Introduction
Heart disease threatens the health of humans
worldwide; however, it is often difficult to detect at the level of
transcriptional regulation. Abnormal transcriptomic regulation is
complicated and affected by a number of complex mechanisms at both
a transcriptional and post-transcriptional level (1,2).
Transcription factors (TFs) determine the level of gene expression
by recognizing specific DNA sequences in diverse cell types
(3). MicroRNAs (miRNAs) are
important gene expression regulators, which bind with their target
mRNAs, and repress or degrade them (4). An increasing number of studies have
emphasized that miRNAs are essential in numerous types of heart
diseases. Therefore, constructing a clear map of the network
between miRNAs, their target genes and TFs may be beneficial for
identifying dysregulated genes and signaling pathways. This would
then increase the understanding on the roles of specific genes in
heart disease pathogenesis.
TFs, miRNAs and their shared target genes form
miRNA-associated feed-forward loops (miR-FFLs), in which miRNAs and
TFs co-ordinate to regulate gene expression (5). The regulatory units within the
miR-FFL network consist of an miRNA, a TF and their shared target
genes (6,7). FFLs govern a number of biological
processes, such as cell differentiation, and cause the development
of certain diseases, including cancer (8,9).
However, the varied roles of FFLs in certain common heart diseases
have not been globally studied in a systematic manner.
Cardiovascular disease is an important cause of
mortality worldwide and affects a great number of individuals
annually (10). Cardiac
hypertrophy is typically an inherited cardiovascular disease caused
by abnormal gene mutations (11).
Transcriptome reprogramming in the diseased heart can result in the
development of abnormal pathological features (12). Several previous studies have
demonstrated the association between miRNAs and cardiac hypertrophy
(13-15).
Acute myocardial infarction (AMI) is a pathological
and life-threatening condition, in which blood is unable to flow
into the heart due to the blockage of a coronary artery, leading to
the death of a part of the myocardial muscle (16). AMI and its associated risk factors
require urgent assessment (17).
A number of previous studies have also demonstrated the essential
roles of miRNAs in AMI (18-20). Furthermore, recent studies have
reported an association between cardiac hypertrophy and AMI. AMI
treatment may be influenced by cardiac hypertrophy, as it appears
to decrease the efficiency of ischemic preconditioning (21). Left ventricular (LV) hypertrophy
and remodeling subsequent to MI are vital predictors of a patient's
prognosis (22). However, the
association between the two diseases on the miR-FFL level has yet
to be globally investigated.
In the present study, a global miR-FFL network was
constructed and its topological features were analyzed. In
addition, a comprehensive method was designed that collects
regulatory interaction and high-throughput expression profiles for
miRNAs, TFs and target genes in order to characterize common or
specifically dysregulated miR-FFL motifs in cardiac hypertrophy and
AMI. The dysregulated miR-FFLs were revealed to be associated with
cardiac-associated functions and signaling pathways. The genes and
miRNAs in these miR-FFLs were also demonstrated to be vital drug
targets. In conclusion, the present study highlighted the effect of
dysregulated miR-FFL motifs in cardiac hypertrophy and AMI, which
revealed their possibility as novel biomarkers and treatment
targets in heart disease.
Materials and methods
Collecting high-throughput data for
miRNAs, TFs and genes
The gene expression profiles of cardiac hypertrophy
and AMI were downloaded from the Gene Expression Omnibus database
(www.ncbi.nlm.nih.gov/geo). Two studies
in which the samples provided both miRNA and gene expression
profiles were extracted. The cardiac hypertrophy expression data
included three diseases samples and three control samples
(accession no. GSE60291) (23).
The AMI expression data included four disease samples and one
control sample (accession no. GSE24591; unpublished data).
Establishing a genome-wide miR-FFL
network
miR-FFL motifs are three-gene modules consisting of
an miRNA, a TF and their shared target gene. The miRNA is regulated
by the TF, while the target gene is regulated by both the miRNA and
TF. Three types of regulatory associations were used to construct a
comprehensive miR-FFL network. Firstly, the TF-miRNA association
was obtained from a public database known as TransmiR, which
contains experimentally validated information regarding TFs and
miRNAs (24). Secondly, TF-gene
regulatory pairs were obtained from the TRANSFAC Professional
database (release date, February 2014) (24). Finally, experiments supporting the
miRNA-gene regulatory association were obtained from TarBase v6.0,
which is a high quality and widely used database (25). The data were merged, and the TFs
and gene names were mapped to gene symbols, while the miRNA names
were mapped to miRBase accession numbers for mature miRNAs
(http://www.mirbase.org/).
Dissecting topological features for the
miR-FFL network
Four measurements were used to assess the entire
miR-FFL network, including degrees, topological coefficients,
neighborhood connectivity and the clustering coefficients of nodes.
All analyses were performed using Cytoscape 3.0 (http://www.cytoscape.org/).
Identifying dysregulated miR-FFLs in
cardiac hypertrophy and AMI
An integrative method was designed to identify
dysregulated miR-FFL motifs in cardiac hypertrophy and AMI, which
used the miR-FFL regulatory networks and the expression data.
Initially, Student's t-test was performed to compare the
differences in expression between the TFs, miRNAs and genes in the
patients with disease and the corresponding controls, for each
miR-FFL motif. Next, for each interacting pair in the miR-FFL motif
(TF-miRNA, TF-gene and miRNA-gene), the Pearson correlation
coefficients (PCCs) and the statistical difference between them in
the disease and control samples were calculated. The association
between regulatory interactions was represented using the absolute
difference of PCCs between the disease and control samples. In
addition, the integrated comprehensive scores (Sdif and
Spcc) for the FFLs were determined using the following
equations for the differential expression of the P-value and
PCCs:
Sdif=Pm×PT×Pg
Spcc=|(DTm−CTm)×(DTg−CTg)×(Dmg−Cmg)|
In these equations, Pm, PT and
Pg refer to the P-values of the miRNAs, TFs and genes,
respectively, in each miR-FFL motif that were derived from the
t-test. Sdif is the difference between the expression
level of the miR-FFL motif between the patients with disease and
the corresponding controls. DTm, DTg and
Dmg correspond to the PCCs of the three regulatory
pairs, including the TF-miRNA, TF-gene and miRNA-gene interactions
for the disease samples, respectively. CTm,
CTg and Cmg represent the PCCs of the same
three regulatory interaction pairs for the control samples,
respectively. Spcc is the absolute distinction of the
PCC score between the disease and control samples in the entire
miR-FFL motif. An integrative and equally-weighted method was also
used to rank all the FFL motifs according to the Sdif
and Spcc scores (26).
Once two ranked lists were obtained, the ranking positions of the
two lists were integrated to calculate the final ranking score list
for each miR-FFL motif. A higher-ranking score represented an
increased level of the dysregulated motif in the disease compared
with the control. To obtain the significant P-value for each
miR-FFL motif, each final motif ranking score was compared with the
permutation-based final ranking score list, which was generated
after randomly disturbing all sample labels in expression profiles
1,000 times. Finally, the significantly dysregulated motifs
(P<0.05) for cardiac hypertrophy and AMI were obtained (27). All aforementioned analyses were
performed using R software (version 3.2.3; https://www.r-project.org/).
Gene Ontology (GO) enrichment
analysis
Enriched GO terms (P<0.01) were obtained from
Database for Annotation, Visualization and Integrated Discovery web
server using the default parameters (28).
Drug target analysis for miRNAs and
genes
SM2miR (29) and
DrugBank (30) databases were
used to investigate the association between drugs and genes, and
drugs and miRNAs, respectively.
Results
Characterizing topological properties of
the miR-FFL network
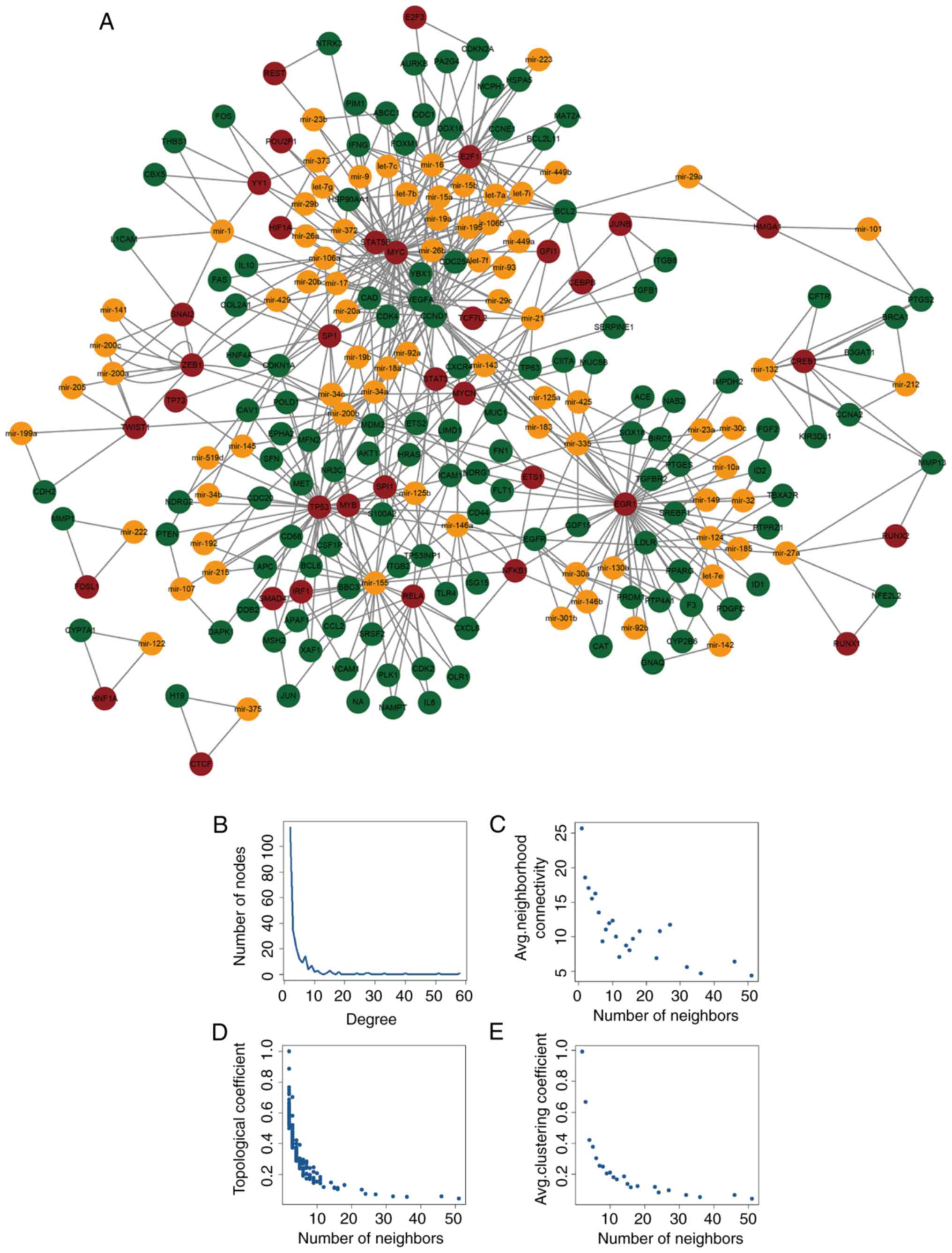
A total of 429 miR-FFL network motifs were obtained
using multiple data resources. Each network motif consisted of a
TF, an miRNA and their shared target gene. A genome-wide miR-FFL
network was established by integrating the network motifs (Fig. 1A), which included 235 nodes (122
genes, 35 TFs and 78 miRNAs) and 578 edges. Similar to the majority
of biological networks, the transcription regulatory network
obtained in the present study had a scale-free distribution
(Fig. 1B). In addition, other
topological features of all nodes were identified, including
connectivity, topological coefficient and clustering coefficient
(Fig. 1C–E), all of which
displayed scale-free properties. The scale-free network
demonstrated that the miR-FFL network was similar to a small-world
network (31). Neighborhood
connectivity was measured based on the average connectivity of
certain neighbors (neighbors = 0, 1, 2…n). In the current study, a
decreased degree distribution of the network was found to be
accompanied by a decrease in the topological coefficient, which
suggests that there was a hierarchical modularity phenomenon within
the network. In addition, the nodes with a high and low degree were
linked by the majority of the edges in the network, and these edges
followed the decreased distribution, suggesting sub-networks were
present within the network (32).
The results also revealed that other types of disease networks,
which shared similar topological properties, generally exhibited
the same features as the miR-FFL network reported in the present
study (33).
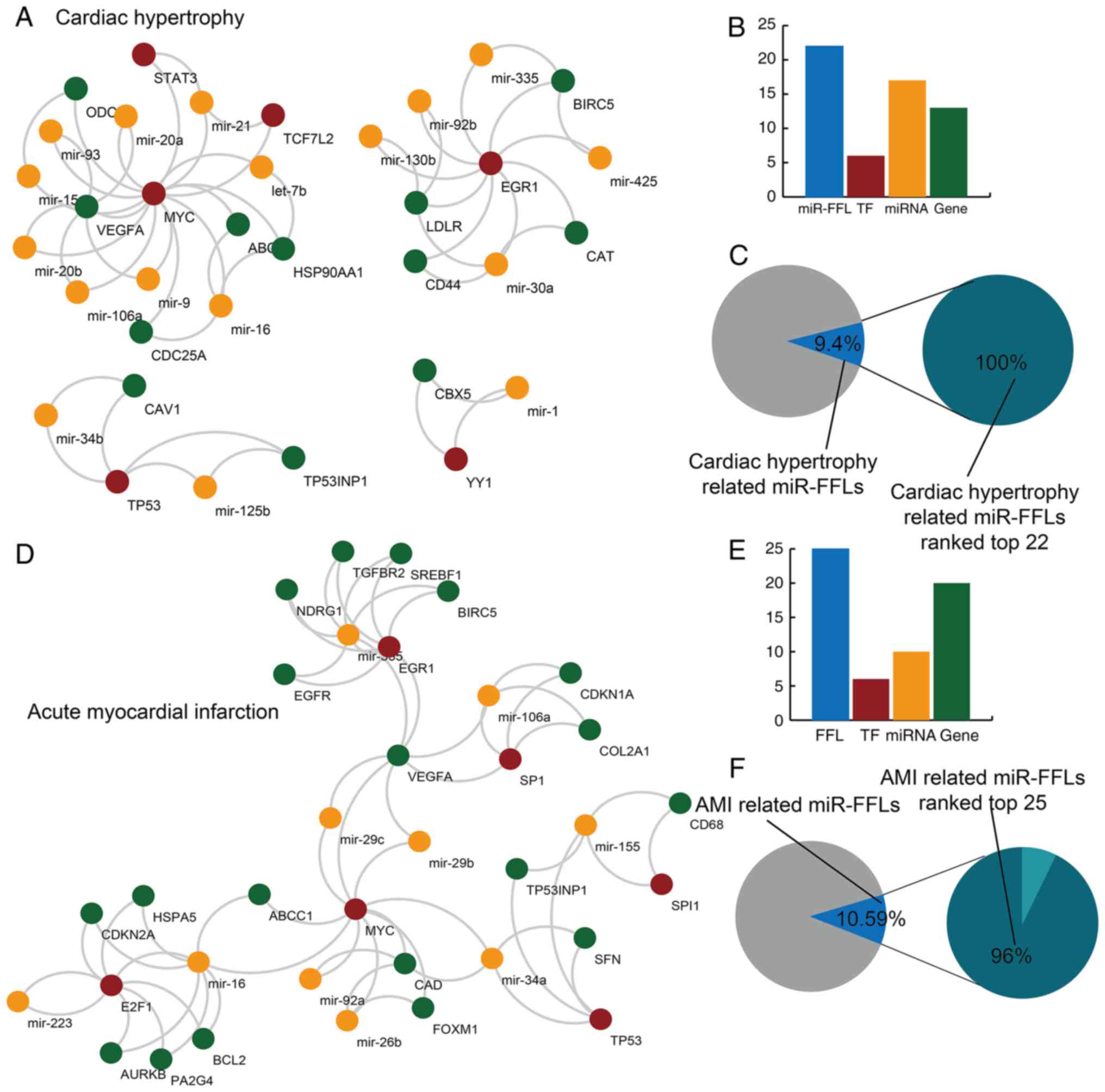
Specific miR-FFL motifs are significantly
dysregulated in cardiac hypertrophy and AMI
The significantly dysregulated miR-FFL network
motifs were characterized globally to determine the effect of
miR-FFL motifs in cardiac hypertrophy and AMI. Next, specific
dysregulated miR-FFL sub-networks were established for cardiac
hypertrophy and AMI (Fig. 2).
Among all background miR-FFLs, there were 22 miR-FFL motifs (9.4%)
dysregulated in cardiac hypertrophy that included 13 genes, 6 TFs
and 17 miRNAs (Fig. 2A and B).
These dysregulated cardiac hypertrophy-associated miR-FFLs were
ranked in the top 22 of all the background miR-FFLs following
permutation testing (Fig. 2C).
For AMI, there were 25 dysregulated miR-FFLs with 20 genes, 6 TFs
and 10 miRNAs, which represented 10.59% of all background miR-FFLs
(Fig. 2D and E). In total, 96% of
these dysregulated AMI-associated miR-FFLs were ranked in the top
25 background miR-FFLs following permutation testing. For both
cardiac hypertrophy and AMI, almost all the dysregulated miR-FFLs
identified by the method reported in the current study were
revealed to be statistically significant following permutation
testing (P<0.05).
In the identified dysregulated miR-FFLs, certain
miRNAs have previously been reported to serve essential roles in
heart disease. For instance, miR-21 can affect the hypertrophic
response process through exosome-associated transmission (34). In addition, miR-34a has a critical
effect in heart development, and the inhibition of miR-34a is
beneficial for cardiac function (35). Certain genes have also been
reported to be strongly associated with heart disease. For
instance, TP53 is a prominent tumor suppressor gene, and its
downregulation in cardiac fibroblasts is regulated by miR-155
(36).
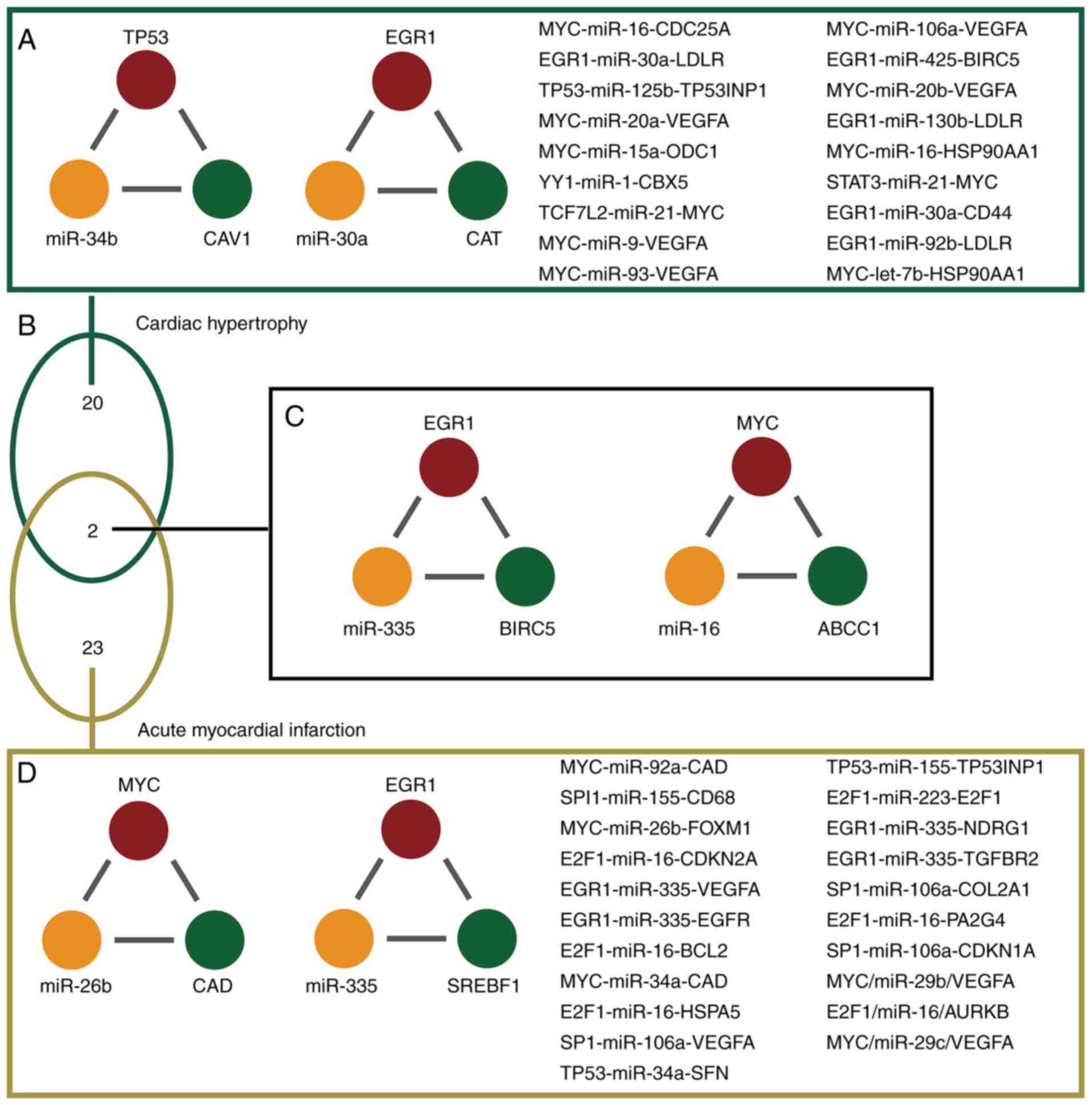
Common miR-FFLs motifs between cardiac
hypertrophy and AMI revealing the disease mechanism
The results of the present study demonstrated that
the majority of dysregulated miR-FFLs were specific in cardiac
hypertrophy and AMI. A total of 20 miR-FFLs were dysregulated in
cardiac hypertrophy alone; 23 miR-FFLs were dysregulated in AMI
alone (Fig. 3A and D). For
example, miR-FFL, consisting of TP53, mir-34b and CAV1, was only
demonstrated to be dysregulated in cardiac hypertrophy (Fig. 3A). In addition, EGR1 was
differentially expressed in both cardiac hypertrophy and AMI;
however, it also forms miR-FFL with diverse miRNAs and genes
(Fig. 3D). The present study
analysis revealed two common miR-FFLs between cardiac hypertrophy
and AMI (Fig. 3B). The first
common miR-FFL consisted of epidermal growth receptor (EGR1),
miR-335 and baculoviral IAP repeat-containing protein 5 (BIRC5).
This miR-FFL ranked nos. 15 and 16 regarding the top dysregulated
miR-FFLs associated with hypertrophy and AMI, respectively. In the
present study, miR-335 was differentially expressed between normal
and AMI samples. Furthermore, EGR1 and BIRC5 were differentially
expressed between normal and cardiac hypertrophy samples (Fig. 3C). In addition, patients suffering
from myocardial infarction have lower miR-335 expression, thus,
miR-335 may serve as a novel treatment strategy for AMI (37). Furthermore, BIRC5 was
downregulated in AMI patients (38). These results suggested that EGR1,
miR-335 and BIRC5 were dysregulated and could form a miR-FFL during
cardiac hypertrophy and AMI. Another common miR-FFL involved MYC,
miR-16 and ABCC1. This miR-FFL ranked no. 20 and 19 regarding the
top dysregulated miR-FFLs associated with hypertrophy and AMI,
respectively. MYC and miR-16 were differentially expressed between
normal and cardiac hypertrophy samples. These findings indicate
that a number of the dysregulated FFLs identified in the present
analysis were associated with heart disease, particularly the
common FFLs, which were almost all associated with cardiac
hypertrophy and AMI. Therefore, the authors suggest that numerous
miRNAs, TFs and genes may have roles in cardiac hypertrophy and AMI
via formation of FFLs.
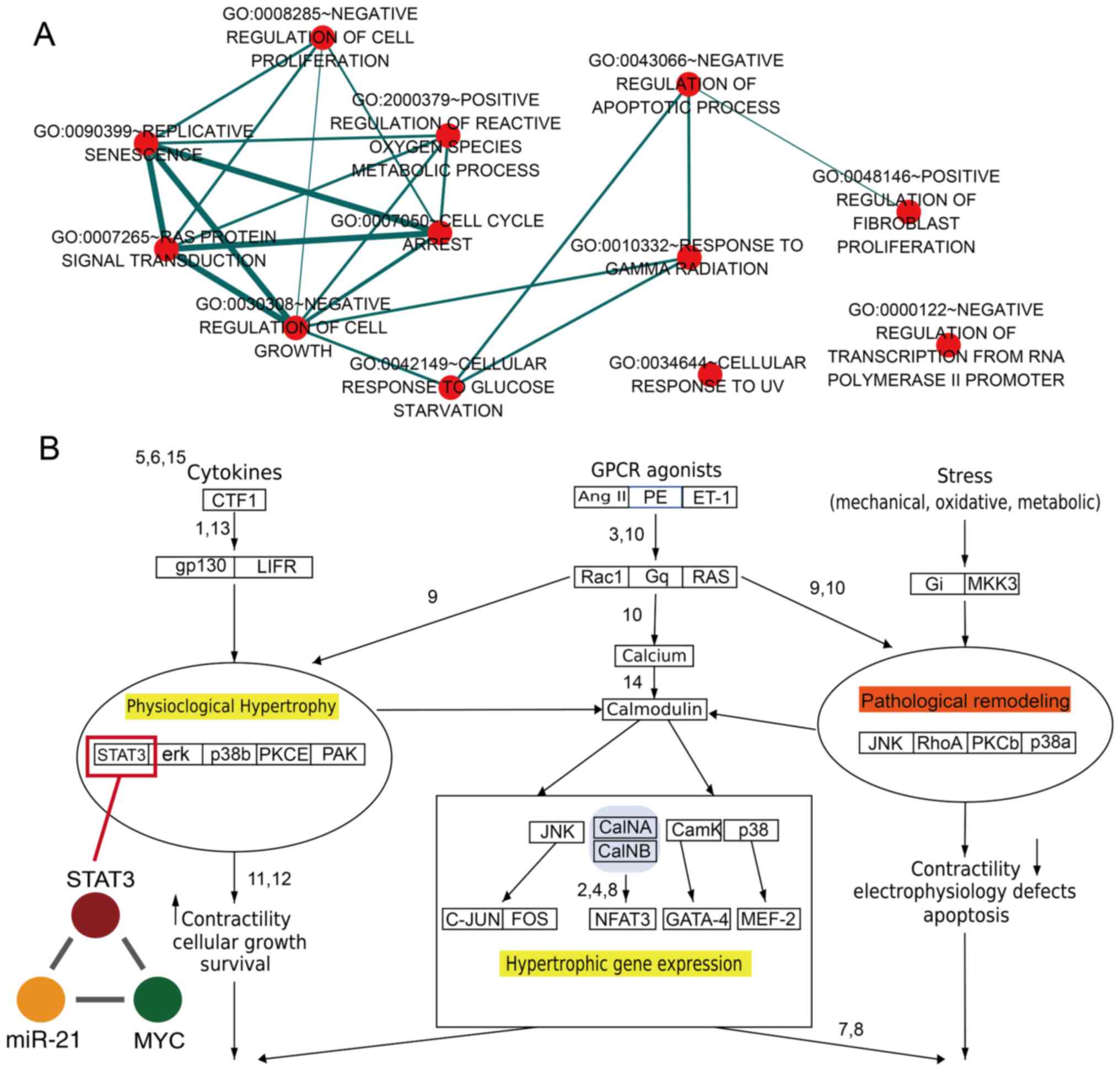
Functional analysis revealing the roles
of miR-FFL motifs in cardiac disease
GO analysis was performed based on all the TFs and
genes in dysregulated miR-FFLs for cardiac hypertrophy and AMI. It
was observed that these dysregulated genes and TFs were enriched in
specific GO terms associated with cell proliferation, fibroblast
proliferation and reactive oxygen species metabolism (Fig. 4A). A previous study reported that
basic fibroblast growth factor promotes human cardiosphere-derived
cell engraftment in order to enhance cardiac repair, thus
representing a potential treatment target for myocardial infarction
(39). In addition, the result of
the present study revealed that numerous GO terms shared common
genes (Fig. 4A). For example, the
'replicative senescence' and 'negative regulation of cell growth'
GO terms shared numerous genes. The results demonstrated that a
single gene may participate in diverse functions via formation of
diverse miR-FFLs involving different miRNAs and TFs. The current
study results also revealed that STAT3 was a key gene for the
pathological and physiological processes within the heart (Fig. 4B). Previously, it has been
observed that STAT3 may be a novel therapeutic biomarker for
cardiac hypertrophy treatment, and downregulation of STAT3
expression resulted in increased collagen synthesis and the
limitation of hypertrophy (40).
In addition, ANG II type 1 receptor activation mediated STAT3 gene
regulation, resulting in the nuclear accumulation of U-STAT3, and
this process was notably associated with the development of cardiac
hypertrophy (41). In present
study, the results revealed that STAT3, miR-21 and MYC formed a
miR-FFL. It has also been reported that interferon (IFN) is able to
greatly induce miR-21 expression based on the STAT3-associated
signaling pathway, and miR-21 was characterized as a new target
that exerts negative feedback on the IFN-induced apoptosis effect
(42). Therefore, the results of
the present study suggest the presence of an association among
STAT3, miR-21 and heart disease.
miR-FFLs contain the drug target miRNAs
and genes
Subsequently, the association of the TFs, genes and
miRNAs in the dysregulated miR-FFLs with a potential drug target
was further explored. Initially, the study searched for miR-FFLs
that were common between cardiac hypertrophy and AMI, including
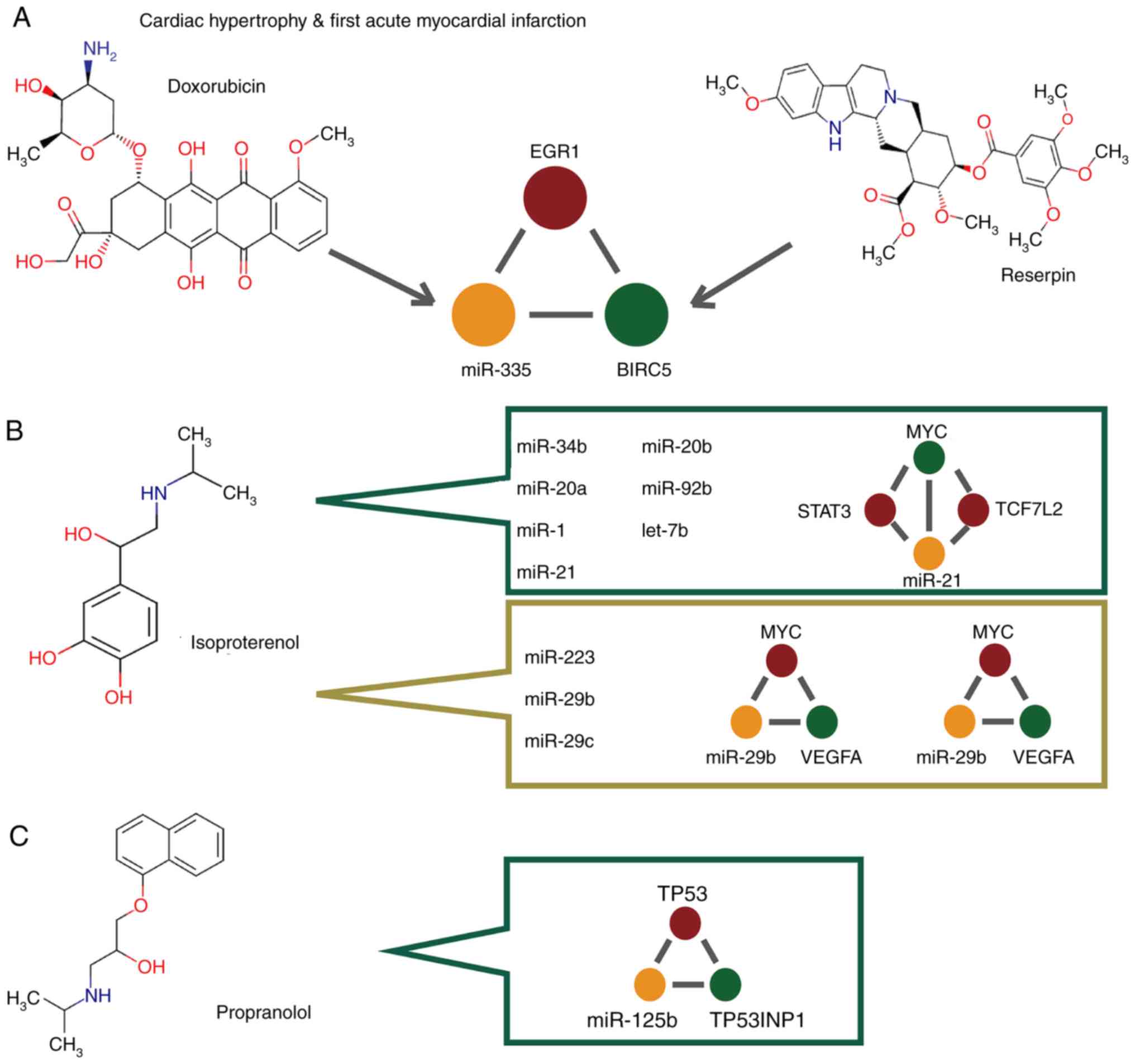
EGR1, miR-335 and BIRC5, using the DrugBank and SM2miR databases
(Fig. 5A). SM2miR database is an
integrated database of the experimentally validated effects of
small molecules on miRNA expression (29).
MiR-335 was found to be associated with doxorubicin,
a chemotherapy drug used for the treatment of a number of cancer
types (Fig. 5A). Furthermore,
BIRC5 was observed to be a target of the drug reserpine (Fig. 5A), which is a type of
antiadrenergic agent occasionally used to control high blood
pressure and treat schizophrenia. However, this drug is rarely used
in clinical practice due to its side effects.
All the miRNAs investigated in the current study
were found to be associated with isoproterenol or propranolol
(Fig. 5B and C). Isoproterenol is
generally used to treat heart diseases, such as abnormal
heartbeats, heart block and heart failure. It was observed that 7
miRNAs were associated with isoproterenol in cardiac hypertrophy,
while 3 miRNAs in AMI were associated with isoproterenol. The
miR-FFL that included MYC, STAT3 and miR-21, as well as the miR-FFL
network that included MYC, TCF7L2 and miR-21, were observed to form
a more complex and larger motif by sharing miR-21 and MYC (Fig. 5B). Furthermore, the miR-FFL
network involving MYC, miR-29b and VEGFA, along with the miR-FFL
that consisted of MYC, miR-29c and VEGFA, also formed a more
complex motif by sharing VEGFA and MYC. Therefore, it may be
suggested that miR-FFL motifs dysregulate significant biological
pathways and networks, and trigger important biological responses,
thereby severely affecting disease mechanisms and influencing the
effect of drugs. Additionally, propranolol is a β-blocker that has
an effect on human heart function and is widely used to treat high
blood pressure and abnormal heart conditions. In the current
analysis, the miR-FFL network consisting of TP53, miR-125b and
TP53INP1 was found to be associated with propranolol in cardiac
hypertrophy. Taken together, the aforementioned findings indicate
that dysregulated miR-FFLs may be a novel target for drug treatment
in future studies investigating hypertrophy and AMI.
Discussion
Various transcripts, including TFs, miRNAs and
genes, can form complex regulatory associations, such as FFLs,
which may influence pathogenic mechanisms in certain heart
diseases. Therefore, the present study investigated TF-miRNA-gene
FFLs motifs and used a comprehensive method to detect their
features by integrating multiple regulatory associations and
expression profiles from large repositories. Dysregulated miR-FFL
motifs were found to be present in cardiac hypertrophy and AMI,
which are two diseases with a high prevalence worldwide. In
addition, certain dysregulated miR-FFLs that were shared or
specific to the two diseases were identified, focusing on
tissue-specific analyses conducted in previous studies (43). Disease-specific miR-FFL motifs may
be beneficial for drug discovery, and may increase the therapeutic
effects of drugs. As in other similar studies, the disease and drug
target-associated research conducted in the present study revealed
the potential functionality of certain miR-FFL motifs, which may
serve as novel biomarkers in the treatment of heart diseases
(27). The current study also
revealed that miR-FFLs may provide another method for studying
heart disease.
In addition, it was observed that certain miR-FFLs
share common miRNAs, genes or TFs, forming larger complexes that
regulate the biological network. These results indicated that,
although miR-FFLs may serve as unitary motifs and participate in
disease progression, complex associations within the disease
network may also have a specific function. The present study also
demonstrated that cardiac hypertrophy and AMI are two complex
diseases with numerous miRNAs, genes, TFs and other factors that
participate in the process of disease development. These factors
may form various different motifs to serve their specific roles.
There are also other types of miRNA-mediated FFLs, which differ
from the ones focused on in the present study, such as those
involving miRNA-regulated TFs (44). These alternative miR-FFLs have
also been reported to participate in the development of several
types of cancer (8). Therefore,
the authors recognize that the effects of certain miR-FFLs may
strongly depend on the cell type and context. Future studies should
investigate an increased number of cell types in a variety of
contexts to validate the accuracy and stability of the method used
in the present study. Finally, our method identified novel
candidates associated with disease development, which require
further investigation and experimental validation.
In conclusion, the present study examined and
highlighted the potential functional mechanism of miRNAs, genes and
TFs within cardiac hypertrophy and AMI. Dysregulated miR-FFLs were
identified, and miR-FFLs common to the two diseases were further
investigated. The functional and drug analyses revealed the
essential role of miR-FFLs in heart diseases. The present study
also provided a systematic and novel approach that revealed the
dysregulated cross-talk in cardiac hypertrophy and AMI by
identifying specific functional motifs.
Acknowledgments
Not applicable.
Funding
This work was supported by a grant from the National
Natural Science Foundation of China Project (grant no. 81670381
awarded to Bingchen Liu).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XC, BL and WQ conceived and designed the present
study. FZ, SM and SZ performed the experiments. SS, YZ and LS
analyzed the data. WQ, BL and XC wrote and revised the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Matsuoka R: Mutations of transcription
factors in human with heart disease for understanding the
development and mechanisms of congenital cardiovascular heart
disease. Adv Exp Med Biol. 565:349–415. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu H: Forkhead box transcription factors
in embryonic heart development and congenital heart disease. Life
Sci. 144:194–201. 2016. View Article : Google Scholar
|
|
3
|
Latchman DS: Transcription factors: An
overview. Int J Biochem Cell Biol. 29:1305–1312. 1997. View Article : Google Scholar
|
|
4
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shalgi R, Lieber D, Oren M and Pilpel Y:
Global and local architecture of the mammalian
microRNA-transcription factor regulatory network. PLoS Comput Biol.
3:e1312007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsang J, Zhu J and van Oudenaarden A:
MicroRNA-mediated feedback and feedforward loops are recurrent
network motifs in mammals. Mol Cell. 26:753–767. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Cassidy JJ, Reinke CA, Fischboeck S
and Carthew RW: A microRNA imparts robustness against environmental
fluctuation during development. Cell. 137:273–282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan Z, Shah PK, Amin SB, Samur MK, Huang
N, Wang X, Misra V, Ji H, Gabuzda D and Li C: Integrative analysis
of gene and miRNA expression profiles with transcription
factor-miRNA feed-forward loops identifies regulators in human
cancers. Nucleic Acids Res. 40:e1352012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang HM, Kuang S, Xiong X, Gao T, Liu C
and Guo AY: Transcription factor and microRNA co-regulatory loops:
Important regulatory motifs in biological processes and diseases.
Brief Bioinform. 16:45–58. 2015. View Article : Google Scholar
|
|
10
|
Mendis S, Davis S and Norrving B:
Organizational update: The world health organization global status
report on noncommunicable diseases 2014 one more landmark step in
the combat against stroke and vascular disease. Stroke.
46:e121–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maron BJ and Maron MS: Hypertrophic
cardiomyopathy. Lancet. 381:242–255. 2013. View Article : Google Scholar
|
|
12
|
Sala V, Gallo S, Leo C, Gatti S, Gelb BD
and Crepaldi T: Signaling to cardiac hypertrophy: Insights from
human and mouse RASopathies. Mol Med. 18:938–947. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Diniz GP, Huang ZP, Liu J, Chen J, Ding J,
Fonseca RI, Barreto-Chaves ML, Donato J Jr, Hu X and Wang DZ: Loss
of microRNA-22 prevents high-fat diet induced dyslipidemia and
increases energy expenditure without affecting cardiac hypertrophy.
Clin Sci. 131:2885–2900. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heggermont WA, Papageorgiou AP, Quaegebeur
A, Deckx S, Carai P, Verhesen W, Eelen G, Schoors S, van Leeuwen R,
Alekseev S, et al: Inhibition of MicroRNA-146a and overexpression
of its target dihydrolipoyl succinyltransferase protect against
pressure overload-induced cardiac hypertrophy and dysfunction.
Circulation. 136:747–761. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagalingam RS, Sundaresan NR, Gupta MP,
Geenen DL, Solaro RJ and Gupta M: A cardiac-enriched microRNA,
miR-378, blocks cardiac hypertrophy by targeting Ras signaling. J
Biol Chem. 292:51232017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shafei AE, Ali MA, Ghanem HG, Shehata AI,
Abdelgawad AA, Handal HR, Talaat KA, Ashaal AE and El-Shal AS:
Mesenchymal stem cells therapy: A promising cell based therapy for
treatment of myocardial infraction. J Gene Med. Dec;2017.Epub ahead
of print. View
Article : Google Scholar
|
|
17
|
Liang H, Qiu H and Tian L: Short-term
effects of fine particulate matter on acute myocardial infraction
mortality and years of life lost: A time series study in Hong Kong.
Sci Total Environ. 615:558–563. 2018. View Article : Google Scholar
|
|
18
|
Chen Y, Zhao Y, Chen W, Xie L, Zhao ZA,
Yang J, Chen Y, Lei W and Shen Z: MicroRNA-133 overexpression
promotes the therapeutic efficacy of mesenchymal stem cells on
acute myocardial infarction. Stem Cell Res Ther. 8:2682017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bayoumi AS, Park KM, Wang Y, Teoh JP,
Aonuma T, Tang Y, Su H, Weintraub NL and Kim IM: A
carvedilol-responsive microRNA, miR-125b-5p protects the heart from
acute myocardial infarction by repressing pro-apoptotic bak1 and
klf13 in cardiomyocytes. J Mol Cell Cardiol. 114:72–82. 2018.
View Article : Google Scholar
|
|
20
|
Liu X, Zhang Y, Du W, Liang H, He H, Zhang
L, Pan Z, Li X, Xu C, Zhou Y, et al: MiR-223-3p as a novel MicroRNA
regulator of expression of voltage-gated K+ channel Kv4.2 in acute
myocardial infarction. Cell Physiol Biochem. 39:102–114. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takeuchi T, Ishii Y, Kikuchi K and Hasebe
N: Ischemic preconditioning effect of prodromal angina is
attenuated in acute myocardial infarction patients with
hypertensive left ventricular hypertrophy. Circ J. 75:1192–1199.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Esen O, Agus HZ, Guler GB, Açar G, Avci A,
Güler E, Karaca O, Geçmen C, Bulut M, Emiroğlu Y, et al:
Relationship between circulating soluble Fas ligand and preexisting
left ventricular hypertrophy in the setting of left ventricular
remodeling after acute myocardial infarction. Coron Artery Dis.
22:294–298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aggarwal P, Turner A, Matter A, Kattman
SJ, Stoddard A, Lorier R, Swanson BJ, Arnett DK and Broeckel U: RNA
expression profiling of human iPSC-derived cardiomyocytes in a
cardiac hypertrophy model. PLoS One. 9:e1080512014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Lu M, Qiu C and Cui Q: TransmiR: A
transcription factor-microRNA regulation database. Nucleic Acids
Res. 38:D119–D122. 2010. View Article : Google Scholar
|
|
25
|
Vergoulis T, Vlachos IS, Alexiou P,
Georgakilas G, Maragkakis M, Reczko M, Gerangelos S, Koziris N,
Dalamagas T and Hatzigeorgiou AG: TarBase 6.0: Capturing the
exponential growth of miRNA targets with experimental support.
Nucleic Acids Res. 40:D222–D229. 2012. View Article : Google Scholar :
|
|
26
|
Aerts S, Lambrechts D, Maity S, Van Loo P,
Coessens B, De Smet F, Tranchevent LC, De Moor B, Marynen P, Hassan
B, et al: Gene prioritization through genomic data fusion. Nat
Biotechnol. 24:537–544. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang W, Mitra R, Lin CC, Wang Q, Cheng F
and Zhao Z: Systematic dissection of dysregulated transcription
factor-miRNA feed-forward loops across tumor types. Brief
Bioinform. 17:996–1008. 2016. View Article : Google Scholar
|
|
28
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu X, Wang S, Meng F, Wang J, Zhang Y,
Dai E, Yu X, Li X and Jiang W: SM2miR: A database of the
experimentally validated small molecules' effects on microRNA
expression. Bioinformatics. 29:409–411. 2013. View Article : Google Scholar
|
|
30
|
Wishart DS, Feunang YD, Guo AC, Lo EJ,
Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, et al:
DrugBank 5.0: A major update to the DrugBank database for 2018.
Nucleic Acids Res. 46:D1074–D1082. 2018. View Article : Google Scholar :
|
|
31
|
Amaral LA, Scala A, Barthelemy M and
Stanley HE: Classes of small-world networks. Proc Natl Acad Sci
USA. 97:11149–11152. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simos T, Georgopoulou U, Thyphronitis G,
Koskinas J and Papaloukas C: Analysis of protein interaction
networks for the detection of candidate hepatitis B and C
biomarkers. IEEE J Biomed Health Inform. 19:181–189. 2015.
View Article : Google Scholar
|
|
33
|
Xu J, Li Y, Lu J, Pan T, Ding N, Wang Z,
Shao T, Zhang J, Wang L and Li X: The mRNA related ceRNA-ceRNA
landscape and significance across 20 major cancer types. Nucleic
Acids Res. 43:8169–8182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Indolfi C and Curcio A: Stargazing
microRNA maps a new miR-21 star for cardiac hypertrophy. J Clin
Invest. 124:1896–1898. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang Y, Cheng HW, Qiu Y, Dupee D, Noonan
M, Lin YD, Fisch S, Unno K, Sereti KI and Liao R: MicroRNA-34a
plays a key role in cardiac repair and regeneration following
myocardial infarction. Circ Res. 117:450–459. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He W, Huang H, Xie Q, Wang Z, Fan Y, Kong
B, Huang D and Xiao Y: MiR-155 knockout in fibroblasts improves
cardiac remodeling by targeting tumor protein p53-inducible nuclear
protein 1. J Cardiovasc Pharmacol Ther. 21:423–435. 2016.
View Article : Google Scholar
|
|
37
|
Liang J, Bai S, Su L, Li C, Wu J, Xia Z
and Xu D: A subset of circulating microRNAs is expressed
differently in patients with myocardial infarction. Mol Med Rep.
12:243–247. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Y, Li Z, Liu T, Xue X, Jiang H, Huang
J and Wang H: Impaired cardioprotective function of transplantation
of mesenchymal stem cells from patients with diabetes mellitus to
rats with experimentally induced myocardial infarction. Cardiovasc
Diabetol. 12:402013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takehara N, Tsutsumi Y, Tateishi K, Ogata
T, Tanaka H, Ueyama T, Takahashi T, Takamatsu T, Fukushima M,
Komeda M, et al: Controlled delivery of basic fibroblast growth
factor promotes human cardiosphere-derived cell engraftment to
enhance cardiac repair for chronic myocardial infarction. J Am Coll
Cardiol. 52:1858–1865. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mir SA, Chatterjee A, Mitra A, Pathak K,
Mahata SK and Sarkar S: Inhibition of signal transducer and
activator of transcription 3 (STAT3) attenuates interleukin-6
(IL-6)-induced collagen synthesis and resultant hypertrophy in rat
heart. J Biol Chem. 287:2666–2677. 2012. View Article : Google Scholar :
|
|
41
|
Yue H, Li W, Desnoyer R and Karnik SS:
Role of nuclear unphosphorylated STAT3 in angiotensin II type 1
receptor-induced cardiac hypertrophy. Cardiovasc Res. 85:90–99.
2010. View Article : Google Scholar
|
|
42
|
Yang CH, Yue J, Fan M and Pfeffer LM: IFN
induces miR-21 through a signal transducer and activator of
transcription 3-dependent pathway as a suppressive negative
feedback on IFN-induced apoptosis. Cancer Res. 70:8108–8116. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ivey KN and Srivastava D: MicroRNAs as
regulators of differentiation and cell fate decisions. Cell Stem
Cell. 7:36–41. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fang Z, Xu C, Li Y, Cai X, Ren S, Liu H,
Wang Y, Wang F, Chen R, Qu M, et al: A feed-forward regulatory loop
between androgen receptor and PlncRNA-1 promotes prostate cancer
progression. Cancer Lett. 374:62–74. 2016. View Article : Google Scholar : PubMed/NCBI
|