Introduction
Hypertension may induce brain damage primarily in
the hippocampus and cortex area (1), which clinically manifest as
cognitive decline. One of the main events that contribute toward
this damage is the formation of reactive oxidative species (ROS)
and reactive nitrogen species (RNS), and subsequent redox signaling
disruption in mitochondria (2,3),
thereby resulting in enhanced oxidative stress. In fact, an
enhancement of oxidative stress may decrease the level of neuronal
activity and cause the influx of calcium ions into neurons, thereby
activating the proapoptotic mechanisms (4), eventually resulting in neuronal
apoptosis. However, there are no pharmacological approaches to
limit or prevent this.
Peroxisome proliferator-activated receptor γ
(PPAR-γ) is a nuclear receptor family member, which regulates
carbohydrate and lipid metabolism, and exerts antioxidative and
anti-apoptotic actions in the central and peripheral nervous
systems (5). A previous study
indicated that PPAR-γ ligands, including rosiglitazone and
piglitazone, possess beneficial effects in cardiovascular disease,
particularly in hypertension (6).
Its well-characterized protective effects on hypertension (7) and target organs, including the brain
(8), kidneys (9), heart (7) and vessels (10) prompted the use of PPAR-γ agonists
on hypertension-related organ damage. However, few studies have
assessed the neuroprotective effect of rosiglitazone on
hypertension-related cognitive decline in aged SHRs.
The results of our previous studies demonstrated
that hypertension enhanced oxidative stress injury and exacerbated
brain damage in the hippocampi of SHRs. Downregulated PPAR-γ
expression may be involved in this process (11). Furthermore, the present study
aimed to examine whether the PPAR-γ agonist, rosiglitazone, may
exert neuroprotective effects in aged SHRs and investigate its
underlying mechanisms of this.
Materials and methods
Animals
A total of sixteen 56-week-old male SHRs and sixteen
56-week-old male Wistar-Kyoto (WKY) rats weighing ~400 g (Shanghai
Slac Laboratory Animal Center) were randomly assigned to control
groups and rosiglitazone groups (n=8 per group). The rats received
gavage administration of vehicle (0.9% saline) or rosiglitazone (5
mg/kg/day) for eight weeks. All rats were housed in a room at 23°C,
60% humidity under a 12 h light/dark cycle with free access to food
and water. All the procedures were performed according to
guidelines established by the Institutional Animal Care and Use
Committee and the National Institutes of Health. The experimental
protocols were approved by the Ethics Committee of Xi'an Jiaotong
University College of Medicine (Xi'an, China) and followed the
guidelines of the Declaration of Helsinki.
Blood pressure measurements
The indirect tail-cuff method (BP-2000; Visitech
Systems, Inc., Apex, NC, USA) was used to measure systolic blood
pressure (SBP). In brief, rats that had not been anesthetized were
placed in a holding device mounted on a thermostatically-controlled
warming plate to make the pulsations of the tail artery detectable.
Tail cuffs were fixed on animals. SBP was measured at the end of
the treatment period. Blood pressure was accurately recorded for
each rat and averaged from ≥3 consecutive readings.
Brain tissue preparation
Rats were anesthetized with 10% chloral hydrate (400
mg/kg) through intraperitoneal injection. No rats exhibited signs
of peritonitis. For terminal deoxynucleotidyl transferase-mediated
dUTP end-labeling (TUNEL) and immunohistochemistry assays,
anesthetized rats (n=4) were perfused transcardially with 0.9%
saline (pH 7.4), followed by 4% paraformaldehyde (PFA). Next, the
brains were post-fixed in 4% PFA overnight. Targeted brain pieces
were dehydrated, embedded in paraffin and cut into 10-µm-thick
sections. Three sections containing the cortex and hippocampus were
selected from each rat. For western blot analysis, anesthetized
rats (n=4) were perfused transcardially with 0.9% saline (pH 7.4),
prior to brains being removed and stored in sample protectors
(Takara Biotechnology Co., Ltd., Dalian, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the hippocampus using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and the RNA samples were reverse transcribed to
cDNA using the PrimeScript RT Master mix kit (Takara Biotechnology
Co., Ltd.). All the procedures were performed according to the
manufacturer's instructions. RT-qPCR was performed using the SYBR
ExScript RT-PCR kit (Takara Biotechnology Co., Ltd.). The primer
sequences for rat PPAR-γ (forward, 5'-GGA GCC TAA GTT TGA GTT TGC
TGT G-3' and reverse, 5'-TGC AGC AGG TTG TCT TGG ATG-3'); caspase-3
(forward, 5'-GAG ACA GAC AGT GGA ACT GAC GAT G-3' and reverse,
5'-GGC GCA AAG TGA CTG GAT GA-3'); B-cell lymphoma 2 (Bcl-2;
forward, 5'-GAC TGA GTA CCT GAA CCG GCA TC-3' and reverse, 5'-CTG
AGC AGC GTC TTC AGA GAC A-3'); Bcl-2-associated X protein (Bax;
forward, 5'-GCG TCC ACC AAG AAG CTG A-3' and reverse, 5'-ACC ACC
CTG GTC TTG GAT CC-3'); and β-actin (forward, 5'-GGA GAT TAC TGC
CCT GGC TCC TA-3' and reverse, 5'-GAC TCA TCG TAC TCC TGC TTG
CTG-3') were designed and synthesized by Takara Biotechnology Co.,
Ltd. Amplification was performed at 95°C for 30 sec, followed by 40
cycles of 95°C for 3 sec and 60°C for 30 sec. Cycle threshold
values were obtained from the Bio-Rad iQ5 2.0 Standard Edition
optical System software (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Relative quantification was performed using the comparative
method (2−ΔΔCq) (12),
and data are presented as the mean ± standard deviation of three
separate experiments performed in triplicate.
Immunohistochemistry
Sections were deparaffinized with serial xylene
washes and rehydrated with serial concentrations of ethanol at room
temperature (Xylene I, Xylene II, 100% alcohol I and 100% alcohol
II, 10 min each. Then, 95% ethanol, 90% ethanol, 80% ethanol, 70%
ethanol for 10 min each and distilled water). Then the sections
were treated with 3% hydrogen peroxide for 20 min, prior to
incubation with 0.3% Triton X-100, followed by incubation with 10%
goat serum for 1 h. The slides were then incubated overnight at 4°C
with the rabbit polyclonal anti-glial fibrillary acidic protein
(GFAP) antibody (1:4,000; cat. no. ab7260; Abcam, Cambridge, UK).
Following three washes with PBS, the sections were incubated with a
biotinylated anti-rabbit immunoglobulin G secondary antibody
(1:1,000; cat. no. ab150077; Abcam). 3,3'-diaminobenzidine was used
to detect the immune reaction. For negative controls, the primary
antibody was replaced with PBS. The slides were photographed under
a light microscope (magnification, ×400; Olympus Corporation,
Tokyo, Japan). Digited images of tissue sections containing
hippocampus were displayed on the video screen under identical
lighting conditions. Labeled astrocytes were investigated in the
left and right hippocampus from five sections per rat.
Western blot analysis
Radioimmunoprecipitation assay lysis buffer was used
to extract total proteins from brain samples, followed by the
measurement of protein concentrations using a bicinchoninic acid
kit (Beyotime Institute of Biotechnology, Haimen, China). Equal
amounts (20 µg) of protein were separated on 12% SDS polyacrylamide
gels, transferred to polyvinylidene difluoride membranes, and
blocked in 10% skimmed milk for 2 h at 37°C. Membranes were
incubated with rabbit polyclonal antibodies against Bax, Bcl-2,
gp47phox and caspase-3 (1:800; cat. nos. BS2538, BS6421,
BS3261, P42574, respectively; Bioworld Technology, Inc., St. Louis
Park, MN, USA), a rabbit polyclonal antibody against PPAR-γ (1:400;
cat. no. ab209350; Abcam) and a mouse monoclonal antibody against
iNOS (1:500; cat. no. sc-7271; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) overnight at 4°C. Following three washes, the
membranes were incubated with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit or goat anti-mouse antibody
(1:10,000; cat. no. BS13278 and BS12478, respectively, Bioworld
Technology, Inc.) for 2 h at 37°C. Protein bands were detected
using chemiluminescent HRP substrate (SuperSignal West Pico; Thermo
Fisher Scientific, Inc.) for 5 min in a dark room and exposed to
X-ray film (Fujifilm Corporation, Tokyo, Japan). The signal
intensity of primary antibody binding was analyzed using Quantity
One software 4.6.2 (Bio-Rad Laboratories, Inc.) and normalized to
β-actin.
TUNEL assay
A cell death detection kit was used to perform the
TUNEL assay (Promega Corporation, Madison, WI, USA) according to
the manufacturer's protocols. In brief, the sections were dewaxed
and rehydrated as described above. Then sections were fixed with 4%
paraformaldehyde prepared in 0.1 M PBS for 15 min at room
temperature and washed in PBS for three times. The sections were
then processed with 20 µg/ml fresh proteinase-K (diluted 1:500 in
PBS) for 20 min at room temperature, followed by three washes in
PBS. Next, sections were treated with equilibration buffer for 10
min at room temperature, followed by incubation with 50 µl rTdT
incubation buffer for 1 h at 37°C in the dark. Then slides were
immersed in 2X SSC buffer for 15 min to terminate the reaction,
followed by three washes with PBS. Sections were then
counterstained with 4',6'-diamino-2-phenylindole (DAPI; dilution,
1:1,000; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Fluorescence microscopy was performed using an Olympus BX51
microscope equipped with a mercury lamp power supply
(magnification, ×400). Cells with bright green nuclei were
identified as TUNEL-positive cells. TUNEL-positive cells were
normalized to DAPI-stained cells. Immunoreactive cells from 9
randomly selected fields (3 samples per group, 3 fields per sample)
were counted using a ×20 objective lens by an observer blinded to
the treatment groups.
Statistical analysis
Statistical analysis was performed using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). The quantitative data are
presented as the mean ± standard deviation and analyzed using SPSS
16.0 software. Differences were detected by two-way analysis of
variance (ANOVA), and then one-way ANOVA, followed by the least
significant difference test was used to identify significant
differences between the groups. Levene's test was used to test the
homogeneity of variance and the distribution of normality.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Rosiglitazone does not significantly
reduce SBP in SHRs
The SBP in the 64-week-old SHR group averaged
193.8±11.2 mmHg, which was significantly different to WKY group
(110.8±9.7 mmHg; P<0.01). The mean SBP levels were 106.5±4.3 and
187.5±12.6 mmHg in the rosiglitazone-treated WKY and SHR groups,
respectively. However, rosiglitazone treatment did not have a
significant effect on SBP (P>0.05). Therefore, rosiglitazone at
the dose used in the present study did not decrease the SBP of the
SHR group throughout the treatment period.
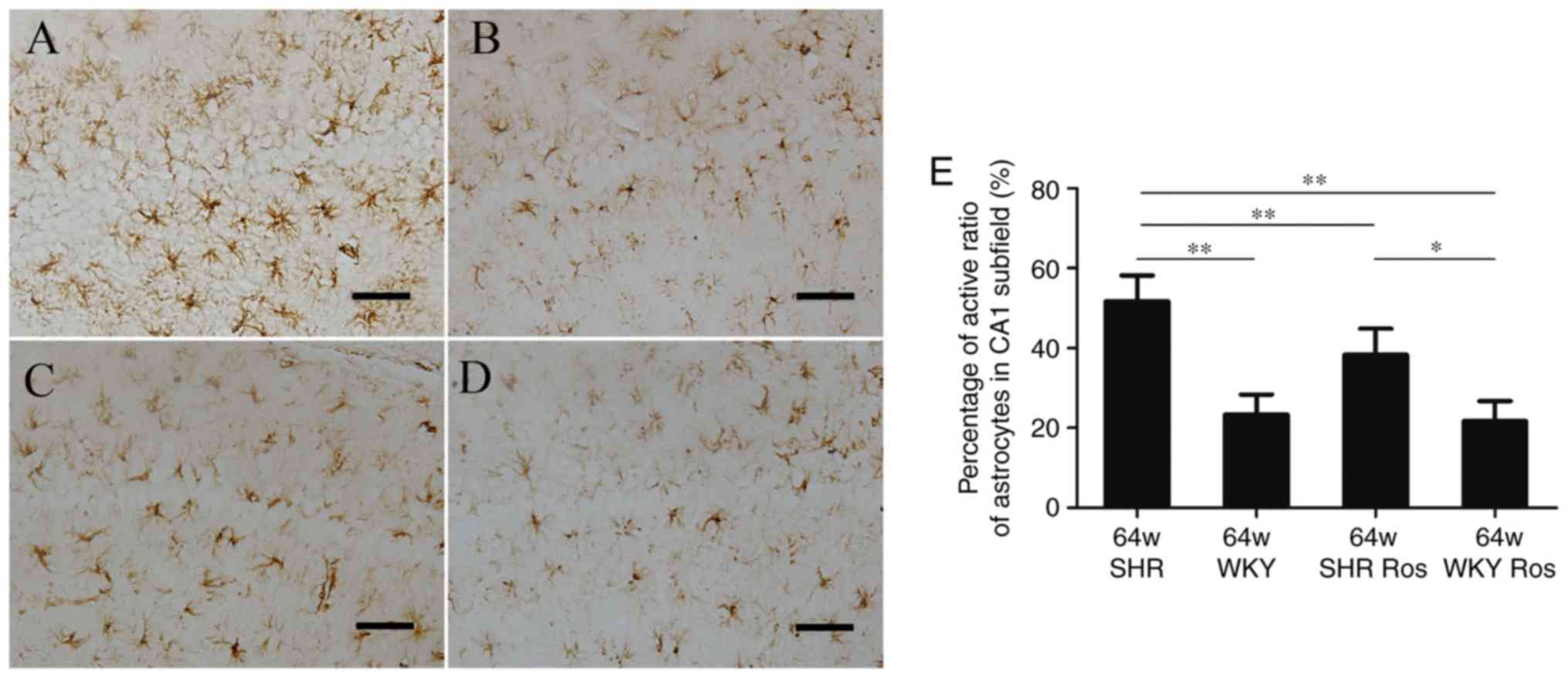
Rosiglitazone decreases the activated
ratio of astrocytes in SHRs
As demonstrated in Fig. 1, the WKY group exhibited thin,
shallow, dark-brown astrocytes with slender branches (Fig. 1B), while the SHR group developed
more activated astrocytes with thick branches and large cell bodies
(Fig. 1A). It was revealed that
the two groups exhibited a similar number of astrocytes. However,
the activated ratio of GFAP-positive astrocytes was increased in
the CA1 subfield of the SHR group, compared with the WKY group
(51.0 and 22.5%, respectively; P<0.01). The parameter was
decreased by rosiglitazone in the SHR group (37.8%; P<0.01;
Fig. 1E), but not in the WKY
group (21.0%; P>0.05; Fig.
1E).
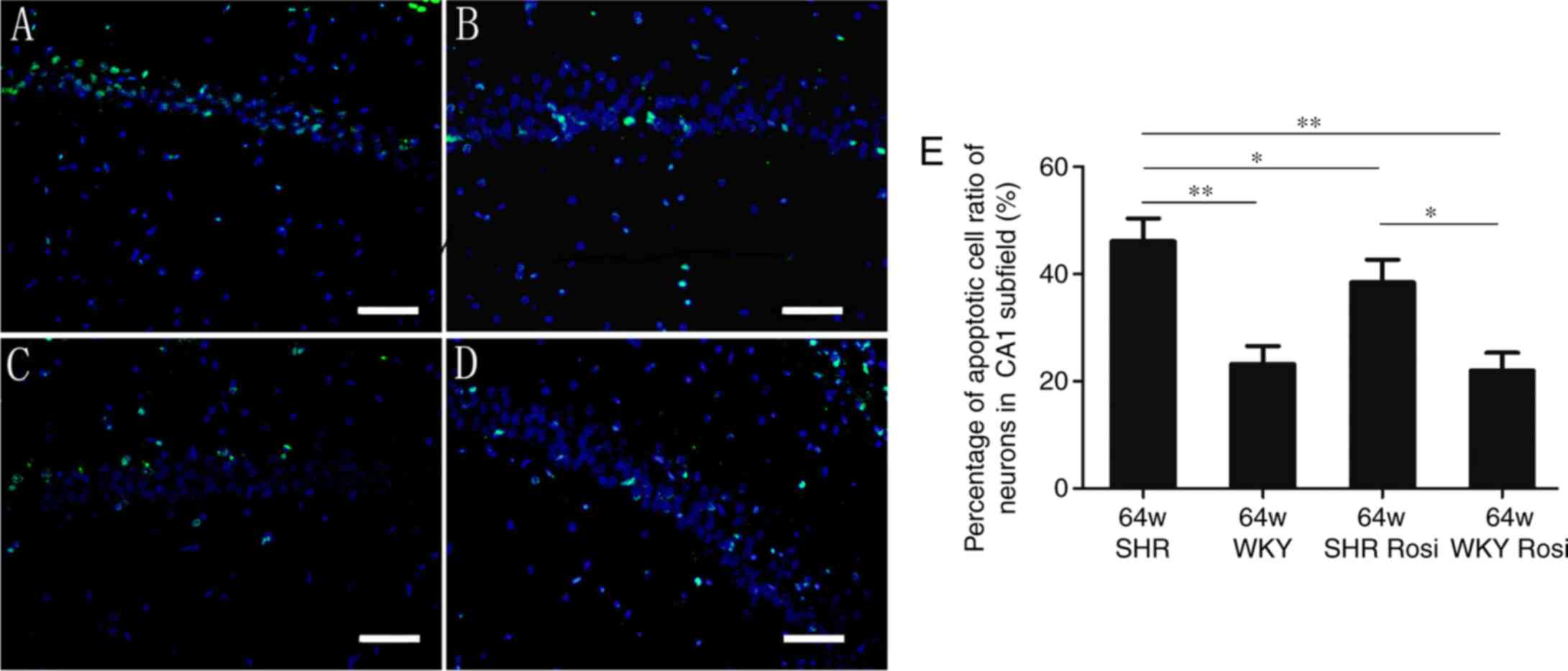
Quantitative analysis of TUNEL-positive
cells
As demonstrated in Fig. 2, neurons in the SHR group
exhibited microanatomical characteristics of damaged cells,
manifested with irregular cellular and nuclear profiles. A total of
46.1% TUNEL-positive nuclei were observed in the SHR group
(Fig. 2A). Rosiglitazone
decreased this number to 38.4% (Fig.
2C), which revealed significant differences with the SHR group
(P<0.05). In the hippocampi of the WKY group, ~23.2% of the
nuclei were TUNEL-positive (Fig.
2B). The parameter was decreased to 22.0% by rosiglitazone
(Fig. 2D). The differences
between the two groups were not statistically significant
(P>0.05; Fig. 2E).
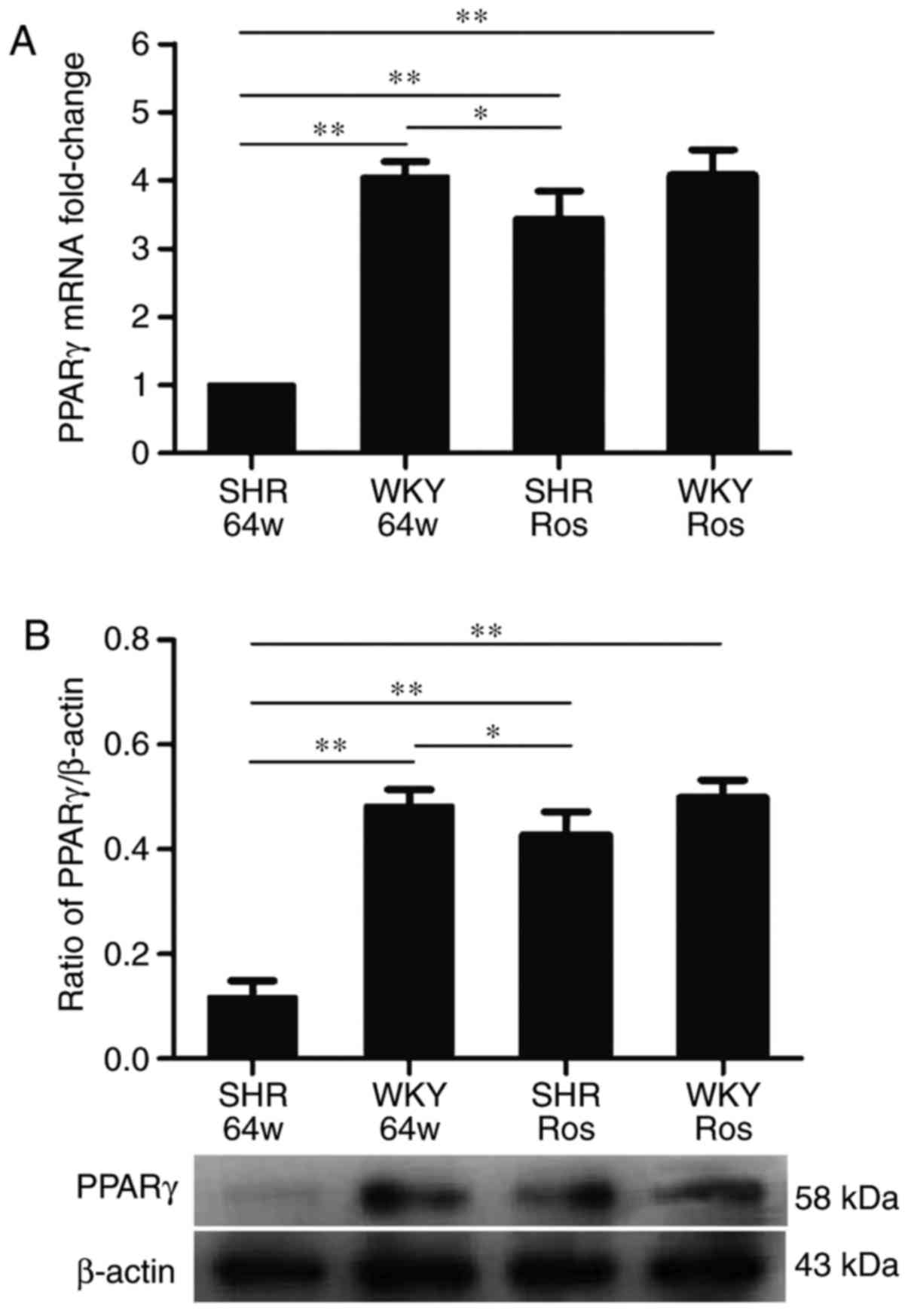
Rosiglitazone increases PPAR-γ expression
in the hippocampi of SHRs
As demonstrated in Fig. 3, hypertension and rosiglitazone
treatment have significant effects on PPAR-γ expression
(P<0.01). Compared with the WKY group, the SHR group exhibited
significantly decreased mRNA and protein expression of PPAR-γ
(P<0.01). Rosiglitazone significantly increased PPAR-γ mRNA and
protein expression in the SHR group (P<0.01), but not in the WKY
group (P>0.05).
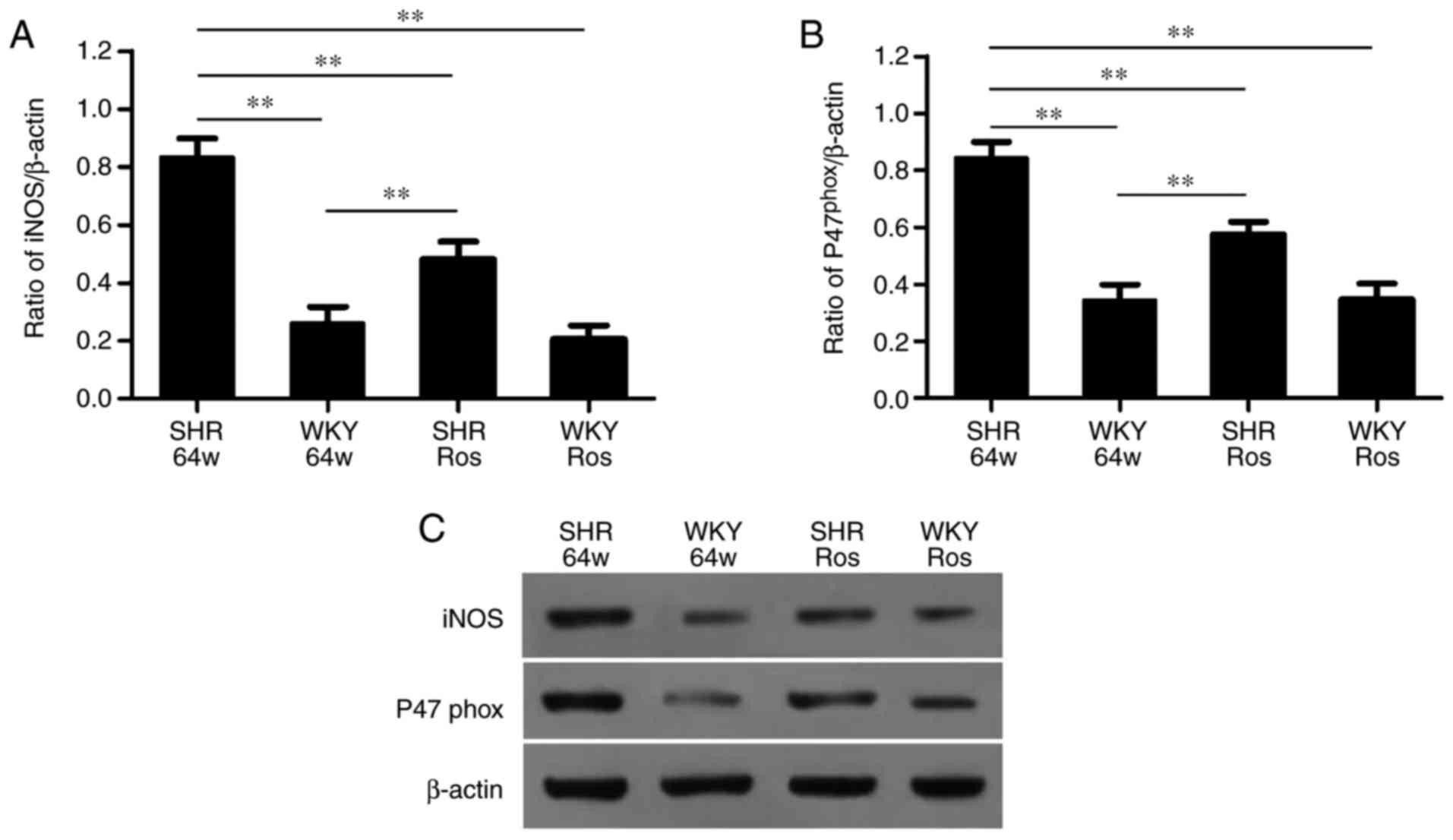
Rosiglitazone attenuates oxidative stress
injury in SHRs
As demonstrated in Fig. 4, hypertension and rosiglitazone
treatment altered the protein expression of iNOS and
gp47phox (P<0.01). The SHR group exhibited
significantly upregulated protein expression of iNOS and
gp47phox compared with the WKY group (P<0.01).
Rosiglitazone significantly decreased iNOS and gp47phox
expression in the SHR group (P<0.01), but not in the WKY group
(P>0.05).
Rosiglitazone exerts anti-apoptotic
effects in SHRs
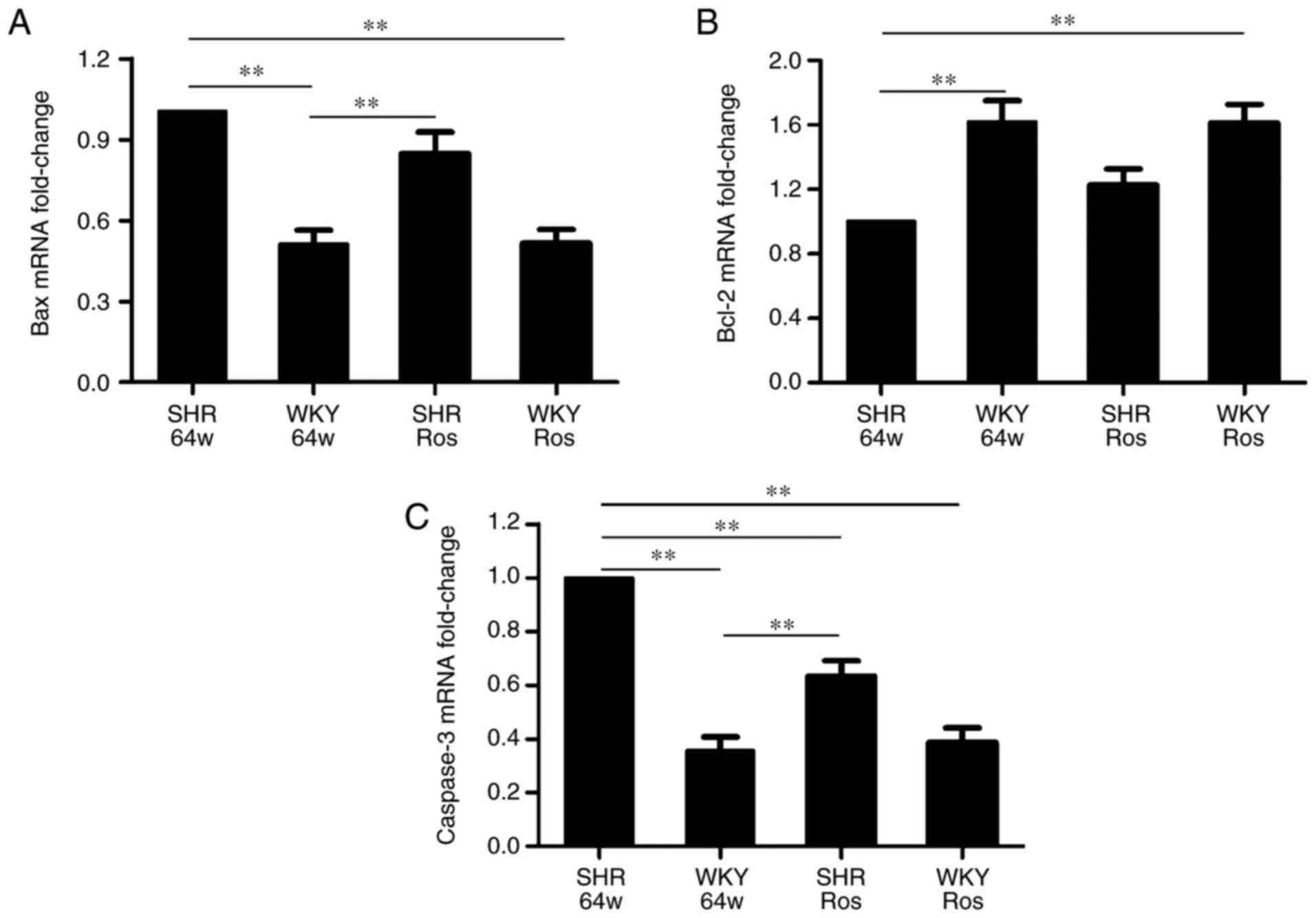
As demonstrated in Fig. 5, compared with the WKY group,
Bcl-2 mRNA expression was downregulated, while Bax and caspase-3
mRNA expression were upregulated in the SHR group (P<0.01).
Rosiglitazone did not alter Bax and Bcl-2 mRNA expression
(P>0.05), but significantly decreased caspase-3 mRNA expression
(P<0.01) in the SHR group.
The SHR group exhibited increased protein expression
of Bax and caspase-3, and upregulated the ratio of Bax/Bcl-2
(Fig. 6). The indexes in the SHR
group all significantly differed from the WKY group (P<0.01). By
contrast, the protein expression of Bcl-2 was decreased in the SHR
group (P<0.01). Rosiglitazone significantly decreased Bax,
caspase-3 protein expression (P<0.05 and P<0.01,
respectively) and the Bax/Bcl-2 expression ratio (P<0.01).
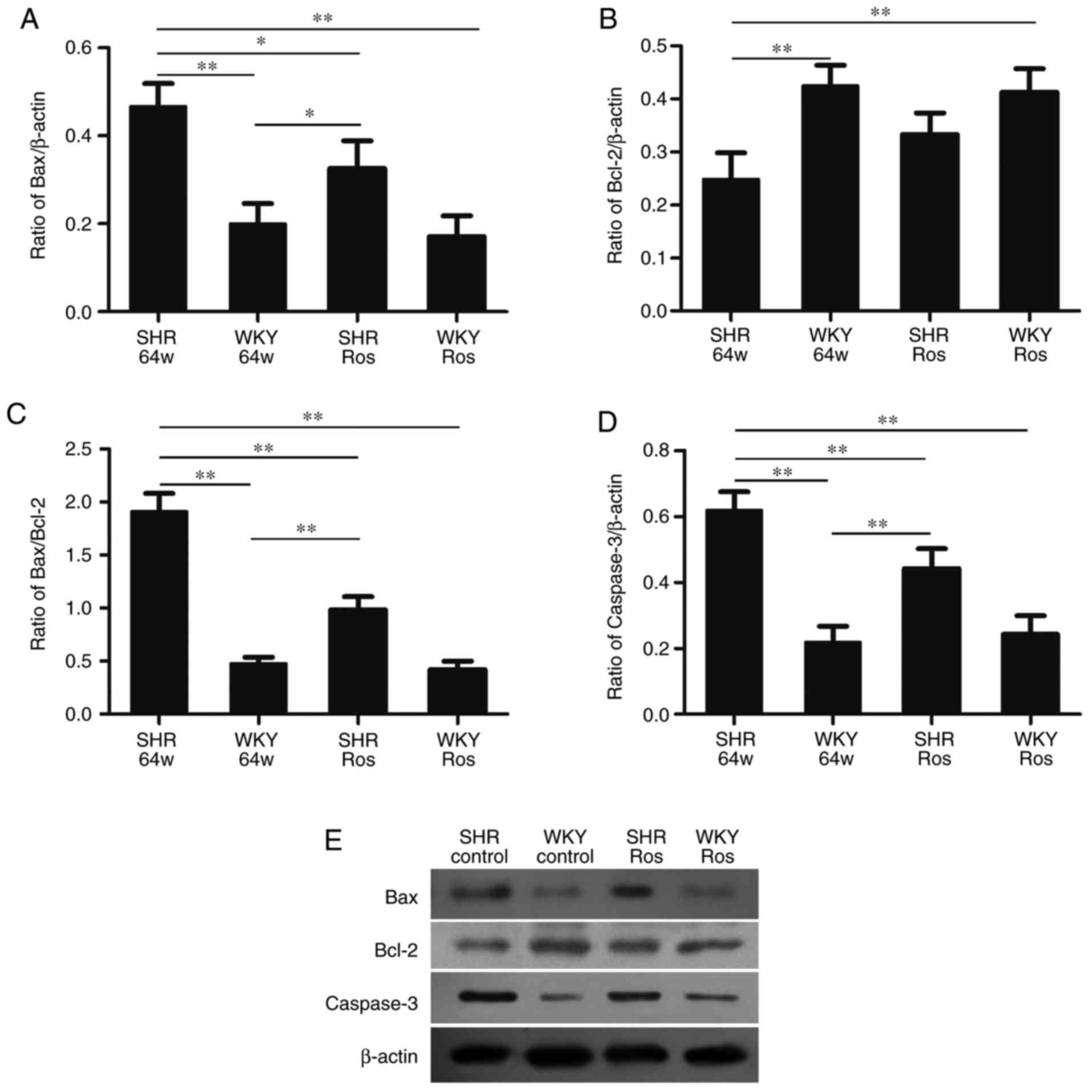 | Figure 6Protein expression of Bcl-2, Bax and
caspase-3 in four groups. SHR group exhibited (B) downregulated
Bcl-2 protein, and (A) upregulated Bax, (D) caspase-3 protein
expression and (C) Bax/Bcl-2 expression ratio. Rosiglitazone
decreased (A) Bax and (D) caspase-3 protein expression and (C)
Bax/Bcl-2 expression ratio, but did not increase (B) Bcl-2 protein
expression in the SHR or WKY groups. (E) Representative western
blotting image, displaying Bax, Bcl-2 and caspase-3 protein
expression. Columns and error bars represent the mean ± standard
error of the mean. *P<0.05, **P<0.01.
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; SHR,
spontaneously hypertensive rats; WKY, Wistar-Kyoto; ROS,
rosiglitazone. |
Discussion
The results of our previous study demonstrated that
SHRs exhibited an age-dependent increase in TUNEL-positive cells in
the CA1 subfield of the hippocampus, which was accompanied with
increased expression of oxidative stress markers and decreased mRNA
and protein expression of PPAR-γ (11). Therefore, we hypothesized that
downregulated PPAR-γ expression may be the underlying mechanism of
hypertension-related brain damage. In order to verify this
hypothesis, 64-week-old SHRs and age-matched WKY rats were used to
study whether the PPAR-γ agonist, rosiglitazone, may exert
neuroprotective effects on aged SHRs and the underlying mechanism
of this was investigated. To the best of our knowledge,
rosiglitazone has been extensively studied in vessels (13), kidneys (13) and the heart (14) of SHRs. Few studies have
investigated the effect of rosiglitazone on hypertensive brain
damage in aged SHRs. The main results of the present study were
that aged SHRs exhibited exacerbated oxidative stress injury in the
hippocampi, and that the PPAR-γ agonist, rosiglitazone, exerts
neuroprotective effects through antioxidative and anti-apoptotic
pathways in preventing SHRs from brain damage, which was
independent of blood pressure control.
Oxidative stress results from an imbalance of
production over degradation of ROS and is associated with
hypertension (15). It is also
one of the main pathophysiological mechanisms of hypertensive brain
damage. It is well known that ROS derives mainly from NADPH oxidase
enzymatic activity in brain tissues (16). Therefore, the present study
evaluated NADPH oxidase subunit gp47phox expression in
the SHR and WKY groups in order to analyze the impact of
hypertension on the activation of NADPH oxidase in the hippocampus
and its role in oxidative stress. Additionally, iNOS is another
important marker of oxidative stress, is observed in neurons and is
selectively distributed in different brain regions. Once induced,
iNOS can generate toxic levels of nitric oxide, resulting in cell
damage and death in the brain (17). The present study revealed that the
expression of gp47phox and iNOS was significantly
increased in the hippocampi of the SHR group. The increased protein
expression levels of the two parameters provided additional support
for increased ROS production and aggravated oxidative stress injury
in the hippocampi of aged SHRs. It is well known that oxidative
stress damages neurons. Excessive ROS may attack cellular
components, including proteins, lipids and DNA, and may cause cell
death via necrosis or apoptosis (18). We hypothesized that this state of
oxidative stress may increase the susceptibility of neurons to
apoptosis and represent a risk factor for neuronal death.
In line with the occurrence of aggravated oxidative
stress changes in the hippocampi of SHRs, similar results were also
observed following TUNEL staining, which was used to detect the
rupture of cell nuclei DNA during the early apoptotic process of
tissue cells. However, it was suggested that the specificity of the
TUNEL technique for apoptosis may also identify necrotic nuclei
(19). In the present study, the
SHR group exhibited a significantly increased number of
TUNEL-positive cells, compared with the WKY group. Although it is
not possible to conclude whether or not cells of the CA1 subfield
undergo apoptosis and/or necrosis based on the present TUNEL data,
the observation of more TUNEL-positive nuclei in the CA1 subfield
of the SHR group indicated the occurrence of neurodegenerative
changes in the hippocampi of aged SHRs. It is well known that the
nerve cells in the hippocampus have the ability to receive, process
and store information (20). The
observation of fewer cells or more TUNEL-positive cells in the aged
SHRs suggested that brain function may undergo a chronic
degenerative changes.
Astrocytes are activated in the condition of brain
injury by increasing the number of their intermediate filaments and
stain strongly for GFAP (21).
Therefore, increased expression of immunoreactivity of GFAP is
considered to be an indicator of brain injury. In the present
study, the SHR group exhibited an increased percentage of activated
astrocytes with thick branches and large cell bodies. The presence
of more activated astrocytes in 64-week-old SHRs probably indicated
that the hippocampus was impaired in the hypertensive state and the
increase in the activated astrocytes maybe a compensatory
protection method for prevent damage to brain tissue.
PPAR-γ is a ligand-activated transcription factor,
which serves beneficial roles in inhibiting oxidative stress in the
central nervous system (22).
Once activated by its agonist, PPAR-γ can protect QZG cells against
oxidative stress injury (23).
Therefore, PPAR-γ agonists may exert beneficial effects in
oxidative stress-related brain injury. Our previous study
demonstrated that SHRs exhibit an age-dependent reduction in PPAR-γ
expression in the hippocampus (11), which suggested that downregulated
PPAR-γ expression may underline hypertensive brain damage. In the
present study, rosiglitazone administration upregulated PPAR-γ mRNA
and protein expression in aged SHRs, which was accompanied by
markedly decreased expression of oxidative stress markers (iNOS and
gp47phox) and pro-apoptotic markers (Bax and caspase-3).
Rosiglitazone also decreased the activated ratio of astrocytes and
decreased the number of TUNEL-positive cells in the hippocampi of
aged SHRs. These results indicated that the PPAR-γ agonist,
rosiglitazone, may protect the neuronal microenvironment and
preserve nerve cells, thereby exerting neuroprotective effects
through antioxidative and anti-apoptotic mechanisms.
It is noteworthy that in the present study,
rosiglitazone did not significantly decrease SBP in the SHR group.
Accumulating evidence has indicated that rosiglitazone possesses
potent blood pressure-lowering effects in patients (24) and animal models with metabolic
syndrome (25). The underlying
mechanisms of this have not yet been fully elucidated, including
inhibiting the proliferation of arterial smooth muscle cells
(26), protecting
endothelial-dependent vasodilation (27), increasing nitrous oxide production
availability (28) and
alleviating oxidative stress in the rostral ventrolateral medulla
of the SHRs (29). A possible
limitation of the present study is that the dose used was 5
mg/kg/day, which was different from previous studies, which used 20
mg/kg/day (29) or 50 mmol/kg/day
(30). It was concluded that the
effect of rosiglitazone is dose-dependent and that the dose in the
present study was insufficient to result in a blood
pressure-lowering effect.
In conclusion, the results of the present study
demonstrated that aged SHRs exhibited increased apoptotic cells in
the hippocampus, accompanied by increased levels of oxidative
stress markers and pro-apoptotic markers, suggesting the important
role of oxidative stress injury in hypertensive brain damage. The
PPAR-γ agonist, rosiglitazone, may protect neuronal
microenvironment and preserve nerve cells in the CA1 subfield of
hippocampi of SHRs through antioxidative and anti-apoptotic
pathways, which are independent of blood pressure control. Another
are that5 requires further investigation is to assess whether other
glial cell populations, besides astrocytes, participate in the
mechanism of hypertensive brain damage.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81070219).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available as the data form part of
an ongoing study, but are available from the corresponding author
on reasonable request.
Authors' contributions
YL and XN conceived the study. YL, TZ and WL
performed the experiments and collected the data. SSh, LL, GY, JL
and SSu analyzed and interpreted the data. YL drafted the paper. YL
and SSh edited and revised the manuscript. YL reviewed the
manuscript. All the authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The methods of specimen collection were conducted in
accordance with the guidelines of National Institutes of Health.
The experimental protocols were approved by the Ethics Committee of
Xi'an Jiaotong University College of Medicine (Xi'an, China) and
were performed according to the guidelines of the Declaration of
Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Professor Yi Zhu for
providing technical assistance.
References
|
1
|
Selvetella G, Notte A, Maffei A, Calistri
V, Scamardella V, Frati G, Trimarco B, Colonnese C and Lembo G:
Left ventricular hypertrophy is associated with asymptomatic
cerebral damage in hypertensive patients. Stroke. 34:1766–1770.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Turer AT and Hill JA: Pathogenesis of
myocardial ischemia-reper-fusion injury and rationale for therapy.
Am J Cardiol. 106:360–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:1391–1401.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mohammadi MT and Dehghani GA: Acute
hypertension induces brain injury and blood-brain barrier
disruption through reduction of claudins mRNA expression in rat.
Pathol Res Pract. 210:985–990. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Corona JC and Duchen MR: PPARgamma as a
therapeutic target to rescue mitochondrial function in neurological
disease. Free Radic Biol Med. 100:153–163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kvandova M, Majzunova M and Dovinova I:
The role of PPARgamma in cardiovascular diseases. Physiol Res.
65:S343–S363. 2016.PubMed/NCBI
|
|
7
|
Chaudhry A, Carthan KA, Kang BY, Calvert
J, Sutliff RL and Michael Hart C: PPARgamma attenuates
hypoxia-induced hypertrophic transcriptional pathways in the heart.
Pulm Circ. 7:98–107. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lan LF, Zheng L, Yang X, Ji XT, Fan YH and
Zeng JS: Peroxisome proliferator-activated receptor-gamma agonist
pioglitazone ameliorates white matter lesion and cognitive
impairment in hypertensive rats. CNS Neurosci Ther. 21:410–416.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Afzal S, Sattar MA, Johns EJ, Abdulla MH,
Akhtar S, Hashmi F and Abdullah NA: Interaction between irbesartan,
peroxisome proliferator-activated receptor (PPAR-gamma), and
adiponectin in the regulation of blood pressure and renal function
in spontaneously hypertensive rats. J Physiol Biochem. 72:593–604.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maccallini C, Mollica A and Amoroso R: The
positive regulation of eNOS signaling by PPAR agonists in
cardiovascular diseases. Am J Cardiovasc Drugs. 17:273–281. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Liu J, Gao D, Wei J, Yuan H, Niu X
and Zhang Q: Age-related changes in hypertensive brain damage in
the hippocampi of spontaneously hypertensive rats. Mol Med Rep.
13:2552–2560. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
13
|
Imig JD, Walsh KA, Hye Khan MA, Nagasawa
T, Cherian-Shaw M, Shaw SM and Hammock BD: Soluble epoxide
hydrolase inhibition and peroxisome proliferator activated receptor
gamma agonist improve vascular function and decrease renal injury
in hypertensive obese rats. Exp Biol Med (Maywood). 237:1402–1412.
2012. View Article : Google Scholar
|
|
14
|
Kadiiska MB, Bonini MG, Ruggiero C,
Cleland E, Wicks S and Stadler K: Thiazolidinedione treatment
decreases oxidative stress in spontaneously hypertensive heart
failure rats through attenuation of inducible nitric oxide
synthase-mediated lipid radical formation. Diabetes. 61:586–596.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paravicini TM and Touyz RM: Redox
signaling in hypertension. Cardiovasc Res. 71:247–258. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patel M, Li QY, Chang LY, Crapo J and
Liang LP: Activation of NADPH oxidase and extracellular superoxide
production in seizure-induced hippocampal damage. J Neurochem.
92:123–131. 2005. View Article : Google Scholar
|
|
17
|
Jiang T, Gao L, Shi J, Lu J, Wang Y and
Zhang Y: Angiotensin-(1-7) modulates renin-angiotensin system
associated with reducing oxidative stress and attenuating neuronal
apoptosis in the brain of hypertensive rats. Pharmacol Res.
67:84–93. 2013. View Article : Google Scholar
|
|
18
|
Ramalingam M and Kim SJ: Reactive
oxygen/nitrogen species and their functional correlations in
neurodegenerative diseases. J Neural Transm (Vienna). 119:891–910.
2012. View Article : Google Scholar
|
|
19
|
Charriaut-Marlangue C and Ben-Ari Y: A
cautionary note on the use of the TUNEL stain to determine
apoptosis. Neuroreport. 7:61–64. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Napoleone P, Ferrante F, Ghirardi O,
Ramacci MT and Amenta F: Age-dependent nerve cell loss in the brain
of Sprague-Dawley rats: Effect of long term acetyl-L-carnitine
treatment. Arch Gerontol Geriatr. 10:173–185. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beach TG, Walker R and McGeer EG: Patterns
of gliosis in Alzheimer's disease and aging cerebrum. Glia.
2:420–436. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kvandová M, Majzúnová M and Dovinová I:
The role of PPARgamma in cardiovascular diseases. Physiol Res.
65:S343–S363. 2016.PubMed/NCBI
|
|
23
|
Li WL, Liang X, Wang X, Zhang XD, Liu R,
Zhang W, Chen HL, Qin XJ, Bai H and Hai CX: Protective effect of
the peroxisome proliferator-activated receptor (PPAR)-gamma, ligand
rosiglitazone on tertbutyl hydroperoxide-induced QZG cell injury.
Exp Toxicol Pathol. 63:527–533. 2011. View Article : Google Scholar
|
|
24
|
Raji A, Seely EW, Bekins SA, Williams GH
and Simonson DC: Rosiglitazone improves insulin sensitivity and
lowers blood pressure in hypertensive patients. Diabetes Care.
26:172–178. 2003. View Article : Google Scholar
|
|
25
|
Dobrian AD, Schriver SD, Khraibi AA and
Prewitt RL: Pioglitazone prevents hypertension and reduces
oxidative stress in diet-induced obesity. Hypertension. 43:48–56.
2004. View Article : Google Scholar
|
|
26
|
de Dios ST, Bruemmer D, Dilley RJ, Ivey
ME, Jennings GL, Law RE and Little PJ: Inhibitory activity of
clinical thiazolidinedione peroxisome proliferator activating
receptor-gamma ligands toward internal mammary artery, radial
artery, and saphenous vein smooth muscle cell proliferation.
Circulation. 107:2548–2550. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang TD, Chen WJ, Cheng WC, Lin JW, Chen
MF and Lee YT: Relation of improvement in endothelium-dependent
flow-mediated vasodilation after rosiglitazone to changes in
asymmetric dimethylarginine, endothelin-1, and C-reactive protein
in nondiabetic patients with the metabolic syndrome. Am J Cardiol.
98:1057–1062. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Polikandriotis JA, Mazzella LJ, Rupnow HL
and Hart CM: Peroxisome proliferator-activated receptor gamma
ligands stimulate endothelial nitric oxide production through
distinct peroxisome proliferator-activated receptor gamma-dependent
mechanisms. Arterioscler Thromb Vasc Biol. 25:1810–1816. 2005.
View Article : Google Scholar
|
|
29
|
Chan SH, Wu KL, Kung PS and Chan JY: Oral
intake of rosiglitazone promotes a central antihypertensive effect
via upregulation of peroxisome proliferator-activated
receptor-gamma and alleviation of oxidative stress in rostral
ventrolateral medulla of spontaneously hypertensive rats.
Hypertension. 55:1444–1453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Walker AB, Chattington PD, Buckingham RE
and Williams G: The thiazolidinedione rosiglitazone (BRL-49653)
lowers blood pressure and protects against impairment of
endothelial function in Zucker fatty rats. Diabetes. 48:1448–1453.
1999. View Article : Google Scholar : PubMed/NCBI
|