Introduction
Endometrial carcinoma is one of the most common
malignant tumors in women, having the fourth highest prevalence and
the eighth highest mortality rate worldwide (1). The treatment for this tumor includes
surgery/chemotherapy/radiotherapy and high-dose progestational
hormone (2). However, local or
distant recurrences, which result from treatment-resistant cancer
cells present an issue for gynecological oncologists (3). To investigate the underlying
mechanisms of its carcinogenesis or progression, and to explore the
potential target for an effective therapy stimulates the interests
of many researchers.
MicroRNAs (miRNAs or miRs) are a group of
evolutionarily conserved regulatory RNAs, which have been suggested
to exert regulatory functions in carcinogenesis and used as
therapeutic targets for cancer treatment (4). It has previously been reported that
miRNAs exert a pivotal role in cell proliferation and metastasis in
endometrial carcinoma (5). Ramón
et al (6) have reported
that specific miRNAs were overexpressed in endometrial carcinoma,
which was positively correlated with VEGF expression. miR-200c was
reported to be associated with the occurrence of endometrial
carcinoma by regulating BRD7 expression (7). miR-205 was overexpressed in
endometrial cancer cells and promoted tumor metastasis by
downregulation of JPH4 expression (8). miR-130b (9), miR-200b (10) and miR-200 family (11) all contributed to the development
of endometrial carcinoma through mediating epithelial-mesenchymal
transition related protein activation. Although miRNAs act as
oncogenes or anti-oncogenes in carcinogenesis and progression of
endometrial carcinoma, the specific role and mechanism of miR-320a
in endometrial carcinoma still remains to be elucidated.
Obesity is a high risk for endometrial carcinoma
(12). Insulin is involved in the
formation of obesity, and its function is exerted partly by a
signal pathway mediated by insulin like growth factor receptor-1
(IGF-1R), as there is a cross link between insulin receptor and
IGF-1R (13). To explore the
potential role of miR-320a on endometrial carcinoma and clarify its
underlying correlation with IGF-1R, the miR-320a expression level
in human endometrial carcinoma tissues and cells was investigated,
then functional analysis to detect the role of miR-320a in HEC-1A
and Ishikawa cells was performed. The present results demonstrated
that miR-320a was a tumor suppressor gene, which exerted anti-tumor
effects through several aspects, predominantly by regulating IGF-1R
expression and disturbing the phosphorylated (p)-protein kinase B
(Akt)/p-mechanistic target of rapamycin (mTOR) signaling pathway.
These results verified the impact of miR-320a on the occurrence of
endometrial carcinoma, which may provide theoretical evidence for
the application of target therapy.
Materials and methods
Clinical specimens and cell culture
A total of 50 endometrial carcinoma samples and 10
normal endometrium tissues were obtained from patients at the
Department of Gynecology and Obstetrics of the First Affiliated
Hospital of Jinan University (Guangzhou, China) from April 2015 to
March 2017. Normal endometrium tissue samples were harvested from
patients who experienced hysterectomy for benign uterine disease.
The age of patients ranged from 42-65 years old. None of the
patients had experienced chemotherapy or radiotherapy prior to
surgery. All the surgically excised tissues were pathologically
confirmed and stored at −80°C until analysis. Ishikawa, HEC-1A,
HEC-1B, HEC-251, AN3CA and RL95-2 were bought from American Type
Culture Collection (ATCC, Manassas, VA, USA). Normal endometrial
cells were also used. To isolate normal cells from endometrial
tissues, the endometrium was rinsed 2-3 times with D-Hank's
solution containing penicillin and streptomycin. Tissue samples (~1
cm3) were administered with 2 ml 0.125% trypsin. The
mixture was digested in a 37°C incubator for 1.5 h. The left tissue
was allowed to pass through a stainless steel sieve for the second
digestion. The digested cells were centrifuged at 72 × g for 10 min
at room temperature, and the supernatant was discarded. The cells
were centrifuged twice and cultured in serum-containing medium.
All cells were cultured at 37°C and 5%
CO2 with Dulbecco's modified Eagle's medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), which was
supplied with 10% fetal calf serum (Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) and 1% penicillin-streptomycin (Hyclone;
GE Healthcare Life Sciences). The present study was performed with
the approval of the Human Ethics Committee of Jinan University in
accordance with the Declaration of Helsinki. All of the enrolled
patients provided written informed consent.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract RNA from tissues and cells,
then Bulge-Loop™ miRNA qRT-PCR primer kits (Guangzhou RiboBio Co.,
Ltd., Guangzhou, China) and All-in-One™ qPCR mix (GeneCopoeia,
Inc., Rockville, MD, USA) were used to detect the expression level
of miR-320a with U6 snRNA as the internal control. The primers used
were as follows: miR-320a forward, 5′-ATC CAG TGC AGG GTC CGA GG-3′
and reverse, 5′-CGC GGT TAA AAG CTG GGT TGA GA-3′; U6 forward,
5′-CTC GCT TCG GCA GCA CA-3′ and reverse, 5′-AAC GCT TCA CGA ATT
TGC GT-3′. The 2−ΔΔCq method (14) was used to determine the relative
expression of miR-320a in tissues and cells.
Oligonucleotide transfection and
generation of stably transfected cell lines
Cells were seeded into 6-well plates at the density
of 2×105 cells/well. Lipofactamine™ RNAi MAX
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to transfect
the cells with miR-320a mimics or controls (50 nM; Shanghai
GenePharma Co., Ltd., Shanghai, China). The miR-320a mimics
sequence was 5′-AAA AGC UGG GUU GAG AGG GCG A-3′. The sequence for
the control was 5′-CAG UAC UUU UGU GUA GUA CAA-3′. Meanwhile,
pcDNA-IGF-1R (100 nM; Invitrogen; Thermo Fisher Scientific, Inc.)
and pcDNA-control were co-transfected using Lipofactamine 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). At 48 h
following transfection, pGCSIL-GFP vector with the A-geI and EcoR I
enzyme sites was inserted via the target sequences. Following
recombination using pCMV and pBR322 vectors (Shanghai GeneChem Co.,
Ltd., Shanghai, China), lentiviral vectors (Lv) expressing
targeting gene were constructed, which was simultaneously
transfected into 293T cells (ATCC) coupled with packaging vectors.
At 48 h following transfection, supernatants containing lentivirus
were harvested. Ultracentrifugation (4°C for 2 h; 210,000 × g) was
used to determine the titer of lentivirus.
MTT and colony formation analysis
Endometrial carcinoma cells were seeded in 96-well
plates at 5,000 cells per well. MTT assay was used to determine the
viability of cells at 24, 48 and 72 h. Absorbance at 490 nm was
determined by spectrophotometer. For colony formation assay,
transfected cells were plated in culture plates and cultured for 14
days at 500 cells per plate. Colonies were fixed with methanol at
room temperature for 15 min and stained with crystal violet at room
temperature for 5 min. The numbers of colonies were counted
manually.
Western blot analysis
The total cell protein was obtained using
radioimmunoprecipitation assay lysis and extraction buffer (Pierce;
Thermo Fisher Scientific, Inc.) and the concentration was
determined by BCA protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.). A total of 25 µg protein was applied per lane
and separated by 10-12% SDS-PAGE, then transferred to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). Membranes were incubated overnight at 4°C with antibodies
against IGF-1R (sc-81167; 1:1,500; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), p-Akt (cat. no. 4060; 1:1,000), total (t)-Akt
(cat. no. 9272; 1:1,000; Cell Signaling Technology, Inc., Danvers,
MA, USA), p-mTOR (sc-101738; 1:2,000; Santa Cruz Biotechnology,
Inc.), t-mTOR (sc-8319; 1:2,000; Santa Cruz Biotechnology, Inc.)
and B cell lymphoma-2-associated death promoter (Bad; ab32245;
1:1,200; Abcam, Cambridge, UK). The membrane was washed and
incubated with horseradish peroxidase-conjugated secondary antibody
(ab205718; 1:3,000) for 1 h at room temperature. The
protein-antibody complex was determined by an enhanced
chemiluminescence system, CL-XPosure Film (Pierce; Thermo Fisher
Scientific, Inc.). The relative expression of protein was
quantified by Quantity One software (version 4.6.6, Bio-Rad
Laboratories, Inc., Hercules, CA, USA) with GAPDH (sc-47724;
1:2,000; Santa Cruz Biotechnology, Inc.) used as the internal
control. Each experiment was repeated three times to assess the
consistency of the results.
Luciferase reporter assay
TargetScan database (http://www.targetscan.org/) was used to predict the
possible target of miR-320a. It was demonstrated that IGF-1R may be
the potential target, then the IGF-1R-untranslated region (UTR) was
ligated into the pMIR-GLO luciferase vector (Promega Corporation,
Madison, WI, USA) to produce recombinant pMIR-IGF-1R-UTR-Wt.
Another pMIR-GLO luciferase construct containing the miR-320a
mutation site in IGF-1R-UTR (CAGCUUUU to CAGCUUGU) was named
IGF-1R-UTR-Mut and used as the control. Subsequently, 293T cells
were simultaneously transfected with pMIR-IGF1R-UTR-Wt or
pMIR-IGF1R-UTR-Mut using Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.). Cells were harvested following incubation at
37°C for 48 h. Luciferase activity was determined and normalized
with Renilla luciferase assay system (Promega
Corporation).
Cell cycle distribution analysis
HEC-1A and Ishikawa cells transfected with
Lv-miR-320a or Lv-control Cells were trypsinized and then the
distribution of cell cycle was determined by DNA Reagent kit
(Becton, Dickinson and Company, Franklin, Lakes, NJ, USA).
According to the manufacturer's recommendation, the collected cells
were washed and incubated with different solutions on ice in the
dark for 10 min, then flow cytometry was used to measure the
relative proportions of transfected cells in different phases by
FACSCalibur™ Flow Cytometry (BD Accuri™ C6; Becton, Dickinson and
Company).
Detection of apoptosis by flow
cytometry
Apoptosis analysis was performed according to the
manufacturer's protocol (Becton, Dickinson and Company). The HEC-1A
and Ishikawa cells transfected with Lv-miR-320a or Lv-control were
washed and incubated at room temperature for 15 min with Annexin
V-PE and 7-AAD in the dark. Flow cytometry was then performed to
detect the apoptotic rate in the transfected cells.
Statistics analysis
SPSS 13.0 (SPSS, Inc., Chicago, IL, USA) was adopted
to perform the statistical analyses. Data were expressed as the
mean ± standard deviation. Comparison between two groups was
analyzed with Student's t-test, and the association between more
than two groups were determined with one-way analysis of variance
with Tukey's post hoc test. P<0.05 was considered to indicate a
statistically different difference.
Results
miR-320a expression is decreased in
endometrial carcinoma tissues and cells
The relative expression of miR-320a among
endometrial carcinoma cell lines and normal endometrial cells was
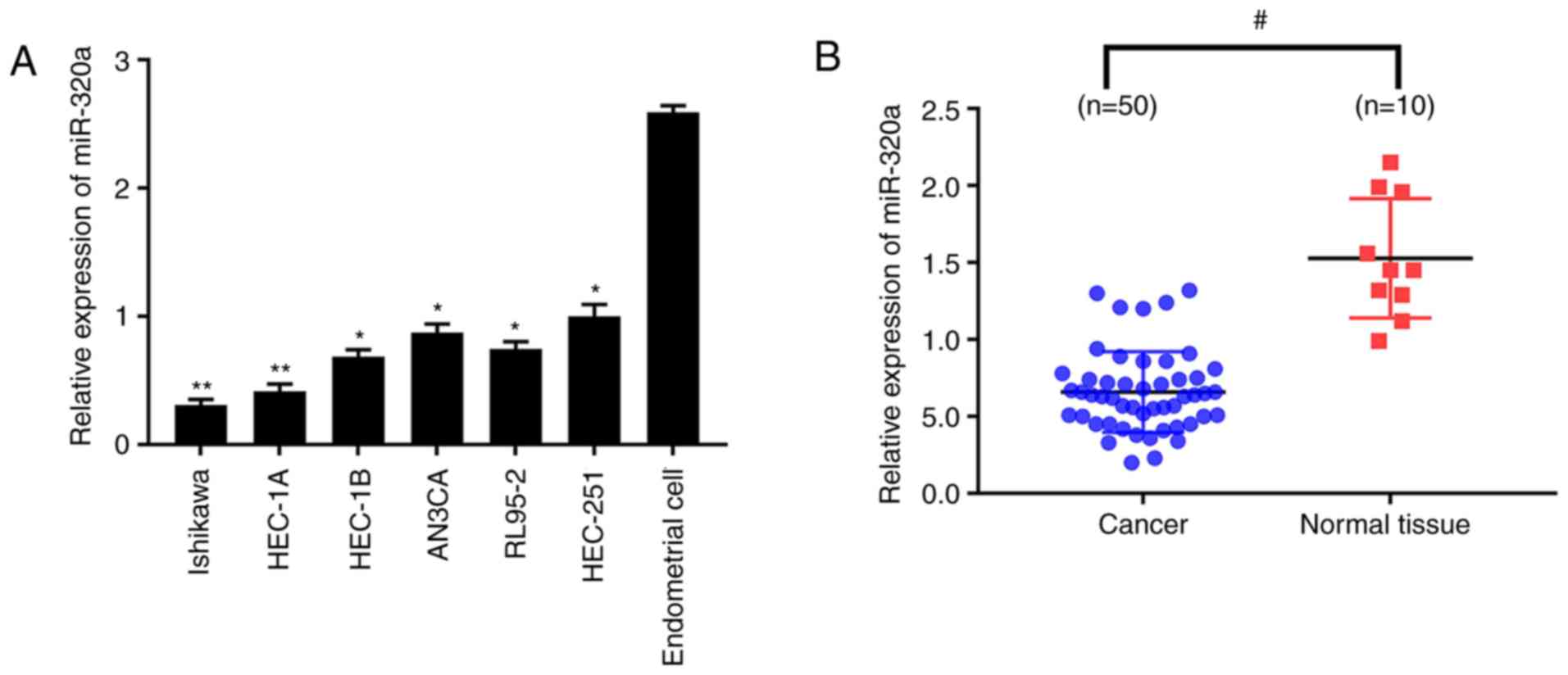
quantitatively analyzed. The results demonstrated that miR-320a
expression was significantly reduced in endometrial carcinoma cells
compared with normal endometrial cells (Fig. 1A), which was more marked in HEC-1A
and Ishikawa cell lines. miR-320a expression was then explored in
50 endometrial carcinoma and 10 normal endometrial tissues. In
accordance with the results obtained from cell lines, miR-320a
expression was significantly lower in endometrial carcinoma than
normal endometrium (Fig. 1B).
These results demonstrated that the expression of miR-320a was
dramatically decreased in endometrial carcinoma.
miR-320a induces proliferation inhibition
in endometrial carcinoma cells
In order to explore the role of miR-320a on cell
growth, HEC-1A and Ishikawa cells were stably transfected with
Lv-miR-320a or Lv-control, then cell proliferation and colony
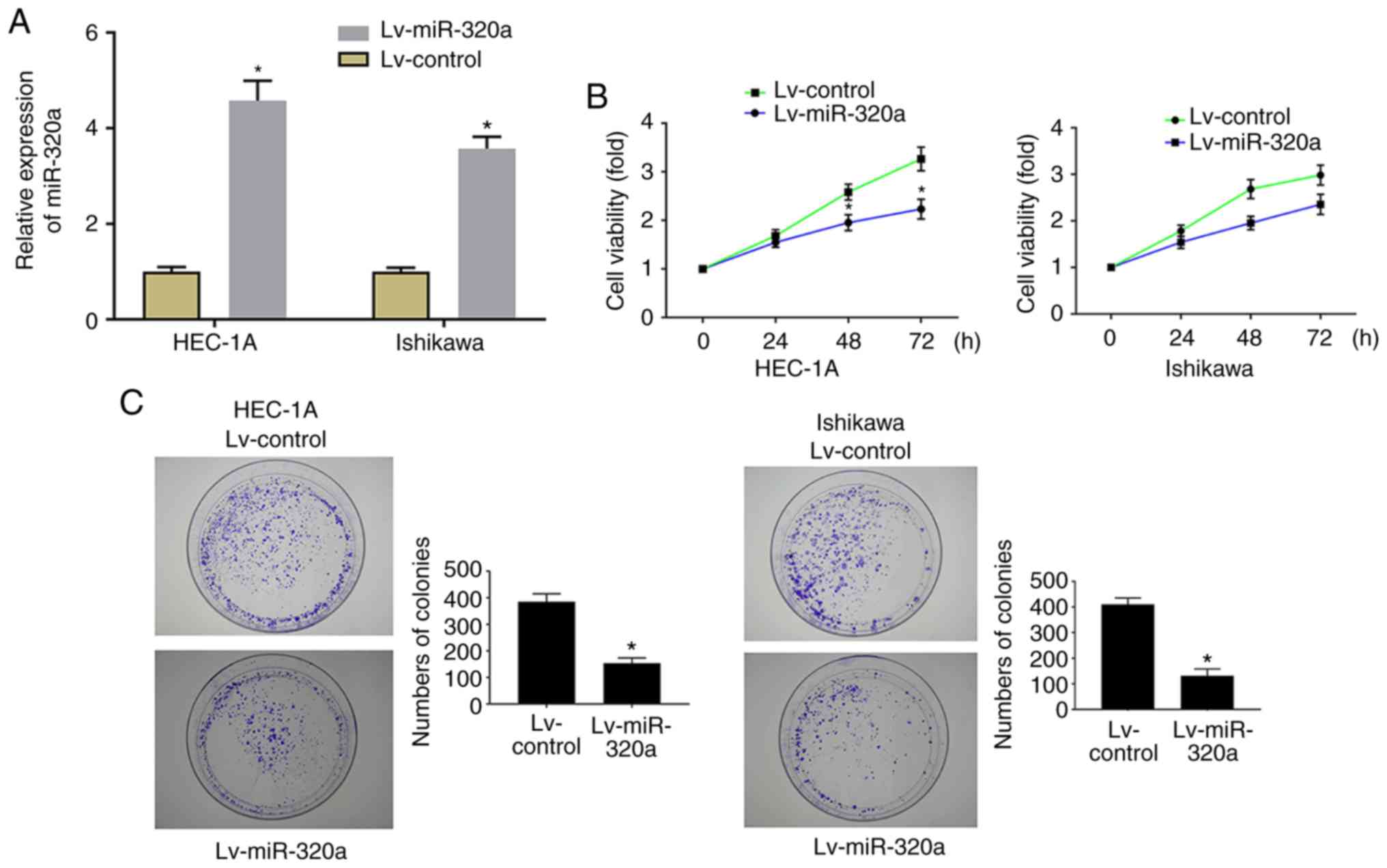
formation ability were examined. RT-qPCR results demonstrated that
miR-320a expression was significantly increased in Lv-miR-320a
transfected cells, which confirmed that miR-320 was successfully
transfected (Fig. 2A). As
presented in Fig. 2B, MTT results
demonstrated that upregulation of miR-320a significantly inhibited
cell proliferation. Meanwhile, the analysis of colony formation
suggested that the number of colonies was significantly decreased
in Lv-miR-320a transfected cells (Fig. 2C), which verified the far-reaching
role of miR-320a on cell growth.
miR-320a induces G2/M phase
arrest and promoted cell apoptosis
It was speculated that the decreased growth in
miR-320a-overexpressed endometrial carcinoma cells may result from
altered cell cycle and increased cell apoptosis. To explore the
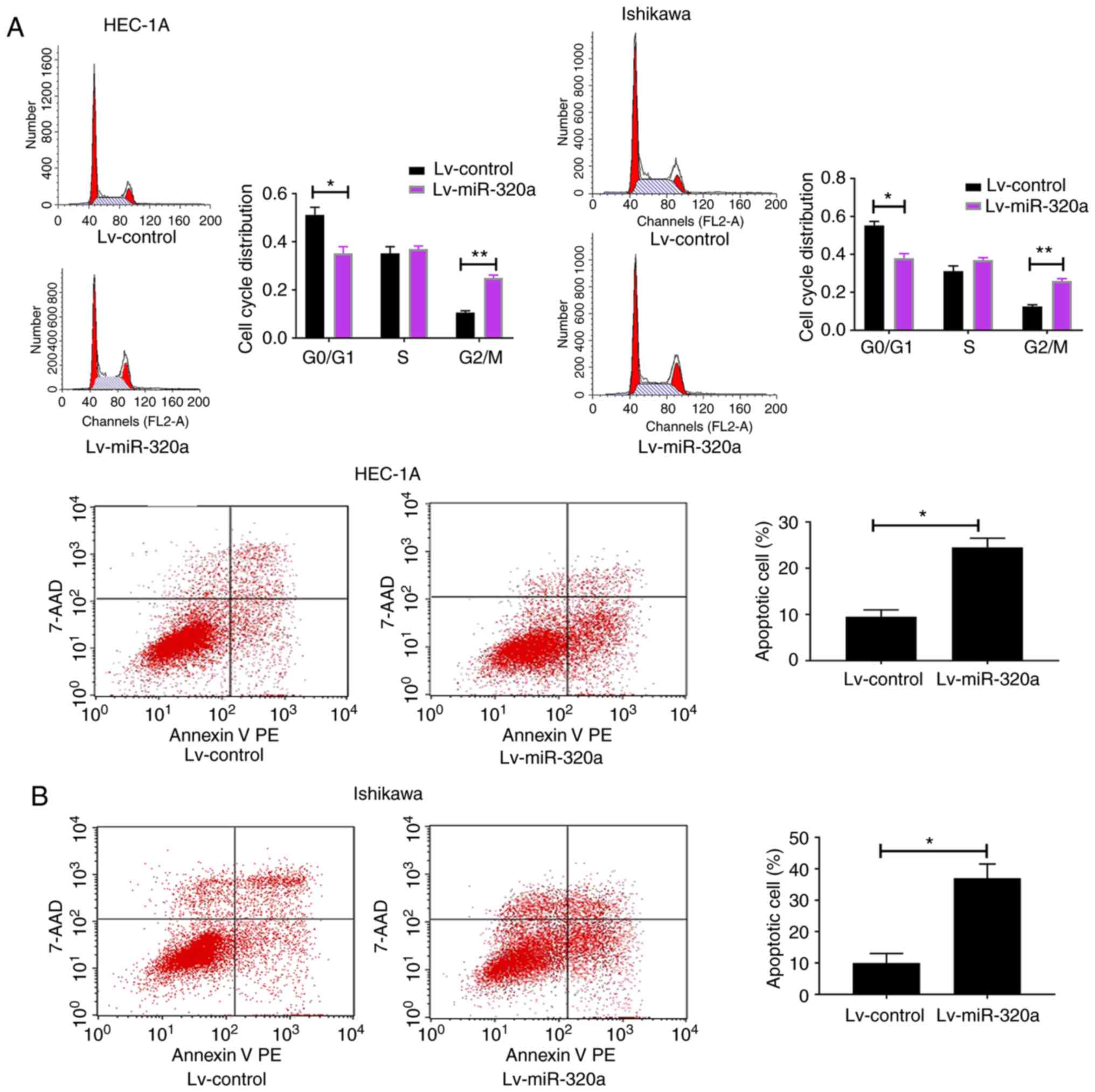
present hypothesis, flow cytometry was used to detect cell cycle
and apoptosis. The results demonstrated that the G2/M
phases were blocked in Lv-miR-320a trans-fected cells, accompanied
with reduction in G0/G1 phases (Fig. 3A). Meanwhile, compared with the
control, it was demonstrated that miR-320a-overexpressed cells
exhibited an increased apoptosis rate (Fig. 3B), which suggested that miR-320a
exerts a negative mediator in endometrial carcinoma by arresting
cell cycle progression and promoting cell apoptosis.
IGF-1R gene is a possible target of
miR-320a in endometrial carcinoma
As miR-320a exerted an important role on endometrial
carcinoma cell growth, it was inferred that the genes regulated by
miR-320a may mainly function in this course. By using the
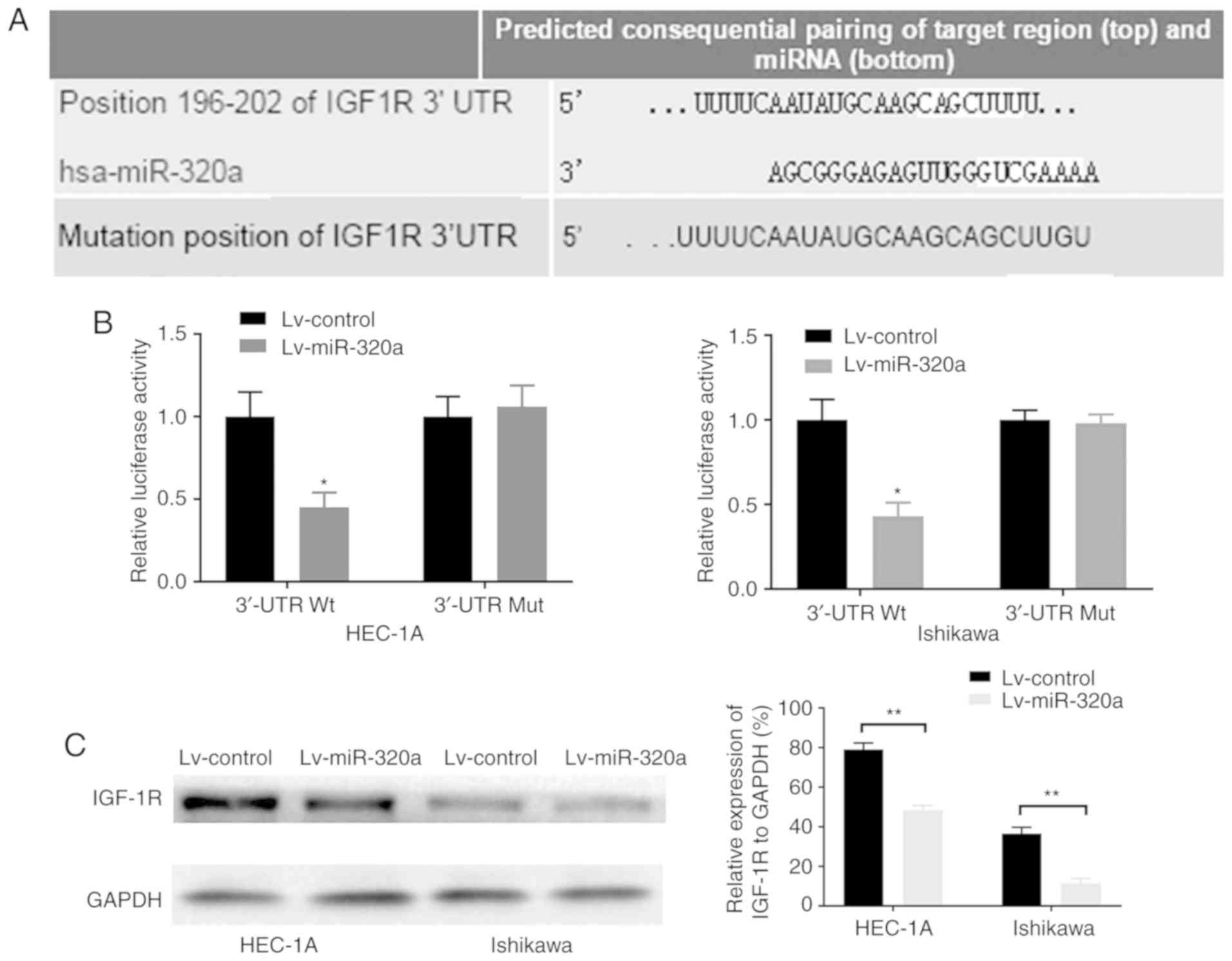
TargetScan database, it was demonstrated that the 3′-UTR of IGF-1R
contains the binding site of miR-320a (Fig. 4A). Luciferase reporters were
constructed to detect whether miR-320a can bind to the 3′-UTR of
IGF-1R. The results demonstrated that luciferase activity in
wild-type IGF-1R 3′-UTR-transfected HEC-1A and Ishikawa cells was
significantly decreased by 55 and 57%, respectively (Fig. 4B). However, a mutation in the
miR-320a binding site to IGF-1R 3′-UTR completely eliminated this
repression, which suggested that miR-320a may directly bind to this
site. Consistently, overexpression of miR-320a significantly
downregulated the protein expression of IGF-1R (Fig. 4C). These results suggested that
the potential target of miR-320a was IGF-1R, and that miR-320a was
inversely associated with IGF-1R.
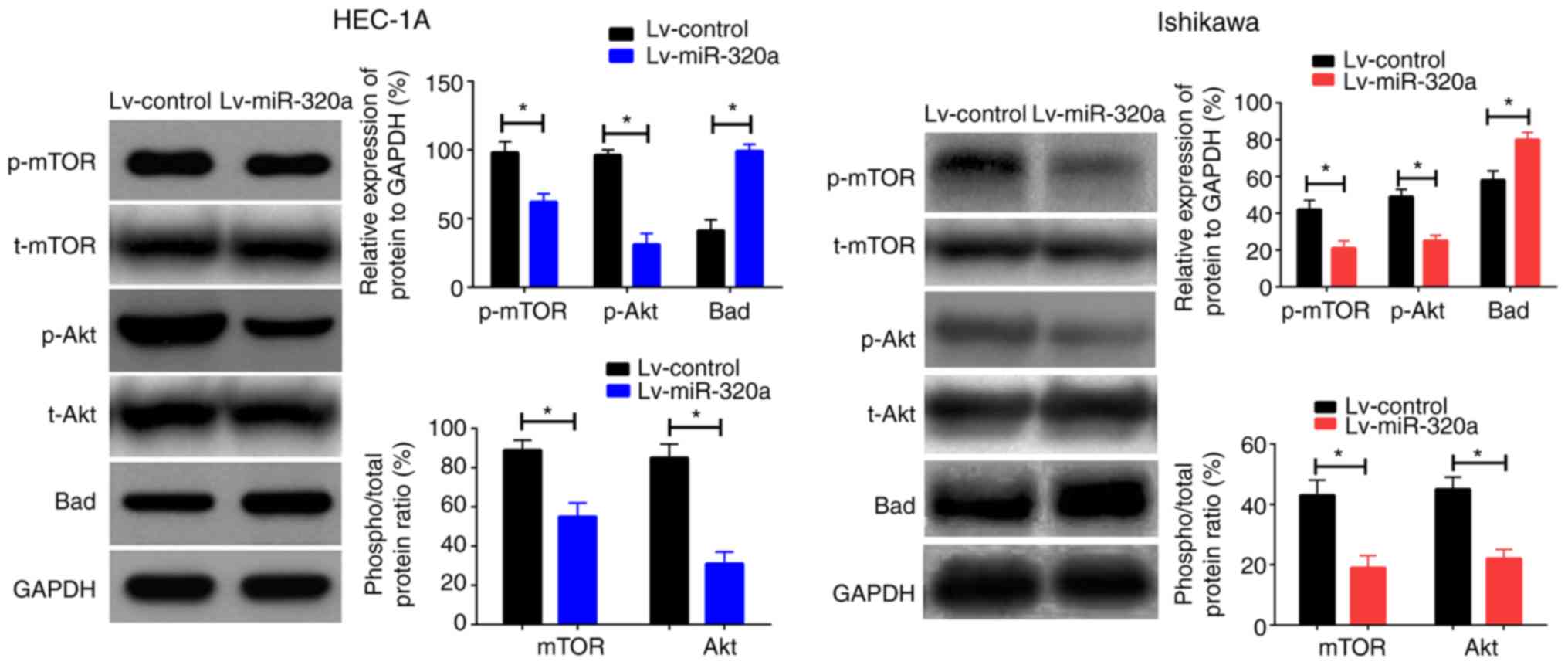
Overexpression of miR-320a inhibits
IGF-1R-mediated transcriptional activity
Given that IGF-1R gene was the direct target of
miR-320a, it was reasonable to infer that suppression of downstream
protein mediated by IGF-1R such as Akt, mTOR and Bad may be the
underlying mechanism for the suppressive effect of miR-320a on cell
proliferation. To verify the present assumption, western blotting
was performed to examine the protein expression of Akt, mTOR and
Bad in cells transfected with Lv-miR-320a or Lv-control. As
presented in Fig. 5, upregulation
of miR-320a reduced p-Akt and p-mTOR expression with no significant
effect on t-Akt or t-mTOR, but increased Bad expression. Taken
together, these results suggested that miR-320a inhibited cell
growth by regulating the phosphorylated protein expression mediated
by IGF-1R.
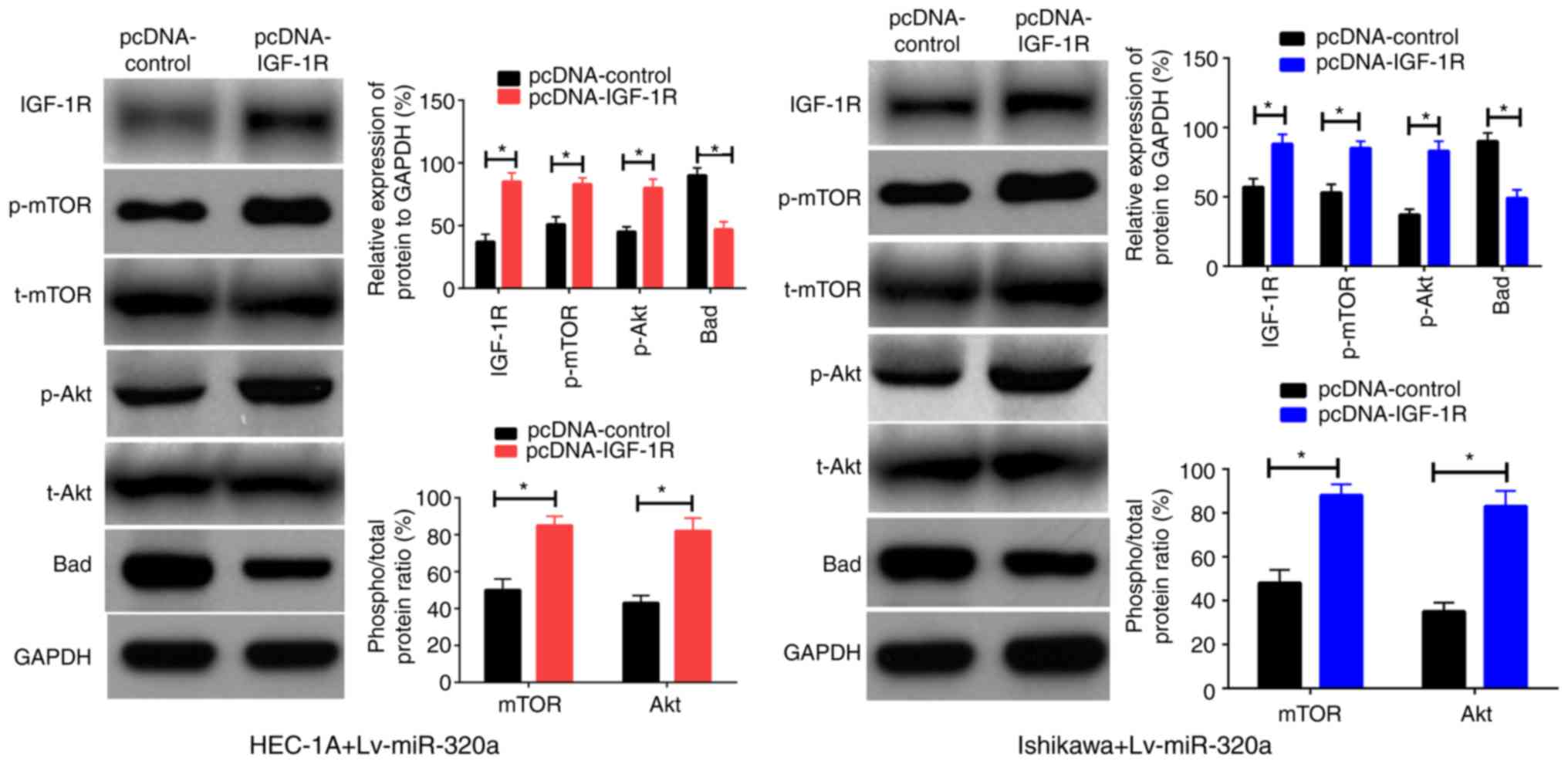
IGF-1R is a functional mediator for
miR-320a in endometrial carcinoma
Previously, it was verified that IGF-1R was a direct
target of miR-320a in endometrial carcinoma, thus it was inferred
that reintroduction of IGF-1R into Lv-miR-320a transfected cells
may be able to antagonize the impact of miR-320a on cell functions.
To verify this assumption, IGF-1R or control were first transferred
into Lv-miR-320a endometrial carcinoma cells, which was confirmed
by western blotting. As presented in Fig. 6A, inreasing the expression of
IGF-1R partly recovered the expression of p-Akt, p-mTOR and Bad,
but had no significant impact on t-Akt and t-mTOR expression. These
results demonstrated that IGF-1R was a direct mediator for miR-320a
in endometrial carcinoma.
Discussion
It is proposed that miR-320a is dysregulated in many
solid tumors, and its underling mechanism has been partly
investigated. It has been reported that miR-320a is implicated in
the metastasis of colorectal cancer to the liver (15). It has been reported that miR-320a
post-transcriptionally regulated hepatocellular carcinoma migration
and invasion by targeting GNAI1 (16). It was suggested that the
expression pattern of miR-320a was tissue-specific and the function
was cell content-dependent (17),
although miR-320a was reported to modulate non-small cell lung
cancer proliferation and invasion (18) and glioma cell functions (19) by directly targeting IGF-1R. Many
miRNAs are reported to have different roles in endometrial
carcinoma (20), but the function
of miR-320a in endometrial carcinoma and the potential mechanisms
are rarely reported.
In the present research, it was demonstrated that
the expression of miR-320a was dramatically decreased in
endometrial carcinoma tissues and cell lines. Overexpression of
miR-320a significantly inhibited cell proliferation and colony
formation in HEC-1A and Ishikawa cells. Furthermore, upregulation
of miR-320a lead to higher apoptosis and G2/M phrase
blockage. These results suggested that miR-320a had an anti-tumor
role in endometrial carcinoma. The underlying mechanism by which
miR-320a exerts its impact on the progression of endometrial
carcinoma was explored. It has been reported that the biological
features of the specific target gene of a certain miRNA are
dependent on its biological system (21). It has been reported that in
leukemia cells, miR-320a increased survivin expression but in
fibroblast cells, it downregulated ETS-1 expression (22,23). In addition, miR-26a exerted the
anti-tumor role in hepatocellular carcinoma by mediating cyclin-D2
and cyclin-E2 expression (24),
but in malignant glioblastoma, miR-26a served as an oncogene by
regulating PTEN function (25).
So, it is important to ascertain the potential target gene of
particular miRNAs in a cell-dependent pattern. In our previous
study, it was demonstrated that IGF-1R is associated with the
carcinogenesis and chemotherapy sensitivity of endometrial
carcinoma (26,27). It was speculated that miR-320a may
function in the tumorigenesis of endometrial carcinoma through
targeting IGF-1R. To detect this hypothesis, TargetScan database
was used to predict the possible target gene of miR-320a, and it
was demonstrated that the IGF-1R gene was the potential target of
miR-320. A rescue experiment was then performed to determine
whether IGF-1R is the exact regulator for miR-320a in endometrial
carcinoma. The present results demonstrated that IGF-1R was the
direct target of miR-320a and reintroduction of IGF-1R into
miR-320a-overexpressed cells antagonized the role of miR-320a on
IGF-1R downstream protein, such as p-Akt/p-mTOR and Bad. Taken
together, these results suggested that IGF-1R is a functional
regulator for miR-320a in endometrial carcinoma.
In conclusion, the present study demonstrated that
miR-320a was an anti-tumor miRNA in endometrial carcinoma, although
it is required to further investigate the role of miR-320a in the
malignant transformation process from normal endometrium to
proliferative or secreting phase endometrium to endometrial
carcinoma. It was also demonstrated that miR-320a exerted
anticancer effects through suppression of the signal pathway
mediated by IGF-1R. Although miRNA-based targeted treatments are
still in an initial stage, the present findings suggest that
miR-320a could be an effective target for the treatment of
endometrial carcinoma in future.
Funding
The present study was funded by National Natural
Science Foundation of China (grant no. 81672496), Guangzhou Science
and Technology Project (grant no. 2018040100420) and the National
College Students Innovation and Entrepreneurship Training Program
(grant no. CX18024).
Availability of data and materials
The authors declare that all of the data and
material are freely accessible on reasonable request.
Authors' contributions
RL and SS developed the project. MX, XG, SC and LZ
performed experiments and wrote the manuscript. XL supervised the
work and edit the manuscript. All authors agreed with the final
version of manuscript.
Ethics approval and consent to
participate
All human tissues were obtained with informed
consent, and protocols were approved by the Human Ethics Committee
of Jinan University (Guangzhou, China) in accordance with the
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
SS, XG, SC and RL, Department of Gynecology and
Obstetrics, The First Affiliated Hospital of Jinan University, 613
Huangpu Road West, Guangzhou, Guangdong 510630, P.R. China. XL,
Department of Hematology, Guangzhou Institute of Pediatrics,
Guangzhou Women and Children's Medical Center, 9 Jinsui Road,
Guangzhou, Guangdong 510630, P.R. China. MX, Department of Medical
Ultrasound, Institute of Diagnostic and Interventional Ultrasound,
The First Affiliated Hospital of Sun Yat-Sen University, 58 Zhong
Shan Second Road, Guangzhou, Guangdong 510080, P.R. China. LZ,
Department of Clinic Medicine, Medical College of Jinan University,
613 Huangpu Road West, Guangzhou, Guangdong 510630, P.R. China
Acknowledgments
Not applicable
References
|
1
|
Sanni OB, Mc Menamin ÚC, Cardwell CR,
Sharp L, Murray LJ and Coleman HG: Commonly used medications and
endometrial cancer survival: A population-based cohort study. Br J
Cancer. 117:432–438. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pautier P and Pommeret F: Systemic therapy
for advanced endometrial cancer. Bull Cancer. 104:1046–1053.
2017.In French. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ballester M, Bendifallah S and Daraï E:
European guidelines (ESMO-ESGO-ESTRO consensus conference) for the
management of endometrial cancer. Bull Cancer. 104:1032–1038.
2017.In French. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stope MB, Koensgen D, Weimer J, Paditz M,
Burchardt M, Bauerschlag D and Mustea A: The future therapy of
endometrial cancer: microRNA's functionality, capability, and
putative clinical application. Arch Gynecol Obstet. 294:889–895.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ramón LA, Braza-Boïls A, Gilabert J,
Chirivella M, España F, Estellés A and Gilabert-Estellés J:
microRNAs related to angiogenesis are dysregulated in endometrioid
endometrial cancer. Hum Reprod. 27:3036–3045. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park YA, Lee JW, Choi JJ, Jeon HK, Cho Y,
Choi C, Kim TJ, Lee NW, Kim BG and Bae DS: The interactions between
MicroRNA-200c and BRD7 in endometrial carcinoma. Gynecol Oncol.
124:125–133. 2012. View Article : Google Scholar
|
|
8
|
Chung TK, Cheung TH, Huen NY, Wong KW, Lo
KW, Yim SF, Siu NS, Wong YM, Tsang PT, Pang MW, et al: Dysregulated
microRNAs and their predicted targets associated with endometrioid
endometrial adenocarcinoma in Hong Kong women. Int J Cancer.
124:1358–1365. 2009. View Article : Google Scholar
|
|
9
|
Li BL, Lu W, Lu C, Qu JJ, Yang TT, Yan Q
and Wan XP: CpG island hypermethylation-associated silencing of
microRNAs promotes human endometrial cancer. Cancer Cell Int.
13:442013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dai Y, Xia W, Song T, Su X, Li J, Li S,
Chen Y, Wang W, Ding H, Liu X, et al: MicroRNA-200b is
overexpressed in endometrial adenocarcinomas and enhances MMP2
activity by downregulating TIMP2 in human endometrial cancer cell
line HEC-1A cells. Nucleic Acid Ther. 23:29–34. 2013. View Article : Google Scholar :
|
|
11
|
Snowdon J, Zhang X, Childs T, Tron VA and
Feilotter H: The microRNA-200 family is upregulated in endometrial
carcinoma. PLoS One. 6:e228282011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hendriks SH, Schrijnders D, van Hateren
KJ, Groenier KH, Siesling S, Maas AHEM, Landman GWD, Bilo HJG and
Kleefstra N: Association between body mass index and
obesity-related cancer risk in men and women with type 2 diabetes
in primary care in the Netherlands: A cohort study (ZODIAC-56). BMJ
Open. 8:e0188592018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Flannery CA, Saleh FL, Choe GH, Selen DJ,
Kodaman PH, Kliman HJ, Wood TL and Taylor HS: Differential
expression of IR-A, IR-B and IGF-1R in endometrial physiology and
distinct signature in adenocarcinoma. J Clin Endocrinol Metab.
101:2883–2891. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
15
|
Zhang Y, He X, Liu Y, Ye Y, Zhang H, He P,
Zhang Q, Dong L, Liu Y and Dong J: [Corrigendum] MicroRNA-320a
inhibits tumor invasion by targeting neuropilin 1 and is associated
with liver metastasis in colorectal cancer. Oncol Rep. 33:20932015.
View Article : Google Scholar
|
|
16
|
Yao J, Liang LH, Zhang Y, Ding J, Tian Q,
Li JJ and He XH: GNAI1 suppresses tumor cell migration and invasion
and is post-transcriptionally regulated by Mir-320a/c/d in
hepatocellular carcinoma. Cancer Biol Med. 9:234–241. 2012.
|
|
17
|
van der Kolk JH, Pacholewska A and Gerber
V: The role of microRNAs in equine medicine: A review. Vet Q.
35:88–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Shi C, Wang J, Cao L, Zhong L and
Wang D: MicroRNA-320a is downregulated in non-small cell lung
cancer and suppresses tumor cell growth and invasion by directly
targeting insulin-like growth factor 1 receptor. Oncol Lett.
13:3247–3252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo T, Feng Y, Liu Q, Yang X, Jiang T,
Chen Y and Zhang Q: MicroRNA-320a suppresses in GBM patients and
modulates glioma cell functions by targeting IGF-1R. Tumour Biol.
35:11269–11275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sianou A, Galyfos G, Moragianni D,
Andromidas P, Kaparos G, Baka S and Kouskouni E: The role of
microRNAs in the pathogenesis of endometrial cancer: A systematic
review. Arch Gynecol Obstet. 292:271–282. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carroll AP, Goodall GJ and Liu B:
Understanding principles of miRNA target recognition and function
through integrated biological and bioinformatics approaches. Wiley
Interdiscip Rev RNA. 5:361–379. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Diakos C, Zhong S, Xiao Y, Zhou M,
Vasconcelos GM, Krapf G, Yeh RF, Zheng S, Kang M, Wiencke JK, et
al: TEL-AML1 regulation of survivin and apoptosis via miRNA-494 and
miRNA-320a. Blood. 116:4885–4893. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bronisz A, Godlewski J, Wallace JA,
Merchant AS, Nowicki MO, Mathsyaraja H, Srinivasan R, Trimboli AJ,
Martin CK, Li F, et al: Reprogramming of the tumour
microenvironment by stromal PTEN-regulated miR-320. Nat Cell Biol.
14:159–167. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen L, Zheng J, Zhang Y, Yang L, Wang J,
Ni J, Cui D, Yu C and Cai Z: Tumor-specific expression of
microRNA-26a suppresses human hepatocellular carcinoma growth via
cyclin-dependent and -independent pathways. Mol Ther. 19:1521–1528.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huse JT, Brennan C, Hambardzumyan D, Wee
B, Pena J, Rouhanifard SH, Sohn-Lee C, le Sage C, Agami R, Tuschl T
and Holland EC: The PTEN-regulating microRNA miR-26a is amplified
in high-grade glioma and facilitates gliomagenesis in vivo. Genes
Dev. 23:1327–1337. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shu S, Yang Y, Li X, Li T, Zhang Y, Xu C,
Liang C and Wang X: Down-regulation of IGF-1R expression inhibits
growth and enhances chemosensitivity of endometrial carcinoma in
vitro. Mol Cell Biochem. 353:225–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shu S, Li X, Yang Y, Zhang Y, Li T, Liang
C and Wan J: Inhibitory effect of siRNA targeting IGF-1R on
endometrial carcinoma. Int Immunopharmacol. 11:244–249. 2011.
View Article : Google Scholar
|