Introduction
Polymeric conjugates of conventional drugs, a
conjugation of a drug with a polymer, have several advantages over
their low molecular weight precursors. Polymer-drug conjugates can
increase the solubility of low solubility or insoluble drugs,
enhancing drug bioavailability (1-3).
In addition, polymeric conjugates can provide passive and/or active
targeting of the drug specifically to the site of its action
leading to a reduction in antigenic activity of the drug (4-7).
Polymer-drug conjugates have introduced a new era of polymeric drug
delivery systems with these advantages over the free form of a
drug.
Several polymers with desirable biological
properties have been employed as a drug delivery platform. Poly
(β-malic acid), referred to as PMLA, which is an aliphatic
polyester based on malic acid, has a preferable biodegradability
and lack of toxicity. PMLA can be degraded into malic acid, an
intermediate of tricarboxylic acid cycle, and subsequently degraded
into water and CO2 (8,9).
PMLA has free pendant carboxyl groups on each monomer, which can be
covalently bound to small-molecule drugs and other functional
moieties (10-12). Multi-modification makes PMLA an
efficient carrier, making it well-suited as a scaffold for tailored
nanoconjugate chemistry.
Polymeric-drug conjugates based on PMLA have been
examined over the course of the last two decades (13). Ljubimova et al (14) synthesized a targeted polymeric
delivery system based on PMLA, named Polycefin, which is
constructed of antisense oligonucleotides targeting Laminin-8 and
monoclonal anti-transferrin receptor antibody, to target brain
tumors and breast cancer. Polycefin was found to accumulate in
U87MG brain tumor tissue and had no toxic effects on normal tissue
(14-16). Controlled molecular weight PMLA
was synthesized with a high yield in our previous study, and the
antitumor agent 10-hydroxycamptothecin was attached to PMLA in
order to enhance its water solubility and bioavailability (17,18).
To further improve delivery efficiency and
cancer-targeting specificity, environment-responsive nanoconjugates
have been developed by employing external stimuli, including
pH-responsiveness. The application of pH-sensitivity is based on
the fact that increased aerobic glycolysis in cancer cells leads to
a lower extracellular pH of cancer cells (pH 6.5-7.2) than that in
normal tissues (19,20). In addition, once taken up by
cells, drug carriers experience a gradient pH moving from endosomes
(pH 5.0-6.0) to lysosomes (pH 4.0-4.5) (21-23). One approach is to introduce
cleavable bonds, which can be broken to release the drugs
conjugated to or encapsulated in the carrier (24-26). In the case of polymer-drug
conjugates, pH-sensitive linkages, including hydrazone, hydrazide
and acetal linkages, have been used to directly attach drug
molecules to polymers (19,27–29).
In the case of polymer-drug conjugates, pH-sensitive
linkages, including hydrazone, hydrazide, and acetal linkages, have
been used to directly attach drug molecules to polymers (24). Based on our previous study, a
pH-triggered drug release profile based on PMLA,
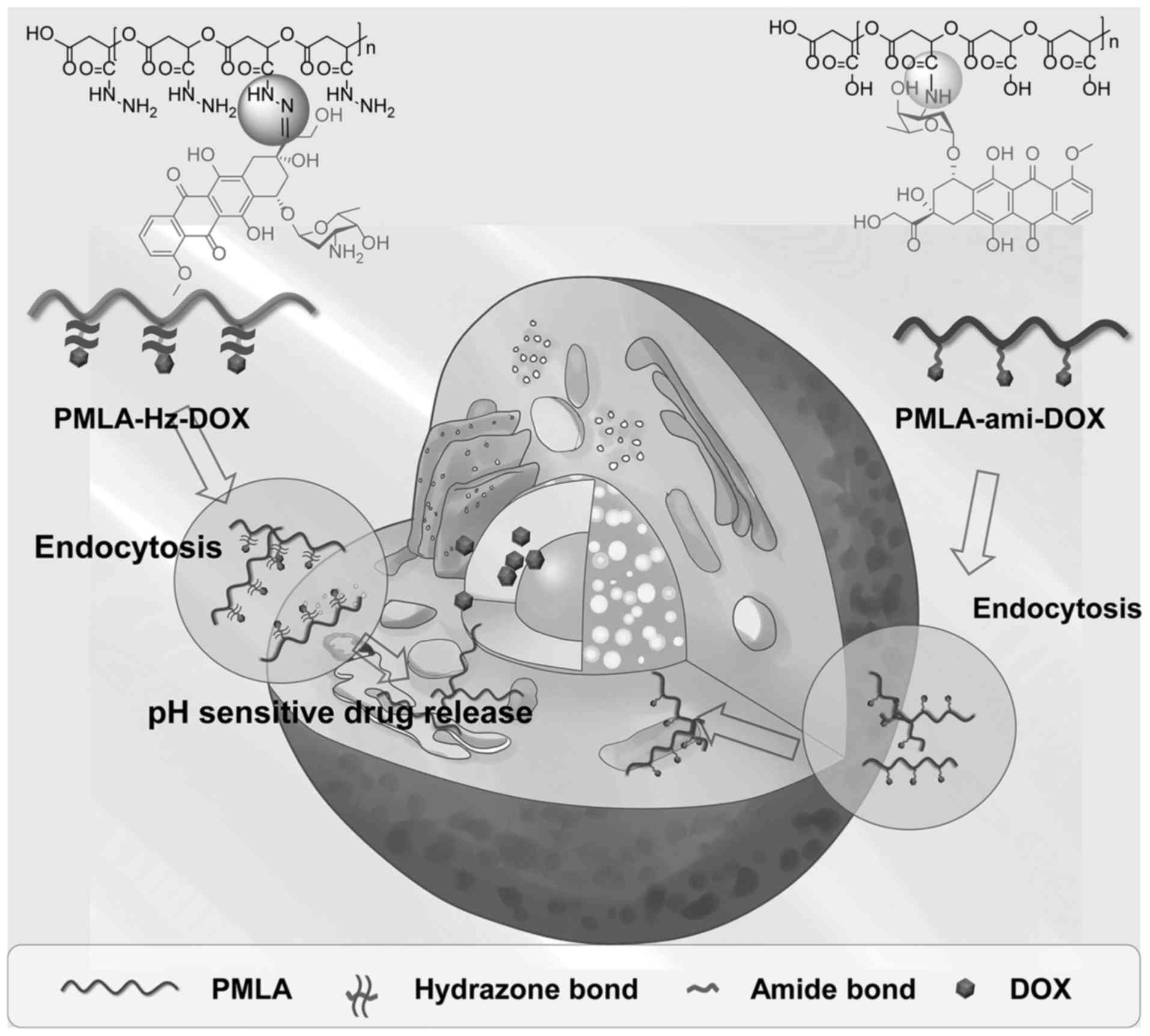
PMLA-Hz-doxorubicin (DOX), was examined in the present study. The
anticancer drug DOX was chemically attached to the polymer backbone
via a pH-responsive hydrazone bond. By contrast, another conjugate
linking DOX via an amide bond was also prepared. The polymeric
conjugation protected DOX from being released during systemic
circulation, however, once the polymeric drug was internalized by
the cancer cells and taken up by the endo/lysosomes, the hydrazone
linkage was ruptured due to the acidic microenvironment and DOX was
released from the PMLA backbone (Fig.
1). It is anticipated that the pH-triggered release of the
nanoconjugate may enhance the drug delivery efficiency and offer a
promising therapeutic outcome.
Materials and methods
Materials
L-aspartic acid and trifluoroacetic anhydride were
obtained from Aladdin Chemical Co., Ltd. (Shanghai, China)
Doxorubicin hydrochloride (DOX·HCl) was purchased from HVSF United
(Beijing, China). 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide
hydrochloride (EDC·HCl) and N-hydroxysuccinimide (NHS) were
purchased from TCI (Shanghai, China). HPLC-grade acetonitrile and
methanol were purchased from Merck KGaA (Darmstadt, Germany).
Minimum Eagle's medium (MEM) and trypsin were purchased from
HyClone; GE Healthcare Life Sciences (Logan, UT, USA) and Gibco;
EMD Millipore (Billerica, MA, USA), respectively. The Cell Counting
Kit-8, fetal bovine serum (FBS) and 4',6-diamidino-2-phenylindole
(DAPI) were purchased from ZETA life (Shanghai, China). All other
chemicals used in the present study were obtained from Sinopharm
Chemical Reagent Co., Ltd. (Shanghai, China), and all reagents were
of analytical grade without further purification.
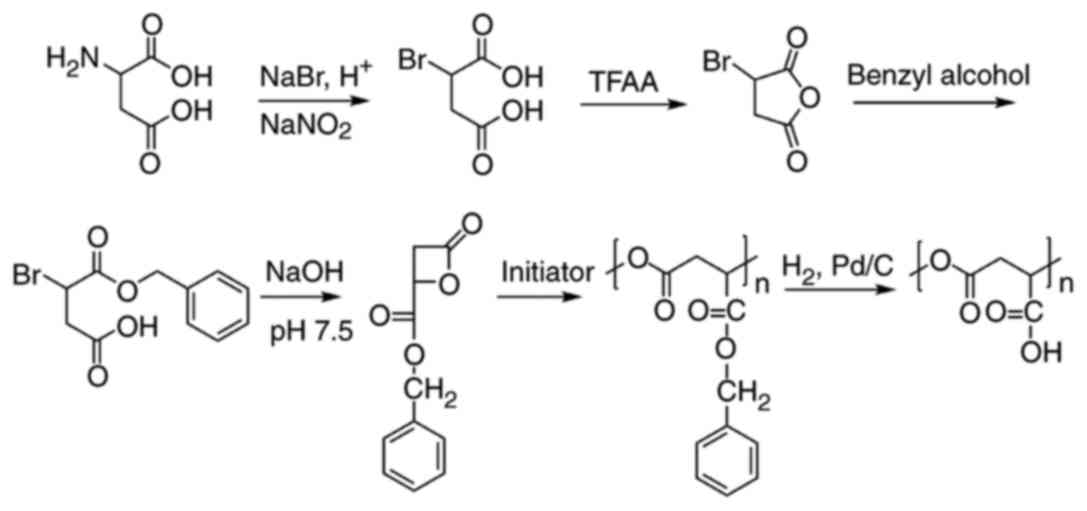
Synthesis of PMLA
PMLA was synthesized as described previously by
ring-opening polymerization, starting from L-aspartic acid
(17,18). PMLA with a molecular weight of 10
kDa was used in the present study, which is a suitable molecular
weight for drug conjugates. The yield of PMLA was 5.6%. The
synthetic route for PMLA is illustrated in Fig. 2.
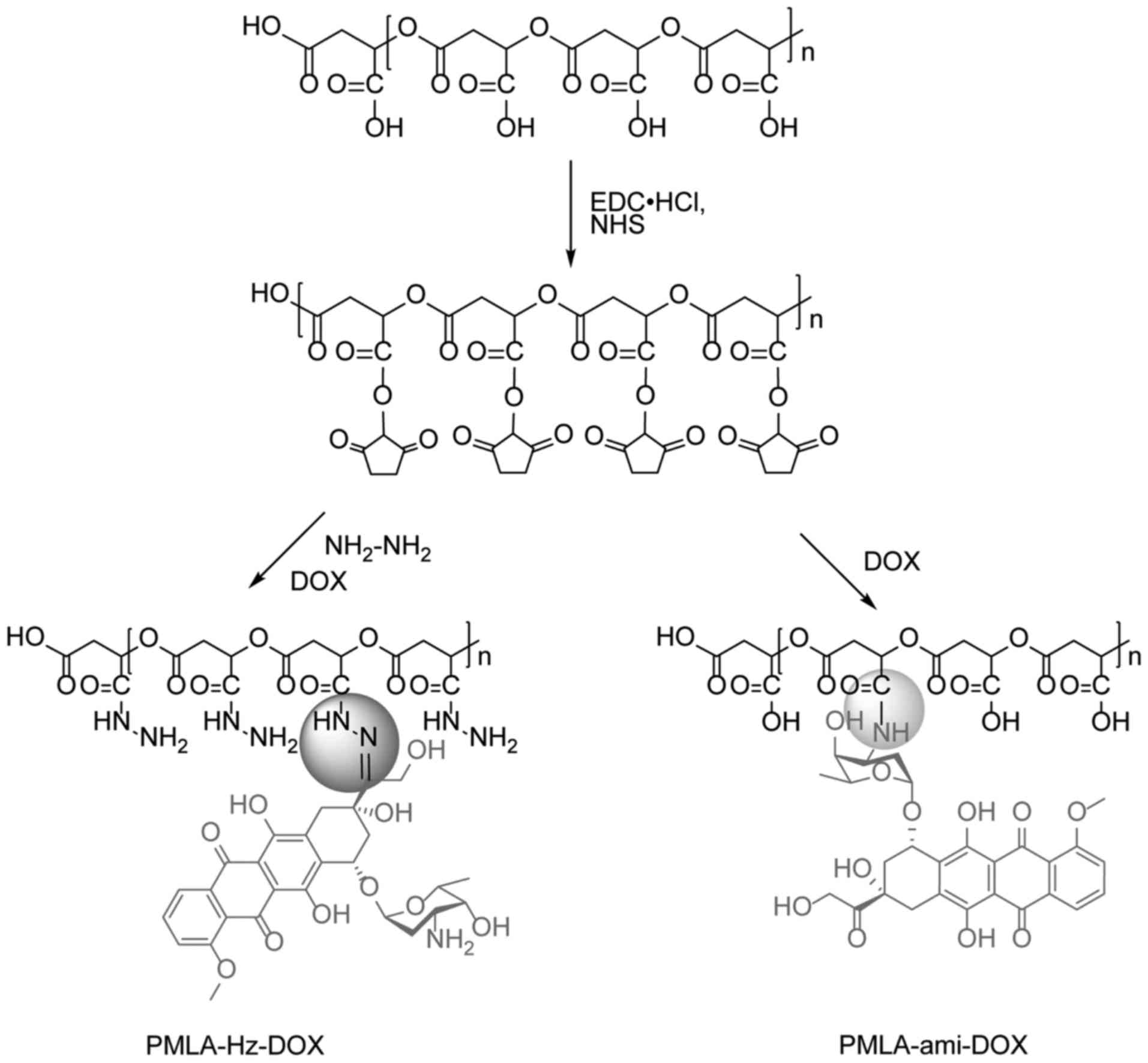
Preparation of PMLA-Hz-DOX and
PMLA-ami-DOX
PMLA (116 mg, 1 mmol with regard to repeat units),
NHS (1 mmol) and EDC·HCl (1.5 smmol) were dissolved in 15 ml of
DMSO and stirred at room temperature for 6 h to activate the
carboxyl group. Subsequently, 2 mmol N2H4·H2O
was added. After 6 h, 0.2 mmol DOX was added to the solution and
stirred at room temperature for another 6 h in the dark. The
solution was dialyzed (MWCO: 5 kDa) against DMSO for 24 h to remove
unreacted and produced small molecules and then dialyzed (MWCO: 5
kDa) against deionized water at 4˚C in the dark for 24 h. Finally,
the solution was lyophilized to yield PMLA-Hz-DOX as red powder,
the yield was 64.5%.
The carboxyl group was activated, as above, and then
0.2 mmol DOX was added and stirred at room temperature overnight in
the dark. The solution was dialyzed (MWCO: 5 kDa) against DMSO for
24 h to remove unreacted regent and produced small molecules and
then dialyzed (MWCO: 5 kDa) against deionized water at 4˚C in the
dark for 24 h. Finally, the solution was lyophilized to yield
PMLA-ami-DOX as red powder with a yield of 76.6%. The synthetic
routes for these two conjugates are illustrated in Fig. 3.
UV-vis spectroscopy (Shimadzu Corporation, Tokyo,
Japan) was used to quantify the quantity of DOX conjugated on the
polymers. Briefly, the DOX-conjugated polymers were dissolved in
deionized water and the absorbance of the solutions at 254 nm was
measured. Using a calibration curve obtained by measuring the
absorbance of different concentrations of free DOX in deionized
water at 254 nm, the DOX content in the polymer was calculated. The
DOX loading rate was calculated follows: DOX loading rate
(%)=(weight of DOX in conjugate/weight of conjugate) x100%; and the
conjugating rate was calculated as follows: Conjugating rate
(%)=(weight of DOX in conjugate/weight of DOX input) x100%.
Characterization of PMLA-Hz-DOX and
PMLA-ami-DOX
The chemical structures of the synthesized
PMLA-Hz-DOX and PMLA-ami-DOX were determined by FT-IR using a KBr
disc (Shimadzu FTIR-8400S) and 1H NMR using Varian 400
mHz spectrometer (Avance, Bruker Corporation, Billerica, MA, USA).
The samples were prepared from freeze-dried products by dissolving
in DMSO-d6 (20 mg/ml) at 25˚C. Chemical shifts were determined in δ
units relative to the tetramethyl silane signal as an internal
reference.
The ζ potential of these two conjugates was
determined by dynamic light scattering (DLS) following dissolving
in water.
In vitro release of DOX from the
conjugates
The in vitro drug release profiles were
obtained by a dynamic dialysis method (30). The release experiments were
performed at 37˚C. Typically, the conjugate solution of 200
µg/ml equivalent DOX concentration was dialyzed into 100 ml
of 0.1 M phosphate-buffered saline (PBS; pH 5.6, 6.0, 6.8, and 7.4)
with magnetic stirring at 200 rpm. At hourly intervals, 0.5 ml was
removed from the release medium for each sample, and the same
volume and temperature of PBS was added to the release medium. The
released DOX was determined by HPLC (Agilent 1260), according to
Chinese Pharmacopoeia 2015 (ChP 2015) (31). The results of the triplicate tests
were used to calculate the accumulated drug release.
In vitro cytotoxicity
HT1080 fibrosarcoma cells, which were provided by
Shanghai Zhongqiaoxinzhou Biotechnology Co., Ltd. (Shanghai,
China), were used as in vitro models. The cells were
cultured in MEM containing 10% heat-activated FBS and 100 IU/ml
penicillin and 100 µg/ml streptomycin. They were incubated
in a 37˚C water-jacketed incubator equilibrated with 5%
CO2 and maintained at ~99% relative humidity. The medium
was replenished every other day until confluence was achieved. The
cells were then washed with PBS and harvested with 0.125%
trypsin-EDTA solution.
The HT1080 fibrosarcoma cells were seeded at a
density of 5x104 cells/well in a 96-well transparent
plate and incubated for 24 h. All growth medium was prepared by
supplementing MEM with 10% FBS and sterilized with a 0.2 µm
filter prior to use. The medium was then replaced with PMLA-Hz-DOX,
PMLA-ami-DOX or free DOX at various drug concentrations (0.005,
0.01, 0.05, 0.1, 0.5 and 1 µg/ml) in medium at pH 6.0 or
7.4. The cells were then incubated for another 48 h prior to
replacing the medium with 0 1 ml of fresh growth medium containing
10% CCK-8. Following incubation for another 2 h, the plates were
vigorously shaken prior to measuring the relative color intensity
at 450 nm using a microplate reader. Cell viability was determined
as a percentage of the intensity of the controls ± standard
deviation. Each experiment was repeated five times at each polymer
concentration.
The cell viability with PMLA alone was also
investigated at various concentrations equivalent to those for the
conjugate.
Confocal laser scanning microscopy (CLSM)
observation
The HT1080 cells were maintained in a 6-well plate
at 5x105 cells/well for 24 h and treated with
PMLA-Hz-DOX, PMLA-ami-DOX or free DOX for 2 h at 37˚C. Following
incubation, the cells were washed with PBS three times to remove
excess conjugates. The concentration of DOX was 5 µg/ml. The
cells were the washed with PBS three times, fixed in precooled 4%
paraformaldehyde for 20 min, and stained with DAPI nuclear stain (5
µg/ml) for 5 min at 4˚C in the dark. The cells were washed
with PBS, and fluorescent images of cells were analyzed using an
FV1000 confocal microscope (Olympus Corporation, Tokyo, Japan).
Flow cytometric analysis
As described in detail previously (32), flow cytometry was used to confirm
the uptake of conjugates by HT1080 cells. Similar to the confocal
study, the HT1080 cells were seeded in a 6-well plate at
5x105 cells/well for 24 h and treated with PMLA-Hz-DOX,
PMLA-ami-DOX or free DOX for 2 h at 37˚C. Following incubation, the
cells were washed with PBS three times. The cells were then
harvested by trypsinization, centrifuged at 352 x g for 5 min, at
4˚C, resuspended in 500 µl PBS medium and examined by flow
cytometry using a FACScan instrument (BD Biosciences, Franklin
Lakes, NJ, USA).
Statistical analysis
All experiments were performed in triplicate and the
obtained data were processed using GraphPad Prism 7 (GraphPad
Software, Inc., La Jolla, CA, USA) and Origin 2018 (OriginLab,
Northampton, MA, USA) software. Differences between the two groups
were analyzed using the unpaired t-test in these software programs.
P<0.05 was considered to indicate a statistically significant
difference.
Results and Discussion
Drug loading and conjugation rate of
PMLA-Hz-DOX and PMLA-ami-DOX
The DOX loading rates of the PMLA-Hz-DOX and
PMLA-ami-DOX conjugates were 20.09±2.64 and 19.13±3.30 wt%,
respectively, and the DOX conjugating rates were 69.28±9.11 and
65.96±11.39%, respectively. A large number of suspended carboxyl
groups on the PMLA backbone makes it a high drug loading carrier
compared with other polymers, including PEG, PLA, PCL and other
polyesters that have no active groups on the main chains, meaning
drugs may only be encapsulated with a loading rate of <5%
(1,2).
Characterization of PMLA-Hz-DOX and
PMLA-ami-DOX
In PMLA-Hz-DOX, the carbonyl group at the C-13
position of DOX reacts with the hydrazide of the PMLA side chain to
form a hydrazone bond whereas the amino group at the 3' position of
the DOX pyran ring in PMLA-ami-DOX forms an amide bond with the
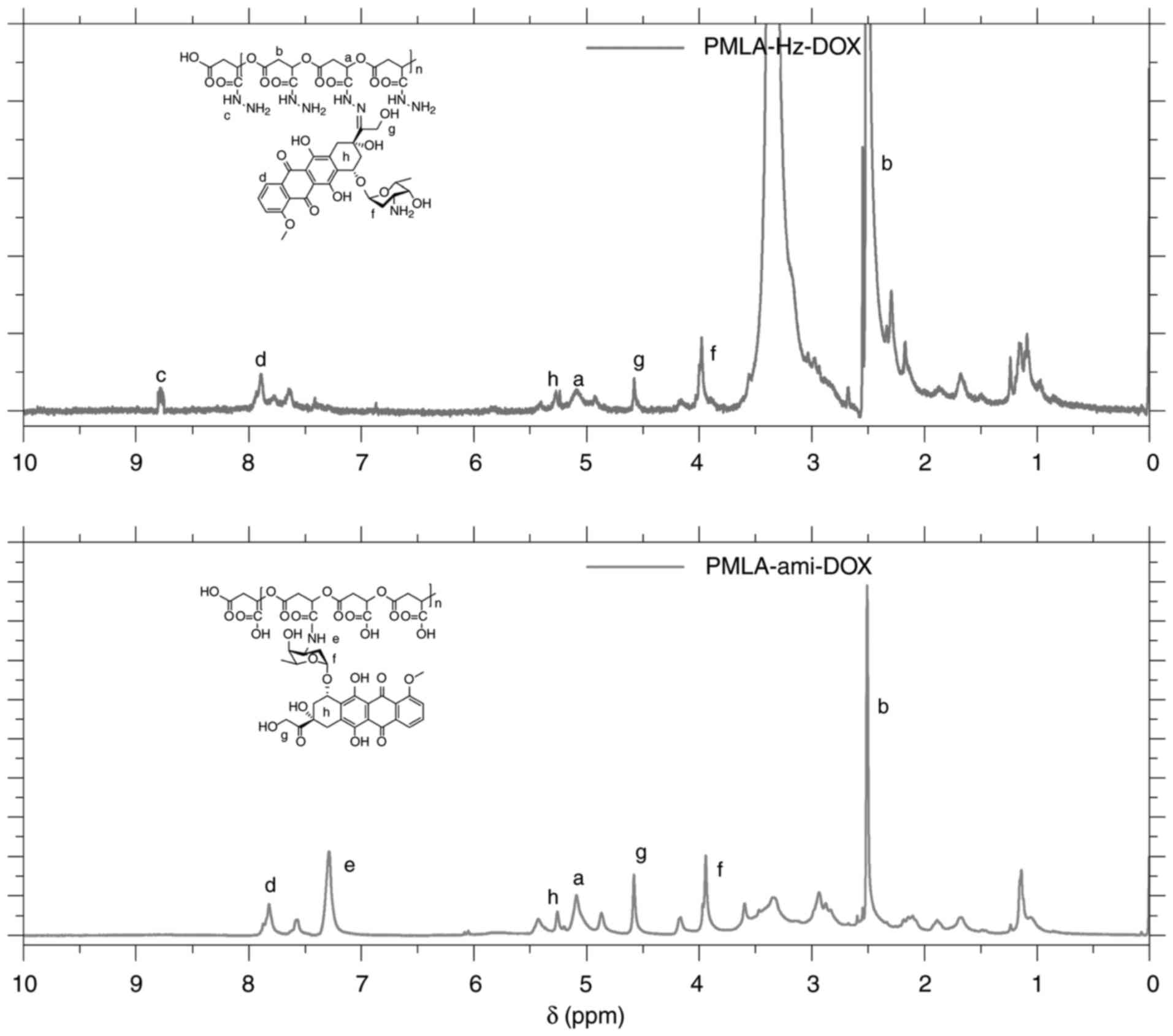
carboxyl group on PMLA. 1H NMR in DMSO-d6 was
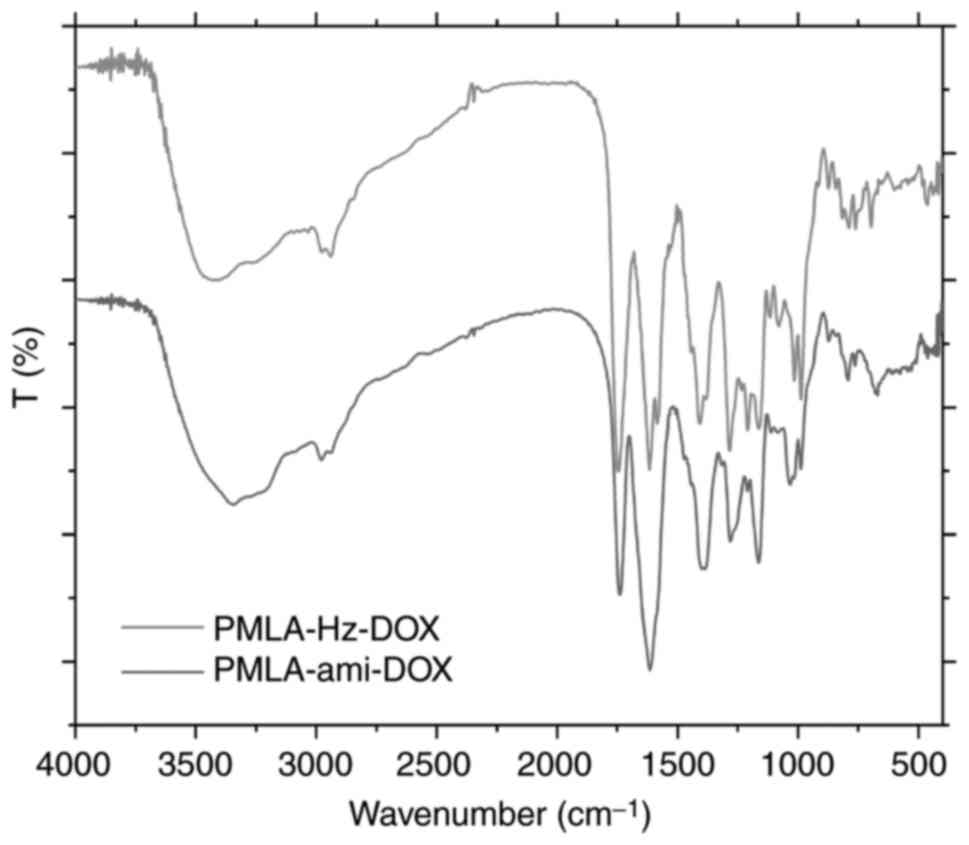
used to confirm the product identity. The IR spectra of these two
conjugates are shown in Fig. 4.
IR (v, cm-1): 1,585 (-NH2, δ), 1,381
(C-N, st). The peak close to 1,540 cm-1 in the IR
spectrum of PMLA-Hz-DOX was δNH (-CO-NH-). The
1H-NMR spectrum of PMLA-Hz-DOX and PMLA-ami-DOX
structures are shown in Fig. 5,
in which the peaks have been identified and marked. The peaks at
different positions of a-h correspond to different protons on
DOX.
The ζ potential of these two conjugates was
determined by DLS (Table I). PMLA
without the DOX connection had negative ζ potential and, once DOX
was conjugated, it showed positive ζ potential. The ζ potential of
PMLA-Hz-DOX was 20.25±0.36 mV due to the modification of the amino
group on the carboxyl group and the introduction of DOX, whereas
the ζ potential of PMLA-ami-DOX was 10.57±0.42 mV. The positively
charged nanoconjugates effectively interact with the negatively
charged cell membrane by electrostatic attraction, triggering
efficient cell internalization (33,34).
 | Table Iζ potential of PMLA-ami-DOX and
PMLA-Hz-DOX. |
Table I
ζ potential of PMLA-ami-DOX and
PMLA-Hz-DOX.
| Conjugate | ζ potential
(mV) |
|---|
| PMLA-Hz-DOX | 20.25±0.36 |
| PMLA-ami-DOX | 10.57±0.42 |
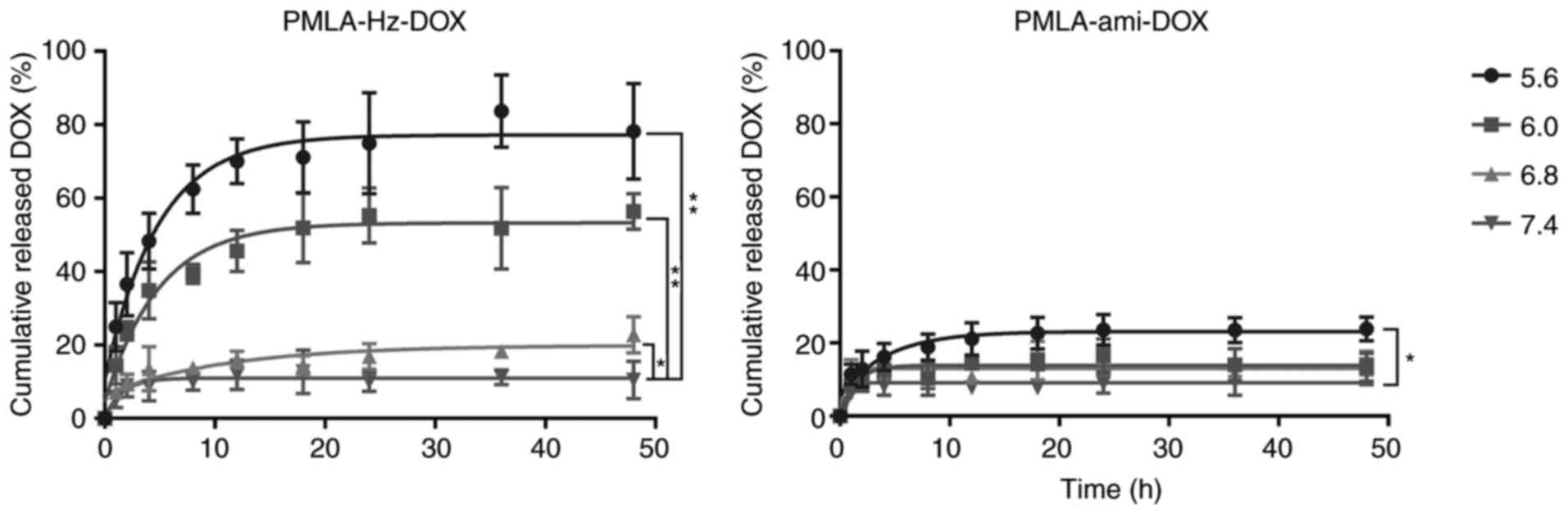
In vitro release of DOX from the
conjugates
The in vitro release of the drug from
PMLA-Hz-DOX and PMLA-ami-DOX conjugates was measured at various pH
conditions. As shown in Fig. 6,
the DOX release showed no marked initial burst in PMLA-Hz-DOX. It
was, however, significantly pH-dependent; the lower the pH was, the
faster the drug released. Specifically, the drug released rapidly
from the PMLA-Hz-DOX conjugate at pH 5.6 and pH 6.0, reaching 70.0
and 54.9%, respectively, at 24 h, whereas DOX release at pH 6.8 and
pH 7.4 was markedly slower at 16.87 and 9.93% in the same period,
respectively. For PMLA-ami-DOX, DOX release was slower than that of
PMLA-Hz-DOX, and there was no pH dependence. Using this polymeric
drug design, the conjugates can stably preserve drugs under
physiological conditions and selectively degrade and release them
by responding to the tumor extracellular pH (pHe),
endosomes (pH 5-6) or lysosomes (pH 4-5) (25,35,36). The in vitro drug release
experiments showed that the release of DOX was pH-dependent. When
the conjugate reached the tumor tissue, DOX was released, and the
free and grafted DOX entered the tumor cells by diffusion or
endocytosis (Fig. 1). In the
tumor cell endosome, in which pH was as low as 4-5, DOX release was
increased, although the release rate of DOX in tumor tissues
requires further improvement.
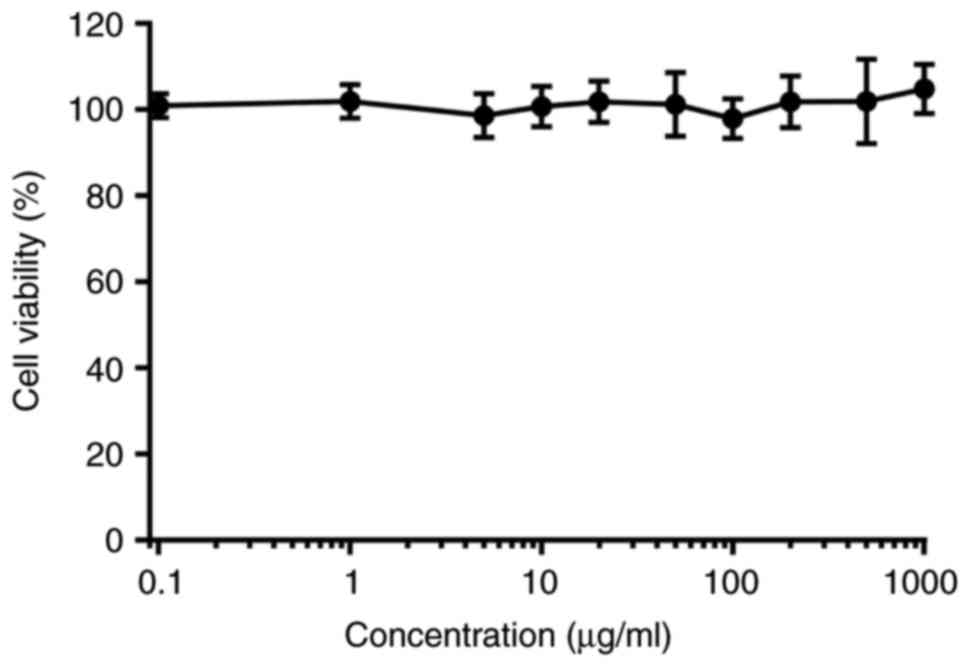
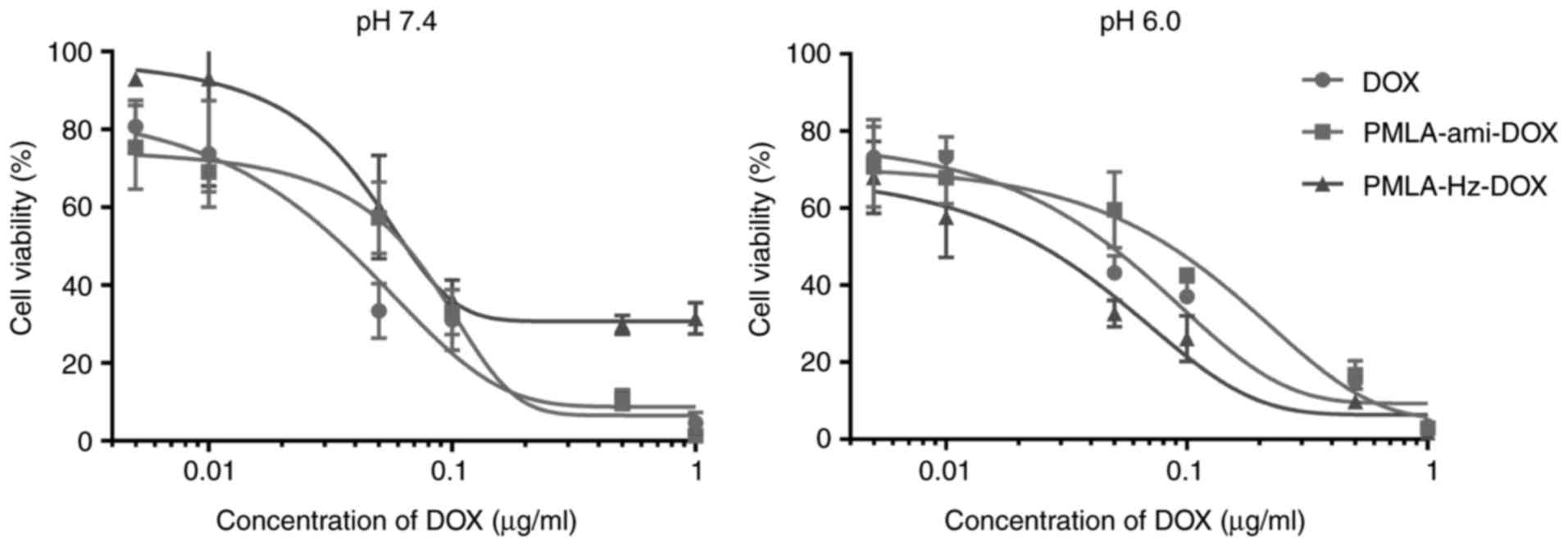
In vitro cytotoxicity
The in vitro antitumor activities of various
DOX formulations were evaluated using a CCK-8 assay of HT1080
fibrosarcoma cells, and the 50% inhibitory concentration
(IC50) was determined. PMLA did not show cytotoxicity up
to a concentration of 1 mg/ml, making it safe to use as a drug
carrier (Fig. 7). The in
vitro cytotoxicity at different pH values exhibited
pH-dependent cytotoxic effects (Fig.
8). At pH 7.4, the two conjugates showed lower IC50
values than free DOX, suggesting that DOX grafted to PMLA is likely
to have lower side effects in systemic circulation. At pH 6.0, the
pH of cell endosomes, PMLA-Hz-DOX had a higher cell cytotoxicity
(IC50=0.026 µg/ml); however, the cell viability
of PMLA-ami-DOX was markedly higher (IC50= 0.31
µg/ml) (P<0.05). According to the structure-activity
association of DOX, the carbonyl of C-13 may interact with the DNA
double helix by hydrogen bond, which is crucial for maintaining its
activity. Following linking to PMLA via a hydrazone bond, the
antitumor activity reduced. These results suggested that the
cytotoxicity of polymeric conjugates against HT1080 cells was lower
than free DOX under physiological pH.
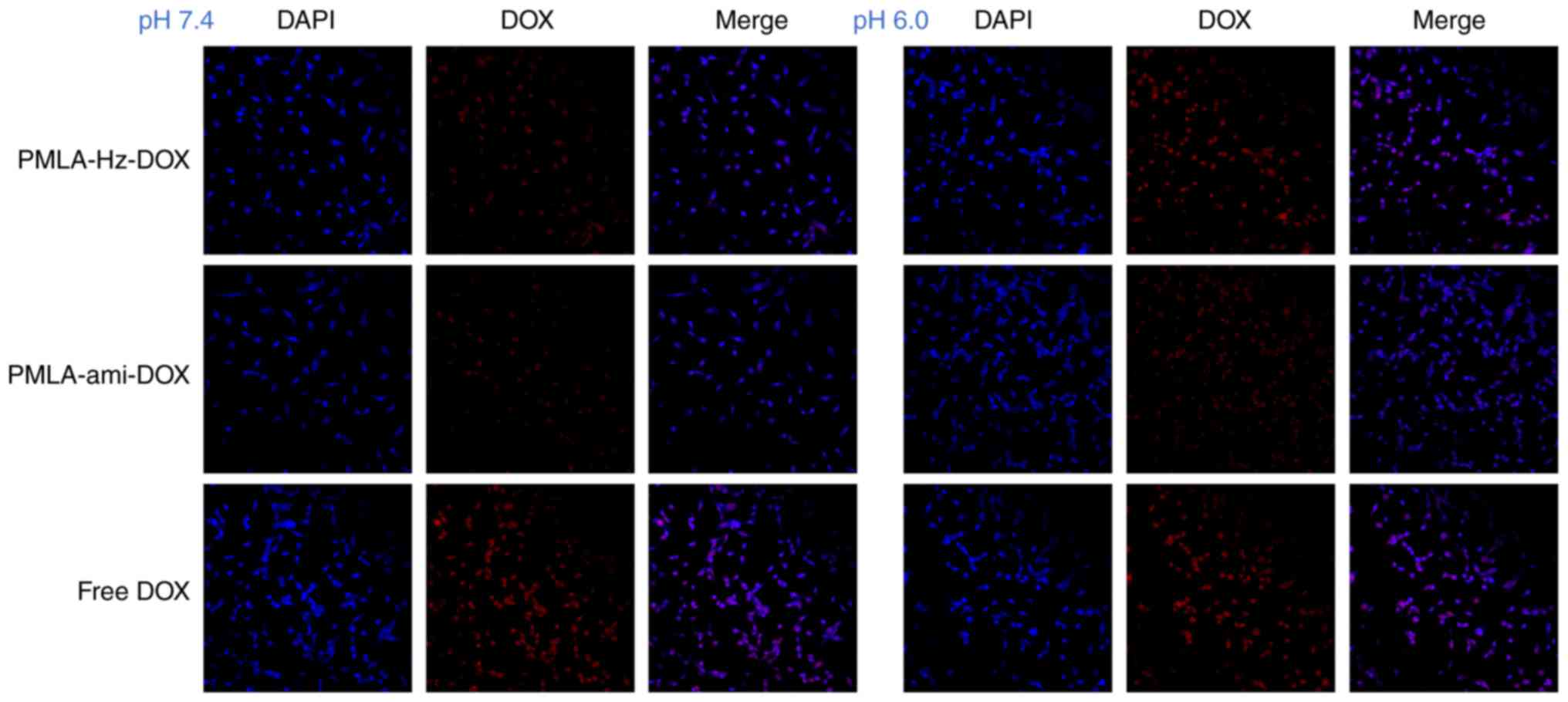
Cellular uptake measured by CLSM and flow
cytometry
CLSM and flow cytometry were performed to
investigate the effects of pH-sensitivity on the cellular uptake of
nanoconjugates. As shown in Fig.
9, the PMLA-ami-DOX conjugate showed the weakest intracellular
fluorescence of DOX at pH 7.4 and 6.0, indicating the DOX grafted
to PMLA via an amide bond had reduced cell uptake. Compared with
PMLA-ami-DOX, PMLA-Hz-DOX showed higher intracellular fluorescence
of DOX at pH 6.0 than that at pH 7.4, suggesting that PMLA-Hz-DOX
was more efficient following uptake by cells.
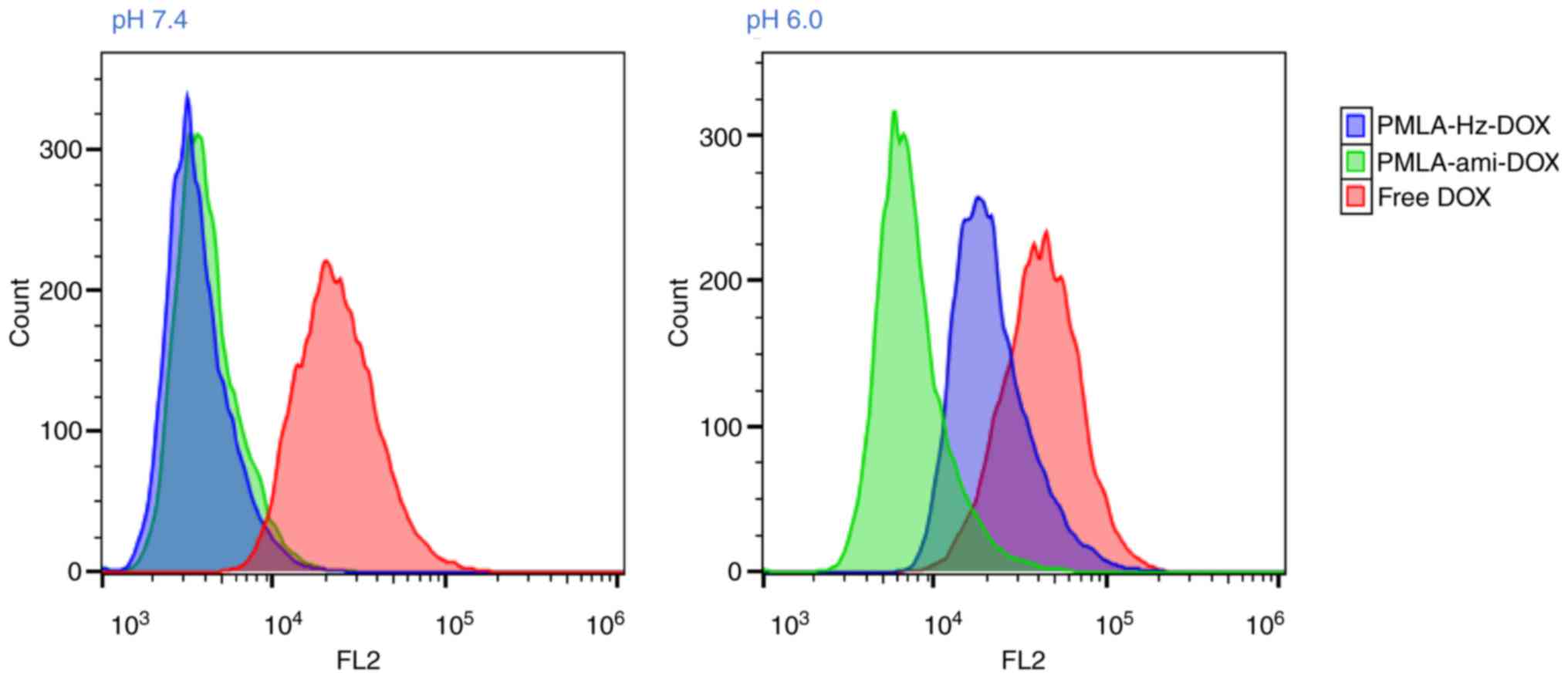
Flow cytometry was used to further examine the
cellular uptake of the DOX-loaded nanoconjugates. As shown in
Fig. 10, following incubation
with HT1080 cells for 2 h, the two nanoconjugates showed relatively
low cell internalization at pH 7.4. When the pH decreased to 6.0,
PMLA-ami-DOX showed similar cellular uptake, however, the cellular
uptake of PMLA-Hz-DOX was increased (P<0.05). These results were
consistent with the CLSM analysis.
PMLA has preferable biocompatibility and
biodegradability and is non-toxic, therefore, it is considered a
promising drug carrier material. In the present study, the
antitumor drug DOX was attached to PMLA via a hydrazine bond to
obtain a pH-sensitive drug delivery conjugate. Drug release
experiments at different pH conditions showed that the release of
DOX was pH-dependent in PMLA-Hz-DOX, whereas the release behavior
of PMLA-ami-DOX did not alter with the decrease of pH. The in
vitro biological experiments showed that grafting DOX onto PMLA
reduced the toxicity of DOX. However, following uptake by cancer
cells, DOX released from the PMLA-Hz-DOX conjugate due to the
tumor-specific pH environment. PMLA-Hz-DOX, with its pH-responding
drug delivery properties, is expected to become a novel type of
controlled release drug platform.
Acknowledgements
Not applicable.
Funding
The present study was partially funded by grants
from the National Natural Science Foundation of China (grant nos.
81571786, 31771087 and 31671015) and the Shaanxi Science &
Technology Co-ordination & Innovation Project (grant no.
2015KTCL03-12).
Availability of data and materials
The datasets used or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BL and YP performed the experiments and drafted the
manuscript. EJ analyzed the data. YQ performed the experiments and
analysed the data. HW made substantial contributions to the design
of the present study and wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
References
|
1
|
Kopecek J: Polymer-drug conjugates:
Origins, progress to date and future directions. Adv Drug Deliv
Rev. 65:49–59. 2013. View Article : Google Scholar
|
|
2
|
Larson N and Ghandehari H: Polymeric
conjugates for drug delivery. Chem Mater. 24:840–853. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li J, Yu F, Chen Y and Oupicky D:
Polymeric drugs: Advances in the development of pharmacologically
active polymers. J Control Release. 219:369–382. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khandare J and Minko T: Polymer-drug
conjugates: Progress in polymeric prodrugs. Prog Polym Sci.
31:359–397. 2006. View Article : Google Scholar
|
|
5
|
Li C and Wallace S: Polymer-drug
conjugates: Recent development in clinical oncology. Adv Drug Deliv
Rev. 60:886–898. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng Q and Tong R: Anticancer
nanoparticulate polymer-drug conjugate. Bioeng Transl Med.
1:277–296. 2016.PubMed/NCBI
|
|
7
|
Canal F, Sanchis J and Vicent MJ:
Polymer-drug conjugates as nano-sized medicines. Curr Opin
Biotechnol. 22:894–900. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee BS, Vert M and Holler E: Water-soluble
aliphatic polyesters: Poly(malic acid)s. Wiley-VCH Verlag GmbH
& Co KGaA. 2005.
|
|
9
|
Braud C, Bunel C and Vert M: Poly(β-malic
acid): A new polymeric drug-carrier. Polymer Bulletin. 13:293–299.
1985. View Article : Google Scholar
|
|
10
|
Loyer P and Cammas-Marion S: Natural and
synthetic poly(malic acid)-based derivates: A family of versatile
biopolymers for the design of drug nanocarriers. J Drug Target.
22:556–575. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fernández CE, Mancera M, Holler E, Galbis
JA and Muñoz-Guerra S: High molecular weight methyl ester of
microbial poly(β,l-malic acid): Synthesis and crystallization.
Polymer. 47:6501–6508. 2006. View Article : Google Scholar
|
|
12
|
Martinez Barbosa ME, Cammas S, Appel M and
Ponchel G: Investigation of the degradation mechanisms of
poly(malic acid) esters in vitro and their related cytotoxicities
on J774 macrophages. Biomacromolecules. 5:137–143. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Segal E and Satchi-Fainaro R: Design and
development of polymer conjugates as anti-angiogenic agents. Adv
Drug Deliv Rev. 61:1159–1176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ljubimova JY, Portilla-Arias J, Patil R,
Ding H, Inoue S, Markman JL, Rekechenetskiy A, Konda B, Gangalum
PR, Chesnokova A, et al: Toxicity and efficacy evaluation of
multiple targeted polymalic acid conjugates for triple-negative
breast cancer treatment. J Drug Target. 21:956–967. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee BS, Fujita M, Khazenzon NM, Wawrowsky
KA, Wachsmann-Hogiu S, Farkas DL, Black KL, Ljubimova JY and Holler
E: Polycefin, a new prototype of a multifunctional nano-conjugate
based on poly(beta-L-malic acid) for drug delivery. Bioconjug Chem.
17:317–326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ding H, Inoue S, Ljubimov AV, Patil R,
Portilla-Arias J, Hu J, Konda B, Wawrowsky KA, Fujita M, Karabalin
N, et al: Inhibition of brain tumor growth by intravenous poly
(beta-L-malic acid) nanobioconjugate with pH-dependent drug release
[corrected]. Proc Natl Acad Sci USA. 107:18143–18148. 2010.
View Article : Google Scholar
|
|
17
|
Qiao YB, Duan X, Fan L, Li W, Wu H and
Wang YK: Synthesis of controlled molecular weight poly (beta-malic
acid) and conjugation with HCPT as a polymeric drug carrier. J
Polym Res. 21:3972014. View Article : Google Scholar
|
|
18
|
Yang T, Li W, Duan X, Zhu L, Fan L, Qiao Y
and Wu H: Preparation of two types of polymeric micelles based on
poly(beta-L-malic acid) for antitumor drug delivery. PLoS One.
11:e01626072016. View Article : Google Scholar
|
|
19
|
Guo X, Shi C, Wang J, Di S and Zhou S:
pH-triggered intracellular release from actively targeting polymer
micelles. Biomaterials. 34:4544–4554. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen B, Dai W, He B, Zhang H, Wang X, Wang
Y and Zhang Q: Current multistage drug delivery systems based on
the tumor microenvironment. Theranostics. 7:538–558. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang X, Liao W, Zhang G, Kang S and Zhang
CY: pH-sensitive micelles self-assembled from polymer brush
(PAE-g-cholesterol)- b-PEG-b-(PAE-g-cholesterol) for anticancer
drug delivery and controlled release. Int J Nanomedicine.
12:2215–2226. 2017. View Article : Google Scholar :
|
|
22
|
Wang X, Yang Y, Zhuang Y, Gao P, Yang F,
Shen H, Guo H and Wu D: Fabrication of pH-responsive nanoparticles
with an AIE feature for imaging intracellular drug delivery.
Biomacromolecules. 17:2920–2929. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao D, Xu JQ, Yi XQ, Zhang Q, Cheng SX,
Zhuo RX and Li F: pH-activated targeting drug delivery system based
on the selective binding of phenylboronic acid. ACS Appl Mater
Interfaces. 8:14845–14854. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun T, Zhang YS, Pang B, Hyun DC, Yang M
and Xia Y: Engineered nanoparticles for drug delivery in cancer
therapy. Angew Chem Int Ed Engl. 53:12320–12364. 2014.PubMed/NCBI
|
|
25
|
Karimi M, Eslami M, Sahandi-Zangabad P,
Mirab F, Farajisafiloo N, Shafaei Z, Ghosh D, Bozorgomid M,
Dashkhaneh F and Hamblin MR: pH-sensitive stimulus-responsive
nanocarriers for targeted delivery of therapeutic agents. Wiley
Interdiscip Rev Nanomed Nanobiotechnol. 8:696–716. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qian CG, Chen YL, Feng PJ, Xiao XZ, Dong
M, Yu JC, Hu QY, Shen QD and Gu Z: Conjugated polymer nanomaterials
for ther-anostics. Acta Pharmacol Sin. 38:764–781. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu YJ and Chen F: pH-responsive
drug-delivery systems. Chem Asian J. 10:284–305. 2015. View Article : Google Scholar
|
|
28
|
Wang Z, Deng X, Ding J, Zhou W, Zheng X
and Tang G: Mechanisms of drug release in pH-sensitive micelles for
tumour targeted drug delivery system: A review. Int J Pharm.
535:253–260. 2018. View Article : Google Scholar
|
|
29
|
Lee ES, Oh KT, Kim D, Youn YS and Bae YH:
Tumor pH-responsive flower-like micelles of poly(L-lactic
acid)-b-poly(ethylene glycol)-b-poly(L-histidine). J Control
Release. 123:19–26. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang H, Li F, Yi J, Gu C, Fan L, Qiao Y,
Tao Y, Cheng C and Wu H: Folate-decorated maleilated
pullulan-doxorubicin conjugate for active tumor-targeted drug
delivery. Eur J Pharm Sci. 42:517–526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
National Pharmacopoeia Committee:
Pharmacopoeia of People's Republic of China. China Medical Science
Press, Beijing Part. 2:9552015.
|
|
32
|
Zhou Q, Hou Y, Zhang L, Wang J, Qiao Y,
Guo S, Fan L, Yang T, Zhu L and Wu H: Dual-pH sensitive
charge-reversal nanocomplex for tumor-targeted drug delivery with
enhanced anticancer activity. Theranostics. 7:1806–1819. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mintzer MA and Simanek EE: Nonviral
vectors for gene delivery. Chem Rev. 109:259–302. 2009. View Article : Google Scholar
|
|
34
|
Aoshima Y, Hokama R, Sou K, Sarker SR,
Iida K, Nakamura H, Inoue T and Takeoka S: Cationic amino acid
based lipids as effective nonviral Gene delivery vectors for
primary cultured neurons. ACS Chem Neurosci. 4:1514–1519. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao G, Long L, Zhang L, Peng M, Cui T,
Wen X, Zhou X, Sun L and Che L: Smart pH-sensitive nanoassemblies
with cleavable PEGylation for tumor targeted drug delivery. Sci
Rep. 7:33832017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Y, Li D, Guo X, Xu H, Li Z, Zhang Y,
Song C, Fan R, Tang X and Zhang Z: A pH-responsive prodrug delivery
system of 10-HCPT for controlled release and tumor targeting. Int J
Nanomedicine. 12:2227–2242. 2017. View Article : Google Scholar : PubMed/NCBI
|