Introduction
Inflammatory bowel disease (IBD) is multifactorial
inflammatory disease, the most common forms of which include
Crohn's disease and ulcerative colitis. IBD is a complex autoimmune
disease, which is associated with genetic predisposition,
intestinal epithelial barrier injury, intestinal flora and adaptive
immunity, however, the pathogenic mechanisms involved in the
development and progression of IBD remain to be fully elucidated
(1). Increasing evidence has
demonstrated that IBD is an intestinal barrier disease and that
injury of the intestinal barrier usually results in disease
occurrence (2,3). Although advances in therapeutic
strategies have been achieved using glucocorticoids, due to
differences between individuals, diverse levels of effectiveness
and the side effects of treatment confound IBD therapy. Therefore,
it is necessary to develop novel strategies for more effective
diagnosis and treatment of IBD.
Long non-coding RNAs (lncRNAs) are a class of
transcripts >200 nucleotides in length with limited
protein-coding capability. The human genome encodes ~10,000 lncRNA
genes (4); lncRNAs have
significant roles in differentiation, proliferation, apoptosis and
various biological processes by serving as regulatory factors for
gene expression. lncRNAs have been found to be tightly linked to a
diverse range of human diseases, including IBD (5). For example, the overexpression of
lncRNA H19 was found to increase Caco-2 monolayer permeability and
decrease the expression of tight junction proteins by increasing
the expression of microRNA (miR)-675, ultimately causing the
destruction of intestinal epithelial barrier function (6). Nuclear paraspeckle assembly
transcript 1 (NEAT1) is a recently identified nuclear-restricted
lncRNA, which localizes exclusively in subnuclear structures,
termed paraspeckles, and serves as an essential architectural
component (7,8). Accumulating evidence has suggested
that NEAT1 may be crucial in regulating gene expression and
consequently influence physiological and pathophysiological
processes (9). Previous studies
have reported that NEAT1 is associated with several types of
cancer, including breast cancer (7), prostate cancer (10,11), acute promyelocytic leukemia
(12) and colorectal cancer
(13,14), by promoting tumor growth through
genetic or epigenetic mechanisms. In addition, NEAT1 is a key
component for establishing the ribo-nucleoprotein complex to
regulate of DNA-mediated activation of the innate immune response
(15). NEAT1 was also revealed to
be associated with the innate immune response, and it may function
as a regulatory factor of inflammatory cytokines (16). However, to date, there have been
few reports on the role of NEAT1 in IBD, and investigations of the
expression pattern and clinical significance of NEAT1 in IBD are
warranted.
Previous investigations (17–21) showed that the knockdown of NEAT1
impaired the integrity and increased the permeability of the
blood-tumor barrier, accompanied by the downregulation of the
expression of the tight junction proteins Zonula occludens-1
(ZO-1), Occludin and Claudin-5 in glioma endothelial cells. In
addition, these results suggested that NEAT1 is important in
regulating the permeability of the blood-tumor barrier. An
ineffective intestinal epithelial barrier is usually regarded as a
significant cause of IBD. Therefore, it was hypothesized that NEAT1
may regulate the permeability of epithelial cells and that an
imbalance in the expression of NEAT1 may be present in IBD.
Exosomes are 40–150 nm microvesicles secreted by
various cell types via the endosomal compartment in multivesicular
bodies and thus express endosomal markers, including CD9, CD61 and
Heat-shock protein (Hsp)90/β (22). Exosomes belong to a group of
naturally secreted extracellular vesicles, which mediate short- and
long-distance intercellular communication and deliver different
types of biologically active cargo to recipient cells (23,24). In addition, exosomes are involved
in other biological processes, including material transportation,
cell division and apoptosis, and circulating exosomes have been
demonstrated to be involved in regulation of the immune response
(25). Exosomes have also been
confirmed to be involved in the pathogenesis of IBD through the
modulation of p38 and extracellular signal-regulated kinase (ERK)
phosphorylation and the production of tumor necrosis factor-α to
regulate macrophage activity (26).
As several studies have reported that NEAT1 is
tightly connected to the inflammatory response, in the present
study, it was hypothesized that the expression of NEAT1 in IBD is
imbalanced, and that this imbalance may ultimately induce
intestinal epithelial barrier injury. In addition, it was
hypothesized that the imbalance in the expression of NEAT1 may
occur through exosome delivery, which is an important intermediary
in the crosstalk between epithelial cells and macrophages. In the
present study, the expression levels of NEAT1 were detected in IBD
cells and animal models, and the change in intestinal epithelial
cell permeability following the inhibition of lncRNA NEAT1
expression was examined. The molecular mechanisms by which NEAT1
mediates the inflammatory response were also investigated. The
results of the present study may reveal a potential strategy of
targeting NEAT1 for IBD therapy.
Materials and methods
Cell culture
RAW264.7 cell lines were purchased from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China) and cultured in 1640 culture medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) plus 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.) at 37°C. The HT-29
colon cancer cell line and the NCM460 normal immortalized colon
epithelial cell line, which represents a reliable model of the
human intestine, were obtained from the Cell Center of Xiangya
School of Medicine, Central South University (Hunan, China). The
cells were routinely cultured in high glucose DMEM (Gibco; Thermo
Fisher Scientific, Inc.) with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in an incubator with a humidified
atmosphere containing 5% CO2.
Animal experiments
All animal experiments were performed in accordance
with the guidelines of the Animal Experimentation Ethics Committee
of Central South University. Male C57BL/6 mice (n=15, 6-week-old,
25–30 g and 5 mice/group) purchased from the Laboratory Animal
Center of Xiangya Hospital of Central South University and housed
at 25°C with a 12/12 h light/dark cycle, 40–70% humidity and ad
libitum access to food and water. The animals were treated with
5% dextran sulfate sodium (DSS) in their drinking water for 2
weeks, following which intestinal mucosa and serum were harvested
for further analysis. In addition, 6-week-old Sprague-Dawley rats
(n=25, 12 males and 13 females; Central South University) weighing
~180±10 g (5 mice/group) were treated with 5% DSS for 2 weeks, and
10 rats were transfected with 100 µl of 293 T cells (titer of 1,000
ng/p24) carrying a lentiviral vector encoding shRNA or shRNA-NEAT1
via rectal perfusion. The weight, diet, feces, and hair status of
all animals were recorded. At the end of the experiments, the rats
were anaesthetized and sacrificed, and whole colon tissues were
harvested for further analysis.
Western blot assay
Total proteins were harvested from exosomes, HT-29,
RAW264.7 and NCM460 cells or mouse colon tissues. Samples were
lysed in radioimmunoprecipitation assay buffer (cat. no. P002A;
Auragene, Changsha, China) containing proteinase inhibitors.
Following a 20 min incubation on ice, total protein was extracted
via centrifugation at 1,000 x g for 20 min at 4°C. A bicinchoninic
acid assay was used to determine protein concentration prior to the
sample being diluted into 2 mg/ml with sodium dodecyl
sulfate-loading buffer (cat. no. P003B; Auragene). Protein samples
(20 µg) were subsequently separated by 12% SDS-PAGE and transferred
onto polyvinylidene fluoride membranes by electroblotting. The
membranes were blocked with 5% non-fat milk and then probed with
primary antibodies against the following proteins at 4°C overnight:
CD9 (cat. no. ab92726; 1:2,000; Abcam, Cambridge, UK), Hsp90α/β
(cat. no. ab203126; 1:10,000; Abcam), ZO-1 (cat. no. ab96587;
1:2,000; Abcam), Claudin-5 (cat. no. ab131259; 1:5,000; Abcam),
Occludin (cat. no. ab222691; 1:500; Abcam), Interleukin (IL)23
(cat. no. ab190356; 1:1,000; Abcam), Inducible nitric oxide
synthase (iNOS; cat. no. ab204017; 1:1,000; Abcam), IL10 (cat. no.
ab34843; 1:500; Abcam), Arginase-1 (cat. no. ab124917; 1:5,000;
Arg-1; Abcam), CD206 (cat. no. ab64693; 1:4,000; Abcam) and β-actin
(cat. no. ab8226; 1:15,000; Abcam). This was followed by incubation
with HRP-conjugated anti-mouse IgG antibodies (cat. no. a5278;
1:15,000; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at room
temperature for 40 min. Finally, the immunosignals were visualized
using chemiluminescence reagents following exposure to X-ray film,
and the expression levels of target proteins were analyzed using
Image J software (version 1.80; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted using TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.) and then analyzed via RT-qPCR
analysis using the Real-Time Quantitative PCR SYBR-Green detection
reagent (Takara Bio, Inc., Tokyo, Japan) according to the
manufacturer's protocol. RT was performed using cDNA (1.0 µl),
ddH2O (7.0 µl), forward primers (10 µmol/l) and reverse
primers (10 µmol/l). The temperature protocol used to perform RT
was 42°C for 1 h followed by 70°C for 10 min. The fluorophore used
for qPCR was 2X SYBR-Green PCR Mix (Bio-Rad Laboratories, Inc.) and
the thermocycling conditions used were as follows: Initial
denaturation at 95°C for 3 min; followed by 40 cycles denaturation
at 95°C for 10 sec; followed by annealing/elongation at 60°C for 30
sec. The primer sequences were as follows: lncRNA NEAT1, forward
5′-CCAGGGTGGTGGCAGTGC-3′ and reverse 5′-CCCAGCCTCAGCGGGAAG-3′;
Monocyte chemoattractant protein 1 (MCP-1), forward
5′-ACTTCACCAATAGGAAGATCTCAG T-3′ and reverse
5′-TGAAGATCACAGCTTCTTTGG-3′; IL-23, forward
5′-GGGACACATGGATCTAAGAG-3′ and reverse 5′-CGATCCTAGCAGCTTCTCAT-3′;
and β-actin, forward 5′-AGGGGCCGGACTCGTCATACT-3′ and reverse
5′-GGCGGCACCACCATGTACCCT-3′. The relative expression of each target
gene was calculated using the 2−∆∆Cq formula (27) relative to the expression level of
β-actin.
Exosome isolation
Prior to purification of the exosomes, the blood
serum supernatant was isolated from 1–1.5 ml of mouse blood, which
was maintained in a low-temperature environment for 1 h and then
centrifuged at 1,000 × g for 10 min at 4°C. The exosomes were
isolated using the exosome isolation reagent (Guangzhou RiboBio
Co., Ltd., Guangzhou, China) according to the manufacturer's
protocol with modification. Briefly, the blood serum was
transferred to the tubes, and centrifuged at 3,000 x g for 30 min
at 4°C. The supernatant was then collected and filtered using a
0.22-µm filtrator, and was then transferred into an ultrafiltration
tube (30-kDa), and centrifuged at 6,000 x g for 10 min at 4°C. The
mixture in the upper chamber was resuspended with PBS and an
appropriate volume of 8% PEG6000 solution was added, followed by
centrifugation at 10,000 x g for 60 min at 4°C. Finally, the
precipitate was resuspended with PBS. The expression of Hsp90α/β
and CD9 (27) in the concentrated
exosome fractions was analyzed by western blot analysis, and
positive expression for Hsp90α/β and CD9 was recognized when the
exosomes had been successfully obtained.
Lentivirus production and
transduction
The sequence of lentiviral short hairpin (sh)RNA
targeting NEAT1 was designed though Block-iTä RNAi Designer online
software (http://rnaidesigner.thermofisher.com/rnaiexpress).
The shRNA-NEAT1 was cloned into the apLV-H1TetO-GFP-Puro vector
(Auragene) according to the manufacturer's protocol, followed by
the transduction of 293 T cells (Auragene) with the recombinant
vector. The viruses were packaged in 293 T cells and harvested 72 h
later. The lentiviruses carrying shRNA-NEAT1 were termed
LV-shNEAT1, and the empty lentiviral vector, termed LV-shRNA, was
used as a control. The HT-29 and NCM460 cells were infected with
virus particles using 6 µg/ml of polybrene, and the medium was
replaced following 24 h of incubation. After ~2 days, the target
cells were harvested for further analysis.
Trans-epithelial electrical resistance
(TEER) measurement
The barrier properties of the HT-29 and NCM460 cells
were assessed by measuring the TEER using a Millicell®
ERS Multimeter (EMD Millipore) as previously described (28). The cells were seeded on Millicell
hanging cell culture inserts with a 0.4-µm pore size polyethylene
terephthalate membrane at a density of 12,0000 cells/ml. Following
~21 days of culture in the inserts, the HT-29 and NCM460 cell
monolayer states were obtained. TEER was measured 2 h following
replacement of the culture medium. The resistance of the cell
monolayer samples measured in the assays, minus the resistance of
the blank membrane insert, indicated the resistance of the
intestinal cell monolayer.
Fluorescein isothiocyanate (FITC)-conjugated dextran
(cat. no. 53379, FITC-dextran; Sigma; EMD Millipore) was used to
detected the cell permeability. The cells were cultivated on a
Transwell (0.33 cm2) to cell monolayers. Sterile
bicarbonate-buffered Ringer's solution was used to wash the cells,
following which FITC-Dextran-FITC (100 µM) was added to the upper
chambers for 2 h. The basolateral chambers were collected in 96
plates, and detected using a fluorescent plate reader (cat. no.
3916, Costar; Corning Incorporated, Corning, NY, USA) with 480 nm
excitation and 520 emission filters (29). The paracellular flux was
determined from a standard curve and normalized to the negative
control group.
Immunofluorescence
The cells were fixed with 4% paraformaldehyde and
permeabilized using 0.2% Triton X-100 for 10 min following being
grown on coated coverslips. The cells were washed three times with
PBS and then blocked with 3% bovine serum albumin (Gibco; Thermo
Fisher Scientific, Inc.) for 1 h at room temperature, followed by
incubation with monoclonal anti-occludin antibody (cat. no.
ab222691; 1:500; Abcam) at 4°C overnight. Subsequently, the cells
were washed and then incubated with Alexa-conjugated secondary
antibody (PV-8000; One-step universal method kit; OriGene
Technologies, Inc., Rockville, MD, USA) for 1 h at room
temperature. The cells were then counterstained with
4′,6-diamidino-2-phenylindole (Invitrogen; Thermo Fisher
Scientific, Inc.) at room temperature for 15 min, and finally, the
coverslips were treated with ProLong Gold antifade reagent (Thermo
Fisher Scientific, Inc.), and fluorescence signals were visualized
using a Leica TCS SP5 confocal microscope.
Flow cytometry
The HT-29 and NCM460 cells (5×105
cells/ml) transfected with LV-shNEAT1 were harvested by
trypsinization at 37°C following treatment with TNF-α for 48 h at
37°C. The cells were then centrifuged at 600 x g for 5 min at 37°C,
washed twice using PBS and incubated with monoclonal anti-CD206
(cat. no. 12-2069-42; 1:200; Thermo Fisher Scientific, Inc.)
antibody for 60 min. The cells were washed again and incubated with
FITC-conjugated secondary antibody (cat. no. 400109; 1:500;
BioLegend, Inc., San Diego, CA, USA) in the dark for 60 min at room
temperature. Finally, the cell population was washed and
resuspended using PBS, following which the cells were analyzed
using a flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA).
Haemotoxylin and eosin (H&E) staining
assay
Tissues were immediately fixed in 10% formalin
solution for 12 h at room temperature and embedded in paraffin.
Embedded paraffin blocks were subsequently cut into 4 µm thick
sections. Sections were stained with eosin at room temperature for
10 min and then stained with hematoxylin for >15 min at room
temperature according to a previously published protocol (30), and then visualized under a light
microscope (magnifications, ×100 and ×200).
Statistical analysis
Quantitative data are presented as the mean ±
standard deviation, and statistical analysis was performed using
SPSS 20.0 (IBM SPSS, Armonk, NY, USA) and GraphPad Prism 5 software
(GraphPad Software, Inc., La Jolla, CA, USA). Significant
differences between groups were compared using one-way analysis of
variance and Student's t-test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Expression of NEAT1 is high in IBD mice
and inflammatory cell lines
To investigate whether abnormal expression levels of
NEAT1 were present in IBD, 30 acute and chronic colitis male
C57BL/6 mice were induced as a model by feeding 5% DSS. The
expression levels of NEAT1 in the mouse intestinal mucosa and serum
were compared between the control and DSS-induced group using
RT-qPCR analysis. The mRNA expression of NEAT1 was significantly
higher in the colitis mice than in the non-colitis mice in the
intestinal mucosa (Fig. 1A) and
serum (Fig. 1B).
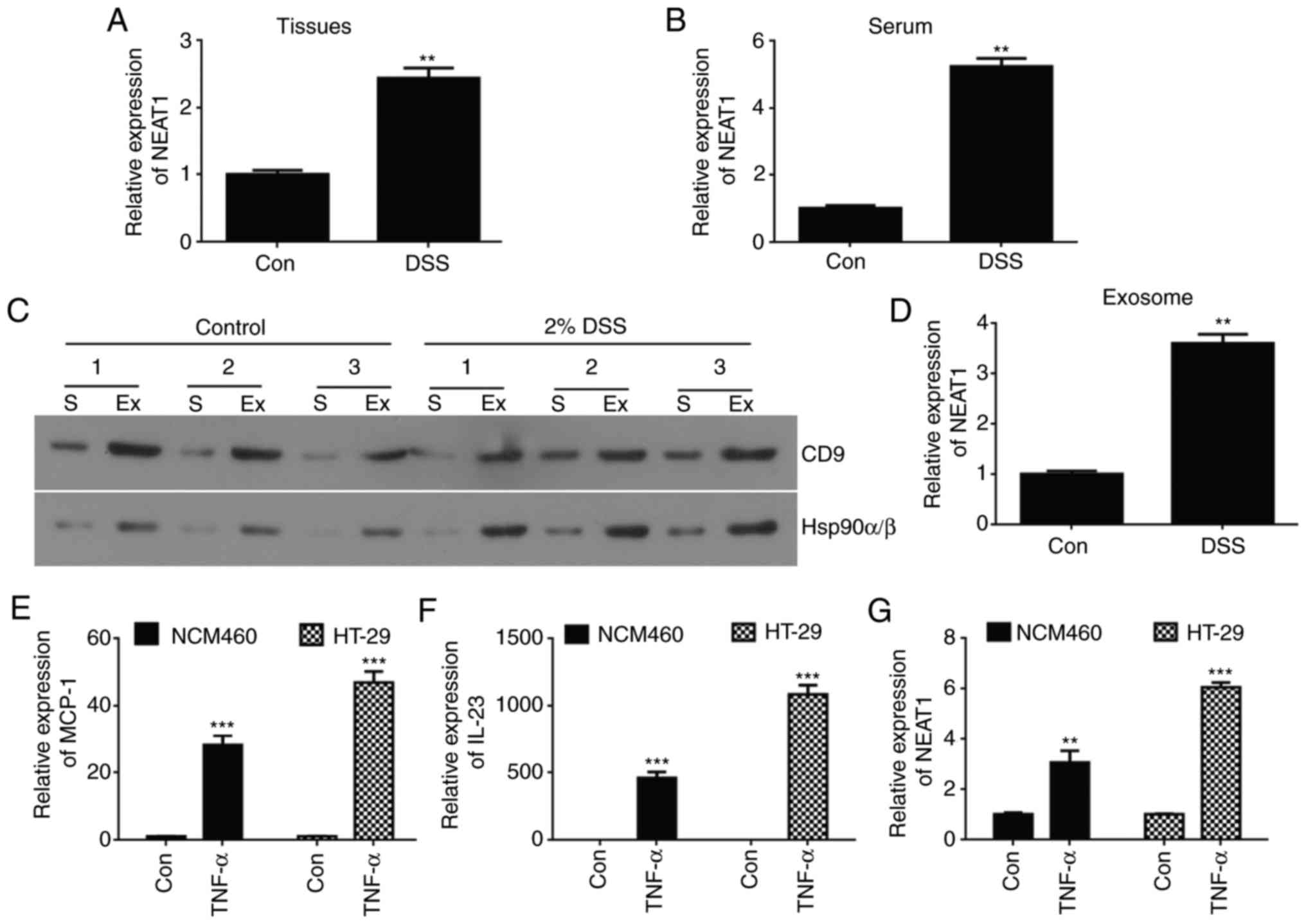 | Figure 1Expression of NEAT1 is high in an
inflammatory bowel disease mouse model and inflammatory cell line
models. Relative expression levels of NEAT1 were compared in (A)
intestinal mucosa tissue and (B) serum from DSS-induced colitis
mice between normal control mice using RT-qPCR analysis. (C)
Western blot analyses of Hsp90α/β and CD9 protein expression in
exosome-rich fractions and serum. (D) Quantification of western
blot results. (E) RT-qPCR analyses of mRNA expression of NEAT1 in
exosomes from DSS-induced colitis mice and normal control mice. The
relative expression of inflammatory factors (E) MCP-1 and (F) IL23
was detected in HT-29 and NCM460 TNF-α-induced inflammatory cell
lines using RT-qPCR analysis. (G) RT-qPCR analyses of the relative
mRNA expression of NEAT1 in TNF-α-induced HT-29 and NCM460
inflammatory cell lines. The data are shown as the mean ± standard
deviation; **P<0.01 and ***P<0.001.
NEAT1, Nuclear paraspeckle assembly transcript 1; Con, control;
DSS, dextran sulfate sodium; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; Ex,
exosome-rich fraction; S, serum; TNF-α, tumor necrosis factor-α;
ZO-1, Zonula occludens-1; MCP-1, Monocyte chemoattractant protein
1; IL-23, Interleukin-23. |
Exosomes are reported to be important in mediating
the immune response though regulation of macrophage activity
(26). In the present study, it
was hypothesized that the expression of NEAT1 may be dysregulated
in exosomes isolated from DSS-induced colitis mouse models.
Exosomes were successfully extracted from DSS-induced mouse serum
as previously described (31)
(Fig. 1C). In addition, the
expression levels of NEAT1 were determined in exosomes from
DSS-induced colitis mouse serum and normal control mice using
RT-qPCR analysis. It was revealed that the expression of NEAT1 was
high in serum exosomes from the DSS-induced colitis mice compared
with those from the control group (Fig. 1D).
To further support the results that the expression
of NEAT1 was high in colitis mouse models, cell experiments were
performed to verify these data in inflammatory cell models. The
HT-29 and NCM460 cells were induced using 20 ng/ml of TNF-α for 24
h to establish the IBD cell models, and the inflammatory cell
models were confirmed via RT-qPCR analysis by detecting the
inflammatory factors MCP-1 and IL23 (32). The analysis suggested that the
relative mRNA expression levels of MCP-1 and IL23 were
significantly higher in the TNF-α-induced HT-29 (P<0.001;
Fig. 1E) and NCM460 (P<0.001;
Fig. 1F) cell lines than in their
control group cells. Similarly, RT-qPCR analyses were used to
examine the relative expression levels of NEAT1 in HT-29 and NCM460
inflammatory cells, and the data showed that the expression of
NEAT1 was high in TNF-α-induced HT-29 (P<0.001) and NCM460
(P<0.01) cell lines compared with that in the control epithelial
cell line (Fig. 1G).
Taken together, the above results showed that the
expression of NEAT1 was high in the DSS-induced colitis mice and in
the TNF-α-induced HT-29 and NCM460 inflammatory cell models, and
was hypothesized that the expression levels of NEAT1 may be
connected to the occurrence of inflammation.
Knockdown of NEAT1 reverses the
TNF-α-induced perme- ability increase in inflammatory HT-29 and
NCM460 cells
To examine the regulatory role of NEAT1 in HT-29 and
NCM460 cell lines, a lentivirus carrying shRNA-NEAT1 was packaged,
and RT-qPCR analysis was used to verify the effectiveness of the
lentiviral vector for NEAT1 interference (Fig. 2A). The TEER value is associated
with the barrier function and permeability of monolayer cells. In
the present study, the TEER value was measured to evaluate the
effects of NEAT1 inhibition in TNF-α-treated NCM460 and HT-29 cells
on monolayer integrity. The TEER value was decreased in the
TNF-α-treated NCM460 and HT-29 cells; however, the TEER value was
increased when the NCM460 and HT-29 cells were treated with TNF-α
and LV-shNEAT1 compared with the cells treated with TNF-α only
(Fig. 2B).
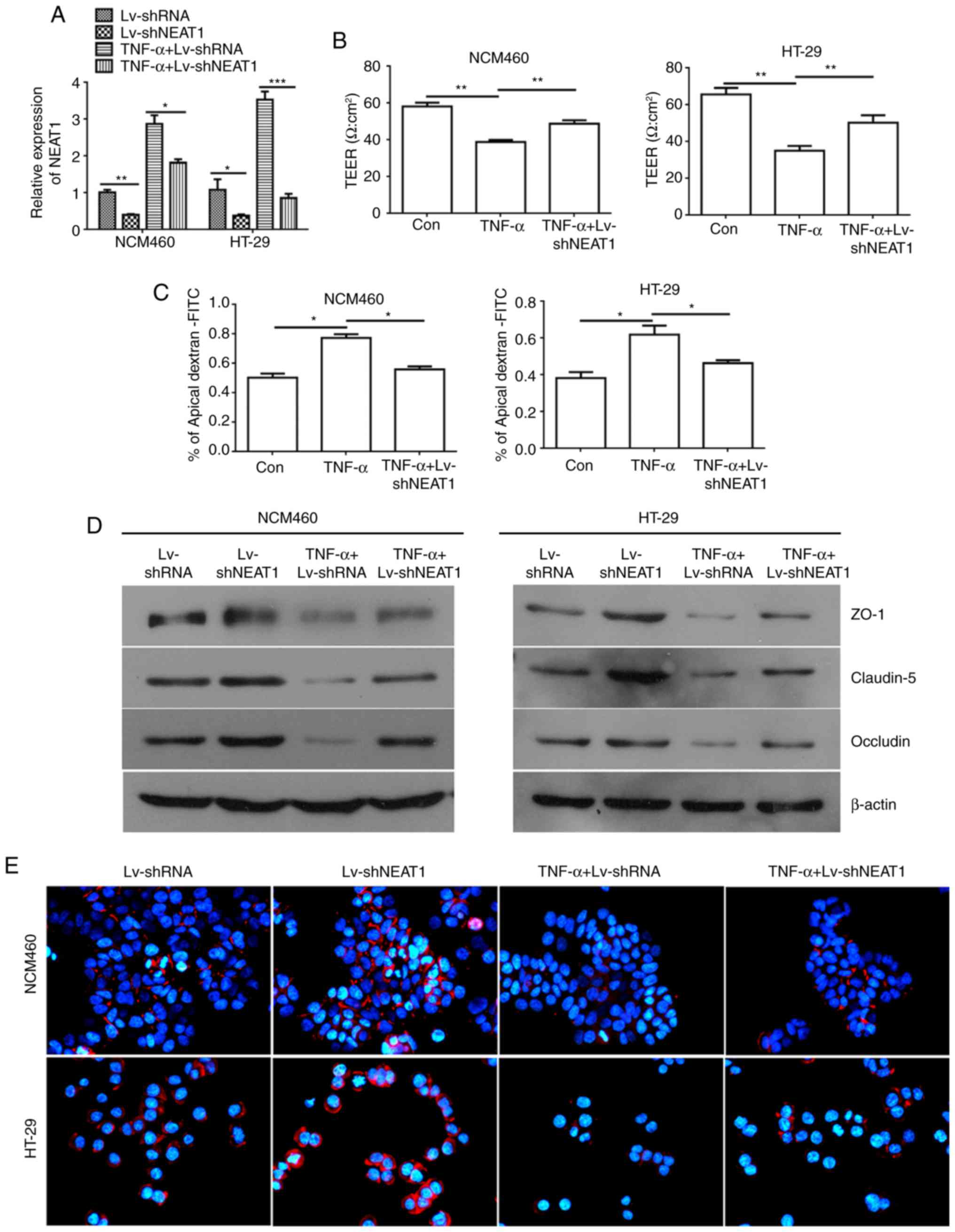 | Figure 2NEAT1 inhibition reverses the
TNF-α-induced permeability increase in inflammatory HT-29 and
NCM460 cells. (A) Relative expression of NEAT1 was detected in
NCM460 and HT-29 cells treated with LV-shNEAT1 and TNF-α using
RT-qPCR analysis. (B) TEER values were monitored in NCM460 and
HT-29 cells treated with LV-shNEAT1 and TNF-α. (C) Permeability
assays were performed to detect the barrier state in NCM460 and
HT-29 cells. (D) Western blot analysis was used to detect the
expression of ZO-1, Claudin-5 and Occludin. (E) Immunofluorescence
analyses of the protein expression of Occludin in NCM460 and HT-29
cells (magnification, x400). *P<0.05,
**P<0.01 and ***P<0.001. NEAT1, nuclear
paraspeckle assembly transcript 1; shRNA, short hairpin RNA; Con,
control; TEER, trans-epithelial electrical resistance; TNF-α, tumor
necrosis factor-α; ZO-1, zonula occludens-1; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; FITC,
fluorescein isothiocyanate. |
To further support the above results, permeability
assays were preformed to examine the change in the barrier property
following TNF-α induction and the downregulation of NEAT1 in NCM460
and HT-29 monolayers by detecting the FITC-dextran concentration;
the percentage of apical FITC-dextran was negatively correlated
with the cell barrier property. The results of the permeability
assays showed that the percentage of apical FITC-dextran was
increased in the TNF-α-induced NCM460 and HT-29 cells compared with
that in the control cells, and the ratio was decreased when the
NCM460 and HT-29 cells were treated with TNF-α and LV-shNEAT1
(Fig. 2C).
According to the TEER and permeability assay
results, in the inflammatory cells (cells treated with TNF-α),
NEAT1 was associated with the barrier property, which is determined
by junction complexes among cells that provide a physical and
biochemical barrier to regulate the passage of ions and small
molecules. The ZO-1, Claudin-5 and Occludin proteins were detected
by western blot analysis and were found to be expressed at high
levels in the NEAT1-inhibited NCM460 and HT-29 cells, and only
marginally expressed in TNF-α-induced inflammatory cells compared
with the negative control cells. Similarly, the connexins ZO-1,
Claudin-5 and Occludin were expressed at higher levels in the
NEAT1-inhibited inflammatory NCM460 and HT-29 cells than in the
inflammatory NCM460 and HT-29 cells (Fig. 2D). Similar results were obtained
in the immunofluorescence assays used to detect the protein
expression of Occludin, which is an integral trans-membrane protein
that has been confirmed to have important functions in the
regulation of the paracellular permeability of epithelial
monolayers (33) (Fig. 2E).
Taken together, these data suggested that TNF-α
mediated the permeability increase in NCM460 and HT-29 cells;
however, NEAT1 inhibition had an adverse function and reversed the
TNF-α-induced increase in the permeability of inflammatory
cells.
Knockdown of NEAT1 suppresses the
DSS-induced permeability increase in colon tissues from IBD
mice
For an improved understanding of the biology and
regulatory functions of NEAT1 in IBDs in vivo, further
experiments in IBD mouse models induced by 5% DSS were performed.
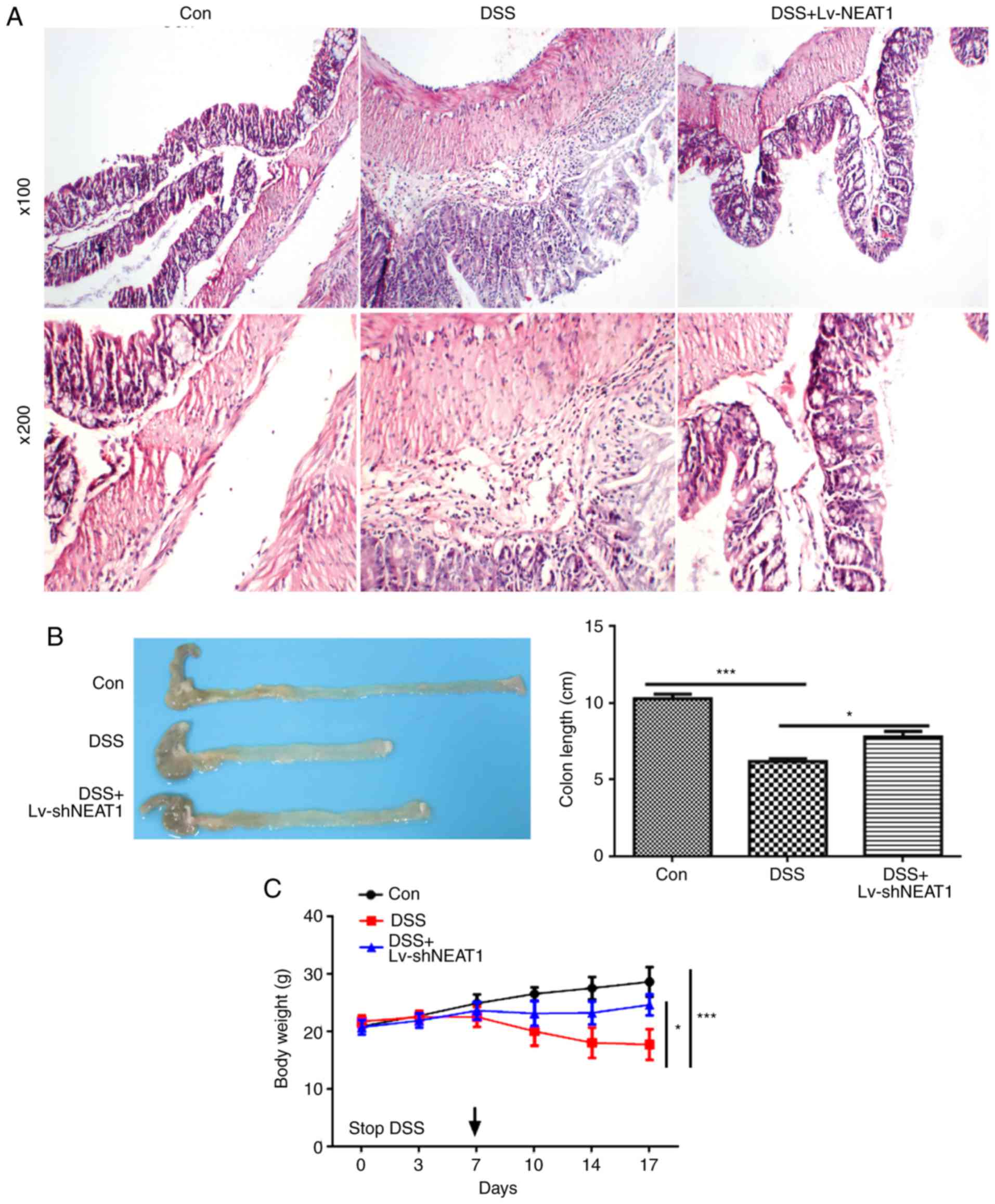
The results of H&E staining (Fig.
3A) suggested that the intestinal epithelial tissues were
damaged in the DSS-induced IBD mice, whereas the intestinal
epithelial tissues of the DSS-induced IBD mice treated with
LV-shNEAT1 were less damaged, compared with those in the group of
DSS-induced IBD mice. Furthermore, the DSS-induced IBD mice had a
lower body weight than the control healthy mice, and the
NEAT1-inhibited IBD mice had significantly higher body weights
compared with the IBD mice; similar results were acquired on
measuring colon length (Fig. 3B and
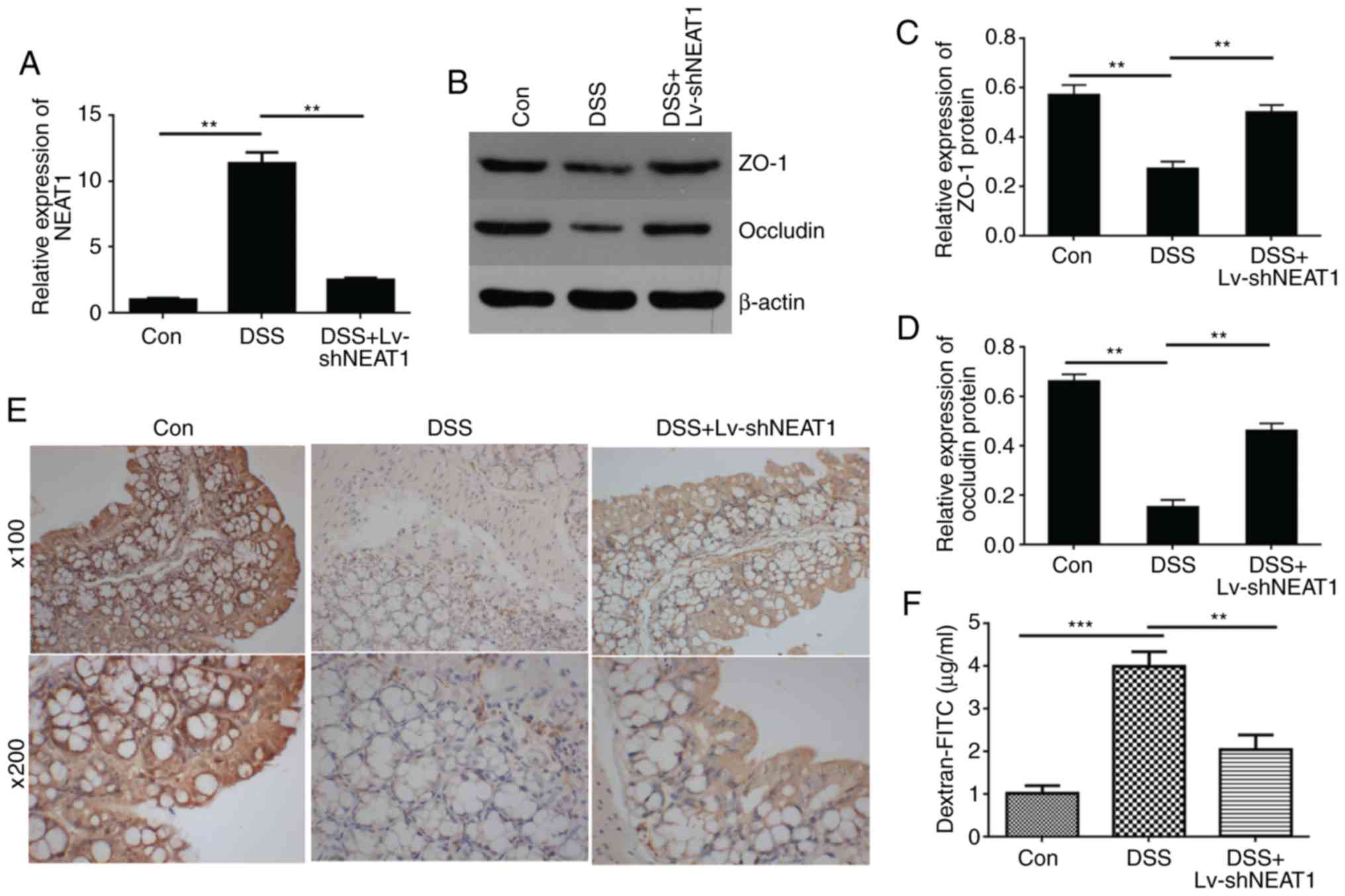
C). The relative expression of NEAT1 was detected in the colon
tissue of IBD mice using RT-qPCR analysis, and the results
suggested that the expression of NEAT1 was high in the DSS-induced
colon tissue, and when the IBD mice were treated with LV-shNEAT1,
the relative expression of NEAT1 was reduced compared with that in
the IBD mice (Fig. 4A).
To detect whether NEAT1 is associated with the
permeability of colon tissue in vivo, western blot analysis
(Fig. 4B–D) and
immunohistochemical experiments (Fig.
4E) were performed to detect the expression of connexins,
including ZO-1 and Occludin. The results showed that the expression
levels of ZO-1 and Occludin was decreased in the DSS-induced mouse
colon tissue compared with those in the negative control mice, and
when the IBD mice were treated with LV-shNEAT1, the expression
levels of ZO-1 and Occludin was increased compared with those in
the DSS-induced IBD mice. Furthermore, permeability assays were
performed to examine changes in the barrier property of intestinal
epithelial tissues from mouse models (Fig. 4F). The data indicated that the
FITC-dextran concentration, representing colon permeability, was
significantly increased in colon tissues from DSS-induced mice,
compared with the negative control group, whereas the permeability
decreased in the DSS-induced IBD mice treated with LV-shNEAT1
compared with the DSS-induced IBD mice, and these results
demonstrated that the inhibition of NEAT1 suppressed the
permeability increase in colon tissues from DSS-induced IBD
mice.
Together, these results showed that DSS induced a
permeability increase in colon tissues, and NEAT1 inhibition
reversed the effect of DSS and decreased the permeability of colon
tissues from IBD mice.
NEAT1 inhibition suppresses the
inflammatory reaction and may be associated with the function of
exosomes
An improved understanding of the regulatory
functions of NEAT1 in IBDs is important for developing novel
strategies for IBD therapy by targeting NEAT1. The previous section
examined whether NEAT1 was associated with inflammation occurrence,
and NEAT1 inhibition was found to suppress the TNF-α-induced
increase in epithelial cell permeability. Additional experiments
were preformed to understand the molecular mechanism of NEAT1 in
the inflammatory response. In this experiment, the RAW246.7
macrophage cell line was treated with exosomes purified from
DSS-induced or control male C57BL/6(B6) mouse blood serum, and the
protein expression of classically activated (M1) macrophage
markers, including IL23 and iNOS, and alternatively activated (M2)
macrophage markers, including IL10, CD206 and Arg-1, were
determined using western blot assays. The results showed that the
expression levels of IL23 and iNOS were high in RAW246.7 cells
treated with DSS-induced serum exosomes. By contrast, the M2
macrophage markers IL10, CD206 and Arg-1 were only marginally
expressed in RAW246.7 cells treated with DSS-induced serum exosomes
compared with the negative control exosome-treated group (Fig. 5A). The data suggested that DSS
induced macrophage activation and activated the inflammatory
response.
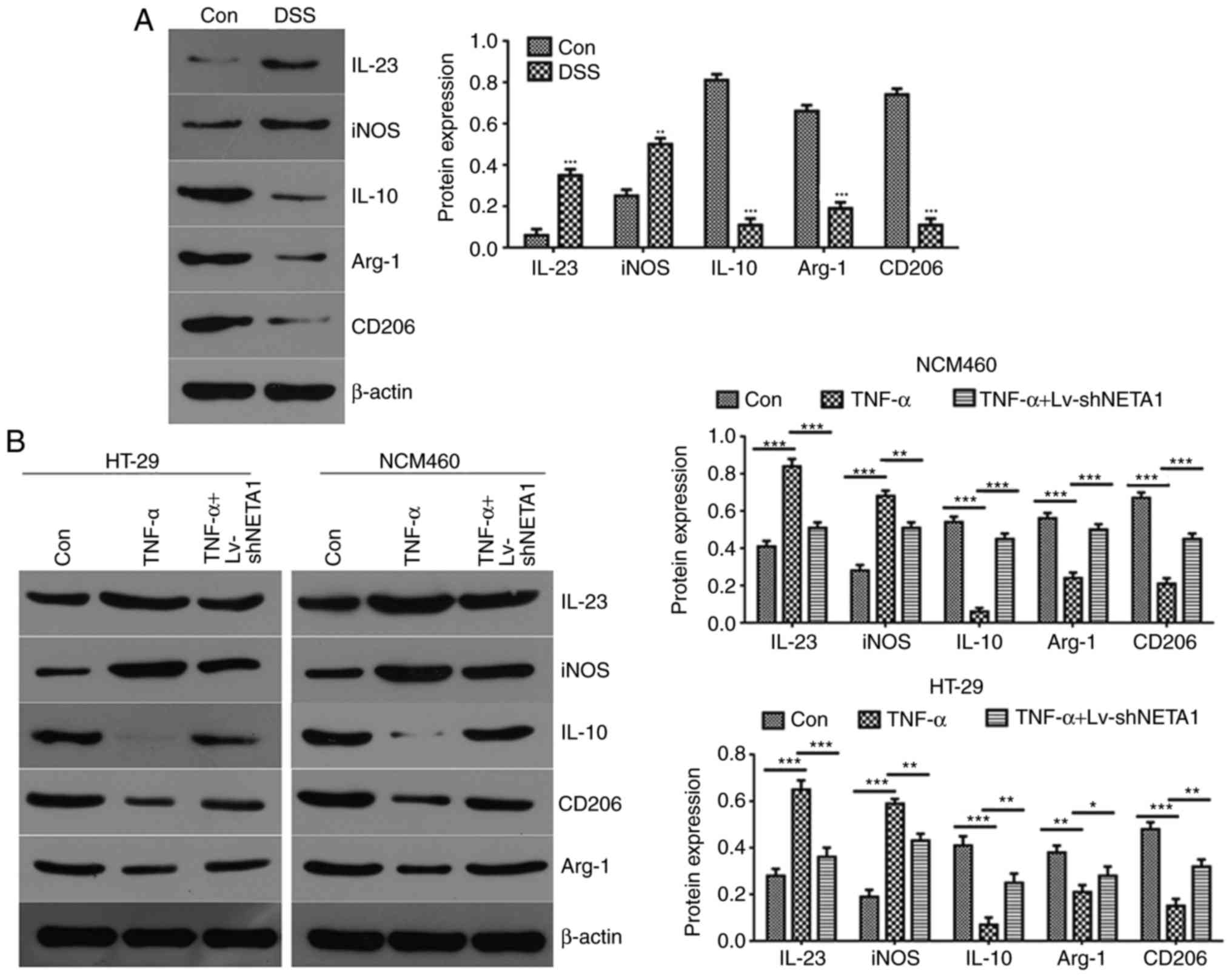 | Figure 5NEAT1 inhibition suppresses the
inflammatory reaction, which may be associated with the function of
exosomes. (A) Western blot analysis was used to detect the
expression of IL23, iNOS, IL10, CD206 and Arg-1 in RAW246.7 cells
treated with DSS-induced serum exosomes. (B) Expression of IL23,
iNOS, IL10, CD206 and Arg-1 was detected by western blot analysis
following NEAT1 inhibition in TNF-α-induced NCM460 and HT-29 cells.
(C) Flow cytometric analyses of the expression of iNOS and CD206 in
TNF-α-induced NEAT1-inhibited epithelial cells.
*P<0.05, **P<0.01 and
***P<0.001. NEAT1, Nuclear paraspeckle assembly
transcript 1; Con, control; DSS, dextran sulfate sodium; shNEAT1,
short hairpin RNA targeting NEAT1; TNF-α, tumor necrosis factor-α;
IL, Interleukin; iNOS, Inducible nitric oxide synthase; Arg-1,
Arginase-1. |
To further detect the function of NEAT1 in the
inflammatory response, the cell supernatants of LV-shNEAT1-treated
and TNF-α-induced inflammatory NCM460 and HT-29 cells were
obtained, and western blot analysis was performed to examine the
protein expression levels of the macrophage markers IL23, iNOS,
IL10, CD206 and Arg-1. The results suggested that the expression
levels of M1 macrophage markers IL23 and iNOS were high and those
of the M2 macrophage markers IL10, CD206 and Arg-1 were low in the
TNF-α-induced NCM460 and HT-29 cells compared with the negative
control group (Fig. 5B). Compared
with the TNF-α-induced NCM460 and HT-29 cells, the expression of
levels M1 macrophage markers IL23 and iNOS were decreased and the
expression levels of the M2 macrophage markers IL10, CD206 and
Arg-1 were increased when NCM460 and HT-29 cells were treated with
TNF-α and LV-shNEAT1 (Fig. 5B).
Together, these data showed that TNF-α promoted macrophage
transformation to M1 and activated the inflammatory response,
whereas NEAT1 inhibition promoted macrophage transformation to M2
and suppressed the inflammatory response in TNF-α-induced NCM460
and HT-29 cells. The protein expression of iNOS and CD206 was
verified by flow cytometry, and the results suggested that the
expression of CD206 was increased and the expression of iNOS was
decreased in the TNF-α-induced epithelial cells, and the opposite
results were obtained when TNF-α-induced epithelial cells were
treated with LV-shNEAT1 (Fig.
5C).
Taken together, these data supported the hypothesis
that NEAT1 inhibition promotes macrophage M1 transformation to M2
and suppresses the inflammatory reaction. These communication
processes between epithelial cells and macrophages may be dependent
on the effect of exosomes.
Discussion
There is increasing evidence that lncRNAs are
critical in immune system regulation, and numerous lncRNAs have
been found to be deregulated in IBD (5). In addition, increasing evidence
supports the hypothesis that NEAT1 is associated with the immune
response in various ways (34,35). A report by Imamura et al
(16) demonstrated that NEAT1 is
important in the innate immune response by facilitating the
expression of antiviral genes, including the cytokine IL8. However,
the effect of NEAT1 in IBD has not been determined previously.
Therefore, the present study investigated the function of NEAT1 in
IBD. It was found that the expression of NEAT1 was high in
DSS-induced mouse intestinal tissues, serum and exosomes, and was
also high in TNF-α-induced HT-29 and NCM460 inflammatory cell
models compared with control group cells. These results suggested
that the expression of NEAT1 is high in IBD, and the data revealed
that NEAT1 may be important in regulation of the inflammatory
response in IBD. The present study revealed the possible benefits
of examining the mechanisms underlying the pathogenesis of IBD,
suggest the future use of NEAT1 as a biomarker for the diagnosis
and treatment of IBD.
The intestinal barrier, which acts as the mammalian
host defense against the external microenvironment and exhibits
selective permeability for the absorption of nutrients, is
attracting increased attention as a critical factor in the
occurrence of IBD (36). In
addition, the intestinal barrier is covered by a cell monolayer
composed of different intestinal epithelial cell subtypes that
maintains intestinal homeostasis by controlling the crosstalk
between microbiota and subjacent immune cells (37). The epithelial monolayer acts as a
protective physical barrier and is mediated by the formation of a
web of tight junctions, which regulate paracellular permeability
and barrier integrity (38).
Dysfunction of the intestinal epithelial barrier is mainly caused
by epithelial cell apoptosis and the loss of function of tight
junctions, increasing the chances of microorganism invasion through
intestinal mucous membranes. Consistently, dysfunction of the
intestinal epithelial barrier is associated with the pathogenesis
of IBD (39,40). Therefore, further investigations
to expand current knowledge of the intestinal epithelial barrier
are important for IBD treatment. NEAT1 was found to be expressed at
high levels in inflammatory cells in the present study, and it was
hypothesized that it may influence the integrity and permeability
of the intestinal epithelial barrier in IBD. In TNF-α-induced HT-29
and NCM460 cells, it was found that cell permeability was increased
compared with that in control group cells; however, when the
expression of NEAT1 in was inhibited in TNF-α-induced HT-29 and
NCM460 inflammatory cells, the permeability decreased compared with
that in the TNF-α-induced cells. Similar results were obtained in
the DSS-induced IBD mouse models. Together, these results suggested
that permeability was increased in the TNF-α- and DSS-induced IBD
models, and that the inhibition of NEAT1 reversed the effect of
TNF-α and DSS and reduced the permeability of epithelial cells.
These data indicated that TNF-α and DSS disrupted the integrity of
the intestinal epithelial barrier and finally led to the occurrence
of IBD, whereas the inhibition of NEAT1 enhanced intestinal
epithelial barrier integrity.
NEAT1 has been verified to be involved in the
inflammatory response by regulating the intestinal epithelial
barrier in IBD. In the inflammatory process, inflammatory cytokines
secreted by epithelial cells trigger the immune response.
Furthermore, T cell and macrophage recruitment to the region of
intestinal tissue injury is another way to defend against a harmful
micro-environment (41,42). However, whether NEAT1 influences
the function of macrophages remains to be fully elucidated. In the
present study, a DSS-induced acute colitis mouse model was used to
investigate the effect of serum exosomes, which contain high levels
of lncRNANEAT1, on TNF-α-induced macrophages. RAW246.7 macrophages
were treated with serum exosomes isolated from DSS-induced mice. It
was found that the expression levels of M1 macrophage markers were
high and those of M2 macrophage marker expression were low compared
with levels in cells treated with exosomes isolated from control
mice. The results suggested that DSS induced M1 macrophage
activation. In the TNF-α-induced inflammatory NCM460 and HT-29
cells, similar results were obtained, suggesting that TNF-α induced
M1 macrophage activation. However, when the expression of NEAT1 was
inhibited in TNF-α-induced cells, M1 macrophage activation was
suppressed. The present study revealed for the first time, to the
best of our knowledge, that NEAT1 in exosomes affects the phenotype
transformation between epithelial cells and macrophages.
In conclusion, the present study demonstrated that
the expression of NEAT1 was high in IBD and was involved in the
inflammatory response by regulating the intestinal epithelial
barrier and exosome-mediated polarization of macrophages in IBD.
Further knowledge of the role of NEAT1 in IBD contributes to an
improved understanding of the pathogenesis of IBD and provides
further insight into the potential and efficacy of NEAT1-based
prevention and treatment options.
Funding
The study was supported by the Foundation of Hunan
Provincial Science and Technology Department, China (grant no.
2016JC2051).
Availability of data and materials
The data used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
RL designed the study, collected data, interpreted
data and prepared manuscript, and was a major contributor in
writing the manuscript. SS designed the study, interpreted data,
performed literature search and obtained funding. AT and XC
performed statistical analysis. XW collected data. LZ prepared the
manuscript. ZX interpreted data. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
All procedures performed in the present study
involving human participants were in accordance with the ethical
standards of the Ethical Meeting of Biomedical Research, The Third
Xiangya Hospital of Central South University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank the reviewers for
their helpful comments on this manuscript.
References
|
1
|
Sairenji T, Collins KL and Evans DV: An
update on inflammatory bowel disease. Prim Care. 44:673–692. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Antoni L, Nuding S, Wehkamp J and Stange
EF: Intestinal barrier in inflammatory bowel disease. World J
Gastroenterol. 20:1165–1179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jäger S, Stange EF and Wehkamp J:
Inflammatory bowel disease: An impaired barrier disease.
Langenbecks Arch Surg. 398:1–12. 2013. View Article : Google Scholar
|
|
4
|
Guttman M, Amit I, Garber M, French C, Lin
MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, et al:
Chromatin signature reveals over a thousand highly conserved large
non-coding RNAs in mammals. Nature. 458:223–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zacharopoulou E, Gazouli M, Tzouvala M,
Vezakis A and Karamanolis G: The contribution of long non-coding
RNAs in inflammatory bowel diseases. Dig Liver Dis. 49:1067–1072.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen SW, Wang PY, Liu YC, Sun L, Zhu J,
Zuo S, Ma J, Li TY, Zhang JL, Chen GW, et al: Effect of long
noncoding RNA H19 overexpression on intestinal barrier function and
its potential role in the pathogenesis of ulcerative colitis.
Inflamm Bowel Dis. 22:2582–2592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choudhry H, Albukhari A, Morotti M, Haider
S, Moralli D, Smythies J, Schödel J, Green CM, Camps C, Buffa F, et
al: Tumor hypoxia induces nuclear paraspeckle formation through
HIF-2α dependent transcriptional activation of NEAT1 leading to
cancer cell survival. Oncogene. 34:45462015. View Article : Google Scholar
|
|
8
|
Clemson CM, Hutchinson JN, Sara SA,
Ensminger AW, Fox AH, Chess A and Lawrence JB: An architectural
role for a nuclear noncoding RNA: NEAT1 RNA is essential for the
structure of paraspeckles. Mol Cell. 33:717–726. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakagawa S, Shimada M, Yanaka K, Mito M,
Arai T, Takahashi E, Fujita Y, Fujimori T, Standaert L, Marine JC
and Hirose T: The lncRNA Neat1 is required for corpus luteum
formation and the establishment of pregnancy in a subpopulation of
mice. Development. 141:4618–4627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin D: Commentary on 'The oestrogen
receptor alpha-regulated lncRNA NEAT1 is a critical modulator of
prostate cancer' Chakravarty D, Sboner A, Nair SS, Giannopoulou E,
Li R, Hennig S, Mosquera JM, Pauwels J, Park K, Kossai M, MacDonald
TY, Fontugne J, Erho N, Vergara IA, Ghadessi M, Davicioni E,
Jenkins RB, Palanisamy N, Chen Z, Nakagawa S, Hirose T, Bander NH,
Beltran H, Fox AH, Elemento O, Rubin MA, University of
Washington-Urology, Seattle, WA Nat Commun 2014; 5: 5383. Urol
Oncol. 34:5222016.PubMed/NCBI
|
|
11
|
Chakravarty D, Sboner A, Nair SS,
Giannopoulou E, Li R, Hennig S, Mosquera JM, Pauwels J, Park K,
Kossai M, et al: The oestrogen receptor alpha-regulated lncRNA
NEAT1 is a critical modulator of prostate cancer. Nat Commun.
5:53832014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zeng C, Xu Y, Xu L, Yu X, Cheng J, Yang L,
Chen S and Li Y: Inhibition of long non-coding RNA NEAT1 impairs
myeloid differentiation in acute promyelocytic leukemia cells. Bmc
Cancer. 14:6932014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng W, Wang Z and Fan H: LncRNA NEAT1
impacts cell proliferation and apoptosis of colorectal cancer via
regulation of Akt signaling. Pathol Oncol Res. 23:651–656. 2017.
View Article : Google Scholar
|
|
14
|
Li Y, Li Y, Chen W, He F, Tan Z, Zheng J,
Wang W, Zhao Q and Li J: NEAT expression is associated with tumor
recurrence and unfavorable prognosis in colorectal cancer.
Oncotarget. 6:27641–27650. 2015.PubMed/NCBI
|
|
15
|
Morchikh M, Cribier A, Raffel R, Amraoui
S, Cau J, Severac D, Dubois E, Schwartz O, Bennasser Y and
Benkirane M: HEXIM1 and NEAT1 long non-coding RNA form a
multi-subunit complex that regulates DNA-mediated innate immune
response. Mol Cell. 67:387–399.e5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Imamura K, Imamachi N, Akizuki G, Kumakura
M, Kawaguchi A, Nagata K, Kato A, Kawaguchi Y, Sato H, Yoneda M, et
al: Long noncoding RNA NEAT1-dependent SFPQ relocation from
promoter region to paraspeckle mediates IL8 expression upon immune
stimuli. Mol Cell. 53:393–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo J, Cai H, Zheng J, Liu X, Liu Y, Ma J,
Que Z, Gong W, Gao Y, Tao W and Xue Y: Long non-coding RNA NEAT1
regulates permeability of the blood-tumor barrier via
miR-181d-5p-mediated expression changes in ZO-1, occludin, and
claudin-5. Biochim Biophys Acta. 1863:2240–2254. 2017. View Article : Google Scholar
|
|
18
|
Zhang F, Wu L, Qian J, Qu B, Xia S, La T,
Wu Y, Ma J, Zeng J, Guo Q, et al: Identification of the long
noncoding RNA NEAT1 as a novel inflammatory regulator acting
through MAPK pathway in human lupus. J Autoimmun. 75:96–104. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang X, Zhou Y, Sun AJ and Xue JL: NEAT1
contributes to breast cancer progression through modulating miR-448
and ZEB1. J Cell Physiol. 2018. View Article : Google Scholar
|
|
20
|
Xiong W, Huang C, Deng H, Jian C, Zen C,
Ye K, Zhong Z, Zhao X and Zhu L: Oncogenic non-coding RNA NEAT1
promotes the prostate cancer cell growth through the SRC3/IGF1R/AKT
pathway. Int J Biochem Cell Biol. 94:125–132. 2018. View Article : Google Scholar
|
|
21
|
Li W, Zhang Z, Liu X, Cheng X, Zhang Y,
Han X, Zhang Y, Liu S, Yang J, Xu B, et al: The FOXN3-NEAT1-SIN3A
repressor complex promotes progression of hormonally responsive
breast cancer. J Clin Invest. 127:3421–3440. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cosenza S, Ruiz M, Maumus M, Jorgensen C
and Noël D: Pathogenic or therapeutic extracellular vesicles in
rheumatic diseases: Role of mesenchymal stem cell-derived vesicles.
Int J Mol Sci. 18:E8892017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maas SLN, Breakefield XO and Weaver AM:
Extracellular vesicles: Unique intercellular delivery vehicles.
Trends Cell Biol. 27:172–188. 2017. View Article : Google Scholar :
|
|
24
|
Colombo M, Raposo G and Théry C:
Biogenesis, secretion, and intercellular interactions of exosomes
and other extracellular vesicles. Annu Rev Cell Dev Biol.
30:255–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu AT, Lu JT, Ran ZH and Zheng Q: Exosome
in intestinal mucosal immunity. J Gastroenterol Hepatol.
31:1694–1699. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wong WY, Lee MM, Chan BD, Kam RK, Zhang G,
Lu AP and Tai WC: Proteomic profiling of dextran sulfate sodium
induced acute ulcerative colitis mice serum exosomes and their
immuno-modulatory impact on macrophages. Proteomics. 16:1131–1145.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
28
|
Espiña B, Otero P, Louzao MC, Alfonso A
and Botana LM: 13-Desmethyl spirolide-c and 13,19-didesmethyl
spirolide-c trans-epithelial permeabilities: Human intestinal
permeability modelling. Toxicology. 287:69–75. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ren DY, Li C, Qin YQ, Yin RL, Du SW, Ye F,
Liu HF, Wang MP, Sun Y, Li X, et al: Lactobacilli reduce chemokine
IL-8 production in response to TNF-alpha and Salmonella challenge
of Caco-2 cells. Biomed Res Int. 2013:9252192013. View Article : Google Scholar
|
|
30
|
Yin J, Wu M, Duan J, Liu G, Cui Z, Zheng
J, Chen S, Ren W, Deng J, Tan X, et al: Pyrrolidine dithiocarbamate
inhibits NF-KappaB activation and upregulates the expression of
Gpx1, Gpx4, occludin, and ZO-1 in DSS-induced colitis. Appl Biochem
Biotechnol. 177:1716–1728. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leoni G, Neumann PA, Kamaly N, Quiros M,
Nishio H, Jones HR, Sumagin R, Hilgarth RS, Alam A, Fredman G, et
al: Annexin A1-containing extracellular vesicles and polymeric
nanoparticles promote epithelial wound repair. J Clin Invest.
125:1215–1227. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Paranavitana C, Zelazowska E, Izadjoo M
and Hoover D: Interferon-gamma associated cytokines and chemokines
produced by spleen cells from Brucella-immune mice. Cytokine.
30:86–92. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Al-Sadi R, Khatib K, Guo S, Ye D, Youssef
M and Ma T: Occludin regulates macromolecule flux across the
intestinal epithelial tight junction barrier. Am J Physiol
Gastrointest Liver Physiol. 300:G1054–G1064. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hirose T, Virnicchi G, Tanigawa A,
Naganuma T, Li R, Kimura H, Yokoi T, Nakagawa S, Bénard M, Fox AH
and Pierron G: NEAT1 long noncoding RNA regulates transcription via
protein sequestration within subnuclear bodies. Mol Biol Cell.
25:169–183. 2014. View Article : Google Scholar :
|
|
35
|
Zhang Q, Chen CY, Yedavalli VS and Jeang
KT: NEAT1 long noncoding RNA and paraspeckle bodies modulate HIV-1
post-transcriptional expression. MBio. 4:e00596–12. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu P, Becker H, Elizalde M, Masclee A and
Jonkers D: Intestinal organoid culture model is a valuable system
to study epithelial barrier function in IBD. Gut. pii:
gutjnl-2017-315685. 2017.PubMed/NCBI
|
|
37
|
Dupaul-Chicoine J, Dagenais M and Saleh M:
Crosstalk between the intestinal microbiota and the innate immune
system in intestinal homeostasis and inflammatory bowel disease.
Inflamm Bowel Dis. 19:2227–2237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Peterson LW and Artis D: Intestinal
epithelial cells: Regulators of barrier function and immune
homeostasis. Nat Rev Immunol. 14:141–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kayama H and Takeda K: Regulation of the
human gut homeostasis by anti-inflammatory CD14+
CD163high CD160high myeloid cells. Nihon
Rinsho Meneki Gakkai Kaishi. 39:441–447. 2016.Japanese. View Article : Google Scholar
|
|
40
|
Pott J and Hornef M: Innate immune
signalling at the intestinal epithelium in homeostasis and disease.
EMBO Rep. 13:684–698. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mao F, Wu Y, Tang X, Kang J, Zhang B, Yan
Y, Qian H, Zhang X and Xu W: Exosomes derived from human umbilical
cord mesenchymal stem cells relieve inflammatory bowel disease in
mice. Biomed Res Int. 2017:53567602017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Doe WF and Dorsman B: Chronic inflammatory
bowel disease-increased plasminogen activator secretion by
mono-nuclear phagocytes. Clin Exp Immunol. 48:256–260.
1982.PubMed/NCBI
|