Introduction
Lung cancer is the most common malignancy and the
leading cause of cancer-associated mortality worldwide (1). In the USA, there were 222,500 newly
diagnosed cases of lung cancer and 155,870 mortalities due to lung
cancer in 2017, according to statistics reported by Siegel et
al (2). Currently, there are
two recognized subtypes of lung cancer, non-small cell lung cancer
(NSCLC) and small cell lung cancer. Clinically, NSCLC accounts for
>80% of the total incidence of lung cancer (3). Chemotherapy is one of the most
effective solutions for lung cancer. However, due to the frequent
alterations in chemotherapy regimens, chemotherapy resistance is a
major problem in clinical practice and, importantly, its underlying
mechanisms remain to be elucidated (4).
Sesamin, a type of lignan, is a biologically active
compound extracted in large quantities from Sesamum indicum
(5). Sesamin has various valuable
biological functions, including protection against oxidative
stress, anti-inflammation and the inhibition of carcinogenesis
(6). A previous study revealed
that sesamin decreased the frequency of chemical induction of
breast tumors and enhanced liver detoxification (5). In addition, sesamin reduced
lipopolysaccharide (LPS)-induced acute lung injury in mice
(7) and inhibited LPS-induced
cell proliferation and invasion in prostate cancer cells (8). However, the effect of sesamin on
lung cancer and the molecular mechanism implicated in this process
remain largely unknown.
Cyclooxygenase 2 (COX2) is one of the two subtypes
of COX, which is considered to be involved in inflammation and
carcinoma progression (9). COX2
may be induced by various stimuli, including growth factors,
oncogenes, cytokines and hormones (10). It has been reported that COX2 may
serve an important role in lung carcinogenesis. Overexpression of
COX2 is common in NSCLC and appears to be associated with tumor
progression and metastasis (11).
However, whether COX2 regulates chemosensitivity toward sesamin in
lung cancer cells and the mechanisms associated with this process,
are still under investigation.
The present study investigated the effect of COX2 on
sesamin-induced apoptosis and the cell cycle in lung cancer cells
via the Akt/PI3K signaling pathways. The present results may
provide a valuable insight into the mechanism of sesamin-induced
progression and development of lung cancer.
Materials and methods
Cell culture
The lung cancer cell lines A549, NCI-H446 and H1299
were purchased from the American Type Culture Collection (Manassas,
VA, USA). The human normal lung epithelial cell line BEAS-2B was
obtained from the Stem Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). All cell lines were cultured in RPMI-1640 medium
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA) containing
10% fetal bovine serum (ExCell Bio, Inc., Shanghai, China) and
incubated at 37°C with 5% CO2. Lung cancer cells were
treated with 25 µM CAY10404 (a COX2 inhibitor; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) for 2 h. Subsequently, the
cells were incubated for additional 24 h with 50 µM sesamin
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China). In the case of A549 cells, these were treated with 10
µM LY294002 (a PI3K inhibitor; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) for 24 h (12).
Cell Counting Kit (CCK)-8 assay
Cell proliferation was detected by CCK-8 analysis
(Dojindo Molecular Technologies, Inc., Rockville, MD, USA).
Briefly, lung cancer cells (5×103 cells/100
µl/well) were seeded in a 96-well plate (Corning
Incorporated, Corning, NY, USA). After 24 h, the cells were treated
with 0, 10, 50, 100 and 150 µM sesamin for 0, 24, 48 and 72
h. Subsequently, 10 µl CCK-8 solution was added to each well
and the absorbance at 450 nm was read following incubation for 3 h
at 37°C (3).
Cell apoptosis assay
Cell apoptosis was analyzed using an apoptosis
detection kit [Multisciences (Lianke) Biotech Co., Ltd., Hangzhou,
China] according to the manufacturer’s protocol. In brief, treated
lung cancer cells were resus-pended in 500 µl 1X Binding
Buffer and incubated with 10 µl propidium iodide (PI) and 5
µl Annexin V-fluorescein isothiocyanate (FITC). Following 5
min of incubation in the dark, flow cytometric analysis was
conducted using a FACScan flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA) and a FlowJo 7.6 software (1997-2008; FlowJo LLC,
Ashland, OR, USA) (13).
Cell cycle assay
Cell cycle was determined with a cell cycle
detection kit [Multisciences (Lianke) Biotech Co., Ltd.] according
to the manufacturer’s protocol. In brief, treated lung cancer cells
were resuspended in 10 µl permeabilization solution and 1 ml
DNA staining solution. Following 30 min of staining at room
temperature, flow cytometric analysis was conducted using a FACScan
flow cytometer (BD Biosciences) (14).
Western blot assay
The cells were lysed in radioimmunopre-cipitation
assay buffer (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China). Protein concentration was determined with a
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology, Haimen, China). Proteins (40 µg/sample) were
separated by 12% SDS-PAGE and transferred to a polyvinylidene
fluoride membrane (0.2 µM; EMD Millipore, Billerica, MA,
USA). The membranes were blocked in 5% bovine serum albumin
(Beijing Solarbio Science & Technology Co., Ltd.) in TBS with
0.1% Tween-20 for 2 h at room temperature and incubated with
primary antibodies at 4°C overnight. Antibodies against COX2 (cat.
no. 12282), Bcl-2 (cat. no. 15071), Bax (cat. no. 5023), cyclin D1
(cat. no. 2922), cyclin B1 (cat. no. 12231), cyclin A2 (cat. no.
4656), interleukin (IL)1β (cat. no. 12242), IL6 (cat. no. 12153),
tumor necrosis factor (TNF)α (cat. no. 3707), AKT (cat. no. 9272),
pAKT (cat. no. 4060), PI3K (cat. no. 4257), mammalian target of
rapamycin (mTOR; cat. no. 2972) and GAPDH (cat. no. 5174; 1:1,000;
Cell Signaling Technology, Inc., Danvers, MA, USA) were used. Upon
washing, the membranes were incubated with secondary antibodies
[Goat anti-rabbit immunoglobulin (Ig)G-horseradish peroxidase (HRP)
cat. no. BA1054 or goat anti-mouse IgG-HRP cat. no. BA1050;
1:5,000; Wuhan Boster Biological Technology, Ltd., Wuhan, China]
for 2 h at room temperature. The protein bands were detected by
chemiluminescence (Western blotting detection kit, cat. no.
K-12045-D10; Advansta, San Jose, CA, USA). Densitometric analysis
was performed by Tanon GIS version 4.1.2 software (Tanon Science
and Technology Co., Ltd., Shanghai, China) (15).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNA was extracted from lung cancer cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). The concentration of RNA was quantified using NanoDrop 2000
(NanoDrop Technologies; Thermo Fisher Scientific, Inc., Wilmington,
DE, USA) and the RNA was then reverse-transcribed into cDNA using
an RT kit (Thermo Fisher Scientific, Inc.). The procedure for
reverse transcription included an annealing step of 10 min at 65°C,
followed 1 h at 42°C, 5 min at 85°C and immediately placed in ice.
RT-qPCR was performed using LightCycler 480 SYBR-Green I Master
(16) (Roche Applied Science,
Madison, WI, USA) to detect COX2 mRNA expression. GAPDH was used as
the internal control. The primer sequences used in RT-qPCR were as
follows: COX2, 5′-TCA AAA CCG AGG TGT A-3′ (sense) and 5′-GTG GGT
AAG TAT GTA GTG C-3′ (antisense); and GAPDH, 5′-AAG CCT GCC GGT GAC
TAA C-3′ (sense) and 5′-GCATCACCCGGAGGAGAAAT-3′ (antisense). The
procedure for qPCR included an initial denaturation of 15 min at
95°C, followed by 40 cycles of 10 sec at 95°C, 22 sec at 55°C and
30 sec at 72°C (17).
Statistical analysis
All experiments were repeated three times. One-way
analysis of variance and Fisher’s exact test was performed to
evaluate the differences between groups using SPSS 18.0 (SPSS,
Inc., Chicago, IL, USA). The data are expressed as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Cytotoxic effect of sesamin against human
lung cancer cells
To evaluate the effect of sesamin on lung cancer
cells, A549, NCI-H446 and H1299 cells were treated with 0, 10, 50,
100 and 150 µM sesamin for 0, 24, 48 and 72 h. Sesamin
significantly inhibited the proliferation of lung cancer cells A549
and NCI-H446 in a concentration-dependent manner compared with the
cells treated with 0 µM sesamin, which served as the control
(P<0.05; Fig. 1A). In H1299
inhibition was only significant at 100 µM or over.
Furthermore, the results of the flow cytometry analysis of Annexin
V-FITC and PI double staining indicated that sesamin promoted the
apoptosis of lung cancer cells in a concentration-dependent manner
compared with the control (Fig.
1B). Therefore, these results suggested that sesamin possesses
an antitumor role in lung cancer cells.
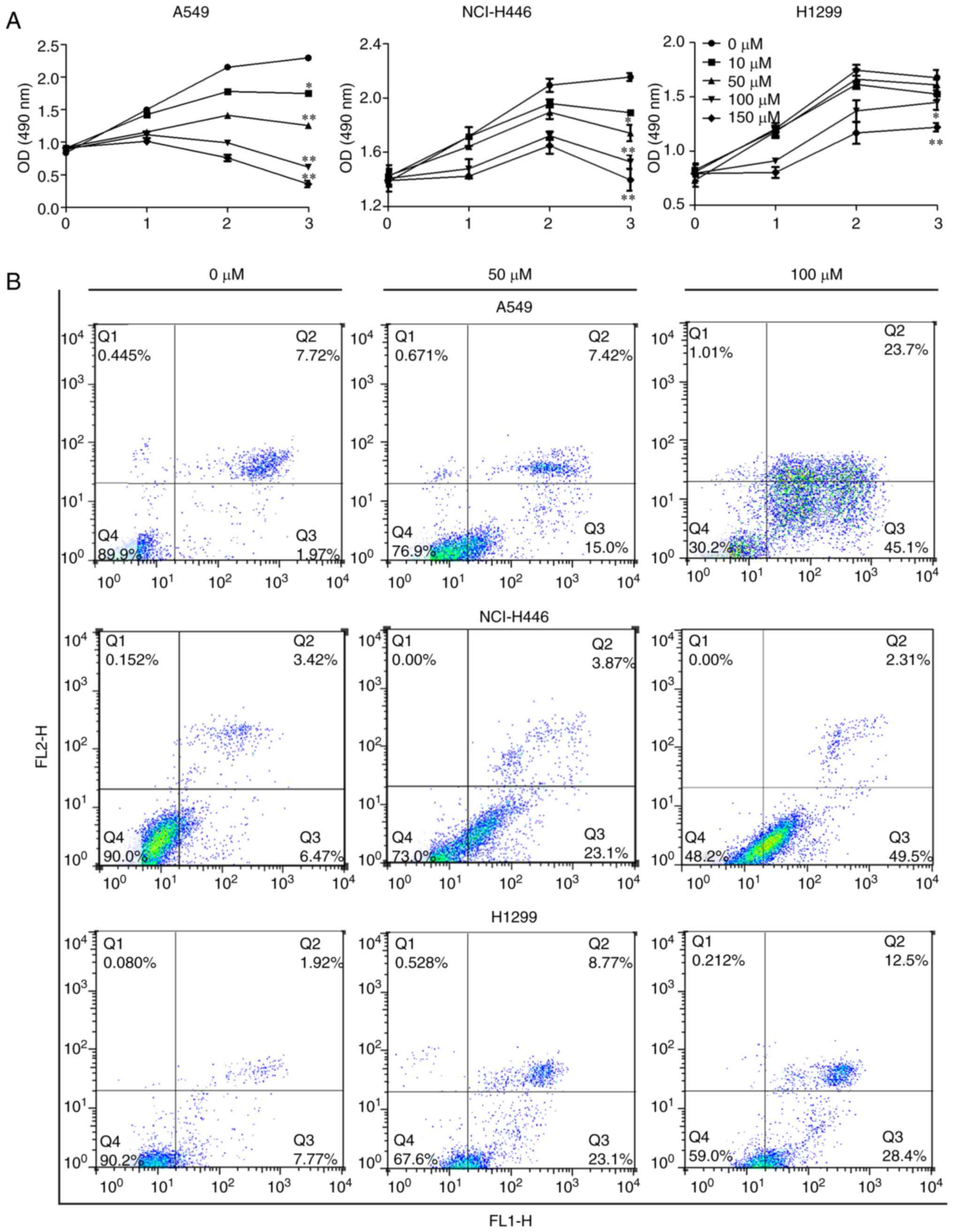 | Figure 1Sesamin inhibits the proliferation and
promotes the apoptosis of lung cancer cells. (A) A549, NCI-H446 and
H1299 cells were treated with 0, 10, 50, 100 and 150 µM
sesamin for 0, 24, 48 and 72 h, and the cytotoxicity of sesamin was
detected by Cell Counting Kit-8 assay. (B) The above three cell
lines were treated with the indicated concentrations of sesamin for
24 h and flow cytometry analysis was performed to examine
sesamin-induced apoptosis. *P<0.05 and **P<0.01
vs. 0 µM. p-Akt, phosphorylated protein kinase B, PI3K,
phosphoinositide 3 kinase; mTOR, mammalian target of rapamycin;
COX2, cyclooxygenase 2. |
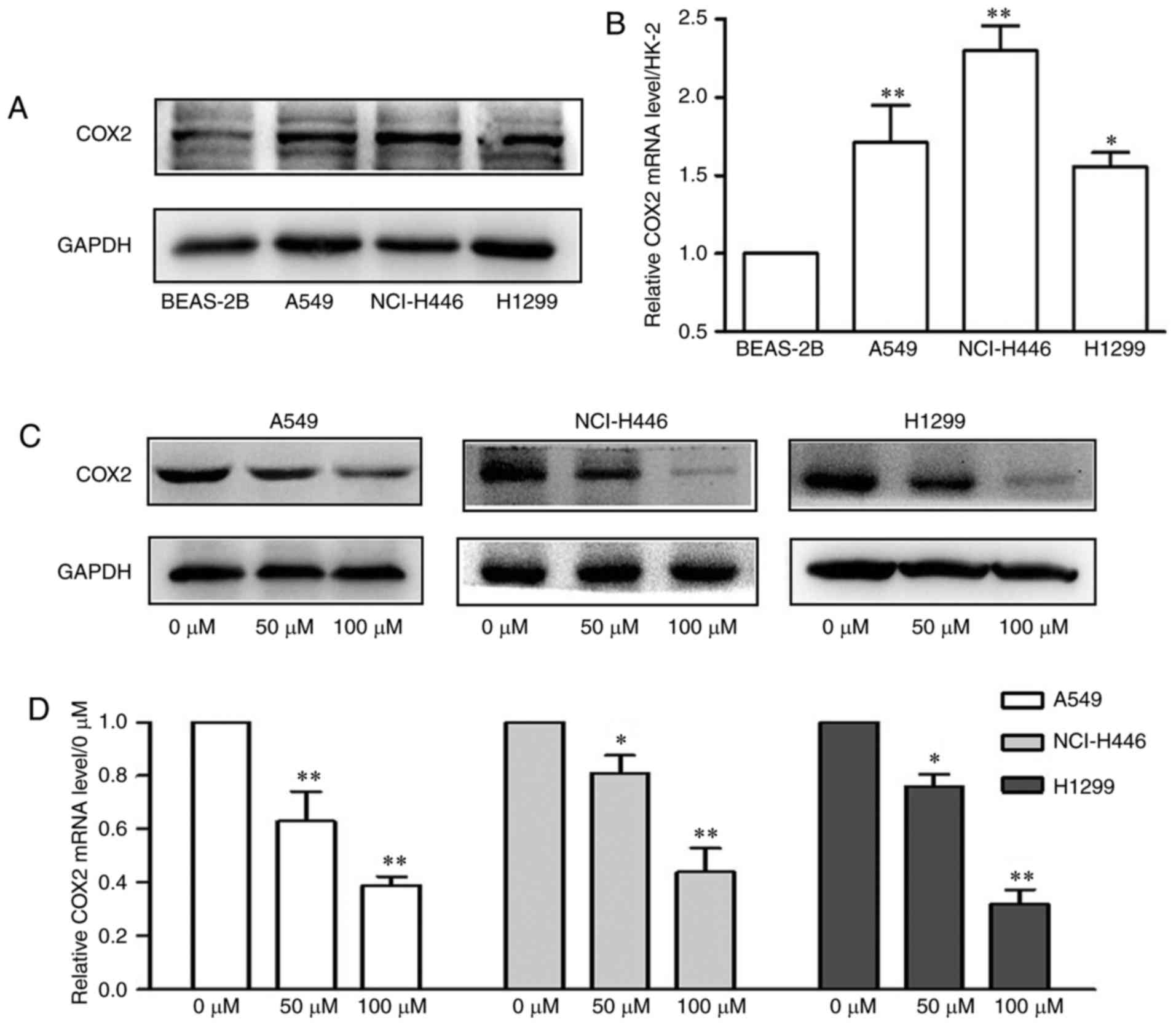
Sesamin regulates the expression of COX2
in lung cancer cells
To investigate the role of COX2 in the cytotoxic
effect of sesamin on A549, NCI-H446 and H1299 cells, the expression
of COX2 was detected in the above lung cancer cell lines. As
presented in Fig. 2A and B, the
protein and mRNA expression levels of COX2 were markedly
upregulated in lung cancer cells compared with BEAS-2B cells.
Furthermore, as presented in Fig. 2C
and D, sesamin decreased the protein and significantly
decreased the mRNA expression levels of COX2 in A549, NCI-H446 and
H1299 cells in a dose-dependent manner (P<0.05). These results
suggested that sesamin serves an antitumor role by regulating the
levels of COX2 in lung cancer cells.
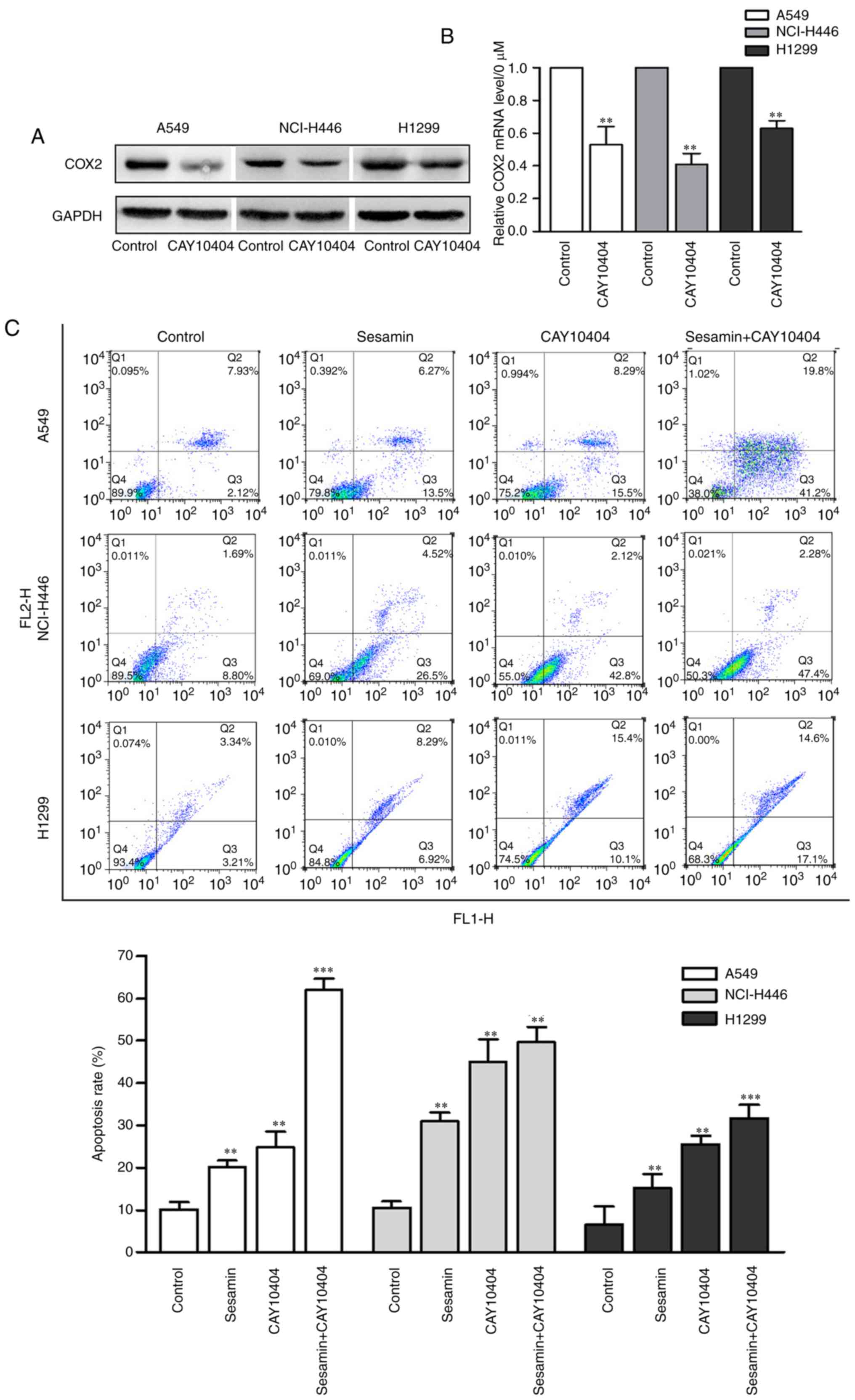
Inhibition of COX2 expression enhances
sesamin-induced lung cancer cells apoptosis
To clarify the role of COX2 expression inhibition in
sesamin-induced insensitivity of lung cancer cells, CAY10404, a
COX2 inhibitor, was used. As presented in Fig. 3A and B, CAY10404 repressed the
mRNA and protein level of COX2 compared with the control. In
addition, lung cancer cells were treated with sesamin in the
presence or absence of CAY10404. As presented in Fig. 3C and D, cotreatment with CAY10404
and sesamin significantly increased the apoptosis of lung cancer
cells (P<0.01) and the expression of the pro-apoptotic protein
Bax, whereas it decreased the expression of the anti-apoptotic
protein Bcl-2. These results suggested that selective inhibition of
COX2 enables sesamin to induce apoptosis in lung cancer cells.
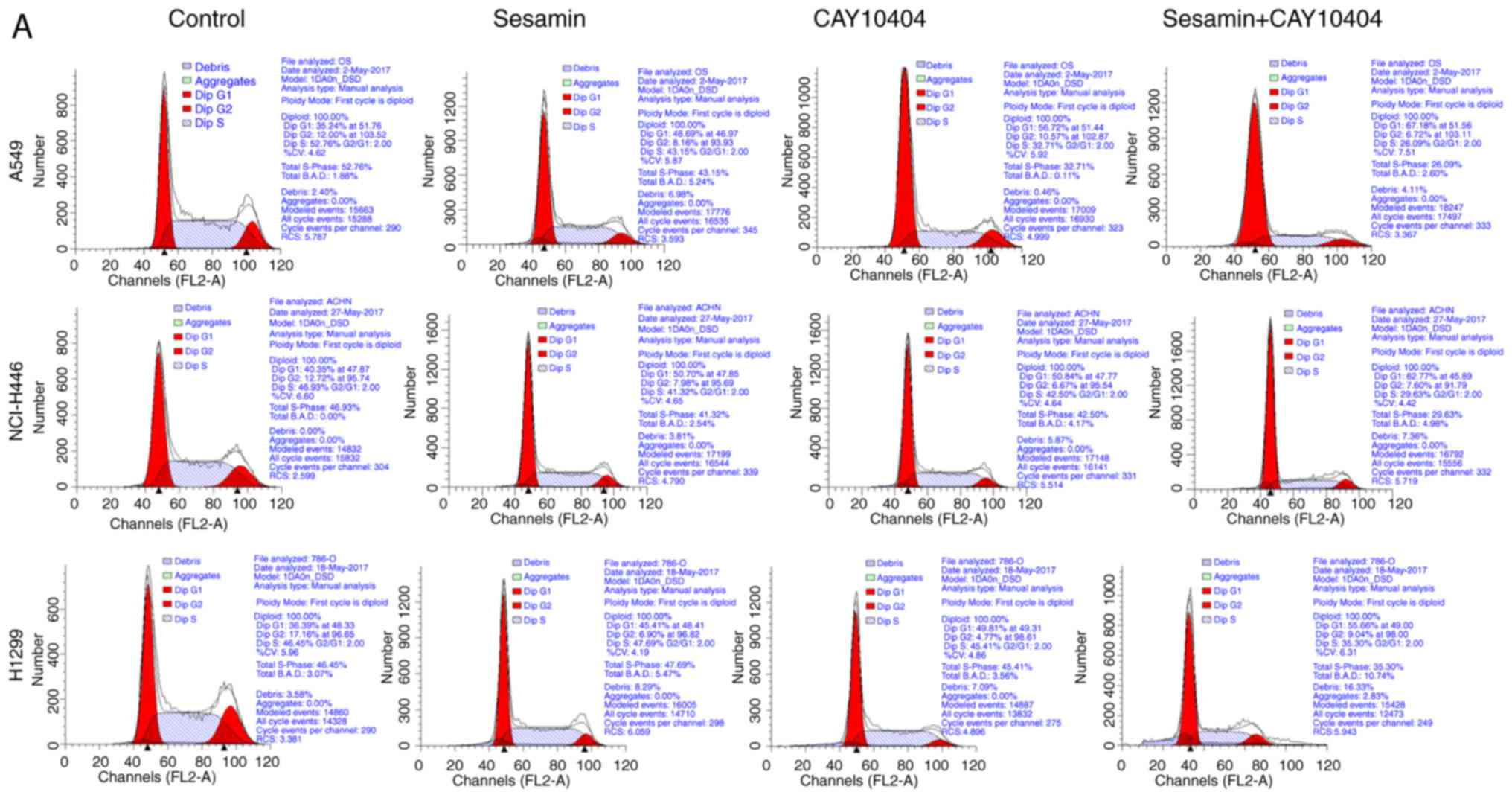
Inhibition of COX2 expression enhances
sesamin-induced cell cycle arrest
The present study determined that COX2 expression
served a role in sesamin-induced cell cycle arrest in lung cancer
cells. The percentage of G1-phase cells increased upon cotreatment
with sesamin and CAY10404 (Fig.
4A). In addition, cotreatment with CAY10404 and sesamin
significantly repressed expression of the G1-phase protein cyclin
D1 (P<0.05) and the G2/M-phase protein cyclin B1, but stimulated
expression of the S-phase protein cyclin A2 (Fig. 4B). These results indicated that
downregulated COX2 expression increased sesamin-induced G1-phase
arrest, which was associated with the altered expression of cell
cycle associated proteins.
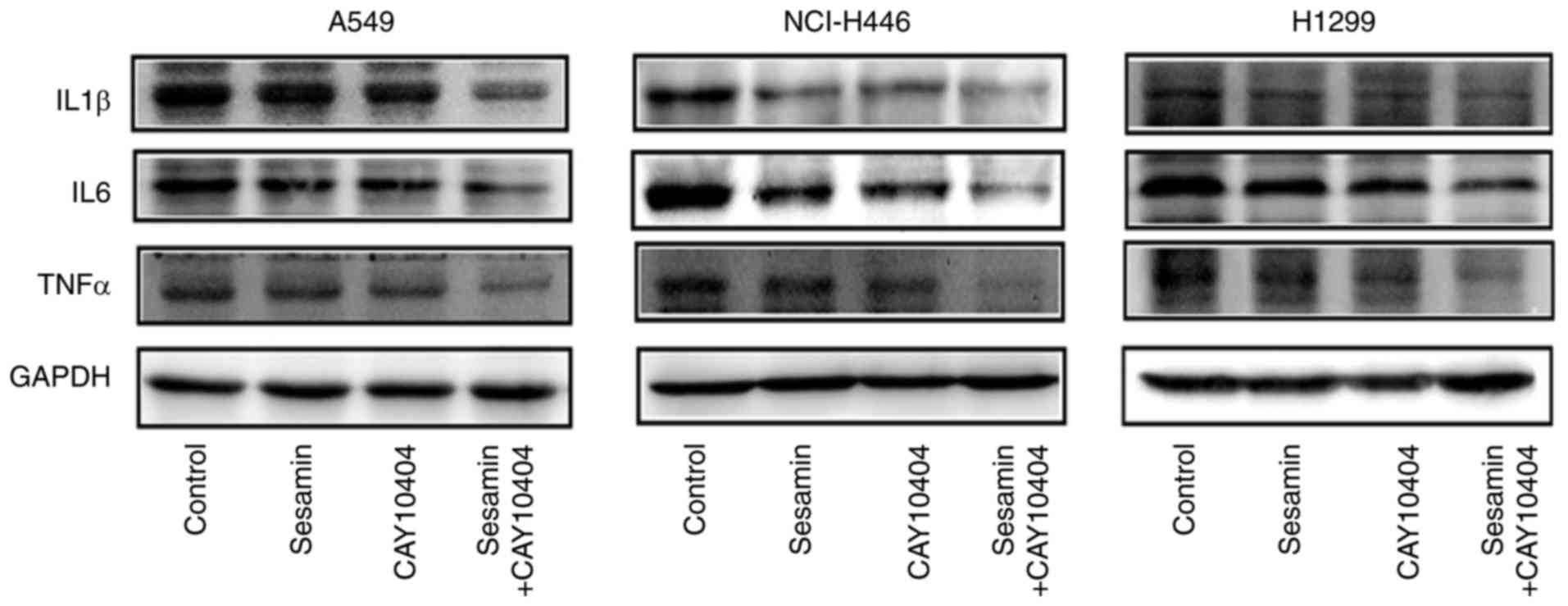
Cotreatment with CAY10404 and sesamin
strengthens the inhibition of the expression of COX2 downstream
molecules
IL1β, IL6 and TNFα are downstream molecules of COX2
(18). To further elucidate the
involvement of COX2 in the sesamin-mediated regulation of lung
cancer cells, the expression of the above downstream proteins was
detected. The results indicated that the levels of these molecules
were down-regulated, particularly upon cotreatment with CAY10404
and sesamin (Fig. 5). These
results suggested that sesamin exhibits an anticancer role in lung
cancer cells and that downregulation of COX2 expression potentiates
its antitumor effect.
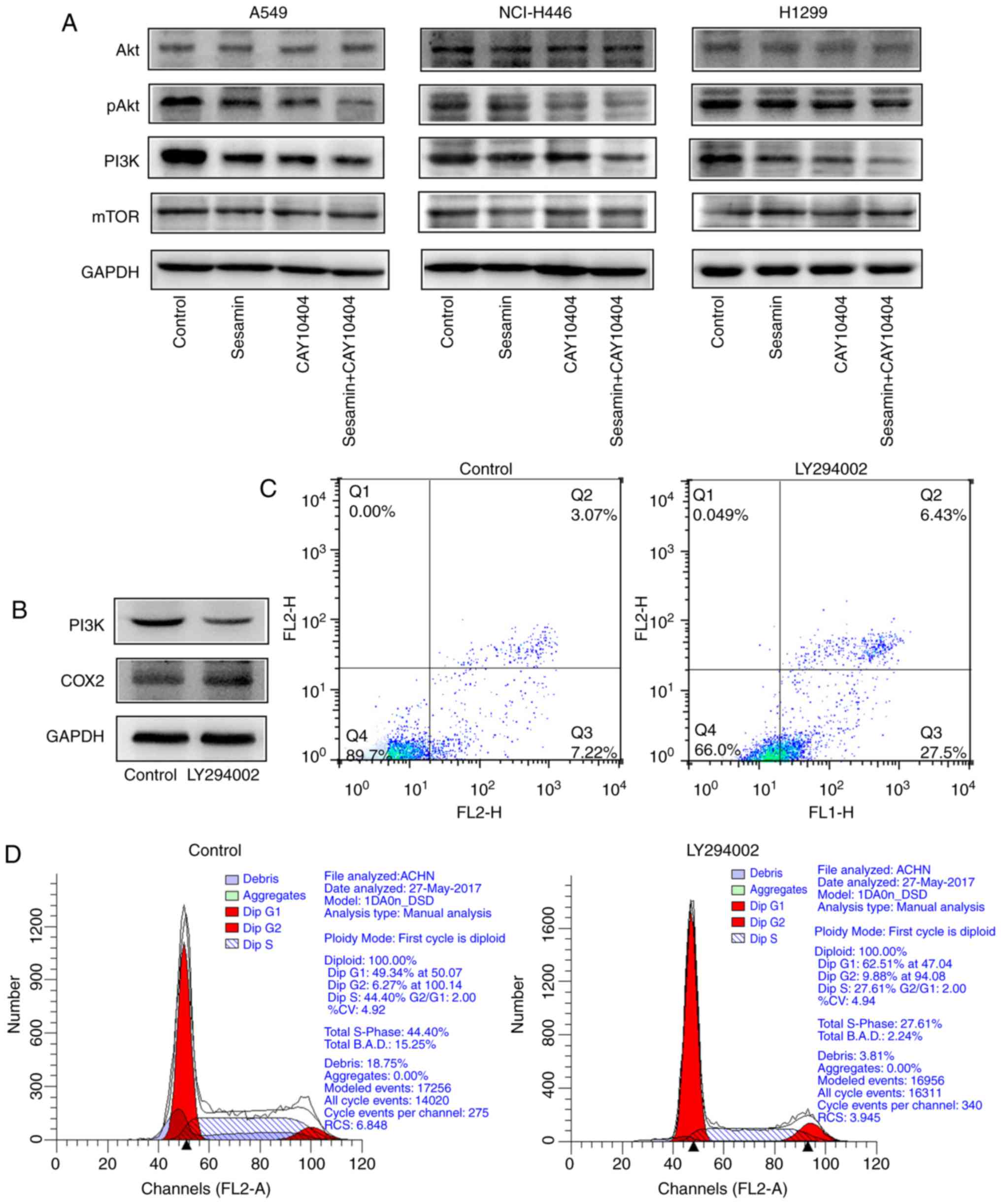
Sesamin inhibits the pAkt-PI3K signaling
pathway by inhibition of COX2 expression
To investigate the molecular mechanism of
sesamin-induced apoptosis via COX2, the expression levels of Akt,
pAkt, PI3K and mTOR in lung cancer cells treated with sesamin
and/or CAY10404 were determined by western blotting. As presented
in Fig. 6A, cotreatment with
sesamin and CAY10404 markedly reduced the levels of pAkt and PI3K
in three lung cancer cell lines. The PI3K inhibitor LY294002
decreased the expression of PI3K while partially upregulating the
expression of COX2. However, the effect was not statistically
significant in A549 cells (Fig.
6B). These results indicated that PI3K was under the control of
COX2 and possibly formed a negative feedback loop. In addition,
inhibition of PI3K expression induced apoptosis (Fig. 6C) and G1-phase arrest (Fig. 6D) in A549 cells. These results
suggested that sesamin inhibits the pAkt-PI3K signaling pathway by
decreasing the expression of COX2, which leads to cell cycle arrest
and the induction of apoptosis in vitro.
Discussion
Lung cancer is the leading cause of
cancer-associated mortality in males and females worldwide
(19). Plant-derived agents are
widely applied as adjuvant or supplemental agents in cancer
therapy. Sesamin has gained attention recently due to its
anti-tumor effects (20). The
present study identified that sesamin is able to suppress the
proliferation and promote the apoptosis of lung cancer cells in a
concentration-dependent manner, indicating that sesamin is
relatively effective in lung cancer, similar to the effects
reported in other cancer types, including breast cancer (21), human hepatocellular carcinoma
(22), colon cancer, prostate
cancer and pancreatic cancer (23). Previous studies have demonstrated
that COX2 expression is strongly associated with cancer progression
in various human tumor types (10,24). COX2 been reported to be one of the
important target molecules in tumor treatment (25). Shimizu et al (26) observed that COX2 transcriptional
activities decreased by 50% in the presence of 100 µM
sesamol (one of the lignans in sesame seeds), while other compounds
in sesame seeds, including sesamin, did not exhibit significant
inhibition of COX2 transcriptional activity at ≤100 µM in
colon cancer cells. However, the present study noted that COX2 was
highly expressed in lung cancer cells and that sesamin
dose-dependently decreased the protein and mRNA levels of COX2.
Therefore, it is reasonable to consider that sesamin inhibits lung
cancer development at least partially by decreasing the expression
of COX2.
Furthermore, upregulation of COX2 expression may be
a cause of cancer development, metastasis and chemoresistance
(27). The present study revealed
that inhibition of COX2 with CAY10404 enhanced the sensitivity of
lung cancer cells toward sesamin by inducing apoptosis and G1-phase
arrest. In addition, IL1β, IL6 and TNFα were confirmed as
downstream molecules of COX2 (18). In the present study, cotreatment
with CAY10404 and sesamin downregulated the levels of these
molecules, indicating that COX2 is an important target of sesamin
in lung cancer and that downregulated COX2 can improve the
antitumor effect of sesamin. Li et al (28) observed that increased COX2
expression was associated with chemosensitivity and poor prognosis
in cervical cancer, and that upregulated COX2 impeded
chemosensitivity to dichloroacetate (DCA), while the combination of
the COX2 inhibitor celecoxib with DCA enhanced the chemosensitivity
to DCA in cervical cancer cells. Other studies have suggested that
knocking down COX2 expression effectively increases the
chemosensitivity of human gastric cancer cells (29) and laryngeal carcinoma cells
(27). By contrast, a previous
study indicated that COX2 is regulated by positive and negative
mechanisms (30). Therefore, it
is necessary to investigate the role of the COX2 network in the
sesamin-induced apoptosis of lung cancer cells to fully understand
the oncogenic mechanisms of COX2.
A previous study reported that COX2 affects Akt
activation, which is involved in bladder development (31). In the present study, the reduction
in COX2 resulted in an increase in sesamin-induced Akt activity and
a decrease in PI3K. In addition, the present results revealed that
inhibition of PI3K with LY294002 improved apoptosis and induced
cell cycle arrest at the G1 phase. Importantly, LY294002 decreased
the expression of PI3K, while partially upregulating the expression
of COX2, although this effect was not statistically significant in
A549 cells. These results indicated that sesamin inhibits the
pAkt-PI3K signaling pathway by decreasing the expression of COX2,
therefore regulating cell cycle arrest and inducing apoptosis in
vitro. In addition, COX2 and PI3K possibly form a negative
feedback loop. However, further studies unraveling the detailed
downstream effects of these molecules on cancer cells must be
conducted.
In conclusion, the present study demonstrated that
inhibition of COX2 expression enhanced the antitumor activity of
sesamin via the Akt-PI3K signaling pathway in lung cancer cells.
Therefore, a potential feedback loop comprising COX2 and PI3K that
coordinates the apoptosis of lung cancer cells is proposed, which
may pave the way for the development of potential treatment
strategies for lung cancer using the combination of sesamin and a
COX2 inhibitor.
Acknowledgments
Not applicable.
Funding
The present study was funded by the Natural Science
Foundation of Ningbo (grant no. 2017A610245).
Availability of data and materials
The datasets supporting the conclusions of the
present study are included within this article. The datasets used
and/or analyzed during the current study are available from the
corresponding author or the first author on reasonable request.
Authors’ contributions
QW conceived and designed the study. QF wrote the
manuscript. YZ was involved in revising manuscript critically for
important intellectual content and given final approval of the
version to be published. QF, MS, GG and ZZ conducted experiments,
collected the data and performed statistical analysis. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Salskov A, Hawes SE, Stern JE, Feng Q,
Jordan CD, Wiens L, Rasey J, Lu H, Kiviat NB and Vesselle H:
Hypermethylation of CCND2 may reflect a smoking-induced
precancerous change in the lung. J Oncol. 2011:9501402011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bao M, Song Y, Xia J, Li P, Liu Q and Wan
Z: miR-1269 promotes cell survival and proliferation by targeting
tp53 and caspase-9 in lung cancer. Onco Targets Ther. 11:1721–1732.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng L, Zhou H, Guo L, Xu X, Zhang S, Xu
W and Mao W: Inhibition of NIPBL enhances the chemosensitivity of
non-small-cell lung cancer cells via the DNA damage response and
autophagy pathway. Onco Targets Ther. 11:1941–1948. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thuy TD, Phan NN, Wang CY, Yu HG, Wang SY,
Huang PL, Do YY and Lin YC: Novel therapeutic effects of sesamin on
diabetes-induced cardiac dysfunction. Mol Med Rep. 15:2949–2956.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fan D, Yang Z, Yuan Y, Wu QQ, Xu M, Jin YG
and Tang QZ: Sesamin prevents apoptosis and inflammation after
experimental myocardial infarction by JNK and NF-κB pathways. Food
Funct. 8:2875–2885. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qiang L, Yuan J, Shouyin J, Yulin L,
Libing J and Jian-An W: Sesamin attenuates
lipopolysaccharide-induced acute lung injury by inhibition of TLR4
signaling pathways. Inflammation. 39:467–472. 2016. View Article : Google Scholar
|
|
8
|
Xu P, Cai F, Liu X and Guo L: Sesamin
inhibits lipopolysaccha-ride-induced proliferation and invasion
through the p38-MAPK and NF-kappaB signaling pathways in prostate
cancer cells. Oncol Rep. 33:3117–3123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuang W, Deng Q, Deng C, Li W, Shu S and
Zhou M: Hepatocyte growth factor induces breast cancer cell
invasion via the PI3K/Akt and p38 MAPK signaling pathways to
up-regulate the expression of COX2. Am J Transl Res. 9:3816–3826.
2017.PubMed/NCBI
|
|
10
|
Chun KS and Surh YJ: Signal transduction
pathways regulating cyclooxygenase-2 expression: Potential
molecular targets for chemoprevention. Biochem Pharmacol.
68:1089–1100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spano JP, Chouahnia K and Morere JF:
Cyclooxygenase 2 inhibitors and lung carcinoma. Bull Cancer.
91(S109-S112)2004.In French.
|
|
12
|
Yang CL, Zheng XL, Ye K, Ge H, Sun YN, Lu
YF and Fan QX: MicroRNA-183 acts as a tumor suppressor in human
non-small cell lung cancer by down-regulating MTA1. Cell Physiol
Biochem. 46:93–106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yin H, Ma J, Chen L, Piao S, Zhang Y,
Zhang S, Ma H, Li Y, Qu Y, Wang X and Xu Q: MiR-99a enhances the
radiation sensitivity of non-small cell lung cancer by targeting
mTOR. Cell Physiol Biochem. 46:471–481. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang SX, Qi B, Yao WJ, Gu CW, Wei XF,
Zhao Y, Liu YZ and Zhao BS: Berberine displays antitumor activity
in esophageal cancer cells in vitro. World J Gastroenterol.
23:2511–2518. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao H, Liu Y, Liang P, Wang B, Tan H,
Zhang Y, Gao X and Gao J: TP53TG1 enhances cisplatin sensitivity of
non-small cell lung cancer cells through regulating miR-18a/PTEN
axis. Cell Biosci. 8:232018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Zhuang H, Meng X, Li Y, Wang X, Huang S,
Liu K, Hehir M, Fang R, Jiang L, Zhou JX, et al: Cyclic AMP
responsive element-binding protein promotes renal cell carcinoma
proliferation probably via the expression of spindle and
kinetochore-associated protein 2. Oncotarget. 7:16325–16337. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoon KY, Kim KJ, Youn HS, Oh SR and Lee
BY: Brazilin suppresses inflammation via the down-regulation of
IRAK4 in LPS-stimulated Raw264.7 Macrophage. J Food Nutri Res.
3:575–580. 2015. View Article : Google Scholar
|
|
19
|
Alam SK, Astone M, Liu P, Hall SR, Coyle
AM, Dankert EN, Hoffman DK, Zhang W, Kuang R, Roden AC, et al:
DARPP-32 and t-DARPP promote non-small cell lung cancer growth
through regulation of IKKα-dependent cell migration. Commun Biol.
1:432018. View Article : Google Scholar
|
|
20
|
Kong X, Ma MZ, Zhang Y, Weng MZ, Gong W,
Guo LQ, Zhang JX, Wang GD, Su Q, Quan ZW and Yang JR:
Differentiation therapy: Sesamin as an effective agent in targeting
cancer stem-like side population cells of human gallbladder
carcinoma. BMC Complement Altern Med. 14:2542014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Akl MR, Ayoub NM and Sylvester PW:
Mechanisms mediating the synergistic anticancer effects of combined
γ-tocotrienol and sesamin treatment. Planta Med. 78:1731–1739.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deng P, Wang C, Chen L, Wang C, Du Y, Yan
X, Chen M, Yang G and He G: Sesamin induces cell cycle arrest and
apoptosis through the inhibition of signal transducer and activator
of transcription 3 signalling in human hepatocellular carcinoma
cell line HepG2. Biol Pharm Bull. 36:1540–1548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harikumar KB, Sung B, Tharakan ST, Pandey
MK, Joy B, Guha S, Krishnan S and Aggarwal BB: Sesamin manifests
chemopreventive effects through the suppression of NF-kappa
B-regulated cell survival, proliferation, invasion, and angiogenic
gene products. Mol Cancer Res. 8:751–761. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dhakal HP, Naume B, Synnestvedt M, Borgen
E, Kaaresen R, Schlichting E, Wiedswang G, Bassarova A, Holm R,
Giercksky KE and Nesland JM: Expression of cyclooxygenase-2 in
invasive breast carcinomas and its prognostic impact. Histol
Histopathol. 27:1315–1325. 2012.PubMed/NCBI
|
|
25
|
Karavitis J, Hix LM, Shi YH, Schultz RF,
Khazaie K and Zhang M: Regulation of COX2 expression in mouse
mammary tumor cells controls bone metastasis and PGE2-induction of
regulatory T cell migration. PLoS One. 7:e463422012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shimizu S, Fujii G, Takahashi M, Nakanishi
R, Komiya M, Shimura M, Noma N, Onuma W, Terasaki M, Yano T and
Mutoh M: Sesamol suppresses cyclooxygenase-2 transcriptional
activity in colon cancer cells and modifies intestinal polyp
development in Apc (Min/+) mice. J Clin Biochem Nutr. 54:95–101.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang R, Wang X, Lin F, Gao P, Dong K and
Zhang HZ: shRNA-targeted cyclooxygenase (COX)-2 inhibits
proliferation, reduces invasion and enhances chemosensitivity in
laryngeal carcinoma cells. Mol Cell Biochem. 317:179–188. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li B, Li X, Xiong H, Zhou P, Ni Z, Yang T,
Zhang Y, Zeng Y, He J, Yang F, et al: Inhibition of COX2 enhances
the chemosensitivity of dichloroacetate in cervical cancer cells.
Oncotarget. 8:51748–51757. 2017.PubMed/NCBI
|
|
29
|
Chan MW, Wong CY, Cheng AS, Chan VY, Chan
KK, To KF, Chan FK, Sung JJ and Leung WK: Targeted inhibition of
COX-2 expression by RNA interference suppresses tumor growth and
potentiates chemosensitivity to cisplatin in human gastric cancer
cells. Oncol Rep. 18:1557–1562. 2007.PubMed/NCBI
|
|
30
|
Liu S, Zhang C, Zhang K, Gao Y, Wang Z, Li
X, Cheng G, Wang S, Xue X, Li W, et al: FOXP3 inhibits cancer stem
cell self-renewal via transcriptional repression of COX2 in
colorectal cancer cells. Oncotarget. 8:44694–44704. 2017.PubMed/NCBI
|
|
31
|
Shimada K, Anai S, Marco DA, Fujimoto K
and Konishi N: Cyclooxygenase 2-dependent and independent
activation of Akt through casein kinase 2alpha contributes to human
bladder cancer cell survival. BMC Urol. 11:82011. View Article : Google Scholar
|