Introduction
Interstitial lung disease represents a group of
diseases involving diffuse lung parenchyma injury, alveolar
inflammation and interstitial fibrosis as the basic pathological
lesions. These diseases are characterized by a gradual
deterioration of pulmonary functions in the majority of patients,
eventually developing into extensive pulmonary fibrosis leading to
respiratory failure (1). The
pathogenesis involves a variety of cells, including alveolar
epithelial cells and lung fibroblasts, inflammatory cytokines, as
well as transforming growth factor (TGF)-β1 signal transduction
(2-5). The incidence rate per 100,000 people
has been reported to range between 10 and 60 cases in the United
States and appears to increase with age, but the pathogenesis
remains elusive (6,7). Due to the varied clinical
manifestations and pathological types, clinical treatment is
unsatisfactory. Once extensive fibrosis occurs, the treatment
options are further limited, increasing the risk of mortality
(8,9). Therefore, the pathogenesis
underlying and potential therapeutic targets of pulmonary fibrosis
require further investigation.
Lysyl oxidase (LOX) is a copper-dependent amine
oxidase that consists of four members (LOX-like 1-4) located in the
extracellular space and nucleus, among which LOX-like 2 (LOXL2)
serves an essential role in matrix remodeling and fibrogenesis
(10-12). A previous study demonstrated that
LOXL2 is involved in epithelial-mesenchymal transition,
extracellular matrix deposition, and occurrence and progression of
fibrosis-associated diseases (13). Barry-Hamilton et al
(14) reported that the
expression of LOXL2 elevated significantly in pulmonary tissues of
mice with pulmonary fibrosis, whereas the fibrosis process was
inhibited by LOXL2-specific antibody treatment. Another study
demonstrated that LOXL2 was highly expressed in the pulmonary
fibroblasts of patients with idiopathic pulmonary fibrosis, and
essential in the transition of fibroblasts to myofibroblasts
(15). Although LOXL2 is involved
in the occurrence and progression of pulmonary fibrosis, its
specific roles and mechanisms require further investigation.
A large amount of extracellular matrix is produced
by fibroblasts and the transition of fibroblasts to myofibroblasts
is one of the primary characteristics of pulmonary fibrosis
(16). The TGF-β1 signaling
pathway has been implicated in the development of pulmonary
fibrosis, with the classical TGF-β1/Smad being one of the most
important pathways (17). TGF-β1
acts on target cells through the transmembrane receptors, leading
to the phosphorylation of Smad2 and Smad3. Phosphorylated Smad2/3
binds to Smad4 to enter the nucleus. Following nuclear
translocation, the Smad protein compound interacts with
transcription factor Snail to regulate the transcription of
fibrosis-associated genes in the downstream. Inhibitory smads
(Smad6 and Smad7) promote the degradation of TGF-β1 and inhibit the
activation of the TGF-β1 signaling pathway (18,19). A previous study revealed that
following the knockout of LOX in C57BL/6 mice, the expression of
TGF in the bronchoalveolar lavage fluid (BALF) is downregulated and
the proportion of pSmad2/3-positive cells in the pulmonary tissues
decreases, suggesting an association between LOX and the TGF-β
signaling pathway (18). Although
LOXL2 is implicated in the occurrence and development of pulmonary
fibrosis, and LOX may act through the TGF-β signaling pathway,
studies on the specific effects of LOXL2 on the proliferation and
fibrosis progression of lung fibroblasts of bleomycin (BLM)-induced
pulmonary fibrosis in mice, as well as the involvement of LOXL2 in
the progression of pulmonary fibrosis through the TGF-β1/Smad
signaling pathway, are limited (20,21).
Therefore, mice models with BLM-induced pulmonary
fibrosis were used in the present study, and the expression of
LOXL2 in serum, lung homogenate and pulmonary tissues was observed.
Then, the pulmonary tissues of mice were extracted to culture
primary MLFs, and the cells were transfected using LOXL2 short
interfering RNA (siRNA). The effects of LOXL2 silencing on the
proliferative ability, and the expression of inflammatory factors
interleukin (IL)-6 and extracellular matrix type 1 collagen α1
(COL1A1) involved in fibrosis progression were determined in
vitro. In addition, the effects of LOXL2 siRNA on the
expression of key factors in the TGF-β1 signaling pathway were also
noted. Further investigations were performed to examine the effect
of LOXL2 on the occurrence of pulmonary fibrosis and its regulatory
mechanism in the TGF-β/Smad signaling pathway, providing
experimental evidence for the identification of potential
therapeutic targets of pulmonary fibrosis.
Materials and methods
Major reagents
C57BL/6 mice were purchased from Beijing Vital River
Laboratory Animal Technology Co., Ltd. (Beijing, China), and 293
cells and the adenovirus vector PGMAV-S1 were provided by Lemo
Biotechnology (Hebei, China). BLM was purchased from Nippon Kayaku
Co., Ltd. (Tokyo, Japan). The RNA simple Total RNA kit (cat. no.
DP419) was purchased from Tiangen Biotech Co., Ltd. (Beijing,
China). The Prime Script™ RT reagent kit, SYBR® Prime
Script plus RT-PCR kit, DNA ligase, Taq DNA polymerase and
restriction endonucleases were purchased from Takara Bio, Inc.
(Otsu, Japan). Taq polymerase was purchased from New England Bio
Labs, Inc. (Ipswich, MA, USA). The QIA prep Spin Mini prep kit was
purchased from Qiagen GmbH (Hilden, Germany). The DNA Gel
Extraction kit was purchased from Generay Biotech Co., Ltd.
(Shanghai, China). The NucleoSpin® 96 Plasmid Core kit
was purchased from Macherey-Nagel GmbH (Düren, Germany).
Lipofectamine® 2000 was purchased from Thermo Fisher
scientific, Inc. (Invitrogen; Waltham, MA, UsA). LOXL2 (cat. no.
ab96233), TGF-β1 (cat. no. ab92486), phosphorylated (p)Smad2/3
(cat. no. ab63399) and Smad4 (cat. no. ab40759) rabbit anti-mouse
primary antibodies were purchased from Abcam (Cambridge, UK). Smad7
(cat. no. sc-365846), Snail (cat. no. sc-271977) mouse monoclonal
antibodies, and the mouse IgGκ binding protein (cat. no. sc-516102)
and goat anti-rabbit IgG (cat. no. sc-2030) HRP-conjugated
secondary antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). The ProteoPrep® Total Extraction
Sample and Bicinchoninic Acid kits was purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). The LOXL2 ELISA kit
(cat. no. A95552Mu02) was purchased from shanghai Wuhao trade Co.,
Ltd. (shanghai, China), the IL-6 ELIsA kit (cat. no. M6000B) from
R&D systems, Inc. (Minneapolis, MN, USA) and the COL1A1 ELISA
kit (cat. no. sEA350Mu) from Wuhan USCN Business Co., Ltd. (Wuhan,
China). Primer synthesis was performed by sangon Biotech Co., Ltd.
(shanghai, China). The Hematoxylin Staining kit and Masson
Tri-color Staining kit were purchased from Beijing solarbio science
& technology Co., Ltd. (Beijing, China). Plasmid sequencing was
performed by Thermo Fisher scientific, Inc. (Invitrogen).
Construction of LOXL2 siRNA adenovirus
vectors
The present study was approved by the Experimental
Animal Welfare Committee of Capital Medical University (Beijing,
China; approval no. AEEI-2016-005). Two siRNAs and one scramble
siRNA (negative control) were designed for cDNA sequence of LOXL2
gene (serial no. NM_033325.2), as shown in Table I.
 | Table IsiRNA oligo sequences. |
Table I
siRNA oligo sequences.
| siRNA | | OligoDNA sequence
(5′-3′) |
|---|
| LOXL2 siRNA1 | Forward |
gatccGCCAGAAGAGGAAGCACAATGTTCAAGAGACATTGTGCTTCCTCTTCTGGCTTTTTTa |
| Reverse |
agcttAAAAAAGCCAGAAGAGGAAGCACAATGTCTCTTGAACATTGTGCTTCCTCTTCTGGCg |
| LOXL2 siRNA2 | Forward |
gatccGGAAGCAGATCTGCAACAAACTTCAAGAGAGTTTGTTGCAGATCTGCTTCCTTTTTTa |
| Reverse |
agcttAAAAAAGGAAGCAGATCTGCAACAAACTCTCTTGAAGTTTGTTGCAGATCTGCTTCCg |
| Control siRNA | Forward |
gatccGGTACTGGCATGGAAATATCTTTCAAGAGAAGATATTTCCATGCCAGTACCTTTTTTa |
| Reverse |
agcttAAAAAAGGTACTGGCATGGAAATATCTTCTCTTGAAAGATATTTCCATGCCAGTACCg |
Four single-strand DNAs were synthesized, which
formed double-strand DNA (dsDNA) via annealing. The dsDNA was bound
to linearize adenovirus vector PGMAV-S1 following double-enzyme
digestion by BamHI and HindIII. The ligations were
transformed into Escherichia coli DH5α, and positive clones
were selected. Plasmids were extracted and gene sequencing was
performed to identify the recombinants.
A large number of recombinant plasmids were
prepared, and 293 cells were then transfected with siRNA through
mediation by liposomes using Lipofectamine 2000. Following the
purification and determination of the virus titer, the recombinant
adenovirus was packaged and stored at -80°C for subsequent use.
Eventually, a total of 1.0x1010 plaque-forming units
(PFUs)/ml LOXL2 siRNA adenovirus vectors together with
1.1x109 PFUs/ml negative control siRNA were
harvested.
Construction and identification of animal
models with pulmonary fibrosis
A total of 16 C57BL/6 male mice, aged 7-8 weeks and
weighing 17-20 g, were pre-fed a normal diet with standard feed in
a new environment for 1 week and kept in an environment of 20-22°C
with 50-60% relative humidity. Furthermore, the mice were housed in
a 12-h light/12-h dark cycle, the background noise was kept <60
decibels, and the ammonia concentration was kept <20 ppm. The
mice had free access to fresh and clean water as well as adequate
feedstuff with balanced nutrition. They were randomly divided into
control (saline injection) and BLM groups (intratracheal injection
was performed once at a dose of BLM 5.0 mg/kg), with eight mice in
each group. Pulmonary fibrosis models were identified by imaging
examination and pathological morphology 14 days after completing
BLM treatment. The serum of mice was used to prepare lung
homogenate following the successful construction of the models for
subsequent experiments. For imaging evaluation, mice anesthetized
with a 5% 300 mg/kg chloral hydrate intraperitoneal injection were
placed in the fixed device of the computed tomography (Ct) machine
(Siemens AG, Munich, Germany) in a supine position. The CT machine
was set to an appropriate position to perform the spiral scan of
the whole lung with the parameters including layer thickness of
0.625 mm, 120 kV and 100 mA. The scanning conditions and parameters
were consistent in the two groups, and Mimics 15.0 software
(Materialise NV, Leuven, Belgium) was used for analysis. For
pathological morphology analysis, a section of the pulmonary
tissues was fixed with 4% paraformaldehyde overnight at room
temperature, dehydrated in varying concentrations of ethanol,
paraffin-embedded, and sliced into 3-5-mm thick sections. The
tissues were then stained with hematoxylin and eosin (H&E), and
Masson's trichrome staining. For H&E staining, tissue slices
were stained with 90% hematoxylin dye solution for 10-15 min,
allowed to differentiate for 30 sec and then stained with 0.5%
eosin dye solution for 2 min. For Masson's staining, tissue slices
were stained with 98% Weigert hematoxylin for 10 min,
differentiated with 1% phospho molybdate solution for 10 sec and
returned to blue using 1% Masson blue liquor for 5 min. Slides were
then dyed with 1% Li Chunhong's magenta for 5 min followed by 2%
aniline blue dyeing solution for 1-2 min. All of the aforementioned
staining procedures were performed at room temperature.
Subsequently, pulmonary tissue inflammation, morphological changes
and fibrosis degree were observed with an optical microscope using
x20 magnification (Olympus BX43; Olympus Corporation, Tokyo,
Japan).
Culture and identification of primary
MLFs
Pulmonary tissues were selected from the control and
BLM model groups. The pleura and blood vessels were removed, and
the tissues were cut into tissue blocks of the size of ~1
mm3. A 25-cm2 culture flask was coated with
Dulbecco's modified Eagle's medium (DMEM) (Invitrogen; thermo
Fisher scientific, Inc.) containing 10% fetal bovine serum
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA), and the
1-mm3 tissue blocks were attached to the bottom of the
culture flask evenly. Then, 5 ml of DMEM/F-12 medium containing 10%
fetal bovine serum was added to cover the tissue blocks following
adhesion in 5% CO2 at 37°C for 12 h. Once the cells
reached the fusion state, 0.25% trypsin was added, and the cells
were passaged at a ratio of 1:3 until the third generation. The
cells were cultured until 90% confluent, fixed with 4%
paraformaldehyde for 30 min, incubated with 0.2% triton X-100 for 5
min and blocked 5% bovine serum albumin (Wuhan Boster Biological
technology, Ltd., Wuhan, China) for 30 min (all performed at room
temperature). Cells were then incubated with the PBS-diluted
α-smooth muscle actin (α-SMA) mouse monoclonal antibody (cat. no.
A5228; 1:300; Sigma-Aldrich; Merck KGaA) at 37°C for 1 h, followed
by incubation with the goat anti-rabbit phycoerythrin-conjugated
secondary antibody (cat. no. 11828681001; 1:1,000; Sigma-Aldrich;
Merck KGaA) for 1 h at room temperature. Then, the cells were
observed under an inverted fluorescence microscope (Olympus IX71;
Olympus Corporation) at magnifications x200 and x400 to identify
MLFs.
Infection of MLFs with adenovirus
siRNA
The MLF fusion degree in the control and BLM groups
was 60-80%. Plating was performed using a 6-well plate at the
inoculation density of 1x105 cells/ml. MLFs of the BLM
model group were divided into four groups: BLM, control siRNA,
LOXL2 siRNA1, and LOXL2 siRNA2. The cells in the latter three
groups were transfected with control siRNA, LOXL2 siRNA1, and LOXL2
siRNA2, respectively, with a multiplicity of infection of 100 for 2
h at 37°C. The cells were continuously cultured for 48 h after
transferring them to the fresh culture medium (DMEM). A FACScan
system (BD Biosciences, San Jose, CA, USA) was used in flow
cytometry to observe the infection efficiency. The infection
efficiency was >70%. The cells and supernatant were
collected.
Detection of cell proliferation using the
Cell Counting kit-8 assay
The cells were counted and inoculated into 96-well
plates with 100 µl of cell suspension/well (1x103
cells/well). Each sample was repeated in triplicate. The cells were
precultured in an inoculator containing 5% CO2 at 37°C
for 24 h, and 10 µl of CCK-8 solution was added to each well,
followed by incubation for 1-4 h. The absorbance values at 450 nm
were measured using a microplate reader. Three biological
replicates were performed and each experiment was repeated three
times.
Detection of gene expression in MLFs
using reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
Total RNA was extracted using the RNAsimple Total
RNA kit according to the manufacturer's protocol following
trans-fection of MLFs for 48 h. Then, it was reverse transcribed to
synthesize cDNA using the PrimeScript RT reagent kit using the
following temperature protocol: 37°C for 15 min; 85°C for 5 sec;
and 4°C for 5 min. The mRNA level was amplified and detected using
SYBR PrimeScript plus RT-PCR kit according to the manufacturer's
protocol. The primers were designed and synthesized (Table II). The thermocycling conditions
for qPCR were as follows: 95°C for 30 sec; 60°C for 30 sec; and
72°C for 30 sec, with a total of 40 cycles. RT-qPCR was performed
using ABI Prism 7500 (Applied Biosystems; Thermo Fisher scientific,
Inc.). GAPDH was used as the internal reference and the relative
expression of genes was calculated using the 2-ΔΔCq
method (22). The experiments
were performed at least three times.
 | Table IIPrimers used for polymerase chain
reaction. |
Table II
Primers used for polymerase chain
reaction.
| Gene | Sequences
(5′-3′) |
|---|
| LOXL2 | F:
ATGACAGCAGGAGCGTGAGGT |
| R:
GGTTTAGAGCAGCAGAGAAGGGTAAG |
| TGF-β1 | F:
AGCAACAATTCCTGGCGTTACCTT |
| R:
CCTGTATTCCGTCTCCTTGGTTCAG |
| Smad2 | F:
GCTTAGTCCTGTGAGAGTTCCTGTTG |
| R:
ACTGACAACCAAGGCGTGATGAAG |
| Smad3 | F:
AGAACACCGATTCCACTCAACTAAGG |
| R:
AAGCCACCAGAACAGAAGCCATC |
| Smad4 | F:
TCAGGTGTGGCTCAGTGCTTGA |
| R:
GCCGACTCCTCCATACAGAACCA |
| Smad7 | F:
CGGACAGCTCAATTCGGACAACA |
| R:
CAGTGTGGCGGACTTGATGAAGAT |
| Snai1 | F:
ACCTGGTTCCTGCTTGGCTCTC |
| R:
AGTGGGTTGGCTTTAGTTCTATGGC |
| GAPDH | F:
AGAAGGtGGtGAAGCAGGCAtCt |
| R:
CGGCATCGAAGGTGGAAGAGTG |
Detection of protein expression in MLFs
using western blotting
Protein was extracted from MLFs by pyrolysis using
the ProteoPrep Total Extraction Sample kit and detected using the
Bicinchoninic Acid kit according to the manufacturers' protocols.
The protein samples were adjusted to the same concentration. Equal
amounts of total protein (50 µg/10 µl/lane) were separated using on
10% sDs-PAGE gels and then electro transferred to a nitrocellulose
membrane. Next, membranes were blocked with 5% skimmed milk powder
for 1 h at room temperature. The proteins were incubated with the
primary antibodies against rabbit anti-mouse LOXL2 (1:2,000),
TGF-β1 (1:500), Smad2/3 (1:500), Phospho-Smad2/3 (1:500), Smad4
(1:500), smad7 (1:500), snail (1:500) and GAPDH (1:5,000) at 4°C
overnight. Then, the membranes were incubated with the horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G (1:5,000)
for 1 h at room temperature, followed by exposure and development
using Immobilon ECL Ultra Western HRP substrate (Merck KGaA).
Detecting the expression of LOXL2 in
serum and lung homogenate, and the IL-6 and COL1A1 levels in cell
supernatant using ELISA
The serum of mice was collected and lung homogenate
was prepared (0.5 g tissues were mixed with 5 ml of precooled PBS).
The tissues were thoroughly ground in a glass homogenizer on ice.
After a freeze-thaw cycle (freezing at -20°C and then dissolving at
room temperature three times), the cell membrane was cracked and
centrifuged at 1,006 x g for 5 min at 4°C followed by the removal
of the supernatant. The supernatant was retained for the ELISA cell
experiments. ELISA was performed according to the protocols of the
LOXL2, IL-6 and COL1A1 ELISA kits.
Detection of the expression of LOXL2 in
pulmonary tissues in mice using immunohistochemical analysis
The paraffin-embedded slices were baked at 67°C,
dewaxed in xylene, and then rehydrated in a descending ethanol
series. The slices were washed with PBS. Antigen retrieval was
performed by boiling at 95°C. The activation of endogenous
peroxidase was blocked by incubation of slices in 50 µl of
peroxidase at room temperature for 10 min. Following washing with
PBS, the slices were blocked with goat serum (Wuhan Boster
Biological Technology, Ltd.) at room temperature for 10 min. Then,
the serum was removed, and 50 µl of primary antibody against LOXL2
(1:100) was added and incubated overnight at 4°C. Following washing
with PBs, slices were incubated with 1-2 drops of goat anti-rabbit
IgG secondary antibody (1:5,000) at room temperature for 10 min.
Then, streptavidin biotin-peroxidase solution was added for 30 min
at room temperature, followed by the addition of 100 µl of
3,3′-diami-nobenzidine coloring solution for 3-10 min.
Subsequently, 90% hematoxylin re-staining was performed for 5 min,
followed by gradient dehydration (70% ethanol, 1 min; 80% ethanol,
1 min; 95% ethanol, 2 min), 0.1% HCl differentiation (3 min), 0.1%
ammonia treatment (1-2 min) and drying, which were all performed at
room temperature. The slices were observed with an optical
microscope at x20 magnification (Olympus BX43; Olympus
Corporation).
Statistical analysis
The experimental data were analyzed using the SPSS
17.0 statistical software (SPSS, Inc., Chicago, IL, USA). The
results are presented as the mean ± standard deviation. Two groups
were compared using the independent-samples t-test, while multiple
groups were compared using one-way analysis of variance followed by
the Fisher's least significant difference or Tamhane's T2 post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Construction of mice model with
BLM-induced pulmonary fibrosis
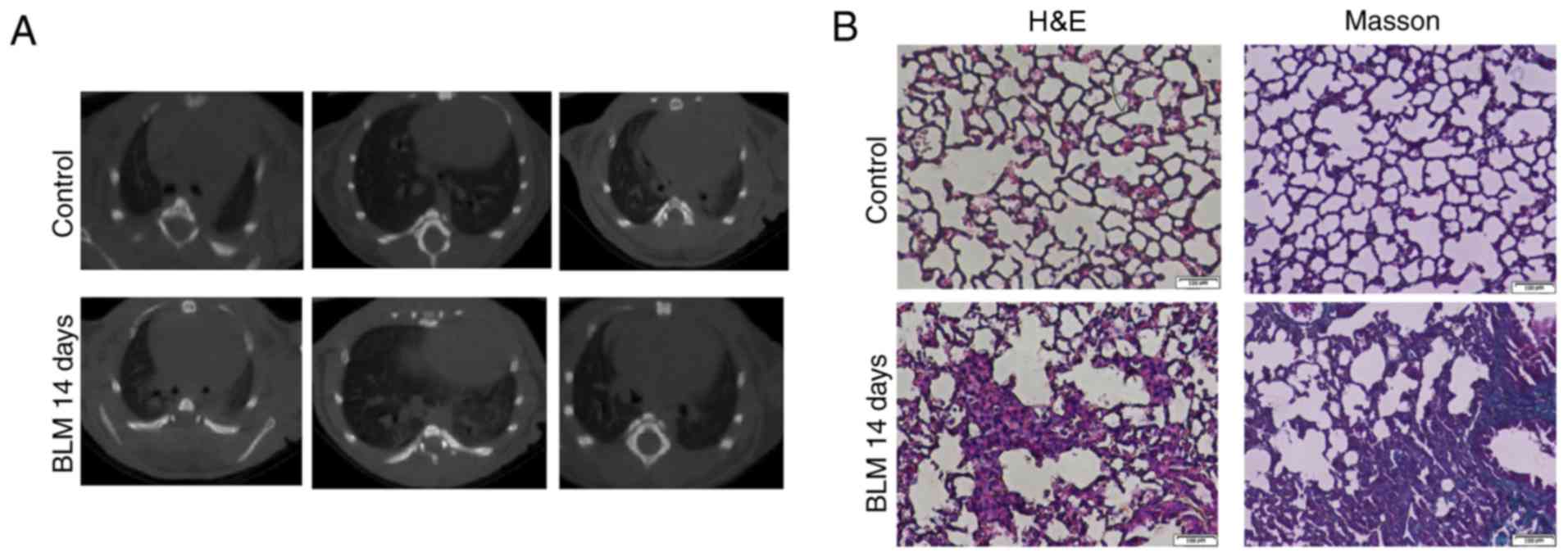
To verify the BLM-induced model of pulmonary
fibrosis in mice, a pulmonary CT scan and histopathological
analysis were performed. The pulmonary CT scan revealed that the
lung markings of the mice in the control group were regular, with
no significant abnormality in the pulmonary parenchyma. However,
the Ct manifestations in BLM at 14 days were as follows: Increased
bronchovascular shadows; patch lesions; diffuse dot-like patchy
shadows; inhomogeneous high-density areas; blurred margins; and
involvement of both lungs primarily in the lower field of the lungs
(Fig. 1A). H&E staining
demonstrated complete alveolar structure, normal alveolar septum as
well as no evident inflammatory infiltration in lungs from mice of
control group (Fig. 1B). In the
BLM model, the alveolar septum was widened, the alveolar wall was
collapsed or disappeared, and interstitial infiltration was evident
with a large number of inflammatory cells. In addition, Masson's
trichrome staining revealed that the density of collagen fibers in
the alveolar epithelium and bronchial area was markedly increased
in the BLM group compared with the control group (Fig. 1B). Histological analysis revealed
BLM-induced architecture destruction and collagen deposition in
lungs from mice, suggesting that the mice model of pulmonary
fibrosis was constructed successfully.
Expression of LOXL2 in mice of
BLM-induced pulmonary fibrosis
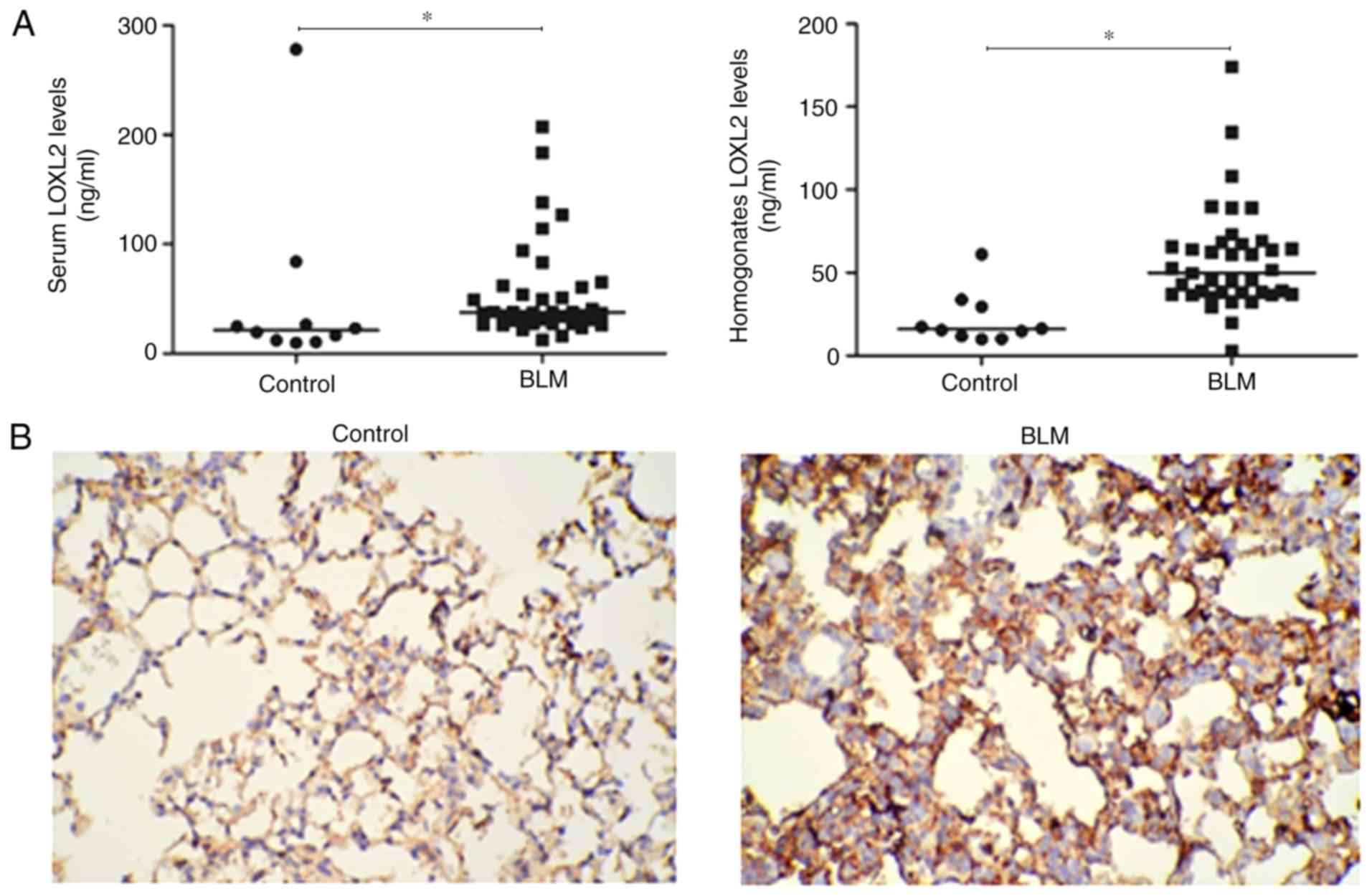
As LOXL2 is essential in mediating collagen
crosslinking and deposition (13), whether LOXL2 is involved in
BLM-induced lung fibrosis was investigated. First, LOXL2 expression
in serum, lung homogenate and pulmonary tissues in mice of
BLM-induced pulmonary fibrosis was detected by ELISA and
immunohistochemical analysis. As expected, BLM significantly
induced LOXL2 expression in the serum and lung homogenate of mice
compared with the control group (Fig.
2A). In addition, immunohistochemical analysis revealed that
LOXL2 expression was low in the alveolar epithelial cells and
partial macrophage cytoplasm in lung tissues of the control group,
while in the BLM group, evident expression of LOXL2 was observed in
certain alveolar epithelial cells in areas of severe lesions
(Fig. 2B). Thus, LOXL2 may be
associated with BLM-induced pulmonary fibrosis.
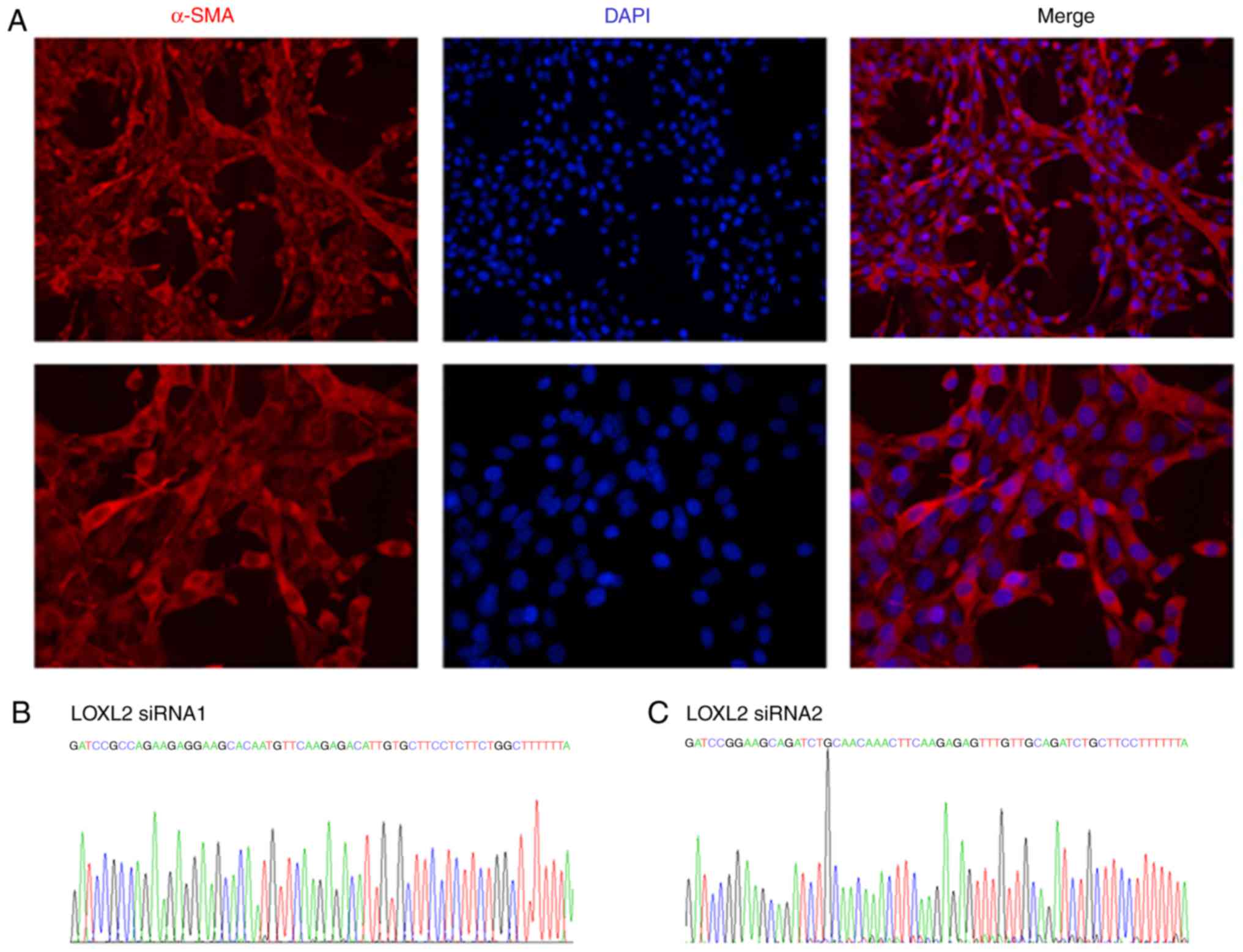
Transfection efficiency and silencing
effect of LOXL2 siRNA adenovirus vectors on MLFs
To further investigate the specific role of LOXL2 in
BLM-induced experimental lung fibrosis, LOXL2 silencing was
performed on MLFs from mice with pulmonary fibrosis by
administration of adenovirus-based LOXL2 siRNA. Following BLM
modeling for 14 days, primary MLFs from the lung tissue in the BLM
and control groups were isolated and cultured to form a stable cell
line. Cell morphology analysis revealed primary long spindles,
polygonal in shape. Immunofluorescence analysis indicated that red
fluorescence protein-labeled α-SMA was extensively expressed in the
cytoplasm of primary fibroblasts, conforming to the characteristics
of fibroblasts (Fig. 3A). The
results of gene sequencing from the primary fibroblasts were
consistent with the two sequences of LOXL2 siRNA and the control
siRNA sequence designed in the present study, thus indicating that
they were stably expressed (Fig.
3B-D). Flow cytometry demonstrated that the transfection
efficiency of LOXL2 siRNA in MLFs was >70% (Fig. 3E). Rt-qPCR demonstrated that LOXL2
mRNA and protein levels in the BLM group were significantly higher
compared with that in the control group (Fig. 3F). In addition, the expression of
LOXL2 was significantly decreased in the BLM group following the
knockdown with the two siRNAs compared with the BLM only and
BLM+siControl groups, and there were no difference between the BLM
only and BLM+siControl groups. The two siRNAs exhibited a marked
silencing effect on LOXL2 expression; both decreased LOXL2
expression by >60% (Fig. 3F)
following treatment with LOXL2 siRNA. Similar to findings with mRNA
expression, LOXL2 siRNA transfection markedly decreased LOXL2
protein expression in MLFs compared with the BLM and control siRNA
groups (Fig. 3G), indicating that
the LOXL2 siRNA constructed in the present study efficiently
transfected MLFs and silenced LOXL2.
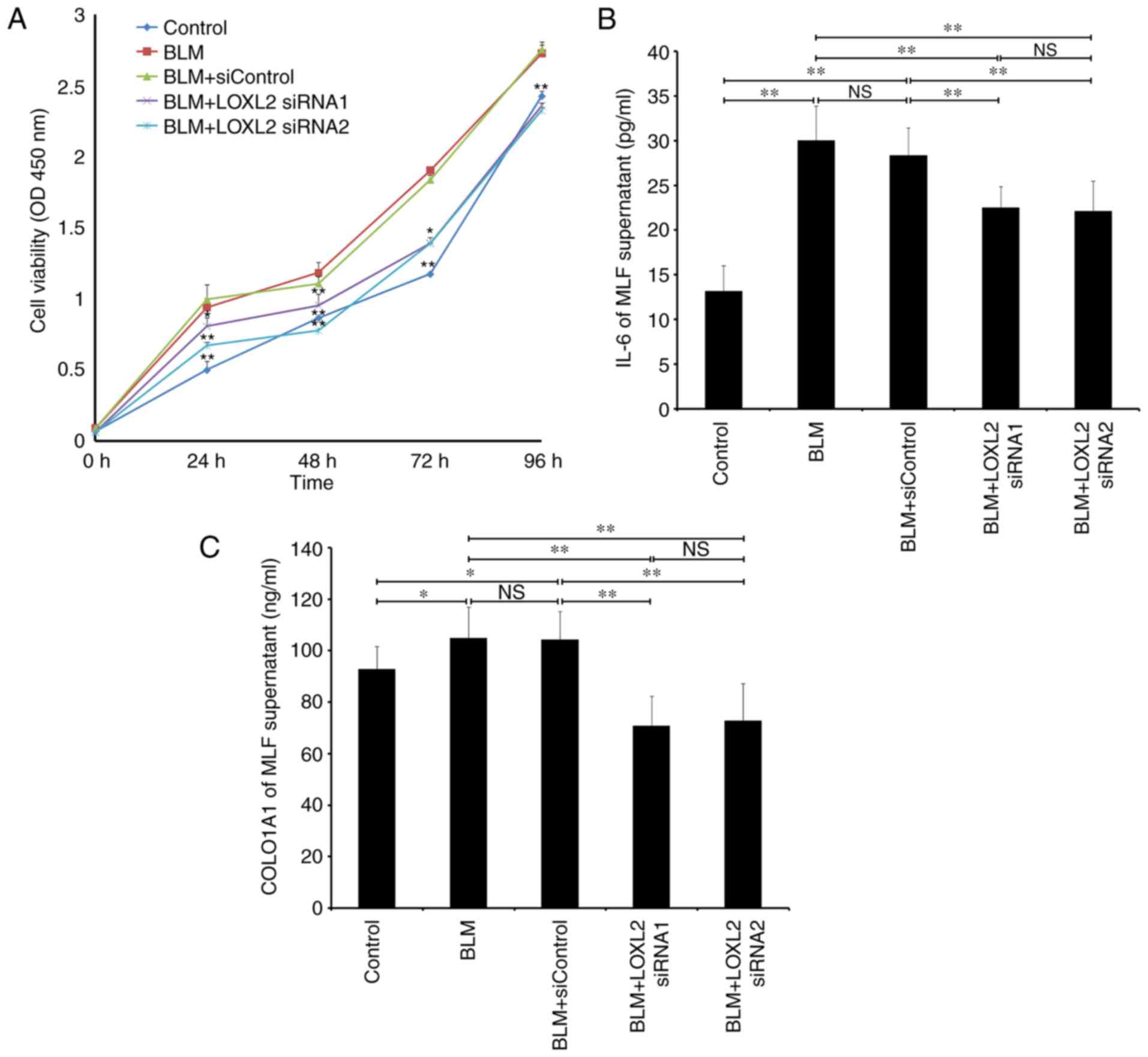
Effects of LOXL2 siRNA on MLF
proliferation and expression of fibrosis-associated factors
As MLFs rapidly proliferate and differentiate into
myofibroblasts with the progression of fibrosis, the effect of
LOXL2 siRNA on MLF proliferation was assessed. The proliferation
rate of MLFs from mice with BLM-induced pulmonary fibrosis elevated
significantly at 24, 48, 72 and 96 h compared with that of MLFs
from the BLM control group (P<0.01; Fig. 4A). Following LOXL2 siRNA
infection, the proliferation of MLFs in the BLM group declined
significantly, particularly after 48 h, compared with the BLM only
and control siRNA groups (both P<0.01; Fig. 4A). In addition, the effects of
LOXL2 siRNA on the expression of inflammatory factors IL-6 and
extracellular matrix COL1A1 involved in fibrosis progression were
observed. IL-6 and COL1A1 in the MLF supernatant from the BLM group
were upregulated significantly compared with MLFs from the control
group (P<0.01 and P<0.05, respectively; Fig. 4B and C). Furthermore, LOXL2 siRNA
significantly inhibited the expression of IL-6 and COL1A1 in the
MLF supernatant from mice with BLM-induced pulmonary fibrosis
compared with the BLM only and BLM+siControl groups (both
P<0.01; Fig. 4B and C),
suggesting that LOXL2 serves an essential role in fibrosis
progression via MLFs in BLM-induced pulmonary fibrosis.
Effects of LOXL2 siRNA on the expression
of key factors in the TGF-β/Smad signaling pathway
The aforementioned results represent an association
between LOXL2 and BLM-induced pulmonary fibrosis, thus LOXL2
required further investigation as a potential biomarker for
pulmonary fibrosis. The effect of LOXL2 siRNA on the TGF-β
signaling pathway in MLFs was assessed (Fig. 5). RT-qPCR demonstrated that MLFs
from mice with BLM-induced pulmonary fibrosis exhibited
signifi-cant upregulation of TGF-β1 and Snail mRNA (P<0.05 and
P<0.01, respectively; Fig. 5A and
F), whereas Smad7 mRNA was significantly downregulated compared
with the control groups (P<0.01; Fig. 5E). Following LOXL2 siRNA
transfection, the difference in Smad4 mRNA expression between the
LOXL2 siRNAs and BLM only groups was significant (P<0.01);
however, only LOXL2 siRNA1 induced a signifi-cant difference
compared with the BLM+siControl group, whereas LOXL2 siRNA2 did not
induce a significant difference compared with the BLM+siControl
group. (P<0.05 and P>0.05, respectively; Fig. 5D). Moreover, the mRNA expression
levels of snail were significantly decreased in the siRNAs groups
compared with the BLM only and BLM+siControl groups (both
P<0.01; Fig. 5F), whereas
Smad7 mRNA expression was significantly increased (P<0.01;
Fig. 5E). In addition, LOXL2
siRNA had no significant effect on the mRNA expression of TGF-β1
and Smad2/3 in MLFs from the BLM group compared with BLM only and
BLM+siControl groups (P>0.05; Fig.
5A-C). Similar to the mRNA findings, MLFs from the BLM group
demonstrated markedly elevated protein levels of TGF-β1, pSmad2/3
and Snail compared with the MLFs from the control group. Following
transfection with LOXL2 siRNA, the protein expression of pSmad2/3,
Smad4 and Snail in the MLFs decreased markedly compared with the
BLM only and control siRNA groups, while the expression of smad7
increased (Fig. 5G). However, no
marked reduction was observed in the protein expression of Smad2/3.
Together, these data suggest that LOXL2 siRNA regulated the
expression of key factors in the TGF-β/Smad signaling pathway.
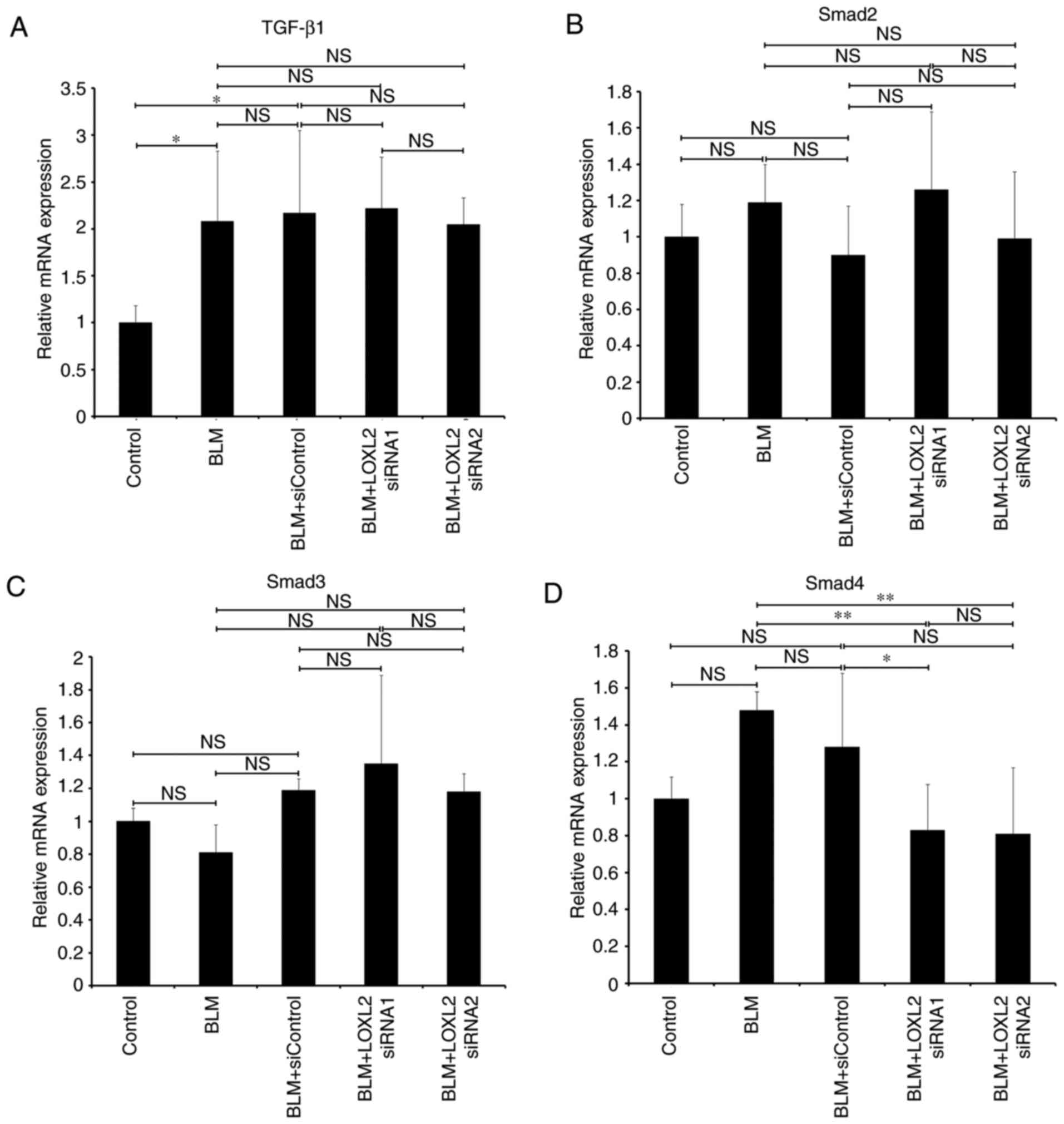 | Figure 5Expression of key factors of
TGF-β/smad pathway in MLFs from mice with BLM-induced pulmonary
fibrosis following LOXL2 siRNA transfection. The mRNA expression of
(A) TGF-β1, (B) Smad2, (C) Smad3, (D) Smad4, (E) Smad7 and (F)
Snail was detected using reverse transcription-quantitative
polymerase chain reaction and normalized to the expression of GADPH
mRNA. (G) Expression of TGF-β1, Smad2/3, pSmad2/3, Smad4, Smad7 and
Snail proteins was detected using western blotting. Protein
expression was normalized to the expression of β-actin. MLFs from
C57BL/6 wild-type mice were used as the control group.
*P<0.05 and **P<0.01. LOXL2, Lysyl
oxidase-like 2; TGF-β1, tumor growth factor-β; siRNA, small
interfering RNA; MLFs, mouse lung fibroblasts; BLM, bleomycin. |
Discussion
Collagen matrix deposition and interstitial
transition of epithelial cells are the primary features of
pulmonary fibrosis occurrence and development. The LOX family is
involved in the production of collagen matrix, regulation of
extracellular matrix deposition and transition of epithelial cells
to stroma cells (23). LOXL2 has
been proposed to be structurally and functionally similar to LOX,
which has been associated with disease-associated fibrogenesis by
promoting the cross-linking of elastin and collagen fibers
(24,25). Barker et al (26) demonstrated that LOXL2 mediated the
crosslinking of the extracellular matrix to promote the activation
of fibroblasts and large-scale production of α-SMA. Chien et
al (24) reported that serum
levels of LOXL2 in patients with idiopathic pulmonary fibrosis were
associated with the progression of the disease. In addition, it has
been reported that inhibition of LOXL2 with an inhibitory
monoclonal antibody is efficacious at fibrosis inhibition in murine
models of fibrotic disease, including lung fibrosis and liver
fibrosis (14). However, the
exact effects of LOXL2 on the pathogenesis of pulmonary fibrosis,
particularly its association with pulmonary fibrosis-associated
signaling pathways, require further investigation.
Consistent with the aforementioned findings,
substantial elevation of LOXL2 was observed in the serum and lung
homogenate of mice with BLM-induced pulmonary fibrosis.
Furthermore, the immunohistochemical analysis revealed BLM induced
LOXL2 expression in pulmonary tissues (alveolar wall and
interstitium) and the lesion area. Notably, Choi et al
(27) revealed that LOXL2 was
present in the fibrous interstitium and infiltrating mononuclear
cells in a mouse model of tubulointerstitial fibrosis,
demonstrating one possible mechanism that inflammatory milieu of
LOXL2 contributes to fibrosis progression. However, the
immunohistochemical results of the present study did not observe
evident LOXL2 expression in inflammatory cells. In combination with
the current understanding of the role of LOXL2 in pulmonary
fibrosis, these findings suggest that LOXL2 may be associated with
BLM-induced pulmonary fibrosis. Next, LOXL2 was silenced using
siRNA transfection in MLFs from mice with BLM-induced pulmonary
fibrosis in order to further investigate the involvement of LOXL2
in pulmonary fibrosis. This enabled the in vitro observation
of the effects of LOXL2 on the pathogenesis and progression of
pulmonary fibrosis through the proliferation of MLFs, and the
expression of factors involving in fibrosis progression in cells.
In addition, the effects of LOXL2 silencing on the expression of
key factors in the TGF-β/Smad signaling pathway in MLFs were
observed, and relevant mechanisms were further investigated.
Following the observation of high levels of LOXL2 in
murine models of pulmonary fibrosis, whether LOXL2 is able to
facilitate fibroblast proliferation and activation, and hence serve
a role in fibrosis progression was investigated. Fibroblast
proliferation and their transition to myofibroblasts as well as the
large amount of extracellular matrix produced by fibroblasts, are
the primary characteristics for the occurrence of pulmonary
fibrosis (28), and are
implicated in the maintenance and reconstruction of extracellular
matrix. Furthermore, pro-inflammatory factor IL-6 and extracellular
matrix Col I of myofibroblasts are involved in the proliferation,
transformation and stromal deposition of fibroblasts, which are
associated with the development of pulmonary fibrosis (29,30). Notably, it has been demonstrated
that LOXL2 is widely expressed in fibroblasts and serves an
important role in fibroblasts activation (26). In the present study, primary MLFs
from mice in the control and BLM-induced pulmonary fibrosis groups
were selectively cultured and identified using α-SMA detection.
Then, MLFs from mice models of pulmonary fibrosis were transfected
with LOXL2 siRNA. The data demonstrated that LOXL2 silencing in
MLFs from BLM-induced fibrosis exhibited a significant inhibitory
effect on MLF proliferation as well as the expression of IL-6 and
COL1A1 compared with MLFs from the BLM group. These results support
our previous finding that LOXL2 is involved in fibrosis
progression, contributing to the fibrotic lung diseases.
In addition, whether LOXL2 acted synergistically
with the TGF-β/Smad signaling pathway was assessed in order to
investigate the underlying mechanism. The classical TGF-β1/Smad
signaling pathway is one of the signaling pathways involved in the
occurrence of pulmonary fibrosis (17). TGF-β1 acts on the target cells
through the transmembrane receptor, thus leading to the
phosphorylation of Smad2 and Smad3. Subsequently, phosphorylated
Smad2/3 binds to Smad4 and enters the nucleus to form a Smad
protein compound that interacts with the transcription factor
Snail, regulating the transcription of fibrosis-associated genes
downstream; however, the activation of this pathway is inhibited by
smad7 (17-19). Whether LOXL2 is the upstream
molecule of the TGF-β1/Smad signaling pathway remains
controversial. A previous study revealed that the expression of
LOXL2 was significantly increased following the stimulation of
airway epithelial cells by TGF-β (15). Silencing LOXL2 completely
abrogated the inhibitory effects of corilagin on TGF-β1-induced
epithelial-mesenchymal transition in A549 cells (21). However, another study reported
that LOXL2 inhibition impaired fibroblast activation in in
vivo models of fibrosis, and led to reductions in TGF-β
signaling (14). In addition,
Millanes-Romero et al (13) demonstrated that LOXL2 affected the
transcription of Snail, subsequently inducing the transition of
epithelial cells to stromal cells. Peinado et al (31) revealed that LOXL2 downregulated
the expression of E-cadherin in coordination with transcription
factor Snail to promote the epithelial-mesenchymal transition. The
aforementioned studies suggested that LOXL2 is associated with the
TGF-β1/Smad signaling pathway. In the present study, the gene and
protein expression of the aforementioned factors in the TGF-β1/Smad
signaling pathway were detected, and the effects of LOXL2 on these
factors were observed. The results indicated that silencing LOXL2
in MLFs from mice with BLM-induced fibrosis inhibited the gene and
protein expression of Smad4 and Snail, as well as the protein
expression of pSmad2/3, thereby upregulating the expression of
inhibitory factor smad7. However, no significant effects were
observed on the expression of TGF-β1 and Smad2/3. These results
suggested that LOXL2 regulated Smad2/3 phosphorylation, and the
expression of Smad4 and Snail in the TGF-β1 signaling pathway,
subsequently causing an effect on the expression of inhibitory
factor smad7. However, LOXL2 did not regulate the expression of
TGF-β, suggesting that LOXL2 may be located downstream of TGF-β or
that they interact indirectly. Furthermore, the results of the
present study demonstrated that silencing LOXL2 did not affect the
expression of Smad2/3, while it affected its phosphorylation level,
indicating that Smad2/3 phosphorylation rather than its own
expression was a key factor in the pathogenesis of fibrosis. These
data revealed an association between LOXL2 and the TGF-β/smad
signaling pathway in fibrotic progression.
The current study demonstrated that the expression
of LOXL2 in serum and pulmonary tissues of mice with BLM-induced
pulmonary fibrosis were significantly elevated compared with
control mice. MLFs from mice with BLM-induced pulmonary fibrosis
were used in subsequent experiments. LOXL2 siRNA inhibited the
proliferation of MLFs in BLM-induced fibrosis and regulated the
expression of important factors, including pSmad2/3, Smad4, Smad7
and Snail in the TGF-β/Smad signaling pathway, suggesting that
LOXL2 was involved in the fibrosis progression through regulation
of the TGF-β/Smad signaling pathway. Furthermore, this study
provided an experimental basis for an in-depth study of LOXL2 in
the pathogenesis of pulmonary fibrosis and its potential as the
therapeutic target. However, numerous questions remain to be
addressed, including whether LOXL2 is involved in inflammation
associated to BLM-induced fibrosis, how it interacts with TGF-β1,
whether TGF-β1 regulates LOXL2 or LOXL2 regulates TGF-β1, and what
the underlying mechanism of LOXL2 is. In addition, the association
between LOXL2 and TGF-β non-Smad signaling pathways requires
further investigation.
In conclusion, the present study investigated the
effects of LOXL2 expression in pulmonary fibrosis, and effect on
the proliferation, activation and fibrosis process of MLFs.
Furthermore, these results indicate a regulatory role for LOXL2 in
fibrogenesis via the TGF-β/Smad signaling pathway. Based on these
findings, we hypothesize that LOXL2 participates in the
pathogenesis of pulmonary fibrosis and may be a simultaneous
therapeutic target.
Acknowledgements
Not applicable.
Funding
The project was supported by the National Natural
Science Foundation of China (grant no. 81471616), the Research
Funds of Baotou Medical College (grant no. BYJJ-YF 2016100) and the
Natural Science Foundation of Inner Mongolia [grant no. 2017Ms
(LH)0812].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ contributed to experimental design, coordination
and revised drafts of the manuscript. XW performed animal
experiment, analyzed and interpreted the data. YL performed the
cell experiments, analyzed the results and drafted the manuscript.
YB and ML performed the histological examinations and ELISA
analysis. QF participated in construction of LOXL2 siRNA and cell
culture. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Animals experiments involving included studies have
been approved by the Experimental Animal Welfare Committee of
Capital Medical University (approval no. AEEI-2018-068) in
compliance with applied principles of experimental animals.
Patient consent for publication
Not applicable.
Competing interests
The authors have declared that they have no
competing interests.
Abbreviations:
|
BLM
|
bleomycin
|
|
CCK-8
|
cell counting kit 8
|
|
CT
|
computed tomography
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
dsDNA
|
double-strand DNA
|
|
H&E
|
hematoxylin and eosin
|
|
LOX
|
Lysyl oxidase
|
|
MLFs
|
mouse lung fibroblasts
|
|
PCR
|
polymerase chain reaction
|
|
PE
|
phycoerythrin
|
|
PFUs
|
plaque-forming units
|
|
siRNA
|
short interfering RNA
|
|
SMA
|
smooth muscle actin
|
|
TGF-β
|
transforming growth factor-β
|
References
|
1
|
Churg A, Muller NL and Wright JL:
Respiratory bronchiolitis/interstitial lung disease: Fibrosis,
pulmonary function, and evolving concepts. Arch Pathol Lab Med.
134:27–32. 2010.PubMed/NCBI
|
|
2
|
Yoshida M, Sakuma J, Hayashi S, Abe K,
Saito I, Harada S, Sakatani M, Yamamoto S, Matsumoto N, Kaneda Y,
et al: A histologically distinctive interstitial pneumonia induced
by overexpression of the interleukin 6, transforming growth factor
beta 1, or platelet-derived growth factor B gene. Proc Natl Acad
Sci USA. 92:9570–9574. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gross TJ and Hunninghake GW: Idiopathic
pulmonary fibrosis. N Engl J Med. 345:517–525. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Todd NW, Luzina IG and Atamas SP:
Molecular and cellular mechanisms of pulmonary fibrosis.
Fibrogenesis tissue Repair. 5:112012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kurundkar A and Thannickal VJ: Redox
mechanisms in age-related lung fibrosis. Redox Biol. 9:67–76. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lederer DJ and Martinez FJ: Idiopathic
pulmonary fibrosis. N Engl J Med. 378:1811–1823. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bagnato G and Harari S: Cellular
interactions in the pathogenesis of interstitial lung diseases. Eur
Respir Rev. 24:102–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Raghu G, Collard HR, Egan JJ, Martinez FJ,
Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et
al: An official Ats/ERS/JRS/ALAT statement: Idiopathic pulmonary
fibrosis: Evidence-based guidelines for diagnosis and management.
Am J RespirCrit Care Med. 183:788–824. 2011. View Article : Google Scholar
|
|
9
|
GBD 2013 Mortality and Causes of Death
Collaborators: Global, regional, and national age-sex specific
all-cause and cause-specific mortality for 240 causes of death,
1990-2013: A systematic analysis for the Global Burden of Disease
Study 2013. Lancet. 385:117–171. 2015. View Article : Google Scholar :
|
|
10
|
Lucero HA and Kagan HM: Lysyl oxidase: An
oxidative enzyme and effector of cell function. Cell Mol Life Sci.
63:2304–2316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kagan HM and Li W: Lysyl oxidase:
Properties, specificity, and biological roles inside and outside of
the cell. J Cell Biochem. 88:660–672. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Magdaleno F and Trebicka J: Selective
LOXL2 inhibition: Potent antifibrotic effects in ongoing fibrosis
and fibrosis regression. Gut. 66:1540–1541. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Millanes-Romero A, Herranz N, Perrera V,
Iturbide A, Loubat- Casanovas J, Gil J, Jenuwein T, García de
Herreros A and Peiró S: Regulation of heterochromatin transcription
by Snail1/LOXL2 during epithelial-to-mesenchymal transition. Mol
Cell. 52:746–757. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barry-Hamilton V, Spangler R, Marshall D,
McCauley S, Rodriguez HM, Oyasu M, Mikels A, vaysberg M, Ghermazien
H, Wai C, et al: Allosteric inhibition of lysyl oxidase-like-2
impedes the development of a pathologic microenvironment. Nat Med.
16:1009–1017. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aumiller V, Strobel B, Romeike M, Schuler
M, Stierstorfer BE and Kreuz S: Comparative analysis of lysyl
oxidase (like) family members in pulmonary fibrosis. Sci Rep.
7:1492017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hinz B, Phan SH, Thannickal VJ, Galli A,
Bochaton-Piallat ML and Gabbiani G: The myofibroblast: One
function, multiple origins. Am J Pathol. 170:1807–1816. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-β family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee CM, Park JW, Cho WK, Zhou Y, Han B,
Yoon PO, Chae J, Elias JA and Lee CG: Modifiers of TGF-β1 effector
function as novel therapeutic targets of pulmonary fibrosis. Korean
J Intern Med. 29:281–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Medici D, Potenta S and Kalluri R:
Transforming growth factor-β2 promotes Snail-mediated
endothelial-mesenchymal transition through convergence of
Smad-dependent and Smad-independent signalling. Biochem J.
437:515–520. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng T, Liu Q, Zhang R, Zhang Y, Chen J,
Yu R and Ge G: Lysyl oxidase promotes bleomycin-induced lung
fibrosis through modulating inflammation. J Mol Cell Biol.
6:506–515. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wei Y, Kim TJ, Peng DH, Duan D, Gibbons
DL, Yamauchi M, Jackson JR, Le Saux CJ, Calhoun C, Peters J, et al:
Fibroblast-specific inhibition of TGF-β1 signaling attenuates lung
and tumor fibrosis. J Clin Invest. 127:3675–3688. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Mäki JM, Sormunen R, Lippo S,
Kaarteenaho-Wiik R, Soininen R and Myllyharju J: Lysyl oxidase is
essential for normal development and function of the respiratory
system and for the integrity of elastic and collagen fibers in
various tissues. Am J Pathol. 167:927–936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chien JW, Richards TJ, Gibson KF, Zhang Y,
Lindell KO, Shao L, Lyman SK, Adamkewicz JI, Smith V, Kaminski N
and O'Riordan T: serum lysyl oxidase-like 2 levels and idiopathic
pulmonary fibrosis disease progression. Eur Respir J. 43:1430–1438.
2014. View Article : Google Scholar
|
|
25
|
Moon HJ, Finney J, Ronnebaum T and Mure M:
Human lysyl oxidase-like 2. Bioorg Chem. 57:231–241. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barker HE, Bird D, Lang G and Erler JT:
Tumor-secreted LOXL2 activates fibroblasts through FAK signaling.
Mol Cancer Res. 11:1425–1436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi SE, Jeon N, Choi HY, Shin JI, Jeong
HJ and Lim BJ: Lysyl oxidase-like 2 is expressed in kidney tissue
and is associated with the progression of tubulointerstitial
fibrosis. Mol Med Rep. 16:2477–2482. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Senavirathna LK, Huang C, Yang X, Munteanu
MC, Sathiaseelan R, Xu D, Henke CA and Liu L: Hypoxia induces
pulmonary fibroblast proliferation through NFAt signaling. Sci Rep.
8:27092018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hansen NU, Karsdal MA, Brockbank S, Cruwys
S, Rønnow S and Leeming DJ: Tissue turnover of collagen type I, III
and elastin is elevated in the PCLS model of IPF and can be
restored back to vehicle levels using a phosphodiesterase
inhibitor. Respir Res. 17:762016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
O'Reilly S, Ciechomska M, Cant R and van
Laar JM: Interleukin-6 (IL-6) trans signaling drives a
STAT3-dependent pathway that leads to hyperactive transforming
growth factor-β (TGF-β) signaling promoting sMAD3 activation and
fibrosis via Gremlin protein. J BiolChem. 289:9952–9960. 2014.
|
|
31
|
Peinado H, Del Carmen Iglesias-de la Cruz
M, Olmeda D, Csiszar K, Fong KS, Vega S, Nieto MA, Cano A and
Portillo F: A molecular role for lysyl oxidase-like 2 enzyme in
snail regulation and tumor progression. EMBO J. 24:3446–3458. 2005.
View Article : Google Scholar : PubMed/NCBI
|