Introduction
Acute lung injury (ALI) is pulmonary inflammation
with severe disorders, resulting in diffuse alveolar damage,
leading to hypoxemia, pulmonary edema and respiratory failure
(1,2). The incidence of acute lung injury in
children is high worldwide (3).
ALI is associated with pediatric intensive care unit admissions,
causing increased pediatric intensive care unit deaths (4). Previous studies in children and
adults show strong relationship between the positive fluid balance
and the worse outcomes, and death is involved, in patients with
respiratory failure and/or ALI (5,6).
Additionally, the percentage of fluid overload is linked with worse
oxygenation, enhanced duration of mechanical ventilation, and
upregulation of hospital length stay according to a previous study
(7). Only a small number of
children with acute lung injury was successfully treated to inhibit
fluid overload (8,9). The mortality rate in patients with
ALI is high due to the slow progress in understading the molecular
mechanisms regarding the disease pathogenesis, as well as the poor
therapeutic strategy. Further, lipopolysaccharide (LPS) results in
symptoms in animal models, closely resembling ALI in human,
highlighting strategies to investigate the pathogenesis of ALI
(10,11). Thus, finding effective methods and
exploring the molecular mechanism are necessary.
Juglanin is a natural compound belonging to
flavonoids, it is extracted from crude 'Polygonum
aviculare', exhibiting inhibitory activity against the
inflammation response as well as cancer growth (12–14). Flavonoids are common plant
secondary metabolites, well known for having several biological
activities in vitro and in vivo among which we can
highlight their remarkable antioxidant activity (15,16). In addition, flavonoids also have
interesting anti-inflammatory activities against several markers
(17–19). For instance, quercetin and
astragalin are two important flavonoids (20,21). Quercetin has been reported to have
anti-inflammatory activity in various diseases (22–24). Thus, we supposed that juglanin may
be also effective for suppressing inflammatory response in acute
lung injury, and potentially provide a new therapeutic strategy for
acute lung injury treatment. In addition, previous data also
suggested that juglanin inhibits apoptosis and inflammatory
response through TLR4-modulated MAPK/nuclear factor-κB (NF-κB) and
JAK2/STAT3 signaling pathways respectively in rats with hepatitis
(25). Also, juglanin could
decrease the level of reactive oxygen species in senescent cells
induced by adriamycin treatment (26). Furthermore, juglanin displayed
inhibitory activities against LPS-induced cytokine production in
cells of macrophage RAW 264.7, such as interleukin-1β (IL-1β),
tumor necrosis factor-α (TNF-α), and IL-6 (27). In consideration of the valid
function of juglanin in various disease treatments with little
toxicity, we supposed that it may be utilized for patients of
children with lung injury, which could be useful for the
development of dietary supplements that alleviate lung injury for
children.
In the present study, experimental mice were given
LPS to induce acute lung injury with severe fibrosis and
inflammation response. Then, juglanin at different concentrations
were administered to mice. Following, the fibrosis and inflammation
response in vivo were calculated through α-smooth
muscle-actin (α-SMA), collagen type I and III as well as
NF-κB-related signaling pathway to illustrate the role of juglanin
in acute lung injury, supplying a new and effective treatment for
children with acute lung injury in future.
Materials and methods
Animal treatment
Sixty male, 6-week-old C57BL6 mice, weight range
20–22 g, were purchased from experimental animals center of Nanjing
Medical University of laboratory animal center (Nanjing, China).
All the mice were carefully maintained at room temperature on a
12:12 h/light:dark cycle, 35% humidity, with free access to food
and water in the cages. All animal experiments were performed to
minimize animal suffering according to the Guide for the Care and
Use of Laboratory Animals which was issued by the National
Institutes of Health in 1996. This study was approved by the Ethics
Committee on Animal Research at the Department of Pediatrics,
Huai'an First People's Hospital, Nanjing Medical University,
Nanjing, China. The mice were divided into 6 groups: the control
group without any treatment (Con, n=10); LPS-treated group (15
mg/kg, LPS, n=10); 10 mg/kg juglanin-treated group after LPS
teratment (JL, n=10); 20 mg/kg juglanin-treated group after LPS
(JH, n=10); 10 mg/kg juglanin-treated group without LPS treatment
(Con-JL, n=10); 20 mg/kg juglanin-treated group without LPS
(Con-JH, n=10). After 10 days adaptation, mice from LPS group were
treated by intraperitoneal injection with 15 mg/kg body weight LPS.
The control group was administered with the same volume of saline.
Then, the mice were consecutively administered with different
concentrations of juglanin via oral gavage for 3 weeks. Juglanin,
purchased from Yuanye Biotech (Shanghai, China), was dissolved in
distilled water. Then, all experimental mice were sacrificed.
Eyeball blood was collected and centrifuged at 12,000 × g for 15
min at 4°C for following study, and the whole lung and liver
tissues were carefully harvested on 4°C glacial table, and then
immediately frozen in liquid N2, and stored at −80°C for
the following studies.
Cells culture and treatment
The human lung cell line, BEAS-2B, was purchased
from American Type Culture Collection (ATCC, Rockville, MD, USA),
and human liver cell line L02, was obtained from KeyGen Biotech
(Nanjing, China). The cells were maintained in a monolayer culture
of 95% air and 5% CO2 at 37°C in Dulbecco's modified
Eagle's medium (DMEM) with 10% fetal bovine serum (FBS), 0.012%
penicillin G, 0.027% streptomycin, 0.022% sodium pyruvate and 0.26%
sodium bicarbonate. Juglanin stock solution was prepared in
dimethyl sulfoxide (DMSO) and diluted to the desired final
concentration (0, 1.25, 5, 10, 20, 40 and 80 µM) in culture
medium for 24 h before use. The final concentration of DMSO did not
exceed 0.1% (v/v).
Cell viability analysis
The lung and liver cells were planted in 96-well
plates with a density of 5×103 cells/well. Then, they
were treated with different concentrations (0 to 80 µM) of
juglanin for different times ranging from 0 to 72 h for cell
viability analysis through Cell Counting kit-8 (CCK-8) (Zoman
Biotechnology Co., Ltd., Beijing, China) to examine cell viability
according to the instructions of the manufacturer.
Inflammatory cell counts of BALF
The samples from BALF were centrifuged at 3,000 rpm
for 10 min at 4°C to pellet the cells. Then, the cell pellets were
re-suspended in phosphate-buffered saline (PBS) for the total cell
number with a hemacytometer, and the cytospins were prepared for
other differential cell number through Wright-Giemsa staining.
Chemical index measurement
The serum tumor necrosis factor-α (TNF-α) (cat. no.
MTA00B), interleukin-1β (IL-1β) (cat. no. MLB00C), IL-4 (cat. no.
M400B), IL-18 (cat. no. 7625), IL-6 (cat. no. M600B), and IL-17
(cat. no. M1700) (R&D Systems, Minneapolis, MN, USA) were
measured according to the manufacturer's protocol using an
enzyme-linked immunosorbent assay (ELISA) kit. Total serum and BAL
IgE and IgA levels were determined by a mouse IgE (cat. no. DY1197;
R&D Systems) and IgA (cat. no. F00295; Lengton Biological
Technology, Shanghai, China) ELISA set. For the determination of
cytokine levels, blood samples from the rats were obtained after
sacrifice and stored at -80°C until use. Lung tissue samples were
isolated from the mice immediately after sacrifice, and was
snap-frozen in liquid nitrogen and stored at -80°C for later
analysis. Lung tissue samples were collected were homogenized, and
the proteins were extracted using hypotonic buffer (25 mM Tris-HCl,
pH 8.0, 1 mM EDTA, 5 µg/ml leupeptin, 1 mM Pefabloc SC, 50
µg/ml aprotinin, 5 µg/ml soybean trypsin inhibitor, 4
mM benzamidine) to yield a homogenate. Then the final supernatants
were obtained by centrifugation at 12,000 rpm for 20 min. Protein
concentration was determined by BCA protein assay kit (Thermo
Fisher Scientific, Waltham, MA, USA) with bovine serum albumin as a
standard. Next, the pro-inflammatory cytokine levels, including
TNF-α, IL-1β, IL-18, IL-6, IL-4 and IL-17 were measured using the
commercial ELISA kits, respectively, following manufacturer's
instruction.
Immunohistochemical analysis
Formalin-fixed, paraffin-embedded mouse lung tissue
samples were processed and then stained based on routine protocol
of hematoxylin and eosin (H&E) (28). The stained slides, sectioned at 4
µM, were then observed with the light microscope and the
digital micrographs of slides, which were taken for analyzing. The
inflammation score was determined by a pathologist as mild,
moderate, or severe and degree of inflammation annotated as
inflammatory score from 0 to 4 (minimum, 0; maximum, 4; 0, no
injury; 1, injury up to 25% of the field; 2, injury up to 50% of
the field; 3, injury up to 75% of the field; and 4, diffuse injury)
was calculated as an index of the degree of lung injury for each
sample described.
For periodic acid-Schiff (PAS) staining methods, the
lung tissue samples were fixed in 95% alcohol for 10 min, prior to
being washed, dried, stained by 1% periodic acid for 15 min and
then washed by chilled PBS and dried twice. The tissue samples were
then stained using Schiff reagent for 1 h, washed and dried, and
stained subsequently with hematoxylin for 5 min, washed and dried.
Finally, the tissue samples were observed with light microscopy.
Wright-Giemsa staining was also carried out, following standard
instructions. For the analysis of collagen deposition, Sirius red
staining was performed by Shanghai Zhenda Biotechnology, Co., Ltd.
(Sanghai, China).
For immunohistochemistry analysis, the fresh lung
tissue samples were fixed in formalin for 48 h. Then the tissue
block was put into paraffin and next cut into slides for the
desired thickness in a microtome, and was then fixed into a slide.
After washing, the samples were prepared for blocking and
incubating with antibody α-SMA (1:200) and transforming growth
factor-β1 (TGF-β1) (1:200), which were diluted in 5% horse serum
with chilled PBS at 4°C overnight. Isotype-matched IgG was used
instead of primary antibody as a negative control of the staining.
Sections were then incubated with diluted streptavidin-peroxidase
HRP at room temperature with a staining kit, following the
manufacturer's instructions. The sections were then stained with
hematoxylin for 5 min and mounted and observed with a
phase-contrast microscope.
Fluorescence imaging
Tissue samples were washed twice with PBS and fixed
with 3.7% (v/v) formaldehyde in PBS for 15 min. Cells were
permeabilised for 5 min with 0.1% Triton X-100. For p-NF-κB
staining, 50 µg/ml mouse anti-caspase-3 (1:200) antibodies
were employed followed by staining with 2 µg/ml Alexa Fluor
488 goat anti-mouse secondary antibodies. DAPI (Sigma-Aldrich) was
used in this part of the studied. Images were acquired by confocal
laser scanning via epifluorescence microscopy (Sunny Biotech Co.,
Ltd., Shanghai, China). After observation and obtaining the
representative images, ImageJ software, focused on biological-image
analysis, was used to calculate the fluorescent intensity,
representing the number of positive cells (29).
Western blot assays
The lung tissue samples and lung cells were
homogenized into 10% (wt/vol) hypotonic buffer (25 mM Tris-HCl, pH
8.0, 1 mM EDTA, 5 µg/ml leupeptin, 1 mM pefa-bloc SC, 50
µg/ml aprotinin, 5 µg/ml soybean trypsin inhibitor, 4
mM benzamidine) to yield a homogenate. The final supernatants were
obtained by centrifugation at 12,000 rpm for 20 min. Protein
concentration was determined by BCA protein assay kit (Thermo
Fisher Scientific) with bovine serum albumin as a standard. The
total protein extract was also used for western blot assays. Equal
amounts (30 µM) of total protein of tissues were subjected
to 10 or 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) followed by immunoblotting using the
following primary polyclonal antibodies: rabbit anti-glyceraldehyde
3-phosphate dehydrogenase (GAPDH), IL-1β, P-NF-κB, NF-κB, p-IKKα,
IκBα, α-SMA, TGF-β1, collagen type I and collagen type III.
Immunoreactive bands were visualized by ECL immunoblot detection
system (Pierce Biotechnology, Inc., Rockford, IL, USA) and exposed
to Kodak (Eastman Kodak Co., Rochester, NY, USA) X-ray film. Each
protein expression level was defined as grey value (version 4.2b,
Mac OS X, ImageJ; National Institutes of Health, Bethesda, MD, USA)
and standardized to housekeeping gene (GAPDH) and expressed as a
fold of control. The specific information of primary antibodies
used here is listed in Table
I.
 | Table IPrimary antibodies for western blot
analysis. |
Table I
Primary antibodies for western blot
analysis.
| Primary
antibodies | Dilution ratio | Corporation |
|---|
| Rabbit
anti-NF-κB | 1:1,000 | Abcam,
ab207297 |
| Rabbit
anti-p-NF-κB | 1:1,000 | Abcam, ab86299 |
| Rabbit
anti-IκBα | 1:2,000 | Abcam, ab7212 |
| Rabbit
anti-IL-1β | 1:1,000 | Abcam, ab9722 |
| Rabbit
anti-p-IKKα | 1:500 | Abcam, ab38515 |
| Rabbit
anti-α-SMA | 1:1,000 | Abcam, ab5694 |
| Rabbit
anti-collagen type I | 1:1,000 | Abcam, ab34710 |
| Rabbit
anti-TGF-β1 | 1:1,000 | Cell Signaling
Technology, ab3711 |
| Collagen type
III | 1:1,000 | Cell Signaling
Technology, ab7778 |
| GAPDH | 1:1,000 | Cell Signaling
Technology, ab5174 |
RT-qPCR analysis
Total RNA from tissue samples and cultured cells was
isolated through the mirVana miRNA isolation kit (Ambion, Foster
City, CA, USA) based on the manufacturer's instruction. Then the
cDNA was synthesized from total RNA with the TaqMan miRNA reverse
transcription kit (Applied Biosystems, Foster City, CA, USA).
Real-time PCR was conducted using the Applied Biosystems 7500
sequence detection system with iQ™ SYBR-Green SuperMix (Bio-Rad
Laboratories, Hercules, CA, USA) containing 5 ng cDNA and 10 pM of
each primer. The data were normalized to the geometric mean of
housekeeping gene GAPDH and calculated using the 2−ΔΔCT
method. Fold changes in mRNA levels of target gene relative to the
endogenous GAPDH control were calculated. In brief, the cycle
threshold (Ct) values of each target gene was subtracted from Ct
values of housekeeping gene cyclophilin (ΔCt). The target gene ΔΔCt
was calculated as ΔCt of target gene minus ΔCt of control. The fold
change in mRNA expression was evaluated as 2−ΔΔCt. All
sequences involved in this study are shown as follows: sense
primers of TGF-β1, 5′-GAA GGT GAG AGG ATG G-3′ and antisense
primers of TGF-β1, 5′-AGA CCT AGA CTA GGC CAA GT-3′; sense primers
of collagen type I, 5′-AAA GGT AAC AAG ACG TGG-3′ and antisense
primers of collagen type I, 5′-TCG TAT ACT GTC AGG GAG AGA T-3′;
sense primers of collagen type III 5′-GAA GGC AGA GAG AAG GTA G-3′
and antisense primers of collagen type III, 5′-AGA CGG AAT GGA GAA
GAC ACA T-3′; sense primers of α-SMA, 5′-AGC TAG TGA ACA ACA GTG
CG-3′ and antisense primers of α-SMA, 5′-TAA GCT CAG AGG CTA TCG
TAT-3′; sense primers of GAPDH, 5′-AAT CAT AAC GCG AGG CCG GA-3′
and antisense primers of GAPDH, 5′-CCA TAT ACA GTC ACC CGA CAC
AC-3′.
Data analysis
Data were expressed as mean ± standard error of the
mean (SEM). Statistical analyses were performed using GraphPad
Prism (version 6.0; GraphPad Software, Inc., La Jolla, CA, USA) by
ANOVA with Dunnet's least significant difference post-hoc tests. A
p-value <0.05 was considered significant.
Results
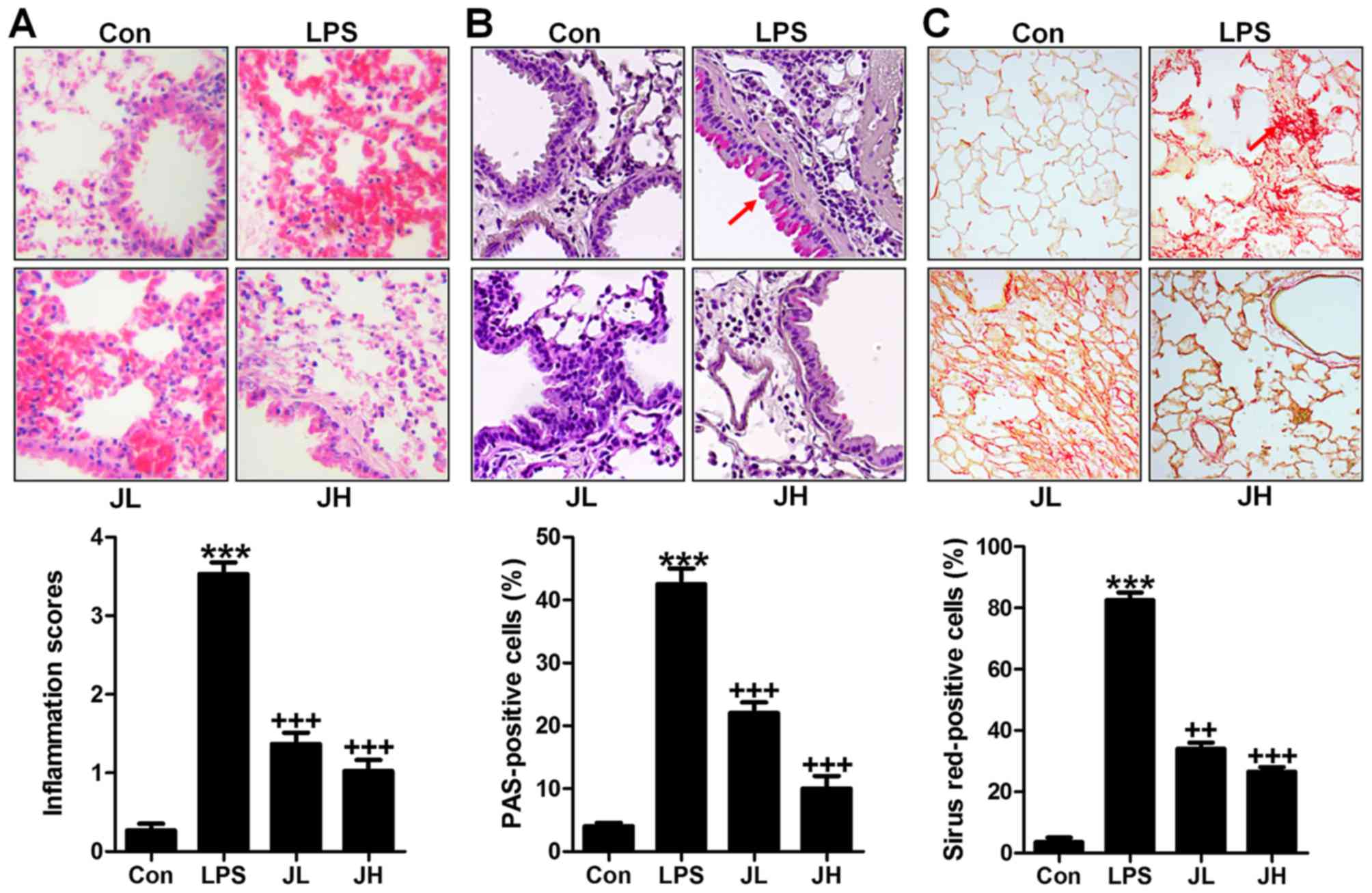
LPS-induced acute lung injury in mice is
ameliorated after juglanin administration
LPS induction is well known to cause injury in
different organs, including liver, renal and heart, even the lung,
and has been investigated before (30). In this study, preliminary
experiment was performed to verify that the inflammatory-mouse
model was set up correctly through evaluating the number of
macrophages, neutrophils and lymphocytes, as well as the total
cells in BALF, and H&E staining. The data indicated that after
15 mg/kg LPS treatment for 6 h, the number of macrophages,
neutrophils and lymphocytes, as well as the total BALF was
significantly higher than that in the control group, which is a
hallmark for ALI. In addition, H&E staining analysis also
indicated that LPS induced lung injury in mice compared to the Con
group. After the pre-experiments mentioned above, we considered
that ALI models were successfully established (data not shown). In
order to prove whether juglanin has potential value on airway
inflammation in children, LPS was also involved to cause acute lung
injury in mice. In this regard, H&E staining was carried out to
observe the injured condition of lung tissue samples. We found that
after LPS treatment in mice, the lung tissue sample showed higher
inflammation score compared to the Con group with significant
difference (Fig. 1A). After,
juglanin treatment, the inflammatory response was attenuated.
Additionally, PAS analysis also indicated that the PAS positive
cell levels were significantly increased with LPS exposure, while
decreased by juglanin administration in a dose-dependent manner
(Fig. 1B). Further, Sirius red
assays illustrated that the fibrosis levels were more severe in
LPS-treated lung tissue samples from mice. Of note, juglanin
treatment indicated that the Sirius red positive cells were highly
reduced ameliorating fibrosis progression (Fig. 1C). The data above suggested that
LPS indeed resulted in severe lung injury, and juglanin showed
protective role against acute lung injury caused by LPS in
mice.
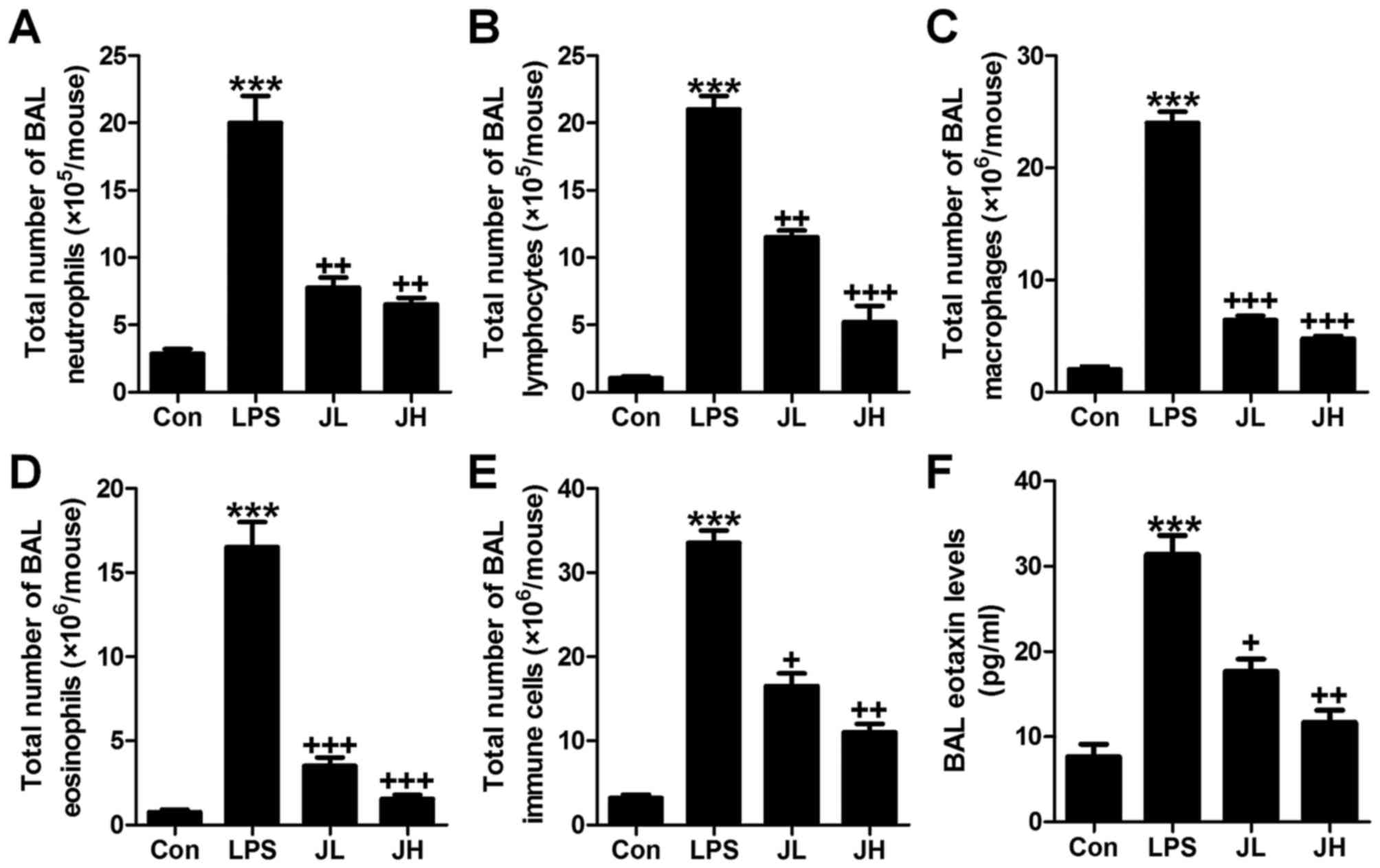
Juglanin reduces inflammatory cell
infiltrate in LPS-induced mice with acute lung injury
In this regard, the role of juglanin in inflammation
regulation was explored. First of all, the bronchoalveolar lavage
(BAL) was conducted and investigated. As shown in Fig. 2, LPS treatment contributed to
acceleration of neutrophils (Fig.
2A), lymphocytes (Fig. 2B),
macrophages (Fig. 2C), and
eosinophils (Fig. 2D), and
elevation of the total number of BAL eventually (Fig. 2E). Juglanin significantly reduced
the total number of neutrophils (Fig.
2A), lymphocytes (Fig. 2B),
macrophages (Fig. 2C), and
eosinophils (Fig. 2D), as well as
the total number of immune cells in BAL (Fig. 2E). The whole process was in a
dose-dependent manner. Furthermore, in the lung tissue samples
after LPS induction, eotaxin level in BAL was apparently
downregulated, which was attenuated by juglanin treatment (Fig. 2F). In conclusion, the data above
indicated that juglanin has a potential role in controlling
inflammation and cell infiltration in mice with acute lung injury
induced by LPS.
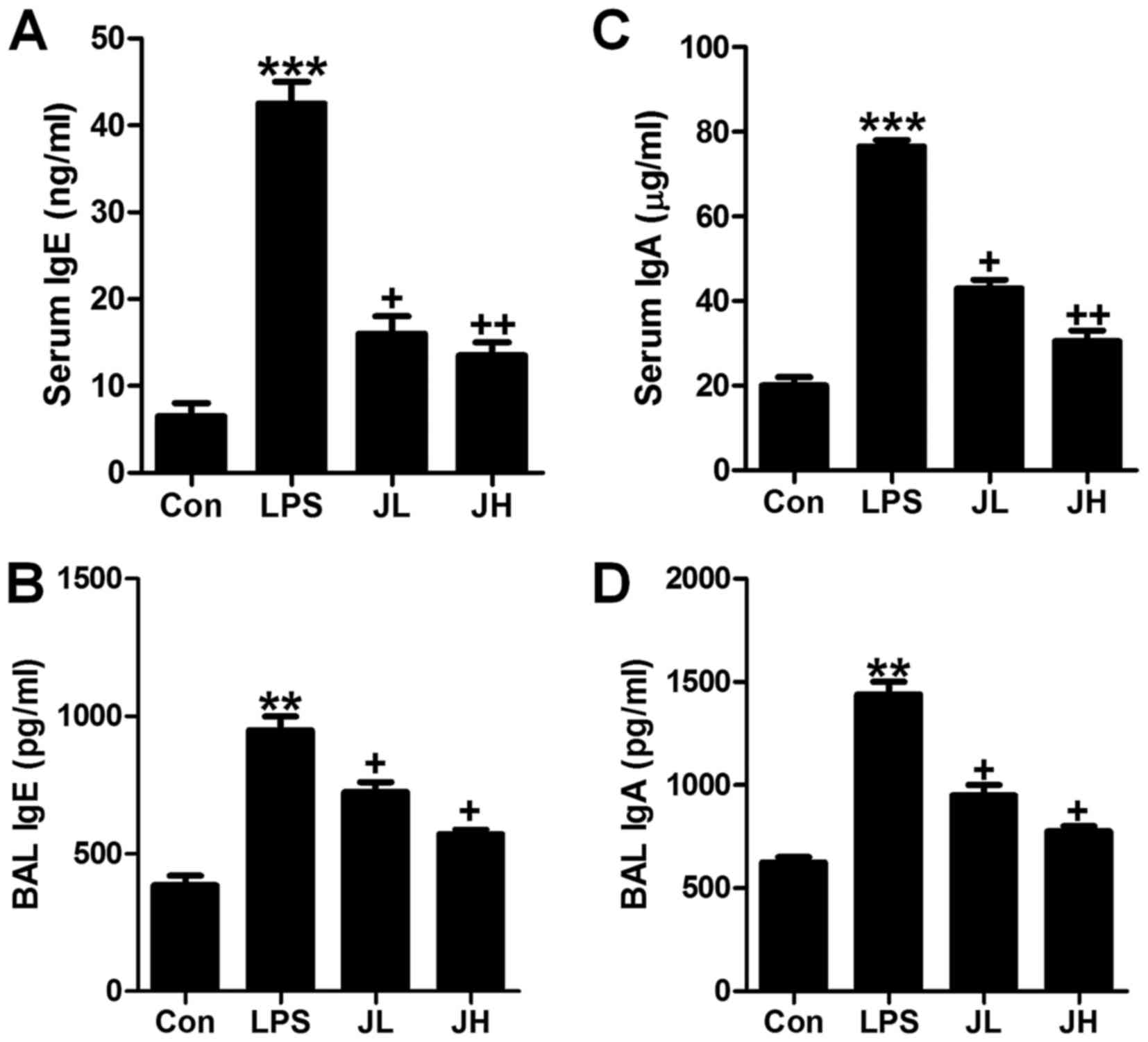
Juglanin downregulates lgE and lgA in
serum and BAL in LPS-induced mice
In order to further investigate if juglanin could
ameliorate LPS-induced acute lung injury, lgE and lgA levels in
serum and BAL were calculated. Accroding to a previous study, lgE
and lgA high expression were markers contributing to various
diseases (31). As shown in
Fig. 3A and B, the lgE levels in
serum and BAL were significantly upregulated in LPS-treatment.
Juglanin administration reversed lgE expression levels in a
dose-dependent manner. Consistent with lgE alteration, lgA levels
in serum and BAL were also found to be highly expressed for
LPS-induction, and were inhibited in serum and BAL (Fig. 3C and D). The results above
indicated that juglanin has a role in suppressing lgE and lgA
expression levels to improve acute lung injury induced by LPS in
mice.
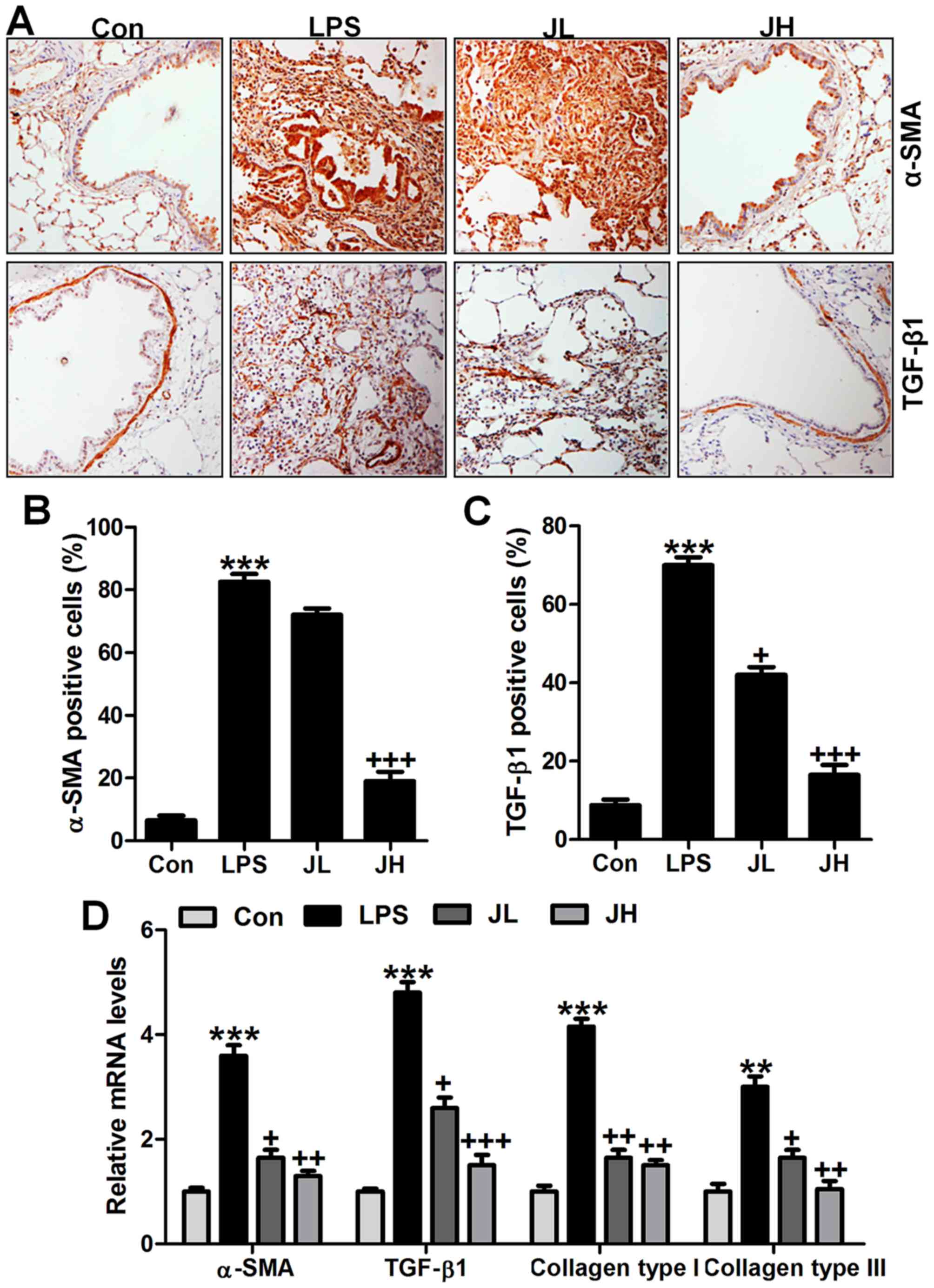
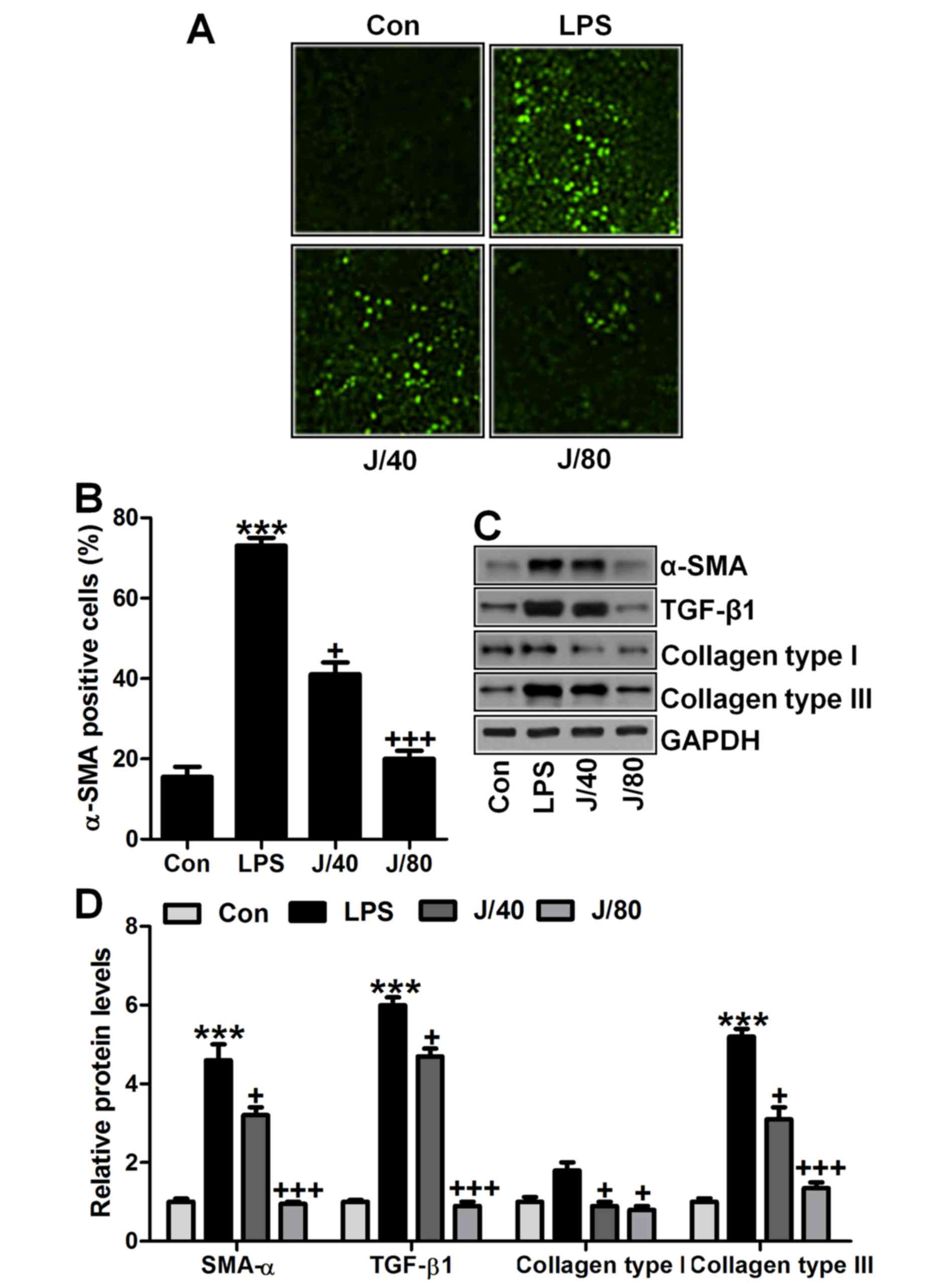
Juglanin-ameliorated fibrosis progression
in mice induced by LPS
Lung fibrosis is known as a significant injury
induced by LPS (32). In our
study, to estimate the extent of fibrosis, we calculated α-SMA,
TGF-β1, collagen type I and collagen type III through different
analysis. As shown in Fig. 4A,
α-SMA and TGF-β1 were significantly upregulated in LPS treatment in
line with previous studies (33).
Of note, juglanin administration significantly downregulated α-SMA
and TGF-β1 levels in LPS-treated mice through immunohistochemical
analysis (Fig. 4B and C).
IgG-isotype control staining showed no positive signal by
immunohistochemical analysis. Similarly, α-SMA and TGF-β1 mRNA
levels, as well as collagen type I and collagen type III gene
levels were also upregulated with significant difference compared
to the Con ones after LPS treatment. Interestingly, juglanin at two
concentrations reduced α-SMA and TGF-β1, collagen type I and III
apparently compared to the LPS alone-treated group through RT-qPCR
analysis (Fig. 4D). Taken
together, the results in this part indicated that fibrosis was
induced in mice in LPS exposure, and juglanin exhibited suppressive
role in fibrosis progression in acute lung injury in mice after LPS
induction.
Juglanin improves pro-inflammatory
cytokine release caused by LPS
Inflammation response is known as a major reason to
cause tissue injury in different organs after LPS treatment, such
as liver and renal injury (34).
Also, previous studies reported that LPS induction resulted in lung
injury, which was also attributed to inflammatory response
(35). As further confirmation,
serum and lung tissue samples of pro-inflammatory cytokines of
TNF-α, IL-1β, IL-18, IL-6, IL-4 and IL-17 were determined. As shown
in Fig. 5, we found that
pro-inflammatory cytokines of TNF-α (Fig. 5A), IL-1β (Fig. 5B), IL-18 (Fig. 5C), IL-6 (Fig. 5D), IL-4 (Fig. 5E) and IL-17 (Fig. 5F) were significantly upregulated
in LPS treatment, which was in line with previous studies (36). Of note, after juglanin
administration, these pro-inflammatory cytokines were highly
downregulated compared to the LPS ones. In addition, the
pro-inflammatory cytokines in lung tissue samples were also found
to be upregulated with higher expression of TNF-α (Fig. 5G), IL-1β (Fig. 5H), IL-18 (Fig. 5I), IL-6 (Fig. 5J), IL-4 (Fig. 5K) and IL-17 (Fig. 5L), which were also reduced in
juglanin treatment. The data above indicated that juglanin has a
role in suppressing inflammation response in LPS-induced acute lung
injury in mice.
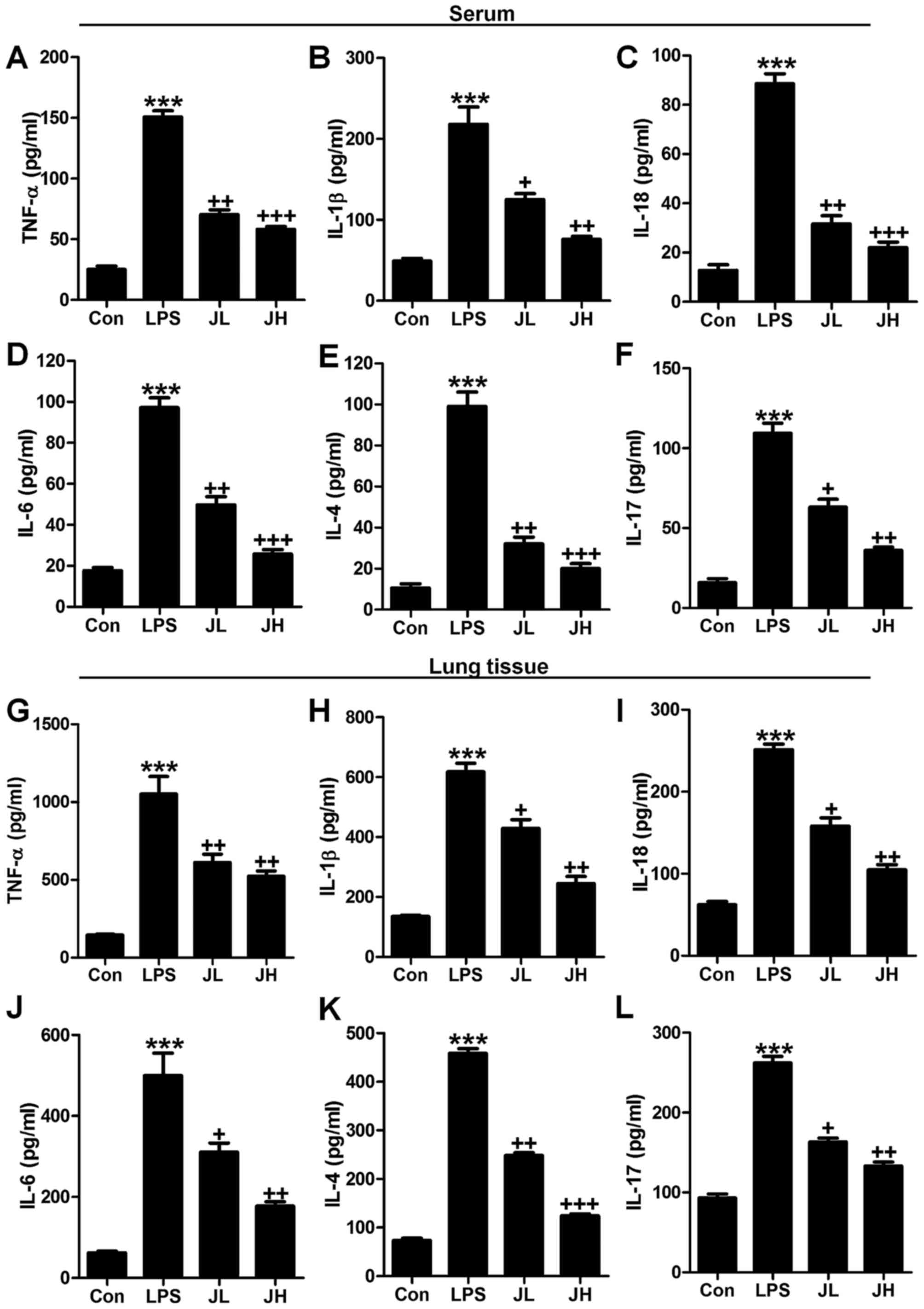 | Figure 5Juglanin improves pro-inflammatory
cytokine releases caused by lipopolysaccharide (LPS). Serum
pro-inflammatory cytokines were measured, including (A) TNF-α, (B)
interleukin-1β (IL-1β), (C) IL-18, (D) IL-6, (E) IL-4 and (F)
IL-17. Pro-inflammatory cytokines of (G) TNF-α, (H) IL-1β, (I)
IL-18, (J) IL-6, (K) IL-4 and (L) IL-17 in lung tissue samples
induced by LPS were measured after juglanin administration. The
data are shown as mean ± SEM (n=10). *p<0.05 and
**p<0.001 vs. the control (Con);
+p<0.05, ++p<0.01 and
+++p<0.001 vs. LPS-induced mice (LPS). |
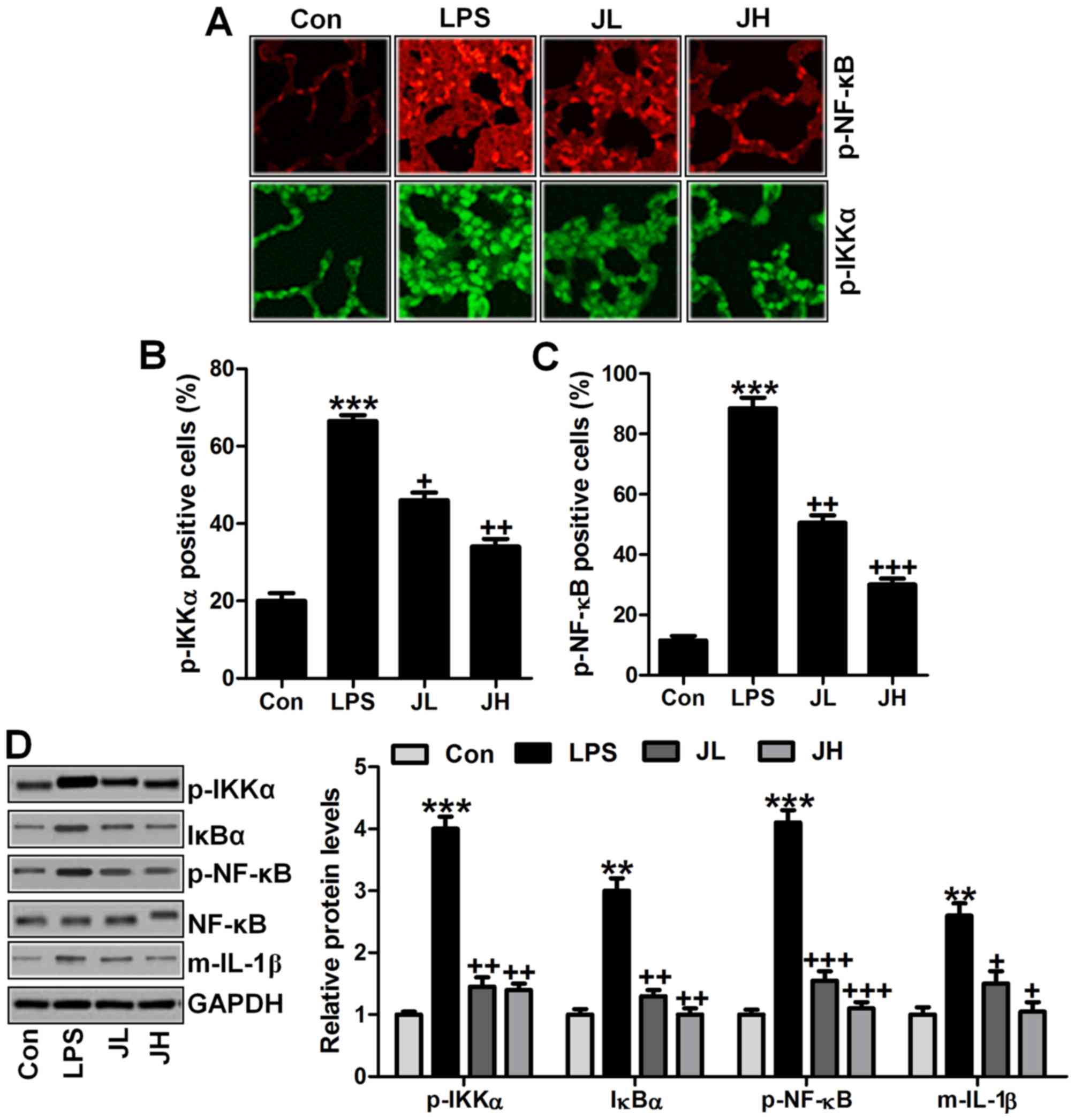
NF-κB signaling pathway was involved in
juglanin-ameliorated inflammation response in mice with acute lung
injury induced by LPS
As mentioned above, inflammation response has been
observed in LPS-induced mice with acute lung injury. Thus, here we
attempted to explore how juglanin altered LPS-induced acute lung
injury in mice. NF-κB signaling pathway is a key to modulate
pro-inflammatory cytokine releases (37). Thus, in our study, this classic
pathway was also investigated to prove whether NF-κB was involved
in our study. As shown in Fig.
6A, immunofluorescent analysis was carried out to explore how
phosphorylated NF-κB and IKKα are changed in LPS-treated mice with
or without juglanin treatment. The data indicated that NF-κB and
IKKα phosphorylation levels were dramatically upregulated in LPS
treatment, which were then significantly downregulated for juglanin
treatment in a dose-dependent manner (Fig. 6B and C). In addition, IgG-isotype
control staining suggested that no positive signal was observed by
immunofluorescent analysis. Furthermore, western blot analysis was
used to prove that IKKα phosphorylated levels, IκBα expression, and
NF-κB activity were found to be stimulated greatly by LPS treatment
compared to the Con group, which was downregulated in the
juglanin-treated groups, contributing to pro-inflammatory cytokine
of IL-1β decrease (Fig. 6D). The
data here indicated that juglanin could attenuate LPS-induced
inflammation response through pro-inflammatory cytokine release via
IKKα/NF-κB signaling pathway suppression.
The effects of juglanin on the toxicity
in vitro and in vivo
We evidenced in vivo that juglanin showed
suppressive role in acute lung injury progression in LPS-treated
mice. As further confirmation in vitro studies were carried
out. The cell viability was estimated for juglanin treatment in
BEAS-2B cells (Fig. 7A). The
cells were treated with different concentrations of juglanin
ranging from 0 to 80 µM for 24 h. The cells were collected
for CCK-8 assays. No significant difference was observed after
juglanin administration, even at the highest dose of juglanin.
Also, 80 µM juglanin was used to treat cells for different
times as indicated, and no difference was shown (Fig. 7B). In order to further ensure that
juglanin was non-toxic on cells, liver normal L02 cells were used.
As shown in Fig. 7C, L02 cells
were treated with juglanin (0, 1.25, 2.5, 5, 10, 20, 40 and 80
µM) for 24 h. The cell viability was not changed between the
groups. Also, after various times of treatment, no significant
difference was observed among the groups with 80 µM
treatment (Fig. 7D). Further,
in vivo, the experimental mice without LPS induction were
administered with juglanin at different concentrations. After
sacrifice, the liver, renal and spleen tissue specimens were
isolated for H&E staining. As shown in Fig. 7E, no significant difference was
observed among the Con and the Con with juglanin treatment groups,
indicating that juglanin induced little toxicity in vivo.
The data above indicated that juglanin shows no toxicity on lung
cells or liver cells. Thus, 40 and 80 µM juglalnin was used
for the following studies in vitro.
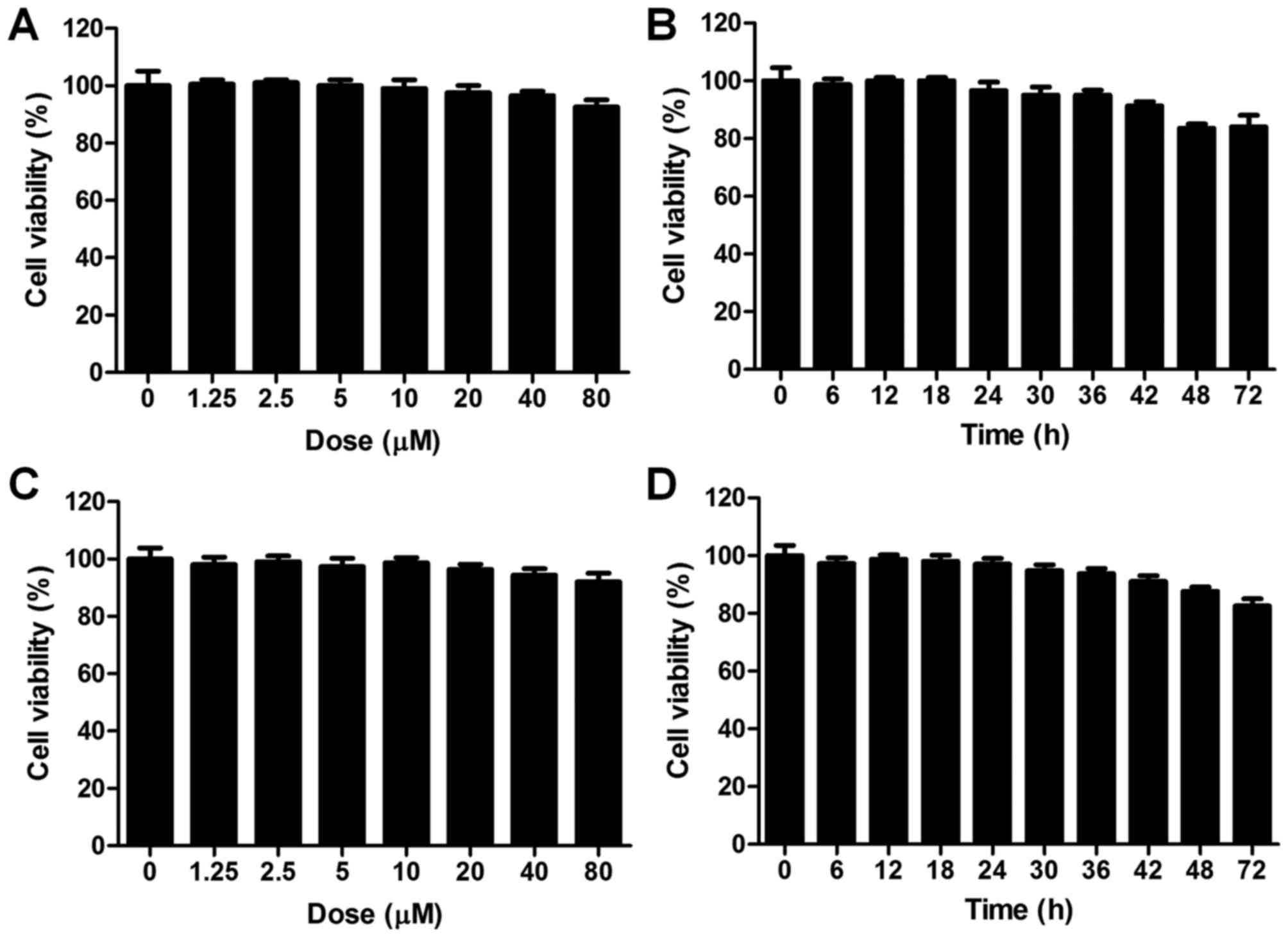 | Figure 7The effects of juglanin on the cell
viability in vitro. (A) Cell counting kit-8 (CKK-8) assays
were used to calculate normal lung cell BEAS-2B viability. The
cells were treated with different concentrations (0, 1.25, 2.5, 5,
10, 20, 40 and 80 µM) of juglanin for 24 h, followed by
CCK-8 analysis. (B) The BEAS-2B cells were treated with 80
µM juglanin for different times as indicated. Then, the cell
viability was measured. (C) The liver normal cells, L02, were
treated with different concentrations of juglanin ranging from 0 to
80 µM for 24 h. Then, CCK-8 analysis was conducted to
calculate the cell viability. (D) The L02 cells were treated with
80 µM juglanin for various times (0 to 72 h). Then, the L02
cell viability was determined. |
Juglanin suppresses fibrosis development
in lung cells in vitro
Fibrosis expression levels were further explored to
prove that juglanin is useful for acute lung injury improvement by
fibrosis inhibition. As shown in Fig.
8A and B, α-SMA was highly upregulated in 100 ng/ml LPS
treatment, whereas downregulated by juglanin administration, which
was in agreement with the results above in vivo. Next,
western blot analysis further indiated that α-SMA, TGF-β1, collagen
type I and collagen type III were dramatically upregulated in LPS
treatment. Of note, juglanin administration showed extremely
suppressive role for these fibrosis markers compared to the LPS
alone-treated group (Fig. 8C and
D). The data above illustrate that juglanin ameliorated
LPS-induced fibrosis development in lung cells in vitro.
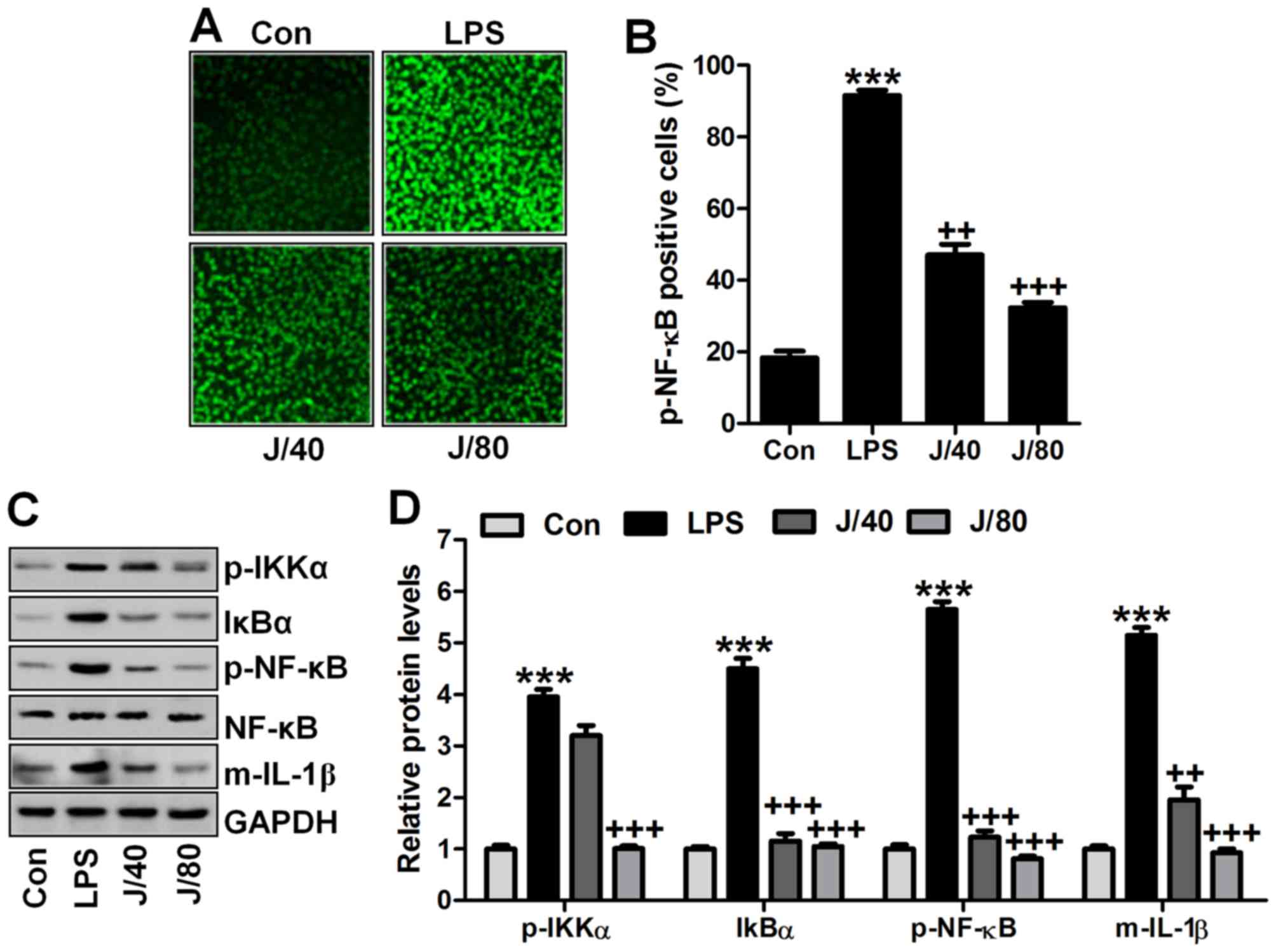
Juglanin inhibits inflammation response
in lung cells induced by LPS in vitro
In vivo studies, we evidenced that
inflammation response was induced for LPS. Thus, here we attempted
to determine if juglanin could improve acute lung injury from
inhibiting inflammatory response in lung cells of BEAS-2B. First,
immunofluorescent analysis was carried out to calculate
phosphorylated NF-κB levels. As shown in Fig. 9A and B, high intensity of
phospho-NF-κB was observed in LPS-treated cells, while being
downregulated in juglanin administration in a dose-dependent
manner. Furthermore, western blot analysis indicated that
phospho-IKKα, IκBα and phospho-NF-κB were dramatically upregulated
in LPS-treated cells. Juglanin treatment at different
concentrations could downregulate expression of these factors. In
agreement with this, pro-inflammatory cytokines of IL-1β was
apparently accelerated for LPS induction, which was reduced greatly
for juglanin (Fig. 9C and D).
Here, this study indicated that juglanin in vivo showed
inhibitory role in inflammation response induced by LPS in
vitro to attenuate lung injury.
Discussion
Acute lung injury in children has been reported
previously (1,38). Currently, though treatments have
been investigated and advanced, short-term morbidity as well as
mortality still always occur in patients. Many patients are unable
to suppress lung injury due to severe tissue damage (39). Additionally, molecular mechanism
still needs clarification on acute lung injury triggered by LPS,
resembling the disease in human, leading to unusual metabolism
(40,41). Accumulating evidence has suggested
that LPS treatment could result in lung dysfunction with serious
injury (42). Juglanin is a
natural compound extracted from the crude 'Polygonum
aviculare', showing bioactivity against inflammation response,
as well as cancer growth (43).
Recently, the anti-inflammation and anti-oxidative activity of
juglanin has been reported, suggesting that juglanin may possess
effects on suppressing LPS-induced diseases (44). Therefore, in our present study,
juglanin was investigated to clarify if it could be used as a
therapeutic strategy in acute lung injury induced by LPS. This is
the first time that juglanin was included in a study of acute lung
injury from the aspects of fibrosis and inflammation
regulation.
In our study, mice exposed to LPS were protected
from acute lung injury due to juglanin administration. Mice exposed
to LPS alone showed higher inflammation infiltration in BAL and lgE
and lgA both in serum and in BAL. Neutrophils and macrophages were
the main inflammatory cells in acute lung injury. They infiltrated
into the lung tissues, releasing enzymes phagocytizing the pathogen
(45,46). Moreover, these inflammatory cells
were the fundamental source of inflammatory mediators in
vivo (47). In LPS-induced
inflammation, neutrophils and macrophages were activated. After
activation, neutrophils and macrophages were recruited to the
inflammation site (48).
Lymphocytes have drawn increased attention, and there is
accumulating evidence indicating that lymphocytes play important
roles in the development and progression of acute lung injury
(49,50). It has been suggested that
lymphocytes contribute to the progression of autoimmune and
inflammatory diseases (51).
Thus, these cells levels could be an indicator supporting the role
of LPS on acute lung injury, and juglanin could reduce these cells
to prevent inflammation response, revealing the anti-inflammatory
effect of juglanin on lung injury treatment. Previous studies
reported that abundant abnormal collagen accumulation in lung
tissue samples was observed after examination of patients with lung
injury (52–54). In addition, the collagen type III
accumulation may occur in lung disease of acute injury (55). In addition, α-SMA expression is
closely related to fibrosis accumulation (56). Further, LPS induction upregulated
markers of fibrosis, such as α-SMA, collagen type I and collagen
type III, as well as TGF-β1, which have been indicated as
significant markers, inducing fibrosis development. TGF-β1 is known
to modulate various cellular responses (57). Significantly, TGF-β1 is a key
fibrotic cytokine, regulating organ fibrosis and dysfunction.
During the progression of fibrosis, TGF-β1 stimulates a number of
fibrogenic genes, such as α-SMA (58). In this study, we found that those
markers were dramatically upregulated via western blot and RT-qPCR
analysis, which was in line with previous studies (59). The results above indicated that
LPS treatment, indeed, induced lung fibrosis development and
progression in mice. Notably, juglanin has potential role in
reducing fibrosis markers both in vitro and in vivo,
improving fibrosis accumulation in the lung tissue samples of
mice.
Inflammation is known to be involved in fibrosis
development, accompanied with pro-inflammatory cytokine release,
including IL-18, IL-6, IL-1β and TNF-α (60,61). NF-κB signaling pathway is of great
importance in inflammation response through transfection activation
(62). NF-κB phosphorylation
could be activated following IκBα activity improvement (63,64). IKKα, as an up-stream signal of
IκBα/NF-κB, was found to be upregulated in LPS-treated mice,
activating IκBα and NF-κB expression. Pro-inflammatory cytokines of
IL-6, IL-1β and TNF-α expression levels were also promoted
subsequently, eventually contributing to inflammatory responses. Of
note, juglanin exhibited anti-inflammatory role in LPS-induced
acute lung injury, which was in agreement with previous studies.
Similarly, in vitro juglanin downregulated pro-inflammatory
cytokine release, as well as IKKα, and IκBα/NF-κB expression
induced by LPS induction.
In conclusion, the data above suggested that LPS
induced acute lung injury in mice with upregulation of α-SMA,
TGF-β1 and collagen, which could be ameliorated by juglanin.
Inflammatory response in mice and lung cells stimulated by LPS was
also inhibited by juglanin through suppressing NF-κB signaling
pathway. Significantly, our study suggested the possible role of
juglanin in preventing acute lung injury by fibrosis formation and
inflammation inhibition, which is of great value as useful
assistant dietary supplements and for finding new and effective
therapeutic strategy in acute lung injury for children in
future.
Acknowledgments
The authors would like to thank the reviewers for
their comments and help to promote this work.
Notes
[1]
Funding
No funding was received.
[2] Availability
of data and material
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
ZWD designed and performed the experiments. YFY
wrote the manuscript. Both authors read and approved the final
manuscript.
[4] Ethics
approval and consent to participate
This study was approved by the Ethics Committee on
Animal Research at the Department of Pediatrics, Huai'an First
People's Hospital, Nanjing Medical University, Nanjing, China.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Saguil A and Fargo M: Acute respiratory
distress syndrome: Diagnosis and management. Am Fam Physician.
85:352–358. 2012.PubMed/NCBI
|
|
2
|
Cortés I, Peñuelas O and Esteban A: Acute
respiratory distress syndrome: Evaluation and management. Minerva
Anestesiol. 78:343–357. 2012.PubMed/NCBI
|
|
3
|
Brun-Buisson C, Minelli C, Bertolini G,
Brazzi L, Pimentel J, Lewandowski K, Bion J, Romand JA, Villar J,
Thorsteinsson A, et al ALIVE Study Group: Epidemiology and outcome
of acute lung injury in European intensive care units. Results from
the ALIVE study. Intensive Care Med. 30:51–61. 2004. View Article : Google Scholar
|
|
4
|
Ware LB: Clinical year in review IV: Acute
respiratory distress syndrome, radiology in the intensive care
unit, nonpulmonary critical care, and pulmonary infections in the
immunocompromised host. Proc Am Thorac Soc. 5:755–760. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mofidi A, Bader A and Pavlica S: The use
of erythropoietin and its derivatives to treat spinal cord injury.
Mini Rev Med Chem. 11:763–770. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matthay MA and Zemans RL: The acute
respiratory distress syndrome: Pathogenesis and treatment. Annu Rev
Pathol. 6:147–163. 2011. View Article : Google Scholar :
|
|
7
|
Li B, Yang J, Huang Q, Zhang Y, Peng C,
Zhang Y, He Y, Shi J, Li W, Hu J, et al: Biodistribution and
pulmonary toxicity of intratracheally instilled graphene oxide in
mice. NPG Asia Mater. 5:e442013. View Article : Google Scholar
|
|
8
|
Spragg RG, Bernard GR, Checkley W, Curtis
JR, Gajic O, Guyatt G, Hall J, Israel E, Jain M, Needham DM, et al:
Beyond mortality: Future clinical research in acute lung injury. Am
J Respir Crit Care Med. 181:1121–1127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rubenfeld GD, Caldwell E, Peabody E,
Weaver J, Martin DP, Neff M, Stern EJ and Hudson LD: Incidence and
outcomes of acute lung injury. N Engl J Med. 353:1685–1693. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muñoz NM, Meliton AY, Meliton LN, Dudek SM
and Leff AR: Secretory group V phospholipase A2 regulates acute
lung injury and neutrophilic inflammation caused by LPS in mice. Am
J Physiol Lung Cell Mol Physiol. 296:L879–L887. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mei SH, McCarter SD, Deng Y, Parker CH,
Liles WC and Stewart DJ: Prevention of LPS-induced acute lung
injury in mice by mesenchymal stem cells overexpressing
angiopoietin 1. PLoS Med. 4:e2692007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang HH, Hwangbo K, Zheng MS, Son JK, Kim
HY, Baek SH, Choi HC, Park SY and Kim JR: Inhibitory effects of
juglanin on cellular senescence in human dermal fibroblasts. J Nat
Med. 68:473–480. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang XY, Duan QY, Liu JX and Di DL:
Determination of a novel diarylheptanoid (Juglanin B) from green
walnut husks (Juglans regia L.) in rat plasma by high-performance
liquid chromatography. Biomed Chromatogr. 24:307–311. 2010.
|
|
14
|
Zhong J, Yang Y, Xiao Z, Chen J, Aisa HA
and Zhang T: Separation and purification of three flavonoids from
the petal of Rosa rugosa Thunb. by HSCCC. Asian J Tradit Med.
4:220–227. 2009.
|
|
15
|
Procházková D, Boušová I and Wilhelmová N:
Antioxidant and prooxidant properties of flavonoids. Fitoterapia.
82:513–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Agati G, Azzarello E, Pollastri S and
Tattini M: Flavonoids as antioxidants in plants: Location and
functional significance. Plant Sci. 196:67–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parhiz H, Roohbakhsh A, Soltani F, Rezaee
R and Iranshahi M: Antioxidant and anti-inflammatory properties of
the citrus flavonoids hesperidin and hesperetin: An updated review
of their molecular mechanisms and experimental models. Phytother
Res. 29:323–331. 2015. View
Article : Google Scholar
|
|
18
|
Cho N, Choi JH, Yang H, Jeong EJ, Lee KY,
Kim YC and Sung SH: Neuroprotective and anti-inflammatory effects
of flavonoids isolated from Rhus verniciflua in neuronal HT22 and
microglial BV2 cell lines. Food Chem Toxicol. 50:1940–1945. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu Y, Chen J, Li YJ, Zheng YF and Li P:
Antioxidant and anti-inflammatory activities of six flavonoids
separated from licorice. Food Chem. 141:1063–1071. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Berlier G, Gastaldi L, Ugazio E, Miletto
I, Iliade P and Sapino S: Stabilization of quercetin flavonoid in
MCM-41 mesoporous silica: Positive effect of surface
functionalization. J Colloid Interface Sci. 393:109–118. 2013.
View Article : Google Scholar
|
|
21
|
Soromou LW, Chen N, Jiang L, Huo M, Wei M,
Chu X, Millimouno FM, Feng H, Sidime Y and Deng X: Astragalin
attenuates lipopolysaccharide-induced inflammatory responses by
downregulating NF-κB signaling pathway. Biochem Biophys Res Commun.
419:256–261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Askari G, Ghiasvand R, Feizi A, Ghanadian
SM and Karimian J: The effect of quercetin supplementation on
selected markers of inflammation and oxidative stress. J Res Med
Sci. 17:637–641. 2012.
|
|
23
|
Bakhshaeshi M, Khaki A, Fathiazad F, Khaki
AA and Ghadamkheir E: Anti-oxidative role of quercetin derived from
Allium cepa on aldehyde oxidase (OX-LDL) and hepatocytes apoptosis
in streptozotocin-induced diabetic rat. Asian Pac J Trop Biomed.
2:528–531. 2012. View Article : Google Scholar
|
|
24
|
Lee CC, Shen SR, Lai YJ and Wu SC: Rutin
and quercetin, bioactive compounds from tartary buckwheat, prevent
liver inflammatory injury. Food Funct. 4:794–802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chae HS, Kim YM, Lee EJ, Song HH, Oh SR,
Choi YH and Chin YW: Corilagin with inhibitory activity against NO
production from Euphorbia supina. Nat Prod Sci. 20:126–129.
2014.
|
|
26
|
Zhou GY, Yi YX, Jin LX, Lin W, Fang PP,
Lin XZ, Zheng Y and Pan CW: The protective effect of juglanin on
fructose-induced hepatitis by inhibiting inflammation and apoptosis
through TLR4 and JAK2/STAT3 signaling pathways in fructose-fed
rats. Biomed Pharmacother. 81:318–328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li C, Liu JX, Zhao L, Di DL, Meng M and
Jiang SX: Capillary zone electrophoresis for separation and
analysis of four diarylheptanoids and an α-tetralone derivative in
the green walnut husks (Juglans regia L.). J Pharm Biomed Anal.
48:749–753. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
El-Salhy M, Lomholt-Beck B and Gundersen
D: The prevalence of celiac disease in patients with irritable
bowel syndrome. Mol Med Rep. 4:403–405. 2011.PubMed/NCBI
|
|
29
|
Jensen EC: Quantitative analysis of
histological staining and fluorescence using ImageJ. Anat Rec
(Hoboken). 296:378–381. 2013. View Article : Google Scholar
|
|
30
|
Kim HA, Park JH, Lee S, Choi JS, Rhim T
and Lee M: Combined delivery of dexamethasone and plasmid DNA in an
animal model of LPS-induced acute lung injury. J Control Release.
156:60–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hague AS: Allergic bronchopulmonary
aspergillosis. Pakistan J Chest Med. 11:19–26. 2015.
|
|
32
|
Martin TR and Matute-Bello G: Experimental
models and emerging hypotheses for acute lung injury. Crit Care
Clin. 27:735–752. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reiss LK, Uhlig U and Uhlig S: Models and
mechanisms of acute lung injury caused by direct insults. Eur J
Cell Biol. 91:590–601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Su X, Bai C, Hong Q, Zhu D, He L, Wu J,
Ding F, Fang X and Matthay MA: Effect of continuous hemofiltration
on hemodynamics, lung inflammation and pulmonary edema in a canine
model of acute lung injury. Intensive Care Med. 29:2034–2042. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li B, Dong C, Wang G, Zheng H, Wang X and
Bai C: Pulmonary epithelial CCR3 promotes LPS-induced lung
inflammation by mediating release of IL-8. J Cell Physiol.
226:2398–2405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thorley AJ, Ford PA, Giembycz MA,
Goldstraw P, Young A and Tetley TD: Differential regulation of
cytokine release and leukocyte migration by
lipopolysaccharide-stimulated primary human lung alveolar type II
epithelial cells and macrophages. J Immunol. 178:463–473. 2007.
View Article : Google Scholar
|
|
37
|
Wong ET and Tergaonkar V: Roles of
NF-kappaB in health and disease: Mechanisms and therapeutic
potential. Clin Sci (Lond). 116:451–465. 2009. View Article : Google Scholar
|
|
38
|
Bhatia M, Zemans RL and Jeyaseelan S: Role
of chemokines in the pathogenesis of acute lung injury. Am J Respir
Cell Mol Biol. 46:566–572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Matthay MA, Ware LB and Zimmerman GA: The
acute respiratory distress syndrome. J Clin Invest. 122:2731–2740.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee JP, Li YC, Chen HY, Lin RH, Huang SS,
Chen HL, Kuan PC, Liao MF, Chen CJ and Kuan YH: Protective effects
of luteolin against lipopolysaccharide-induced acute lung injury
involves inhibition of MEK/ERK and PI3K/Akt pathways in
neutrophils. Acta Pharmacol Sin. 31:831–838. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kabir K, Gelinas JP, Chen M, Chen D, Zhang
D, Luo X, Yang JH, Carter D and Rabinovici R: Characterization of a
murine model of endotoxin-induced acute lung injury. Shock.
17:300–303. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rojas M, Woods CR, Mora AL, Xu J and
Brigham KL: Endotoxin-induced lung injury in mice: Structural,
functional, and biochemical responses. Am J Physiol Lung Cell Mol
Physiol. 288:L333–L341. 2005. View Article : Google Scholar
|
|
43
|
Chen G, Pi XM and Yu CY: A new
naphthalenone isolated from the green walnut husks of Juglans
mandshurica Maxim. Nat Prod Res. 29:174–179. 2015. View Article : Google Scholar
|
|
44
|
Yoo G, Park SJ, Lee TH, Yang H, Baek YS,
Kim N, Kim YJ and Kim SH: Flavonoids isolated from Lespedeza
cuneata G. Don and their inhibitory effects on nitric oxide
production in lipopolysaccharide-stimulated BV-2 microglia cells.
Pharmacogn Mag. 11:651–656. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q,
Monsel A, Qu JM, Matthay MA and Lee JW: Human mesenchymal stem cell
microvesicles for treatment of Escherichia coli endotoxin-induced
acute lung injury in mice. Stem Cells. 32:116–125. 2014. View Article : Google Scholar :
|
|
46
|
Bhatia M, Zemans RL and Jeyaseelan S: Role
of chemokines in the pathogenesis of acute lung injury. Am J Respir
Cell Mol Biol. 46:566–572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kovarova M, Hesker PR, Jania L, Nguyen M,
Snouwaert JN, Xiang Z, Lommatzsch SE, Huang MT, Ting JP and Koller
BH: NLRP1-dependent pyroptosis leads to acute lung injury and
morbidity in mice. J Immunol. 189:2006–2016. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhu T, Wang DX, Zhang W, Liao XQ, Guan X,
Bo H, Sun JY, Huang NW, He J, Zhang YK, et al: Andrographolide
protects against LPS-induced acute lung injury by inactivation of
NF-κB. PLoS One. 8:e564072013. View Article : Google Scholar
|
|
49
|
Ci X, Chu X, Wei M, Yang X, Cai Q and Deng
X: Different effects of farrerol on an OVA-induced allergic asthma
and LPS-induced acute lung injury. PLoS One. 7:e346342012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xiao M, Zhu T, Zhang W, Wang T, Shen YC,
Wan QF and Wen FQ: Emodin ameliorates LPS-induced acute lung
injury, involving the inactivation of NF-κB in mice. Int J Mol Sci.
15:19355–19368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Garibaldi BT, D'Alessio FR, Mock JR, Files
DC, Chau E, Eto Y, Drummond MB, Aggarwal NR, Sidhaye V and King LS:
Regulatory T cells reduce acute lung injury fibroproliferation by
decreasing fibrocyte recruitment. Am J Respir Cell Mol Biol.
48:35–43. 2013. View Article : Google Scholar :
|
|
52
|
Tsukada S, Westwick JK, Ikejima K, Sato N
and Rippe RA: SMAD and p38 MAPK signaling pathways independently
regulate α1(I) collagen gene expression in unstimulated and
transforming growth factor-β-stimulated hepatic stellate cells. J
Biol Chem. 280:10055–10064. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dempsey OJ and Miller D: Idiopathic
pulmonary fibrosis. BMJ. 347:f65792013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mahendran S and Sethi T: Treatments in
idiopathic pulmonary fibrosis: Time for a more targeted approach?
QJM. 105:929–934. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ghaffari AR, Noshad H, Ostadi A and
Hasanzadeh N: Effect of pulse therapy with glucocorticoid and
cyclophosphamide in lung fibrosis due to paraquat poisoning in
rats. Saudi Med J. 32:249–253. 2011.PubMed/NCBI
|
|
56
|
Ghazi-Khansari M, Mohammadi-Karakani A,
Sotoudeh M, Mokhtary P, Pour-Esmaeil E and Maghsoud S: Antifibrotic
effect of captopril and enalapril on paraquat-induced lung fibrosis
in rats. J Appl Toxicol. 27:342–349. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen CM, Chou HC, Hsu HH and Wang LF:
Transforming growth factor-β1 upregulation is independent of
angiotensin in paraquat-induced lung fibrosis. Toxicology.
216:181–187. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Son JY, Kim SY, Cho SH, Shim HS, Jung JY,
Kim EY, Lim JE, Park BH, Kang YA, Kim YS, et al: TGF-β1 T869C
polymorphism may affect susceptibility to idiopathic pulmonary
fibrosis and disease severity. Lung. 191:199–205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Madhyastha R, Madhyastha H, Pengjam Y,
Nakajima Y, Omura S and Maruyama M: NFkappaB activation is
essential for miR-21 induction by TGFβ1 in high glucose conditions.
Biochem Biophys Res Commun. 451:615–621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bastard JP, Jardel C, Bruckert E, Blondy
P, Capeau J, Laville M, Vidal H and Hainque B: Elevated levels of
interleukin 6 are reduced in serum and subcutaneous adipose tissue
of obese women after weight loss. J Clin Endocrinol Metab.
85:3338–3342. 2000.PubMed/NCBI
|
|
61
|
Lu CN, Yuan ZG, Zhang XL, Yan R, Zhao YQ,
Liao M and Chen JX: Saikosaponin a and its epimer saikosaponin d
exhibit anti-inflammatory activity by suppressing activation of
NF-κB signaling pathway. Int Immunopharmacol. 14:121–126. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Nieto-Vazquez I, Fernández-Veledo S,
Krämer DK, Vila-Bedmar R, Garcia-Guerra L and Lorenzo M: Insulin
resistance associated to obesity: The link TNF-alpha. Arch Physiol
Biochem. 114:183–194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang HH, Halbleib M, Ahmad F, Manganiello
VC and Greenberg AS: Tumor necrosis factor-alpha stimulates
lipolysis in differentiated human adipocytes through activation of
extracellular signal-related kinase and elevation of intracellular
cAMP. Diabetes. 51:2929–2935. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wang SL, Li Y, Wen Y, Chen YF, Na LX, Li
ST and Sun CH: Curcumin, a potential inhibitor of upregulation of
TNF-alpha and IL-6 induced by palmitate in 3T3-L1 adipocytes
through NF-kappaB and JNK pathway. Biomed Environ Sci. 22:32–39.
2009. View Article : Google Scholar : PubMed/NCBI
|