Introduction
Papillary thyroid carcinoma (PTC), accounting for
~80% of all thyroid cancers, is the most common malignant tumor of
the thyroid (1,2). Detected with the use of ultrasound
screening, the annual incidence of PTC has increased dramatically
(2). Poor prognosis is closely
associated with clinical characteristics such as tumor size and
lymph node metastasis (3-5). Therefore, understanding the
molecular mechanisms of PTC may be helpful in the identification of
novel therapeutic targets (6).
MicroRNAs (miRNAs) are small, noncoding
single-chained RNAs, which comprise a series of regulators by
targeting mRNAs, and causing their translation or degradation
(7). Notably, a large amount of
evidence indicate that miRNAs have important functions in adjusting
biological behaviors, such as cell proliferation, differentiation
and apoptosis (8,9). Actually, miRNAs control multiple
genes, which can provide new thinking for the development of human
disease treatment, especially for cancer. Targeting specific
miRNAs, such as miR-125a-5p and miR-337, has demonstrated promising
inhibitory roles on cancer progression (10,11). miR-214 is significantly
downregulated in multiple types of human cancer, including
hepatocellular, breast and cervical cancer (12-14), and displays the behavior of a
tumor suppressor (15). However,
the regulation of miR-214 and its mechanism in PTC remain
unclear.
Proteasome 26S subunit non-ATPase 10 (PSMD10), also
known as gankyrin, was originally cloned as an ingredient of the
26S proteasome (16). It has been
identified as an overexpressed protein and has complex functions in
hepatocellular carcinoma (17).
Previous studied have demonstrated that dysregulation of PSMD10 is
conducive to the tumor progression, and has significant effects on
tumor cell proliferation and metastasis (18,19). It was reported that PSMD10 can
regulate negatively numerous tumor types (20). However, the role of PSMD10 in PTC
has remained unexplored.
The present study explored the role of miR-214 in
PTC, and revealed that the expression of miR-214 was significantly
decreased in PTC tissues and cells. Overexpression of miR-214
suppressed cell proliferation, invasion, migration and
epithelial-mesenchymal transition (EMT) in the CGTH W-3 and PTC-uc3
PTC cell lines, via targeting PSMD10. Altogether, the present
results demonstrated that miR-214 might be a potential target for
the treatment of PTC.
Materials and methods
PTC tissue sample collection
Human clinical PTC tissues from 30 patients were
collected at Jiangsu Cancer Hospital (Nanjing, China) between
January 2015 and December 2016. The stage following surgery was
evaluated according to the 7th edition of American Joint Committee
on Cancer Tumor Node Metastasis staging system (21). The adjacent normal tissues were
isolated at the same time as matched controls. Written informed
consent was obtained from all the patients and the research
protocols were approved by the Ethics Committee of Jiangsu Cancer
Hospital.
Cell culture
The human PTC cell lines (CGTH W-3 and PTC-uc3) and
human thyroid follicular epithelial cells (Nthyori 3-1) were
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China). Cells were cultured in Roswell Park
Memorial Institute (RPMI)-1640 medium (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin,
and 100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), and incubated at 37°C in a humidified
incubator with 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted from tissues or cells using TRIzol
reagent (Thermo Fisher Scientific, Inc.) following the
manufacturer’s protocol. Total RNA (2 µg) was reversed
transcribed into cDNA using the TaqMan microRNA Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.). qPCR was performed in a Bio-Rad CFX96 Real-Time PCR System
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) using SYBR Premix
Ex Taq kit (Takara Biotechnology Co., Ltd., Dalian, China) or
TaqMan probes (Applied Biosystems; Thermo Fisher Scientific, Inc.),
according to the manufacturer’s instructions. The thermo-cycling
conditions were as follows: 95°C for 5 min followed by 45 cycles at
95°C for 10 sec and 55°C for 30 sec, then a melting curve analysis
every 0.2°C from 55°C to 95°C for 2 min was obtained. Expressions
were normalized to the levels of the internal reference gene GAPDH.
Specific PCR primers were synthesized by Invitrogen and the
sequences were as follows: miR-214 forward, 5′-TAT ACA TCA AAC AGC
AGG CAC A-3′ and reverse, 5′-CAT TCG ATC TTC TCC ACA GTC TC-3′;
PSMD10 forward, 5′-CGA GCT CTG GAA GGT TAA ACA GCT TGG A-3′ and
reverse, 5′-GCT CTA GAA GAC TCA CAA CAG CCA CAG AA-3′; GAPDH
forward, 5′-AAG AAG GTG GTG AAG CAG GC-3′ and reverse, 5′-TCC ACC
ACC CTG TTG CTG TA-3′. The expression levels of miR-214 and PSMD10
were assessed using the 2−∆∆Cq method (22).
Cell transfection
miR-214 mimics (5′-ACA GCA GGC ACA GAC AGG CAG U-3′)
miR-214 inhibitors (5′-ACU GCC UGU CUG UGC CUG CUG U-3′) mimics
negative control (NC; 5′-UUC UCC GAA CGU GUC ACG UTT-3′) inhibitors
NC (5′-CAG UAC UUU UGU GUA GUA CAA-3′) and PDMS10 small interfering
(si) RNA (PDMS10-siRNA; 5′-CTG GCC GGG ATG AGA TTG TAA AAG-3′) were
obtained from GenePharma Co., Ltd. (Shanghai, China). Cells were
transfected at a final concentration of 50 nM using Lipofectamine
2000 (Thermo Fisher Scientific, Inc.) for 24 h, according to the
manufacturer’s protocols.
Cell proliferation assay
Cell Counting Kit-8 (CCK-8) assays were performed to
evaluate cell proliferation, following the manufacturer’s protocol.
Briefly, transfected cells were added to 96-well plates
(1×104 cells/well) and then CCK-8 solution (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) was added. The
optical density at 450 nm was recorded on a microplate reader
(Multiscan FC; Thermo Fisher Scientific, Inc.) at different
times.
Cell apoptosis assay
Flow cytometry analysis (C6 type flow cytometer; BD
Biosciences, San Jose, CA, USA) using a FACSCalibur (version 6.0;
BD Biosciences) was conducted to evaluate cells apoptosis. For
apoptosis analysis, cells were collected 48 h post-transfection and
stained with fluorescein isothiocyanate (FITC)-Annexin V (5
µl; Nanjing KeyGen Biotech Co., Ltd., Nanjing, China), and
propidium iodide (PI; 5 µl).
Cell cycle analysis
Following transfection for 48 h, cells were
harvested, washed with PBS and fixed in 70% ethanol over-night at
4°C. Then, 10 mg/l RNase A was added and incubated in 37°C for 30
min. The cells were subsequently incubated in 10 g/ml PI at 4°C for
30 min in the dark. Cell cycle distribution was analyzed by flow
cytometry. The results were analyzed using ModFit software v3.3 (BD
Biosciences).
Wound-healing assay
Cells were seeded in 6-well plates and grown to ~80%
confluency. Cell monolayers were wounded (width, 2 mm) with sterile
plastic pipette tips and washed with PBS to remove cell debris.
Images with a magnification of ×40 were captured at 0, 24 and 48 h
following wounding using a light microscope. A total of 5 fields
were captured per sample and the distance of cell migration into
the wound area was calculated using Image J software 1.48u
(National Institutes of Health, Bethesda, MD, USA).
Cell invasion assay
Diluted matrigel (BD Biosciences) was incubated for
2 h at 37°C in the upper chamber of Transwell chambers (Corning
Incorporated, Corning, NY, USA). Then, cells
(1×105/well) were added into the upper chamber. The
bottom chamber was filled with medium containing 20% FBS and
incubated for 24 h. Then, noninvasive cells on the upper surface
were gently wiped off with cotton swabs. Invaded cells were fixed
with 4% paraformaldehyde (Sigma-Aldrich; Merck KGaA) and stained in
10% crystal violet (Sigma-Aldrich; Merck KGaA). Images of the
invasive cells at the bottom of the chamber were captured under an
inverted light microscope (TE 2000-S; Nikon Corporation, Tokyo,
Japan); the quantification of the invaded cells used the mean
number of cells in five fields.
Dual luciferase reporter assay
TargetScan (www.targetscan.og) (23) was used to predict the binding site
of miR-214 and PSMD10 3′ untranslated region (UTR) using miR-214 as
a keyword on the 12th February 2017. The wild-type or mutant 3′UTR
of PSMD10 (Shanghai GeneChem Co., Ltd., Shanghai, China) was cloned
into pGL3 vector (Promega Corporation, Madison, WI, USA) to confirm
direct target association. The wild-type contained binding sites of
PSMD10 3′UTR with miR-214. Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.) was used for transfection for 24 h at 37°C. Cells
(1×104/well) were incubated for 48 h. Then the
luciferase activity was measured after 48 h using a dual-luciferase
reporter assay system (Promega Corporation). Luciferase ratio was
calculated for each construct. The activity was normalized to the
corresponding Renilla luciferase activity.
Western blot analysis
RIPA lysis buffer was used to extract total
proteins. The supernatant was collected by centrifuging at 13,282 x
g at 4°C for 10 min. Protein concentration was tested using the
Pierce BCA Protein Assay kit (Thermo Fisher Scientific, Inc.). The
samples (30 µg) were separated on SDS-PAGE (10%) and then
blotted onto a polyvinylidene fluoride membrane. The membrane was
blocked with 5% non-fat milk at 25°C for 1 h, and then incubated
with primary antibodies overnight at 4°C, including matrix
metalloproteinase (MMP)-2 (cat. no. 40994; Cell Signaling
Technology Inc., Danvers, MA, USA; 1:1,000), MMP-9 (cat. no. 13667;
Cell Signaling Technology; 1:1,000), E-cadherin (cat. no. 14472;
Cell Signaling Technology; 1:2,000), Vimentin (cat. no. 5741; Cell
Signaling Technology; 1:1,000), Fibronectin (cat. no. 4705; Cell
Signaling Technology; 1:1,000), PSMD10 (cat. no. 12985; Cell
Signaling Technology; 1:1,000), phosphorylated (p)-protein kinase B
(AKT; cat. no. 4060; Cell Signaling Technology; 1:2,000), AKT (cat.
no. 2920; Cell Signaling Technology; 1:1,000), p-glycogen synthase
kinase (GSK)-3 β (cat. no. 9323; Cell Signaling Technology;
1:1,000), GSK-3 β (cat. no. 5676; Cell Signaling Technology;
1:1,000) and GAPDH (cat. no. 5174; Cell Signaling Technology;
1:2,000). The membrane was incubated with the corresponding
horseradish peroxidase-conjugated secondary antibody (cat. no.
4410; Cell Signaling Technology; 1:5,000) at 25°C for 2 h. The
immunoreactivity was visualized using the ECL Western blotting kit
(Beyotime Institute of Biotechnology, Beijing, China). The amount
of protein was normalized to GAPDH and analyzed using ImageJ
software 1.48u (National Institutes of Health).
Colony formation assay
PTC cells (1×104 cells/well) were plated
in 24-well culture plates. After 12 days, cells were washed and
fixed with 4% paraformaldehyde at 25°C for 30 min, and then stained
in 10% crystal violet. The number of colonies containing ≥50 cells
was counted under a light microscope.
Statistical analysis
Data were represented as means ± standard deviation
(n=3). GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA,
USA) was used to perform all the statistical analyses. When only
two groups were compared, Student’s t-test was conducted. One-way
analysis of variance followed Bonferroni’s correction as a post-hoc
test was applied to compare differences between multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-214 is downregulated in PTC tissues
and cell lines
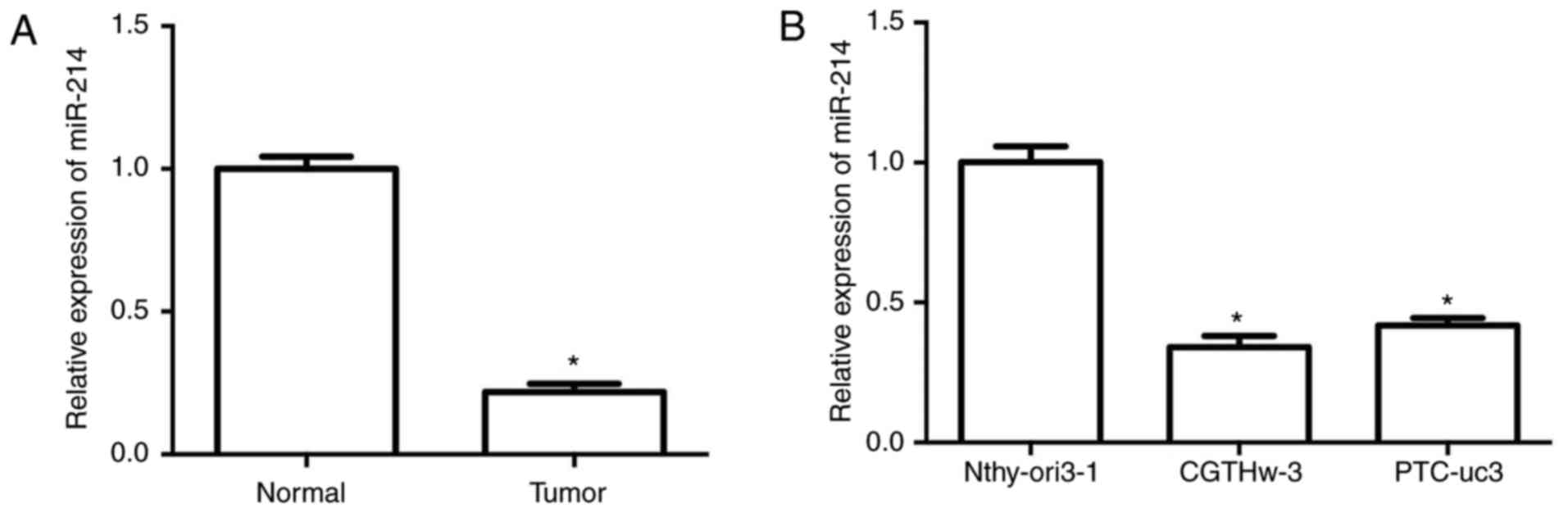
To explore the expression status of miR-214 in PTC,
the expression levels of miR-214 were examined in 30 pairs of human
PTC tissues and matched adjacent normal tissues by RT-qPCR. The
results demonstrated that miR-214 expression was significantly
downregulated in PTC tissues compared with adjacent normal tissues
(Fig. 1A). In addition, the
correlation of miR-214 expression to various clinical features,
including age, gender, tumor size, lymph node metastasis and TNM
stage, was investigated in PTC. The results revealed that decreased
expression of miR-214 was associated with lymph node metastasis,
tumor size and advanced TNM stage (Table I).
 | Table IAssociation of miR-214 expression
levels and patient clinical characteristics. |
Table I
Association of miR-214 expression
levels and patient clinical characteristics.
| Variable | Number of
patients | Relative
expression | P-value |
|---|
| Age (years) | | | 0.6140 |
| ≤60 | 15 | 0.25 | |
| >60 | 15 | 0.23 | |
| Sex | | | 0.2540 |
| Male | 12 | 0.21 | |
| Female | 18 | 0.25 | |
| Tumor size | | | <0.0001 |
| T1+T2 | 12 | 0.33 | |
| T3+T4 | 18 | 0.17 | |
| Lymphatic
metastasis | | | <0.0001 |
| Positive | 14 | 0.16 | |
| Negative | 16 | 0.32 | |
| TNM stage | | | <0.0001 |
| ≤II | 10 | 0.34 | |
| >II | 20 | 0.18 | |
Next, the expression levels of miR-214 were
determined in one human thyroid follicular epithelial cell line
(Nthyori 3-1) and two PTC cell lines (CGTH W-3 and PTC-uc3). The
results demonstrated that miR-214 was markedly downregulated in the
PTC cell lines (CGTH W-3 and PTC-uc3) compared with the normal
Nthyori 3-1 cell line (Fig. 1B).
These data indicated that the expression of miR-214 was decreased
in both PTC tissues and cells, suggesting that miR-214 may be
important in PTC development and metastasis.
miR-214 overexpression suppresses cell
proliferation, and induces cell apoptosis and cell cycle arrest in
PTC cells
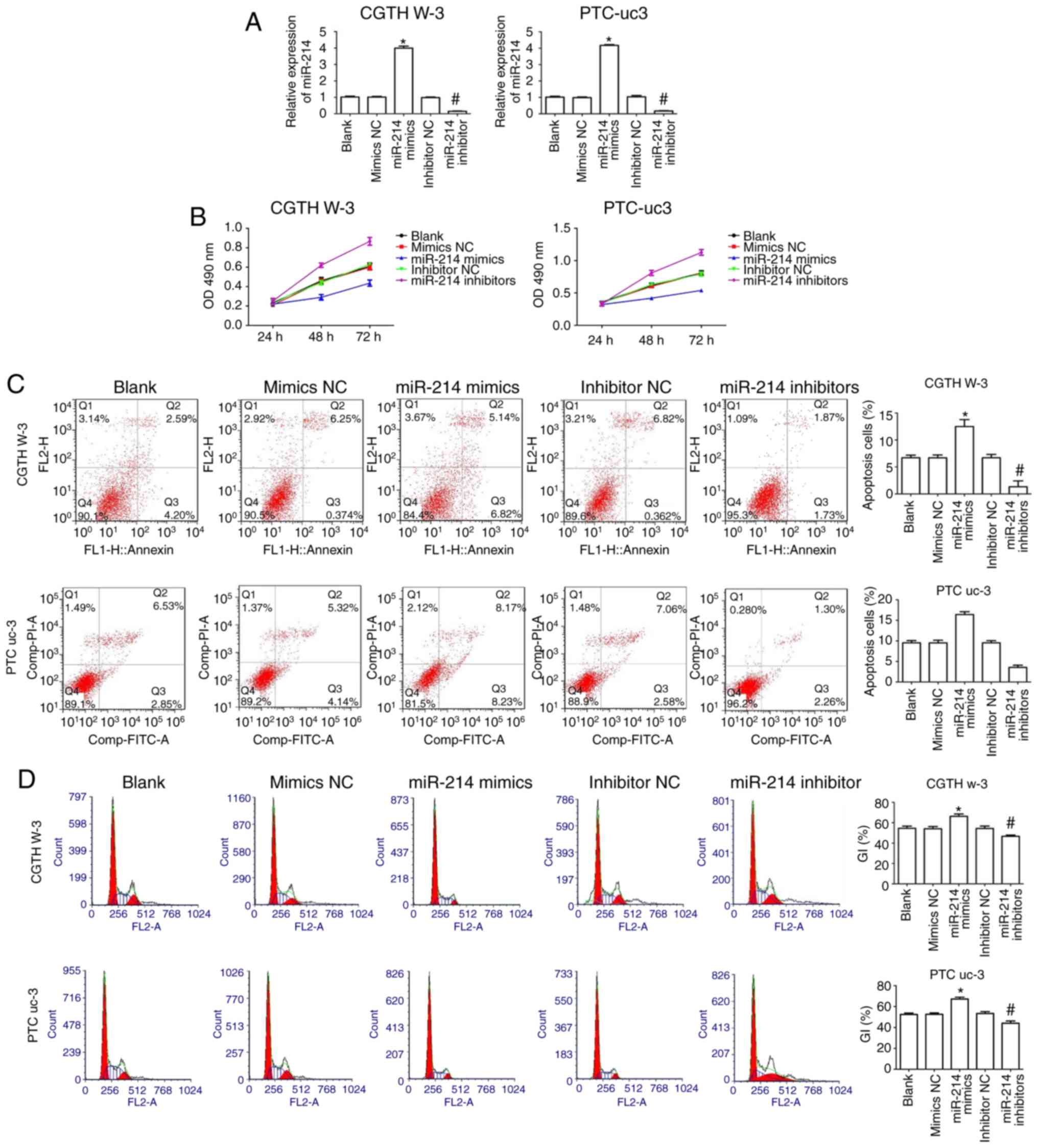
CGTH W-3 and PTC-uc3 cells were transfected with
miR-214 mimics or miR-214 inhibitors or corresponding negative
control (NC) to evaluate the effect of miR-214 in PTC. The results
indicated that miR-214 mimics increased expression of miR-214
compared with the mimics NC group in CGTH W-3 and PTC-uc3 cells,
while its expression was significantly decreased in the miR-214
inhibitors-transfected group compared with the inhibitors NC group
(Fig. 2A).
CCK-8 assay results demonstrated that transfection
with miR-214 mimics significantly decreased the proliferation of
CGTH W-3 and PTC-uc3 cells compared with the mimics NC group
(Fig. 2B). By contrast,
transfection of miR-214 inhibitors resulted in the opposite effect
(Fig. 2B). Flow cytometry
analysis demonstrated that miR-214 mimics clearly promoted cell
apoptosis compared with the mimics NC group, while miR-214
inhibitors markedly decreased apoptosis compared with the
inhibitors NC group (Fig. 2C). In
addition, cell cycle phase distribution analysis revealed that
miR-214 mimics significantly increased the % of cells in the
G0/G1 phases of the cell cycle in CGTH W-3
and PTC-uc3 cells compared with the mimics NC group (Fig. 2D). By contrast, inhibition of
miR-214 decreased the % of cells in the G0/G1
phases in both CGTH W-3 and PTC-uc3 cells compared with the
inhibitors NC group (Fig. 2D),
suggesting that miR-214 affects PTC cell cycle regulation.
miR-214 decreases PTC cell migration and
invasion and inhibits EMT
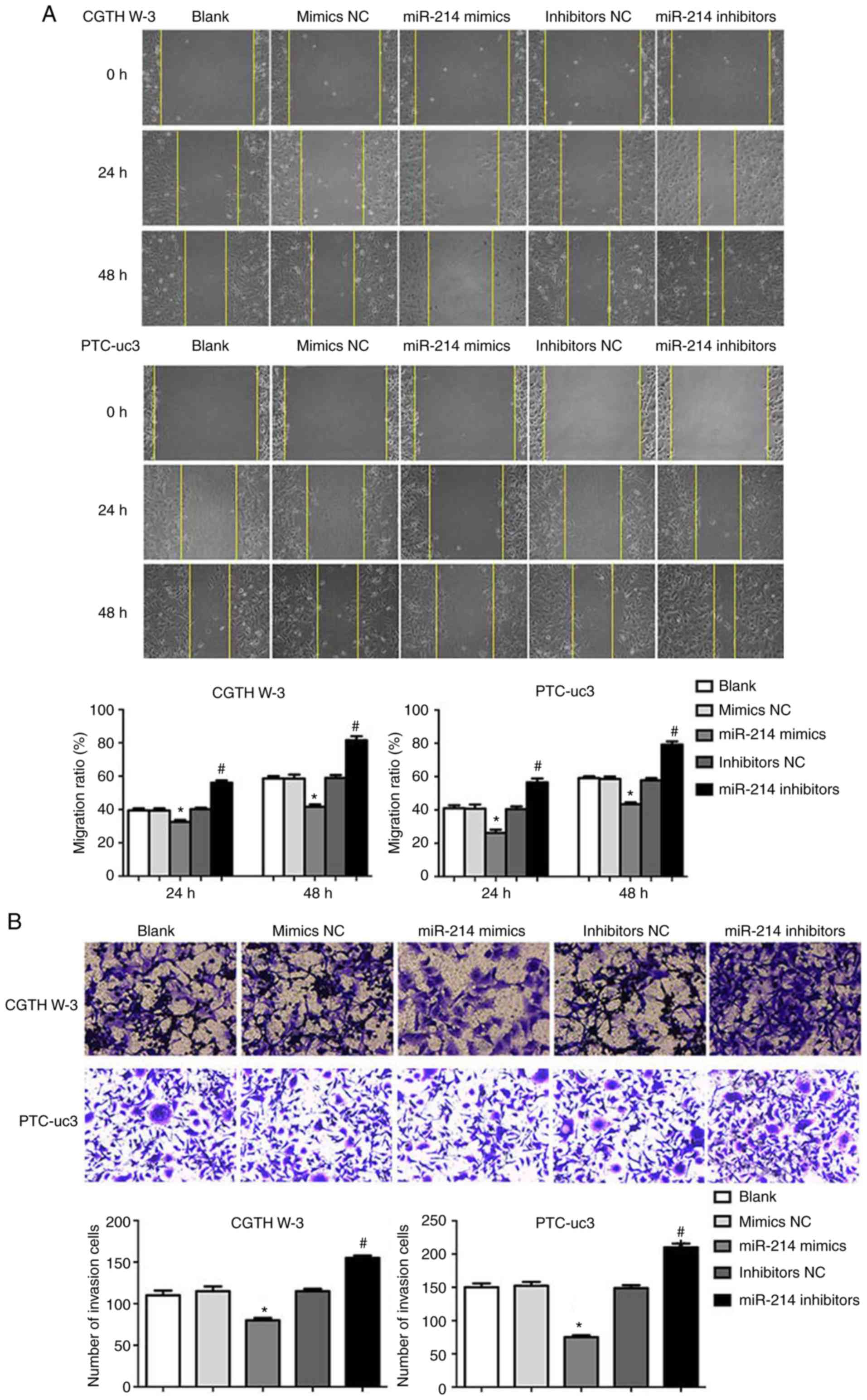
The effects of miR-214 on PTC cell migration and
invasion were evaluated next. The results demonstrated that miR-214
mimics decreased cell migration in CGTH W-3 and PTC-uc3 cells,
while depletion of miR-214 resulted in a significant increase in
cell migration, compared with the respective NC group (Fig. 3A). Similarly, miR-214 mimics
significantly decreased CGTH W-3 and PTC-uc3 cell invasion, while
inhibition of miR-214 decreased cell invasion, compared with the
respective NC group (Fig.
3B).
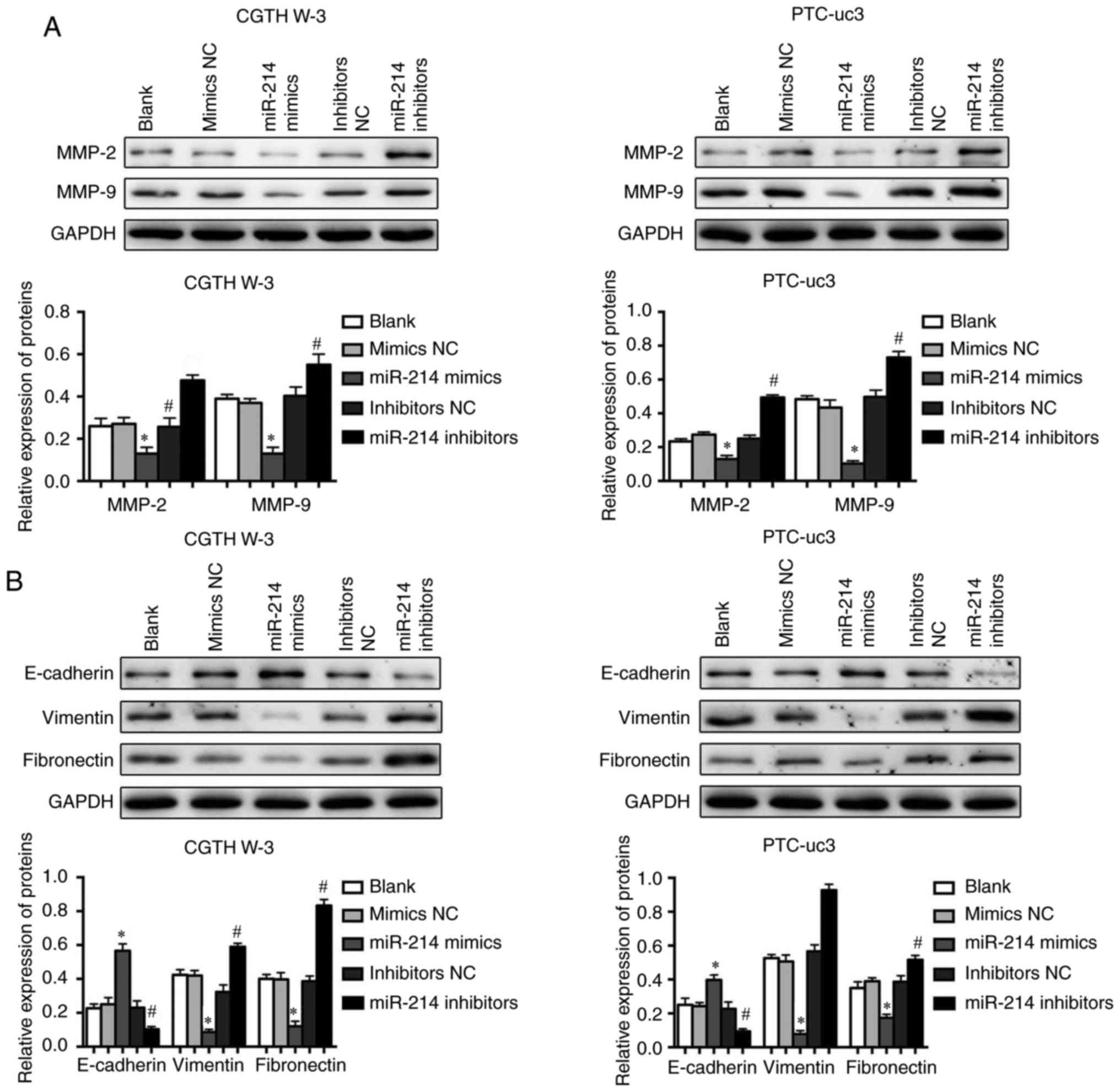
Furthermore, the expression of proteins associated
with tumor cell metastasis was examined in CGTH W-3 and PTC-uc3
cells by western blotting. The results demonstrated that miR-214
mimics significantly decreased the expression levels of matrix
metallopeptidase (MMP)-2 and MMP-9 (Fig. 4A). However, inhibition of miR-214
promoted the upregulation of MMP-2 and MMP-9 in both CGTH W-3 and
PTC-uc3 cells (Fig. 4A). In
addition, the results revealed that overexpression of miR-214
reversed EMT-associated protein expression in CGTH W-3 and PTC-uc3
cells; miR-214 mimics increased the expression of E-cadherin and
inhibited the expression of N-cadherin and vimentin compared with
the mimics NC group (Fig. 4B).
However, the EMT-associated expression displayed opposite patterns
in the miR-214 inhibitor-transfected groups (Fig. 4B).
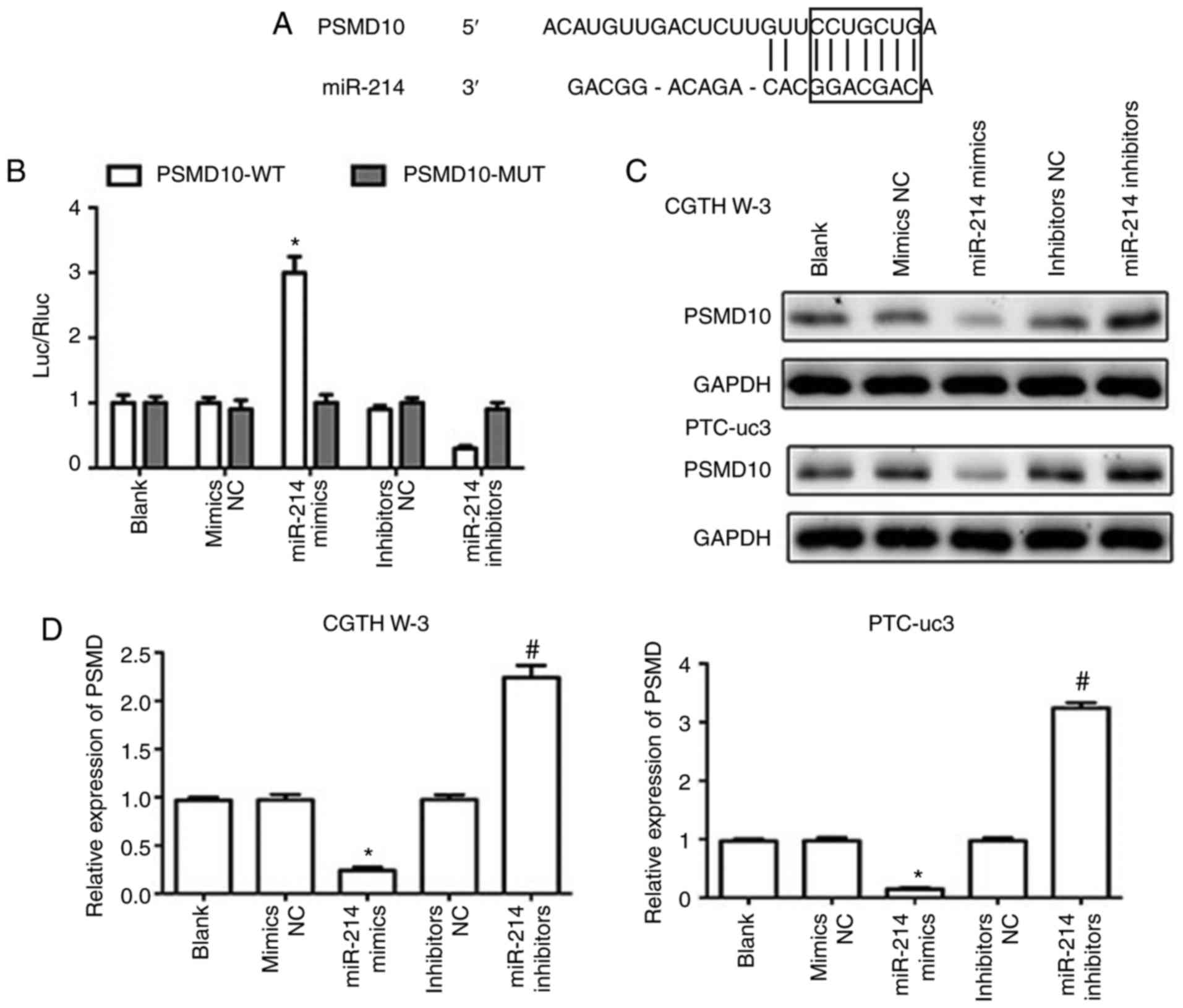
PSMD10 is a direct target of miR-214
The TargetScan software was utilized to analyze
potential gene targets for miR-214 (23), and PSMD10 was predicted to be a
target gene of miR-214 (Fig. 5A).
Dual luciferase reporter assays were then employed to test the
hypothesis that miR-214 directly targets PSMD10. The results
indicated that miR-214 mimics reduced the luciferase activity of a
PSMD10-3′-UTR wild-type reporter plasmid (Fig. 5B), while the luciferase activity
of a PSMD10-3′-UTR mutant reporter was not affected. In addition,
miR-214 mimics downregulated the expression of PSMD10 in CGTH W-3
and PTC-uc3 cells at the mRNA and protein levels, while miR-214
inhibitors markedly increased the expression of PSMD10 compared
with the inhibitors NC group (Fig. 5C
and D). These results demonstrated that PSMD10 was a direct
target of miR-214.
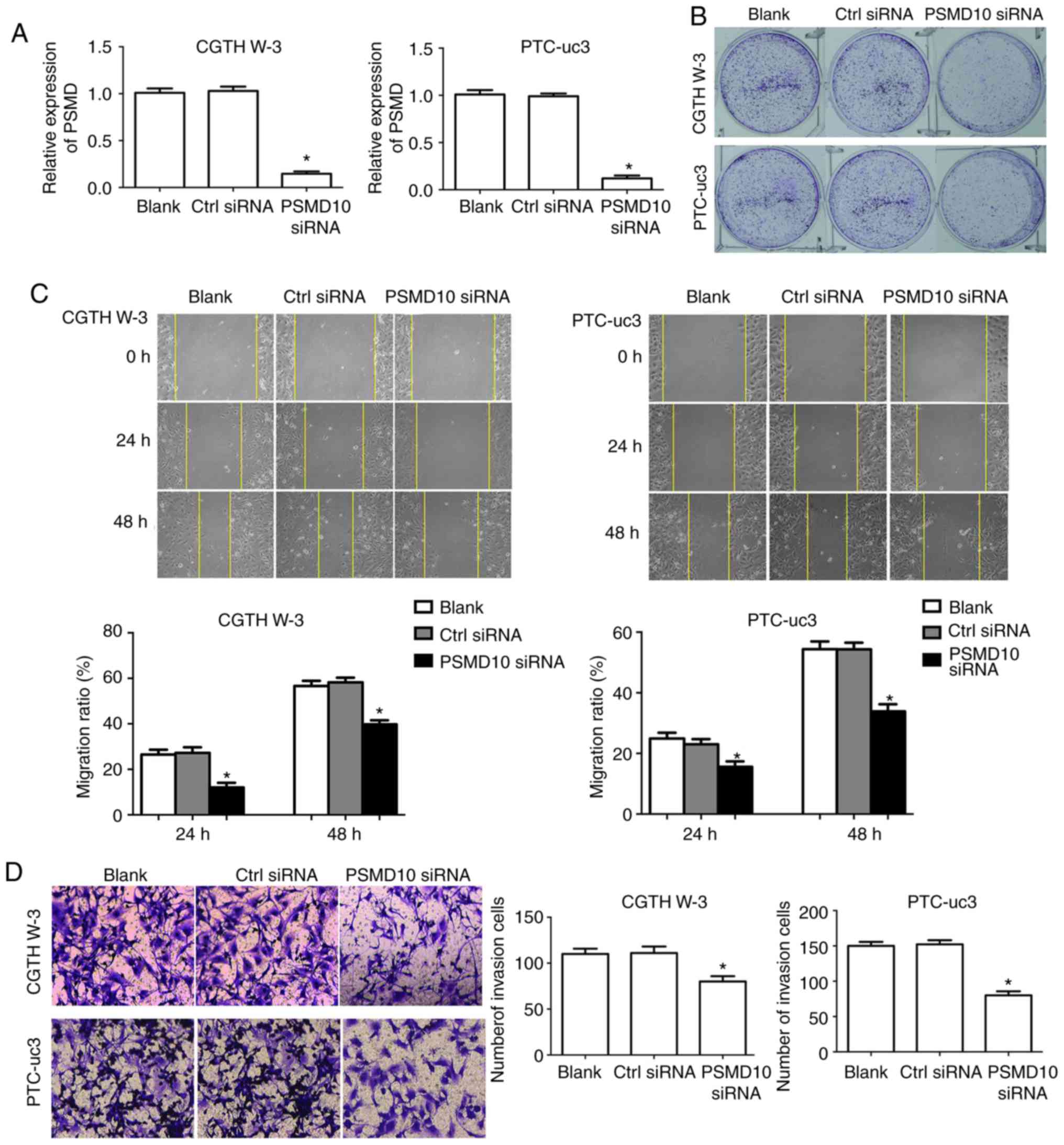
PSMD10 knockdown reduces the
proliferation, migration and invasion potential of PTC cells
First, RT-qPCR was used to verify that PSMD10 levels
were successfully silenced by siRNA transfection compared with
control, in both CGTH W-3 and PTC-uc3 cells (Fig. 6A). Then, the effects of PSMD10
silencing were evaluated in the PTC cells. Proliferation capacity
was determined by colony formation assay. The results demonstrated
that colony formation in the PSMD10-siRNA transfection group was
markedly decreased compared with the control group, in both CGTH
W-3 and PTC-uc3 cells (Fig. 6B).
In addition, cell migration was significantly decreased in CGTH W-3
and PTC-uc3 cells transfected with PSMD10-siRNA (Fig. 6C). Finally, the cell invasion rate
was significantly decreased in CGTH W-3 and PTC-uc3 cells in the
PSMD10-siRNA group compared with the control group (Fig. 6D).
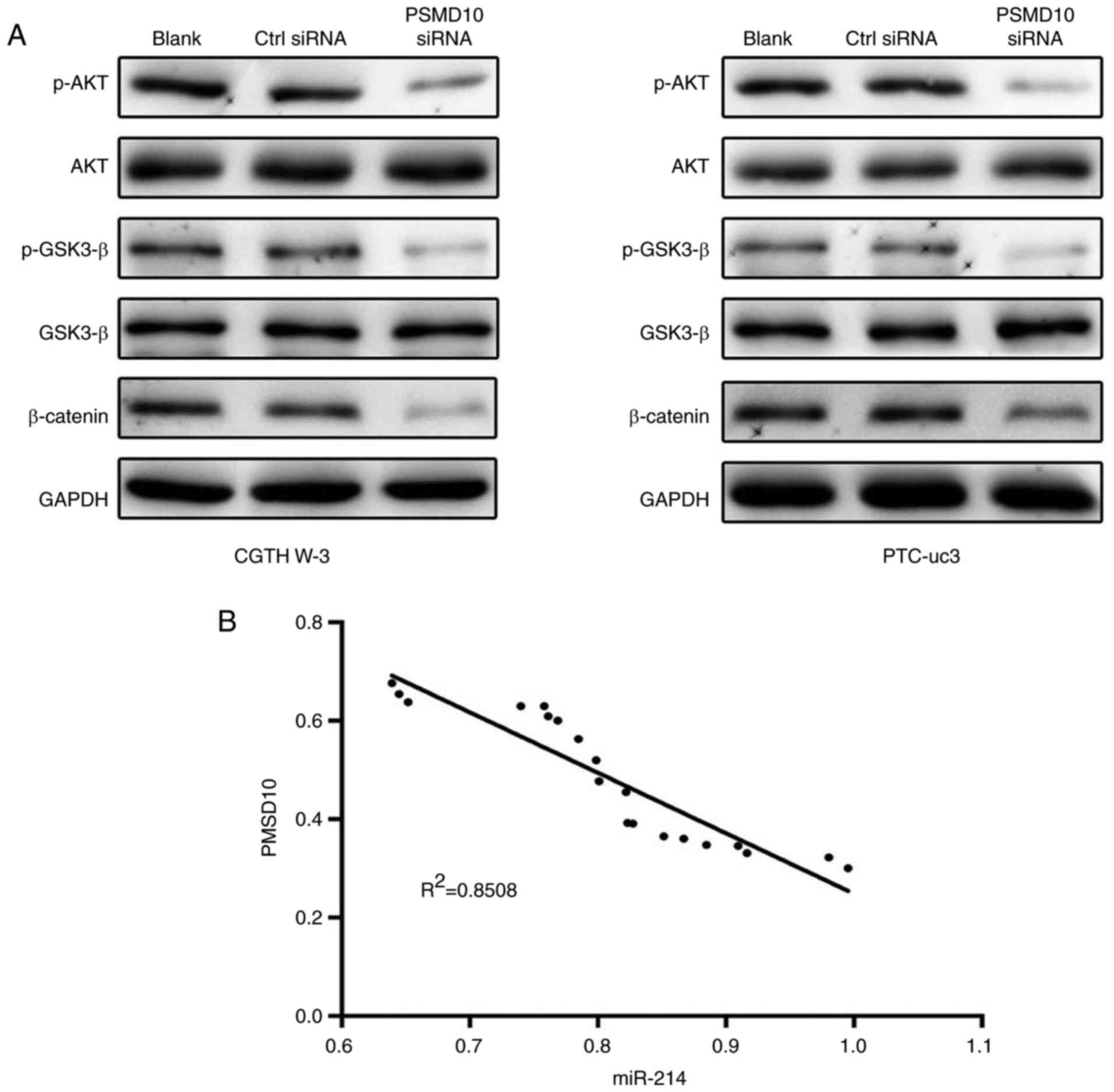
miR-214 expression is inversely
correlated with PSMD10 expression in PTC tissues
To further investigate the molecular basis of
PSMD10-mediated antitumor effects, the role of PSMD10 was examined
in regulating glycogen synthase kinase (GSK)-3β/β-catenin signaling
in CGTH W-3 and PTC-uc3 cells. The results demonstrated that PSMD10
downregulation significantly inhibited phosphorylation of GSK-3β
and β-catenin (Fig. 7A).
Furthermore, the activity of AKT was markedly clearly decreased in
the PSMD10-siRNA group. These data indicated that PSMD10 repressed
GSK-3β/β-catenin and AKT signaling in PTC cells.
Next, the correlation between the expression levels
of miR-214 and PSMD10 was evaluated in PTC tissues. The results
revealed that the expression of miR-214 was inversely correlated
with the expression of PSMD10 in PTC tissues (Fig. 7B).
Discussion
PTC represents one of the most common thyroid
malignancies, which is characterized by frequent recurrence and
high mortality due to fast growth and metastasis. The present study
focused on the role of miRNA in regulating the growth and
metastasis of PTC.
Evidence has indicated that the expression of
miR-214 is significantly decreased in various types of cancer,
including hepatocellular (24),
ovarian (25), cervical (26) and prostate cancer (27). In the present study, miR-214 was
also identified as a downregulated miRNA in PTC. This is the first
study to demonstrate that miR-214 is dysregulated in PTC. In
addition, the dysregulated expression of miR-214 has also been
associated with invasion, metastasis and TNM stage in the gastric
cancer tissues (28). In the
present study, downregulation of miR-214 was associated with lymph
node metastasis, tumor size and TNM stage.
The dysregulated expression of miR-214 in cancer
caused researchers to explore the effects of its tumor specificity
(16). miR-214 inhibits cell
proliferation in breast cancer and hepatic carcinomas (29,30). Furthermore, miR-214 inhibits DNA
replication and apoptosis by upregulating the expression of p53,
p21 and BCL2 associated X (Bax) (31). Similarly, the present study
demonstrated that overexpression of miR-214 inhibited PTC cell
proliferation, induced PTC cell apoptosis and arrested PTC cell
cycle. miR-214 has been reported to significantly suppress
hepatocellular carcinoma cell invasion by downregulating β-catenin
and enhancer of zeste 2 poly-comb repressive complex 2 subunit
(Ezh2) (24). In addition,
β-catenin leads to malignancy in breast and hepatic cancer by
regulating the expression of its downstream genes, cyclin D1 and
E-cadherin (24). Notably,
miR-214 inhibits the expression of Ezh2 and decreases esophageal
squamous carcinoma cell migration and invasion (32), which indicates that miR-214 has a
key role in regulating cancer invasion and metastasis. The present
study suggested that upregulation of miR-214 could inhibit PTC cell
migration, invasion and EMT in PTC cells.
Identifying the targets of miRNAs may help elucidate
the pathogenesis of cancer (26).
It has been reported that miR-214 has several validated gene
targets, including transcription factor AP-2α (TFAP2), phosphatase
and tensin homolog (PTEN), EZH2 and fibroblast growth factor
receptor 1 (FGFR1) (33-35). In the present study, upregulation
of miR-214 decreased the expression levels of PSMD10 in PTC cells.
Furthermore, the present study demonstrated that inhibition of
PSMD10 decreased PTC cell proliferation, migration and invasion.
PSMD10 is a regulator of colorectal cancer by phosphoinositide
3-kinase (PI3K)/GSK-3β/β-catenin pathway activation (36). The present study demonstrated that
PSMD10 downregulation repressed GSK-3β/β-catenin and AKT signaling
in PTC cells.
In conclusion, the present study is the first report
that miR-214 functions as a tumor suppressor in PTC cells by
targeting PSMD10, suggesting that it may be a potential target for
the treatment of PTC.
Funding
This work was supported by the Key Projects of
Nanjing Science and Technology Commission (grant no. 201402006) and
the Key Projects of Jiangsu Provincial Science and Technology
Department (grant no. BE2016796).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors’ contributions
FL and YZhang conceived and designed the
experiments. FL, KL, XZ and JZ performed the experiments. WC, YQ
and YZhao analyzed the data. FL, YZhu and YZhang wrote the paper.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all the
patients and the research protocols were approved by the Ethics
Committee of Jiangsu Cancer Hospital.
Patient consent for publication
Informed consent was obtained from the subjects for
participation in this study.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Chen AY, Jemal A and Ward EM: Increasing
incidence of differentiated thyroid cancer in the United States,
1988–2005. Cancer. 115:3801–3807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davies L and Welch HG: Increasing
incidence of thyroid cancer in the United States, 1973–2002. JAMA.
295:2164–2167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Voutilainen PE, Multanen MM, Leppäniemi
AK, Haglund CH, Haapiainen RK and Franssila KO: Prognosis after
lymph node recurrence in papillary thyroid carcinoma depends on
age. Thyroid. 11:953–957. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chou CK, Chen RF, Chou FF, Chang HW, Chen
YJ, Lee YF, Yang KD, Cheng JT, Huang CC and Liu RT: miR-146b is
highly expressed in adult papillary thyroid carcinomas with high
risk features including extrathyroidal invasion and the BRAF(V600E)
mutation. Thyroid. 20:489–494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brown RL, de Souza JA and Cohen EE:
Thyroid cancer: Burden of illness and management of disease. J
Cancer. 2:193–199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chou CK, Yang KD, Chou FF, Huang CC, Lan
YW, Lee YF, Kang HY and Liu RT: Prognostic implications of miR-146b
expression and its functional role in papillary thyroid carcinoma.
J Clin Endocrinol Metab. 98:E196–E205. 2013. View Article : Google Scholar
|
|
7
|
Jazdzewski K, Liyanarachchi S, Swierniak
M, Pachucki J, Ringel MD, Jarzab B and de la Chapelle A:
Polymorphic mature microRNAs from passenger strand of pre-miR-146a
contribute to thyroid cancer. Proc Natl Acad Sci USA.
106:1502–1505. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li M, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar
|
|
9
|
Vriens MR, Weng JL, Suh I, Huynh N,
Guerrero MA, Shen WT, Duh QY, Clark OH and Kebebew E: MicroRNA
expression profiling is a potential diagnostic tool for thyroid
cancer. Cancer. 118:3426–3432. 2012. View Article : Google Scholar
|
|
10
|
Zhong L, Sun S, Shi J, Cao F, Han X and
Chen Z: MicroRNA-125a 5p plays a role as a tumor suppressor in lung
carcinoma cells by directly targeting STAT3. Tumor Biol.
39:10104283176975792017. View Article : Google Scholar
|
|
11
|
Dong W, Li B, Wang J, Song Y, Zhang Z and
Fu C: MicroRNA-337 inhibits cell proliferation and invasion of
cervical cancer through directly targeting specificity protein 1.
Tumor Biol. 39:10104283177113232017. View Article : Google Scholar
|
|
12
|
Wang P, Chen S, Fang H, Wu X, Chen D, Peng
L, Gao Z and Xie C: miR-214/199a/199a* cluster levels
predict poor survival in hepatocellular carcinoma through
interference with cell-cycle regulators. Oncotarget. 7:929–945.
2016.
|
|
13
|
Zhang J, Su B, Gong C, Xi Q and Chao T:
miR-214 promotes apoptosis and sensitizes breast cancer cells to
doxorubicin by targeting the RFWD2-p53 cascade. Biochem Bioph Res
Co. 478:337–342. 2016. View Article : Google Scholar
|
|
14
|
Peng R, Men J, Ma R, Wang Q, Wang Y, Sun Y
and Ren J: miR-214 down-regulates ARL2 and suppresses growth and
invasion of cervical cancer cells. Biochem Bioph Res Co.
484:623–630. 2017. View Article : Google Scholar
|
|
15
|
Penna E, Orso F and Taverna D: miR-214 as
a key hub that controls cancer networks: Small player, multiple
functions. J Invest Dermatol. 135:960–969. 2015. View Article : Google Scholar
|
|
16
|
Hori T, Kato S, Saeki M, DeMartino GN,
Slaughter CA, Takeuchi J, Toh-e A and Tanaka K: cDNA cloning and
functional analysis of p28 (Nas6p) and p40.5 (Nas7p), two novel
regulatory subunits of the 26S proteasome. Gene. 216:113–122. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Higashitsuji H, Itoh K, Nagao T, Dawson S,
Nonoguchi K, Kido T, Mayer RJ, Arii S and Fujita J: Reduced
stability of retinoblastoma protein by gankyrin, an oncogenic
ankyrin-repeat protein overexpressed in hepatomas. Nat Med.
6:96–99. 2000. View
Article : Google Scholar
|
|
18
|
Zhen C, Chen L, Zhao Q, Liang B, Gu YX,
Bai ZF, Wang K, Xu X, Han QY, Fang DF, et al: Gankyrin promotes
breast cancer cell metastasis by regulating Rac1 activity.
Oncogene. 32:3452–3460. 2000. View Article : Google Scholar
|
|
19
|
Meng Y, He L, Guo X, Tang S, Zhao X, Du R,
Jin J, Bi Q, Li H, Nie Y, et al: Gankyrin promotes the
proliferation of human pancreatic cancer. Cancer Lett. 297:9–17.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Uddin MH, Choi MH, Kim WH, Jang JJ and
Hong ST: Involvement of PSMD10, CDK4, and tumor suppressors in
development of intrahepatic cholangiocarcinoma of syrian golden
hamsters induced by clonorchis sinensis and N-Nitrosodimethylamine.
PLoS Negl Trop Dis. 9:e00040082015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edge SB, Byrd DR, Compton CC, Fritz AG and
Greene FL: AJCC cancer staging manual. 7th edition. Springer; New
York, NY: 2010
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−delta delta C(T)) methods. Methods. 25:420–408. 2001.
View Article : Google Scholar
|
|
23
|
Agarwal V, Bell GW, Nam J and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:e050052015. View Article : Google Scholar :
|
|
24
|
Xia H, Ooi LL and Hui KM: MiR-214 targets
β-catenin pathway to suppress invasion, stem-like traits and
recurrence of human hepatocellular harcinoma. PLoS One.
7:e442062012. View Article : Google Scholar
|
|
25
|
Mitra AK, Zillhardt M, Hua Y, Tiwari P,
Murmann AE, Peter ME and Lengyel E: MicroRNAs reprogram normal
fibroblasts into cancer-associated fibroblasts in ovarian cancer.
Cancer Discov. 2:1100–1108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang F, Liu M, Li X and Tang H: MiR-214
reduces cell survival and enhances cisplatin-induced cytotoxicity
via down-regulation of Bcl2l2 in cervical cancer cells. FEBS Lett.
587:488–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Srivastava A, Goldberger H, Dimtchev A,
Ramalinga M, Chijioke J, Marian C, Oermann EK, Uhm S, Kim JS, Chen
LN, et al: MicroRNA profiling in prostate cancer-the diagnostic
potential of urinary miR-205 and miR-214. PLoS One. 8:e769942013.
View Article : Google Scholar
|
|
28
|
Yang TS, Yang XH, Wang XD, Wang YL, Zhou B
and Song ZS: MiR-214 regulate gastric cancer cell proliferation,
migration and invasion by targeting PTEN. Cancer Cell Int.
13:682013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Derfoul A, Juan AH, Difilippantonio MJ,
Palanisamy N, Ried T and Sartorelli V: Decreased microRNA-214
levels in breast cancer cells coincides with increased cell
proliferation, invasion and accumulation of the Polycomb Ezh2
methyltransferase. Carcinogenesis. 32:1607–1614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Duan Q, Wang X, Gong W, Ni L, Chen C, He
X, Chen F, Yang L, Wang P and Wang DW: ER stress negatively
modulates the expression of the miR-199a/214 cluster to regulates
tumor survival and progression in human hepatocellular cancer. PLoS
One. 7:e315182012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Misiewicz-Krzeminska I, Sarasquete ME,
Quwaider D, Krzeminski P, Ticona FV, Paino T, Delgado M, Aires A,
Ocio EM, Garcia-Sanz R, et al: Restoration of microRNA-214
expression reduces growth of myeloma cells through positive
regulation of P53 and inhibition of DNA replication. Haematologica.
98:640–648. 2013. View Article : Google Scholar :
|
|
32
|
Huang SD, Yuan Y, Zhuang CW, Li BL, Gong
DJ, Wang SG, Zeng ZY and Cheng HZ: MicroRNA-98 and microRNA-214
post-transcriptionally regulate enhancer of zeste homolog 2 and
inhibit migration and invasion in human esophageal squamous cell
carcinoma. Mol Cancer. 11:512012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Penna E, Orso F, Cimino D, Tenaglia E,
Lembo A, Quaglino E, Poliseno L, Haimovic A, Osella-Abate S, De
Pittà C, et al: microRNA-214 contributes to melanoma tumour
progression through suppression of TFAP2C. EMBO J. 30:1990–2007.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen DL, Wang ZQ, Zeng ZL, Wu WJ, Zhang
DS, Luo HY, Wang F, Qiu MZ, Wang DS, Ren C, et al: Identification
of microRNA-214 as a negative regulator of colorectal cancer liver
metastasis via regulation of fibroglast growth factor 1 expression.
Hepatology. 60:598–609. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He F, Chen H, Yang P, Wu Q, Zhang T, Wang
C, Wei J, Chen Z, Hu H, Li W and Cao J: Gankyrin sustains
PI3K/GSK-3β/β-catenin signal activation and promotes colorectal
cancer aggressiveness and progression. Oncotarget. 7:81156–81171.
2016. View Article : Google Scholar : PubMed/NCBI
|