Introduction
Leukoderma, also named vitiligo, is an acquired
disfiguring pigmentary anomaly of the skin manifested by
depigmented white patches surrounded by a normal or hyper-pigmented
border (1). Vitiligo affects 1–2%
of the world population without racial or sex difference (2). Hypopigmentation may be the source of
severe psychological distress, diminished quality of life, and
increased risk of psychiatric morbidity (3). However, the effective medicines are
largely absent from the clinical treatment of the disease.
Melanin synthesis in the skin plays an important
evolutionary role in hypopigmentation therapy. In mammals, melanin
biosynthesis is catalyzed by three melanocyte-specific enzymes:
tyrosinase (TYR), tyrosinase-related protein 1 (TRP1), and TRP2
(4). TYR is the rate-limiting
enzyme in melanogenesis (5),
catalyzing the hydroxylation of tyrosine to produce
3,4-dihydroxyphenylalanine (DOPA) and the oxidation of DOPA to DOPA
quinone. TRP-1 and TRP-2 function in the biosynthesis of melanin
downstream of TYR (4).
Microphthalmia-associated transcription factor (MITF) has a crucial
role in the transcription of melanogenic genes, binding a highly
conserved motif termed M-box within the TYR promoter. Thereby MITF
plays an important role in increasing melanogenesis (6,7).
Many approaches have been used to help clarify the
specific mechanism controlling melanin biosynthesis via tyrosinase
regulation. The mitogen-activated protein kinases (MAPKs) are key
signaling molecules related to the regulation of melanogenesis
(8), including extracellular
signal-regulating kinase (ERK), stress-activated protein kinase
(SAPK)/JNK and p38 mitogen-activated protein kinase (p38 MAPK)
signaling cascades. Previous literature showed that phosphorylation
of p38 can lead to the activation of MITF via the phosphorylation
of cyclic adenosinemonophosphate (cAMP) responsive element binding
(CREB) protein. Some Chinese medicine extracts such as methyl
3,5-di-caffeoylquinate have been shown to have melanogenesis
activity through activating the p38 signaling pathway (9).
Another signaling pathway involved in melanogenesis
regulation is protein kinase A (PKA). PKA can be activated by the
elevation of cellular cAMP and cAMP stimulation results in the
elevation of MITF protein levels and subsequent activation of the
TYR, TRP-1 and TRP-2 promoters by binding with M-box or E-box
consensus motif (10,11).
Previous literature also showed that the inhibition
of the PI3K/AKT pathway increases the production of melanin by MITF
activation and induction of tyrosinase expression (12).
As an active compound, psoralen is present in a
variety of traditional Chinese medicinal plants, such as
Psoralea corylifolia L., Glehnia littoralis Fr.
Schmidr ex Miq, Heracleum lanatum Michx., Ruta
graveolens L. and Ficus carica L. Recent studies have
revealed that it possesses significant pharmacological activities
in dermatosis treatment, including vitiligo, psoriasis and alopecia
areata. Similarly, the extract of Psoralea corylifolia L.
seeds was one of the most popular Uygur medicines used for vitiligo
and initially recorded in 'Yao Yong Zong Ku' around 300 years ago
(13–15). In 1930s, 8-methoxypsoralen (8-MOP)
and 5-methoxypsoralen (5-MOP) were isolated from the Psoralea
corylifolia L. (16,17). Later, other psoralens, such as
4,5,8-trimethylpsoralen (TMP) was synthesized as well. Continuous
research proved that these compounds show strong activity in the
treatment of vitiligo (13,14). Among them, 8-MOP is considered as
a better therapeutic agent against vitiligo in consideration of low
doses and toxicity. However, it is still accompanied by some
undesired side effects in clinical therapy, such as gene mutation,
skin phototoxicity and risk of skin cancer (18,19). So, it is necessary to find
substitutions for enhancing skin hyperpigmentation.
Our group has been dedicated to the drug development
of vitiligo for many years (20–23). Recently, a new series of
furocoumarin derivatives were designed and synthesized by our
research team (20), and
biologically evaluated for activity on tyrosinase and melanin
synthesis in murine B16 cells.
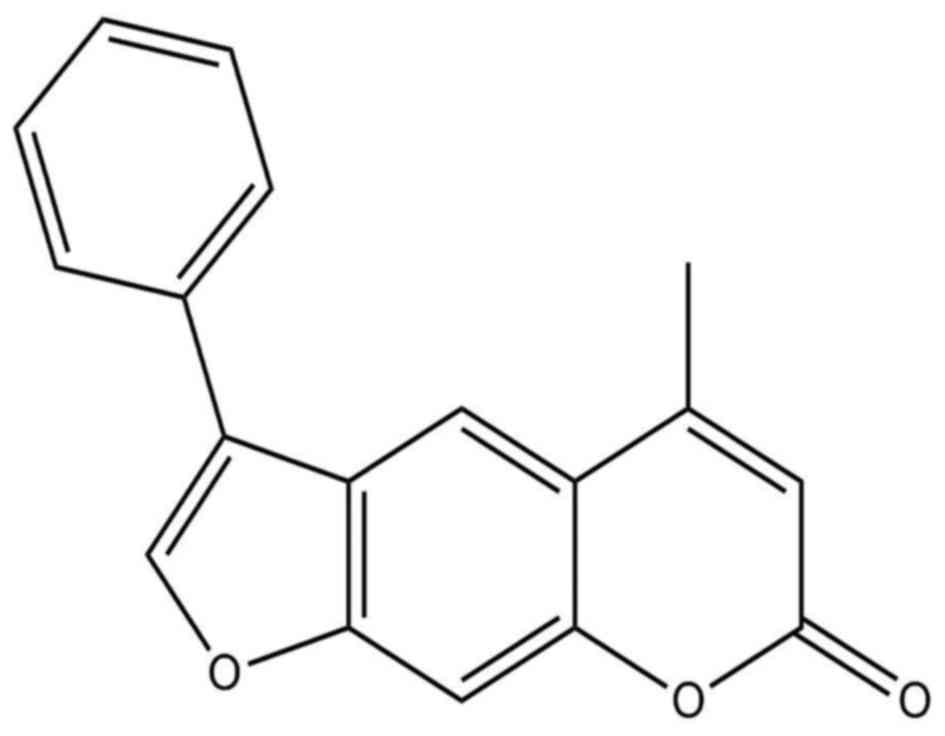
4-Methyl-6-phenyl-2H-furo[3,2-g]chromen-2-one (MPFC)
(Fig. 1) is recognized as one of
the most promising candidate compound with an effect on melanin
synthesis and tyrosinase activity much better than the positive
control 8-MOP. We speculate that better melanogenesis activity of
MPFC may result from the different structural modifications
compared with 8-MOP. In this study, we evaluated the activity of
MPFC on melanogenesis and provide solid evidence showing that p38
MAPK and PKA pathway are targets of this compound to active melanin
biosynthesis.
Materials and methods
Reagents
Dimethylsulfoxide (DMSO) was from Sigma (St. Louis,
MO, USA), [2-(2-methoxy-4-nitrophenyl)-3-(4-nit
rophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium] (CCK-8) was
purchased from TransGen Biotechnology (Beijing, China). CERB,
phospho-CREB (Ser133), AKT, p-AKT (Thr308), p38, p-p38
(Thr180/Tyr182), ERK, p-ERK (Thr202/Tyr204), JNK, p-JNK
(Thr183/Tyr185) and β-actin antibodies were purchased from Cell
Signaling Technology (Danvers, MA, USA). Antibodies against
tyrosinase, TRP1 and TRP2 were from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Anti-MITF antibody was purchased from Millipore
(Billerica, MA, USA). Anti-mouse, anti-goat and anti-rabbit IgG
anti-bodies (horseradish peroxidase conjugated) were purchased from
Santa Cruz Biotechnology, Inc.
Preperation of MPFC (20)
Four percent ethanol potassium hydroxide solution
(70 ml) was added to an ethanolic solution (500 ml) of intermediate
4-methyl-7-(2-oxo-2-phenylethoxy)-2H-chromen-2-one (10 mmol), and
the mixture was refluxed for 4 h. After cooling, the solution was
acidified with 1 M hydrochloric acid and extracted with ethyl
acetate three times. The organic phase was dried overnight and
evaporated under reduced pressure. The resulting residue was
purified by silica gel chromatography with petroleum ether/ethyl
acetate to obtain MPCF. Yield 97%, light yellow solid, m.p.
171-173°C; purity 98.70%; 1H NMR (400 MHz, CDCl3)
δ 7.98 (s, 1H), 7.83 (s, 1H), 7.64 (dd, J = 8.2, 1.1 Hz,
2H), 7.50–7.56 (m, 3H), 7.44 (td, J = 7.4, 1.1 Hz, 1H), 6.29 (s,
1H), 2.52 (s, 3H). 13C NMR (101 MHz, CDCl3)
δ 160.99 (s), 157.10 (s), 152.64 (s), 151.80 (s), 142.88
(s), 131.06 (s), 129.25 (s), 128.06 (s), 127.58 (s), 123.96 (s),
122.32 (s), 116.80 (s), 115.77 (s), 113.64 (s), 100.23 (s), 19.26
(s); IR (KBr) v: 2925, 1735, 1611, 1447, 1280, 1125, 1063, 831
cm−1; HRMS (ESI) calcd for
C18H13O3 [M+H]+
277.0865, found 277.0853. MPFC was dissolved in DMSO and stored at
−20°C as a stock solution (50 mM).
Cell culture
The murine B16 melanoma cell line (acquired from
Chinese Academy of Sciences, Beijing, China) were grown in
Dulbecco's modified Eagle's medium (DMEM) containing 10%
heat-inactivated fetal bovine serum (FBS), penicillin G (100 U/ml)
and streptomycin (100 mg/ml) (Gibco-BRL, Grand Island, NY, USA) at
37°C in a humidified atmosphere of 5% CO2.
Cell morphology and cell viability
measurement
Cell morphology was examined under a LEICA DMI8
microscope (LEICA Microsystems CMS GmbH, Wetzlar, Germany). Cell
viability was assayed by adding CCK-8 (TransGen Biotech) solution.
Briefly, B16 cells were plated in 96-well dishes at a density of
5×103 cells/well and allowed to adhere for 24 h. Test
samples were added and the cells were incubated for 24 h. After
discarding the culture medium of the cells, 10 μl of CCK-8
solution was added into each well and cells were incubated at 37°C
for another 2 h. The absorbance was deter-mined at 450 nm using a
Spectra Max M5 (Molecular Devices, Sunnyvale, CA, USA). Absorbance
of cells without treatment was regarded as 100% of cell survival.
Each treatment was performed in triplicate and each experiment was
repeated three times.
Tyrosinase activity assay
Tyrosinase activity was estimated by measuring the
rate of L-DOPA oxidation. Briefly, B16 cells were seeded in a
6-well plate at a density of 2×105 cells/well and
allowed to attach for 24 h. Test samples were then added to
individual wells. After a 24 h incubation, cells were washed with
ice-cold phosphate-buffered saline (PBS) twice, lysed with 1%
Triton X-100 solution containing 1% sodium deoxycholate for 30 min
at −80°C, then the lysate was centrifuged at 12,000 x g for 15 min
to obtain the supernatant. A reaction mixture containing 10 mM
L-DOPA in PBS (pH 6.8) was added and then, the cells were incubated
at 37°C in dark for 60 min. The dopachrome was monitored by
measuring the absorbance at 490 nm using an enzyme-linked
immunosorbent assay (ELISA) reader and the treated cells were
presented as percentage against the untreated cells. Each treatment
was repeated three times.
Melanin measurement
B16 cells were seeded in a 6-well plate at a density
of 2×105 cells/well and allowed to attach for 24 h. Then
adding test samples to individual wells, cells were incubated for
48 h and washed with PBS. After cells were lysed according to the
method previously described (9),
lysate was put in a 96-well microplate, and measured
spectro-photometrically at 405 nm by a multi-plate reader. Protein
concentration of each sample was determined by BCA Protein assay
kit (Biomed, Beijing, China). The melanin amount expressed as
abs/μg protein was shown as percentage value. The percentage
value of the treated cells were calculated with respect to the
untreated cells. Each experiment was repeated three times.
Measurement of cAMP concentration
The cAMP level was measured using a cAMP ELISA kit
(Cell Biolabs, Inc., San Diego, CA, USA) B16 cells were plated in a
6-well plate at a density of 5×105 cells/well and
allowed to adhere for 24 h, then test samples were added to
individual wells. After incubating for 12 h, B16 cells were
harvested and lysed in lysis buffer. Supernatants were collected
after centrifuging to determine cAMP levels using a commercially
available cAMP ELISA kit. cAMP levels were normalized to total
protein content. Each experiment was repeated three times.
Western blot analysis
The treated cells were lysed in cold RIPA buffer (pH
7.4) containing protease and protease inhibitor cocktail [1 M
4-nitrophenyl phosphate disodium salt hexahydrate (PNPP), 1 M
sodium fluoride (NaF), 10 mM phenylmethanesulfonyl fluoride (PMSF),
100 mM benzamidine, 100 mM DL-Dithiothreitol (DTT), 200 mM sodium
orthovanadate (OV)]. The whole-cell lysate was collected and
regarded as a protein sample. Its concentration was measured by BCA
Protein assay kit (Biomed), 60 μg of individual protein
samples were separated by 10% sodium dodecyl sulfate (SDS)
polyacrylamide gels at 100 V and transferred onto membranes for 2 h
at 400 mA. Following electrotransfer to polyvinylidene fluoride
(PVDF) membranes (Merck Millipore Ltd., Darmstadt, Germany)
membrane blocking was performed with 5% skim milk solution for 1 h,
then they were incubated with the primary antibodies at 4°C
overnight. Equal loading was assessed using anti-β-actin antibody
to normalize the amounts of total protein. After three washes with
TBS containing 0.2% Tween-20 (TBST), the membranes were incubated
with horseradish peroxidase-conjugated secondary antibodies at a
dilution of 1:2,000 for 1 h at room temperature. The targeted
proteins were detected by ECL western blot detection reagents (GE
Healthcare, Pittsburgh, PA, USA), and visualized after exposure to
chemiluminescence film (X-OMAT BT film; Carestream Health, Inc.,
Xiamen, China). Western blot assay results reported here are
representative of at least three experiments.
Statistical analysis
Data were expressed as the mean ± SD and statistical
analysis was performed with one-way ANOVA followed by Tukey post
hoc test for multiple comparison tests. A P-value of <0.05 was
considered of significant difference.
Results
Morphological changes of melanoma cells
induced by MPFC
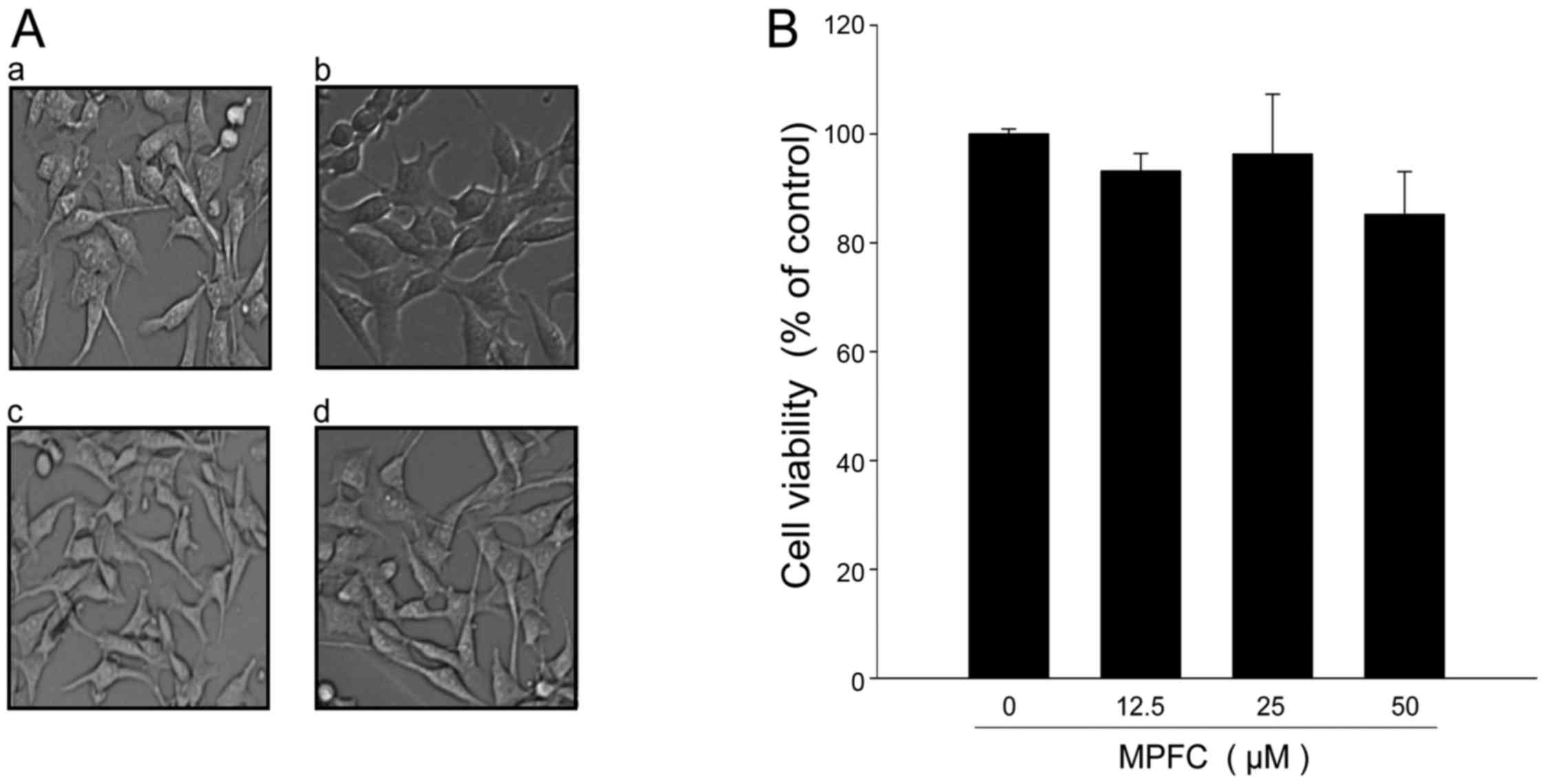
Our results showed that murine melanoma B16 cells
treated with MPFC for 24 h did not induce any change in cell
morphology when compared with the untreated cells (Fig. 2A) and did not show any increase in
cytotoxicity (Fig. 2B). Thus,
dosages at 0–50 μM were chosen to determine the effects of
MPFC on tyrosinase activity and melanin synthesis.
Treatment with MPFC stimulates tyrosinase
activity and melanin content in B16 cells at non-cytotoxic
dosages
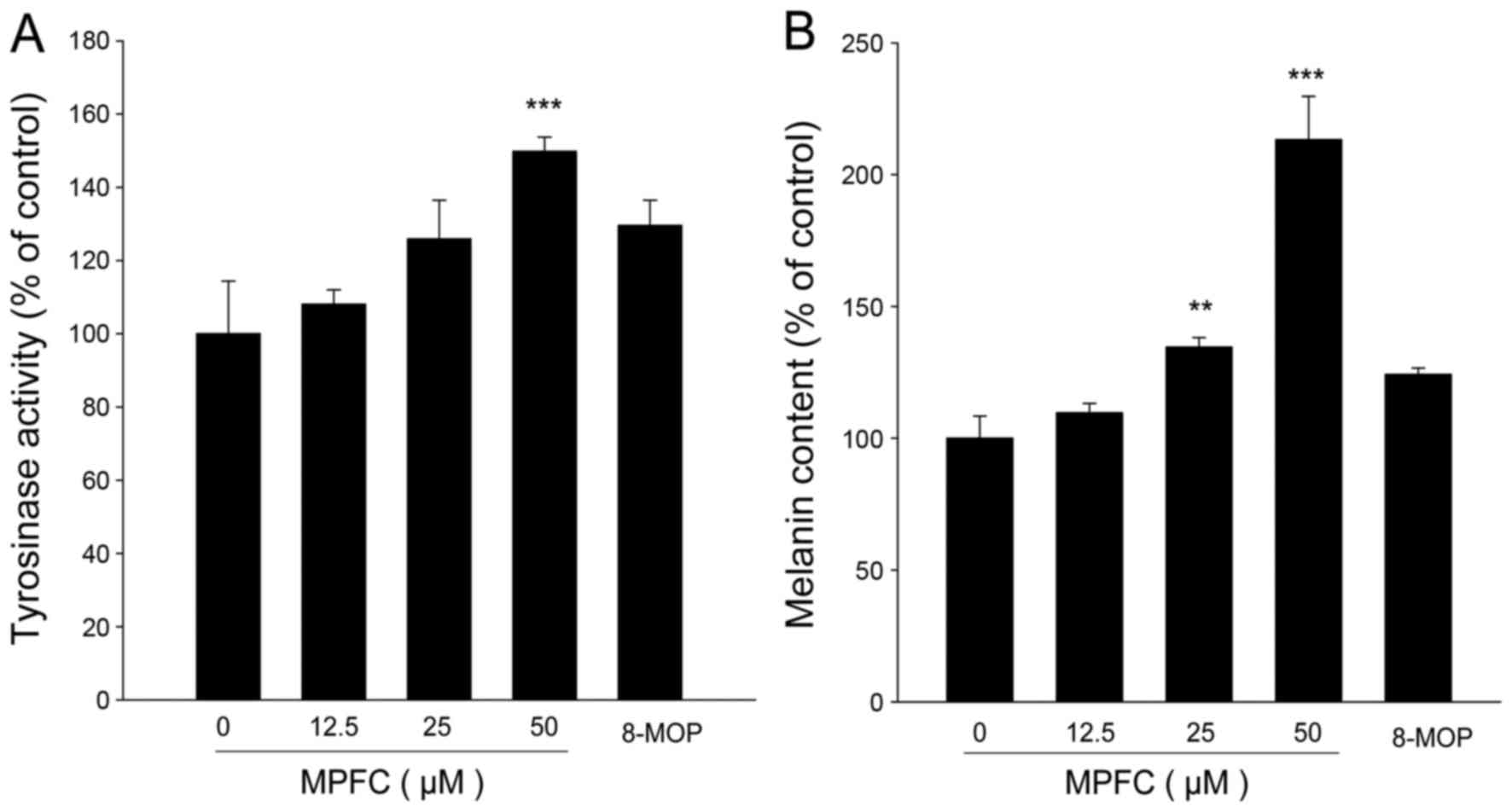
Treatment with MPFC demonstrated the increased
tyrosinase activity in a dose-dependent manner. At the same
concentration of 50 μM, the tyrosinase activity of MPFC was
increased by 20% compared with 8-MOP (0 μM, 100±14.4%; 12.5
μM, 108.1±3.9%; 25 μM, 125.9±10.6%; 50 μM,
149.8±3.9%; 8-MOP, 50 μM, 129.6±6.9%) (Fig. 3A). As shown in Fig. 3B, melanin amount showed the same
increasing trend in response to MPFC treatment, and the melanin
content of MPFC was increased 90% more than 8-MOP at 50 μM
(0 μM, 100±8.4%; 12.5 μM, 109.7±3.5%; 25 μM,
134.6±3.6%; 50 μM, 213.3±16.4%; 8-MOP, 50 μM,
124.2±2.4%). These results provide a pharmacological basis for the
traditional use of MPFC instead of 8-MOP in melanogenesis.
Effect of MPFC on the expression of
TRPs
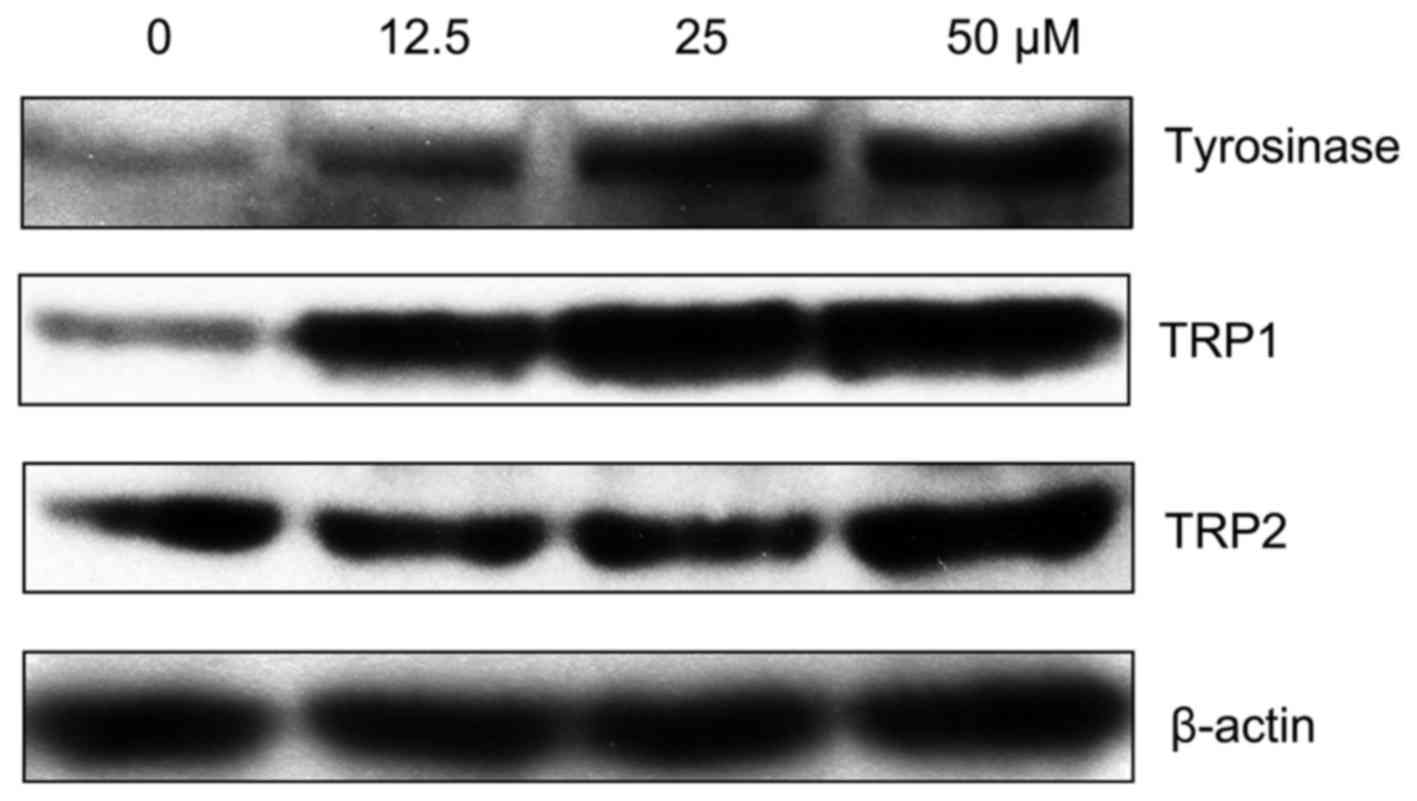
Since MPFC increased tyrosinase activity and melanin
production, we explored the melanogenic signaling pathway related
to the stimulatory activity of MPFC. After treatment with MPFC, the
expression of melanogenesis-related proteins (MRPs) such as
tyrosinase, TRP1, and TRP2 was examined by western blotting. MRP
expression was clearly increased after treatment with MPFC in a
dose-dependent manner (Fig.
4).
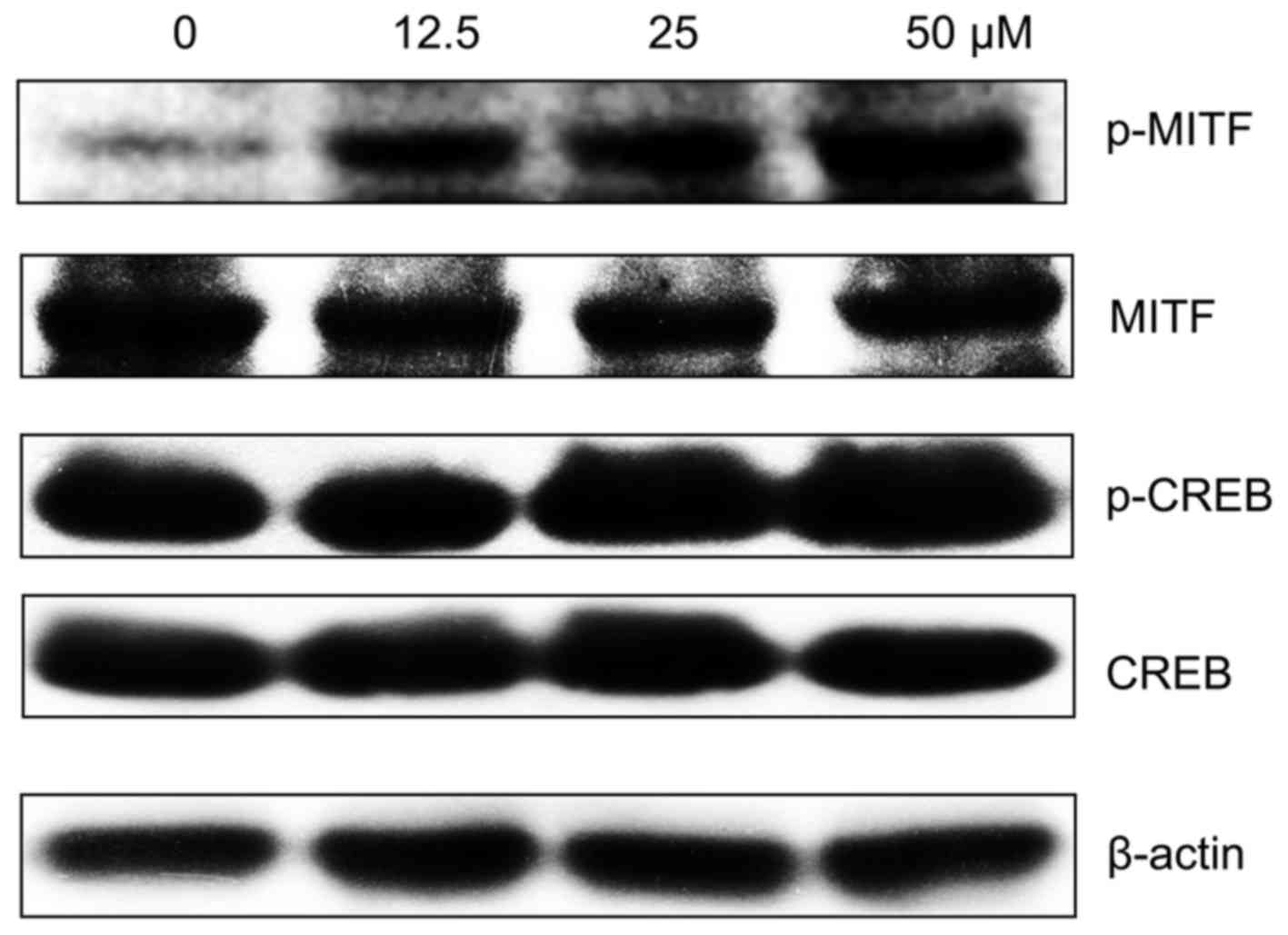
MPFC induces CREB activation and enhances
the expression of p-MITF
In order to elucidate how MPFC activates melanin
synthesis, both CREB and MITF were hypothesized to be involved in
MPFC-induced melanogenesis. As expected, the expression of
phosphorylated MITF by MPFC treatment for 48 h had a significant
increase. Our results also showed that phosphorylation of CREB was
clearly enhanced for 1 h (Fig. 5)
compared with cells treated with 0.1% DMSO only, which suggested
that MPFC-mediated elevation of the MITF may be cAMP-dependent.
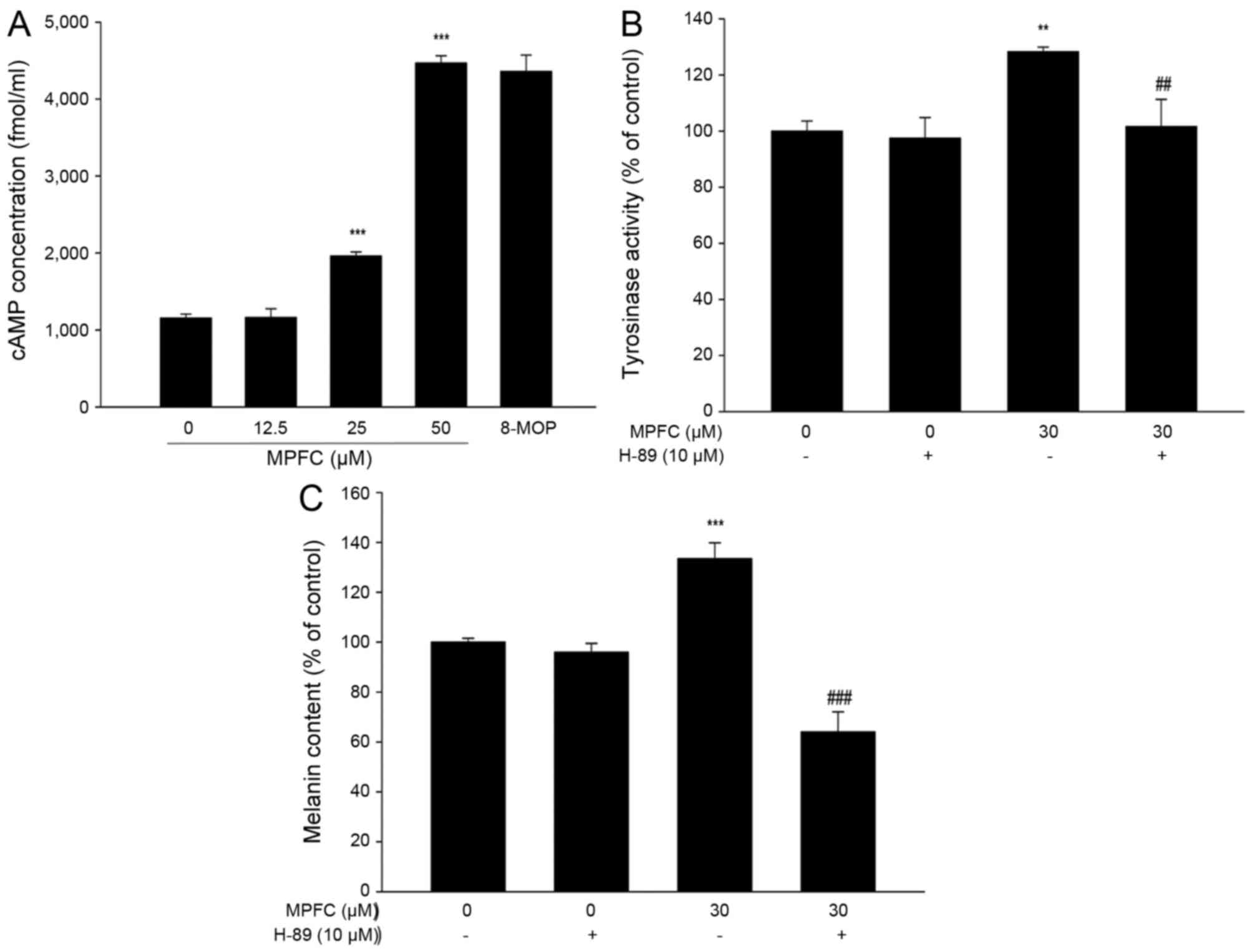
MPFC induces melanin synthesis through
intracellular cAMP accumulation and melanogenesis-related signaling
pathways PKA
To evaluate the hypothesis above, we explored
whether MPFC affects the accumulation of cAMP, which is a vital
step in melanogenesis. As seen in Fig. 6A, 12 h after MPFC addition, the
level of cAMP was increased. cAMP-related biological effects depend
on PKA, which has a direct influence on melanogenesis. Thus, we
evaluated the effect of H-89 (Beyotime Biotechnology, Shanghai,
China), an inhibitor of cAMP-dependent PKA, on the MPFC-mediated
induction of tyrosinase activity and melanin content. As shown in
Fig. 6B, MPFC-induced enhancement
of tyrosinase activity on incubation for 24 h was abrogated by
H-89. In addition, the melanin content after MPFC treatment for 48
h also reduced by H-89 compared with untreated cells (Fig. 6C). Generally, these results
revealed the critical involvement of cAMP/PKA signaling in
MPFC-mediated melanogenesis in B16 melanoma cells.
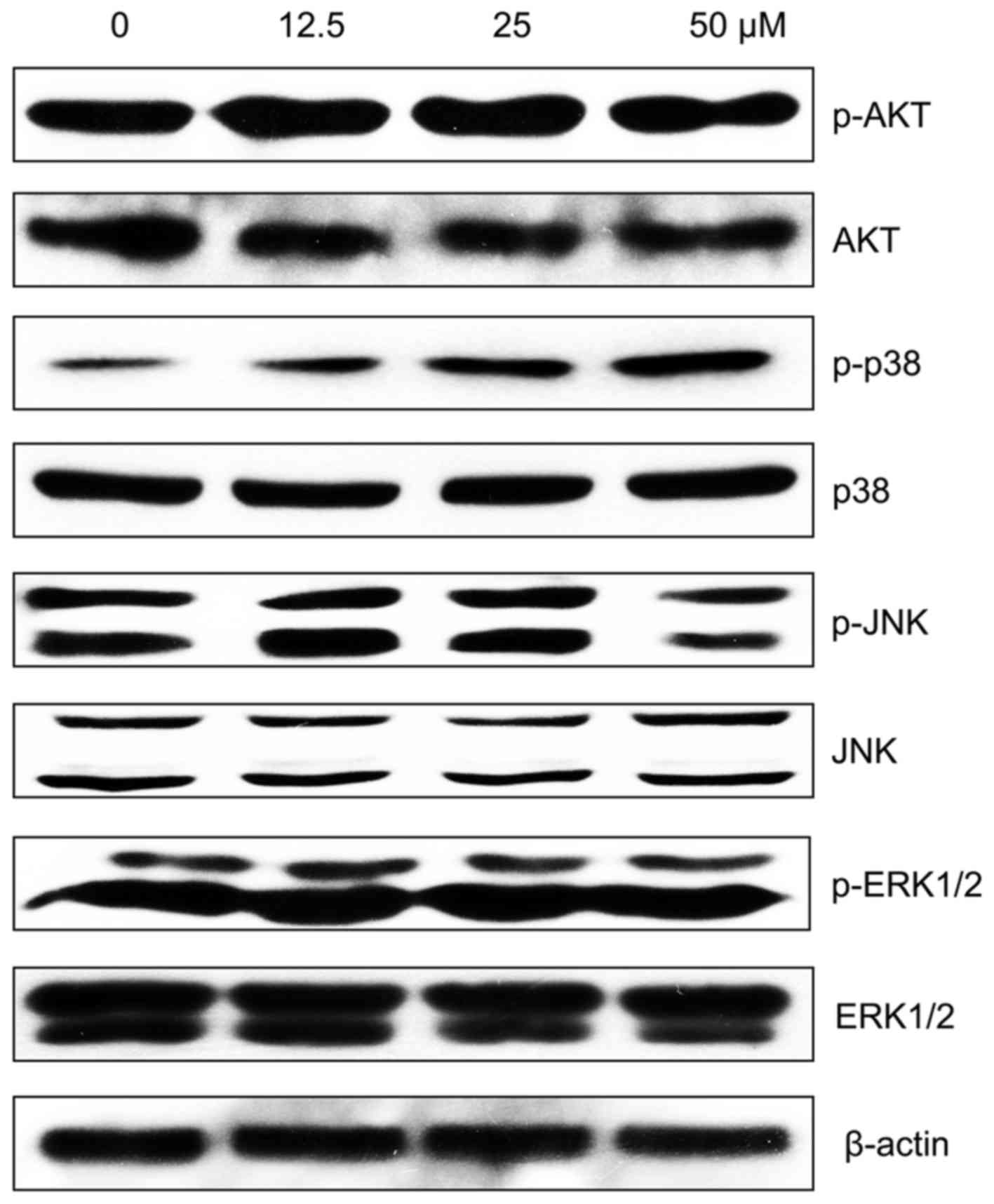
MPFC induces activation of p38 MAPK
The phosphorylation of MAPK or inhibition of
PI3K/AKT activation was reported to be the signaling process in
hyperpigmentation. Thus, we performed western blot analysis the
impact of MPFC on p38, ERK, JNK and AKT phosphorylation. As shown
in Fig. 7, phosphorylation of p38
MAPK was significantly increased after 1 h at different
concentrations of MPFC treatment compared with untreated cells. In
contrast, no significant upregulation of AKT phosphorylation was
induced by MPFC.
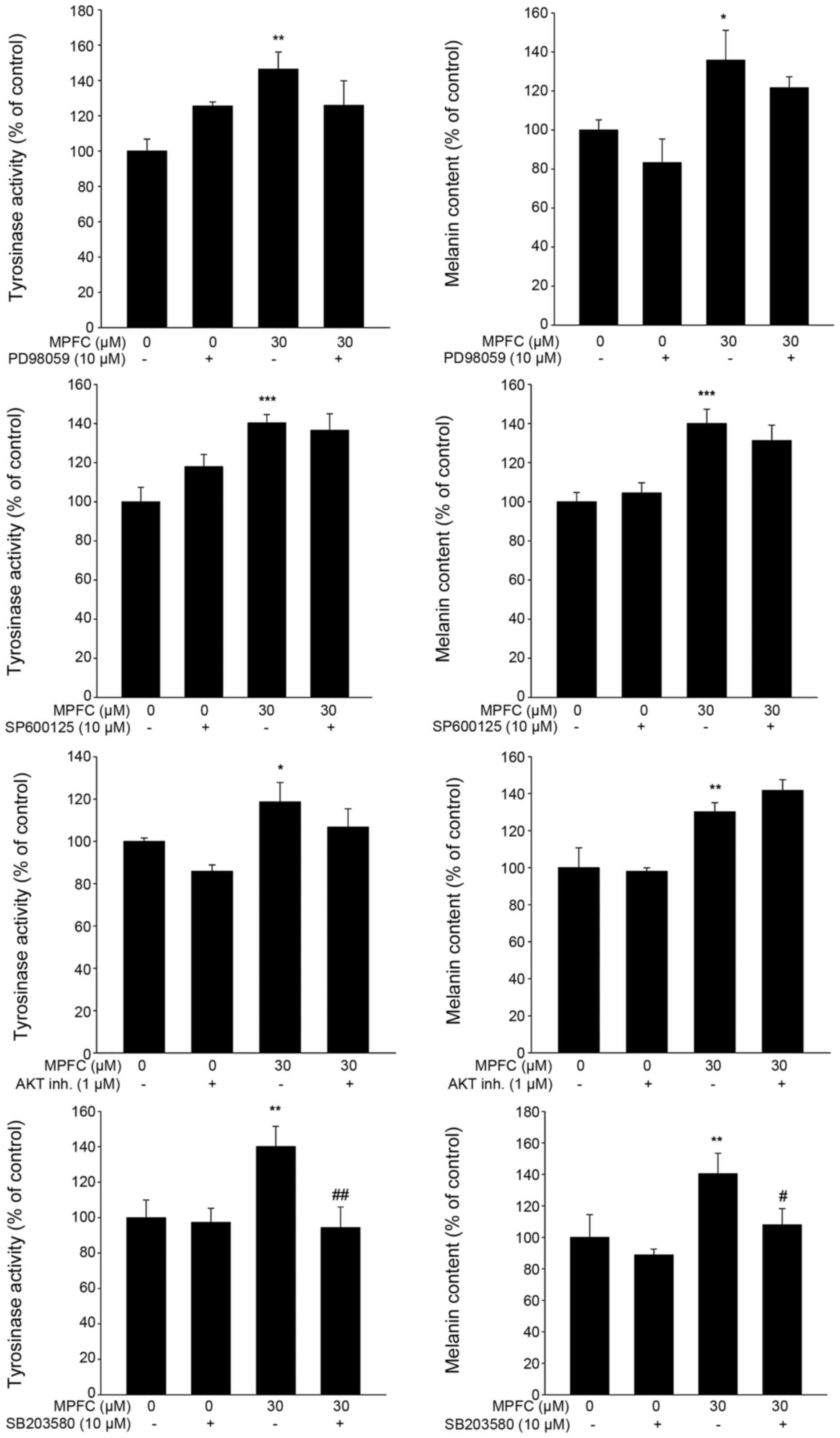
Effects of inhibitors of MAPKs and AKT on
MPFC-induced tyrosinase activity and melanin content
Even co-treatment with ERK inhibitor (PD98059), JNK
inhibitor (SP600125) (Beyotime Biotechnology) or AKT inhibitor IV
(EMD Biosciences, Inc., Madison, WI, USA), MPFC-induced tyrosinase
activity and melanin content were not influenced. However, the p38
MAPK inhibitor SB203580 (Beyotime Biotechnology) significantly
reduced MPFC-triggered tyrosinase activity and melanin content
(Fig. 8). These observations
reveal that p38, but not ERK, JNK or AKT pathway, was directly
involved in the upstream pathway of melanogenesis mediated by
MPFC.
Discussion
In hypopigmentation therapy (24,25), the induction of melanin production
was the focus of study to develop effective treatments (2,3,26,27). Natural resources have been
screened and active compounds have been synthesized for the
development of pigmentation agents by our research group, including
chlorogenic acid (22), kaliziri
extracts (23), furocoumarin
derivatives (20) and isoxazole
chalcone derivatives (21).
Recently, Niu et al (20) synthesized a series of furocoumarin
derivatives and discovered that many of them have strong activities
in melanogenesis. In consideration of the generally low
cytotoxicities of these compounds, we tested all of the derivatives
in B16 melanoma cells, and identified that MPFC, a psoralen
derivative, was an effective tyrosinase activitor in our recent
report. The melanin content and tyrosinase activity increased by 90
and 20%, respectively, in B16 cells treated with MPFC compared with
8-MOP treated controls in our research. We infer that the in
vitro melanin synthesis evaluation of these structurally
diverse analogues attributed to an outline of structure-activity
relationship. Studies (31,32) have reported that 8-MOP leads to
dramatic increases in melanin production through activating the
protein kinase A and/or protein kinase C signaling pathways. By
comparison, the regulation of MPFC in melanin synthesis results
from cross-talk between several different signaling pathways.
As mentioned in the introduction, phosphorylation of
MAPK (including ERK, JNK and p38 MAPKs) or inhibition of PI3K/AKT
activation has been reported as one of the signaling processes in
hyperpigmentation (33). It has
been shown that p38 MAPK activates MITF through the phosphorylation
of CREB, which in turn upregulates the expression of tyrosinase,
TRP-1 and TRP-2, resulting in melanin production (34,35). Activations of the ERK signaling
(36) and the JNK/SAPK pathways
(37) are related to the
downregulation of melanogenesis. Another signaling pathway involved
in melanogenesis regulation includes phosphatidylinositol 3-kinase
(PI3K)/AKT signaling, which phosphorylates MITF and promotes its
activation, leading to melanogenesis enhancement (38,39). In our experiments, treatment with
MPFC did not affect the total protein levels of ERK, JNK, AKT or
p38. However, it significantly promoted the levels of p-p38,
although not p-ERK, p-JNK and p-AKT in B16 cells. To verify whether
p38 MAPK signaling factors are responsible for MPFC-induced
activation effects on melanogenesis, co-incubation with p38 MAPK
inhibitor SB203580 clearly abrogated MPFC-stimulated melanin
content and tyrosinase activity. Unlike its effect on p38 MAPK,
other inhibitors did not influence the MPFC-stimulated melanogenic
process. These results suggested that p38 MAPK is responsible for
the pigmentation process mediated by MPFC in melanoma cells among
the upstream pathways involved in melanogenesis. The activation
effects on melanogenesis of MPFC and phosphorylation of p38
demonstrated in our research are consistent with the above
mentioned role of p38 signaling pathway in hyperpigmentation.
The PKA-dependent signaling pathway has also been
reported as one of the signaling processes in hyperpigmentation
(40). There is evidence that
intracellular cAMP promotes MITF expression via phosphorylating the
CREB family transcription factors. Once phosphorylated, CREB can
upregulate MITF and subsequently results in the indirect activation
of the tyrosinase promoter by MITF (41). In accordance with previous
studies, we observed that MPFC induces the phosphorylation of CREB
and enhances the production of cAMP compared with untreated cells.
It is noteworthy that 8-MOP showed the same increasing trend in
response to MPFC treatment in cAMP level, the results agreed with
the experimental results from literature (31,32). H-89, an inhibitor of protein
kinase A, completely abolished tyrosinase expression in B16 cells
induced by MPFC, indicating that MPFC-mediated MITF activation
relies on PKA signaling pathway.
In conclusion, MPFC enhanced melanin synthesis and
tyrosinase activity through accelerating p38 MAPK and PKA signaling
pathways. These results provide a molecular function for psoralen
derivative components in melanogenesis and will help expand our
knowledge of clinical therapy for enhancing skin
hyperpigmentation.
Acknowledgments
Not applicable.
Notes
[1]
Funding
This study was funded by the Projects of
International Science & Technology Cooperation of the Xinjiang
Uyghur Autonomous Region (no. 20146020), the Key Research and
Development Project Ofxinjiang Autonomous Region (no. 2016B03038-3)
and Personalized Medicines-Molecular Signature-based Drug Discovery
and Development, Strategic Priority Research Program of the Chinese
Academy of Sciences (no. xDA12050301).
[2] Availability
of data and material
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
LY, GP and CN conceived and designed the experiments
and wrote the paper. LY and CN performed the experiments.GP, HAA
and JD analyzed the data. HAA revised the paper. All authors read
and approved the final manuscript.
[4] Ethics
approval and consent to participate
Not applicable.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Donata, Kesavan M, Austin, Mohan KS,
Rajagopalan K and Kuttan R: Clinical trial of certain ayurvedic
medicines indicated in vitiligo. Anc Sci Life. 9:202–206.
1990.PubMed/NCBI
|
|
2
|
Lotti T, Zanardelli M and D'Erme AM:
Vitiligo: What's new in the psycho-neuro-endocrine-immune
connection and related treatments. Wien Med Wochenschr.
164:278–285. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alikhan A, Felsten LM, Daly M and
Petronic-Rosic V: Vitiligo: A comprehensive overview Part I.
Introduction, epidemiology, quality of life, diagnosis,
differential diagnosis, associations, histopathology, etiology, and
work-up. J Am Acad Dermatol. 65:473–491. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Silpa-Archa Narumol, Nitayavardhana
Sunatra, Thanomkitti Kanchalit, Chularojanamontri Leena, Varothai
Supenya and Wongpraparut Chanisada: Comparison of the efficacy and
safety of 0.1% tacrolimus ointment and 0.1% mometasonefuroate cream
for adult vitiligo: A single-blinded pilot study. Dermatologica
Sinica. 34:177–179. 2016. View Article : Google Scholar
|
|
5
|
Park SH, Kim DS, Kim WG, Ryoo IJ, Lee DH,
Huh CH, Youn SW, Yoo ID and Park KC: Terrein: A new melanogenesis
inhibitor and its mechanism. Cell Mol Life Sci. 61:2878–2885. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hearing VJ: Biochemical control of
melanogenesis and melanosomal organization. J Investig Dermatol
Symp Proc. 4:24–28. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Screaton RA, Conkright MD, Katoh Y, Best
JL, Canettieri G, Jeffries S, Guzman E, Niessen S, Yates JR III,
Takemori H, et al: The CREB coactivator TORC2 functions as a
calcium- and cAMP-sensitive coincidence detector. Cell. 119:61–74.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou J, Shang J, Ping F and Zhao G:
Alcohol extract from Vernonia anthelmintica (L.) willd seed
enhances melanin synthesis through activation of the p38 MAPK
signaling pathway in B16F10 cells and primary melanocytes. J
Ethnopharmacol. 143:639–647. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim HJ, Kim JS, Woo JT, Lee IS and Cha BY:
Hyperpigmentation mechanism of methyl 3,5-di-caffeoylquinate
through activation of p38 and MITF induction of tyrosinase. Acta
Biochim Biophys Sin (Shanghai). 47:548–556. 2015. View Article : Google Scholar
|
|
10
|
Hemesath TJ, Price ER, Takemoto C,
Badalian T and Fisher DE: MAP kinase links the transcription factor
microphthalmia to c-Kit signalling in melanocytes. Nature.
391:298–301. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Price ER, Ding HF, Badalian T,
Bhattacharya S, Takemoto C, Yao TP, Hemesath TJ and Fisher DE:
Lineage-specific signaling in melanocytes. C-kit stimulation
recruits p300/CBP to microphthalmia. J Biol Chem. 273:17983–17986.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen T, Heo SI and Wang MH: Involvement of
the p38 MAPK and ERK signaling pathway in the anti-melanogenic
effect of methyl 3,5-dicaffeoyl quinate in B16F10 mouse melanoma
cells. Chem Biol Interact. 199:106–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
El Mofty AM: Vitiligo and Psoralens.
Pergamon Press; Oxford: pp. 1147–1195. 1968
|
|
14
|
Fitzpatrick TB, Parrish JA and Pathak MA:
Phototherapy of vitiligo (idiopatic leukodermia). Sunlight and Man:
Normal and abnormal photobiologic responses. Tokyo University
Press; Tokyo: pp. 783–791. 1974
|
|
15
|
Parrish JA, Fitzpatrick TB, Shea C and
Pathak MA: Photochemotherapy of vitiligo. Use of orally
administered psoralens and a high-intensity long-wave ultraviolet
light system. Arch Dermatol. 112:1531–1534. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jois HS, Manjunath BL and Venkatarao SJ:
Chemical examination of the seeds of Psoralea corylifolia. J Indian
Chem Soc. 10:411933.
|
|
17
|
Späth E and Kainrath P: Über Bergamottin
und über die Auffindung von Limettin im Bergamottöl (XXXIV.
Mitteil. über natürliche Cumarine). Ber Dtsch Chem Ges.
70:2272–2276. 1937.In German. View Article : Google Scholar
|
|
18
|
Felsten LM, Alikhan A and Petronic-Rosic
V: Vitiligo: a comprehensive overview Part II: treatment options
and approach to treatment. J Am Acad Dermatol. 65:493–514. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tippisetty S, Goudi D, Mohammed AW and
Jahan P: Repair efficiency and PUVA therapeutic response variation
in patients with vitiligo. Toxicol In Vitro. 27:438–440. 2013.
View Article : Google Scholar
|
|
20
|
Niu C, Pang GX, Li G, Dou J, Nie LF, Himit
H, Kabas M and Aisa HA: Synthesis and biological evaluation of
furocoumarin derivatives on melanin synthesis in murine B16 cells
for the treatment of vitiligo. Bioorg Med Chem. 24:5960–5968. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Niu C, Yin L, Nie LF, Dou J, Zhao JY, Li G
and Aisa HA: Synthesis and bioactivity of novel isoxazole chalcone
derivatives on tyrosinase and melanin synthesis in murine B16 cells
for the treatment of vitiligo. Bioorg Med Chem. 24:5440–5448. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li HR, Habasi M, Xie LZ and Aisa HA:
Effect of chlorogenic acid on melanogenesis of B16 melanoma cells.
Molecules. 19:12940–12948. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tuerxuntayi A, Liu YQ, Tulake A, Kabas M,
Eblimit A and Aisa HA: Kaliziri extract upregulates tyrosinase,
TRP-1, TRP-2 and MITF expression in murine B16 melanoma cells. BMC
Complement Altern Med. 14:166–174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Westerhof W and d'Ischia M: Vitiligo
puzzle: The pieces fall in place. Pigment Cell Res. 20:345–359.
2007.PubMed/NCBI
|
|
25
|
Spritz RA: The genetics of generalized
vitiligo and associated autoimmune diseases. Pigment Cell Res.
20:271–278. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guerra L, Dellambra E, Brescia S and
Raskovic D: Vitiligo: Pathogenetic hypotheses and targets for
current therapies. Curr Drug Metab. 11:451–467. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Taieb A, Alomar A, Böhm M, Dell'anna ML,
De Pase A, Eleftheriadou V, Ezzedine K, Gauthier Y, Gawkrodger DJ,
Jouary T, et al Vitiligo European Task Force (VETF); European
Academy of Dermatology and Venereology (EADV); Union Europeenne des
Medecins Specialistes (UEMS): Guidelines for the management of
vitiligo: the European Dermatology Forum consensus. Br J Dermatol.
168:5–19. 2013. View Article : Google Scholar
|
|
28
|
Eun JS, Kim KS, Kim HN, Park SA, Ma TZ,
Lee KA, Kim DK, Kim HK, Kim IS, Jung YH, et al: Synthesis of
psoralen derivatives and their blocking effect of hKv1.5 channel.
Arch Pharm Res. 30:155–160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grass JA, Hei DJ, Metchette K, Cimino GD,
Wiesehahn GP, Corash L and Lin L: Inactivation of leukocytes in
platelet concentrates by photochemical treatment with psoralen plus
UVA. Blood. 91:2180–2188. 1998.
|
|
30
|
Chakraborty DP, Roy S and Chakraborty AK:
Vitiligo, psoralen, and melanogenesis: Some observations and
understanding. Pigment Cell Res. 9:107–116. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kanof NB: Melanin formation in
vitiliginous skin under the influence of external applications of
8-methoxypsoralen. J Invest Dermatol. 24:5–10. 1955. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lei TC, Virador V, Yasumoto K, Vieira WD,
Toyofuku K and Hearing VJ: Stimulation of melanoblast pigmentation
by 8-methoxypsoralen: The involvement of microphthalmia-associated
transcription factor, the protein kinase a signal pathway, and
proteasome-mediated degradation. J Invest Dermatol. 119:1341–1349.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park HY and Gilchrest BA: Signaling
pathways mediating melanogenesis. Cell Mol Biol (Noisy-le-grand).
45:919–930. 1999.
|
|
34
|
Bellei B, Maresca V, Flori E, Pitisci A,
Larue L and Picardo M: p38 regulates pigmentation via proteasomal
degradation of tyrosinase. J Biol Chem. 285:7288–7299. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ye Y, Chu JH, Wang H, xu H, Chou GX, Leung
AK, Fong WF and Yu ZL: Involvement of p38 MAPK signaling pathway in
the anti-melanogenic effect of San-bai-tang, a Chinese herbal
formula, in B16 cells. J Ethnopharmacol. 132:533–535. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim DS, Jeong YM, Park IK, Hahn HG, Lee
HK, Kwon SB, Jeong JH, Yang SJ, Sohn UD and Park KC: A new
2-imino-1,3-thiazoline derivative, KHG22394, inhibits melanin
synthesis in mouse B16 melanoma cells. Biol Pharm Bull. 30:180–183.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bu J, Ma PC, Chen ZQ, Zhou WQ, Fu YJ, Li
LJ and Li CR: Inhibition of MITF and tyrosinase by
paeonol-stimulated JNK/SAPK to reduction of phosphorylated CREB. Am
J Chin Med. 36:245–263. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Oka M, Nagai H, Ando H, Fukunaga M,
Matsumura M, Araki K, Ogawa W, Miki T, Sakaue M, Tsukamoto K, et
al: Regulation of melanogenesis through phosphatidylinositol
3-kinase-Akt pathway in human G361 melanoma cells. J Invest
Dermatol. 115:699–703. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Khaled M, Larribere L, Bille K, Aberdam E,
Ortonne JP, Ballotti R and Bertolotto C: Glycogen synthase kinase
3beta is activated by cAMP and plays an active role in the
regulation of melanogenesis. J Biol Chem. 277:33690–33697. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hirata N, Naruto S, Ohguchi K, Akao Y,
Nozawa Y, Iinuma M and Matsuda H: Mechanism of the melanogenesis
stimulation activity of (−)-cubebin in murine B16 melanoma cells.
Bioorg Med Chem. 15:4897–4902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ganss R, Schütz G and Beermann F: The
mouse tyrosinase gene. Promoter modulation by positive and negative
regulatory elements. J Biol Chem. 269:29808–29816. 1994.PubMed/NCBI
|