Introduction
Colon cancer is the third most common cancer and the
fourth leading cause of cancer-related death worldwide (1). The presence of liver metastasis at
early stages, even at the time of diagnosis, is the predominant
reason. The use of adjuvant chemotherapy for colorectal liver
metastases (CRLM) has increased resection rates and improved
outcomes (2,3). Oxaliplatin is one of the most
commonly used chemotherapeutic agents. Studies have revealed that
after six months of oxaliplatin-based chemotherapy, patients with
potentially resectable liver metastases showed significantly
improved overall and disease-free survival rates (4-6).
However, oxaliplatin-induced liver injury is a
limiting factor for the otherwise highly effective use of the drug
in patients with CRLM (7). In
on-going studies, it has been proved that there is
oxaliplatin-associated hepatotoxicity in a considerable portion of
patients (8,9). Liver injury leads to an increased
risk of peri-operative morbidity and mortality in liver resection
cases (10). Previous global gene
microarray analyses have demonstrated that oxaliplatin-induced
liver injury correlated with elevated oxidative stress, activation
of the interleukin-6 (IL-6) pathway and overactivation of the
coagulation cascade (11,12). To overcome these side-effects, a
medication that protects the liver against damage caused by
oxaliplatin is urgently needed.
Magnesium isoglycyrrhizinate (MgiG) is a magnesium
salt of 18-α glycyrrhizic acid stereoisomer. The full name of MgiG
is tetrahydrate magnesium 18α, 20β-hydroxy-11-oxon
orolean-12-en-3β-yl-2-O-β-D-glucopyranurosyl-α-D-glucopy
ranosiduronate (13). Previous
studies have demonstrated that MgiG attenuates liver injury caused
by toxins (carbon tetrachloride and D-galactosamine) and other
factors, including free fatty acid, ethanol and
ischemia/reperfusion (14). MgiG
exerts a greater liver-protecting effect than glycyrrhizin or
β-glycyrrhizic acid (15-17). Recently, there have been reports
of MgiG possessing hepatoprotective effects against
chemotherapeutic agents, such as cyclophosphamide, by attenuating
oxidative stress and cytokines, such as IL-6, in liver cells
(18). A recent in vivo
study reported that reduced glutathione alleviates
oxaliplatin-induced acute liver injury (19). However, there has not been a study
regarding whether MgiG could be used for the prevention or
treatment of oxaliplatin-associated liver injury.
In the present study, the protective functions of
MgiG against oxaliplatin-induced liver injury were investigated
in vitro and in vivo. To better imitate the
pathogenic process of oxaliplatin-induced liver injury, a liver
damage animal model was established by direct oxaliplatin
administration, where the drug was administered to animals based on
a treatment schedule comparable to human patients receiving
chemotherapy. The mechanisms underlying the hepatoprotective
effects of MgiG were also investigated, including oxidative stress,
inflammatory responses and the coagulation cascade.
Materials and methods
Reagents
Magnesium isoglycyrrhizinate (MgiG) was purchased
from Chia Tai Tianqing Pharmaceutical Group Co., Ltd. (Jiangsu,
China). Oxaliplatin was obtained from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). ELISA kits for IL-6 (KGEMC138) and von
Willebrand factor (vWF) (KGEMC146) were obtained from Nanjing
KeyGEN Biotech. Co., Ltd. (Nanjing, China). The alanine
aminotransferase (ALT), aspartate aminotransferase (AST),
glutathione (GSH) and malondialdehyde (MDA) detection kits were
obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing,
China). Unless indicated otherwise, chemicals used in experiments
were purchased from Sigma-Aldrich (Merck KGaA).
Animals and treatments
Forty male, 10-week-old C57Bl/6 mice, weighing 18-22
g, were obtained from the Laboratory Animal Center of the School of
Medicine, Shandong University (Shandong, China). Animals were kept
under standard conditions of 55% humidity, 20–25°C, and a
controlled 12-h light/dark cycle, and allowed free access to
standard food and water. All C57Bl/6 mice were randomly divided
into 4 groups. Group I mice were intraperitoneally (i.p.) injected
with vehicle (normal saline) as control (n=10). Group II mice
received 10 mg/kg intraperitoneal oxaliplatin on a weekly basis for
5 weeks (n=10). Group III mice received an low dose of MgiG [15
mg/kg/day, intraperitoneal (i.p.)] together with intraperitoneal
oxaliplatin (n=10). Group IV mice received high-dose MgiG (45
mg/kg/day, i.p.) together with intraperitoneal oxaliplatin (n=10).
The drug dosing schedule was based on previously published studies
and our preliminary experiments (18,20–22). Mice were sacrificed one week
following the final dose of chemotherapy under isoflurane
anesthesia by cardiac puncture. The present study was conducted in
accordance with Chinese laws and National Institute of Health
publications on the use and care of laboratory animals and using a
protocol approved by the Institutional Animal Care and Use
Committee of Shandong University (Shandong, China).
Cell culture
The human hepatic cell line LO2 and the human colon
cancer cell line HT-29 were purchased from the American Type
Culture Collection (Manassas, VA, USA). The cells were maintained
in RPMI1640 medium for LO2 (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and DMEM for HT-29 (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin, and 100 µg/ml
streptomycin (Amresco, Inc.; VWR International, Radnor, PA, USA) at
37°C in a 5% CO2 humidified atmosphere.
Analysis of liver enzymes and other
indicators
AST, ALT, GSH and MDA commercial kits were used to
measure the indicators, according to the manufacturers'
instructions. In brief, liver homogenates, serum or cell
supernatant substrate solutions were added to the reacting system,
and the final products were spectrophotometrically measured with
various wavelengths. The concentrations of the indicator were
calculated according to standard curves. The concentrations of IL-6
and vWF in serum and liver homogenates were measured with ELISA
kits, according to the manufacturer's instructions.
Cell Counting Kit-8 (CCK-8) assay for
cell viability
Cell viability was determined with a CCK-8 assay.
Briefly, cells were plated in 96-well plates at a density of 3,000
cells per well. After 10 h, the medium was replaced with fresh
medium containing 300 µM oxaliplatin, with or without other
reagents. The doses of oxaliplatin and other reagents were
determined based on previous studies and our preliminary
experiments (18,23). Experimental groups were treated
with various concentrations of MgiG. Untreated cells were used as
control. Following incubation for 24 h, the cells were exposed to
CCK-8 (100 µl/ml; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) followed by incubation at 37°C for another 2 h.
Subsequently, the absorbance of the resulting color in each well
was measured using a microplate spectrophotometer at a wavelength
of 450 nm.
Histological assessment
Formalin-fixed and paraffin-embedded liver tissues
were cut into 5 µm-thick slices. Hematoxylin and eosin
(H&E) and Sirius red stains were used. The pathological changes
of both stained slices were evaluated by a pathologist blinded to
the experimental group. The histological changes were scored
according to the following criteria: 0, absent; 1, mild; 2,
moderate; and 3, severe.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA of liver homogenates and cells was
extracted and purified using TRIzol reagent (Thermo Fisher
Scientific, Inc.). The reverse transcription of 1 µg RNA to
cDNA was established using the PrimeScript RT reagent kit (cat. no.
RR036A; Takara Bio, Inc., Otsu, Japan). qPCR was run on a Bio-Rad
iQ5 optical module (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Cycling conditions were: 95°C for 2 min as initial denaturation, 40
cycles of denaturation at 95°C for 15 sec, and annealing/extension
at 60°C for 40 sec. Melt curve analysis was set between 65 and 95°C
with 0.5°C increments at 5 sec per step. Quantitative values were
obtained by the threshold cycle (Cq) value. Relative mean fold
change in expression ratios was calculated by the 2−ΔΔCq
method (24). GAPDH was used as
internal control. The primer sequences used in the present study
were as follows: GAPDH, forward 5′-GCACAGTCAAGGCCGAGAAT-3′ and
reverse 5′-GCCTTCTCCATGGTGGTGAA-3′; metallothionein 1 (Mt1),
forward 5′-GCTGCTGCTCCTGCTGTCCC-3′ and reverse
5′-CAGCACGTGCACTTGTCCGC-3′; peroxiredoxin 1 (PRDX1), forward
5′-TGTTTCCCCAGCATGTGTACC-3′ and reverse
5′-TGCTCTTATAGAAGACCCAGGTTC-3′; superoxide dismutase 2 (SOD2),
forward 5′-CGTGACTTTGGGTCTTTTGAG-3′ and reverse
5′-TAGAGCAGGCAGCAATCTGTAA-3′; IL-6, forward
5′-GAGGATACCACTCCCAACAGACC-3′ and reverse
5′-AAGTGCATCATCGTTGTTCATACA-3′; signal transducer and activator of
transcription 3 (STAT3), forward 5′-CCTTCTTGTTCTACGGCTTGC-3′ and
reverse 5′-TCGCCTATCTTCTCAACCAGG-3′; vWF, forward
5′-CGGGAAGAGTGTGATGGTTGAC-3′ and reverse
5′-AGCATCTCCCACAGCATTCACC-3′; plasminogen activator inhibitor-1
(PAI-1), forward 5′-GATGCTATGGGATTCAAAGTCA-3′ and reverse
5′-TCCACCTGTTTCACCATAGTCT-3′.
Protein extraction and western
blotting
Mice liver samples or cells were lysed in
radioimmunoprecipitation assay lysis solution (Beyotime Institute
of Biotechnology, Haimen, China) containing phosphatase inhibitors
and PMSF. A BCA Protein Assay kit (Beyotime Institute of
Biotechnology) was used to determine the protein concentrations. A
total of 30 µg protein extracts were resolved by 8 and 15%
SDS-PAGE. The proteins were transferred onto polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA), blocked
with 5% nonfat dry milk at room temperature for 1 h and incubated
with primary antibodies at 4°C overnight. The following antibodies
were used: Metallothionein 1 (cat. no. ab12228; 1:2,000; Abcam,
Cambridge, MA, USA), peroxiredoxin 1 (cat. no. 8499; 1:5,000; Cell
Signaling Technology, Inc., Danvers, MA, USA), superoxide dismutase
2 (cat. no. 13141; 1:500; Cell Signaling Technology, Inc.), STAT3
(cat. no. 9132; 1:1,000; Cell Signaling Technology, Inc.); vWF
(cat. no. 65707; 1:500; Cell Signaling Technology, Inc.), PAI-1
(cat. no. 11907; 1:2,000; Cell Signaling Technology, Inc.). and
GAPDH (cat. no. ab22555; 1:2,500; Abcam). The blots were then
incubated with horseradish peroxidase-conjugated secondary
antibodies at room temperature for 1.5 h (cat. nos. BA1054 and
BA1050; 1:50,000; Wuhan Boster Biological Technology, Ltd., Wuhan,
China), and visualized by enhanced chemiluminescence detection.
GAPDH was used as the loading control. The optical density was
analyzed with ImageJ 10.0 software (National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
Three independent repeats were performed for each
experiment. Experimental data were expressed as the mean ± standard
deviation. Analyses were performed using GraphPad Prism 5 (GraphPad
Software, Inc., La Jolla, CA, USA). Comparisons among datasets were
made using one-way analysis of variance followed by Tukey's post
hoc test or unpaired Student's t-test. P<05 was considered to
indicate a statistically significant difference.
Results
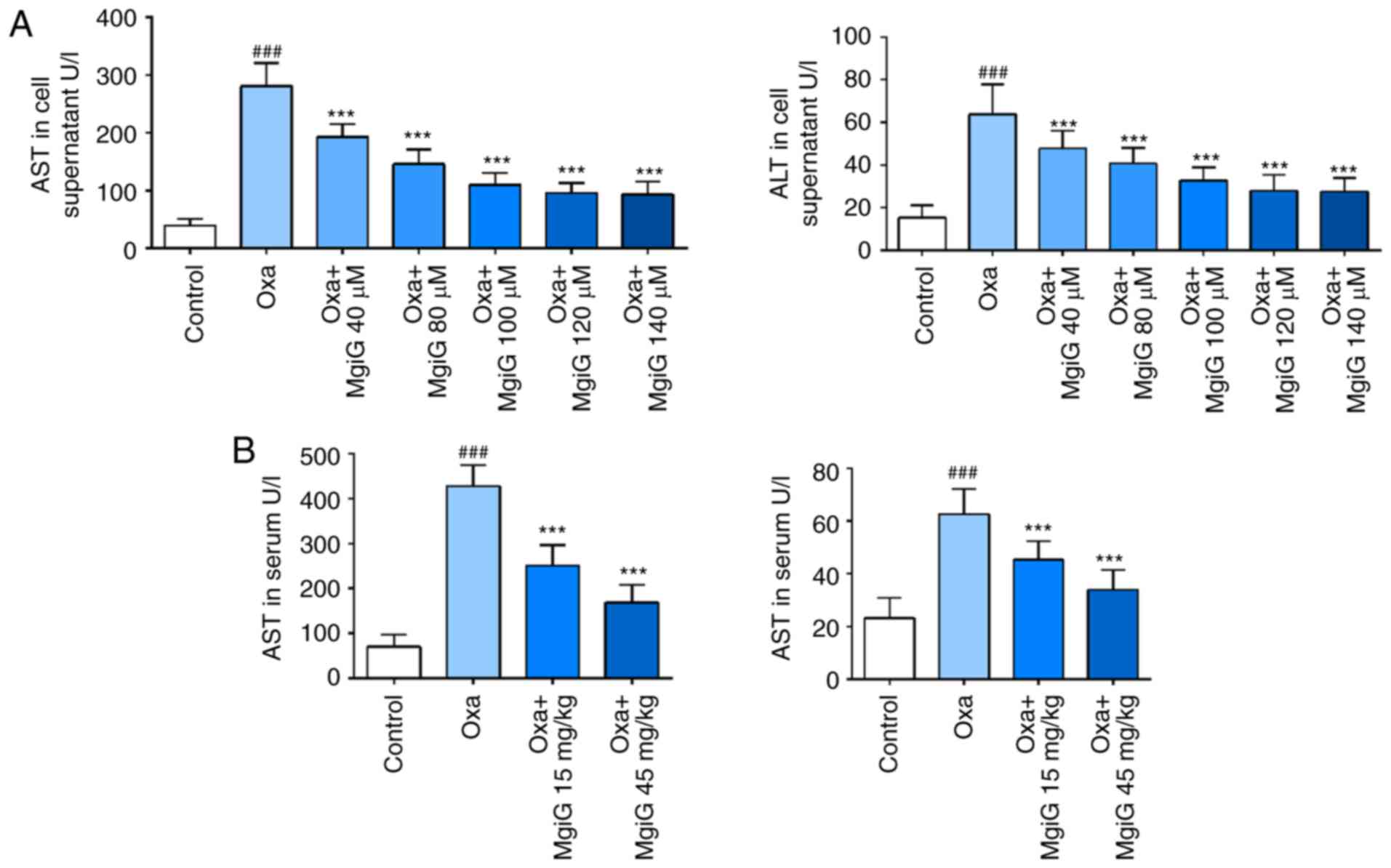
MgiG attenuates the aminotransferase
increase caused by oxaliplatin
Aminotransferase levels are diagnostic markers of
liver damage. LO2 cells exposed to oxaliplatin were cultured in
medium supplemented with or without MgiG, and mice receiving
oxaliplatin were treated with or without MgiG. AST and ALT activity
in serum and cell supernatants was then measured. Following
oxaliplatin challenge, both indicators significantly increased in
cell supernatants (Fig. 1A). In
the MgiG-treated groups, the increase in indicators was
significantly attenuated (Fig.
1A). AST and ALT levels continued decreasing when the MgiG
concentration was below 100 µM. AST and ALT activity in
mouse serum also displayed a significant increase following
oxaliplatin administration (Fig.
1B). MgiG effectively attenuated the oxaliplatin-induced
changes and this effect was dose-dependent with a more significant
reduction observed in the high-dose group (Fig. 1B).
MgiG improves oxaliplatin-induced
pathological changes in liver parenchyma
To assess the histological changes in liver
parenchyma, H&E-stained liver sections were blindly reviewed by
a pathologist and graded according to the degree of histological
damage. A large area of hepatocyte necrosis, extensive
vacuolization, and inflammatory cell infiltration were observed in
the oxaliplatin-treated group (Fig.
1C). MgiG treatment significantly attenuated the pathological
damage in the liver of the experimental animals (Fig. 1C). High-dose MgiG had a more
significant effect in protecting the liver against
oxaliplatin-induced damage (Fig.
1C). Sirius red staining was also performed on the tissue
sections to estimate fibrosis in the liver. Collagen deposition was
observed within the injured hepatic sinusoids in the liver of
oxaliplatin-treated mice (Fig.
1D). This pathological progress was significantly attenuated in
the liver of MgiG-treated mice (Fig.
1D).
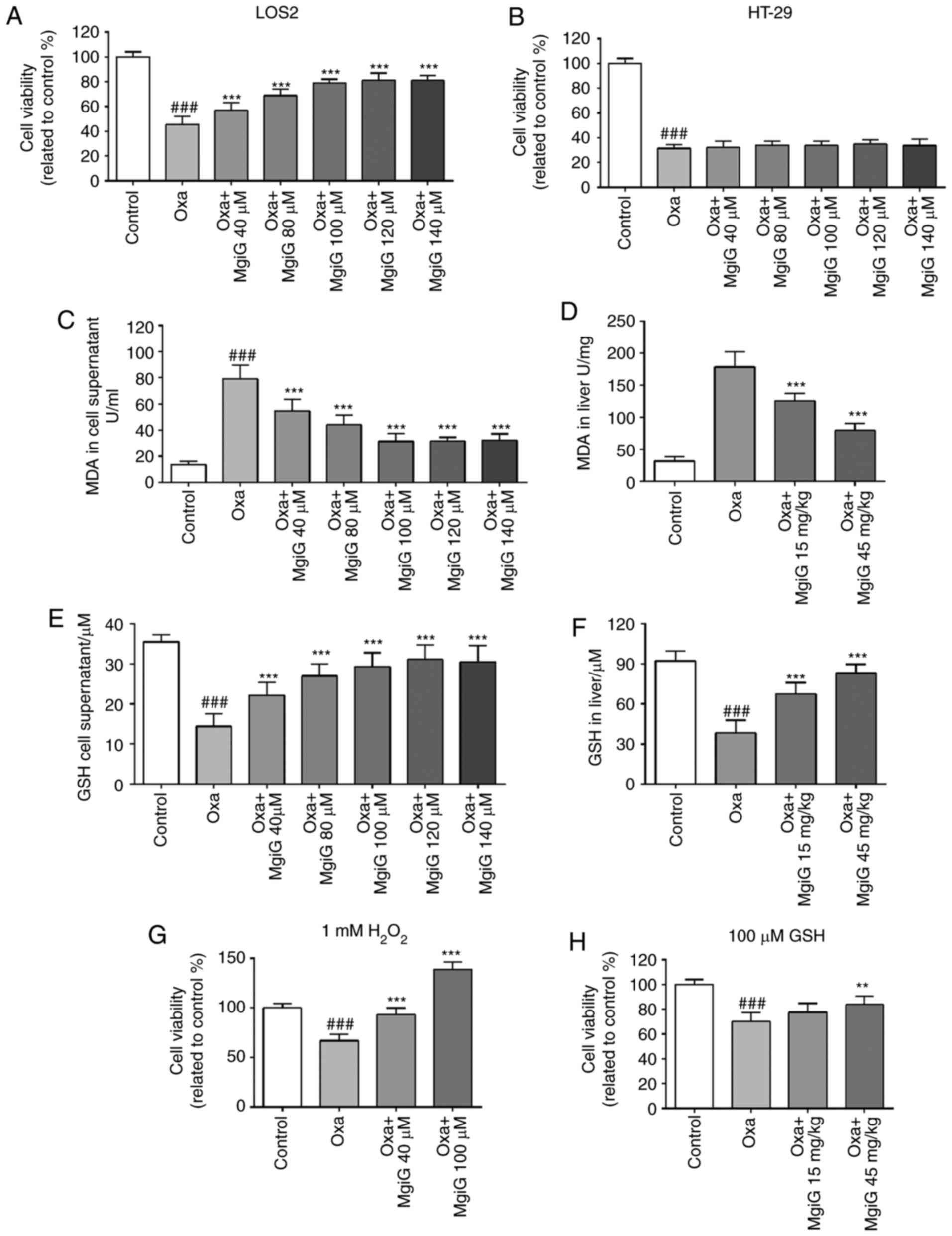
MgiG improves cell viability after
oxaliplatin challenge in hepatic cells
A cell viability assay was performed on LO2 cells to
estimate their response to oxaliplatin challenge and treatment of
MgiG at various concentrations. Cell viability decreased by 57% in
the oxaliplatin-treated group compared with the control group
(Fig. 2A). There was an increase
in cell viability in MgiG-treated groups (Fig. 2A). When the concentration of MgiG
was >100 µM, the rise in viability occurred in a
dose-dependent manner (Fig. 2A).
The same experiment was repeated using the colon cancer cell line
HT-29. Cell viability also decreased dramatically following
oxaliplatin exposure, but MgiG treatment had no effect in the
oxaliplatin-mediated loss of viability in the colon cancer cells
(Fig. 2B).
MgiG protects liver cells by attenuating
oxaliplatin-induced oxidative stress
It has been well-demonstrated in previous studies
that increased oxidative stress is one of the major mechanisms
inducing sinusoidal obstruction syndrome (SOS) in patients and
animals receiving oxaliplatin treatment (11,21,25,26). To estimate oxidative stress,
contents of MDA and GSH in liver homogenates and cell supernatants
were measured (27). In LO2 cell
supernatants, the content of MDA significantly increased following
oxaliplatin exposure, and MgiG treatment significantly attenuated
MDA levels (Fig. 2C). In liver
homogenates from experimental mice, MDA increased 5.04-fold
following oxaliplatin administration, and MgiG treatment remarkably
attenuated this effect (Fig. 2D).
In addition, a remarkable reduction in the concentration of GSH in
LO2 cell supernatants was observed with oxaliplatin exposure, and
MgiG significantly restored this (Fig. 2E). In vivo, the GSH levels
in the liver homogenates of oxaliplatin-injected mice decreased by
59% compared with control, and MgiG treatment significantly
increased the GSH levels by 74 and 113% at low and high-dose,
respectively (Fig. 2F).
To explore the role of oxidative stress in the
pathogenic process of oxaliplatin-induced liver injury and the
mechanism of the protective function of MgiG, a cell viability
assay was performed on LO2 cells in the presence of supplementary 1
mM H2O2 or 100 µM GSH in the medium.
The dose of H2O2 for creating oxidative
stress in vitro was based on previous studies and our
preliminary experiments (28-30). When H2O2 was
added in the medium, cell viability decreased by 34% in the
oxaliplatin group, while high-dose MgiG treatment improved
viability by 109, 38% higher than the control group (Fig. 2G). In the viability assay with
supplementary GSH, oxaliplatin challenge attenuated cell viability
by 30%. The cell viability in MgiG-treated groups also increased
(Fig. 2H). High-dose MgiG
treatment improved cell viability by 20%, and there was no
significant difference between the viabilities of the
oxaliplatin-treated group and the low-dose MgiG group (Fig. 2H).
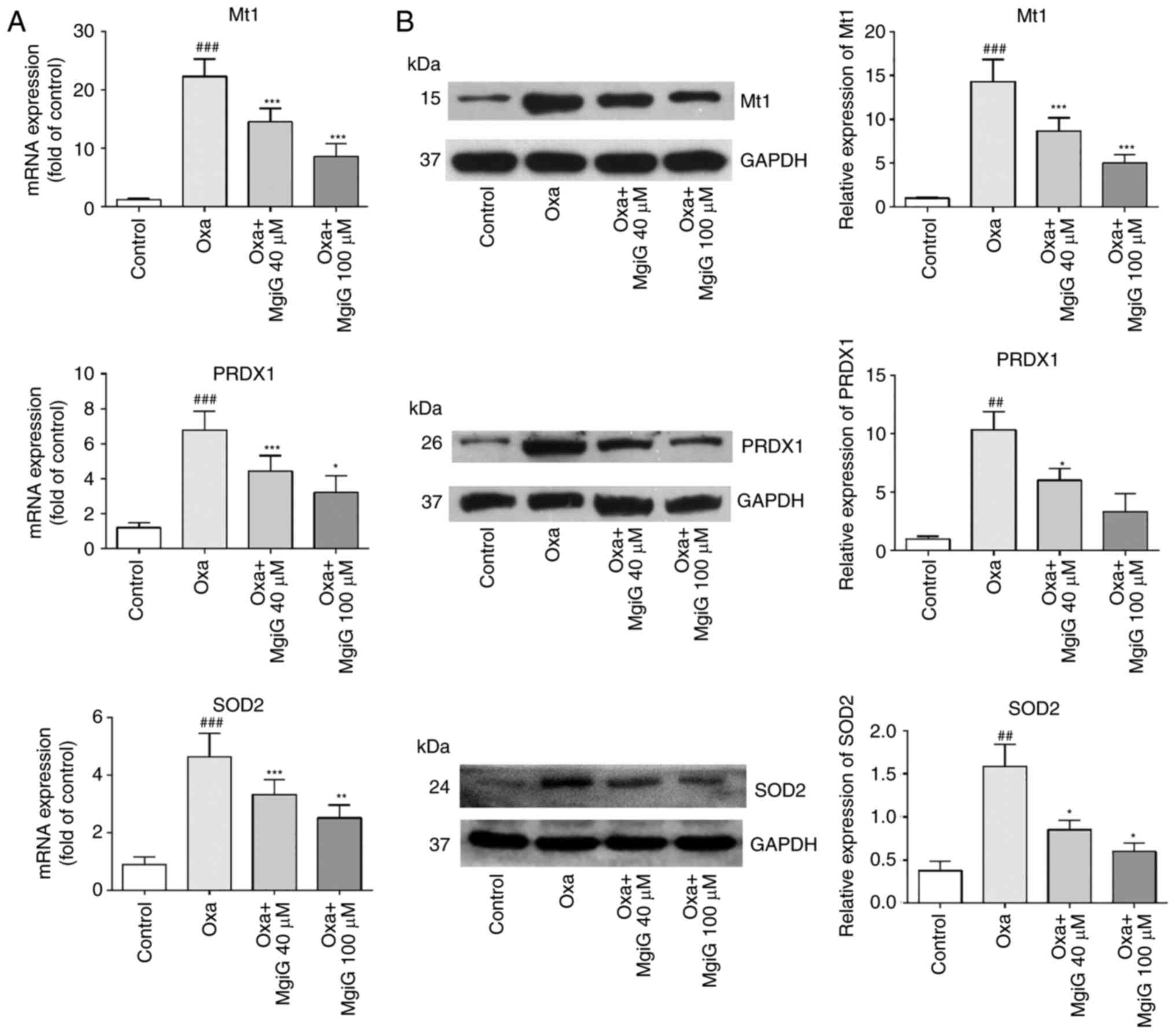
Next, the expression of genes classically associated
with anti- and pro-oxidative functions was investigated (27). The expression levels of vital
genes implicated in the response to oxidative stress, Mt1, PRDX1
and SOD2, were measured in the liver of oxaliplatin-treated mice by
RT-qPCR and western blotting. The results demonstrated that both
mRNA and protein expression levels of these genes were increased in
the livers of oxaliplatin-treated mice compared with control mice
(Fig. 3). MgiG treatment,
however, effectively attenuated the increased expression of these
genes (Fig. 3). In addition, all
three indicators were more improved with high-dose MgiG than with
low-dose MgiG (Fig. 3).
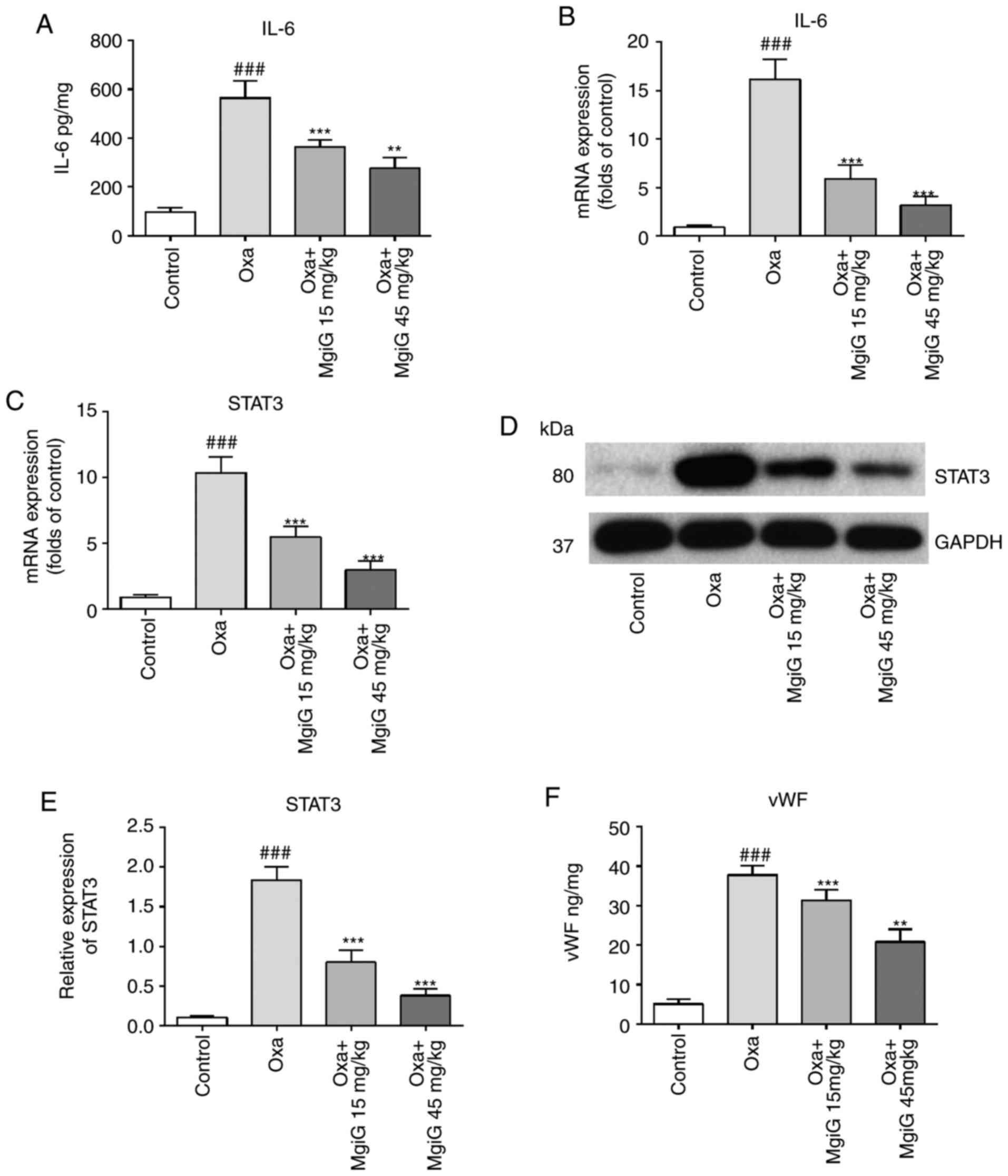
MgiG inhibits IL-6 pathway activation
induced by oxaliplatin
Previous studied have reported that the IL-6 pathway
is a core signaling pathway activated during oxaliplatin-related
liver damage (11,21). IL-6 levels in liver homogenates
were measured by ELISA and RT-qPCR. The secreted levels and mRNA
expression of IL-6 in liver homogenates were increased following
oxaliplatin administration and this effect was significantly
attenuated by MgiG treatment (Fig. 4A
and B). The levels of IL-6 in the high-dose group were lower
compared with the low-dose group. STAT3 is one of the most
important transcription factors downstream of IL-6. Its expression
in liver homogenates was also tested by RT-qPCR and Western blot.
The RT-qPCR results demonstrated that STAT3 expression was
increased by 3.4-fold in the oxaliplatin-administered group, and
that this increase was reversed by MgiG treatment (Fig. 4C). The western blot analysis
demonstrated similar results (Fig. 4D
and E).
MgiG reverses the pro-thrombotic
environment within oxaliplatin-injured hepatic sinusoids
In most previous studies of oxaliplatin-induced
liver injury, activation of the coagulation system was one of the
most-discussed mechanisms (11,12,21,26,31). In the present study, ELISA results
revealed that levels of vWF, a key component in platelet adhesion,
were significantly increased in the livers of animals administered
with oxaliplatin (Fig. 4F). MgiG
treatment significantly attenuated this increase (Fig. 4F). This effect was also evident at
the mRNA and protein level, as measured by RT-qPCR and western
blotting (Fig. 4G–I). Expression
of PAI-1 (also known as SERPINE1) in the livers of experimental
animals was also increased as a result of oxaliplatin exposure, as
measured by RT-qPCR (Fig. 4J).
MgiG treatment notably inhibited this increase (Fig. 4J). Western blott analysis revealed
similar results at the protein level (Fig. 4K and L). In both experiments,
high-dose MgiG had an improved protective effect compared with
low-dose MgiG.
Discussion
Oxaliplatin-induced liver injury is a primary
limiting factor affecting the efficacy of oxaliplatin-based
chemotherapy in patients with CRLM (7). Medications that can protect liver
cells against damage caused by oxaliplatin are urgently needed.
However, relevant studies are very rare. There is only one known
study regarding the use of reduced glutathione in
oxaliplatin-induced acute liver injury in vivo (19). To the best of our knowledge, the
present study is the first report of a medication to treat or
prevent oxaliplatin-caused liver injury using both in vivo
and in vitro experiments. The current study presented novel
insights into the mechanisms of oxaliplatin-induced liver injury
and might represent a potential strategy for its treatment in the
future.
It has been demonstrated that MgiG has an improved
liver-protective effect compared with either glycyrrhizin or
β-glycyrrhizic acid in carbon tetrachloride- and
D-galactosamine-induced liver damage animal models. Previous
studies have also demonstrated that MgiG attenuates liver injury
caused by toxins and other factors, such as free fatty acids,
ethanol and ischemia/reperfusion (13-15,20,32). MgiG has already been clinically
used as a drug in the treatment of hepatitis and toxin-associated
liver injury. In addition, a previous study has reported the
hepatoprotective effect of MgiG in chemotherapy reagent-induced
liver injury caused by cyclophosphamide (18).
In most previous studies, chemotherapy-associated
liver injury animal models were created by administration of toxic
reagents (33-35), such as tetrachloromethane,
D-galactosamine and monocrotaline. However, there was a great risk
that the underlying molecular mechanisms leading to the
pathological changes in the liver of experimental animals would be
different from those of patients receiving chemotherapy, because of
the different drugs used to cause it. The side-effects induced by
toxic agents are also a difficulty, including the renal toxicity of
tetrachloromethane and pulmonary hypertension as a result of
monocrotaline exposure (36,37). More valuable information could be
derived from studying the liver-protecting effect against
chemotherapy in an animal model induced by a chemotherapy reagent,
rather than toxins. The liver injury animal model in the present
study was established by intraperitoneal injection of oxaliplatin,
and the drug was administered to the animals based on a treatment
schedule comparable with that of human patients receiving
chemotherapy in the clinic. In this way, the aim of the present
study was to more accurately mimic the pathogenic process of
oxaliplatin-associated liver injury.
AST and ALT are the most reliable biochemical
markers of liver damage. The present results revealed significant
increases in transaminase levels both in serum and in LO2 cell
supernatants following oxaliplatin exposure. Grading results from
H&E and Sirius red staining demonstrated pathological
alterations within the livers of experimental animals, including
vast areas of cellular necrosis, extensive vacuolization,
inflammatory cell infiltration and fibrosis. The experimental
findings indicated the occurrence of severe liver injury caused by
oxaliplatin. MgiG treatment significantly ameliorated the injury,
accompanied by an improvement in transaminase levels both in
vivo and in vitro and reversion of the pathological
changes.
Potential limitations of the current study may be
that MgiG served its protective role by directly inactivating the
chemotherapy drug or by protecting other types of cells, such as
the cancer cells, from oxaliplatin. While the cell viability assay
revealed a substantial protective effect of MgiG in LO2 cells,
there was no significant difference in Ht-29 cell viability with or
without MgiG treatment, suggesting that MgiG specifically protected
hepatic cells from oxaliplatin without attenuating its efficacy on
colon cancer cells. This is a very interesting phenomenon, and
several reasons might explain it. Firstly, liver is the primary
source of GSH production in the human body. There are studies
indicating that GSH is closely associated with drug inactivation,
and GSH is elevated in a number of drug-resistant tumor cell lines
(38-40). In addition, alleviated oxidative
stress is one of the mechanisms by which oxaliplatin functions.
MgiG might, by an unknown mechanism, promote the synthesis of GSH
by liver cells. Other explanations can be considered; a previous
study has demonstrated that glycyrrhetinic acid (GA) administration
increased the expression and activity of UDP-glucuronyltransferases
(UGTs) in the liver of experimental animals while simultaneously
attenuating UGT activity in the intestine (41). UGTs are known as the primary
enzymatic family affecting the pharmacokinetic and pharmacodynamic
properties of xenobiotics. Theoretically, MgiG is also a derivative
of glycyrrhizin, as is GA, suggesting that they might share some
metabolic process in the body. Therefore, increasing UGT activity
in the liver might also be a potential mechanism by which MgiG
attenuated oxaliplatin-induced liver injury.
Previous studies indicated that increased oxidative
stress is an important cause of oxaliplatin-induced hepatotoxicity
(11,21,25). As the end-product of lipid
breakdown, MDA is recognized as a reliable indicator sensitive to
oxidative stress in hepatic lesions (42). The present study demonstrated that
MgiG eliminated the changes in oxidative stress indicators (GSH and
MDA) caused by oxaliplatin exposure. When additional oxidative
stress was present, the hepatic toxicity of oxaliplatin was
partially abolished, and MgiG restored cell viability to even
higher levels than those of the control group. With the presence of
extra reductant, the hepatic toxicity of oxaliplatin was also
partially abolished, and the protective effect of MgiG decreased
simultaneously. Taken together, these data suggested that
oxaliplatin caused liver injury by increasing oxidative stress, and
MgiG protected liver cells by attenuating oxidative stress. The
increased expression of a variety of genes implicated in responses
to oxidative stress (Mt1, PRDX1 and SOD2) was also diminished
following MgiG treatment at both the transcriptional and the
translational level.
A broad spectrum of studies suggested that
oxaliplatin induced inflammatory responses in the liver.
Oxaliplatin has been reported to cause overproduction of various
pro-inflammation cytokines, including CXC motif chemokine ligand 1
(CXCL1; also known as IL-8), monocyte chemoattractant protein-1
(MCP-1) and IL-6 (11,21). Although inflammation is unlikely
to be the main initiator of oxaliplatin-induced liver injury, the
IL-6 pathway might become activated in response to toxic damage or
ischemic injury (43). As a vital
transcription factor downstream of IL-6, STAT3 mediates the
function of anti-apoptosis, reducing oxidative stress and
maintaining capillary integrity. The present study demonstrated
that oxaliplatin stimulation increased IL-6 and STAT3 expression,
while this effect was significantly antagonized by MgiG, confirming
the anti-inflammatory role of MgiG in oxaliplatin-induced hepatic
injury.
Coagulation pathways have been implicated in the
pathogenic process of oxaliplatin-induced liver injury (11,12,21). More support for this hypothesis
came from the observation that patients taking aspirin tended to be
at lower risk of developing oxaliplatin-induced SOS (44). A possible explanation regarding
how a prothrombotic environment contributes to the development of
SOS involves the generation of micro-thrombi within hepatic
sinusoids that subsequently become occluded, exacerbating the
elevated oxidative stress. Both vWF and PAI-1 are principle markers
of the coagulation cascade. The present results demonstrated that
vWF and PAI-1 were overexpressed following oxaliplatin exposure,
and MgiG treatment effectively restored the levels of these
factors. The changes in PAI-1 mRNA levels measured by RT-qPCR were
much higher than the changes in protein levels measured by western
blot analysis. PAI-1 is a primary inhibitor of the plasminogen
activators. The activity of PAI-1 is regulated by many factors,
including circadian rhythm, blood glucose levels, insulin and
insulin-like molecules, and even its own genetic variation at
polymorphic loci is associated with differences in plasma PAI-1
levels (45-50). Therefore, there might be other
regulatory mechanisms occurring following the transcription of the
protein, leading to smaller changes in protein levels compared with
mRNA levels. In addition, the hepatocyte is the main source of
PAI-1 synthesis in the body, and the produced protein is then
secreted into the serum (51).
This process could also result in smaller changes in liver PAI-1
protein levels compared with mRNA levels.
There are some limitations in the present study. The
phenomenon of MgiG specifically protecting hepatocytes but having
no influence on the effectiveness of oxaliplatin in colon cancer
cells is very interesting and requires further investigation.
Deeper exploration of the detailed mechanism by which MgiG
functions is needed, particularly the metabolic processes of MiG in
the body. Our group will continue this line of research in future
studies.
In summary, the present study demonstrated that MgiG
effectively attenuated oxaliplatin-induced liver injury in
vitro and in vivo. The protective effect of MgiG on
oxaliplatin-induced liver injury was correlated with the
attenuation of oxidative stress, the IL-6 pathway and the
coagulation pathway. The present findings revealed that MgiG may
have potential value as a treatment or prevention agent against
oxaliplatin-induced liver injury in clinical practice.
Acknowledgments
We are grateful to Dr Su and his colleague from the
Department of Pathology, Qilu Hospital for the assistance for the
construction and analysis of tissue slices.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81572414) and the Natural
Science Foundation of Shandong Province (grant no.
ZR2018BH023).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
JN was responsible for designing of the study and
critical review of manuscript. XZ was responsible for designing and
performing the study, literature research and manuscript drafting.
YW participated in the animal model establishment, cell culturing
and statistical analysis. BW and CP participated in the design of
the study and sequence alignment. ZN and ZL participated in
sequence alignment and immunoassays. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present research was approved by the
Institutional Animal Care and Use Committee of Shandong University
(Jinan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maughan TS, Adams RA, Smith CG, Meade AM,
Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL,
et al: Addition of cetuximab to oxaliplatin-based first-line
combination chemotherapy for treatment of advanced colorectal
cancer: Results of the randomised phase 3 MRC COIN trial. Lancet.
377:2103–2114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beppu T, Sakamoto Y, Hayashi H and Baba H:
Perioperative chemotherapy and hepatic resection for resectable
colorectal liver metastases. Hepatobiliary Surg Nutr. 4:72–75.
2015.PubMed/NCBI
|
|
4
|
André T, Boni C, Mounedji-Boudiaf L,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: Oxaliplatin, fluorouracil, and leucovorin
as adjuvant treatment for colon cancer. N Engl J Med.
350:2343–2351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
André T, Boni C, Navarro M, Tabernero J,
Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F
and de Gramont A: Improved overall survival with oxaliplatin,
fluorouracil, and leucovorin as adjuvant treatment in stage II or
III colon cancer in the MOSAIC trial. J Clin Oncol. 27:3109–3116.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Portier G, Elias D, Bouche O, Rougier P,
Bosset JF, Saric J, Belghiti J, Piedbois P, Guimbaud R, Nordlinger
B, et al: Multicenter randomized trial of adjuvant fluorouracil and
folinic acid compared with surgery alone after resection of
colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin
Oncol. 24:4976–4982. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rubbia-Brandt L, Lauwers GY, Wang H, Majno
PE, Tanabe K, Zhu AX, Brezault C, Soubrane O, Abdalla EK, Vauthey
JN, et al: Sinusoidal obstruction syndrome and nodular regenerative
hyperplasia are frequent oxaliplatin-associated liver lesions and
partially prevented by bevacizumab in patients with hepatic
colorectal metastasis. Histopathology. 56:430–439. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Slade JH, Alattar ML, Fogelman DR, Overman
MJ, Agarwal A, Maru DM, Coulson RL, Charnsangavej C, Vauthey JN,
Wolff RA and Kopetz S: Portal hypertension associated with
oxaliplatin administration: Clinical manifestations of hepatic
sinusoidal injury. Clin Colorectal Cancer. 8:225–230. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hubert C, Fervaille C, Sempoux C, Horsmans
Y, Humblet Y, Machiels JP, Zech F, Ceratti A and Gigot JF:
Prevalence and clinical relevance of pathological hepatic changes
occurring after neoadjuvant chemotherapy for colorectal liver
metastases. Surgery. 147:185–194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Robinson SM, Wilson CH, Burt AD, Manas DM
and White SA: Chemotherapy-associated liver injury in patients with
colorectal liver metastases: A systematic review and meta-analysis.
Ann Surg Oncol. 19:4287–4299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rubbia-Brandt L, Tauzin S, Brezault C,
Delucinge-Vivier C, Descombes P, Dousset B, Majno PE, Mentha G and
Terris B: Gene expression profiling provides insights into pathways
of oxaliplatin-related sinusoidal obstruction syndrome in humans.
Mol Cancer Ther. 10:687–696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Agostini J, Benoist S, Seman M, Julié C,
Imbeaud S, Letourneur F, Cagnard N, Rougier P, Brouquet A,
Zucman-Rossi J and Laurent-Puig P: Identification of molecular
pathways involved in oxaliplatin-associated sinusoidal dilatation.
J Hepatol. 56:869–876. 2012. View Article : Google Scholar
|
|
13
|
Sun L, Shen J, Pang X, Lu L, Mao Y and
Zeng M: Phase I safety and pharmacokinetic study of magnesium
isoglycyrrhizinate after single and multiple intravenous doses in
chinese healthy volunteers. J Clin Pharmacol. 47:767–773. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu Q, Wang J, Chen F, Lin K, Zhu M, Chen
L, Zhou X, Li C and Zhu H: Protective role of magnesium
isoglycyrrhizinate in non-alcoholic fatty liver disease and the
associated molecular mechanisms. Int J Mol Med. 38:275–282. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng Y, Zhang J, Shang J and Zhang L:
Prevention of free fatty acid-induced hepatic lipotoxicity in HepG2
cells by magnesium isoglycyrrhizinate in vitro. Pharmacology.
84:183–190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao HJ, Xiao L, Glizila B, Zhang H, Mao
R, Xiong Y, Xu L, Shu MY, Bai YW and Bao YX: The effect and
comparison of commonly used liver-protection drugs for irradiated
HL-7702 by X. Zhonghua Gan Zang Bing Za Zhi. 25:612–617. 2017.In
Chinese. PubMed/NCBI
|
|
17
|
Wang Y, Zhang Z, Wang X, Qi D, Qu A and
Wang G: Amelioration of ethanol-induced hepatitis by magnesium
isoglycyrrhizinate through inhibition of neutrophil cell
infiltration and oxidative damage. Mediators Inflamm.
2017:35269032017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang W, Liu J, Li P, Lu Q, Pei X, Sun Y,
Wang G and Hao K: Magnesium isoglycyrrhizinate shows
hepatoprotective effects in a cyclophosphamide-induced model of
hepatic injury. Oncotarget. 8:33252–33264. 2017.PubMed/NCBI
|
|
19
|
Lin Y, Li Y, Hu X, Liu Z, Chen J, Lu Y,
Liu J, Liao S, Zhang Y, Liang R, et al: The hepatoprotective role
of reduced glutathione and its underlying mechanism in
oxaliplatin-induced acute liver injury. Oncol Lett. 15:2266–2272.
2018.PubMed/NCBI
|
|
20
|
He Y, Zeng F, Liu Q, Ju W, Fu H, Hao H, Li
L and Xie Y: Protective effect of magnesium isoglycyrrhizinate on
ethanol-induced testicular injuries in mice. J Biomed Res.
24:153–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Robinson SM, Mann J, Vasilaki A, Mathers
J, Burt AD, Oakley F, White SA and Mann DA: Pathogenesis of FOLFOX
induced sinusoidal obstruction syndrome in a murine chemotherapy
model. J Hepatol. 59:318–326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiong W, Ren ZG, Qiu SJ, Sun HC, Wang L,
Liu BB, Li QS, Zhang W, Zhu XD, Liu L, et al: Residual
hepatocellular carcinoma after oxaliplatin treatment has increased
metastatic potential in a nude mouse model and is attenuated by
Songyou Yin. BMC Cancer. 10:2192010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kelley MR, Jiang Y, Guo C, Reed A, Meng H
and Vasko MR: Role of the DNA base excision repair protein, APE1 in
cisplatin, oxaliplatin, or carboplatin induced sensory neuropathy.
PLoS One. 9:e1064852014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Kweekel DM, Gelderblom H and Guchelaar HJ:
Pharmacology of oxaliplatin and the use of pharmacogenomics to
individualize therapy. Cancer Treat Rev. 31:90–105. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McWhirter D, Kitteringham N, Jones RP,
Malik H, Park K and Palmer D: Chemotherapy induced hepatotoxicity
in metastatic colorectal cancer: A review of mechanisms and
outcomes. Crit Rev Oncol Hematol. 88:404–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hua W, Huang HZ, Tan LT, Wan JM, Gui HB,
Zhao L, Ruan XZ, Chen XM and Du XG: CD36 mediated fatty
acid-induced podocyte apoptosis via oxidative stress. PLoS One.
10:e01275072015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang S, Li X, Jourd'heuil FL, Qu S,
Devejian N, Bennett E, Jourd'heuil D and Cai C: Cytoglobin promotes
cardiac progenitor cell survival against oxidative stress via the
upregulation of the NFκB/iNOS signal pathway and nitric oxide
production. Sci Rep. 7:107542017. View Article : Google Scholar
|
|
29
|
Arafa E, Bondzie PA, Rezazadeh K, Meyer
RD, Hartsough E, Henderson JM, Schwartz JH, Chitalia V and Rahimi
N: TMIGD1 is a novel adhesion molecule that protects epithelial
cells from oxidative cell injury. Am J Pathol. 185:2757–2767. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Madesh M and Hajnóczky G: VDAC-dependent
permeabilization of the outer mitochondrial membrane by superoxide
induces rapid and massive cytochrome c release. J Cell Biol.
155:1003–1015. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu Z, Cheng D, Yin J, Wu R, Zhang G, Zhao
Q, Wang N, Wang F and Liang M: Antithrombin III protects against
contrast-induced nephropathy. EBioMedicine. 17:101–107. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gu X, Dong L, Liu Y and Yu F: Protective
effect of magnesium isoglycyrrhizinate on acute hepatic injury
induced by CCl4 in mice. China Pharmacist. 12:37–39. 2009.
|
|
33
|
DeLeve LD, McCuskey RS, Wang X, Hu L,
McCuskey MK, Epstein RB and Kanel GC: Characterization of a
reproducible rat model of hepatic veno-occlusive disease.
Hepatology. 29:1779–1791. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma JQ, Ding J, Zhao H and Liu CM: Puerarin
attenuates carbon tetrachloride-induced liver oxidative stress and
hyperlipidaemia in mouse by JNK/c-Jun/CYP7A1 pathway. Basic Clin
Pharmacol Toxicol. 115:389–395. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakamura K, Hatano E, Miyagawa-Hayashino
A, Okuno M, Koyama Y, Narita M, Seo S, Taura K and Uemoto S:
Soluble thrombomodulin attenuates sinusoidal obstruction syndrome
in rat through suppression of high mobility group box 1. Liver Int.
34:1473–1487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schultze AE and Roth RA: Chronic pulmonary
hypertension-the monocrotaline model and involvement of the
hemostatic system. J Toxicol Environ Health B Crit Rev. 1:271–346.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hassan MH, Bahashawan SA, Abdelghany TM,
Abd-Allah GM and Ghobara MM: Crocin abrogates carbon
tetrachloride-induced renal toxicity in rats via modulation of
metabolizing enzymes and diminution of oxidative stress, apoptosis,
and inflammatory cytokines. J Biochem Mol Toxicol. 29:330–339.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bray TM and Taylor CG: Tissue glutathione,
nutrition, and oxidative stress. Can J Physiol Pharmacol.
71:746–751. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu SC: Regulation of hepatic glutathione
synthesis: Current concepts and controversies. FASEB J.
13:1169–1183. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mulcahy RT, Bailey HH and Gipp JJ:
Up-regulation of gamma-glutamylcysteine synthetase activity in
melphalan-resistant human multiple myeloma cells expressing
increased glutathione levels. Cancer Chemother Pharmacol. 34:67–71.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li FY, Xie H, Weng L, Wang H, Cao LJ, Hao
HP and Wang GJ: Effects of diammonium glycyrrhizinate on hepatic
and intestinal UDP-Glucuronosyltransferases in rats: Implication in
herb-drug interactions. Chin J Nat Med. 14:534–540. 2016.PubMed/NCBI
|
|
42
|
Liu M, Xu H, Zhang L, Zhang C, Yang L, Ma
E, Liu L and Li Y: Salvianolic acid B inhibits myofibroblast
transdifferentiation in experimental pulmonary fibrosis via the
up-regulation of Nrf2. Biochem Biophys Res Commun. 495:325–331.
2018. View Article : Google Scholar
|
|
43
|
Wu R, Liu X, Yin J, Wu H, Cai X, Wang N,
Qian Y and Wang F: IL-6 receptor blockade ameliorates diabetic
nephropathy via inhibiting inflammasome in mice. Metabolism.
83:18–24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Brouquet A, Benoist S, Julie C, Penna C,
Beauchet A, Rougier P and Nordlinger B: Risk factors for
chemotherapy-associated liver injuries: A multivariate analysis of
a group of 146 patients with colorectal metastases. Surgery.
145:362–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Angleton P, Chandler WL and Schmer G:
Diurnal variation of tissue-type plasminogen activator and its
rapid inhibitor (PAI-1). Circulation. 79:101–106. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Maiello M, Boeri D, Podesta F, Cagliero E,
Vichi M, Odetti P, Adezati L and Lorenzi M: Increased expression of
tissue plas-minogen activator and its inhibitor and reduced
fibrinolytic potential of human endothelial cells cultured in
elevated glucose. Diabetes. 41:1009–1015. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cagliero E, Roth T, Roy S, Maiello M and
Lorenzi M: Expression of genes related to the extracellular matrix
in human endothelial cells. Differential modulation by elevated
glucose concentrations, phorbol esters, and cAMP. J Biol Chem.
266:14244–14250. 1991.PubMed/NCBI
|
|
48
|
Nordt TK, Schneider DJ and Sobel BE:
Augmentation of the synthesis of plasminogen activator inhibitor
type-1 by precursors of insulin. A potential risk factor for
vascular disease. Circulation. 89:321–330. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Alessi MC, Juhan-Vague I, Kooistra T,
Declerck PJ and Collen D: Insulin stimulates the synthesis of
plasminogen activator inhibitor 1 by the human hepatocellular cell
line Hep G2. Thromb Haemost. 60:491–494. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lijnen HR: Pleiotropic functions of
plasminogen activator inhibitor-1. J Thromb Haemostasis. 3:35–45.
2005. View Article : Google Scholar
|
|
51
|
Kooistr T: The use of cultured human
endothelial cells and hepatocytes as an in vitro model system to
study modulation of endogenous fibrinolysis = Fibrinolysis. 4(Suppl
2): S33–S39. 1990.
|