates
metastasis by EIF3I/Akt/MMP13 signaling in hepatocellular
carcinoma. Oncotarget. 6:2903–2916. 2015.PubMed/NCBI
|
|
28
|
Xiu P, Dong XF, Li XP and Li J: Clusterin:
Review of research progress and looking ahead to direction in
hepatocellular carcinoma. World J Gastroenterol. 21:8262–8270.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Niu ZH, Wang Y, Chun B, Li CX and Wu L:
Secretory clusterin (sCLU) overexpression is associated with
resistance to preoperative neoadjuvant chemotherapy in primary
breast cancer. Eur Rev Med Pharmacol Sci. 17:1337–1344.
2013.PubMed/NCBI
|
|
30
|
Hassan MK, Watari H, Han Y, Mitamura T,
Hosaka M, Wang L, Tanaka S and Sakuragi N: Clusterin is a potential
molecular predictor for ovarian cancer patient's survival:
Targeting clusterin improves response to paclitaxel. J Exp Clin
Cancer Res. 30:1132011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zheng W, Sai W, Yao M, Gu H, Yao Y, Qian Q
and Yao D: Silencing clusterin gene transcription on effects of
multidrug resistance reversing of human hepatoma HepG2/ADM cells.
Tumour Biol. 36:3995–4003. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang Y, Liu F, Zheng C, Sun S and Jiang Y:
Knockdown of clusterin sensitizes pancreatic cancer cells to
gemcitabine chemotherapy by ERK1/2 inactivation. J Exp Clin Cancer
Res. 31:732012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma G, Cai H, Gao L, Wang M and Wang H:
sCLU regulates cisplatin chemosensitivity of lung cancer cells in
vivo. World J Surg Oncol. 13:802015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang B, Liu ZM, Hao FG and Wang M:
siRNA-directed clusterin silencing promotes cisplatin antitumor
activity in human non-small cell lung cancer xenografts in
immunodeficient mice. Eur Rev Med Pharmacol Sci. 18:1595–1601.
2014.PubMed/NCBI
|
|
35
|
Huang H, Wang L, Li M, Wang X and Zhang L:
Secreted clusterin (sCLU) regulates cell proliferation and
chemosensitivity to cisplatin by modulating ERK1/2 signals in human
osteosarcoma cells. World J Surg Oncol. 12:2552014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dong F, Tian H, Yan S, Li L, Dong X, Wang
F, Li J, Li C, Cao Z, Liu X and Liu J: Dihydroartemisinin inhibits
endothelial cell proliferation through the suppression of the ERK
signaling pathway. Int J Mol Med. 35:1381–1387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Scherzad A, Steber M, Gehrke T, Rak K,
Froelich K, Schendzielorz P, Hagen R, Kleinsasser N and Hackenberg
S: Human mesenchymal stem cells enhance cancer cell proliferation
via IL-6 secretion and activation of ERK1/2. Int J Oncol.
47:391–397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu SQ, Xu CY, Qin MB, Tan L, Zhuge CF,
Mao YB, Lai MY and Huang JA: Ginkgo biloba extract enhances
chemotherapy sensitivity and reverses chemoresistance through
suppression of the KSR1-mediated ERK1/2 pathway in gastric cancer
cells. Oncol Rep. 33:2871–2882. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang LJ, Han SX, Bai E, Zhou X, Li M, Jing
GH, Zhao J, Yang AG and Zhu Q: Dose-dependent effect of tamoxifen
in tamoxifen-resistant breast cancer cells via stimulation by the
ERK1/2 and AKT signaling pathways. Oncol Rep. 29:1563–1569. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chou TY, Chen WC, Lee AC, Hung SM, Shih NY
and Chen MY: Clusterin silencing in human lung adenocarcinoma cells
induces a mesenchymal-to-epithelial transition through modulating
the ERK/Slug pathway. Cell Signal. 21:704–711. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shim YJ, Kang BH, Jeon HS, Park IS, Lee
KU, Lee IK, Park GH, Lee KM, Schedin P and Min BH: Clusterin
induces matrix metal-loproteinase-9 expression via ERK1/2 and
PI3K/Akt/NF-κB pathways in monocytes/macrophages. J Leukoc Biol.
90:761–769. 2011. View Article : Google Scholar : PubMed/NCBI
|