Introduction
Glioma accounts for 80% of malignant brain tumors
and has one of the highest mortality rates among all types of
cancer (1). The current standard
glioma treatment comprises surgical excision followed by
radiotherapy and chemotherapy (2). However, as glioma cells infiltrate
into the brain parenchyma, combination therapy can only minimally
prolong survival time and almost all patients relapse (3-5).
The high risk of recurrence following combination therapy is a
major therapeutic challenge. Thus, it is necessary to investigate
novel therapeutic approaches to glioma and the underlying mechanism
of the invasion and metastasis of glioma cells.
Tumor metastasis is a multi-step process that
comprises neovascularization and cell adhesion, invasion, migration
and proliferation, in which the crucial step is degradation of the
extracellular matrix (ECM) and basement membrane (6,7). A
growing body of evidence supports the perspective that tumor
progression is associated with the overexpression of matrix
metalloproteinases (MMPs) in tumor cells, particularly that of
MMP-2 and -9, which are able to modulate the tumor microenvironment
(8). Apart from their role in the
degradation of the ECM and cancer metastasis, MMPs also regulate
inflammatory processes, cell proliferation and angiogenesis
(9). These functions of MMPs
provide an alternative method for targeting cancer therapy. There
are multiple methods to regulate the activities of MMPs, including
regulation of the following: MMP mRNA expression, conversion from
the zymogen to the active enzyme, and tissue inhibitors of
metalloproteinases (TIMPs) (9). A
number of studies have demonstrated that overexpression of TIMPs
can reduce tumor metastasis (10-12). In the TIMP family, TIMP-1 and -2
are able to suppress the activities of most known MMPs (11).
Peptide toxins have offered a novel approach for
targeted treatment of glioma. Chlorotoxin (CTX), a 36-amino-acid
peptide that is isolated from the venom of Leiurus
quinquestriatus (13), has
been considered to be a novel blocker of small conductance chloride
ion channels (14) and MMP-2
(15). It has an anti-invasive
effect that is due to inhibition of the enzymatic activity of MMP-2
and reduced expression of MMP-2 via its interactions with MMP-2
(15). Furthermore,
131I-labeled CTX is considered to be a promising agent
for imaging or therapy of glioma (16-18) and has been used in clinical trials
(19,20). Similarly, a CTX-like peptide
purified from Buthus martensii Karsch (BmK CT), which
possesses similar biological features to CTX, has been demonstrated
to inhibit glioma cell proliferation, migration and invasion
(21). Our previous studies have
revealed that BmK CT can specifically bind to C6 glioma cells and
suppress cell invasion through downregulation of MMP-2 expression
(22-24), while not affecting mRNA expression
of MMP-2 at the genetic level (24). However, these studies only
investigated the MMP-2 protein. Thus, the aim of the present study
was to further investigate the potential anti-metastasis mechanism
of BmK CT on glioma cells and the possibility of
131I-labeled BmK CT (131I-BmK CT) as a novel
targeted therapeutic agent for glioma.
In the present study study, Cell Counting Kit-8
(CCK-8) and plate colony formation assays demonstrated that BmK CT
had no influence on cell proliferation at concentrations of 0-1
mg/ml, while a significant reduction in proliferation was observed
in the glioma cells treated with 131I-BmK CT.
Furthermore, the results of the cell cycle assay demonstrated that
only 131I-BmK CT could induce evident changes in the
cell cycle and arrest glioma cells at the S and G2/M
phases. Transwell invasion and wound healing assays demonstrated
that 131I-BmK CT possessed a stronger effect than BmK CT
in terms of inhibiting cell migration and invasion. Furthermore,
western blotting, ELISA, immunofluorescence and a gelatin
zymography assay demonstrated that both 131I-BmK CT and
BmK CT were able to downregulate the expression of MMP-2 and -9
proteins, and upregulate that of TIMP-2 protein. Notably,
131I-BmK CT was more effective than BmK CT at inhibiting
cell migration and invasion and the enzyme activity of MMP-2 and
-9, but not at inhibiting the protein expression of MMP-2, MMP-9
and TIMP-2. The results indicate that BmK CT inhibits the
metastasis of U87MG cells via regulation of TIMP-2 expression and
that 131I-BmK CT has the potential to be a novel
targeted drug for glioma therapy.
Materials and methods
Materials
U87MG cells (CVCL_0022), human glioblastoma cells of
unknown origin, were purchased from American Type Culture
Collection (Manassas, VA, USA). Dulbecco's modified Eagle's medium
(DMEM), fetal bovine serum (FBS), streptomycin, penicillin and
CCK-8 were purchased from Shanghai DoBio Biotech Co., Ltd.
(Shanghai, China). Rabbit anti-MMP-2 (cat. no. 40994), -MMP-9 (cat.
no. 13667) and -TIMP-2 (cat. no. 5738) monoclonal antibodies were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
PI/RNase staining buffer and mouse anti-β-actin antibody were
obtained from Beyotime Institute of Biotechnology (Haimen, China).
BmK CT was purchased from Chinese Peptide Company, Ltd. (Hangzhou,
China). Na131I solution was supplied by Shanghai GMS
Pharmaceutical Co., Ltd. (Shanghai, China). Disposable PD-10
desalting columns were procured from GE Healthcare (Chicago, IL,
USA). All other chemicals and solvents were supplied by Sinopharm
Chemical Reagent Co., Ltd. (Shanghai, China).
Synthesis of 131I-BmK CT
For the 131I radiolabeling, a tyrosine
was modified in the N-terminus of BmK CT that could be labeled with
131I easily using the chloramine-T method. Briefly,
sterile Na131I solution (370-740 MBq; 0.1-0.2 ml) was
mixed with 0.2 ml PBS containing BmK CT (200 µg) and
chloramine-T (100 µg). The mixture was continuously stirred
for 5 min at room temperature and then 100 µg
Na2S2O5 was added to stop the
reaction. Subsequently, 131I-BmK CT was purified from
the reaction mixture using disposable PD-10 desalting columns. In
the follow-up experiments, the specific activity was 10 mCi/mg.
Cell proliferation assay
U87MG cells were cultured in DMEM supplemented with
10% FBS, 1% streptomycin and 1% penicillin at 37°C with 5%
CO2 as described previously (25,26). Cell viability was examined using a
CCK-8. Briefly, U87MG cells were seeded in 96-well plates (5,000
cells/well) and treated with different concentrations of BmK CT (0,
0.1, 1, 10, 100 and 1,000 µg/ml) or 131I-BmK CT
(0, 12.5, 25, 50, 100 and 200 µCi/ml) for 24, 48 and 72 h at
37°C. CCK-8 solution (10 µl) was then added to each well and
cells were incubated for a further 1 h at 37°C. Subsequently, the
absorbance at 450 nm was measured using a Gen5 micro-plate reader
(BioTek Instruments, Inc., Winooski, VT, USA).
Plate colony formation assay
In order to evaluate colony formation, 400 log-phase
U87MG cells were seeded in 6-well plates and then treated with BmK
CT (0, 0.05, 0.5, 5, 10 and 20 µg/ml) or 131I-BmK
CT (0, 0.5, 5, 50, 100 and 200 µCi/ml) for 24 h.
Subsequently, the culture medium was replaced with fresh culture
medium. Following incubation at 37°C for 10 days, the cells were
fixed in methyl alcohol and stained with crystal violet for 15 min
at room temperature. The colonies were then counted and images of
the plates were captured.
Cell cycle analysis
U87MG cells were seeded at a density of
2×105 cells/ml in 6-well culture plates and then were
treated with different concentrations of BmK CT (0, 5, 10 and 20
µg/ml) or 131I-BmK CT (0, 50, 100 and 200
µCi/ml). Following incubation for 72 h at 37°C, the cells
were trypsinized, washed twice with PBS and fixed with 70% ethanol
overnight at 4°C. The cells were then resuspended in PI/RNase
staining buffer for 30 min at 37°C and analyzed using flow
cytometry (BD Accuri C6 Software; version 1. 0. 264. 21; BD
Biosciences, San Jose, CA, USA).
Transwell invasion assay
Transwell inserts (Corning Incorporated, Corning,
NY, USA) were coated with Matrigel (BD Biosciences) to form a thin
layer of gel and incubated overnight at 37°C. U87MG cells were
treated with PBS, Na131I (0.5, 5 and 50 µCi/ml),
BmK CT (0.05, 0.5 and 5 µg/ml) or 131I-BmK CT
(0.5, 5 and 50 µCi/ml) for 24 h. A total of 5×104
cells were seeded in the upper chamber with serum-free DMEM medium
and then the chambers were placed into a 24-well plate containing
complete medium. Following incubation for 24 h at 37°C, cells on
the upper surface of the chambers were removed with a cotton swab
and the chambers were washed 3 times with PBS. Subsequently, the
cells on the lower surface of the chambers were fixed with methanol
for 15 min and stained with crystal violet solution for 30 min at
37°C. The number of invaded cells was counted under an inverted
microscope (magnification, ×200).
Wound healing assay
U87MG cells were seeded at a density of 4
×105 cells/ml in a 6-well plate to form a confluent
monolayer. A wound was made with a pipette tip and then fresh
medium containing the treatment (PBS, 50 µCi/ml
Na131I, 5 µg/ml BmK CT or 50 µCi/ml
131I-BmK CT) was added to the wells. Then the cells were
incubated at 37°C. Images of the cells were captured at 0, 12 and
24 h with an inverted phase contrast microscope, and cell migration
was assessed by measuring the size of the gap in multiple fields.
The microscope images were quantified by a distance measurement.
The magnification of the microscope was set as ×100.
Western blotting assays
The primary antibodies used were rabbit anti-MMP-2,
-MMP-9 and -TIMP-2 (1:1,000). Horseradish peroxidase-conjugated
goat anti-mouse or anti-rabbit secondary antibodies (cat. no. 7076
and 7074; 1:10,000; Cell Signaling Technology, Inc.) were used to
detect the primary antibodies on the western blots. Briefly, cells
were incubated with PBS, BmK CT (5 µg/ml), Na131I
or 131I-BmK CT (50 µCi/ml) for 72 h at 37°C.
Total protein from the U87MG cells was isolated using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology) and the protein concentration was measured using a
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology). A total of 20 µg total protein was separated
by 10% SDS-PAGE and transferred onto a polyvinylidene difluoride
(PVDF) membrane. The PVDF membranes weres then blocked in PBS
containing 5% skimmed milk and 0.1% Tween-20 for 2 h at room
temperature. Subsequently, the membranes were incubated at 4°C
overnight with the primary antibodies, followed by incubation with
the corresponding secondary antibody for 1 h at room temperature.
The bands were visualized with enhanced chemiluminescence reagents
(Beyotime Institute of Biotechnology). The expression of the
proteins was quantified using ImageJ software (version 1.52a;
National Institutes of Health, Bethesda, MD, USA) and compared with
that of the control (β-actin).
Confocal microscopy
U87MG cells were seeded at a density of
5×104 cells/ml in glass bottom cell culture dishes. The
cells were initially incubated in medium containing the treatment
for 48 h and the incubation conditions were the same as those of
the western blotting assay. Subsequently, cells were fixed with 4%
paraformaldehyde solution for 15 min at room temperature. The cells
were then washed 3 times with PBS, permeabilized with 0.2% Triton
X-100 for 15 min at 37°C and blocked with 5% bovine serum albumin
(cat. no. P0260; Shanghai DoBio Biotech Co., Ltd.) in PBS for 30
min at room temperature. Following blocking, cells were incubated
with the appropriate primary antibody (MMP-2, MMP-9 and TIMP-2;
1:250) overnight at 4°C. The cells were then washed 3 times with
PBS and stained with fluorescein isothiocyanate-labelled goat
anti-rabbit immunoglobulin G (cat. no. SAB3700873; 1:300;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 1 h at 37°C.
Subsequently, the cells were washed 3 times with PBS and were then
stained with DAPI for 2 min at room temperature. Images were
captured using a confocal microscope (FV300; Olympus Corporation,
Tokyo, Japan).
ELISA
Rat MMP-2, MMP-9 and TIMP-2 ELISA kits were
purchased from Sigma-Aldrich (cat. nos. RAB1916, RAB1112 and
RAB1156, respectively; Merck KGaA). U87MG cells were treated with
BmK CT (0.05, 0.5 and 5 µg/ml), Na131I or
131I-BmK CT (0.5, 5 and 50 µCi/ml) for 72 h at
37°C. The secreted proteins MMP-2, MMP-9 and TIMP-2, which are
produced by U87MG cells, in the supernatant from each well were
evaluated via ELISA. Briefly, 96-well ELISA plates were coated with
the appropriate antibodies (MMP-2, MMP-9 and TIMP-2) at 4°C
overnight. Subsequently, the supernatants of the cell cultures (100
µl) collected by centrifugation (3,661 x g; 15 min; 4°C)
were added to the plates and the plates were incubated for 1 h at
37°C. The plates were washed 5 times with PBS and then incubated
with the horseradish peroxidase-conjugated detection antibodies
provided with each kit for 1 h at 37°C. The plates were washed a
further 5 times and were then incubated for 15 min at 37°C with
tetramethyl benzidine dilution. The reaction was stopped with 2N
H2SO4 and the absorbance at 450 nm was
determined using a microplate reader (BioTek Instruments,
Inc.).
Gelatin zymography assay
The activities of MMP-2 and -9 were examined using a
gelatin zymography assay. U87MG cells were treated with PBS, BmK CT
(0.05, 0.5 and 5 µg/ml) or 131I-BmK CT (0.5, 5
and 50 µCi/ml) for 72 h at 37°C. Protein was isolated and
the concentration determined as in the western blotting protocol.
Total protein (10 µg) was separated using 10% SDS-PAGE that
included 1% gelatin (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) without prior heating or reduction. The gel was
washed with 2.5% Triton-X 100 to strip the SDS and incubated with
developing buffer (50 mM Tris-HCl, 150 mM NaCl and 5 mM
CaCl2) for 16 h at 37°C. Following staining with 0.5%
Coomassie Brilliant Blue R-250 (Sigma-Aldrich; Merck KGaA) for 3 h
at room temperature, the gel was destained and photographed. Signal
intensities were measured by densitometry using Bio-1D software,
version 15.01 (Vilber Lourmat Deutschland GmbH, Eberhardzell,
Germany).
Statistical analysis
Data are presented as the mean ± standard deviation.
All results were analyzed using SPSS software, version 20.0 (IBM
Corp., Armonk, NY, USA). Student's t-test was used to analyze the
variances between two groups. Multi-group comparisons of the means
were carried out by one-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
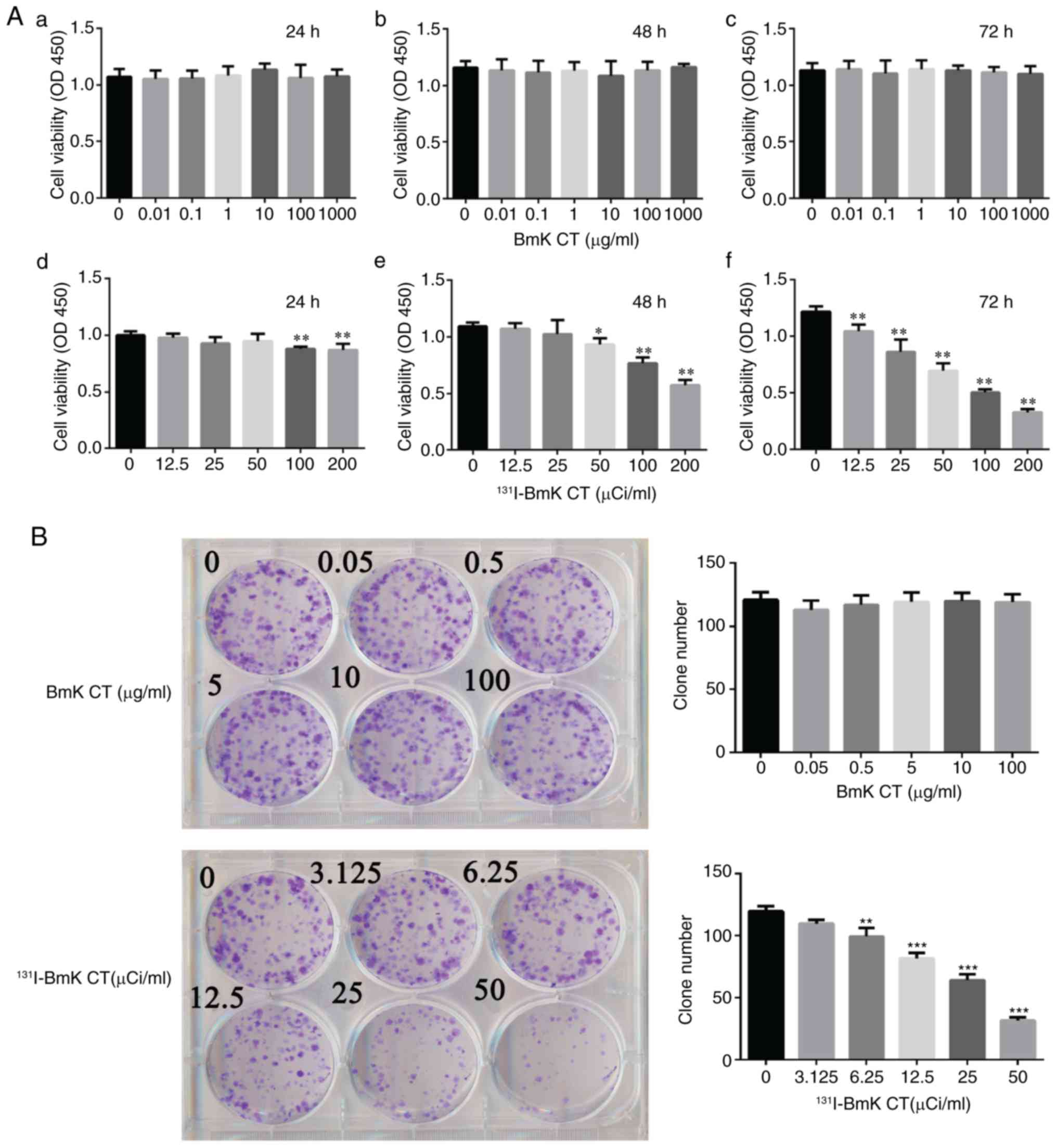
Results
131I-BmK CT inhibits U87MG
cell proliferation
In order to investigate the proliferation of U87MG
cells treated with BmK CT or 131I-BmK CT, CCK-8 and
plate colony formation assays were performed. The viability of the
U87MG cells did not change significantly following treatment with
BmK CT at concentrations of 0-1,000 µg/ml (Fig. 1Aa-c). However, there was
significant inhibition in the growth of the cells in the
131I-BmK CT group. This effect was time- (24-72 h) and
dose-dependent (0-200 µCi/ml; Fig. 1Ad-f). The half maximal inhibitory
concentration of the 131I-BmK CT cells at the 72-h time
point was 50 µCi/ml. In addition, the colony formation assay
demonstrated that 131I-BmK CT reduced colony formation
compared with the blank control (Fig.
1B). These results demonstrated that 131I-BmK CT
could inhibit U87MG cell proliferation, whereas BmK CT had no
significant influence on cell viability at a concentration of
≤1,000 µg/ml.
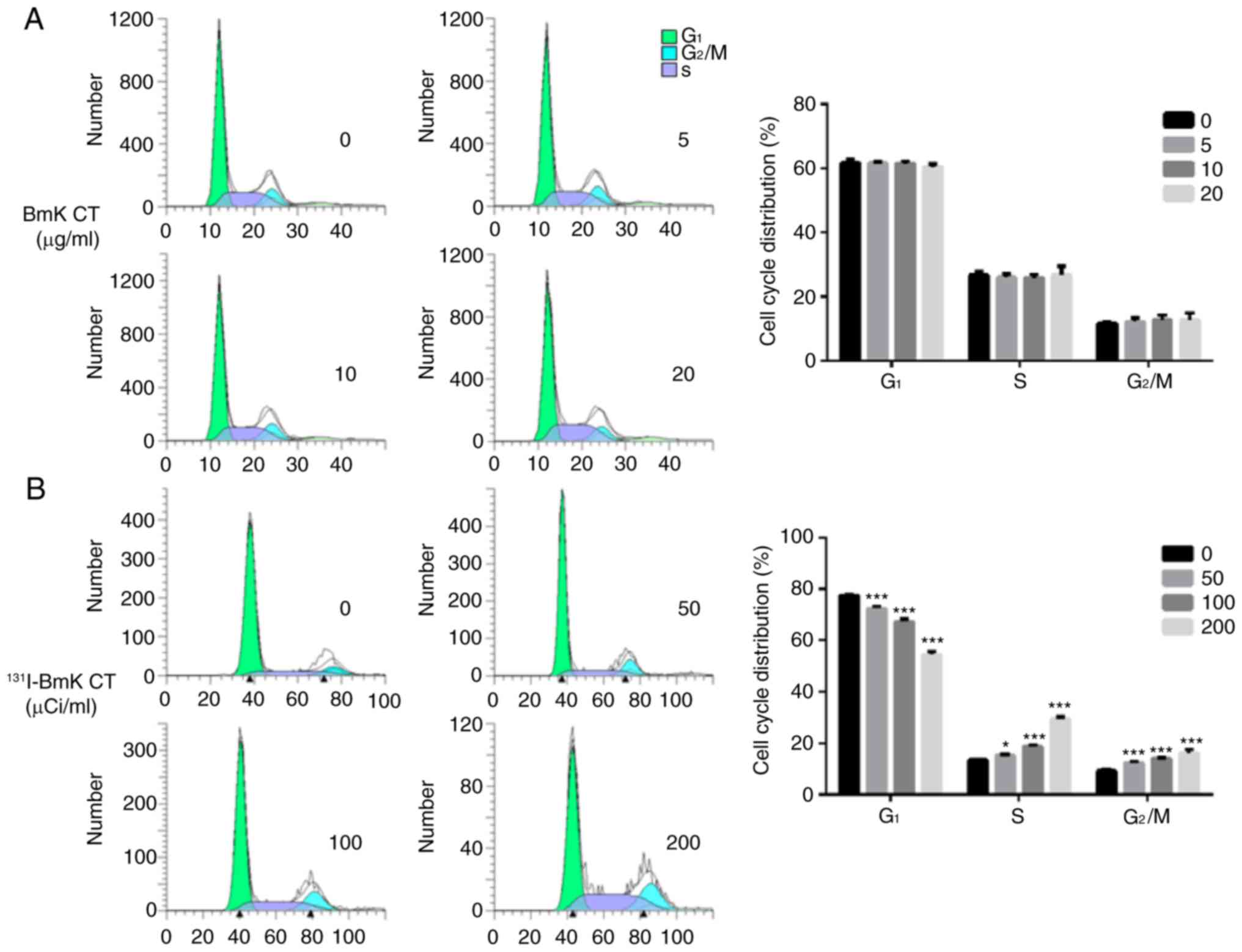
131I-BmK CT induces changes in
the cell cycle of U87MG cells
As presented in Fig.
2A, BmK CT (0, 5, 10 and 20 µg/ml) did not significantly
affect the cell cycle progression of U87MG cells. In contrast to
blank control, 131I-BmK CT (50, 100 and 200
µCi/ml) significantly increased the proportion of U87MG
cells in the S and G2/M phases and reduced the
proportion of cells in the G1 phase (Fig. 2B). These results suggest that
131I-BmK CT inhibits cell proliferation by inducing cell
cycle arrest at the S and G2/M phases.
131I-BmK CT inhibits U87MG
cell invasion and migration
Tumor metastasis depends on the capacity of tumor
cells to invade and migrate to distant sites. Transwell invasion
assays demonstrate the invasive capacity of tumor cells. The
results in the present study demonstrated that the number of cells
that passed through the membrane was reduced following treatment
with BmK CT and 131I-BmK CT. As the treatment
concentration increased, a significant reduction in the cell number
was observed (Fig. 3A). As
presented in Fig. 3B, the results
of wound healing assay in vitro demonstrated that 24 h
following scraping, the cells in the PBS group had migrated across
the wound. The percentage of wound closure in the
Na131I, BmK CT and 131I-BmK CT groups was
78.3±19.6, 56.6±10.0 and 45.0±8.2%, respectively, and these results
were statistically significant compared with the blank control. The
Transwell and wound healing assays demonstrated that BmK CT can
inhibit the invasion and migration of U87MG cells, and that
131I-BmK CT has a more marked effect than BmK CT at the
same concentration.
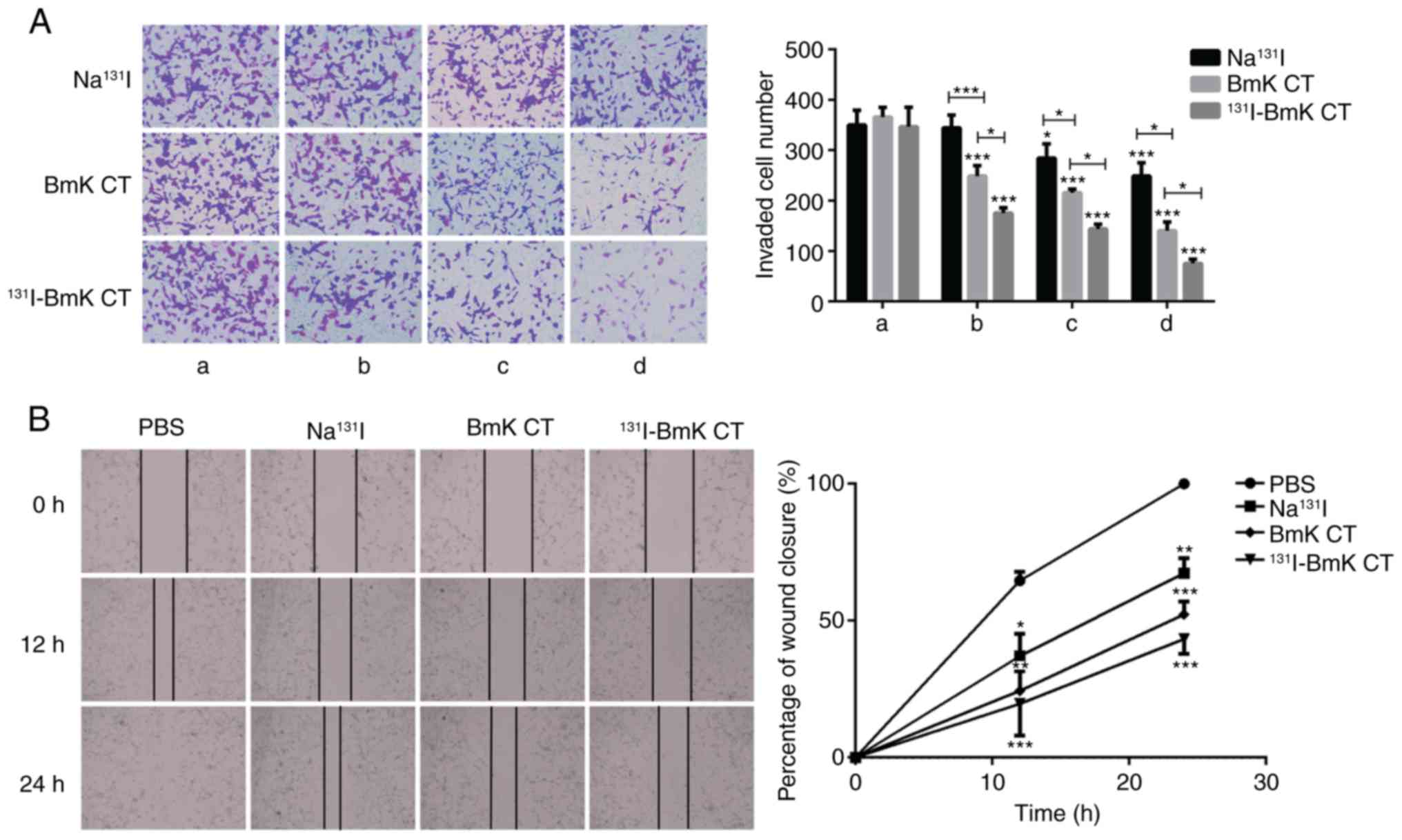 | Figure 3BmK CT inhibited U87MG cell invasion
and migration. (A) The number of invaded cells was significantly
lower in the groups treated with BmK CT or 131I-BmK CT.
The concentration of group BmK CT was (a) 0, (b) 0.05, (c) 0.5 and
(d) 5 µg/ml. The concentration of group 131I-BmK
CT was (a) 0, (b) 0.5, (c) 5 and (d) 50 µCi/ml (The specific
activity was 10 mCi/ml). The concentration of group
Na131I was (a) 0, (b) 0.5, (c) 5 and (d) 50
µCi/ml. Magnification, ×200. (B) Migration of U87MG cells
following the different treatments was detected using a
wound-healing assay (magnification, ×100). *P<0.05,
**P<0.01 and ***P<0.001. BmK CT,
Buthus martensii Karsch chlorotoxin; 131I-BmK CT,
131I-labeled BmK CT. |
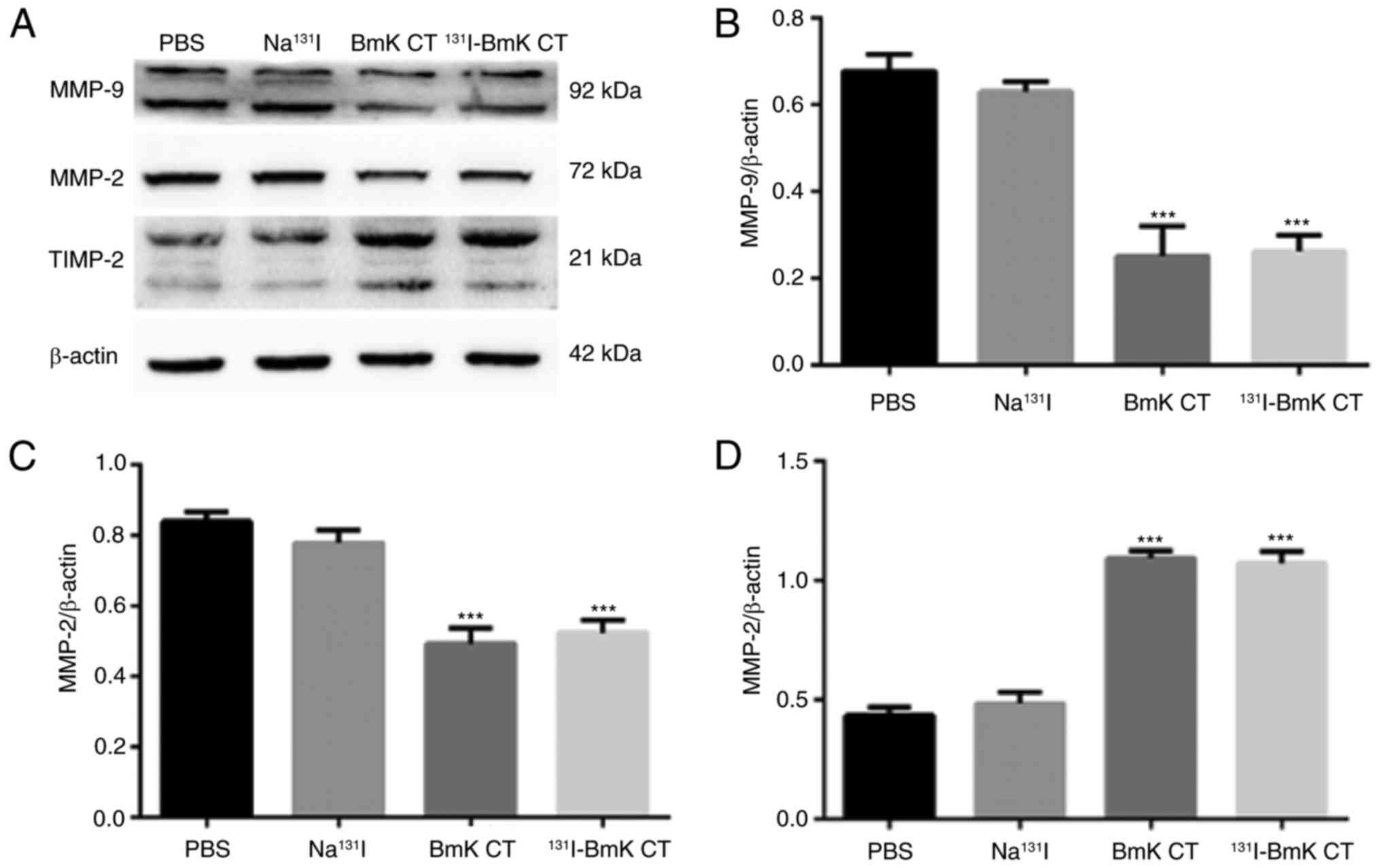
131I-BmK CT regulates the
expression of MMP-2, MMP-9 and TIMP-2 in U87MG cells
Western blotting demonstrated that BmK CT
upregulated TIMP-2 and downregulated MMP-2 and -9 in U87MG cells
compared with the blank control (Fig.
4). These results indicate that BmK CT upregu-lates TIMP-2 and
downregulates MMP-2 and -9. Furthermore, 131I-BmK CT was
not significantly more effective than BmK CT. The upregulation of
TIMP-2 and downregulation of MMP-2 and -9 were also visualized by
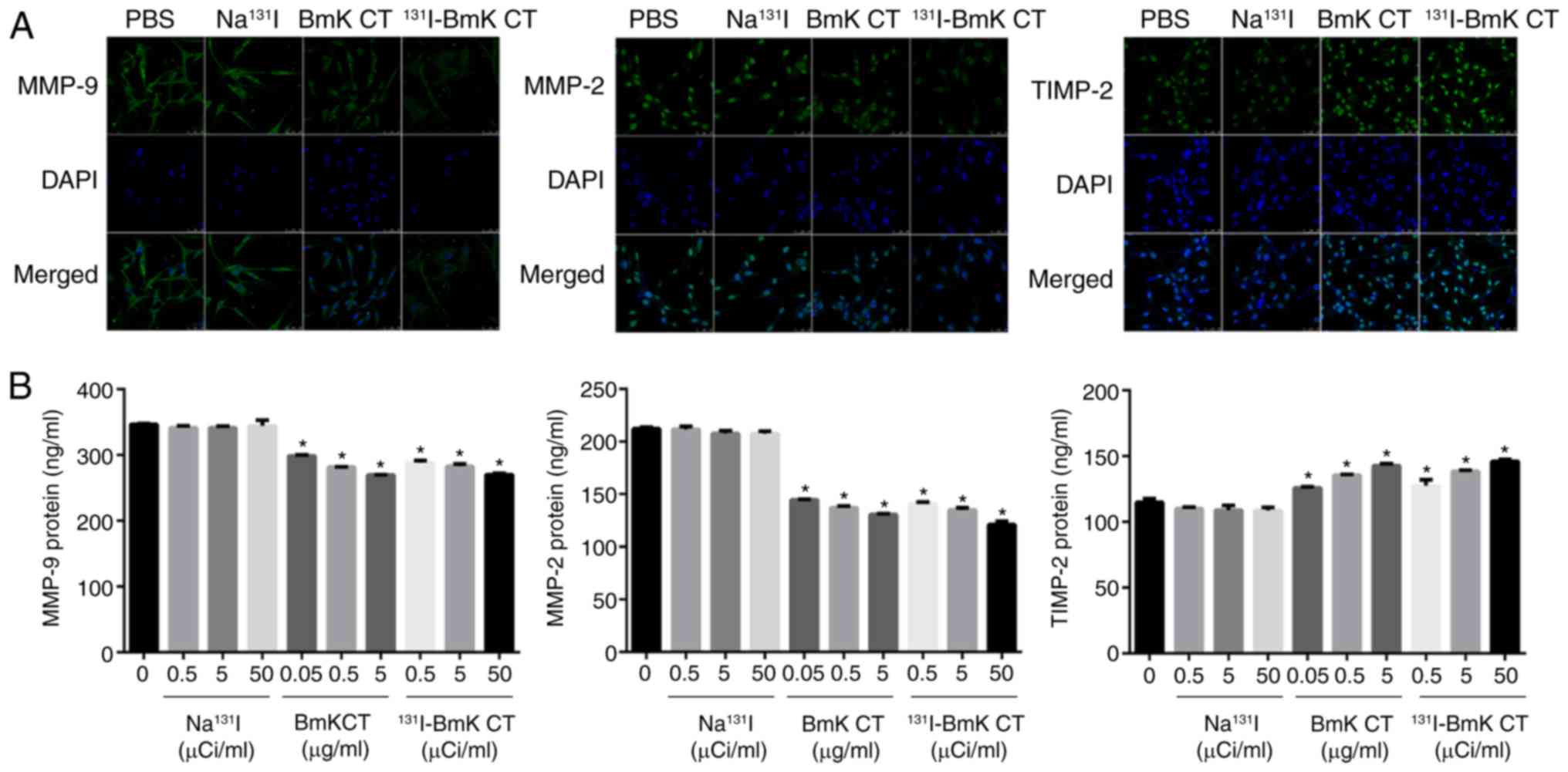
confocal imaging of the U87MG cells (Fig. 5A).
131I-BmK CT stimulates the
secretion of TIMP-2 and suppresses the expression of MMP-2 and
-9
ELISA was used to investigate the effect of BmK CT
and 131I-BmK CT on the secretion of MMP-2, MMP-9 and
TIMP-2 in U87MG cells. The results demonstrated that BmK CT and
131I-BmK CT inhibited the secretion of MMP-2 and -9, and
stimulated the secretion of TIMP-2 in glioma cells (Fig. 5B). These results were in
accordance with those of the western blotting.
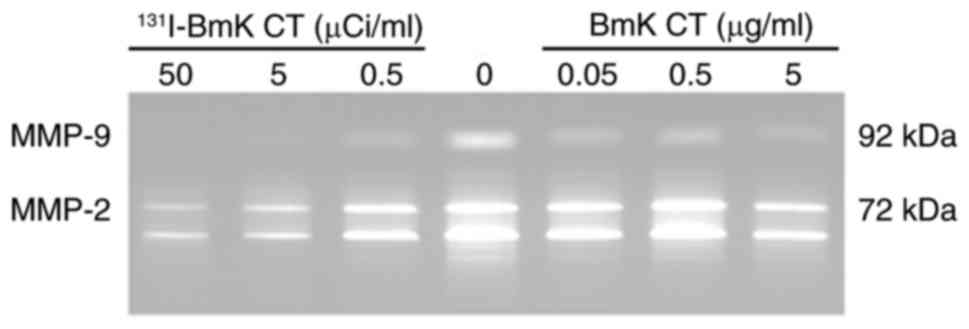
131I-BmK CT reduces the
enzymatic activities of MMP-2 and -9 that are secreted by U87MG
cells
The enzymatic activities of MMP-2 and -9 in the
treated groups were analyzed and compared with the blank control
using gelatin zymography. The activities of MMP-2 and -9 were
reduced in the treated groups in a dose-dependent manner (Fig. 6), which may partially explain the
inferior ability of cells to migrate and invade following treatment
with BmK CT or 131I-BmK CT.
Discussion
As malignant glioma is difficult to treat and recurs
easily, novel therapies are urgently required. BmK CT has been
identified as a small peptide that can specifically bind to glioma
cells and inhibit their metastasis (21,27). Certain previous studies also
demonstrated that BmK CT can inhibit proliferation glioma cells
(21,24), but similar results were not
obtained in the present study. The proliferation of U87MG cells
treated with BmK CT (0-1,000 µg/ml) was assessed. Although
the results do not demonstrate outcomes such as its
anti-proliferative effect, the data demonstrated that BmK CT did
not affect cell survival following 24, 48 and 72 h of treatment.
These results suggest that BmK CT may be non-toxic at
concentrations of ≤1,000 µg/ml. The results of the plate
colony formation assay and flow cytometric analysis also supported
this. The invasion and migration of U87MG cells was subsequently
investigated. The Transwell invasion assay indicated that BmK CT
exposure (0.05-5 µg/ml) for 24 h inhibited the invasiveness
of U87MG cells in a dose-dependent manner. The wound-healing assay
demonstrated that BmK CT exposure significantly inhibited U87MG
cell migration. Notably, the data demonstrated that the
anti-invasive effect of BmK CT on U87MG cells was not due to its
cytotoxicity and that 131I-BmK CT was more effective
than BmK CT at inhibiting the invasion and migration of U87MG
cells.
A previous study demonstrated that BmK CT displayed
anti-metastatic ability and inhibited glioma cell invasion via
MMP-2 (28). Invasion and
metastasis are the later stages of tumor progression and are the
major cause of treatment failure in patients with cancer due to the
spreading of tumor cells from the localized stage to distant sites
in order to form a secondary tumor (29). ECM degradation is a fundamental
step in the process of tumor metastasis (30). MMPs that can degrade and remodel
the ECM serve a major role in tumor growth, angiogenesis and
metastasis. Without MMPs, tumor cells would be unable to cross
through the ECM barriers that restrict their migration (31). MMP-2 and -9 are the key enzymes in
the MMP family due to their critical role in ECM degradation
(32). The enzymatic activity of
MMPs is fundamental to their physiological role in tumor
progression. BmK CT has been demonstrated to downregulate MMP-2
expression (28). In the present
study, the western blotting results, laser scanning confocal images
and ELISA results demonstrated that the levels of MMP-2 and -9 in
or secreted by U87MG cells were reduced. The enzyme activities of
MMP-2 and -9 were measured by gelatin zymography and the results
indicated that the enzyme ability of MMP-2 and -9 to degrade
gelatin was reduced.
MMPs can be regulated by TIMPs. TIMPs are the
natural endogenous inhibitors of MMP proteins and the family
contains four members (TIMP-1, -2, -3 and -4) (33). Among the TIMP family, TIMP-2
serves an essential role in regulating the activities of MMP-2 and
-9. Furthermore, increasing the expression of TIMP-2 has been
demonstrated to suppress cell invasion (34,35). Therefore, an aim of the present
study was to investigate whether BmK CT could reduce the expression
of MMP-2 and -9 through TIMP-2. The western blotting results
demonstrated that TIMP-2 was increased in the U87MG cells compared
with the control group, which was further supported by the laser
scanning confocal images and the ELISA results, suggesting that
TIMP-2 has a significant influence on the expression of MMP-2 and
-9 and the invasiveness of U87MG cells. These results indicate that
the BmK CT may downregulate MMP-2 and -9 by upregulating
TIMP-2.
131I has been widely used for the therapy
of patients with cancer (36-38). Its therapeutic effect is due to
its β radiation, whereas the γ rays can be used for SPECT imaging.
The β emission results in the production of free radicals and toxic
substances that can damage crucial macromolecules, including DNA,
cell membranes and enzymes, and can cause cell death (39). These functions allow the
combination of targeted tumor-specific agents to this radionuclide.
Due to the high affinity to thyroid, 131I has been
routinely used in radionuclide therapy and imaging of thyroid
diseases, especially thyroid cancer (37,40), and 131I whole-body
SPECT imaging has been developed as a diagnostic method in
detecting recurrences or metastases from thyroid carcinoma
(41). Clinical trails have
demonstrated the safety of 131I-labeled CTX used in the
treatment of glioma patients (19,20). As a CTX-like peptide,
131I-BmK CT will likely not affect normal tissues.
However, some relative experiments should be performed to elucidate
the safety and radiation dose in vivo which will be reported
in our further work. In the present study, it was demonstrated that
not only can 131I-BmK CT inhibit metastasis, but it can
also induce cell apoptosis. Although there was no obvious advantage
in the effectiveness of 131I-BmK CT at downregulating
MMP-2 and -9 by upregulating TIMP-2 compared with BmK CT, it was
more effective in reducing the enzyme abilities of MMP-2 and -9.
The CCK-8 and plate colony formation assays demonstrated that
131I-BmK CT had a marked effect on U87MG cell
proliferation. The effect of 131I-BmK CT on the cell
cycle was analyzed and the cells were demonstrated to be arrested
in the S phase. The main reason that 131I-BmK CT
demonstrated a stronger effect than BmK CT was due to the presence
of 131I emitting the β minus that may induce cell
apoptosis and inhibit the invasion and migration of U87 cells. This
finding is consistent with a previous study, which demonstrated
that 131I-BmK CT significantly induced cell apoptosis
(22). Induction of cell
apoptosis by 131I-BmK CT is a good explanation for its
superior inhibitory effect on glioma cell invasion. In the present
study, the effectiveness of BmK CT and 131I-BmK CT at
regulating MMP-2, MMP-9 and TIMP-2 were similar due to the presence
of BmK CT. Following 131I radiolabeling,
131I-BmK CT induced apoptosis and fewer cells passing
through the membrane in the Transwell invasion assay were observed,
which was in accordance with the results of the wound healing
assay.
Tumor-specific radionuclide therapy ensures the
preferential accumulation of radiopharmaceuticals in tumor cells
and not in normal tissue. As a monotherapy, BmK CT could inhibit
metastasis and 131I-BmK CT could be used in imaging and
therapy of malignant glioma. Following BmK CT binding to glioma
cells (15), the β radiation from
131I induces cell death. This therapeutic method could
increase the radiation reaching tumor tissues, decrease the
radioactive damage to surrounding tissues and reduce the chance of
relapse. However, the effect of 131I-BmK CT in glioma
therapy in vivo requires investigation in future
studies.
In conclusion, the present study demonstrated that
BmK CT inhibits U87MG cell metastasis, invasion and migration by
downregulating MMP-2 and -9 expression and upregulating TIMP-2
expression. Additionally, BmK CT and 131I-BmK CT are
able to suppress the activities of MMP-2 and -9. Therefore,
131I-BmK CT may be a promising therapeutic treatment for
malignant glioma. 131I is suitable for imaging and dose
determination purposes and is simple to conjugate. In order to
further determine the mechanism of the effect of BmK CT on glioma
cell invasion, in vivo studies are required.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81671712 and
81401440), Shanghai Sailing Program (grant no. 16YF1409300) and
West China First-Class Disciplines Basic Medical Sciences at
Ningxia Medical University (grant no. NXYLXK2017B07). The present
study was also supported by the platform of Shandong Co-Innovation
Center of Classic TCM Formula, Scientific Innovation Team of
Shandong University of Traditional Chinese Medicine, Shandong
Province University Scientific Research Project (grant no.
J18KZ014).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
WLQ and JHZ conceived and designed the experiments.
SW, KM, LZZ and MLZ performed the experiments. CCL and LLG analyzed
the data. YX provided the analysis tools. SW and LZZ wrote the
manuscript. MLZ and JHZ edited and proofread this manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Goodenberger ML and Jenkins RB: Genetics
of adult glioma. Cancer Genet. 205:613–621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cuddapah VA, Robel S, Watkins S and
Sontheimer H: A neuro-centric perspective on glioma invasion. Nat
Rev Neurosci. 15:455–465. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Franceschi E, Tosoni A, Girardi F and
Brandes AA: Bevacizumab in brain tumors: Ready for primetime.
Future Oncol. 5:1183–1184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu Z, Zhao G, Zhang Z, Li Y, Chen Y, Wang
N, Zhao Z and Xie G: Efficacy and safety of bevacizumab for the
treatment of glioblastoma. Exp Ther Med. 11:371–380. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hou LC, Veeravagu A, Hsu AR and Tse VC:
Recurrent glioblastoma multiforme: A review of natural history and
management options. Neurosurg Focus. 20:E52006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coussens LM, Fingleton B and Matrisian LM:
Matrix metalloproteinase inhibitors and cancer: Trials and
tribulations. Science. 295:2387–2392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fidler IJ, Kim SJ and Langley RR: The role
of the organ micro-environment in the biology and therapy of cancer
metastasis. J Cell Biochem. 101:927–936. 2007. View Article : Google Scholar
|
|
8
|
Stetler-Stevenson WG: Dynamics of matrix
turnover during pathologic remodeling of the extracellular matrix.
Am J Pathol. 148:1345–1350. 1996.PubMed/NCBI
|
|
9
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Figueira RC, Gomes LR, Neto JS, Silva FC,
Silva ID and Sogayar MC: Correlation between MMPs and their
inhibitors in breast cancer tumor tissue specimens and in cell
lines with different metastatic potential. BMC Cancer. 9:202009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimoda M, Jackson HW and Khokha R: Tumor
suppression by stromal TIMPs. Mol Cell Oncol. 3:e9750822016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Groblewska M, Siewko M, Mroczko B and
Szmitkowski M: The role of matrix metalloproteinases (MMPs) and
their inhibitors (TIMPs) in the development of esophageal cancer.
Folia Histochem Cytobiol. 50:12–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
DeBin JA and Strichartz GR: Chloride
channel inhibition by the venom of the scorpion Leiurus
quinquestriatus. Toxicon. 29:1403–1408. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lyons SA, O'Neal J and Sontheimer H:
Chlorotoxin, a scorpion-derived peptide, specifically binds to
gliomas and tumors of neuroectodermal origin. Glia. 39:162–173.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deshane J, Garner CC and Sontheimer H:
Chlorotoxin inhibits glioma cell invasion via matrix
metalloproteinase-2. J Biol Chem. 278:4135–4144. 2003. View Article : Google Scholar
|
|
16
|
Soroceanu L, Gillespie Y, Khazaeli MB and
Sontheimer H: Use of chlorotoxin for targeting of primary brain
tumors. Cancer Res. 58:4871–4879. 1998.PubMed/NCBI
|
|
17
|
Hockaday DC, Shen S, Fiveash J,
Raubitschek A, Colcher D, Liu A, Alvarez V and Mamelak AN: Imaging
glioma extent with 131I-TM-601. J Nucl Med. 46:580–586.
2005.PubMed/NCBI
|
|
18
|
Shen S, Khazaeli M, Gillespie GY and
Alvarez VL: Radiation dosimetry of 131I-chlorotoxin for
targeted radiotherapy in glioma-bearing mice. J Neurooncol.
71:113–119. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mamelak AN, Rosenfeld S, Bucholz R,
Raubitschek A, Nabors LB, Fiveash JB, Shen S, Khazaeli MB, Colcher
D, Liu A, et al: Phase I single-dose study of
intracavitary-administered iodine-131-TM-601 in adults with
recurrent high-grade glioma. J Clin Oncol. 24:3644–3650. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng YJ, Zhao JH, Qiao WL and Chen K:
Recent advances in diagnosis and treatment of gliomas using
chlorotoxin-based bioconjugates. Am J Nucl Med Mol Imaging.
4:385–405. 2014.PubMed/NCBI
|
|
21
|
Fan S, Sun Z, Jiang D, Dai C, Ma Y, Zhao
Z, Liu H, Wu Y, Cao Z and Li W: BmKCT toxin inhibits glioma
proliferation and tumor metastasis. Cancer Lett. 291:158–166. 2010.
View Article : Google Scholar
|
|
22
|
Zhao JH, Qiao WL, Zhang Y and Shao X:
Preparation and in vitro evaluation of 131I-BmK CT as a
glioma-targeted agent. Cancer Biother Radiopharm. 25:353–359. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qiao WL, Zhao JH, Shao X, Zhang Z, Liu X,
Wang T, Jin W and Yao Y: Preparation of 131I-BmK CT and
bio-distribution and imaging in glioma-bearing rats. Nucl Tech.
34:213–216. 2011.
|
|
24
|
Fu YJ, Yin LT, Liang AH, Zhang CF, Wang W,
Chai BF, Yang JY and Fan XJ: Therapeutic potential of
chlorotoxin-like neurotoxin from the Chinese scorpion for human
gliomas. Neurosci Lett. 412:62–67. 2007. View Article : Google Scholar
|
|
25
|
Fu YJ, An N, Chan KG, Wu YB, Zheng SH and
Liang AH: A model of BmK CT in inhibiting glioma cell migration via
matrix metalloproteinase-2 from experimental and molecular dynamics
simulation study. Biotechnol Lett. 33:1309–1317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mignatti P and Rifkin DB: Biology and
biochemistry of proteinases in tumor invasion. Physiol Rev.
73:161–195. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sternlicht MD and Werb Z: How matrix
metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol.
17:463–516. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim SK, Kang SW, Park HJ, Ban JY, Oh CH,
Chung JH, Oh IH, Cho KB and Park MS: Meta-analysis of association
of the matrix metalloproteinase 2 (-735 C/T) polymorphism with
cancer risk. Int J Clin Exp Med. 8:17096–17101. 2015.
|
|
30
|
Schulz R: Intracellular targets of matrix
metalloproteinase-2 in cardiac disease: Rationale and therapeutic
approaches. Annu Rev Pharmacol Toxicol. 47:211–242. 2007.
View Article : Google Scholar
|
|
31
|
Valente P, Fassina G, Melchiori A,
Masiello L, Cilli M, Vacca A, Onisto M, Santi L, Stetler-Stevenson
WG and Albini A: TIMP-2 over-expression reduces invasion and
angiogenesis and protects B16F10 melanoma cells from apoptosis. Int
J Cancer. 75:246–253. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Su Y, Wan D and Song W: Dryofragin
inhibits the migration and invasion of human osteosarcoma U2OS
cells by suppressing MMP-2/9 and elevating TIMP-1/2 through
PI3K/AKT and p38 MAPK signaling pathways. Anticancer Drugs.
27:660–668. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schlumberger M, Catargi B, Borget I,
Deandreis D, Zerdoud S, Bridji B, Bardet S, Leenhardt L, Bastie D,
Schvartz C, et al: Strategies of radioiodine ablation in patients
with low-risk thyroid cancer. N Engl J Med. 366:1663–1673. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gruenwald F and Ezziddin S:
131I-Metaiodobenzylguanidine therapy of neuroblastoma
and other neuroendocrine tumors. Semin Nucl Med. 40:153–163. 2010.
View Article : Google Scholar
|
|
35
|
Klutz K, Schaffert D, Willhauck MJ,
Gruenwald GK, Haase R, Wunderlich N, Zach C, Gildehaus FJ,
Senekowitsch-Schmidtke R, Goeke B, et al: Epidermal growth factor
receptor-targeted 131I-therapy of liver cancer following
systemic delivery of the sodium iodide symporter gene. Mol Ther.
19:676–685. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kayano D and Kinuya S: Current consensus
on I-131 MIBG therapy. Nucl Med Mol Imaging. 52:254–265. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sgouros G, Kolbert KS, Sheikh A, Pentlow
KS, Mun EF, Barth A, Robbins RJ and Larson SM: Patient-specific
dosimetry for 131I thyroid cancer therapy using
124I PET and 3- dimensional-internal dosimetry (3D-ID)
software. J Nucl Med. 45:1366–1372. 2004.PubMed/NCBI
|
|
38
|
Zhang X, Liu DS, Luan ZS, Zhang F, Liu XH,
Zhou W, Zhong SF and Lai H: Efficacy of radioiodine therapy for
treating 20 patients with pulmonary metastases from differentiated
thyroid cancer and a meta-analysis of the current literature. Clin
Transl Oncol. 20:928–935. 2018. View Article : Google Scholar :
|
|
39
|
Hosseinimehr SJ: Flavonoids and genomic
instability induced by ionizing radiation. Drug Discov Today.
15:907–18. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Leo M, Sabini E, Ionni I, Sframeli A,
Mazzi B, Menconi F, Molinaro E, Bianchi F, Brozzi F, Santini P, et
al: Use of low-dose radioiodine ablation for Graves' orbitopathy:
Results of a pilot, perspective study in a small series of
patients. J Endocrinol Invest. 41:357–361. 2018. View Article : Google Scholar
|
|
41
|
Spanu A, Solinas ME, Chessa F, Sanna D,
Nuvoli S and Madeddu G: 131I SPECT/CT in the follow-up
of differentiated thyroid carcinoma: Incremental value versus
planar imaging. J Nucl Med. 50:184–90. 2009. View Article : Google Scholar : PubMed/NCBI
|