Introduction
Solar ultraviolet (UV) radiation is the primary
source of environmental damage for human skin. UV radiation is
divided into two spectral regions: UVA (320-400 nm) and UVB
(290-320 nm) constituting 96 and 4%, respectively, and each are
responsible for UV overexposure-associated skin pathologies,
including premature skin aging and skin cancer (1). Accordingly, UVA, which penetrates
the dermal layers of skin more deeply compared with UVB, serves a
principal role in these skin pathologies by generating reactive
oxygen species (ROS) in the resident dermal fibroblasts and
extracellular structures (2). A
growing body of evidence additionally indicates that the
overexposure of skin cells to UV rays triggers various cellular
alterations, including cell cycle arrest, cell membrane disruption
and nuclear DNA damage, leading to skin cell loss and/or apoptosis
(3-6). Therefore, there is an urgent
requirement to identify novel substances that may be potential
skin-improving and cosmeceutical materials against UV insult, and
may inhibit or lessen the cellular alterations triggered by UV
overexposure.
Apoptosis is the process of programmed cell death,
which involves a series of morphological and biochemical
alterations, including cell detachment, cell shrinkage and nuclear
DNA fragmentation (7,8). This process is regulated by the
action of a variety of proteins and/or factors. For example,
activation of the caspase family members, including caspase-9,
caspase-8, and caspase-3, is crucial for apoptosis induction in
UV-irradiated dermal fibroblasts or keratinocytes (9-11).
In addition, members of the B-cell lymphoma-2 (Bcl-2) family,
including Bcl-2 and Bcl-2-associated X protein (Bax), are involved
in UV-induced apoptosis of dermal fibroblasts or keratinocytes
(10,12). It is known that Bcl-2, as an
anti-apoptotic protein, and Bax, as a pro-apoptotic protein, have
important roles in competitively regulating the mitochondrial
membrane integrity and the mitochondria-initiated caspase
activation pathway (13). In
addition, previous studies have demonstrated that a decrease or
loss of the mitochondrial membrane potential (ΔΨm) is an early
event of apoptosis and cytochrome c release from the
mitochondria to the cytosol additionally contributes to apoptosis
induction by activating members of the caspase family, which
cleaves critical proteins for cell survival and growth (14,15).
The genus Stachys is comprised of ~300
species worldwide, representing one of the largest genera of the
Lamiaceae. Importantly, a number of Stachys species have
been exploited in traditional medicine as astringent,
wound-healing, anti-diarrheal, anti-nephritic and anti-inflammatory
agents (16-18). Notably, there is additionally
evidence demonstrating an anti-allergic effect of the aqueous
extract of Stachys riederi var. japonica (19). However, at present, the
antioxidant and cytoprotective effects of Stachys riederi
var. japonica on dermal cells remain unclear.
In the present study, the antioxidant and
cytoprotective effects of Stachys riederi var.
japonica ethanol extract (SREE) on UVA-irradiated human
dermal fibroblasts (HDFs) were investigated. To the best of our
knowledge, this is the first study to suggest that SREE has
antioxidant and cytoprotective effects on UVA-irradiated HDFs,
which may be mediated through regulation of ROS production, the
ΔΨm, expression of Bcl-2 and Bax, caspase-3 activity and
apoptosis.
Materials and methods
Materials and apparatus
Dimethyl sulfoxide (DMSO; cat. no. D8418), ascorbic
acid (AA; cat. no. A5960) and MTT (cat. no. M2128) were purchased
from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Dulbecco's
modified Eagle's medium (DMEM; cat. no. BE12-604F) and
penicillin/streptomycin (P/S) cocktail (cat. no. BW17-718R) were
purchased from Lonza Group Ltd., (Basel, Switzerland). Fetal bovine
serum (FBS; cat. no. S001-01) was purchased from WELGENE, Inc.,
(Gyeongsan, Republic of Korea). The anti-Bcl-2 (cat. no. ab7973)
and anti-Bax (cat. no. ab7977) primary antibodies were obtained
from Abcam (Cambridge, MA, USA). The anti-β-actin (cat. no.
sc-81178) primary antibody was obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Horseradish peroxidase
(HRP)- conjugated goat anti-rabbit immunoglobulin G (IgG; H+L; cat.
no. 111-035-045) and goat anti-mouse IgG (H+L; cat. no.
115-035-062) secondary antibodies were purchased from Jackson
ImmunoResearch Laboratories, Inc. (West Grove, PA, USA). UV
irradiation was performed with a UVA sunlamp (Sankyo Denki,
Hiratsuka, Japan). An inverted microscope (CKX41; Olympus
Corporation, Tokyo, Japan) was used for observation of cell growth
and a CO2 incubator (MCO-17AC; Sanyo Electric Co., Ltd.,
Osaka, Japan) was used for cell culture. A rotary vacuum
concentrator (BÜCHI R-205; BÜCHI Labortechnik AG, Flawil,
Switzerland) was used for sample extraction.
Preparation of SREE
The root of Stachys riederi var.
japonica was purchased from DONG UI Chosukjam Farmers, Inc.
(Sancheong, Korea). A voucher specimen was deposited in the
herbarium of our laboratory at the Department of Public Health,
Faculty of Food and Health Sciences (Keimyung University, Daegu,
Korea) with the plant identification no. KMU/J/638. A pulverized
sample (50 g) was put into a flask and extracted in 500 ml 80%
ethanol three times for 24 h each at 25°C. The extract was filtered
and concentrated using rotary vacuum evaporator followed by
lyophilization (yield 46.7%).
Cell culture
HDFs (Amore Pacific Company, Seoul, Korea) were
grown in DMEM supplemented with 10% heat-inactivated FBS and 1% P/S
cocktail in a humidified atmosphere of 95% air and 5%
CO2 at 37°C.
Measurement of cell viability
The effect of SREE on the viability of HDFs was
assessed by MTT assay. HDFs were seeded in a 96-well plate
(1×104 cells/100 µl/well) overnight. HDFs were
treated with different concentrations of SREE (25, 50, 100 and 200
µg/ml) for 48 h. Subsequently, MTT (0.5 mg/ml) was added to
each well of the 96-well plate and the cells were incubated at 37°C
for an additional 3 h. The 96-well plate was centrifuged 25°C at
168 × g for 10 min. Following removal of the supernatant, 200
µl DMSO was added to each well. Subsequent to dissolving the
formazan with DSMO in the cells with a plate-shaker for 15 min, the
absorbance at 540 nm was read using an ELISA plate reader.
UVA irradiation
HDFs were cultivated in a culture dish
(1.5×105 cells/ml) until they reached ~80% confluence.
Following removal of the culture medium, HDFs were washed with PBS
and exposed to 6.3 J/cm2 UVA in DMEM without FBS prior
to treatment with SREE or AA.
Measurement of cellular ROS levels
Cellular ROS levels were measured using
dichlorodihydro-fluorescein-diacetate (DCFH-DA). Following UVA
irradiation, HDFs were incubated at 37°C with freshly prepared
pre-warmed 10 mM DCFH-DA for 20 min. HDFs were subsequently rinsed
twice with PBS and levels of green fluorescence, corresponding to
the levels of cellular ROS, were detected through a 520 nm
long-pass filter on an FV-1000 laser fluorescence microscope
(Olympus Corporation; magnification, ×100). The levels of cellular
ROS were quantified by a fluoro-photometer with DataMax version 2.2
and GRAMS/32 software (HORIBA Jobin Yvon, Inc., Edison, NJ, USA),
using an excitation wavelength of 480 nm and an emission wavelength
of 530 nm.
Semi quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
Following treatment, total RNA from the conditioned
HDFs was isolated using TRIzol® (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol. Equal amounts of total RNA (5 µg) were reverse
transcribed in a 40 µl reaction mixture containing 8
µl Molony Murine Leukemia Virus Reverse Transcriptase (M-MLV
RT) 5X buffer, 3 µl 10 mM dNTPs, 0.45 µl 40
U/µl RNase inhibitor, 0.3 µl 200 U/µl M-MLV RT
(Promega Corporation, Madison, WI, USA) and 3.75 µl 20
µM oligo dT (Bioneer Corporation, Daejeon, Korea).
Single-stranded cDNA was amplified by PCR using 4 µl 5X
Green Go-Taq® Flexi reaction buffer, 0.4 µM 10 mM
dNTPs, 0.1 µl 5 U/µl Taq polymerase, 1.2 µl 25
mM MgCl2 (Promega Corporation), and 0.4 µl primer
(20 pM/µl). The primer sequences used for PCR were as
follows: Bcl-2 forward, 5′-CGACGACTTCTCCCGCCGCTACCGC-3′ and
reverse, 5′-CCGCATGCTGGGCCGTACAGTTCC-3′; Bax forward,
5′GGCAATCTGACCTTCAACTG-3′ and reverse, 5′-AGTCTCTTGAGGACCCAACC-3′;
β-actin forward, 5′-CCCACTAACATCAAATGGGG-3′ and reverse,
5′-ACACATTGGGGGTAGGAACA-3′. The PCR thermocycler conditions were as
follows: Bcl-2, 38 cycles of denaturation at 94°C for 1 min,
annealing at 56°C for 90 sec and extension at 72°C for 2 min; Bax,
35 cycles of denaturation at 94°C for 1 min, annealing at 58°C for
1 min and extension at 72°C for 2 min. The expression level of
β-actin mRNA was used as an internal control to evaluate the
relative mRNA expression of Bcl-2 and Bax. PCR products were
visualized using ethidium bromide staining on a 1.2% agarose gel.
DNA band density was semi-quantitatively analyzed using a Kodak Gel
Logic 100 image analysis system with Kodak molecular imaging
software, version 4.0 (Eastman Kodak Company, Rochester, NY,
USA).
Preparation of whole cell lysates
Following treatment, HDFs were washed with PBS and
lysed on ice for 15 min using 0.1 M Tris-HCl (pH 7.2) buffer
containing 1% Nonidet P-40, 0.01% SDS and a protease inhibitor
cocktail (Roche Diagnostics GmbH, Mannheim, Germany). Following
centrifugation at 12,074 × g for 20 min at 4°C, the supernatants
were collected, and protein concentrations were determined by
bicinchoninic acid assay (Pierce; Thermo Fisher Scientific,
Inc.).
Western blot analysis
Equal amounts of protein (10 µg) were
separated by 10% SDS-PAGE and transferred onto nitrocellulose
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
washed with TBS (10 mM Tris-Cl, 150 mM NaCl; pH 7.5) with 0.05%
(v/v) Tween-20 (TBST) followed by blocking for 2 h at 25°C with
TBST containing 5% (w/v) non-fat dried milk. The membranes were
incubated overnight with antibodies specific for Bcl-2 (1:1,000),
Bax (1:500) or β-actin (1:10,000) at 4°C. The membranes were
subsequently exposed to HRP-conjugated secondary antibodies of a
goat anti-rabbit IgG (H+L; 1:2,000) or goat anti-mouse IgG (H+L;
1:2,000) for 2 h at room temperature and additionally washed three
times with TBST. Immunoreactivity was detected using an enhanced
chemiluminescence reagent according to the manufacturer's protocol
(Amersham; GE Healthcare, Chicago, IL, USA). Band intensity was
semi-quantified using ImageJ software (version 1.8.0; National
Institutes of Health, Bethesda, MD, USA). β-actin was used as an
internal control.
Measurement of the ΔΨm
The ΔΨm was visualized by using
5,5′,6,6′-tetrachloro-1,1′3,3′-tetrathylbenzimidazolyl-carbocyanine
iodide (JC-1) staining. JC-1 aggregates and monomers represent high
and low ΔΨm, as indicated by red and green fluorescence,
respectively. Following treatment, HDFs were incubated with 5
µg/ml JC-1 for 30 min at 37°C. JC-1 monomers and JC-1
aggregates were observed at an emission wavelength of 488 nm
(green) and 561 nm (red), respectively. Images were acquired using
an FV-1000 laser fluorescence microscope (Olympus Corporation;
magnification, ×400).
Measurement of cellular caspase-3
activity
Following treatment, caspase-3 activity in HDFs was
determined using a colorimetric caspase-3 assay kit (cat. no.
APT165; EMD Millipore) according to the manufacturer's protocol.
Following UVA irradiation, HDFs were harvested and lysed in cell
lysis buffer (cat. no. 90065) provided in the assay kit. Assays
were performed in 96-well microtiter plates by incubating 20
µg cell lysates in 100 µl reaction buffer (1% NP-40,
20 mM Tris-HCl, pH 7.5, 137 mM NaCl, 10% glycerol) containing a
caspase substrate
(Asp-Glu-Val-Aspchromophore-p-nitroanilide). Lysates were
incubated at 37°C for 90 min. Subsequently, the absorbance at 405
nm was measured with a spectrophotometer.
DNA fragmentation assay
Following treatment, HDFs were washed with PBS and
lysed in a lysis buffer [0.3 M Tris-HCl (pH 7.5), 0.1 M NaCl, 0.01
M EDTA and 0.2 M sucrose] followed by the addition of RNase A (0.5
µg/ml) for an additional 18 h at 55°C. The lysates were
centrifuged at 25°C, 10,000 × g for 20 min, and nuclear DNA in the
supernatant was extracted with an equal volume of neutral
phenol-chloroform-isoamyl alcohol mixture (25:24:1) and analyzed by
electrophoresis on a 1.7% agarose gel. DNA was visualized and
images were captured under UV illumination following staining with
ethidium bromide (0.1 µg/ml). All observations made
concerning this data were made visually.
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. Differences between groups were
evaluated by one-way analysis of variance followed by a Duncan
multiple range test for post-hoc comparison using SPSS 21.0 (IBM
Corp., Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
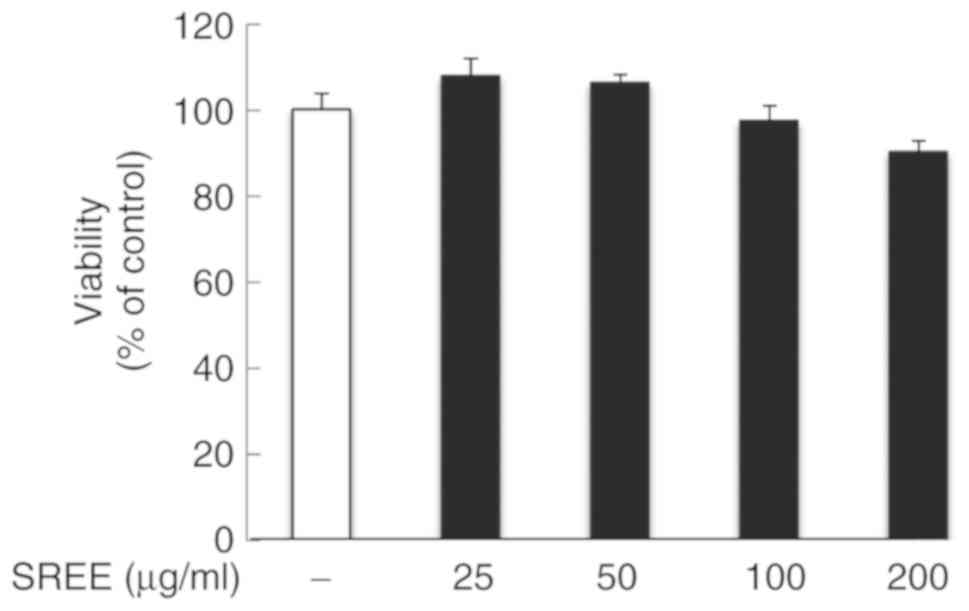
SREE has no or low cytotoxic effects on
HDFs
Initially, the effect of treatment with SREE at
different concentrations for 48 h on the growth of HDFs was
evaluated by an MTT assay. Notably, compared with the control
(vehicle), treatment with SREE at 25 and 50 µg/ml slightly
increased the viability of HDFs (Fig.
1). In addition, treatment with SREE at 100 and 200 µg/m
only decreased the viability of HDFs by 2.7 and 9.9%, respectively.
These results suggest that SREE at the concentrations examined has
no or little cytotoxicity to HDFs.
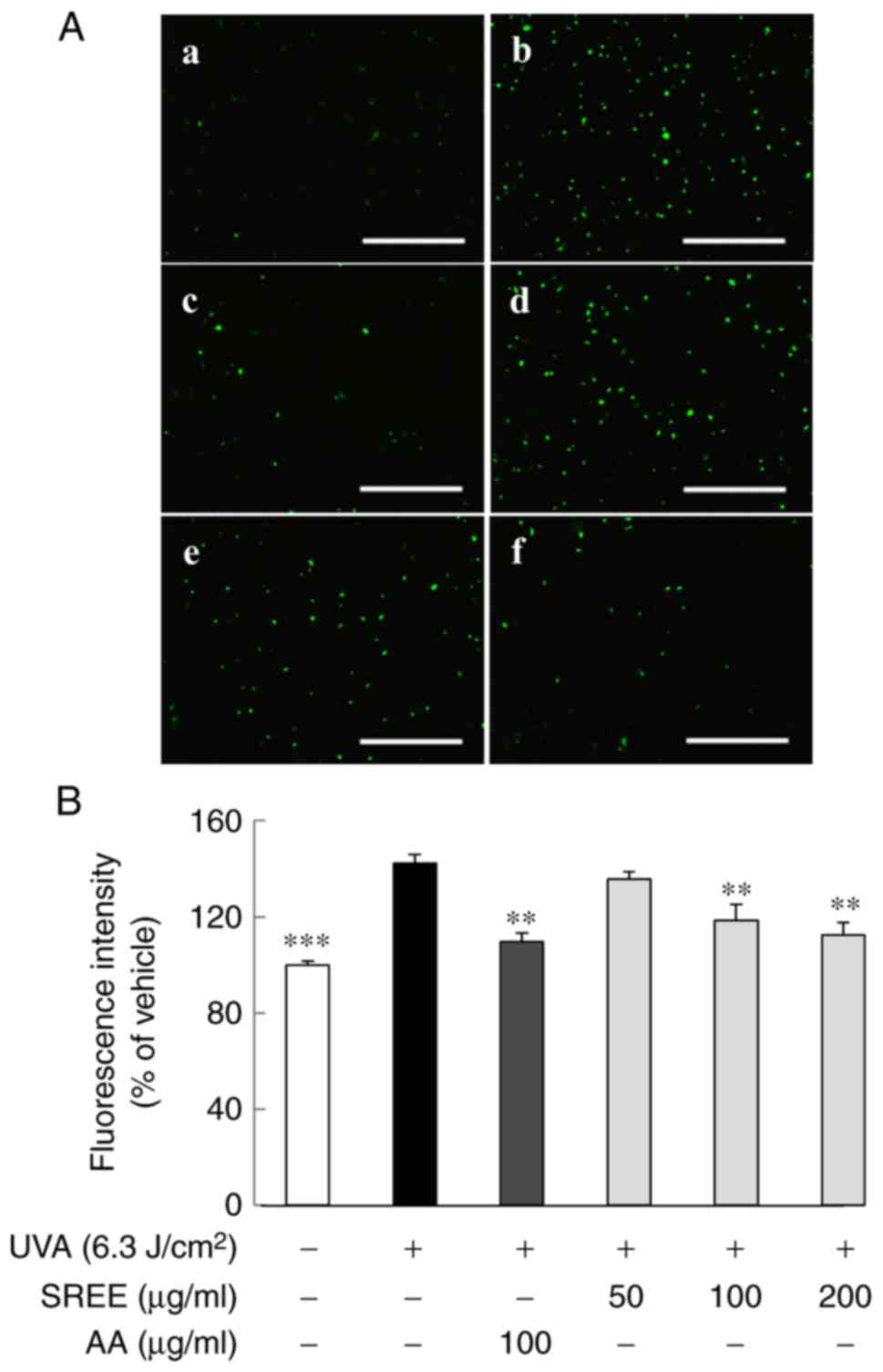
SREE inhibits cellular ROS production in
UVA-irradiated HDFs
To identify any antioxidant activity of SREE, the
effect of treatment with SREE at different concentrations for 24 h
on ROS production in UVA-irradiated HDFs was examined using
fluorescence microscopy. For this, the fluorescein derivative
DCFH-DA, a redox indicator, was used. As demonstrated in Fig. 2A, compared with the control (no
UVA), HDFs exposed to UVA exhibited extensive green fluorescence
staining, indicative of a marked increase in cellular ROS
generation following UVA exposure. However, treatment with SREE led
to a concentration-dependent decrease in green fluorescence
staining in UVA-irradiated HDFs. As hypothesized, 100 µg/ml
treatment with AA, used as an antioxidant positive control,
additionally markedly decreased UVA-induced green fluorescence
staining in HDFs. The quantitative fluorescence intensity is
presented in Fig. 2B. Compared
with UVA-treated HDFs, treatment with SREE at 50, 100 and 200
µg/ml decreased ROS generation by 6.7, 24.0 and 30.1%,
respectively. It was additionally observed that 100 µg/ml AA
treatment decreased ROS generation, similar to the inhibition by
SREE at 200 µg/ml.
SREE inhibits loss of the ΔΨm in
UVA-irradiated HDFs
Given that disruption of the ΔΨm is crucial for
UV-induced apoptosis of human keratinocytes (20), the present study aimed to examine
whether UVA altered the ΔΨm in HDFs, and if SREE was involved,
using fluorescence microscopy. The ΔΨm was visualized by JC-1
staining, in which high and low ΔΨm were indicated by JC-1
aggregates (red) and JC-1 monomers (green), respectively. As
demonstrated in Fig. 3, control
HDFs (no UVA) exhibited marked red staining. However, HDFs that
were exposed to UVA exhibited extensive green staining, suggesting
a decrease of the ΔΨm in UVA-irradiated cells. Notably, while
treatment with SREE at 50 µg/ml had no effect on UVA-induced
decrease of the ΔΨm in HDFs, SREE treatment at 100 or 200
µg/ml led to a concentration-dependent increase of red
staining in the cells. At 100 µg/ml, AA treatment
additionally markedly inhibited the UVA-induced decrease of the ΔΨm
in HDFs.
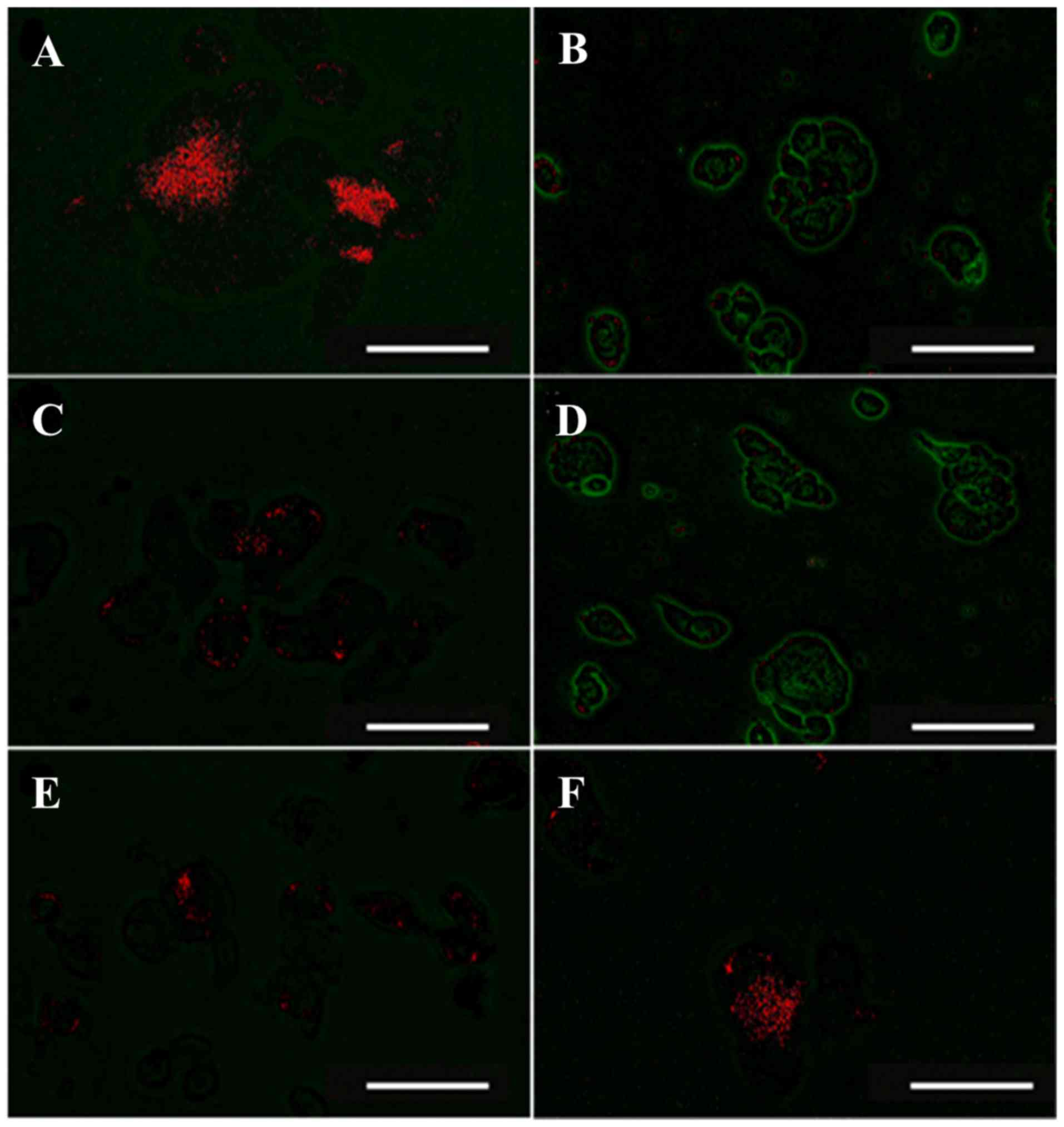 | Figure 3Effect of SREE on the mitochondrial
membrane potential in UVA-irradiated HDFs. Fluorescence detection
of the mitochondrial membrane potential in UVA-irradiated HDFs in
the absence or presence of SREE or AA at the indicated
concentrations for 24 h. (A) no SREE or AA with JC-1. (B) 6.3
J/cm2 UVA with JC-1. (C) 6.3 J/cm2 UVA plus
100 µg/ml AA with JC-1. (D) 6.3 J/cm2 UVA plus 50
µg/ml SREE with JC-1. (E) 6.3 J/cm2 UVA plus 100
µg/ml SREE with JC-1. (F) 6.3 J/cm2 UVA plus 200
µg/ml SREE with JC-1. The mitochondrial membrane potential
staining by JC-1 monomers and JC-1 aggregates is indicative of low
(green) and high (red) mitochondrial membrane potential,
respectively. Representative fluorescent images of the conditioned
cells were detected with Olympus FV-1000 laser fluorescence
microscope. Magnification, ×400 (scale bar, 150 µm). SREE,
Stachys riederi var. japonica ethanol extract; AA,
ascorbic acid; UVA, ultraviolet A; HDFs, human dermal fibroblasts;
JC-1,
5,5′,6,6′-tetrachloro-1,1′3,3′-tetrathylbenzimidazolyl-carbocyanine
iodide. |
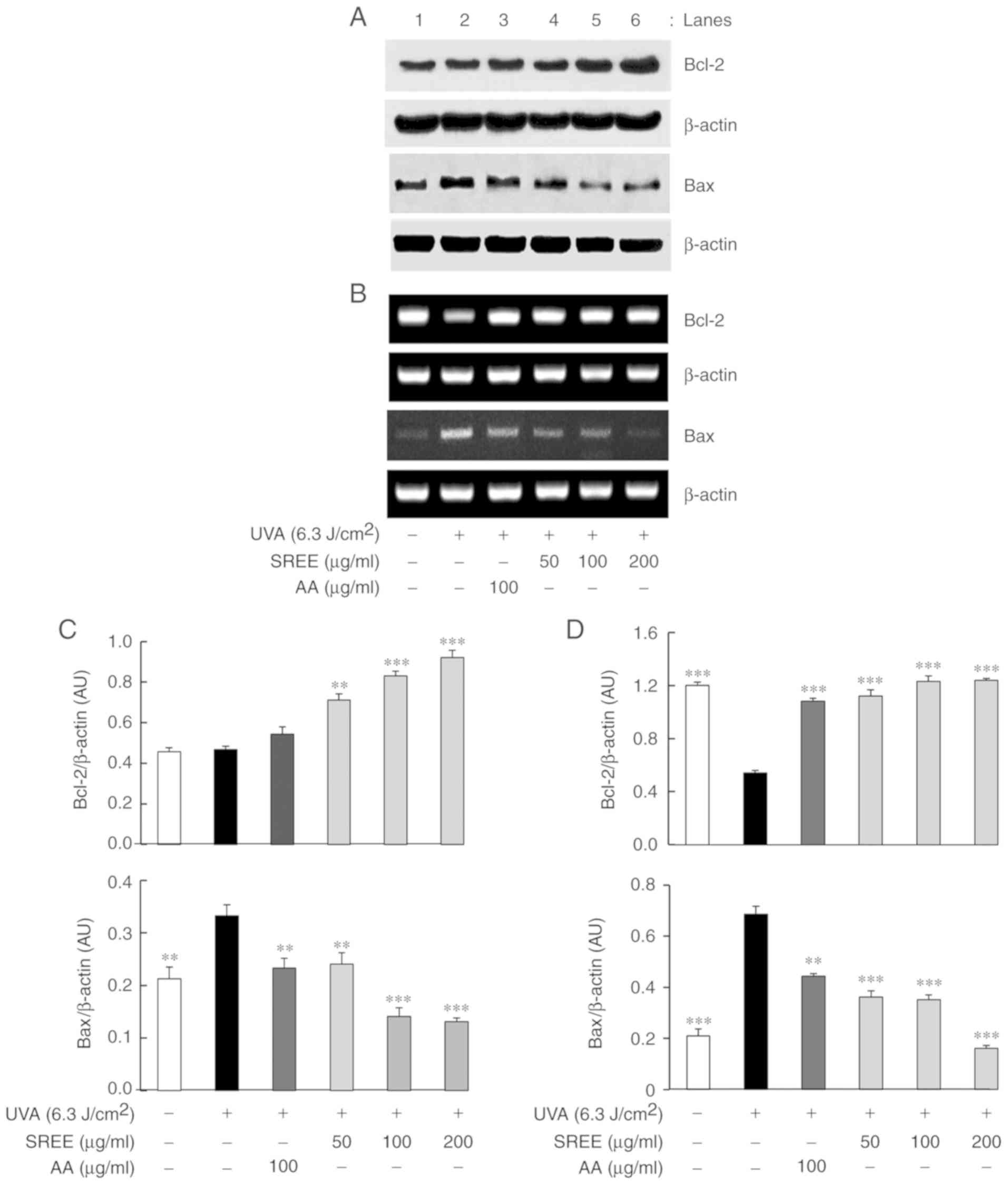
SREE increases protein and mRNA
expression levels of Bcl-2; however, decreases those of Bax in
UVA-irradiated HDFs
The effect of SREE treatment at different
concentrations for 24 h on the protein expression levels of Bcl-2
and Bax, mitochondrial membrane-associated proteins, was
subsequently determined in UVA-irradiated HDFs using western blot
analysis. As demonstrated in Fig.
4A, compared with the control (no UVA), UVA irradiation did not
significantly affect the protein expression of Bcl-2 in HDFs.
However, UVA irradiation markedly increased the protein expression
of Bax in HDFs. Notably, SREE treatment concentration-dependently
increased the protein expression of Bcl-2 in UVA-irradiated HDFs.
In addition, SREE treatment concentration-dependently inhibited the
ability of UVA to increase Bax protein expression in HDFs. At 100
µg/ml, treatment with AA additionally led to a slight
increase in Bcl-2 protein expression and decreased expression
levels of Bax protein in UVA-irradiated HDFs, compared with cells
exposed to only UVA. RT-PCR assays were performed to examine
whether SREE affected mRNA expression levels of Bcl-2 and Bax in
UVA-irradiated HDFs. As presented in Fig. 4B, compared with the control (no
UVA), UVA irradiation markedly downregulated mRNA expression of
Bcl-2 while upregulated that of Bax in HDFs. Notably, SREE
treatment at the concentrations examined markedly attenuated the
ability of UVA to downregulate Bcl-2 mRNA expression in HDFs. In
addition, SREE treatment concentration-dependently inhibited
UVA-induced Bax mRNA upregulation in HDFs. Compared with the cells
exposed to only UVA, AA treatment at 100 µg/ml additionally
markedly inhibited the ability of UVA to decrease the mRNA
expression of Bcl-2 and increase that of Bax in HDFs.
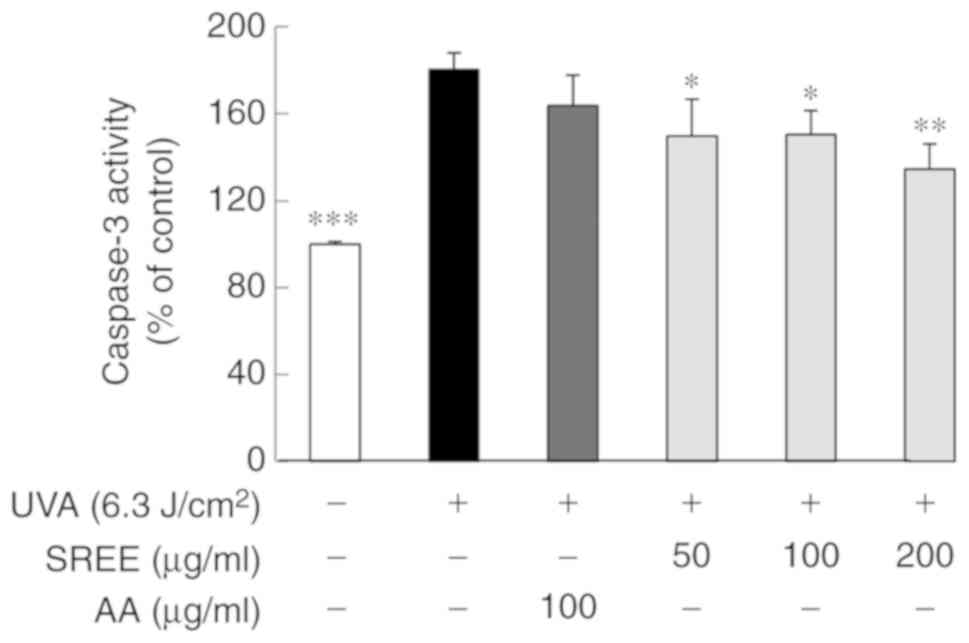
SREE inhibits the activation of caspase-3
in UVA-irradiated HDFs
The effects of SREE treatment at different
concentrations for 24 h on the activity of caspase-3, a member of
the caspase family involved in UV-induced apoptosis (20), was investigated in UVA-irradiated
HDFs. As demonstrated in Fig. 5,
compared with the control (no UVA), UVA irradiation substantially
increased the activity of caspase-3 in HDFs. SREE treatment at the
concentrations examined significantly attenuated the ability of UVA
to activate caspase-3 in HDFs. However, treatment with AA at 100
µg/ml did not significantly inhibit UVA activation of
caspase-3 in HDFs.
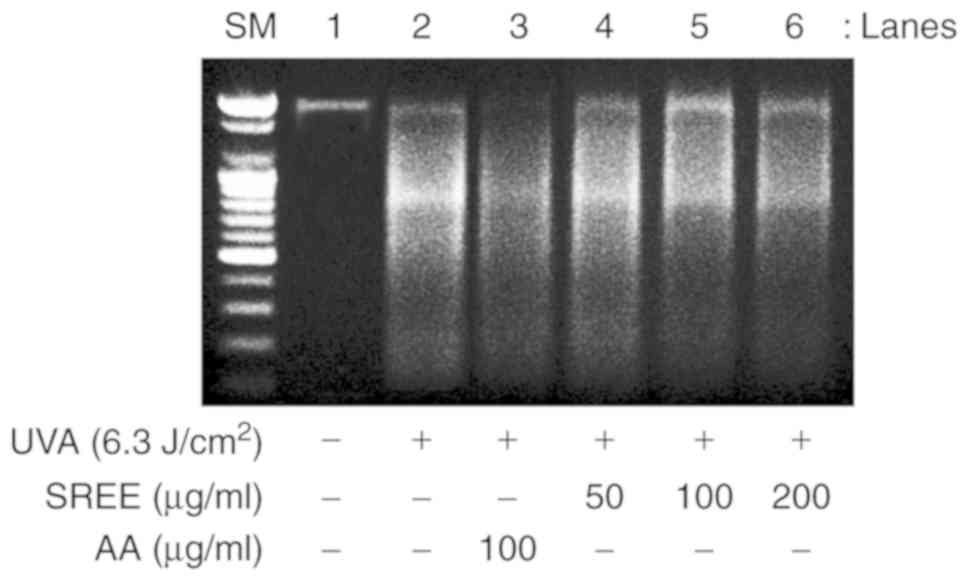
SREE inhibits UVA-induced DNA
fragmentation HDFs
Given that nuclear DNA fragmentation is a hallmark
of apoptosis (7,21), whether UVA induces DNA
fragmentation in HDFs, and whether SREE is involved in the
mechanism, was examined using a DNA fragmentation assay. As
indicated in Fig. 6, compared
with the control (no UVA), UVA irradiation markedly induced DNA
fragmentation in HDFs. Notably, SREE treatment inhibited
UVA-induced DNA fragmentation in HDFs in a concentration-dependent
manner. AA treatment at 100 mg/ml additionally markedly inhibited
UVA-induced DNA fragmentation in HDFs.
Discussion
The aqueous extract of Stachys riederi var.
japonica is known for its anti-allergic effects (19). To gain additional insight into the
potential use of Stachys riederi as novel skin improving
and/or cosmeceutical material, SREE was prepared and its
antioxidant and cytoprotective effects on UVA-irradiated HDFs were
investigated the present study. To the best of our knowledge, the
present study demonstrated for the first time that SREE exhibited
antioxidant and cytoprotective effects on UVA-irradiated HDFs
through regulation of ROS production, the ΔΨm, expression of Bcl-2
and Bax, caspase-3 activity and apoptosis.
It has been demonstrated that the exposure of UVA
into skin cells rapidly and markedly increases the amounts of
cellular ROS (2), suggesting
UVA-induced oxidative stress. Excessive ROS causes cellular damage
to a number of biological molecules including lipids, proteins and
DNA (22). At present, the
mechanism of SREE regulation of UVA-induced ROS production in
dermal cells remains unclear. Notably, the results of the
fluorescence microscopy in the present study demonstrated that SREE
markedly decreased the cellular ROS levels in UVA-irradiated HDFs,
suggesting the antioxidant effects of SREE.
It has been indicated that impairment of the
mitochondrial function leads to cellular ROS generation in skin
cells in response to UVA exposure (23). It has been established that
mitochondrial membrane integrity is markedly affected by the
expression levels of the Bcl-2 family proteins (24). For example, Bcl-2 is a
mitochondrial membrane-associated protein that inhibits apoptosis
(25,26). The anti-apoptotic role of Bcl-2
against cell death is supported by previous studies, which
demonstrated that Bcl-2 overexpression abrogates UVB-induced
apoptosis in human keratinocytes in vitro and in vivo
(10,27). Conversely, an additional member of
the Bcl-2 family Bax, usually identified in the cytosol, is a
pro-apoptotic protein that perturbs the barrier function of
mitochondrial membrane and induces apoptosis, primarily by
translocating from the cytosol to the outer membrane of
mitochondria (28). There is
additionally evidence that Bax serves as a sentinel for cellular
damage, as cytotoxic signals induce Bax translocation to the outer
membrane of mitochondria, where it promotes mitochondrial
dysfunction and triggers apoptosis (28,29). Notably, the present study
demonstrated that SREE markedly interfered with the ability of UVA
to downregulate mRNA and protein expressions of Bcl-2 in HDFs;
however, it was additionally demonstrated that markedly attenuated
UVA-induced increases in Bax expression at the mRNA and protein
expression levels in the cells. These results suggest that SREE
exhibited a protective effect on the mitochondrial membrane
integrity in UVA-irradiated HDFs through regulation of the
expression of Bcl-2 and Bax, and that the SREE-induced up- and
downregulation of Bcl-2 and Bax expression in these cells occurs at
the transcriptional level.
The opening of mitochondrial permeability transition
pore is closely associated with increased permeability and loss of
the ΔΨm (30). Previously,
ROS-induced depolarization of the ΔΨm and cytochrome c
release from the mitochondria to the cytosol have been proposed
(31). The present study
demonstrated the ability of SREE to significantly inhibit
UVA-induced loss of the ΔΨm in HDFs. These data suggested that SREE
may have the ability to prevent depolarization of the ΔΨm and to
inhibit release of cytochrome c from the mitochondria to the
cytosol in UVA-irradiated HDFs, which is in part attributable to
the antioxidant properties of SREE. Cytochrome c is a
well-characterized mobile electron transport protein that is
essential to energy conversion in all aerobic organisms. However,
in cells undergoing apoptosis, high levels of cytochrome c
are released from the mitochondrial membrane to the cytosol, where
they subsequently trigger activation of the caspase family members
(32,33). Among those, caspases-3 is at the
core of the execution phase of apoptosis and its activation is
regarded as one of the biochemical hallmarks of apoptosis (7). Accumulating evidence additionally
indicates that cells undergoing apoptosis are characterized by
nuclear DNA fragmentation (28,34). As SREE markedly suppressed the
activation of caspase-3 and DNA fragmentation induced by UVA in
HDFs, it was hypothesized that SREE exerted its cytoprotective
effect on UVA-irradiated HDFs by inhibiting caspase-3 and
apoptosis.
In conclusion, to the best of our knowledge, the
present study is the first to indicate that SREE exhibited
antioxidant and cytoprotective effects on UVA-irradiated human
dermal fibroblasts, which may be mediated through the suppression
of ROS generation, inhibition of the loss of the ΔΨm, Bcl-2
upregulation and Bax downregulation, caspase-3 inactivation and
inhibition of apoptosis.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JYH and AKY performed experiments. BCJ and YCK
analysed the data and wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Sarasin A: The molecular pathways of
ultraviolet-induced carcinogenesis. Mutat Res. 428:5–10. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wondrak GT, Roberts MJ, Cervantes-Laurean
D, Jacobson MK and Jacobson EL: Proteins of the extracellular
matrix are sensitizers of photo-oxidative stress in human skin
cells. J Invest Dermatol. 121:578–586. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jin GH, Liu Y, Jin SZ, Liu XD and Liu SZ:
UVB induced oxidative stress in human keratinocytes and protective
effect of antioxidant agents. Radiat Environ Biophys. 46:61–68.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pavey S, Russell T and Gabrielli B: G2
phase cell cycle arrest in human skin following UV irradiation.
Oncogene. 20:6103–6110. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sung LY and Sung KL: Ultraviolet
radiation: DNA damage, repair, and human disorders. Mol Cell
Toxicol. 13:21–28. 2017. View Article : Google Scholar
|
|
6
|
Sheikh MS, Antinore MJ, Huang Y and
Fornace AJ Jr: Ultraviolet-irradiation-induced apoptosis is
mediated via ligand independent activation of tumor necrosis factor
receptor 1. Oncogene. 17:2555–2563. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Savitskaya MA and Onishchenko GE:
Mechanisms of apoptosis. Biochemistry (Mosc). 80:1393–1405. 2015.
View Article : Google Scholar
|
|
8
|
He B, Lu N and Zhou Z: Cellular and
nuclear degradation during apoptosis. Curr Opin Cell Biol.
21:900–912. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sitailo LA, Tibudan SS and Denning MF:
Activation of caspase-9 is required for UV-induced apoptosis of
human keratinocytes. J Biol Chem. 277:19346–19352. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Assefa Z, Garmyn M, Vantieghem A, Declercq
W, Vandenabeele P, Vandenheede JR and Agostinis P: Ultraviolet B
radiation-induced apoptosis in human keratinocytes: Cytosolic
activation of procaspase-8 and the role of Bcl-2. FEBS Lett.
540:125–132. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salucci S, Burattini S, Battistelli M,
Baldassarri V, Maltarello MC and Falcieri E: Ultraviolet B (UVB)
irradiation-induced apoptosis in various cell lineages in vitro.
Int J Mol Sci. 14:532–546. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lei L, Li H and Zhen-Zhen Z: Activation of
JNK/Bim/Bax pathway in UV-induced apoptosis. Proc SPIE 7900,
Biophotonics Immune Responses VI. 79000I. pp. 79000I2011
|
|
13
|
Assefa Z, Van Laethem A, Garmyn M and
Agostinis P: Ultraviolet radiation-induced apoptosis in
keratinocytes: On the role of cytosolic factors. Biochim Biophys
Acta. 755:90–106. 2005.
|
|
14
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu X, Kim CN, Yang J, Jemmerson R and
Wang X: Induction of apoptotic program in cell-free extracts:
Requirement for dATP and cytochrome c. Cell. 86:147–157. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tundis R, Peruzzi L and Menichini F:
Phytochemical and biological studies of Stachys species in relation
to chemo-taxonomy: A review. Phytochemistry. 102:7–39. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sadeghi H, Zarezade V, Sadeghi H,
AkbartabarToori M, Jafari Barmak M, Azizi A, Ghavamizadeh M and
Mostafazadeh M: Anti-inflammatory activity of Stachys Pilifera
Benth. Iran Red Crescent Med J. 16:e192592014. View Article : Google Scholar
|
|
18
|
Hajhashemi V, Ghannadi A and Sedighifar S:
Analgesic and anti-inflammatory properties of the hydroalcoholic,
polyphenolic and boiled extracts of Stachys lavandulifolia. Res
Pharm Sci. 1:92–98. 2007.
|
|
19
|
Shin TY: Stachys riederi inhibits mast
cell-mediated acute and chronic allergic reactions. Immunopharmacol
Immunotoxicol. 26:621–630. 2004. View Article : Google Scholar
|
|
20
|
Denning MF, Wang Y, Tibudan S, Alkan S,
Nickoloff BJ and Qin JZ: Caspase activation and disruption of
mitochondrial membrane potential during UV radiation-induced
apoptosis of human keratinocytes requires activation of protein
kinase C. Cell Death Differ. 9:40–52. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gorczyca W, Gong J and Darzynkiewicz Z:
Detection of DNA strand breaks in individual apoptotic cells by the
in situ terminal deoxynucleotidyl transferase and nick translation
assays. Cancer Res. 53:1945–1951. 1993.PubMed/NCBI
|
|
22
|
Phaniendra A, Jestadi DB and Periyasamy L:
Free radicals: Properties, sources, targets, and their implication
in various diseases. Indian J Clin Biochem. 30:11–26. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Genova ML, Pich MM, Bernacchia A, Bianchi
C, Biondi A, Bovina C, Falasca AI, Formiggini G, Castelli GP and
Lenaz G: The mitochondrial production of reactive oxygen species in
relation to aging and pathology. Ann N Y Acad Sci. 1011:86–100.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adams JM and Cory S: The BCL-2 arbiters of
apoptosis and their growing role as cancer targets. Cell Death
Differ. 25:27–36. 2018. View Article : Google Scholar
|
|
25
|
Borner C: The Bcl-2 protein family: Sensor
and checkpoints for life-or-death decisions. Mol Immunol.
39:615–647. 2003. View Article : Google Scholar
|
|
26
|
Shamas-Din A, Kale J, Leber B and Andrews
DW: Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb
Perspect Biol. 5:a0087142013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takahashi H, Honma M, Ishida-Yamamoto A,
Namikawa K, Miwa A, Okado H, Kiyama H and Iizuka H: In vitro and in
vivo transfer of bcl-2 gene into keratinocytes suppresses
UVB-induced apoptosis. Photochem Photobiol. 74:579–586. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gross A, Jockel J, Wei MC and Korsmeyer
SJ: Enforced dimerization of BAX results in its translocation,
mitochondrial dysfunction and apoptosis. EMBO J. 17:3878–3885.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Banerjee G, Gupta N, Kapoor A and Raman G:
UV induced by stander signaling leading to apoptosis. Cancer Lett.
223:275–284. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bonora M and Pinton P: The mitochondrial
permeability transition pore and cancer: Molecular mechanisms
involved in cell death. Front Oncol. 4:3022014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gogvadze V, Orrenius S and Zhivotovsky B:
Multiple pathways of cytochrome c release from mitochondria in
apoptosis. Biochim Biophys Acta. 1757:639–647. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Orrenius S, Gogvadze V and Zhivotovsky B:
Calcium and mitochondria in the regulation of cell death. Biochem
Biophys Res Commun. 460:72–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schuler M, Bossy-Wetzel E, Goldstein JC,
Fitzgerald P and Green DR: p53 induces apoptosis by caspase
activation through mitochondrial cytochrome c release. J Biol Chem.
275:7337–7342. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kitazumi I and Tsukahara M: Regulation of
DNA fragmentation: The role of caspases and phosphorylation. FEBS
J. 278:427–441. 2011. View Article : Google Scholar
|