Introduction
Traditional Chinese Medicines (TCMs), especially
herbal medicines, have been used as the main approaches in treating
various diseases in China for >2000 years. TCMs are gaining
increasing attention worldwide for their thousands of years of
practice and the potential therapeutic applications. To obtain
synergistic effects or diminish potential adverse effects, TCMs are
commonly prescribed in combination (1). To our knowledge, the synergistic and
therapeutic effects of TCMs are based on the joint contribution of
multi-components (2). Generally,
due to differences in cultivation areas, practices, plant origins,
climate conditions and processing protocols among others, the
chemical composition of herbal formulations may vary in a larger
scale (3–5). Because of the multicomponent and
multi-target nature of therapy, it is difficult to control the
quality of the TCM preparation effectively. Thus, establishing an
effective and feasible method which can control the quality of
individual herbs or multiple herbs is necessary.
Ailanthus altissima Swingle (Simaroubaceae,
AA), the tree-of-heaven, is native to China and was introduced to
Europe around the end of 18th century. AA has been used in Chinese
traditional medicine to treat various diseases including epilepsy,
asthma, diarrhea, dysentery, heat ailments, and also as an
astringent (6). Various
biological activities has been observed in the stem bark of AA,
including anti-plasmodial, anti-viral, anti-proliferative,
cytotoxic and anti-malarial (7,8).
It has been reported that AA water decoction decreased the
production of inflammatory cytokines including interleukin-6
(IL-6), tumor necrosis factor and IL-8, and the expression of
nuclear factor-κB in HMC-1 human mast cell line (9). Further study also showed that the
EtOH extract of AA inhibited the generation of the cyclooxygenase-2
(COX-2) in BMMC cells (10). In
previous phytochemical studies, quassinoids, indole alkaloids,
lipids, fatty acids, phenolic derivatives, and volatile compounds
from AA have been characterized (11–14). Extracts of AA and some isolated
chemical compounds have shown some medicinal properties (15). Ailanthone (AT), one of the primary
potent quassinoids in AA, has various biological activities,
including anti-malarial, anti-inflammatory, anti-allergic,
anti-HIV, antiulcer and antimicrobial activities (16–18). It inhibited growth of several
cancer cell lines, including Jurkat, Hep3B, HepG2, R-HepG2, MCF-7,
HeLa and A549 cells in vitro (17–20). However, details of their effect in
inhibiting brain glioma growth is scarce.
There have been limited reports describing assays
for simultaneous determination of the active components in AA. Only
a few reports that separated some quassinoids from AA by
high-performance liquid chromatography (HPLC) methods (21,22), but only one study reported the
determination of AT in soil, which was not suitable for biological
analysis (23). A highly
sensitive LC-MS/MS method has been used to analyse AT levels in
rats plasma, however, these procedures require specialized
equipment and are not suitable for chemical systems. For routine
analysis in the production process of AA and its preparations, a
quick and simple HPLC analytical method would be highly desirable.
Here, a simple and feasible method for the quantitative
determination of the active components in AA was developed to
improve the QC. Cellular pharmacology experiments were also
undertaken to assess the effect of different AA samples.
Materials and methods
Chemicals and herbal materials
The reference standard for AT was purchased from
National Institute for the Control of Pharmaceutical and Biological
Products (NICPP, China). The purity was determined to be >98%
based on a peak area normalization method by HPLC analysis.
HPLC-grade acetonitrile and methanol were obtained from Burdick
& Jackson (Muskegon, MI, USA). Formic acid was purchased from
Beijing Chemical Factory (Beijing, China). Purified water was
prepared from a Millipore water purification system (Millipore,
Billerica, MA, USA). Other reagents were from Sigma-Aldrich (St.
Louis, MO, USA) and analytical grade.
Crude plant materials (lot no. S1–S10) were
collected from ten regions (Henan, Shanxi, Anhui, Hebei, Guizhou,
Guangxi, Sichuan, Yunnan, Zhejiang, Jiangsu, respectively) and met
the requirements of Beijing Food and Drug Administration. All
herbal medicines were identified by Professor Zhishu Tang of the
School of Pharmacy, Shaanxi University of Chinese Medicine and
voucher specimens were deposited in the Institute of Materia
Medica, School of Pharmacy, the Fourth Military Medical
University.
Preparation of the extracts
The stem bark of AA was air-dried and powdered. Each
powdered sample was precisely weighed (1 kg) and extracted with 75%
EtOH three times at room temperature, and then the solution was
evaporated in vacuo. For further use, all extracts were
stored at 4°C. Extracts were diluted if necessary.
Chromatographic conditions
HPLC analysis was performed on a Shimadzu LC-2010A
HT HPLC equipped with a UV detector. The analytes were separated on
a Kromasil 100-5-C18 column (250×4.6 mm, 5 µm). The mobile
phase consisted of A (1% acetonitrile) and B (water containing 1%
formic acid). The linear gradient conditions were optimized as
follows: 0–40 min, 3–20% A; 40–45 min, 20–25% A; 45–50 min, 25–30%
A; 50–60 min, 30–60% A; 60–65 min, 60–65% A. The flow rate was 0.8
ml/min at 25°C. The UV detection wavelength was set at 250 nm and
sample injection volume was 10 µl.
Preparation of stock and working
solutions
Stock solutions were prepared by dissolving accurate
amounts of AA and AT in water, and stored at 4°C. Then the stock
standard solution was serially diluted with water in order to
prepare calibration standard solutions of 0.825, 0.412, 0.206,
0.103, 0.052, 0.026, 0.013 and 0.0065 mg/ml, respectively. For
establishing the calibration curves, 10 µl of the above
working solutions were used. For preparing the calibration curve,
the peak area of the analyte was taken as the Y-axis and the
concentration of AT (mg/ml) was taken as the X-axis.
Method validation
According to the guidelines of the China Food and
Drug Administration (CFDA), HPLC fingerprinting analysis was
developed and its stability, precision, and repeatability were
validated. To evaluate sample stability, the same sample AA-S1
solution at different time-points (0, 0.5, 1, 2, 4, 6, 8, 12 and 24
h) were analyzed. Five replicates of a sample of an AA-S1 solution
were performed to determine the precision on the same day. To
confirm the repeatability of results, five different sample
solutions prepared from the same sample (AA-S1) were analyzed. To
measure the relative recoveries, six concentrations (11.0108,
11.0160, 6.0012, 6.0511, 2.8875 and 2.9438 mg) of AT were added
into the same sample (AA-S1). Five replicates of the resultant
samples were analyzed with the HPLC method established above. The
measured to the added amount ratio was used to calculate the
recovery. The LOD and LOQ were determined from the S/n level of
three and ten, respectively.
Pharmacological studies
The human brain glioma cell line U87 was obtained
from the American Type Culture Collection (Rockville, MD, USA).
Cells were cultured in Dulbecco's modified essential medium (DMEM)
supplemented with 10% fetal bovine serum (FBS), and
penicillin/streptomycin (Gibco, Carlsbad, CA, USA) in an atmosphere
of 5% CO2 at 37°C. For drug treatments, the U87 cell
lines were treated with the vehicle DMEM or various concentrations
of AA for ~1–3 days. For some experiments, the AA-treated cells
were also incubated with or without 20 µM of Z-VAD-fmk, a
broad-spectrum caspase inhibitor, or the antioxidant reagent,
N-acetyl cysteine (NAC, 2.5 mM). After different treatments, cell
survival rate was measured by MTT. ROS and MDA levels were measured
by corresponding kits according to the instructions.
To measure protein expression, U87 cells were
collected and dissolved in lysis buffer containing protease
inhibitors (Sigma-Aldrich), and then centrifuged at 10,000 rpm for
20 min. The supernatant was collected for protein analysis and
stored at −80°C until used. Thirty micrograms of the cell lysates
was separated by using 10% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE). Then, transferred to polyvinylidene
fluoride membrane (PVDF), and immunoblotted with antibodies against
cleaved-caspase-8, cleaved-caspase-3, p-PERK, PERK, elF2α, p-elF2α,
Bax, Bcl-2 and β-actin (Santa Cruz Biotechnology, Inc., Dallas, TX,
USA). Bands were visualized using the ECL Plus detection system (GE
Healthcare, Freiburg, Germany). Densitometric analyses were
performed using Image-Pro Plus.
Data analysis
The data analysis of chromatographic fingerprinting
was conducted by Similarity Evaluation System for Chromatographic
Fingerprint of Traditional Chinese Medicine software (version
2008), which was recommended by the State Food and Drug
Administration of China. One AA sample from one origin was chosen
and evaluated through the proposed pharmacological method. Data are
expressed as the mean ± standard deviation of triplicate
measurements. The comparison was done using the Student's t-test.
P<0.05 was considered significant.
Results
Optimization of chromatographic
conditions
To determine the best separation mechanism and to
obtain as much chemical information as possible in chromatograms,
different HPLC parameters including different columns, detection
wavelengths, mobile phases, and gradient elution conditions were
examined and compared. Yilite Hypersil base deactivated silica C18
column, Yilite SinoChrom ODS-BP C18 column, and Kromasil
100-5 C18 column were investigated and compared. Kromasil 100-5 C18
column had good peak separation and sharp peaks, so Kromasil 100-5
C18 column was chosen in the further study. The effect of mobile
phase composition (acetonitrile-water and methanol-water together
with different organic solvents including formic acid, acetic acid,
triethylamine, and phosphoric acid) on chromatographic separation
was investigated. Adding 1% formic acid in the mobile phase B and
1% methanol-water in the mobile phase A, provided a better
resolution and separation, and resulted in high precision
sensitivity and selectivity. The detection wavelengths at 210, 230,
250 and 280 nm were selected according to the maximum absorption
and full-scan experiment, and compared the peak number and peak
resolution. Finally, the wavelength was set at 250 nm. The
injection volume was set at 10 µl, the flow rate was set at
0.8 ml/min and the column temperature was kept at 25°C.
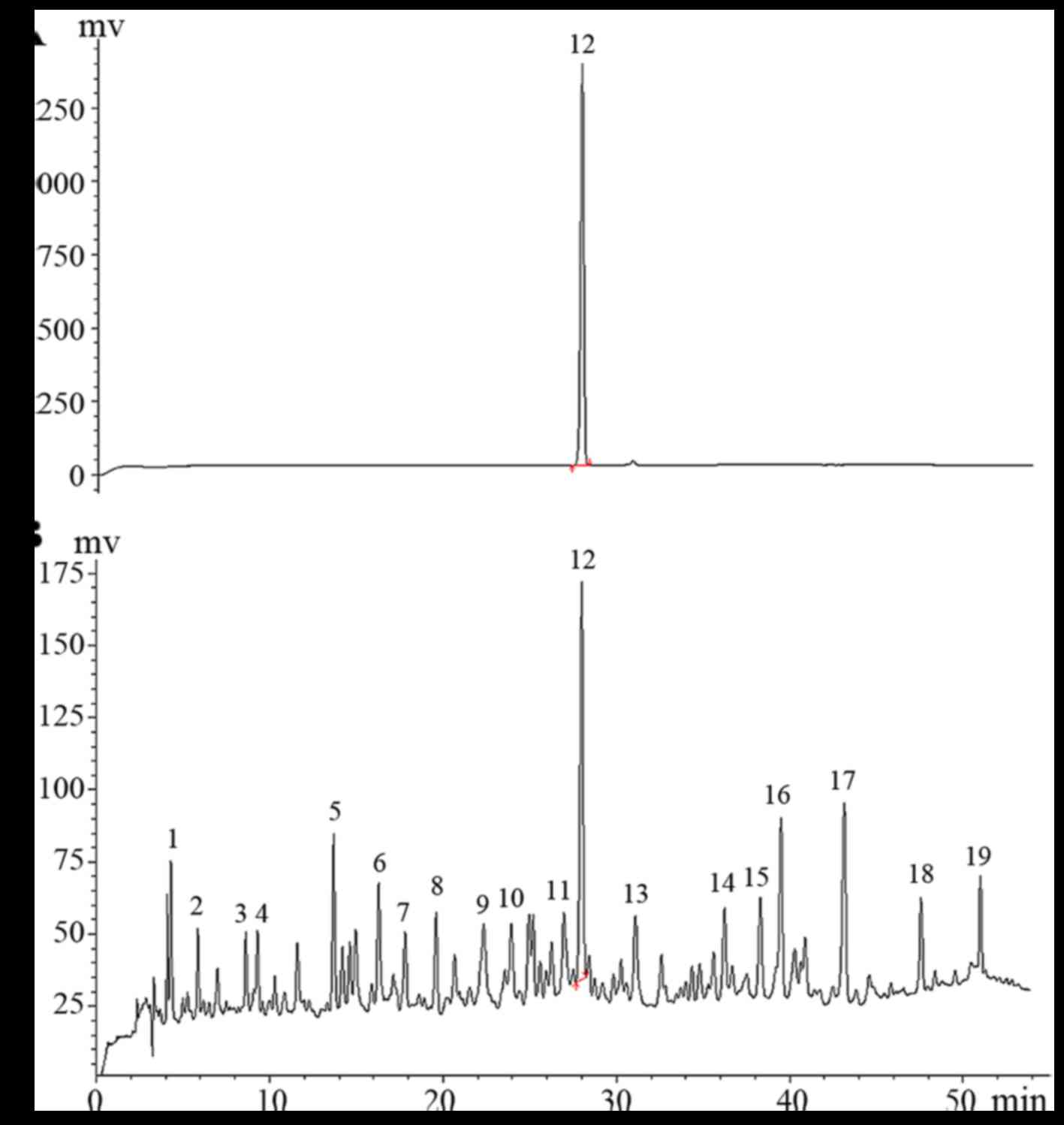
Representative HPLC chromatogram is shown in Fig. 1.
Analytical method validation
Analytical method validation, which includes the
linearity, repeatability, precision, LOD, LOQ, stability, and
recovery, was performed according to the guidance of International
Union of Pure and Applied Chemistry. The linearity was determined
by eight concentration levels of AT ranging from 6.44–825
µg/ml. The results showed the calibration curve of AT was
y=2.2x–20.9, and exhibited excellent linear regressions with a high
determination coefficient (r2=0.9992). We also found
that the calibration range adequately covered the amounts of AT in
the investigated samples. The LOD, defined as three times the
baseline noise, was 0.32 µg/ml. The LOQ, defined as 10 times
the baseline noise, was 0.45 µg/ml. The results of
precision, repeatability, recovery, and stability showed that the
relative standard deviation (RSD) values were all <2.3%. The
recovery of the method was in the range of 93–102%, with an RSD
<2.3%. These results demonstrated that the system and the HPLC
method was accurate enough for determination of AT and the AA
samples. The data are shown in Table
I.
 | Table ICalibration curve parameters,
precision, repeatability, stability and recovery of AT. |
Table I
Calibration curve parameters,
precision, repeatability, stability and recovery of AT.
| No. | Item | AT |
|---|
| 1 | Calibration
curves | y=2.2x–20.9 |
| 2 | Linear range
(µg/ml) | 6.44–825
µg/ml |
| 3 | Correlation
coefficient, r2 | 0.9992 |
| 4 | Precision RSD, %,
n=6 | 2.1 |
| 5 | Repeatablity RSD,
%, n=6 | 1.9 |
| 6 | Stability RSD, %,
n=6 | 2.2 |
| 7 | Recovery, mean,
RSD, %, n=3 | 1.8 |
| 8 | Retention time,
min | 32.26 |
| 9 | LOD
(µg/ml) | 0.32 |
| 10 | LOQ
(µg/ml) | 0.45 |
Sample analysis
The newly established analytical method was used to
quantify the contents of AT in 10 batches of AA from different
provinces in China. The results showed that all the AA samples were
rich in AT, however, their contents were significantly different.
The average content in 10 batches of AT was 0.613 mg/g, while the
total contents of AT in different samples ranged from 0.21–1.78
mg/g. These results suggested remarkable differences between
different samples. In the point of origin analysis, Henan province
reached the highest level, whereas it was only 0.21 mg/g in S8,
which was from Yunnan province (Table II). These data provide an
important reference for the quality of AA used as herbal medicine
for the treatment of cancers or as a material to obtain the AT for
further use.
 | Table IIContents of AT in 10 batches of AA
samples (n=3). |
Table II
Contents of AT in 10 batches of AA
samples (n=3).
| No. | Origin | Batch no. | Content (mg/g) | RSD (%) |
|---|
| S1 | Henan | 150317 | 1.78 | 0.13 |
| S2 | Shanxi | 141109 | 0.63 | 0.08 |
| S3 | Anhui | 150502 | 0.75 | 0.11 |
| S4 | Hebei | 150104 | 0.52 | 0.06 |
| S5 | Guizhou | 141208 | 0.48 | 0.15 |
| S6 | Guangxi | 150106 | 0.45 | 0.03 |
| S7 | Sichuan | 150212 | 0.24 | 0.13 |
| S8 | Yunnan | 150411 | 0.21 | 0.08 |
| S9 | Zhejiang | 140219 | 0.35 | 0.12 |
| S10 | Jiangsu | 150101 | 0.72 | 0.08 |
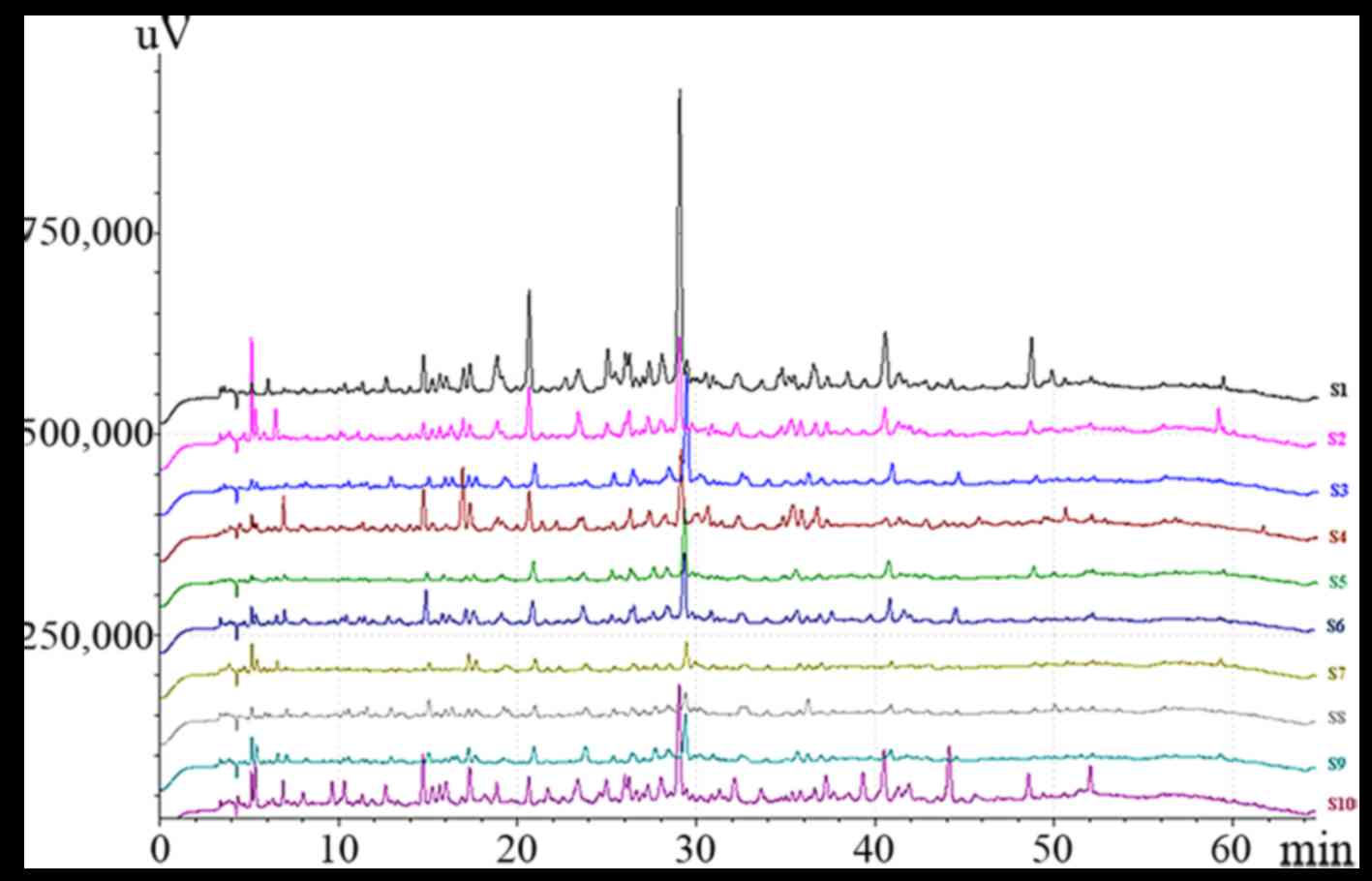
HPLC fingerprint of AA
Altogether, fingerprints of the 10 batches of
samples were analyzed. AT peak which is the main component of AA,
was set as the reference peak. The simulated mean chromatogram was
generated by the Computer-Aided Similarity Evaluation System (CASE)
for Chromatographic Fingerprint of TCM (China Committee of
Pharmacopeia, 2008). Nineteen common peaks were selected as
characteristic peaks (Fig. 2).
The relative retention time of each peak was calculated (Table III). The RSD of the relative
retention time of common peaks for all 10 batches was <1.5%,
which is in line with the requirements of fingerprints by the HPLC
method.
 | Table IIIThe relative retention time of each
peak. |
Table III
The relative retention time of each
peak.
| Peak no. |
Batches |
|---|
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | RSD/% |
|---|
| 1 | 0.1765 | 0.1769 | 0.1744 | 0.1761 | 0.1747 | 0.1752 | 0.1748 | 0.1745 | 0.1750 | 0.1769 | 0.57 |
| 2 | 0.2389 | 0.2381 | 0.2404 | 0.2375 | 0.2381 | 0.2380 | 0.2368 | 0.2409 | 0.2409 | 0.2374 | 0.63 |
| 3 | 0.3562 | 0.3554 | 0.3588 | 0.3553 | 0.3572 | 0.3558 | 0.3583 | 0.3593 | 0.3591 | 0.3553 | 0.47 |
| 4 | 0.3901 | 0.4055 | 0.3931 | 0.3892 | 0.3916 | 0.3902 | 0.3930 | 0.3938 | 0.3935 | 0.3899 | 1.20 |
| 5 | 0.5240 | 0.5243 | 0.5112 | 0.5230 | 0.5093 | 0.5074 | 0.5114 | 0.5118 | 0.5117 | 0.5246 | 1.38 |
| 6 | 0.5386 | 0.5393 | 0.5422 | 0.5443 | 0.5408 | 0.5394 | 0.5424 | 0.5434 | 0.5435 | 0.5506 | 0.64 |
| 7 | 0.5966 | 0.5953 | 0.5869 | 0.5931 | 0.5846 | 0.5834 | 0.5870 | 0.5871 | 0.5874 | 0.5966 | 0.86 |
| 8 | 0.7106 | 0.7109 | 0.7122 | 0.7087 | 0.7115 | 0.7108 | 0.7128 | 0.7133 | 0.7119 | 0.7106 | 0.19 |
| 9 | 0.8053 | 0.8061 | 0.8090 | 0.8114 | 0.8068 | 0.8072 | 0.8089 | 0.8109 | 0.8095 | 0.8051 | 0.28 |
| 10 | 0.8617 | 0.8613 | 0.8626 | 0.8694 | 0.8621 | 0.8621 | 0.8634 | 0.8633 | 0.8630 | 0.8623 | 0.27 |
| 11 | 0.9653 | 0.9667 | 0.9674 | 0.9692 | 0.9662 | 0.9667 | 0.9670 | 0.9580 | 0.9673 | 0.9647 | 0.31 |
| 12 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 0.00 |
| 13 | 1.2096 | 1.2338 | 1.2173 | 1.2312 | 1.2120 | 1.2152 | 1.2152 | 1.2171 | 1.2137 | 1.2334 | 0.76 |
| 14 | 1.2890 | 1.2841 | 1.2817 | 1.2799 | 1.2805 | 1.2813 | 1.2566 | 1.2806 | 1.2811 | 1.2982 | 0.81 |
| 15 | 1.3957 | 1.3961 | 1.3909 | 1.3940 | 1.3892 | 1.3917 | 1.3898 | 1.3903 | 1.3907 | 1.3947 | 0.18 |
| 16 | 1.4346 | 1.4445 | 1.4210 | 1.4316 | 1.4291 | 1.4309 | 1.4271 | 1.4218 | 1.4278 | 1.4426 | 0.54 |
| 17 | 1.5218 | 1.5216 | 1.5170 | 1.5388 | 1.5163 | 1.5175 | 1.5128 | 1.5156 | 1.5157 | 1.5203 | 0.48 |
| 18 | 1.6767 | 1.6770 | 1.6643 | 1.6778 | 1.6652 | 1.6672 | 1.6631 | 1.6639 | 1.6641 | 1.6729 | 0.37 |
| 19 | 2.0456 | 2.0391 | 2.0124 | 2.0290 | 2.0188 | 2.0210 | 2.0145 | 2.0146 | 2.0178 | 2.0478 | 0.66 |
The State Food and Drug Administration (SFDA)
suggested that all herbal chromatograms should be evaluated in
terms of similarity by calculating the correlation coefficient
and/or angle cosine value of the original data (24–26). In this study, SA was conducted
based on the standard fingerprints, and the results are shown in
Table IV. The relative retention
time similarities of communal peaks were all >0.99, which
indicates that different samples had similar constituents. However,
peak area similarities of communal peaks were significantly
different because of the production process and the contents of
main bioactive constituents.
 | Table IVRelative retention time and peak area
similarities of AA. |
Table IV
Relative retention time and peak area
similarities of AA.
| Items |
Batches |
|---|
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 |
|---|
| Relative retention
time similarities | 0.999 | 0.999 | 0.998 | 0.998 | 0.999 | 0.999 | 0.998 | 0.999 | 0.999 | 0.999 |
| Peak areas
similarities | 0.912 | 0.897 | 0.954 | 0.838 | 0.911 | 0.834 | 0.753 | 0.682 | 0.869 | 0.934 |
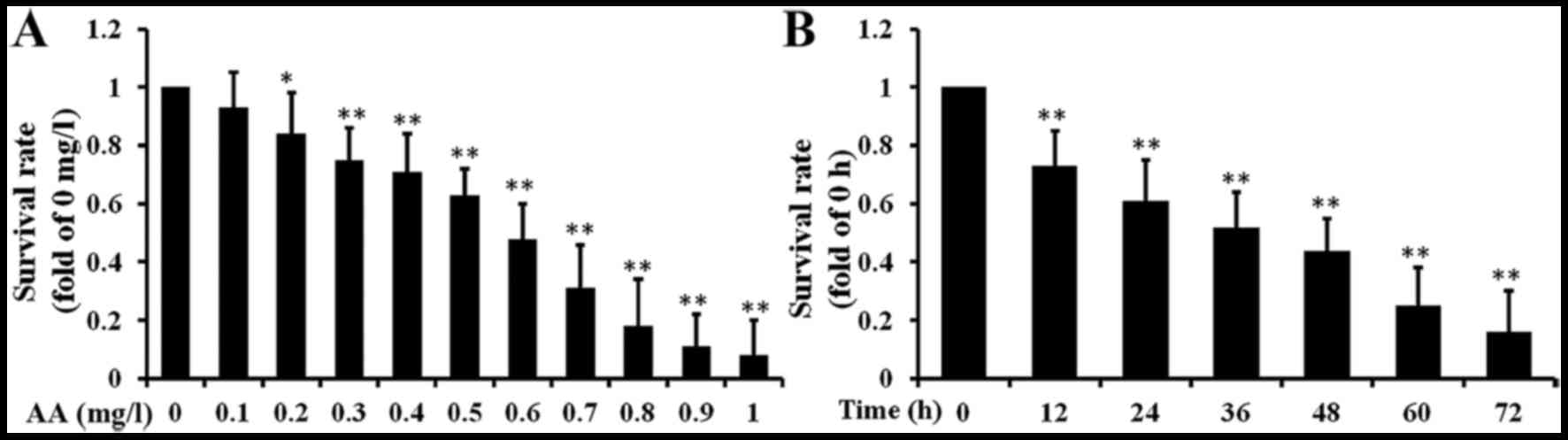
Cell growth inhibition effects of AA
In our previous study, we investigated the
AA-induced cytotoxicity and apoptosis in hepatoma carcinoma cells.
To evaluate the cytotoxicity of AA against brain glioma cells, U87
cell line was treated with 0.1–1 mg/l AA for 1–3 days. The cell
viability was measured by using the MTT assay. AA potently
inhibited the growth of U87 in a concentration-dependent manner
(Fig. 3A). Further, we also found
AA inhibited cell proliferation of U87 in a time-dependent manner
(Fig. 3B). Thus, 0.3, 0.6 and 0.9
mg/l AA were selected for further study.
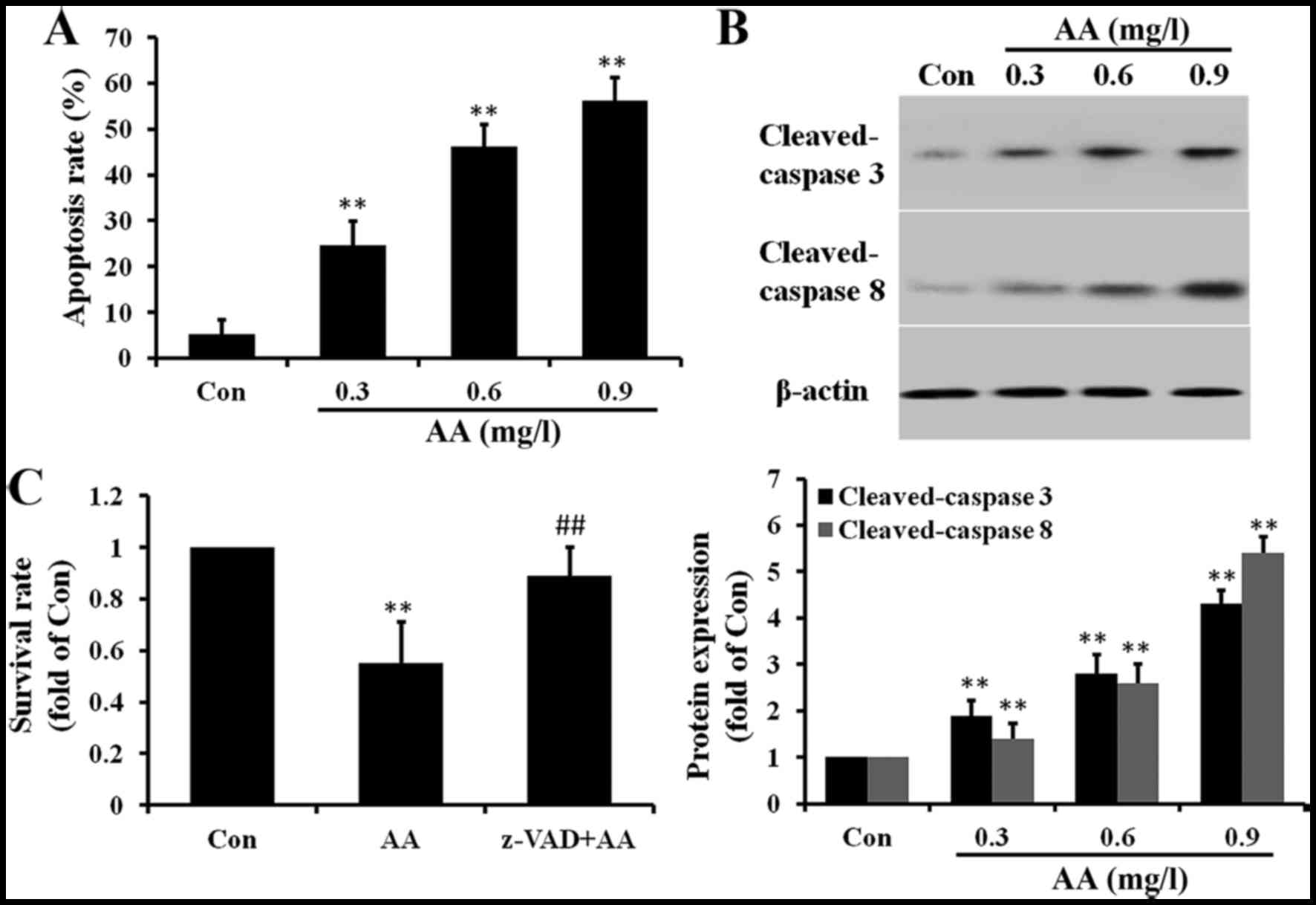
AA induces apoptosis in brain glioma
cells
AA caused U87 cell death and, therefore, the role of
apoptosis in the process was determined subsequently. The
occurrence of apoptosis was performed by an Annexin
V-FITC/propidium iodide (PI) double-staining assay. As shown in
Fig. 4A, the percentage of
apoptotic cells (including early and late apoptotic cells)
increased significantly from 5.3 to 56.2%. As caspase activation
plays an important role in the process of apoptosis, western
blotting was performed to examine caspase activation. As shown in
Fig. 4B, cleaved caspase-8 and
caspase-3 were clearly increased in AA-treated cells. To evaluate
the role of caspases in cell apoptosis, U87 cells were pretreated
with the pan-caspase inhibitor z-VAD-fmk for 3 h prior to
treatment. As shown in Fig. 4C,
z-VAD-fmk pretreatment significantly reduced cell death caused by
AA. These results confirmed that AA may promote apoptosis via a
caspase-dependent mechanism.
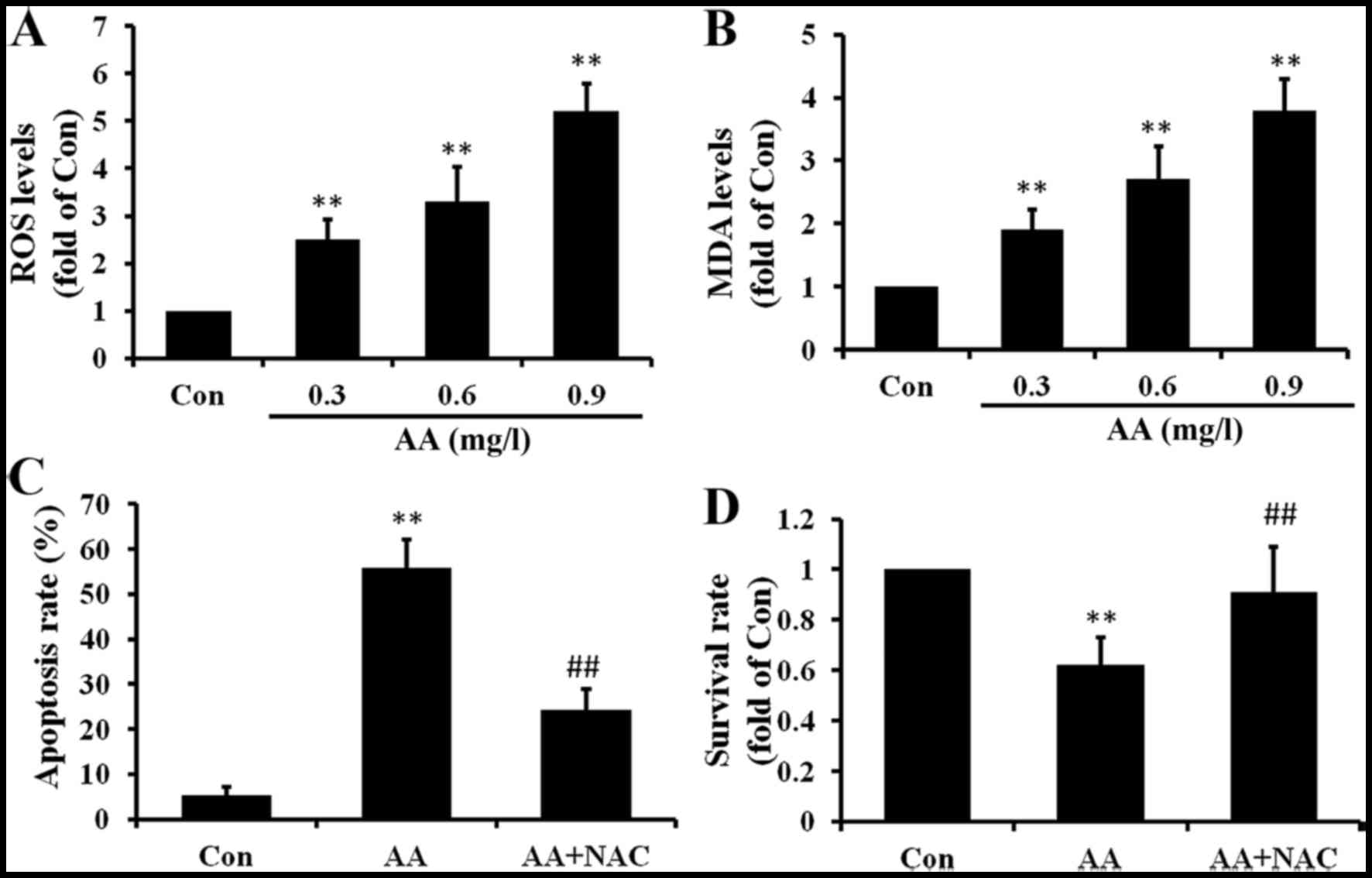
AA induces oxidative stress in brain
glioma cells
To further investigate whether oxidative stress was
involved in the AA-induced apoptosis, ROS and MDA levels in U87
cells were measured. As shown in Fig.
5A, AA induced the production of ROS in U87 cells in a
dose-dependent manner. ROS always induce lipid peroxidation of
cells, so we also measured the MDA levels. As the results show in
Fig. 5B, MDA levels were induced
by AA treatment and also in a dose-dependent manner. To evaluate
the role of ROS in cell apoptosis, U87 cells were pretreated with
the ROS scavenger, NAC. Cotreatment with NAC attenuated both
AA-induced apoptosis and growth inhibition in U87 cells (Fig. 5C and D). These results suggested
that ROS production occurs upstream of AA-induced apoptosis in U87
cells.
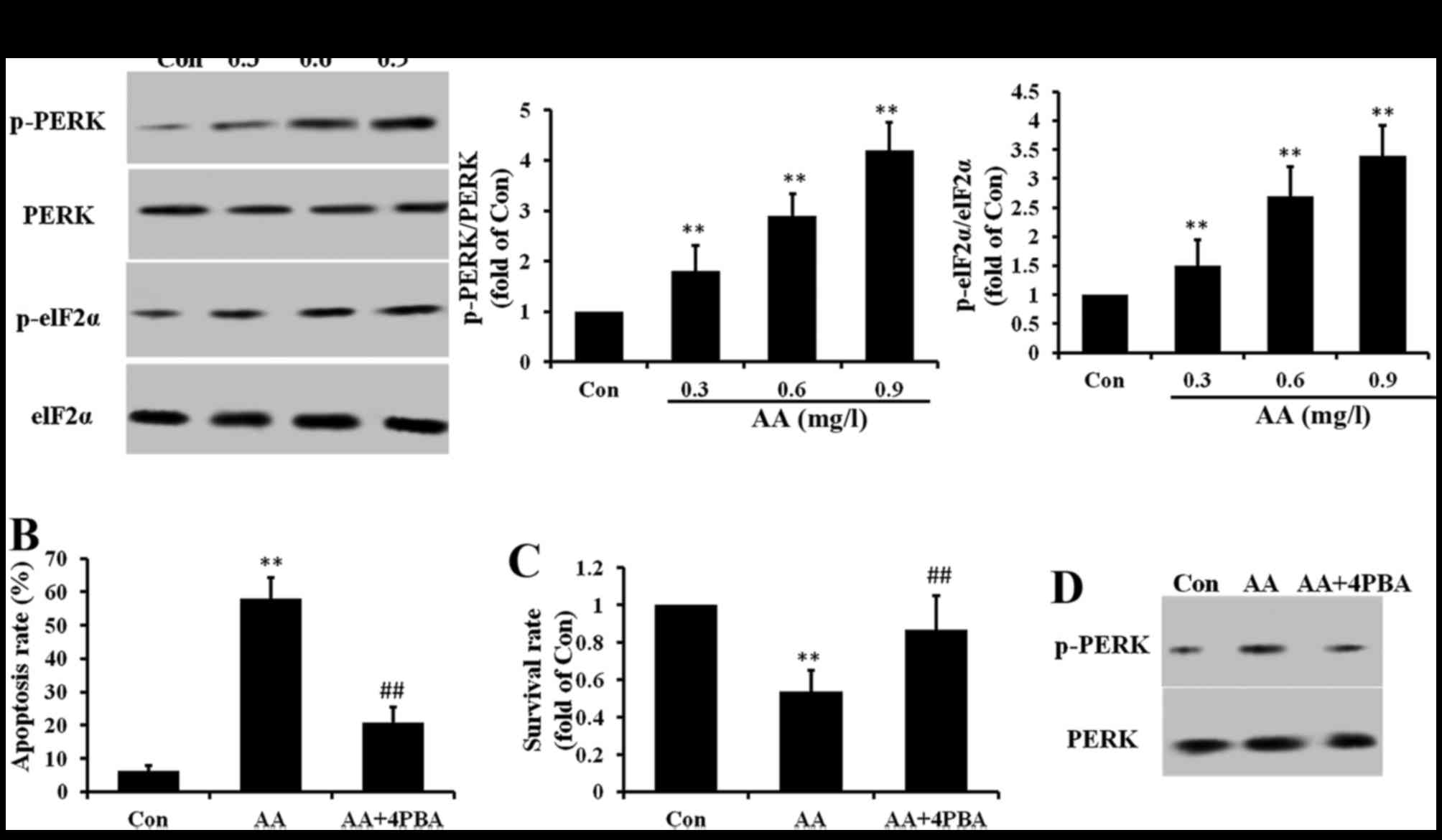
AA induces ER stress in brain glioma
cells
To further investigate whether other signaling
molecules are involved in the AA-induced apoptosis, ER stress was
measured. Our results showed that ER stress makers PERK and elF2α
were phosphorylated by AA treatment (Fig. 6A). To evaluate the role of ER
stress in cell apoptosis, 4PBA (10 mM), an ER stress inhibitor was
used. Cotreatment with 4PBA inhibited both AA-induced apoptosis and
growth inhibition in U87 cells (Fig.
6B and C). To make clear the relationship between ER stress and
oxidative stress in the apoptosis process, NAC was used. We found
that cotreatment with NAC decreased the phosphorylation of PERK
caused by AA (Fig. 6D). These
results suggested that AA induced ER stress through oxidative
stress.
Discussion
AA a Chinese herbal medicine, has shown potent
activities in inhibiting cancer (21). AT one of the primary active
quassinoids in AA, has also been reported to possess some
inhibiting properties in various cancers (20). However, no HPLC analytical method
which could be used in the routine analysis to improve the quality
control has been reported. In this study, a quick and simple HPLC
analytical method was build and its pharmacological studies were
also studied.
Different HPLC conditions including mobile phase,
column and detection wavelength were investigated to select the
optimization of chromatographic conditions. Three different types
of LC columns were investigated: Yilite Hypersil base deactivated
silica C18 column, Yilite SinoChrom ODS-BP C18 column,
Kromasil 100-5 C18 column. Kromasil 100-5 C18 column were selected
because they provided better separation efficiency. The HPLC mobile
phase in methanol-water, acetonitrile-water and the different
concentrations of modifiers (formic acid, acetic acid,
triethylamine and phosphoric acid) were investigated. Finally, 1%
formic acid and 1% methanol-water was applied for the separation of
chemical constituents in AA. The monitoring wavelength was set at
250 nm for fingerprinting analysis.
Analytical method validation was performed to test
the reliability. The linearity of AT was 6.44–825 µg/ml, and
the determination coefficient was 0.9992, which indicated the
regression model for the calibration curves confirmed the good
linearity of the method. The low LOD and LOQ values indicated that
the newly established method provides adequate sensitivity. The
results of precision, repeatability, recovery, and stability showed
that the relative standard deviation (RSD) values were all
<2.3%. These results indicated that the proposed method is well
validated and suitable for quantitatively detecting AT in AA.
Toxic, bioactive, characteristic, synergistic, main,
correlative and general components were often used as the quality
control markers of TCM (27). The
chemical marker is useful for those herbal medicines of limited and
known chemical composition. In this study, the content of AT was
selected as the quality control. Ten batches of AA from different
provinces in China were detected, and we found that the contents of
AT ranged from 0.21–1.78 mg/g in different origins. Henan province
had the highest level, and Yunnan province had the lowest level.
These data provide an important reference for the quality of AA
used as herbal medicine.
Chromatographic fingerprinting, a rational and
powerful approach to characterize a multi-herb formulation, has
been widely used in TCM. There are many analytical methods
including X-ray diffraction (XRD), gas chromatography (GC),
high-performance liquid chromatography (HPLC), and capillary
electrophoresis (CE), used in the fingerprinting analysis (28). HPLC has been proven to be an
economical, stable, and reliable method for fingerprinting analysis
(29). In this study, 10 batches
of AA collected from 10 different regions were analyzed by the HPLC
fingerprinting method according to a standard protocol. Nineteen
common peaks were selected as characteristic peaks. Results
obtained through similarity evaluation software showed that the
similarity of 10 batches is <1.5%. The relative retention time
similarities of communal peaks were all >0.99, and the peak area
similarities of communal peaks were significantly different
(0.682–0.954). These results demonstrated that different samples
had similar constituents and the contents of main bioactive
constituents varied in a large range. Therefore, HPLC
fingerprinting combined with simultaneous quantification of AT
could be taken into the consideration for quality control of
AA.
Glioblastoma is the most lethal form of cancer in
adults, affecting >10,000 patients each year in USA (30). Glioblastoma is characterized by
abnormal regulation of glial cell differentiation and its
invasiveness and aggressive progression. Once diagnosed with
glioblastoma, a median survival is <2 years (31). In the past decades, treatment
options and medications have remained limited with few advances or
success (32). Thus, screening
anti-glioblastoma drugs from TCMs may be an effective method. Even
through AA has shown good inhibiting activity in treating liver
cancer, human breast cancer and cervical cancer, however, the
effects of AA on glioblastoma is not known. In this study, U87
cells were used and treated with AA, and then therapeutic effect
and action mechanism were investigated. We found that AA inhibited
the growth of U87 in a concentration- and time-dependent manner. In
addition, we found apoptosis was induced by AA treatment through
caspase activation. Oxidative stress and ER stress were also found
in AA treated U87 cells, and they all play important roles in
apoptosis. The relationships between oxidative stress, ER stress
and caspases in cell apoptosis were also investigated by their
specific inhibitors. Our results demonstrated that AA induced
oxidative stress first in U87 cells, then induced ER stress,
finally activated the caspases which caused cell apoptosis.
In conclusion, the results of this study indicated
that the combination of chromatographic fingerprint and
quantitative analysis could be readily used as a quality control
approach for AA. In addition, AA-induced oxidative stress, ER
stress and cell apoptosis in U87 cells. Our study demonstrated that
AA may be a potential candidate herb for further development as
prescription or adjuvant treatment for glioblastoma.
Acknowledgments
Not applicable.
Notes
[1]
Funding
This study was supported by the Shaanxi Science and
Technology Innovation Project Plan (no. 2015HBGC-16) and the Key
Research Laboratory of Traditional Chinese Medicine and Natural
Medicine in Shaanxi Province (no. 2015-164).
[2] Availability
of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
QH and SW conceived and designed the study. JL, YL,
MJ and FW performed the experiments. HX participated in the work of
the HPLC fingerprint analysis of the experiment. YZ and WW provided
the materials. QH and SW wrote the paper. JL, YL, MJ and FW
reviewed and edited the manuscript. All authors read and approved
the manuscript.
[4] Ethics
approval and consent to participate
Not applicable.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Li H, Wang SW, Zhang BL, Xie YH, Yang Q,
Cao W and Wang JB: Simultaneous quantitative determination of 9
active components in traditional Chinese medicinal preparation
ShuangDan oral liquid by RP-HPLC coupled with photodiode array
detection. J Pharm Biomed Anal. 56:820–824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
SW W: Molecular composition theory of
Chinese medicinal materials and modern traditional Chinese
medicine. J Asia-Pac Tradit. 4:9–12. 2008.
|
|
3
|
Xie PS and Leung AY: Understanding the
traditional aspect of Chinese medicine in order to achieve
meaningful quality control of Chinese materia medica. J Chromatogr
A. 1216:1933–1940. 2009. View Article : Google Scholar
|
|
4
|
Zhang Z, Li Q, Li Q, Du S, Zhou Y, Lv C,
Zhao Y, Wang Y and Zhang N: Simultaneous determination of nineteen
major components in Qi She Pill by ultra-high-performance liquid
chromatography-tandem mass spectrometry. Acta Pharm Sin B.
4:384–393. 2014. View Article : Google Scholar
|
|
5
|
Khan IA: Issues related to botanicals.
Life Sci. 78:2033–2038. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim HM, Lee JS, Sezirahiga J, Kwon J,
Jeong M, Lee D, Choi JH and Jang DS: A new canthinone-type alkaloid
isolated from Ailanthus altissima Swingle. Molecules. 21:E6422016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Howard JL: Ailanthus altissima; Fire
Effects Information System. US Department of Agriculture, Forest
Service, Rocky Mountain Research Station, Fire Sciences Laboratory;
Fort Collins, CO, USA: 2004. 2014
|
|
8
|
Kowarik I and Saumel I: Biological flora
of central Europe: Ailanthus altissima (Mill.) swingle. Perspect
Plant Ecol Evol Syst. 8:302007. View Article : Google Scholar
|
|
9
|
Jin MH, Yook J, Lee E, Lin CX, Quan Z, Son
KH, Bae KH, Kim HP, Kang SS and Chang HW: Anti-inflammatory
activity of Ailanthus altissima in ovalbumin-induced lung
inflammation. Biol Pharm Bull. 29:884–888. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang TH, Choi IY, Kim SJ, Moon PD, Seo JU,
Kim JJ, An NH, Kim SH, Kim MH, Um JY, et al: Ailanthus altissima
swingle has anti-anaphylactic effect and inhibits inflammatory
cytokine expression via suppression of nuclear factor-kappaB
activation. In Vitro Cell Dev Biol Anim. 46:72–81. 2010. View Article : Google Scholar
|
|
11
|
Casinovi CG, Ceccherelli P, Fardella G and
Grandolini G: Isolation and structure of a quassinoid from
Ailanthus glandulosa. Phytochemistry. 22:2871–2873. 1983.
View Article : Google Scholar
|
|
12
|
Kubota K, Fukamiya N, Hamada T, Okano M,
Tagahara K and Lee KH: Two new quassinoids, ailantinols A and B,
and related compounds from Ailanthus altissima. J Nat Prod.
59:683–686. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kosuge K, Mitsunaga K, Koike K and Ohmoto
T: Studies on the constituents of Ailanthus integrifolia. Chem
Pharm Bull (Tokyo). 42:1669–1671. 1994. View Article : Google Scholar
|
|
14
|
Hwang SG, Lee HC, Kim CK, Kim DG, Lee GO,
Yun YG and Jeon BH: Effect of Ailanthus altissima water extract on
cell cycle control genes in Jurkat T lymphocytes. Yakhak Hoechi.
46:18–23. 2002.
|
|
15
|
Tamura S, Fukamiya N, Mou XY, Mukainaka T,
Tokuda H, Nishino H, Tagahara K, Koike K, Lee KH and Okano M:
Conversion of quassinoids for enhancement of inhibitory effect
against Epstein-Barr virus early antigen activation. Introduction
of lipophilic side chain and esterification of diosphenol. Chem
Pharm Bull (Tokyo). 48:876–878. 2000. View Article : Google Scholar
|
|
16
|
Okunade AL, Bikoff RE, Casper SJ, Oksman
A, Goldberg DE and Lewis WH: Antiplasmodial activity of extracts
and quassinoids isolated from seedlings of Ailanthus altissima
(Simaroubaceae). Phytother Res. 17:675–677. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fukamiya N, Lee KH, Muhammad I, Murakami
C, Okano M, Harvey I and Pelletier J: Structure-activity
relationships of quassinoids for eukaryotic protein synthesis.
Cancer Lett. 220:37–48. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rosati A, Quaranta E, Ammirante M, Turco
MC, Leone A and De Feo V: Quassinoids can induce mitochondrial
membrane depolarisation and caspase 3 activation in human cells.
Cell Death Differ. 11(Suppl 2): S216–S218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Wang WJ, Su C, Zhang DM, Xu LP, He
RR, Wang L, Zhang J, Zhang XQ and Ye WC: Cytotoxic quassinoids from
Ailanthus altissima. Bioorg Med Chem Lett. 23:654–657. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang XL, Yuan YL, Zhang DM, Li F and Ye
WC: Shinjulactone O, a new quassinoid from the root bark of
Ailanthus altissima. Nat Prod Res. 28:1432–1437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ishibashi M, Tsuyuki T and Takahashi T:
Bitter principles of Ailanthus altissima SWingLE. Conversion of
ailanthone into shinjulactone C. Bull Chem Soc Jpn. 58:2357–2360.
1985. View Article : Google Scholar
|
|
22
|
Jaziri M, Diallo B and Vanhaelen M:
Separation of quassinoids from Ailanthus altissima by high-speed
counter-current chromatography. J Chromatogr A. 538:227–229. 1991.
View Article : Google Scholar
|
|
23
|
Furey A, Moriarty M, Bane V, Kinsella B
and Lehane M: Ion suppression; a critical review on causes,
evaluation, prevention and applications. Talanta. 115:104–122.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin XF, Lu YH, Wei DZ and Wang ZT:
Chemical fingerprint and quantitative analysis of Salvia plebeia
R.Br. by high-performance liquid chromatography. J Pharm Biomed
Anal. 48:100–104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang D, Yang D, Tang A, Gao Y, Jiang X,
Mou J and Yin X: Simultaneous chemical fingerprint and quantitative
analysis of Ginkgo biloba extract by HPLC-DAD. Anal Bioanal Chem.
396:3087–3095. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei H, Sun L, Tai Z, Gao S, Xu W and Chen
W: A simple and sensitive HPLC method for the simultaneous
determination of eight bioactive components and fingerprint
analysis of Schisandra sphenanthera. Anal Chim Acta. 662:97–104.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu EH, Qi LW, Li K, Chu C and Li P:
Recent advances in quality control of traditional Chinese
medicines. Comb Chem High Throughput Screen. 13:869–884. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhong XK, Li DC and Jiang JG:
Identification and quality control of Chinese medicine based on the
fingerprint techniques. Curr Med Chem. 16:3064–3075. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li T, Zhuang S, Wang Y, Wang Y, Wang W,
Zhang H, Chen L, Wang D, Zhou Z and Yang W: Flavonoid profiling of
a traditional Chinese medicine formula of Huangqin Tang using high
performance liquid chromatography. Acta Pharm Sin B. 6:148–157.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ostrom QT, Gittleman H, de Blank PM,
Finlay JL, Gurney JG, McKean-Cowdin R, Stearns DS, Wolff JE, Liu M,
Wolinsky Y, et al: American brain tumor association adolescent and
young adult primary brain and central nervous system tumors
diagnosed in the United States in 2008–2012. Neuro Oncol. 18(Suppl
1): i1–i50. 2016. View Article : Google Scholar
|
|
31
|
Adamson C, Kanu OO, Mehta AI, Di C, Lin N,
Mattox AK and Bigner DD: Glioblastoma multiforme: A review of where
we have been and where we are going. Expert Opin Investig Drugs.
18:1061–1083. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Levin VA, Tonge PJ, Gallo JM, Birtwistle
MR, Dar AC, Iavarone A, Paddison PJ, Heffron TP, Elmquist WF,
Lachowicz JE, et al: CNS anticancer drug discovery and development
conference white paper. Neuro Oncol. 17(Suppl 6): vi1–vi26. 2015.
View Article : Google Scholar : PubMed/NCBI
|