Introduction
The degradation of articular cartilage is a major
characteristic of several arthritic diseases, including
osteoarthritis (OA) and rheumatoid arthritis (RA), which eventually
result in joint destruction (1,2).
The biosynthetic activities of chondrocytes contribute to the
stability of articular cartilage (3-5),
which contains extracellular matrix (ECM) molecules that confer
mechanical elasticity. By contrast, chondrocyte dysfunction
enhances cartilage ECM degradation and cartilage degeneration
(6,7). Chondrocyte senescence is also
important in articular cartilage degeneration (8), as it is associated with declines in
chondrocyte numbers and contributes to the development of arthritic
diseases (9,10). It has been established that
senescent chondrocytes accumulate in the articular cartilages of
patients with arthritic diseases (11-13), however, the factors that govern
chondrocyte decisions regarding senescence remain to be fully
elucidated.
Protein kinase casein kinase 2 (CK2) is a
constitutively active and highly conserved serine/threonine protein
kinase consisting of two catalytic (α and/or α′) and two regulatory
(β) subunits (14). CK2 is
ubiquitously expressed in subcellular compartments in all
eukaryotes and phosphorylates over 300 substrates (15), and regulates several different
metabolic events as a result. Several studies have demonstrated the
links between CK2 and cell growth (16,17), transformation (18) and apoptosis (19), and between CK2 and inflammatory
diseases (16,20). However, despite research efforts
on various aspects of CK2, its function in chondrocytes is only
partly understood. Previously, CK2 was reported to be involved in
the regulation of rat articular chondrocyte apoptosis by
phosphorylating certain apoptosis-related factors (21). In another study, it was suggested
that inhibition of the activity of CK2 facilitates tumor necrosis
factor-mediated chondrocyte death through apoptosis, and that the
activity of CK2 is downregulated in the chondrocytes of aged
articular cartilage (22).
In our previous study, it was shown that CK2 is
associated with the expression of heme oxygenase-1 (HO-1) in
articular chondrocytes (23). In
the present study, it was observed that inhibiting the activity of
CK2 induces the senescence of primary articular chondrocytes. In
addition, it was found that CK2 inhibition-mediated senescence is
associated with regulation of the expression of HO-1 in
chondrocytes.
Materials and methods
Reagents
The 4,5,6,7-terabromo-2-azabenzimidazole (TBB),
5,6-dichlorobenzimidazole 1-β-D-ribofuranoside (DRB),
3-morpholinosydnonimine hydrochloride (SIN-1), protease inhibitor
cocktail, trypan blue solution (0.4%),
5-bromo-4-chloroindol-3-indolyl β-D-galactopyranoside, potassium
ferrocyanide, and potassium ferricyanide were purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Fetal bovine serum
(FBS), Dulbecco’s modified Eagle’s medium (DMEM) and other culture
reagents were purchased from GE Healthcare Life Sciences (Logan,
UT, USA). Anti-CK2 (sc-373894), HO-1 (sc-10789), type II collagen
(sc-52658), β-catenin (sc-7199), and β-actin (sc-1616-R) antibodies
were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Secondary horseradish peroxidase (HRP)-conjugated antibodies
(anti-rabbit, NA934; anti-mouse, NA931) and the enhanced
chemiluminescence (ECL) western blotting kit were obtained from GE
Healthcare Life Sciences.
Animals and cell culture of articular
chondrocytes
Five-week-old female Sprague-Dawley rats were
purchased from Samtako Bio Korea, Co. (Osan, Korea). All animal
experiments and protocols were approved by the Pusan National
University Institutional Animal Care and Use Committee (Miryang,
Korea) and performed in accordance with the institutional and
national guidelines for the care and use of laboratory animals. The
knee joint cartilages were collected shortly upon arrival, however
if necessary, the rats were housed under controlled conditions at
23±2°C and 50±10% humidity on a 12 h light/dark cycle and had free
access to a standard chow diet and water.
A total of 30 rats were used in the present study
and their weight range was 110-130 g. To obtain primary articular
chondrocytes, the knee joint cartilages from three rats per
experiment were cut into ~1-mm3 sections, and the
samples were incubated for 1 h at 37°C with 0.2% type II
collagenase in DMEM. Primary chondrocytes were collected by
centrifugation (300 × g; 5 min; room temperature) and resuspended
in DMEM supplemented with 10% (v/v) heat-inactivated FBS and
antibiotics (50 U/ml penicillin, 50 µg/ml streptomycin) at
37°C in a 5% CO2/95% air atmosphere. The cells were then
plated on culture dishes at 5×104 cells/cm2
and cultured until confluent for ~4-5 days (medium was replaced
every 2 days during culture). Following subculture (the cells were
designated as passage 1, which was used for all experiments), cells
were plated at a density of 1×105 cells/cm2
and cultured for 24 h in 37°C incubator under a humidified
atmosphere conditions with 5% CO2. Then, the cells were
treated with various concentrations (2, 10, and 50 µM) of
TBB or DRB in the presence or absence of SIN-1 for 48 h. The cell
viabilities were determined using a trypan blue exclusion assay
with a hemocytometer.
Senescence-associated β-galactosidase
(SA-β-gal) staining assay
The cells were washed in phosphate-buffered saline
(PBS), fixed for 5 min at room temperature in 0.2%
glutaraldehyde/2% formaldehyde, washed with PBS, and incubated with
SA-β-gal staining solution [1 mg/ml
5-bromo-4-chloro-indol-3-indolyl β-D-galactopyranoside, 40 mM
citrate/sodium phosphate buffer (pH 6.0), 5 mM potassium
ferrocyanide, 5 mM potassium ferricyanide, 150 mM NaCl, and 2 mM
MgCl2] for 12 h at 37°C. The percentages of senescent
cells were determined by counting the numbers of blue-stained cells
per 200 cells in randomly selected areas at ×20 magnification under
an optical microscope.
Western blot analysis
The cells were lysed in ice-cold RIPA buffer with
protease inhibitors, and protein concentrations in the whole cell
lysates were determined using a colorimetric method with
bicinchoninic acid protein dye reagent (Pierce; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Subsequently, the proteins (20
µg) were loaded onto 12% SDS-polyacrylamide gels,
electrophoresed, and transferred onto nitrocellulose membranes
using an electroblotting apparatus (Bio-Rad Laboratories, Inc.,
Richmond, CA, USA). Following blocking with 5% skim milk in TBS-T,
the membranes were incubated with primary antibodies targeting CK2α
(1:250), HO-1 (1:250), type II collagen (1:250), β-catenin (1:250),
and β-actin (1:500) at 4°C overnight, washed with TBS-T, incubated
with HRP-conjugated secondary antibodies (1:2,000) at room
temperature for 2 h, and developed using the ECL detection system.
The blots were visualized and quantified using a LAS-3000
Luminescent Image Analyzer (FujiFilm, Tokyo, Japan). β-actin was
used as an internal loading control.
Measurement of CK2 kinase activity
The activity of CK2 was measured in cell lysates
using a CK2 kinase assay kit (CycLex Co., Ltd., Ina, Japan).
Briefly, the purified recombinant CK2 (for standard curve) and cell
lysates (2×105 cells each) from CK2α small interfering
(si)RNA or SCR-transfected cells were added to the wells of a CK2
substrate (recombinant p53)-precoated plate. Following the addition
of kinase reaction buffer, the wells were washed and incubated with
HRP-conjugated detection antibody at room temperature for 30 min.
The phosphorylation activities were assessed by adding
tetramethylbenzidine and measuring the absorbances at 450 nm. All
quantifications were performed in triplicate.
Transfection of CK2α siRNA
To knock down CK2 gene expression, a 21-nucleotide
RNA duplex containing 3′-dTdT overhangs (GE Healthcare Dharmacon,
Inc., Lafayette, CO, USA) was used to target rat CK2α mRNA
(sequence, 5′-CTGGGTGGGTGTCTCATTCAA-3′). At 24 h after plating, the
chondrocytes at ~70% confluence were transfected with 30 nM of CK2α
or negative control siRNA in wells using DharmaFECT® Duo
transfection reagent, according to the manufacturer’s protocol.
Preparation of HO-1 expression vector and
transfection
The pcDNA3 mammalian cell expression vector
containing full-length rat HO-1 cDNA was used as previously
described (24). The chondrocytes
(1×105 cells/cm2) were trans-fected with 10
µg of the HO-1 construct or control plasmid complexed with
Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), incubated for 24 h in a 37°C incubator, placed in fresh
medium, and then treated with TBB. The cells were also analyzed to
monitor the expression level of HO-1.
Statistical analysis
All experiments were repeated at least three times.
Student’s t-test (two-tailed) or one-way analysis of variance were
used to analyze the data by using GraphPad Prism software (GraphPad
Software, Inc., La Jolla, CA, USA). Dunnett’s or Tukey’s honest
significant difference post hoc tests were used. The results are
presented as the mean ± standard deviation, and P<0.05 was
considered to indicate a statistically significant difference.
Results
TBB and DRB induces primary rat
chondrocyte senescence
To determine whether the activity of CK2 is
associated with cellular senescence in chondrocytes, the present
study first examined the effect of TBB and DRB (pharmacological
inhibitors of CK2) in primary rat chondrocytes. Isolated cells were
treated with various concentrations (2, 10 and 50 µM) of TBB
or DRB for 48 h and then subjected to trypan blue dye exclusion
assay and SA-β-gal staining assay. As shown in Fig. 1, TBB marginally reduced cell
proliferation without affecting cell viability and significantly
increased SA-β-gal staining compared with the untreated controls.
The DRB-treated chondrocytes exhibited significant decreases in
cell proliferation, although DRB also increased cellular senescence
of chondrocytes (Fig. 1A-D).
Therefore, TBB was only used as a specific inhibitor to
downregulate the activity of CK2 in further experiments.
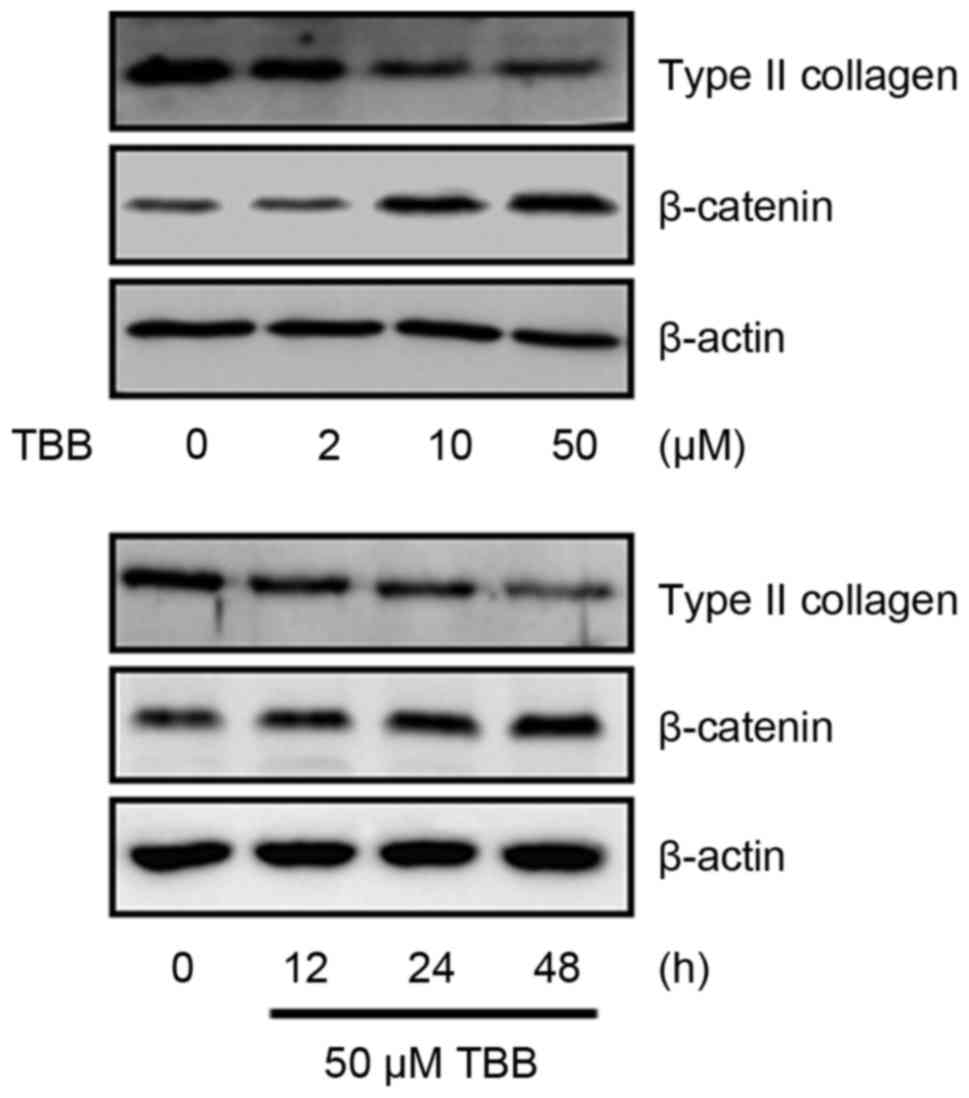
Subsequently, the effect of TBB on the expression of phenotypic
markers were examined, including type II collagen and β-catenin,
which regulate chondrocyte functions. The primary chondrocytes were
treated with different concentrations (2, 10 and 50 µM) of
TBB and for different time intervals (12, 24 or 48 h). As shown in
Fig. 2, TBB significantly
suppressed the expression of type II collagen and enhanced the
expression of β-catenin. These results show that TBB induced
changes in the expression of phenotypic markers of the
differentiation and dedifferentiation of primary chondrocytes.
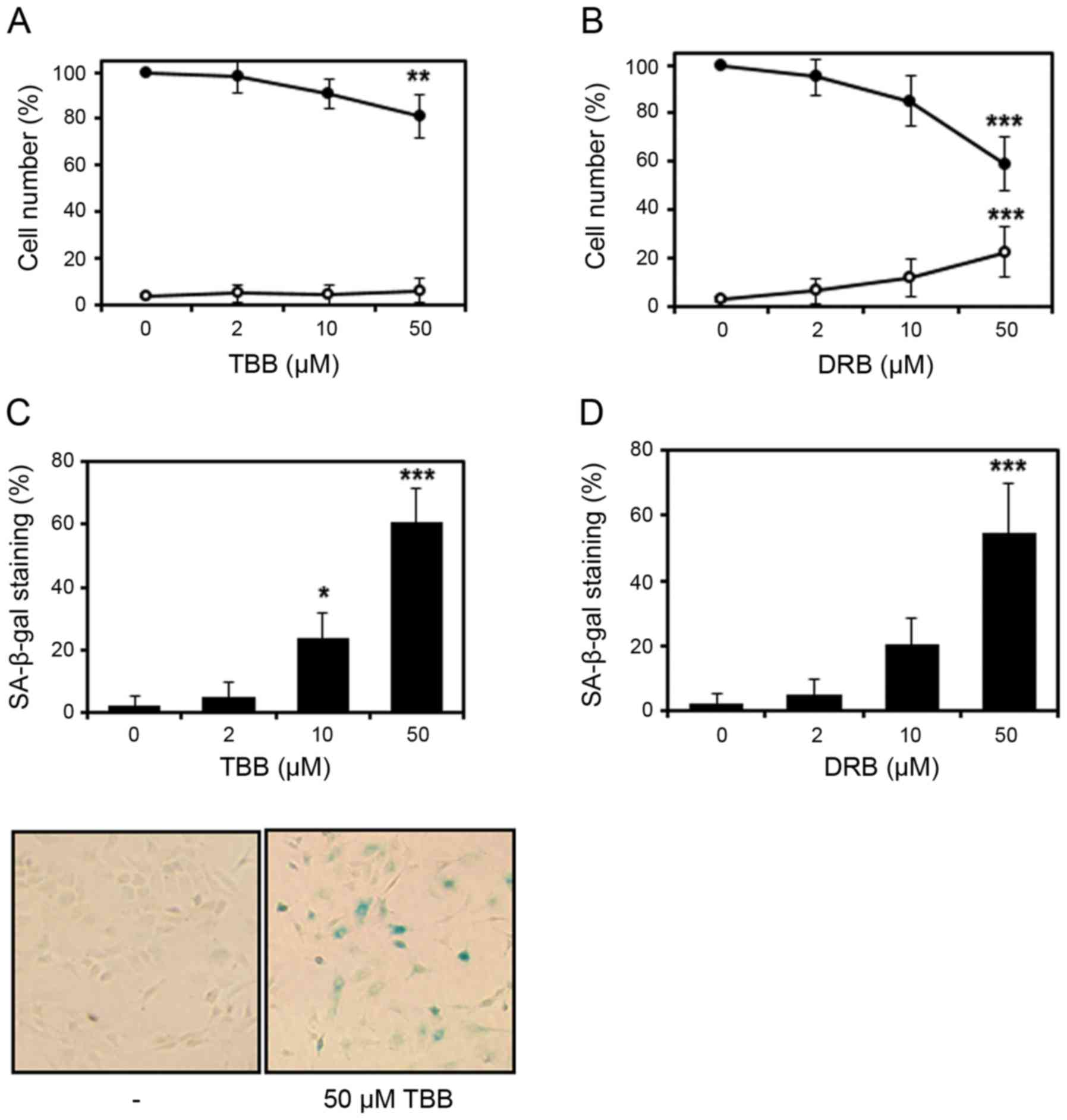 | Figure 1Effect of CK2 inhibitors on
chondrocyte senescence. Primary rat articular chondrocytes were
treated with different concentrations (2, 10 and 50 µM) of
TBB or DRB for 48 h. Cells treated with (A) TBB and (B) DRB were
assessed for viability using the trypan blue dye exclusion method
(closed circles represent live cells, open circles represent dead
cells). Values are shown as the percentages of control cells.
Chondrocyte senescence was measured in cells treated with (C) TBB
and (D) DRB using the SA-β-gal assay. Percentages of
SA-β-gal-positive cells were calculated from numbers of
blue-stained cells per 200 cells in randomly selected areas.
Representative images were captured at ×20 magnification. The
P-value was calculated by one-way analysis of variance followed by
Dunnett’s post hoc test. *P<0.05,
**P<0.01 and ***P<0.001 vs. 0 µM
of TBB or DRB. CK2, protein kinase casein kinase 2; TBB,
4,5,6,7-terabromo-2-azabenzimidazole; DRB,
5,6-dichlorobenzimidazole 1-β-D-ribofuranoside; SA-β-gal,
senescence-associated β-galactosidase. |
Specific inhibition of the activity of
CK2 enhances chondrocyte senescence
To confirm that the activity of CK2 is associated
with chondrocyte senescence, CK2 was knocked down by transfecting
chondrocytes with a CK2 siRNA duplex or a non-specific control for
24 h and then incubating the cells for a further 48 h. Transfection
was found to effectively inhibit CK2 enzyme activity and to
significantly reduce the protein levels of CK2 in chondrocytes
(Fig. 3A and B). Transfection
with control siRNA did not affect SA-β-gal staining significantly
compared with the non-transfected controls. CK2 knockdown
significantly increased SA-β-gal staining (Fig. 3C), and reduced the protein levels
of type II collagen and enhanced β-catenin in chondrocytes. These
observations suggest that CK2 activity is required to maintain
chondrocyte phenotype.
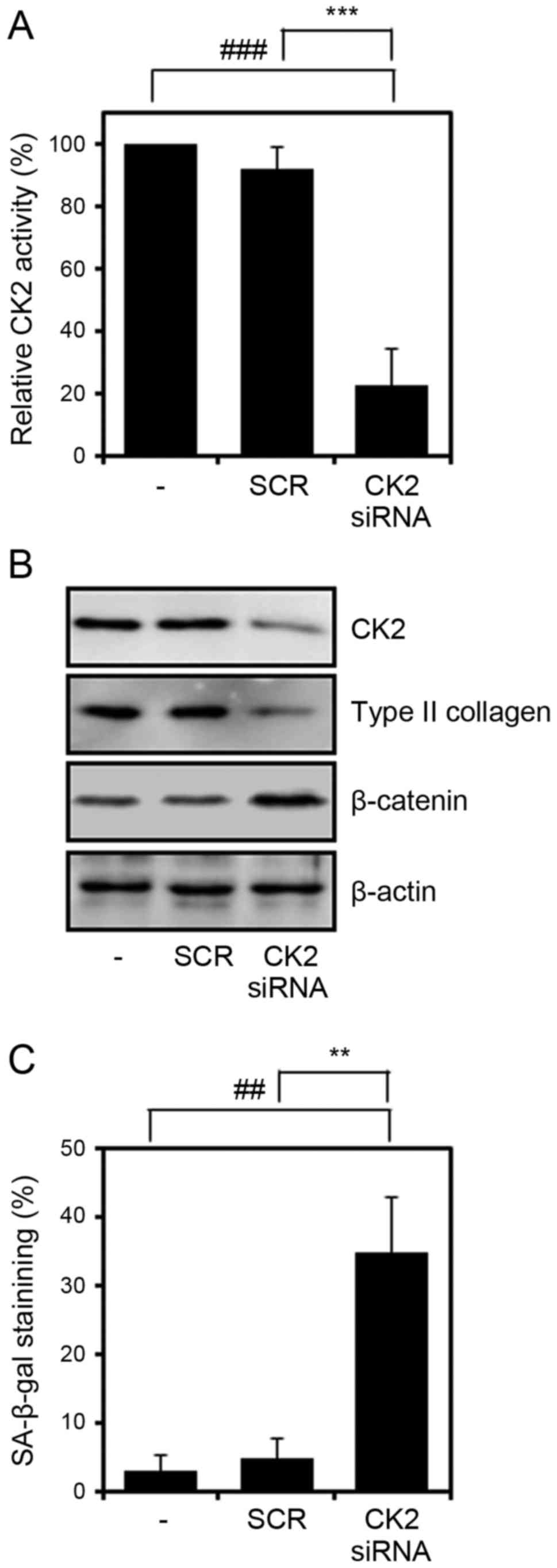 | Figure 3Effect of CK2 inhibition on
chondrocyte senescence. Cells were transfected with CK2α siRNA,
incubated for 48 h, and subjected to the (A) CK2 activity assay,
(B) western blot analysis and (C) SA-β-gal assay. Values are
presented as the mean ± standard deviation of three independent
experiments. The P-value was calculated by one-way analysis of
variance followed by Tukey’s honest significant difference post hoc
test. ##P<0.01 and ###P<0.001 vs.
negative control. **P<0.01 and
***P<0.001 vs. SCR. The difference between the
negative control and SCR was not significant. siRNA, small
interfering RNA; CK2, protein kinase casein kinase 2; TBB,
4,5,6,7-terabromo-2-azabenzimidazole; SA-β-gal,
senescence-associated β-galactosidase; SCR, scrambled siRNA
negative control. |
SIN-1 modulates TBB-induced cellular
senescence in chondrocytes
Subsequently, the present study investigated the
effect of the peroxynitrite generator SIN-1, which is widely used
to increase the expression and activity of HO-1 in several cell
types, on TBB-induced chondrocyte senescence. In our previous
study, it was reported that CK2 modulates SIN-1-induced HO-1
expression in chondrocytes (23),
and as a result it was hypothesized that TBB-induced chondrocyte
senescence was caused by the inhibition of HO-1. In the present
study, the chondrocytes were cultured for 6 h either in medium
alone or in medium containing 200 µM SIN-1 and stimulated
with TBB (50 µM) for 48 h. As shown in Fig. 4A, pretreatment with SIN-1
suppressed TBB-induced cellular senescence, which resulted from the
induction of the expression of HO-1 by pretreatment with SIN-1
(Fig. 4B). However, when the
cells were treated with SIN-1 following TBB, no significant
reduction in SA-β-gal staining was observed (Fig. 4C). In addition, as expected,
pretreatment with TBB inhibited the induction of HO-1 by SIN-1
(Fig. 4D), indicating that the
effect of SIN-1 on the TBB-induced senescence of chondrocytes is
associated with the expression of HO-1.
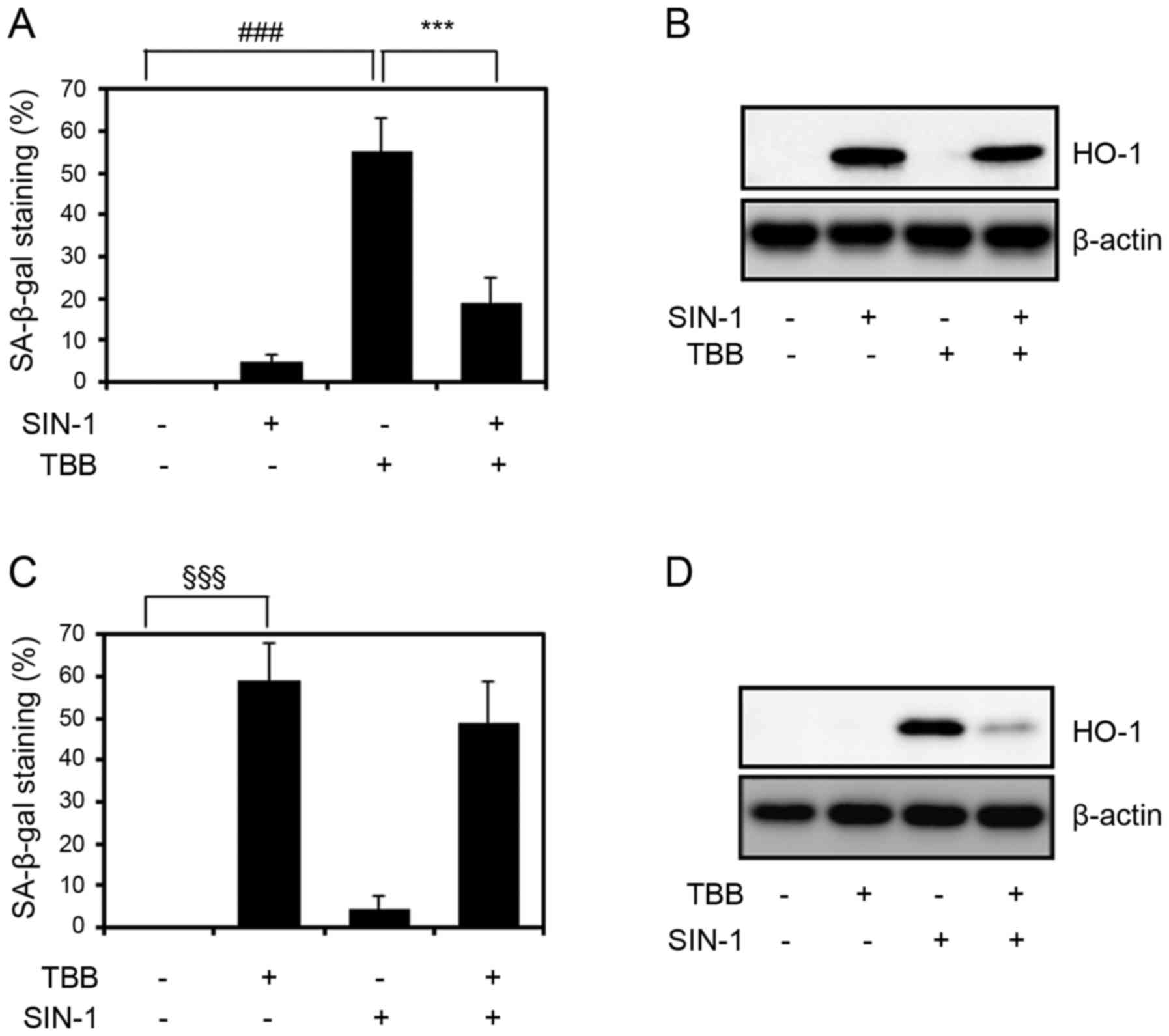 | Figure 4Effect of SIN-1 on TBB-induced
chondrocyte senescence. (A) SA-β-gal assay and (B) western blot
analysis of chondrocytes pretreated with 200 µM SIN-1 for 6
h, stimulated with 50 µM TBB, and incubated for 48 h.
###P<0.001: negative control vs. TBB;
***P<0.001: TBB vs. SIN-1 + TBB. P-values among the
other groups were also analyzed (negative control vs. SIN-1: ns;
negative control vs. SIN-1 + TBB: P<0.05; SIN-1 vs. TBB:
P<0.001; SIN-1 vs. SIN-1 + TBB: P<0.05). (C) SA-β-gal assay
and (D) western blot analysis of chondrocytes stimulated with 50
µM TBB for 6 h, and incubated for another 42 h in the
absence or presence of SIN-1 (200 µM).
§§§P<0.001: negative control vs. TBB. P-values among
the other groups were also analyzed (negative control vs. SIN-1:
ns; negative control vs. TBB + SIN-1: P<0.001; TBB vs. SIN-1:
P<0.001; TBB vs. TBB + SIN-1: ns; SIN-1 vs. TBB + SIN-1:
P<0.001). Values are presented as the mean ± standard deviation
of three independent experiments. P-values were calculated by
one-way analysis of variance followed by Tukey’s honest significant
difference post hoc test. For western blotting, β-actin was used as
an internal control. SIN-1, 3-morpholinosydnonimine hydrochloride;
TBB, 4,5,6,7-terabromo-2-azabenzimidazole; SA-β-gal,
senescence-associated β-galactosidase; ns, not significant; HO-1,
heme oxygenase-1. |
HO-1 inhibits TBB-induced cellular
senescence in chondrocytes
Based on the above-mentioned results, the
enhancement of chondrocyte senescence by TBB treatment appeared to
be associated with the expression of HO-1, therefore, an
HO-1-overexpression vector was used to determine whether HO-1
directly modulates the senescence of TBB-treated chondrocytes. The
chondrocytes were transfected with the HO-1 expression vector or
control pcDNA vector and the protein overexpression of HO-1 was
assessed by western blot analysis (Fig. 5A). As shown in Fig. 5B, the overexpression of HO-1
significantly reduced the senescence-specific SA-β-gal staining of
TBB-treated chondrocytes. These results suggest that the forced
overexpression of HO-1 decreased the CK2 inhibition-mediated
senescence of chondrocytes.
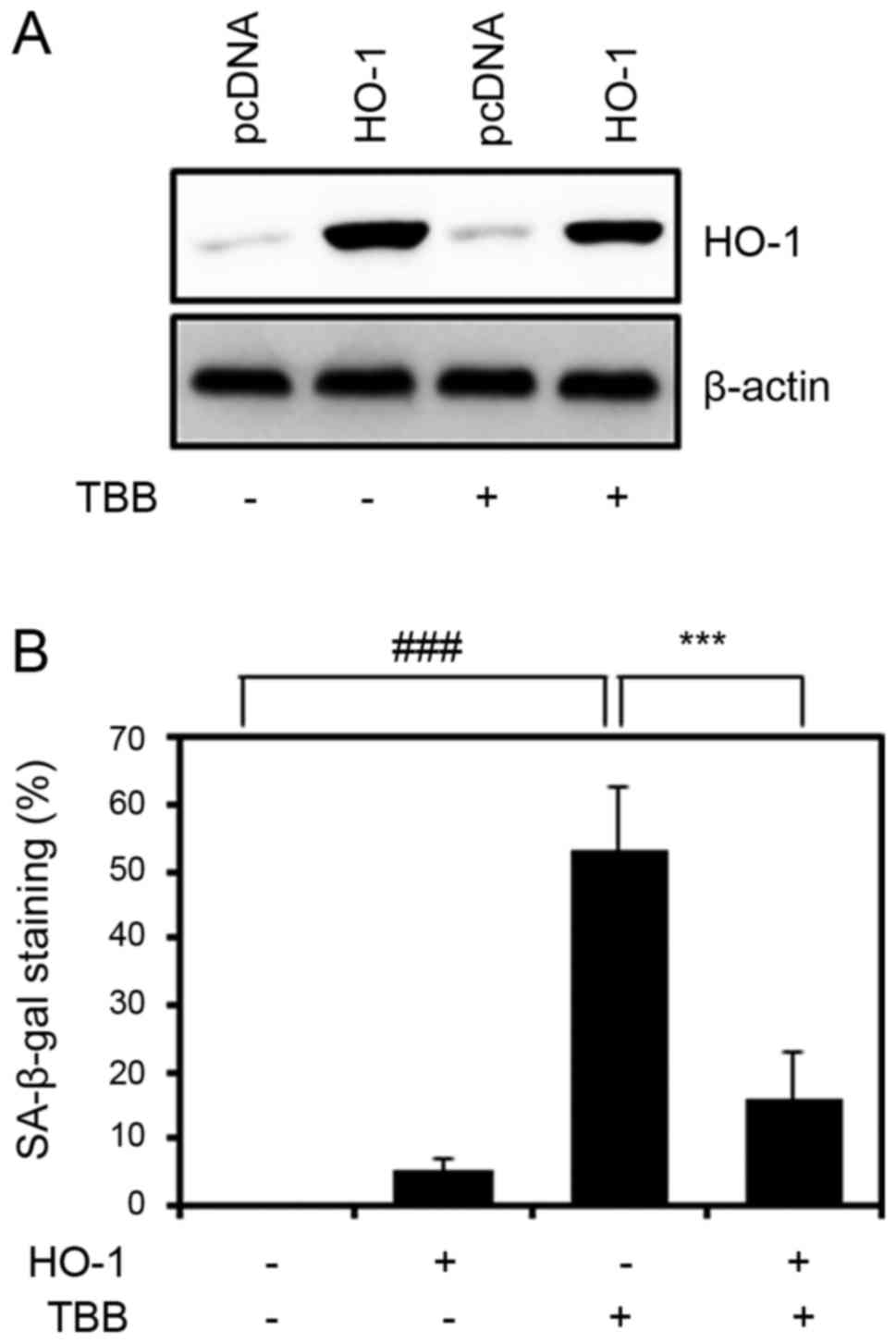 | Figure 5Effect of the overexpression of HO-1
on TBB-induced chondrocyte senescence. (A) Cells were subjected to
western blot analysis to show the effi-ciency of HO-1 expression.
β-actin was used as an internal control. (B) Cells were transfected
with the HO-1 expression vector, incubated for 24 h, stimulated
with 50 µM TBB for 48 h, and subjected to a SA-β-gal assay.
Values are presented as the mean ± standard deviation of three
independent experiments. P-values were calculated by one-way
analysis of variance followed by Tukey’s honest significant
difference post hoc test. ###P<0.001: negative
control vs. TBB, ***P<0.001: TBB vs. HO-1 + TBB.
P-values among other groups are also analyzed (negative control vs.
HO-1: ns; negative control vs. HO-1 + TBB: ns; HO-1 vs. TBB:
P<0.001; HO-1 vs. HO-1 + TBB: ns). TBB,
4,5,6,7-terabromo-2-azabenzimidazole; SA-β-gal,
senescence-associated β-galactosidase; ns, not significant; HO-1,
heme oxygenase-1. |
Discussion
Under physiological conditions, chondrocytes have
rare proliferative properties and have a central role in the make
up of articular cartilage, which contains an avascular
cartilage-specific matrix (25).
Chondrocytes produce and maintain extracellular matrix, which is
composed of type II collagen and sulfated proteoglycans, by
facilitating the cell-matrix interactions responsible for several
of the functions of cartilage (4,26).
Although articular cartilage is hypoxic in nature, chondrocytes
produce ROS to enable metabolism adapted to anaerobic conditions,
and is exposed to abnormal levels of ROS produced by immune cells
under pathological conditions, including OA, rheumatoid arthritis
and gout (27-29). Chondrocytes constitutively express
well-coordinated antioxidant enzymes, including superoxide
dismutase (SOD; cytosolic Cu/Zn SOD and mitochondrial Mn SOD),
glutathione peroxidase (GPX) and catalase (30,31).
Elevated levels of ROS and antioxidant depletion
have been reported under pathological conditions, including those
associated with inflammatory diseases (32). Oxidative stress can damage the
components of the ECM by upregulating matrix metalloproteinases
(MMPs) and inflammatory mediators, including inducible nitric oxide
synthase and cyclooxygenase-2 (33,34), and by doing so can cause the
apoptosis and senescence of chondrocytes in articular cartilage and
destroy articular cartilage (9,13,35). Furthermore, age-related changes in
the intracellular redox status of chondrocytes has been shown to be
associated with the development of cell alterations (36,37). Apoptotic chondrocyte death is
widely observed in degenerated cartilage of OA, and chondrocyte
senescence may contribute to the reduction in chondrocyte numbers
in articular cartilage; senescent chondrocytes accumulate with age
and in articular cartilage of patients with OA (8,11-13).
Unlike SOD, GPX, and catalase, HO-1 is adaptively
induced to protect chondrocytes and cartilage against the
destructive effects of oxidative stress (10). It has also been reported that HO-1
inhibits catabolic enzymes and inflammatory cytokines that may
contribute to the pathogeneses of cartilage diseases (5), which suggests that HO-1 is a
component of defense systems that protect articular cartilage
(27,38). In addition, it has been shown that
HO-1 contributes significantly to protection against cartilage
degeneration in humans (39).
However, the mechanisms responsible for the regulation and
activities of HO-1 in the contexts of chondrocyte maintenance,
senescence and apoptosis in articular cartilage remain to be fully
elucidated.
CK2 is critical in the control of cell
proliferation, transformation and apoptosis (17-19), and reportedly, loss of CK2
activity is involved in chromatin structural alteration and changes
in gene expression (40,41). Therefore, it appears that CK2 can
stimulate the phosphorylation of several proteins required for DNA
replication and transcription (15,42,43). In our previous study, it was
reported that CK2 mediates the expression of HO-1 by
phosphorylating and inducing the nuclear translocation of Nrf2 in
chondrocytes exposed to oxidative stress (23). In the present study, it was found
that the activity of CK2 is associated with the senescence of
primary articular chondrocytes, and that the downregulation of CK2
induces cellular senescence by inhibiting the expression of HO-1.
Furthermore, inhibition of CK2 activity, achieved using TBB (a
specific CK2 inhibitor) or by silencing the CK2 gene, caused
specific SA-β-gal staining of chondrocytes and altered the
expression of type II collagen and β-catenin (phenotypic markers of
chondrocyte differentiation). Subsequently, the present study
investigated whether HO-1 is involved in TBB-induced chondrocyte
senescence using the pharma-cologic inducer (SIN-1) and by the
transfection-induced overexpression of HO-1. Chondrocytes
overexpressing HO-1 exhibited significant inhibition of TBB-induced
cellular senescence, which provides evidence that the activity of
HO-1 is associated with the CK2 inhibition-mediated senescence of
chondrocytes, and suggests that HO-1 may act to inhibit chondrocyte
senescence.
However, reports indicate that HO-1 has a dual role
in pathological states, as high levels of HO-1 are frequently
detected in pathological tissues (44,45). In parallel, chondrocytes obtained
by serial subculture exhibit passage number-dependent increases in
cellular senescence and expression of HO-1 (unpublished data),
which may reflect age-related changes in intracellular redox status
during the development of cellular senescence. This observation
encourages the suggestion that the level of HO-1 in articular
cartilage may be a useful biomarker of the degree of articular
cartilage degeneration. Based on the results obtained in the
present study, it can be concluded that the activity of CK2 may act
as an anti-aging factor by inducing the expression of HO-1 to
counteract the effects of chondrocyte senescence in articular
cartilage.
Funding
The present study was supported by the 2013
Specialization Project Research Grant Funded by Pusan National
University.
Availability of data and materials
All the datasets generated and analysed during the
present study are available from the corresponding author on
reasonable request.
Authors’ contributions
KMK, KK and YCP made contributions to the conception
and design of the study. KMK, DHS and YCP performed the
experiments. DHS, KK, and YCP performed the statistical analysis.
DHS and YCP wrote and revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments and protocols were approved
by the Pusan National University Institutional Animal Care and Use
Committee and performed in accordance with the institutional and
national guidelines for the care and use of laboratory animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Abbreviations:
|
CK2
|
protein kinase casein kinase 2
|
|
TBB
|
4,5,6,7-terabromo-2-azabenzimidazole
|
|
DRB
|
5,6-dichloro benzimidazole
1-β-D-ribofuranoside
|
|
SA-β-gal
|
senescence-associated
β-galactosidase
|
|
HO-1
|
heme oxygenase-1
|
|
SIN-1
|
3-morpholino-sydnonimine
hydrochloride
|
|
ECM
|
extracellular matrix
|
|
ROS
|
reactive oxygen species
|
|
OA
|
osteoarthritis
|
References
|
1
|
Sandell LJ and Adler P: Developmental
patterns of cartilage. Front Biosci. 4:D731–D742. 1999. View Article : Google Scholar
|
|
2
|
DeLise AM, Fischer L and Tuan RS: Cellular
interactions and signaling in cartilage development. Osteoarthritis
Cartilage. 8:309–334. 2000. View Article : Google Scholar
|
|
3
|
Goldring MB: The role of the chondrocyte
in osteoarthritis. Arthritis Rheum. 43:1916–1926. 2000. View Article : Google Scholar
|
|
4
|
Roughley PJ: Articular cartilage and
changes in arthritis: Noncollagenous proteins and proteoglycans in
the extracellular matrix of cartilage. Arthritis Res. 3:342–347.
2001. View Article : Google Scholar
|
|
5
|
Guillén M, Megías J, Gomar F and Alcaraz
M: Haem oxygenase-1 regulates catabolic and anabolic processes in
osteoarthritic chondrocytes. J Pathol. 214:515–522. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cawston T, Billington C, Cleaver C,
Elliott S, Hui W, Koshy P, Shingleton B and Rowan A: The regulation
of MMPs and TIMPs in cartilage turnover. Ann N Y Acad Sci.
878:120–129. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim HA and Song YW: Apoptotic chondrocyte
death in rheumatoid arthritis. Arthritis Rheum. 42:1528–1537. 1999.
View Article : Google Scholar
|
|
8
|
Aigner T and Kim HA: Apoptosis and
cellular vitality: Issues in osteoarthritic cartilage degeneration.
Arthritis Rheum. 46:1986–1996. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jallali N, Ridha H, Thrasivoulou C,
Underwood C, Butler PE and Cowen T: Vulnerability to ROS-induced
cell death in ageing articular cartilage: The role of antioxidant
enzyme activity. Osteoarthritis Cartilage. 13:614–622. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zwerina J, Tzima S, Hayer S, Redlich K,
Hoffmann O, Hanslik-Schnabel B, Smolen JS, Kollias G and Schett G:
Heme oxygenase 1 (HO-1) regulates osteoclastogenesis and bone
resorption. FASEB J. 19:2011–2013. 2005. View Article : Google Scholar
|
|
11
|
Loeser RF: Aging and osteoarthritis: The
role of chondrocyte senescence and aging changes in the cartilage
matrix. Osteoarthritis Cartilage. 17:971–979. 2009. View Article : Google Scholar :
|
|
12
|
Martin JA and Buckwalter JA: Aging,
articular cartilage chondrocyte senescence and osteoarthritis.
Biogerontology. 3:257–264. 2002. View Article : Google Scholar
|
|
13
|
McCulloch K, Litherland GJ and Rai TS:
Cellular senescence in osteoarthritis pathology. Aging Cell.
16:210–218. 2017. View Article : Google Scholar
|
|
14
|
Litchfield DW: Protein kinase CK2:
Structure, regulation and role in cellular decisions of life and
death. Biochem J. 369:1–15. 2003. View Article : Google Scholar
|
|
15
|
Meggio F and Pinna LA:
One-thousand-and-one substrates of protein kinase CK2. FASEB J.
17:349–368. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pinna LA: Casein kinase 2: An ’eminence
grise’ in cellular regulation? Biochim Biophys Acta. 1054:267–284.
1990. View Article : Google Scholar
|
|
17
|
Ahmed K, Gerber DA and Cochet C: Joining
the cell survival squad: An emerging role for protein kinase CK2.
Trends Cell Biol. 12:226–230. 2002. View Article : Google Scholar
|
|
18
|
Faust RA, Gapany M, Tristani P, Davis A,
Adams GL and Ahmed K: Elevated protein kinase CK2 activity in
chromatin of head and neck tumors: Association with malignant
transformation. Cancer Lett. 101:31–35. 1996. View Article : Google Scholar
|
|
19
|
Ahmad KA, Wang G, Unger G, Slaton J and
Ahmed K: Protein kinase CK2 - a key suppressor of apoptosis. Adv
Enzyme Regul. 48:179–187. 2008. View Article : Google Scholar
|
|
20
|
Drygin D, Ho CB, Omori M, Bliesath J,
Proffitt C, Rice R, Siddiqui-Jain A, O’Brien S, Padgett C, Lim JK,
et al: Protein kinase CK2 modulates IL-6 expression in inflammatory
breast cancer. Biochem Biophys Res Commun. 415:163–167. 2011.
View Article : Google Scholar
|
|
21
|
Yoon YM, Kim SJ, Oh CD, Ju JW, Song WK,
Yoo YJ, Huh TL and Chun JS: Maintenance of differentiated phenotype
of articular chondrocytes by protein kinase C and extracellular
signal-regulated protein kinase. J Biol Chem. 277:8412–8420. 2002.
View Article : Google Scholar
|
|
22
|
Lee SW, Song YS, Lee SY, Yoon YG, Lee SH,
Park BS, Yun I, Choi H, Kim K, Chung WT, et al: Downregulation of
protein kinase CK2 activity facilitates tumor necrosis
factor-α-mediated chondrocyte death through apoptosis and
autophagy. PLoS One. 6:e191632011. View Article : Google Scholar
|
|
23
|
Kim KM, Song JD, Chung HT and Park YC:
Protein kinase CK2 mediates peroxynitrite-induced heme oxygenase-1
expression in articular chondrocytes. Int J Mol Med. 29:1039–1044.
2012.PubMed/NCBI
|
|
24
|
Kim KM, Park SE, Lee MS, Kim K and Park
YC: Induction of heme oxygenase-1 expression protects articular
chondrocytes against cilostazol-induced cellular senescence. Int J
Mol Med. 34:1335–1340. 2014. View Article : Google Scholar
|
|
25
|
Muir H: The chondrocyte, architect of
cartilage. Biomechanics, structure, function and molecular biology
of cartilage matrix macromolecules. BioEssays. 17:1039–1048. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bau B, Gebhard PM, Haag J, Knorr T,
Bartnik E and Aigner T: Relative messenger RNA expression profiling
of collagenases and aggrecanases in human articular chondrocytes in
vivo and in vitro. Arthritis Rheum. 46:2648–2657. 2002. View Article : Google Scholar
|
|
27
|
Henrotin YE, Bruckner P and Pujol JP: The
role of reactive oxygen species in homeostasis and degradation of
cartilage. Osteoarthritis Cartilage. 11:747–755. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Phillips DC, Dias HK, Kitas GD and
Griffiths HR: Aberrant reactive oxygen and nitrogen species
generation in rheumatoid arthritis (RA): Causes and consequences
for immune function, cell survival, and therapeutic intervention.
Antioxid Redox Signal. 12:743–785. 2010. View Article : Google Scholar
|
|
29
|
Martin WJ, Herst PM, Chia EW and Harper
JL: Sesquiterpene dialdehydes inhibit MSU crystal-induced
superoxide production by infiltrating neutrophils in an in vivo
model of gouty inflammation. Free Radic Biol Med. 47:616–621. 2009.
View Article : Google Scholar
|
|
30
|
Deahl ST II, Oberley LW, Oberley TD and
Elwell JH: Immunohistochemical identification of superoxide
dismutases, catalase, and glutathione-S-transferases in rat femora.
J Bone Miner Res. 7:187–198. 1992. View Article : Google Scholar
|
|
31
|
Borsiczky B, Szabó Z, Jaberansari MT, Mack
PP and Röth E: Activated PMNs lead to oxidative stress on
chondrocytes: A study of swine knees. Acta Orthop Scand.
74:190–195. 2003. View Article : Google Scholar
|
|
32
|
Matés JM, Pérez-Gómez C and Núñez de
Castro I: Antioxidant enzymes and human diseases. Clin Biochem.
32:595–603. 1999. View Article : Google Scholar
|
|
33
|
Stadler J, Stefanovic-Racic M, Billiar TR,
Curran RD, McIntyre LA, Georgescu HI, Simmons RL and Evans CH:
Articular chondrocytes synthesize nitric oxide in response to
cytokines and lipopolysaccharide. J Immunol. 147:3915–3920.
1991.
|
|
34
|
Pelletier JP, McCollum R, DiBattista J,
Loose LD, Cloutier JM and Martel-Pelletier J: Regulation of human
normal and osteo-arthritic chondrocyte interleukin-1 receptor by
antirheumatic drugs. Arthritis Rheum. 36:1517–1527. 1993.
View Article : Google Scholar
|
|
35
|
Henrotin Y, Kurz B and Aigner T: Oxygen
and reactive oxygen species in cartilage degradation: Friends or
foes? Osteoarthritis Cartilage. 13:643–654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Carlo MD Jr and Loeser RF: Increased
oxidative stress with aging reduces chondrocyte survival:
Correlation with intracellular glutathione levels. Arthritis Rheum.
48:3419–3430. 2003. View Article : Google Scholar
|
|
37
|
Murray MM, Zurakowski D and Vrahas MS: The
death of articular chondrocytes after intra-articular fracture in
humans. J Trauma. 56:128–131. 2004. View Article : Google Scholar
|
|
38
|
Clérigues V, Guillén MI, Gomar F and
Alcaraz MJ: Haem oxygenase-1 counteracts the effects of
interleukin-1β on inflammatory and senescence markers in
cartilage-subchondral bone explants from osteoarthritic patients.
Clin Sci (Lond). 122:239–250. 2012. View Article : Google Scholar
|
|
39
|
Fernández P, Guillén MI, Gomar F and
Alcaraz MJ: Expression of heme oxygenase-1 and regulation by
cytokines in human osteoarthritic chondrocytes. Biochem Pharmacol.
66:2049–2052. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Villeponteau B: The heterochromatin loss
model of aging. Exp Gerontol. 32:383–394. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu S, Wang H, Davis A and Ahmed K:
Consequences of CK2 signaling to the nuclear matrix. Mol Cell
Biochem. 227:67–71. 2001. View Article : Google Scholar
|
|
42
|
Lorenz P, Ackermann K, Simoes-Wuest P and
Pyerin W: Serum-stimulated cell cycle entry of fibroblasts requires
undisturbed phosphorylation and non-phosphorylation interactions of
the catalytic subunits of protein kinase CK2. FEBS Lett.
448:283–288. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
St-Denis NA and Litchfield DW: Protein
kinase CK2 in health and disease: From birth to death: the role of
protein kinase CK2 in the regulation of cell proliferation and
survival. Cell Mol Life Sci. 66:1817–1829. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Otterbein LE, Kolls JK, Mantell LL, Cook
JL, Alam J and Choi AM: Exogenous administration of heme
oxygenase-1 by gene transfer provides protection against
hyperoxia-induced lung injury. J Clin Invest. 103:1047–1054. 1999.
View Article : Google Scholar
|
|
45
|
Bauer M and Bauer I: Heme oxygenase-1:
Redox regulation and role in the hepatic response to oxidative
stress. Antioxid Redox Signal. 4:749–758. 2002. View Article : Google Scholar : PubMed/NCBI
|