Introduction
Podocytes are crucial to maintain the normal
function of the glomerular filtration barrier. Several kidney
diseases, including minimal change disease, focal segmental
glomerular sclerosis and membranous nephropathy (1–3),
are associated with podocyte injury. Therefore, the treatment of
kidney diseases by protecting podocytes has become a popular
research topic.
Tripterygium wilfordii Hook F (TwHF), a
widely used Chinese herb, is a member of the Celastraceae family of
perennial vine-like plants. Tripterygium glycoside (TG), extracted
and purified from the root xylem of TwHF, is the active component
of TwHF. TG has anti-inflammatory and immunosuppressive effects,
and has been used extensively to treat autoimmune and inflammatory
diseases, including rheumatoid arthritis, systemic lupus
erythematosus and nephrotic syndrome (4,5).
For example, TG exhibited promising therapeutic effects on
idiopathic membranous nephropathy, which is one of the most common
causes of nephrotic syndrome in adults. The injury and apoptosis of
podocytes is associated with this typical kidney disease (6–8).
In another study of chronic kidney disease (CKD), a systematic
meta-analysis showed that therapy with tripterygium preparations
significantly decreased proteinuria and serum creatinine levels in
patients with CKD (9). However,
the mechanism underlying this therapeutic effect remains to be
fully elucidated.
In the present study, a puromycin aminonucleoside
(PAN)-induced podocyte injury model was used to evaluate the effect
of TG on podocyte injury. The study aimed to test the hypothesis
that TG protects against PAN-induced podocyte injury by
upregulating autophagy via the phosphatidylinositol 3-kinase
(PI3K)/AKT pathway.
Materials and methods
Reagents and antibodies
TG was purchased from Zhejiang Deende Pharmaceutical
Co., Ltd. (Zhejiang, China; cat. no. 14002219121). The Annexin V
Apoptosis Detection kit was purchased from eBioscience, Inc. (San
Diego, CA, USA; cat. no. 88-8007). The Cell Counting Kit-8 (CCK-8)
was purchased from Beyotime Institute of Biotechnology (Shanghai,
China; cat. no. C0038). Chloroquine (CQ) was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany; cat. no. C6628) and
LY294002 was purchased from Selleck Chemicals (Selleck, Houston,
TX, USA; cat. no. S1105). The antibodies used in the present study
included antibodies against microtubule-associated protein
1A/1B-light chain 3 (LC3)II (cat. no. 12135-1-AP), p62 (cat. no.
18420-1-AP) (both from Wuhan Sanying Biotechnology, Wuhan, China),
PI3K [cat. no. Ab151549; Abcam Trading (Shanghai) Co. Ltd.,
Shanghai, China], AKT (cat. no. Sc-5298; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), phosphorylated (p-)AKT (cat. no. AF0908),
caspase-3 (cat. no. 19677-1-AP) and cleaved-caspase-3 (cat. no.
25546-1-AP) (all from Wuhan Sanying Biotechnology).
Cell culture and drug treatment
Conditionally immortalized differentiated mouse
podocyte cells (MPC5) were provided by the Cell Resource Center of
the Shanghai Institute for Biological Sciences of the Chinese
Academy of Sciences (Shanghai, China). The podocytes were cultured
in Roswell Park Memorial Institute (RPMI)-1640 medium (cat. no.
SH30809.01B; HyClone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS; cat. no. 16000-044;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml
penicillin G, and 100 mg/ml streptomycin. The podocytes were
maintained and expanded at 33°C with 100 U/ml interferon-γ in
medium. For podocytes to acquire a differentiated phenotype, the
cells were grown under 'restrictive conditions' at 37°C. To induce
injury, podocytes were treated with PAN (50 µg/ml) for 24 h.
Different concentrations of TG (0.31, 0.63, 1.25, 2.5, 5 and 10
µg/ml) were added 1 h prior to PAN treatment. CQ (20
µmol/l) and LY294002 (20 µmol/l) pretreatment were
performed 1 h before PAN treatment.
Cell viability assay
Cell viability was measured using a CCK-8 assay
(cat. no. C0038; Beyotime Institute of Biotechnology). The
podocytes were seeded in 96-well plates at a concentration of
105 cells/ml. The cells were incubated with different
concentrations of TG (0.31, 0.63, 1.25, 2.5, 5 and 10 µg/ml)
at 37°C, 5% CO2 for 24 h. Following TG treatment, 10
µl of CCK-8 was added to each well, and the cells were
incubated at 33°C for 1 h. The absorbance was detected at 450 nm
using a microplate reader (Thermo Fisher Scientific, Inc., Waltham,
MA, USA).
Western blot analysis
The cells were washed three times with ice-cold
phosphate-buffered saline (PBS) and lysed with cell lysis buffer
(radioimmunoprecipitation assay; cat. no. R0010; Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China), and the total
proteins were extracted for western blot analysis. A BCA protein
assay (Thermo Fisher Scientific, Inc.) was used for protein
quantitation. The soluble material (50 µg) was subjected to
SDS-PAGE with a 7.0% acrylamide gel and transferred onto a
nitrocellulose membrane by electrophoretic transblotting for 90 min
using Trans-Blot SD (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The membranes were blocked at room temperature with 5% skim
milk in Tris-buffered saline Tween-20 for 1 h and then probed at
4°C overnight with the following primary antibodies: Anti-LC3II
(1:1,000), anti-p62 (1:500); anti-PI3K (1:600), anti-AKT (1:1,000),
anti-p-AKT (1:1,000) and anti-Caspase-3 (1:500). The membranes were
then incubated with the horseradish peroxidase-conjugated goat
anti-rabbit secondary antibody (1:1,000; cat. no. A0208; Beyotime
Institute of Biotechnology) for 1 h at room temperature. Following
enhanced chemiluminescence detection, the membranes were stripped
and proteins were rehybridized with anti-β-actin antibody (1:1,000,
cat. no. 4970) or anti-glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) antibody (1:1,000; cat. no. 5174) (both from CST Biological
Reagents Co., Ltd., Shanghai, China). The protein levels were
determined as the protein/β-actin or protein/GAPDH ratio to
minimize differences in sample loading. Blots were analyzed using
the ImageJ software version 1.8.0–112 (National Institutes of
Health, Bethesda, MD, USA).
Analysis of apoptosis by flow
cytometry
A fluorescein isothiocyanate-Annexin V Apoptosis
Detection kit (BD Biosciences, San Diego, CA, USA) was used to
analyze podocyte apoptosis. The cells were washed twice with cold
PBS, following which 1×106 cells were suspended in 195
µl of Annexin V-APC binding buffer. Subsequently, the
solution was transferred to a 5 ml culture tube, and 5 µl of
Annexin V and 5 µl of propidium iodide (PI) were added; the
mixture was gently vortexed and incubated for 15 min at room
temperature (25°C) in the dark. Subsequent flow cytometric analysis
(BD Biosciences) was performed within 1 h.
Transmission electronic microscopy
The cell culture media were centrifuged for 10 min
at 714 × g and 25°C The supernatant was discarded and the cells
were fixed in 2.5% glutaraldehyde, dehydrated with graded ethanol,
and embedded in Epon 812 using standard laboratory procedures.
Ultrathin sections (1 µm) were subsequently cut, mounted on
nickel grids, and stained with lead citrate for transmission
electron microscopy (JSM-IT300LV; JEOL, Ltd., Tokyo, Japan).
Statistical analysis
All statistical analyses were performed using SPSS
19.0 software (IBM SPSS, Armonk, NY, USA). The results are
expressed as the mean ± standard deviation. Group means were
compared using one-way analysis of variance followed by the least
significant difference test for independent data. All P-values were
two-tailed, and P<0.05 was considered to indicate a
statistically significant difference.
Results
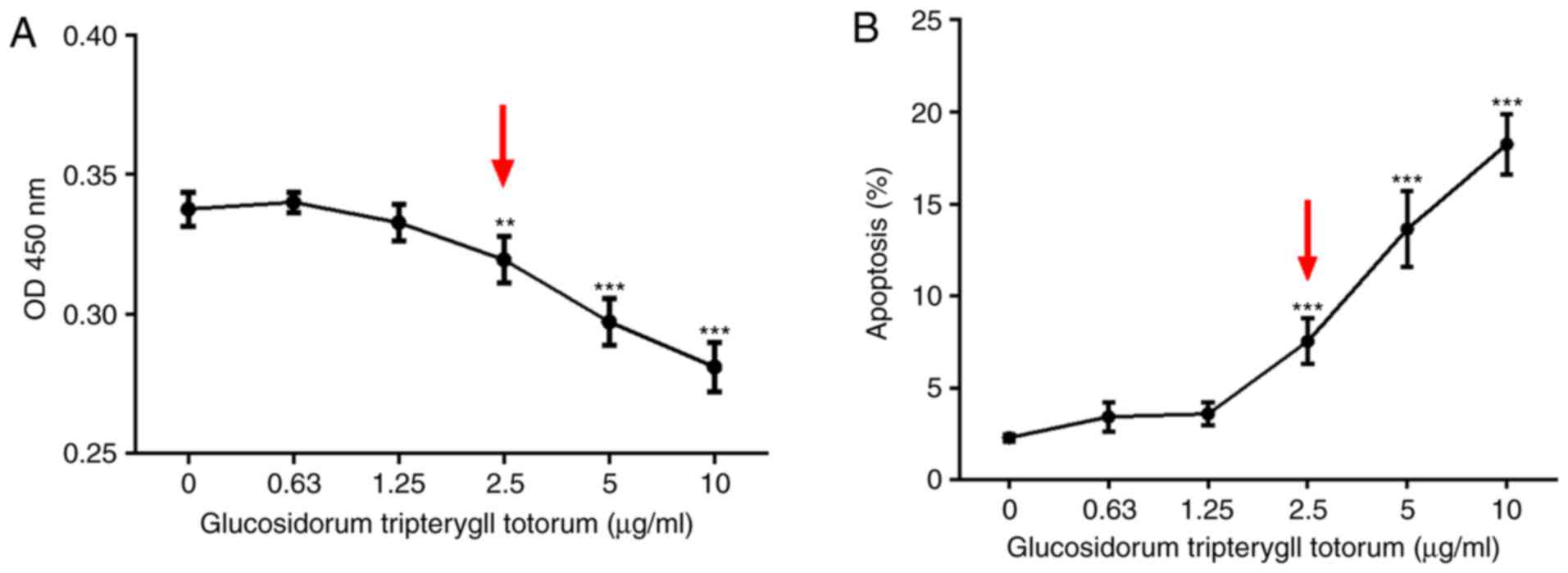
Cytotoxic effect of TG on podocytes
CCK-8 and Annexin V/PI assays was performed to
detect the cytotoxic effect of TG on the cultured podocytes. The
results showed that podocyte viability declined with increasing
concentrations of TG. At a concentration of 2.5 µg/ml,
podocyte viability was significantly decreased (Fig. 1A). Similarly, the percentage of
apoptotic cells was increased when treated with TG at 2.5
µg/ml (Fig. 1B). No
apparent effects of TG on the proliferation or apoptosis of
podocytes were observed below a concentration of 2.5
µg/ml.
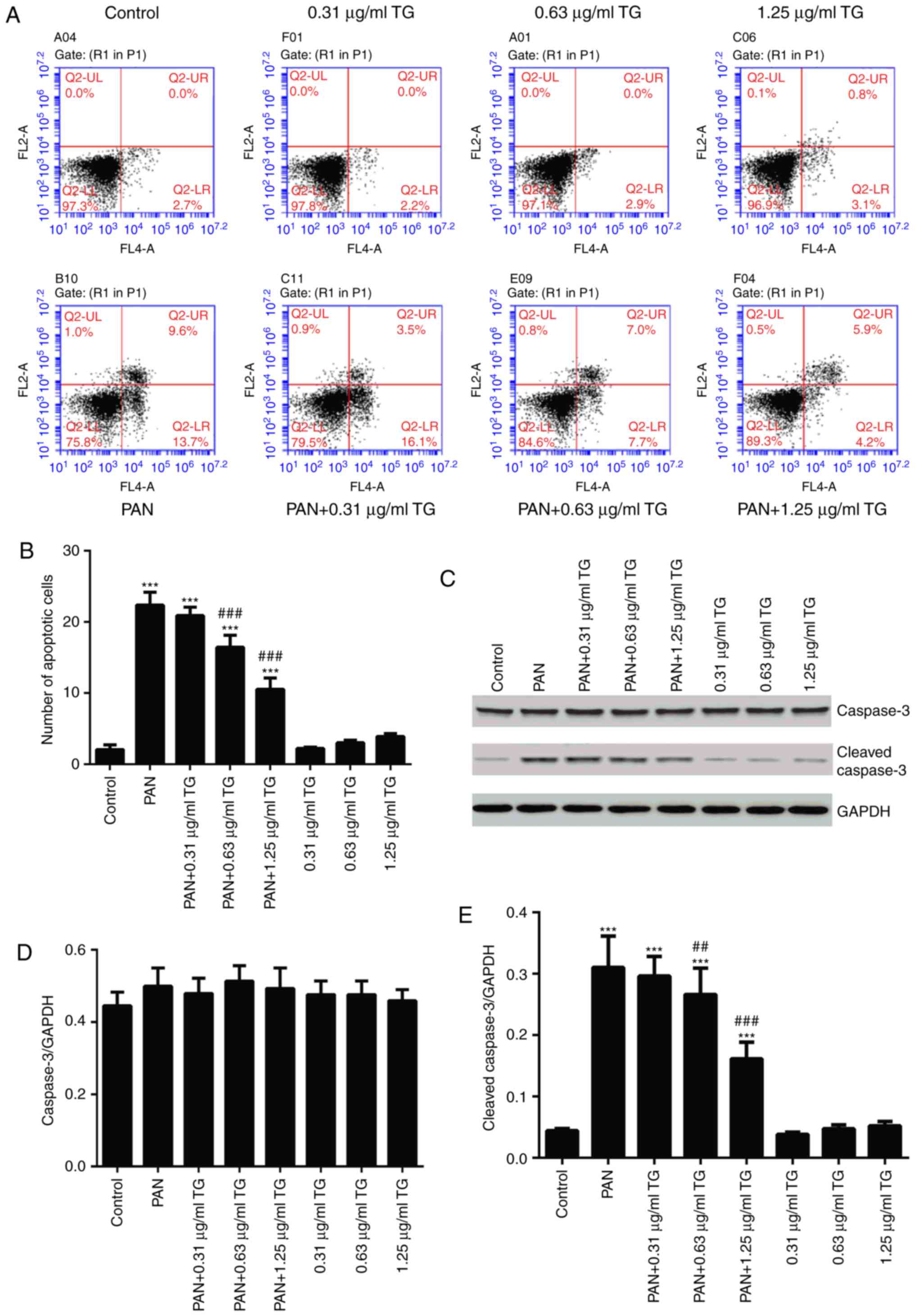
Protective effect of TG on PAN-induced
podocyte apoptosis
To observe the effects of TG on PAN-induced podocyte
apoptosis in the present study, podocyte apoptosis was examined
using flow cytometry and western blot analysis following treatment
with PAN and different concentrations of TG. The in vitro
experiments showed that, compared with the control group, the
number of apoptotic cells was significantly increased in the PAN
group (P<0.001). In the PAN+TG groups, apoptosis was inhibited
by TG when the dose of TG added was ≥0.63 µg/ml, compared
with that in the PAN group (Fig.
2A and B). The levels of cleaved-caspase-3 and caspase-3 were
also detected by western blot analysis to evaluate the apoptosis of
the podocytes in all groups. As shown in Fig. 2C–E, the level of cleaved-caspase-3
was increased significantly in the PAN-treated group, compared with
that in the control group, whereas TG treatment significantly
decreased the level of cleaved-caspase-3 in the PAN-treated
podocytes.
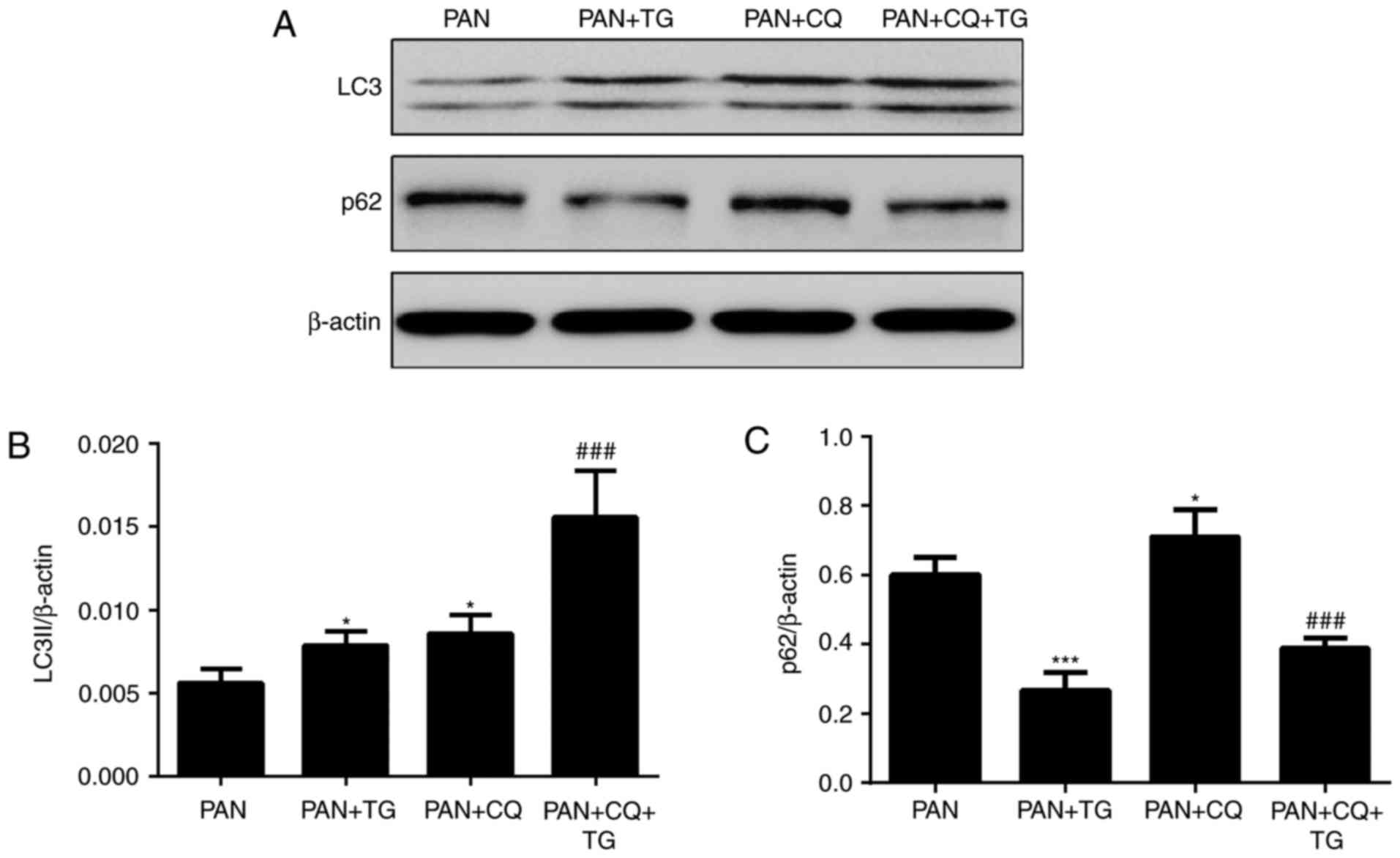
TG protects against podocyte injury by
upregulating cell autophagy
To evaluate the role of autophagy in the protective
effect of TG against PAN-induced podocyte injury, the expression of
autophagic markers, LC3II and p62, in podocytes were detected by
western blot analysis following treatment with PAN, TG and
chloroquine (CQ), a widely used inhibitor of autophagy. As shown by
the levels of LC3II and p62, a protein degraded by autophagy
pathways, autophagy was activated in the podocytes following
treatment by TG and was inhibited by CQ treatment (Fig. 3).
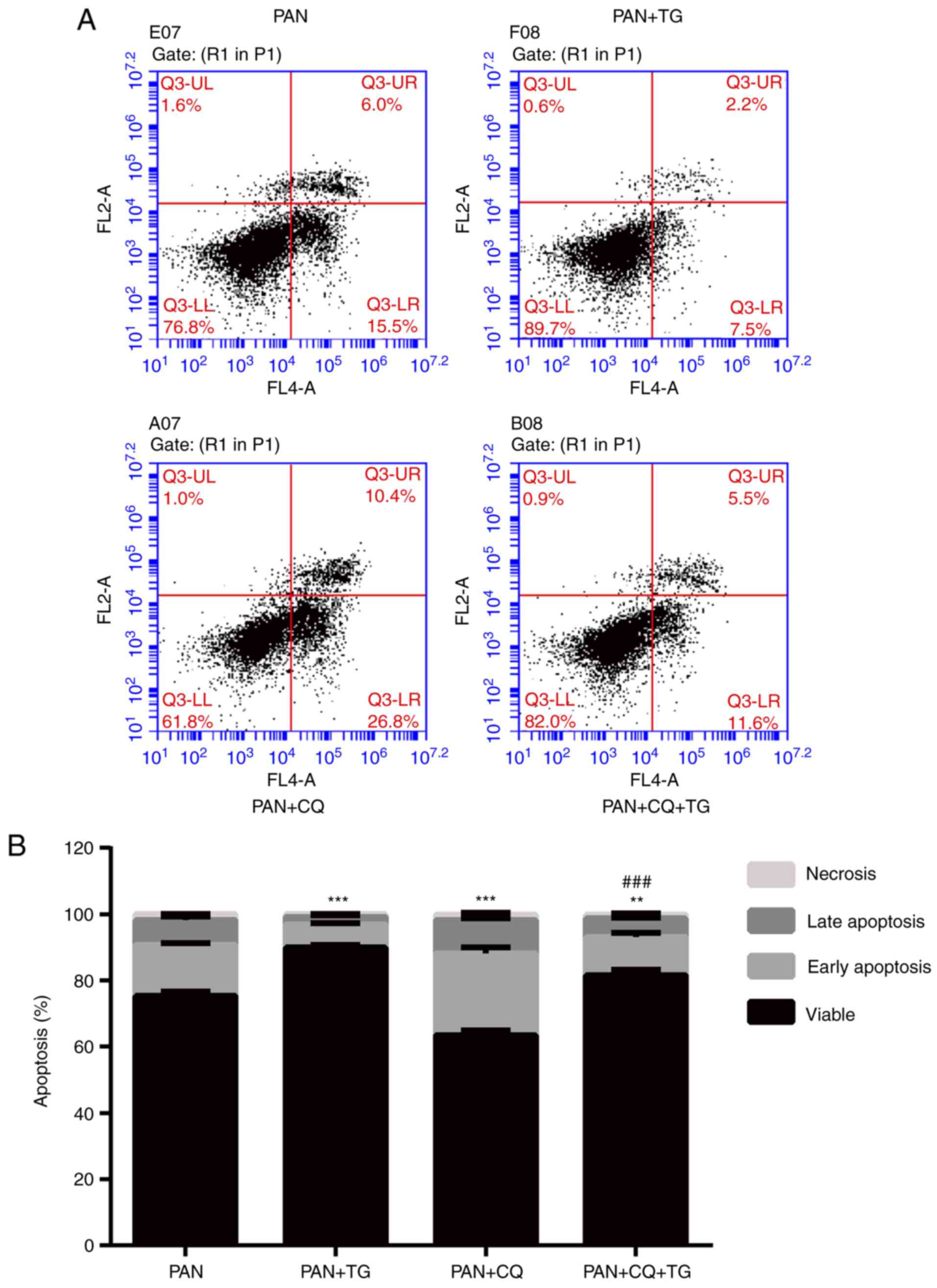
The apoptosis of podocytes was detected by Annexin
V/PI following treatment with PAN, TG and CQ. As shown in Fig. 4, the apoptotic rate of the
podocytes was negatively correlated with the level of autophagy.
The apoptosis of podocytes induced by PAN treatment was reversed by
TG treatment. When CQ was added, the protective effect of TG was
inhibited. Therefore, an apparent increase in apoptosis in the
PAN+CQ+TG group was observed. These results suggested that TG
protected the podocytes from apoptosis by upregulating
autophagy.
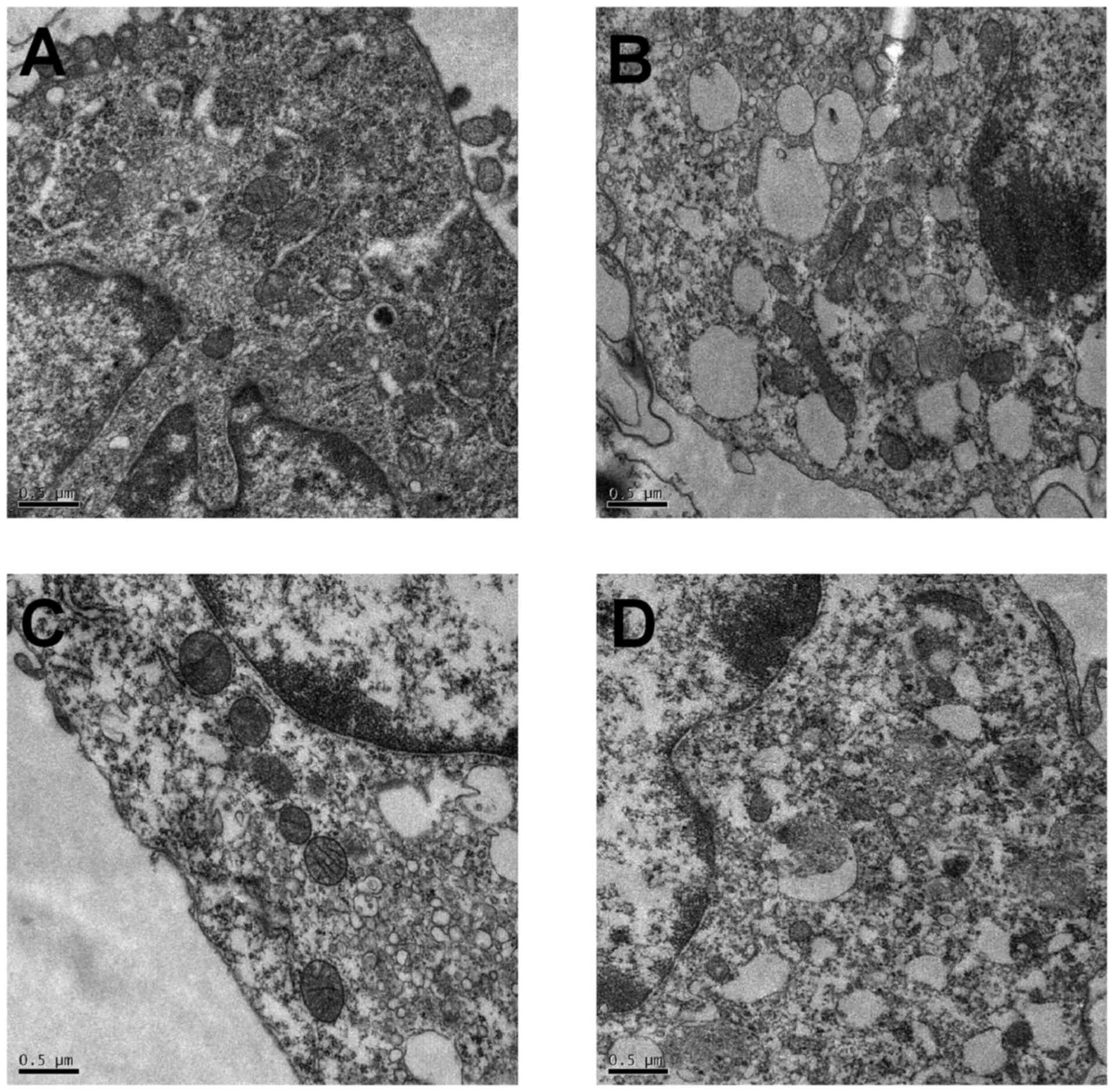
Assessment of autophagosomes by
transmission electron microscopy
Transmission electronic microscopy was used to
observe the effect of PAN and TG on autophagy. As shown in Fig. 5, in the control group, the
membrane surface of the podocytes had protrusions, the nuclei were
irregular, the endoplasmic reticulum structure was clear and
autophagosomes were visualized in the cytoplasm. However, in the
PAN-treated podocytes, the cytoplasm contained a large number of
vacuoles and few autophagosomes. The number of autophagosomes in
the cytoplasm of the podocytes increased when treated with TG.
TG induces autophagy by activating the
PI3K III signaling pathway
The class III PI3K inhibitor, LY294002, was used to
assess the relevance of PI3K pathway activation in the protective
effect of TG. The results of the western blot analysis showed that
the level of p-AKT was reduced by PAN treatment and was rescued by
TG treatment. The level of p-AKT was significantly reduced by
LY294002 treatment (Fig. 6A and
B) in the LY294002+PAN and LY294002+TG+PAN groups. However, no
statistically significant difference was found in the levels of AKT
when the podocytes were treated with PAN and TG (Fig. 6A and C). The levels of autophagy
makers, LC3II and p62, were also measured by western blot analysis.
The level of autophagy was also reduced by PAN treatment and
rescued by TG treatment. Treatment with LY294002 also decreased the
level of autophagy in the podocytes (Fig. 6A, D and E). The levels of
apoptosis of the podocytes were measured by Annexin V/PI analysis.
The protective effect of TG on PAN-induced podocyte injury was
counteracted by LY294002 treatment (Fig. 6F).
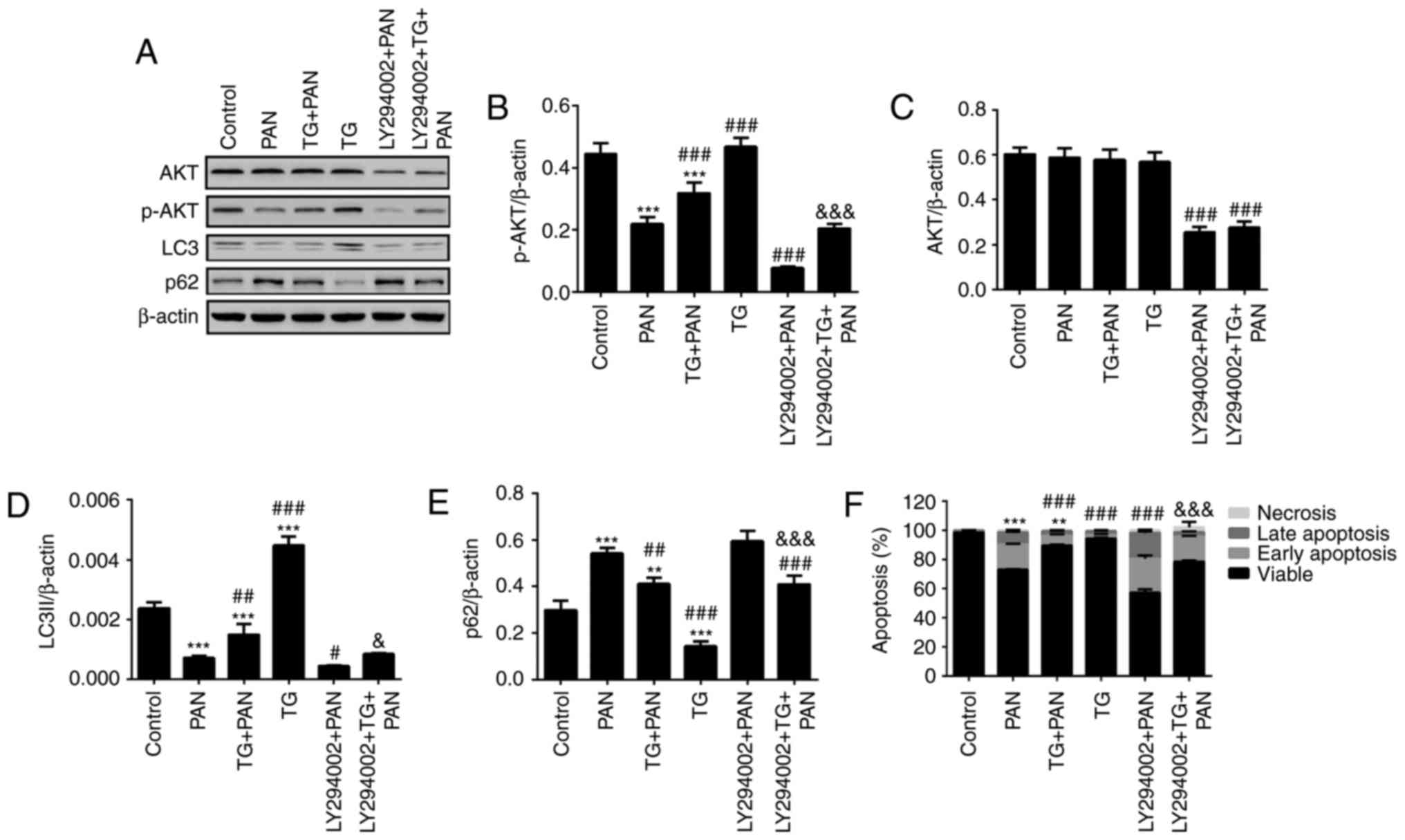 | Figure 6TG promotes the activation of
autophagy by PI3K signaling during podocyte injury. (A)
Representative bands of AKT, p-AKT, LC3 and p62 proteins in the
individual groups. Quantitative evaluation of the levels of (B)
p-AKT, (C) AKT, (D) LC3 and (E) p62 in the individual groups. The
expression of p-AKT was markedly inhibited by LY294002. The
expression of p-AKT was markedly increased in the TG+PAN and
LY294002+TG+PAN groups, compared with the expression in the PAN
group and LY294002+PAN group, respectively. TG promoted the
expression of LC3II and decreased the expression of p62. (F)
Apoptotic rates in the groups. Data are presented as the mean ±
standard deviation (n=3). **P<0.01 and
***P<0.001 vs. control group; #P<0.05,
##P<0.01 and ###P<0.001 vs. PAN group;
&P<0.05 and &&&P<0.001
vs. LY294002+PAN group. TG, tripterygium glycoside; PAN, puromycin
aminonucleoside; PI3K, phosphatidylinositol 3-kinase; p-,
phosphorylated; LC3, microtubule-associated protein 1A/1B-light
chain 3. |
Discussion
Previous studies have revealed that TG has
therapeutic effects against various nephropathies, including IgA
nephropathy, diabetic nephropathy and membranous nephropathy.
Treatment with the autophagy activator rapamycin has been reported
to reduce the progression of proteinuria and alleviate pathological
lesions in IgA nephropathy rats (10).
Podocytes, as post-mitotic differentiated cells,
show limited capacity for regeneration once they are damaged.
Podocyte injury can lead to marked proteinuria, and cause several
glomerulopathies, beginning with glomerulosclerosis and chronic
progression, and eventually leading to end-stage renal disease. The
protection of podocytes is one of the most important therapeutic
targets for nephrotic syndrome. A number of studies have been
performed to examine the therapeutic effect of TG on podocyte
injury (8,11); however, the mechanisms remain to
be fully elucidated.
In the present study, the classical PAN-induced
podocyte injury model was used to evaluate the effect of TG on
podocytes. As shown in a previous study (12), PAN induced the apoptosis of
podocytes. TG treatment resulted in a reduction in podocyte
apoptosis. Further investigation indicated that TG treatment
effectively upregulated autophagy and increased the number of
autophagosomes. In addition, the levels of PI3K/AKT pathway-related
proteins were detected in the various groups, and the results
revealed that TG treatment significantly increased the level of
p-AKT. This suggested that activation of the PI3K/AKT pathway may
be important in the regulation of autophagy and in the protective
effect of TG in podocyte injury.
Autophagy, a major intracellular lysosomal
degradation system, performs homeostatic functions linked to
metabolism and organelle turnover. The renoprotective functions of
autophagy in podocytes are predominantly mediated by the clearance
of affected mitochondria and the removal of protein aggregates,
which may trigger inflammation and cell death (13). A previous study suggested that the
podocyte foot processes were widely effaced following the
prevention of normal autophagic pathways in the nephrons of mice
(14). Another study showed that
the activation of autophagy ameliorated human podocyte injury
induced by high glucose (15). In
the present study, it was shown that TG protected podocytes from
apoptosis, which was accompanied by increased autophagic activity,
as evidenced by an increase in the level of LC3 and a reduction in
the level of p62 (Figs. 3 and
4). The effect of TG in
upregulating autophagy was confirmed by transmission electronic
microscopy. As shown in Fig. 5,
the number of autophagosomes in the cytoplasm of podocytes
increased significantly when the cells were treated with TG.
The present study also revealed that the level of
cellular apoptosis decreased following TG treatment in a
dose-dependent manner within the safe concentration range (Figs. 1 and 2). The podocytes were treated with
different concentrations of TG and no marked induction of apoptosis
was observed in the normal podocytes when the concentration of TG
was <2.5 µg/ml, whereas TG markedly inhibited PAN-induced
podocyte apoptosis. These results suggested that the optimal dose
of TG was able to ameliorate the injury induced by PAN. CQ, a
well-known inhibitor of autophagy, was used to verify the role of
TG-induced autophagy. Analysis of the protein levels of LC3-II and
p62 revealed that CQ inhibited TG-induced autophagy. TG and CQ
increased the levels of LC3; however, CQ inhibited the binding
process of autophagosomes and their substrate proteins, resulting
in the accumulation of p62. Furthermore, apoptosis increased when
CQ inhibited autophagy in the PAN group at 24 h. In particular,
early apoptosis significantly increased, which may be associated
with the duration of cultivation. However, the rates of apoptosis
and necrosis were markedly alleviated by TG, and accompanied by the
activation of autophagy. It has been reported that autophagy
eliminates dysfunctional mitochondria to decrease the generation of
cytochrome c, which activates apoptosis (16). Autophagy maintains the function of
the endoplasmic reticulum via the digestion of protein aggregates
and misfolded proteins, thereby inhibiting apoptosis (17). In the future, investigations aim
to examine how TG upregulates autophagy and protects against
podocyte apoptosis.
PAN increased the generation of reactive oxygen
species and activated the cytochrome c-caspase-9-caspase-3
apoptotic signaling pathway (18,19). The cysteinyl aspartate specific
proteinase (caspase) family of proteases are pivotal in the
execution of apoptosis. They function as initiators and
executioners in the process of apoptosis. Caspase-3, a typical
effector caspase, is responsible for the cleavage of a number of
death substrates and leads to cell death (20). The present study showed that TG at
a concentration of 1.25 µg/ml markedly alleviated
PAN-induced podocyte apoptosis, accompanied by a reduction in
cleaved-caspase-3. This indicated that inhibiting the activation of
caspase-3 may be involved in the anti-apoptotic effect of TG.
The PI3K/AKT signaling pathway, a well-known cell
survival pathway, is critical in the regulation of cell autophagy
and apoptosis (21,22). To determine the contribution of
the PI3K/AKT signaling pathway in the TG-mediated anti-apop-totic
effect in podocytes, the present study examined the phosphorylation
and expression of AKT. The results showed that PAN downregulated
the level of p-AKT, accompanied by a reduction in LC3 and an
increase in p62, whereas TG significantly upregulated the level of
p-AKT and the activation of autophagy (Fig. 6). LY294002, a classical PI3K
inhibitor, significantly downregulated the levels of PI3K, AKT and
p-AKT, and inhibited the autophagic activity of the podocytes.
Prospectively, TG significantly improved the inhibition of
autophagy and decreased the apoptosis of podocytes (Fig. 6). These data demonstrated that the
PI3K/AKT pathway is important in the regulation of TG-mediated
podocyte autophagy. In the present study, no significant difference
in the level of total AKT was found between cells treated with TG
and the untreated cells. This suggested that TG promoted the
phosphorylation of AKT, rather than promoting the formation of AKT,
when it mediates the autophagy of podocytes.
In conclusion, the findings of the present study
indicated that TG provided protection against podocyte injury via
apoptosis, and that this effect was mediated by the concomitant
activation of autophagy. The PI3K/AKT pathway was shown to be
important in the regulation of autophagy and apoptosis. These
findings provide novel insights into understanding the effects of
TG. In addition to its anti-inflammatory effect, TG can reduce
proteinuria and serum creatinine levels in CKD; the results of the
present study identified novel therapeutic targets for the
treatment of podocytopathy and glomerular diseases.
Acknowledgments
Not applicable.
References
|
1
|
Cara-Fuentes G, Clapp WL, Johnson RJ and
Garin EH: Pathogenesis of proteinuria in idiopathic minimal change
disease: Molecular mechanisms. Pediatr Nephrol. 31:2179–2189. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mallipattu SK and He JC: The podocyte as a
direct target for treatment of glomerular disease. Am J Physiol
Renal Physiol. 311:F46–F51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagata M: Podocyte injury and its
consequences. Kidney Int. 89:1221–1230. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu X, Li QJ, Xia S, Wang MM and Ji W:
Tripterygium glycosides for treating late-onset rheumatoid
arthritis: A systematic review and meta-analysis. Altern Ther
Health Med. 22:32–39. 2016.PubMed/NCBI
|
|
5
|
Chen Y, Gong Z, Chen X, Tang L, Zhao X,
Yuan Q and Cai G: Tripterygium wilfordii Hook F (a traditional
Chinese medicine) for primary nephrotic syndrome. Cochrane Database
Syst Rev. 8:CD0085682013.
|
|
6
|
Petermann AT, Krofft R, Blonski M,
Hiromura K, Vaughn M, Pichler R, Griffin S, Wada T, Pippin J,
Durvasula R, et al: Podocytes that detach in experimental
membranous nephropathy are viable. Kidney Int. 64:1222–1231. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu S, Li X, Li H, Liang Q and Chen J and
Chen J: Comparison of tripterygium wilfordii multiglycosides and
tacrolimus in the treatment of idiopathic membranous nephropathy: A
prospective cohort study. BMC Nephrol. 16:2002015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen ZH, Qin WS, Zeng CH, Zheng CX, Hong
YM, Lu YZ, Li LS and Liu ZH: Triptolide reduces proteinuria in
experimental membranous nephropathy and protects against
C5b9-induced podocyte injury in vitro. Kidney Int. 77:974–988.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu B, Wang Y, Jardine M, Jun M, Lv JC,
Cass A, Liyanage T, Chen HY, Wang YJ and Perkovic V: Tripterygium
preparations for the treatment of CKD: A systematic review and
meta-analysis. Am J Kidney Dis. 62:515–530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu D, Liu Y, Chen G, He L, Tang C, Wang
C, Yang D, Li H, Dong Z and Liu H: Rapamycin enhances repressed
autophagy and attenuates aggressive progression in a rat model of
IgA nephropathy. Am J Nephrol. 45:293–300. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hao L, Pan MS, Zheng Y and Wang RF: Effect
of Cordyceps sinensis and Tripterygium wilfordii polyglycosidium on
podocytes in rats with diabetic nephropathy. Exp Ther Med.
7:1465–1470. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu SY and Qi R: Role of bad in podocyte
apoptosis induced by puromycin aminonucleoside. Transplant Proc.
45:569–573. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fougeray S and Pallet N: Mechanisms and
biological functions of autophagy in diseased and ageing kidneys.
Nat Rev Nephrol. 11:34–45. 2015. View Article : Google Scholar
|
|
14
|
Kawakami T, Gomez IG, Ren S, Hudkins K,
Roach A, Alpers CE, Shankland SJ, D'Agati VD and Duffield JS:
Deficient autophagy results in mitochondrial dysfunction and FSGS.
J Am Soc Nephrol. 26:1040–1052. 2015. View Article : Google Scholar :
|
|
15
|
Xin W, Li Z, Xu Y, Yu Y, Zhou Q, Chen L
and Wan Q: Autophagy protects human podocytes from high
glucose-induced injury by preventing insulin resistance.
Metabolism. 65:1307–1315. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goldman SJ, Taylor R, Zhang Y and Jin S:
Autophagy and the degradation of mitochondria. Mitochondrion.
10:309–315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fernández A, Ordóñez R, Reiter RJ,
González-Gallego J and Mauriz JL: Melatonin and endoplasmic
reticulum stress: Relation to autophagy and apoptosis. J Pineal
Res. 59:292–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu L, Liu Y, Wu Y, Liu Q, Feng J, Gu X,
Xiong Y, Fan Q and Ye J: Smad3/Nox4-mediated mitochondrial
dysfunction plays a crucial role in puromycin
aminonucleoside-induced podocyte damage. Cell Signal. 26:2979–2991.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ha TS, Park HY, Seong SB and Ahn HY:
Puromycin aminonucleoside increases podocyte permeability by
modulating ZO-1 in an oxidative stress-dependent manner. Exp Cell
Res. 340:139–149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Portt L, Norman G, Clapp C, Greenwood M
and Greenwood MT: Anti-apoptosis and cell survival: A review.
Biochim Biophys Acta. 1813:238–259. 2011. View Article : Google Scholar
|
|
21
|
Zhao GX, Pan H, Ouyang DY and He XH: The
critical molecular interconnections in regulating apoptosis and
autophagy. Ann Med. 47:305–315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heras-Sandoval D, Pérez-Rojas JM,
Hernández-Damián J and Pedraza-Chaverri J: The role of
PI3K/AKT/mTOR pathway in the modulation of autophagy and the
clearance of protein aggregates in neurodegeneration. Cell Signal.
26:2694–2701. 2014. View Article : Google Scholar : PubMed/NCBI
|