Introduction
Aerobically growing bacteria utilize oxygen species
for energy metabolism. Reactive oxygen species (ROS), including
superoxide anions (O2−) and hydrogen peroxide
(H2O2), are generated during this process
(1,2). These ROS impair cellular functions
by damaging DNA, proteins, and lipid membranes. Aerobic bacteria
have evolved oxidative stress responses, including enzymes to
scavenge ROS (catalases, superoxide dismutases and alkyl
hydroperoxide reductases) (3,4)
and the rec system for DNA repair (5). However, oxidative stress that
overwhelms the capacity of these stress response systems kills the
bacteria. Therefore, oxidative antimicrobials are widely used for
sanitation purposes in healthcare facilities.
A compromised DNA repair system has been reported to
render microbes vulnerable to oxidative stress (3,5).
In the present study, L-histidine suppressed the proliferation of
recA-deficient Escherichia coli laboratory strains
under aerobic conditions, but not anaerobic conditions. This
indicated that L-histidine enhances the oxidative DNA damage of
E. coli cells. L-histidine has been reported to enhance the
genotoxicity of hydrogen peroxide (H2O2) in
mammalian cells by increasing ROS production (6–9).
H2O2 is produced in hostile
eukaryotic hosts (10) or from
resident microbes (11), and
eradicates pathogenic bacteria or competitors in the same habitat,
respectively. H2O2 is a non-polar molecule
that penetrates lipid membranes and oxidizes intracellular
molecules (12,13). H2O2
generates hydroxyl radicals (OH·) through reacting with iron in a
process known as the Fenton reaction (14). OH· is a powerful oxidant that
reacts with a wide range of organic substances (1).
In previous decades, the microbicidal activity of
H2O2 has been utilized for wastewater
disinfection and the sanitation of hospital environments. For
example, H2O2 vapor is used for bedroom
sanitation in hospitals, and treatment of rooms occupied by
patients colonized with multidrug-resistant microorganisms (MDROs)
reduces the risk of carriage of these MDROs by 64% (15). In addition, a combination of
H2O2 and low concentrations of anionic and/or
nonionic surfactants has been commercialized as a disinfectant for
healthcare settings (16)
H2O2 is recognized as an environment-friendly
reagent that does not leave unfavorable environmental effects
(17–19).
The main disinfection mechanism of
H2O2 involves hydroxyl or hydroperoxyl
radical production (20). The
combination of H2O2 and metallic ions,
including ferrous iron (Fe2+) or copper
(Cu2+) enhances the disinfection potential via the
Fenton reaction, or Fenton-like reactions (21). H2O2 combined
with copper or silver synergistically enhances the disinfection
efficiency of wastewater (22,23). In addition, modifications of the
disinfection efficacy of H2O2 through
combination with other components have been reported, including
surfactant (16), chlorhexidine
(24), iodine (25), sodium bicarbonate (26), hypothiocyanate (27), rifampicin (28), organic acids (29), neucopropine (30), and UV-irradiation (31). In the present study, L-histidine
was also demonstrated to augment the bactericidal activity of
H2O2 against Gram-negative bacteria.
Materials and methods
Reagents
H2O2 (50% w/v in
H2O) and L-histidine were purchased from Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany) and Wako Chemicals GmbH (Neuss,
Germany), respectively.
Bacterial strains and culture
conditions
Laboratory strains of E. coli MG1655
(American Type Culture Collection, Manassas, VA, USA), JM109 (New
England BioLabs, Inc., Ipswich, MA, USA), and DH5α (Thermo Fisher
Scientific, Inc., Waltham, MA, USA), Pseudomonas aeruginosa
PAO1 (Japan Collection of Microorganisms, Tsukuba, Ibaraki, Japan)
and clinical isolates of multidrug resistant P. aeruginosa
(MDRP) TUP1 from human blood (Tokushima University Hospital,
Tokushima, Japan), methicillin-resistant Staphylococcus
aureus (MRSA) TUM1 from human sputum (Tokushima University
Hospital), extended-spectrum β-lactamase (ESBL)-producing E.
coli KUM1 (corresponding to the isolate E6 reported by Uemura
et al (Kagawa University Hospital, Miki, Japan) (32), and vancomycin-resistant
Enterococcus faecium (VRE) FN1 (Dr Koichi Tanimoto, Gunma
University, Maebashi, Japan) (33) were used in the present study.
Glycerol stocks (−70°C) of these strains were streaked onto brain
heart infusion (BHI; Eiken Chemical Co., Ltd., Tokyo, Japan) agar
plates, which were incubated at 37°C for 24 h in the dark. A single
colony from the BHI agar plate was inoculated into 3 ml BHI broth
and incubated aerobically at 37°C for 16 h. Then, 1 ml of the
culture was centrifuged (10,000 × g, 5 min, 4°C), and the collected
cells were washed once with 2.5 ml phosphate-buffered saline (PBS;
pH 7.4). The bacterial cells were then suspended in 4 ml PBS and
used as the inoculum in the bactericidal test. Ten-fold serial
dilutions of bacterial suspension were prepared and 0.1 ml of this
suspension was spread on BHI agar plates to enumerate the viable
cell counts in the suspension.
Monitoring of E. coli cell proliferation
in the presence of L-histidine
The E. coli K12 strain MG1655 and the
recA-deficient strains JM109 and DH5α were cultured in 3 ml
BHI with shaking at 37°C overnight in the dark. Each overnight
culture (30 μl) was used to inoculate two sets of 3 ml BHI
supplemented with or without 1% L-histidine. One of the tubes was
grown aerobically with shaking at 37°C in the dark, and the other
was grown anaerobically at 37°C in the dark, using an anaerobic
chamber conditioned with mixed gas (N2, 80%;
H2, 10%; CO2, 10%). The proliferation of
E. coli strains was monitored every 2 h by checking the
optical density at 600 nm (OD600) until 10 h following
inoculation.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of recA genes
Total RNA was extracted using the hot-phenol method
(34) from the
mid-logarithmic-phase cultures (OD660, 0.4–0.6) of E.
coli MG1655 strains with or without exposure to 10 mM
H2O2 (30 min at 37°C) and/or 1% L-histidine
in the dark. The RNA was further purified using the RNeasy CleanUp
kit (Qiagen GmbH, Hilden, Germany) and was treated with TURBO
DNA-free (Ambion; Thermo Fisher Scientific, Inc.) to remove
contaminating DNA. Total RNA (400 ng) was reverse transcribed using
a PrimeScript RT reagent kit (Takara Bio, Inc., Otsu, Japan) with
random hexamers at 37°C for 15 min. Reverse transcription was
terminated by heating the mixture at 85°C for 5 sec. The cDNA
products were subsequently amplified using SYBR Premix Ex Taq II
(Takara Bio, Inc.) under the following conditions: Preheating at
95°C for 10 sec, followed by 40 cycles of 95°C for 5 sec and 60°C
for 34 sec in a StepOne Plus system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The oligonucleotide primers used for
monitoring recA expression were as follows: Forward,
5′-GTGAAGAACAAAATCGCTGC-3′ and reverse, 5′-TCTGCTACGCCTTCGCTAT-3′
(35). All samples were run in
triplicate. Threshold cycle values were normalized to the levels of
rrnH transcripts, and changes were calculated using the
2−ΔΔCq method (36).
The PCR primers used for rrnH were as follows: Forward,
5′-AGTCGAACGGTAACAGGAAGA-3′ and reverse,
5′-GCAATATTCCCCACTGCTG-3′.
Assessment of DNA damage
E. coli MG1655 were statically incubated in
10 ml BHI for 16 h at 37°C in the dark. The culture was centrifuged
(10,000 × g, 5 min, 4°C) and suspended in 10 ml PBS (pH 7.4) or PBS
containing 1% L-histidine. H2O2 was added to
the tubes (1, 10 or 100 mM) and incubated for 30 min at 37°C in the
dark. Distilled water was used as a control in place of
H2O2. Following incubation, chromosomal DNA
fragmentation of the treated E. coli cells was assessed by
pulsed-field gel electrophoresis (PFGE) as follows: Treated cells
were embedded into 1% agarose to make sample plugs. The E.
coli cells were then lysed within the agarose plug using the
CHEF Bacterial DNA Genome kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) according to the manufacturer's protocol.
Genomic DNA was prepared in situ in agarose blocks. The DNA
was resolved by PFGE in 1% agarose (SeaKem GTG agarose, Lonza Japan
Ltd., Tokyo, Japan) in 0.5x Tris-borate-EDTA buffer with a CHEF-DR
II system (Bio-Rad Laboratories, Inc.) at 14°C under 6 V/cm of
electric field. Pulse time was set to 1–30 sec for the first 17 h,
and changed to 5–9 sec for the last 6 h. Following electrophoresis,
the gel was stained with 0.5 μg/ml ethidium bromide for 20
min at room temperature and washed with distilled water. The length
of the smear was compared between samples treated with distilled
water or H2O2 (1, 10 or 100 mM) with or
without 1% L-histidine.
Measurement of ROS
Intracellular ROS levels were measured using the
OxiSelect Intracellular ROS assay kit (Cell Biolabs, Inc., San
Diego, CA, USA). E. coli MG1655 was cultured aerobically in
3 ml BHI to the mid-logarithmic phase and collected by
centrifugation (10,000 × g, 5 min, 4°C). The cells were washed once
with PBS and resuspended in PBS containing ROS substrate at 37°C
for 60 min. The cells absorbing ROS substrate were collected,
washed once with PBS and resuspended in PBS containing 2%
L-histidine. An equal volume of 20 mM H2O2
was added to the cell suspension and incubated for 10 min at 30°C.
Following incubation, 2′,7′-dichlorodihydrofluorescein (DCF)
generated by the oxidative degradation of the substrate was
monitored at 480 nm excitation/530 nm emission.
Free radical analysis using electron
paramagnetic resonance (EPR) spectroscopy
Generation of free radicals in E. coli MG1655
following exposure to H2O2 and L-histidine
was confirmed using an EPR-spin trapping method at room
temperature. The spin trapping reagent,
5,5-dimethyl-1-pyrrorine-N-oxide (DMPO), was obtained from Labotec
Co., Ltd. (Tokyo, Japan). The E. coli MG1655 cell suspension
[0.1 ml; 5.6×108 colony forming units (CFU)] containing
2% L-histidine and 500 mM DMPO was incubated for 5 min at room
temperature, and 0.1 ml 200 mM H2O2 was then
added. After 5 min, the mixture was centrifuged at 2,000 × g for 1
min at room temperature and the cell pellets were resuspended in
0.1 ml PBS. EPR measurement was performed at room temperature. The
sample was transferred to three sections of glass capillaries (10
μl; Drummond Scientific Co., Inc., Broomall, PA, USA) and
set into the EPR cavity for the measurements. A Bruker EMXPlus EPR
spectrometer (Bruker Corporation, Billerica, MA, USA) with an
X-band cavity (ER 4103TM) was used to collect the EPR signal of the
DMPO spin adducts. The typical instrumental conditions were as
follows: 10 mW microwave power, 2.0-Gauss modulation amplitude,
0.08-sec time constant, 120-sec scan time, and 100-Gauss scan
range. Hyperfine coupling constants and radical concentrations were
obtained using the computer program Winsim (37).
H2O2 bactericidal
test
The final concentrations of
H2O2 used for the bactericidal test against
Gram-negative (MDRP TUP1 and ESBL-producing E. coli KUM1)
and Gram-positive (MRSA TUM1 and VRE FN1) bacteria were 100 and 200
mM, respectively. Each bacterial suspension (0.25 ml) was added to
0.5 ml of 2% histidine or sterilized distilled water. The
suspension was mixed with 0.25 ml H2O2 (400
or 800 mM) and incubated at 25°C for 15 min. Following incubation,
the mixture (15 μl) was transferred to 1.5 ml catalase
solution (10 mg/ml; Sigma-Aldrich; Merck KGaA) to inactivate
H2O2. Serial 10-fold dilutions of the mixture
were prepared and 0.1 ml of each dilution was spread onto BHI
plates in triplicate. Following incubation of the plates at 37°C
for 24 h, the number of surviving cells was calculated from the
colony counts grown on the BHI plates.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analysis of the data was performed with StatFlex 6.0
(Artech Co., Ltd., Osaka, Japan) using one-way analysis of variance
to compare the means of all groups, followed by Tukey's test to
compare the means of two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
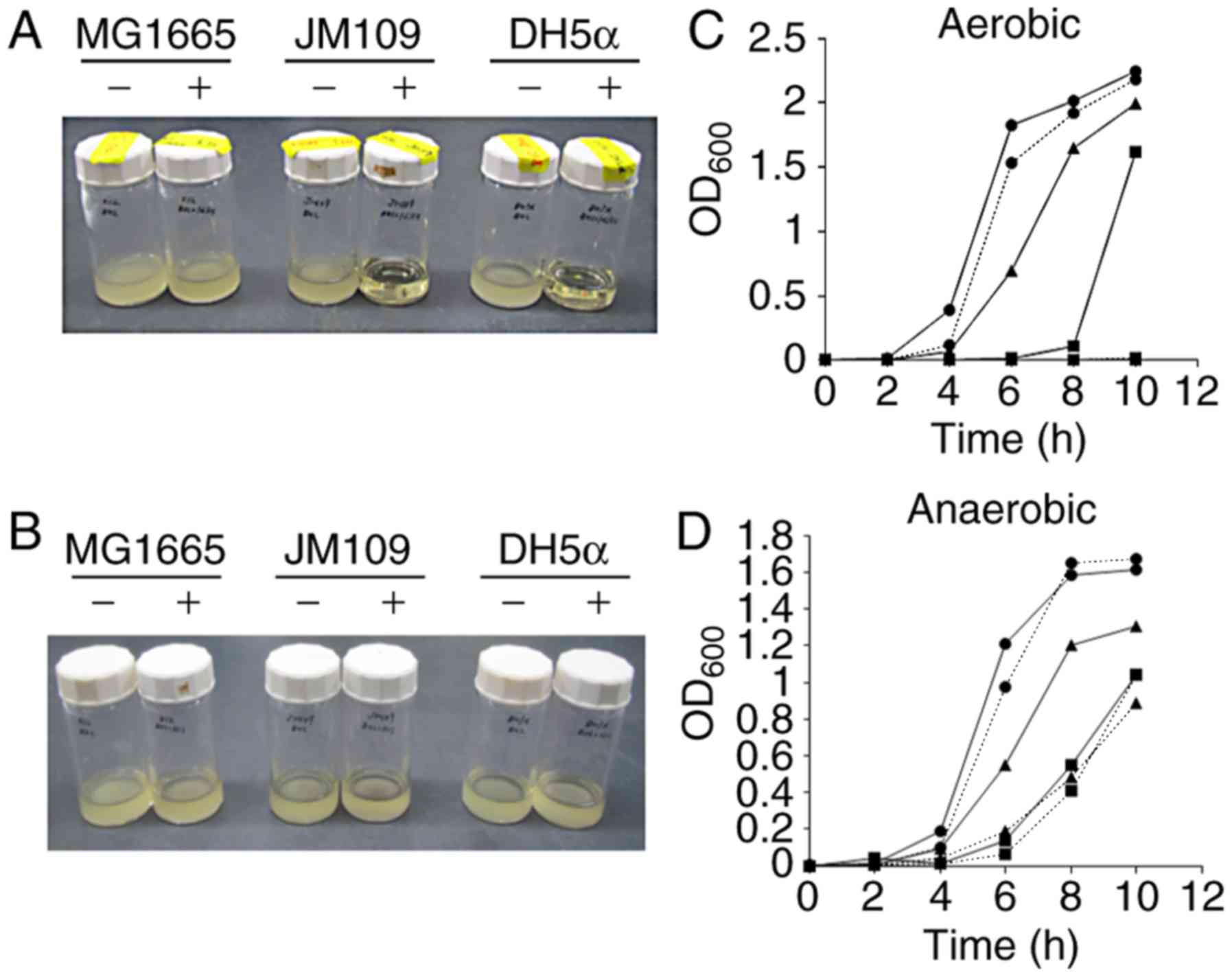
Effect of L-histidine on the aerobic
proliferation of recA-deficient E. coli strains
The MG1655 E. coli strain and the
recA-deficient strains JM109 and DH5α were cultured in BHI
broth supplemented with 1% L-histidine. As presented in Fig. 1, E. coli MG1655 grew well
in the presence of 1% L-histidine under aerobic and anaerobic
conditions. In contrast, JM109 and DH5α did not grow under aerobic
conditions in the presence of 1% L-histidine, while they were able
to grow under anaerobic conditions in the presence of this amino
acid. As RecA plays a crucial role in DNA repair, these results
indicated that L-histidine may induce oxidative DNA damage in the
presence of oxygen, resulting in a lethal effect on the bacterial
strains with impairments in the DNA repair system.
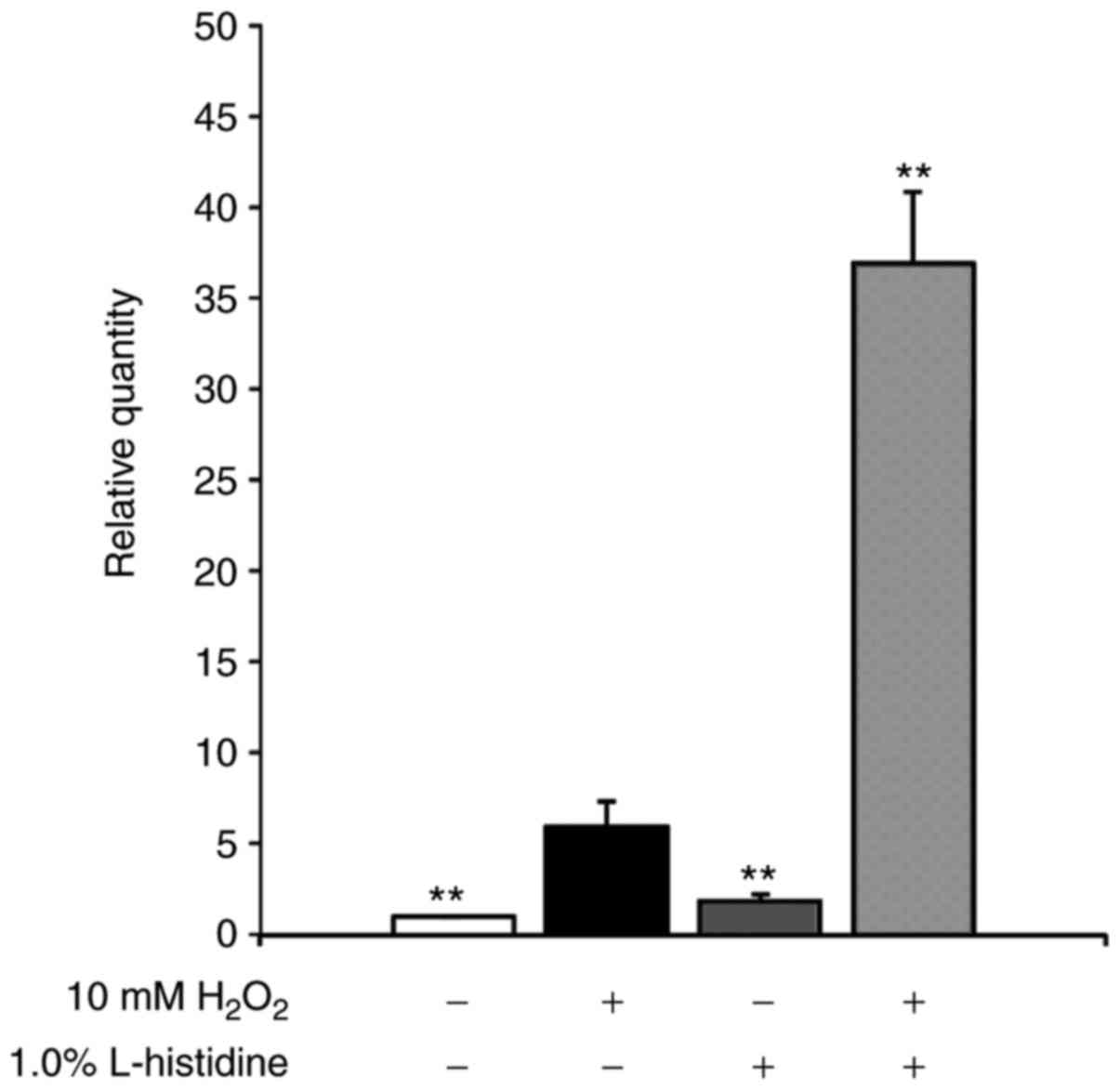
SOS response in E. coli induced by
H2O2
DNA damage induces the expression of SOS stress
response genes in order to repair the injury. The recA gene
is a representative SOS stress response gene. To assess the level
of DNA damage induced by H2O2 in the presence
of L-histidine, recA gene expression in aerobically grown,
mid-logarithmic phase E. coli MG1655 cells was measured by
RT-qPCR (Fig. 2). The relative
recA expression levels following treatment with 10 mM
H2O2 increased ~5-fold compared with the
distilled water control. The combination treatment with 10 mM
H2O2 and 1% L-histidine induced a ~7-fold
increase in recA gene expression compared with 10 mM
H2O2 alone. Treatment with 1% L-histidine
alone did not increase recA expression.
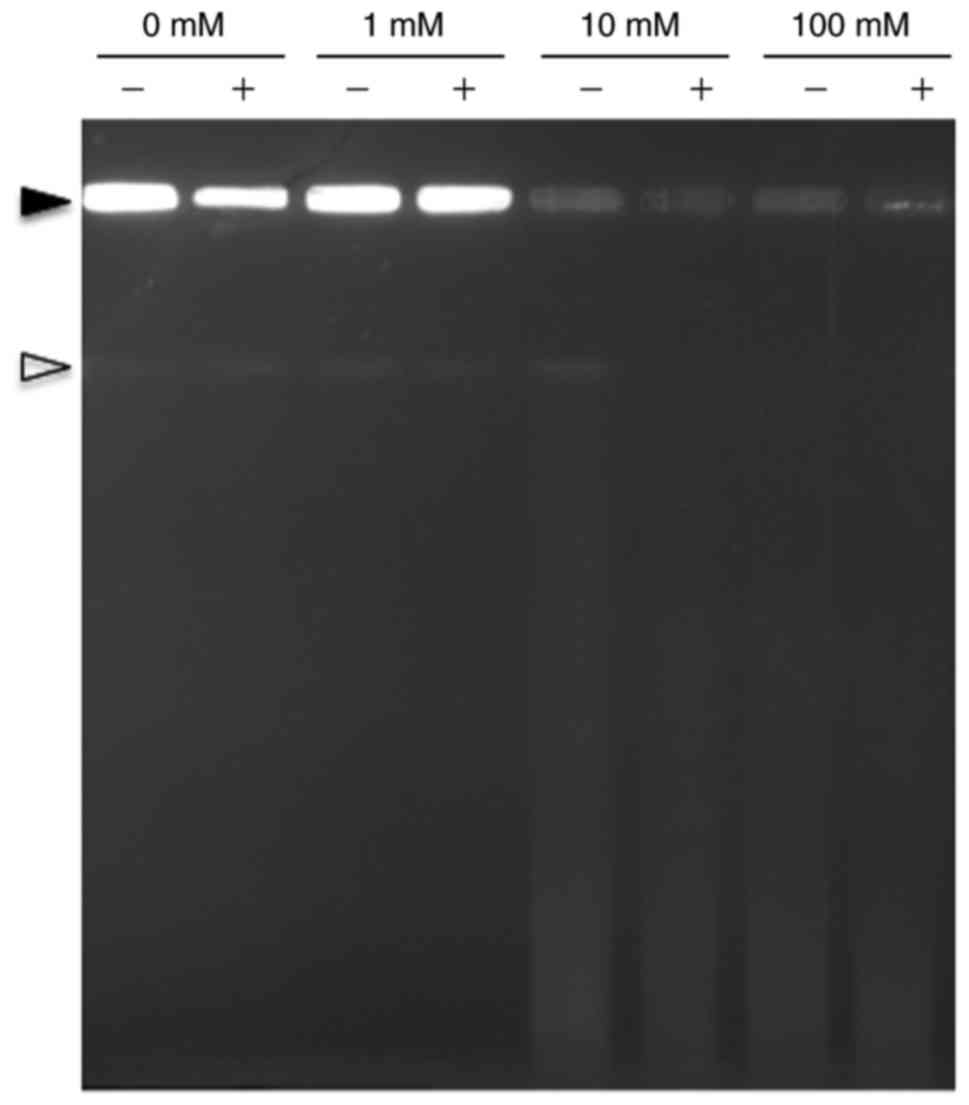
Assessment of DNA damage following
exposure to H2O2
DNA damage induced by H2O2 and
by the H2O2/L-histidine combination in
statically grown E. coli MG1655 cells was assessed by PFGE.
As presented in Fig. 3, treatment
of E. coli MG1655 cells with 1% L-histidine alone did not
induce DNA damage. DNA degradation was not observed in the E.
coli cells treated with 1 mM H2O2
regardless of the presence of L-histidine. In contrast, a clear
difference in DNA degradation appeared when >10 mM
H2O2 was used, as E. coli DNA was
degraded more extensively when 1% L-histidine was combined with 10
or 100 mM H2O2 compared with
H2O2 treatment alone.
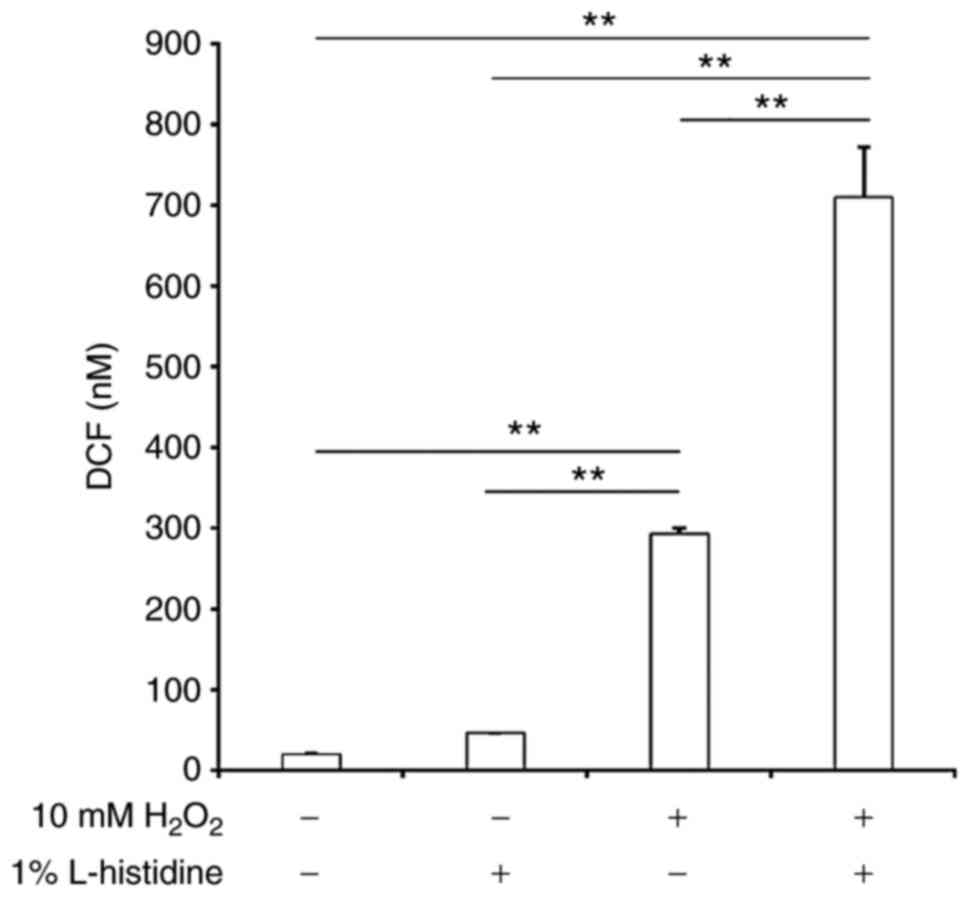
ROS generation in E. coli cells following
exposure to H2O2
Intracellular ROS levels in E. coli MG1655
cells grown to mid-logarithmic phase under aerobic conditions and
exposed to H2O2 were compared in conditions
with or without 1% L-histidine. As presented in Fig. 4, intracellular ROS levels were low
without H2O2. Addition of 1% L-histidine
alone did not induce ROS generation. In contrast, exposure to 10 mM
H2O2 significantly induced ROS generation in
E. coli MG1655 cells compared with the negative control. In
addition, ROS levels in E. coli significantly increased when
the cells were treated with a combination of 10 mM
H2O2 and 1% L-histidine compared with the
other groups.
EPR analysis
EPR analysis was performed to identify which types
of free radical were generated in E. coli cells following
exposure to 100 mM H2O2 and 1% L-histidine.
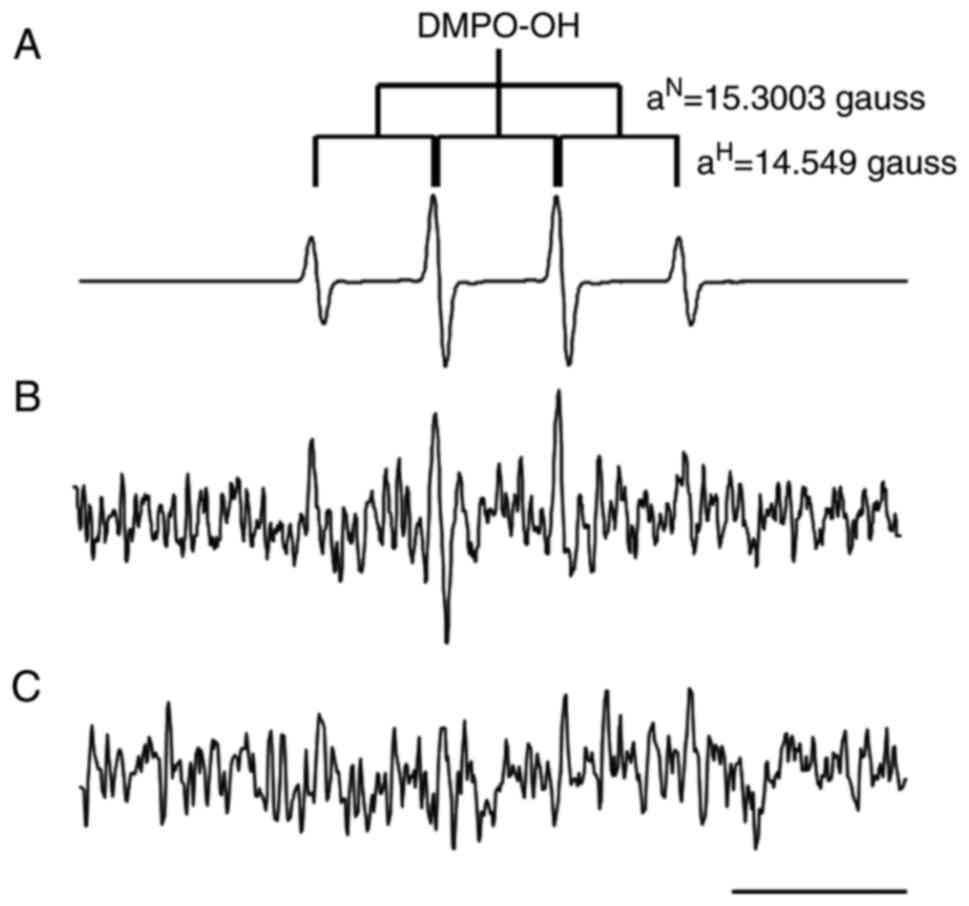
As presented in Fig. 5A, a
typical four-line EPR signal attributed to DMPO-OH spin-adduct with
an intensity ratio of 1:2:2:1 (hyperfine splitting constant;
aN=15.300 and aH=14.549 gauss) (38) was observed by Fenton reaction
systems containing 20 mM Fe2+, 100 mM
H2O2 and 500 mM DMPO. The EPR spectrum
representing DMPO-OH was observed in E. coli cells exposed
to 100 mM H2O2 and 1% L-histidine (Fig. 5B). On the other hand, this EPR
signal was not observed in E. coli cells exposed to 100 mM
H2O2 alone (Fig. 5C). These data indicated that
L-histidine promoted intracellular hydroxyl radical formation by
H2O2 in E. coli cells.
Effect of L-histidine on the bactericidal
activity of H2O2 against Gram-positive
MDROs
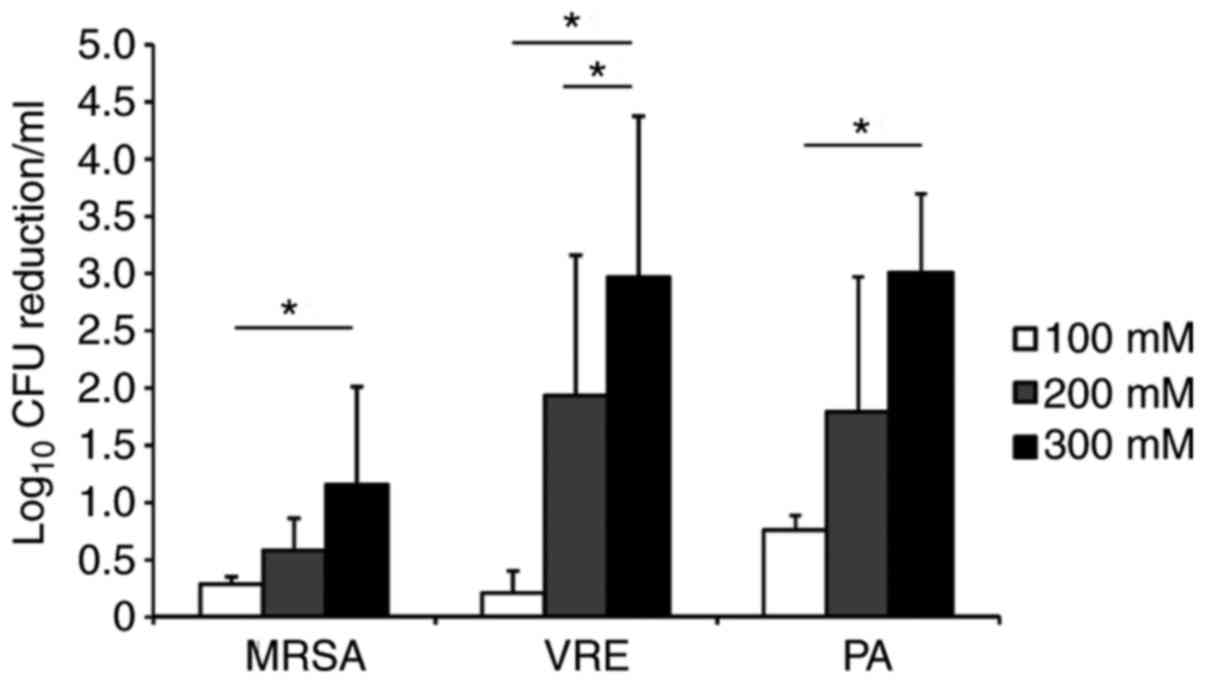
To examine the sensitivity of MRSA and VRE to
H2O2, aerobically-grown overnight cultures of
these MDROs were exposed to 100, 200 and 300 mM
H2O2 for 15 min. As presented in Fig. 6, H2O2
reduced the number of viable MRSA cells in a dose-dependent manner,
and 100, 200 and 300 mM H2O2 achieved
reductions of 0.29±0.07, 0.58±0.27 and 1.15±0.85 log10
CFU/ml in MRSA. H2O2 also reduced the viable
numbers of VRE cells dose-dependently, and 100, 200 and 300 mM
H2O2 achieved reductions of 0.31±0.19,
1.94±1.22 and 2.97±1.40 log10 CFU/ml in VRE.
Next, the effect of the combination of L-histidine
and 200 mM H2O2 on the viability of these
Gram-positive bacteria was assessed. As presented in Table I, no bactericidal effect was
observed in the presence of 1% L-histidine alone. Addition of 200
mM H2O2 alone resulted in 0.89±0.35 and
1.22±0.74 log10 CFU/ml reductions in MRSA and VRE, while
200 mM H2O2 with 1% L-histidine reduced cell
numbers only by 0.40±0.14 and 0.92±0.63 log10 CFU/ml,
respectively. Thus, L-histidine did not improve the bactericidal
effect of H2O2 against MRSA and VRE.
 | Table IEffect of L-histidine on the
bactericidal activity of H2O2 against
multidrug-resistant microorganisms. |
Table I
Effect of L-histidine on the
bactericidal activity of H2O2 against
multidrug-resistant microorganisms.
Treatment
| Organisms
(Log10 CFU/ml reduction)
|
|---|
|
H2O2 (mM) | 1% L-histidine | MRSA | VRE | MDRP | ESBL E.
coli |
|---|
| − | + | −0.01±0.13a,b | −0.01±0.09a,b | 0.00±0.12d,e | −0.07±0.09d,e |
| 100 | − | NT | NT | 0.24±0.16e,f | 0.76±0.08e,f |
| + | NT | NT | 1.12±0.36d,f | 1.14±0.02d,f |
| 200 | − | 0.89±0.35b,c | 1.21±0.74c | NT | NT |
| + | 0.40±0.14a,c | 0.91±0.63c | NT | NT |
Effect of L-histidine on the bactericidal
activity of H2O2 against Gram-negative
MDROs
The bactericidal activities of 100 mM
H2O2 against aerobically grown overnight
cultures of MDRP or ESBL-producing E. coli were assessed in
the presence or absence of 1% histidine (Table I). H2O2
alone achieved 0.24±0.16 and 0.76±0.08 log10 CFU/ml
reductions in MDRP and ESBL-producing E. coli cells,
respectively. Addition of 100 mM H2O2 along
with 1% L-histidine reduced cell counts by 1.12±0.36 and 1.14±0.02
log10 CFU/ml in these bacteria, respectively. These
results indicated that L-histidine significantly enhanced the
bactericidal action of H2O2 against MDRP and
ESBL-producing E. coli (P<0.01).
Discussion
In the present study, L-histidine supplementation
in the culture media was revealed to suppress the proliferation of
the recA-deficient E. coli laboratory strains JM109
and DH5α, but this amino acid did not influence the proliferation
of strain MG1655, which possesses an intact rec system. In
addition, this suppression was not apparent when the strains were
cultured anaerobically. These results indicate that L-histidine may
increase oxidative DNA damage to E. coli, because RecA
serves a crucial function in DNA repair. This hypothesis was
supported by the experimental data, which revealed that recA
gene expression in E. coli MG1655 cells exposed to 10 mM
H2O2 plus 1% L-histidine increased 7-fold
compared with that in cells exposed to 10 mM
H2O2 alone. In addition, DNA degradation was
more evident in the E. coli cells exposed to 10–100 mM
H2O2 plus 1% L-histidine than in cells
treated with the same concentration of H2O2
alone.
The augmenting effect of L-histidine on
H2O2-induced DNA damage may be mediated by a
Fenton-like reaction initiated by the contact of oxidative agents
with metal ions, generating ROS (21). Since the imidazole group in
L-histidine is known to bind to metal ions, including
Ni+ or Co+, this characteristic has been
applied to histidine-tagged recombinant protein purification. Prior
work has demonstrated that L-histidine increased the genotoxicity
of H2O2 against Chinese hamster ovary (CHO)
cells (8,9) and that this is dependent on the
L-histidine transporting activity of the CHO cells (39). Therefore, metal ions may be
co-transported inside the E. coli cells with L-histidine,
and these metal ions may increase the
H2O2-derived DNA damage by a Fenton-like
reaction. In fact, our group detected hydroxyl radicals in E.
coli cells following treatment with H2O2
and L-histidine, but an EPR signal was not observed when the cells
were treated with H2O2 alone. Schubert et
al (7) reported that
L-hisitidine and H2O2 readily form a stable
adduct. Their group indicated that L-hisitidine-peroxide adduct may
enter bacterial cells more rapidly than H2O2
alone, or that the rate of breakdown of the adduct by catalase is
slower.
Consistent with the augmenting effect of
L-histidine on H2O2-derived DNA damage in
E. coli, L-histidine increased the killing activity against
Gram-negative MDROs: ESBL-producing E. coli and MDRP. Wide
penetration of multidrug resistance (MDR) in Gram-negative bacilli
is a serious, global public health concern (40). MDR in highly virulent bacteria
including fluoroquinolone-resistant Salmonella (41) or ESBL-producing Shiga-toxigenic
E. coli (42,43) is emerging. In addition, the
dissemination of carbapenemase-producing Enterobacteriaceae
has made it difficult to treat certain infectious diseases with
antibiotics (44). Thus,
transmission control of Gram-negative bacilli with MDR is an urgent
issue. Disinfection techniques using a combination of
H2O2 and L-histidine may contribute to the
efficient eradication of these MDR Gram-negative bacteria from
hospital environments. However, H2O2 is a
highly reactive oxidizing agent and has genotoxic effects. Oosterik
et al (45) reported that
nebulization of 2% H2O2 renders chickens more
susceptible to avian pathogenic E. coli, potentially due to
epithelial damage by hydroxyl radicals. The simultaneous use of
L-histidine may decrease the H2O2
concentration necessary to eradicate Gram-negative pathogens.
In contrast to Gram-negative MDROs, L-histidine did
not increase the microbicidal activity of
H2O2 against Gram-positive bacteria (MRSA and
VRE). The reason for this selective augmentation remains unclear
but L-histidine transporting activity may be different between
Gram-negative and Gram-positive bacteria. This selective effect of
L-histidine on the bactericidal activity of
H2O2 may be advantageous in certain
instances, including during the elimination of foodborne pathogens
(for example, Salmonella and pathogenic E. coli from
fermented food made with lactic acid bacteria) or for
bioremediation of contaminated soil by Gram-positive bacteria. The
physiological benefit of resident microflora for human health has
also been increasingly recognized. For example, a resident skin
microbe, Staphylococcus epidermidis, produces an Esp
protease that degrades S. aureus biofilms, thereby
conferring colonization resistance to MRSA (46). Thus, the use of the
H2O2/L-histidine combination may be
applicable for treatment of skin lesions, including decubitus
ulcers infected with P. aeruginosa.
H2O2/L-histidine is expected to exert
enhanced toxicity against Gram-negative pathogens, while having a
less detrimental effect on colonization resistance by normal
resident skin flora.
In the present study, our group demonstrated the
selective augmenting effect of L-histidine on the microbicidal
activity of H2O2 against Gram-negative
bacteria. This effect is potentially derived from DNA damage by ROS
generated through Fenton-like reactions between
H2O2 and metal ions bound to the imidazole
group of L-histidine. The H2O2/L-histidine
combination reduces the H2O2 concentration
necessary to inactivate Gram-negative pathogens. In addition, this
selectivity to Gram-negative bacteria may be useful in sanitation
processes required to protect Gram-positive bacteria, including
lactic acid bacteria in fermented foods or resident skin
microbiota. Taken together, the results of the present study may
provide valuable information to develop novel
H2O2-based disinfection techniques.
Acknowledgments
The authors would like to thank Dr Koichi Tanimoto
(Gunma University, Maebashi, Japan) for the gift of E.
faecium FN1, and Mr. Hitoshi Yamaoka (Honbu Sankei Co. Ltd.,
Osaka, Japan) for technical assistance.
Notes
[1]
Funding
The present study was supported by a Grant-in-Aid
from the Japan Society for the Promotion of Science KAKEN (grant
no. 16K12012).
[2] Availability
of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
[3] Authors'
contributions
TN and HNI analyzed and interpreted the effect of
the test reagents on E. coli growth. AT and HNI performed
recA qPCR and ROS measurement. ME and HY performed PFGE
analysis. MH and KT performed EPR analysis. TN, ET and KO examined
the bactericidal effect of test reagents. TN and TK were major
contributors in writing the manuscript. All authors read and
approved the final manuscript.
[4] Ethics
approval and consent to participate
Not applicable.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Imlay JA: The molecular mechanisms and
physiological consequences of oxidative stress: Lessons from a
model bacterium. Nat Rev Microbiol. 11:443–454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iuchi S and Weiner L: Cellular and
molecular physiology of Escherichia coli in the adaptation to
aerobic environments. J Biochem. 120:1055–1063. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gort AS and Imlay JA: Balance between
endogenous superoxide stress and antioxidant defenses. J Bacteriol.
180:1402–1410. 1998.PubMed/NCBI
|
|
4
|
Park S, You X and Imlay JA: Substantial
DNA damage from submicromolar intracellular hydrogen peroxide
detected in Hpx− mutants of Escherichia coli. Proc Natl
Acad Sci USA. 102:9317–9322. 2005. View Article : Google Scholar
|
|
5
|
Touati D, Jacques M, Tardat B, Bouchard L
and Despied S: Lethal oxidative damage and mutagenesis are
generated by iron in delta fur mutants of Escherichia coli:
Protective role of superoxide dismutase. J Bacteriol.
177:2305–2314. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oya Y and Yamamoto K: The biological
activity of hydrogen peroxide. IV. Enhancement of its clastogenic
actions by co-administration of L-histidine. Mutat Res.
198:233–240. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schubert J, Watson JA and Baecker JM:
Formation of a histidine-peroxide adduct by
H2O2 or ionizing radiation on histidine:
Chemical and microbiological properties. Int J Radiat Biol Relat
Stud Phys Chem Med. 14:577–583. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sestili P, Cattabeni F and Cantoni O: The
L-histidine-mediated enhancement of hydrogen peroxide-induced DNA
double strand breakage and cytotoxicity does not involve metabolic
processes. Biochem Pharm. 50:1823–1830. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tachon P, Deflandre A and Giacomoni PU:
Modulation by L-histidine of H2O2-mediated
damage of cellular and isolated DNA. Carcinogenesis. 15:1621–1626.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McRipley RJ and Sbarra AJ: Role of the
phagocyte in host-parasie interactions. XII. Hydrohgen
peroxide-myeloperoxidase bactericidal system in the phagocyte. J
Bacteriol. 94:1425–1430. 1967.PubMed/NCBI
|
|
11
|
Eschenbach DA, Davick PR, Williams BL,
Klebanoff SJ, Young-Smith K, Critchlow CM and Holmes KK: Prevalence
of hydrogen peroxide-producing Lactobacillus species in normal
women and women with bacterial vaginosis. J Clin Microbiol.
27:251–256. 1989.PubMed/NCBI
|
|
12
|
Seaver LC and Imlay JA: Alkyl
hydroperoxide reductase is the primary scavenger of endogenous
hydrogen peroxide in Escherichia coli. J Bacteriol. 183:7173–7181.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seaver LC and Imlay JA: Hydrogen peroxide
fluxes and compartmentalization inside growing Escherichia coli. J
Bacteriol. 183:7182–7189. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Walling C: Fenton's reagent revisited.
Accounts Chem Res. 8:125–131. 1975. View Article : Google Scholar
|
|
15
|
Passaretti CL, Otter JA, Reich NG, Myers
J, Shepard J, Ross T, Carroll KC, Lipsett PL and Perl TM: An
evaluation of environmental decontamination with hydrogen peroxide
vapor for reducing the risk of patient acquisition of
multidrug-resistant organisms. Clin Infect Dis. 56:27–35. 2013.
View Article : Google Scholar
|
|
16
|
Rutala WA, Gergen MF and Weber DJ:
Efficacy of improved hydrogen peroxide against important
healthcare-associated pathogens. Infect Control Hosp Epidemiol.
33:1159–1161. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ronen Z, Guerrero A and Gros A: Graywater
disinfection with the environmentally friendly hydrogen peroxide
plus (HPP). Chemosphere. 78:61–65. 2010. View Article : Google Scholar
|
|
18
|
Tang WZ and Tassos S: Oxidation kinetics
and mechanisms of trihalomethanes by Fenton's reagent. Water
Research. 31:1117–1125. 1997. View Article : Google Scholar
|
|
19
|
Yu Y, Chan WI, Liao PH and Lo KV:
Disinfection and solubilization of sewage sludge using the
microwave enhanced advanced oxidation process. J Hazard Mater.
181:1143–1147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oya Y, Yamamoto K and Tonomura A: The
biological activity of hydrogen peroxide. I. Induction of
chromosome-type aberrations susceptible to inhibition by scavengers
of hydroxyl radicals in human embryonic fibroblasts. Mutat Res.
172:245–253. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jung YS, Lim WT, Park JY and Kim YH:
Effect of pH on Fenton and Fenton-like oxidation. Environ Technol.
30:183–190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aslani H, Nabizadeh R, Alimohammadi M,
Mesdaghinia A, Nadafi K, Nemati R and Ghani M: Disinfection of raw
wastewater and activated sludge effluent using Fenton like reagent.
J Environ Health Sci Eng. 12:1492014. View Article : Google Scholar
|
|
23
|
Orta De Velásquez MT, Yáñez-Noguez I,
Jiménez-Cisneros B and Luna Pabello VM: Adding silver and copper to
hydrogen peroxide and peracetic acid in the disinfection of an
advanced primary treatment effluent. Environ Technol. 29:1209–1217.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Steinberg D, Heling I, Daniel I and
Ginsburg I: Antibacterial synergistic effect of chlorhexidine and
hydrogen peroxide against Streptococcus sobrinus, Streptococcus
faecalis and Staphylococcus aureus. J Oral Rehabil. 26:151–156.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zubko EI and Zubko MK: Co-operative
inhibitory effects of hydrogen peroxide and iodine against bacteria
and yeast species. BMC Res Notes. 6:2722013. View Article : Google Scholar
|
|
26
|
Miyasaki KT, Genco RJ and Wilson ME:
Antimicrobial properties of hydrogen peroxide and sodium
bicarbonate individually and in combination against selected oral,
Gram-negative, facultative bacteria. J Dent Res. 65:1142–1148.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carlsson J, Edlund MB and Hanstrom L:
Bactericidal and cytotoxic effects of hypothiocyanite-hydrogen
peroxide mixtures. Infect Immun. 44:581–586. 1984.PubMed/NCBI
|
|
28
|
Humphrey TJ: The synergistic inhibition of
Campylobacter jejuni by rifampicin and hydrogen peroxide. Lett
Apple Microbiol. 10:97–100. 1990. View Article : Google Scholar
|
|
29
|
Martin H and Maris P: Synergism between
hydrogen peroxide and seventeen acids against six bacterial
strains. J Appl Microbiol. 113:578–590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Almeida CE, Felício DL, Galhardo RS,
Cabral-Neto JB and Leitão AC: Synergistic lethal effect between
hydrogen peroxide and neocuproine (2,9-dimethyl
1,10-phenanthroline) in Escherichia coli. Mutat Res. 433:59–66.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shama G: Inactivation of Escherichia coli
by ultraviolet light and hydrogen peroxide in a thin film
contactor. Lett Appl Microbiol. 15:259–260. 1992. View Article : Google Scholar
|
|
32
|
Uemura M, Imataki O, Uchida S,
Nakayama-Imaohji H, Ohue Y, Matsuka H, Mori H, Dobashi H, Kuwahara
T and Kadowaki N: Strain-specific transmission in an outbreak of
ESBL-producing Enterobacteriaceae in the hematooncology care unit:
A cohort study. BMC Infect Dis. 17:262017. View Article : Google Scholar
|
|
33
|
Fujita N, Yoshimura M, Komori T, Tanimoto
K and Ike Y: First report of the isolation of high-level
vancomycin-resistant Enterococcus faecium from a patient in Japan.
Antimicrob Agents Chemother. 42:25101998.
|
|
34
|
Rocha ER and Smith CJ: Regulation of
Bacteroides fragilis katB mRNA by oxidative stress and carbon
limitation. J Bacteriol. 179:7033–7039. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim YS, Min J, Hong HN, Park JH, Park KS
and Gu MB: Gene expression analysis and classification of mode of
toxicity of polycyclic aromatic hydrocarbons (PAHs) in Escherichia
coli. Chemosphere. 66:1243–1248. 2007. View Article : Google Scholar
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Method. 25:402–408. 2001.
View Article : Google Scholar
|
|
37
|
Oya-Ohta Y, Ueda A, Ochi T, Harada M and
Yamamoto K: The biological activity of hydrogen peroxide VII.
L-Histidine increases incorporation of H(2)O(2) into cells and
enhances formation of 8-oxodeoxyguanosine by UV-C plus H(2)O(2) but
not by H(2)O(2) alone. Mutat Res. 478:119–127. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Duling DR: Simulation of multiple
isotropic spin-trap EPR spectra. J Magn Reson B. 104:105–110. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Buettner GR: Spin trapping: ESR parameters
of spin adducts. Free Radic Biol Med. 3:259–303. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kaye KS and Pogue JM: Infections caused by
resistant gram-negative bacteria: Epidemiology and management.
Pharmacotherapy. 35:949–962. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sjölund-Karlsson M, Folster JP, Pecic G,
Joyce K, Medalla F, Rickert R and Whichard JM: Emergence of
plasmid-mediated quinolone resistance among non-typhi Salmonella
enterica isolates from humans in the United States. Antimicrob
Agents Chemother. 53:2142–2144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bielaszewska M, Mellmann A, Zhang W, Köck
R, Fruth A, Bauwens A, Peters G and Karch H: Characterisation of
the Escherichia coli strain associated with an outbreak of
haemolytic uraemic syndrome in Germany, 2011: A microbiological
study. Lancet Infect Dis. 11:671–679. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mellmann A, Harmsen D, Cummings CA, Zentz
EB, Leopold SR, Rico A, Prior K, Szczepanowski R, Ji Y, Zhang W, et
al: Prospective genomic characterization of the German
enterohemorrhagic Escherichia coli O104:H4 outbreak by rapid next
generation sequencing technology. PLoS One. 6:e227512011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tzouvelekis LS, Markogiannakis A,
Psichogiou M, Tassios PT and Daikos GL: Carbapenemases in
Klebsiella pneumoniae and other Enterobacteriaceae: An evolving
crisis of global dimensions. Clin Microbiol Rev. 25:682–707. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Oosterik L, Tuntufye HN, Janssens S,
Butaye P and Goddeeris BM: Disinfection by hydrogen peroxide
nebulization increases susceptibility to avian pathogenic
Escherichia coli. BMC Res Notes. 8:3782015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Iwase T, Uehara Y, Shinji H, Tajima A, Seo
H, Takada K, Agata T and Mizunoe Y: Staphylococcus epidermidis Esp
inhibits Staphylococcus aureus biofilm formation and nasal
colonization. Nature. 465:346–349. 2010. View Article : Google Scholar : PubMed/NCBI
|