Introduction
As pivotal events involved in central nervous system
(CNS) disorders, neuroimmune variations are rather pivotal to the
deterioration of neurodegenerative diseases, including Parkinson's
disease and Alzheimer's disease (1,2).
Neuroinflammatory responses are well-known to be associated with
the progression of these diseases (3), and proinflammatory cytokines,
including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6),
have been widely identified as convincing hallmarks of inflammation
(4,5). Astrocytes, a major component of the
neural network, are increasingly being recognized for their
importance in neuroimmunity, including responsiveness to IL-1
(6), removal of old cells and
secretion of TNF-α/IL-6 (7,8).
Impaired astrocytic function is regarded as a critical issue
implicated in neuroinflammation (9); however, the underlying molecular
mechanism remains obscure.
Aquaporin-4 (AQP4), the predominant isoform of AQPs
in the adult brain, is primarily expressed on astrocytic foot
processes throughout the CNS (10,11). It has been well documented that
AQP4 is intimately involved in the modulation of astrocyte function
(12), but the majority of
knowledge on the transporter is limited to water balance (13), glial scar formation (14) and neuroexcitation (15). Little focus has been placed on the
area of neuroinflammation until recently (9). As found by Manley et al
(16), AQP4 deletion attenuates
inflammation-related brain edema, providing a novel perspective on
AQP4 in the management of neuroinflammation. Furthermore, several
studies provided direct evidence to show the contribution of AQP4
to neuroinflammatory responses, although AQP4 was shown to serve
distinct roles in different stages of neuroinflammation (17,18). In addition, multiple studies
provided evidence that the involvement of AQP4 in the
neuroinflammatory cascade may be dependent on the mitogen-activated
protein kinase (MAPK) pathway (17), but its precise control of
intracellular signals are not well understood.
The role of sphingolipids in neuroinflammation has
been well established. Sphingosine kinase 1 (SHPK1) and
sphingosine-1-phosphate receptor 1 (S1P1) are predominantly
distributed in astrocytes (18).
Abnormal sphingolipid metabolism is observed during
neuroinflammation progression. As previous studies have
demonstrated, SPHK modulates the function of neurocytes by
regulating sphingolipid metabolism. The SPHK inhibitor was shown to
prevent glutamate uptake in astrocytes (19), SPHK1 activation was demonstrated
to correlate with the histological grade of astrocytomas (20) and genetic deletion of SPHK1 led to
the inhibition of glial cell proliferation (21). More recently, a patient suffering
from neuromyelitis optica spectrum disorder developed a fulminant
course of multiple white-matter lesions following a treatment with
S1P mimics, which may be connected with the presence of AQP4
antibodies (22). Although
preliminary evidence from clinical data has implied a link between
AQP4 and sphingolipids, little is known about the role of AQP4 in
sphingolipid metabolism.
The present study aimed to assess the role of AQP4
in cytokine release, with an emphasis on the SPHK1/MAPK/protein
kinase B (AKT) pathway. Thus, AQP4 knockout (KO) mice was used to
elucidate the potential effect of AQP4 on nureo-inflammation by
assessing the levels of inflammation associate kinases in LPS
treated primary astrocyte cultures isolated from mouse embryos.
Materials and methods
AQP4-knockout mice
AQP4-deficient mice were generated as previously
described (11) (generation and
phenotype of a transgenic knockout mouse lacking the
mercurial-insensitive water channel AQP4). CD-1 mice were kept
under environmentally controlled conditions (3-day-old mouse
embryos, ambient temperature, 22°C; humidity, 40%) on a 12-h
light/dark cycle with food and water ad libitum. All
experiments were approved by the Institutional Animal Care and Use
Committee, Nanjing Medical University (Nanjing, China). All efforts
were made to minimize animal suffering and to reduce the number of
animals used for the experiments.
Primary cultures of astrocytes and
treatments
Primary astrocytic cultures were prepared from
cerebral cortices of 20 AQP4+/+ and 20
AQP4−/− 3-day-old mouse embryos. Following mechanical
dissociation, pooled dissected cortices were digested with 0.25%
trypase (Amresco, Inc., Solon, OH, USA) at 37°C for 10 min and
maintained in Dulbecco's modified Eagle's medium (DMEM)/F-12
containing 10% fetal bovine serum (both Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), penicillin (200 U/ml) and
streptomycin (200 μg/ml; Gibco; Thermo Fisher Scientific,
Inc.). Following centrifugation at 400 × g for 5 min at room
temperature, the cell pellets were resuspended and plated on a
polylysine-treated (Sigma-Aldrich; Merck KGaA, Darmstadt, Germant)
flask. The cultures were maintained at 37°C in a humidified
incubator with 5% CO2. Half of the medium was replaced
with fresh substratum 24 h after plating and then changed every 2-3
days. Astrocytes were treated with serum-free DMEM for 24 h and
then exposed to LPS (100 ng/ml; Sigma-Aldrich; Merck KGaA).
Cell viability assay
Cell viability was measured by the Cell Counting
Kit-8 (CCK-8) assay. Astrocytes were collected from flasks and
plated in 96-well plates at a density of 5×103
cells/well in growth medium and allowed to adhere for 48 h prior to
the growth media being replaced with serum-free DMEM. Cells were
treated with 100 ng/ml LPS, and CCK8 (Obio Technology, Shanghai,
China) was applied after 1, 3, 6 and 24 h. The absorbance was
measured at a wavelength of 490 nm.
Measurement of cytokines
The quantitative determination of mouse TNF-α and
IL-6 in cell culture supernatants was assessed by double antibody
sandwich enzyme-linked immunosorbent assay (ELISA) (cat. nos.
MTA00B and M6000B; R&D Systems, Inc., Minneapolis, MN, USA)
following the manufacturer's protocols. The total protein
concentrations of the viable cells were determined using the
Bradford reagent. Total amounts of the TNF-α and IL-6 in media were
normalized to the total protein amounts of the viable cell pellets
and expressed as pg/mg proteins.
Western blot analysis
Astrocytes were lysed by RIPA buffer supplemented
with protease and phosphatase inhibitors (Roche, Indianapolis, IN,
USA). Lysates were centrifuged (400 x g for 10 min at 4°C) and the
protein concentration in the extracts was determined by the
Bradford assay. Samples in the extracts were denatured with a SDS
sample buffer. A total of 200 μg of protein was loaded into
each well and separated by 12% sodium dodecyl sulfate
polyacrylamide gel electrophoresis. Gels were transferred to a
polyvinylidene difluoride membrane (Pierce; Thermo Fisher
Scientific, Inc.) and immunoblotted, followed by blocking of the
membranes with 5% skimmed milk dissolved in TBST [(pH 7.5), 10 mM
Tris-HCl, 150 mM NaCl and 0.1% Tween-20] at room temperature for 1
h. Subsequent to being washed three times with TBST buffer, the
membranes were incubated with the following primary antibodies at
4°C over one night: Phosphorylated (phospho)-Akt (Ser473) (1:1,000,
cat. no. 4060), phospho-p44/42 MAPK [p-extracellular
signal-regulated protein kinases 1 and 2 (ERK1/2)] (Thr202/Tyr204)
(1:1000, cat. no. 9106), phospho-p38 MAPK (Thr180/Tyr182) (1:1,000,
cat. no. 4511), AKT (1:1,000, cat. no. 9272), p44/42 MAPK (ERK1/2)
(1:1,000, cat. no. 4695) and p38 MAPK (1:1,000, cat. no. 9212; all
Cell Signaling Technology, Inc., Danvers, MA, USA), and SPHK1
antibody (1:1,000, cat. no. ab71700; Abcam, Cambridge, MA, USA),
which were visualized by reaction with horseradish
peroxidase-linked secondary antibodies for 1 h at room temperature
(cat. nos. 29-0382-77 and 29-0382-78; GE Healthcare, Chicago, IL,
USA) and an enhanced chemiluminescence (ECL; cat. no. 32106; Thermo
Fisher Scientific, Inc.) or ECL-plus detection system (Quantity One
Quantitation software, Bio-Rad Laboratories, Hercules, CA, USA).
GAPDH (1:1,000, cat. no. D16H11; Cell Signaling Technology, Inc.)
was used as an internal control.
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical analysis between AQP4+/+ and
AQP4−/− was performed with a two-tailed indirect
Student's t-test using SPSS version 10.0 for Windows (SPSS, Inc.,
Chicago, IL, USA). Statistical analysis for multiple comparisons
was performed by a one-way analysis of variance with Bonferroni's
corrections. The level of statistical significance was defined as
P<0.05.
Results
LPS has no significant effect on
astrocyte viability
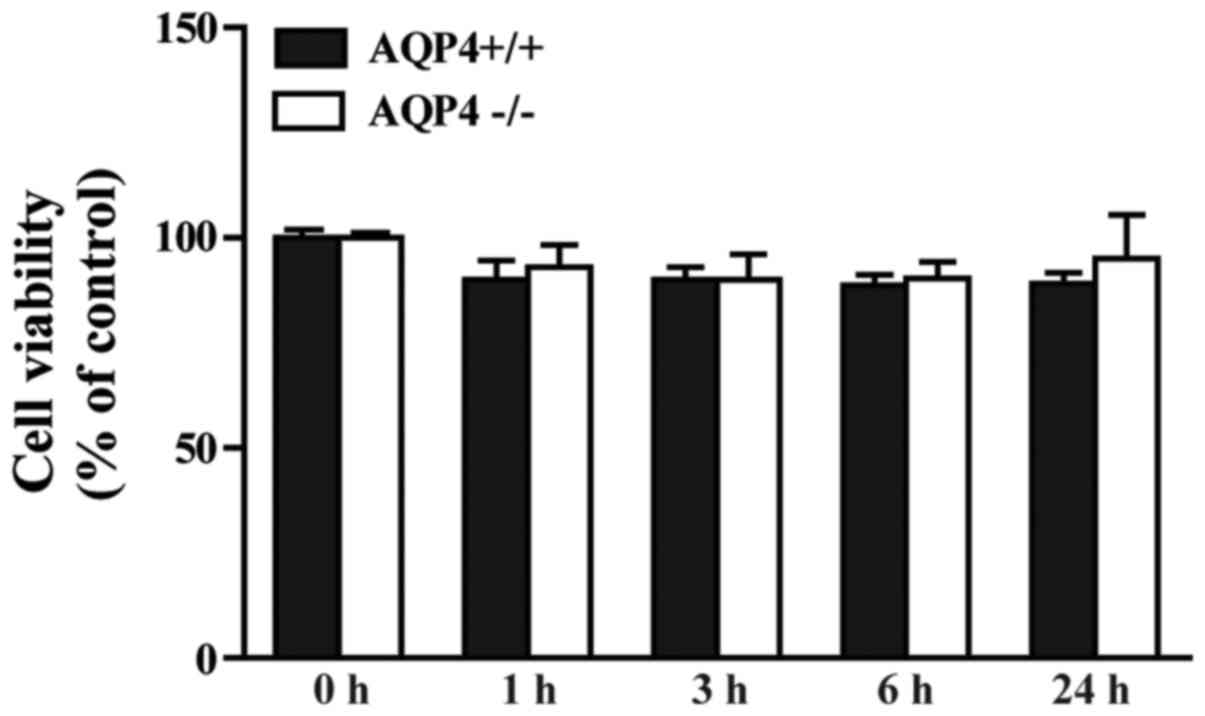
As shown in Fig.
1, LPS at 100 ng/ml exhibited no significant effect on
astrocyte viability within 24 h in the AQP4+/+ and
AQP4−/− groups. Furthermore, no clear difference was
observed between the two genotypes. The results of the CCK-8 assay
indicate that the alterations in the cytokines and pathways found
in the following experiments were not associated with LPS-induced
cell damage.
AQP4-knockout reduces LPS-induced
cytokine secretion in astrocyte cultures
To investigate the role of AQP4 in the inflammatory
response of astrocytes, measurements of cytokine release were
performed on differentiated primary astrocyte cultures from the
brain cortex of neonatal AQP4+/+ and AQP4−/−
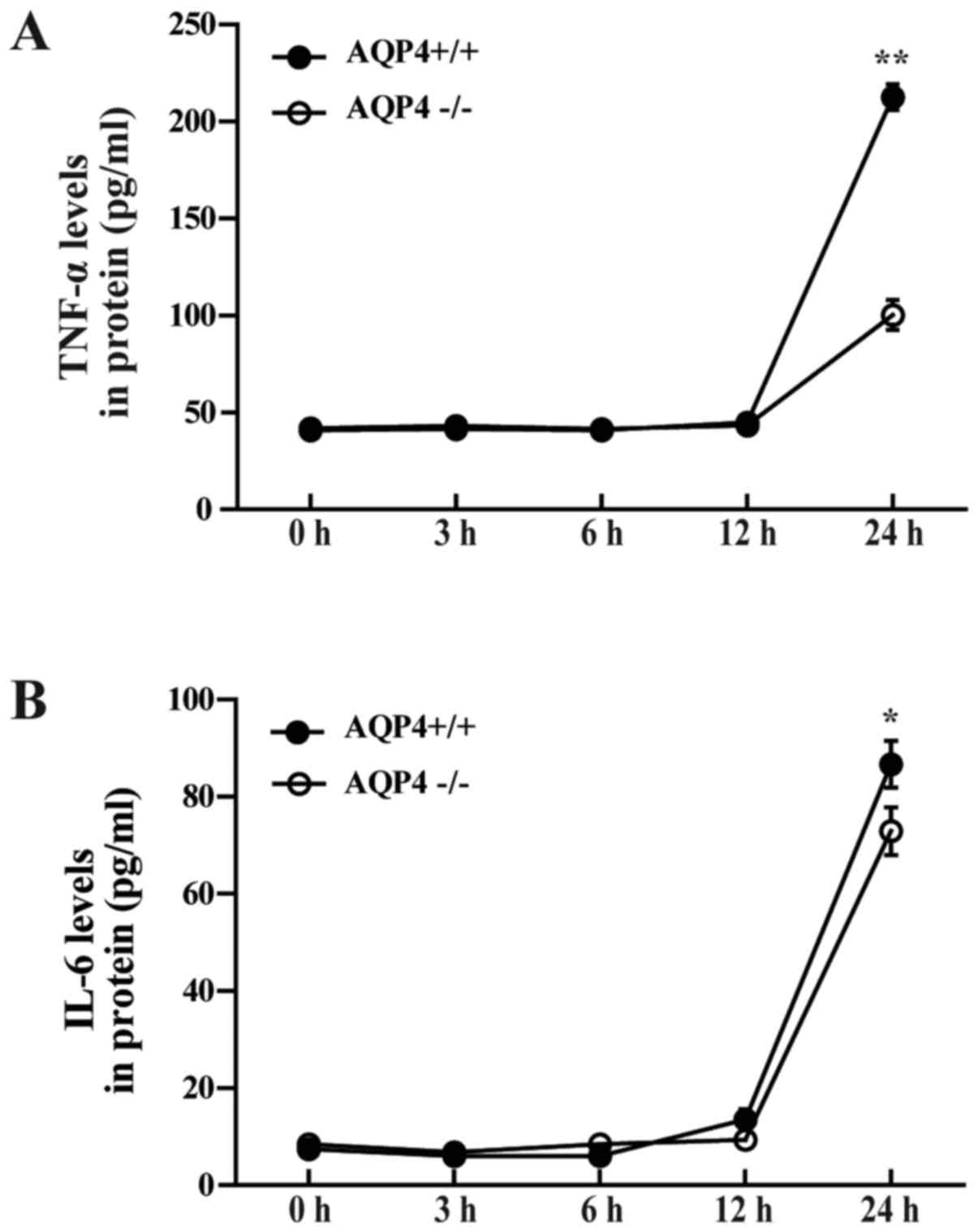
mice. As shown in Fig. 2,
AQP4-knockout attenuated TNF-α and IL-6 concentrations in cell
culture supernatant following 12 h of treatment with LPS. Compared
with the AQP4−/− genotype, AQP4+/+ showed
only a slight elevation in IL-6 at 12 h after LPS administration
(Fig. 2B), while the secretion of
TNF-α in AQP4+/+ astrocytes was robustly elevated at 24
h after LPS addition in astrocyte cultures (Fig. 2A). This supports the hypothesis
that knockout of AQP4 alleviates LPS-induced cytokine secretion in
astrocytes.
AQP4-knockout attenuates LPS-induced
SPHK1 generation in astrocytes
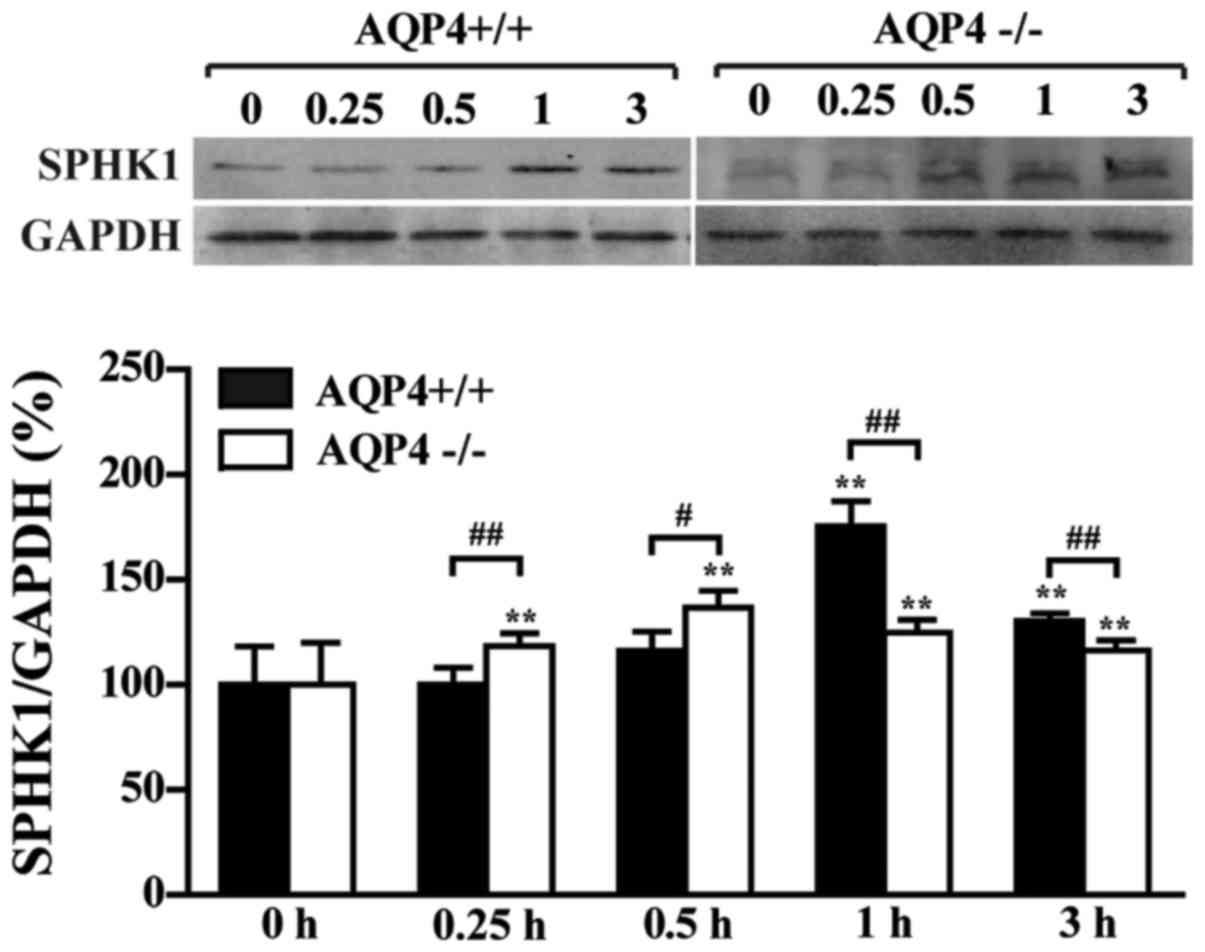
Western blot analysis by semi-quantitative analysis
showed that the expression of SPHK1 was induced by LPS in
AQP4+/+ and AQP4−/− astrocytes, and peaked at
1 and 0.5 h, respectively (Fig.
3). However, the induction of SPHK1 was attenuated in the
AQP4−/− group compared with that in the
AQP4+/+ group, demonstrating that AQP4 mediated the
induction of SPHK1 expression by LPS in astrocytes.
AQP4-knockout alleviates LPS-induced AKT
pathway activation in astrocytes
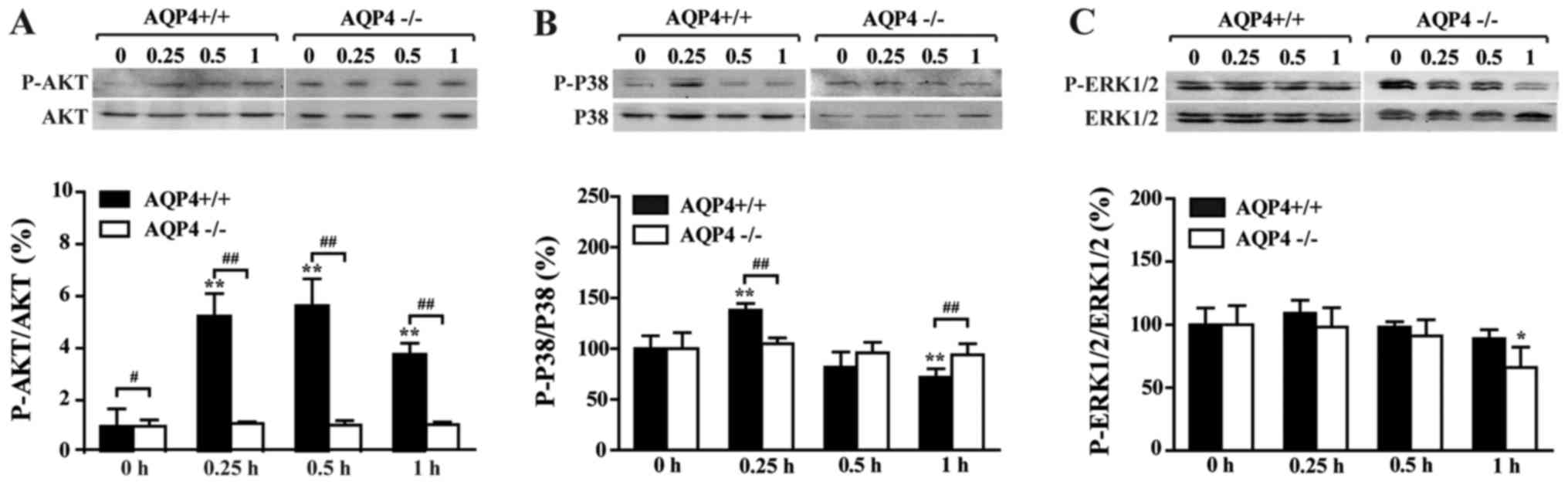
As shown in Fig.
4A, the phosphorylation of AKT was elevated by LPS after 0.25 h
and peaked at 0.5 h in the AQP4+/+ group, while in the
AQP4−/− group, the phosphorylation of AKT was not
affected following LPS administration within 1 h. It was
demonstrated that AKT signaling in the AQP4−/− genotype
is more stable than that in the AQP4+/+ gentoype with
regard to inflammatory stimulation.
AQP4-knockout alleviates LPS-induced MAPK
pathway activation in astrocytes
Next, the phosphorylation ratio of p38 and ERK was
determined in order to investigate the involvement of the MAPK
pathway. As observed in Fig. 4B and
C, the phosphorylation of p38 and ERK peaked at 0.25 h in the
AQP4+/+ group and no clear elevation was observed in the
AQP4−/− group. A decline was actually found in the
phosphorylation of ERK following the administration of LPS. Taken
together, these results demonstrated that lack of AQP4 may
interfere with the phosphorylation of ERK. Furthermore, a marked
decline was found in the phosphorylation of p38 after 0.25 h, and
the phosphorylation ratios at 0.5 and 1 h were lower than the basic
level (0 h), which indicates an exhaustion of phosphorylation. The
data suggests a decrease in MAPK signaling over time in
AQP4−/− astrocytes.
Discussion
Neuroinflammatory responses have long been observed
to be associated with neuroimmune changes outside and inside the
brain. Apart from reactive peripheral immune cells that traverse
the blood-brain barrier (23),
glia cells in the CNS are also implicated in initiating an
inflammatory cascade (24).
Microglia are often assumed to serve the most vital role in brain
inflammation (25). However,
since astrocytes occupy nearly half of the total cells in the CNS
(26) and exhibit important
immune regulatory properties, the role of astrocytes in
inflammation should not be neglected. AQP4 has been demonstrated to
be dispersed in the cytoplasm of reactive astrocytes, particularly
when inflammation occurs (27).
This AQP holds the key to our understanding of brain inflammation.
Astrocytes also express toll-like receptors (TLRs) that
specifically recognize LPS, which may stimulate the release of
proinflammatory cytokines and oxidative stress (28). It was previously found that TLR4
activation regulates AQP4 expression, and that IL-6 leads to an
increase in AQP4 (29). In turn,
AQP4 may modulate astrocyte-to-microglia communication during the
development of inflammation (30). All these findings suggest an
important role of AQP4 in neuro-inflammation, but the underlying
mechanism remains obscure.
As is known, it takes time to synthesize and
transport cytokines, such as IL-6 and TNF-α, to the cell surface
upon TLR4 activation. Previous studies have implied a different
amplification loop formed in AQP4−/− and
AQP4+/+ genotypes once cytokines are secreted (31-33). The signaling between these two
groups is distinct and complicated. The present study established a
cell model to determine the role of AQP4 in the early phase of
inflammation. From LPS administration to cytokine secretion, it was
revealed that cytokines were not secreted during the first 12 h,
yet intracellular pathways central to inflammation were activated.
In addition, AQP4−/− astrocyte signaling (SPHK/MAPK/AKT)
was decreased over time.
SPHKs serve an important role in sphingolipid
metabolism and are heavily linked to inflammatory diseases
(34). Compelling evidence from
clinical trials has indicated a link between AQP4 and SPHK1, with
an emphasis on their role in inflammation (25). Attention has become focused on
SPHK1 in LPS-induced cytokine production (35), which could lead to a rapid
increase in S1P, which has been hypothesized to be asso ciated with
the transactivation of different inflammatory pathways (36). The present study found that SPHK1
expression in AQP4+/+ reached a peak at 1 h and then
declined rapidly,while the increase in SPHK1 in the AQP4-knockout
genotype was attenuated despite an earlier peak time (0.5 h). The
level of SPHK1 in AQP4−/− astrocytes challenged by LPS
was much lower than that in AQP4+/+ astrocytes, implying
that SPHK1 signaling may inhibited when AQP4 is deleted.
As previously reported, S1P is able to bind to S1PR
and activate pathways such as those of MAPK, AKT and phospholipase
C, which are generally considered as the triggers of inflammation
and cell proliferation (37);
their activation drive the transcription factor, nuclear factor
(NF)-κB, to launch proinflammatory cytokine transcription. To date,
in colon diseases, it has been found that upregulation of S1P
promotes a persistent MAPK/NF-κB/IL-6/STAT3/S1PR amplification loop
that is critical to chronic inflammation development (36). ERK1/2 has also been confirmed to
mediate the activation of SPHK1 in turn (38). There is a potential clue among
SPHK1/MAPK/AKT signaling involved in cytokine release from
astrocytes (Fig. 5). We
hypothesized that the attenuated increase of SPHK1 observed in the
AQP4−/− genotype may result from the inhibited
activation of MAPK. Attenuation of the phosphorylation of MAPK and
AKT in LPS-induced AQP4−/− astrocytes was indeed
found.
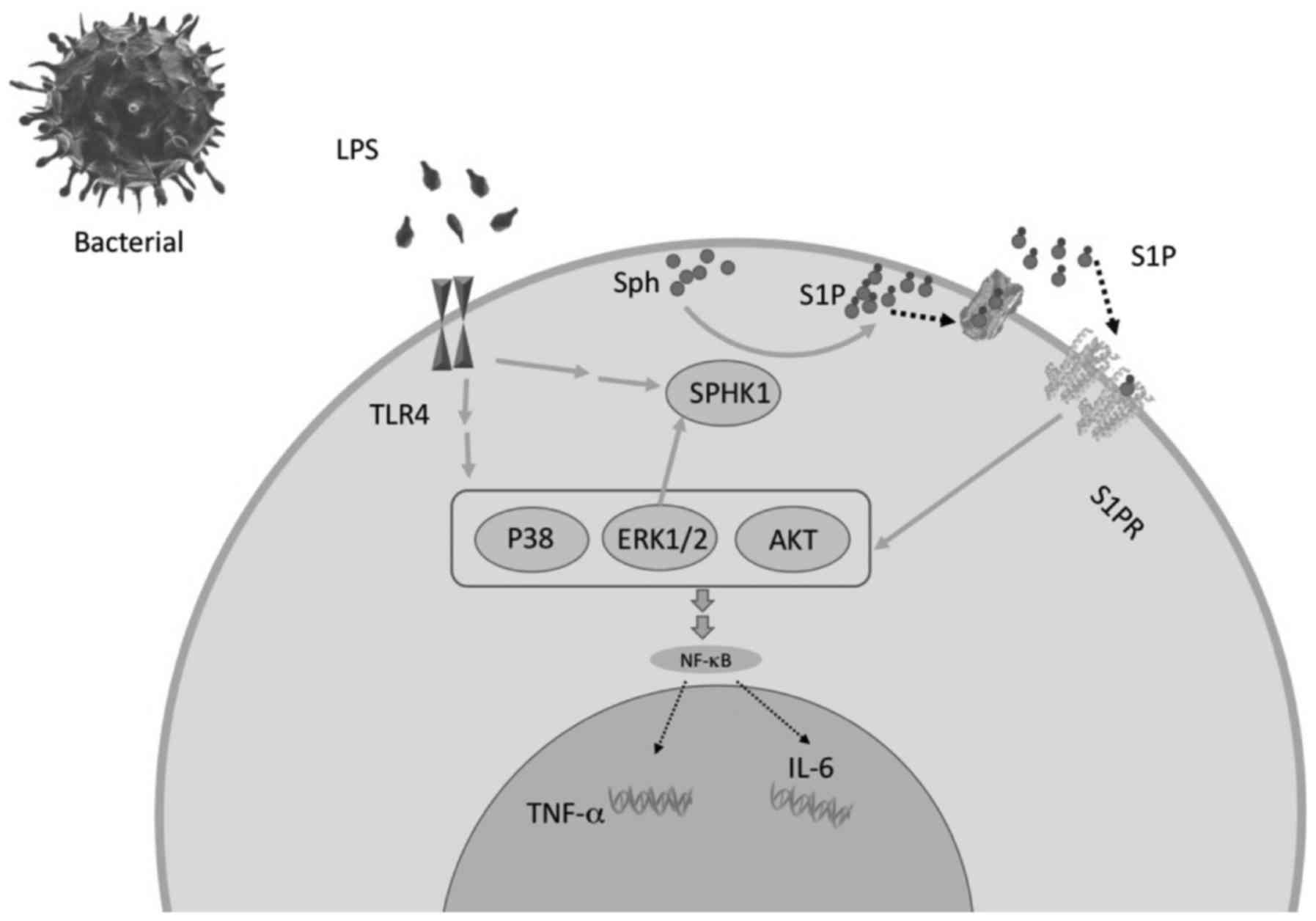 | Figure 5The potential mechanism by which LPS
induces proinflammatory cytokine release from astrocytes via
SPHK1/MAPK/AKT signaling. LPS derived from bacteria activate TLR 4
and strengthen the SPHK1/S1PR/MA PK/SPHK1 loop through ER K1/2,
which contributes to the expression of TNF-α and IL-6.
AQP4-knockout alleviates LPS-induced cytokine secretion through
inhibition of SPHK1/MA PK/AKT signaling. The present model enabled
the examination of the interaction of signaling pathways involved
in LPS-induced inflammation. It was found that LPS significantly
increased TNF-α and IL-6 levels in the astrocyte culture
supernatants. LPS derived from bacteria activate TLR 4 and
strengthen the SPHK1 and MA PK signals, which ultimately contribute
to the expression of TNF-α and IL-6. As previously described,
ERK1/2 was confirmed to activate SPHK1, and SPHK1 modulates the
MAPK/AKT pathway through the S1P/S1PR signal in turn; an
amplification loop is formed when TLR4 is activated. AQP4,
aquaporin-4; LPS, lipopolysaccharide; SPHK1, sphingosine kinase 1;
MAPK, mitogen-activated protein kinase; AKT, protein kinase B;
ERK1/2, extracellular signal-regulated protein kinases 1 and 2;
TLR4, toll-like receptor 4; TNF-α, tumor necrosis factor-α; IL-6,
interleukin 6; S1P, sphingosine-1-phosphate. |
The highly conserved MAPK family transfers
extracellular signals to nucleus in eukaryotic organisms (39,40). MAPK family is serine/threonine
protein kinases belonging to p38 MAPKs, ERK1/2 and Jun
amino-terminal kinases (JNKs) (41). LPS leads to the activation of p38
MAPKs and ERK, which contribute to the production of
proinflammatory cytokines (42,43). The present study compared the
level of MAPK phosphorylation in AQP4+/+ and
AQP4−/− astrocytes. The findings showed that the
phosphorylation levels of p38 MAPKs and ERK peaked at 0.25 h in the
AQP4+/+ group, while the levels declined markedly in the
AQP4−/− group. As previous studies implied, AQP4
overexpression may be associated with the activation of p38 MAPKs
and ERK when inflammation occurs (20). The present study found a
significant increase in MAPKs in the AQP4+/+ group, but
not in the AQP4−/− group, suggesting an AQP4-dependent
mechanism underlying the LPS-initiated MAPK activation.
Furthermore, AKT phosphorylation in AQP4−/− astrocytes
remained at a very low level at 1 h comp ared with that in
AQP4+/+ astrocytes, also hinting at stable signaling of
AKT in the absence of AQP4.
The role of AQP4 in inflammation has been
investigated, but as the pathological conditions vary, as does the
role of AQP4 in the inflammatory response. Previously, Li et
al (31) revealed that AQP4
deficiency alleviated LPS-induced TNF-α production in astrocytes,
which is consistent with the present findings. In an overhydration
model, AQP4 deficiency presented the opposite effect (44,45), highlighting a distinct role of
AQP4 when the type of stress changes. As previous studies reported,
AQP4 deficiency alleviated LPS-induced TNF-α production, but
augmented MPTP-induced TNF-α generation (46,47). This may be due to the distinct
downstream signals. Further studies are required to investigate the
signaling alterations under different pathological conditions.
However, in the present study, it was indicated that AQP4-knockout
could evoke inhibition of MAPKs and AKT, and attenuate the
fluctuation of SPHK1, which ultimately contributed to the secretion
of cytokines. Further studies are required to confirm the
SPHK1/MAPK/AKT loop.
In conclusion, the present study identified a
specific role of AQP4 in regulating the initial astrocytic
inflammatory response. It was found that astrocytes with AQP4
ablation are less responsive to inflammatory stress. This study
indicates the importance of AQP4 in astrocyte activation and
provides mechanistic insight into understanding the different
kinase pathways involved in this response.
Acknowledgments
Not applicable.
Funding
This study was supported by the Jiangsu Province
Special Program for Young Medical Talent (grand. no. QNRC2016599),
the National Natural Science Foundation of China (grant no.
81570522), the Open Subject of Jiangsu Province Health Department
(grant no. XK14_200904), the Jiangsu Province Scientific Research
Innovation Project of University Graduate Students (grant no.
KYLX15_0979) and a Project Funded by the Priority Academic Program
Development of Jiangsu Higher Education Institutions (grant no.
JX10231802).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
XZe and XZh conceived and designed the experiments;
WD and JY performed the experiments; WD and GC analyzed the data;
GH provided the AQP4 knockout mice; WD, JY and XZe wrote the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Institutional
Animal Care and Use Committee, Nanjing Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Connolly BS and Lang AE: Pharmacological
treatment of Parkinson disease: a review. JAMA. 311:1670–1683.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Strooper B and Karran E: The cellular
phase of Alzheimer's disease. Cell. 164:603–615. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Procaccini C, Santopaolo M, Faicchia D,
Colamatteo A, Formisano L, de Candia P, Galgani M, De Rosa V and
Matarese G: Role of metabolism in neurodegenerative disorders.
Metabolism. 65:1376–1390. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sastre M, Klockgether T and Heneka MT:
Contribution of inflammatory processes to Alzheimer's disease:
molecular mechanisms. Int J Dev Neurosci. 24:167–176. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Winklewski PJ, Radkowski M,
Wszedybyl-Winklewska M and Demkow U: Brain inflammation and
hypertension: the chicken or the egg? J Neuroinflammation.
12:852015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hennessy E, Griffin ÉW and Cunningham C:
Astrocytes are primed by chronic neurodegeneration to produce
exaggerated chemokine and cell infiltration responses to acute
stimulation with the cytokines IL-1β and TNF-α. J Neurosci.
35:8411–8422. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cho ML, Min SY, Chang SH, Kim KW, Heo SB,
Lee SH, Park SH, Cho CS and Kim HY: Transforming growth factor beta
1 (TGF-beta1) down-regulates TNFalpha-induced RANTES production in
rheumatoid synovial fibroblasts through NF-kappaB-mediated
transcriptional repression. Immunol Lett. 105:159–166. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song TT, Bi YH, Gao YQ, Huang R, Hao K, Xu
G, Tang JW, Ma ZQ, Kong FP, Coote JH, et al: Systemic
pro-inflammatory response facilitates the development of cerebral
edema during short hypoxia. J Neuroinflammation. 13:632016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ikeshima-Kataoka H: Neuroimmunological
implications of AQP4 in astrocytes. Int J Mol Sci. 17:172016.
View Article : Google Scholar
|
|
10
|
De Pittà M, Brunel N and Volterra A:
Astrocytes: orchestrating synaptic plasticity? Neuroscience.
323:43–61. 2016. View Article : Google Scholar
|
|
11
|
Yool AJ: Aquaporins: multiple roles in the
central nervous system. Neuroscientist. 13:470–485. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Szu JI and Binder DK: The role of
astrocytic aquaporin-4 in synaptic plasticity and learning and
memory. Front Integr Nuerosci. 10:82016. View Article : Google Scholar
|
|
13
|
Eckhard A and Löwenheim H: Water
regulation in the cochlea: do molecular water channels facilitate
potassium-dependent sound transduction? HNO. 62:423–431. 2014.In
German. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Q, Zhang YJ, Gao JY, Li XM, Kong H,
Zhang YP, Xiao M, Shields CB and Hu G: Aquaporin-4 mitigates
retrograde degeneration of rubrospinal neurons by facilitating
edema clearance and glial scar formation after spinal cord injury
in mice. Mol Neurobiol. 49:1327–1337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Katada R, Akdemir G, Asavapanumas N,
Ratelade J, Zhang H and Verkman AS: Greatly improved survival and
neuroprotection in aquaporin-4-knockout mice following global
cerebral ischemia. FASEB J. 28:705–714. 2014. View Article : Google Scholar :
|
|
16
|
Manley GT, Fujimura M, Ma T, Noshita N,
Filiz F, Bollen AW, Chan P and Verkman AS: Aquaporin-4 deletion in
mice reduces brain edema after acute water intoxication and
ischemic stroke. Nat Med. 6:159–163. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim EK and Choi EJ: Compromised MAPK
signaling in human diseases: an update. Arch Toxicol. 89:867–882.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee DH, Jeon BT, Jeong EA, Kim JS, Cho YW,
Kim HJ, Kang SS, Cho GJ, Choi WS and Roh GS: Altered expression of
sphingosine kinase 1 and sphingosine-1-phosphate receptor 1 in
mouse hippocampus after kainic acid treatment. Biochem Biophys Res
Commun. 393:476–480. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee YK, Kim HL, Kim YL and Im DS: Multiple
actions of dimethylsphingosine in 1321N1 astrocytes. Mol Cells.
23:11–16. 2007.PubMed/NCBI
|
|
20
|
Li J, Guan HY, Gong LY, Song LB, Zhang N,
Wu J, Yuan J, Zheng YJ, Huang ZS and Li M: Clinical significance of
sphingosine kinase-1 expression in human astrocytomas progression
and overall patient survival. Clin Cancer Res. 14:6996–7003. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu YP, Mizugishi K, Bektas M, Sandhoff R
and Proia RL: Sphingosine kinase 1/S1P receptor signaling axis
controls glial proliferation in mice with Sandhoff disease. Hum Mol
Genet. 17:2257–2264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Izaki S, Narukawa S, Kubota A, Mitsui T,
Fukaura H and Nomura K: A case of neuromyelitis optica spectrum
disorder developing a fulminant course with multiple white-matter
lesions following fingolimod treatment. Rinsho Shinkeigaku.
53:513–517. 2013.In Japanese. View Article : Google Scholar
|
|
23
|
Lopes Pinheiro MA, Kooij G, Mizee MR,
Kamermans A, Enzmann G, Lyck R, Schwaninger M, Engelhardt B and de
Vries HE: Immune cell trafficking across the barriers of the
central nervous system in multiple sclerosis and stroke. Biochim
Biophys Acta. 1862:461–471. 2016. View Article : Google Scholar
|
|
24
|
Jangra A, Sriram CS and Lahkar M:
Lipopolysaccharide-induced behavioral alterations are alleviated by
sodium phenylbutyrate via attenuation of oxidative stress and
neuroinflammatory cascade. Inflammation. 39:1441–1452. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park SY, Kim YH and Park G:
Anti-neuro-inflammatory effects of Nardostachys chinensis in
lipopolysaccharide-and lipoteichoic acid-stimulated microglial
cells. Chin J Nat Med. 14:343–353. 2016.PubMed/NCBI
|
|
26
|
Chung WS, Allen NJ and Eroglu C:
Astrocytes control synapse formation, function, and elimination.
Cold Spring Harb Perspect Biol. 7:a0203702015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nagelhus EA and Ottersen OP: Physiological
roles of aquaporin-4 in brain. Physiol Rev. 93:1543–1562. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rannikko EH, Weber SS and Kahle PJ:
Exogenous α-synuclein induces toll-like receptor 4 dependent
inflammatory responses in astrocytes. BMC Neurosci. 16:572015.
View Article : Google Scholar
|
|
29
|
Laird MD, Shields JS, Sukumari-Ramesh S,
Kimbler DE, Fessler RD, Shakir B, Youssef P, Yanasak N, Vender JR
and Dhandapani KM: High mobility group box protein-1 promotes
cerebral edema after traumatic brain injury via activation of
toll-like receptor 4. Glia. 62:26–38. 2014. View Article : Google Scholar
|
|
30
|
Sun H, Liang R, Yang B, Zhou Y, Liu M,
Fang F, Ding J, Fan Y and Hu G: Aquaporin-4 mediates communication
between astrocyte and microglia: implications of neuroinflammation
in experimental Parkinson's disease. Neuroscience. 317:65–75. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li L, Zhang H, Varrin-Doyer M, Zamvil SS
and Verkman AS: Proinflammatory role of aquaporin-4 in autoimmune
neuroinflammation. FASEB J. 25:1556–1566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ikeshima-Kataoka H, Abe Y, Abe T and Yasui
M: Immunological function of aquaporin-4 in stab-wounded mouse
brain in concert with a pro-inflammatory cytokine inducer,
osteopontin. Mol Cell Neurosci. 56:65–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alexander JJ, Jacob A, Cunningham P,
Hensley L and Quigg RJ: TNF is a key mediator of septic
encephalopathy acting through its receptor, TNF receptor-1.
Neurochem Int. 52:447–456. 2008. View Article : Google Scholar
|
|
34
|
Maceyka M, Harikumar KB, Milstien S and
Spiegel S: Sphingosine-1-phosphate signaling and its role in
disease. Trends Cell Biol. 22:50–60. 2012. View Article : Google Scholar :
|
|
35
|
Pchejetski D, Nunes J, Coughlan K, Lall H,
Pitson SM, Waxman J and Sumbayev VV: The involvement of sphingosine
kinase 1 in LPS-induced Toll-like receptor 4-mediated accumulation
of HIF-1α protein, activation of ASK1 and production of the
pro-inflammatory cytokine IL-6. Immunol Cell Biol. 89:268–274.
2011. View Article : Google Scholar
|
|
36
|
Liang J, Nagahashi M, Kim EY, Harikumar
KB, Yamada A, Huang WC, Hait NC, Allegood JC, Price MM, Avni D, et
al: Sphingosine-1-phosphate links persistent STAT3 activation,
chronic intestinal inflammation, and development of
colitis-associated cancer. Cancer Cell. 23:107–120. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Grin'kina NM, Karnabi EE, Damania D,
Wadgaonkar S, Muslimov IA and Wadgaonkar R: Sphingosine kinase 1
deficiency exacerbates LPS-induced neuroinflammation. PLoS One.
7:e364752012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pitson SM: Regulation of sphingosine
kinase and sphingolipid signaling. Trends Biochem Sci. 36:97–107.
2011. View Article : Google Scholar
|
|
39
|
Arthur JS and Ley SC: Mitogen-activated
protein kinases in innate immunity. Nat Rev Immunol. 13:679–692.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Peti W and Page R: Molecular basis of MAP
kinase regulation. Protein Sci. 22:1698–1710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sabio G and Davis RJ: TNF and MAP kinase
signalling pathways. Semin Immunol. 26:237–245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee M, McGeer E, Kodela R, Kashfi K and
McGeer PL: NOSH-aspirin (NBS-1120), a novel nitric oxide and
hydrogen sulfide releasing hybrid, attenuates neuroinflammation
induced by microglial and astrocytic activation: a new candidate
for treatment of neurodegenerative disorders. Glia. 61:1724–1734.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lo JY, Kamarudin MN, Hamdi OA, Awang K and
Kadir HA: Curcumenol isolated from Curcuma zedoaria suppresses
Akt-mediated NF-κB activation and p38 MAPK signaling pathway in
LPS-stimulated BV-2 microglial cells. Food Funct. 6:3550–3559.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bloch O, Papadopoulos MC, Manley GT and
Verkman AS: Aquaporin-4 gene deletion in mice increases focal edema
associated with staphylococcal brain abscess. J Neurochem.
95:254–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Papadopoulos MC, Manley GT, Krishna S and
Verkman AS: Aquaporin-4 facilitates reabsorption of excess fluid in
vasogenic brain edema. FASEB J. 18:1291–1293. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fan Y, Kong H, Shi X, Sun X, Ding J, Wu J
and Hu G: Hypersensitivity of aquaporin 4-deficient mice to
1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine and astrocytic
modulation. Neurobiol Aging. 29:1226–1236. 2008. View Article : Google Scholar
|
|
47
|
Sun H, Liang R, Yang B, Zhou Y, Liu M,
Fang F, Ding J, Fan Y and Hu G: Aquaporin-4 mediates communication
between astrocyte and microglia: implications of neuroinflammation
in experimental Parkinson's disease. Neuroscience. 317:65–75. 2016.
View Article : Google Scholar : PubMed/NCBI
|