Introduction
Primary liver cancer is the main diagnosis of
hepatocellular carcinoma cell (HCC). The mortality of HCC is ranked
second in cancer-related death worldwide, there are 695,900 death
and 748,300 new cases every year (1,2).
Although the 5-year survival rate is >70% when the patients are
diagnosed as early stage and have good prognosis, The vast majority
of patients are diagnosed as late stage, and the 5-year survival
rate is <16% (1). The
treatment of HCC includes surgery and adjuvant chemoradiation
(1). Due to the limitation of
surgical therapy with tumor size, intra-hepatic metastases and
hepatic functional reserve sufficiency, few patients are suitable
to receive this treatment (3).
Chemotherapy is the final choice of the vast majority of patients,
however, the HCC appear moderately tolerated, reducing the effect
of chemotherapy (1,3). Therefore, exploring the mechanisms
of chemotherapy agents that are tolerated and to select the
appropriate adjuvant chemotherapy is very important.
Licochalcone A (LicA) is a chalcone constituent of
licorice, it has anti-inflammatory, antitumor and anti-bacterial
ability (4). LicA is a potent
inhibitor of Bcl-2 protein expression which is the anti-apoptotic
proteins in various tumors, LicA also can induce apoptosis in
several cancer cell lines contributing to the antitumor effect
(5,6). In human HCCs, the migration and
invasion can be suppressed by LicA. LicA has anti-inflammatory
ability through suppressing nuclear factor-κB (NF-κB) and activator
protein-1 (AP-1), and tumor angiogenesis is closely linked to
inflammation, LicA can also inhibit tumor angiogenesis (7,8).
These results suggest that LicA may have potential as antitumor
therapy.
Autophagy is a protective mechanism, many
chemotherapy drugs can induce autophagy, and reduce the effect of
chemotherapy (9). Few reports
exist on LicA inducing autophagy in HCCs, in this study, LicA
induced autophagy in HCCs through activating ULK1/Atg13 complex and
reactive oxygen species (ROS), ULK1/Atg13 complex is upstream of
autophagy, and ROS was upstream of autophagy-related molecule.
Moreover, the antioxidant N-Acetyl-L-cysteine (NAC) can inhibit
LicA-induced autophagy through suppressing ROS in HCCs, promoting
apoptosis and enhancing cell death rate, suggesting LicA may be a
good chemotherapy drugs for HCC, especially as co-treatment with an
antioxidant.
Materials and methods
Cell culture
Human HCCs HuH7 and HepG2 were purchased from
American Type Culture Collection (ATCC, Manassas, VA, USA). The
cells were cultured in DMEM conditioned medium, including 2 mM
L-glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin and 10%
fetal bovine serum (FBS), in the normal culture environment with
37°C and 5% CO2, and all the cell culture regents were
purchased from Gibco Laboratories (Grand Island, NE, USA).
Western blot analysis
For each sample, all the cells were lysed for 30 min
in lyses buffer (Beyotime, Bejing, China) on ice, and the cell
debris was centrifuged at 12,000 × g for 12 min at 4°C, protein
concentration were detected by BCA (Beyotime), using 8–15% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to
separate the proteins with 12 V, the separated proteins were
blotted onto PVDF membrane, blocking 1 h at room temperature with
5% nonfat drymilk in 0.05% Tween-20 in PBS (PBST), incubating the
antibody for 24 h at 4°C with 1:1,000 concentration, washing PVDF
membrane 3 times for 10 min in PBST, incubating secondary antibody
at 37°C for 1 h with the 1:10,000 concentration, washing PVDF
membrane 3 times for 10 min in PBST. All the antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA).
Acridine orange (AO) staining
Cells were treated with drugs, slightly washed 3
times with PBS, using 4% faraformaldehyde to fix for 10 min,
stained with 1 mg/l AO dye liquor for 20 min at 37°C in the dark,
and then observed under fluorescence microscope (Nikon TE2000;
Nikon, Tokyo, Japan).
GFP-LC3 transfection
HuH7 and HepG2 cells were transfected with GFP-LC3
vector (GeneChem, Shanghai, China) through Lipofectamine 2000
(Invitrogen Life Technology, Carlsbad, CA, USA). After 18 h, cells
were treated with drugs for 24 h, using 4% faraformaldehyde to fix
for 10 min, washed 3 times with PBS, and then observed under
fluorescence microscope (Nikon TE2000; Nikon).
Co-immunoprecipitation experiments
HepG2 cells were treated with drugs for 12 h, lysing
cells in hypotonic lyses buffer (Beyotime) on ice for 30 min,
collecting the supernatant to pellet nuclei in whole-cell lysate at
a low speed (1,000 × g), preclearing whole-cell lysate with protein
A-agarose, adding ULK1 or Atg13 antibodies for 1 h at 4°C. Then the
immunoprecipitates were captured on protein A-agarose and detected
by immunoblotting with ULK1 or Atg13 antibodies, respectively.
Cell viability assay
Cell viability was detected by cell counting kit-8
(CCK-8) agent. Cells on a 96-well plate were left to attach
overnight, then treated with drugs for 24 h. Medium was removed the
and washed 3 times with PBS, then 90 µl DMEM and 10
µl CCK-8 was added into each well, and incubated for 1.5 h
at 37°C in the dark. OD values were measured by microplate reader
at 450 nm.
ROS detection
ROS level was measured by 2,7-dichlorofluo-rescein
diacetate (DCFH-DA) (Beyotime). Cells on a 96-well plate were let
to attached overnight, then treated with drugs for 24 h. The medium
was removed and washed 3 times with PBS, then incubated with
DCFH-DA at 37°C for 30 min, measuring the ROS levels through
fluorescence microplate reader with 488 nm excitation wavelength
and 525 nm emission wavelength.
Cell death assay
Cell death ratio was detected by trypan blue. The
cells were treated with drugs, and then digested adding 0.4% (w/v)
trypan blue solution into cell suspension at volume ratio 1:9.
Counting the dead cells and total cells with a microscope, the dead
cells cannot exclude the dye, total death rate = (the number of
dead cell/total cell) ×100%.
Transmission electron microscopy
(TEM)
Samples were processed with the standard protocol
for TEM. TEM was performed on a JEOL 1230 TEM at an accelerating
voltage of 80 kV. Images were acquired with an AMT Advantage Plus
2K×2K digital camera connected to the TEM.
Transfection experiments
siRNA transient transfections were performed with
Lipofectamine 2000 (Invitrogen Life Technology) according to the
manufacturer's protocol. After 36 h transfection, drugs were added,
and after an additional 24 h the cells were collected for western
blot analysis.
Apoptosis detection
Apoptosis was detected by FACScan flow cytometer.
Treating the cell with drugs, cells were incubated with Annexin
V-FITC and propidium-iodide (PI) (both from Beyotime) at room
temperature for 10 min. FACScan flow cytometer was used to analyses
the apoptosis ratio.
Statistic analysis
The data are represented as the mean ± SD from
triplicate experiments. Two-way ANOVA was used to analyze the
variance of different groups. A threshold of P<0.05 was defined
as statistically significant.
Results and Discussion
When autophagy occurs, the cytosolic LC3 will be
cleaved to short peptide hydrolysis LC3 II which is located on the
membrane of autophagosome, therefore, LC3 II is the marker of
autophagy that can reflect the level of autophagy. Because the
lower PH value around autophagosome, AO can penetrate into acidic
organelles and appear as red fluorescence, staining with AO, and
the red fluorescence intensity also can reflect the level of
autophagy. Moreover, TEM is the gold standard of autophagosome.
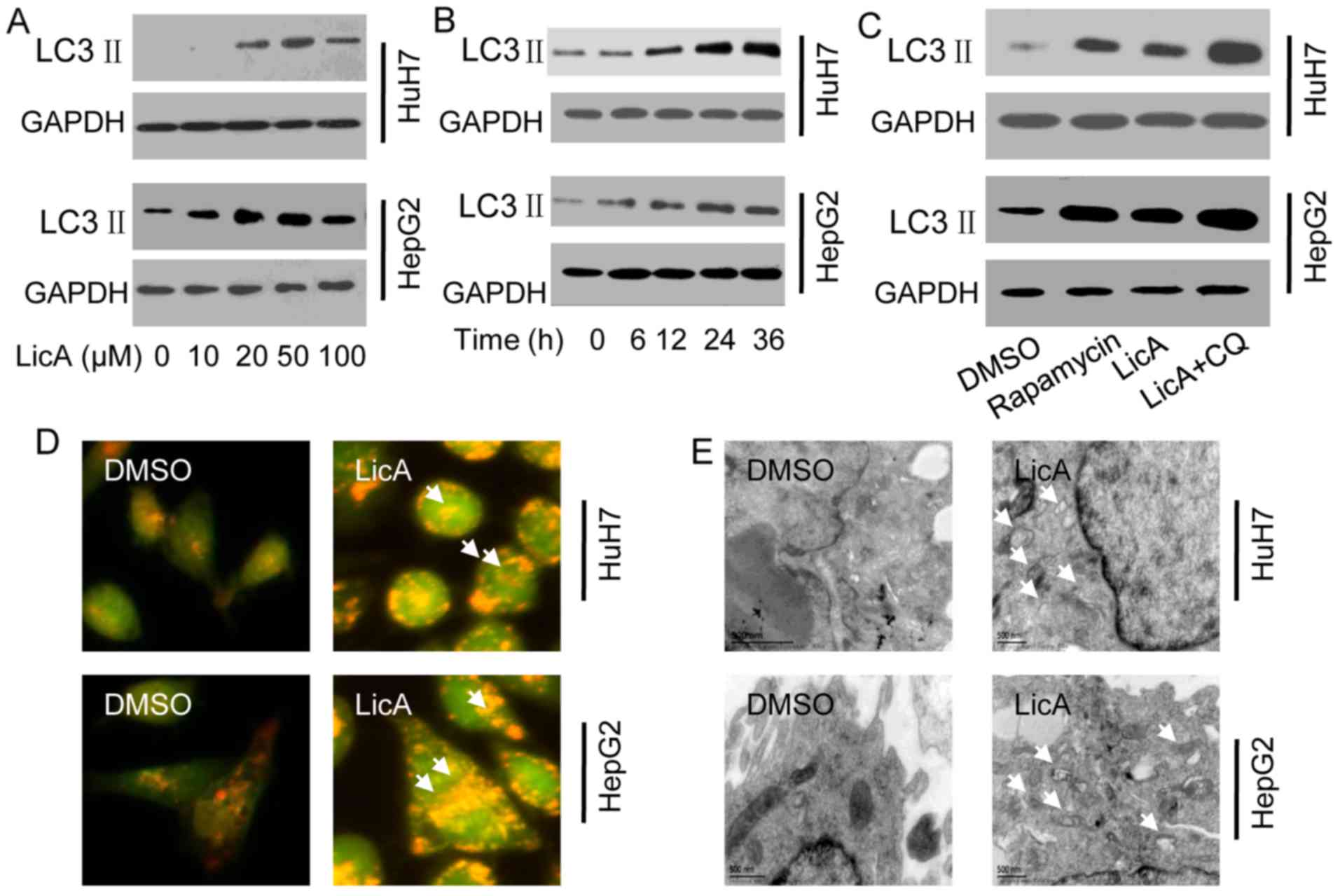
LicA can significant enhance the expression of LC3 II, the induced
ability was time-dependent and dose-dependent, and the best dose
was 50 µM, the best time was 24 h (Fig. 1A and B). Compared to the positive
control rapamycin treatment, the level of induced-LC3 II has no
significant difference, when LicA is co-treated with chloroquine
(CQ) which can block the lysosome and engulf the autophagosome and
contribute to LC3-II accumulation, the level of induced-LC3 II was
significantly increased (Fig.
1C). AO staining show that the red fluorescence intensity of
cells which were treated with 50 µM LicA was significant
higher than the cells which were treated with 0.1% dimethyl
sulfoxide (DMSO) (Fig. 1D). The
TEM results also prove that the number of autophagosome in the
cells which were treated with 50 µM LicA was significant
higher than the cells which were treated with 0.1% DMSO (Fig. 1E). The above results means that
LicA can significantly induce autophagy in HCCs.
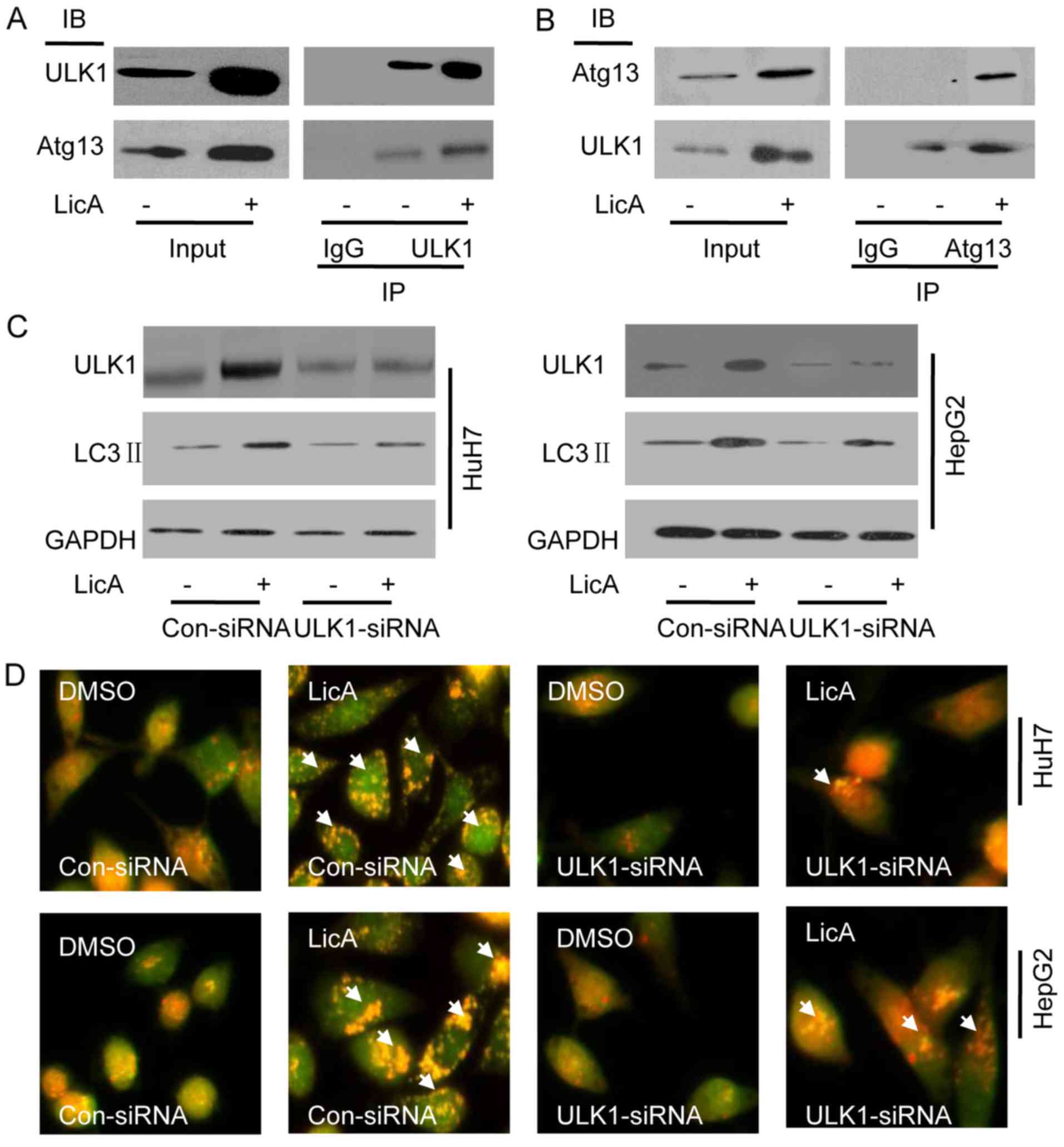
ICo-immunoprecipitation (Co-IP) pull-down assay was
used to investigate the mechanism of LicA-induced-autophagy. HepG2
cells were treated with 50 µM LicA or not,
immunoprecipitation of ULK1 can pull down Atg13, and
immunoprecipitation of Atg13 also can pull down ULK1, that means
ULK1 and Atg13 were bound to each other forming a complex in HCCs,
and LicA can activate the complex which is upstream of autophagy
(Fig. 2A and B). Knocking down
ULK1 by ULK1-siRNA in HCCs, the level of LicA-induced LC3 II was
significant lower than co-siRNA, but still higher than DMSO
treatment (Fig. 2C), similarly,
staining with AO, the red fluorescence intensity was significant
lower than co-siRNA, but still higher than DMSO treatment (Fig. 2D), thus ULK1/Atg13 complex is one
of the regulating molecules of LicA-induced autophagy, not the
unique regulating molecule.
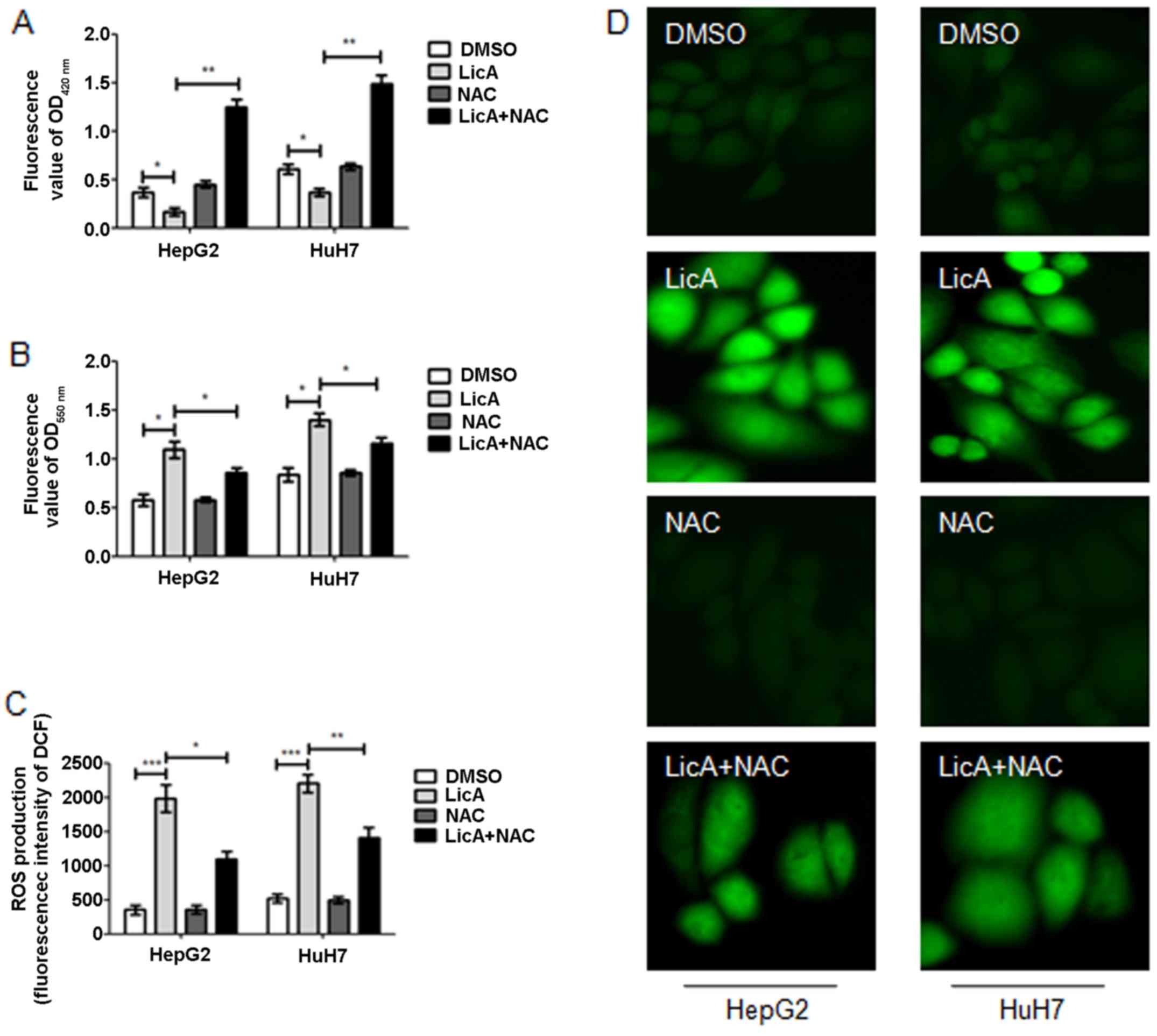
ROS is one of the survival and proliferation
regulator factors of cancer cells, and also regulate autophagy.
LicA can suppress the glutathione (GSH) generation (Fig. 3A) and promote the
O2− generation (Fig. 3B), finally contributing to ROS
generation (Fig. 3C and D) in
HCCs. Moreover, when HCCs were cotreated with LicA and antioxidant
NAC, the level of GSH, O2− and ROS reversed
partly (Fig. 3A–D). These results
show that LicA can promote ROS generation in HCCs, and the NAC can
inhibit the phenomena.
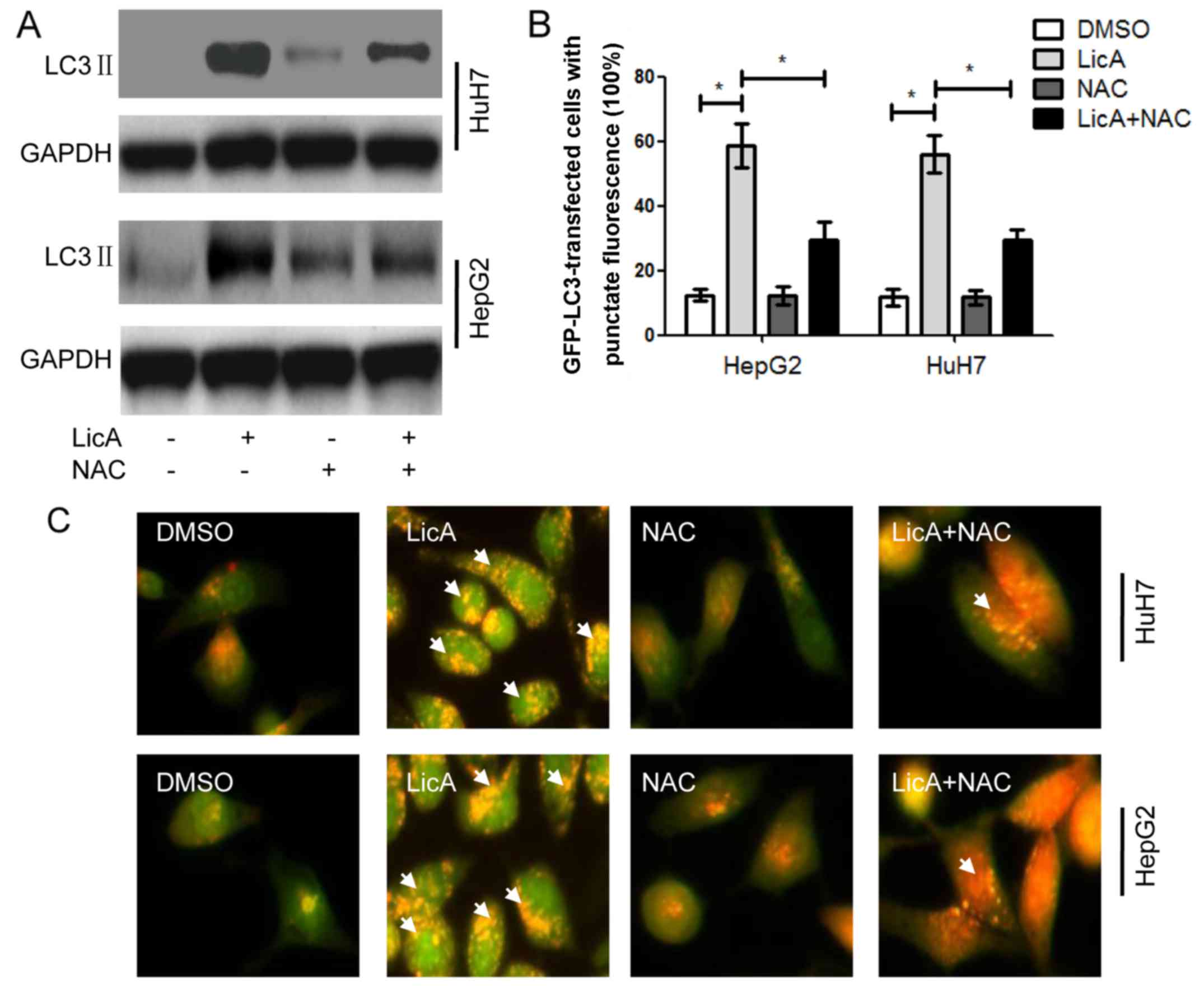
The above results have proved that LicA can promote
ROS generation and NAC can reverse the effect of LicA in HCCs. When
HCCs were cotreated with LicA and NAC, the level of LicA-induced
LC3 II was lower than HCCs treated with only LicA, but also higher
than DMSO treatment (Fig. 4A). At
the same time the quantity of LC3-GFP puncta and the red
fluorescence intensity with AO staining in HCCs was cotreated with
LicA and NAC were lower than those treated with LicA alone, but
also higher than DMSO treatment (Fig.
4B and C). These results indicated that LicA can induce
ROS-related autophagy, and NAC can inhibit the same. Previous
studies have reported that ROS was upstream of many molecules, and
ROS was not the direct inducing factor of autophagy. In order to
investigate the downstream ROS pro-autophagy pathways, other
regulated factors were detected in HCCs. Sarbassov et al
(10) have reported that TSC1/2
complex was upstream of mTORC1, and mTORC1 was a universal
regulator of autophagy (11). So
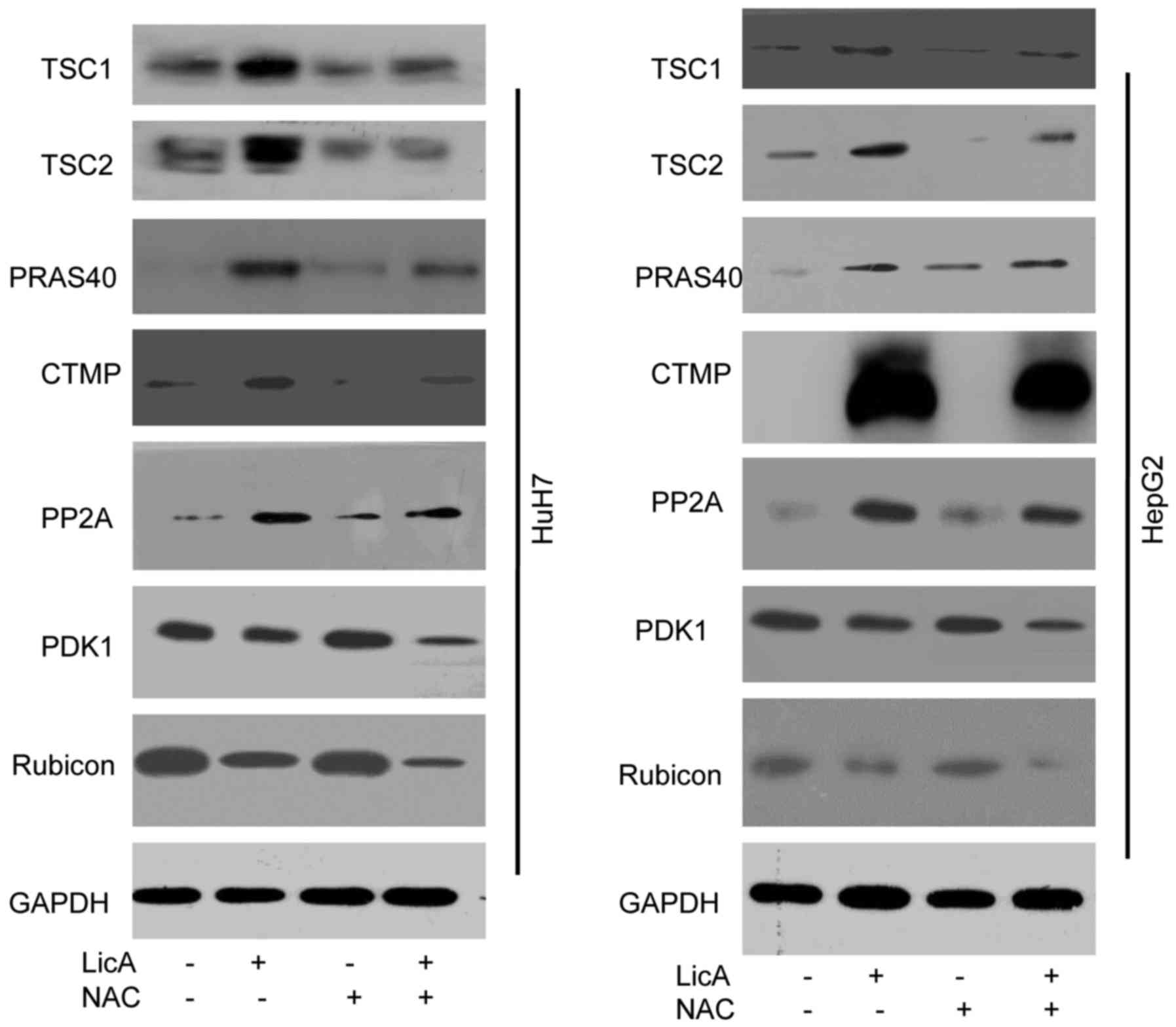
TSC1/2 complex was detected in this work, the results show that
TSC1/2 complex can be activated by LicA through ROS pathway, and
can be inhibited by NAC that can suppress ROS generation (Fig. 5). PRAS40 is a metabolism protein
bound to mTOR complex, and acts as the receptor of mTOR, PRAS40
also can regulate the proliferation in various cell lines (12,13). The results of this work indicated
that PRAS40 can be regulated by LicA-induced-ROS in HCCs, and when
HCCs were cotreated with LicA and NAC, the change of PRAS40
expression level was inhibited (Fig.
5). Carboxy-terminal modulator protein (CTMP), as an endogenous
inhibitor of Akt, also can influence the phosphoinositide 3-kinase
(PI3K) pathway through activating protein kinase B (PKB) signaling,
leading to insulin signaling regulation (14). These studies support our results
that CTMP can regulate autophagy, moreover, CTMP was downstream in
LicA-induced autophagy via promoting ROS generation in HCCs,
similarly, the phenomenon can be changed by NAC (Fig. 5). Protein phosphatase 2A (PP2A) is
a key regulator of cell cycle, also the target for anticancer
drugs, PP2A can regulate the autophagy through AMPK
phosphorylation. These studies showed PP2A may be an important
regulator in LicA-induced autophagy (15,16) the results in our study proved the
hypothesis as PP2A acted as the regulator of LicA-induced autophagy
through promoting ROS generation, and ROS inhibitor NAC can block
this progress (Fig. 5). PDK1 is
an AGC kinase of the PKB family which contain a PH domain
regulating cell proliferation, apoptosis and differentiation, PDK1
is upstream of Akt, and impair the autophagic effect through
targeting activated c-Src (p-Src) (17,18). LicA can inhibit the PDK1
expression through ROS in LicA-induced autophagy, and NAC reversed
this (Fig. 5). Rubicon is a
subunit of PI3KC3, containing cysteine-rich protein, acting as a
regulator of Beclin1-UVRAG-Vps34 complex to regulate autophagy
(19). LicA-induced ROS can
inhibit Rubicon expression in LicA-induced autophagy, and NAC is
the inhibitor of this program (Fig.
5). These results indicated that ROS is the pathway of
LicA-induced autophagy in HCCs.
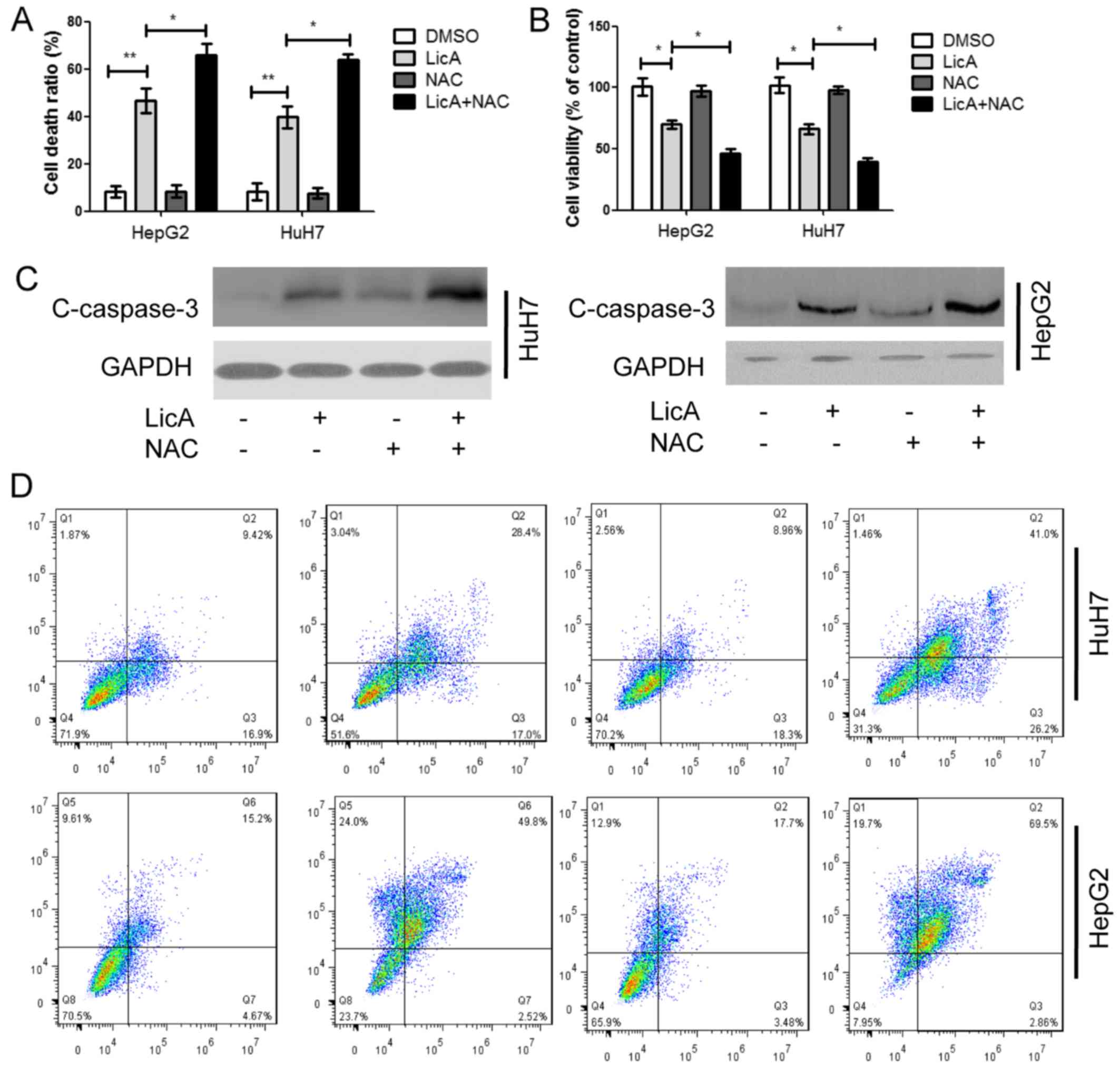
LicA can induce autophagy in HCCs through promoting
ROS generation, and autophagy is a protective mechanism for cells,
the antioxidant NAC can inhibit ROS generation, moreover, LicA is
the activator of apoptosis in various cancer cells. Therefore, in
order to investigate the function of ROS-mediated autophagy in
LicA-induced apoptosis for HCCs, HCCs were cotreated with LicA and
NAC or treated with LicA alone, the cell death ratio and cell
viability ratio were detected, LicA can induced cell death and
decrease cell viability in HCCs, and NAC can enhance the effect of
LicA in killing HCCs (Fig. 6A and
B). The apoptosis marker caspase-3 was detected, LicA can
promote caspase-3 expression and NAC enhance the effect, which
means that NAC could enhance LicA-induced apoptotic in HCCs
(Fig. 6C). Annexin V-FITC and PI
results indicated that LicA NAC enhanced the LicA-induced cell
apoptosis ratio (Fig. 6D). Thus,
the above results indicated that suppression of ROS-mediated
autophagy induced by LicA enhanced LicA-induced apoptosis.
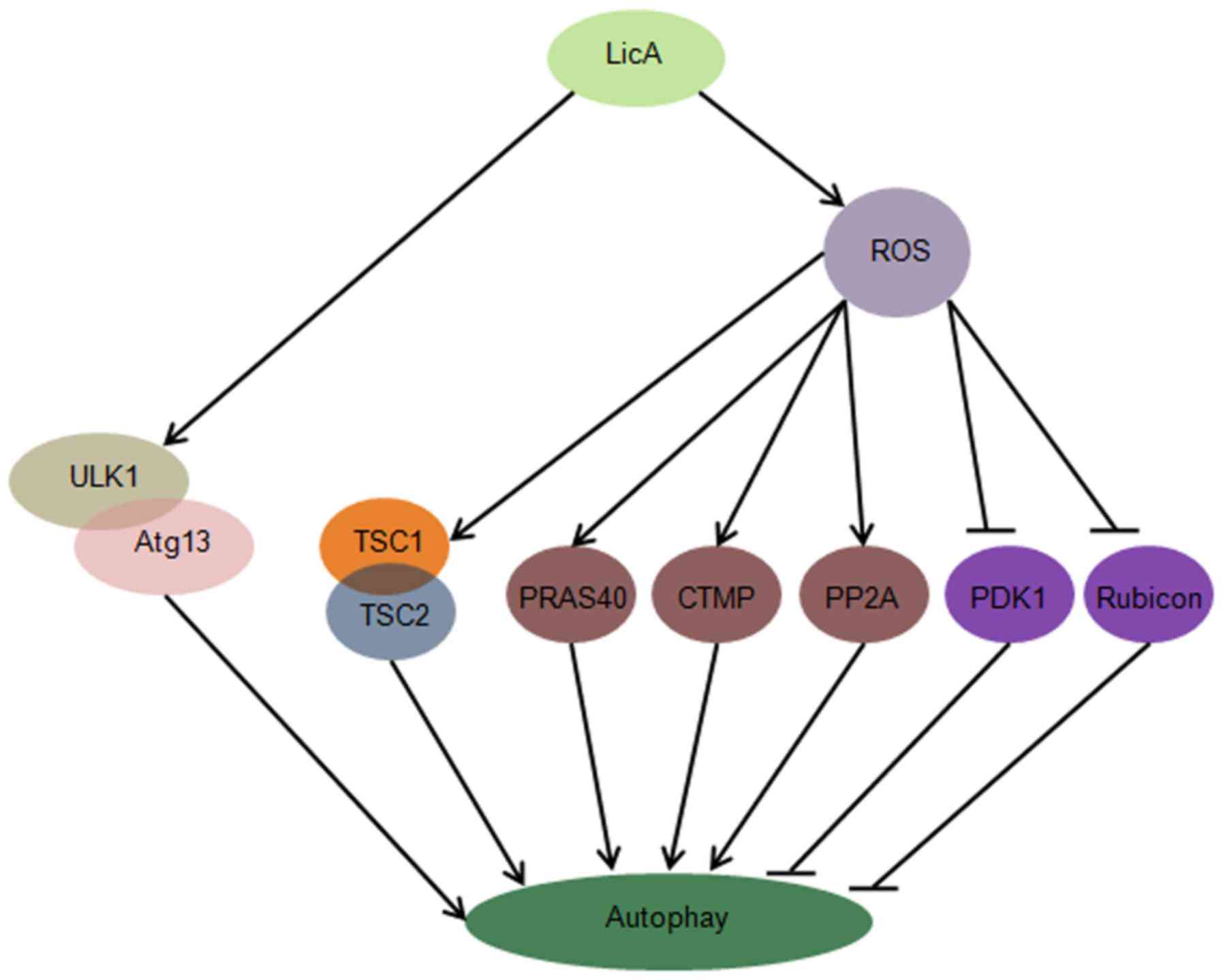
In this study, we first report that LicA can induce
autophagy through ULK1/Atg13 and ROS pathway in HCCs, suppression
of LicA-induced ROS through antioxidant NAC can enhance
LicA-induced apoptosis, promoting the function of LicA killing
HCCs. LicA can activate the ULK1/Atg13 complex which is upstream of
autophagy, LicA can promote ROS generation, ROS trigger the
expression level of TSC1/2 complex, PRAS40, CTMP, PP2A, PDK1 and
Rubicon change, these molecules are upstream of autophagy (Fig. 7).
LicA has been reported to have antitumor,
anti-angiogenesis and anti-inflammatory ability, and considered as
Bcl-2 inhibitor (4,5,6,20).
LicA can block cell cycle progression through regulating
extracellular signal-regulated kinase 1/2 (ERK1/2) and Rb
phosphorylation (21). Animal
experiments show that, LicA significantly inhibits tumor growth,
and reduces the cell nuclear antigen, cyclooxygenase-2 (COX-2) and
inducible nitric oxide synthase (iNOS), contributing to survival.
Moreover, previously it has been reported that LicA can inhibit
matrix metalloproteinase-9 (MMP-9) and vascular endothelial growth
factor receptor-2 (VEGFR-2) expression in various cancer cells,
indicating that LicA can mediate metabolism and angiogenesis
(8,20).
Autophagy is a double membrane structure
autophagosome formed in the no ribosome attachment region of
reticulum membrane, engulfing the waste proteins or aged
organelles, fused with lysosomes and forming autolysosome,
degradation its contents. Therefore, autophagy is necessary for
cell metabolism, and contributes to cell survival. LicA is the
Bcl-2 inhibitor, and Bcl-2 is the anti-apoptosis protein,
previously reported have demonstrated that apoptosis and autophagy
occur simultaneously. The antitumor function of LicA was mainly
through inducing apoptosis. The results have proven that
LicA-induced autophagy in HCCs depend on the dose and time. ROS has
negative influence on normal cells, but have dual role for cancer
cells. ROS can promote tumor growth through inducing DNA damage and
activating oncogenes. DeNicola et al reported that Nrf2 was
in high expression state in various cancer cells, and Nrf2 could
decrease the intracellular ROS generation, remove the free radicals
in cells, moreover, cancer cells could not proliferate without Nrf2
expression, therefore, ROS also can inhibit cancer cell
proliferation (22). ULK1/Atg13
complex assembled by RB1CC1, FIP200 and Atg101, forms a
macroautophagy, and participate in the autophagy mediation through
mTOR and AMPK. Our results indicated that LicA could activate the
ULK1/Atg13 complex, inducing autophagy in HCCs. Furthermore, LicA
also can promote the O2− generation and
suppress GSH generation, contribute to ROS generation in HCCs, and
the antioxidant NAC can inhibit the ROS generation induced by LicA
in HCCs. Previous studies have reported that tuberous sclerosis
complex (TSC) is the mutational production of TSC1 or TSC2 gene,
occuring in hamartomas and neurological manifestations. TSC1/2
complex can negatively regulate the mTORC1 complex, contribute to
protein synthesis, cell growth and autophagy mediation (23). PRAS40 is the substrate of PKB/Akt,
it can also bind to mTORC1 complex, possessing various
phosphorylation sites, including Thr246 mediated by Akt, Ser183,
Ser212 and Ser221 mediated by mTORC1 complex (12). CTMP is an endogenous inhibitor of
Akt, a key mediator of insulin signals, and insulin signals mediate
autophagy pathway (14,24). PP2A has an important role in the
mediation of cell cycle, and PTEN-induced kinase 1 can increase the
phosphorylation of PP2A at Y307, moreover, Bcl-2 is downstream of
PP2A in PTEN-induced kinase 1 pathway, which participates in the
autophagy mediation (15,25). PDK1 is upstream of Akt, and
inhibits autophagy through targeting c-Src, Rubicon as a PI3KC3
subunit can communicate with Beclin 1, contribute to autophagosome
suppression (17,19). In this study, ROS induced by LicA
can activate TSC1/2 complex, PRAS40, CTMP and PP2A signals, and
inhibit PDK1 and Rubicon signals, contributing to autophagy
progress triggering. Moreover, the antioxidant can inhibit ROS
generation in LicA treated HCCs. Cotreatment with LicA and NAC,
inhibited and promoting cell death, and enhanced LicA-induced
apoptosis in HCCs. Thus, our results may provide a new direction on
rational design of clinical trials, LicA and antioxidant NAC may be
a good combined regimen in HCC chemotherapy.
Abbreviations:
|
LicA
|
licochalcone A
|
|
ROS
|
reactive oxygen species
|
|
NAC
|
N-Acetyl-L-cysteine
|
|
HCCs
|
human hepatocellular carcinoma
cells
|
|
GSH
|
glutathione
|
|
CTMP
|
carboxy-terminal modulator protein
|
|
PKB
|
protein kinase B
|
|
PP2A
|
protein phosphatase 2A
|
|
TSC
|
tuberous sclerosis complex
|
Acknowledgments
All the authors are very thankful to Professor Hong
Zhao and Wei Yang for their support.
Notes
[1]
Funding
No funding was received.
[2] Availability
of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
QN, WZ, WD and DZ contributed to the conception and
design of the study. QN, WZ, GW, KW and DZ developed the
methodology. QN, WZ, JW and CL acquired the data. TY, WL, GW, TZ
and KW analyzed and interpreted the data. QN, WZ, WD and DZ wrote,
reviewed and revised the manuscript. DZ supervized the study.
[4] Ethics
approval and consent to participate
Not applicable.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yo YT, Shieh GS, Hsu KF, Wu CL and Shiau
AL: Licorice and licochalcone-A induce autophagy in LNCaP prostate
cancer cells by suppression of Bcl-2 expression and the mTOR
pathway. J Agric Food Chem. 57:8266–8273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee CK, Son SH, Park KK, Park JH, Lim SS,
Kim SH and Chung WY: Licochalcone A inhibits the growth of colon
carcinoma and attenuates cisplatin-induced toxicity without a loss
of chemotherapeutic efficacy in mice. Basic Clin Pharmacol Toxicol.
103:48–54. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rafi MM, Rosen RT, Vassil A, Ho CT, Zhang
H, Ghai G, Lambert G and DiPaola RS: Modulation of bcl-2 and
cytotoxicity by licochalcone-A, a novel estrogenic flavonoid.
Anticancer Res. 20:2653–2658. 2000.PubMed/NCBI
|
|
7
|
Kwon HS, Park JH, Kim DH, Kim YH, Park JH,
Shin HK and Kim JK: Licochalcone A isolated from licorice
suppresses lipopolysaccharide-stimulated inflammatory reactions in
RAW264.7 cells and endotoxin shock in mice. J Mol Med (Berl).
86:1287–1295. 2008. View Article : Google Scholar
|
|
8
|
Kim YH, Shin EK, Kim DH, Lee HH, Park JH
and Kim JK: Antiangiogenic effect of licochalcone A. Biochem
Pharmacol. 80:1152–1159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anding AL and Baehrecke EH: Autophagy in
cell life and cell death. Curr Top Dev Biol. 114:67–91. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sarbassov DD, Ali SM and Sabatini DM:
Growing roles for the mTOR pathway. Curr Opin Cell Biol.
17:596–603. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Høyer-Hansen M and Jäättelä M:
AMP-activated protein kinase: A universal regulator of autophagy.
Autophagy. 3:381–383. 2007. View Article : Google Scholar
|
|
12
|
Kazi AA and Lang CH: PRAS40 regulates
protein synthesis and cell cycle in C2C12 myoblasts. Mol Med.
16:359–371. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wong CP, Seki A, Horiguchi K, Shoji T,
Arai T, Nugroho AE, Hirasawa Y, Sato F, Kaneda T and Morita H:
Bisleuconothine a induces autophagosome formation by interfering
with AKT-mTOR signaling pathway. J Nat Prod. 78:1656–1662. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park J, Li Y, Kim SH, Yang KJ, Kong G,
Shrestha R, Tran Q, Park KA, Jeon J, Hur GM, et al: New players in
high fat diet-induced obesity: LETM1 and CTMP. Metabolism.
63:318–327. 2014. View Article : Google Scholar
|
|
15
|
Yin X, Zhang N and Di W: Regulation of
LC3-dependent protective autophagy in ovarian cancer cells by
protein phosphatase 2A. Int J Gynecol Cancer. 23:630–641. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gong FR, Wu MY, Shen M, Zhi Q, Xu ZK, Wang
R, Wang WJ, Zong Y, Li ZL, Wu Y, et al: PP2A inhibitors arrest G2/M
transition through JNK/Sp1- dependent down-regulation of CDK1 and
autophagy-dependent up-regulation of p21. Oncotarget.
6:18469–18483. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng Q, Yu X, Xiao L, Hu Z, Luo X, Tao Y,
Yang L, Liu X, Chen H, Ding Z, et al: Neoalbaconol induces energy
depletion and multiple cell death in cancer cells by targeting
PDK1-PI3-K/Akt signaling pathway. Cell Death Dis. 4:e8042013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karlsson I, Zhou X, Thomas R, Smith AT,
Bonner MY, Bakshi P, Banga AK, Bowen JP, Qabaja G, Ford SL, et al:
Solenopsin A and analogs exhibit ceramide-like biological activity.
Vasc Cell. 7:52015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhong Y, Wang QJ, Li X, Yan Y, Backer JM,
Chait BT, Heintz N and Yue Z: Distinct regulation of autophagic
activity by Atg14L and Rubicon associated with Beclin
1-phosphatidylinositol-3-kinase complex. Nat Cell Biol. 11:468–476.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JK, Shin EK and Park JH, Kim YH and
Park JH: Antitumor and antimetastatic effects of licochalcone A in
mouse models. J Mol Med (Berl). 88:829–838. 2010. View Article : Google Scholar
|
|
21
|
Park JH, Lim HJ, Lee KS, Lee S, Kwak HJ,
Cha JH and Park HY: Anti-proliferative effect of licochalcone A on
vascular smooth muscle cells. Biol Pharm Bull. 31:1996–2000. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
DeNicola GM, Karreth FA, Humpton TJ,
Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES,
et al: Oncogene-induced Nrf2 transcription promotes ROS
detoxification and tumorigenesis. Nature. 475:106–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Di Nardo A, Wertz MH, Kwiatkowski E, Tsai
PT, Leech JD, Greene-Colozzi E, Goto J, Dilsiz P, Talos DM, Clish
CB, et al: Neuronal Tsc1/2 complex controls autophagy through
AMPK-dependent regulation of ULK1. Hum Mol Genet. 23:3865–3874.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Y, Nie H, Tian L, Tong L, Deng J,
Zhang Y, Dong H and Xiong L: Sevoflurane preconditioning-induced
neuroprotection is associated with Akt activation via
carboxy-terminal modulator protein inhibition. Br J Anaesth.
114:327–335. 2015. View Article : Google Scholar
|
|
25
|
Qi Z, Yang W, Liu Y, Cui T, Gao H, Duan C,
Lu L, Zhao C, Zhao H and Yang H: Loss of PINK1 function decreases
PP2A activity and promotes autophagy in dopaminergic cells and a
murine model. Neurochem Int. 59:572–581. 2011. View Article : Google Scholar : PubMed/NCBI
|