Introduction
Post-natal mesenchymal stem cells derived from
dental tissues, such as dental pulp stem cells (DPSCs), stem cells
from human exfoliated deciduous teeth (SHEDs) and stem cells from
apical papilla (SCAP) exhibit great multi-plasticity to
differentiate into mesodermal lineages (1-3).
Capability of differentiation into osteo/odontogenic, adipogenic
and neurogenic lineages are inclusively used to confirm the
stemness of mesenchymal stem cells. Notably, DPSCs and SHEDs have
been successfully induced into endothelial cells in vitro
and in vivo (4–7), along with bone marrow mesenchymal
stem cells (BMMSCs) and human adipose-derived stem cells (ASCs)
(8,9).
It is well-known that vascular endothelial growth
factor (VEGF), an endothelial cell mitogen is the best-defined
regulator for inducing endothelial lineage differentiation from
mesenchymal stem cells. Indeed, VEGF-A combined with its cognate
VEGF receptor 2, effectively increases BMMSCs, ASCs, DPSCs and
SHEDs differentiation into endothelial cells (4, 5, 8, and 9). The
VEGF-Notch-EphrinB2 pathway was reported to regulate
arterial-venous specification (10). During vessel sprouting, activation
of VEGFR2 by VEGF induces tip cell migration and upregulates Notch
ligand Delta-like 4 (DLL4) expression, which in turn interacts
Notch receptors on neighboring endothelial cells (ECs),
subsequently upregulates VEGFR1, metabolizing VEGF (11).
Substrate elasticity and biomechanical force are
important factors of stem cell niche. Mechanical cues could
modulate stem cell differentiation, division, survival, and
motility (12). Studies have
shown that shear stress can activate Notch signaling, triggering
endothelial differentiation into the arterial phenotype (13). For example, shear stress induced
arterial specification of embryonic stem (ES)-derived ECs and
endothelial progenitor cells (14,15). Shear stress or combination with
VEGF induced BMMSC differentiation into endothelial cells (16).
To the best of our knowledge, there are no reports
on shear stress on the endothelial differentiation of SHEDs. In
this study, we investigated: i) The cell sternness of SHEDs, ii)
the impact of shear stress on the endothelial differentiation, and
iii) the molecular mechanism by which shear stress induces
endothelial differentiation.
Materials and methods
Culture and characterization of
SHEDs
The 3rd passage SHEDs were a gift from Dr Jianguang
Xu (The University of Hong Kong, Hong Kong, SAR, China). Cells were
cultured in α-MEM supplemented with 10% (v/v) FBS and 1% (v/v)
penicillin/streptomycin at 37°C in a 5% CO2 humidified
incubator to 70% confluence, 4th to 6th passage cells were used in
later experiments. Cell 'stemness' of SHEDs were evaluated before
induction. The expression of cell surface markers, CD90, CD73,
CD45, STRO-1, and CD105 was assessed by flow cytometry (BD
Biosciences, Franklin Lakes, CA, USA). Additionally,
osteo/odontogenic, adipogenic, and neurogenic differentiation
assays were conducted in the respective induction media.
Shear stress experiments
Glass slides (Flexcell International, Hillsborough,
NC, USA) were coated with Cultrex® Rat Collagen I
(R&D Systems, Inc., Minneapolis, MN, USA) at a concentration of
5 μg/cm2 and incubated at 37°C for 1 h before
usage. SHEDs grown to 70% confluence were trypsinized and counted.
Before shear stress treatment, SHEDs (3×105 cells/ml)
were seeded in glass slides (Flexcell International) for 4 h,
followed by transfer to 4-well sterile dishes (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with 4 ml α-MEM supplemented
with 15% (v/v) FBS to achieve 80–90% confluence. Then, the glass
slides were transferred to Streamer® Shear Stress Device
(Flexcell International). After shear stress values of 4 and 16
dynes/cm2 for 2 h, supernatants were collected for later
ELISA assay and cells were harvested for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
protein analyses. Cells without treatment were used as control
groups.
Combine shear stress with VEGF
SHEDs on glass slides after shear stress treatment
were transferred to 4-well sterile dishes (Thermo Fisher
Scientific, Inc.), 4 ml FBS-free α-MEM supplemented with VEGF (50
ng/ml) (R&D Systems, Inc.) was added to each well. Cells were
cultured for 12 h before being harvested for RT-qPCR and western
blot analysis. Cells without VEGF treatment were used as control
groups.
In vitro Matrigel angiogenesis assay
To assess whether shear stress induced SHEDs could
promote angiogenesis, the in vitro Matrigel angiogenesis
assay was conducted as described previously (17). Briefly, different groups of cells
(4 dynes/cm2 shear stress induced SHEDs, 16
dynes/cm2 shear stress induced SHEDs and primary SHEDs
groups) were seeded at 70,000 cells per well of labeled and 120
μl liquid Matrigel (cat. no. 354230; BD Biosciences,
Franklin Lakes, NJ, USA) pre-coated 48-well plates. After being
incubated for 2, 4, 6 and 8 h at 37°C and 5% CO2. Images
were captured using an inverted microscope (Olympus, Tokyo, Japan).
In addition, to investigate whether VEGF-DLL4/Notch-EphrinB2
signaling pathway is involved, SHEDs were transfected with EphrinB2
small interfering RNA (EphrinB2-siRNA) (Thermo Fisher Scientific,
Inc.). Briefly, SHEDs were seeded on 24-well plates to 80%
confluence, RNA-lipid complexes was prepared by mixing 50 μl
Opti-MEM Medium (Thermo Fisher Scientific, Inc.) and 3 ml
Lipofectamine and RNAiMAX reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) together. siRNA 1 μl (10 pmol) was diluted
in 50 μl Opti-MEM Medium before being mixed with diluted
Lipofectamine and RNAiMAX reagent at a ratio of 1:1. Then the
siRNA-lipid complex was incubated for 5 min at room temperature.
SHEDs were incubated with the siRNA-lipid complex for 24 h before
the shear stress experiments and in vitro Matrigel
angiogenesis assay were performed. Quantification of vessel-like
structures performed using the Nikon NIS-Elements AR 3.1 imaging
software (Kawasaki, Kanagawa, Japan). The tubule length and
branching points were measured on x4 magnification images
(n=3).
RT-qPCR
Total RNA of different time points were extracted
with the RNeasy Plus mini kit (Qiagen GmbH, Hilden, Germany)
(18). Sample of total RNA (1.0
μg) was used to synthesize complementary DNA by cDNA
Synthesis (SuperScript™ III Reverse Transcriptase, Invitrogen;
Thermo Fisher Scientific, Inc.) RT-qPCR was performed with the ABI
Prism 7000 Sequence Detection System using fast-SYBR Green (Applied
Biosystems; Thermo Fisher Scientific, Inc.), Standards and samples
were run in triplicates. Primer sequences were shown in Table I.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Genes | Primers |
|---|
| VEGF | F:
TTCATGGATGTCTATCAGCG
R: CATCTCTCCTATGTGCTGGC |
| VEGFR2 | F:
TCAGAAGAACTGAAAACTTTGGAA
R: GAGCCTTCAGATGCCACACACT |
| DLL4 | F:
TGCAACTGCCCTTATGGCTTTGTG
R: ACAAGTTGTTCATGGCTTCCCTGC |
| Notch1 | F:
TCCACCAGTTTGAATGGTCA
R: AGCTCATCATCTGGGACAGG |
|
EphrinB2 | F:
TGGATCACCTGAAAATGCTG
R: CGAAATCCCAAACTCCGATA |
| Hey1 | F:
TGGATCACCTGAAAATGCTG
R: CGAAATCCCAAACTCCGATA |
| Hey2 | F:
GTGCGGCTTGTGTCTCATCTC
R: CTGCTGCTGCTGCGTTTG |
| EphB4 | F:
AGAGGCCGTACTGGGACATGAG
R: TCCAGCATGAGCTGGTGGAG |
| CD31 | F:
CCCAGCCCAGGATTTCTTAT
R: ACCGCAGGATCATTTGAGTT |
| GAPDH | F:
GGCATGGACTGTGGTCATGAG
R: TGCACCACCAACTGCTTAGC |
Western blot analysis
Cells grown in glass slides were treated with shear
stress for 2 h. Then total protein was obtained by using M-PER
protein extraction buffer, and quantified using a BCA kit (Thermo
Fisher Scientific, Inc.) and separated on 7.5 or 12% polyacrylamide
gel followed by transfer onto an ImmunBlot PVDF membrane (GE
Healthcare Life Sciences, Little Chalfont, UK). The membranes were
blocked for 1 h with 5% BSA in Tris-phosphate buffer containing
0.05% Tween-20 (TBS-T). It was further incubated overnight at 4°C
with anti-Notch1 (1:2,000; ab52627), anti-DLL4 (1:1,000; ab7280),
anti-Hey1 (2 μg/ml, ab22614), anti-Hey2 (2 μg/ml;
ab25404) all from Abcam (MA, USA); anti-EphrinB2 (1:1,000;
sc-398735) and anti-EphB4 (1:1,000; sc-130081) both from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA); anti-VEGFR2 (1:2,000;
ab39256) and anti-CD31 (1:500; ab28364) both from Abcam, primary
antibodies. After three washes (5 min) with TBS-T, membranes were
further incubated with HRP-conjugated secondary antibodies:
anti-rabbit (cat. no. 7074) or anti-mouse (cat. no. 7076) both from
Cell Signaling Technology, Inc., (Danvers, MA, USA) for 1-2 h and
followed by three washes with TBS-T. The target protein signal was
detected and digitized using ECL (Thermo Fisher Scientific,
Inc.).
ELISA
The above collected supernatants were used for VEGF
secreted protein detection. The secreted levels of VEGF were
quantified using corresponding Human VEGF ELISA kits (DY293B;
R&D Systems, Inc.). Briefly, supernatants were centrifuged at
3,500 x g for 5 min, at 4°C to remove any impurity. Microplate
strips were removed from the plate frame. Assay Diluent RD1W (50
μl) (R&D Systems, Inc.) was added to each well. Then,
200 μl of VEGF standards, controls, or samples were added to
each well. Wells were covered with a plate sealer and incubated at
room temperature for 2 h. After 3 washes, 200 μl VEGF
conjugate was added and incubated at room temperature for 20 min in
the dark followed by adding 50 μl of Stop Solution (R&D
Systems, Inc.) to each well. Light absorption values were read
within 30 min using a microplate reader (Molecular Devices, San
Jose, USA) (450 nm).
Statistical analysis
All experiments were performed in triplicates and
repeated at least 3 times. Data are expressed as mean ± standard
deviation (SD). One way ANOVA analysis of variance with Tukey's
post hoc test was utilized in multiple comparisons between the
groups. P-value <0.05 was considered to be statistically
significant.
Results
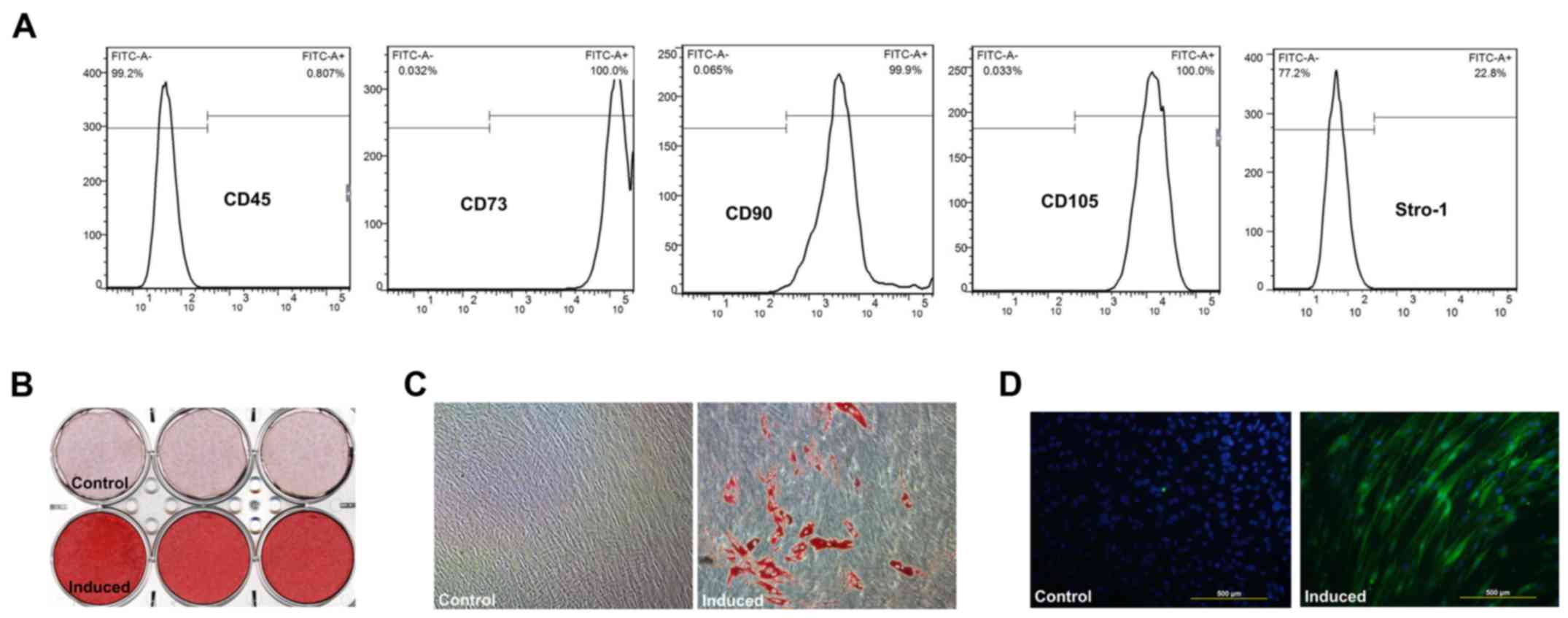
SHEDs show stem cell characteristics
SHEDs displayed a fibroblastic spindle or stellate
shape in α-MEM. Flow cytometric analyses revealed high expression
levels of CD90, CD73, and CD105, but low expression levels of CD45;
22.8% of the SHED population expressed STRO-1 (Fig. 1A). Mineralization (Fig. 1B), lipid droplets (Fig. 1C) and neurogenic marker
βIll-tubulin expression (Fig. 1D)
were verified after induction in osteogenic, adipogenic and
neurogenic media, respectively.
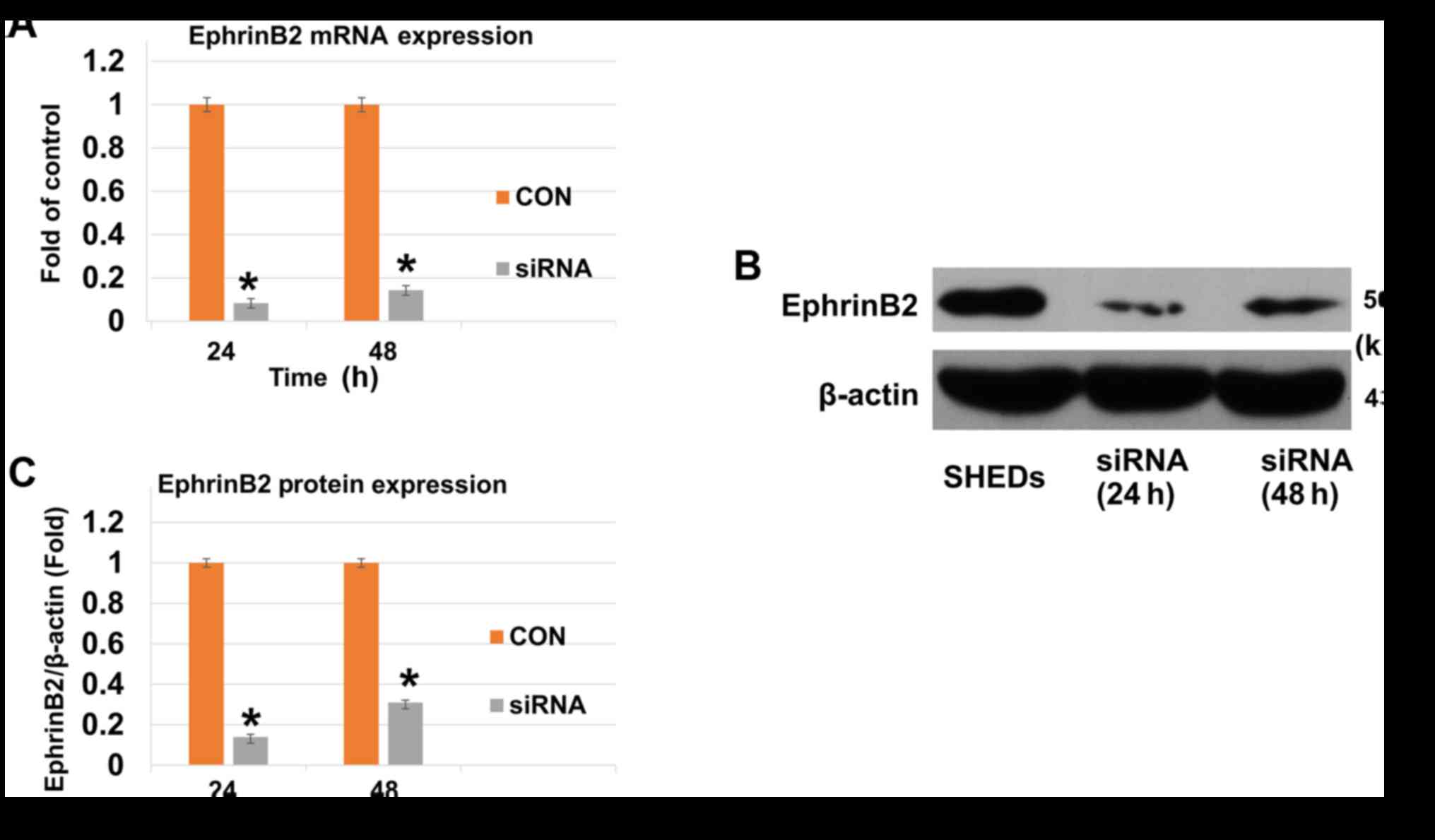
EphrinB2-siRNA suppresses mRNA and
protein expression of EphrinB2
To determine the EphrinB2 expression levels of SHEDs
after being incubated with EphrinB2-siRNA, RT-qPCR and western blot
analysis were performed. When SHEDs were incubated with
EphrinB2-siRNA for 24 and 48 h, both mRNA and protein expression of
EphrinB2 was significantly decreased (Fig. 2A–C).
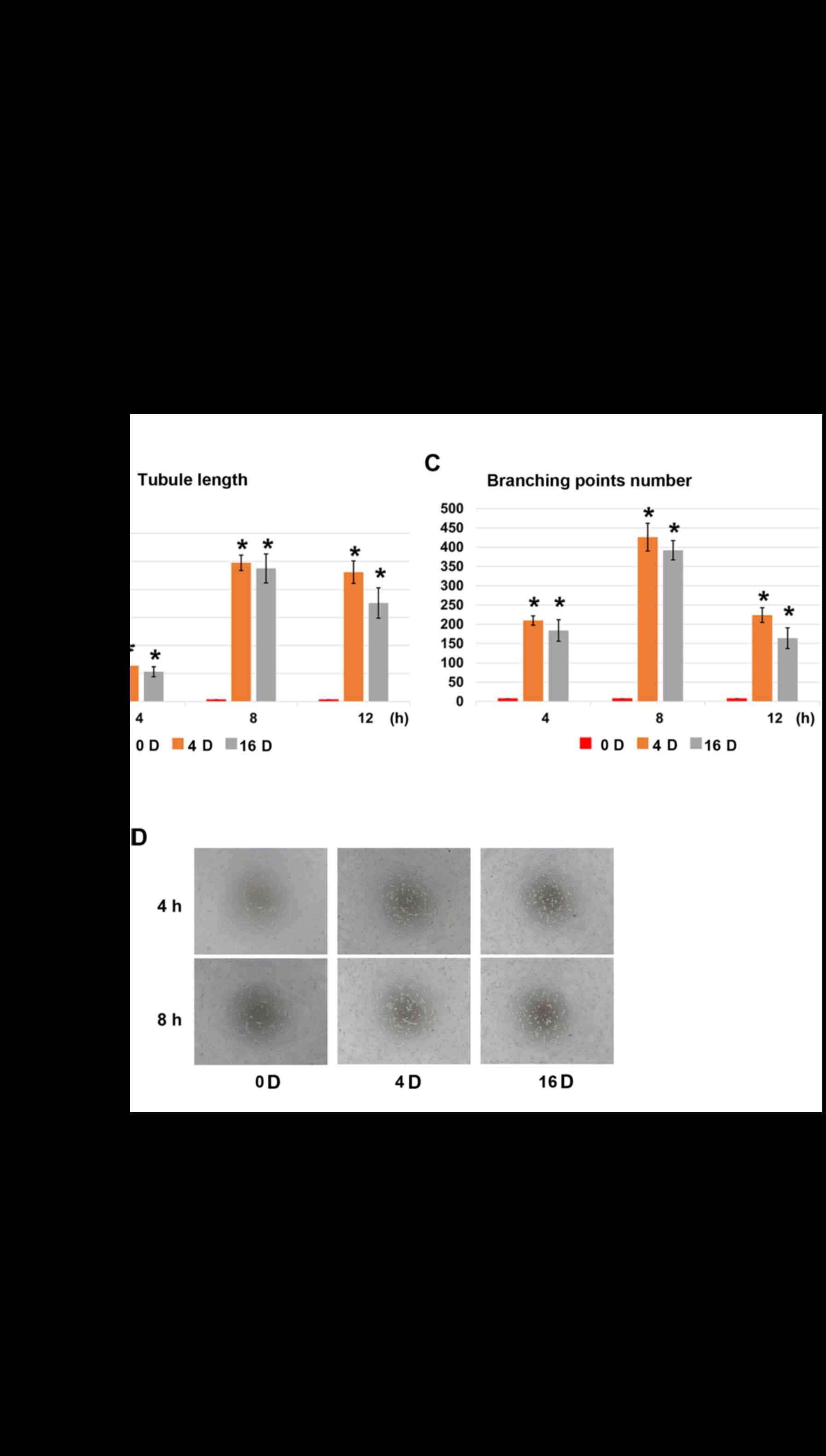
Shear stress enhances the formation of
vessel-like structures of SHEDs
Untreated SHEDs seeded on Matrigel cannot form
vessel-like structures at any time points (Fig. 3A). Groups treated with shear
stress (4 and 16 dynes/cm2) formed a small number of
vessel-like structures at 4 h (Fig.
3A). The average total tubule length was more than 5,000
μm (Fig. 3B), and the
number of branching points was more than 200 (Fig. 3C). The average total tubule
reached about 25,000 μm at 8 h in both treated groups and
began to degrade afterwards. When SHEDs were treated with EphrinB2
siRNA (SHEDs-EphrinB2-RNAi), the vessel-like structure formation
capacity was restrained (Fig.
3D), with an average total tubule length less than 3,500
μm in each group and the number of branching points less
than 120 at 8 h (Fig. 3E and
F).
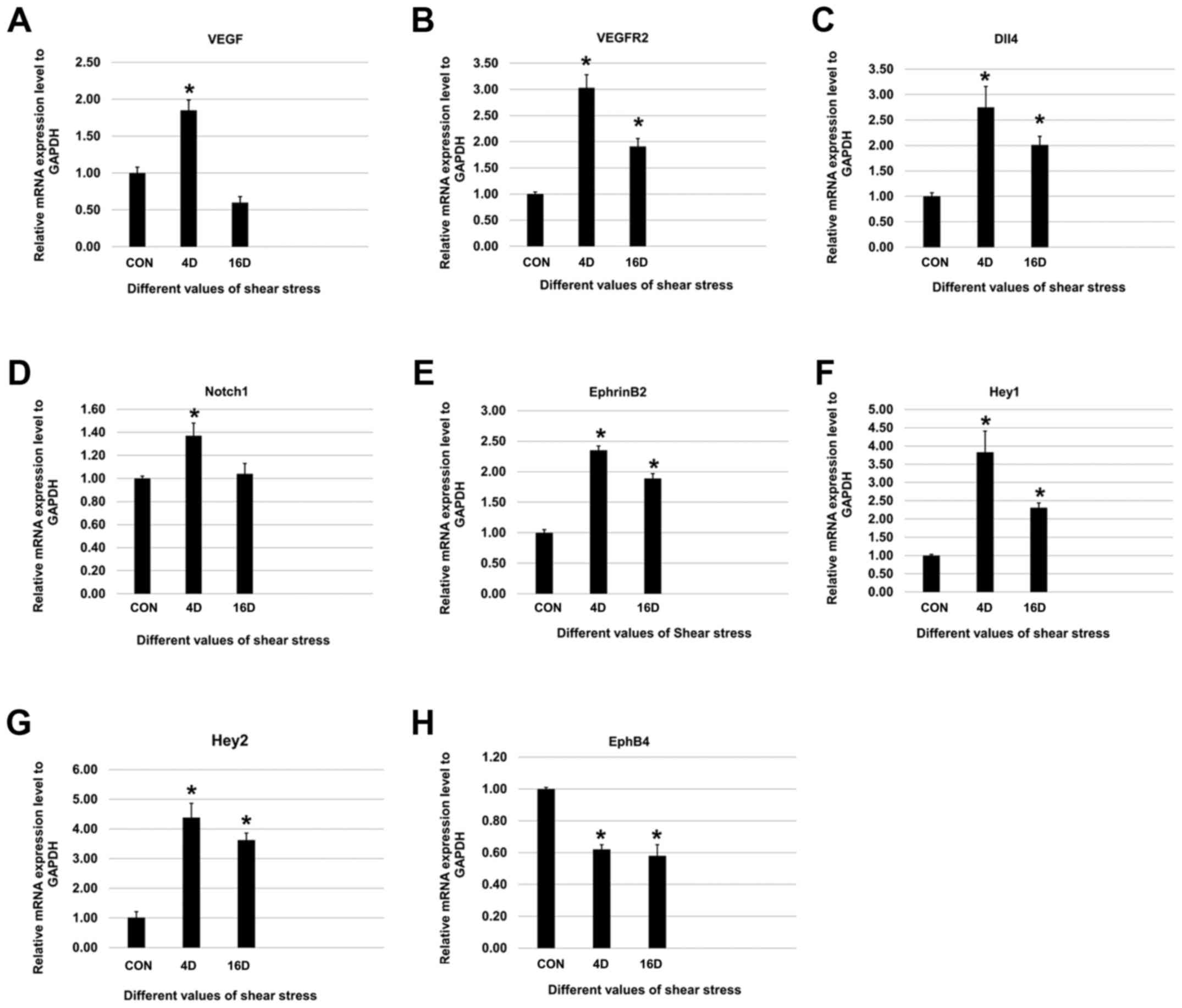
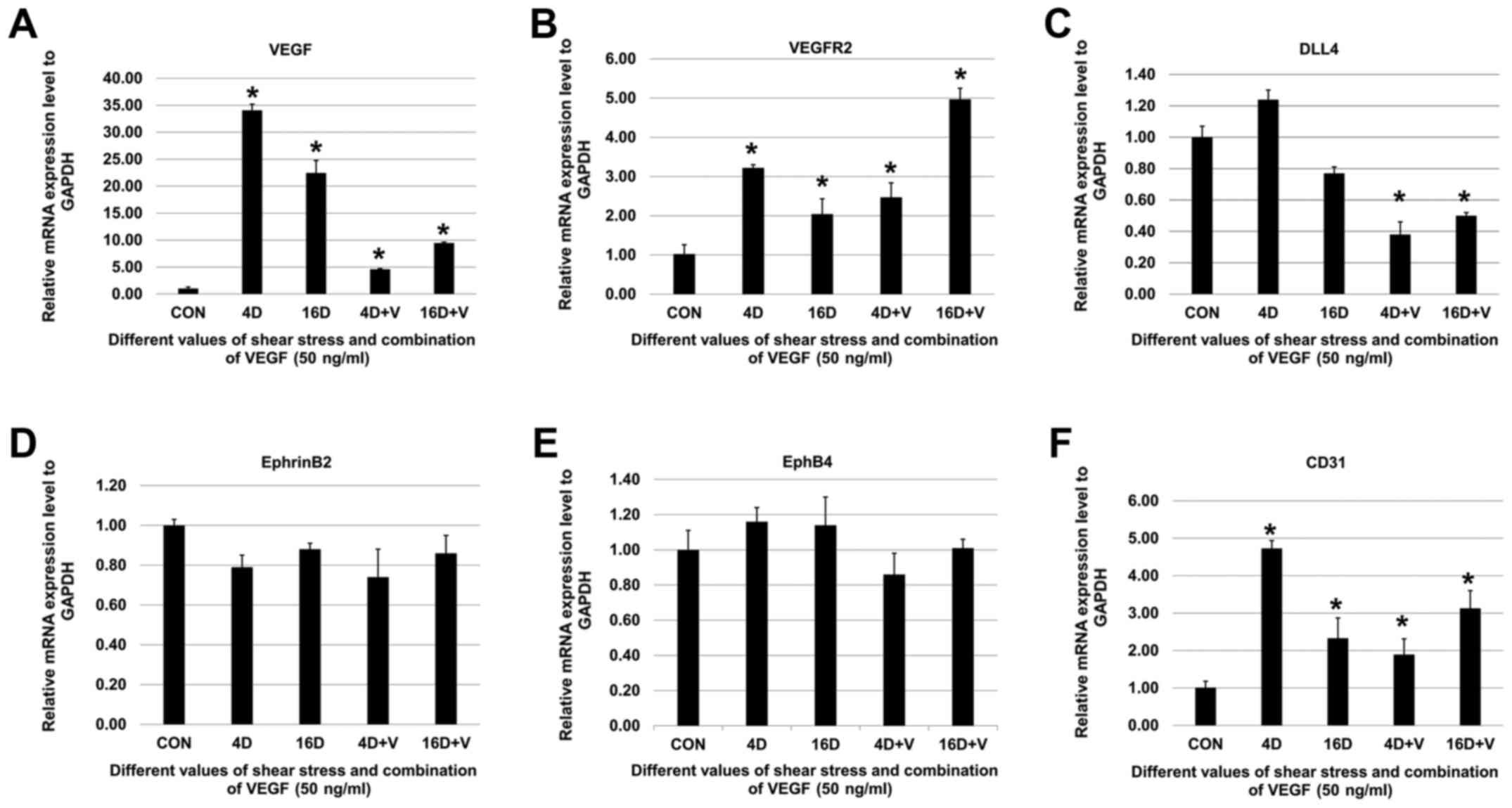
Effect of shear stress on the mRNA
expression levels of VEGF, VEGFR2, DLL4, Notchl, Heyl, Hey2,
EphrinB2, EphB4 and CD31
Shear stress (4 and 16 dynes/cm2)
upregulated the mRNA expression levels of endothelial cell markers
(VEGF, VEGFR2, and DLL4) (Fig.
4A–C) and arterial endothelial cell specific markers (Notchl,
EphrinB2, Heyl and Hey2) (Fig.
4D–G). However, the venous endothelial cell specific marker
expression (EphB4) was downregulated (Fig. 4H). Of note, compared to control
groups, mRNA expression levels of VEGF and Notch-1 increased
significantly when SHEDs were exposed to 4 dynes/cm2
groups while there was no significant difference in 16
dynes/cm2 groups (Fig. 4A
and D). After shear stress treatments, SHEDs were cultured in
FBS-free α-MEM supplemented with VEGF (50 ng/ml) for 12 h. The mRNA
expression levels of VEGF, VEGFR2, and CD31 were upregulated
significantly (Fig. 5A, B and F)
while there were no significant changes in mRNA expression levels
of EphrinB2 and EphB4 (Fig. 5D and
E). Noteworthy, mRNA expression levels of DLL4 decreased
significantly when VEGF was added (Fig. 5C).
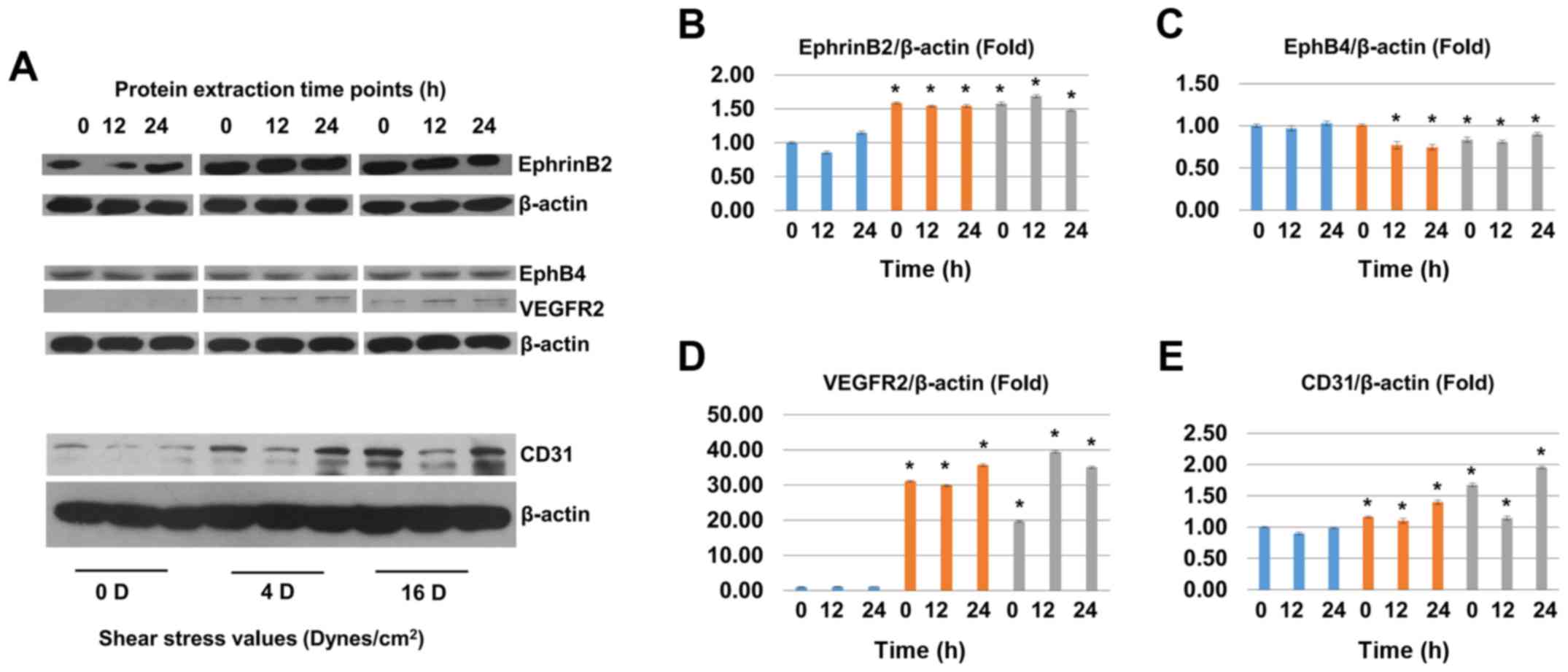
Effect of shear stress on the protein
expression levels of EphrinB2, VEGFR2, EphB4, and CD31 in
SHEDs
After shear stress (4 and 16 dynes/cm2)
treatment for 2 h, we chose three different protein extraction time
points (0, 12, and 24 h) to perform western blot assay. The protein
expression level of EphrinB2 was upregulated significantly in shear
stress groups (Fig. 6A and B).
The protein level of VEGFR2 cannot be detected in non-induced
groups but was significantly upregulated in shear stress treatment
groups while the protein level of EphB4 was downregulated in shear
stress groups (Fig. 6A, C, and
D). The protein level of CD31 was significantly upregulated in
shear stress treatment groups (Fig.
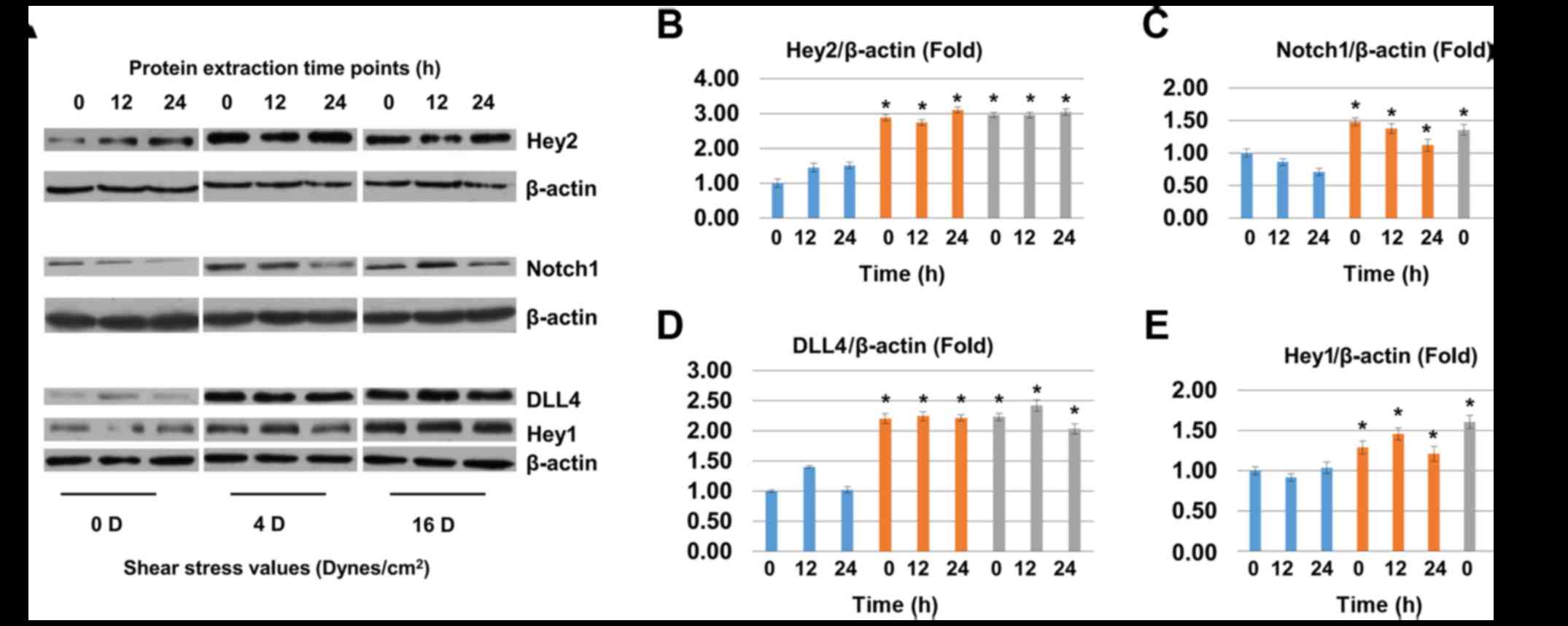
6A and E). The protein level of Hey2, Notchl, DLL4 and Heyl
were upregulated significantly in shear stress groups (Fig. 7A–E).
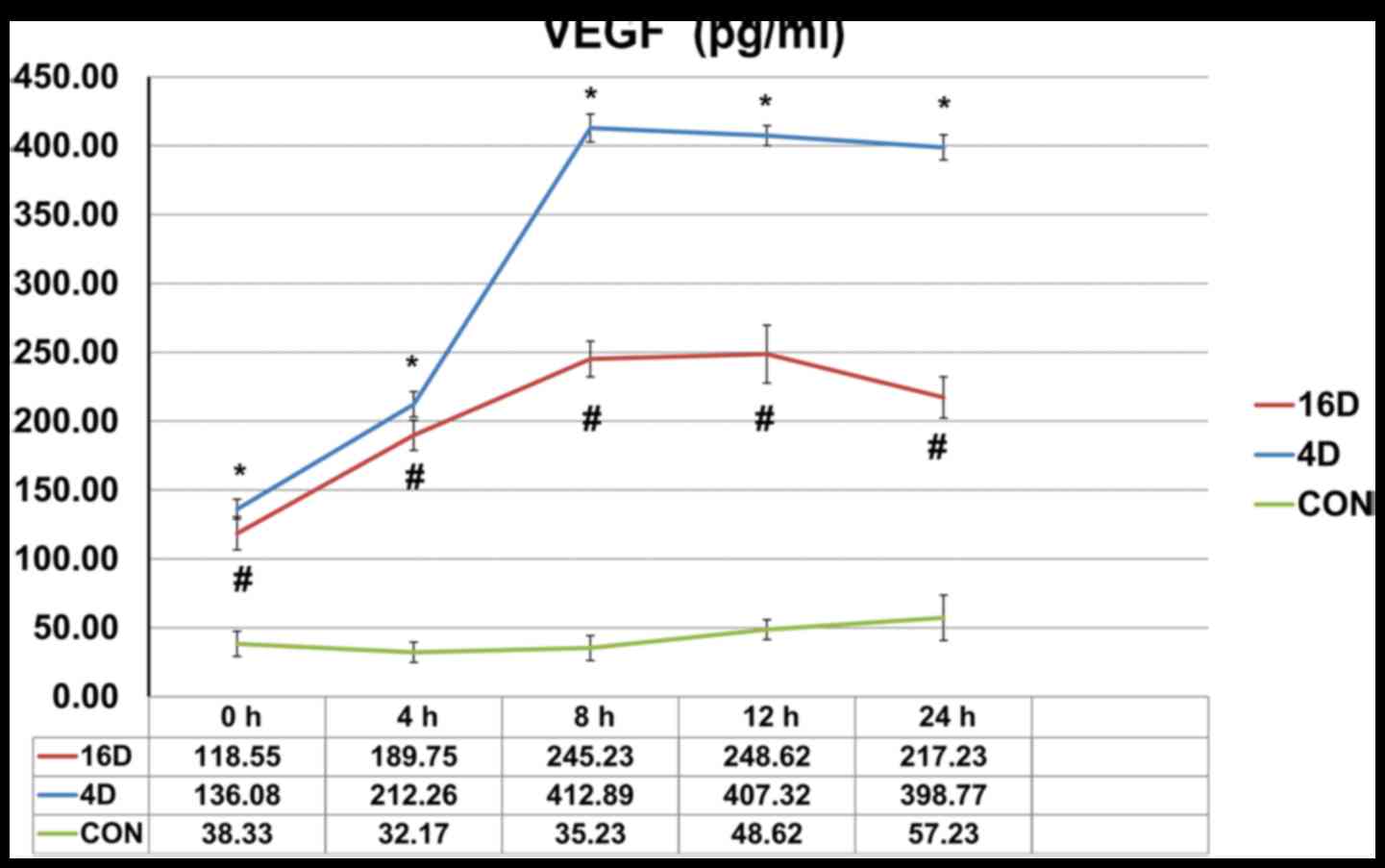
The secreted levels of VEGF in
supernatants
To investigate the mechanisms by which shear stress
in promoting the endothelial differentiation of SHEDs, ELISA assay
was performed using the Human VEGF Quantikine ELISA kit. The ELISA
results showed that the average VEGF protein concentration in
supernatants secreted by untreated SHEDs groups was no more than 60
pg/ml. When SHEDs were treated with 4 dynes/cm2 (16
dynes/cm2) shear stress, the secreted levels of VEGF
increased to 136.08 pg/ml (118.55 pg/ml), 212.26 pg/ml (189.75
pg/ml), 412.89 pg/ml (245.23 pg/ml), 407.32 pg/ml (248.62 pg/ml),
398.77 pg/ml (217.23 pg/ml) at 0, 4, 8, 12 and 24 h time points
(Fig. 8).
Discussion
Previous studies suggested that shear stress could
induce arterial specification of endothelial cells derived from
human induced pluripotent stem cells (hiPSC-ECs) and
SHH-VEGF-Notch-DLL4-EphrinB2 signaling is involved in this process
(10,13,14,19). However, some other studies also
argued that shear stress failed to induce endothelial
differentiation of human adipose tissue mesenchymal stem cells
(hASC) (20). Besides, previous
research demonstrated that two-dimension (2D) and three-dimension
(3D) environments had a different effect on cell differentiation,
drug metabolism and gene and protein expression levels. 3D chitos
and conduit together with dynamic culture system promoted the cells
aggregated into neurosphere-like cells while this effect was not
found in 2D culture environment (21,22,23). Our previous studies revealed that
TGF-β1 induced differentiation of SHEDs into vascular smooth muscle
cells (vSMCs) (24). Also, the
decellularized extracellular matrix of human umbilical vein
endothelial cells (HUVECs) promoted endothelial differentiation of
SHEDs (25). However, the effect
of biomechanical force on the differentiation of SHEDs was yet to
be demonstrated.
In the present study, we found SHEDs possess unique
stem cell characteristics, as high as 22.8% of the SHED population
expressed STRO-1, which is the widely-known mesenchymal stem cell
(MSC) marker (26). Besides,
mineralization, lipid droplets, and neurogenic marker βIII-tubulin
expression were verified after induction in osteogenic, adipogenic
and neurogenic media, respectively. These findings suggested SHEDs
might be a favorable candidate for our study.
To investigate the impact of shear stress on the
endothelial differentiation of SHEDs, in vitro matrigel
angiogenesis assay was performed as previously described (17). Untreated groups, shear stress (2
h, 4 dynes/cm2) treated groups and shear stress (2 h, 16
dynes/cm2) treated groups were seeded on Matrigel,
respectively, tubule length at 4, 8 and 12 h was quantified.
Untreated SHEDs seeded on Matrigel cannot form vessel-like
structures at any time points, whereas groups treated with shear
stress (4 dynes/cm2) formed a small number of
vessel-like structures at 4 h. When SHEDs were treated with
EphrinB2 siRNA, the vessel-like structure formation capacity was
restrained, which indicated that EphrinB2 signaling pathway is
involved in the endothelial differentiation process of SHEDs. Of
note, compared with 4 dynes/cm2 groups, groups treated
with shear stress (16 dynes/cm2) formed less vessel-like
structures, which implied that shear stress values of 4
dynes/cm2 performed more effectively in this
process.
To further evaluate the endothelial differentiation
of SHEDs, RT-qPCR and western blot analysis were performed. After
shear stress induction for 2 h, the mRNA expression of VEGF,
VEGFR2, EphrinB2, DLL4, Notch1, Hey1 and Hey2 (arterial markers) in
SHEDs increased significantly, whereas the expression of EphB4
(venous mark) decreased. The protein expression of Hey1, Hey2,
Notch1, DLL4, EphrinB2, VEGFR2 and CD31 was upregulated
significantly after shear stress treatment while the protein
expression of EphB4 was downregulated, these findings were
consistent with previous studies (13,14). When the SHEDs were cultured in
FBS-free aMEM combined with or without VEGF (50 ng/ml) for 12 h
after shear stress, the mRNA expression of VEGF, VEGFR2, and CD31
was upregulated significantly, while the expression levels of
EphrinB2 and EphB4 had no significant changes. We speculated that
this might be related to the limited plasticity of post-natal
mesenchymal stem cells (27). In
addition, arterial marker EphrinB2 and venous marker EphB4 are
known to act downstream of the SHH-VEGF-Notch-DLL4-EphrinB2 cascade
in arterial-venous specification (10,13,14,19,28). Shear stress for 2 h could not
maintain the persistent high expression of mRNA of EphrinB2 and
EphB4, even though VEGF (50 ng/ml) was added to the medium.
Contrary to Shear stress per treatment, the mRNA expression levels
of DLL4 was down-regulated significantly when VEGF was added,
suggesting that a negative-feedback loop between VEGF-DLL4/Notch
signaling may exist as previously reported (29).
Based on these results, we assumed
VEGF-DLL4/Notch1-EphrinB2 cascade took place in this process as
previously reported (10,19). To prove our hypothesis, the
secreted levels of VEGF at 0, 4, 8, 12, and 24 h in SHED culture
supernatants after shear stress were quantified. Results showed
that the concentration of VEGF in culture supernatants increased
significantly after shear stress. VEGF is known as a strong
stimulator of angiogenesis (30,31), it stimulates cellular responses by
binding to tyrosine kinase receptors (the VEGFRs) on the cell
surface. Among all the VEGFRs, VEGFR-2 (also known as KDR or Flk-1)
mediate most of the known cellular responses when activated by VEGF
(32). In this study, the protein
level of VEGFR2 cannot be detected in non-induced groups but
increased significantly in shear stress treatment groups, which
implied the signaling pathway mediated by VEGF was activated
(30,32,33).
VEGF was reported as an upstream regulator of
EphrinB2/EphB4 (34). Both
EphrinB2 and EphB4 are expressed on endothelia and mural cells.
EphrinB2 exists mainly on arterial endothelia and mural cells,
while EphB4 prefers venous ECs. The EphrinB2/Eph4 receptor
signaling pathway is known to play a key role in embryonic vascular
development (35,36), some regulatory genes including
notch-1, VEGF, and DLL4 were reported to act as the upstream
regulators of EphrinB2/EphB4 pathway activation (34). VEGF was reported to promote the
protein expression of EphrinB2 in two ways: i) VEGF promotes
expression of EphrinB2 directly, but can not promote EphrinB2
phosphorylation (37,38); and ii) VEGF activate DLL4/Notch
pathway and selectively promote the expression of EphrinB2
(14), thus suggesting that there
may exist a cascade among VEGF-DLL4/Notch-EphrinB2 in angiogenesis
(39,40). According to previous studies, VEGF
was able to downregulate the expression levels of EphB4 in
embryonic stem cells, HUVECs and adult ECs (14,33), and the process was reported to be
MAPK/ERK-dependent (33). Also,
Notch signaling was also reported to inhibit EphB4 expression by
overexpressing HERPs (32,42).
Besides, inhibition of EphB4 forward signaling was sufficient to
inhibit VEGF-induced angiogenesis in vivo (43). Herein, the
VEGF-DLL4/Notch-EphrinB2 cascade may inhibit VEGF-induced
angiogenesis through EphB4 forward signaling (44), suggesting that EphB4 forward
signaling may involve in this negative feedback loop, but more
studies have to be done to clarify the mechanisms.
In summary, our data confirmed that shear stress
could induce arterial endothelial differentiation of SHEDs.
Additionally, VEGF-DLL4/Notch1-EphrinB2 cascade took place in this
process as previously demonstrated (10). This finding not only benefits the
dental pulp tissue regeneration but also promotes the development
of other subjects, such as the treatment of ischemic heart
diseases.
Acknowledgments
Not applicable.
Funding
This work was supported by National Natural Science
Foundation Youth Science Foundation project, no. 81700954.
Availability of data and materials
The datasets generated or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
PW, SZ and ZL designed the study and conducted the
experiments. LW contributed to the acquisition and collection of
the data. CY and JX were responsible for analyzing and interpreting
the data. PW, CY, JX and SZ drafted the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gronthos S, Mankani M, Brahim J, Robey PG
and Shi S: Postnatal human dental pulp stem cells (DPSCs) in vitro
and in vivo. Proc Natl Acad Sci USA. 97:13625–13630. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miura M, Gronthos S, Zhao M, Lu B, Fisher
LW, Robey PG and Shi S: SHED: Stem cells from human exfoliated
deciduous teeth. Proc Natl Acad Sci USA. 100:5807–5812. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang GT-J, Sonoyama W, Liu Y, Liu H, Wang
S and Shi S: The hidden treasure in apical papilla: The potential
role in pulp/dentin regeneration and bioroot engineering. J Endod.
34:645–651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Z, Nör F, Oh M, Cucco C, Shi S and
Nör JE: Wnt/β-catenin signaling determines the vasculogenic fate of
post-natal mesenchymal stem cells. Stem Cells. 34:1576–1587. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bento LW, Zhang Z, Imai A, Nör F, Dong Z,
Shi S, Araujo FB and Nör JE: Endothelial differentiation of SHED
requires MEK1/ERK signaling. J Dent Res. 92:51–57. 2013. View Article : Google Scholar :
|
|
6
|
Dissanayaka WL, Zhu L, Hargreaves KM, Jin
L and Zhang C: Scaffold-free prevascularized microtissue spheroids
for pulp regeneration. J Dent Res. 93:1296–1303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cordeiro MM, Dong Z, Kaneko T, Zhang Z,
Miyazawa M, Shi S, Smith AJ and Nör JE: Dental pulp tissue
engineering with stem cells from exfoliated deciduous teeth. J
Endod. 34:962–969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang N, Chen B, Wang W, Chen C, Kang J,
Deng SQ, Zhang B, Liu S and Han F: Isolation, characterization and
multi-lineage differentiation of stem cells from human exfoliated
deciduous teeth. Mol Med Rep. 14:95–102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qazi TH, Mooney DJ, Pumberger M, Geissler
S and Duda GN: Biomaterials based strategies for skeletal muscle
tissue engineering: Existing technologies and future trends.
Biomaterials. 53:502–521. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lawson ND, Scheer N, Pham VN, Kim CH,
Chitnis AB, Campos-Ortega JA and Weinstein BM: Notch signaling is
required for arterial-venous differentiation during embryonic
vascular development. Development. 128:3675–3683. 2001.PubMed/NCBI
|
|
11
|
Welti J, Loges S, Dimmeler S and Carmeliet
P: Recent molecular discoveries in angiogenesis and antiangiogenic
therapies in cancer. J Clin Invest. 123:3190–3200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee HJ, Li N, Evans SM, Diaz MF and Wenzel
PL: Biomechanical force in blood development: extrinsic physical
cues drive prohematopoietic signaling. Differentiation. 86:92–103.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sivarapatna A, Ghaedi M, Le AV, Mendez JJ,
Qyang Y and Niklason LE: Arterial specification of endothelial
cells derived from human induced pluripotent stem cells in a
biomimetic flow bioreactor. Biomaterials. 53:621–633. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Masumura T, Yamamoto K, Shimizu N, Obi S
and Ando J: Shear stress increases expression of the arterial
endothelial marker ephrinB2 in murine ES cells via the VEGF-Notch
signaling pathways. Arterioscler Thromb Vasc Biol. 29:2125–2131.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Obi S, Yamamoto K, Shimizu N, Kumagaya S,
Masumura T, Sokabe T, Asahara T and Ando J: Fluid shear stress
induces arterial differentiation of endothelial progenitor cells. J
Appl Physiol 1985. 106:203–211. 2009. View Article : Google Scholar
|
|
16
|
Bai K, Huang Y, Jia X, Fan Y and Wang W:
Endothelium oriented differentiation of bone marrow mesenchymal
stem cells under chemical and mechanical stimulations. J Biomech.
43:1176–1181. 2010. View Article : Google Scholar
|
|
17
|
Yuan C, Wang P, Zhu L, Dissanayaka WL,
Green DW, Tong EH, Jin L and Zhang C: Coculture of stem cells from
apical papilla and human umbilical vein endothelial cell under
hypoxia increases the formation of three-dimensional vessel-like
structures in vitro. Tissue Eng Part A. 21:1163–1172. 2015.
View Article : Google Scholar :
|
|
18
|
Dissanayaka WL, Zhan X, Zhang C,
Hargreaves KM, Jin L and Tong EH: Coculture of dental pulp stem
cells with endothelial cells enhances osteo-/odontogenic and
angiogenic potential in vitro. J Endod. 38:454–463. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lawson ND, Vogel AM and Weinstein BM:
sonic hedgehog and vascular endothelial growth factor act upstream
of the Notch pathway during arterial endothelial differentiation.
Dev Cell. 3:127–136. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bassaneze V, Barauna VG, Lavini-Ramos C,
Kalil J, Schettert IT, Miyakawa AA and Krieger JE: Shear stress
induces nitric oxide-mediated vascular endothelial growth factor
production in human adipose tissue mesenchymal stem cells. Stem
Cells Dev. 19:371–378. 2010. View Article : Google Scholar
|
|
21
|
Su W-T, Shih Y-A and Ko C-S: Effect of
chitosan conduit under a dynamic culture on the proliferation and
neural differentiation of human exfoliated deciduous teeth stem
cells. J Tissue Eng Regen Med. 10:507–517. 2016. View Article : Google Scholar
|
|
22
|
Su WT, Liao YF, Lin CY and Li LT:
Micropillar substrate influences the cellular attachment and
laminin expression. J Biomed Mater Res A. 93:1463–1469. 2010.
|
|
23
|
Su WT: Ex vivo expansion of a
hematopoietic stem cell on a murine stromal cell by 3D micro-pillar
device. Biomed Microdevices. 13:11–17. 2011. View Article : Google Scholar
|
|
24
|
Xu JG, Zhu SY, Heng BC, Dissanayaka WL and
Zhang CF: TGF-β1-induced differentiation of SHED into functional
smooth muscle cells. Stem Cell Res Ther. 8:102017. View Article : Google Scholar
|
|
25
|
Gong T, Heng BC, Xu J, Zhu S, Yuan C, Lo
EC and Zhang C: Decellularized extracellular matrix of human
umbilical vein endothelial cells promotes endothelial
differentiation of stem cells from exfoliated deciduous teeth. J
Biomed Mater Res A. 105:1083–1093. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ning H, Lin G, Lue TF and Lin CS:
Mesenchymal stem cell marker Stro-1 is a 75 kd endothelial antigen.
Biochem Biophys Res Commun. 413:353–357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alison MR, Poulsom R, Otto WR, Vig P,
Brittan M, Direkze NC, Preston SL and Wright NA: Plastic adult stem
cells: Will they graduate from the school of hard knocks? J Cell
Sci. 116:599–603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
dela Paz NG, Walshe TE, Leach LL,
Saint-Geniez M and D'Amore PA: Role of shear-stress-induced VEGF
expression in endothelial cell survival. J Cell Sci. 125:831–843.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Noguera-Troise I, Daly C, Papadopoulos NJ,
Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD and Thurston
G: Blockade of Dll4 inhibits tumour growth by promoting
non-productive angiogenesis. Novartis Found Symp. 283:106–125.
2007. View Article : Google Scholar
|
|
30
|
Liang D, Chang JR, Chin AJ, Smith A, Kelly
C, Weinberg ES and Ge R: The role of vascular endothelial growth
factor (VEGF) in vasculogenesis, angiogenesis, and hematopoiesis in
zebrafish development. Mech Dev. 108:29–43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barleon B, Sozzani S, Zhou D, Weich HA,
Mantovani A and Marmé D: Migration of human monocytes in response
to vascular endothelial growth factor (VEGF) is mediated via the
VEGF receptor flt-1. Blood. 87:3336–3343. 1996.PubMed/NCBI
|
|
32
|
Holmes K, Roberts OL, Thomas AM and Cross
MJ: Vascular endothelial growth factor receptor-2: Structure,
function, intracellular signalling and therapeutic inhibition. Cell
Signal. 19:2003–2012. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang C, Guo Y, Jadlowiec CC, Li X, Lv W,
Model LS, Collins MJ, Kondo Y, Muto A, Shu C, et al: Vascular
endothelial growth factor-A inhibits EphB4 and stimulates
delta-like ligand 4 expression in adult endothelial cells. J Surg
Res. 183:478–486. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bai J, Wang YJ, Liu L and Zhao YL: Ephrin
B2 and EphB4 selectively mark arterial and venous vessels in
cerebral arteriovenous malformation. J Int Med Res. 42:405–415.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Swift MR and Weinstein BM: Arterial-venous
specification during development. Circ Res. 104:576–588. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pasquale EB: Eph-ephrin bidirectional
signaling in physiology and disease. Cell. 133:38–52. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang D, Jin C, Ma H, Huang M, Shi GP, Wang
J and Xiang M: EphrinB2/EphB4 pathway in postnatal angiogenesis: A
potential therapeutic target for ischemic cardiovascular disease.
Angiogenesis. 19:297–309. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hayashi S, Asahara T, Masuda H, Isner JM
and Losordo DW: Functional ephrin-B2 expression for promotive
interaction between arterial and venous vessels in postnatal
neovascularization. Circulation. 111:2210–2218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hainaud P, Contrerès JO, Villemain A, Liu
LX, Plouet J, Tobelem G and Dupuy E: The role of the vascular
endothelial growth factor-Delta-like 4 ligand/Notch4-ephrin B2
cascade in tumor vessel remodeling and endothelial cell functions.
Cancer Res. 66:8501–8510. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Salvucci O and Tosato G: Essential roles
of EphB receptors and EphrinB ligands in endothelial cell function
and angiogenesis. Adv Cancer Res. 114:21–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pitulescu ME and Adams RH: Eph/ephrin
molecules - a hub for signaling and endocytosis. Genes Dev.
24:2480–2492. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xue C, Chen Y, Huang Z, Ge Y, Wang H and
Wang J: EphB4 expression in pterygium is associated with
microvessel density. Int J Clin Exp Med. 7:4008–4015. 2014.
|
|
44
|
Kim I, Ryu YS, Kwak HJ, Ahn SY, Oh JL,
Yancopoulos GD, Gale NW and Koh GY: EphB ligand, ephrinB2,
suppresses the VEGF- and angiopoietin 1-induced
Ras/mitogen-activated protein kinase pathway in venous endothelial
cells. FASEB J. 16:1126–1128. 2002. View Article : Google Scholar : PubMed/NCBI
|