Introduction
Bone defects are caused by several clinical
conditions, including cancer, trauma, congenital malformation, and
infection (1). The costs of
medical treatment, together with the loss of income of those with
these musculoskeletal diseases, in the United States of America are
estimated to be $849,000,000,000, which is ~7.7% of the gross
domestic product from 2002 to 2004 (1). In addition, suitable treatment
options that promote bone healing are still lacking (2). Although autograft is the gold
standard for the treatment of bone defects at present, this
approach has two major drawbacks, namely the limited number of
donor sites and the occurrence of donor site morbidity (3). Furthermore, bone regenerative
ability can also be limited with increase in age (4) and by certain conditions, including
arthritis and osteoporosis (5,6).
Therefore, those patients who require bone grafts the most may not
have the autologous stem cells required for autografting.
Therefore, transplantation of allogeneic stem cells is an
attractive option for engineering bone tissue.
Previous studies have shown that tissues can be
regenerated through tissue engineering approaches (7), and osteogenic differentiation and
bone regeneration can be achieved with stem cells (8). Bone marrow-derived mesenchymal stem
cells (BMSCs) were first separated in the 1970s, and are the source
of several mesenchymal tissues, including fat, bone, and cartilage
(9,10). BMSCs are a type of multipotent
mesenchymal stem cell found in bone marrow. They have a high
capacity for self-renewal and the potential to differentiate into
various cell types, and have long been considered a valuable source
of cells for bone tissue engineering (11-14). Therefore, BMSCs have been
identified as an important source of osteogenic cells for bone
tissue engineering.
Transforming growth factor β1 (TGF-β1) belongs to
the TGF-β superfamily of proteins, which regulate cell growth and
differentiation. The TGF-β family includes activins, inhibins,
Müllerian inhibitory substance, and bone morphogenetic proteins.
Early studies showed that TGF-β is important in inflammation,
tissue repair, and embryonic development (15,16). Subsequent studies have shown that
TGF-β is also crucial in cell growth, differentiation and immune
function (17,18). TGF-β1 as a signal molecule is
important in bone regeneration, and the importance of TGF-β1 in the
migration, growth and differentiation of BMSCs has been established
(19). However, the effects of
TGF-β1 in combination with BMSCs on bone repair remain to be fully
elucidated.
The present study showed that bone formation was
highest in femoral defects treated with TGF-β1-overexpressing BMSCs
of all the experimental groups, and that the combination of BMSCs
and TGF-β1 may be a potential novel therapeutic option for femoral
defects.
Materials and methods
Experimental animals and generation of
the femoral defect model
A total of 42 New Zealand white rabbits (male),
weighing 2.0-2.5 kg each, aged 2-3 months, were purchased from the
Experimental Animal Center at Guangzhou University of Chinese
Medicine (Guangzhou, China). All animal experiments were performed
in accordance with the policies and principles in the Guide for
Care and Use of Laboratory Animals (20). In addition, rabbits were housed as
previously described (20). A
rabbit femoral bone defect model was generated using a custom-made
distraction support, as previously reported (21). Briefly, the rabbit was
anesthetized with sodium pentobarbital (25 mg/kg) and xylazine (8
mg/kg). A 2-mm Kirschner wire was used to penetrate the double bone
cortex (~20 mm), and was fixed with a tension bracket 200 mm from
the skin. The rectus femoris from the vastus lateralis muscle was
then bluntly separated, and the medial rectus muscle was opened,
and a 14-mm defect in length was generated in the femur.
Femoral samples were collected from the rabbits,
which were randomly divided into three experimental groups of 14
rabbits each: Control group (phosphate-buffered saline, PBS), BMSC
group, and BMSC+TGF-β1 group. An osteotomy was generated (1.4 cm in
length), and the speed and quality of new bone formation was then
observed and assessed. The established rabbit femoral defect models
were then treated with PBS, BMSCs, or TGF-β1-overexpressing BMSCs
for 2 or 6 weeks. The study was approved by the Animal Care and Use
Committee of Guangzhou University of Chinese Medicine.
Isolation, culture and verification of
rabbit BMSCs
The BMSCs were obtained from the rabbit tibiae and
femora. Density gradient centrifugation (800 × g, 5 min, 22°C) and
adherent screening were used to isolate BMSCs, as previous
described (10). The tibiae and
femora were flushed with Low glucose-Dulbecco's modified Eagle's
medium (L-DMEM) (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and the BMSCs were cultured in L-DMEM
supplemented with 10% fetal bovine serum (Invitrogen; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin, and incubated
with 5% CO2 at 37°C. BMSCs from the third passage were
used for all experiments in the present study.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from rabbit BMSCs using
TRIzol reagent (cat. no. 15596026, Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol. The TGF-β1 RT reaction was performed with the TaqMan RNA
Reverse Transcription kit (cat. no. 4366596; Applied Biosystems;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. cDNA was reverse-transcribed from 2 µg total RNA
using a Reverse Transcription kit (Takara, Biochemical, Tokyo,
Japan). cDNA was then amplified using SYBR GREEN PCR Master mix
(cat. no. 638320, Takara Biotechnology Co., Ltd., Dalian, China)
according to the manufacturer's protocols and analyzed with an
ABI7500 Real-time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Following the reaction, the Cq value was
obtained, and the results were analyzed using the 2−ΔΔCq
method (22). The expression of
TGF-β1 was normalized to the expression of glyceraldehyde
3-phosphate dehydrogenase (GAPDH). All data are presented as
the mean ± standard deviation of three independent experiments. The
qPCR cycling conditions were as follows: Pre-denaturing at 95°C for
10 min, followed by 40 cycles of denaturing at 95°C for 10 sec,
annealing at 58°C for 20 sec, and extension at 72°C for 10 sec. The
specific PCR primers used were as follows: GAPDH, forward
5′-ATG TCG TGG AGT CTA CTG GC-3; and reverse 5′-TGA CCT TGC CCA CAG
CCT TG-3′; TGF-β1, forward 5′-CAG CAA CAA TTC CTG GCG ATA-3′
and reverse 5′-AAG GCG AAA GCC CTC AAT TT-3′.
Western blot analysis
Proteins were extracted from the rabbit BMSCs with
radio-immunoprecipitation assay buffer (cat. no. R0278;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) containing Protease
Inhibitor Cocktail (Thermo Fisher Scientific, Inc.). The total
protein concentration was determined using the protein assay kit
(Qcbio Science Technologies Co., Ltd., Shanghai, China). Total
proteins (30 µg) were separated by electrophoresis with 10%
SDS-PAGE and transferred onto a polyvinylidene fluoride (EMD
Millipore) membrane. The membrane was blocked with 5% skim milk and
incubated with the respective primary antibodies at 4°C overnight.
The membrane was washed and incubated with horseradish peroxidase
(HRP)-conjugated secondary antibody (1:5,000; cat. no. 115-035-003;
Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) the
following day at room temperature for 2 h, and the blots were
analyzed by enhanced chemiluminescence (ECL) using the ECL
substrate kit (Amersham; GE Healthcare Life Sciences, Chalfont,
UK). The primary antibodies used were as follows: Anti-TGF-β1
(1:1,000; cat. no. ab66043; Abcam, Cambridge, UK) and anti-GAPDH
(cat. no. FL-335, an internal loading control, 1:2,000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA).
Enzyme-linked immunosorbent assay
(ELISA)
The culture supernatant was harvested from rabbit
BMSCs transfected with the TGF-β1 construct or from non-transfected
BMSCs, which served as the negative control (NC). The activity of
alkaline phosphatase (ALP) in the culture medium was detected using
the ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA)
according to the manufacturer's protocol. A 96-well plate was
coated with a monoclonal anti-ALP antibody (1:1,000 dilution;
SAB2500128; Sigma-Aldrich; Merck KGaA) for 1 h at room temperature,
which was then detected with a HRP-conjugated secondary antibody
(1:30,000 dilution; A0545; Sigma-Aldrich; Merck KGaA) for 1 h at
room temperature. The absorbance at 450 nm was determined using a
microtiter plate reader (Multiskan Go microplate reader, Thermo
Fisher Scientific, Inc.).
Masson's staining
Masson's staining was performed to observe the
histopathology of the rabbit femoral defects. The treated rabbit
femoral defects were washed twice with Dulbecco's-PBS, fixed with
cold methanol for 10 min at 4°C, washed twice with sterile water,
and then stained with the Masson stain kit (Diagnostic BioSystems,
Pleasanton, CA, USA). The stained samples were observed and images
were captured under a light microscope.
Alizarin red staining
The cells were stained with Alizarin Red S to detect
calcium deposition (23). The
BMSCs were washed three times with PBS and then fixed with 70%
ethanol for 1 h at 4°C. Following fixation, the cells were washed
with water and stained with 0.2% Alizarin red (Sigma-Aldrich; Merck
KGaA) in 2% ethanol for 15 min, and then washed three times with
water, and dried at 37°C.
Transmission electron microscopy
(TEM)
The samples were prepared and analyzed as previously
described (24). Briefly, the
samples were fixed and sectioned to 60 nm using an MTXL RMC
ultramicrotome (Boeckeler Instruments Inc., Tucson, AZ, USA), and
stained with 1% toluidine blue. The sections were observed using a
Morgagni 268 TEM (FEI Company, Eindhoven, The Netherlands) at 80
kV. Images were captured using MegaView III CCD using iTEM-SIS
software (Olympus, Soft Imaging System GmbH, Münster, Germany).
X-ray microtomography
The rabbit femoral defect model was established and
treated with PBS, BMSCs, or TGF-β1-overexpressing BMSCs for 2 and 6
weeks. The rabbits were anesthetized with xylazine (5 mg/kg) and
ketamine (25 mg/kg). An X-ray source was then used to irradiate the
right forelimb of the rabbits. X-ray irradiation (MI-201, Shimadzu
Corporation, Kyoto, Japan) was used to detect the right ulna,
radius, and surrounding soft tissues.
Statistical analysis
All data are presented as the mean ± standard
deviation. The statistical analysis was performed using SPSS 13.0
(SPSS, Inc., Chicago, IL, USA), and statistical significance was
assessed using Student's t-test or one-way analysis of variance
(ANOVA) test followed by a Student-Newman-Keuls post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characterization of rabbit BMSCs
The rabbit BMSCs were cultured, and the first and
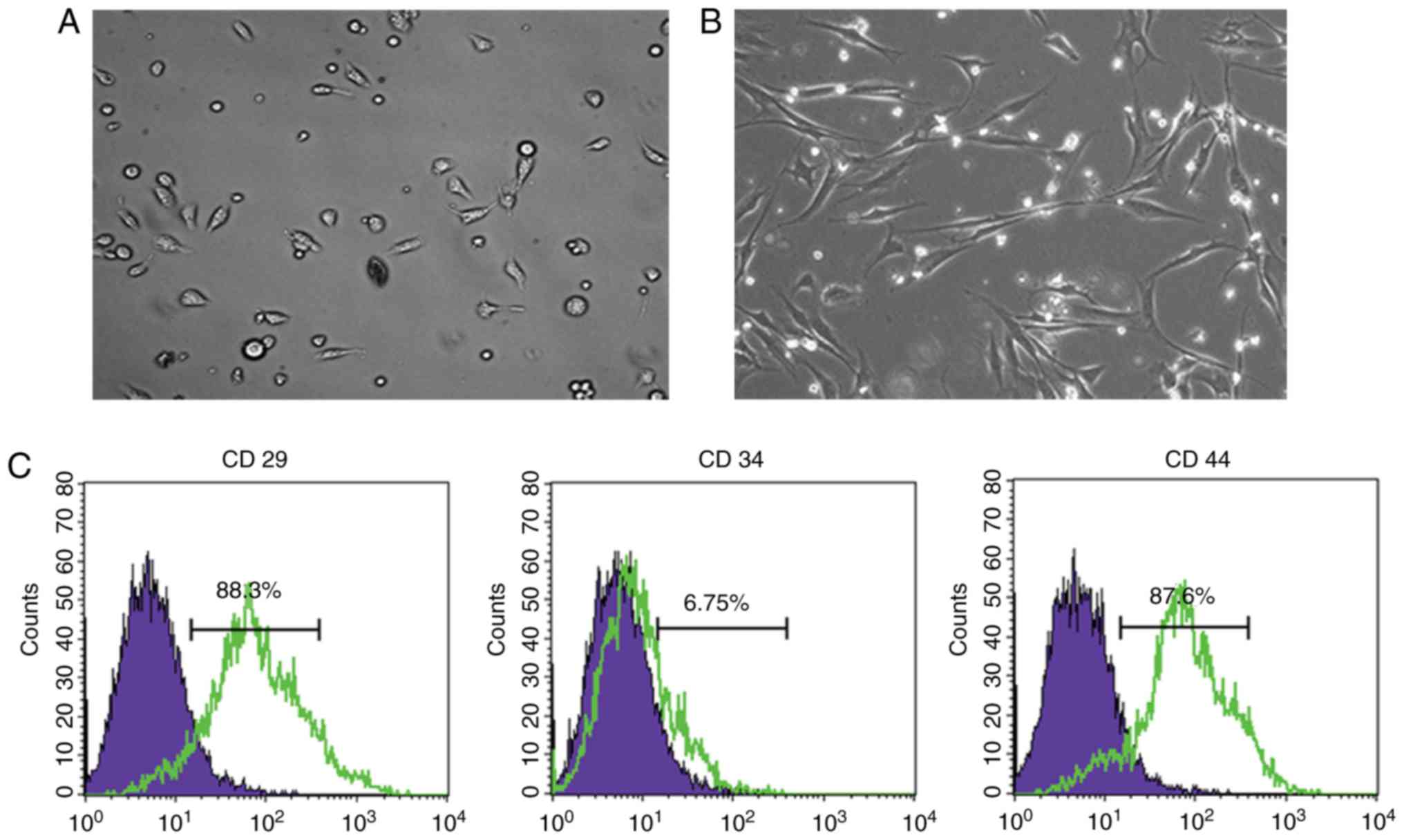
third passages were observed. As shown in Fig. 1A and B, the cultured BMSCs
exhibited a round shape at passage 1 (Fig. 1A) and spindle-shaped morphology at
passage 3 (Fig. 1B). Flow
cytometry was performed to characterize BMSC surface markers; 88.3%
of the BMSCs were CD29-positive and 87.6% of the BMSCs were
CD44-positive, whereas <6.75% of the BMSCs were CD34-positive
(Fig. 1C).
TGF-β1 is stably overexpressed in rabbit
BMSCs and improves osteogenesis
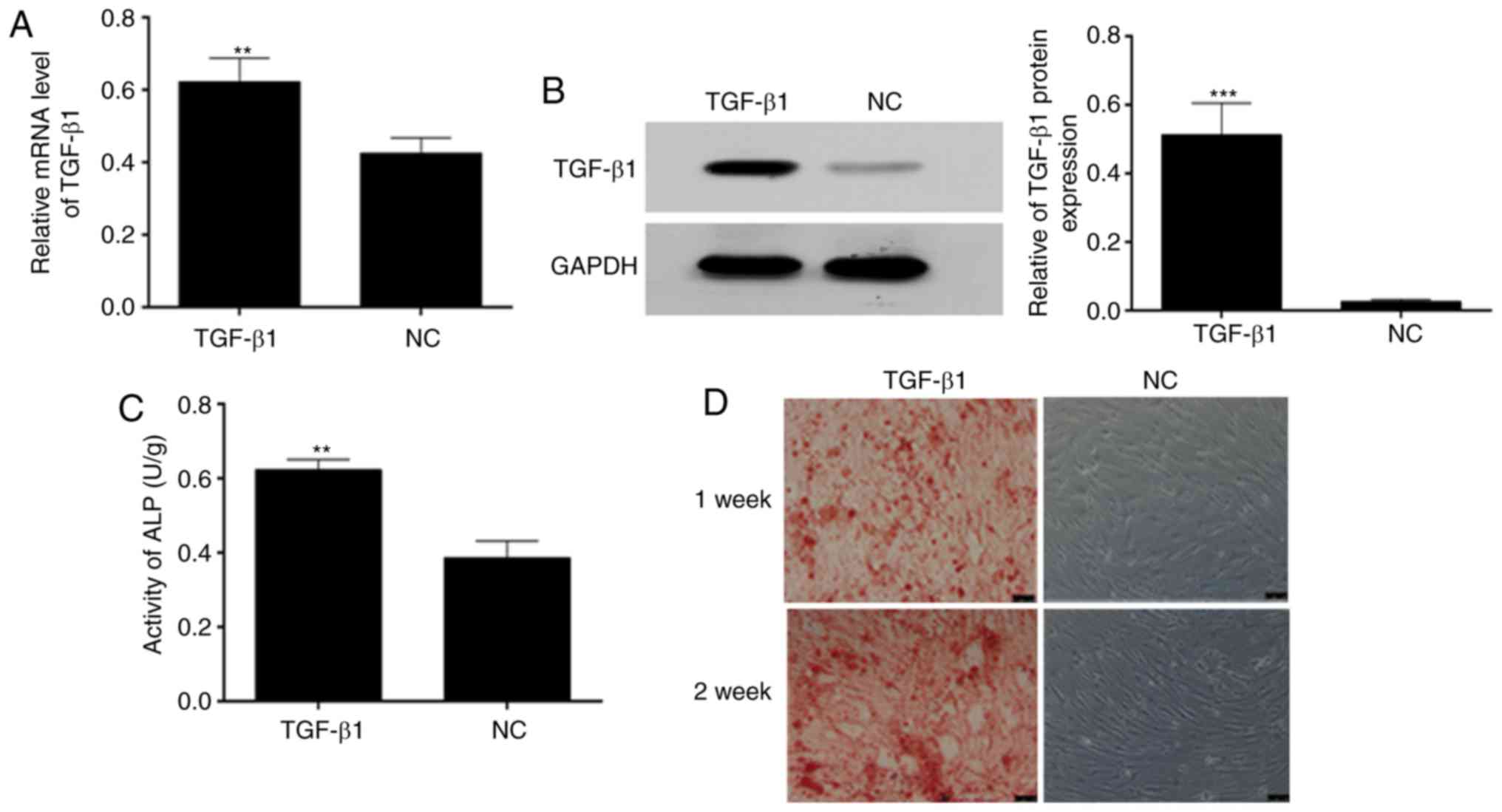
To evaluate whether TGF-β1 was overexpressed in the
BMSCs, RT-qPCR analysis was performed and the results indicated
that the mRNA expression of TGF-β1 was high in the BMSCs
transfected with TGF-β1, compared with that in the NC BMSCs
(Fig. 2A). This was consistent
with the results of western blot analysis (Fig. 2B). In addition, the ELISA results
showed that the activity of ALP was higher in the BMSCs transfected
with TGF-β1 than in the NC BMSCs (Fig. 2C). Furthermore, TGF-β1 improved
the osteogenesis of the BMSCs (Fig.
2D).
Expression of TGF-β1 is high in newly
formed bone of femoral defects treated with TGF-β1-overexpressing
BMSCs
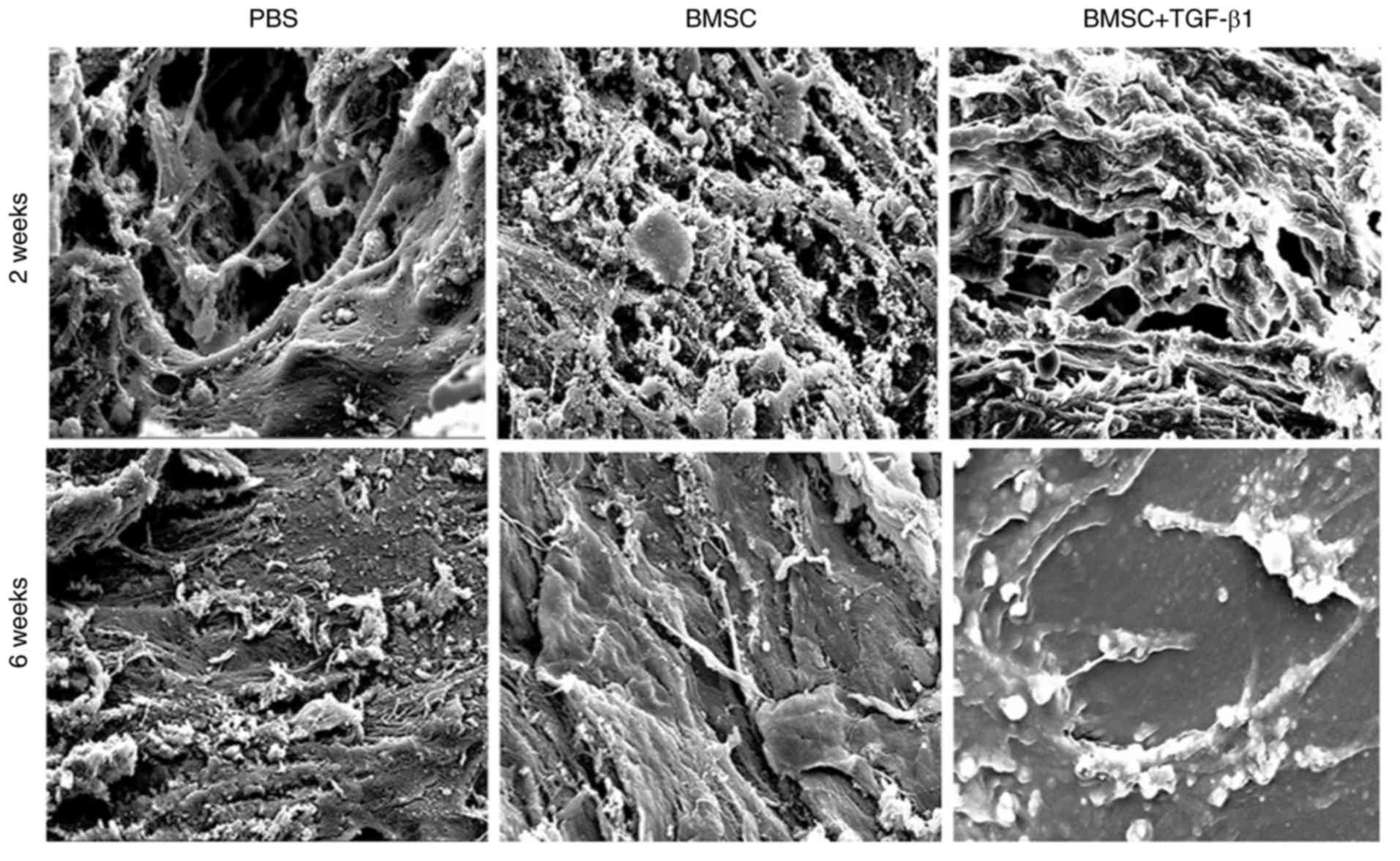
New bone ultrastructure of the rabbit femoral defect
models was determined using electron microscopy. The femoral
defects treated with TGF-β1-overexpressing BMSCs exhibited improved
bone formation (Fig. 3), whereas
in the control groups, there were numerous collagen fiber bundles
arranged in a single direction and fewer new osteoids.
Treatment with TGF-β1-overexpressing
BMSCs promotes new bone formation in femoral defects
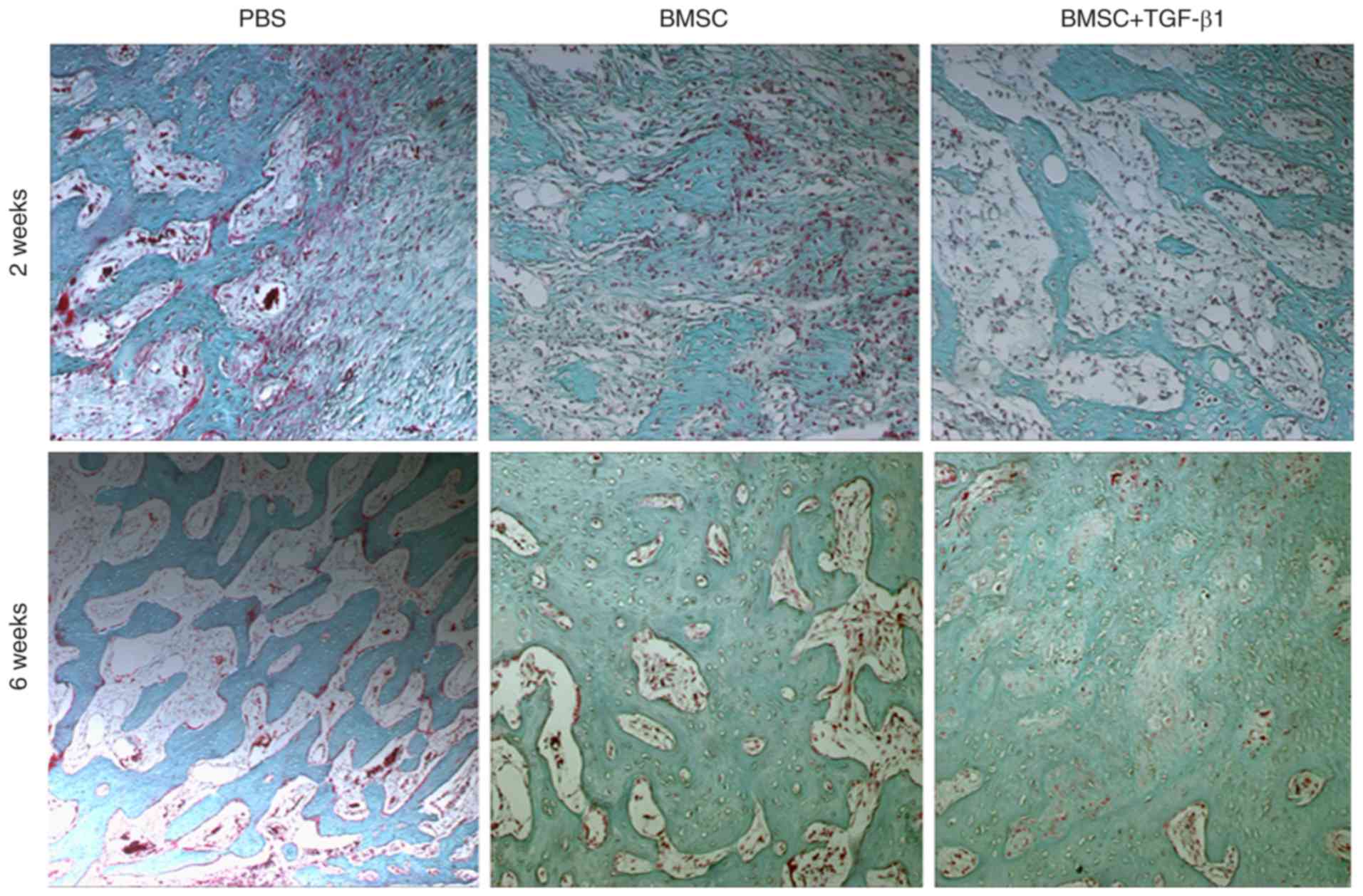
As shown in Table
I, it was found that the maximum torque, failure angle, failure
energy, and torsional rigidity were significantly increased in the
femoral defects treated with the TGF-β1-overexpressing BMSCs,
compared with those in the PBS- and BMSC-treated control groups. In
the BMSC- and TGF-β1-overexpressing BMSC-treated groups, the bone
surface was smooth, the trabecular bone was relatively dense, and
calcification was more complete and closer to that in the normal
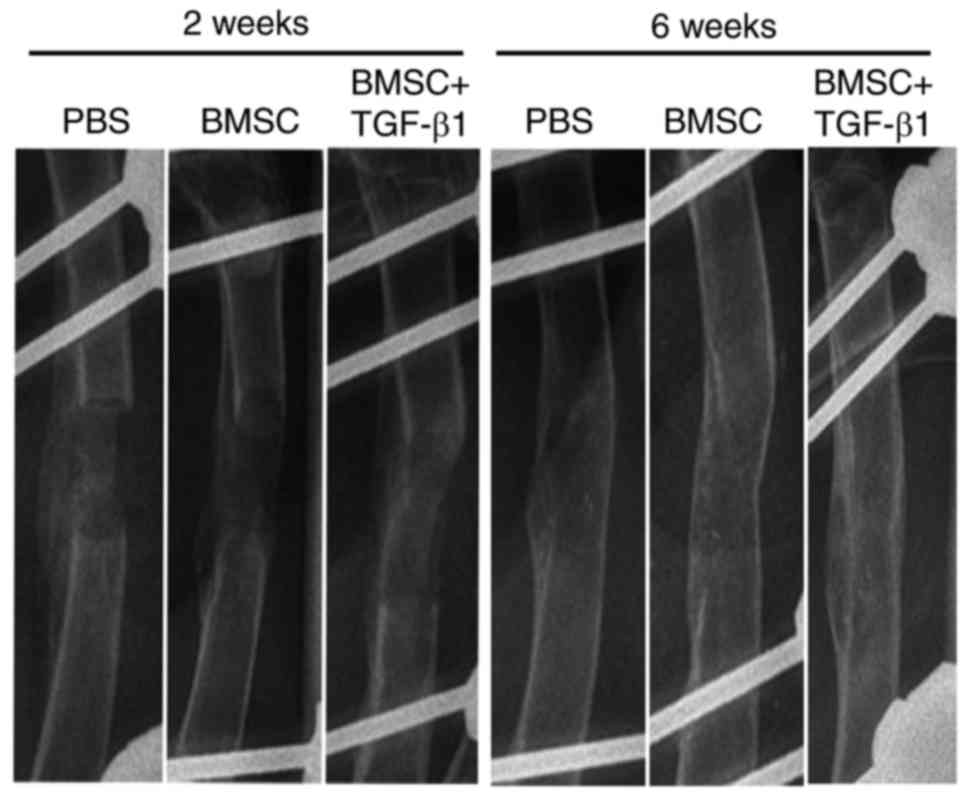
bone surface (Fig. 4). X-ray
analysis further demonstrated that the alignment of the majority of
femurs was well-maintained 2 weeks following surgery, and the
implants were in a stable position in the TGF-β1-overexpressing
BMSC group. However, a number of implants in the control group had
partially collapsed. In all three groups, a cartilaginous callus
had formed and surrounded the majority of the implant, although
gaps were visible. At 6 weeks post-surgery, the cartilaginous
callus began to take shape and was remodeled into a thin cortical
lamellar bone, with the presence of gaps disappearing in all three
groups. However, the remodeled cortical lamellar bone was closer to
the natural bone in the femoral defects treated with
TGF-β1-expressing BMSCs than in that in the control group (Fig. 5).
 | Table IBiomechanical indices of rabbit
femoral defects. |
Table I
Biomechanical indices of rabbit
femoral defects.
| Group | Maximum torque
(Nm) | Failure angle
(radian) | Failure energy (Nm ×
angle) | Torsional rigidity
(Nm/angle) |
|---|
| A | 0.205±0.035 | 3.136±0.112 | 3.215±0.165 | 0.021±0.005 |
| B | 0.403±0.059a | 5.232±0.106a | 4.136±0.325a | 0.027±0.003 |
| C | 0.457±0.048b | 5.243±0.162b | 4.225±0.568a | 0.045±0.006b |
| D | 0.402±0.065 | 5.163±0.157 | 4.207±0.149 | 0.033±0.002 |
| E | 0.654±0.074c | 5.373±0.168 | 6.355±0.186d | 0.052±0.004c |
| F | 0.698±0.046d | 7.454±0.194d | 7.526±0.512e | 0.068±0.005d |
TGF-β1-overexpressing BMSC treatment
increases bone-related markers in the rabbit femoral defect
model
To determine whether TGF-β1-overexpressing BMSCs
increase bone formation, bone-related markers, including SATB
homeobox 2 (Satb2), Runt-related transcription factor 2 (Runx2) and
osteocalcin (OCN), were quantified using RT-qPCR and western blot
analyses. As shown in Fig. 6A and
B, compared with the PBS-treated control group, significant
increases were detected in these three markers following treatment
with TGF-β1-overexpressing BMSCs at weeks 2 and 6.
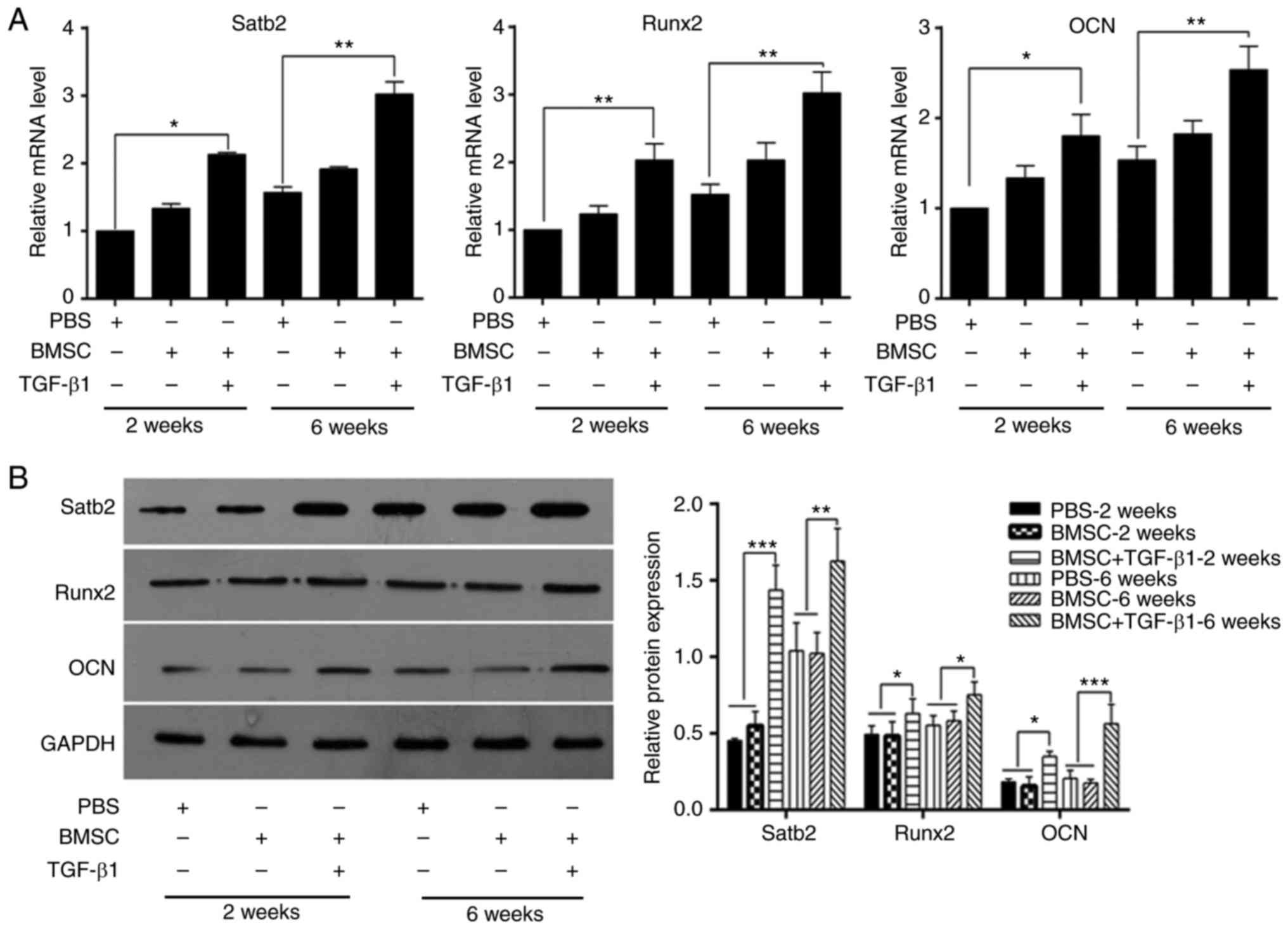 | Figure 6Expression of bone-related markers in
TGF-β1-overexpressing BMSCs treated femoral defects. (A) mRNA
levels of Satb2, Runx2, and OCN in femoral
defects treated with PBS, BMSCs, or TGF-β1-overexpressing BMSCs at
week 2 and week 6, quantified by reverse transcription-quantitative
polymerase chain reaction analysis (*P<0.05;
**P<0.01). (B) Protein expression of Satb2, Runx2,
and OCN in femoral defects treated with PBS, BMSCs, or
TGF-β1-overexpressing BMSCs at week 2 and week 6, measured by
western blot analysis (*P<0.05;
**P<0.01; ***P<0.001). BMSCs, bone
marrow-derived mesenchymal stem cells; PBS, phosphate-buffered
saline; TGF-β1, transforming growth factor-β1; Satb2, SATB homeobox
2; Runx2, Runt-related transcription factor 2; OCN, osteocalcin;
GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
Discussion
Distraction osteogenesis is an effective novel
method for treating bone defects. However, clinical problems
associated with this method, including the long treatment cycle,
remain. Therefore, a large number of studies have been performed to
accelerate the ossification and shorten the treatment process using
various methods. Studies have shown that combining growth factors
with distraction osteogenesis can effectively shorten the duration
of treatment (25,26). However, the delivery of growth
factors into the defective region remains challenging. In addition,
the cost of growth factor treatment is prohibitive for the majority
of patients. Therefore, the development of novel strategies that
are effective and convenient is critical.
Mesenchymal stem cells (MSCs) are the progenitor
cells of connective tissues, including adipose tissue, cartilage
and bone (27). BMSCs are a type
of pluripotent MSC. Under specific induction conditions, BMSCs can
differentiate into various cell types, including bone cells.
Therefore, BMSCs offer potential for treating musculoskeletal
injuries and disorders. In addition, BMSCs can be readily separated
and cultured, with high proliferation capacity and weak
immunogenicity, making them ideal for tissue engineering (28).
TGF-β1 is one of the most important cytokines in the
bone matrix (29). Previous
studies have shown that it is involved in a variety of biological
processes, including development, apoptosis, proliferation,
inflammation, bone formation, differentiation and tumor growth.
TGF-β1 can be activated by dissociation from latency-associated
peptide, when tissue is injured or remolded (30,31). TGF-β then rapidly recruits
perivascular MSCs to the bone surface for osteoblast
differentiation and new bone formation (32). Therefore, TGF-β has significant
effects on bone homeostasis during remodeling by recruiting
MSCs.
In the present study, TGF-β1 was stably
overexpressed in rabbit BMSCs to evaluate the effects of TGF-β1 in
combination with BMSCs on new bone formation in a rabbit femoral
defect model. The results showed that TGF-β1 was expressed at a
high level in the new bone of the femoral defects treated with
TGF-β1-overexpressing BMSCs, and that the combination of BMSCs and
TGF-β1 significantly promoted the formation of new bone. In the
femoral defects treated with TGF-β1-overexpressing BMSCs, the bone
surface was smooth, the trabecular bone was relatively dense, and
the calcification was more complete and closer to that of the
normal bone surface. In addition, in this TGF-β1-overexpressing
BMSC group, the remodeled cortical lamellar bone was closer to
natural bone than that of the control group, and the cartilaginous
callus began to take shape and was remodeled into thin cortical
lamellar bone. Therefore, the results suggested that TGF-β1, in
combination with BMSCs, promoted new bone formation in the rabbit
femoral defect model. Previous studies have shown that TGF-β is
important in the repair of BMSC-mediated subchondral osteoarthritis
(33), as the bone matrix
releases and activates TGF-β, and the active TGF-β recruits MSCs to
the bone resorption pit (34).
Therefore, TGF-β signaling is crucial in the formation of new bone
by BMSCs.
Acknowledgments
Not applicable.
Funding
This study was supported by Shandong Provincial
Natural Science Foundation (grant no. BS2014YY026) and the Foshan
Municipal Health Bureau for Scientific Research (grant nos.
20170161 and 20180184).
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
BYS, BXZ, JYZ, ZPS, YAS and FH made substantial
contributions to the conception and design of the present the
study. BYS, BXZ, JYZ and ZPS performed the experiments. BYS and BXZ
wrote the paper. YAS and FH revised the manuscript critically for
important intellectual content. All authors read and approved the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
and Use Committee of Guangzhou University of Chinese Medicine and
experiments were performed in accordance with the policies and
principles in the Guide for Care and Use of Laboratory Animals
(20).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yelin E, Weinstein S and King T: The
burden of musculoskeletal diseases in the United States. Semin
Arthritis Rheum. 46:259–260. 2016. View Article : Google Scholar
|
|
2
|
Qu D, Mosher CZ, Boushell MK and Lu HH:
Engineering complex orthopaedic tissues via strategic biomimicry.
Ann Biomed Eng. 43:697–717. 2015. View Article : Google Scholar
|
|
3
|
Goodrich JT, Sandler AL and Tepper O: A
review of reconstructive materials for use in craniofacial surgery
bone fixation materials, bone substitutes, and distractors. Childs
Nerv Syst. 28:1577–1588. 2012. View Article : Google Scholar
|
|
4
|
Mendes SC, Tibbe JM, Veenhof M, Bakker K,
Both S, Platenburg PP, Oner FC, de Bruijn JD and van Blitterswijk
CA: Bone tissue-engineered implants using human bone marrow stromal
cells: Effect of culture conditions and donor age. Tissue Eng.
8:911–920. 2002. View Article : Google Scholar
|
|
5
|
Suzuki Y, Kim KJ, Kotake S and Itoh T:
Stromal cell activity in bone marrow from the tibia and iliac crest
of patients with rheumatoid arthritis. J Bone Miner Metab.
19:56–60. 2001. View Article : Google Scholar
|
|
6
|
Rodriguez JP, Montecinos L, Rios S, Reyes
P and Martinez J: Mesenchymal stem cells from osteoporotic patients
produce a type I collagen-deficient extracellular matrix favoring
adipogenic differentiation. J Cell Biochem. 79:557–565. 2000.
View Article : Google Scholar
|
|
7
|
Johnson PC, Mikos AG, Fisher JP and Jansen
JA: Strategic directions in tissue engineering. Tissue Eng.
13:2827–2837. 2007. View Article : Google Scholar
|
|
8
|
Kim K, Dean D, Lu A, Mikos AG and Fisher
JP: Early osteogenic signal expression of rat bone marrow stromal
cells is influenced by both hydroxyapatite nanoparticle content and
initial cell seeding density in biodegradable nanocomposite
scaffolds. Acta Biomater. 7:1249–1264. 2011. View Article : Google Scholar
|
|
9
|
Friedenstein AJ, Chailakhjan RK and
Lalykina KS: The development of fibroblast colonies in monolayer
cultures of guinea-pig bone marrow and spleen cells. Cell Tissue
Kinet. 3:393–403. 1970.
|
|
10
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar
|
|
11
|
Hosseinkhani M, Mehrabani D, Karimfar MH,
Bakhtiyari S, Manafi A and Shirazi R: Tissue engineered scaffolds
in regenerative medicine. World J Plast Surg. 3:3–7. 2014.
|
|
12
|
Kagami H, Agata H, Inoue M, Asahina I,
Tojo A, Yamashita N and Imai K: The use of bone marrow stromal
cells (bone marrow-derived multipotent mesenchymal stromal cells)
for alveolar bone tissue engineering: Basic science to clinical
translation. Tissue Eng Part B Rev. 20:229–232. 2014. View Article : Google Scholar
|
|
13
|
Stroncek DF, Sabatino M, Ren J, England L,
Kuznetsov SA, Klein HG and Robey PG: Establishing a bone marrow
stromal cell transplant program at the National Institutes of
Health Clinical Center. Tissue Eng Part B Rev. 20:200–205. 2014.
View Article : Google Scholar
|
|
14
|
Polymeri A, Giannobile WV and Kaigler D:
Bone marrow stromal stem cells in tissue engineering and
regenerative medicine. Horm Metab Res. 48:700–713. 2016. View Article : Google Scholar
|
|
15
|
Frangogiannis NG: Inflammation in cardiac
injury, repair and regeneration. Curr Opin Cardiol. 30:240–245.
2015. View Article : Google Scholar
|
|
16
|
Frangogiannis NG: The inflammatory
response in myocardial injury, repair, and remodelling. Nat Rev
Cardiol. 11:255–265. 2014. View Article : Google Scholar
|
|
17
|
Katsuno Y, Lamouille S and Derynck R:
TGF-β signaling and epithelial-mesenchymal transition in cancer
progression. Curr Opin Oncol. 25:76–84. 2013. View Article : Google Scholar
|
|
18
|
Cortez VS, Cervantes-Barragan L, Robinette
ML, Bando JK, Wang Y, Geiger TL, Gilfillan S, Fuchs A, Vivier E,
Sun JC, et al: Transforming Growth Factor-β signaling guides the
differentiation of innate lymphoid cells in salivary glands.
Immunity. 44:1127–1139. 2016. View Article : Google Scholar
|
|
19
|
Andrades JA, Han B, Becerra J, Sorgente N,
Hall FL and Nimni ME: A recombinant human TGF-beta1 fusion protein
with collagen-binding domain promotes migration, growth, and
differentiation of bone marrow mesenchymal cells. Exp Cell Res.
250:485–498. 1999. View Article : Google Scholar
|
|
20
|
SN Council: Washington, Bethesda and MD:
Guide for the care and use of Laboratory Animals. Revised edition.
73:1978.
|
|
21
|
Kim J, McBride S, Dean DD, Sylvia VL, Doll
BA and Hollinger JO: In vivo performance of combinations of
autograft, demineralized bone matrix, and tricalcium phosphate in a
rabbit femoral defect model. Biomed Mater. 9:0350102014. View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Zhao Y, Chen J, Dai X, Cai H, Ji X, Sheng
Y, Liu H, Yang L, Chen Y, Xi D, et al: Human glioma stem-like cells
induce malignant transformation of bone marrow mesenchymal stem
cells by activating TERT expression. Oncotarget. 8:104418–104429.
2017. View Article : Google Scholar
|
|
24
|
Kristensen HB, Andersen TL, Marcussen N,
Rolighed L and Delaisse JM: Increased presence of capillaries next
to remodeling sites in adult human cancellous bone. J Bone Miner
Res. 28:574–585. 2013. View Article : Google Scholar
|
|
25
|
Makhdom AM and Hamdy RC: The role of
growth factors on acceleration of bone regeneration during
distraction osteogenesis. Tissue Eng Part B Rev. 19:442–453. 2013.
View Article : Google Scholar
|
|
26
|
Siwicka KA, Kitoh H, Kawasumi M and
Ishiguro N: Spatial and temporal distribution of growth factors
receptors in the callus: Implications for improvement of
distraction osteogenesis. Nagoya J Med Sci. 73:117–127. 2011.
|
|
27
|
Caplan AI: New MSC: MSCs as pericytes are
Sentinels and gatekeepers. J Orthop Res. 35:1151–1159. 2017.
View Article : Google Scholar
|
|
28
|
van Gorp S, Leerink M, Kakinohana O,
Platoshyn O, Santucci C, Galik J, Joosten EA, Hruska-Plochan M,
Goldberg D, Marsala S, et al: Amelioration of motor/sensory
dysfunction and spasticity in a rat model of acute lumbar spinal
cord injury by human neural stem cell transplantation. Stem Cell
Res Ther. 4:572013. View
Article : Google Scholar
|
|
29
|
Hering S, Isken E, Knabbe C, Janott J,
Jost C, Pommer A, Muhr G, Schatz H and Pfeiffer AF: TGFbeta1 and
TGFbeta2 mRNA and protein expression in human bone samples. Exp
Clin Endocrinol Diabetes. 109:217–226. 2001. View Article : Google Scholar
|
|
30
|
Dallas SL, Rosser JL, Mundy GR and
Bonewald LF: Proteolysis of latent transforming growth factor-beta
(TGF-beta)-binding protein-1 by osteoclasts. A cellular mechanism
for release of TGF-beta from bone matrix. J Biol Chem.
277:21352–21360. 2002. View Article : Google Scholar
|
|
31
|
Brauer PR and Yee JA: Cranial neural crest
cells synthesize and secrete a latent form of transforming growth
factor beta that can be activated by neural crest cell proteolysis.
Dev Biol. 155:281–285. 1993. View Article : Google Scholar
|
|
32
|
Pfeilschifter J, Wolf O, Naumann A, Minne
HW, Mundy GR and Ziegler R: Chemotactic response of osteoblastlike
cells to transforming growth factor beta. J Bone Miner Res.
5:825–830. 1990. View Article : Google Scholar
|
|
33
|
Zhen G, Wen C, Jia X, Li Y, Crane JL,
Mears SC, Askin FB, Frassica FJ, Chang W, Yao J, et al: Inhibition
of TGF-β signaling in mesenchymal stem cells of subchondral bone
attenuates osteoarthritis. Nat Med. 19:704–712. 2013. View Article : Google Scholar
|
|
34
|
Crane JL, Xian L and Cao X: Role of TGF-β
signaling in coupling bone remodeling. Methods Mol Biol.
1344:287–300. 2016. View Article : Google Scholar
|