Introduction
Colorectal cancer (CRC) is one of the most common
malignancies worldwide and the third leading cause of
cancer-associated mortality (1).
According to the statistics, there are 1.36 million new cases of
CRC and 694,000 CRC-associated mortalities annually worldwide
(2). Cancer cell metastasis is
one of the major problems that hinders successful CRC treatment
(3,4). Increasing evidence indicated that
cell proliferation, migration and invasion are crucial in CRC
metastasis. However, the potential molecular mechanism underlying
CRC metastasis has yet to be fully elucidated. Therefore,
investigating the molecular mechanisms involved in CRC progression
is crucial in order to develop novel and effective therapies.
MicroRNAs (miRNAs/miRs) are a type of highly
conserved small non-coding RNA with a length of approximately 19–25
nucleotides. miRNA post-transcriptionally regulates the expression
of a target gene through direct interaction with the
3′-untranslated region (3′UTR) of its target mRNA, and is known to
be involved in cell proliferation, migration and invasion (5). To date, miRNAs have emerged as
potential critical regulators of carcinogenesis and tumor
progression (6,7). A variety of miRNAs have been
reported to serve as anti-oncogenes in CRC, including miR-483 and
miR-551 (8). miR-7 is an
evolutionarily conserved miRNA that is involved in the development
of the eye and pancreas in Drosophila (9). Accumulating evidence suggests that
miR-7 simultaneously targets a number of mRNAs that are involved in
various signaling pathways in several types of cancer (9). It has been demonstrated that miR-7
is downregulated in certain human tumors, including CRC, and this
miRNA has been reported to regulate a number of oncogenic signal
transduction pathways, including the epidermal growth factor
receptor signaling pathway, as well as the phosphoinositide
3-kinase/protein kinase B (PI3K/AKT) and RAF/mitogen-activated
protein kinase kinase/extracellular signal-regulated kinase
(RAF/MEK/ERK) pathways, suggesting that it may function as a tumor
suppressor (10,11). The present study aimed to examine
the role of miR-7 in CRC and identify novel targets that may be
clinically useful.
TYRO3, a member of the TAM family (comprising TYRO3,
AXL and MERTK) of tyrosine kinases (12), has been demonstrated to be
abnormally expressed in a wide variety of human tumors, including
CRC, and to be associated with tumor progression and resistance to
targeted therapeutics (13,14). It has been reported that the
receptor tyrosine kinase Axl promotes cell migration and invasion
in CRC (15). In addition,
overexpression of TYRO3 in tumor tissues significantly reduced the
survival of patients with CRC (13). TYRO3, as a novel functional target
of miR-7, was reported to regulate the proliferation, invasion and
migration of Huh-7 cells via the PI3K/AKT pathway (16). This signaling pathway serves a key
role in several cancer processes, including proliferation, tumor
growth and tumorigenesis (17,18). A previous study has reported that
abnormal activation of PI3K/AKT promotes the invasion and
metastasis of numerous tumors, including CRC (18). However, whether miR-7 regulates
cell proliferation, invasion and migration in CRC via TYRO3 and
subsequent PI3K/AKT pathway inhibition has not been elucidated to
date.
In the present study, aimed to investigate the role
of miR-7 in CRC and its potential mechanism. The results
demonstrated that miR-7 was significantly downregulated in CRC.
Overexpression of miR-7 inhibited the proliferation, migration and
invasion of CRC cell lines by directly inhibiting the TYRO3
receptor tyrosine kinase, resulting in the inhibition of PI3K/AKT
pathway, with a significant impact on cancer cell migration,
proliferation and invasion.
Materials and methods
Patients
A total of 30 CRC tissue samples and corresponding
matched adjacent noncancerous tissues were obtained from patients
with CRC at the Affiliated Suzhou Hospital of Nanjing Medical
University (Suzhou, China) between March 2014 and September 2017.
The mean age of the included patients was 63±8 years and the age
range was 34-74 years. The diagnosis of CRC was histologically
confirmed in all patients based on colonoscopy findings (19). No patients had received
chemotherapy or radiation therapy. Each patient provided written
informed consent, and all experimental protocols were approved by
the Ethics Committee of the Affiliated Suzhou Hospital of Nanjing
Medical University (Suzhou, China).
Cell lines and culture
The human colon cancer cell lines LoVo, SW480,
SW620, HCT116 and HT29, as well as 293 cells, were purchased from
the American Type Culture Collection (Manassas, VA, USA). The
normal human colonic epithelium cell line NCM460 was purchased from
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
LoVo, SW480, SW620, HCT116 and HT29 cells were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml
streptomycin in a humidified atmosphere with 5% CO2 at
37°C. NCM460 and 293 cells were cultured in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 10% FBS, 100 U/ml penicillin and 100 mg/ml streptomycin at
37°C in a 5% CO2 incubator.
Reagents
The miR-7 mimic, miR-7 inhibitor, TYRO3 small
interfering RNA (siRNA) (cat. no. stB0004873C-1-5) and the negative
controls (NC) were purchased from Guangzhou RiboBio Co., Ltd.
(Guangzhou, China). The detailed information regarding miR-7 mimic,
miR-7 inhibitor, siRNA and their controls is as follows: i) miR-7
mimic sense, 5′-UGG AAG ACU AGU GAU UUU GUU GU-3′ and antisense,
5′-AAC AAA AUC ACU AGU CUU CCA UU-3′; NC of miR-7 mimic sense,
5′-UUC UCC GAA CGU GUC ACG UTT-3′ and antisense, 5′-ACG UGA CAC GUU
CGG AGA ATT-3′; ii) miR-7 inhibitor, 5′-ACA ACA AAA UCA CUA GUC UUC
CA-3′; NC of miR-7 inhibitor, 5′-CAG UAC UUU UGU GUA GUA CAA-3′;
iii) TYRO3 siRNA sense, 5′-GAG CUU UAC UUG UCU GCG ATT-3′ and
antisense, 5′-UCG CAG ACA AGU AAA GCU CGG-3′. The pmiR-REPORT-TYRO3
3′UTR wild-type (WT) and the pmiR-REPORT-TYRO3 3′UTR mutant (MUT)
sequences were synthesized by GenScript, Inc. (Piscataway, NJ,
USA).
Transfections
LoVo, SW480 and SW620 cells were seeded in 6-well
plates at a concentration of (1–1.5)x105 cells/well
(25–35% confluence) for 48 h before transfection. Subsequently,
Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) was used for transient transfection of cells with miR-7 mimic
(50 nM), miR-7 inhibitor (100 nM) or NC (100 nM), while
Oligofectamine transfection reagent (Thermo Fisher Scientific,
Inc.) was used for transfection with TYRO3 siRNA (200 nmol/l) or
transfection of cells with miR-7 inhibitor + TYRO3 siRNA for 48 h,
following the manufacturer's protocol.
Dual-luciferase reporter assay
The prediction programs TargetScan (http://www.targetscan.org/vert_72/), PicTar
(http://www.pictar.org) and miRanda (http://www.microrna. org) were used to predict the
potential targets of miR-7. Luciferase assays were conducted using
293 cells, which were seeded in 24-well plates at 2×104
cells/well and incubated overnight. The pmiR-REPORT-TYRO3 3′UTR WT
or MUT luciferase plasmid (500 ng) along with 30 nM miR-7 mimic or
miR-NC was co-transfected into 293 cells using Lipofectamine 2000
reagent. Subsequent to transfection for 48 h, cells were harvested,
and a Dual-Luciferase Reporter Assay kit (Promega Corporation,
Madison, WI, USA) was used to measure the luciferase activity
according to the manufacturer's protocol. Luciferase activity was
normalized to Renilla luciferase activity.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the tissues and cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
and an miRNeasy extraction kit (Qiagen, Inc., Valencia, CA, USA),
according to the manufacturer's protocol. The RNA concentration was
qualitied by Uv-vis at 280 nm. Single-stranded cDNA was synthesized
with a QuantiTect Reverse Transcription kit (Qiagen, Inc.). Next,
qPCR was performed using SYBR-Green Master Mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.) in an ABI 7500 thermocycler
(Applied Biosystems; Thermo Fisher Scientific, Inc.), and the
results were normalized to GAPDH expression. The following primer
sequences were used: TYRO3 sense, 5′-GTG TGT GGC TGA CTT CGG AC-3′,
and antisense, 5′-CAC GTC CTC CAT ACA CTC CG-3′; GAPDH sense,
5′-GGA GCC AAA AGG GTC ATC AT-3′, and antisense, 5′-GTG ATG GCA TGG
ACT GTG GT-3′. The reaction conditions were conducted as follows:
40 cycles of predenaturation for 10 min at 95°C, denaturation for
30 sec at 95°C, annealing for 20 sec at 60°C and extension for 35
sec at 72°C. The expression of mature miR-7 was quantified with a
TaqMan microRNA assay kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and was detected relative to U6 small nuclear RNA
expression. The relative expression of each gene was calculated
using the 2−∆∆Cq method (20).
Cell proliferation assay
Cell proliferation was detected using an MTT assay
[also known as
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany] following
transfection with miR-7 mimic, miR-7-inhibitor, NC and TYRO3 siRNA.
At 48 h post-transfection, the LoVo, SW480 and SW620 cells
(3×103 cells/well) were seeded in 96-well culture plates
for 24 h, and then incubated with 20 µl MTT (5 mg/ml) for 4
h at 37°C. Next, the culture medium was removed and dimethyl
sulfoxide (150 µl; Sigma-Aldrich; Merck KGaA) was added into
each well for 30 min until all crystals had been dissolved.
Absorbance was measured at 570 nm using a spectrophotometer
(Ultrospec 2000; GE Healthcare Life Sciences, Little Chalfont,
UK).
Wound healing assay
A wound healing assay was conducted to measure cell
migration. Briefly, LoVo, SW480 and SW620 cells (3×105
cells/well) were seeded into 6-well plates. After 48 h of
transfection, a 10-µl sterile pipette tip was used to scrape
the cell monolayer. The migration path of cells was subsequently
tracked at 0 and 24 h after wounding using a phase contrast
microscope (IX711; Olympus Corporation, Tokyo, Japan). Quantitative
analysis of the wound healing area was performed using ImageJ
software (National Institutes of Health, Bethesda, MA, USA).
Cell invasion assay
The cell invasion assay was performed in 24-well
chambers containing a Transwell membrane filter (Corning
Incorporated, Corning, NY, USA). After 48-h transfection, LoVo,
SW480 and SW620 cells were resuspended in 200 µl serum-free
medium and subsequently seeded into the upper chamber of the
plates. A total of 600 µl medium containing 10% FBS was
placed in the lower chamber. Subsequent to incubation for 24 h,
cells on the upper membrane were removed with a cotton swab. The
number of cell invading the lower chamber was used to evaluate the
invasive capacity, by counting and averaging the cells in five
random fields for each well at a magnification of ×100.
Western blot analysis
Total protein was extracted from the cells using a
protein lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China), and the protein concentration was measured with a
bicinchoninic acid assay kit (Beyotime Institute of Biotechnology).
Protein samples (30 µg each) were then separated by 10%
SDS-PAGE (Sigma-Aldrich; Merck KGaA) and transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked with 5% (w/v) skimmed milk in
Tris-buffered saline/Tween-20 for 1 h at room temperature and
subsequently stained overnight at 4°C with primary antibodies at a
dilution of 1:1,000, as follows: Anti-TYRO3 (cat. no. 5585),
anti-phospho-PI3K (Tyr458; cat. no. 4228), anti-PI3K (cat. no.
4292), anti-AKT (cat. no. 9272), anti-phospho-AKT (Ser473; cat. no.
4060), anti-mammalian target of rapamycin (mTOR; cat. no. 2972),
anti-phospho-mTOR (Ser2448; cat. no. 2971) and anti-GAPDH (14C10;
cat. no. 2118; all purchased from Cell Signaling Technology, Inc.,
Danvers, MA, USA). Membranes were subsequently incubated with
horseradish peroxidase-conjugated anti-rabbit IgG secondary
antibody (1:2,000; cat. no. 7074; Cell Signaling Technology, Inc.)
for 1 h at room temperature. Proteins bands were visualized using
an enhanced chemiluminescence detection system (GE Healthcare Life
Sciences). ImageJ software, version 1.46 (National Institutes of
Health, Bethesda, MD, USA) was applied to quantify the integrated
density of the bands.
Statistical analysis
All data were analyzed using SPSS software, version
13.0 (SPSS, Inc., Chicago, IL, USA). The difference in miR-7
expression between CRC tissues and adjacent normal tissue samples
was determined by paired t-test. Statistical differences between
the groups were assessed by one-way analysis of variance, followed
by Tukey post-hoc test. The data are presented as the mean ±
standard deviation. P<0.05 was considered to denote a
statistically significant difference.
Results
miR-7 is downregulated in CRC tissues and
cell lines
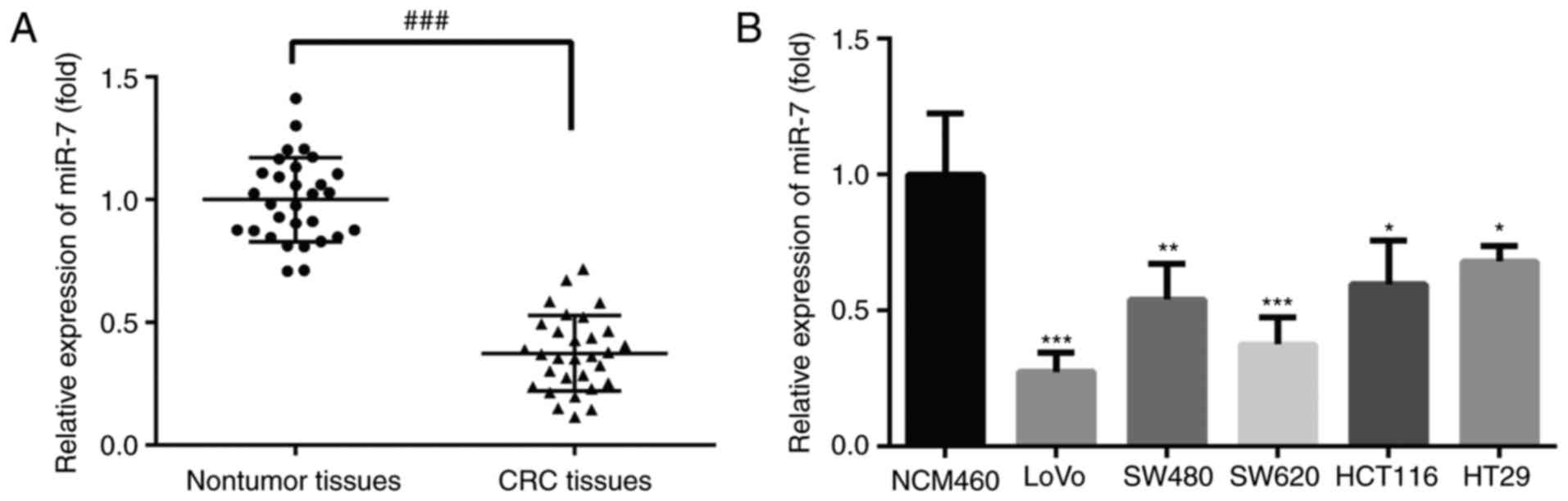
The expression of miR-7 in CRC tissues and CRC cell
lines was detected by RT-qPCR. As shown in Fig. 1A, the expression of miR-7 was
significantly decreased in CRC tissues as compared with that in
adjacent normal tissue samples. In addition, the expression of
miR-7 mRNA was markedly downregulated in all five CRC cell lines
compared with that in normal colonic mucosa epithelial cells
(Fig. 1B). These results
indicated that miR-7 may be a potential tumor suppressor and may be
involved in the development of CRC.
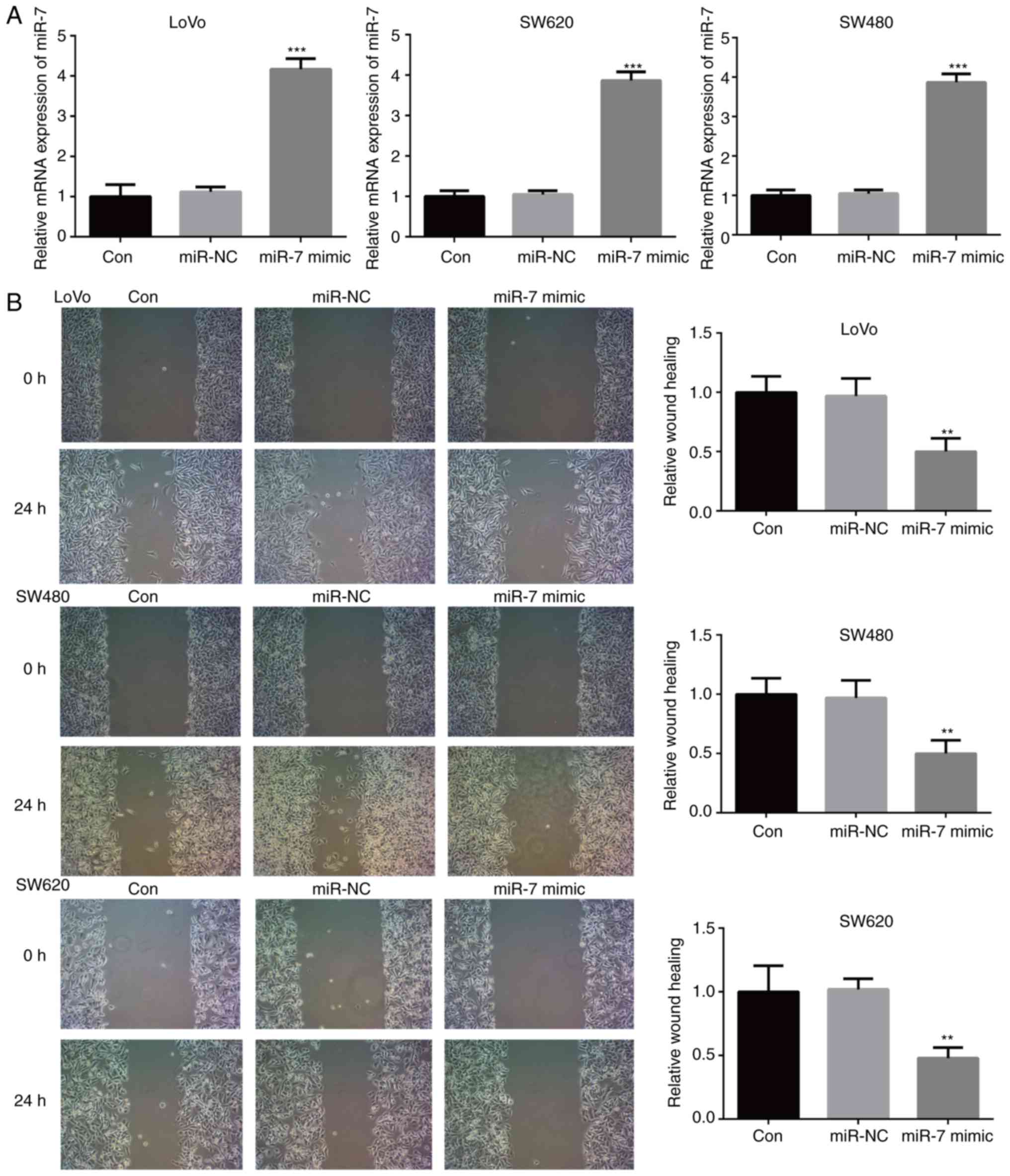
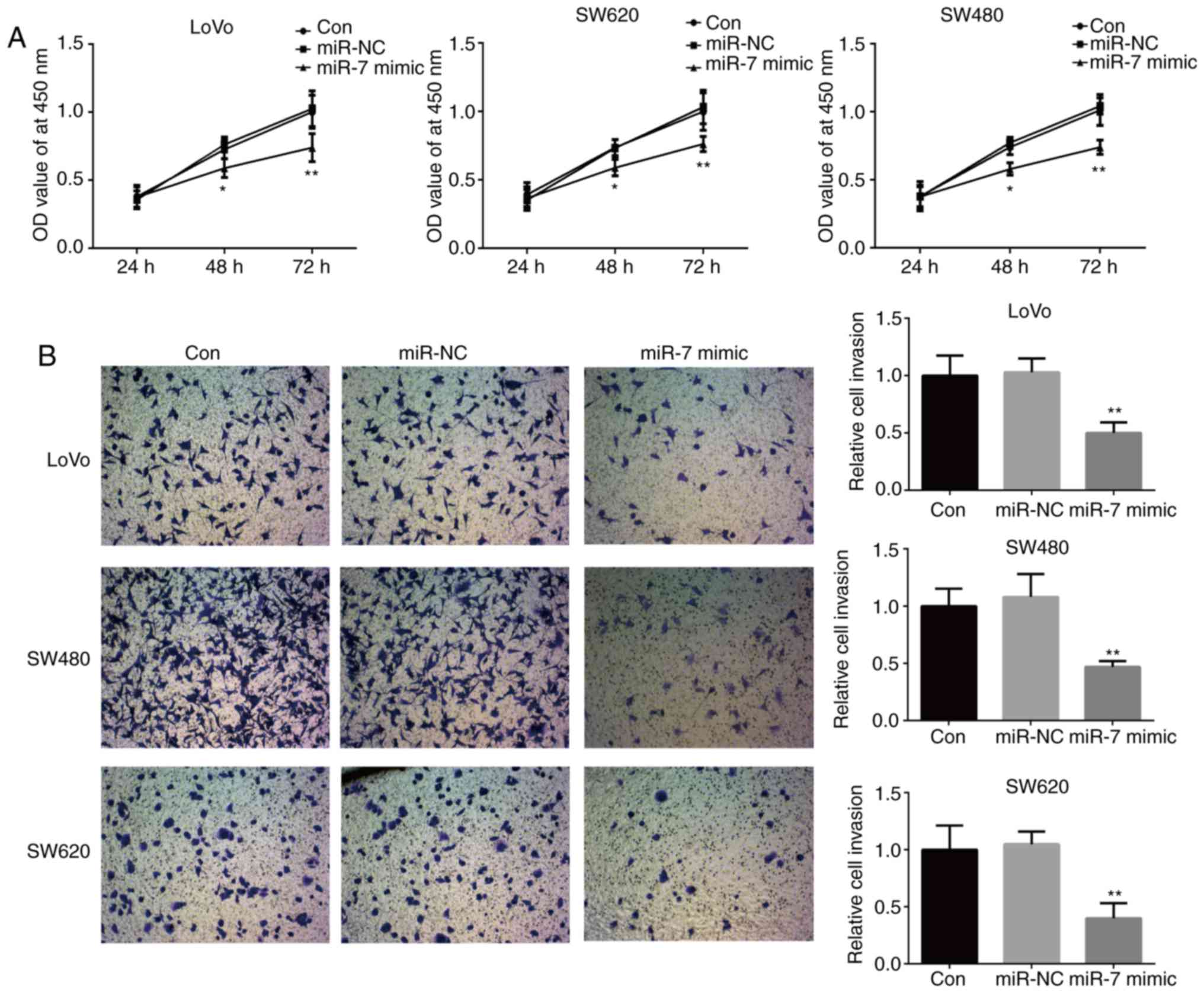
miR-7 overexpression inhibits the
proliferation, migration and invasion of CRC cells
In order to investigate whether miR-7 serves a role
in the development and progression of CRC, the LoVo, SW480 and
SW620 cell lines were transfected with miR-7 mimic or miR-NC.
RT-qPCR analysis demonstrated that miR-7 mimic transfection
effectively increased miR-7 mRNA expression (Fig. 2A). The effect of miR-7 over
expression on the migration of CRC cells was then assessed with a
wound healing assay. As shown in Fig.
2B, transfection with miR-7 mimic significantly inhibited the
migration of LoVo, SW480 and SW620 cells, as compared with that
observed in the control group. MTT and Transwell assays further
revealed that miR-7 overexpression significantly inhibited the
proliferation and invasion of LoVo, SW480 and SW620 cells compared
with the control group (Fig. 3A and
B). Taken together, these results suggested that miR-7 served
an important role in CRC progression.
TYRO3 is upregulated in CRC cells and is
a direct target gene of miR-7
As reported earlier, miR-7 was downregulated in CRC
tissues and cell lines (Fig. 1).
The protein and mRNA expression levels of TYRO3 were subsequently
assessed, and the results revealed that these levels were
significantly increased in CRC cells compared with those in NCM460
cells (Fig. 4A and B). The
prediction programs TargetScan, PicTar and miRanda were used to
predict the potential targets of miR-7, and TYRO3 was predicted to
be a target gene of miR-7. To further confirm whether TYRO3 was a
direct target gene of miR-7, TYRO3 3′UTR WT and MUT were
co-transfected into 293 cells along with miR-7 mimic or miR-NC. As
presented in Fig. 4C, miR-7
reduced the relative luciferase activity of TYRO3 3′UTR WT, whereas
luciferase activity was unaffected in the MUT binding sites. In
addition, the effect of miR-7 mimic transfection on TYRO3
expression in LoVo, SW480 and SW620 cells was determined by western
blot analysis, and the results indicated that miR-7 overexpression
led to a significant decrease in TYRO3 expression in the CRC cells
(Fig. 4D).
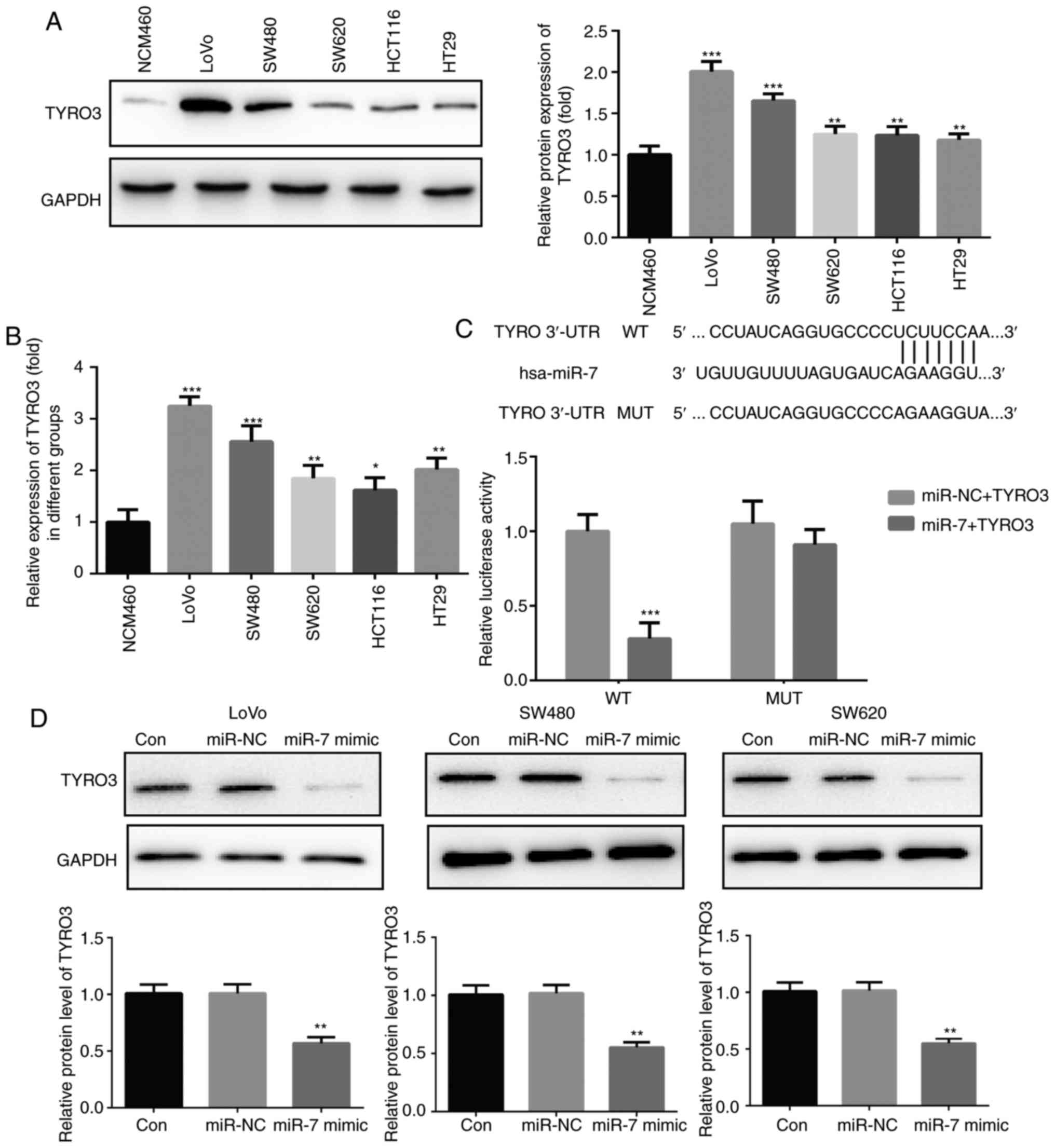 | Figure 4TYRO3 was upregulated in colorectal
cancer cells and is a direct target gene of miR-7. (A) Western blot
analysis and (B) reverse transcription-quantitative polymerase
chain reaction were used to determine the expression of TYRO3 in
LoVo, SW480, SW620, HCT116, HT29 and normal colonic mucosa
epithelial cells. (C) Luciferase activity in cells co-transfected
with TYRO3 3′UTR WT/MUT and miR-7 mimic/mimic control. (D) Protein
expression of TYRO3 in LoVo, SW480 and SW620 cells transfected with
miR-7 mimic or miR-NC was detected by western blot analysis. Data
are expressed as the mean ± standard deviation.
*P<0.05, **P<0.01 and
***P<0.001, vs. corresponding control group. miR,
microRNA; Con, Control; NC, negative control; 3′UTR,
3′-untranslated region; WT, wild-type; MUT, mutant. |
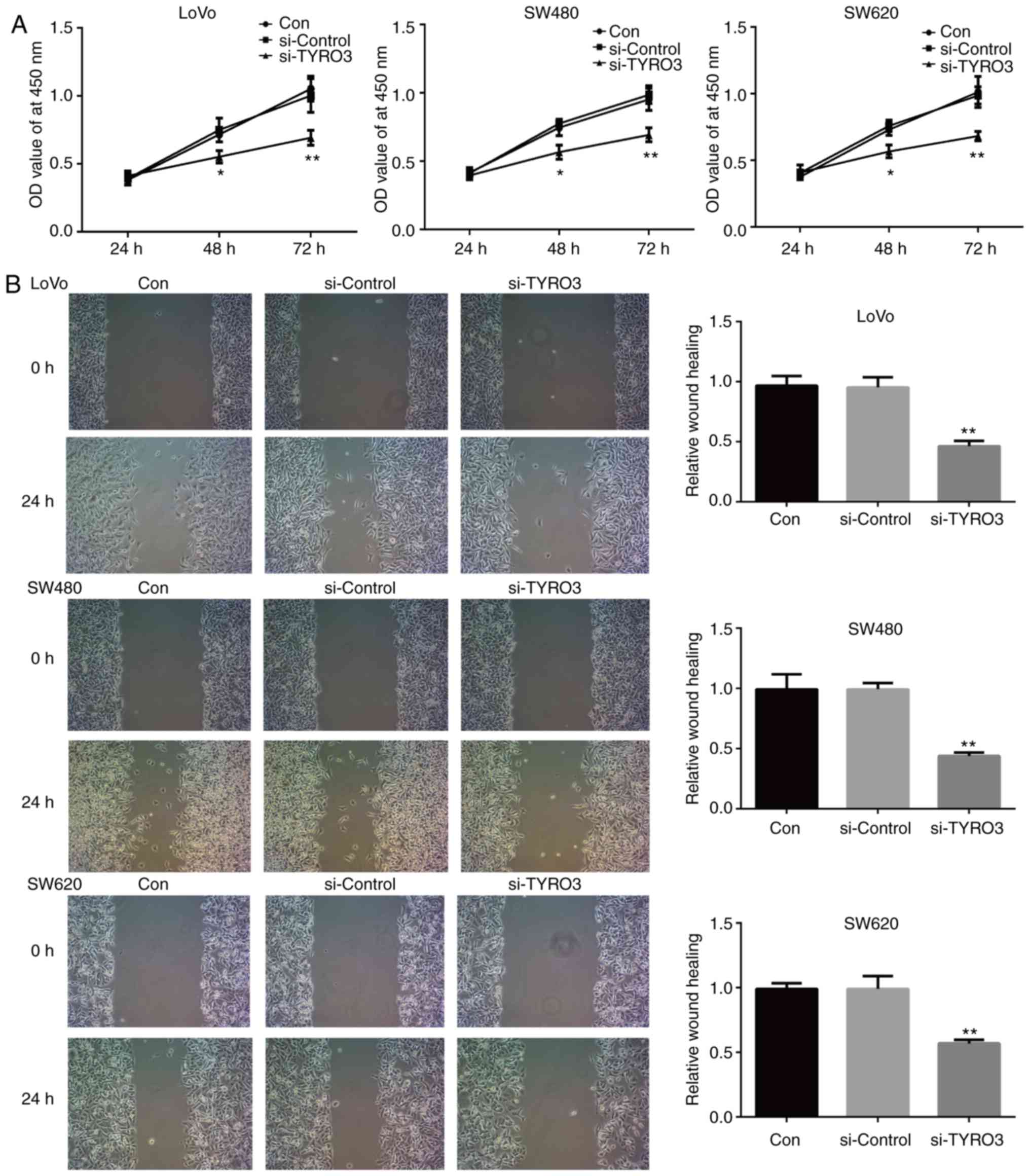
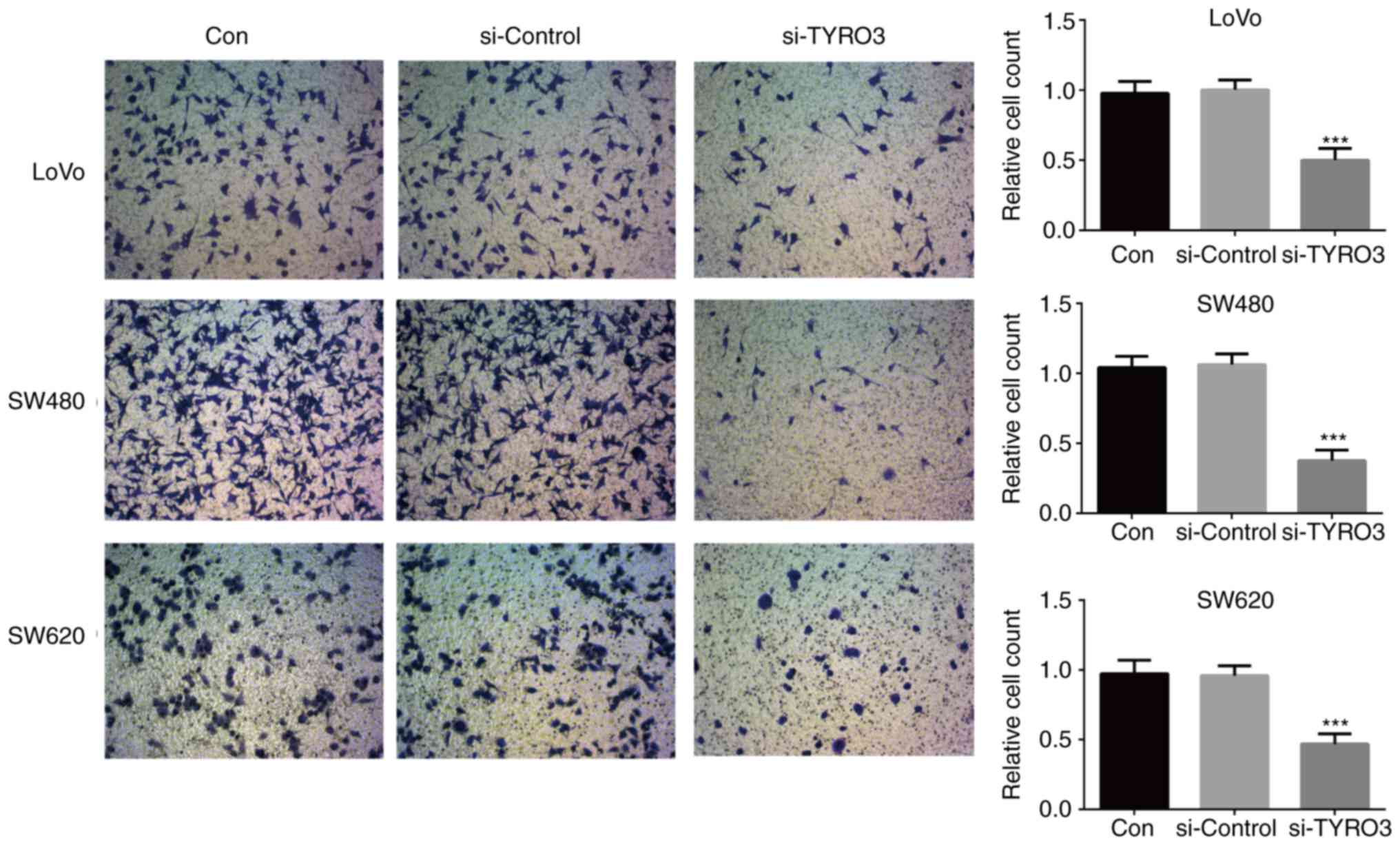
TYRO3 regulates CRC cell proliferation,
migration and invasion via the PI3K/AKT pathway
To evaluate the effects of TYRO3 on CRC, LoVo, SW480
and SW620 cells were transfected with TYRO3 siRNA. Knockdown of
TYRO3 significantly inhibited the proliferation, migration and
invasion of CRC cells when compared with the control group
(Figs. 5A, B and 6). Furthermore, in order to identify the
potential mechanism of TYRO3 in the oncogenic processes of CRC
cells, the activity of PI3K/AKT/mTOR pathway was detected by
western blot analysis. The levels of phosphorylated proteins PI3K,
AKT and mTOR were significantly reduced in TYRO3-deficient CRC
cells (Fig. 7A–C). These data
indicated that the PI3K/AKT/mTOR pathway was involved in the
pro-tumorigenic signaling of TYRO3 in CRC cells.
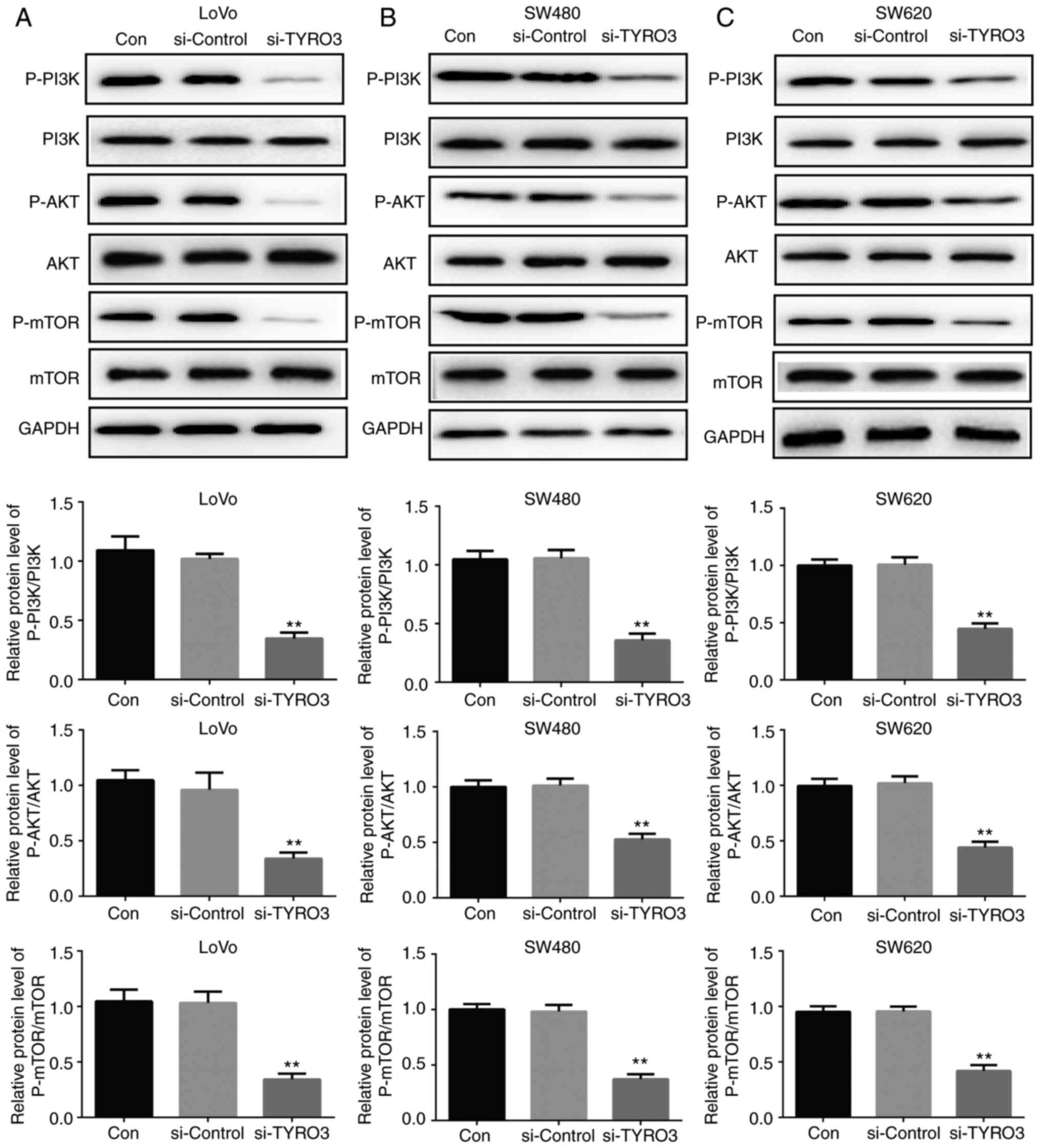 | Figure 7TYRO3 downregulation inhibited the
activation of the PI3K/AKT/mTOR pathway in colorectal cancer cells.
Western blot analysis was used to examine the protein expression
levels of p-PI3K, PI3K, p-AKT, AKT, p-mTOR and mTOR in (A) LoVo,
(B) SW480 and (C) SW620 cells transfected with si-Control or
si-TYRO3. Data are expressed as the mean ± standard deviation.
**P<0.01, vs. Con group. si-, small interfering RNA;
Con, Control; PI3K, phosphoinositide 3-kinase; AKT, protein kinase
B; mTOR, mammalian target of rapamycin; p-, phosphorylated. |
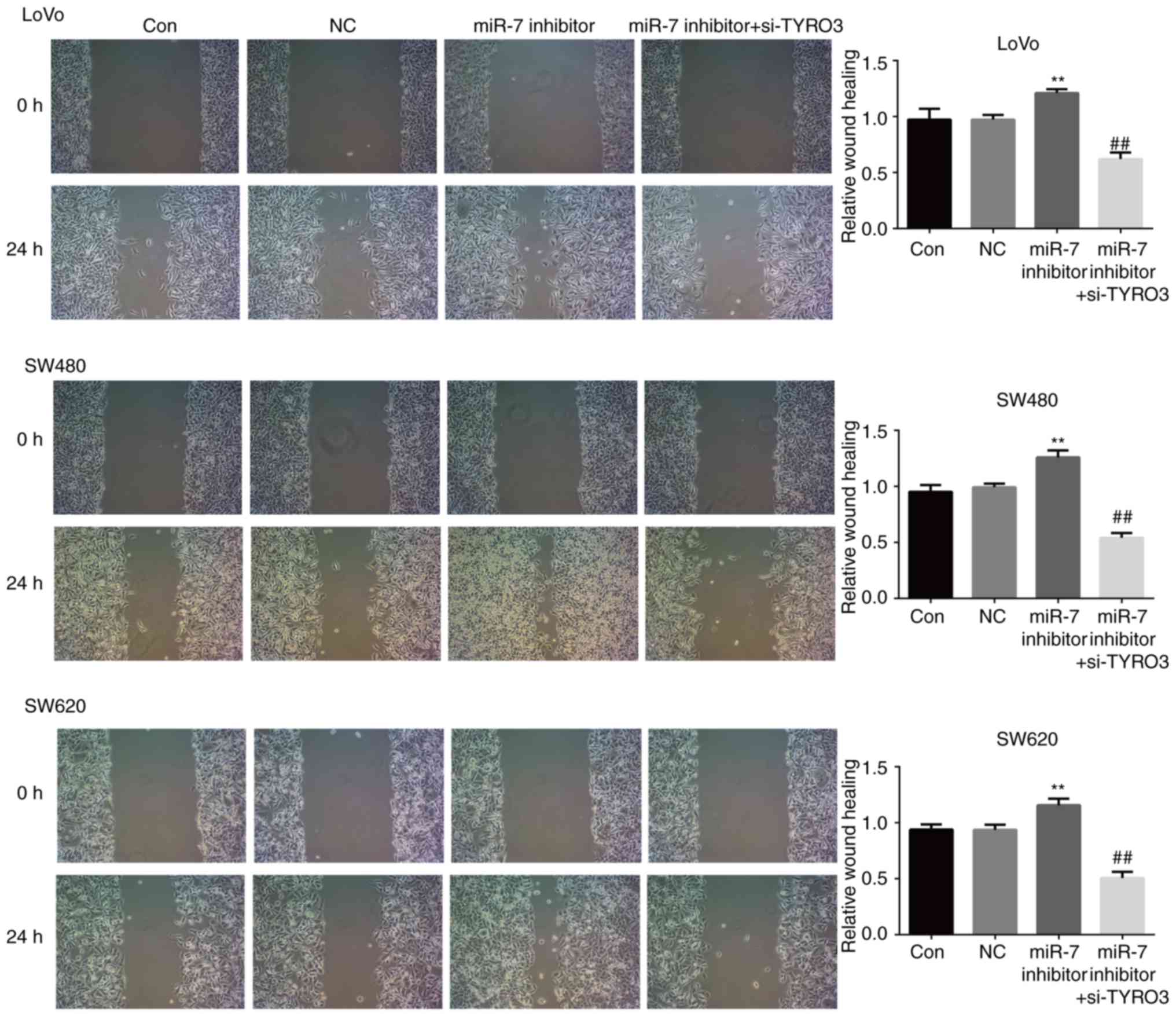
TYRO3 represses the function of miR-7 in
CRC cells
As presented in Fig.
8A, the expression of TYRO3 in LoVo, SW480 and SW620 cells
transfected with miR-7 inhibitor was significantly increased
compared with that in the control group. The effect of co-treatment
with TYRO3-siRNA and miR-7 inhibitor was then examined, and
co-transfection with TYRO3-siRNA and miR-7 inhibitor effectively
reduced TYRO3 expression (Fig.
8A). The results also demonstrated that the proliferation rate
of LoVo, SW480 and SW620 cells co-transfected with the miR-7
inhibitor and TYRO3-siRNA was significantly decreased as compared
with that in the group treated with miR-7 inhibitor alone (Fig. 8B). Furthermore, the migration and
invasion abilities of LoVo, SW480 and SW620 cells co-transfected
with the TYRO3-siRNA and miR-7 inhibitor were significantly
inhibited compared with the group treated with miR-7 inhibitor
alone (Figs. 8C and 9). These results suggested that TYRO3
may repress the effects of miR-7 on CRC cell proliferation,
migration and invasion.
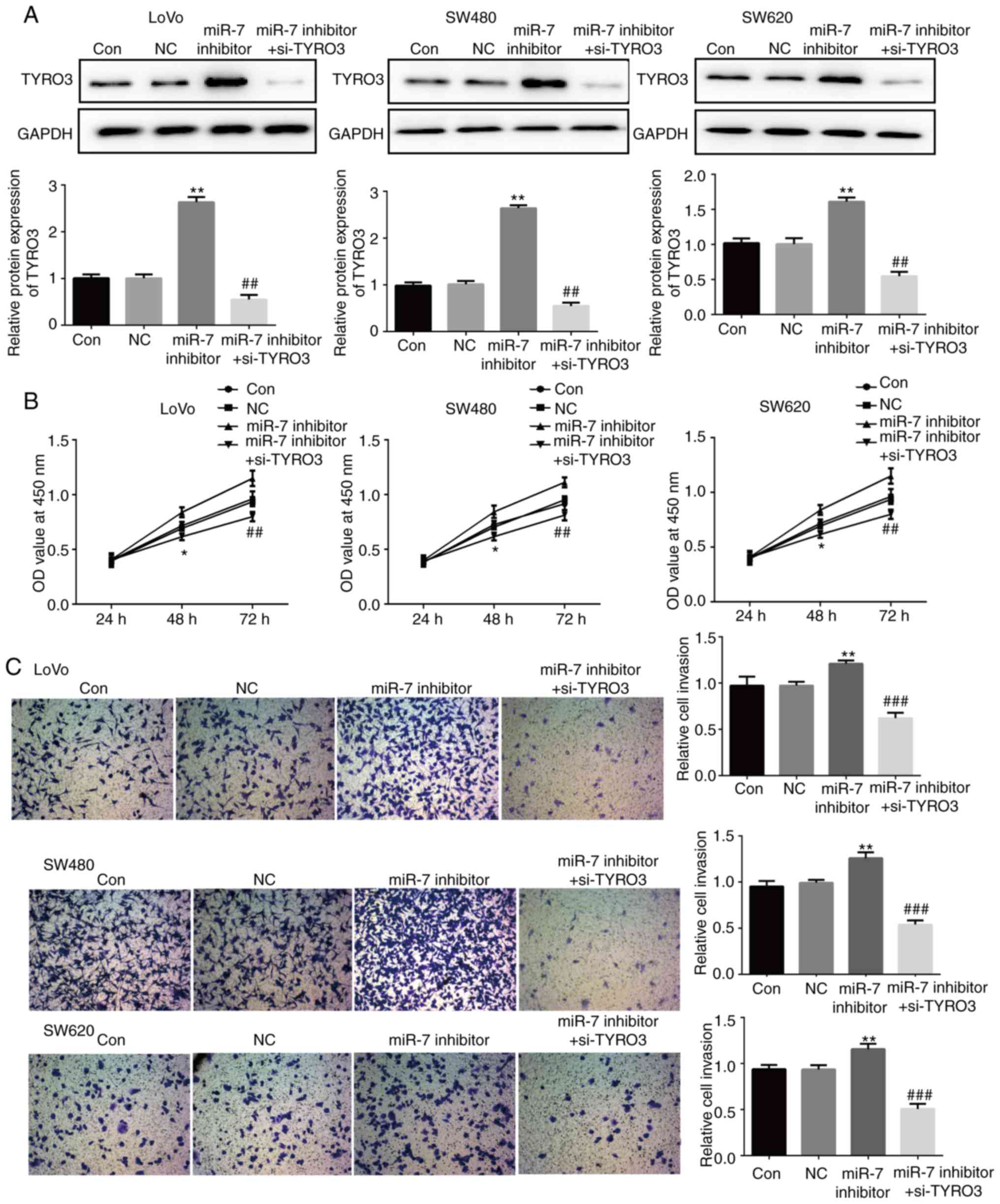 | Figure 8miR-7 downregulation promoted the
proliferation and invasion of colorectal cancer cells via directly
targeting TYRO3, while TYRO3 repressed the function of miR-7 in
these cells. (A) Western blot analysis was used to examine the
protein expression levels of TYRO3 in LoVo, SW480 and SW620 cells
transfected with NC or miR-7 inhibitor, or co-transfected with
miR-7 inhibitor and si-TYRO3. (B) MTT and (C) Transwell assays were
used to examine the cell proliferation and invasion, respectively,
of LoVo, SW480 and SW620 cells in the different groups. Data are
expressed as the mean ± standard deviation. *P<0.05
and **P<0.01, vs. control groups;
##P<0.01 and ###P<0.001, vs. miR-7
inhibitor. Con, Control; NC, inhibitor control; si-TYRO3, TYRO3
small interfering RNA; miR, microRNA. |
miR-7 inhibits the activation of PI3K/AKT
pathway by directly targeting TYRO3 in CRC cells
In order to understand the molecular mechanism of
miR-7 on the biological behavior of CRC cell lines, alterations in
the protein expression levels of p-PI3K, PI3K, p-AKT, AKT, p-mTOR
and mTOR in LoVo, SW480 and SW620 cells transfected with miR-7
inhibitor or with TYRO3-siRNA plus miR-7 inhibitor were assessed by
western blotting. As presented in Fig. 10A-C, compared with the control
group, the protein levels of p-PI3K, p-AKT and p-mTOR were
significantly increased in LoVo, SW480 and SW620 cells transfected
with miR-7 inhibitor, suggesting that the PI3K/AKT pathway was
activated. However, this effect was suppressed by co-transfection
with si-TYRO3 and miR-7 inhibitor. These results indicated that
miR-7 suppressed the activation of the PI3K/AKT/mTOR signaling
pathway via direct targeting of TYRO3 in CRC cells.
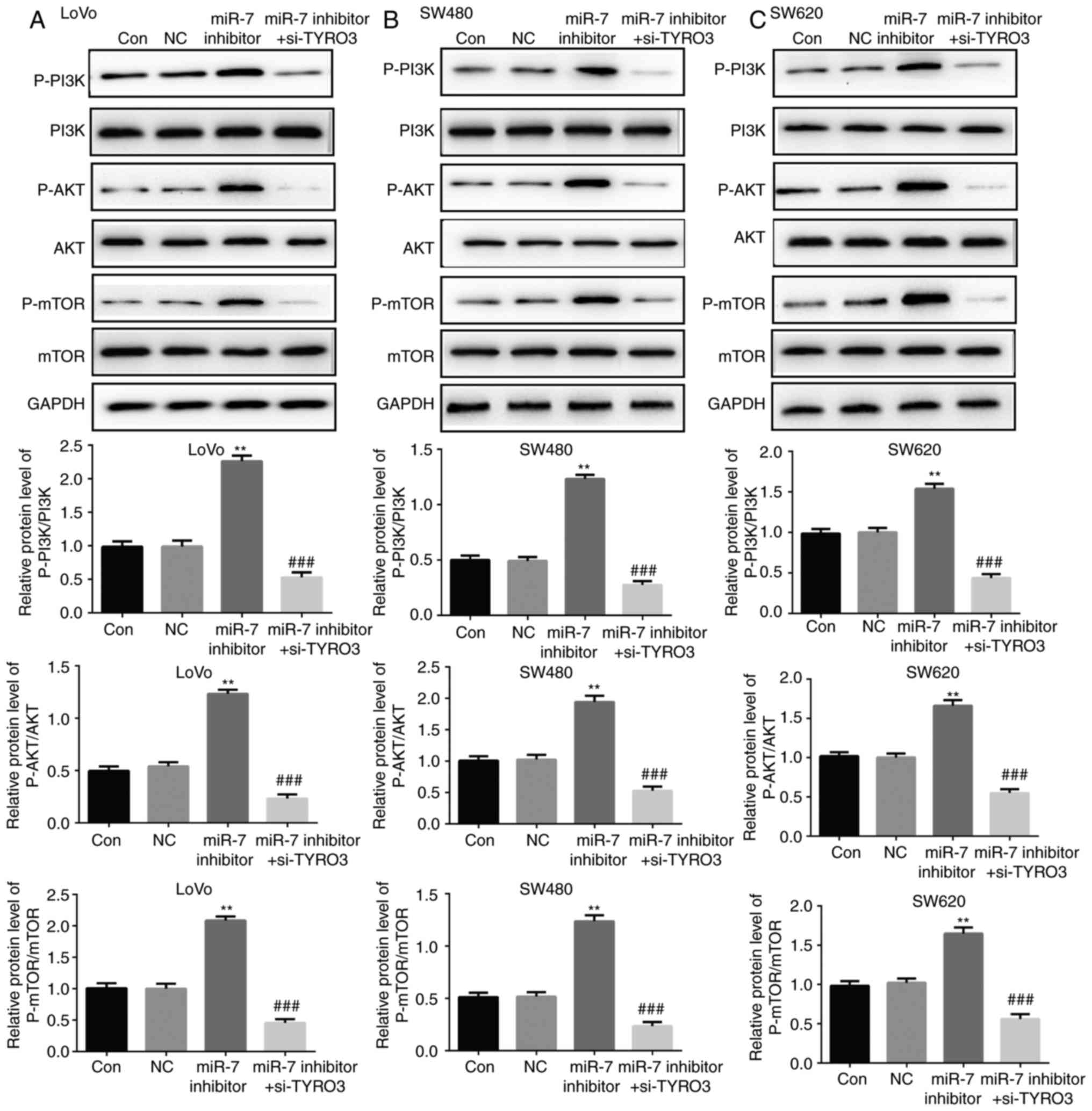 | Figure 10miR-7 downregulation promoted the
activation of PI3K/AKT/mTOR pathway via directly targeting TYRO3 in
colorectal cancer cells. Western blot analysis was used to examine
the protein expression levels of p-PI3K, PI3K, p-AKT, AKT, p-mTOR
and mTOR in (A) LoVo, (B) SW480 and (C) SW620 cells transfected
with NC or miR-7 inhibitor, or co-transfected with miR-7 inhibitor
and si-TYRO3. Data are expressed as the mean ± standard deviation.
**P<0.01, vs. control groups;
###P<0.01, vs. miR-7 inhibitor. miR, microRNA; PI3K,
phosphoinositide 3-kinase; AKT, protein kinase B; mTOR, mammalian
target of rapamycin; p-, phosphorylated; Con, Control; NC,
inhibitor control; si-TYRO3, TYRO3 small interfering RNA. |
Discussion
CRC is one of the most common types of cancer,
posing a serious societal and economic burden worldwide.
Accumulating evidence has suggested that miRNAs serve an important
role in CRC progression. In the present study, it was determined
that miR-7 was downregulated in CRC tissues and cell lines. In
addition, it was demonstrated that miR-7 inhibited the
proliferation, migration and invasion of CRC cells via TYRO3
through the inhibition of the PI3K/AKT/mTOR signaling pathway.
A previous study reported that elevated miR-7
expression was significantly correlated with tumor depth, venous
invasion, lymphatic invasion, lymph node metastasis and liver
metastasis (21). Previous
studies have also reported that miR-7 functions as a tumor
suppressor in several types of human cancer, including CRC
(11,22). High miR-7 expression was also
observed to be significantly associated with poor overall survival
in patients with CRC (P=0.010), while miR-7 expression was an
independent prognostic factor in these patients (hazard ratio,
1.854; 95% confidence interval, 1.016-3.540; P=0.044) (21). Thus, miR-7 functions as an
oncogene in CRC, and is significantly associated with tumor
progression and poor prognosis. In addition, multivariate analysis
indicated that low miR-7 expression was an independent prognostic
factor for poor survival (P=0.0430) (10). miR-7 was reported to be
downregulated in non-small cell lung cancer cell lines, and its
overexpression induces cell apoptosis and suppresses cell
proliferation, migration and tumorigenicity by targeting B-cell
lymphoma-2 (23). In addition, it
has been reported that miR-7 was downregulated in breast cancer,
and associated with epithelial-to-mesenchymal transition and
metastasis (24). Furthermore,
miR-7 inhibits CRC proliferation and induces apoptosis by targeting
X-ray repair cross complementing 2 (22). A miR-7, as a novel miRNA with
tumor suppressive function in CRC, was confirmed in nude mice to
induce apoptosis through regulation of the early cell-cycle
checkpoint (25). The present
study demonstrated that miR-7 was downregulated in CRC cells, and
that miR-7 overexpression inhibited CRC cell proliferation,
invasion and angiogenesis, which was consistent with the findings
of a previous study (25).
Bioinformatics analysis in the current study
predicted that TYRO3 is a potential direct target of miR-7, which
was also consistent with previous findings (16). It was thus suggested that miR-7
may function by interacting with TYRO3. To experimentally validate
this hypothesis, the expression of TYRO3 in human CRC cells was
initially examined. As expected, the results demonstrated that the
levels of TYRO3 expression in CRC cells were elevated and inversely
associated with miR-7 expression. Previous studies have reported
that TYRO3 is overexpressed in patients with CRC, as well as in
liver cancer metastases in comparison with normal liver (13,26). Furthermore, TYRO3 has previously
been identified as a novel target of miR-7 in CRC (16). It has been revealed that high
expression of TYRO3 in tumor tissues significantly reduced the
survival of patients with CRC (13). In the present study, the results
demonstrated that knockdown of TYRO3 with siRNA significantly
inhibited the proliferation, migration and invasion capacity of CRC
cells, indicating that TYRO3 expression was involved in CRC.
However, whether miR-7 exerted its tumor suppressor effects in CRC
by targeting TYRO3 has not been previously reported, to the best of
our knowledge. Therefore, the present study is the first to
demonstrate that TYRO3 repressed the inhibitory effects of miR-7 on
CRC cell proliferation, migration and invasion.
A previous study has reported that TYRO3 modulates
several oncogenic pathways, including those involved in survival,
altered cellular morphology, cell cycle transition, proliferation,
adhesion and motility (27). In
addition, it has previously been observed that TYRO3 binds to
growth-inhibitory specific 6 receptors through the PI3K/AKT/mTOR or
RAF/MEK/ERK1/2 pathways in order to control cell proliferation and
survival (28,29). TYRO3 has also been identified as a
key driver of the oncogenic PI3K/AKT pathway in hepatocellular
carcinoma (30). However, it
remained unclear whether miR-7 functions through interaction with
the TYRO3-dependent PI3K/AKT/mTOR signaling pathway. In the present
report, the results revealed that the inhibition of miR-7 markedly
increased the activity of PI3K/AKT signaling pathway in human CRC
cell lines, while this effect was inhibited by TYRO3 siRNA.
Therefore, the present study clarified a novel mechanism of miR-7
in regulating the proliferation, migration and invasion of CRC
cells. Future studies will aim to confirm this mechanism using
in vivo models.
In conclusion, the present study demonstrated that
miR-7 inhibited the proliferation, migration and invasion of CRC
cell lines via direct inhibition of TYRO3, which may be mediated by
the PI3K/AKT/mTOR signaling pathway. This suggested that TYRO3 and
miR-7 may serve as promising diagnostic and therapeutic targets in
CRC.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AQ wrote the manuscript and analyzed and interpreted
the data. WQ designed the study and revised the manuscript. Both
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Each patient provided written informed consent, and
all experimental protocols were approved by the Ethics Committee of
the Affiliated Suzhou Hospital of Nanjing Medical University
(Suzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Dr Yu Luo and Dr Hai
Huang (Department of General Surgery, The Affiliated Suzhou
Hospital to Nanjing Medical University) for collecting the data
reported in the article.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
3
|
Van Cutsem E, Cervantes A and Nordlinger
B: Metastatic colorectal cancer: ESMO Clinical Practice Guidelines
for diagnosis, treatment and follow-up. Ann Oncol. 25(Suppl 3):
iii1–iii9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Petri A, Lindow M and Kauppinen S:
MicroRNA silencing in primates: Towards development of novel
therapeutics. Cancer Res. 69:393–395. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Landi MT, Zhao Y, Rotunno M, Koshiol J,
Liu H, Bergen AW, Rubagotti M, Goldstein AM, Linnoila I, Marincola
FM, et al: MicroRNA expression differentiates histology and
predicts survival of lung cancer. Clin Cancer Res. 16:430–441.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Loo JM, Scherl A, Nguyen A, Man FY,
Weinberg E, Zeng Z, Saltz L, Paty PB and Tavazoie SF: Extracellular
metabolic energetics can promote cancer progression. Cell.
160:393–406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen WQ, Hu L, Chen GX and Deng HX: Role
of microRNA-7 in digestive system malignancy. World J Gastrointest
Oncol. 8:121–127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suto T, Yokobori T, Yajima R, Morita H,
Fujii T, Yamaguchi S, Altan B, Tsutsumi S, Asao T and Kuwano H:
MicroRNA-7 expression in colorectal cancer is associated with poor
prognosis and regulates cetuximab sensitivity via EGFR regulation.
Carcinogenesis. 36:338–345. 2015. View Article : Google Scholar
|
|
11
|
Li Y, Li Y, Liu Y, Xie P, Li F and Li G:
PAX6, a novel target of microRNA-7, promotes cellular proliferation
and invasion in human colorectal cancer cells. Dig Dis Sci.
59:598–606. 2014. View Article : Google Scholar
|
|
12
|
Ohashi K, Mizuno K, Kuma K, Miyata T and
Nakamura T: Cloning of the cDNA for a novel receptor tyrosine
kinase, Sky, predominantly expressed in brain. Oncogene. 9:699–705.
1994.PubMed/NCBI
|
|
13
|
Schmitz R, Valls AF, Yerbes R, von Richter
S, Kahlert C, Loges S, Weitz J, Schneider M, Ruiz de Almodovar C,
Ulrich A and Schmidt T: TAM receptors Tyro3 and Mer as novel
targets in colorectal cancer. Oncotarget. 7:56355–56370. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vouri M and Hafizi S: TAM receptor
tyrosine kinases in cancer drug resistance. Cancer Res.
77:2775–2778. 2017. View Article : Google Scholar
|
|
15
|
Martinelli E, Martini G, Cardone C,
Troiani T, Liguori G, Vitagliano D, Napolitano S, Morgillo F,
Rinaldi B, Melillo RM, et al: AXL is an oncotarget in human
colorectal cancer. Oncotarget. 6:23281–23296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kabir TD, Ganda C, Brown RM, Beveridge DJ,
Richardson KL, Chaturvedi V, Candy P, Epis M, Wintle L, Kalinowski
F, et al: A microRNA-7/growth arrest specific 6/TYRO3 axis
regulates the growth and invasiveness of sorafenib-resistant cells
in human hepatocellular carcinoma. Hepatology. 67:216–231. 2018.
View Article : Google Scholar
|
|
17
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chaturvedi MM, Sung B, Yadav VR, Kannappan
R and Aggarwal BB: NF-κB addiction and its role in cancer: 'One
size does not fit all'. Oncogene. 30:1615–1630. 2011. View Article : Google Scholar
|
|
19
|
Thorson AG, Christensen MA and Davis SJ:
The role of colonoscopy in the assessment of patients with
colorectal cancer. Dis Colon Rectum. 29:306–311. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nagano Y, Toiyama Y, Okugawa Y, Imaoka H,
Fujikawa H, Yasuda H, Yoshiyama S, Hiro J, Kobayashi M, Ohi M, et
al: MicroRNA-7 is associated with malignant potential and poor
prognosis in human colorectal cancer. Anticancer Res. 36:6521–6526.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu K, Chen Z, Qin C and Song X: miR-7
inhibits colorectal cancer cell proliferation and induces apoptosis
by targeting XRCC2. Onco Targets Ther. 7:325–332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiong S, Zheng Y, Jiang P, Liu R, Liu X
and Chu Y: MicroRNA-7 inhibits the growth of human non-small cell
lung cancer A549 cells through targeting BCL-2. Int J Biol Sci.
7:805–814. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang H, Cai K, Wang J, Wang X, Cheng K,
Shi F, Jiang L, Zhang Y and Dou J: MiR-7, inhibited indirectly by
lincRNA HOTAIR, directly inhibits SETDB1 and reverses the EMT of
breast cancer stem cells by downregulating the STAT 3 pathway. Stem
Cells. 32:2858–2868. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang N, Li X, Wu CW, Dong Y, Cai M, Mok
MT, Wang H, Chen J, Ng SS, Chen M, et al: microRNA-7 is a novel
inhibitor of YY1 contributing to colorectal tumorigenesis.
Oncogene. 32:5078–5088. 2013. View Article : Google Scholar
|
|
26
|
Chien CW, Hou PC, Wu HC, Chang YL, Lin SC,
Lin SC, Lin BW, Lee JC, Chang YJ, Sun HS and Tsai SJ: Targeting
TYRO3 inhibits epithelial-mesenchymal transition and increases drug
sensitivity in colon cancer. Oncogene. 35:5872–5881. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu S, Wurdak H, Wang Y, Galkin A, Tao H,
Li J, Lyssiotis CA, Yan F, Tu BP, Miraglia L, et al: A genomic
screen identifies TYRO3 as a MITF regulator in melanoma. Proc Natl
Acad Sci USA. 106:17025–17030. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gustafsson A, Boström AK, Ljungberg B,
Axelson H and Dahlback B: Gas6 and the receptor tyrosine kinase Axl
in clear cell renal cell carcinoma. PLoS One. 4:e75752009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ou WB, Corson JM, Flynn DL, Lu WP, Wise
SC, Bueno R, Sugarbaker DJ and Fletcher JA: AXL regulates
mesothelioma proliferation and invasiveness. Oncogene.
30:1643–1652. 2011. View Article : Google Scholar
|
|
30
|
Zhong Z, Wang Y, Guo H, Sagare A,
Fernández JA, Bell RD, Barrett TM, Griffin JH, Freeman RS and
Zlokovic BV: Protein S protects neurons from excitotoxic injury by
activating the TAM receptor Tyro3-phosphatidylinositol 3-kinase-Akt
pathway through its sex hormone- binding globulin-like region. J
Neuroscience. 30:15521–15534. 2010. View Article : Google Scholar
|