Introduction
Bone metabolism is coordinated mainly by two types
of functional cells, osteoclasts and osteoblasts (1). Osteoclasts are responsible for bone
resorption, whereas osteoblasts are responsible for bone formation
(1). Skeletal tissue is
constantly regenerated through a sequential process of resorption
and formation, known as ‘bone remodeling’, to preserve sufficient
bone quality and quantity, which is strictly regulated by numerous
hormones and cytokines (2,3).
Metabolic bone diseases, including osteoporosis, are caused by
impairment in the bone remodeling process.
Interleukin-6 (IL-6), a potent and multifuncational
cytokine, is known to perform pivotal physiological actions,
including the promotion of B-cell differentiation and the induction
of acute-phase proteins (4,5).
Regarding bone metabolism, it has been considered that IL-6
stimulates bone resorption and induces osteoclast formation
(5). However, IL-6 is also
reportedly essential in the process of bone fracture repair
(6). Therefore, IL-6 may
osteotropically modulate bone formation under conditions of
increased bone turnover (7).
Heat shock proteins (HSPs), are molecular
chaperones, which function as central regulators of proteostasis
under various stresses, including heat and chemicals (8). HSPs that are responsible for the
quality control of protein folding, facilitate the refolding of
misfolded proteins or assist in their deletion (8). Accumulating evidence indicates that
HSPs are implicated in various physiological cellular functions,
including the immune response and regulation of the cytoskeleton,
in addition to protein folding (8). Among the HSP family, HSP90, a
homodimeric ATPase, is constitutively expressed in various types of
unstressed mammalian cell (9,10).
It is well known that HSP90 is essential in the regulation of
steroid hormone receptor under physiological conditions (8). Furthermore, it has been shown that
the expression of HSP90 is elevated in several types of cancer, and
that client proteins of HSP90 contribute to several oncogenic
signaling pathways (11,12). As such, HSP90 inhibitors,
including 17-allylamino-17demethoxy-geldanamycin (17-AAG) (13),
17-dimethylamino-ethylamino-17-demethoxy-geldanamycin (17-DMAG)
(14) and geldanamycin (15), have received attention for their
potential utility as anticancer chemotherapeutics (11,12).
In terms of the effects of HSP90 inhibitors on bone
metabolism, it has been reported that 17-AAG induces
osteoclastgenesis and potentiates osteolytic bone metastasis in the
bone metastasis of breast cancer cells (16). Additionally, geldanamycin
reportedly induces autophagy and apoptosis in osteosarcoma cells
(17). In our previous study
(18), HSP90 was shown to
negatively regulate the prostaglandin F2α-stimulated
synthesis of IL-6 in osteoblast-like MC3T3-E1 cells, and the effect
of HSP90 on the synthesis of IL-6 was exerted by regulating p38
mitogen-activated protein kinase (MAPK) of the MAPK superfamily
(19). However, the details
regarding the role of HSP90 in osteoblasts remain to be fully
elucidated.
It is firmly established that thrombin, a serine
protease, is key in the blood coagulation cascade, promoting the
cleavage of fibrinogen to fibrin (20). Accumulating evidence suggests that
thrombin also affects the cell functions of various types of cells,
including osteoblasts, through specific receptors on the cell
surface termed protease-activated receptors (21). It has been reported that thrombin
stimulates the proliferation of osteoblasts and secretion of IL-6,
and suppresses alkaline phosphatase activity, a phenotype of
osteoblastic differentiation (21).
In terms of IL-6 synthesis in osteoblasts, our
previous investigations demonstrated that thrombin induces IL-6
synthesis, at least in part, via p38 MAPK and p44/p42 MAPK of the
MAPK superfamily in osteoblast-like MC3T3-E1 cells (22,23). Additionally, it was revealed that
Rho-kinase regulates the thrombin-stimulated synthesis of IL-6 at a
point upstream of p38 MAPK in these cells (23). However, the mechanism underlying
the thrombin-stimulated synthesis of IL-6 in osteoblasts has not
been clarified.
In the present study, the involvement of HSP90 in
thrombin-induced IL-6 synthesis was investigated in
osteo-blast-like MC3T3-E1 cells using HSP90 inhibitors. It was
shown that the thrombin-stimulated synthesis of IL-6 was amplified
by HSP90 inhibitors in osteoblasts, and that the effect of HSP90
inhibitors was exerted at the point between Rho-kinase and p38
MAPK.
Materials and methods
Materials
17-AAG, 17-DMAG and SB203580 were purchased from
Calbiochem; EMD Millipore (Billerica, MA, USA). Thrombin and
geldanamycin were obtained from Sigma-Aldrich; EMD Millipore. A
mouse IL-6 enzyme-linked immunosorbent assay (ELISA) kit was
purchased from R&D Systems, Inc. (Minneapolis, MN, USA).
Phospho-specific p44/p42 MAPK antibodies (cat. no. 9101), p44/p42
MAPK antibodies (cat. no. 9102), phospho-specific p38 MAPK
antibodies (cat. no. 4511), p38 MAPK antibodies (cat. no. 9212) and
phospho-specific myosin phosphatase targeting subunit (MYPT-1)
antibodies (cat. no. 4563) were purchased from Cell Signaling
Technology, Inc. (Beverly, MA, USA). Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) antibodies (cat. no. sc-25778) were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). An ECL
western blot detection system was purchased from GE Healthcare Life
Sciences (Chalfont, UK). Other materials and chemicals were
obtained from commercial sources. 17-AAG, 17-DMAG, geldanamycin and
SB203580 were dissolved in dimethyl sulfoxide. The maximum
concentration of dimethyl sulfoxide was 0.1%, which did not affect
assays for IL-6, reverse transcription-polymerase chain reaction
(RT-PCR) analysis or western blot analysis.
Cell culture
Cloned osteoblast-like MC3T3-E1 cells derived from
newborn mouse calvariae (24)
were provided by Dr. M. Kumegawa (Meikai University, Sakado, Japan)
and maintained as described previously (25). In brief, the cells were cultured
in α-minimum essential medium (α-MEM; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) containing 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a
humidified atmosphere of 5% CO2/95% air. The cells were
seeded into 35-mm diameter dishes (5×104 cells/dish) for
the IL-6 assay and RT-PCR analysis, or in 90-mm diameter dishes
(2×105 cells/dish) for western blot analysis in α-MEM
containing 10% FBS. After 5 days, the medium was replenished with
α-MEM containing 0.3% FBS at 37°C for 48 h. The cells were then
used in the experiments described below.
Assay for IL-6
The cultured MC3T3-E1 cells were stimulated by 1
U/ml thrombin or vehicle in 1 ml of α-MEM containing 0.3% FBS for
48 h and then pretreated with various doses of 17-AAG or 17-DMAG,
or 1 µM of geldanamycin at 37°C for 60 min. Preincubation
with 10 µM of SB203580 or vehicle was performed at 37°C for
60 min prior to pretreatment with 17-AAG. The conditioned medium
was collected at the end of the incubation, and the concentration
of IL-6 in the medium was then measured using the mouse IL-6 ELISA
kit according to the manufacturer’s protocol.
RT-PCR analysis
The cultured MC3T3-E1 cells were pretreated with 1
µM of geldanamycin or vehicle for 60 min, and then
stimulated by 1 U/ml of thrombin or vehicle in α-MEM containing
0.3% FBS for 3 h. Total RNA was isolated and reverse transcribed
into complementary DNA at 37°C for 60 min and then 95°C for 5 min
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
an Omniscript Reverse Transcriptase kit (Qiagen, Inc., Valencia,
CA, USA) and Oligo (dT) 12–18 Primers (Thermo Fisher Scientific,
Inc.), respectively. RT-PCR analysis was performed in capillaries
using a Light Cycler system with Fast Start DNA Master SYBR Green I
(Roche Diagnostics, Basel, Switzerland). Samples (cDNA 2.5
ng/assay, primers 0.5 mM) were subjected to the following PCR
thermocycling conditions: Initial denaturation at 95°C for 10 min;
followed by 40 cycles of denaturation at 95°C for 1 sec, annealing
at 60°C for 5 sec and elongation at 72°C for 7 sec. Primers used
for PCR were purchased from Takara Bio, Inc. (Otsu, Japan) and had
the following sequences: IL-6 forward, 5′-CCATTCACAAGTCGAGGCTTA-3′
and reverse, GCAAGTGCATCATCGTTGTTCATAC; and GAPDH forward,
5′-TGTGTCCGTCGTGGATCTGA-3′ and reverse,
5′-TTGCTGTTGAAGTCGCAGGAG-3′.
Western blot analysis
The cultured MC3T3-E1 cells were pretreated with
various doses of 17-AAG or geldanamycin for 60 min, and then
stimulated by 1 U/ml of thrombin or vehicle in α-MEM containing
0.3% FBS for the indicated periods. The cells were then washed
twice with phosphate-buffered saline, and then lysed, homogenized
and sonicated in a lysis buffer containing 62.5 mM Tris/HCl (pH
6.8), 2% sodium dodecyl sulfate (SDS), 50 mM dithiothreitol and 10%
glycerol. SDS-polyacrylamide gel electrophoresis was performed
using the method of Laemmli (26)
using 10% polyacrylamide gels. Protein quantification was performed
using the Bradford method using a Pierse BCA Protein Assay kit
(Thermo Fisher Scientific, Inc.) according to the manufacturer’s
protocol. The protein (10 µg/lane) was fractionated and
transferred onto an Immun-Blot PVDF membrane (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The membranes were blocked with 5%
fat-free dry milk in Tris-buffered saline-Tween [20 mM Tris-HCl (pH
7.6), 137 mM NaCl and 0.1% Tween-20] for 1 h prior to incubation
with primary antibodies. The western blot analysis was performed as
described previously (27) using
phospho-specific p44/p42 MAPK antibodies, p44/p42 MAPK antibodies,
phospho-specific p38 MAPK antibodies, p38 MAPK antibodies,
phospho-specific MYPT-1 antibodies and GAPDH antibodies as primary
antibodies. Peroxidase-labeled antibodies raised in goat against
rabbit IgG (cat. no. 5220-0336; KPL, Inc., Gaithersburg, MD, USA)
were used as secondary antibodies. The primary and secondary
antibodies were diluted at 1:1,000 with 5% fat-free dry milk in the
Tris-buffered saline-Tween. The peroxidase activity on the PVDF
sheet was visualized on X-ray film using the ECL western blot
detection system.
Densitometric analysis
Densitometric analysis of the western blots was
performed using a scanner and image analysis software program
(Image J version 1.48; NIH, Bethesda, MD, USA). The phosphorylated
protein levels were calculated as follows: Background-subtracted
signal intensity of each phosphorylation signal was respectively
normalized to the total protein signal and plotted as the fold
increase compared with that of the control cells without
stimulation.
Statistical analysis
The data were analyzed using one-way analysis of
variance followed by Bonferroni’s method for multiple comparisons
between pairs using StatView (ver.5.0; SAS Institute, Inc., Cary,
NC, USA). P<0.05 was considered to indicate a statistically
significant difference. All data are presented as the mean ±
standard deviation of triplicate determinations from three
independent cell preparations.
Results
Effects of 17-AAG, 17-DMAG or
geldanamycin on the thrombin-stimulated release of IL-6 in MC3T3-E1
cells
HSP90 inhibitors, 17-AAG, 17-DMAG and geldanamycin,
which are all benzoquinone ansamycin antibiotics, bind to the
N-terminal domain ATP binding site of HSP90, inhibiting the
ATP-dependent HSP90 chaperone activity (13–15). To clarify whether or not HSP90 is
involved in the thrombin-induced synthesis of IL-6 in
osteoblast-like MC3T3-E1 cells, the present study examined the
effects of these HSP90 inhibitors on the release of IL-6. The
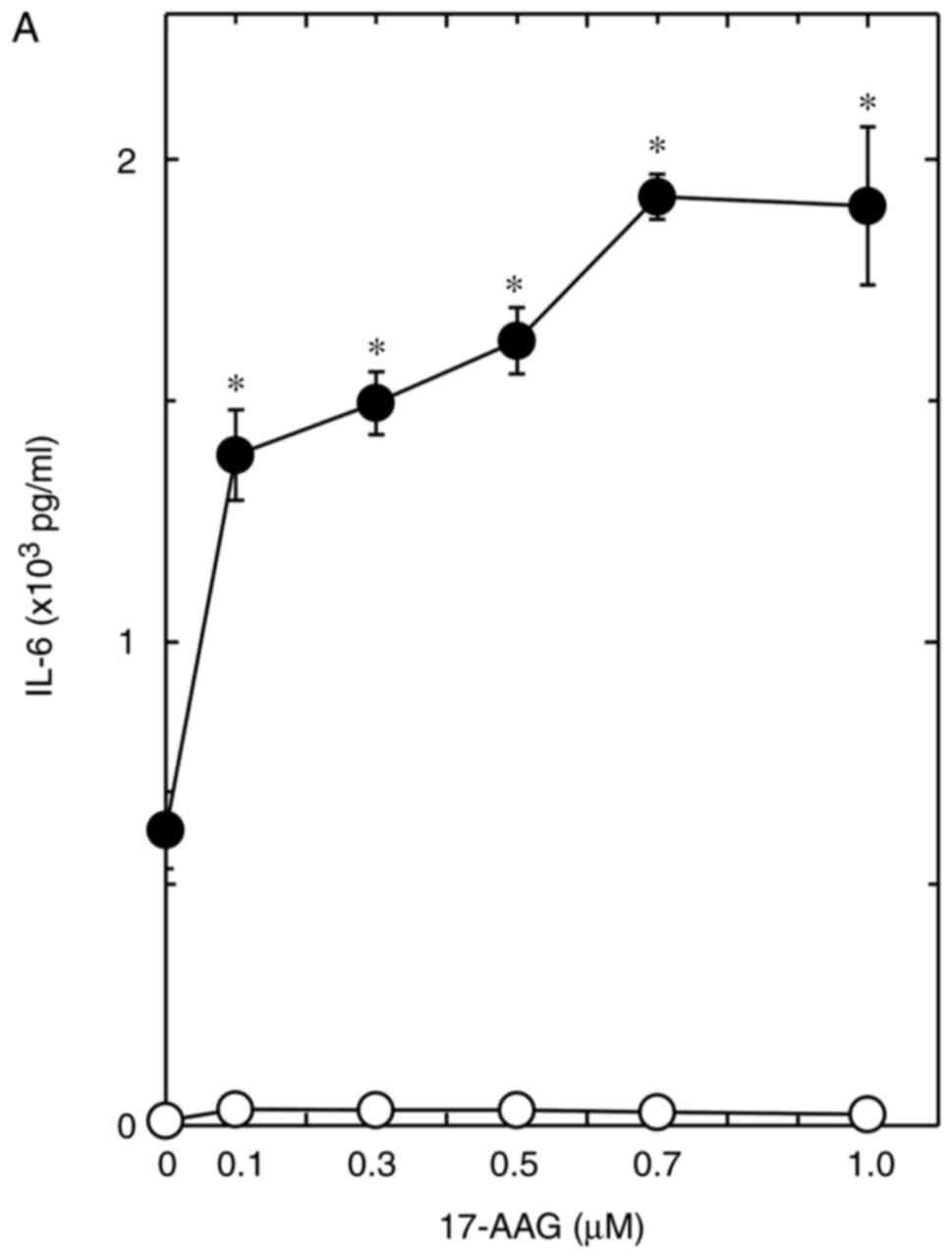
effect of 17-AAG was dose-dependent over the range of 0.1–1
µM, and the maximum effect was observed at 0.7 µM
with 1.9×103 pg/ml IL-6, which induced an increase of
~310% from the effect of thrombin at 0.6×103 pg/ml IL-6
(P=1.6×10−5; Fig. 1A).
Additionally, the effect of 17-DMAG was dose-dependent over the
range of 0.01–0.1 µM, and the maximum effect was observed at
0.1 µM with 3.1×103 pg/ml IL-6, which induced an
increase of ~560% from the effect of thrombin at 0.5×103
pg/ml IL-6 (P=8.0×10−6; Fig. 1B).
The release of IL-6 by thrombin was significantly
increased by geldanamycin (1 µM) with an IL-6 level of
3.8×103 pg/ml, which was an increase of ~700% from the
effect of thrombin at 0.5×103 pg/ml IL-6
(P=3.0×10−4; Table
I).
 | Table IEffect of geldanamycin on the
thrombin-stimulated release of IL-6 in MC3T3-E1 cells. |
Table I
Effect of geldanamycin on the
thrombin-stimulated release of IL-6 in MC3T3-E1 cells.
| Geldanamycin (1
µM) | Thrombin (1
U/ml) | IL-6 (pg/ml) |
|---|
| − | − | 10.0±2.0 |
| − | + | 468.7±65.4a |
| + | − | 102.2±5.5 |
| + | + |
3,757.9±458.3b |
Effect of geldanamycin on the
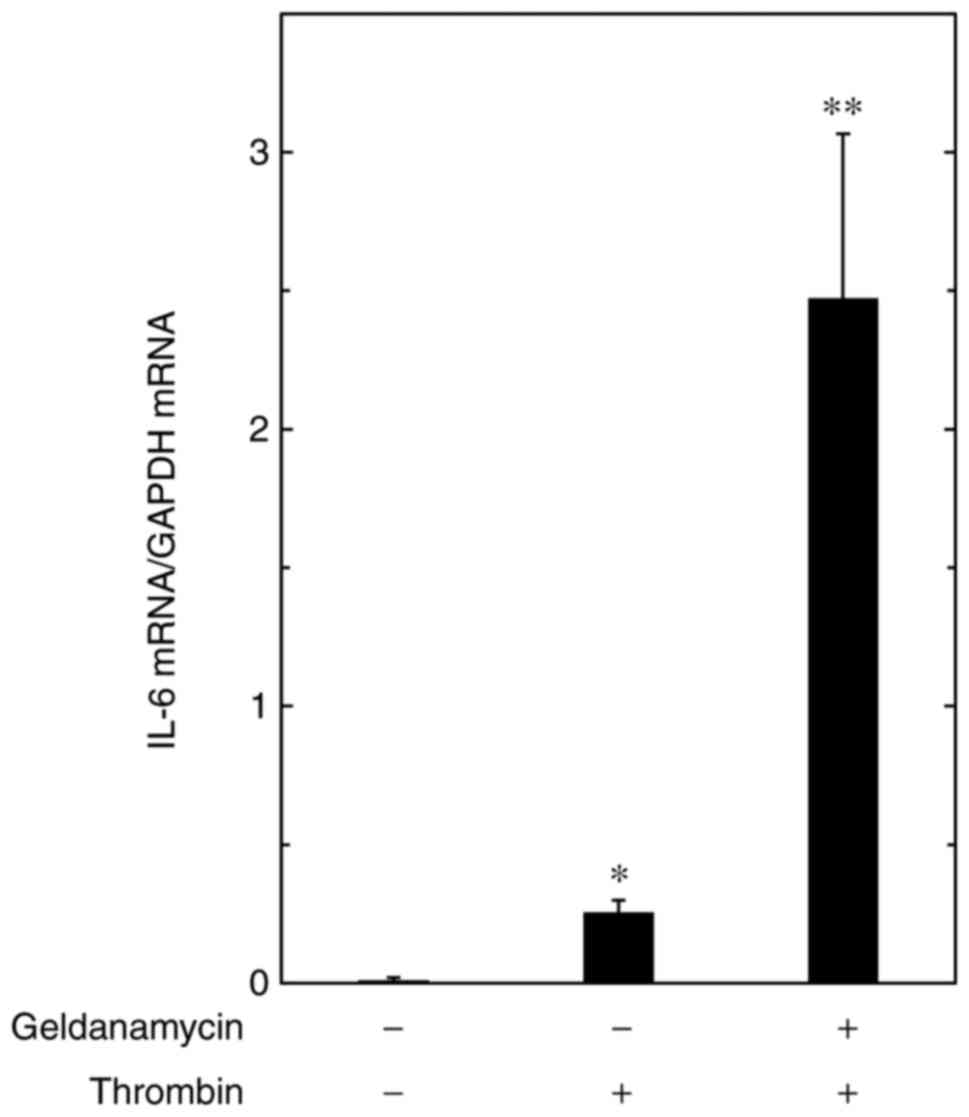
thrombin-induced mRNA expression of IL-6 in MC3T3-E1 cells
To elucidate whether or not the enhancement by HSP90
inhibitors of the thrombin-stimulated release of IL-6 is mediated
through transcriptional events in MC3T3-E1 cells, the present study
examined the effect of geldanamycin on the thrombin-induced mRNA
expression of IL-6. Geldanamycin (1 µM) markedly amplified
the expression of mRNA induced by thrombin (Fig. 2).
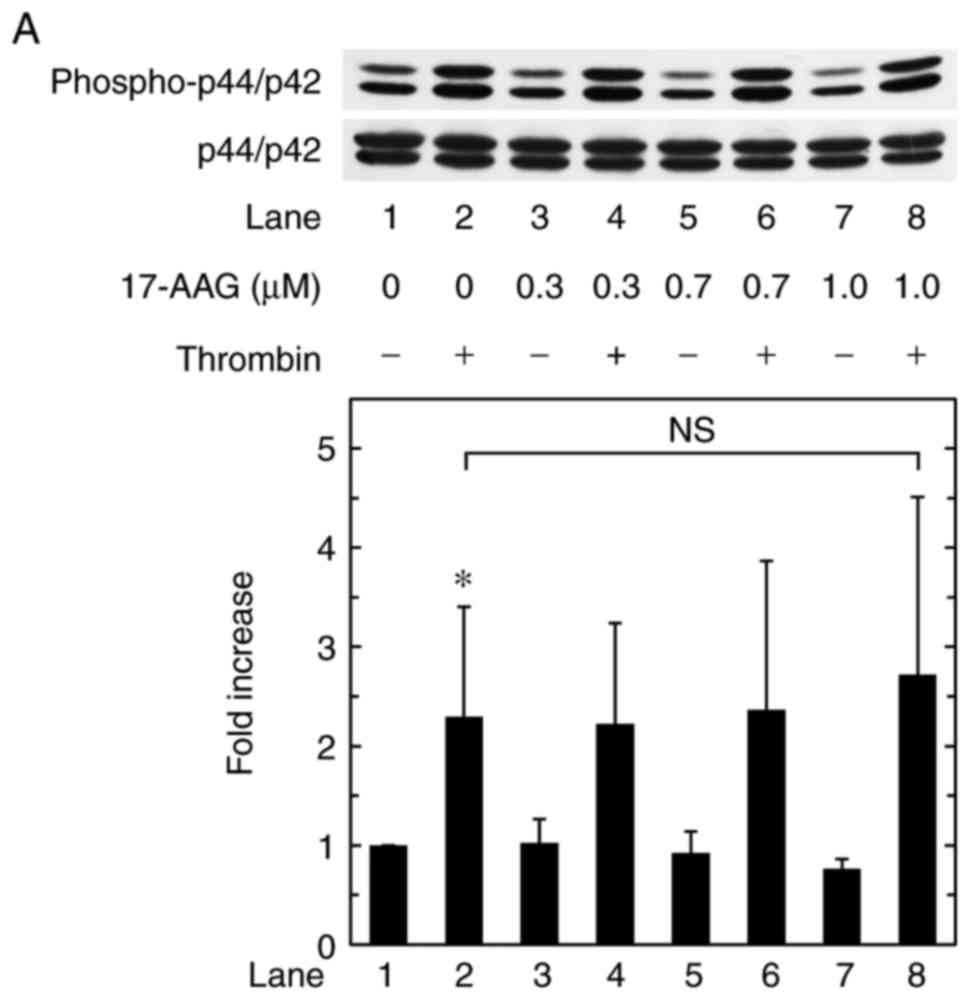
Effects of 17-AAG or geldanamycin on the
thrombin-induced phosphorylation of p44/p42 MAPK in MC3T3-E1
cells
Regarding to the intracellular signaling pathway of
thrombin in osteoblasts, our previous study showed that thrombin
stimulates the synthesis of IL-6, at least in part, via the
activation of p44/p42 MAPK in osteoblast-like MC3T3-E1 cells
(23). Therefore, the present
study examined the effects of 17-AAG or geldanamycin on the
phosphorylation of p44/p42 MAPK induced by thrombin in MC3T3-E1
cells. However, 17-AAG had no significant effect on the
phosphorylation of p44/p42 MAPK (Fig.
3A). Geldanamycin also had no significant effect on the
phosphorylation of p44/p42 MAPK (Fig.
3B).
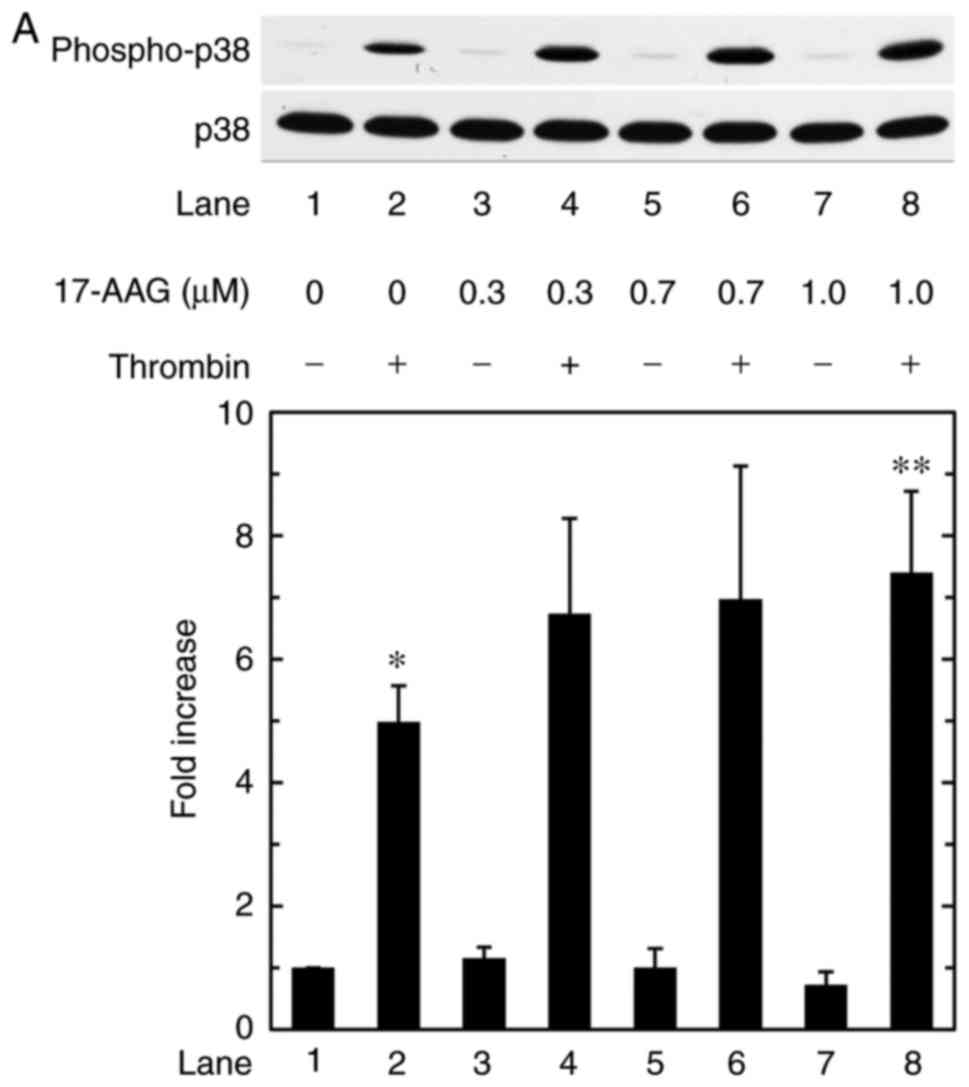
Effects of 17-AAG or geldanamycin on the
thrombin-induced phosphorylation of p38 MAPK in MC3T3-E1 cells
Our previous study showed that p38 MAPK and p44/p42
MAPK act as positive regulators in the thrombin-stimulated
synthesis of IL-6 in osteoblast-like MC3T3-E1 cells (23). To investigate whether or not the
amplification by HSP90 inhibitors of the thrombin-induced synthesis
of IL-6 is exerted through the modulation of p38 MAPK activity in
MC3T3-E1 cells, the present study examined the effects of 17-AAG or
geldanamycin on the phosphorylation of p38 MAPK induced by
thrombin. Only the 17-AAG concentration of 1.0 µM and
geldanamycin concentration of 1.0 µM led to a significant
difference compared with thrombin alone (P=0.04 and P= 0.03,
respectively). The other concentrations had no significant effect
(Fig. 4A and B).
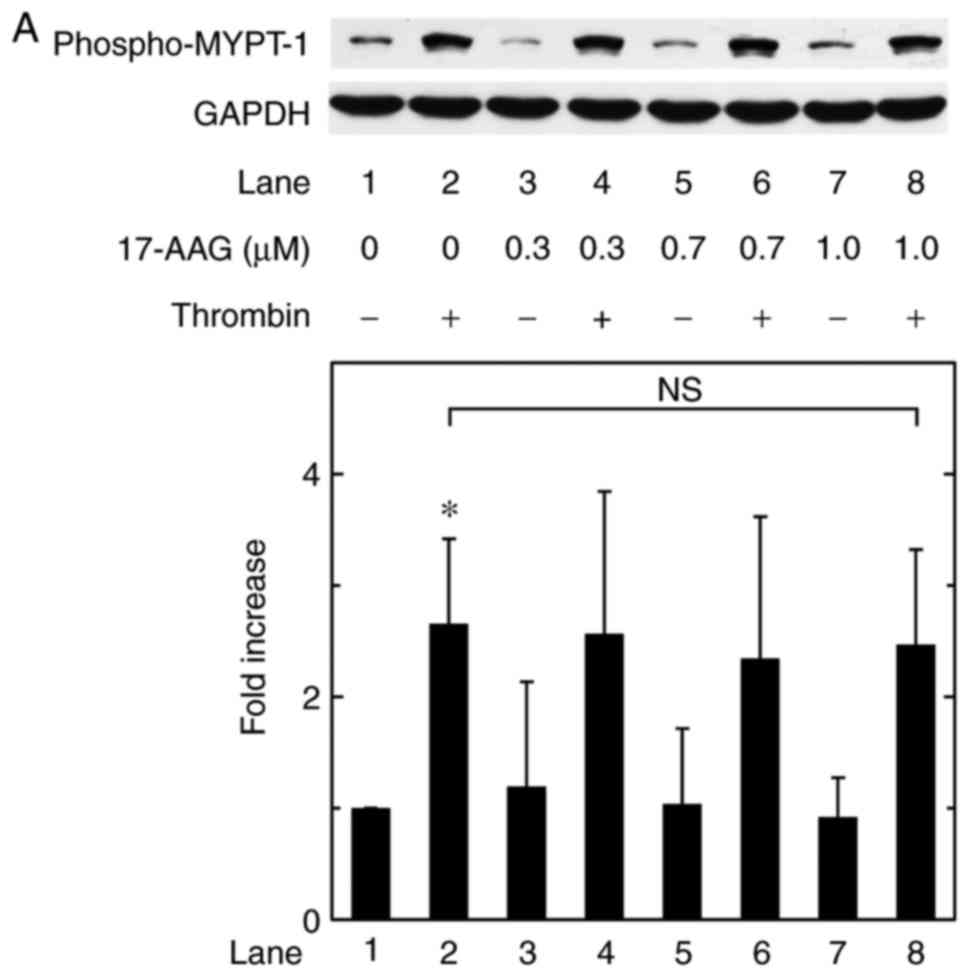
Effects of 17-AAG or geldanamycin on the
thrombin-induced phosphorylation of MYPT-1 in MC3T3-E1 cells
In our previous study (23), it was also shown that Rho-kinase
positively regulates the thrombin-stimulated synthesis of IL-6 at a
point upstream of p38 MAPK, but not p44/p42 MAPK, in
osteoblast-like MC3T3-E1 cells. It is currently recognized that
Rho-kinase phosphorylates MYPT-1, a component of myosin
phosphatase, as a direct downstream substrate (28,29). Therefore, the present study
further examined the effects of 17-AAG or geldanamycin on the
phosphorylation of MYPT-1 induced by thrombin in MC3T3-E1 cells.
However, 17-AAG failed to affect the thrombin-induced
phosphorylation of MYPT-1 up to 1 µM (Fig. 5A). Geldanamycin also had minimal
effect on the thrombin-induced phosphorylation of MYPT-1 up to 1
µM (Fig. 5B).
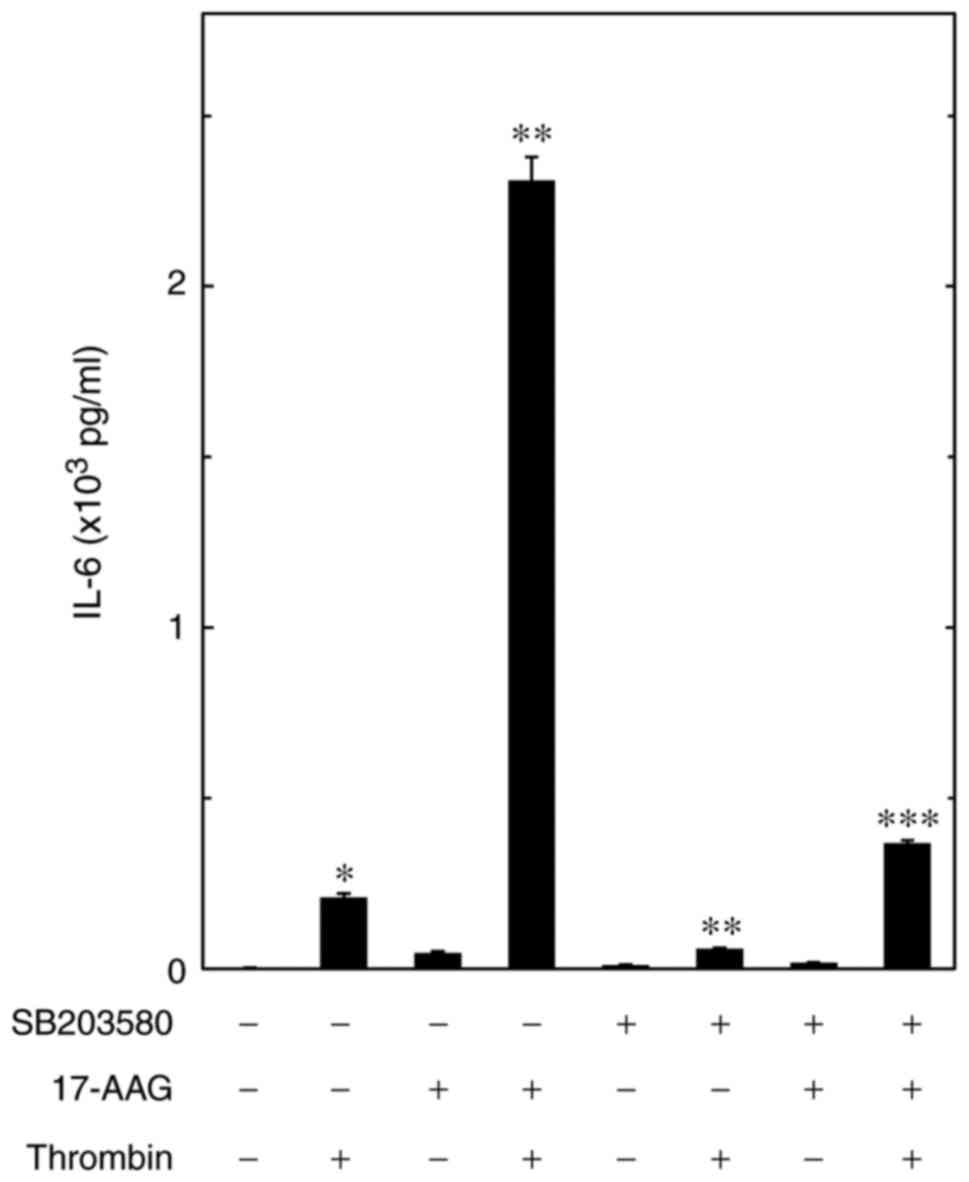
Effect of SB203580 on the amplification
by 17-AAG of the thrombin-stimulated release of IL-6 in MC3T3-E1
cells
The present study further investigated the role of
p38 MAPK in the upregulation by HSP90 inhibitors of the
thrombin-stimulated synthesis of IL-6 in osteoblast-like MC3T3-E1
cells. It was confirmed that SB203580, an inhibitor of p38 MAPK
(30), suppressed the
thrombin-induced release of IL-6, as previously reported (24) (Fig.
6). SB203580 significantly reduced the enhancement by 17-AAG of
the thrombin-stimulated release of IL-6 (0.4×103 pg/ml
IL-6). SB203580 induced a decrease of ~85% in the effect of
thrombin and 17-AAG (2.3×103 pg/ml IL-6;
P=1.0×10−6; Fig.
6).
Discussion
In the present study, it was shown that the
thrombin-stimulated release of IL-6 was significantly amplified by
representative HSP90 inhibitors, including 7-AAG, 17-DMAG and
geldanamycin, in osteoblast-like MC3T3-E1 cells. Additionally, it
was revealed that geldanamycin markedly enhanced the
thrombin-induced mRNA expression of IL-6. These findings suggested
that the HSP90 inhibitors exerted an amplifying effect on the
thrombin-stimulated release of IL-6 through gene transcription in
these cells. It is recognized that geldanamycin and its less toxic
derivatives, 17-AAG and 17-DMAG, inhibit ATP-dependent HSP90
chaperone activity in a common mechanism (13–15). Therefore, it was considered likely
that 17-AAG and 17-DMAG possess a positive effect on the mRNA
expression levels of IL-6 induced by thrombin and geldanamycin.
Taking the findings of the present study into account, it is
possible that HSP90 possesses inhibitory activity on the
thrombin-stimulated synthesis of IL-6 in osteoblast-like MC3T3-E1
cells. Therefore, using HSP90 inhibitors, the present study
investigated the mechanism underlying the suppressive effect of
HSP90 on the synthesis of IL-6 stimulated by thrombin in
osteoblast-like MC3T3-E1 cells.
In terms of the intracellular signal transduction of
thrombin in osteoblasts, our previous study revealed that thrombin
induces the activation of p38 MAPK, p44/p42 MAPK and
stress-activated protein kinase/c-Jun N-terminal kinase in
osteoblast-like MC3T3-E1 cells, and that among the MAPKs, p38 MAPK
and p44/p42 MAPK lead to the stimulation of IL-6 synthesis
(23). Based on these previous
findings, the present study investigated whether or not the
thrombin-induced activation of p38 MAPK and/or p44/p42 MAPK are
affected by HSP90 inhibitors in osteoblast-like MC3T3-E1 cells. It
was found that the phosphorylation of p44/p42 MAPK by thrombin was
not affected by either 17-AAG or geldanamycin. By contrast, the
thrombin-induced phosphorylation of p38 MAPK was significantly
increased by 17-AAG and geldanamycin. It is likely that similar
results can be obtained using 17-DMAG, as geldanamaycin and its
derivatives suppress the ATP-dependent chaperone activity of HSP90
in common mechanism (13–15). Therefore, these results suggested
that the upregulation of p38 MAPK, rather than p44/p42 MAPK, is
implicated in the enhancement by HSP90 inhibitors of
thrombin-stimulated IL-6 synthesis in osteoblast-like MC3T3-E1
cells.
It was noted that HSP90 inhibitors combined with
thrombin treatment resulted in the amplification of IL-6 synthesis
to high levels, compared with those induced by thrombin or the
inhibitors alone, whereas neither geldanamycin nor 17-AAG caused
the enhancement of thrombin-induced phosphorylation of p38 MAPK to
similar levels. It is likely that the sustained enhancement of
thrombin-induced p38 MAPK activity by HSP90 inhibitors results in
high levels of IL-6 synthesis in osteoblasts. However, it has been
reported that 17-AAG itself enhances osteoblastic differentiation
in C3H10T1/2 cells and primary calvarial osteoblasts (28). Therefore, the amplification of
thrombin-induced IL-6 synthesis by HSP90 inhibitors in the present
study may have been affected by the enhancement of osteoblastic
differentiation induced by HSP90 inhibitors.
In our previous study (23), it was also shown that the
thrombin-stimulated synthesis of IL-6 is positively regulated by
Rho-kinase at a point upstream of p38 MAPK in these cells. However,
it was shown in the present study that, unlike p38 MAPK, the
thrombin-induced phosphorylation of MYPT-1 was not affected by
either 17-AAG or geldanamycin. As MYPT-1 is a well-established
substrate of Rho-kinase (29,30), it is unlikely that the amplifying
effect of HSP90 inhibitors on the synthesis of IL-6 stimulated by
thrombin is exerted at a point upstream of Rho-kinase. These
findings suggested that the thrombin-stimulated IL-6 synthesis was
negatively regulated by HSP90 in osteoblast-like MC3T3-E1 cells,
and that HSP90 exerted its inhibitory effect on IL-6 synthesis at
the point between Rho-kinase and p38 MAPK. The present study also
showed that the amplification of the thrombin-stimulated release of
IL-6 by 17-AAG was markedly reduced by SB203580, a p38 MAPK
inhibitor (31), in these cells.
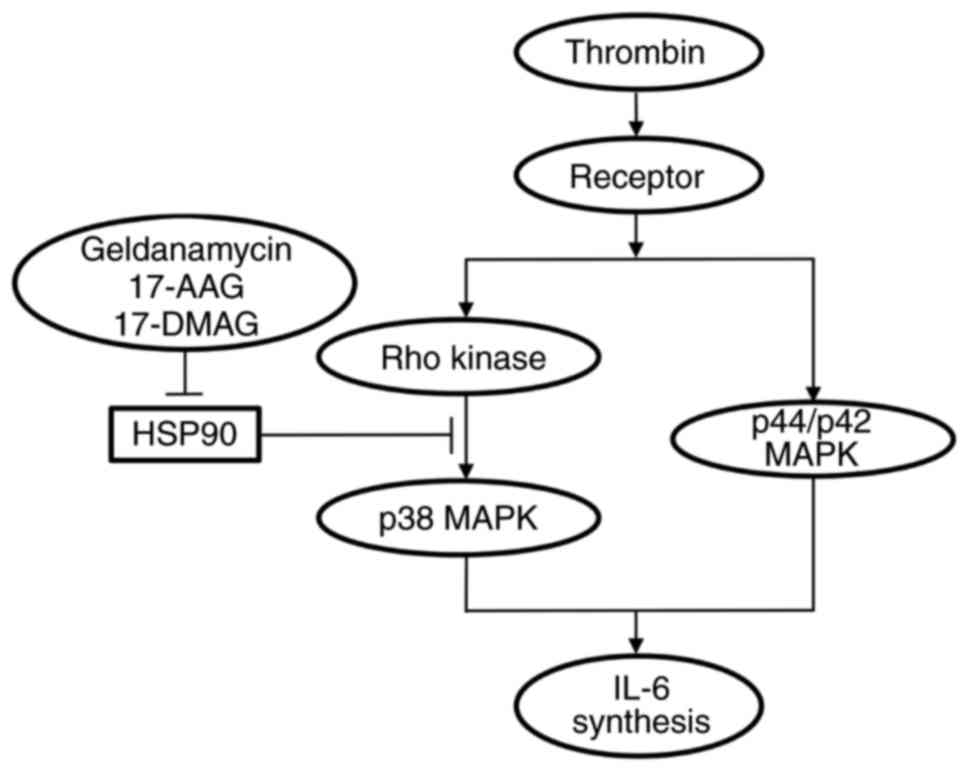
Taken together, these results suggested that the
thrombin-stimulated synthesis of IL-6 was inhibited by HSP90 in the
osteoblast-like MC3T3-E1 cells, and that the suppressive effect of
HSP90 was exerted at the point between Rho-kinase and p38 MAPK. The
potential mechanism underlying amplification of thrombin-stimulated
IL-6 synthesis by HSP90 inhibitors in osteoblasts is summarized in
Fig. 7.
Bone remodeling is initiated with bone resorption,
followed by bone formation (3).
In order to maintain the quantity and quality of the adult
skeleton, the regulation of bone remodeling handled by osteoblasts
and osteoclasts must be well orchestrated. It is generally known
that IL-6 is a potent bone resorptive agent promoting
osteoclastgenesis (5). In
addition, accumulating evidence suggests that IL-6 functions as an
osteotropic factor under the conditions of increased bone turnover,
inducing bone formation (7).
Therefore, IL-6 is currently recognized to act as a bone-remodeling
agent in bone metabolism. In our previous study, it was
demonstrated that HSP90 is expressed at high levels in
osteoblast-like MC3T3-E1 cells, even in their resting state
(32). Therefore, the results of
the present study, showing that thrombin-stimulated IL-6 synthesis
was upregulated by HSP90 inhibitors in MC3T3-E1 cells, support the
physiological function of HSP90 in osteoblasts as a fundamental
bone remodeling modulator. Several HSP90 inhibitors have been
adopted in clinical trials as anticancer agents (33). Taken together, the findings of the
present study suggested the role of HSP90 inhibitors as a
bone-remodeling agent through the amplification of IL-6 synthesis
in osteoblasts.
In the present study, all experiments were performed
using murine osteoblast-like MC3T3-E1 cells. Further investigations
using another types of osteoblastic cells, for example primary
human osteoblasts, are required to clarify the exact mechanism and
the clinical relevance of HSP90 inhibitors in bone metabolism. In
addition, supporting evidence to clarify whether HSP90 inhibitors
affect the synthesis or the activity of HSP90 in osteoblasts is
required.
In conclusion, the results of the present study
suggested that thrombin-stimulated IL-6 synthesis is negatively
regulated by HSP90 in osteoblasts, and that the effect of HSP90 on
the synthesis of IL-6 is exerted at the point between Rho-kinase
and p38 MAPK.
Acknowledgments
The authors would like to thank Mrs. Yumiko Kurokawa
(Department of Pharmacology, Gifu University Graduate School of
Medicine, Gifu, Japan) for her skillful technical assistance.
Funding
This study was supported in part by a Grant-in-Aid
for Scientific Research (grant nos. 26462289, 15K10487 and
17K11002) from the Ministry of Education, Culture, Sports, Science
and Technology of Japan; a Grant-in-Aid for Scientific Research
(grant. no. H25-Aging-General-004) from the Ministry of Health,
Labor and Welfare of Japan; and Research Funding for Longevity
Sciences (grant no. 26-12 and 28-9) from the National Center for
Geriatrics and Gerontology, Japan.
Availability of data and materials
All data generated or analyzed during this study are
included within.
Authors’ contribution
KF, OK and HT conceived and designed the
experiments. KF, TK, SK and GS performed the experiments. KF, TO,
RM-N, OK and HT analyzed the data. KF, OK and HT wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kular J, Tickner J, Chim SM and Xu J: An
overview of the regulation of bone remodeling at the cellular
level. Clin Biochem. 45:863–873. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chim SM, Tickner J, Chow ST, Kuek V, Guo
B, Zhang G, Rosen V, Erber W and Xu J: Angiogenic factors in bone
local environment. Cytokine Growth Factor Rev. 24:297–310. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parfitt AM: Targeted and nontargeted bone
remodeling: Relationship to basic multicellular unit origination
and progression. Bone. 30:5–7. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirano T, Yasukawa K, Harada H, Taga T,
Watanabe Y, Matsuda T, Kashiwamura S, Nakajima K, Koyama K,
Iwamatsu A, et al: Complementary DNA for a novel human interleukin
(BSF-2) that induces B lymphocytes to produce immunoglobulin.
Nature. 324:73–76. 1986. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kwan TS, Padrines M, Théoleyre S, Heymann
D and Fortun Y: IL-6, RANKL, TNF-alpha/IL-1: Interrelations in bone
resorption pathophysiology. Cytokine Growth Factor Rev. 15:49–60.
2004. View Article : Google Scholar
|
|
6
|
Prystaz K, Kaiser K, Kovtun A,
Haffner-Luntzer M, Fischer V, Rapp AE, Liedert A, Strauss G,
Waetzig GH, Rose-John S and Ignatius A: Distinct effects of IL-6
classic and trans-signaling in bone fracture healing. Am J Pathol.
188:474–490. 2018. View Article : Google Scholar
|
|
7
|
Sims NA: Cell-specific paracrine actions
of IL-6 family cytokines from bone, marrow and muscle that control
bone formation and resorption. Int J Biochem Cell Biol. 79:14–23.
2006. View Article : Google Scholar
|
|
8
|
Schopf FH, Biebl MM and Buchner J: The
HSP90 chaperone machinery. Nat Rev Mol Cell Biol. 18:345–360. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chiosis G: Targeting chaperones in
transformed systems-a focus on Hsp90 and cancer. Expert Opin Ther
Targets. 10:37–50. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prodromou C: Mechanisms of Hsp90
regulation. Biochem J. 473:2439–2452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu W and Neckers L: Targeting the
molecular chaperone heat shock protein 90 provides a multifaceted
effect on diverse cell signaling pathways of cancer cells. Clin
Cancer Res. 13:1625–1629. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Whitesell L and Lindquist SL: HSP90 and
the chaperoning of cancer. Nat Rev Cancer. 5:761–772. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schulte TW and Neckers LM: The
benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds
to HSP90 and shares important biologic activities with
geldanamycin. Cancer Chemother Pharmacol. 42:273–279. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jez JM, Chen JC, Rastelli G, Stroud RM and
Santi DV: Crystal structure and molecular modeling of 17-DMAG in
complex with human Hsp90. Chem Biol. 10:361–368. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ochel HJ, Eichhorn K and Gademann G:
Geldanamycin: The prototype of a class of antitumor drugs targeting
the heat shock protein 90 family of molecular chaperones. Cell
Stress Chaperones. 6:105–112. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Price JT, Quinn JM, Sims NA, Vieusseux J,
Waldeck K, Docherty SE, Myers D, Nakamura A, Waltham MC, Gillespie
MT and Thompson EW: The heat shock protein 90 inhibitor,
17-allylamino-17-demethoxygeldanamycin, enhances osteoclast
formation and potentiates bone metastasis of a human breast cancer
cell line. Cancer Res. 65:4929–4938. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mori M, Hitora T, Nakamura O, Yamagami Y,
Horie R, Nishimura H and Yamamoto T: Hsp90 inhibitor induces
autophagy and apoptosis in osteosarcoma cells. Int J Oncol.
46:47–54. 2015. View Article : Google Scholar
|
|
18
|
Fujita K, Tokuda H, Kuroyanagi G, Yamamoto
N, Kainuma S, Kawabata T, Sakai G, Matsushima-Nishiwaki R, Kozawa O
and Otsuka T: HSP90 inhibitors potentiate PGF2α-induced IL-6
synthesis via p38 MAPK in osteoblasts. PLoS One. 12:e01778782017.
View Article : Google Scholar
|
|
19
|
Kyriakis JM and Avruch J: Mammalian
mitogen-activated protein kinase signal transduction pathways
activated by stress and inflammation. Physiol Rev. 81:807–869.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lane DA, Philippou H and Huntington JA:
Directing thrombin. Blood. 106:2605–2612. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mackie EJ, Loh LH, Sivagurunathan LS,
Uaesoontrachoon K, Yoo HJ, Wong D, Georgy SR and Pagel CN:
Protease-activated receptors in the musculoskeletal system. Int J
Biochem Cell Biol. 40:1169–1184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kozawa O, Tokuda H, Kaida T, Matsuno H and
Uematsu T: Thrombin regulates interleukin-6 synthesis through
phosphate-dylcholine hydrolysis by phospholipase D in osteoblasts.
Arch Biochem Biophys. 345:10–15. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kato K, Otsuka T, Matsushima-Nishiwaki R,
Natsume H, Kozawa O and Tokuda H: Rho-kinase regulates
thrombin-stimulated interleukin-6 synthesis via p38
mitogen-activated protein kinase in osteoblasts. Int J Mol Med.
28:653–658. 2011.PubMed/NCBI
|
|
24
|
Sudo H, Kodama HA, Amagai Y, Yamamoto S
and Kasai S: In vitro differentiation and calcification in a new
clonal osteogenic cell line derived from newborn mouse calvaria. J
Cell Biol. 96:191–198. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kozawa O, Tokuda H, Miwa M, Kotoyori J and
Oiso Y: Cross-talk regulation between cyclic AMP production and
phosphoinositide hydrolysis induced by prostaglandin E2 in
osteoblast-like cells. Exp Cell Res. 198:130–134. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kato K, Ito H, Hasegawa K, Inaguma Y,
Kozawa O and Asano T: Modulation of the stress-induced synthesis of
hsp27 and alpha B-crystallin by cyclic AMP in C6 rat glioma cells.
J Neurochem. 66:946–950. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen H, Xing J, Hu X, Chen L, Lv H, Xu C,
Hong D and Wu X: Inhibition of heat shock protein 90 rescues
glucocorticoid-induced bone loss through enhancing bone formation.
J Steroid Biochem Mol Biol. 171:236–246. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fukata Y, Amano M and Kaibuchi K:
Rho-Rho-kinase pathway in smooth muscle contraction and
cytoskeletal reorganization of non-muscle cells. Trends Pharmacol
Sci. 22:32–39. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ito M, Nakano T, Erdori F and Hartshorne
DJ: Myosin phosphatase: Structure, regulation and function. Mol
Cell Biochem. 259:197–209. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cuenda A, Rouse J, Doza YN, Meier R, Cohen
P, Gallagher TF, Young PR and Lee JC: SB 203580 is a specific
inhibitor of a MAPK homologue which is stimulated by cellular
stresses and interleukin-1. FEBS Lett. 364:229–233. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kozawa O, Niwa M, Hatakeyama D, Tokuda H,
Oiso Y, Matsuno H, Kato K and Uematsu T: Specific induction of heat
shock protein 27 by glucocorticoid in osteoblasts. J Cell Biochem.
86:357–364. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Verma S, Goyal S, Jamal S, Singh A and
Grover A: Hsp90: Friends, clients and natural foes. Biochimie.
127:227–240. 2016. View Article : Google Scholar : PubMed/NCBI
|