Introduction
Multiple myeloma (MM) is a common hematological
malignancy caused by the malignant proliferation of plasma cells
(PCs), which accumulate within bone marrow (1,2).
As a consequence, normal hematopoiesis is disrupted. MM ranks as
the second most common of all hematological malignancies around the
world (3). However, no specific
markers have been validated for MM detection or therapy,
representing an obstacle for the investigation of MM. Although
several studies in previous years have revealed certain
therapeutics, such progress remains inadequate, and investigations
of MM are urgently required.
MicroRNAs (miRNAs) are small, non-coding RNAs with a
length of ~22 nucleotides. They are considered to function as mRNA
regulators by interacting with the 3′-untranslated region (3′UTR)
of the targeted mRNA transcript and leading to degradation
(2-5). Several, if not all, biological
processes are reported to be regulated by miRNAs, including cell
cycle, proliferation, metabolism, immune response, hematopoiesis
and differentiation (6-10). It is widely accepted that miRNAs
are vital in the process of tumorigenesis by targeting key factors
(11,12). They are also considered as
potential therapeutic factors for several diseases.
miR-497 is one of the well-studied miRNAs and is
considered to be a tumor-suppressive miRNA in osteosar-coma,
colorectal carcinoma, glioma, breast carcinoma and several other
cancer types, being involving in the regulation of tumor cell
growth, migration, invasion and chemoresistance (11-17). Previous studies have found that
miR-497 was downregulated in patients with MM compared with healthy
donors (18,19), and miR-497 was the one of four
miRNA signatures identified as having differential expression
levels that correlated with lenalidomide/dexzmethasone treatment
response in a primary plasma cell leukemia clinical trial (19). However, the role and underlying
mechanism of miR-497 in regulating the tumorigenesis of leukemia
remain to be fully elucidated.
Multiple genes may be targets of miR-497, and a
well-accepted potential target of miR-497 is B-cell lymphoma 2
(Bcl-2) (15,20-23). Bcl-2 is a protein involved in
modulating cell death through suppressing apoptosis and promoting
cell survival. In several types of cancer, Bcl-2 is reported to be
upregulated (23,24). Its expression is also considered
to be regulated by multiple miRNAs, including miR-497. Bcl-2 family
proteins are also involved in the chemoresistance of MM (9).
Few studies have reported the functional mechanism
of miR-497 and Bcl-2 in MM, particularly whether miR-497 can
directly target Bcl‑2 and influence MM cell cycle and apoptosis. In
addition, as miR-497 and Bcl-2 are involved in chemotherapy of
other types of cancer (20,21,23), it is of interest to evaluate the
possibility of the combination therapy of miR-497 and bortezomib, a
common drug used for MM treatment (25). Although bortezomib promotes the
death of malignant plasma cells and is a conventional clinical
medication used in MM therapy, several patients are intrinstically
or become bortezomib-resistant (26,27). Therefore, RPMI-8226 and U266 MM
cell lines, which are confirmed to be resistant to bortezomib
(26,27), were used in the present study to
verify the function of miR-497. The results may reveal a synergetic
effect of miR-497 in improving bortezomib efficacy.
Materials and methods
Clinical specimens
Bone marrow aspirates of 30 untreated II phase MM
patients (50-70 years old, half men and half women, serum
β-microglobulin=3.5-5.5 mg/l) were collected at the Longgang
District People’s Hospital of Shenzhen (Shenzhen, China) from
September 2016 to December 2017. Normal bone marrow aspirates were
collected from 30 healthy donors with signed statements of informed
consent. The bone marrow aspirates were lysed to obtain the bone
marrow mononuclear cells (BMMCs), and then the total RNA were
extracted by commercial kits for subsequent gene expression
analysis. The ethics committee of the Longgang District People’s
Hospital of Shenzhen approved the present study.
Cell culture
The RPMI-8266 and U266 human MM cell lines were
obtained from American Type Culture Collection (Manassas, VA, USA)
and cultured in RPMI-1640 medium supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) in a humidified atmosphere of 95% air and 5% CO2 at
37°C. The bone mesenchymal stem cells (BMSCs) were isolated from
human bone segments, as described previously, and cultured in a
standard growth medium for stem cells (28).
miRNA transfection and drug
treatment
The human miR-497 mimic (miR-497) and the negative
control (miR-NC) were designed and synthesized by GenePharma
(Shanghai, China). When the cells were cultured to 80% confluence,
the RNA oligonucleotides were transfected into the cells using
Lipo3000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer’s protocol. The two miRNAs were used at 50 nM
unless otherwise indicated.
For the drug susceptibility assay, bortezomib was
dissolved in DMSO at a storage concentration of 10 μM and
administered following miRNA transfection for 24 h with a
concentration range of 0, 5, 10, 15, and 20 nM when cells reached
90% confluency. Following another 24 h incubation with bortezomib
or DMSO at 37°C, the cells were collected for analysis.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
An MTT assay was performed for cell viability
detection. The cells were seeded into a 96-well plate for culture
with serum-free medium for 24 h. Following treatment, the cells
were rinsed with PBS and treated with 20 μl of 5 mg/ml MTT
for 4 h at 37°C, and DMSO was used to dissolve the formazan
precipitation. The absorbance at 570 nm was measured using a plate
reader, and the cell viability was calculated as follows:
Absorbance
(A)=(Atreatemnt−Acontrol)/Acontrol×100%.
PI staining for cell cycle analysis
The cells were rinsed with PBS, trypsinized and
collected by centrifugation at 500 × g for 5 min and room
temperature (25°C). Following discarding of the supernatant, the
cells were resuspended with PBS and rinsed for 3 times, and then
ice-cold ethanol was added to fix the cells at −20°C overnight.
Following centrifugation (500 × g, 5 min, 25°C) and rinse of 3
times, 1×106 cells were added to a mix of 400 μl
RNase (20 μg/ml) together with PI (50 μg/ml) and 0.2%
Triton X-100 in PBS, and then incubated for 30 min at 37°C.
Finally, the cells were collected, rinsed and centrifuged again
(500 × g, 5 min, 25°C), and then resus-pended with PBS for flow
cytometric analysis.
Apoptosis assay
Annexin V-FITC was used for the analysis of
apoptosis assay following the manufacturer’s protocol. The cells
were rinsed with cold PBS three times. Following centrifugation at
500 × g for 5 min at 4°C, the cells were collected and concentrated
to 1×105 cells per ml. The sample solution (0.1 ml) was
mixed with 5 μl FITC-conjugated Annexin V and 5 μl PI
solution. The mixture was incubated for 30 min in the dark at room
temperature, and then analyzed by flow cytometry.
Soft-agar colony formation assay
For the soft-agar colony formation assay, the cells
were mixed with DMEM containing 0.35% low-melting agarose and 10%
FBS, and seeded 500 cells onto a coating of 0.7% low-melting
agarose in DMEM with 10% FBS. The cells were incubated for up to 3
weeks, following which colonies were visualized by 0.4% crystal
violet and imaged using a digital camera (D5600; Nikon Corporation,
Tokyo, Japan). Colonies with >0.1 mm diameter were considered as
positive.
Terminal deoxynucleotidyl transferase
(TdT)-mediated dUTP nick-end-labeling (TUNEL) assay
A TUNEL assay was performed using an in situ
Cell Death Detection kit, Fluorescein (Promega Corporation,
Madison, WI, USA) according to the manufacturer’s protocol. Images
were captured using a fluorescent microscope.
Analysis of caspase-3 activity
Caspase 3 activity was assayed with a fluorometric
kit (R&D Systems, Inc., Minneapolis, MN, USA) following the
manufacturer’s protocol. Briefly, the cells were rinsed with PBS
and lysed with the lysis buffer from the kit. A substrate of
caspase-3 was mixed with the lysate and incubated for 60 min. The
fluorescence signal was analyzed at 405 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total cellular RNA was isolated from the cultured
cells using the RNeasy Mini kit (Qiagen, Inc., Valencia, CA, USA)
according to the manufacturer’s protocol. RT-qPCR analysis was
performed on a 96-well plate ABI Prism 7500 Sequence Detection
system (Applied Biosystems; Thermo Fisher Scientific, Inc.) using a
25 μl reaction system containing 12.5 μl 2X
SYBR-Green PCR Master mix (Takara Bio, Inc., Otsu, Japan), 100 ng
cDNA and 0.4 μM paired primers. The cycling conditions were
as follows: 40 cycles of 94°C for 5 sec, 60°C for 34 sec, and 72°C
for 30 sec. A comparative 2−∆∆Cq method (29) was used to calculate the relative
expression of target genes. The sequences of Bcl-2 primers were
forward, 5′-GGATCCAGGATAACGGAGGC-3′ and reverse,
5′-GATAGGCACCCAGGGTGATG-3′. The relative expression level of Bcl-2
was normalized to the internal reference gene glyceraldehyde
3-phosphate dehydrogenase (GAPDH) with primers were forward,
5′-CCAGGTGGTCTCCTCTGA-3′ and reverse, 5′-GCTGTAGCCAAATCGTTGT-3′.
The level of mature miR-497 was analyzed by Stem-loop qPCR as
described (30) and normalized to
that of U6 with primers were forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse, 5′-AACGCTTCACGAATTTGCGT-3′.
Western blot analysis
The protein was extracted with M-PER (Thermo Fisher
Scientific, Inc.), quantified using a bicin-choninic acid kit
(Thermo Fisher Scientific, Inc.), and 30 μg samples were
separated via 12% SDS-PAGE and then transferred onto polyvinylidene
difluoride membrane at 100 V for 70 min using a mini transfer tank
for electrophoresis (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The membranes were blocked in 2% bovine serum albumin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 1 h at room
temperature, and incubated with primary antibodies (1:1,000) at 4°C
overnight. The membranes were washed in TBS with 0.1% Tween-20
(TBST) three times (5 min each time), and then incubated with
horseradish peroxidase-conjugated goat anti-rabbit IgG secondary
antibody (cat. no. ab97051; 1:3,000) for 1 h at room temperature.
The blots were analyzed using an enhanced chemiluminescence western
blotting detection system (GE Healthcare Life Sciences, Piscataway,
NJ, USA). All primary antibodies were obtained from Abcam
(Cambridge, UK; BCL-2; cat. no. ab32124; BAX; cat. no. ab32503;
caspase-3; cat. no. ab13847; caspase-9; cat. no. ab32539;
cytochrome-c; cat. no. ab133504); GAPDH (cat. no. ab9485) was used
as the internal reference.
Dual luciferase reporter assay
The wild-type 3′ UTR sequence of Bcl-2 containing
the predicted miR-497 binding sites using bioinformatics software
TargetScan (http://www.targetscan.org) and
miRanda (http://cbio.mskcc.org/microrna_data/manual.html) was
amplified by PCR using a human cDNA template and paired primers
(sense, 5′-GAG TTG CTT TAC GTG GCC TG-3′; antisense, 5′-ACA TGT GTT
GGG ATT GCC CT-3′). The corresponding mutated 3′UTR sequence was
generated by a TaKaRa MutanBEST kit (Takara Bio, Inc., Otsu, Japan)
and primers (mutation-introduced-sense, 5′-CCA ATC CTG TCA GAC GTT
CCT GCC AAA AT-3′; antisense, 5′-GTA ACA GTT TCA CTA CTT ATA CCT
TAT A-3′) following the manufacturer’s protocol. The two sequences
were separately inserted into the pMIR-Reporter vector (Ambion;
Thermo Fisher Scientific, Inc.) using restriction enzymes
SacI and HindIII. These constructs, in addition to
pRL-TK (Promega Corporation) and miR-497/miR-NC, were
co-transfected into the cultured cells using Xfect™ Polymer. The
luciferase activity was assayed using the
Dual-Luciferase® Reporter (DLR™) Assay system (Promega
Corporation) following the manufacturer’s protocol. All samples
were assessed in triplicate, and the results were normalized
relative to control Renilla activity.
Statistical analysis
All data values are presented as the mean ± standard
deviation from at least three separate determinations. Comparison
of all other results was performed by one-way analysis of variance
with Tukey’s comparison analysis or Student’s t test, and the
statistical significance was analyzed using these tests. P<0.05
was considered to indicate a statistically significant difference.
GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA) was
used to compare groups.
Results
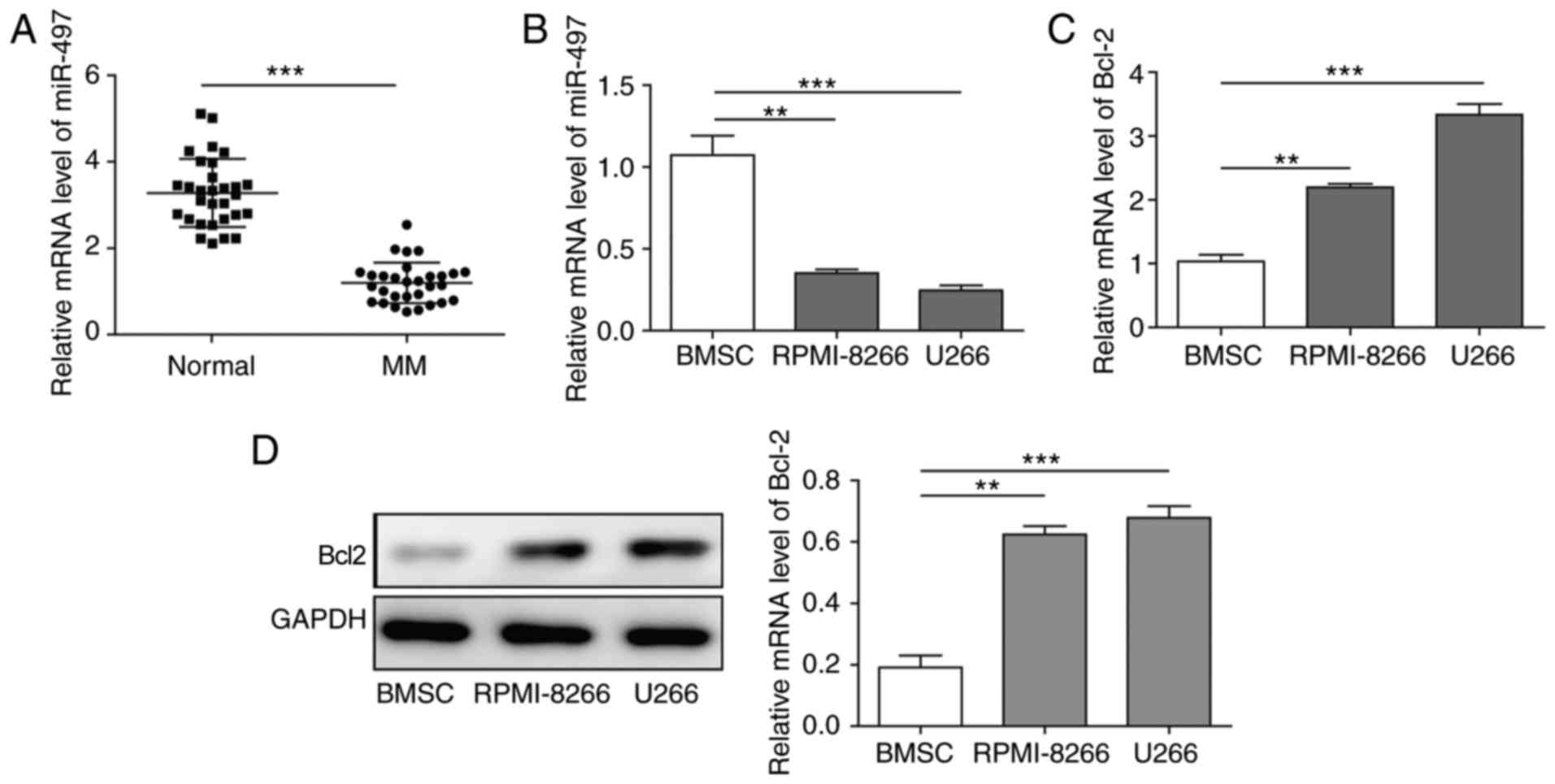
Expression of miR-497 is lower and
expression of Bcl-2 is elevated in MM tissues and cells
To elucidate the association between miR-497 and MM,
RT-qPCR analysis was performed in 46 MM patients/normal
individuals. The expression of miR-497 was lower in patients with
MM than in normal individuals (Fig.
1A). Similar experiments were performed in the RPMI-8226 and
U266 cell lines, and BMSCs used as a normal control. The results
were consistent with the clinical cases, when compared with the
normal BMSCs; the level of miR-497 was significantly lower in MM
cells (Fig. 1B). Subsequently,
the expression of Bcl-2, one of the target genes of miR-497
(20,22,23), detected in MM cells and BMSCs. As
expected, it was found that the expression of Bcl-2, at the mRNA
level and protein level, was elevated in both MM cell lines
(Fig. 1C and D). These results
indicated that miR-497 was downregulated but Bcl-2 was upregulated
in MM cells, a negative correlation between the two.
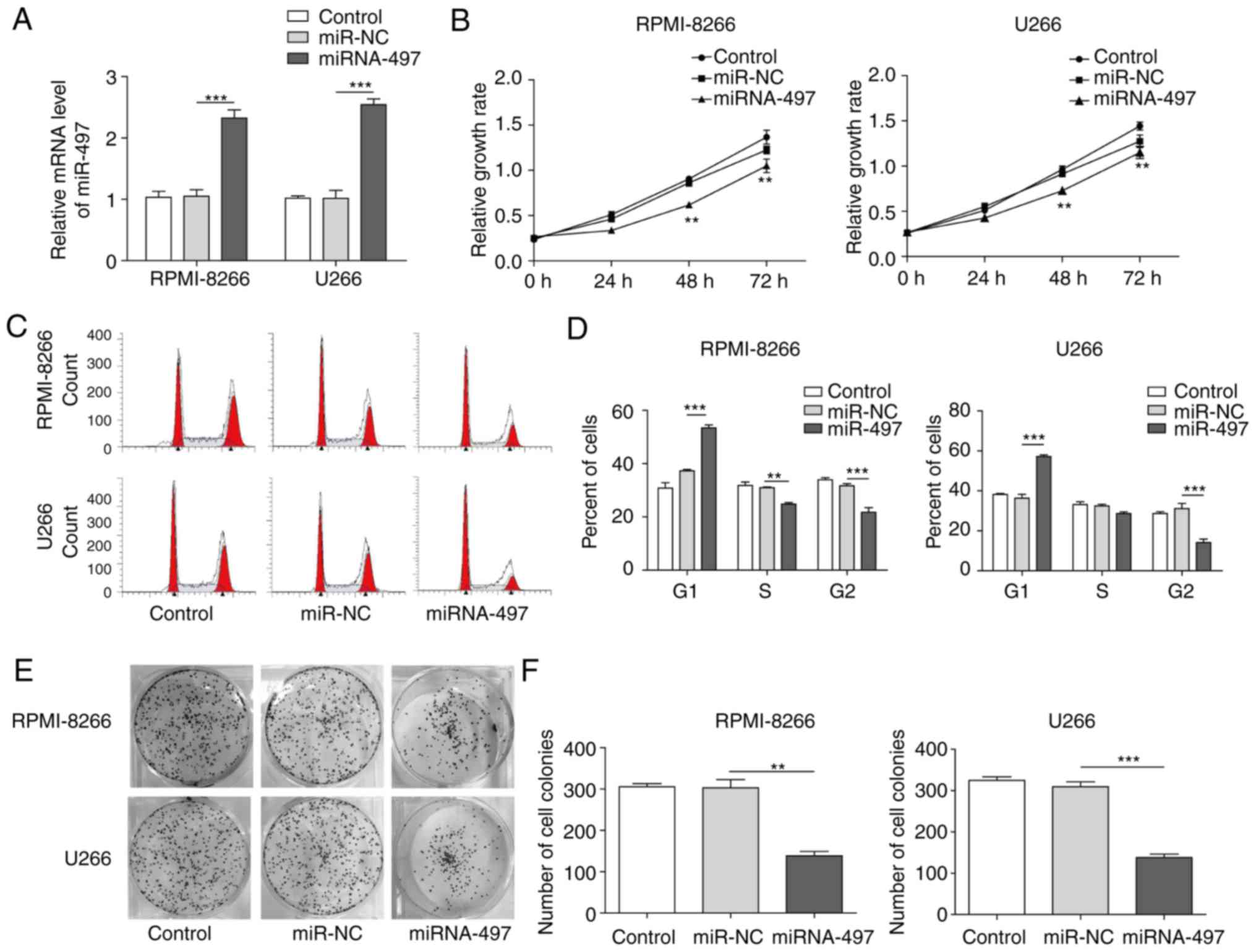
Ectopic overexpression of miR-497
inhibits cell proliferation of MM
It was previously reported that Bcl-2 was closely
associated with myeloma cell proliferation (3,5,15).
To investigate the effects of miR-497 on myeloma cell
proliferation, miR-497 mimic (miR-497) or the matched negative
control (miR-NC) were transfected into the RPMI-8266 and U266 MM
cell lines to overexpress miR-497 (Fig. 2A). The cell viability and cell
cycle were then analyzed through an MTT assay and flow-cytometry,
respectively. The results revealed that the overexpression of
miR-497 in RPMI-8266 and U266 cells led to decreased cell viability
(Fig. 2B) together with altered
cell cycle with the majority of cells arrested at the
G0/G1 phase (Fig. 2C and D).
The soft-agar colony formation assay is another
common assay to examine cell proliferation (28,31). In the RPMI-8266 and U266 cells,
the number of colonies in the group overex-pressing miR-497 was
markedly decreased compared with the miR-NC and control groups
(Fig. 2E). Statistical analysis
of the colony numbers (Fig. 2F)
also indicated that miR-497 disrupted clone formation of MM
cells.
Taken together, the overexpression of miR-497
inhibited MM cell proliferation through cell cycle arrest, and
reductions in viability and colony formation ability.
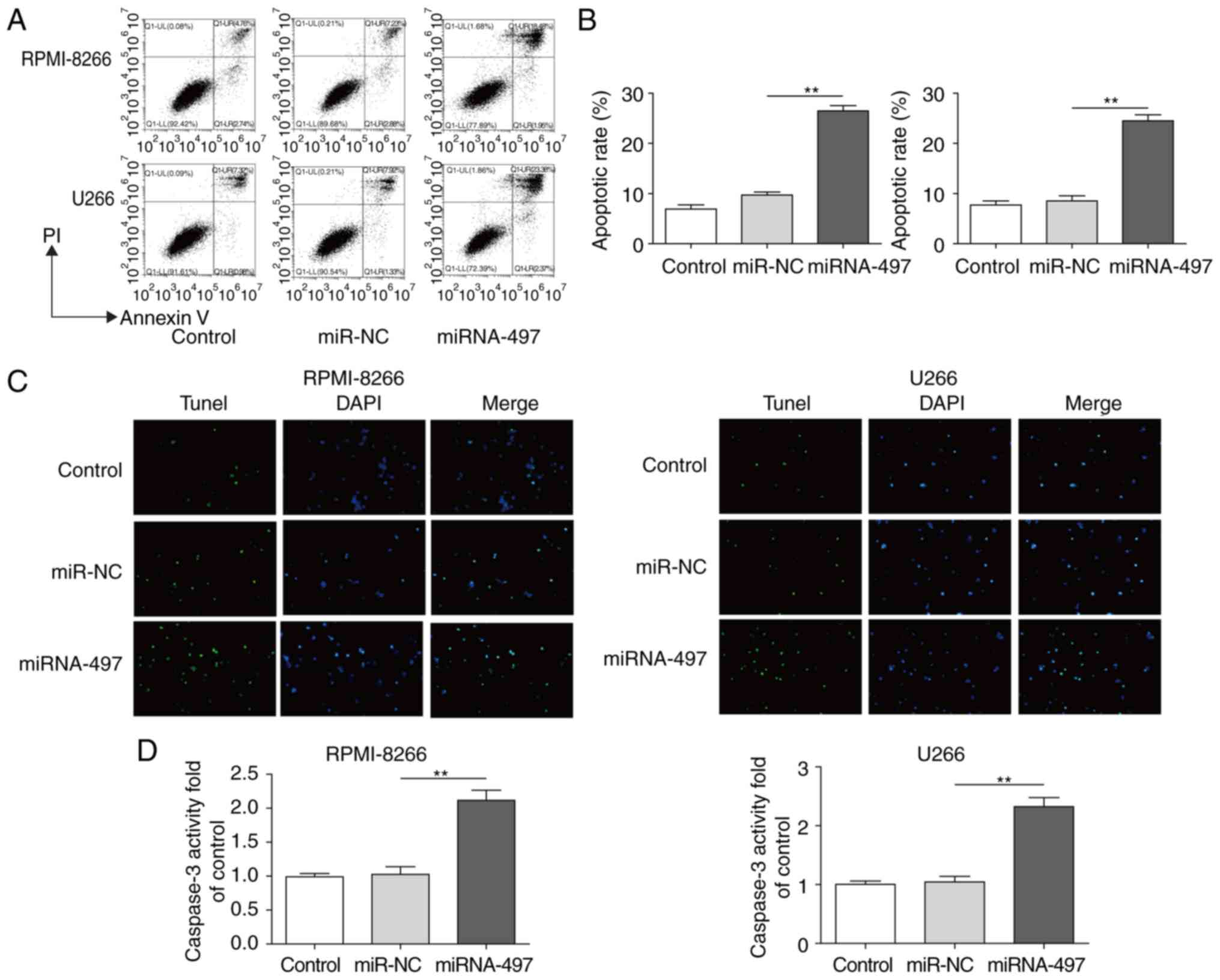
Overexpression of miR-497 induces MM
apoptosis
It was hypothesized that reduced cell viability may
be caused by increased apoptosis (32-34), therefore, subsequent assays of the
apoptotic patterns of cells with ectopic miR-497 were performed.
Cell apoptosis was analyzed by Annexin V/FACS and TUNEL assays in
RPMI-8266 and U266 cells transfected with or without miR-497. The
cells with ectopic miR-497 exhibited increased apoptosis (~2.5
fold) according to FACS results (Fig.
3A and B) and TUNEL staining (Fig. 3C). To confirm these findings, the
caspase-3 activity was also detected as a common marker for
apoptosis (9,31). A consistent result further
suggested that the overexpression of miR-497 induced apoptosis by
markedly increasing the caspase-3 activity (~2.2 fold) (Fig. 3D). These results indicated that
miR-497 promoted MM cell apoptosis.
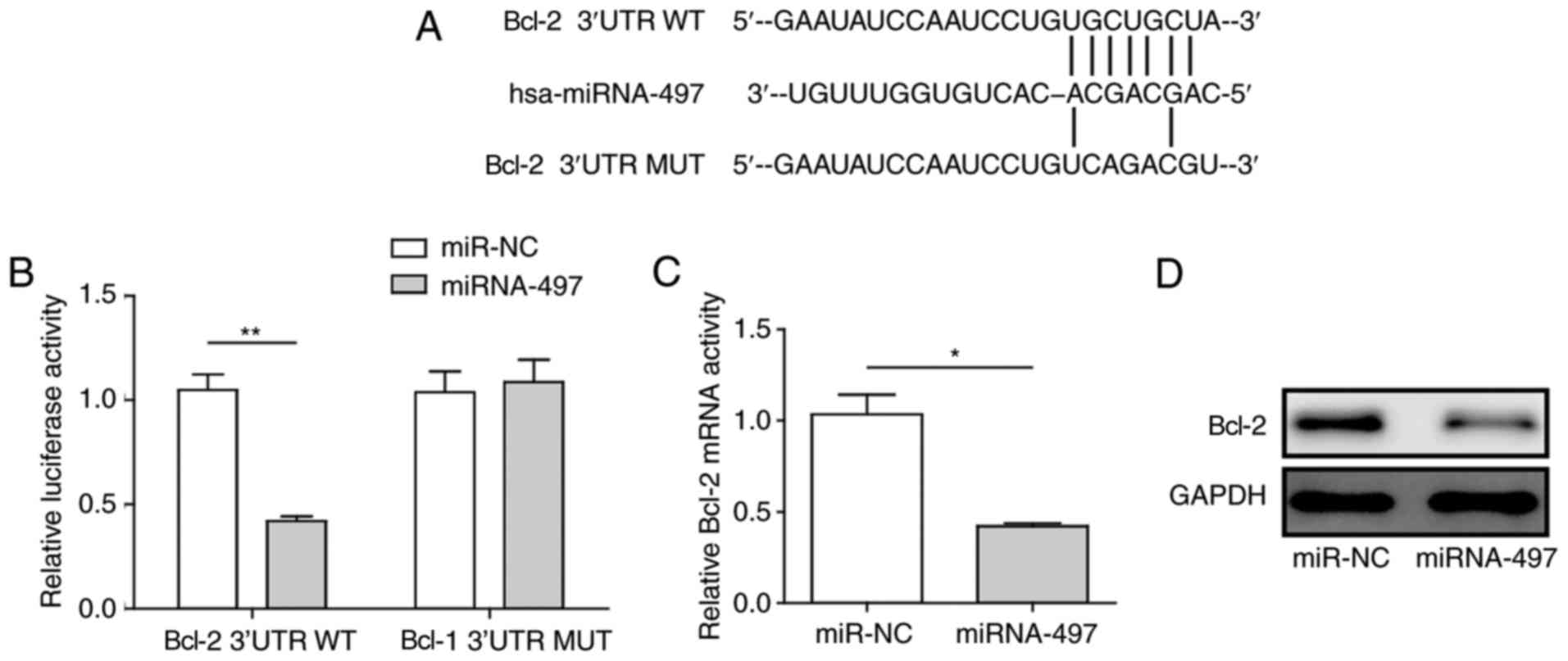
miR-497 directly targets Bcl-2
As a close correlation between Bcl-2 and miR-497 was
described in previous reports (20,23), the present study examined whether
miR-497 directly targeted the expression of Bcl-2. For this, the
Bcl-2 3′UTR and miR-497 sequences were analyzed by bioinformatics
software, including TargetScan, miRTarBase, RNAhybrid, and a strong
interaction region was found (Fig.
4A). To create an miR-497-insensitive Bcl-2 3′UTR, mutations
were introduced into the binding sites (Fig. 4A), and inserted into a luciferase
reporter gene plasmid, in addition to the wild-type Bcl-2 3′UTR. A
dual-luciferase assay was performed to verify the interaction
between miR-497 and Bcl-2. The results revealed that miR-497
significantly decreased the activity of luciferase with the
wild-type Bcl-2 3′UTR, but did not affect the activity of
luciferase with the mutated Bcl-2 3′UTR (Fig. 4B), suggesting that Bcl-2 serves as
a direct target of miR-497, and the interaction site is just as the
prediction. To confirm this finding, the present study detected the
expression levels of Bcl-2 by RT-qPCR and western blot analyses
following miR-497 or miR-NC treatment. The mRNA and protein levels
of Bcl-2 were markedly decreased by the overexpression of miR-497,
with ~3-fold inhibition (Fig. 4C and
D).
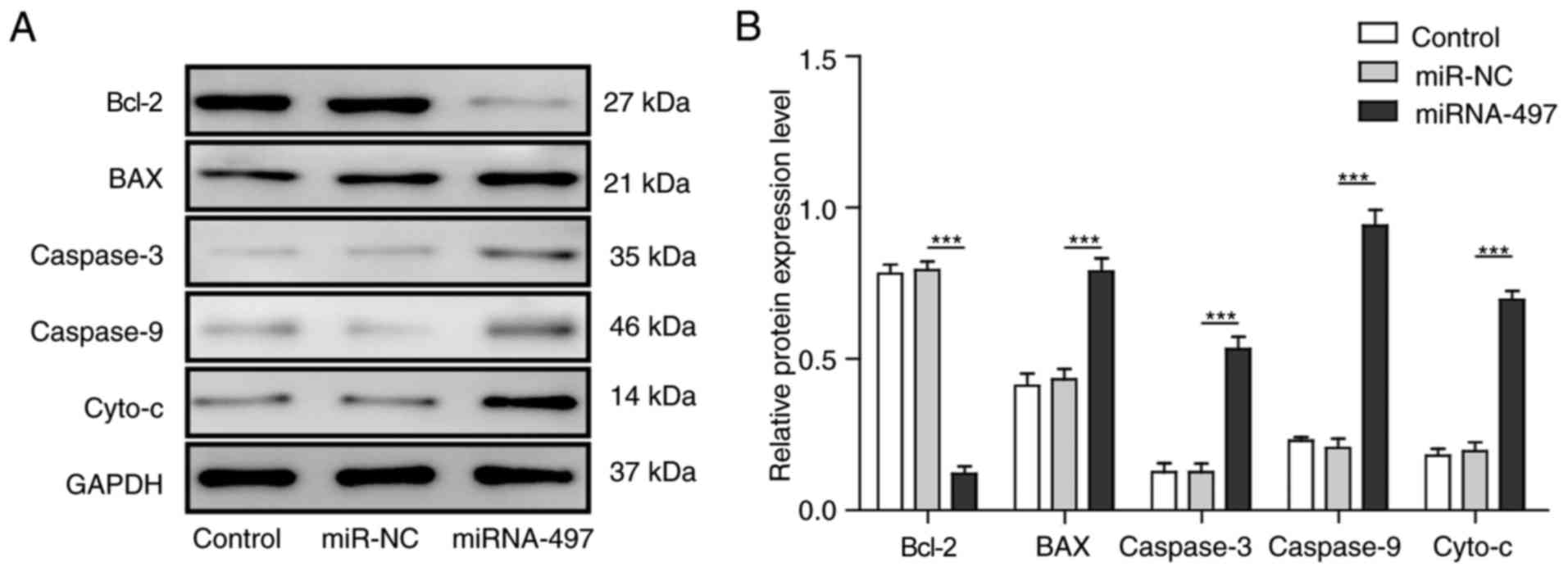
miR-497 regulates the expression of
downstream apoptotic-related proteins by directly targeting
Bcl-2
Aside from the anti-apoptotic gene Bcl-2, other
apoptotic-related proteins also closely associated with Bcl-2 and
cell apoptosis. The present study detected the expression of
several well-known pro-apoptotic proteins, including
Bcl-2-associated X protein (BAX), caspase-3, caspase-9 and
cytochrome c (cyto-c) by western blot analysis. All of these
proteins were upregulated markedly by the overexpression of miR-497
(Fig. 5A and B).
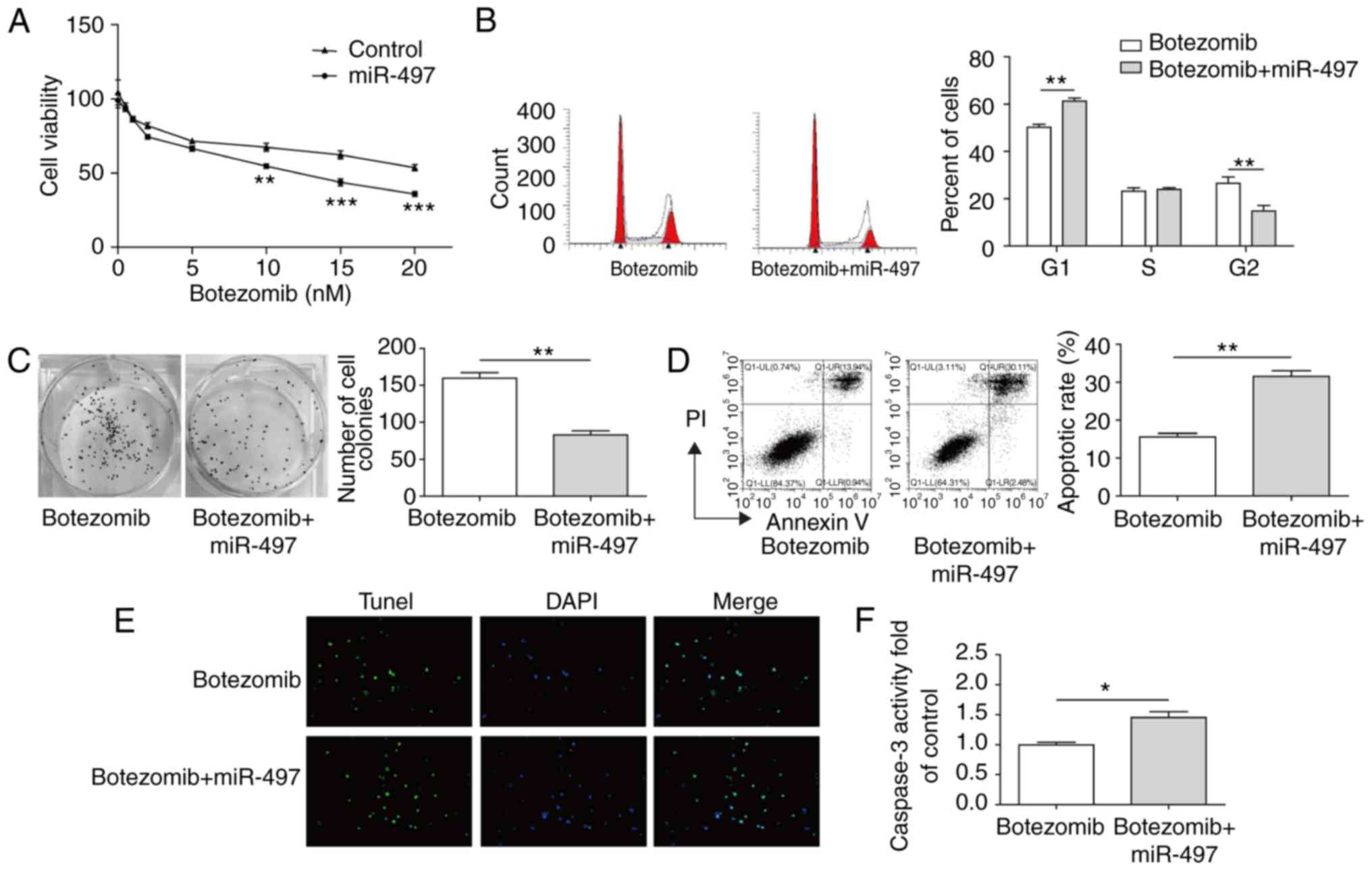
miR-497 enhanced bortezomib
chemosensitivity in MM cells
Bortezomib is a conventional clinical medication in
MM therapy; however, bortezomib-resistance can also occur
intrinsically or can be acquired in several patients (25). According to the findings of the
present study, miR-497 inhibited proliferation and promoted
apoptosis of MM cells, indicating that miR-497 may have a potential
synergetic effect or sensitization to bortezomib. To confirm this
hypothesis, RPMI-8226 and U266 cells, which were have been shown to
be more resistant to bortezomib compared with other MM cell lines
(26,27), were used. Although the cell
viability was decreased when treated with bortezomib treatment
alone, a lower level was detected following treatment with ≥10 nM
bortezomib combined with miR-497 (Fig. 6A). Subsequently, 10 nM bortezomib
treatment for 24 h was selected for the subsequent experiments to
analyze cell cycle, colony formation ability and apoptosis. The
results showed that the cell cycle arrest was enhanced by the
combination of miR-497 and botezomib in the RPMI-8226 and U266
cells (Fig. 6B); the proportion
of cells in the G0/G1 phase increased from
54.12 to 76.25% when the cells were treated with the addition of
miR-497. The colony formation ability was also decreased by the
combination of miR-497 and bortezomib, with 50% inhibition compared
with bortezomib treatment alone (Fig.
6C). The apoptotic signature analysis by Annexin V/FACS and
TUNEL assays demonstrated that miR-497 promoted bortezomib-induced
MM cell apoptosis (Fig. 6D and
E). As another vital hallmark of apoptosis, the activity of
caspase-3 was further detected and, as expected, miR-497 enhanced
the activity of caspase-3 when co-treated with bortezomib (Fig. 6F). Taken together, these results
led to the conclusion that miR-497 enhanced the chemosensitivity of
MM cells to bortezomib, and may serve as a potential therapeutic
candidate in combination with bortezomib.
Discussion
MM comprises up to 10% of all hematological
malignancies, ranking second to leukemia. The susceptibility to MM
increases with aging. With average age increasing in China, the
number of patients with MM also increases each year (1). At present, there is no completely
effective cure for MM, therefore, investigating the molecular
mechanism of MM is important (1).
miR-497 is closely associated with multiple types of cancer,
including breast cancer, ovarian cancer and glioma, by regulating
proliferation, apoptosis and cell cycle (11-17). The investigation of miR-497 in MM
may well provide potentially novel therapeutic ideas. The findings
of the present study revealed decreased expression levels of
miR-497 in MM tissues and cell lines, compared with those in
control groups, supporting this potential.
By transfecting MM cells with miR-497 or miR-NC, the
viability of the two MM cell lines decreased with statistical
significance, as expected, in addition to the cell cycle arrest and
reduced colony formation ability effects of miR-497 on MM cells.
These biological processes are closely associated with
carcinogenesis, and the inhibitory effect of miR-497 on these
processes may lead to decreased cancer burden. The analysis of
apoptotic features by TUNEL assays and Annexin V flow cytometry
verified that miR-497 increased the apoptosis of MM cells. These
results confirmed the hypothesis that miR-497 can have a
therapeutic role against MM.
The present study then aimed to reveal the
underlying mechanism of miR-497 on growth inhibition of MM cells.
The first and most important step is to reveal proteins targeted by
miR-497. Sequencing analyzing indicated that Bcl-2 may be a
potential miR-497 target (22,23), and this was validated by decreased
mRNA and protein levels in MM cells transfected with miR-497. Bcl-2
is a master regulatory factor in apoptotic signaling pathways,
which may regulate other apoptotic proteins levels, including BAX,
caspase-3 and caspase-9. The expression of these apoptosis-related
proteins was altered along with Bcl-2 with the overexpression of
miR-497. These findings provide evidence that miR-497 directly
targeted Bcl-2 and modulated apoptotic signaling in MM. Therefore,
this may explain how miR-497 inhibited MM cell viability.
In consideration of the inhibitory effect of miR-497
on MM cell proliferation and viability, the present study
investigated whether it can be used in MM therapy by combination
with conventional medication. Bortezomib is a common drug used in
MM treatment and has been shown to promote cell death of malignant
plasma cells (25). The
combination of miR-497 and bortezomib in MM cells confirmed a
marked susceptibility increase for bortezomib treatment in the
present study. In the miR-497-overexpressing groups, bortezomib
administration resulted in more marked viability inhibition, more
severe cell cycle arrest, increased apoptosis and more marked
colony formation ability loss, compared with the miR-NC groups.
These findings suggested the therapeutic effect was superior when
miR-497 and bortezomib were combined together against MM cells.
The present study revealed the function of miR-497
in MM; miR-497 was downregulated in MM tissues and cells, and the
overexpression of miR-497 induced MM cell apoptosis by inhibiting
cell proliferation and viability. In addition, the present study
revealed the underlying mechanism of miR-497 by directly targeting
and inhibiting the expression of Bcl-2, upregulating the expression
of pro-apoptotic genes and finally promoting apoptosis. When the
combination treatment of bortezomib with miR-497 was used, enhanced
chemosensitivity was readily detected in MM cells. These findings
may assist in investigating the mechanisms of MM and provide novel
potential therapeutic methods for future clinical applications.
Funding
The present study was funded by the Science and
Technology R&D Fund of Shenzhen (grant no.
JCYJ20170307104838077), the Shenzhen Longgang District Science and
Technology Development Fund (grant no. YLWS 20150514150041453) and
the National Natural Science Foundation of China (grant no.
81472275).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors’ contributions
YZ and WZ performed the cell culture, drugs
treatment, flow cytometry and TUNEL analysis, and were major
contributors in writing the manuscript. JL performed the collection
and management of clinic specimens. MT and XC performed the dual
luciferase reporter assays and molecular expression detections. JJ
analyzed the data. FT designed the experiments, revised the paper
and submitted the final versions. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The use of tissue specimens was based on an informed
and voluntary principle, and was reviewed and approved by the
Ethics Committee of Longgang District People’s Hospital of
Shenzhen.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Abdi J, Rastgoo N, Li L, Chen W and Chang
H: Role of tumor suppressor p53 and micro-RNA interplay in multiple
myeloma pathogenesis. J Hematol Oncol. 10:1692017. View Article : Google Scholar
|
|
2
|
Ahmad N, Haider S, Jagannathan S, Anaissie
E and Driscoll JJ: MicroRNA theragnostics for the clinical
management of multiple myeloma. Leukemia. 28:732–738. 2014.
View Article : Google Scholar
|
|
3
|
Troppan K, Wenzl K, Pichler M, Pursche B,
Schwarzenbacher D, Feichtinger J, Thallinger GG, Beham-Schmid C,
Neumeister P and Deutsch A: miR-199a and miR-497 are associated
with better overall survival due to increased chemosensitivity in
diffuse large β-cell lymphoma patients. Int J Mol Sci.
16:18077–18095. 2015. View Article : Google Scholar :
|
|
4
|
Rastgoo N, Abdi J, Hou J and Chang H: Role
of epigenetics-microRNA axis in drug resistance of multiple
myeloma. J Hematol Oncol. 10:1212017. View Article : Google Scholar
|
|
5
|
Sevcikova S, Kubiczkova L, Sedlarikova L,
Slaby O and Hajek R: Serum miR-29a as a marker of multiple myeloma.
Leuk Lymphoma. 54:189–191. 2013. View Article : Google Scholar
|
|
6
|
Fang T, Wu Q, Zhou L, Mu S and Fu Q:
miR-106b-5p and miR-17-5p suppress osteogenic differentiation by
targeting Smad5 and inhibit bone formation. Exp Cell Res.
347:74–82. 2016. View Article : Google Scholar
|
|
7
|
Xu D, Gao Y, Hu N, Wu L and Chen Q:
miR-365 Ameliorates dexamethasone-induced suppression of
osteogenesis in MC3T3-E1 cells by targeting HDAC4. Int J Mol Sci.
18:2017. View Article : Google Scholar
|
|
8
|
Tang CM, Zhang M, Huang L, Hu ZQ, Zhu JN,
Xiao Z, Zhang Z, Lin QX, Zheng XL, Yang M, et al: CircRNA_000203
enhances the expression of fibrosis-associated genes by
derepressing targets of miR-26b-5p, Col1a2 and CTGF, in cardiac
fibroblasts. Sci Rep. 7:403422017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhai Y, Tyagi SC and Tyagi N: Cross-talk
of MicroRNA and hydrogen sulfide: A novel therapeutic approach for
bone diseases. Biomed Pharmacother. 92:1073–1084. 2017. View Article : Google Scholar
|
|
10
|
Kang H, Chen H, Huang P, Qi J, Qian N,
Deng L and Guo L: Glucocorticoids impair bone formation of bone
marrow stromal stem cells by reciprocally regulating
microRNA-34a-5p. Osteoporos Int. 27:1493–1505. 2016. View Article : Google Scholar
|
|
11
|
Lu Y, Li F, Xu T and Sun J: miRNA-497
Negatively regulates the growth and motility of chondrosarcoma
cells by targeting Cdc25A. Oncol Res. 23:155–163. 2016. View Article : Google Scholar
|
|
12
|
Kong XJ, Duan LJ, Qian XQ, Xu D, Liu HL,
Zhu YJ and Qi J: Tumor-suppressive microRNA-497 targets IKKbeta to
regulate NF-kappaB signaling pathway in human prostate cancer
cells. Am J Cancer Res. 5:1795–804. 2015.
|
|
13
|
Lin Z, Zhao J, Wang X, Zhu X and Gong L:
Overexpression of microRNA-497 suppresses cell proliferation and
induces apoptosis through targeting paired box 2 in human ovarian
cancer. Oncol Rep. 36:2101–2107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ge L, Zheng B, Li M, Niu L and Li Z:
MicroRNA-497 suppresses osteosarcoma tumor growth in vitro and in
vivo. Oncol Lett. 11:2207–2212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei C, Luo Q, Sun X, Li D, Song H, Li X,
Song J, Hua K and Fang L: MicroRNA-497 induces cell apoptosis by
negatively regulating Bcl-2 protein expression at the
posttranscriptional level in human breast cancer. Int J Clin Exp
Pathol. 8:7729–7739. 2015.
|
|
16
|
Shao XJ, Miao MH, Xue J, Xue J, Ji XQ and
Zhu H: The down-regulation of MicroRNA-497 contributes to cell
growth and cisplatin resistance through PI3K/Akt pathway in
osteo-sarcoma. Cell Physiol Biochem. 36:2051–2062. 2015. View Article : Google Scholar
|
|
17
|
Li W, Jin X, Deng X, Zhang G, Zhang B and
Ma L: The putative tumor suppressor microRNA-497 modulates gastric
cancer cell proliferation and invasion by repressing eIF4E. Biochem
Biophys Res Commun. 449:235–240. 2014. View Article : Google Scholar
|
|
18
|
Yu T, Zhang X, Zhang L, Wang Y, Pan H, Xu
Z and Pang X: MicroRNA-497 suppresses cell proliferation and
induces apoptosis through targeting PBX3 in human multiple myeloma.
Am J Cancer Res. 6:2880–2889. 2016.
|
|
19
|
Lionetti M, Musto P, Di Martino MT, Fabris
S, Agnelli L, Todoerti K, Tuana G, Mosca L, Gallo Cantafio ME,
Grieco V, et al: Biological and clinical relevance of miRNA
expression signatures in primary plasma cell leukemia. Clin Cancer
Res. 19:3130–3142. 2013. View Article : Google Scholar
|
|
20
|
Wu R, Tang S, Wang M, Xu X, Yao C and Wang
S: MicroRNA-497 induces apoptosis and suppresses proliferation via
the Bcl-2/Bax-Caspase9-Caspase3 pathway and cyclin D2 protein in
HUVECs. PLoS One. 11:e01670522016. View Article : Google Scholar :
|
|
21
|
Zhu W, Zhu D, Lu S, Wang T, Wang J, Jiang
B, Shu Y and Liu P: miR-497 modulates multidrug resistance of human
cancer cell lines by targeting BCL2. Med Oncol. 29:384–391. 2012.
View Article : Google Scholar
|
|
22
|
Shen L, Li J, Xu L, Ma J, Li H, Xiao X,
Zhao J and Fang L: miR-497 induces apoptosis of breast cancer cells
by targeting Bcl-w. Exp Ther Med. 3:475–480. 2012. View Article : Google Scholar
|
|
23
|
Yadav S, Pandey A, Shukla A, Talwelkar SS,
Kumar A, Pant AB and Parmar D: miR-497 and miR-302b regulate
ethanol-induced neuronal cell death through BCL2 protein and cyclin
D2. J Biol Chem. 286:37347–37357. 2011. View Article : Google Scholar
|
|
24
|
Zhu D, Tu M, Zeng B, Cai L, Zheng W, Su Z
and Yu Z: Up-regulation of miR-497 confers resistance to
temozolomide in human glioma cells by targeting mTOR/Bcl-2. Cancer
Med. 6:452–462. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gandolfi S, Laubach JP, Hideshima T,
Chauhan D, Anderson KC and Richardson PG: The proteasome and
proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev.
36:561–584. 2017. View Article : Google Scholar
|
|
26
|
Huang J, Zhou Y, Thomas GS, Gu Z, Yang Y,
Xu H, Tricot G and Zhan F: NEDD8 inhibition overcomes CKS1B-Induced
drug resistance by upregulation of p21 in multiple myeloma. Clin
Cancer Res. 21:5532–5542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Campanella A, Santambrogio P, Fontana F,
Frenquelli M, Cenci S, Marcatti M, Sitia R, Tonon G and Camaschella
C: Iron increases the susceptibility of multiple myeloma cells to
bortezomib. Haematologica. 98:971–979. 2013. View Article : Google Scholar :
|
|
28
|
Florine EM, Miller RE, Porter RM, Evans
CH, Kurz B and Grodzinsky AJ: Effects of dexamethasone on
mesenchymal stromal cell chondrogenesis and aggrecanase activity:
Comparison of agarose and Self-Assembling peptide scaffolds.
Cartilage. 4:63–74. 2013. View Article : Google Scholar
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
30
|
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee
DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acids Res. 33:1792005. View Article : Google Scholar
|
|
31
|
Wang YG, Han XG, Yang Y, Qiao H, Dai KR,
Fan QM and Tang TT: Functional differences between AMPK alpha1 and
alpha2 subunits in osteogenesis, osteoblast-associated induction of
osteoclastogenesis, and adipogenesis. Sci Rep. 6:327712016.
View Article : Google Scholar
|
|
32
|
Song L, Zhao J, Zhang X, Li H and Zhou Y:
Icariin induces osteoblast proliferation, differentiation and
mineralization through estrogen receptor-mediated ERK and JNK
signal activation. Eur J Pharmacol. 714:15–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun J, Yan P, Chen Y, Chen Y, Yang J, Xu
G, Mao H and Qiu Y: MicroRNA-26b inhibits cell proliferation and
cytokine secretion in human RASF cells via the
Wnt/GSK-3beta/beta-catenin pathway. Diagn Pathol. 10:722015.
View Article : Google Scholar
|
|
34
|
Wang L, Jiang CF, Li DM, Ge X, Shi ZM, Li
CY, Liu X, Yin Y, Zhen L, Liu LZ and Jiang BH: MicroRNA-497
inhibits tumor growth and increases chemosensitivity to
5-fluorouracil treatment by targeting KSR1. Oncotarget.
7:2660–2671. 2016.
|