Introduction
Sepsis is a serious pathogenic infection and can
deteriorate in a generalized immune response to systemic infection,
which is a leading contributor to mortality rates in intensive care
units (1). Candidiasis,
particularly Candida albicans (C. albicans), is one
of the leading worldwide nosocomial infections occurring following
the onset of sepsis, and the mortality rate attributable to
candidemia is ~40% despite the use of antifungal agents (2,3).
In order to improve the treatment of candidemia-induced multiple
organ failure, it is important to first understand its
pathogenesis. A mouse model of disseminated C. albicans
infection has been used extensively to examine the pathological
process, and the kidney is the primary target organ (4). There is increasing evidence
suggesting that the activation of the pro-inflammatory mechanism is
involved in candidiasis-induced acute kidney injury (AKI) (5,6).
Interleukin-1α (IL-1α), IL-1β, IL-6, tumor necrosis factor-α
(TNF-α) and interferon-γ (IFN-γ) have been shown previously to be
important in the pathogenesis of candidemia-induced renal injury
(7,8). In addition, chemokines and their
receptors are reportedly induced in a fatal mouse model of invasive
candidiasis (5,7). These findings suggest that reducing
the excessive production of inflammatory cytokines during
candidemia is beneficial for attenuating AKI.
Itraconazole (ITR) is an azole broad-spectrum
anti-fungal agent, and is orally administered for C.
parapsilosis, C. albicans and C. glabrata
infections (9–11). Generally, the treatment of fungal
infections with ITR may be due not only to its direct antifungal
effect, but also due to the induction of an immunomodulatory effect
on the host defense systems via the regulation of cytokine
production (12). ITR has an
anti-inflammatory effect in vivo and in vitro
(13,14). In human lymphoid cells, ITR
suppresses the accumulation of TNF-α and IFN-γ messenger RNAs
(mRNAs) in the presence of polyhydroxyalkanoate (15). In addition, ITR inhibits the
TNF-α-induced expression of CXCL10 in oral fibroblasts (16). In a murine model of chronic
pulmonary paracoccidioidomycosis, ITR improves tissue lesions and
immunomodulation through the regulation of pro-inflammatory
cytokine levels (17). However,
there has been no report on the protective effect of ITR in mice
with renal injury.
A class of small non-coding RNAs (18–25 nucleotides)
known as microRNAs (miRs), which regulate the translation of target
mRNAs by binding to 3′-untranslated regions (3′-UTRs), have emerged
as multifunctional post-translational modulators correlated with
several diseases (18,19). The association between sepsis and
differentially expressed miRs has become a focus of investigations
(20). C. albicans
infection or bacterial cell surface lipopolysaccharide (LPS)
treatment can induce the upregulation of miRs, including miR-146,
miR-155, miR-455 and miR-125a, in macrophages (21). In our previous study, it was
demonstrated that miR-204 and miR-211 were down-regulated in the
kidneys of septic mice, however, intravenous injection of miR-204
and miR-211 reversed sepsis-induced renal injury (22). Therefore, the identification of
novel therapeutic targets based on an improved understanding of the
molecular pathogenesis is required.
In the present study, ITR was identified as an
antifungal agent against candidiasis-induced AKI through the
inhibition of monocyte chemoattractant protein-1 (MCP-1). However,
information on the effect of post-translational modulators on the
nephroprotection of ITR in septic mice remains limited. Utilizing
online prediction algorithms in the present study, MCP-1 was
identified as a direct target of miR-124. It was recognized that
miR-124/MCP-1 was closely associated with candidiasis-induced AKI,
which may be a valuable molecular target for investigating the
pathogenesis of renal dysfunction.
Materials and methods
Cell culture
293T cells (American Type Culture Collection,
Manassas, VA, USA) were incubated in Dulbecco's modified Eagle's
medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
supplemented with 10% FBS, 100 µg/ml streptomycin and 100
IU/ml penicillin (all from Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany).
Animal treatment
The experiments were approved by the Ethics
Committee of the Second Affiliated Hospital of Guilin Medical
University (Guilin, China) and were performed in accordance with
its guidelines. A total of 120 male 8-week-old C57BL/6J mice (body
weight, 20±2 g) were obtained from Charles River Laboratories
(Wilmington, MA, USA) and were allowed to acclimate to the
environment for 1 week. The mice were provided with free access to
food and tap water, and were individually caged under a controlled
temperature (23±2°C) and humidity (60±5%) with an artificial 12-h
light/dark cycle. The mice were randomly divided into six groups
(n=20 in each group) as follows: NC group injected with normal
saline; CAN group infected with C. albicans via the
injection of 1.0×107 colony-forming units
intraperitoneally; ITR group treated with ITR (50 mg/kg;
Xi'an-Janssen Pharmaceutical, Ltd., Xi'an, China) by intragastric
administration; CAN+ITR group infected with C. albicans and
co-treated with ITR (50 mg/kg); miR-Con group infected with C.
albicans with co-injection of scramble sequence (miR-Con);
miR-124 group infected with C. albicans with co-injection of
miR-124 mimics. The C. albicans strain SC5314 was obtained
from a fungal suspension (American Type Culture Collection) as
described elsewhere (23) and
cultivated in YPD (1% yeast extract, 1% peptone and 2% dextrose)
broth with overnight shaking at 30°C. The systemic fungal infection
was performed as in our previously described method (22).
Hematoxylin and eosin (H&E)
staining
Kidney tissues were collected 7 days following C.
albicans injection via intra-peritoneal injection of sodium
pentobarbital (2%; 150 mg/kg; Sigma-Aldrich; Merck Millipore), and
were fixed with 4% formalin at room temperature for 24 h and
paraffin-embedded. The tissues were then cut into
~5-µm-thick sections, which were stained with H&E at
room temperature for 1–2 min and visualized under a microscope
(Leica DM 2500; Leica Microsystems GmbH, Wetzlar, Germany). Renal
injury was assessed using a previously described 0–4 point scale
(24) as follows: 0, none; 1,
<10%; 2, 10–25%; 3, 25–75%; or 4, >75%.
Enzyme-linked immunosorbent assay
(ELISA)
The levels of inflammatory cytokines, TNF-α (cat.
no. E-EL-M0049c), IL-1β (cat. no. E-EL-M0037c) and IL-6 (cat. no.
E-EL-M0044c) were measured using mouse ELISA kits (Elabscience
Biotechnology Co., Ltd., Wuhan, China) with a SpectraMax M5 ELISA
plate reader (Molecular Devices, LLC., Sunnyvale, CA, USA),
according to the manufacturer's protocol.
Measurement of glomerular filtration rate
(GFR)
Serum cystatin C (CysC) and β2-microglobulin (β2-MG)
are freely filtered by the glomerular membrane, which makes blood
levels good indicators of GFR function. In the present study, CysC
(cat. no. CYS4004) and β2-MG (cat. no. RQ9114) were measured using
a RANDOX enzymatic creatinine assay (Randox Laboratories Ltd.,
Antrim, UK). Blood urea nitrogen (BUN) was measured via an
enzymatic kinetic method using a commercial kit (cat. no. C013-2;
Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Serum
creatinine (SCr) levels were measured using an autoanalyzer
according to the manufacturer's protocol (cat. no. C011-1; Nanjing
Jiancheng Bioengineering Institute).
Recombinant adenoviruses
Recombinant adenoviruses for the expression of
miR-124 or control scrambled short hairpin RNA (miR-Con) were
generated using the BLOCK-iT adenoviral RNAi expression system
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. High-titer stocks of amplified recombinant
adenoviruses were purified as described previously (25). The viruses were diluted in PBS and
administered at a dose of 107 plaque-forming units per
well in 12-well plates, 109 plaque-forming units per
were administered to mice via tail-vein injection every other day
for 7 days.
Transfection with miR-124 mimics and
miR-Con
The sequences of the miR-124 mimics
(5′-UAAGGCACGCGGUGAAUGCC-3′) and miR-Con
(5′-UCCAAACAUCGGUGAAUGCC-3′) were synthesized by Guangzhou RiboBio
Co., Ltd. (Guangzhou, China). The 293T cells were transfected using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) at
a final concentration of 50 nM. At 48 h post-transfection, the
cells were harvested for analysis.
Dual-luciferase reporter gene assay
The wild-type (WT, 5′-GACUCGGACUGUGUGCCUUA-3′) and
mutant-type (MUT, 5′-GACUCGGACUGUGUAUUCCA-3′) 3′-UTR of MCP-1 were
synthesized by PCR, and the fragments were subcloned into the
pmirGLO Dual-Luciferase miRNA target expression vector (Promega
Corp., Madison, WI, USA). The potential binding site between
miR-124 and MCP-1 was obtained using online prediction software
(miRanda-mirSVR; http://www.microrna.org), miRDB (http://www.mirdb.org/miRDB/) and TargetScan
(http://www.targetscan.org/). The 293T
cells were transfected with luciferase reporter vectors containing
the WT and MUT MCP-1-3′-UTR (0.5 µg). The miR-124 mimics or
miR-Con were co-transfected at 50 nM. The luciferase activity was
measured using the Dual Luciferase Reporter® assay
system (cat. no. E1960; Promega Corp.) on a Luminoskan™ Ascent
Microplate Luminometer (Thermo Fisher Scientific, Inc.). The
dual-luciferase reporter gene assay was performed as previously
described (22).
RNA analysis and RT-qPCR analysis
Total RNA was extracted using TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. The cDNA was synthesized by RT reactions with 2 µg
of total RNA using moloney murine leukemia virus reverse
transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The mRNA and miR levels
were determined using the TaqMan RT-qPCR ELISA (Thermo Fisher
Scientific, Inc.) on an ABI 7500 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). A total of 20
µl reaction mixture includes cDNA (100 ng) + DEPC
H2O (9 µl), 300 nM of each primer, 2X buffer (10
µl) and 20X TaqMan Gene Expression Assay (1 µl). The
thermocycling reactions were as follows: 95°C for 10 min, followed
by 40 cycles of 95°C for 15 sec, 60°C for 30 sec, and 72°C for 30
sec. The relative expression levels of miR and mRNA were calculated
using the 2−ΔΔCq method (26) and normalized to the internal
control U6 and glyceraldehyde 3-phosphate dehydrogenase,
respectively. The primers were synthesized by Sangon Biotech Co.,
Ltd. (Shanghai, China) as shown in Table I.
 | Table IPrimers were used for reverse
transcription-quantitative polymerase chain reaction of miRs and
mRNAs. |
Table I
Primers were used for reverse
transcription-quantitative polymerase chain reaction of miRs and
mRNAs.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| miR-124 |
CGTGTTCACAGCGGACCTTG |
TGGTGTCGTGGAGTCG |
| miR-290-5p |
ACACTCCAGCTGGGTTTCACGGGGGTATCA |
TGGTGTCGTGGAGTCG |
| miR-292-5p |
ACACTCCAGCTGGGGTTTTCUCGGGGGUCA |
TGGTGTCGTGGAGTCG |
| U6 |
CGCTTCGGCAGCACATATACTAA |
TATGGAACGCTTCACGAATTTGC |
| MCP-1 |
ATTTCCACACTTCTATGCCTCCT |
ATCCAGTATGGTCCTGAAGATCA |
| TNF-α |
GCCACCACGCTCTTCTGTCTAC |
GGGTCTGGGCCATAGAACTGAT |
| IL-1β |
ACCTTCCAGGATGAGGACATGA |
CTAATGGGAACGTCACACACCA |
| IL-6 |
CACATGTTCTCTGGGAAATCG |
TTGTATCTCTGGAAGTTTCAGATTG |
| GAPDH |
GCACCGTCAAGCTGAGAAC |
TGGTGAAGACGCCAGTGGA |
Western blot analysis
Protein was extracted using Radioimmunoprecipitation
Assay Lysis Buffer (Beyotime Institute of Biotechnology, Haimen,
China). The concentration was determined using the Bicinchoninic
Acid Kit for Protein Determination (Sigma-Aldrich; Merck KGaA).
Samples containing 50 µg of protein were separated using 10%
SDS-PAGE gel and transferred to nitrocellulose membranes (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Following blocking with 5%
(w/v) non-fat dry milk in TBS and 0.1% (w/v) Tween-20 (TBST),
Primary antibodies MCP-1 (cat. no. sc-1784; dilution 1:500) and
β-actin (cat. no. sc-130065; dilution 1:2,000) were obtained from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) and incubated
for 2 h at room temperature. The appropriate horseradish
peroxidase-conjugated secondary antibody (cat. no. sc-516102;
dilution 1:10,000) was purchased from Santa Cruz Biotechnology,
Inc. and incubated for 1 h at room temperature. Western blot
analysis was performed as previously described (22). Protein bands were
densitometrically assessed using Quantity One® software
(version 4.5; Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation
for each group. All statistical analyses were performed using PRISM
version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Inter-group differences were analyzed using one-way analysis of
variance, followed by Tukey's post-hoc test for multiple
comparisons. The log-rank test was used to compare group survival
trends. P<0.05 was considered to indicate a statistically
significant difference.
Results
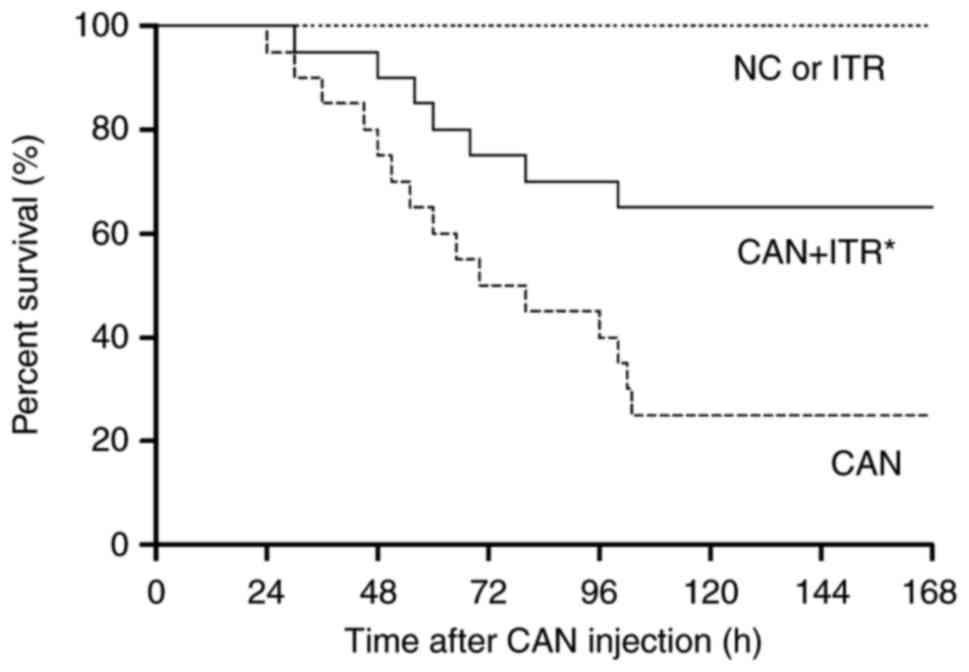
ITR improves survival outcome following
candidiasis
C. albicans injection induced animal death
within 24 h, with 50% mortality by 70 h post-C. albicans
injection, and 25% of animals surviving at day 7. However, in the
septic mice treated with ITR, no death occurred until 30 h
post-C. albicans injection, with 65% of the ITR-treated mice
surviving for the duration of the investigation (Fig. 1).
ITR inhibits systemic and local
inflammatory responses in septic mice
MCP-1 is a member of the CC chemokine family and it
is an important molecule for monocyte recruitment under acute
inflammatory conditions, regulating the progression of inflammation
through the production of pro-inflammatory cytokines (27). A study by Labbe et al
showed that antibody neutralization of MCP-1 prevented the
endotoxin-induced upregulation of IL-1α, IL-1β and IL-6 in the
diaphragm (28). Therefore, the
present study investigated the levels of MCP-1 in septic mice. The
results showed that the mRNA (Fig.
2A) and protein (Fig. 2B)
expression levels of MCP-1 in the kidneys from septic mice were
significantly higher, compared with those in the control group,
which were markedly decreased by ITR (Fig. 2A and B). The serum and renal
levels of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) were
determined. As shown in Fig. 2C and
D, compared with the control group, the serum and renal levels
of pro-inflammatory cytokines were significantly increased in the
septic mice. Treatment with ITR for 7 days resulted in a decrease
of pro-inflammatory cytokines in septic mice, suggesting that ITR
inhibited the excessive systemic and local inflammatory response in
candidiasis-induced AKI mice.
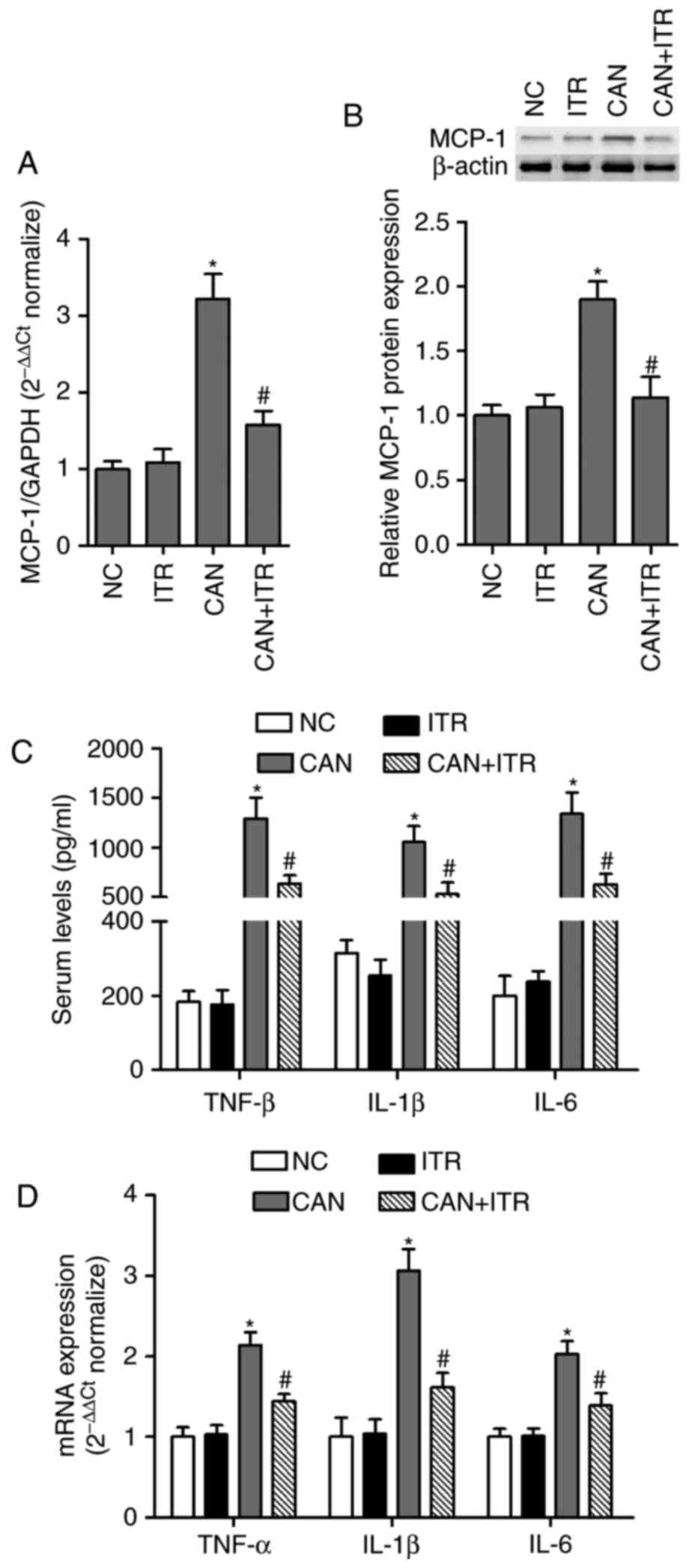 | Figure 2ITR inhibits systemic and local
inflammatory responses in septic mice. mRNA and protein expression
levels were analyzed by (A) RT-qPCR and (B) western blot analyses
in the kidneys of septic mice with or without ITR treatment. (C)
Levels of inflammatory cytokines, TNF-α, IL-1β and IL-6, in serum
were measured using mouse ELISA kits. (D) mRNA levels of TNF-α,
IL-1β and IL-6 were analyzed by RT-qPCR analysis in the kidneys
from septic mice with or without ITR treatment.
*P<0.05 compared with the NC group;
#P<0.05 compared with the CAN group. TNF-α, tumor
necrosis factor-α; IL, interleukin; ITR, itraconazole; CAN,
Candida albicans; NC, negative control; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
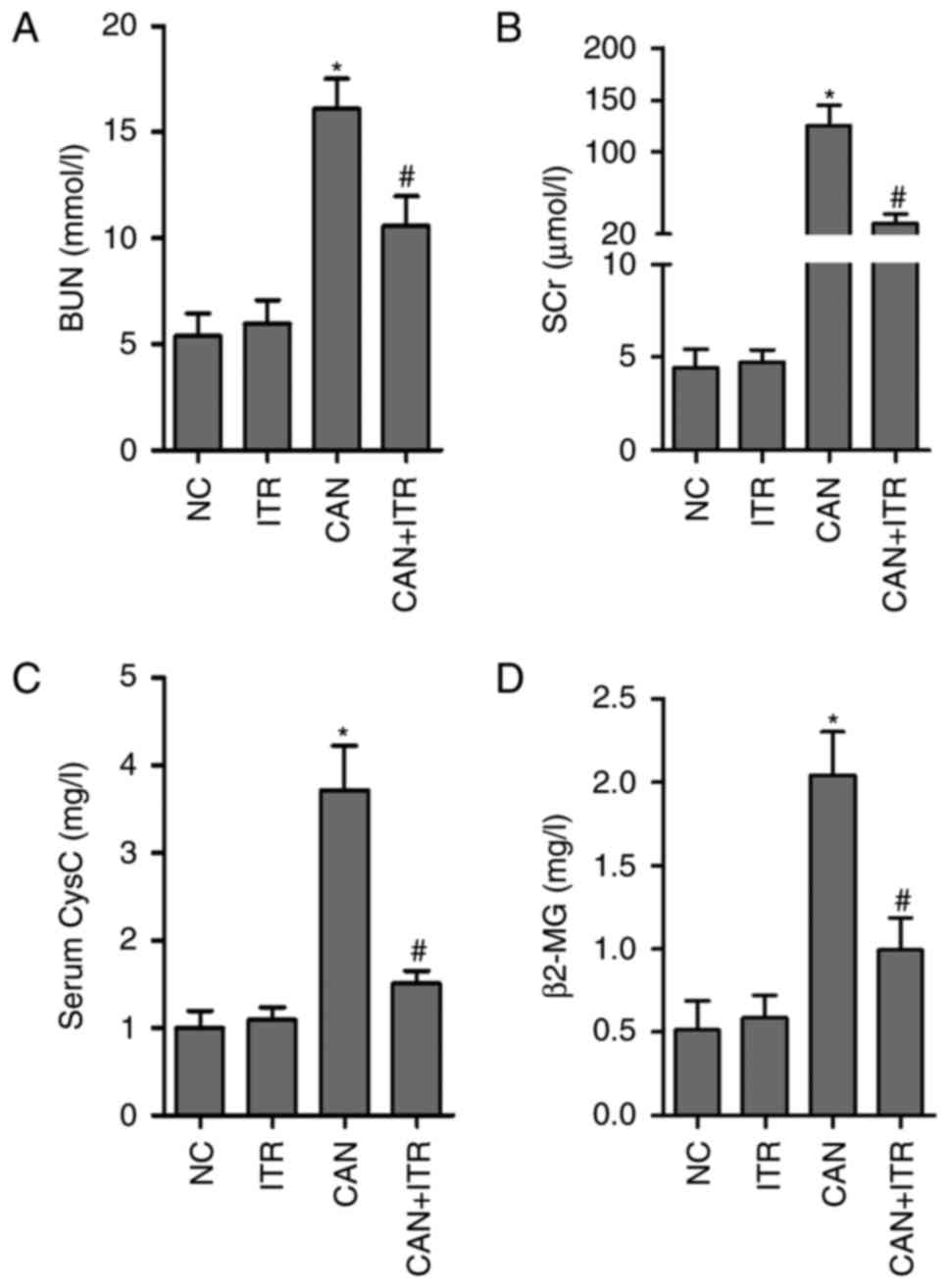
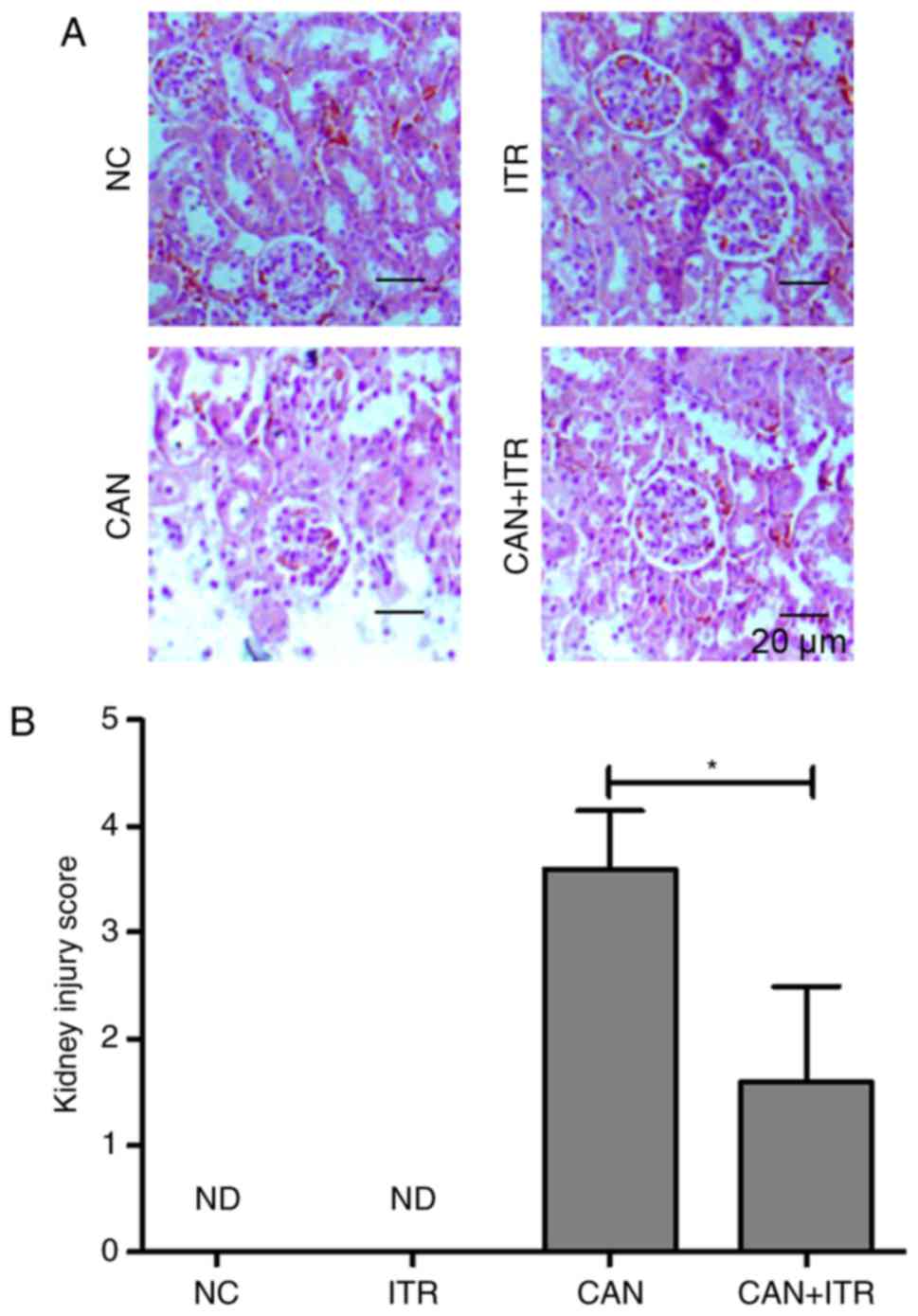
ITR alleviates candidiasis-induced
AKI
BUN is an indicator of kidney impairment, and SCr
represents a good indicator of the severity of renal impairment,
particularly of GFR (29). To
evaluate renal damage, BUN and SCr were measured in healthy and
septic mice with or without ITR treatment. As shown in Fig. 3A and B, candidiasis induced
significant increases in BUN and SCr levels, which were
significantly attenuated by treatment with ITR. In addition, the
present study analyzed the GFR by measuring CysC and serum β2-MG
under different treatment conditions. As shown in Fig. 3C and D, CysC and β2-MG levels were
significantly higher in the septic mice, compared with those in the
control group, however, ITR treatment significantly reduced the
increased levels of CysC and β2-MG in the septic mice. In
accordance with these data, improved renal pathological changes
were observed in the ITR-treated septic mice. As shown in Fig. 4A and B, the induction of
candidiasis produced focal and segmental hyperplasia with diffuse
mesangial proliferation and glomerular lesions. By contrast, the
damage was markedly attenuated in the ITR-treated group.
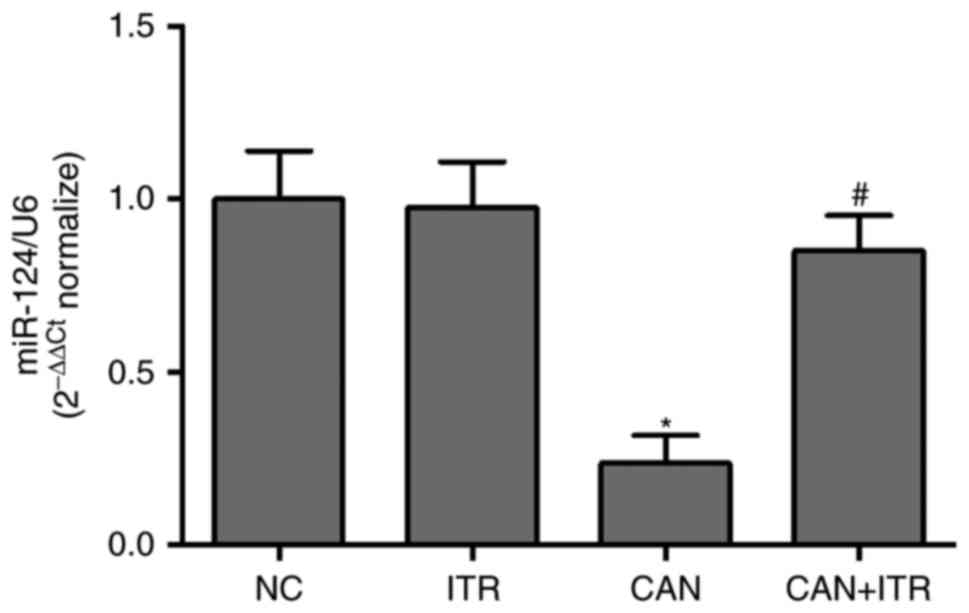
ITR regulates the expression of miR-124
in the kidneys of septic mice
Based on the experiments, MCP-1 was closely
associated with candidiasis-induced AKI. To investigate whether
MCP-1 may be regulated by miRs, online prediction software
(miRanda-mirSVR, miRDB and TargetScan) was used for prediction. It
was found that miR-124 was overlapping in the miRanda-mirSVR and
TargetScan databases, and miR-290-5p and miR-292-5p were
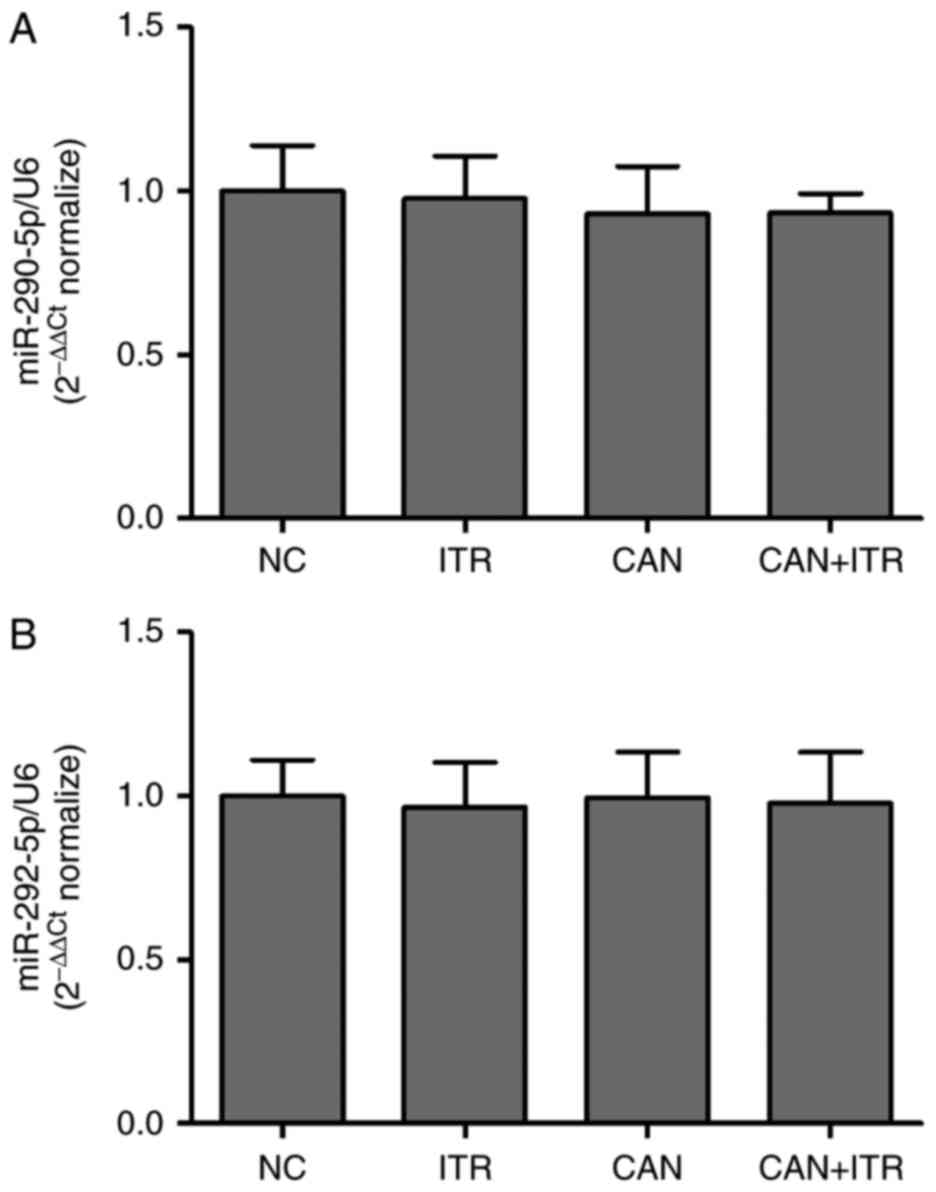
overlapping in the miRanda-mirSVR and miRDB databases. Therefore,
the expression levels of miR-124, miR-290-5p and miR-292-5p were
measured in the kidneys from septic mice. The results demonstrated
that the levels of miR-124 were significantly decreased in the
kidneys from the C. albicans-injected mice, compared with
those in the healthy mice (Fig.
5). However, there was no significant difference in miR-290-5p
or miR-292-5p between the CAN group and NC group (Fig. 6A and B). Of note, ITR-treated
reversed the candidiasis-induced downregulation of miR-124 in the
kidneys of the septic mice (Fig.
5), suggesting that the overexpression of miR-124 contributed
to the improved candidiasis-induced AKI. To investigate whether
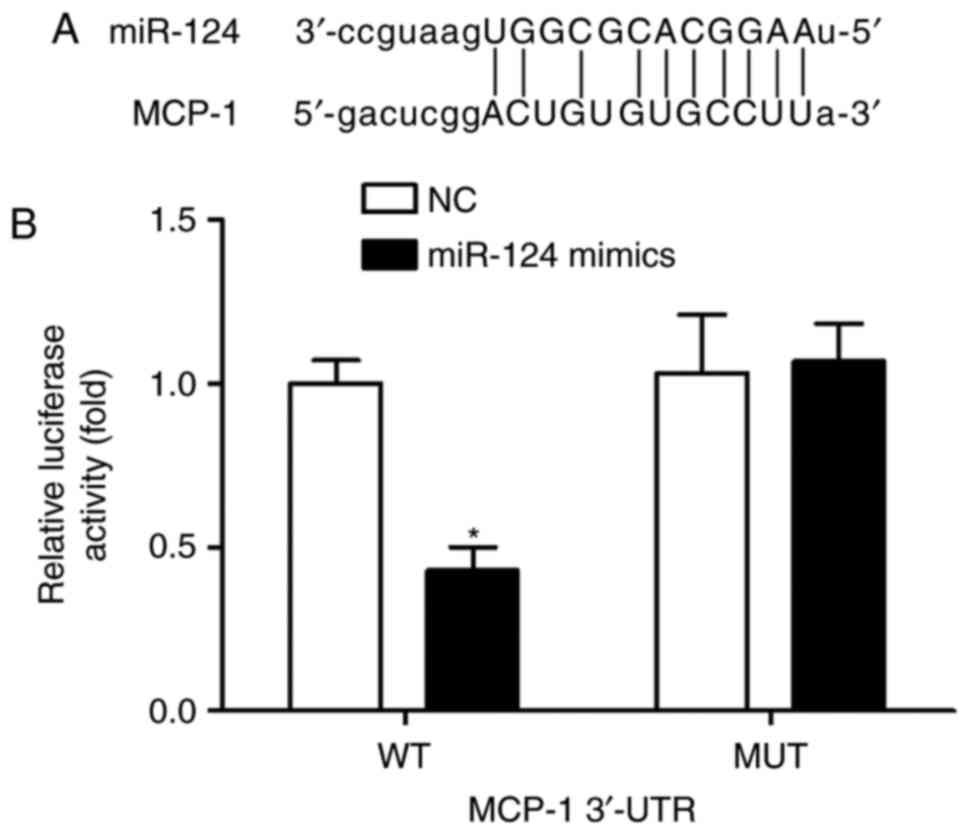
MCP-1 was a direct target of miR-124, the online prediction
software was used for prediction. The results showed that the
3′-UTR of MCP-1 contained one conserved binding site of miR-124
(Fig. 7A). To confirm this, a
luciferase activity assay was performed on the 293T cells. As shown
in Fig. 7B, transfection with
miR-124 mimics significantly reduced the luciferase activity
elicited by the luciferase vector carrying the complementary
sequence with the WT 3′-UTR of MCP-1. Following transfection with
miR-124 mimics, no significant difference was observed in
luciferase enzyme activity between the NC group and the reporter
vector group containing the MUT 3′-UTR of MCP-1. These findings
demonstrated that MCP-1 was a direct target gene of miR-124 by
binding with its 3′-UTR.
Overexpression of miR-124 improves
survival outcome following candidiasis
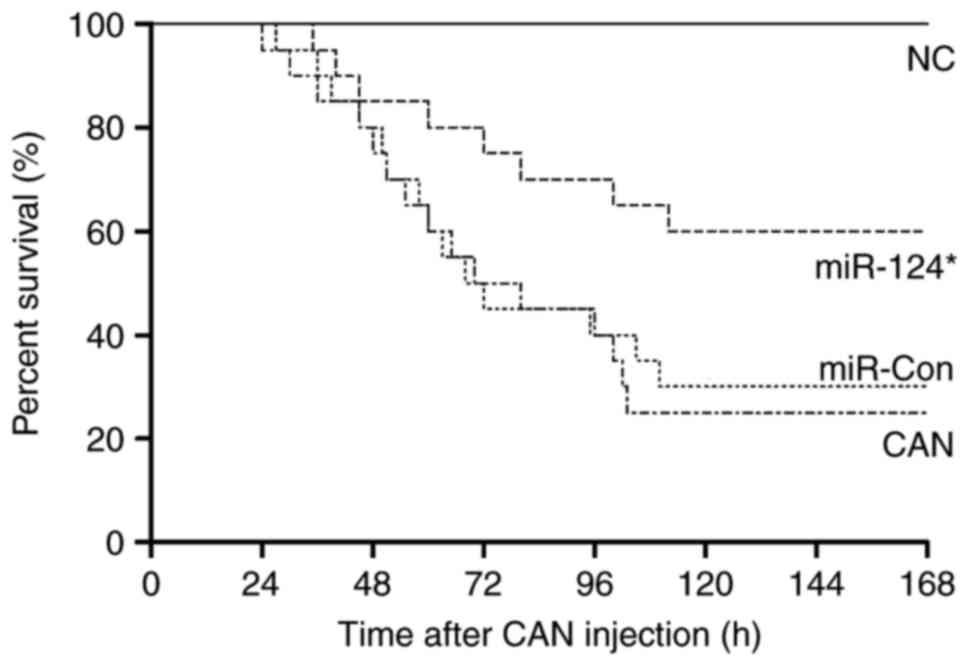
In septic mice, miR-Con transfection had no
significant effect on survival outcome. However, the septic mice
transfected with miR-124 mimics began to die at 35 h, with 60% of
the septic mice surviving for the duration of the investigation
(Fig. 8).
Intravenous injection of miR-124 inhibits
the expression of MCP-1, inflammatory cytokines and AKI in septic
mice
To investigate the effects of miR-124 on the
inflammatory response and sepsis-induced AKI in vivo, male
C57BL/6J mice were infected with miR-124 mimics and miR-Con via
tail-tail injection, and it was found that levels of miR-124 were
significantly increased in the kidneys, compared with those in the
NC group or miR-Con group (Fig.
9A). To determine the effects of miR-124 on inflammatory
signaling, the effects of the overexpression of miR-124 on MCP-1
were examined in the kidneys of the mice. The mRNA and protein
levels of MCP-1 were significantly inhibited in the kidneys of mice
transfected with miR-124, compared with those in the miR-Con group
(Fig. 9B-D). The effects of
miR-124 mimics on the serum levels of inflammatory cytokines
associated with MCP-1 were also examined in septic mice. It was
found that the serum levels of TNF-α, IL-1β and IL-6 were markedly
decreased by miR-124 mimics in septic mice (Fig. 9E). Furthermore, the miR-124 mimics
led to significant decreases in the levels of BUN and SCr in serum
of the septic mice (Fig. 9F and
G), suggesting that miR-124 may improve the inflammatory
response and AKI in septic mice through the inhibiting the
expression of MCP-1.
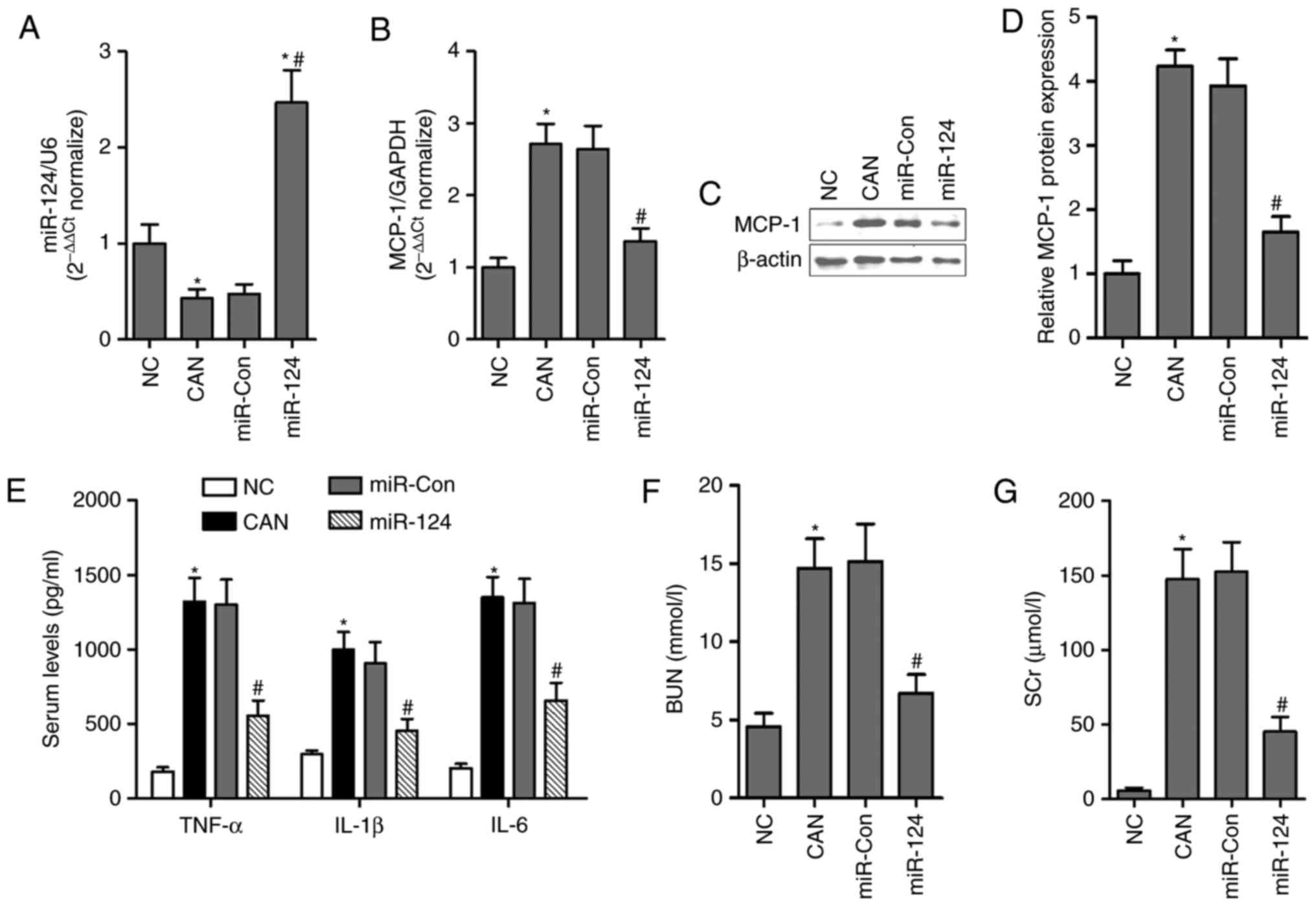 | Figure 9Intravenous injection of miR-124
inhibits the expression of MCP-1, inflammatory cytokines and AKI in
septic mice. (A) Levels of miR-124 were analyzed by RT-qPCR in the
kidneys of septic mice following adenovirus-delivered miR-124
transfection. mRNA and protein expression were analyzed by (B)
RT-qPCR and (C) western blot analyses in the kidneys of septic mice
with or without miR-124 treatment. (D) Quantification of blots. (E)
Serum levels of inflammatory cytokines, TNF-α, IL-1β and IL-6 were
measured using mouse enzyme-linked immunosorbent assay kits in
septic mice with or without miR-124 treatment. Levels of (F) BUN
and (G) SCr were measured in healthy and septic mice with or
without miR-124 treatment. *P<0.05 compared with the
NC group; #P<0.05 compared with the CAN group. miR,
microRNA; MCP-1, monocyte chemoattractant protein-1; TNF-α, tumor
necrosis factor-α; IL, interleukin; BUN, blood urea nitrogen; SCr,
serum creatinine; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; CAN, Candida albicans; NC,
negative control; Con, control. |
Discussion
Experimental and clinical data support that
candidiasis is a dominant factor for AKI (22,30). There are no effective therapies in
candidiasis-induced AKI. In order to improve clinical outcomes,
understanding the exact molecular mechanisms in candidiasis-induced
AKI is considered essential. The present study used a mouse model
of disseminated candidiasis, and the findings indicated that the
upregulation of MCP-1 levels and inhibition of miR-124 were
involved in candidiasis-induced AKI. In addition, a bioinformatics
prediction approach showed that miR-124 was targeted to MCP-1 by
binding with its 3′-UTR. The data revealed molecular insights into
the involvement of miR-124 and its direct target MCP-1 in
modulating sepsis-induced renal injury.
Previous studies have demonstrated that inflammation
is pivotal in the pathogenesis of AKI, and that MCP-1, which is
considered a key inducer of the pro-inflammatory response, has been
shown to be linked with renal perfusion, glomerular filtration and
glomerular lesions (31,32). Munshi et al showed that the
pathologic increase in the expression of MCP-1 originates from the
diseased kidney, and MCP-1 offers potential as a biomarker of AKI
(31). MCP-1 is known to
contribute to the toxic effect of LPS in vivo and in
vitro (33,34); therefore, attenuating a pathologic
increase in the expression of MCP-1 may be important in controlling
the occurrence and development of AKI. The present study provided
evidence that ITR possessed a nephroprotective effect against
candidiasis-induced toxicity via inhibiting the mRNA and protein
expression of MCP-1 in the kidney. In candidiasis-exposed mice,
significant increases in BUN, SCr and mortality rate were observed.
By contrast, treatment with ITR resulted in a significant decrease
of these parameters in septic mice. On histopathological
examination, it was also observed that renal lesions were reduced
significantly in septic mice receiving ITR treatment. Unexpectedly,
the administration of exogenous miR-124 was able to efficiently
restore and ameliorate the damaged kidney in candidemia mice, as
ITR, through the suppression of MCP-1 levels. In addition,
consistent with a previous study (35), miR-124 directly inhibited the
serum levels of TNF-α, IL-1β and IL-6 in septic mice. Therefore,
the evidence showed that miR-124 may be a potential therapeutic
target for the treatment of inflammatory diseases.
In the present study, it was demonstrated that
candidiasis increased the expression of MCP-1 in the kidney and
that this effect occurred with the downregulation of miR-124.
miR-124 is known to directly target MCP-1, and restoration of the
expression of miR-124 by lentiviral infection or formulated miR-124
injection inhibits oral tumor growth in vivo (36). miR-124 is also involved in the
amadori-glycated albumin-induced inflammatory effect in cultured
rat retinal ganglion cells and the inflammatory phenotype of
pulmonary vascular fibroblasts (37,38). The present study confirmed that
miR-124 regulated inflammatory signaling via an MCP-1-dependent
mechanism in septic mice, associated with renal protection. The
results provided confirmation of exogenous miR-124 as a novel
avenue for the treatment of AKI.
In our previous study, it was demonstrated that the
administration of exogenous miR-204 and miR-211 possessed the
ability to efficiently ameliorate candidiasis-induced AKI (22), and is a type of gene therapy that
has been well accepted in clinical practice (39). Zhou et al showed that the
systemic administration of miR-155 mimics attenuated cardiac
dysfunction and improved survival rates in late sepsis (40). The results of a study by Zheng
et al showed that the silencing of miR-195 reduced
multiple-organ injury and improved survival rates in sepsis
(41). All the evidence indicates
that exogenous miRs are involved in sepsis-induced inflammatory
activity and tissues lesions. In the present study,
adenovirus-delivered miR-124 increased by almost 2.5-fold in the
kidneys from septic mice, and the overexpression of miR-124 may
represent a novel therapeutic approach for sepsis-induced AKI.
Taken together, previous results and those of the
present study provide evidence that MCP-1 is involved in the
excessive inflammatory response induced by candidiasis in septic
mice. However, ITR had a potent nephroprotective effect on the
septic mouse model. In addition, an in vivo experiment
demonstrated that adenovirus-delivered miR-124 can be introduced
into the kidney to inhibit the expression of MCP-1 and the
inflammatory response. These results provide a novel perspective on
molecular-targeted therapy for candidiasis-induced AKI.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Leelahavanichkul A, Somparn P, Bootprapan
T, Tu H, Tangtanatakul P, Nuengjumnong R, Worasilchai N,
Tiranathanagul K, Eiam-ong S, Levine M, et al: High-dose ascorbate
with low-dose amphotericin B attenuates severity of disease in a
model of the reappearance of candidemia during sepsis in the mouse.
Am J Physiol Regul Integr Comp Physiol. 309:R223–R234. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pfaller MA and Diekema DJ: Epidemiology of
invasive candidiasis: A persistent public health problem. Clin
Microbiol Rev. 20:133–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lionakis MS, Swamydas M, Fischer BG,
Plantinga TS, Johnson MD, Jaeger M, Green NM, Masedunskas A,
Weigert R, Mikelis C, et al: CX3CR1-dependent renal macrophage
survival promotes Candida control and host survival. J Clin Invest.
123:5035–5051. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spellberg B, Ibrahim AS, Edwards JE Jr and
Filler SG: Mice with disseminated candidiasis die of progressive
sepsis. J Infect Dis. 192:336–343. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ngo LY, Kasahara S, Kumasaka DK, Knoblaugh
SE, Jhingran A and Hohl TM: Inflammatory monocytes mediate early
and organ-specific innate defense during systemic candidiasis. J
Infect Dis. 209:109–119. 2014. View Article : Google Scholar
|
|
6
|
Navarathna DH, Stein EV, Lessey-Morillon
EC, Nayak D, Martin-Manso G and Roberts DD: CD47 promotes
protective innate and adaptive immunity in a mouse model of
disseminated candidiasis. PLoS One. 10:e01282202015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lionakis MS, Fischer BG, Lim JK, Swamydas
M, Wan W, Richard Lee CC, Cohen JI, Scheinberg P, Gao JL and Murphy
PM: Chemokine receptor Ccr1 drives neutrophil-mediated kidney
immunopathology and mortality in invasive candidiasis. PLoS Pathog.
8:e10028652012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Castillo L, MacCallum DM, Brown AJ, Gow NA
and Odds FC: Differential regulation of kidney and spleen cytokine
responses in mice challenged with pathology-standardized doses of
Candida albicans mannosylation mutants. Infect Immun. 79:146–152.
2011. View Article : Google Scholar :
|
|
9
|
Imbert F, Jardin M, Fernandez C, Gantier
JC, Dromer F, Baron G, Mentre F, Van Beijsterveldt L, Singlas E and
Gimenez F: Effect of efflux inhibition on brain uptake of
itraconazole in mice infected with Cryptococcus neoformans. Drug
Metab Dispos. 31:319–325. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carrer DP, Samonis G, Droggiti DI,
Tsaganos T, Pistiki A and Giamarellos-Bourboulis EJ: Intravenous
itraconazole against experimental neutropenic Candida parapsilosis
infection: Efficacy after suppression of bacterial translocation. J
Infect Chemother. 19:1080–1086. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sharifzadeh A, Khosravi AR, Shokri H and
Tari PS: Synergistic anticandidal activity of menthol in
combination with itraconazole and nystatin against clinical Candida
glabrata and Candida krusei isolates. Microb Pathog. 107:390–396.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Inoue H, Iwasaki H, Abe S, Yamaguchi H and
Ueda T: Modulation of the human interleukin-12p40 response by a
triazole antifungal derivative, itraconazole. Scand J Infect Dis.
36:607–609. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wark PA, Hensley MJ, Saltos N, Boyle MJ,
Toneguzzi RC, Epid GD, Simpson JL, McElduff P and Gibson PG:
Anti-inflammatory effect of itraconazole in stable allergic
bronchopulmonary aspergillosis: A randomized controlled trial. J
Allergy Clin Immunol. 111:952–957. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oliveira AH, de Oliveira GG, Carnevale
Neto F, Portuondo DF, Batista-Duharte A and Carlos IZ:
Anti-inflammatory activity of Vismia guianensis (Aubl.) Pers
Extracts and antifungal activity against Sporothrix schenckii. J
Ethnopharmacol. 195:266–274. 2017. View Article : Google Scholar
|
|
15
|
Friccius H, Pohla H, Adibzadeh M,
Siegels-Hubenthal P, Schenk A and Pawelec G: The effects of the
antifungal azoles itraconazole, fluconazole, ketoconazole and
miconazole on cytokine gene expression in human lymphoid cells. Int
J Immunopharmacol. 14:791–799. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ohta K, Ishida Y, Fukui A, Nishi H, Naruse
T, Takechi M and Kamata N: Itraconazole inhibits TNF-α-induced
CXCL10 expression in oral fibroblasts. Oral Dis. 21:106–112. 2015.
View Article : Google Scholar
|
|
17
|
Naranjo TW, Lopera DE, Diaz-Granados LR,
Duque JJ, Restrepo A and Cano LE: Histopathologic and immunologic
effects of the itraconazole treatment in a murine model of chronic
pulmonary paracoccidioidomycosis. Microbes Infect. 12:1153–1162.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu J, Shi K, Chen M, Xu L, Hong J, Hu B,
Yang X and Sun R: Elevated miR-155 expression induces
immunosuppression via CD39(+) regulatory T-cells in sepsis patient.
Int J Infect Dis. 40:135–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan S, Yang X, Jia Y, Li Y, Chen R, Wang
M, Cai D and Zhao R: Intravenous injection of microvesicle-delivery
miR-130b alleviates high-fat diet-induced obesity in C57BL/6 mice
through translational repression of PPAR-γ. J Biomed Sci.
22:862015. View Article : Google Scholar
|
|
20
|
Ge QM, Huang CM, Zhu XY, Bian F and Pan
SM: Differentially expressed miRNAs in sepsis-induced acute kidney
injury target oxidative stress and mitochondrial dysfunction
pathways. PLoS One. 12:e01732922017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Monk CE, Hutvagner G and Arthur JS:
Regulation of miRNA transcription in macrophages in response to
Candida albicans. PLoS One. 5:e136692010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li XY, Zhang K, Jiang ZY and Cai LH:
MiR-204/miR-211 downregulation contributes to candidemia-induced
kidney injuries via derepression of Hmx1 expression. Life Sci.
102:139–144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Venturini J, de Camargo MR, Felix MC,
Vilani-Moreno FR and de Arruda MS: Influence of tumour condition on
the macrophage activity in Candida albicans infection. Scand J
Immunol. 70:10–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fan HY, Qi D, Yu C, Zhao F, Liu T, Zhang
ZK, Yang MY, Zhang LM, Chen DQ and Du Y: Paeonol protects
endotoxin-induced acute kidney injury: potential mechanism of
inhibiting TLR4-NF-κB signal pathway. Oncotarget. 7:39497–39510.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiao F, Huang Z, Li H, Yu J, Wang C, Chen
S, Meng Q, Cheng Y, Gao X and Li J: Leucine deprivation increases
hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways.
Diabetes. 60:746–756. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
He J, Chen Y, Lin Y, Zhang W, Cai Y, Chen
F, Liao Q, Yin Z, Wang Y, Tao S, et al: Association study of MCP-1
promoter polymorphisms with the susceptibility and progression of
sepsis. PLoS One. 12:e01767812017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Labbe K, Danialou G, Gvozdic D, Demoule A,
Divangahi M, Boyd JH and Petrof BJ: Inhibition of monocyte
chemoattractant protein-1 prevents diaphragmatic inflammation and
maintains contractile function during endotoxemia. Crit Care.
14:R1872010. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dorso L, Bigot-Corbel E, Abadie J, Diab M,
Gouard S, Bruchertseifer F, Morgenstern A, Maurel C, Chérel M and
Davodeau F: Long-term toxicity of 213Bi-labelled BSA in mice. PLoS
One. 11:e01513302016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kluger N, Kataja J, Aho H, Ronn AM, Krohn
K and Ranki A: Kidney involvement in autoimmune polyendocrinopath
y-candidiasis-ectodermal dystrophy in a Finnish cohort. Nephrol
Dial Transplant. 29:1750–1757. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Munshi R, Johnson A, Siew ED, Ikizler TA,
Ware LB, Wurfel MM, Himmelfarb J and Zager RA: MCP-1 gene
activation marks acute kidney injury. J Am Soc Nephro. 22:165–175.
2011. View Article : Google Scholar
|
|
32
|
Vianna HR, Soares CM, Silveira KD, Elmiro
GS, Mendes PM, de Sousa Tavares M, Teixeira MM, Miranda DM, Simões
E and Silva AC: Cytokines in chronic kidney disease: Potential link
of MCP-1 and dyslipidemia in glomerular diseases. Pediatr Nephrol.
28:463–469. 2013. View Article : Google Scholar
|
|
33
|
Yu C, Qi D, Sun JF, Li P and Fan HY: Rhein
prevents endotoxin-induced acute kidney injury by inhibiting NF-κB
activities. Sci Rep. 5:118222015. View Article : Google Scholar
|
|
34
|
Chen H, Zhu J, Liu Y, Dong Z, Liu H, Liu
Y, Zhou X, Liu F and Chen G: Lipopolysaccharide induces chronic
kidney injury and fibrosis through activation of mTOR signaling in
macrophages. Am J Nephrol. 42:305–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang D, Shi L, Xin W, Xu J, Xu J, Li Q, Xu
Z, Wang J, Wang G, Yao W, et al: Activation of PPARγ inhibits
pro-inflammatory cytokines production by upregulation of miR-124 in
vitro and in vivo. Biochem Biophys Res Commun. 486:726–731. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li X, Fan Q, Li J, Song J and Gu Y:
MiR-124 down-regulation is critical for cancer associated
fibroblasts-enhanced tumor growth of oral carcinoma. Exp Cell Res.
351:100–108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dong N, Xu B, Shi H and Tang X: Baicalein
inhibits amadori-glycated albumin-induced MCP-1 expression in
retinal ganglion cells via a microRNA-124-dependent mechanism.
Invest Ophthalmol Vis Sci. 56:5844–5853. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang D, Zhang H, Li M, Frid MG, Flockton
AR, McKeon BA, Yeager ME, Fini MA, Morrell NW, Pullamsetti SS, et
al: MicroRNA-124 controls the proliferative, migratory, and
inflammatory phenotype of pulmonary vascular fibroblasts. Circ Res.
114:67–78. 2014. View Article : Google Scholar :
|
|
39
|
Fischer A: Gene therapy: Repair and
replace. Nature. 510:226–227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou Y, Song Y, Shaikh Z, Li H, Zhang H,
Caudle Y, Zheng S, Yan H, Hu D, Stuart C and Yin D: MicroRNA-155
attenuates late sepsis-induced cardiac dysfunction through JNK and
β-arrestin 2. Oncotarget. 8:47317–47329. 2017.PubMed/NCBI
|
|
41
|
Zheng D, Yu Y, Li M, Wang G, Chen R, Fan
GC, Martin C, Xiong S and Peng T: Inhibition of microRNA 195
prevents apoptosis and multiple-organ injury in mouse models of
sepsis. J Infect Dis. 213:1661–1670. 2016. View Article : Google Scholar :
|