Introduction
Bisphosphonate-related osteonecrosis of the jaw
(BRONJ) is a newly reported disease. The first cases of BRONJ
associated with the use of oral bisphosphonates (BPs) were reported
in 2003 (1). Most of the reported
cases were of patients who had received intravenous BPs, including
alendronate, zoledronate (ZON) and ibandronate (2,3).
Numerous studies have attempted to identify the pathogenesis of
BRONJ (2,3). In addition to hard tissue
disturbance and immune system disorders caused by BPs, the
anti-angiogenic action of BPs was also reported as a possible
pathological mechanism of BRONJ (4,5).
The antiangiogenic theory states that the inhibition of
neo-angiogenesis by BPs leads to a loss of blood vessels in bone
tissue and avascular necrosis (1). Although numerous molecules are known
to be associated with the cellular mechanisms of angiogenesis
(6), they have remained to be
fully elucidated.
Angiogenesis, the formation and growth of new
capillary blood vessels, is an important process in numerous
physiological conditions, including embryonic development and wound
healing. A previous study reported that ZON, a new-generation BP
with a heterocyclic imidazole substituent, is a potent inhibitor of
angiogenesis (7). This study also
demonstrated that ZON inhibited the proliferation and regulated the
adhesion and migration of endothelial cells. Furthermore, Yamada
et al (8) reported that
ZON inhibited angiogenic processes, including capillary tube
formation.
Interferon-induced transmembrane protein 1 (IFITM1),
also known as 9–27, CD225 or Leu 13, is a member of the IFITM
family. The protein is a cell surface molecule with several
molecular functions, including cell adhesion and proliferation. In
particular, the IFITM1 protein has been identified as an antiviral
restriction factor for influenza A virus replication (9) and is overexpressed in several types
of cancer, including colorectal, gastrointestinal, head and neck,
and breast cancer (10–12). A recent study demonstrated that
human IFITM1 regulates angiogenesis in human endothelial cells
(13). However, the possible
implication of IFITM1 in the anti-angiogenic activity of ZON has
remained to be elucidated. Therefore, the purpose of the present
study was to investigate the association between the
anti-angiogenic activity of ZON and the IFITM1 protein in human
umbilical vein endothelial cells (HUVECs).
Materials and methods
Cell culture
Immortalized HUVECs were purchased from the American
Type Culture Collection (Manassas, VA, USA) and maintained in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (both from Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA and antibiotics. The cells were cultured at
37°C in a humidified atmosphere with 5% CO2.
Cell proliferation assay
The cells were seeded in 96-well plates at
3×103 cells/well. After 24 h, the culture medium was
replaced with medium containing ZON (1 nM-10 μM) and/or
vascular endothelial growth factor (VEGF; 10 ng/ml; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). After incubation for 3 days, cell
proliferation was analyzed by using an MTS assay kit. The
absorbance was measured at 490 nm on a microplate reader (Spectra
MAX M3; Molecular Devices, Sunnyvale, CA, USA) and the changes in
cell proliferation were expressed as a percentage of the mean value
of the control (untreated cells).
Matrigel tube formation assay
To determine capillary tube formation, a Matrigel
tube formation assay was performed. The prepared liquid Matrigel
(BD Biosciences, Franklin Lakes, NJ, USA) was added into a 96-well
culture plate at 70 μl/well and allowed to polymerize for 1
h at 37°C. Prior to the tube formation assay, the HUVECs were
cultured in serum-free medium for 24 h, seeded on Matrigel
(3×104 cells/well) with VEGF or various concentrations
of ZON, and then incubated at 37°C for 24 h. Subsequently, tube
formation was observed under a microscope (DM IL LED Fluo; Leica
Microsystems, Wetzlar, Germany) and the total tube length was
measured by using Image Pro Plus 6.0 software (Media Cybernetics,
Rockville, MD, USA).
Western blot analysis
Western blot analysis was used to detect IFITM1
expression. In brief, cells were grown in serum-free DMEM for 24 h
and then treated with VEGF or various concentrations of ZON. The
total protein was subsequently obtained by using a cell lysis
buffer (iNtRon Biotechnology; Seongnam, Korea). The protein
concentrations of the sample were determined using a BCA protein
assay kit (Thermo Fisher Scientific, Inc.) and equal amounts (50
μg) of protein were loaded, separated by 12% SDS-PAGE and
transferred onto a nitrocellulose membrane (GE Healthcare; Little
Chalfont, UK). The membranes were blocked with 1% bovine serum
albumin (Sigma-Aldrich; Merck KGaA) in 50 mM Tris (pH 7.5)
containing 100 mM NaCl and 0.1% Tween-20, and incubated with the
primary antibody, anti-IFITM1 (cat. no. 13126S; dilution 1:1,000;
Cell Signaling Technology, Inc., Danvers, MA, USA) for 3 h at room
temperature. After extensive washing in TBS and 0.1% Tween-20, the
horseradish peroxidase-conjugated secondary antibody (cat. no.
7074S; dilution 1:5,000; Cell Signaling Technology, Inc.) was added
and the membranes were incubated for a further 1 h at room
temperature. Finally, the membrane was visualized by using an
enhanced chemiluminescence kit (GE Healthcare).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
To determine the mRNA expression of IFITM1, total
mRNA was extracted using an RNA isolation kit (Ribospin II; Gene
All, Seoul, Korea), and complementary DNA was synthesized by
reverse transcriptase (RT) with oligo(dT) primers using Accu
Power® RTPre Mix (Bioneer Corporation, Daejeon, Korea).
The sequences of the primers used for this application were as
follows: IFITM1 forward, 5′-AGCCAGAAGATGCACAAGGA-3′ and reverse,
5′-GATCACGGTGGACCTTGGAA-3′; β-actin forward,
5′-AATGCTTCTAGGCGGACTATG-3′ and reverse,
5′-TTTCTGCGCAAGTTAGGTTTT-3′. Real-time PCR was performed using an
ABI Step One Plus™ machine (Applied Biosystems; Thermo Fisher
Scientific, Inc.) with SYBR-Green (Fast SYBR™-Green Master mix;
Applied Biosystems). Thermocycling conditions were as follows:
Initial denaturation at 95°C for 10 min for 1 cycle, followed by
denaturation at 92°C for 15 sec and annealing/extension at 60°C for
30 sec, repeated for 40 cycles. The data were normalized to the
geometric means of the reference gene β-actin using a comparative
ΔΔCq method (14).
Zymography
To analyze matrix metalloproteinase (MMP-9)
activation, gelatinase zymography was used. Gelatinolytic activity
of VEGF or/and ZON was visualized on SDS-PAGE gels (10%
polyacrylamide) containing 1 mg/ml gelatine (Sigma-Aldrich; Merck
KGaA). The gels were soaked in 2.5% Triton X100 (Sigma-Aldrich;
Merck KGaA) for 40 min at 37°C, and incubated in 50 mM Tris-HCl (pH
7.5) with 10 mM CaCl2 and 10 mM NaCl overnight at 37°C,
followed by staining with Coomassie brilliant blue (Sigma-Aldrich;
Merck KGaA). The zymography results were quantified using Image J
software version 1.37a (National Institutes of Health, Bethesda,
MD, USA).
Statistical analysis
All experiments were performed a minimum of three
times. The values are expressed as the mean ± standard deviation.
Statistical analyses were performed with GraphPad Prism version 5.3
(GraphPad Software, Inc., La Jolla, CA, USA). Significant
differences among groups were identified by one-way analysis of
variance followed by Dunnett's multiple comparisons test. P<0.05
was considered to indicate a statistically significant
difference.
Results
ZON inhibits VEGF-induced HUVEC
proliferation
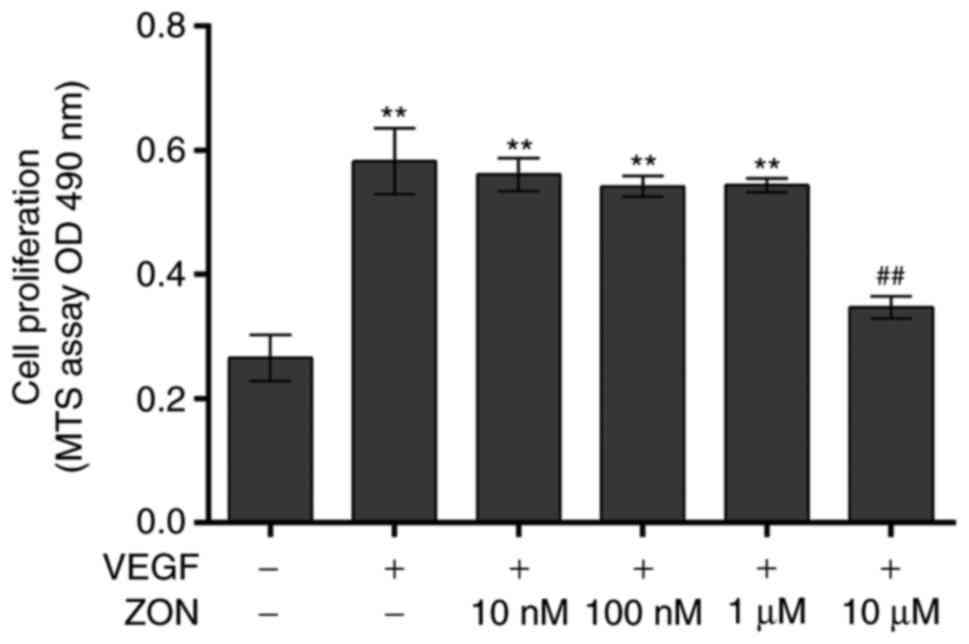
To investigate the effects of ZON on vascular
endothelial cell proliferation, the viability of HUVECs treated
with various concentrations of ZON (10 nM-10 μM) in the
presence of VEGF was first analyzed. The cell proliferation was
significantly increased by 10 ng/ml VEGF (252.1±23.17%). ZON (10
nM-1 μM) did not significantly alter the VEGF-induced
proliferation. However, the proliferation was inhibited by 10
μM ZON (150.2±7.7%) (Fig.
1). These results indicated that ZON inhibited VEGF-induced
HUVEC proliferation in vitro.
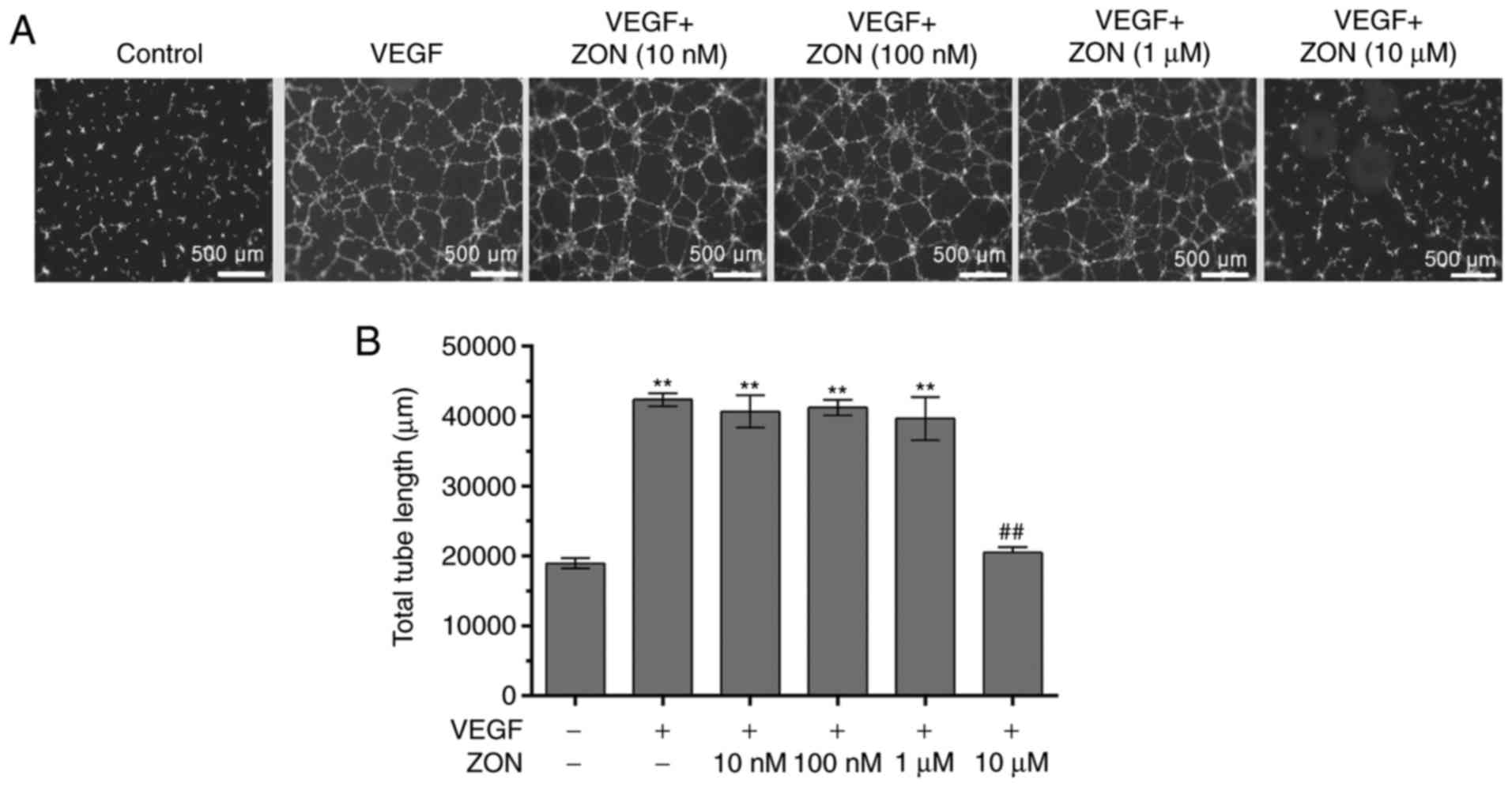
ZON inhibits VEGF-induced tube
formation
To evaluate the effects of ZON on tube formation,
tube formation in Matrigel was evaluated after treatment with VEGF
and/or ZON. The levels of endothelial cell capillary tube and
network formation were markedly increased by 10 ng/ml VEGF
(~2-fold). Although tube formation was not significantly affected
by 10 nM-1 μM ZON (data not shown), the VEGF-induced tube
formation of HUVECs was strongly suppressed by 10 μM ZON
(Fig. 2). These results indicated
that ZON inhibited VEGF-induced capillary tube formation by
HUVECs.
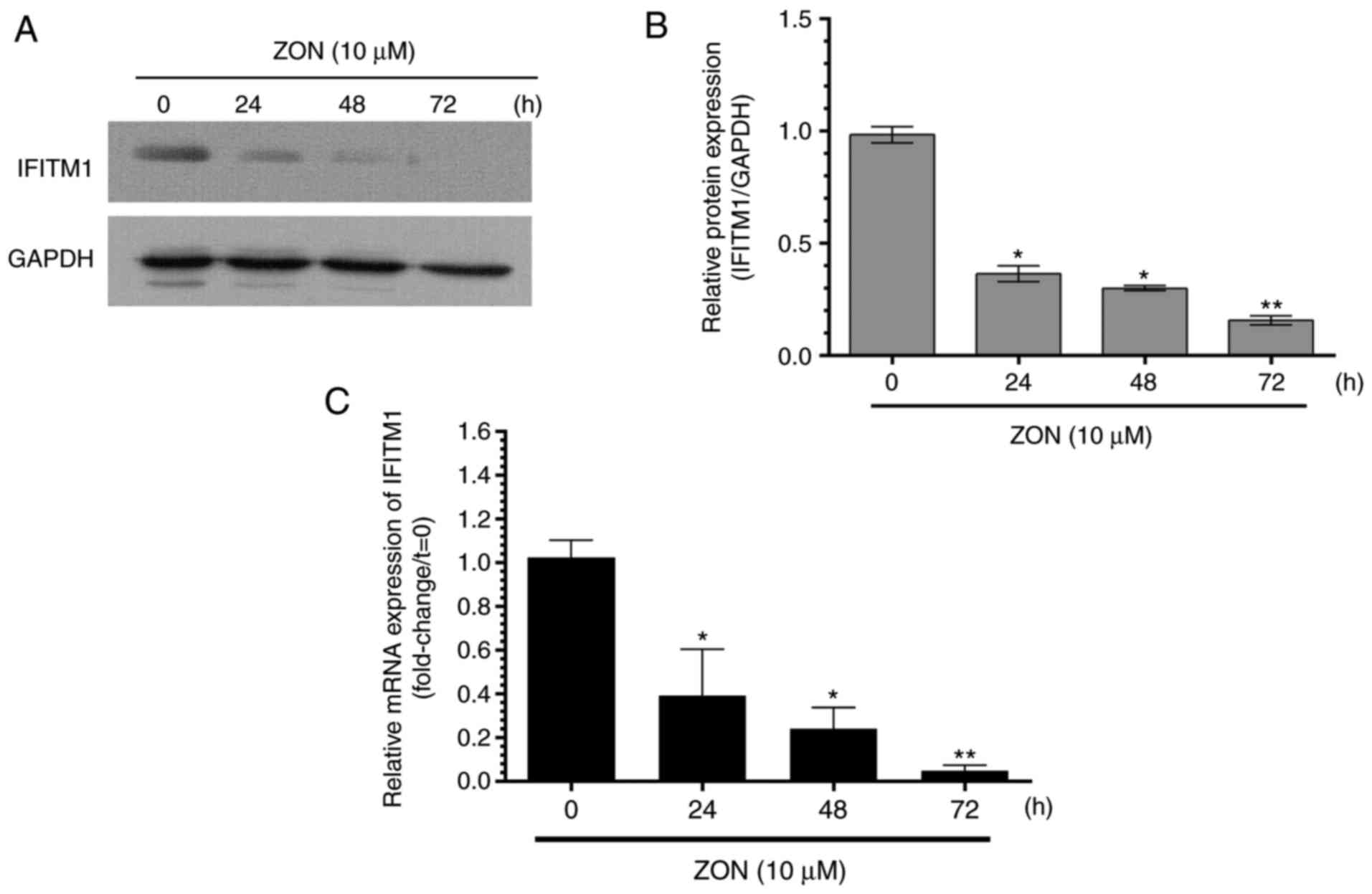
Effect of ZON on IFITM1 expression
The involvement of IFITM1 in ZON-induced inhibition
of cell proliferation and tube formation was then explored.
Fig. 3A and B presents IFITM1
protein expression after treatment with 10 μM ZON for 24, 48
and 72 h. Furthermore, the relative IFITM1 mRNA expression
determined by RT-qPCR is presented in Fig. 3C. In the absence of ZON, IFITM1
was expressed at basal levels. However, IFITM1 protein and mRNA
expression was significantly decreased by ZON in a time-dependent
manner. These results indicated that IFITM1 expression was
inhibited by treatment with ZON. By contrast, IFITM1 expression was
enhanced by VEGF treatment for 24 h.
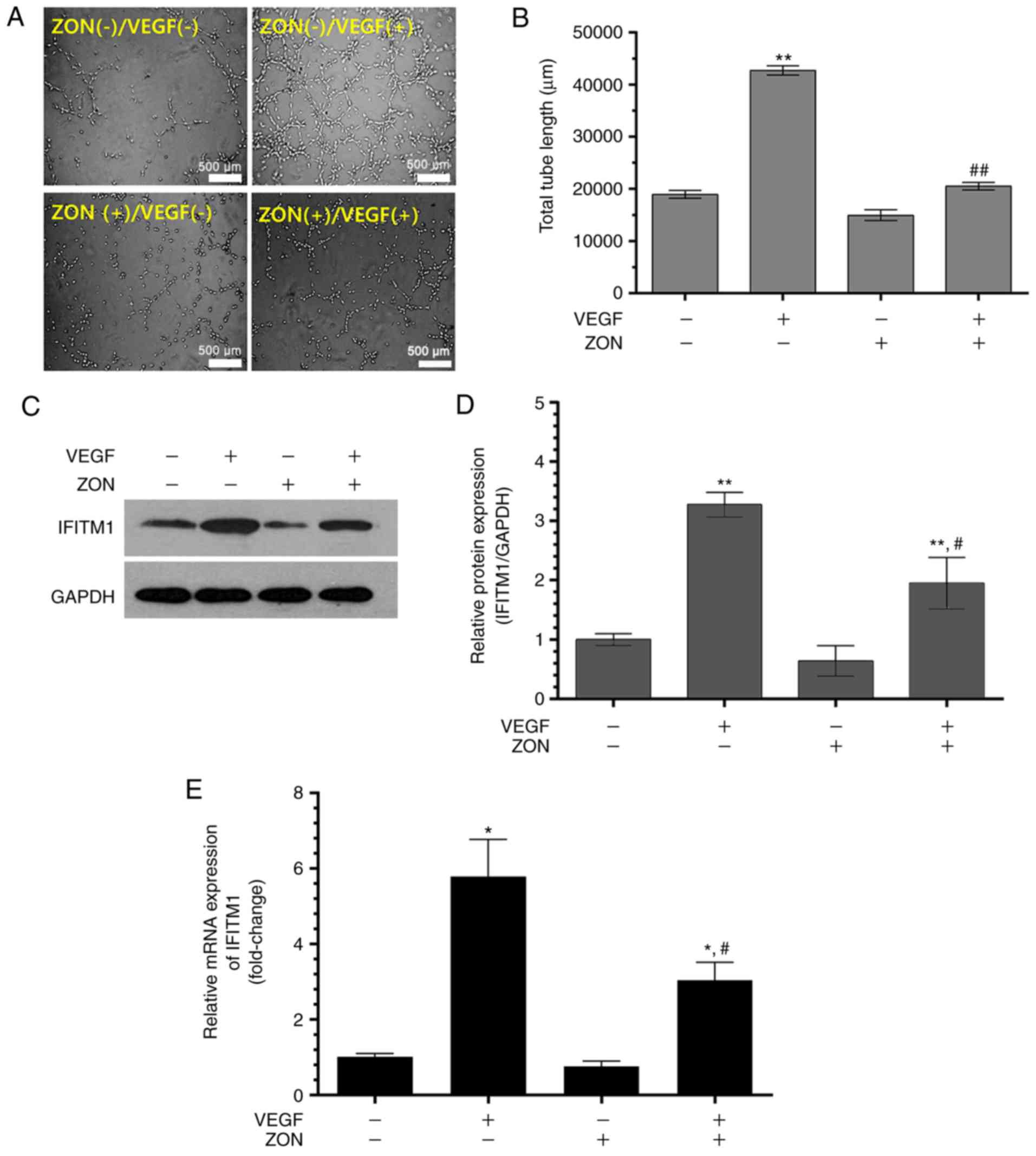
ZON inhibits VEGF-induced tube formation
and IFITM1 expression
To confirm the effect of ZON on VEGF-induced tube
formation and IFITM1 expression, tube formation and IFITM1
expression were evaluated in the presence or absence of 10 ng/ml
VEGF and/or 10 μM ZON. In the ZON(−)/VEGF(+) group, the
total tube length significantly increased (~2-fold). In the
ZON(+)/VEGF(−) group, the tube length was slightly, but not
significantly, decreased in comparison with that in the
ZON(−)/VEGF(−) group. The total tube length was markedly decreased
in the ZON(+)/VEGF(+) group in comparison with that in the
ZON(−)/VEGF(+) group (Fig. 4A and
B). Western blot analysis of IFITM1 indicated that the
expression of IFITM1 was inhibited in the ZON(+)/VEGF(+) group in
comparison with that in the ZON(−)/VEGF(+) group (Fig. 4C and D). Furthermore, RT-qPCR also
indicated that IFITM1 mRNA expression decreased after treatment
with ZON (Fig. 4E). These results
indicated that 10 μM ZON inhibited VEGF-induced tube
formation activity and IFITM1 expression in HUVECs.
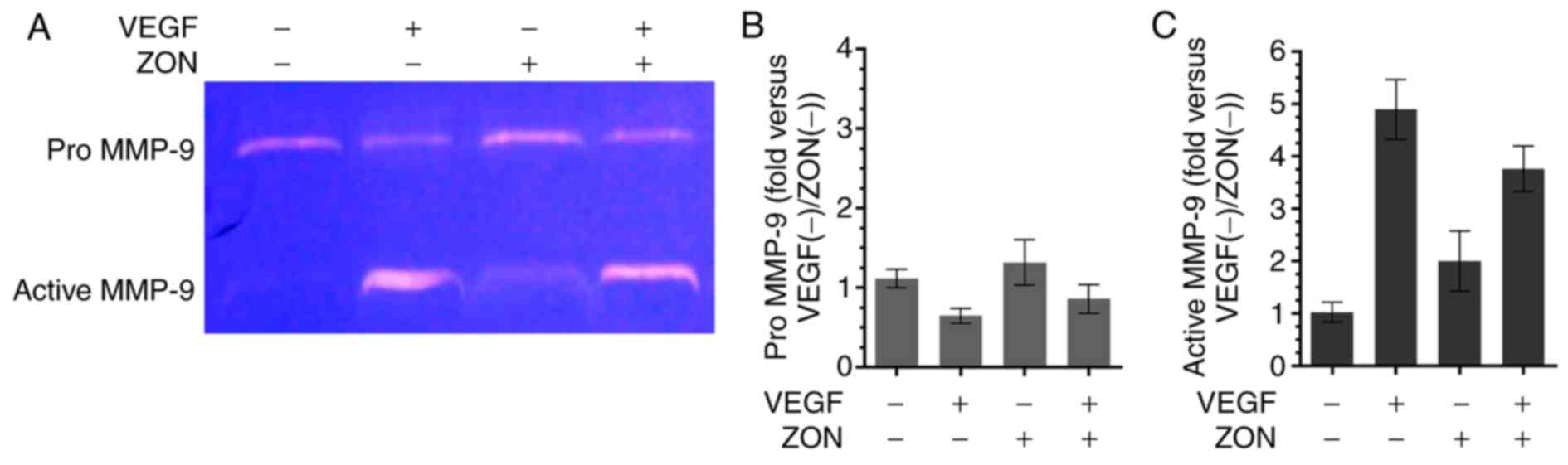
ZON inhibits VEGF-induced MMP-9
activation in HUVECs
To investigate whether the anti-tube formation
effect of ZON was associated with MMP-9, the activation of MMP-9
was determined by zymography (Fig.
5A). The results indicated that pro MMP-9 expression was
reduced by VEGF. Quantitative analysis revealed that the activated
form of MMP-9 was notably increased by VEGF treatment. However, the
VEGF-induced activated form of MMP-9 was slightly decreased by ZON
(Fig. 5B and C). These results
revealed that VEGF-induced MMP-9 activation was suppressed by
ZON.
Discussion
BRONJ is a well-known adverse effect of long-term BP
therapy and represents a challenge for dentists and maxillofacial
surgeons (15). Several studies
have suggested the anti-angiogenic action of BP as a possible
pathological mechanism of BRONJ (16). Although the anti-angiogenic effect
of BPs, including ZON, has been assumed to be responsible for the
pathology of BRONJ, the associated molecular events have remained
to be fully characterized. The present study focused on the role of
IFITM1 expression in the anti-angiogenic activity of ZON in
HUVECs.
The present study first investigated the effect of
ZON on endothelial cell proliferation and observed that ZON
inhibited the rate of VEGF-induced endothelial cell growth. In a
previous study, ZON (~64 μM) decreased the viability of
HUVECs (17). In addition,
another previous study indicated that ZON at doses of 30 and 100
μM exerted a moderate effect in decreasing HUVEC viability
(7). The authors demonstrated
that ZON caused cellular apoptosis and sub-G1 phase arrest. In the
present study, HUVEC proliferation was significantly suppressed by
10 μM ZON in the presence of 10 ng/ml VEGF. Although the
present study did not establish the reason for the dose-dependent
effect of ZON on HUVEC anti-proliferation, the results indicated
that the concentration of 10 μM ZON inhibited VEGF-induced
HUVECs proliferation, which was lower than observed in previous
studies (7,17).
The capillary tube formation assay is one of the
most widely used in vitro assays to model the reorganization
stage of angiogenesis (18). A
previous study reported that ZON also reduced tube formation by
human dermal microvascular endothelial cells (19). Fournier et al (20) also reported that treatment with
ZON decreased capillary tube formation in vitro. Therefore,
to determine whether ZON inhibited angiogenesis, tube formation was
examined in the present study by using the Matrigel tube formation
assay, which demonstrated that the tube formation capacity was
inhibited by treatment with 10 μM ZON. These results were
also in agreement with previous studies on the anti-angiogenic
effects of ZON (7,20,21).
A previous study (22) suggested that the inhibition of
neovascularization was associated with MMP-9, VEGF, endothelial
nitric oxide synthase and AKT signaling in endothelial progenitor
cells. In addition, ZON was reported to reduce the mRNA and protein
expression of VEGF in A549 non-small cell lung cancer cells
(23). ZON was also demonstrated
to inhibit endothelial cell adhesion and migration, and reduce Ras
prenylation (24). In the present
study, ZON inhibited the proliferation and capillary tube formation
of HUVECs. Furthermore, it was observed that the expression of
IFITM1 was reduced during ZON treatment in a time-dependent manner.
Jaffe et al (25)
demonstrated that IFITM1 expression and synthesis were induced by
interferon-γ and interferon-α in cultured human endothelial cells.
In human glioma cells, knockdown of IFITM1 caused an inhibition of
cell proliferation, migration and invasion (26). Tanaka et al (27) reported that IFITM1 regulated the
adhesion and differentiation of mouse primordial germ cells. In
addition, according to Popson et al (13), IFITM1 regulates endothelial lumen
formation during angiogenesis. The study also reported that
endothelial cells lacking IFITM1 failed to form stable cell-cell
contacts (13).
During angiogenesis, MMP-9 activity has important
roles in the degradation of the basement membrane (28). Therefore, the present study
determined MMP-9 activation using gelatin zymography. The results
indicated that MMP-9 activation was markedly increased by VEGF
treatment, but markedly reduced in the presence of ZON. These
results suggested that the anti-angiogenesis effect of ZON may be
mediated through IFITM1 and MMP-9.
Previous studies have reported the
anti-proliferative effect of ZON at high doses and have suggested
that ZON has potential as an anti-angiogenic agent (7,17).
In the present study, the anti-capillary tube formation effects of
ZON (10 μM) in VEGF-treated HUVECs were investigated.
However, at a ZON concentration of 10 μM, VEGF-induced cell
proliferation was also inhibited. Therefore, the possibility that
these anti-angiogenic effects may be due to the cytotoxic effect of
ZON cannot be ruled out. However, during the anti-angiogenic
response, IFITM1 expression increased in response to VEGF, and
VEGF-induced IFITM1 expression was also reduced by treatment with
ZON. These results clearly indicate that IFITM1 is involved in this
effect. Therefore, further functional studies on IFITM1 are
required to identify the association between IFITM1 and
anti-angiogenic activity.
Acknowledgments
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF; grant nos. NRF-2015R1A2A2A01004888 and
NRF-2015R1D1A1A01056748) and partially supported by the X-mind
Corps program of the NRF funded by the Ministry of Science, ICT
& Future Planning (grant no. 2017H1D8A2030449).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Marx RE: Pamidronate (Aredia) and
zoledronate (Zometa) induced avascular necrosis of the jaws: A
growing epidemic. J Oral Maxillofac Surg. 61:1115–1117. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marx RE, Sawatari Y, Fortin M and Broumand
V: Bisphosphonate-induced exposed bone
(osteonecrosis/osteopetrosis) of the jaws: Risk factors,
recognition, prevention, and treatment. J Oral Maxillofac Surg.
63:1567–1575. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pires FR, Miranda A, Cardoso ES, Cardoso
AS, Fregnani ER, Pereira CM, Correa ME, Almeida JP, Alves Fde A,
Lopes MA and de Almeida OP: Oral avascular bone necrosis associated
with chemotherapy and biphosphonate therapy. Oral Dis. 11:365–369.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma D, Ivanovski S, Slevin M, Hamlet S,
Pop TS, Brinzaniuc K, Petcu EB and Miroiu RI:
Bisphosphonate-related osteonecrosis of jaw (BRONJ): Diagnostic
criteria and possible pathogenic mechanisms of an unexpected
anti-angiogenic side effect. Vasc Cell. 5:12013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Petcu EB, Ivanovski S, Wright RG, Slevin
M, Miroiu RI and Brinzaniuc K: Bisphosphonate-related osteonecrosis
of jaw (BRONJ): An anti-angiogenic side-effect? Diagn Pathol.
7:782012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Otrock ZK, Mahfouz RAR, Makarem JA and
Shamseddine AI: Understanding the biology of angiogenesis: Review
of the most important molecular mechanisms. Blood Cells Mol Dis.
39:212–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wood J, Bonjean K, Ruetz S, Bellahcene A,
Devy L, Foidart JM, Castronovo V and Green JR: Novel antiangiogenic
effects of the bisphosphonate compound zoledronic acid. J Pharmacol
Exp Ther. 302:1055–1061. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamada J, Tsuno NH, Kitayama J, Tsuchiya
T, Yoneyama S, Asakage M, Okaji Y, Shuno Y, Nishikawa T, Tanaka J,
et al: Anti-angiogenic property of zoledronic acid by inhibition of
endothelial progenitor cell differentiation. J Surg Res.
151:115–120. 2009. View Article : Google Scholar
|
|
9
|
Brass AL, Huang IC, Benita Y, John SP,
Krishnan MN, Feeley EM, Ryan BJ, Weyer JL, van der Weyden L, Fikrig
E, et al: The IFITM proteins mediate cellular resistance to
influenza A H1N1 virus, West Nile virus, and dengue virus. Cell.
139:1243–1254. 2009. View Article : Google Scholar
|
|
10
|
Hatano H, Kudo Y, Ogawa I, Tsunematsu T,
Kikuchi A, Abiko Y and Takata T: IFN-induced transmembrane protein
1 promotes invasion at early stage of head and neck cancer
progression. Clin Cancer Res. 14:6097–6105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu F, Ng SS, Chow BK, Sze J, Lu G, Poon
WS, Kung HF and Lin MC: Knockdown of interferon-induced
transmembrane protein 1 (IFITM1) inhibits proliferation, migration,
and invasion of glioma cells. J Neurooncol. 103:187–195. 2011.
View Article : Google Scholar
|
|
12
|
Pan Z, Chen S, Pan X, Wang Z, Han H, Zheng
W, Wang X, Li F, Qu S and Shao R: Differential gene expression
identified in uigur women cervical squamous cell carcinoma by
suppression subtractive hybridization. Neoplasma. 57:123–128. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Popson SA, Ziegler ME, Chen X, Holderfield
MT, Shaaban CI, Fong AH, Welch-Reardon KM, Papkoff J and Hughes CC:
Interferon-induced transmembrane protein 1 regulates endothelial
lumen formation during angiogenesis. Arterioscler Thromb Vasc Biol.
34:1011–1019. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vescovi P, Merigo E, Meleti M, Manfredi M,
Guidotti R and Nammour S: Bisphosphonates-related osteonecrosis of
the jaws: A concise review of the literature and a report of a
single-centre experience with 151 patients. J Oral Pathol Med.
41:214–221. 2012. View Article : Google Scholar
|
|
16
|
Bagan JV, Murillo J, Jimenez Y, Poveda R,
Milian MA, Sanchis JM, Silvestre FJ and Scully C: Avascular jaw
osteonecrosis in association with cancer chemotherapy: Series of 10
cases. J Oral Pathol Med. 34:120–123. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Misso G, Porru M, Stoppacciaro A,
Castellano M, De Cicco F, Leonetti C, Santini D and Caraglia M:
Evaluation of the in vitro and in vivo antiangiogenic effects of
denosumab and zoledronic acid. Cancer Biol Ther. 13:1491–1500.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ponce ML: Tube formation: An in vitro
matrigel angiogenesis assay. Methods Mol Biol. 467:183–188. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Michailidou M, Brown HK, Lefley DV, Evans
A, Cross SS, Coleman RE, Brown NJ and Holen I: Microvascular
endothelial cell responses in vitro and in vivo: Modulation by
zoledronic acid and paclitaxel? J Vasc Res. 47:481–493. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fournier P, Boissier S, Filleur S,
Guglielmi J, Cabon F, Colombel M and Clezardin P: Bisphosphonates
inhibit angiogenesis in vitro and testosterone-stimulated vascular
regrowth in the ventral prostate in castrated rats. Cancer Res.
62:6538–6544. 2002.PubMed/NCBI
|
|
21
|
Coxon JP, Oades GM, Kirby RS and Colston
KW: Zoledronic acid induces apoptosis and inhibits adhesion to
mineralized matrix in prostate cancer cells via inhibition of
protein prenylation. BJU Int. 94:164–170. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsai SH, Huang PH, Chang WC, Tsai HY, Lin
CP, Leu HB, Wu TC, Chen JW and Lin SJ: Zoledronate inhibits
ischemia-induced neovascularization by impairing the mobilization
and function of endothelial progenitor cells. PLoS One.
7:e410652012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Di Salvatore M, Orlandi A, Bagala C,
Quirino M, Cassano A, Astone A and Barone C: Anti-tumour and
anti-angiogenetic effects of zoledronic acid on human
non-small-cell lung cancer cell line. Cell Prolif. 44:139–146.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li S and De Souza P: Ras Isoprenylation
and pAkt Inhibition by zoledronic acid and fluvastatin enhances
paclitaxel activity in T24 bladder cancer cells. Cancers (Basel).
3:662–674. 2011. View Article : Google Scholar
|
|
25
|
Jaffe EA, Armellino D, Lam G, Cordon-Cardo
C, Murray HW and Evans RL: IFN-gamma and IFN-alpha induce the
expression and synthesis of Leu 13 antigen by cultured human
endothelial cells. J Immunol. 143:3961–3966. 1989.PubMed/NCBI
|
|
26
|
Yu F, Ng SSM, Chow BKC, Sze J, Lu G, Poon
WS, Kung HF and Lin MCM: Knockdown of interferon-induced
transmembrane protein 1 (IFITM1) inhibits proliferation, migration,
and invasion of glioma cells. J Neurooncol. 103:187–195. 2011.
View Article : Google Scholar
|
|
27
|
Tanaka SS, Yamaguchi YL, Tsoi B, Lickert H
and Tam PP: IFITM/Mil/fragilis family proteins IFITM1 and IFITM3
play distinct roles in mouse primordial germ cell homing and
repulsion. Dev Cell. 9:745–756. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bergers G, Brekken R, McMahon G, Vu TH,
Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z and
Hanahan D: Matrix metalloproteinase-9 triggers the angiogenic
switch during carcinogenesis. Nature Cell Biol. 2:737–744. 2000.
View Article : Google Scholar : PubMed/NCBI
|