Introduction
Obesity is associated with an increased risk of
chronic diseases, and is a major health concern worldwide (1). Clinical data have revealed that
there is a positive correlation between oxidative stress and
obesity (2,3). The effects of oxidative stress on
adipocyte differentiation of mesenchymal stem cells (MSCs) is of
research interest. Previous studies have revealed that oxidative
stress is able to regulate stem cell differentiation into adipocyte
lineage, which is involved in the pathogenesis of obesity, but the
underlying molecular mechanisms remain unclear (4,5).
The elucidation of the molecular mechanisms involved in the
adipocyte differentiation of MSCs induced by oxidative stress is of
vital importance for the development of therapeutics for obesity
and diabetes. It is well-known that MSCs are regulated by a number
of transcription factors and microRNAs (miRNAs) in the process of
differentiation into adipocytes.
Retinoid X receptor γ (RXRγ), a member of the
nuclear receptor superfamily, is expressed in brown adipocytes and
an increased gene expression is observed during brown adipogenesis
in stem cells (6,7). It has an important function in the
control of adipogenic differentiation of MSCs (8), modulating MSC commitment and
differentiation to determine whether cells partake in adipogenesis.
RXR homodimers may promote adipogenesis by activating the target
genes of peroxisome proliferator-activated receptor (PPAR)
(9). RXRγ activates PPARγ
expression, thus promoting the entry of MSCs to the adipocyte
lineage while impeding progression for alternative lineage pathways
(10). The RXR ligand LGD1069
increases the expression of adipogenesis-associated genes (11). The functions of RXR agonists in
adipogenesis are cell type-specific and are based on the
integration of signals from different RXR dimers (12). These results indicated that RXRγ
is a positive regulator of adipogenesis. However, the epigenetic
regulation of RXRγ in adipogenic differentiation in MSCs remains
unclear.
miRNAs have been identified as essential elements of
the epigenetic machinery which post-transcriptionally repress the
expression of target genes, contributing to the regulation of a
number of biological effects (13). Previous studies have revealed that
miRNAs targeting osteoblast-associated genes are involved in
adipogenic differentiation of MSCs. miR-204/211, miR-320 and miR-30
target Runt-related transcription factor 2 to promote adipocytic
differentiation of human MSCs (14). miRNAs targeting
adipocyte-associated genes negatively regulate adipogenic
differentiation of MSCs (15,16). miRNAs targeting cell cycle and
self-renewal-associated genes indirectly regulate adipogenic
differentiation of MSCs, as identified with miR-21 (17). These results strongly suggested
that the regulation of transcription factors by miRNAs is a notable
component of the regulatory machinery. The results of the present
study predicted that the target gene of miR-330-5p is RXRγ, using
bioinformatics to further investigate the function of miR-330-5p in
targeting RXRγ involved in H2O2-induced
adipogenic differentiation of MSCs.
Materials and methods
Culture of MSCs
Sprague-Dawley rat MSCs were purchased from Cyagen
Biosciences Inc. (Guangzhou, China). The cells have been tested for
bacteria, fungi and Mycoplasma, and were assessed for
cell-surface markers. CD29, CD44 and CD90 were markedly expressed,
whereas the expression of CD34, CD45 and CD11 was <5%. Cells
were grown in accordance with the supplier's specifications, seeded
at 37°C under 5% CO2 in low-glucose Dulbecco's modified
Eagle's medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin (mother solution
was 1×104 U/ml; Thermo Fisher Scientific, Inc.). The
cells were passaged at a density of 2.0×104
cells/cm2 or cryopre-served at a density of (1.0–1.5)
×106 cells/ml.
Induction of adipogenic
differentiation
The model was established and optimized as described
previously (18-21). To induce differentiation, 2-day
post-confluent MSCs (designated day 0) were incubated in DMEM
containing 10% fetal bovine serum; differentiation was induced with
10% fetal bovine serum and 100 µM H2O2
for 1 h. After 1 h of H2O2 induction, the
induction medium was replaced with DMEM containing 10% fetal bovine
serum. The medium was replaced every 2-3 days. Cell RNA and protein
were determined on day 3, and Oil Red O staining was performed on
day 7.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was extracted using TRIzol®
(Thermo Fisher Scientific, Inc.) and chloroform. Samples of 2
µl were tested for concentration and purity using an
ultraviolet spectrophotometer (BioSpec-Nano, Shimadzu Corporation,
Kyoto, Japan) at the wavelengths of 260/280 nm. A total of 1-3
µg RNA was used to synthesize first-strand cDNA (miRNA using
specific RT primers and mRNA using random primers). Reverse
transcription was performed using a RevertAid First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol, with specific retrovirus conditions as
follows: 65°C for 5 min, followed by placing on ice, then 42°C for
60 min, heating to 70°C for 5 min and finally cooling to 4°C. The
qPCR adopted the two-step method, with a 20 µl reaction
volume and specific reaction conditions using a Talent fluorescent
quantitative detection kit (SYBR-Green) (Tiangen Biotech Co., Ltd.,
Beijing, China), according to the manufacturer's protocol as
follows: 95°C pre-degeneration for 3 min; then 40 cycles of 95°C
degeneration for 5 sec, 60°C annealing/extension for 15 sec and
fluorescence signal collection. The differences in gene expression
were analyzed using the 2−ΔΔCq method (22). U6 was used as the internal
reference for miRNA detection, and β-actin or GAPDH was used as
internal references for the detection of mRNA. The primer sequences
are presented in Table I.
 | Table IPrimers for RT-qPCR. |
Table I
Primers for RT-qPCR.
| Gene | Sequence | Supplier |
|---|
| β-actin | Forward,
5′-cccatctatgagggttacgc-3′ | Sangon Biotech,
Co., Ltd., Shanghai, China |
| Reverse,
5′-tttaatgtcacgcacgatttc-3′ | |
| PPARγ | Forward,
5′-gtcagtactgtcggtttcagaag-3′ | |
| Reverse,
5′-gcatggtgtagatgatctcatgg-3′ | |
| aP2 | Forward,
5′-tcgtcatccggtcagagagt-3′ | |
| Reverse,
5′-ccagcttgtcaccatctcgt-3′ | |
| RXRγ | Forward,
5′-cgagcagagagtgaggcaga-3′ | |
| Reverse,
5′-acactcatgtcgccgtagga-3′ | |
| U6 | RT-qPCR forward and
reverse primers, sequences unavailable from the supplier; cat. no.
MQP-0201 | Guangzhou RiboBio
Co., Ltd., Guangzhou, China |
| rno-miR-330-5p | RT-PCR forward and
reverse primers, sequences unavailable from the supplier; cat. no.
MQP-0101 | |
Western blotting
Total protein was extracted using
radioimmunoprecipitation assay lysis and extraction buffer (Thermo
Fisher Scientific, Inc.) on ice, and bicinchoninic acid protein
quantification (Beyotime Institute of Biotechnology, Haimen, China)
was used to adjust the sample concentration. Samples were boiled in
loading buffer for denaturation. A total of 30-50 µg/lane
protein was separated by SDS-PAGE (10% gel; conditions, 80 V for 30
min and 120 V for 60 min) prior to wet transfer (conditions, 300 mA
for 80 min) onto polyvinylidene difluoride membranes. Following
blocking each membranes with 5% skimmed milk in Tris-buffered
saline containing 0.01% Tween-20 (TBST) for 2 h at room
temperature, membranes were incubated with primary antibodies at
4°C overnight. Following elution with TBST, membranes were
incubated with secondary antibodies for 1 h at room temperature,
prior to elution with TBST. The enhanced chemiluminescence working
fluid (Beyotime Institute of Biotechnology) was used with the Tanon
5200 Multi Chemiluminescent Imaging system (Tanon Science &
Technology Co., Ltd., Shanghai, China) exposure strip. ImageJ
software (version 1.4; National Institutes of Health, Bethesda, MD,
USA) was used to analyze the gray values of each group. Anti-PPARγ
(cat. no. sc-7237; dilution, 1:200) and anti-CCAAT/enhancer-binding
protein α (C/EBPα; cat. no. sc-365318; dilution, 1:200) antibodies
were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA); anti-GAPDH (cat. no. AC002; dilution, 1:20,000), anti-β-actin
(cat. no. AC004; dilution, 1:20,000), anti-glucose transporter 4
(Glut4; cat. no. A0071; dilution, 1:500), anti-30 kDa adipocyte
complement-related protein (Acrp30; cat. no. A2543; dilution,
1:1,000) and horseradish peroxidase (HRP)-conjugated goat
anti-mouse immunoglobulin G (IgG; cat. no. AS003; dilution,
1:25,000) antibodies were all purchased from ABclonal Biotech Co.,
Ltd. (Woburn, MA, USA); and anti-RXRγ (cat. no. ab15518, dilution,
1:150), anti-adipocyte protein 2 (aP2; cat. no. ab186424; dilution,
1:3,000) and HRP-conjugated goat anti-rabbit IgG (cat. no. ab97051;
dilution, 1:20,000) antibodies were all purchased from Abcam
(Cambridge, MA, USA). Antibodies were diluted with 5% bovine serum
albumin, according to the manufacturers' protocols.
Oil Red O assay
According to a protocol described previously
(23), the conditioned medium was
discarded and rat MSCs were washed with PBS twice. Subsequently,
cells were fixed with 4% paraformaldehyde for 20 min and rinsed
once with ultrapure water. Next, 60% isopropanol was added for a 5
min wash, and Oil Red O working liquid was added at room
temperature for 30 min. Subsequently, 60% isopropanol was used
again two or three times to rapidly wash away the excess dye, with
ultrapure water; hematoxylin (final concentration, 99.25 mM)
staining occurred for 20 sec and tap water was used to wash cells
three times. Finally, cells were sealed with glycerol gelatin.
Using the image analysis system, five random images were captured.
Oil Red O working liquid and hematoxylin were provided by Professor
Saixia Zhang (School of Basic Medical Science, Guangzhou University
of Chinese Medicine, Guangzhou, China).
Bioinformatic prediction of the target
gene of miR-330-5p
According to a protocol described previously
(24), the target gene of
Rattus norvegicus (rno)-miR-330-5p was predicted and
screened using bioinformatics websites, including miRbase
(www.mirbase.org), miRWalk2.0
(zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/index.html),
TargetScan (www.targetscan.org/mmu_71), microRNA.org (www.microrna.org), miRTarBase (mirtarbase.mbc.nctu.edu.tw/php/index.php),
FINDTAR3 (bio.sz.tsinghua.edu.cn). The predicted gene was
selected using a Venn diagram, when the number of genes in the
intersection number >3 were imported into the DAVID
Bioinformatics Resources 6.8 website (david.ncifcrf.gov) for gene identity conversion.
Following protein enrichment and Kyoto Encyclopedia of Genes and
Genomes (www.kegg.jp/kegg/pathway.html) signal pathway
analysis, a series of genes associated with adipose differentiation
were selected as candidate target genes, including RXRγ.
Subsequently, biological products of miR-330-5p and RXRγ were
constructed for verification by western blot assay and
dual-luciferase reporter gene analysis. The sequences of miRNA and
small interfering (si)RNA are presented in Table II.
 | Table IImiRNAs and siRNA sequences. |
Table II
miRNAs and siRNA sequences.
| Name | Sequence |
|---|
| miR-330-5p
mimic |
5′-ucucugggccugugucuuaggc-3′ |
|
5′-agagacccggacacagaauccg-3′ |
| miR-330-5p
inhibitor |
5′-gccuaagacacaggcccagaga-3′ |
| siR-RXRγ
fragments | 001,
actggctccacatccatga |
| 002,
gacagtcatcccagctaca |
| 003,
ctcctccgggaatcaactt |
| miRNA mimic
negative control |
uuuguacuacacaaaaguacug |
| miRNA inhibitor
negative control |
uuuguacuacacaaaaguacug |
| siRNA negative
control | Sequence
unavailable from the supplier; cat. no. siN05815122147-1-5 |
Transient transfection of MSCs with
miR-330-5p mimic, inhibitor and the respective negative controls
(m-NC and i-NC) or siRNA (siR)-RXRγ
On the day before transfection, MSCs were inoculated
into different plates according to certain density (24-well plates,
3.0×104 cells/well; 6-well plates, 1.5×105
cells/well), fusion for cell proliferation to ~55% for
transfection. Transfection of miR-330-5p or siR-RXRγ used a
RiboFect™ CP transfection kit (Guangzhou RiboBio Co., Ltd.,
Guangzhou, China). Preparation of specific transfection complexes
was according to the manufacturer's protocol. The final
concentration of mimic, m-NC and siRNAs was 50 nM, and the final
concentration of inhibitor and i-NC was 100 nM. Western blotting
and RT-qPCR analysis were performed after 48 h.
Co-transfection of fluorescent reporter
plasmid containing RXRγ-3′-untranslated region (UTR) fragments
MSCs were inoculated at 3.0×104
cells/well density in 24-well plates, then co-transfection of
mimic, m-NC, inhibitor and i-NC with pLUC-RXRγ-wild-type (WT)
3′-UTR and pLUC-RXRγ-mutant (MUT) 3′-UTR plasmid (Shenzhen Huaan
Ping Kang Bio Technology, Inc., Shenzhen, China) using
Lipofectamine® 3000 reagent (Thermo Fisher Scientific,
Inc.) was performed according to the manufacturer's protocol. The
final concentration of miR-330-5p mimic and its control was 50 nM,
the final concentration of miR-330-5p inhibitor and its control was
100 nM, and plasmid content was 500 ng. After 48 h, using the
Dual-Luciferase® Reporter assay kit (Promega
Corporation, Madison, WI, USA) according to the manufacturer's
protocol, the degree of activation of the target reporter gene in
different samples was compared on the basis of the ratio obtained.
Using a GloMax®-20/20 Single Tube Luminometer (Promega
Corporation) to obtain the fluorescence ratio at 465 nm
(Renilla luciferase)/560 nm (firefly luciferase). Comparing
the fluorescence ratio of the experimental group and the control
group to determine the accuracy of the target gene.
Co-transfection of the miR-330-5p mimic
and its control with siR-RXRγ and its control
Using a protocol described previously (25), MSCs were inoculated at a density
of 3.0×104 cells/well in a 24-well plate or
1.5×105 cells/well in a 6-well plate. Following
transfection, 1.25 or 5 µl mimic, m-NC, siR-RXRγ-002 and
siRNA negative control (siR-NC) were diluted with 50 or 200
µl 1X riboFECT™ CP buffer (Guangzhou RiboBio Co., Ltd.),
respectively, and mixed with 5 or 20 µl riboFECT™ CP reagent
(Guangzhou RiboBio Co., Ltd.). Cells were mixed evenly and
incubated at room temperature for 15 min. The transfected complex
was added to the medium without antibiotics to a volume of 500
µl or 2 ml. Following transfection for 12 h, the original
cell culture medium was re-added and incubation at 37°C was
performed for 48 h. Subsequently, western blotting and RT-qPCR
assays were performed.
Statistical analysis
Data are presented as the mean ± standard deviation.
Data were analyzed using Excel 2016 (Microsoft Corporation,
Redmond, WA, USA), SPSS 20 (version 20; IBM Corp., Armonk, NY, USA)
and GraphPad Prism (version 7.0; GraphPad Software, Inc., La Jolla,
CA, USA). The comparison between two sample groups was analyzed
using a t-test. The comparison between multiple sample groups was
analyzed using analysis of variance followed by Dunnett's multiple
comparisons test for post hoc analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
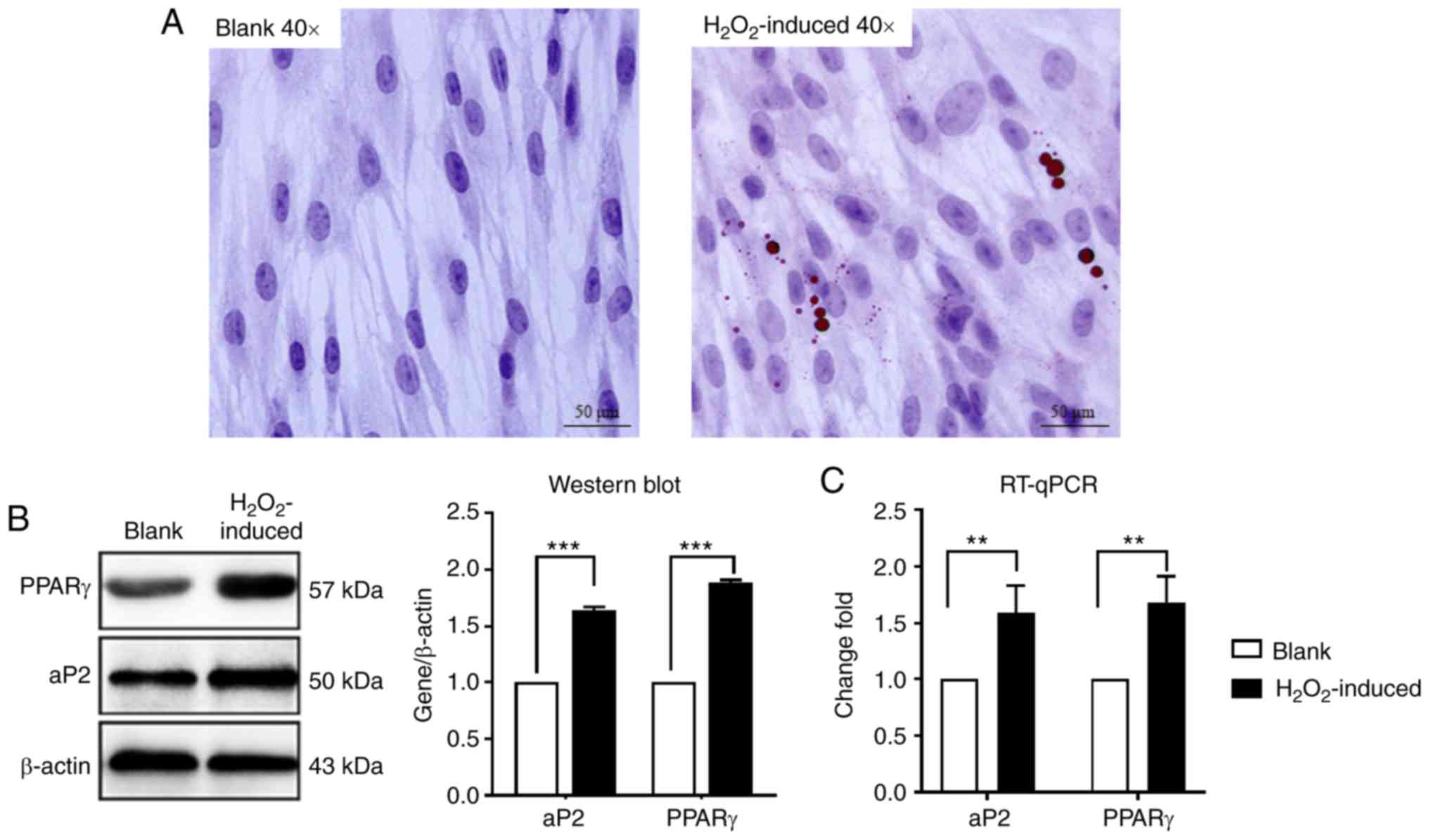
H2O2 induces
adipogenic differentiation of MSCs
To investigate whether H2O2
affects adipocyte differentiation in MSCs,
H2O2 (100 µM) was added to the culture
medium. As presented in Fig. 1A,
H2O2 treatment induced differentiation, as
detected using an Oil Red O assay, and the number of lipid droplets
increased markedly in the H2O2-induced group
compared with the blank group. RT-qPCR and western blot assay also
indicated that the expression of PPARγ and aP2 was also increased
significantly compared with the blank control group (Fig. 1B and C). These data suggested that
H2O2 induces adipogenic differentiation of
MSCs.
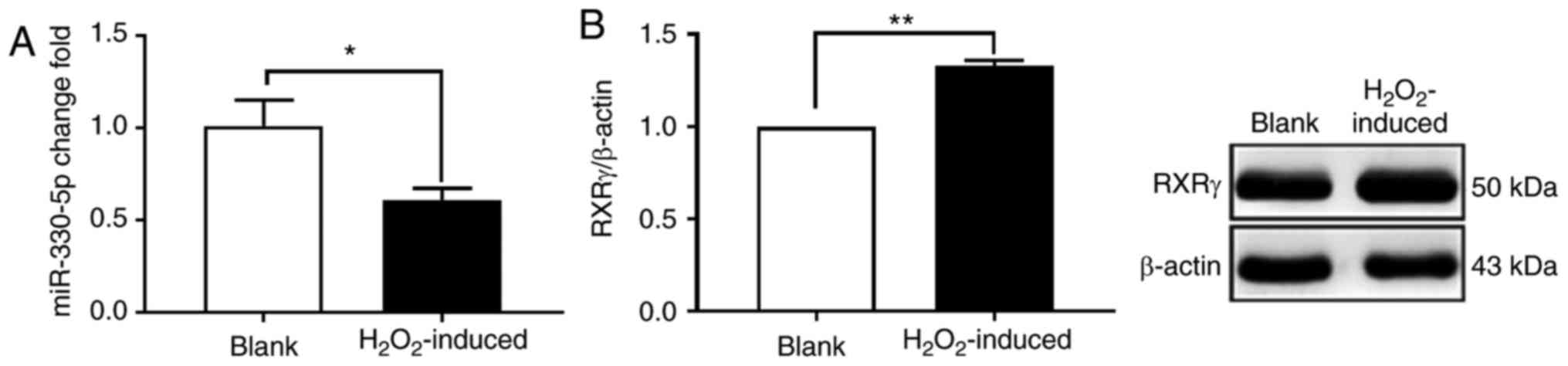
miR-330-5p expression is decreased and
RXRγ expression is increased in the
H2O2-induced adipogenic differentiation of
MSCs
To determine whether miR-330-5p expression changes
during the process of H2O2-induced adipogenic
differentiation, an RT-qPCR assay was performed. When adipogenic
differentiation of MSCs was induced by H2O2,
the expression of rno-miR-330-5p was decreased significantly
compared with the blank group (Fig.
2A). A western blot assay was used to detect the expression of
RXRγ, which identified that the expression of RXRγ was
significantly increased in the H2O2-induced
group (Fig. 2B). The results
indicated that the miR-330-5p expression was decreased and RXRγ
expression was increased in the H2O2-induced
adipogenic differentiation of MSCs.
miR-330-5p inhibits
H2O2-induced adipogenic differentiation of
MSCs
In order to further clarify the function of
miR-330-5p in the H2O2-induced adipogenic
differentiation of MSCs, miR-330-5p mimic or m-NC were used to
transfect MSCs, which were subjected to adipogenic differentiation
when induced with H2O2. An Oil Red O assay
revealed that the number of lipid droplets in the miR-330-5p mimic
group was decreased compared with the
H2O2-induced group and m-NC group, and cell
morphology tended to be in the original form of MSCs (Fig. 3A). Western blotting also indicated
that the expression of PPARγ, aP2, Glut4 and C/EBPα in the
H2O2-induced group and the m-NC group was
significantly increased compared with that in the blank group
(Fig. 3B). In contrast, the
expression of PPARγ, aP2, Glut4 and C/EBPα in the miR-330-5p mimic
group was decreased (Fig. 3B).
These data suggested that miR-330-5p inhibited
H2O2-induced adipogenic differentiation of
MSCs.
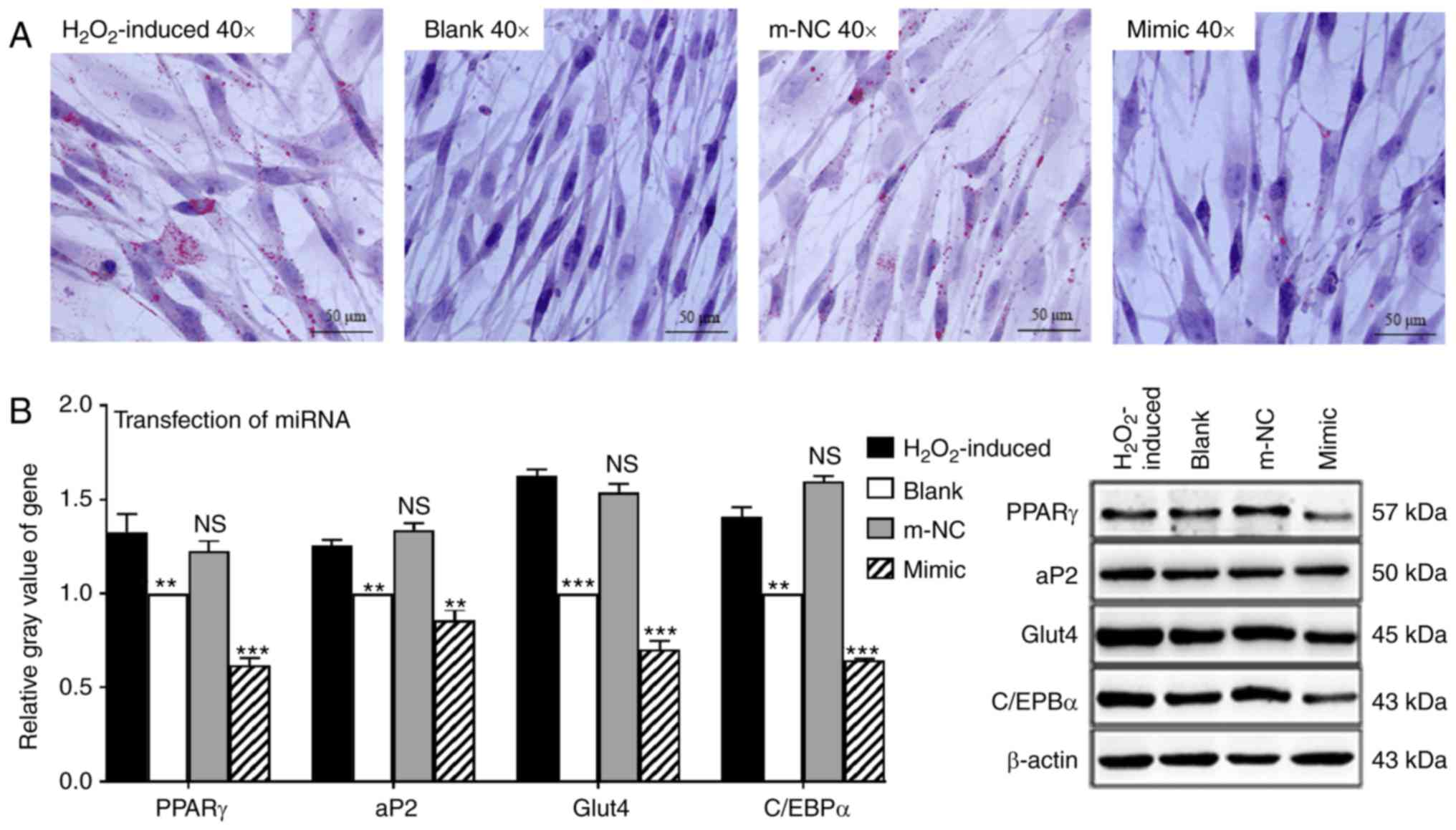 | Figure 3miR-330-5p inhibits the adipogenic
differentiation process of MSCs. (A) An Oil Red O assay identified
that the H2O2-induced group and the m-NC
group contained large numbers of lipid droplets, and overexpression
of miR-330-5p decreased the number of lipid droplets. (B) Western
blotting indicated that the gene expression levels of PPARγ, aP2,
Glut4 and C/EBPα in the H2O2-induced group
and the m-NC group were increased compared with the blank group.
Overexpression of miR-330-5p inhibited expression of the
aforementioned genes. **P<0.01,
***P<0.001 vs. the H2O2-induced
group; NS, not significant. miR, microRNA; MSC, mesenchymal stem
cell; m-NC, mimic negative control; PPARγ, peroxisome
proliferator-activated receptor γ; aP2, adipocyte protein 2; Glut4,
glucose transporter 4; C/EBPα, CCAAT/enhancer-binding protein
α. |
RXRγ is a potential target of
miR-330-5p
The aforementioned results suggested that miR-330-5p
negatively regulated adipogenesis and increased RXRγ.
Bioinformatics predicted the miR-330-5p sequence. GGGUCUC was
associated with the RXRγ gene sequence CCCAGAG (Fig. 4A). To determine whether RXRγ is
targeted to miR-330-5p through this sequence, luciferase reporters
were constructed that had either a WT 3′-UTR or a 3′-UTR containing
mutant sequences of the miR-330-5p-binding site. It was revealed
that overexpression of miR-330-5p significantly inhibited the
luciferase reporter activity of the WT RXRγ 3′-UTR, but not that of
the mutated 3′-UTR or the 3′-UTR of another gene (Fig. 4B). These results indicated that
miR-330-5p may directly regulate RXRγ expression. Further
experiments confirmed that miR-330-5p overexpression significantly
suppressed the expression of RXRγ, at the mRNA and protein levels
compared with the m-NC group (Fig. 4C
and D). These results indicated that RXRγ is a target gene of
miR-330-5p.
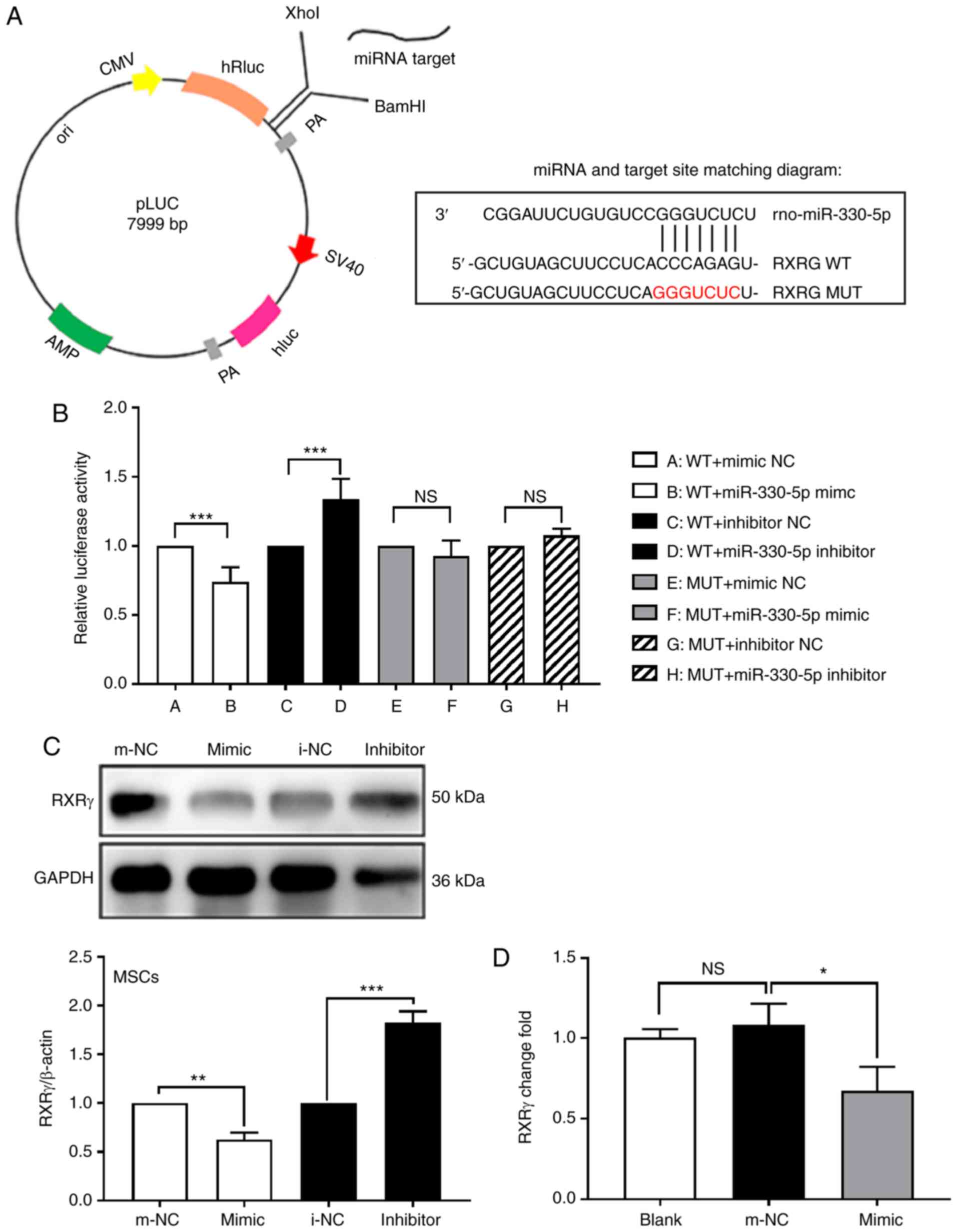 | Figure 4RXRγ is a potential target of
miR-330-5p. (A) RXRγ was predicted by miRbase, miRWalk2.0,
microRNA.org and FINDTAR3 target prediction
websites, and the construction of pLUC-RXRγ-WT and pLUC-RXRγ-MUT
was conducted by Shenzhen Huaan Ping Kang Bio Technology, Inc.,
Shenzhen, China. (B) Dual-luciferase reporter gene results
indicated that there is an association between miR-330-5p and RXRγ.
(C) Western blotting results identified that in MSCs, transfection
of miR-330-5p mimic decreased RXRγ expression compared with the
m-NC group, and transfection of the miR-330-5p inhibitor was
reversed. (D) Reverse transcription-quantitative polymerase chain
reaction results identified that RXRγ mRNA decreased in the
miR-330-5p group. *P<0.05, **P<0.01,
***P<0.001 vs. the respective NC groups; NS, not
significant. RXRγ, retinoid X receptor γ; miR, microRNA; MSC,
mesenchymal stem cell; m-NC, mimic negative control; WT, wild-type;
MUT, mutant; i-NC, inhibitor negative control. |
Silencing RXRγ inhibits adipogenic
differentiation of MSCs
To further understand the effect of RXRγ on MSCs
adipogenesis, siR-RXRγ-001, siR-RXRγ-002 or siR-RXRγ-003 were
transfected into MSCs. Western blotting and RT-qPCR analysis
verified that siR-RXRγ-002 effectively suppressed the expression of
RXRγ at the protein and mRNA levels compared with the siR-NC group
(Fig. 5A and B). The decrease in
RXRγ significantly inhibited the expression levels of the
adipogenic marker genes PPARγ, aP2, Glut4 and C/EBPα compared with
the H2O2-induced group, as indicated by
western blotting (Fig. 5C). The
Oil Red O assay clearly indicated that the downregulation of RXRγ
also resulted in decreases in the accumulation of lipid droplets
(Fig. 5D). The data demonstrated
that the downregulation of RXRγ effectively inhibited
H2O2-induced adipogenic differentiation of
MSCs.
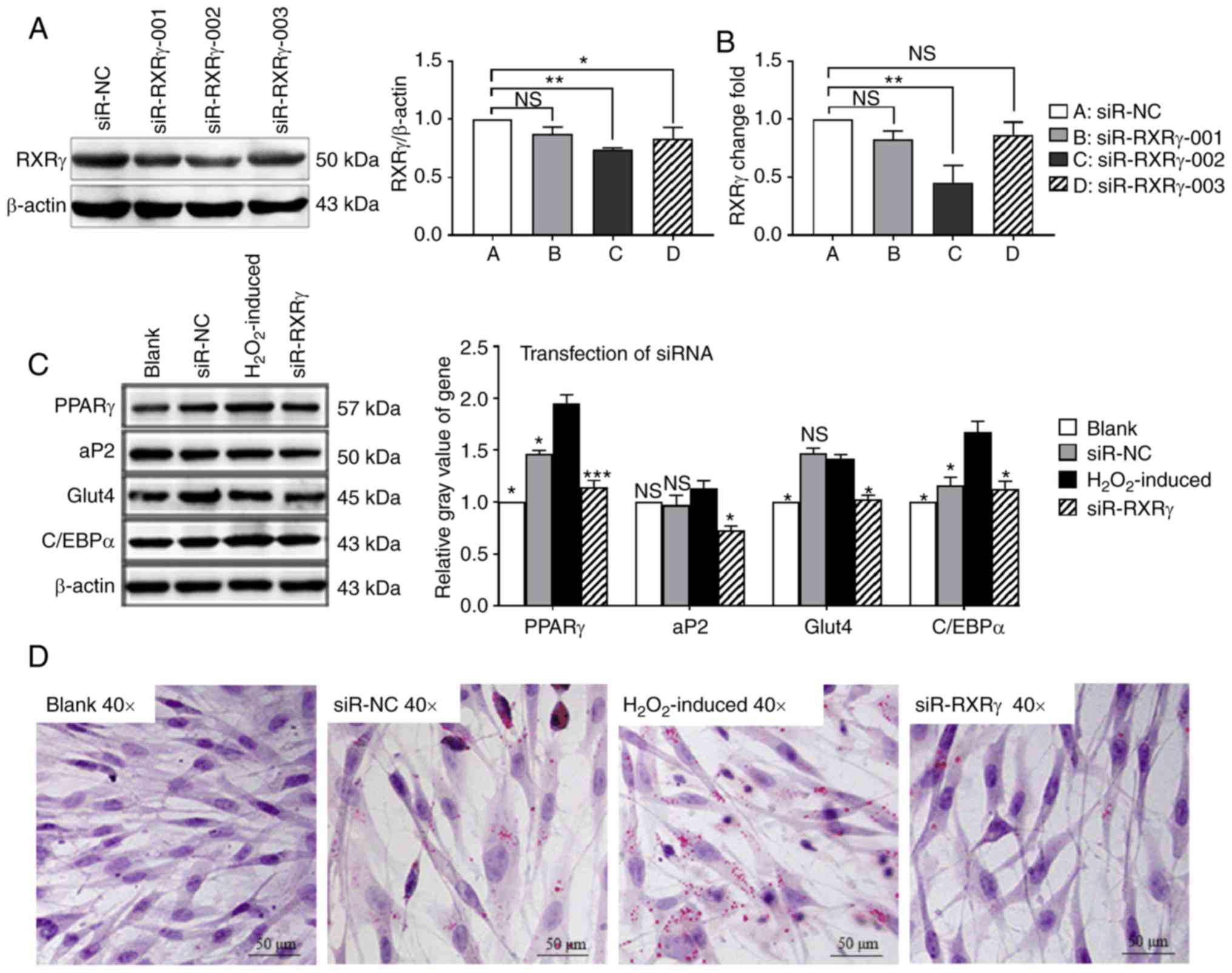 | Figure 5Silencing of RXRγ inhibits adipogenic
differentiation of MSCs. Screening of the optimal silent fragments
by transfection into rat MSCs. The second RXRγ siRNA fragment
silencing effect was selected by (A) western blotting and (B)
reverse transcription-quantitative polymerase chain reaction assay,
and β-actin was used as the internal reference for the two
experiments. *P<0.05, **P<0.01 vs. the
siR-NC group. (C) Western blotting indicated that the expression
levels of PPARγ, aP2, Glut4 and C/EBPα were decreased following
silencing of RXRγ. β-actin was used as an internal reference.
*P<0.05, ***P<0.001 vs. the
H2O2-induced group; NS, not significant. (D)
An Oil Red O assay identified that the silencing of RXRγ decreased
the number of lipid droplets. RXRγ, retinoid X receptor γ; MSC,
mesenchymal stem cell; siRNA/siR, short interfering RNA; NC,
negative control; PPARγ, peroxisome proliferator-activated receptor
γ; aP2, adipocyte protein 2; Glut4, glucose transporter 4; C/EBPα,
CCAAT/enhancer-binding protein α. |
miR-330-5p inhibition of
H2O2-induced adipogenic differentiation of
MSCs is dependent on RXRγ
From the aforementioned experiments, it may be
concluded that miR-330-5p and RXRγ are different in the process of
adipogenic differentiation of MSCs. RXRγ positively regulated the
adipogenic differentiation of MSCs, whereas miR-330-5p negatively
regulated the process. Experiments have confirmed that miR-330-5p
specifically regulated RXRγ expression; it was therefore
hypothesized that miR-330-5p inhibited MSCs differentiation by
directly targeting RXRγ, from the miR-330-5p/RXRγ signaling
pathway. To demonstrate that miR-330-5p inhibited RXRγ-dependent
adipogenic differentiation, miR-330-5p and RXRγ-interfering
fragments were co-transfected into MSCs. The western blot assay
results revealed that when siR-NC was co-transfected with
miR-330-5p mimic, inhibitor and negative control, the expression of
RXRγ in the mimic + siR-NC group was decreased compared with that
of the m-NC + siR-NC group and the effects of the inhibitor +
siR-NC group were the opposite. However, on co-transfection with
miR-330-5p and siR-RXRγ-002, the expression of RXRγ was
significantly less in the mimic + siR-RXRγ-002 group (Fig. 6A). RT-qPCR results also identified
that RXRγ mRNA levels were significantly decreased in the mimic +
siR-RXRγ-002 group (Fig. 6B). The
western blot assay revealed that transfection of mimic, without
silencing of RXRγ (i.e. siR-NC + mimic group), was able to decrease
the expression of PPARγ, C/EBPα and Acrp30, but following
transfection of mimic and silent RXRγ (i.e. siR-RXRγ + mimic
group), the decrease in PPARγ, C/EBPα and Acrp30 was greater.
Silencing RXRγ without transfection of mimic (i.e. siR-RXRγ + m-NC
group) was able to decrease the expression of PPARγ, C/EBPα and
Acrp30; however, the decrease in PPARγ, C/EBPα and Acrp30 was
greater following transfection of mimic and silent RXRγ (i.e.
siR-RXRγ + mimic group). Briefly, co-transfection of miR-330-5p
mimic with siR-RXRγ-002 significantly decreased the expression of
PPARγ, C/EBPα and Acrp30 compared with the
H2O2-induced group, the siR-NC + m-NC group,
the siR-NC + mimic group or the siR- RXRγ + m-NC group (Fig. 6C). The gain of miR-330-5p function
promoted the inhibitory effect on adipogenesis of RXRγ siRNA. These
results indicated that miR-330-5p inhibited
H2O2-induced adipogenic differentiation of
MSCs, dependent on RXRγ.
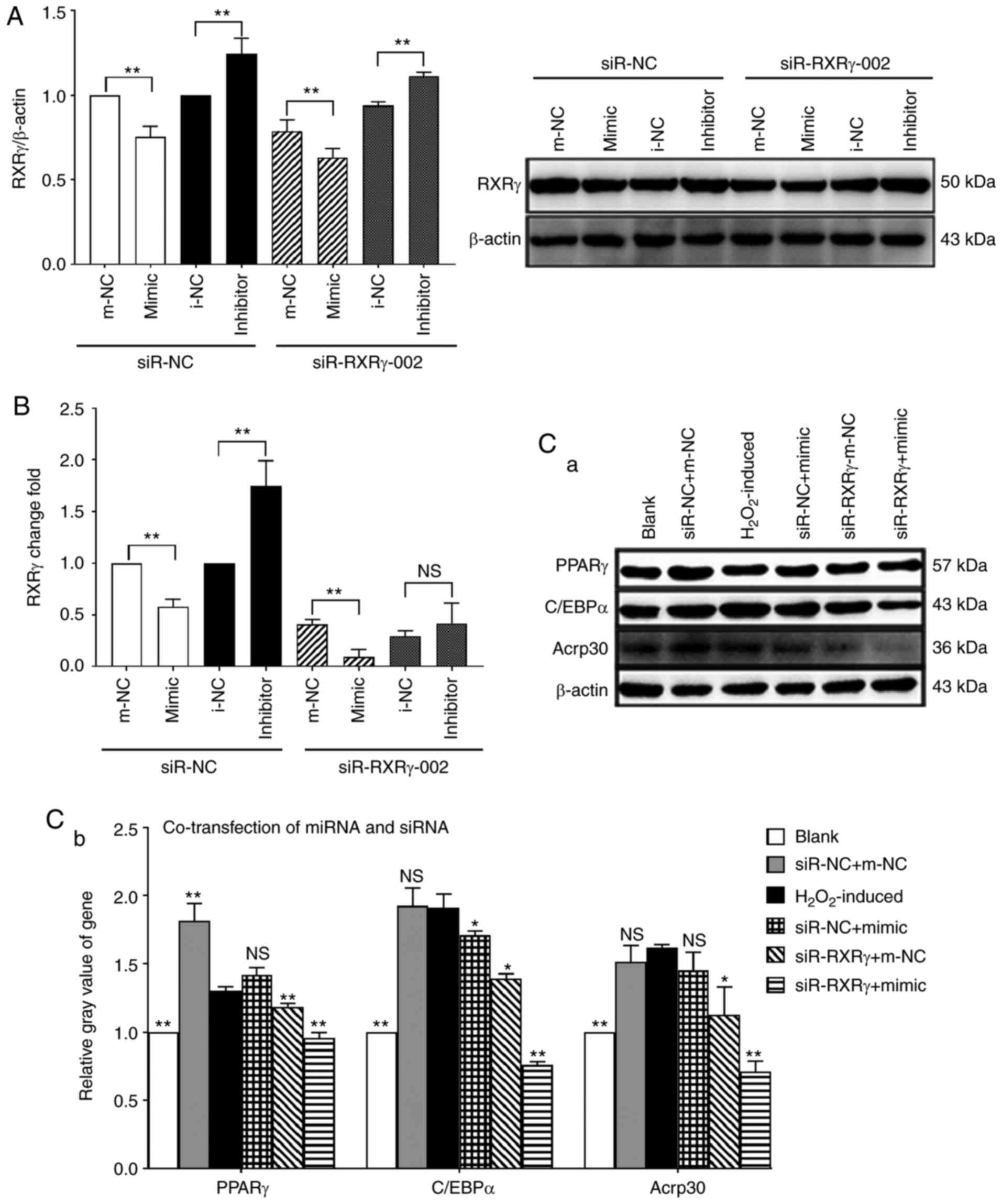 | Figure 6Inhibitory effects of miR-330-5p on
H2O2-induced adipogenic differentiation of
MSCs depend on RXRγ. (A) Western blotting and (B) RT-qPCR detected
the protein and mRNA level of RXRγ, respectively.
**P<0.01 vs. the respective NC groups; NS, not
significant. (C) Western blotting indicated that the expression
levels of PPARγ, C/EBPα and Acrp30 in the siR-RXRγ + mimic group
were significantly decreased compared with the
H2O2-induced group, the siR-NC + m-NC group,
the siR-NC + mimic group or the siR-RXRγ + m-NC group.
*P<0.05, **P<0.01 vs. the
H2O2-induced group; NS, not significant. miR,
microRNA; MSC, mesenchymal stem cell; RXRγ, retinoid X receptor γ;
NC, negative control; PPARγ, peroxisome proliferator-activated
receptor γ; C/EBPα, CCAAT/enhancer-binding protein α; Acrp30, 30
kDa adipocyte complement-related protein; siR, short interfering
RNA. |
Discussion
Oxidative stress has been associated with increased
dysfunctional adipogenesis (5,26-28), but its molecular mechanism is
unclear. Adipogenic differentiation of MSCs contributes greatly to
metabolic diseases. Therefore, it is of the utmost importance to
explore the positive and negative regulators of adipogenic
differentiation of MSCs. The results may provide promising
therapeutic targets for metabolic diseases. The present study used
an H2O2-treated MSC model to simulate a
number of significant characteristics in the process of metabolic
disorders of fat, to identify further the function of miRNAs in
regulating adipogenic differentiation of MSCs induced by oxidative
stress. The principal results of the present study were: i) Low
concentration of H2O2-induced adipogenic
differentiation of MSCs; ii) miR-330-5p was downregulated,
accompanied by upregulated RXRγ during H2O2-
induced adipogenic differentiation of MSCs; iii) miR-330-5p was
demonstrated to be a negative regulator of
H2O2-induced adipogenic differentiation of
MSCs; iv) RXRγ was demonstrated to be a positive regulator of
H2O2-induced adipogenic differentiation of
MSCs; and v) RXRγ was identified as a direct target of miR-330-5p.
These results suggested that the miR-330-5p/RXRγ signaling pathway
is an important part of the regulatory mechanisms involved in early
adipogenesis and that the miR-330-5p/RXRγ signaling pathway may be
a key target for drug development in metabolic diseases.
The key result of the present study is that
miR-330-5p is a negative regulator of
H2O2-induced adipogenic differentiation of
MSCs. A recent study identified that miR-330-5p may be associated
with cancer progression (29) and
may regulate leukemia (30).
However, the regulatory function of miR-330-5p on adipogenic
differentiation of MSCs has not been reported. Compared with the
younger MSCs, miR-330-5p is altered in the aging MSCs (31). Aging is associated with oxidative
stress, and it was hypothesized that miR-330-5p serves a function
in adipogenic differentiation of MSCs induced by oxidative stress.
To confirm the hypothesis, H2O2-induced
adipogenic differentiation of MSCs was used, which is an
established cell model (20).
MSCs have been widely used for elucidating the molecular mechanisms
involved in adipogenesis (32).
In the present study, miR-330-5p was downregulated in
H2O2-induced adipogenic differentiation of
MSCs. Therefore, it was further inferred that miR-330-5p is a
negative regulator of H2O2-induced adipogenic
differentiation of MSCs. Furthermore, the data suggested that
overexpression of miR-330-5p inhibited the process of adipogenic
differentiation induced by H2O2, which
indicated that miR-330-5p is indeed a negative regulator of
H2O2-induced adipogenic differentiation of
MSCs. These results may provide a new regulatory function of
miR-330-5p in the process of H2O2- induced
adipogenic differentiation.
The key molecular mechanism identified in the
present study is that RXRγ is a direct target of miR-330-5p. RXRγ
is initially associated with the development of the animal
(including human) fetus and is used to detect genetic variation
associated with growth, reproduction, selection of metabolic
characteristics and breeding (33). RXRγ, expressed in white and brown
fat cells, increases markedly following the differentiation of
lipids (34). Previous studies
have identified that RXRγ is crucial for fat differentiation since,
in the late stage of adipocyte differentiation, it is able to form
a heterodimer with PPAR (8,10).
The RXRγ-PPARγ association is enriched near the 5′-region of the
transcriptional start site to promote the upregulation of gene
transcription associated with fatty acid and lipid metabolism. RXRγ
is activated to modify the whole genome histone 3 Lys27
trimethylation, promoting adipogenic differentiation. It has been
identified that the expression of the adipose
differentiation-associated protein is regulated by RXRγ, which
accelerates the accumulation of neutral lipid (35). It has also been identified that
RXRγ contributes to the genetic background of familial combined
hyperlipidemia (36). Therefore,
RXRγ is a novel target for the treatment of adipose disease
(37). In view of this,
investigation has focused on RXRγ. In the present study, expression
of RXRγ was positively associated with
H2O2-induced adipogenic differentiation of
MSCs. Using target prediction tools, including miRWalk2.0 and
microRNA.org, it was observed that RXRγ is one target
of miR-330-5p. The luciferase activity result revealed that
overexpression of miR-330-5p mimic suppressed RXRγ expression.
However, this effect was abolished when a luciferase reporter
containing a mutant 3′-UTR of RXRγ was co-transfected with mimic
miR-330-5p, thus confirming the specificity of action. Enforced
expression of miR-330-5p significantly inhibited adipocyte
differentiation by decreasing RXRγ mRNA and protein levels. In
contrast, inhibition of the endogenous miR-330-5p promoted the
formation of lipid droplets by rescuing RXRγ expression.
Furthermore, the effects of the inhibition of silencing RXRγ were
similar to those of overexpression of miR-330-5p on
H2O2-induced adipogenic differentiation from
MSCs. miR-330-5p inhibited H2O2-induced
adipogenic differentiation of MSCs, and this effect was dependent
on RXRγ. Taken together, the results of the present study
identified that miR-330-5p negatively regulates
H2O2-induced adipogenic differentiation of
MSCs by targeting RXRγ.
The potential molecular pathway speculated in the
present study is that RXRγ regulates the adipogenesis of MSCs
through the PPARγ signaling pathway. It was identified that PPARγ,
C/EBPα, aP2 and Glut4 were all altered following silencing of RXRγ.
PPARγ, C/EBPα and aP2 are all key genes for fat differentiation,
and they all exist in the PPAR signaling pathway. Glut4 is involved
in the AMP-activated protein kinase (AMPK) signaling pathway. It
has been identified that the AMPK and PPARγ signaling pathways are
interrelated in the process of 3T3-IL adipocyte differentiation and
co-regulate the formation of adipocytes (38). Considering that RXR is more likely
to form a heteropolymer with PPAR, we hypothesize that RXRγ
regulates H2O2-induced adipogenic
differentiation of MSCs that may be associated with the PPARγ and
AMPK signaling pathways, particularly the PPARγ pathway. However,
additional data are required to confirm this.
The results of the present study have a number
important clinical implications. First, obesity is associated with
increased risk of heart disease, stroke and diabetes. By 2016,
there were >1.9 billion adults >18 years that were overweight
globally, of whom >650 million were obese (39). Obesity and associated disorders
lead to heavy economic burdens (40). Understanding of adipogenic
differentiation of MSCs to develop effective drugs for the
prevention and treatment of obesity and associated disorders is
vital; alterations in adipocyte differentiation of MSCs may lead to
obesity and associated disorders. The earliest symptoms of obesity
and associated disorders present as a relative deficit. Therefore,
sensitive and specific biomarkers for early detection are urgently
required. In the present study, it was demonstrated that miR-330-5p
was decreased significantly in early adipogenic differentiation.
Thus, miR-330-5p may be a potential biomarker for the early
diagnosis of obesity and its associated disorders. Secondly,
currently, there are no therapies to prevent the progression of
obesity or its associated disorders. It was revealed that
miR-330-5p functions as a negative regulator of adipogenesis by
repressing RXRγ expression, which in turn, may result in
suppression of the RXRγ signaling pathway. Therefore, a
pharmacological regulator of miR-330-5p may represent a therapeutic
strategy for obesity. Thirdly, the present study employed an
H2O2-induced adipogenic differentiation of
MSCs and provided an example of highly efficient miRNA
identification and functional dissection of the miRNA/RXRγ
signaling pathway regulating stem cell fate. Further studies on the
identified miRNA/RXRγ network will aid in understanding the
critical molecular switches in adipogenic differentiation of MSCs,
and facilitate the characterization of the miRNA basis of obesity
and associated disorders as well as the development of novel
therapies to treat them.
In conclusion, the present study aimed to detect the
effect of miR-330-5p on the adipogenic differentiation of MSCs
under oxidative stress and searching for its target genes. The
results of the present study revealed that miR-330-5p functions as
a negative regulator of H2O2-induced
adipogenic differentiation of MSCs by repressing RXRγ expression.
miR-330-5p should be considered an important candidate molecular
target of adipogenic differentiation of MSCs for the development of
preventive or therapeutic approaches against obesity and its
associated disorders.
Acknowledgments
The authors thank Professor Saixia Zhang (School of
Basic Medical Science, Guangzhou University of Chinese Medicine,
Guangzhou, China) for providing the materials and methods of Oil
Red O assay.
Funding
The present study was supported by the Guangdong
Provincial Natural Science Foundation of China (grant no.
2017A030312009) and Guangdong Provincial Science and Technology
Plan Project (grant no. 2016A050503039).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
WH performed the experiments and wrote the
manuscript; KL performed the experiments; AL performed experimental
technical guidance; ZY provided the reagents/materials; CH analyzed
the data; DC and HW conceived and designed the experiments. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bidwell AJ: Chronic fructose ingestion as
a major health concern: Is a sedentary lifestyle making it worse? A
review. Nutrients. 9:E5492017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Savini I, Gasperi V and Catani MV:
Oxidative stress and obesity. Springer International Publishing;
2016
|
|
3
|
Morihiro M and Iichiro S: Oxidative stress
and obesity: Their impact on metabolic syndrome. 2013.
|
|
4
|
Tormos KV, Anso E, Hamanaka RB, Eisenbart
J, Joseph J, Kalyanaraman B and Chandel NS: Mitochondrial complex
III ROS regulate adipocyte differentiation. Cell Metab. 14:537–544.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kanda Y, Hinata T, Kang SW and Watanabe Y:
Reactive oxygen species mediate adipocyte differentiation in
mesenchymal stem cells. Life Sciences. 89:250–258. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Evans RM and Mangelsdorf DJ: Nuclear
receptors, RXR, and the Big Bang. Cell. 157:255–266. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Svensson PA, Lindberg K, Hoffmann JM,
Taube M, Pereira MJ, Mohsen-Kanson T, Hafner AL, Rizell M, Palming
J, Dani C and Svensson MK: Characterization of brown adipose tissue
in the human perirenal depot. Obesity (Silver Spring).
22:1830–1837. 2014. View Article : Google Scholar
|
|
8
|
Shoucri BM, Martinez ES, Abreo TJ, Hung
VT, Moosova Z, Shioda T and Blumberg B: Retinoid X receptor
activation alters the chromatin landscape to commit mesenchymal
stem cells to the adipose lineage. Endocrinology. 158:3109–3125.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ijpenberg A, Tan NS, Gelman L, Kersten S,
Seydoux J, Xu J, Metzger D, Canaple L, Chambon P, Wahli W and
Desvergne B: In vivo activation of PPAR target genes by RXR
homodimers. EMBO J. 23:2083–2091. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hamza MS, Pott S, Vega VB, Thomsen JS,
Kandhadayar GS, Ng PWP, Chiu KP, Pettersson S, Wei CL, Ruan Y and
Liu ET: De-Novo identification of PPARgamma/RXR binding sites and
direct targets during adipogenesis. PLoS One. 4:e49072009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Agarwal VR, Bischoff ED, Hermann T and
Lamph WW: Induction of adipocyte-specific gene expression is
correlated with mammary tumor regression by the retinoid X
receptor-ligand LGD1069 (targretin). Cancer Res. 60:6033–6038.
2000.PubMed/NCBI
|
|
12
|
Cao J, Ma Y, Yao W, Zhang X and Wu D:
Retinoids regulate adipogenesis involving the TGFβ/SMAD and
Wnt/β-catenin pathways in human bone marrow mesenchymal stem cells.
Int J Mol Sci. 18:E8422017. View Article : Google Scholar
|
|
13
|
Ebert M and Sharp P: Roles for MicroRNAs
in conferring robustness to biological processes. Cell.
149:515–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hamam D, Ali D, Vishnubalaji R, Hamam R,
Al-Nbaheen M, Chen L, Kassem M, Aldahmash A and Alajez NM:
microRNA-320/RUNX2 axis regulates adipocytic differentiation of
human mesenchymal (skeletal) stem cells. Cell Death Dis.
5:e14992014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim SY, Kim AY, Lee HW, Son YH, Lee GY,
Lee JW, Lee YS and Kim JB: miR-27a is a negative regulator of
adipocyte differentiation via suppressing PPARgamma expression.
Biochem Biophys Res Commun. 392:323–328. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee EK, Lee MJ, Abdelmohsen K, Kim W, Kim
MM, Srikantan S, Martindale JL, Hutchison ER, Kim HH, Marasa BS, et
al: miR-130 suppresses adipogenesis by inhibiting peroxisome
proliferator-activated receptor gamma expression. Mol Cell Biol.
31:626–638. 2011. View Article : Google Scholar
|
|
17
|
Kang M, Yan LM, Zhang WY, Li YM, Tang AZ
and Ou HS: Role of microRNA-21 in regulating 3T3-L1 adipocyte
differentiation and adiponectin expression. Mol Biol Rep.
40:5027–5034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Furukawa S, Fujita T, Shimabukuro M, Iwaki
M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M and
Shimomura I: Increased oxidative stress in obesity and its impact
on metabolic syndrome. J Clin Invest. 114:1752–1761. 2005.
View Article : Google Scholar
|
|
19
|
Lin CH, Li NT, Cheng HS and Yen ML:
Oxidative stress induces imbalance of adipogenic/osteoblastic
lineage commitment in mesenchymal stem cells through decreasing
SIRT1 functions. J Cell Mol Med. 22:786–796. 2018.
|
|
20
|
Lee H, Lee YJ, Choi H, Ko EH and Kim JW:
Reactive oxygen species facilitate adipocyte differentiation by
accelerating mitotic clonal expansion. J Biol Chem.
284:10601–10609. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou J, Li H, Song S, Chen J, Du S, Li Y
and Chen D: Effects of H2O2 on proliferation
of bone marrow mesenchymal stem cell. Guangdong Med J.
26:1199–1200. 2005.In Chinese.
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Hopper N, Wardale J, Howard D, Brooks R,
Rushton N and Henson F: Peripheral blood derived mononuclear cells
enhance the migration and chondrogenic differentiation of
multipotent mesenchymal stromal cells. Stem Cells Int.
2015:3234542015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang H, Zheng Y, Wang G and Li H:
Identification of microRNA and bioinformatics target gene analysis
in beef cattle intramuscular fat and subcutaneous fat. Mol Biosyst.
9:2154–2162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mao C, Zhang J, Lin S, Jing L, Xiang J,
Wang M, Wang B, Xu P, Liu W, Song X and Lv C: miRNA-30a inhibits
AECs-II apoptosis by blocking mitochondrial fission dependent on
Drp-1. J Cell Mol Med. 18:2404–2416. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Turker I, Zhang Y, Zhang Y and Rehman J:
Oxidative stress as a regulator of adipogenesis. FASEB J. 21:A1053.
2007.
|
|
27
|
Youn JY, Siu KL, Lob HE, Itani H, Harrison
DG and Cai H: Role of vascular oxidative stress in obesity and
metabolic syndrome. Diabetes. 63:2344–2355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tumova E, Sun W, Jones PH, Vrablik M,
Ballantyne CM and Hoogeveen RC: The impact of rapid weight loss on
oxidative stress markers and the expression of the metabolic
syndrome in obese individuals. J Obes. 2013:7295152013. View Article : Google Scholar
|
|
29
|
Fu X, Zhang L, Dan L, Wang K and Xu Y:
LncRNA EWSAT1 promotes ovarian cancer progression through targeting
miR-3305pexpression. Am J Transl Res. 9:4094–4103. 2017.
|
|
30
|
Fooladinezhad H, Khanahmad H,
Ganjalikhani-Hakemi M and Doosti A: Negative regulation of TIM-3
expression in AML cell line (HL-60) using miR-3305p. Br J Biomed
Sci. 73:129–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoo JK, Kim CH, Jung HY, Lee DR and Kim
JK: Discovery and characterization of miRNA during cellular
senescence in bone marrow-derived human mesenchymal stem cells. Exp
Gerontol. 58:139–145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim DH, Vanella L, Inoue K, Burgess A,
Gotlinger K, Manthati VL, Koduru SR, Zeldin DC, Falck JR,
Schwartzman ML and Abraham NG: Epoxyeicosatrienoic acid agonist
regulates human mesenchymal stem cell-derived adipocytes through
activation of HO-1-pAKT signaling and a decrease in PPARγ. Stem
Cells Dev. 19:1863–1873. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nair U, Bartsch H and Nair J: Lipid
peroxidation-induced DNA damage in cancer-prone inflammatory
diseases: A review of published adduct types and levels in humans.
Free Radic Biol Med. 43:1109–1120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nielsen S, Åkerström T, Rinnov A, Yfanti
C, Scheele C, Pedersen BK and Laye MJ: The miRNA plasma signature
in response to acute aerobic exercise and endurance training. PLoS
One. 9:e873082014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Suzuki K, Takahashi K, Nishimaki-Mogami T,
Kagechika H, Yamamoto M and Itabe H: Docosahexaenoic acid induces
adipose differentiation-related protein through activation of
retinoid x receptor in human choriocarcinoma BeWo cells. Biol Pharm
Bull. 32:1177–1182. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nohara A, Kawashiri MA, Claudel T, Mizuno
M, Tsuchida M, Takata M, Katsuda S, Miwa K, Inazu A, Kuipers F, et
al: High frequency of a retinoid X receptor gamma gene variant in
familial combined hyperlipidemia that associates with atherogenic
dyslipidemia. Arterioscl Throm Vasc Biol. 27:923–928. 2007.
View Article : Google Scholar
|
|
37
|
Blumberg B: Obesogens, stem cells and the
maternal programming of obesity. J Dev Orig Health Dis. 2:3–8.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang S, Wang W, Miner J and Fromm M:
Cross regulation of sirtuin 1, AMPK, and PPARgamma in conjugated
linoleic acid treated adipocytes. PLoS One. 7:e488742012.
View Article : Google Scholar
|
|
39
|
World Health Organizstion Western Pacific
Region (WPRO): Reports of obesity and overweight. http://www.who.int/media-centre/factsheets/fs311/zh/.
Accessed Jan 27, 2018.
|
|
40
|
Schwartz MW, Seeley RJ, Zeltser LM,
Drewnowski A, Ravussin E, Redman LM and Leibel RL: Obesity
pathogenesis: An endocrine society scientific statement. Endocr
Rev. 38:267–296. 2017. View Article : Google Scholar : PubMed/NCBI
|