Introduction
Myocardial ischemia/reperfusion injury (MI/R), which
occurs following myocardial infarction, is caused by the sudden,
complete interruption of the myocardial blood circulation, which
leads to myocardial necrosis (1).
MI/R is a major health problem in China and its morbidity and
mortality rates increase every year (1). The development of acute heart
failure is the primary cause of mortality following acute MI/R
(2). The advancement of medical
techniques, such as internal medicine thrombolysis, interventional
stent and bypass surgery, have markedly reduced the mortality rate
of patients with acute MI/R that consequently experience acute
heart failure (3). However, MI/R
may develop into a chronic disease as it progresses; the chronic
stage of the disease is dominated by myocardial fibrosis
remodeling, which may induce chronic heart failure and lead to
mortality (4).
Micro (mi)RNA acts on target genes and regulates
expression of these genes, influencing the relevant molecular
signaling pathways of progenitor cell activation, thus regulating
multiple biological and pathological processes (5). MiRNA also serves an important role
in pathological processes, including myocardial ischemia and
ventricular remodeling following ischemia and infarction (6). Furthermore, miRNA serves an
important role in cardiac development and myogenic differentiation,
and protects against myocardial ischemia (6). The neurogenic locus notch homolog
protein (Notch) signaling pathway serves a role in regulating
cardiac development, as well as the proliferation and
differentiation of myocardial cells (7). Therefore, miRNA may regulate cardiac
development, as well as the proliferation and differentiation of
myocardial cells by activating the Notch signaling pathway.
Autophagy occurs in normal cardiac myocardium,
however, it is increased in hearts affected by myocardial
hypertrophy and ischemia (8).
Autophagy has been demonstrated to support the adaptive response of
the heart, thus protecting the heart from hemodynamic overload and
acute ischemic death (9).
However, cell death induced by autophagy also occurs following MI/R
(9). It is therefore important to
identify the impact of myocardial autophagy and the mechanisms by
which it occurs in order to to develop novel therapies to prevent
and treat post-MI/R myocardial remodeling (10).
A number of signaling pathways are involved in the
polarization of inflammation of MI/R (11). The Notch signaling pathway has
been conserved through evolution and serves an important role in
regulating cell growth, development, differentiation and apoptosis,
as well as the post-injury remodeling of tissue (11). The results of a study into the
cardiac development of fruit flies demonstrated that the Notch
signaling pathway primarily regulates cardiac development in two
ways: Asymmetric differentiation and lateral inhibition (12). The Notch signaling pathway induces
the asymmetric division of heart progenitor cells via asymmetric
differentiation and the expression of the Notch signal differs
between the two daughter cells; therefore it induces different
effects in the two daughter cells (13). It has been identified in a
previous study on the cardiac development of mice that receptors of
the Notch signaling pathway were primarily expressed during
different stages of development: Notch1 and Notch2 were highly
expressed during cardiac development, whereas Notch3 exhibited high
expression in smooth muscle and Notch4 was highly expressed in the
vascular endothelium (14).
The downstream molecules of the Notch signaling
pathway possess the characteristic structure of a basic
helix-loop-helix and belong to the Hes and Hey families (15). In mammals, the Hes family is
comprised of seven genes (Hes1-7), of which only Hes1, Hes5 and
Hes7 are regulated by the Notch signaling pathway (15). From the Hey family, Hey1, Hey2 and
Hey L are regulated by the Notch signaling pathway (15). A mouse embryo with a mutation in
the Notch1 gene exhibits cardiac defects, suggesting that the Notch
signaling pathway is essential for the early morphology and
remodeling of the heart in vertebrates (16). During early cardiac development,
the expression of Hes1 is restricted to the atria; by contrast,
Hes2 is expressed in the ventricles (17). Following birth, the expression of
Hes1 and Hes2 in the heart gradually decrease (17).
The protein kinase B (Akt) signaling pathway is
involved in cytophysiological processes, for example, it regulates
the mitochondrion-mediated anti-apoptosis process (16). The results of a recent study
demonstrated that the Akt signaling cascade was involved in the
production of antioxidant enzymes in the mitochondria and suggested
that the Akt signaling cascade may be an important upstream
regulatory mechanism that increases the activity of antioxidant
enzymes in the cytoplasm (18).
Lai et al (19)
demonstrated that miRNA-30e induces cardio-protection in rats with
doxorubicin-induced heart failure via autophagy. Therefore, the
present study examined the cardioprotective mechanisms by which
miRNA-30e protects the heart against MI/R. The specific signaling
pathways that may provide protection and be a potential target for
novel therapies to treat patients with heart disease were also
explored.
Materials and methods
Patients
Peripheral blood samples were collected from 24
patients (all male, aged 47–63 years old) with MI/R admitted to the
Department of Cardiac Surgery at The First Affiliated Hospital of
Medical College of Xi'an Jiaotong University, as well as 24 age-
and sex-matched healthy volunteers (all male, aged 45–65 years old)
between August and September 2016. Peripheral blood was centrifuged
at 2,000 × g for 10 min at 4°C; subsequently, serum was collected
and stored at −80°C. The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Xi'an Jiaotong
University (Xi'an, China). All patients provided written informed
consent prior to the collection of blood samples.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from collected serum or rat
myocardium H9C2 cells (Shanghai Cell Bank of Chinese Academy of
Sciences, Shanghai, China) using TRIzol Reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Subsequently, 1
µg total RNA was reverse-transcribed into cDNA using the
RealMasterMix First Strand cDNA Synthesis kit (Tiangen Biotech Co.,
Ltd., Beijing, China) following the manufacturer's protocol. The
relative expression of miRNA-30e was mixed. qPCR was performed
using SYBR® Premix ExTaq™ (Takara Bio, Inc., Otsu,
Japan) on an Applied Biosystems 7500 real-time PCR system (Thermo
Fisher Scientific, Inc.). The qPCR conditions were as follows: 95°C
for 10 min, 40 cycles of 95°C for 30 sec and 60°C for 30 sec. The
following primers were used: miRNA-30e, forward,
5′-GGCGGTGTAAACATCCTT-3′ and reverse,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCG-3′; U6, forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′.
miRNA-30e expression was quantified using the 2−ΔΔCq
method (20).
Cell culture and transfection
Cardiac myoblast H9C2 cells were cultured in
Dulbecco's modified eagle's medium (DMEM, Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) and 100 U penicillin and
streptomycin at 37°C in a humidified atmosphere containing 5%
CO2. si-miRNA-30e and negative mimics were purchased
from Sangon Biotech Co., Ltd. (Shanghai, China). A total of 200 ng
si-miRNA-30e (5′-AAAGAGCAGTGTTTCGT-3′ and 5′-CCGACCGTCGGTAAA-3′)
and 200 ng negative control (5′-CCCCCCCCCCC-3′ and 5′-CCCCCCCCC-3′)
mimics were co-transfected into H9C2 cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Following 6 h transfection, cells were incubated
in a hypoxic chamber (5% CO2 and 95% nitrogen) at 37°C
for 2 h to establish an in vitro model of MI/R, following a
previously published protocol (21).
Treatment with 3-methyladenine
(3-MA)
Following 6 h transfection, cells were incubated
with 10 µM 3-MA (MedChemExpress, Monmouth Junction, NJ, USA)
in a hypoxic chamber (5% CO2 and 95% nitrogen) at 37°C
for 2 h.
Notch1 treatment
Notch1 recombinant protein was purchased from
Beijing Yiqiao Shenzhou Technology Co. Ltd. (Beijing, China).
Following 6 h transfection, H9C2 cells were treated with 5 ng
Notch1 recombinant protein in a hypoxic chamber (5% CO2
and 95% nitrogen) for 2 h.
Cell proliferation assay
To analyze cell proliferation, 2,000 cells/well were
plated in 96-well plates and 10 µl MTT was added to each
well. The plates were then incubated for 4 h at 37°C. The culture
medium was removed, dimethyl sulfoxide was added to the wells and
the plates were incubated for 20 min at 37°C. A microplate reader
was then used to measure absorbance of formazan at 492 nM.
Flow cytometric assay of apoptosis
To analyze apoptosis, H9C2 cells were transfected
for 48 h and processed using the 10 µl Annexin V-Fluorescein
isothiocyanate/5 µl Propidium Iodide Apoptosis Detection kit
(BD Biosciences, San Jose, CA, USA) for 15 min in the dark.
Analysis was performed using a BD FACSCanto™ II (BD Biosciences)
flow cytometer with FlowJo 7.6.1 software (FlowJo LLC, Ashland, OR,
USA).
Analysis of caspase-3 activity
Prior to analysis of caspase-3 activity, cells were
transfected for 48 h and lysed in radioimmunoprecipitation assay
(RIPA) buffer (Beyotime Institute of Biotechnology, Haimen, China).
Total protein was quantified using a BCA assay (Beyotime Institute
of Biotechnology). Subsequently, 10 µg protein was analyzed
using a caspase-3 assay kit (cat. no., C1115; Beyotime Institute of
Biotechnology) to determine caspase-3 activity. A microplate reader
was used to measure absorbance at 405 nM.
Western blot analysis
Proteins were extracted from H9C2 cells following
transfection for 48 h and subsequently lysed in RIPA buffer. Total
protein was quantified using a BCA assay. A total of 50 µg
protein was separated using 6–12% SDS-PAge and transferred onto
polyvinylidene difluoride membranes. Following blocking for 1 h
with 5% bovine serum albumin (Beyotime Institute of Biotechnology)
and TBS with 0.1% Tween-20 at 37°C, membranes were incubated with
primary antibodies against apoptosis regulator BAX (Bax), inducible
nitric oxide synthase (iNOS, cat. no., sc-649; 1:500; Santa Cruz
Biotechnology), microtubule-associated proteins 1A/1B light chain
3B (LC3, cat. no., 4599; 1:2,000), p62 (cat no., 88588; 1:2,000),
Beclin-1 (cat. no., 3495; 1:2,000), neurogenic locus notch homolog
protein 1 (Notch1, cat. no., 3608; 1:2,000), transcription factor
Hes-1 (Hes1, cat. no., 11988; 1:2,000), phosphorylated (p)-Akt
(cat. no., 4060; 1:2,000) and gAPDH (cat. no., 2118; 1:5,000; all
from Cell Signaling Technology, Inc., Danvers, MA, USA) overnight
at 4°C. Membranes were subsequently incubated with anti-rabbit or
anti-mouse AP-linked secondary antibody (cat. nos., 7054 and 7056,
respectively; 1:5,000; Cell Signaling Technology Inc.) for 1 h at
room temperature. Membranes were labeled with horseradish
peroxidase and detected using the Pierce enhanced Chemiluminescence
kit (Thermo Fisher Scientific, Inc.) and results were analyzed
using Image Lab software 3.0 (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
ELISA assay
Following 48 h transfection, cells were lysed in
RIPA buffer. Total protein was quantified using a BCA assay. A
total of 10 µg total protein was analyzed using ELISA kits
against glutathione (GSH; cat. no., A006-2), GSH-peroxidase (PX;
cat. no., A005) and superoxide dismutase (SOD; cat. no., A001-1-1).
A microplate reader was used to measure absorbance at 450 nM.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Two-way analysis of variance with Bonferroni multiple
comparisons adjustments was used to assess the statistical
significance of differences among groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
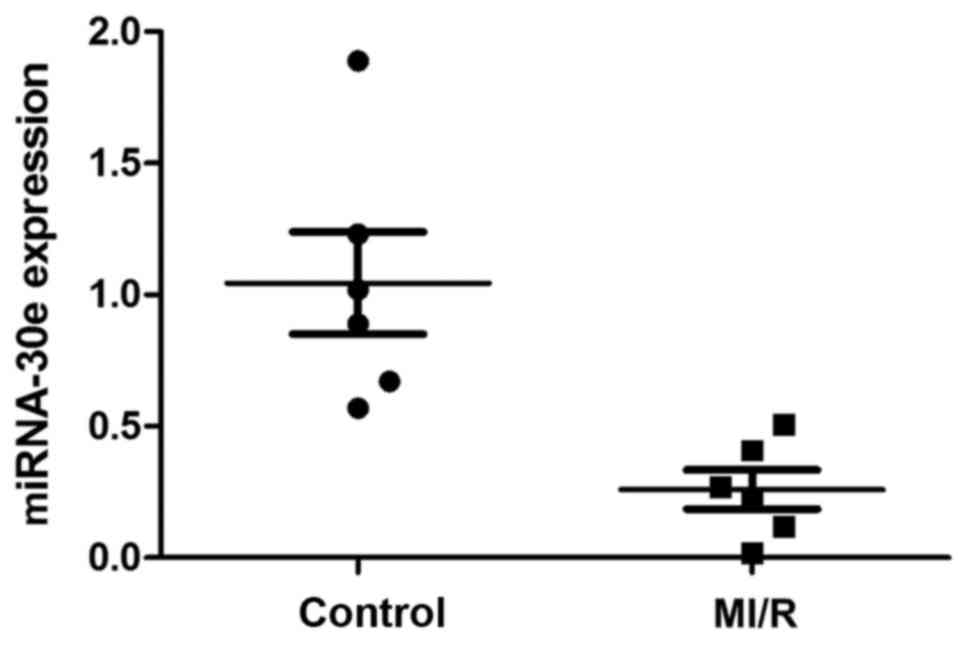
MiRNA-30e expression is decreased in
patients with MI/R
Changes in the expression of miRNA-30e were
investigated in patients with MI/R compared with a control group.
It was demonstrated that miRNA-30e expression was markedly
decreased in the serum of patients with MI/R compared with the
control group (Fig. 1).
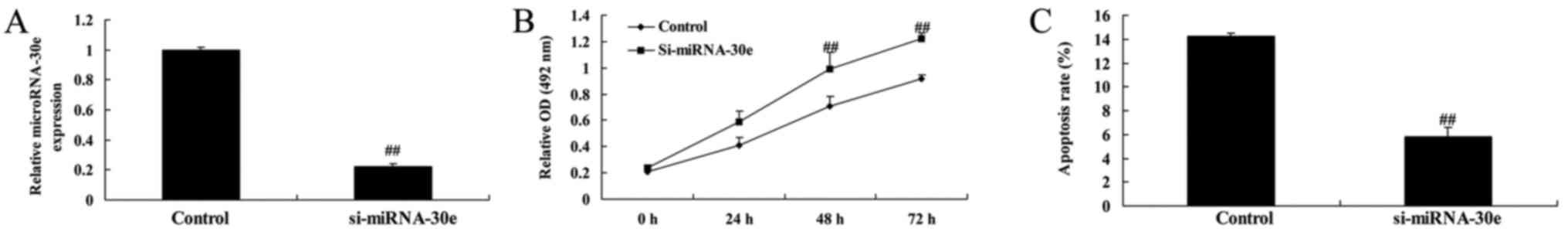
Si-miRNA-30e inhibits apoptosis
To investigate the effects of miRNA-30e on
apoptosis, si-miRNA-30e was used to significantly inhibit miRNA-30e
expression in H9C2 cells (P<0.01; Fig. 2A). The rate of cell proliferation
was significantly increased in cells transfected with si-miRNA-30e
compared with controls (P<0.01; Fig. 2B). Furthermore, the rate of
apoptosis was significantly decreased in cells treated with
si-miRNA-30e compared with controls (P<0.01; Fig. 2C).
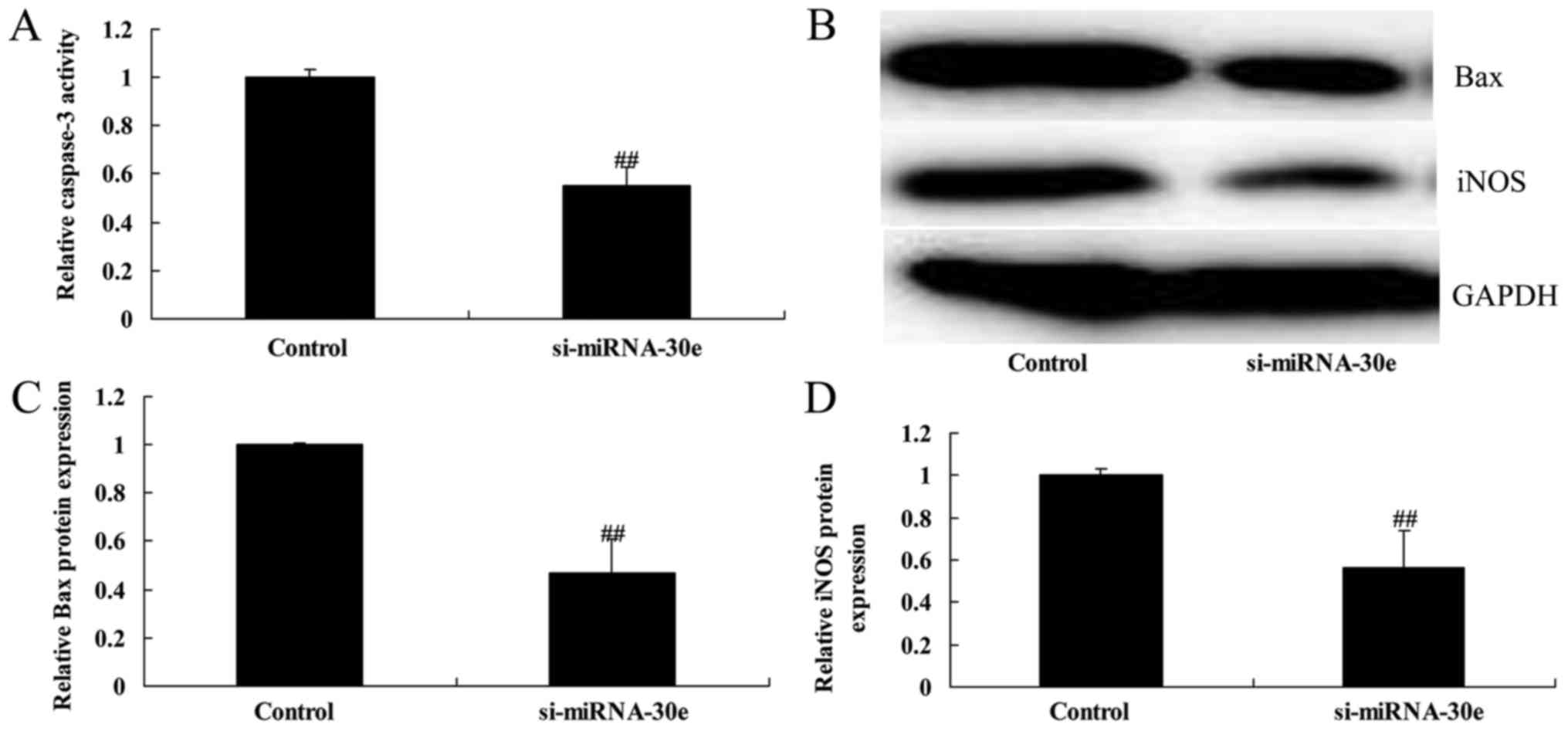
Si-miRNA-30e inhibits iNOS, Bax and
caspase-3 expression
ELISA and western blot analysis was used to evaluate
the effect of miRNA-30e on caspase-3 activity and the expression of
iNOS and Bax in H9C2 cells. Following inhibition of miRNA-30e by
si-miRNA-30e, there was a significant reduction in caspase-3
activity compared with the control group (P<0.01; Fig. 3A). Western blot analysis (Fig. 3B) revealed that the expression of
Bax and iNOS were significantly reduced following inhibition of
miRNA-30e (P<0.01; Fig. 3C and
D).
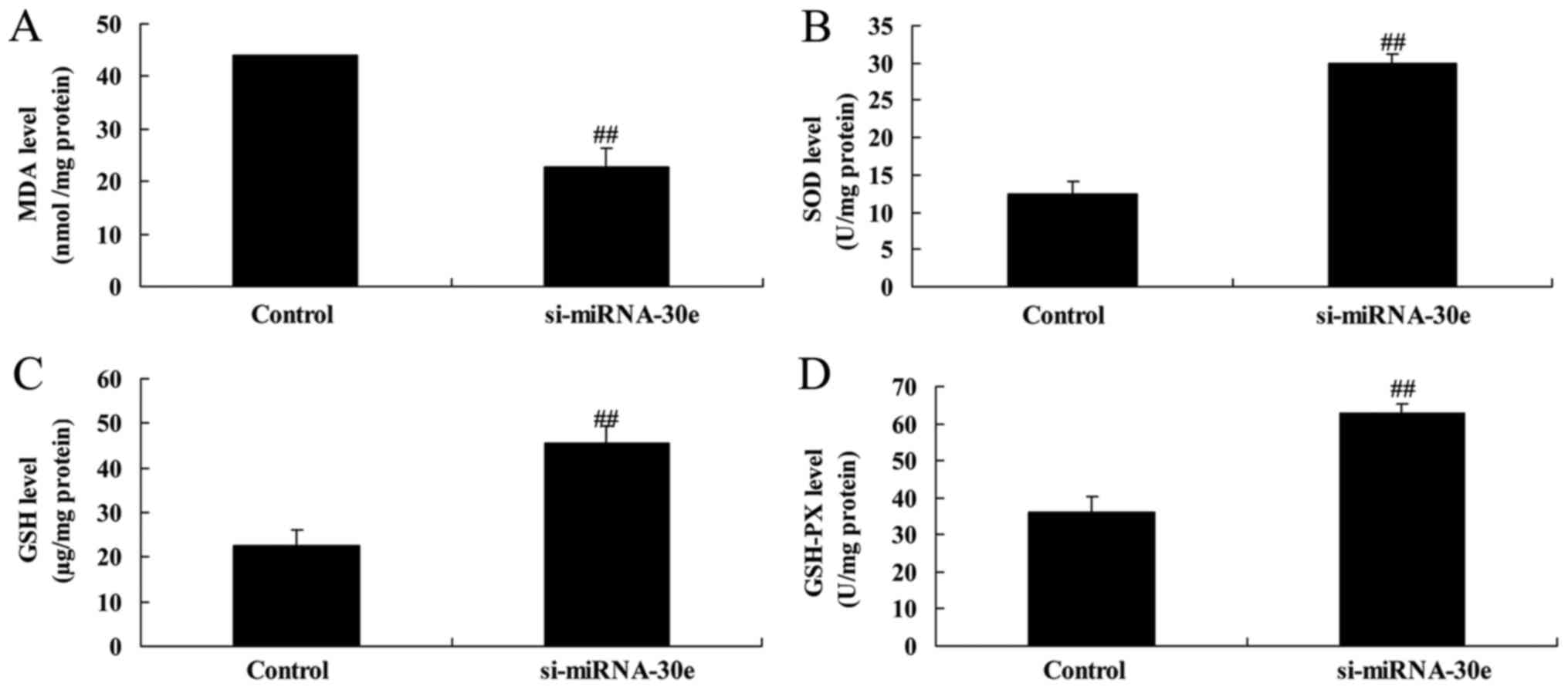
Knockdown of miRNA-30e increases the
activity of proteins associated with oxidative stress
ELISA revealed that malondialdehyde activity was
significantly decreased (P<0.01; Fig. 4A) and that GSH, GSH-PX and SOD
activities were significantly increased (P<0.01; Fig. 4B–D) in H9C2 cells following
inhibition of miRNA-30e expression. These results indicate that
miRNA-30e is able to regulate oxidative stress.
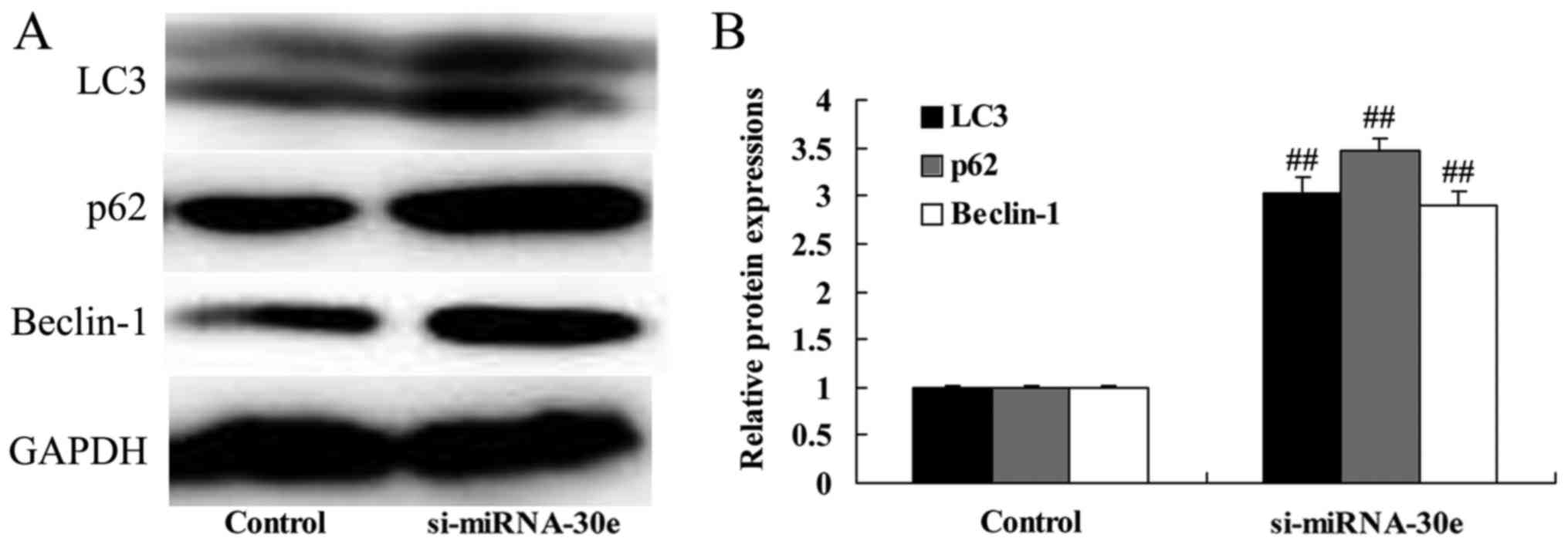
Silencing of miRNA-30e increases LC3, p62
and Beclin-1 expression
Western blot analysis was performed to determine
whether miRNA-30e affects autophagy and the expression of
autophagy-associated proteins in H9C2 cells. The inhibition of
miRNA-30e expression by si-miRNA-30e significantly increased the
expression of LC3, p62 and Beclin-1 compared with the control group
(P<0.01; Fig. 5). These are
autophagy-associated proteins and these results suggest that a
reduction of miRNA-30e expression, as observed in patients with
MI/R, leads to an increase in cell autophagy.
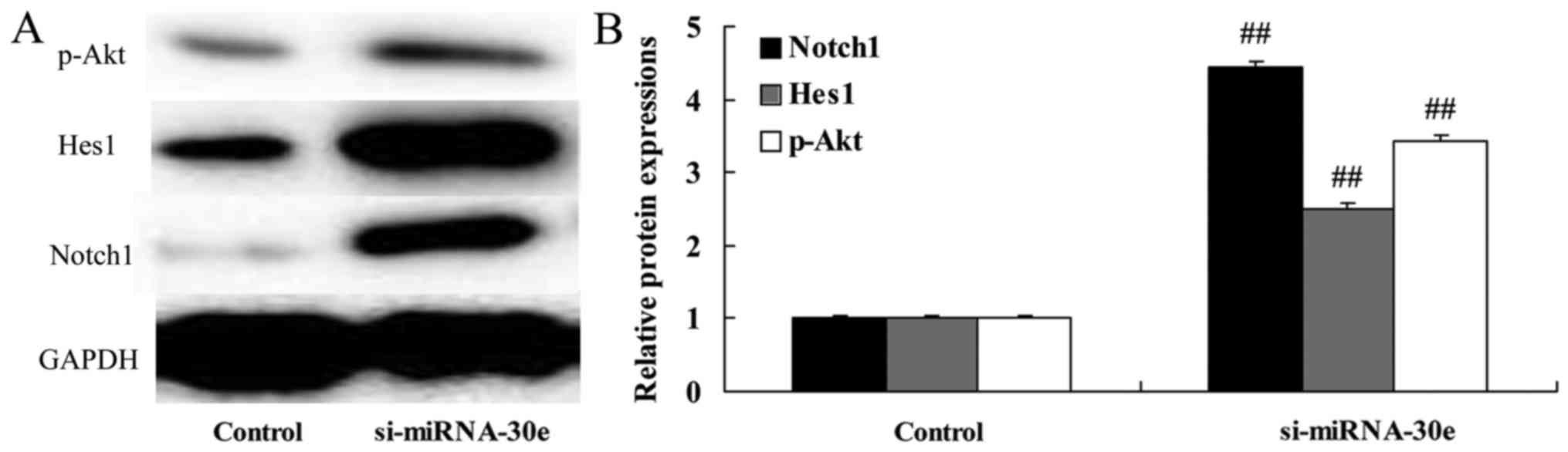
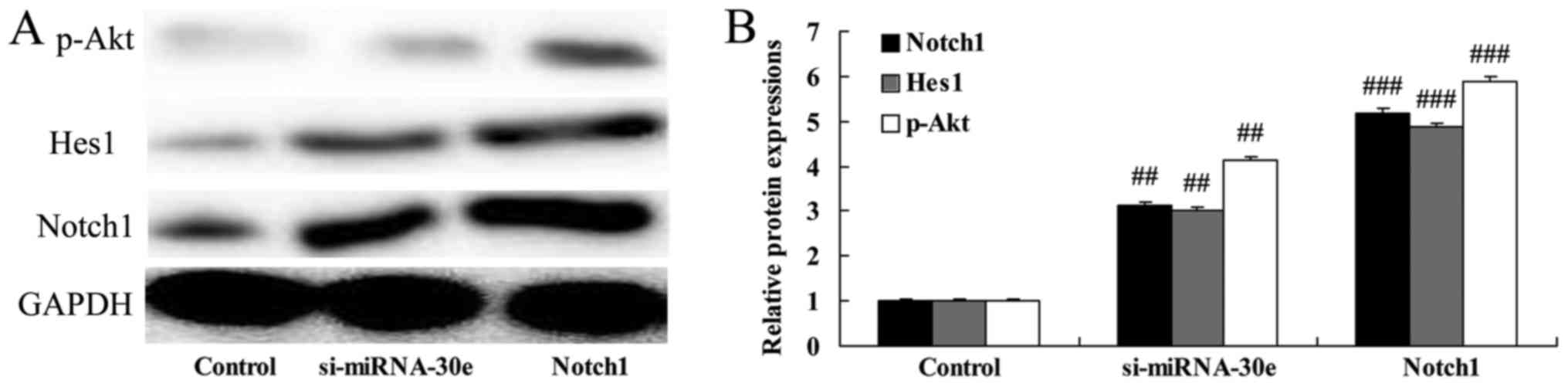
miRNA-30e knockdown leads to a
significant increase in Notch1, Hes1 and p-Akt expression
The effect of si-miRNA-30e on Notch1, Hes1 and p-Akt
signaling was examined in H9C2 cells. Inhibition of miRNA-30e
expression by si-miRNA-30e significantly increased the expression
of Notch1, Hes1 and p-Akt compared with the control group
(P<0.01; Fig. 6). These
results demonstrate that miRNA-30eprotects the heart against I/R
injury via the Notch1/Hes1/Akt signaling pathway.
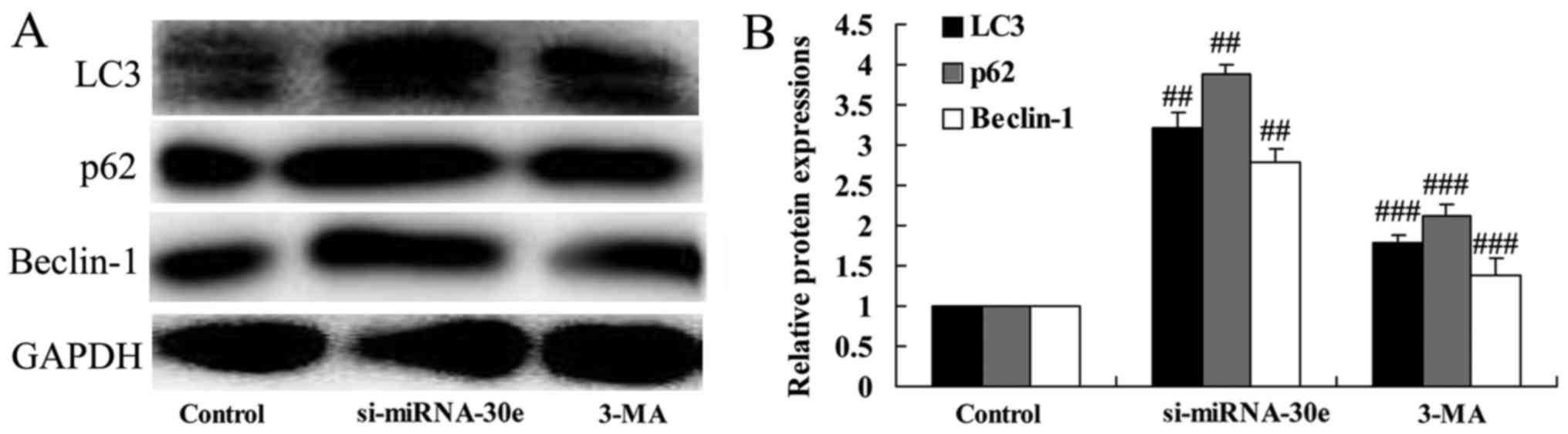
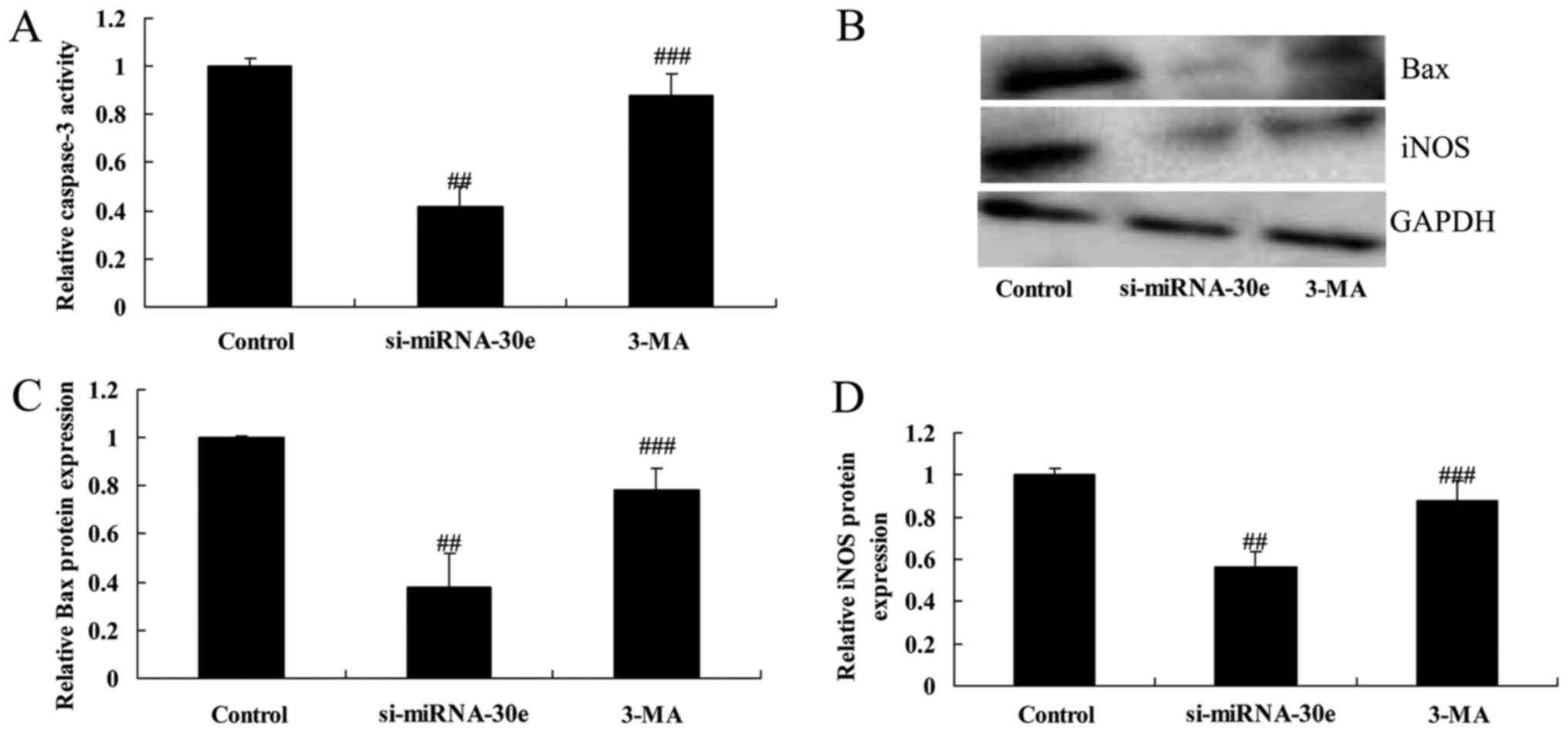
Inhibition of autophagy following
treatment with 3-MA reverses the effect of si-miRNA-30e on LC3, p62
and Beclin-1 expression
To investigate whether inhibition of autophagy
affects the function of si-miRNA-30e, H9C2 cells were treated with
3-MA. In cells treated with 3-MA, the expression of LC3, p62 and
Beclin-1 were significantly reduced compared with the si-miRNA-30e
group (P<0.01; Fig. 7). These
results indicate that treatment with 3-MA is able to suppress
autophagy and reduce the effects of si-miRNA-30e.
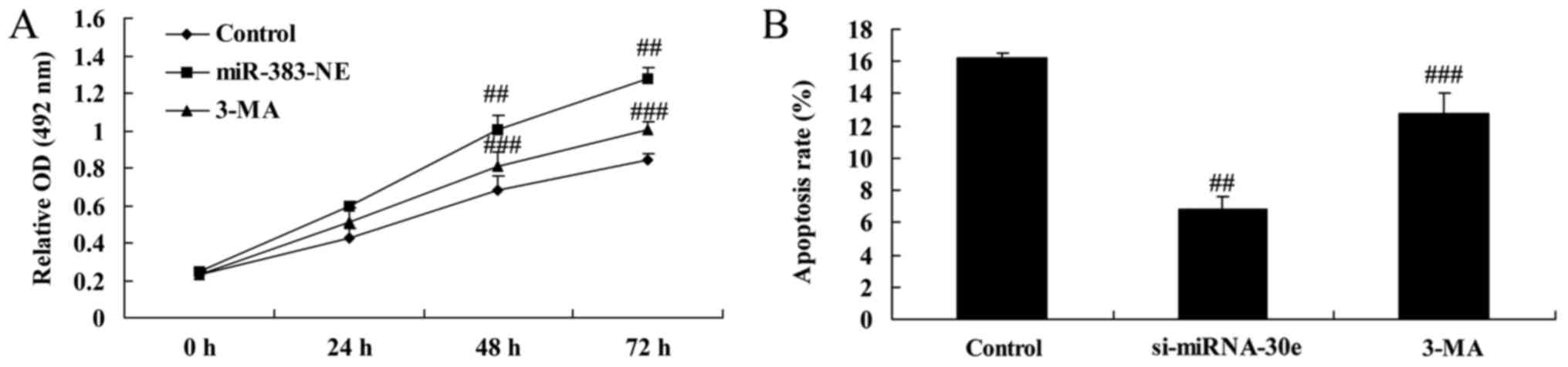
Autophagy inhibition reverses the effect
of si-miRNA-30e on apoptosis
The proliferation and apoptosis of H9C2 cells
treated with either si-miRNA-30e or si-miRNA-30e and 3-MA were
measured. Treatment with 3-MA reversed the effects of si-miRNA-30e
on proliferation, as 3-MA-treated cells exhibited significantly
lower proliferation rates compared with cells transfected with
si-miRNA-30e (P<0.01; Fig.
8A). In addition, 3-MA treated cells exhibited significantly
higher rates of apoptosis compared with si-miRNA-30e cells,
indicating that 3-MA reverses the effects of si-miRNA-30e on
cellular apoptosis (P<0.01; Fig.
8B).
Inhibition of autophagy following
treatment with 3-MA reverses the effect of si-miRNA-30e on the
expression of iNOS and Bax and caspase-3 activity
Inhibiting autophagy following treatment of H9C2
cells with 3-MA significantly reversed the inhibition of caspase-3
activity, as well as the inhibition of Bax and iNOS expression by
si-miRNA-30e (P<0.01; Fig.
9).
Treatment with 3-MA reverses the effect
of si-miRNA-30e on oxidative stress
The effect of autophagy on the effect of
si-miRNA-30e on the expression of proteins in H9C2 cells associated
with oxidative stress was investigated (Fig. 10). Inhibition of autophagy
following treatment with 3-MA significantly reduced the increase of
SOD, GSH and GSH-PX activity compared with the si-miRNA-302 group
(P<0.01; Fig. 10B–D
respectively). Furthermore, treatment with 3-MA significantly
reversed the decrease in MDA activity that occurred following
treatment with si-miRNA-30e alone (P<0.01; Fig. 10A).
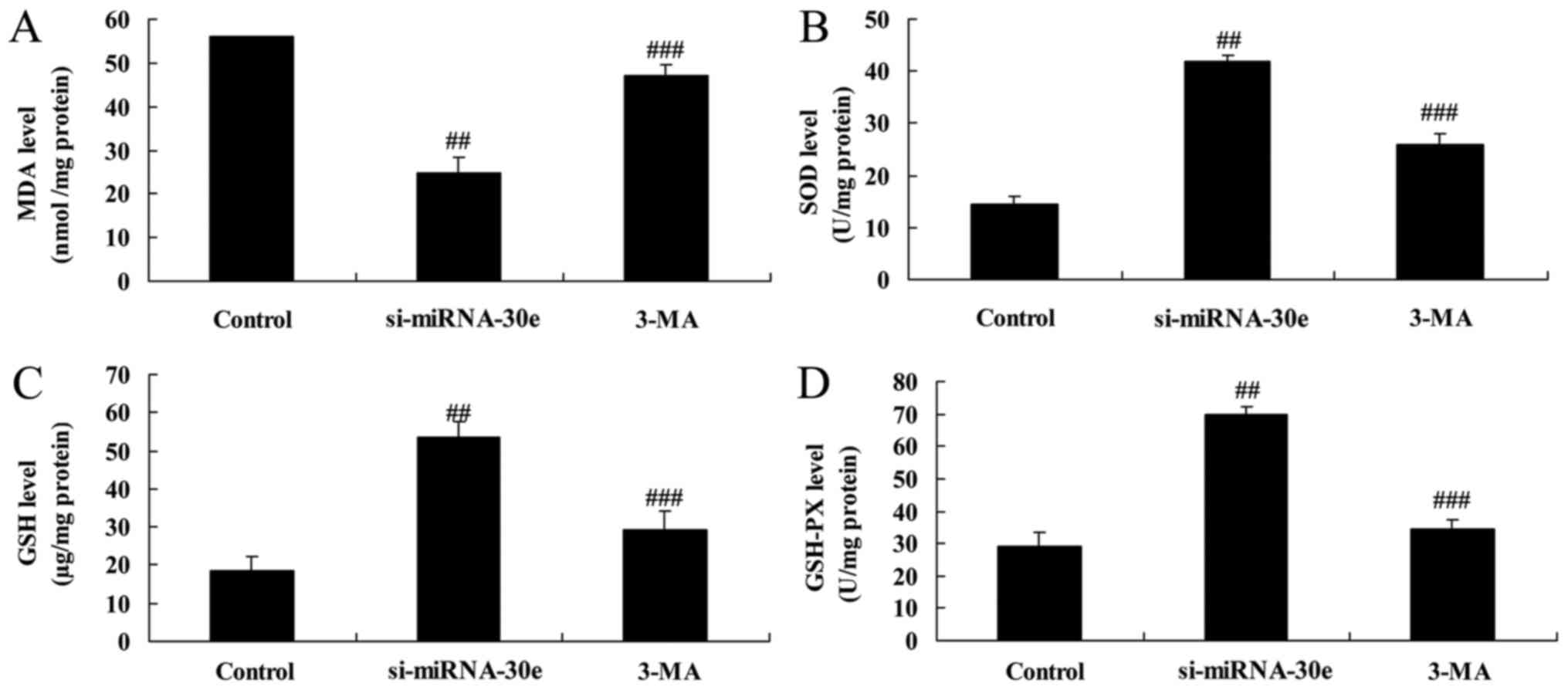 | Figure 10Inhibition of autophagy by 3-MA
reverses the effect of si-miRNA-30e on oxidative stress. ELISA was
used to measure levels of (A) MDA (B), SOD (C), GSH and (D) GSH-PX.
##P<0.01 vs. the control group,
###P<0.01 vs. the si-miRNA-30e group. 3-MA,
3-methyladenine; GSH, glutathione; PX, peroxides; SOD, superoxide
dismutase; MDA, malondialdehyde; si, small interfering; miRNA,
microRNA. |
Notch1 promotion enhances the effect of
si-miRNA-30e on Notch1, Hes1 and p-Akt expression
The effect of Notch1 expression on Notch1, Hes1 and
p-Akt expression was investigated. The Notch1 recombinant protein
was administered to H9C2 cells as well as si-miRNA-30e and the
effect was compared to that of H9C2 cells treated with si-miRNA-30e
alone. The Notch1 recombinant protein significantly enhanced the
effect of si-miRNA-30e on Notch1, Hes1 and p-Akt expression
compared with treatment with si-miRNA-30e alone (P<0.01;
Fig. 11).
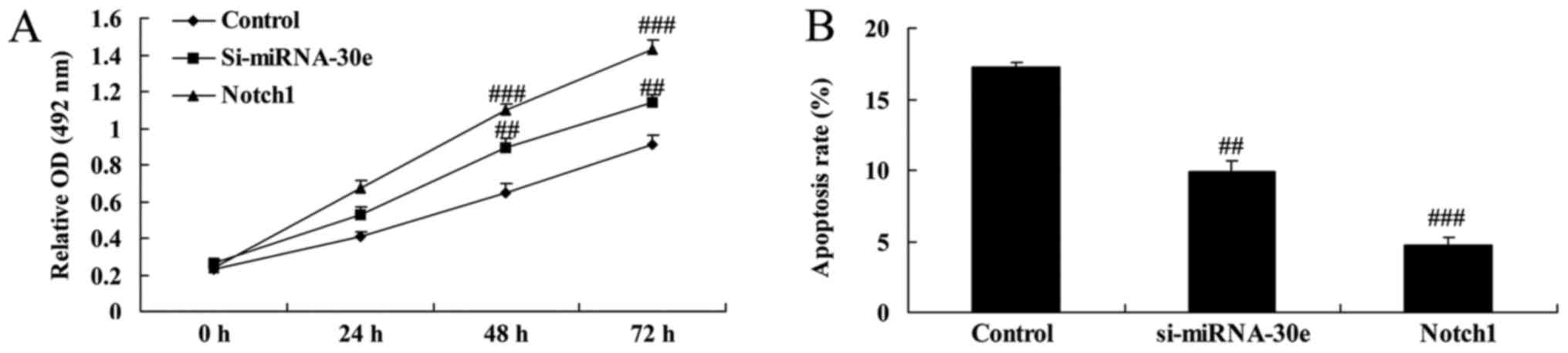
Notch1 promotion increases the effect of
si-miRNA-30e on the apoptosis and proliferation of cells
It was determined whether Notch1 affects the
function of si-miRNA-30e on the apoptosis and proliferation of H9C2
cells. Compared with the si-miRNA-30e group, the promotion of
Notch1 expression significantly increased proliferation (P<0.01;
Fig. 12A) and significantly
decreased apoptosis (P<0.01; Fig.
12B).
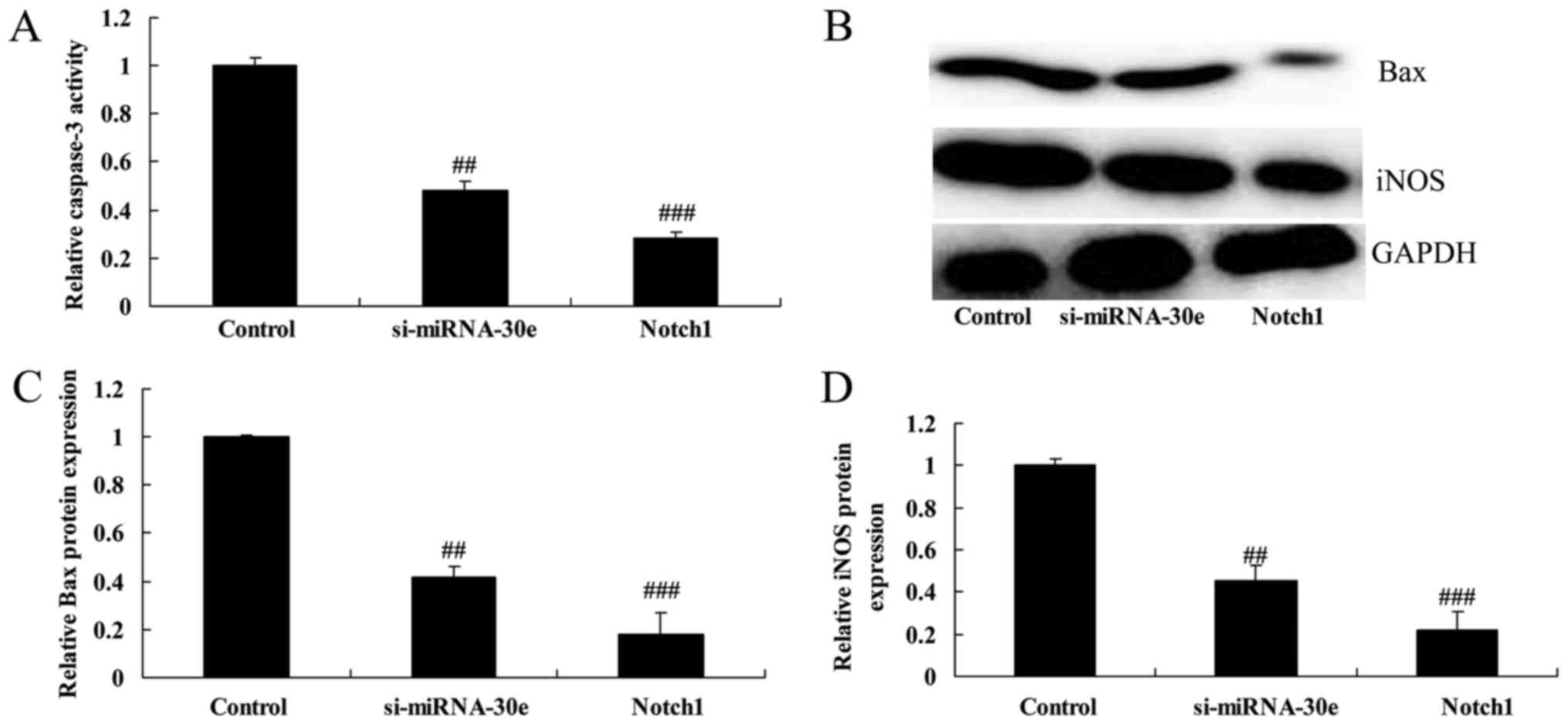
Notch1 promotion enhances the effect of
si-miRNA-30e on iNOS and Bax expression and caspase-3 activity
The promotion of Notch1 expression significantly
increased the effect of si-miRNA-30e on the inhibition of caspase-3
activity, as caspase-3 activity was significantly lower in H9C2
cells treated with Notch1 recombinant protein compared with
si-miRNA-30e treated cells (P<0.01; Fig. 13A). Furthermore, western blot
analysis revealed that treatment with Notch1 recombinant protein
led to a significant reduction in the expression of Bax and iNOS
compared with the si-miRNA-30e group (P<0.01; Fig. 13B–D).
Notch1 promotion increases the effect of
si-miRNA-30e on the activity of proteins associated with oxidative
stress
The effect of Notch1 on the function of si-miRNA-30e
on oxidative stress was investigated. MDA activity was
significantly lower in cells treated with Notch1 recombinant
protein compared with si-miRNA-30e-treated cells (P<0.01;
Fig. 14A). However, the activity
of GSH, GSH-PX and SOD were all significantly increased in cells
treated with Notch1 recombinant protein compared with
si-miRNA-30e-treated cells (P<0.01; Fig. 14B–D).
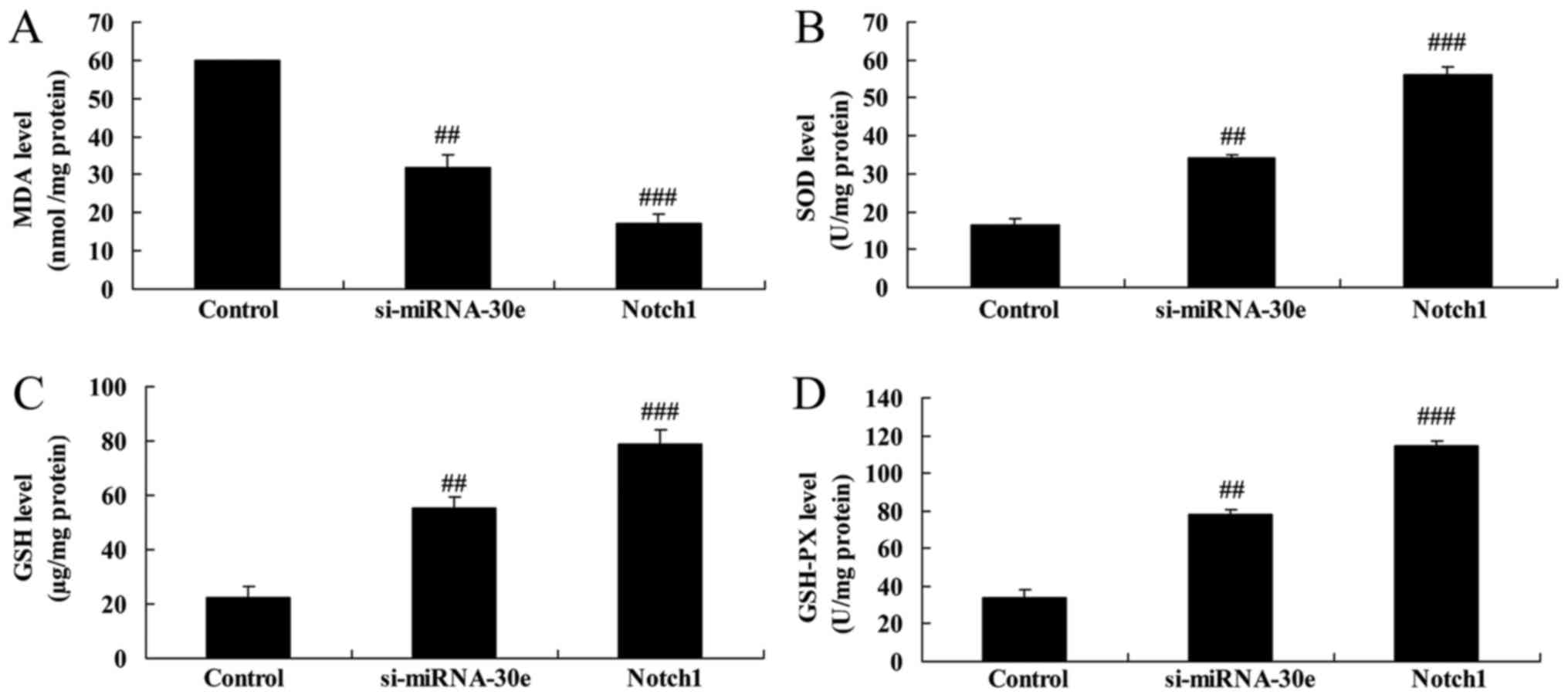 | Figure 14Notch1 promotion increases the effect
of si-miRNA-30e on oxidative stress. ELISA was used to measure the
activity of (A) MDA (B), SOD (C), GSH and (D) GSH-PX.
##P<0.01 vs. the control group,
###P<0.01 vs. the si-miRNA-30e group. Notch,
neurogenic locus notch homolog protein; GSH, glutathione; PX,
peroxides; SOD, superoxide dismutase; MDA, malondialdehyde; si,
small interfering; miRNA, microRNA. |
Discussion
MI/R is a condition that severely affects human
health. Due to the development of novel anticoagulants and
antiplatelet agents, as well as the continuous development of
intervention catheter technology and coronary artery bypass
grafting, the survival rate of patients with MI/R has improved
markedly since 2012 (22).
However, myocardial cells lack the capacity to regenerate, which
results in an irreversible loss of myocardial cells following
necrosis. This reduces cardiac function and may progress to heart
failure, which has a high long-term mortality rate (23). Promoting repair of the MI/R region
is key to preventing post-MI/R heart failure and lowering long-term
mortality rates (23). The
present study revealed that miRNA-30e expression was decreased in
patients with MI/R. Furthermore, it was demonstrated that miRNA-30e
suppression significantly inhibited apoptosis and significantly
reduced Bax protein expression and caspase-3 activity in H9C2
cells.
Studies have indicated that autophagy is involved in
the process of myocardial remodeling. It has been demonstrated that
treatment with the autophagy inducer sirolimus delays the process
of ventricular remodeling (10).
In MI/R, autophagy may increase intracellular energy supplies and
produce sufficient energy for cell survival; it may therefore be
considered a protective process (8). Zeng et al (24) reported that autophagy was enhanced
in animal models of MI/R. Autophagy was activated in the subacute
and chronic phases of MI/R and the expression of the autophagosome
markers LC3-II and p62, as well as the expression of cathepsin,
were upregulated (24). During
reperfusion, a large number of myocardial cells die, which may be
caused by Beclin-1-induced autophagy (25). In the present study, suppression
of miRNA-30e increased LC3, p62 and Beclin-1 expression in H9C2
cells. Lai et al (19)
demonstrated that miRNA-30e exhibited cardioprotection in rats with
doxorubicin-induced heart failure via autophagy.
During the embryonic cardiac developmental phase,
Notch1 is highly expressed in the immature myocardium but its
expression decreases markedly following birth (13). However, the expression of Notch
signaling pathway-related molecules is increased in the adult
myocardium following MI/R, indicating that the Notch signaling
pathway is closely associated with heart injury (12). It has been suggested that the
activated Notch signal pathway restricts the myocardial ischemic
size and improves myocardial function following MI/R (13). Therefore, the increased expression
of Notch signaling pathway-associated molecules in the myocardial
cells of adults following MI/R in the adult myocardium may be a
protective mechanism (26). The
results of the present study revealed that suppression of
miRNA-30e-induced Notch1 expression and promotion of Notch1 in H9C2
cells significantly increased the effect of si-miRNA-30e on cell
apoptosis, Bax and iNOS expression and caspase-3 activity in H9C2
cells.
Notch1 intracellular cytoplasmic domain (NICD) is
translocated into the cell nucleus and regulates Hes1 gene
expression by associating with ubiquitous transcription factors and
centromere-binding protein 1 (17). It has been demonstrated that the
Notch signaling pathway serves an important role in the onset and
development of the cardiovascular system, as well as during
pathophysiological processes (17). The roles of the Notch1 receptor
and its downstream signal molecule Hes1 in ischemic heart disease
have been investigated (27).
Activation of the Notch1 molecule during MI/R may activate the
downstream Hes1 molecule and thus inhibit the expression of
phosphatase and tensin homolog, thereby alleviating MI/R injury
(17). Activation of the
myocardial Notch1 signaling pathway contributes to the alleviation
of MI/R injury-induced oxidative stress and nitrative stress injury
by activating the downstream Hes1 signal, thus protecting the
ischemic myocardium (17). The
Akt signaling pathway serves a crucial role in processes including
cell survival, growth and migration; it may also serve as a
survival signal during myocardial ischemia and activation of this
signal exerts anti-apoptotic effects via up- and downstream
signaling (16,17). The results of the present study
demonstrated that suppression of miRNA-30e increased Hes1 and p-Akt
expression. Shan et al (28) revealed that miRNA-30e regulates
the abnormal differentiation of small intestinal epithelial cells
via the δ-like canonical notch ligand 4/NICD/Hes1 signaling
pathway.
Preventing the obstruction of blood flow in patients
with MI/R is a problem that occurs during coronary artery
reperfusion therapy and requires solving (29). The pathogenesis of blood flow
obstruction is complicated; previous studies have reported that it
occurs due to the combined action of multiple factors, including
ischemic microvascular injury in the infarct area, release of
oxygen radicals, endothelial injury, inflammatory response,
platelet activation and microthrombosis (29,30). In the present study, it was
revealed that suppression of miRNA-30e in H9C2 cells decreased
oxidative stress.
Increased iNOS expression and the resulting
excessive production of NO causes of cell death following MI/R
injury (31). NOS catalyzes
arginine to produce citrulline and release NO (31). Minimal NO is produced under normal
physiological conditions and under these conditions, it inhibits
neutrophil adhesion and platelet aggregation, dilates blood
vessels, regulates vascular tone and blood flow volume and has
protective effects on heart function (32). However, activation of iNOS during
MI/R causes excessive NO production, which may cause the injury or
death of nerve cells (32). In
the present study, suppression of miRNA-30e decreased iNOS
expression and the inhibition of autophagy following treatment with
3-MA reversed the effects of si-miRNA-30e on apoptosis, Bax and
iNOS expression, caspase-3 activity and oxidative stress.
In conclusion, the results of the present study
indicate that miRNA-30e serves a significant role in suppressing
MI/R-induced apoptosis and oxidative stress via autophagy and the
Notch1/Hes1/Akt signaling pathway. Further studies are required to
determine the clinical significance of these effects and to develop
novel therapies for patients with MI/R, as treating this disease
remains clinically challenging.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Science and
Technology Project of Shaanxi Province (grant no. 2007K13-03).
Notes
[1] Availability
of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
[2] Authors'
contributions
All authors have seen the manuscript and approved
submission to your journal. JZ designed the experiment; JL, BK, QY
and TS performed the experiment; JZ and JL analyzed the data; JZ
wrote the manuscript.
[3] Ethics
approval and consent to participate
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Xi'an Jiaotong
University (Xi'an, China). All patients provided written informed
consent prior to the collection of blood samples.
[4] Consent for
publication
Not applicable.
[5] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Gu YL, Kampinga MA, Wieringa WG, Fokkema
ML, Nijsten MW, Hillege HL, van den Heuvel AF, Tan ES, Pundziute G,
van der Werf R, et al: Intracoronary versus intravenous
administration of abciximab in patients with ST-segment elevation
myocardial infarction undergoing primary percutaneous coronary
intervention with thrombus aspiration: The comparison of
intracoronary versus intravenous abciximab administration during
emergency reperfusion of ST-segment elevation myocardial infarction
(CICERO) trial. Circulation. 122:2709–2717. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garcia-Dorado D, García-del-Blanco B,
Otaegui I, Rodríguez-Palomares J, Pineda V, gimeno F, Ruiz-Salmerón
R, Elizaga J, Evangelista A, Fernandez-Avilés F, et al:
Intracoronary injection of adenosine before reperfusion in patients
with ST-segment elevation myocardial infarction: A randomized
controlled clinical trial. Int J Cardiol. 177:935–941. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hausenloy D, Kunst G, Boston-Griffiths E,
Kolvekar S, Chaubey S, John L, Desai J and Yellon D: The effect of
cyclosporin-A on perioperative myocardial injury in adult patients
undergoing coronary artery bypass graft surgery: A randomised
controlled clinical trial. Heart. 100:544–549. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liang Y, Li YP, He F, Liu XQ and Zhang JY:
Long-term, regular remote ischemic preconditioning improves
endothelial function in patients with coronary heart disease. Braz
J Med Biol Res. 48:568–576. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma N, Bai J, Zhang W, Luo H, Luo H, Zhang
X, Liu D and Qiao C: Trimetazidine protects against cardiac
ischemia/reperfusion injury via effects on cardiac miRNA-21
expression, Akt and the Bcl-2/Bax pathway. Mol Med Rep.
14:4216–4222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Ouyang M, Wang Q and Jian Z:
MicroRNA-142-3p inhibits hypoxia/reoxygenation-induced apoptosis
and fibrosis of cardiomyocytes by targeting high mobility group box
1. Int J Mol Med. 38:1377–1386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hendgen-Cotta UB, Messiha D, Esfeld S,
Deenen R, Rassaf T and Totzeck M: Inorganic nitrite modulates miRNA
signatures in acute myocardial in vivo ischemia/reperfusion. Free
Radic Res. 51:91–102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang ZG, Wang Y, Huang Y, Lu Q, Zheng L,
Hu D, Feng WK, Liu YL, Ji KT, Zhang HY, et al: bFGF regulates
autophagy and ubiquitinated protein accumulation induced by
myocardial ischemia/reperfusion via the activation of the
PI3K/Akt/mTOR pathway. Sci Rep. 5:92872015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duan Q, Yang W, Jiang D, Tao K, Dong A and
Cheng H: Spermine ameliorates ischemia/reperfusion injury in
cardiomyocytes via regulation of autophagy. Am J Transl Res.
8:3976–3985. 2016.PubMed/NCBI
|
|
10
|
Song H, Yan C, Tian X, Zhu N, Li Y, Liu D,
Liu Y, Liu M, Peng C, Zhang Q, et al: CREG protects from myocardial
ischemia/reperfusion injury by regulating myocardial autophagy and
apoptosis. Biochim Biophys Acta. 1863:1893–1903. 2017. View Article : Google Scholar
|
|
11
|
Pei H, Song X, Peng C, Tan Y, Li Y, Li X,
Ma S, Wang Q, Huang R, Yang D, et al: TNF-α inhibitor protects
against myocardial ischemia/reperfusion injury via Notch1-mediated
suppression of oxidative/nitrative stress. Free Radic Biol Med.
82:114–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pei H, Yu Q, Xue Q, Guo Y, Sun L, Hong Z,
Han H, Gao E, Qu Y and Tao L: Notch1 cardioprotection in myocardial
ischemia/reperfusion involves reduction of oxidative/nitrative
stress. Basic Res Cardiol. 108:3732013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou XL, Wan L and Liu JC: Activated
Notch1 reduces myocardial ischemia reperfusion injury in vitro
during ischemic postconditioning by crosstalk with the RISK
signaling pathway. Chin Med J. 126:4545–4551. 2013.PubMed/NCBI
|
|
14
|
Boccalini G, Sassoli C, Formigli L, Bani D
and Nistri S: Relaxin protects cardiac muscle cells from
hypoxia/reoxygenation injury: Involvement of the Notch-1 pathway.
FASEB J. 29:239–249. 2015. View Article : Google Scholar
|
|
15
|
Zhou XL, Zhao Y, Fang YH, Xu QR and Liu
JC: Hes1 is upregulated by ischemic postconditioning and
contributes to cardioprotection. Cell Biochem Funct. 32:730–736.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu L, Li F, Zhao G, Yang Y, Jin Z, Zhai M,
Yu W, Zhao L, Chen W, Duan W and Yu S: Protective effect of
berberine against myocardial ischemia reperfusion injury: Role of
Notch1/Hes1-PTEN/Akt signaling. Apoptosis. 20:796–810. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu L, Liang H, Lu Z, Zhao G, Zhai M, Yang
Y, Yang J, Yi D, Chen W, Wang X, et al: Membrane receptor-dependent
Notch1/Hes1 activation by melatonin protects against myocardial
ischemia-reperfusion injury: In vivo and in vitro studies. J Pineal
Res. 59:420–433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lavie L: Oxidative stress in obstructive
sleep apnea and intermittent hypoxia-revisited-the bad ugly and
good: Implications to the heart and brain. Sleep Med Rev. 20:27–45.
2015. View Article : Google Scholar
|
|
19
|
Lai L, Chen J, Wang N, Zhu G, Duan X and
Ling F: MiRNA-30e mediated cardioprotection of ACE2 in rats with
Doxorubicin-induced heart failure through inhibiting cardiomyocytes
autophagy. Life Sci. 169:69–75. 2017. View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods.
25:402–408. 2001. View Article : Google Scholar
|
|
21
|
Xue R, Lei S, Xia ZY, Wu Y, Meng Q, Zhan
L, Su W, Liu H, Xu J, Liu Z, et al: Selective inhibition of PTEN
preserves ischaemic post-conditioning cardioprotection in
STZ-induced Type 1 diabetic rats: Role of the PI3K/Akt and
JAK2/STAT3 pathways. Clin Sci. 130:377–392. 2016. View Article : Google Scholar
|
|
22
|
Liu Z, Zhao L, Hong D and Gao J: Remote
ischaemic preconditioning reduces myocardial ischaemic reperfusion
injury in patients with ST-elevation myocardial infarction
undergoing primary percutaneous coronary intervention. Acta
Cardiol. 71:596–603. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pöss J, Desch S, Eitel C, de Waha S,
Thiele H and Eitel I: Left ventricular thrombus formation after
ST-segment-elevation myocardial infarction: Insights from a cardiac
magnetic resonance multicenter study. Circ Cardiovasc Imaging.
8:e0034172015.PubMed/NCBI
|
|
24
|
Zeng C, Li H, Fan Z, Zhong L, Guo Z, Guo Y
and Xi Y: Crocin-elicited autophagy rescues myocardial
ischemia/reperfusion injury via paradoxical mechanisms. Am J Chin
Med. 44:515–530. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thapalia BA, Zhou Z and Lin X: Autophagy,
a process within reperfusion injury: An update. Int J Clin Exp
Pathol. 7:8322–8341. 2014.
|
|
26
|
Zhou XL, Wan L, Xu QR, Zhao Y and Liu JC:
Notch signaling activation contributes to cardioprotection provided
by ischemic preconditioning and postconditioning. J Transl Med.
11:2512013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang S, Wang P, Ren L, Hu C and Bi J:
Protective effect of melatonin on soluble Aβ1-42-induced
memory impairment, astrogliosis, and synaptic dysfunction via the
Musashi1/Notch1/Hes1 signaling pathway in the rat hippocampus.
Alzheimers Res Ther. 8:402016. View Article : Google Scholar
|
|
28
|
Shan TD, Ouyang H, Yu T, Li JY, Huang CZ,
Yang HS, Zhong W, Xia ZS and Chen QK: miRNA-30e regulates abnormal
differentiation of small intestinal epithelial cells in diabetic
mice by downregulating Dll4 expression. Cell Prolif. 49:102–114.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou Q, Cao J and Chen L: Apelin/APJ
system: A novel therapeutic target for oxidative stress-related
inflammatory diseases (Review). Int J Mol Med. 37:1159–1169. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bagheri F, Khori V, Alizadeh AM,
Khalighfard S, Khodayari S and Khodayari H: Reactive oxygen
species-mediated cardiac-reperfusion injury: Mechanisms and
therapies. Life Sci. 165:43–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang G, Li X, Wang H, Wang Y, Zhang L,
Zhang L, Liu B and Zhang M: Later phase cardioprotection of
ischemic post-conditioning against ischemia/reperfusion injury
depends on iNOS and PI3K-Akt pathway. Am J Transl Res. 7:2603–2611.
2015.
|
|
32
|
Sun N, Wang H and Wang L: Protective
effects of ghrelin against oxidative stress, inducible nitric oxide
synthase and inflammation in a mouse model of myocardial
ischemia/reperfusion injury via the HMGB1 and TLR4/NF-κB pathway.
Mol Med Rep. 14:2764–2770. 2016. View Article : Google Scholar : PubMed/NCBI
|