Introduction
Skin flap surgery has been increasingly used in
plastic and reconstructive surgery of a number of skin defects, and
flap necrosis is the least common but most serious problem
following reconstructive flap surgery. Ischemia-reperfusion (I/R)
injury is a leading cause of surgical skin flap compromise and
organ dysfunction. I/R injury occurs when the circulation is
abruptly restored following prolonged ischemia, and the mechanisms
underlying I/R injury are complex. Evidence shows that high levels
of calcium and tissue neutrophil accumulation cause cellular damage
(1), the generation of high level
of reactive oxygen species (ROS) during reperfusion, and the
induction of marked epithelial apoptosis are critical in the
pathogenesis of various types of I/R injury in tissue damage and
organ dysfunction (2). As skin
flaps are vulnerable to surgical skin flap-induced I/R injury,
reducing I/R injury in the necrotizing flaps has long been a
clinical challenge.
Luteolin (3′,4′,5,7-tetrahydroxyflavone) is a
naturally occurring polyphenol flavonoid found in several
vegetables, fruits, and tea. It has been reported to exert varied
pharmacological activities, including antioxidant, antimutagenic,
anti-inflammatory, anti-allergic and antihypertensive activities
(3). The beneficial effects of
luteolin have been reported in various pathological conditions,
including lipopolysaccharide-induced acute lung injury,
endotoxin-induced uveitis (4),
intestinal inflammation (5),
acetaminophen-induced hepatotoxicity and asthma (6,7).
Luteolin has also been found to exert protective
effects during the process of I/R injury; it has been demonstrated
to protect by the alleviation of myocardial, kidney and intestinal
I/R injury in animal models or clinical studies (8-10).
However, whether luteolin can be used for the treatment of the
cutaneous I/R injury in skin flap surgery is of interest and
warrants investigation. Therefore, the present study aimed to
evaluate the anti-ischemic effect of luteolin in skin flap surgery
and investigate its potential mechanism.
Materials and methods
Reagents and antibodies
Luteolin was purified and provided by the Key
Laboratory of Bioactive Substances and Resources Utilization of
Chinese Herbal Medicine, Ministry of Education (Beijing, China) and
the purity of the product was >98%, detected by HPLC (UV). The
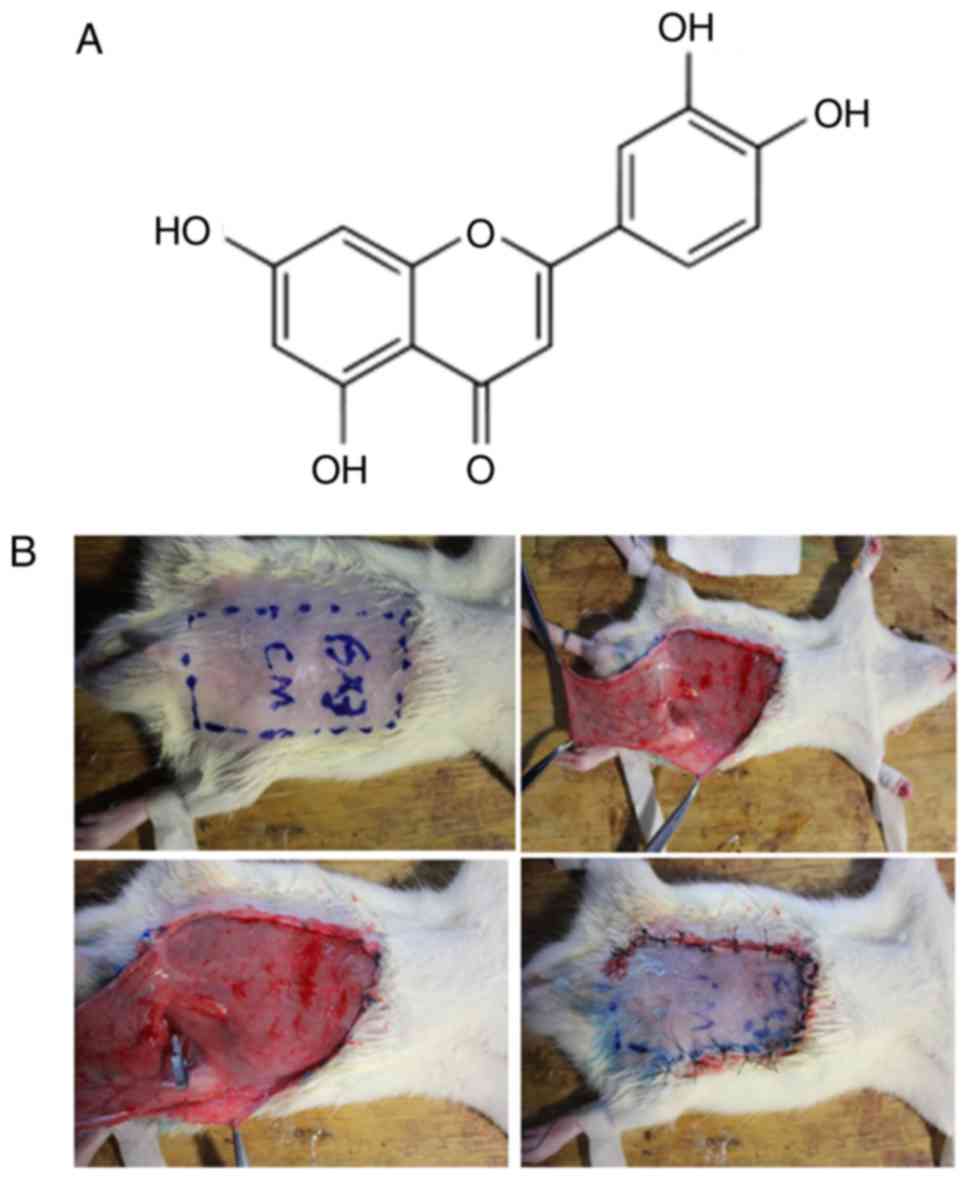
molecular structure is shown in Fig.
1A. Monoclonal antibodies against protein kinase B (AKT; no.
4691), phosphor-AKT (Ser473; no. 4060) and horseradish
peroxidase-conjugated anti-mouse or anti Rabbit IgG (nos. 7076 and
7074) were purchased from Cell Signaling Technology, Inc. (Boston,
MA, USA). Monoclonal antibodies against heme oxygenase-1 (HO-1; no.
ab13243), B-cell lymphoma 2 (BCL-2; no. ab692), BCL-2-associated X
protein (BAX; no. ab32503), activated caspase-3 (no. ab2302) and
GAPDH (no. ab9484) were purchased from Abcam (Cambridge, MA, USA).
Alexa 488-conjugated goat anti-rabbit secondary antibodies (no.
A-11078) were purchased from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). The Pierce BCA Protein Assay kit was purchased
from Thermo Fisher Scientific, Inc.
Cell culture
Cultured immortalized HaCaT human keratinocyte cells
were obtained from Nanjing KeyGen Biotech Co., Ltd. (Nanjing,
China) (11). The cells were
grown in DMEM (Thermo Fisher Scientific, Inc.) supplemented with
10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.), 100
U/ml penicillin, and 100 µg/ml streptomycin, cultured at
37°C in 5% CO2. When 80% confluence was achieved, the
HaCaT cells were treated with hydrogen peroxide (100 µM) for
2 h with or without the pretreatment of indicated concentrations
(3, 6, 12, 25 µg/ml) of luteolin for 12 h at 37°C in 5%
CO2.
Cell viability and apoptosis assay
Cell viability was assessed using standard MTS
methods. Briefly, the cells were seeded in triplicate at a density
of 1×105 cells/ml in a 96-well plate, and cell viability
assays were performed using the CellTiter 96 Aqueous One Solution
Cell Proliferation Assay kit (Promega Corporation, Madison, WI,
USA), the absorbance at 490 nm was measured using a microplate
reader (BioTek Instruments, Inc., Winooski, VT, USA).
For the apoptosis assays, a fluorescein-conjugated
Annexin V (Annexin V-FITC) staining assay kit (BD Biosciences, San
Jose, CA, USA) was used to quantitatively assess the level of
induced cell apoptosis. Briefly, the cells were washed with PBS and
stained with 5 µl of Annexin V-FITC and 5 µl of
propidium iodide (PI) in each sample to quantify the cell number at
different stages of cell death. Following incubation at room
temperature in the dark for 15 min, the percentages of apoptotic
cells were quantified as a percentage of the Annexin V-positive
population with a FACSCalibur flow cytometer (BD Biosciences), and
the data were analyzed with FlowJo Version 7.6.2 software (Tree
Star, Inc., Ashland, OR, USA).
Measurement of mitochondrial membrane
potential (MMP)
The MMP values were determined using the
dual-emission potential-sensitive probe
6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanineiodide
(JC-1; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Briefly, the
cells were seeded in the camber slides at a density of
5×105 cells/well in 200 µl culture medium and
incubated with 10 µM of JC-1 for 20 min at 37°C in the dark.
The JC-1 was then removed, and the cells were washed with cold PBS
to remove unbound dye. The quantity of JC-1 retained in the cells
was assessed with a laser scanning confocal microscope system
(Olympus Corporation, Tokyo, Japan).
Western blot analysis
Skin keratinocytes were incubated for a period of
time following hydrogen peroxide exposure, following which cell
lysates were collected by lysis in RIPA buffer, and the
concentration of protein was detected using a Pierce BCA protein
kit purchased from Thermo Fisher Scientific, Inc. Equal quantities
of proteins (20 µg) were mixed with loading buffer and
subjected to electrophoresis using 10% SDS-polyacrylamide gels. The
separated proteins were transferred onto polyvinylidene fluoride
membranes and non-specific bindings were blocked with 5% (w/v) skim
milk dissolved in tris-buffered saline with Tween. The membranes
were then subjected to immunoblot analysis with the appropriate
antibodies (1;1,000 dilution for the primary antibodies for 2 h at
room temperature; 1:2,000 dilution for the secondary antibodies for
1 h at room temperature). The immune-reactive protein bands were
visualized using an enhanced chemiluminescence detection system (GE
Healthcare Life Sciences, Chalfont, UK) followed by
autoradiography. A G:Box Bioimaging system (Syngene, Frederick, MD,
USA) was used to assess autoradiographic signals and bands were
quantified using GeneTools Image Analysis Software version 4.3.7
(Syngene).
Animal preparation and experimental
groups
The animal experiments were performed in the
Experimental Animal Laboratory of Nanjing University School of
Medicine (Nanjing, China), and approved by the Institutional Animal
Care and Use Committee of Nanjing University. A total of 18 male
8-10 weeks-old Sprague-Dawley rats weighing 220-280 g were
purchased from Research Institute of Model Organisms at Nanjing
University, the rats were housed in separate cages at 25°C and in a
12-h light/dark lighting system. All animals has free access to
food and water. The rats were randomly allocated into three groups:
Mock control group (Ctl), I/R injury group (I/R), and I/R injury
with luteolin treatment group (I/R + Luo).
Development of the ischemic flap rat
model
The rats were anesthetized with an intraperitoneal
(i.p.) injection of ketamine (100 mg/ml) and xylazine (20 mg/ml) at
a total dose of 0.2 ml/100 g of body weight. Abdominal hair was
removed with an electric clipper, and all surgical procedures were
performed under sterile conditions. The borders of the flaps were
outlined on the abdomen using a template measuring 3×6 cm. The flap
was raised with the base at the left inferior epigastric artery,
including the skin and the intimately attached panniculus carnosus,
as previously described (11,12). Ischemia was induced by applying a
single microvascular clamp across the femoral vascular pedicle, and
the flap was sutured to the donor bed using a 4/0 polypropylene
suture. Following 4 h of ischemia, the clamps were removed. The
rats in the mock control group received the flap elevation and
vessel dissection but no ischemic insult. The surgical procedure is
shown in Fig 1B. To assess the
functions of phosphoinositide-3-kinase (PI3K)/AKT and HO-1 in
luteolin-mediated tissue protection, HO-1 inhibitor (ZnPP) or
PI3K/AKT inhibitor (LY294002) was administrated to the rats on day
1 and day 4 post-I/R injury at doses of 3 and 100 mg/kg body weight
(i.p.). The surviving area of the skin flap was then measured.
Assessment of skin flap survival
Following surgery, all animals were sacrificed on
post-operative day 7. Gross observation was performed to identify
the line of demarcation between the viable and the necrotic tissue.
The dorsum of the rat in each group was covered with clear paper in
anesthesia to accurately measure the surviving or necrotic areas of
the flap, and the flap was cut into two sections: Viable and
necrotic. The entire flap and the necrotic and viable regions were
measured using two-dimensional planimetry in a blinded-manner. The
surviving proportions of the flaps were determined as a percentage
of the entire flap area (surviving flap proportion=viable flap
area/total area ×100%). Following assessment, the rats were
sacrificed with an overdose of sodium pentothal.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The transcriptional expression of interleukin
(IL)-1β, tumor necrosis factor (TNF)-α and IL-6 were detected by
RT-qPCR analysis. Total RNA was extracted from the skin flap tissue
on day 1 post-I/R using an RNAeasy Micro kit (Qiagen GmbH, Hilden,
Germany) according to the manufacturer’s protocol. mRNA was reverse
transcribed into cDNA with PrimeScript RT Master mix (Takara Bio,
Inc., Otsu, Japan). SYBR green qPCR was performed using PCR Master
mix (Thermo Fisher Scientific, Inc.). Each cDNA reaction was
prepared from 1 µg RNA, diluted to 100 µl of the
final volume and 1 µl cDNA was subsequently used for each
PCR reaction, and the reaction mixture had a total volume of 20
µl containing 10 µl PCR Master Mix (2X), 0.5
µl PCR forward primer (10 mM), 0.5 µl PCR reverse
primer (10 mM) and 8 µl H2O. The PCR conditions
were as follows: 95°C for 30 sec for pre-incubation, 95°C for 5 sec
and 60°C for 30 sec for amplification; 95°C for 10 sec and 65°C for
10 sec to melting curve, and 40°C for 30 sec for cooling. The
following primer pairs were used: IL-1β, 5′-GGA ACC CGT GTC TTC CTA
AAG-3′ (forward) and 5′-CTG ACT TGG CAG AGG ACA AAG-3′ (reverse);
TNF-α, 5′-CCA ACA AGG AGG AGA AGT TCC-3′ (forward) and 5′-CTC TGC
TTG GTG GTT TGC TAC-3′ (reverse); IL-6, 5′-GAA AGT CAA CTC CAT CTG
CC-3′ (forward) and 5′-CAT AGC ACA CTA CGT TTG CC-3′ (reverse);
first measure-actin, 5′-AAC CCT AAG GCC AAC CGT GAA AAG-3′
(forward) and 5′-TCA TGA GGT AGT CTG TCA GGT-3′ (reverse).
The relative expression of target genes was
determined to β-actin and was calculated using the
2−ΔΔCq method. The relative mRNA expression was
quantified as described previously (13).
Assessment of oxidative stress
status
The oxidative stress status of the flaps was
assessed by measuring the superoxide dismutase (SOD) activity and
the content of myeloperoxidase (MPO) and malondialdehyde (MDA) in
the skin flap tissue. Tissue samples (1×1 cm) were separated from
the central area of the surgical flaps in each group; these samples
were weighed, homogenized, and diluted to 10% (v/v) in an ice bath.
The homogenate was then centrifuged at 600 × g for 15 min at 4°C
and the supernatant solution was collected. The activity of SOD and
the levels of MPO and MDA in the homogenate were then determined
using a commercial kit following the protocol suggested by the
manufacturer (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China).
Immunofluorescent staining
Tissue specimens were embedded in paraffin following
fixation in 10% formalin. The sections (4-6 µm) were
deparaffinized in xylene and hydrated with ethanol. The sections
were incubated with 1% bovine serum albumin (Sigma-Aldrich; Merck
KGaA) in PBS/Tween to block unspecific binding of the antibodies.
Following overnight incubation at 4°C, the sections were incubated
with primary antibodies (1:500 for P-AKT, HO-1 and active
Caspase-3) for 2 h followed by the secondary anti-bodies (1:1,000)
for 30 min in a dark room at 25°C. Nuclei were stained using DAPI
solution (Molecular Probes, Thermo Fisher Scientific, Inc.).
Fluorescent images were captured and visualized using an Olympus
fluorescence microscope (Olympus Corporation).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). One-way
analysis of variance or Student’s t-test was used to test for
significant difference. P<0.05 was considered to indicate a
statistically significant difference.
Results
Luteolin treatment protects human skin
keratinocytes from hydrogen peroxide-induced cytotoxicity
Prior to investigating the protective properties of
luteolin against hydrogen peroxide-induced cell death, the
cytotoxic effects of hydrogen peroxide were first examined in the
HaCaT cells. The MTT assay indicated that the treatments with
various doses of hydrogen peroxide resulted in cytotoxic effects,
and cell viability was significantly decreased at a concentration
of 100 µM. Therefore, 100 µM of hydrogen peroxide was
selected as the optimum concentration for the subsequent in
vitro assay. To measure the protective effect of luteolin, the
HaCaT cells were sham-exposed or received treatment with various
doses of luteolin. MTT assays revealed that the hydrogen
peroxide-induced reduction of cell viability was effectively
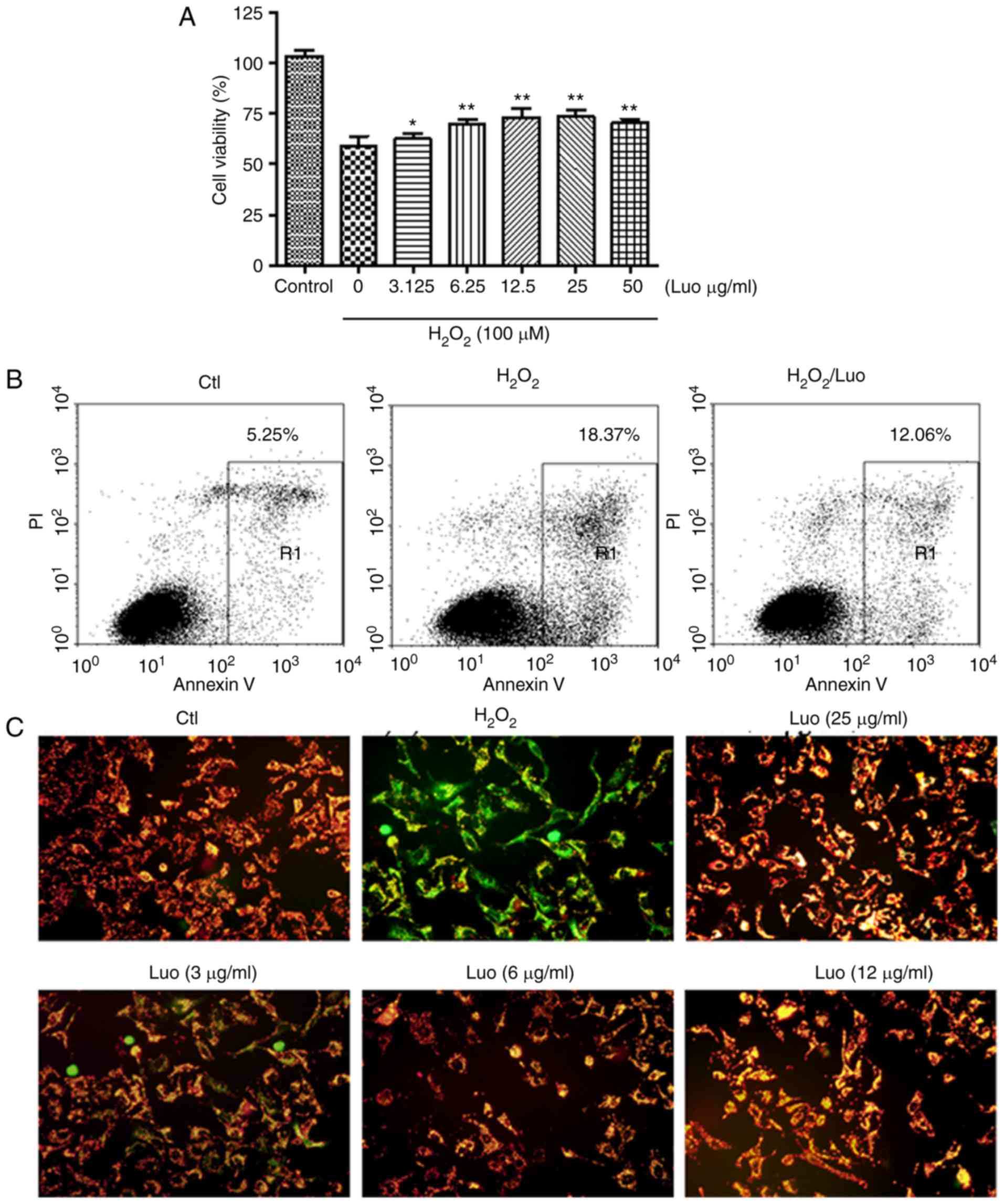
prevented by pretreatment with luteolin (Fig. 2A).
To further evaluate the effect of luteolin on
hydrogen peroxide-induced apoptosis, the HaCaT cells were stained
with Annexin V and PI, and cell apoptosis was measured by FACS, as
shown in Fig. 2B. Cells with a
high expression of Annexin V and not expressing PI were considered
early apoptotic cells, whereas cells with a high expression of
Annexin V and expressing PI were classified as late apoptotic
cells. The results showed that HaCaT cells exposed to hydrogen
peroxide treatment had a high level of cell apoptosis, and
treatment of these cells with luteolin significantly reduced cell
apoptosis; the percentage of apoptotic cells was 12.06±2.32%
(luteolin treatment group), vs. 18.37±1.92% (untreated group). This
suggested that luteolin may inhibit hydrogen peroxide-induced
keratinocyte apoptosis.
A change in MMP was examined as mitochondria are
major sites of oxidative phosphorylation and ROS production, and
they are involved in the initiation of apoptosis through membrane
permeabilization (14). As shown
in Fig. 2C, compared with the
control group, the hydrogen peroxide-treated cells exhibited a loss
of MMP; increased fluorescence intensity was observed in the
hydrogen peroxide-treated HaCaT cells following JC-1 dye staining,
indicative of mitochondrial depolarization. However, pretreatment
with luteolin significantly inhibited the loss of MMP in the
hydrogen peroxide-treated cells. Altered cell morphology was also
observed in response to hydrogen peroxide treatment, however, the
cell morphology was maintained and the overall cell shape was
maintained following treatment with increased dosed of luteolin,
indicating the protective effect of luteolin against hydrogen
peroxide-induced cell death.
Luteolin inhibits hydrogen
peroxide-induced keratinocyte apoptosis through the PI3K/AKT
pathway
To assess the mechanism by which luteolin treatment
can protect against hydrogen peroxide-induced keratinocyte
apoptosis, the present study investigated whether luteolin
pretreatment was associated with varied intracellular signaling
pathway activation. As shown in Fig.
3, treatment of the keratinocytes with hydrogen peroxide
resulted in a significant inhibition of PI3K/AKT pathway
activation, which indicated the inhibition of cell growth and
differentiation. Consistent with this observation, there was
increased expression of BAX and decreased expression of BCL-2, and
the BCL-2/BAX ratio were decreased, which suggested that these
cells underwent apoptosis once exposed to hydrogen peroxide
treatment.
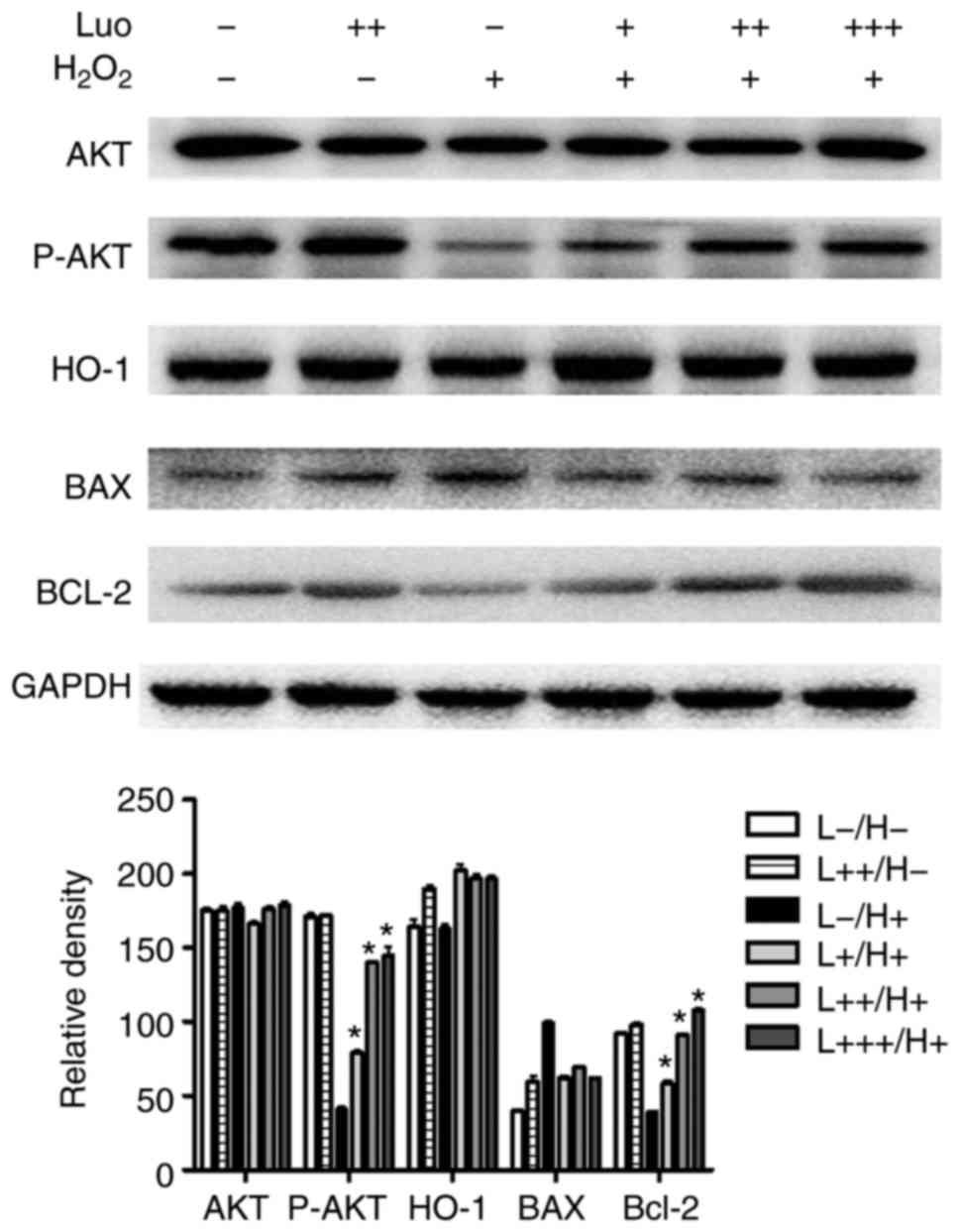 | Figure 3Effect of luteolin on hydrogen
peroxide-induced keratinocyte apoptosis. The protein levels of
markers of apoptosis, BAX, caspase 3 and BCL-2, were evaluated
using a western blot assay. The HaCaT cells were exposed to 100
µM of hydrogen peroxide in the presence or absence of
increased concentration of luteolin. +, 3 µg/ml; ++, 6
µg/ml; +++, 12 µg/ml; AKT, protein kinase B; P-AKT,
phosphorylated AKT; HO-1, heme oxygenase-1; BCL-2, B-cell lymphoma
2; BAX, BCL-2-assocated X protein; I/R, Luo, luteolin.
*P<0.05, vs H2O2 treatment. |
Luteolin pretreatment not only significantly
restored the cell viability, but also decreased the apoptotic rate,
upregulated the expression of BCL-2, downregulated the expression
of BAX and increased the BCL-2/BAX ratio. In addition, luteolin
pretreatment increased the phosphorylation of AKT in a
dose-dependent manner, as the PI3K/AKT pathway is one of the most
important intracellular survival signaling pathways. This result
suggested the protective effect of luteolin in I/R injury may be
associated with PI3K/AKT pathway activation.
Luteolin treatment protects against skin
damage during the process of I/R
From the observations in vitro, it was
hypothesized that luteolin may have a protective effect in I/R
injury during skin flap surgery. To evaluate this hypothesis, the
present study successfully established a cutaneous I/R injury rat
model. To assess the effect of luteolin on I/R injury in the skin
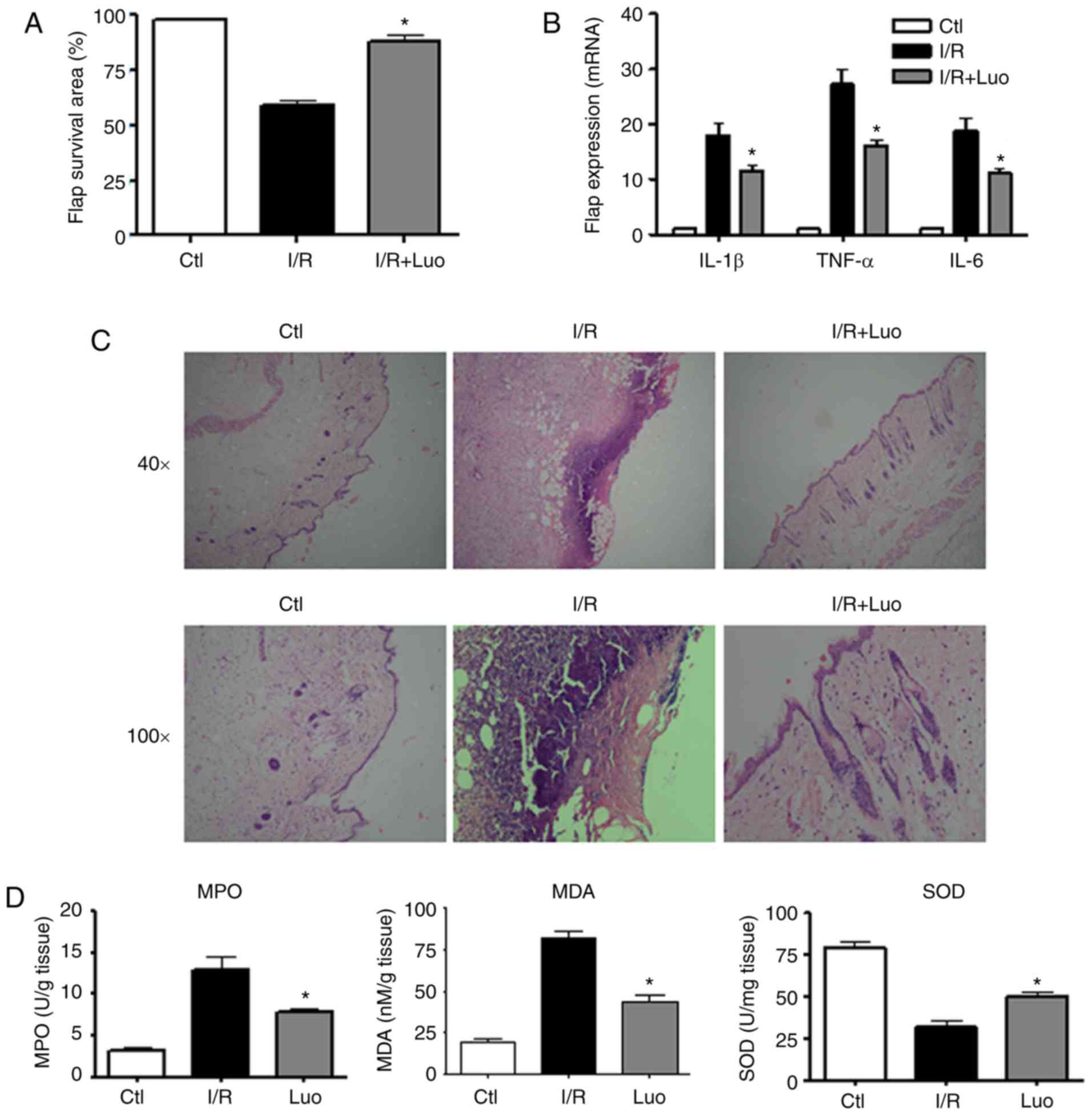
flap model, the surviving areas of the flaps were measured 7 days
following surgery. It was observed that the I/R injury group
exhibited smaller surviving flap areas compared with the mock
control groups. The rats that received luteolin pretreatment had
larger surviving flap areas than those in the I/R injury group at 7
days following surgery (Fig.
4A).
It was also observed that luteolin treatment
suppressed the mRNA levels of pro-inflammatory cytokines and
chemokines. The expression of pro-inflammatory cytokines in the
biopsied skin samples were examined using an RT-qPCR assay. As
shown in Fig. 4B, the expression
levels of TNF-α, IL-6 and IL-1β were significantly decreased in the
luteolin-treated groups compared with those in the I/R injury
groups. The histopathological examination indicated that the
severity of tissue injury in the luteolin pretreatment groups was
markedly reduced; there was less inflammatory cell infiltration and
the damaged skin area was significantly decreased (Fig. 4C).
To assess the I/R injury induced oxidative stress
damage, the levels of MPO, MDA, and SOD were examined in the
surgical flaps. There was increased production of MPO and MDA in
the ischemia group compared with the control group, and luteolin
treatment significantly decreased the expression of these enzymes.
The decreased expression of SOD following I/R injury was recovered
in response to luteolin treatment. These results indicated the
oxidative stress scavenging effects of luteolin (Fig. 4D).
Protective effect of luteolin on
I/R-induced skin damage is partly mediated through activation of
the PI3K/AKT pathway
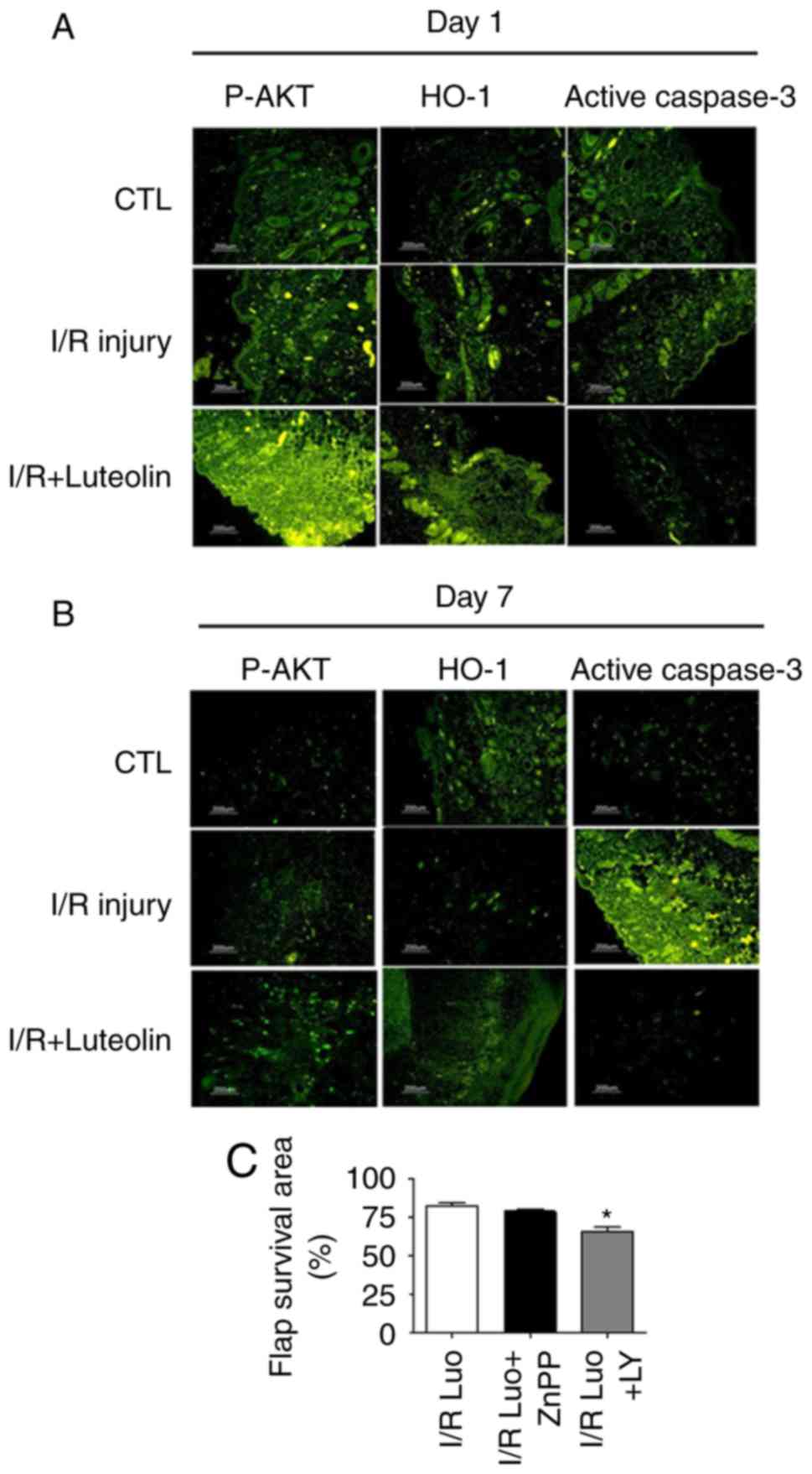
Using immunofluorescent staining (Fig. 5A and B), it was observed that
there was increased activation of caspase-3 protein in the ischemia
group compared with the control group and the luteolin treatment
group. Luteolin treatment reduced the level of cell apoptosis
induced by I/R injury, as the protein expression of activated
caspase-3 was significantly reduced. The levels of phospho-AKT and
antioxidant enzyme HO-1 were significantly increase in response to
luteolin treatment, indicating that these two molecules may be
involved in the protective function of luteolin.
To determine whether luteolin-induced skin
protection was mediated by the PI3K/AKT pathway, the PI3K inhibitor
LY294002 (14) was used. The
inhibition of AKT activity markedly reduced the luteolin-induced
protection and inhibition of apoptosis in skin I/R injury (Fig. 5C). These results suggested that
luteolin inhibited apoptosis and improved skin flap survival at
least partly through the PI3K/AKT pathway in skin flap surgery. By
contrast, using the HO-1 inhibitor ZnPP (15), no significant change in
luteolin-induced protection was observed; the surviving flap areas
and neutrophil infiltration levels were similar to those in the
luteolin treatment groups, suggesting that HO-1 may not be a
crucial molecule in regulation of skin damage during the I/R injury
caused by skin flap surgery.
Discussion
During skin flap surgery, the skin tissue is lifted
from a donor site and moved to a recipient site with an intact
blood supply, and I/R injury is the main factor reducing the
survival rate of flaps following grafting (15). I/R injury refers to the tissue and
microvasculature injury that is observed despite the restoration of
blood flow following an initial ischemic insult, often affecting
free flaps. The mechanisms of I/R injury include hypoxia,
inflammation and oxidative damage, characterized by microvascular
vasoconstriction, ROS release, oxidant/anti-oxidant imbalance and
neutrophil adhesion/infiltration (16).
Flavonoids are a group of natural products currently
receiving attention for their anti-reactive stress and
anti-inflammatory effects. Several studies have reported that
certain flavonoids, including kaempferol and quercetin, exert
antioxidant effects and can also inhibit tissue damage (17). Luteolin is a common flavonoid that
exists in several types of plants including fruits, vegetables, and
medicinal herbs; it has antioxidant, anti-inflammatory, antitumor
or other physiological beneficial effects. Studies have
demonstrated that luteolin may exert protective effects in I/R
injury in various pathological conditions, including myocardial and
cerebral I/R injury (18,19). In our previous study, it was shown
that luteolin protected HUVECs from TNF-α-induced oxidative stress
and inflammation (20). In the
present study, an in vivo animal model was used to
investigate the protective effect of luteolin against cutaneous I/R
injury during the process of skin flap surgery. It was found that
luteolin pretreatment conferred a skin protective effect, as
evidenced by improvement following I/R injury and reduced skin
keratinocyte apoptosis. Of note, PI3K/AKT signaling was shown to be
key in this process. The findings indicate that luteolin protected
against skin tissue damage in rats that underwent skin flap
surgery.
There are diverse mechanisms of cell death,
including necrosis, apoptosis, mitotic catastrophe and pyroptosis
(21). Apoptosis is an active
form of cell death that is initiated by a variety of stimuli,
including ROS (22). Several
studies have established that I/R injury can induce cell apoptosis,
resulting in the deregulation of associated functions (23-25). ROS-induced cell apoptosis has been
shown to be one of the important pathological features of cutaneous
I/R injury.
To understand the cytotoxic protective effect of
luteolin in I/R injury, the present study first measure the
protective effect of luteolin using the illustrative human
keratinocyte HaCaT cell line as an in vitro skin model, as
skin keratinocytes are the predominant cell type in the epidermis,
constituting 90% of the cells found in the outermost layer of the
skin (26). During skin I/R
injury, ROS-induced skin keratinocyte apoptosis has been considered
to be the major pathological cause for the tissue damage (27). In the present study, by analyzing
the hydrogen peroxide-induced skin HaCaT cell apoptosis, the
results showed that luteolin pretreatment significantly inhibited
the hydrogen peroxide-induced apoptosis, indicating the
anti-apoptotic property of luteolin.
To further delineate the mechanism, the present
study also measured the expression of apoptosis regulatory
components. Apoptosis is mediated by two evolutionarily conserved
path-ways: Intrinsic and extrinsic cell death pathways, which are
respectively represented by the Bcl-2 family and the caspase
family. The Bcl-2 family proteins, consisting of death antagonists
(Bcl-2) and agonists (Bax), are crucial in the regulation of
ROS-induced cell death (28). It
has been found that, during ischemia and particularly when combined
with reperfusion, Bax protein is triggered and translocated into
the outer mitochondrial membrane, resulting in elevated Bax levels
and a reduced Bcl-2/Bax ratio (29). It is well known that this ratio is
involved in MMP. The downregulation of the Bcl-2/Bax ratio
indicates that mitochondria-dependent pathways are involved in
hydrogen peroxide-induced apoptosis (30). It has been shown that the
overexpression of Bcl-2 decreases cell apoptosis in multiple types
of I/R injury (31). In the
present study, it was detected that luteolin pretreatment
significantly elevated the expression of BCL-2 and decreased the
expression of BAX, which corresponded to the increased BCL-2/BAX
ratio. Therefore changes in the ratio of pro-apoptotic to
anti-apoptotic proteins may contribute to the observed
anti-apoptotic mode of action of luteolin.
Caspase-3 are cysteine proteases are central in the
execution of the apoptotic program. Caspase-3 interacts with
caspase-8 and caspase-9, therefore, caspase-3 is activated in the
apoptotic cell by extrinsic (death ligand) and intrinsic
(mitochondrial) pathways (32).
In the present study, marked Caspase-3 activation was observed in
the healing skin tissue following skin flap surgery, indicating
that ROS-induced apoptosis contributed to the I/R injury-induced
tissue damage, The anti-apoptotic action of luteolin alleviated the
tissue damage during the cutaneous I/R injury, and the in
vitro experiments support this conclusion.
Cutaneous I/R injuries also cause the development of
inflammatory responses (33). In
the present study, the increased expression of pro-inflammatory
cytokines IL-1β and TNF-α suggested the induced acute inflammation
upon cutaneous I/R injury. It was noted that luteolin treatment
attenuated the acute inflammation by decreasing the expression of
these pro-inflammatory cytokines, suggesting that the
anti-inflammatory properties of luteolin may accelerate the wound
healing process. Neutrophil infiltration is characteristic of acute
inflammation, which has been suggested to aggravate the reperfusion
injury induced by leukocyte activation, and the expression of
adhesion molecules contributed by ROS. In the present study, the
increased expression of MPO, MDA and inflammatory factors in the
injured skin tissue during cutaneous I/R injury were significantly
ameliorated, and the decreased release of ROS and increased
production of antioxidant enzymes SOD and HO-1 upon luteolin
treatment support the anti-inflammatory and ROS scavenging function
of luteolin.
The PI3K/AKT pathway is one of the well-documented
pathways involved in protection against oxidative stress and is
critical in promoting wound healing (34). The activation of PI3K/AKT has been
shown to have a beneficial effect on multiple types of I/R injury,
including the gut, liver, heart and cerebral regions (35-39). The phosphorylation of AKT has been
shown to suppress apoptosis and promote cell survival in I/R injury
(40). AKT regulates cell
survival by phosphorylating different substrates that directly or
indirectly regulate apoptosis. It has been found that the
phosphorylation of AKT prevents cytochrome c release by
inhibiting the interaction of BCL-2 family apoptotic proteins,
including BCL-extra large, with other apoptosis regulating
molecules, including BCL-2-associated death promoter; AKT also
phosphorylates caspase-9 on Ser196, which inhibits its proteolytic
activity via a conformation change (41). Previous studies have demonstrated
that activation of the PI3K/AKT signaling pathway improved wound
healing in human and animal models; the increased PI3K/AKT
activation during the wound healing process was time
course-dependent, and was mainly observed in the early period
during wound healing (21,42).
In the present study, it was found that luteolin pretreatment
significantly upregulated the protein phosphorylation of PI3K and
AKT. The increased activation of the PI3K/AKT signaling pathway in
the luteolin treatment group, which occurred mainly on Day 1 rather
than Day 7, suggested that activation of the PI3K/AKT pathway was
an early event for tissue regeneration. Therefore, the protective
effect of luteolin was due, at least in part, to its ability to
upregulate the activation of the PI3K/AKT signaling pathway.
To understand whether the protective effects of
luteolin were mediated via the PI3K/AKT pathway, the rats received
pretreatment with PI3K/AKT inhibitors during the in vivo
administration of luteolin. The results showed that, in the
presence of LY294002, the cytoprotective activity of luteolin was
significantly reduced, suggesting the involvement of the PI3K/AKT
pathway in the regulation of cutaneous I/R injury.
In conclusion, the present study demonstrated that
luteolin pretreatment attenuated cutaneous I/R injury by scavenging
of extracellular ROS and regulating apoptosis. Therefore, the
administration of luteolin may represent a promising therapeutic
strategy for the treatment of ROS-related cutaneous I/R injury and
improve skin flap survival.
Funding
This study was supported by a grant from the Natural
Science Foundation of Jiangsu Province China (grant nos. BK20141505
and BK20171347), the Key Laboratory of Acupuncture and Medicine
Research (grant no. 201710zykf01), Qinglan Project of Jiangsu
Province and the Research Projects in Traditional Chinese Medicine
Industry of China (grant no. 201507004-2).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
HW and YS contributed to planning and implementing
the experiments, interpretation of data and writing the manuscript.
GC, HS and LZ contributed to implementing the experiments. ZS and
JH contributed to planning the experiments, interpreting data, and
writing the manuscript. All authors reviewed the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee of Nanjing University
(Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Wang X, Takahashi N, Uramoto H and Okada
Y: Chloride channel inhibition prevents ROS-dependent apoptosis
induced by ischemia-reperfusion in mouse cardiomyocytes. Cell
Physiol Biochem. 16:147–154. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park TH and Park YJ: The effect of
botulinum toxin a on ischemia-reperfusion injury in a rat model.
Biomed Res Int. 2017.1074178:2017.
|
|
3
|
Nabavi SF, Braidy N, Gortzi O,
Sobarzo-Sanchez E, Daglia M, Skalicka-Woźniak K and Nabavi SM:
Luteolin as an anti-inflammatory and neuroprotective agent: A brief
review. Brain Res Bull. 119:1–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JP, Li YC, Chen HY, Lin RH, Huang SS,
Chen HL, Kuan PC, Liao MF, Chen CJ and Kuan YH: Protective effects
of luteolin against lipopolysaccharide-induced acute lung injury
involves inhibition of MEK/ERK and PI3K/Akt pathways in
neutrophils. Acta Pharmacol Sin. 31:831–838. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nunes C, Almeida L, Barbosa RM and
Laranjinha J: Luteolin suppresses the JAK/STAT pathway in a
cellular model of intestinal inflammation. Food Funct. 8:387–396.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Das M, Ram A and Ghosh B: Luteolin
alleviates bronchocon-striction and airway hyperreactivity in
ovalbumin sensitized mice. Inflamm Res. 52:101–106. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shanmugam S, Thangaraj P, Lima B, Lima
BDS, Chandran R, de Souza Araújo AA, Narain N, Serafini MR and
Júnior LJQ: Effects of luteolin and quercetin 3-β-d-glucoside
identified from Passiflora subpeltata leaves against acetaminophen
induced hepatotoxicity in rats. Biomed Pharmacother. 83:1278–1285.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hong X, Zhao X, Wang G, Zhang Z, Pei H and
Liu Z: Luteolin treatment protects against renal
ischemia-reperfusion injury in rats. Mediators Inflamm.
2017.9783893:2017.
|
|
9
|
Yu D, Li M, Tian Y, Liu J and Shang J:
Luteolin inhibits ROS-activated MAPK pathway in myocardial
ischemia/reperfusion injury. Life Sci. 122:15–25. 2015. View Article : Google Scholar
|
|
10
|
Karakas BR, Davran F, Elpek GO, Akbas SH,
Gulkesen KH and Bulbuller N: The effects of luteolin on the
intestinal ischemia/reperfusion injury in mice. J Invest Surg.
27:249–255. 2014. View Article : Google Scholar
|
|
11
|
Gideroglu K, Yilmaz F, Aksoy F, Bugdayci
G, Saglam I and Yimaz F: Montelukast protects axial pattern rat
skin flaps against ischemia/reperfusion injury. J Surg Res.
157:181–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim HJ, Xu L, Chang KC, Shin SC, Chung JI,
Kang D, Kim SH, Hur JA, Choi TH, Kim S and Choi J:
Anti-inflammatory effects of anthocyanins from black soybean seed
coat on the keratinocytes and ischemia-reperfusion injury in rat
skin flaps. Microsurgery. 32:563–570. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang Y, Zhang Y, Wang Z, Wang S, Gao M, Xu
R, Liang C and Zhang H: Attenuation of acute phase injury in rat
intracranial hemorrhage by cerebrolysin that inhibits brain edema
and inflammatory response. Neurochem Res. 41:748–757. 2016.
View Article : Google Scholar
|
|
14
|
Yoon JJ, Jeong JW, Choi EO, Kim MJ,
Hwang-Bo H, Kim HJ, Hong SH, Park C, Lee DH and Choi YH: Protective
effects of Scutellaria baicalensis Georgi against hydrogen
peroxide-induced DNA damage and apoptosis in HaCaT human skin
keratinocytes. EXCLI J. 16:426–438. 2017.PubMed/NCBI
|
|
15
|
Birk-Sorensen L, Kerrigan CL and Jensen
GS: E-selectin and L-selectin blockade in pure skin flaps exposed
to ischaemia and reperfusion injury. Scand J Plast Reconstr Surg
Hand Surg. 32:365–371. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Francis A and Baynosa RC: Hyperbaric
oxygen therapy for the compromised graft or flap. Adv Wound Care
(New Rochelle). 6:23–32. 2017. View Article : Google Scholar
|
|
17
|
Kumar AD, Bevara GB, Kaja LK, Badana AK
and Malla RR: Protective effect of 3-O-methyl quercetin and
kaempferol from semecarpus anacardium against H2O2 induced
cytotoxicity in lung and liver cells. BMC Complement Altern Med.
16:3762016. View Article : Google Scholar :
|
|
18
|
Zhang X, Du Q, Yang Y, Wang J, Dou S, Liu
C and Duan J: The protective effect of Luteolin on myocardial
ischemia/reperfusion (I/R) injury through TLR4/NF-κB/NLRP3
inflammasome pathway. Biomed Pharmacother. 91:1042–1052. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang S, Qi Y, Xu Y, Han X, Peng J, Liu K
and Sun CK: Protective effect of flavonoid-rich extract from rosa
laevigata michx on cerebral ischemia-reperfusion injury through
suppression of apoptosis and inflammation. Neurochem Int.
63:522–532. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun GB, Sun X, Wang M, Ye JX, Si JY, Xu
HB, Meng XB, Qin M, Sun J, Wang HW and Sun XB: Oxidative stress
suppression by luteolin-induced heme oxygenase-1 expression.
Toxicol Appl Pharmacol. 265:229–240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kroemer G, Galluzzi L, Vandenabeele P,
Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS,
Golstein P, Green DR, et al: Classification of cell death:
Recommendations of the nomenclature committee on cell death 2009.
Cell Death Differ. 16:3–11. 2009. View Article : Google Scholar :
|
|
22
|
Miyata Y, Matsuo T, Sagara Y, Ohba K,
Ohyama K and Sakai H: A mini-review of reactive oxygen species in
urological cancer: Correlation with NADPH oxidases, angiogenesis,
and apoptosis. Int J Mol Sci. 18:E22142017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu H, Jing X, Dong A, Bai B and Wang H:
Overexpression of TIMP3 protects against cardiac
ischemia/reperfusion injury by inhibiting myocardial apoptosis
through ROS/mapks pathway. Cell Physiol Biochem. 44:1011–1023.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Uchiyama A, Yamada K, Perera B, Ogino S,
Yokoyama Y, Takeuchi Y, Ishikawa O and Motegi S: Topical
betamethasone butyrate propionate exacerbates pressure ulcers after
cutaneous ischemia-reperfusion injury. Exp Dermatol. 25:678–683.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uchiyama A, Yamada K, Perera B, Ogino S,
Yokoyama Y, Takeuchi Y, Ishikawa O and Motegi SI: Protective effect
of MFG-E8 after cutaneous ischemia-reperfusion injury. J Invest
Dermatol. 135:1157–1165. 2015. View Article : Google Scholar
|
|
26
|
Johansen C: Generation and culturing of
primary human keratinocytes from adult skin. J Vis Exp.
130:e568632017.
|
|
27
|
Cai Y, Wang W, Liang H, Sun L, Teitelbaum
DH and Yang H: Keratinocyte growth factor improves epithelial
structure and function in a mouse model of intestinal
ischemia/reperfusion. PLoS One. 7:e447722012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeng C, Jiang W, Zheng R, He C, Li J and
Xing J: Cardioprotection of tilianin ameliorates myocardial
ischemia-reperfusion injury: Role of the apoptotic signaling
pathway. PLoS One. 13:e1938452018. View Article : Google Scholar
|
|
29
|
Xiao YD, Liu YQ, Li JL, Ma XM, Wang YB,
Liu YF, Zhang MZ, Zhao PX, Xie F and Deng ZX: Hyperbaric oxygen
preconditioning inhibits skin flap apoptosis in a rat
ischemia-reperfusion model. J Surg Res. 199:732–739. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roy AM, Baliga MS and Katiyar SK:
Epigallocatechin-3-gallate induces apoptosis in estrogen
receptor-negative human breast carcinoma cells via modulation in
protein expression of p53 and Bax and caspase-3 activation. Mol
Cancer Ther. 4:81–90. 2005.PubMed/NCBI
|
|
31
|
Liu YQ, Liu YF, Ma XM, Xiao YD, Wang YB,
Zhang MZ, Cheng AX, Wang TT, Li JL, Zhao PX, et al: Hydrogen-rich
saline attenuates skin ischemia/reperfusion induced apoptosis via
regulating Bax/Bcl-2 ratio and ASK-1/JNK pathway. J Plast Reconstr
Aesthet Surg. 68:e147-e1562015. View Article : Google Scholar
|
|
32
|
Salvesen GS: Caspases: Opening the boxes
and interpreting the arrows. Cell Death Differ. 9:3–5. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ju J, Wu J and Hou R: Role of the p38
mitogen-activated protein kinase signaling pathway in
estrogen-mediated protection following flap ischemia-reperfusion
injury. Cell Biochem Funct. 34:522–530. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang B, Zhao Z, Meng X, Chen H, Fu G and
Xie K: Hydrogen ameliorates oxidative stress via PI3K-Akt signaling
pathway in UVB-induced HaCaT cells. Int J Mol Med. 41:3653–3661.
2018.PubMed/NCBI
|
|
35
|
Mahajan UB, Patil PD, Chandrayan G, Patil
CR, Agrawal YO, Ojha S and Goyal SN: Eplerenone pretreatment
protects the myocardium against ischaemia/reperfusion injury
through the phosphatidylinositol 3-kinase/Akt-dependent pathway in
diabetic rats. Mol Cell Biochem. 446:91–103. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiao S, Zhu H, He P and Teng J: Betulinic
acid protects against cerebral ischemia/reperfusion injury by
activating the PI3K/Akt signaling pathway. Biomed Pharmacother.
84:1533–1537. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kai-lan W and Si Z: Pretreatment with
erythropoietin attenuates intestinal ischemia reperfusion injury by
further promoting PI3K/Akt signaling activation. Transplant Proc.
47:1639–1645. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arslan F, Lai RC, Smeets MB, Akeroyd L,
Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA,
Pasterkamp G, et al: Mesenchymal stem cell-derived exosomes
increase ATP levels, decrease oxidative stress and activate
PI3K/Akt pathway to enhance myocardial viability and prevent
adverse remodeling after myocardial ischemia/reperfusion injury.
Stem Cell Res. 10:301–312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mason S, Hader C, Marlier A, Moeckel G and
Cantley LG: Met activation is required for early cytoprotection
after ischemic kidney injury. J Am Soc Nephrol. 25:329–337. 2014.
View Article : Google Scholar :
|
|
40
|
Thokala S, Inapurapu S, Bodiga VL, Vemuri
PK and Bodiga S: Loss of ErbB2-PI3K/Akt signaling prevents zinc
pyri-thione-induced cardioprotection during ischemia/reperfusion.
Biomed Pharmacother. 88:309–324. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nath S and Mandal C, Chatterjee U and
Mandal C: Association of cytosolic sialidase Neu2 with plasma
membrane enhances Fas-mediated apoptosis by impairing
PI3K-Akt/mTOR-mediated pathway in pancreatic cancer cells. Cell
Death Dis. 9:2102018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu T, Gao M, Yang P, Pei Q, Liu D, Wang D,
Zhang X and Liu Y: Topical insulin accelerates cutaneous wound
healing in insulin-resistant diabetic rats. Am J Transl Res.
9:4682–4693. 2017.PubMed/NCBI
|