Introduction
Inflammation, an innate response in the immune
system, is triggered through the release of specific cytokines,
noxious stimuli and tissue injury (1). An inflammatory response may occur
due to damage in tissues or organs, and diseases, including cancer,
cardiovascular disease, diabetes, obesity, rheumatoid arthritis,
depression and Parkinson’s disease (2,3).
Therefore, the inhibition of overactivated inflammation is
considered one of the most efficient strategies for treating
inflammatory diseases (2,3).
In inflammation, primary mediators, including nitric
oxide (NO) and chemotactic cytokines, including tumor necrosis
factor-α (TNF-α), interleukin (IL)-1β and IL-6, are stimulated by
lipopolysaccharide (LPS), which is a component of the gram-negative
bacterial cell wall (3). These
mediators react as a toxic agent against infectious organisms and
are associated with modulation of cellular functions and
homeostasis in the innate immune response. However, the
overproduction of NO leads to the production of a number of
proteins, including the mitogen-activated protein kinases (MAPKs)
and nuclear factor κ-light-chain-enhancer of activated B cells
(NF-κB) pathway, which are implicated in chronic inflammatory
reactions (4,5).
MAPKs consist of three principal components,
including extracellular signal-regulated kinases (ERK), c-Jun
N-terminal kinase/stress activated protein kinases (JNK/SAPK) and
p38 (6). These proteins are
closely associated with a wide range of signaling cascades and
serve a key role in the regulation of synthesis of inflammation
mediators at the transcriptional and translational levels.
Therefore, regulation of MAPK activity is considered a target for
anti-inflammatory therapeutics (6,7).
NF-κB, one of the downstream components in the MAPK signaling
pathway, is translocated to the nucleus by upstream stimuli. It
promotes the transcription of pro-inflammatory genes producing the
pro-inflammatory enzymes, including cyclooxygenase-2 (COX-2) and
inducible nitric oxide synthase (iNOS) (8,9).
COX-2 is one of the pro-inflammatory enzymes, which converts
arachidonic acid to prostaglandins E2 (PGE2) and contributes to the
progression of chronic inflammatory diseases (10). In addition, the expression of
COX-2 is implicated in the generation of reactive oxygen species in
response to LPS stimulation (10,11).
Angelica decursiva is a traditional medicinal
plant in Korea, which demonstrates curative effects for cough,
thick phlegm and asthma (12).
6-Formyl umbelliferone (6FU), isolated from Angelica
decursiva, is one of the uncommon coumarin derivatives in
nature. It has been demonstrated that coumarin and its derivatives
have numerous pharmacological activities, including anticoagulant,
vasodilator, anthelmintic, antimicrobial and antifungal capacity;
however, at present, the biological activities of 6FU have not been
extensively studied (12).
The aim of the present study was to investigate the
therapeutic potential of 6FU in inflammation. LPS-stimulated RAW
264.7 murine macrophages were used to monitor anti-inflammatory
activity by regulating the production of inflammatory mediators by
suppressing expression of the MAPKs and NF-κB signaling
pathway.
Materials and methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS) and penicillin/streptomycin solution were
purchased from Corning, Inc. (Corning, NY, USA). LPS from
Escherichia coli O111:B4, Griess reagent, Triton X-100 and
2-mercaptoethanol were obtained from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Rabbit primary antibodies [iNOS (cat. no.
13120), COX-2 (cat. no. 12282), phosphorylated (p)-ERK1/2
(Thr202/Tyr204; cat. no. 4370), p38 (cat. no. 8690), p-p38
(Thr180/Tyr182; cat. no. 4511), JNK/SAPK (cat. no. 9252),
p-JNK/SAPK (Thr183/Tyr185; cat. no. 9255), NF-κB p65 (cat. no.
8242) and GAPDH (cat. no. 5174)], horseradish peroxidase
(HRP)-conjugated anti-rabbit immunoglobulin G (IgG) secondary
antibody (cat. no. 7074) and anti-rabbit IgG (Heavy+Light; H+L),
F(ab′)2 fragment (Alexa Fluor® 488 conjugate; cat. no.
4412) were purchased from Cell Signaling Technology, Inc. (Danvers,
MA, USA). Anti-ERK1/2 rabbit primary antibody (cat. no. sc-514302)
and rabbit normal serum were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). DAPI was purchased from
Roche Diagnostics GmbH (Mannheim, Germany) and formaldehyde was
bought from Junsei Chemical Co., Ltd. (Tokyo, Japan).
ProLong® Gold Anti-fade Reagent was obtained from
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Isolation of 6FU from A. decursiva
Isolated 6FU was provided by Ali et al
(12); the isolation process was
performed as previously described. Whole A. decursiva powder
was refluxed in methanol for 3 h and filtered. Subsequently, the
filtrate was dried in a vacuum at 40°C for concentrating, followed
by suspension in distilled water. This extract was partitioned by
ethyl acetate (EtOAc), and the EtOAc fraction was served to silica
gel chromatography using dichloromethane
(CH2Cl2)-MeOH (10:1→0:1, gradient). Following
chromatography, 20 subfractions (F-1 to F-20) were obtained, F-6
was partitioned with a silica gel chromatography using
CH2Cl2-MeOH column (20:1→0:1, gradient).
Following these processes, 6FU was obtained. The presence of a
single compound that was 6FU were confirmed by nuclear magnetic
resonance studies.
Cell culture
The murine RAW 264.7 macrophage cell line and the
293 human kidney cell line were obtained from American Type Culture
Collection (Manassas, VA, USA) and incubated with DMEM containing
10% FBS and 1% penicillin/streptomycin solution at 37°C with 5%
CO2 and a humidified atmosphere.
Cell viability
The cell viability of 6FU in RAW 264.7 and 293 cells
were measured by a WST-1 assay (13-15). In total, 1×104 RAW
264.7 and 293 cells were seeded in each well of 96-well cell
culture plates. RAW 264.7 cells were cultured for 24 h with or
without 1 µg/ml LPS and 50 or 100 µM 6FU. 293 cells
were incubated with or without 25, 50 and 100 µM 6FU for 24
h. Following incubation, 10 µl EZ-cytox Cell Viability Assay
Solution WST-1® (Daeil Lab Service Co., Ltd., Seoul,
Korea) was added to each well and incubated for 3 h. Subsequently,
the absorbance was measured using a micro-plate reader (Molecular
Devices, LLC, Sunnyvale, CA, USA) at 460 nm.
Nitrite assay
The nitrite concentration in the medium was measured
with the Griess reaction (13-15). RAW 264.7 cells were seeded in
24-well cell culture plates (5×104 cells/well) and
pre-treated with 10, 25 and 50 µM 6FU for 2 h and further
incubated with 1 µg/ml LPS for 24 h. The supernatant of each
well (100 µl) was transferred to 96-well plates and Griess
reagent was added in the dark. Absorbance was measured at 540 nm
and the calculated nitrate concentration was considered as an
indicator of NO production.
Western blot analysis
To perform western blot analysis, RAW 264.7 cells
were pre-treated with 6FU for 2 h and stimulated with or without
LPS (1 µg/ml) for 6 and 24 h. Whole cells were harvested and
lysed with the cell lysis buffer [50 mM Tris-Cl (pH 7.5), 150 mM
NaCl, 1 mM DTT, 0.5% NP-40, 1% Triton X-100, 1% deoxycholate and
0.1% SDS; Intron Biotechnology, Inc., Seongnam, Korea] and the
lysates were subsequently centrifuged at 18,000 × g for 20 min.
Separate nuclear and cytoplasmic proteins were obtained using
NE-PER® nuclear and cytoplasmic extraction reagents
(Thermo Fisher Scientific, Inc.) according to the manufacturer’s
protocol. The protein concentration in the cell lysates were
measured using Bradford reagent (Biosesang, Inc., Seongnam, Korea).
An equal amount (20 µg) of the prepared proteins were
separated by 12% SDS-PAGE and subsequently transferred to a
nitrocellulose membrane (Pall Life Sciences, Ann Harbor, MI, USA).
Following blocking with 1X PBST buffer containing 5% skim milk for
2 h at room temperature, the membranes were incubated overnight
with primary rabbit antibodies [1:1,000 with 1X PBS containing 5%
bovine serum albumin (BSA; BioShop Canada, Inc., Burlington, ON,
Canada) and 0.1% Tween-20] at 4°C. The membranes were washed three
times with PBST, followed by incubation with HRP-conjugated
anti-rabbit IgG secondary antibodies (1:1,000 with 1X PBS
containing 5% BSA and 0.1% Tween-20) for 1 h at room temperature.
The membranes were developed on X-ray film for visualization using
an enhanced chemiluminescent detection solution (Pierce; Thermo
Fisher Scientific, Inc.).
Immunofluorescence (IF) staining
In total, 1×105 cells were plated at
cover-glass bottom dishes (SPL Life Sciences, Pocheon, Korea) and
pre-treated with 25 µM 6FU for 30 min (16). To investigate the nuclear
translocation activity of p-ERK1/2 or NF-κB, LPS was treated for 6
or 24 h respectively. Subsequently, for pre-fixing, cells were
stained with 1 µg/ml DAPI diluted in methanol (99.8%) and
incubated for 15 min at 37°C, followed by washing with PBS buffer
and fixed with 4% formaldehyde for 15 min at room temperature.
Following incubation, these cells were blocked with 5% rabbit
normal serum containing 0.3% Triton X-100 in 1× PBS for 1 h in the
dark and incubated with the anti-ERK1/2 (Thr202/Tyr204) or -NF-κB
p65 primary antibody (1:2,000 with 1× PBS containing 0.3% Triton
X-100) at 4°C overnight. Following the reaction, the cells were
washed with 1× PBS and incubated for 50 min with anti-rabbit IgG
(H+L), F(ab′)2 fragment (Alexa Fluor® 488 conjugate) as
secondary antibodies (0.5 µg/ml with 1× PBS containing 0.3%
Triton X-100) at room temperature in the dark. Following staining,
cells were mounted using ProLong® Gold Anti-fade
Reagent. Stained cells were observed using Carl Zeiss LSM 710
confocal laser scanning microscope (Carl Zeiss AG, Oberkochen,
Germany; magnification, ×400).
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
For total RNA extraction, RAW 264.7 cells were
pre-treated with 6FU (25 and 50 µM) prior to stimulation
with LPS (1 µg/ml), subsequently RNA was extracted with
2-mercaptoethanol the and RNeasy plus mini kit, according to the
manufacturer’s protocol (Qiagen GmbH, Hilden, Germany). The
concentration of total RNA was measured using a nanodrop (Mecasys
Co., Ltd., Daejeon, Korea) and 2 µg RNA was synthesized to
cDNA using a SuPrimeScript RT Premix (GeNet Bio, Inc., Daejeon,
Korea) under the following conditions; 50°C for 60 min and 70°C for
10 min. The cDNA was amplified using Prime Taq Premix (GeNet Bio,
Inc.) with specific primers presented in Table I, according to the manufacturer’s
protocols (95°C for 30 sec, 55°C for 30 sec, and 72°C for 30 sec,
30 cycles). Amplified PCR products were stained with ethidium
bromide (Sigma-Aldrich; Merck KGaA) and visualized in a 2% agarose
gel using ImageMaser® VDS Software version 3.0 in
ImageMaster® VDS GE Healthcare, Chicago, IL, USA).
 | Table IPrimer sequences for reverse
transcription-polymerase chain reaction. |
Table I
Primer sequences for reverse
transcription-polymerase chain reaction.
| Genes | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| TNF-α |
ACGGCATGGATCTCAAAGAC |
CGGACTCCGCAAAGTCTAAG |
| IL-1β |
CAGGCAGGCAGTATCACTCA |
AGGCCACAGGTATTTTGTCG |
| IL-6 |
AACGATGATGCACTTGCAGA |
CTCTGAAGGACTCTGGCTTTG |
| GAPDH |
AACTTTGGCATTGTGGAAGG |
CACATTGGGGGTAGGAACAC |
Statistical analysis
One-way analysis of variance with Dunnett’s multiple
comparison tests were used for determining the statistical
significance of differences between experimental and control
groups. Analysis was performed using Prism 5.0 software (GraphPad
Software, Inc., La Jolla, CA, USA). Results are presented as the
mean ± standard deviation and all experiments were performed in
triplicates independently. P<0.05 was considered to indicate a
statistically significant difference.
Results
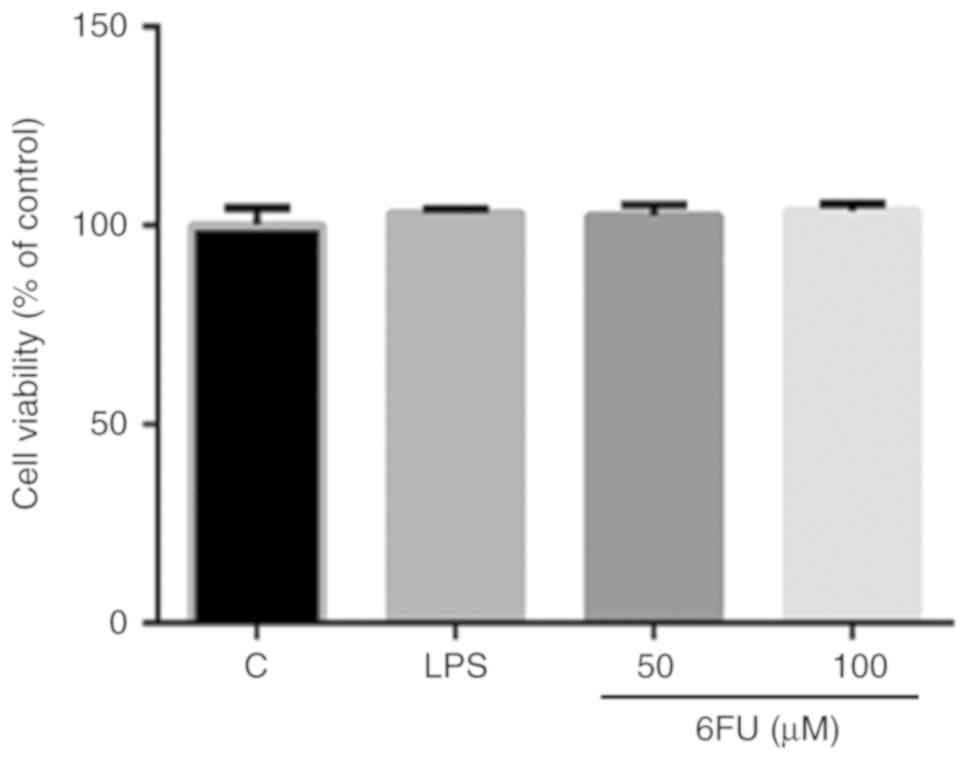
Effect of 6FU on cell viability in RAW
264.7 and 293 cells
The cell viability of RAW 264.7 and 293 cells was
measured by a WST-1 assay. RAW 264.7 cells were treated with or
without 6FU (50 and 100 µM) and LPS (1 µg/ml) for 24
h. 293 cells were incubated with or without various concentrations
of 6FU (25, 50 and 100 µM) for 24 h. As presented in
Fig. 1, 6FU and LPS did not
demonstrate any cytotoxicity on RAW 264.7 cells. Additionally, in
293 cells, the cell viability of 6FU treated cells was 99.8, 93.9
and 88.1% at 25, 50 and 100 µM concentrations, respectively
(data not shown). Therefore, <100 µM 6FU was used for
investigating its anti-inflammatory capacity in the absence of
cytotoxicity.
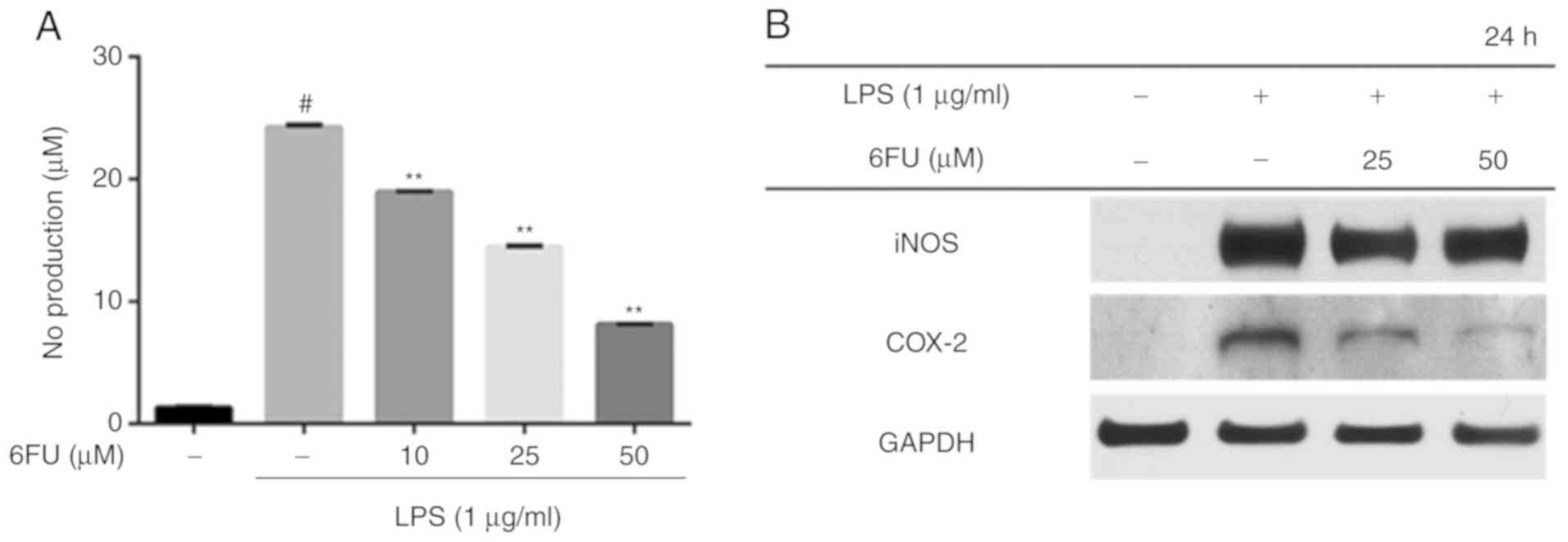
Effect of 6FU on NO production level in
RAW 264.7 cells
RAW 264.7 cells were pretreated with or without 6FU
for 2 h and subsequently stimulated with LPS for 24 h in order to
evaluate the NO production level. The level of NO secretion was
significantly increased in LPS-stimulated cells compared with
non-stimulated cells (Fig. 2A;
P<0.01). However, the expression level of NO was decreased by
treatment with 6FU in a dose-dependent manner (Fig. 2A). Western blot analysis was
performed to investigate whether 6FU has an ability to modulate the
expression of pro-inflammatory enzymes, including iNOS and COX-2.
The results demonstrated that 6FU downregulated the expression of
iNOS and COX-2 in contrast with LPS only-treated cells (Fig. 2B).
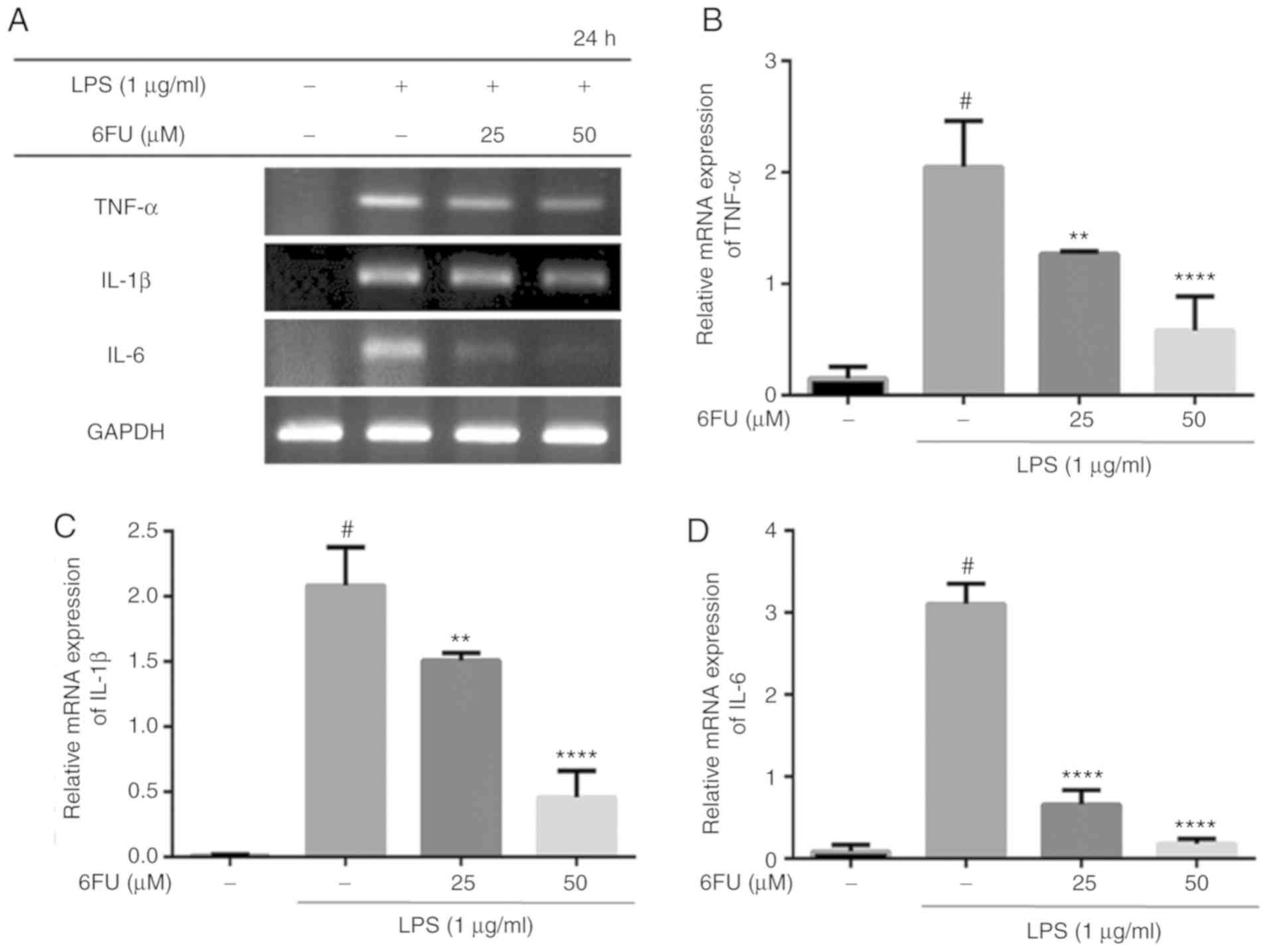
Effect of 6FU on mRNA expression of
pro-inflammatory cytokines
As presented in Fig.
3, 6FU significantly suppressed the mRNA expression of
pro-inflammatory cytokines, including IL-6, TNF-α and IL-1β,
compared with LPS-stimulated RAW264.7 cells (P<0.01).
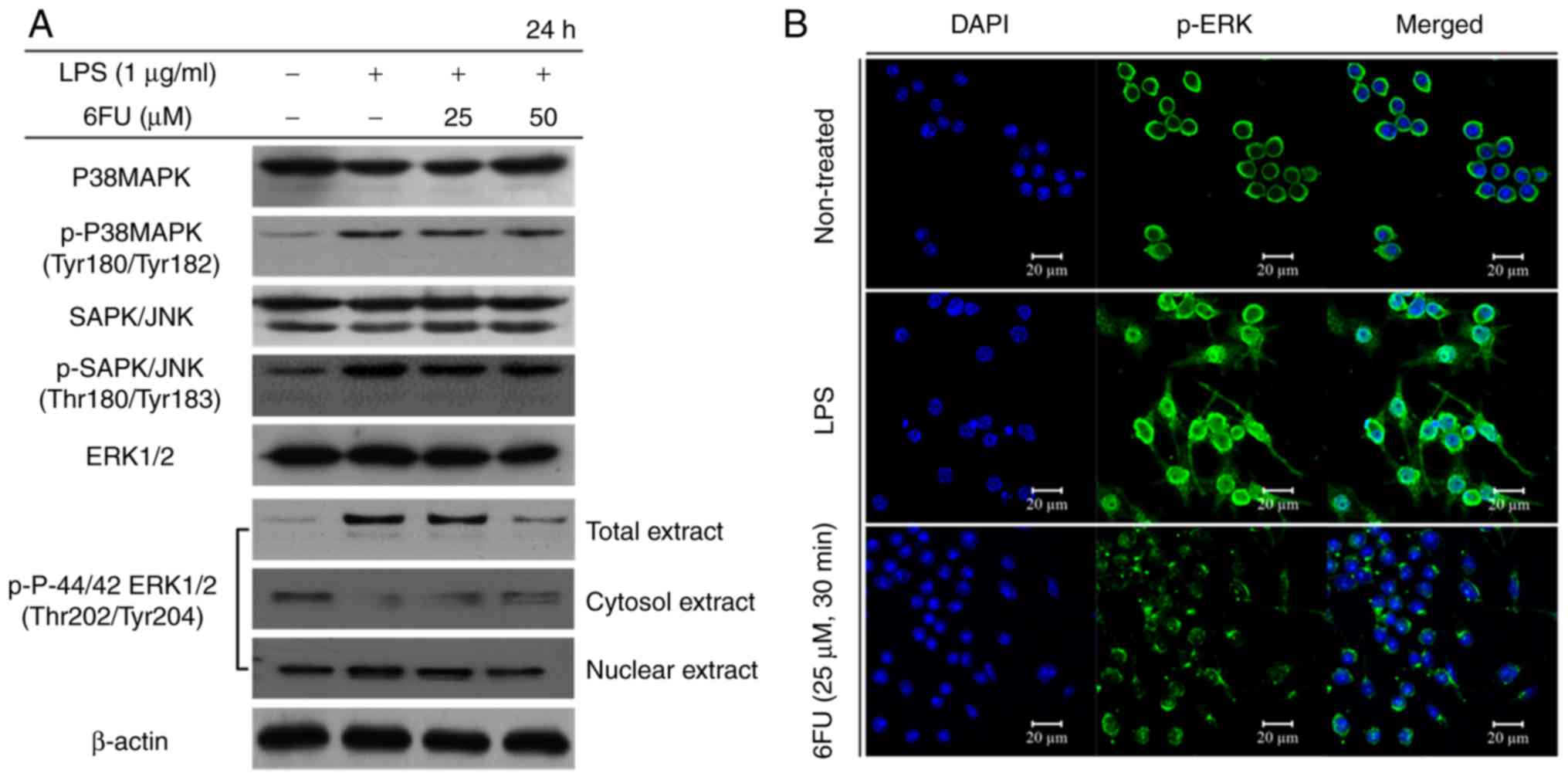
Effect of 6FU on LPS-induced
phosphorylation and activation of MAPKs
The LPS-induced phosphorylation level of MAPKs,
including p-ERK, p38 and JNK were measured by western blot
analysis. In LPS only-treated RAW 264.7 cells, the phosphorylation
level of ERK, p38 and JNK were increased. However, only the
expression of p-ERK1/2 was markedly decreased in the 6FU-treated
LPS-stimulated RAW264.7 cells in a dose dependent manner compared
with the phosphorylation level of p38 and JNK (Fig. 4A). In addition, the translocation
of phosphorylated ERK1/2 to the nucleus was inhibited following
pretreatment with 6FU in LPS-stimulated RAW 264.7 cells by western
blotting and IF staining (Fig.
4).
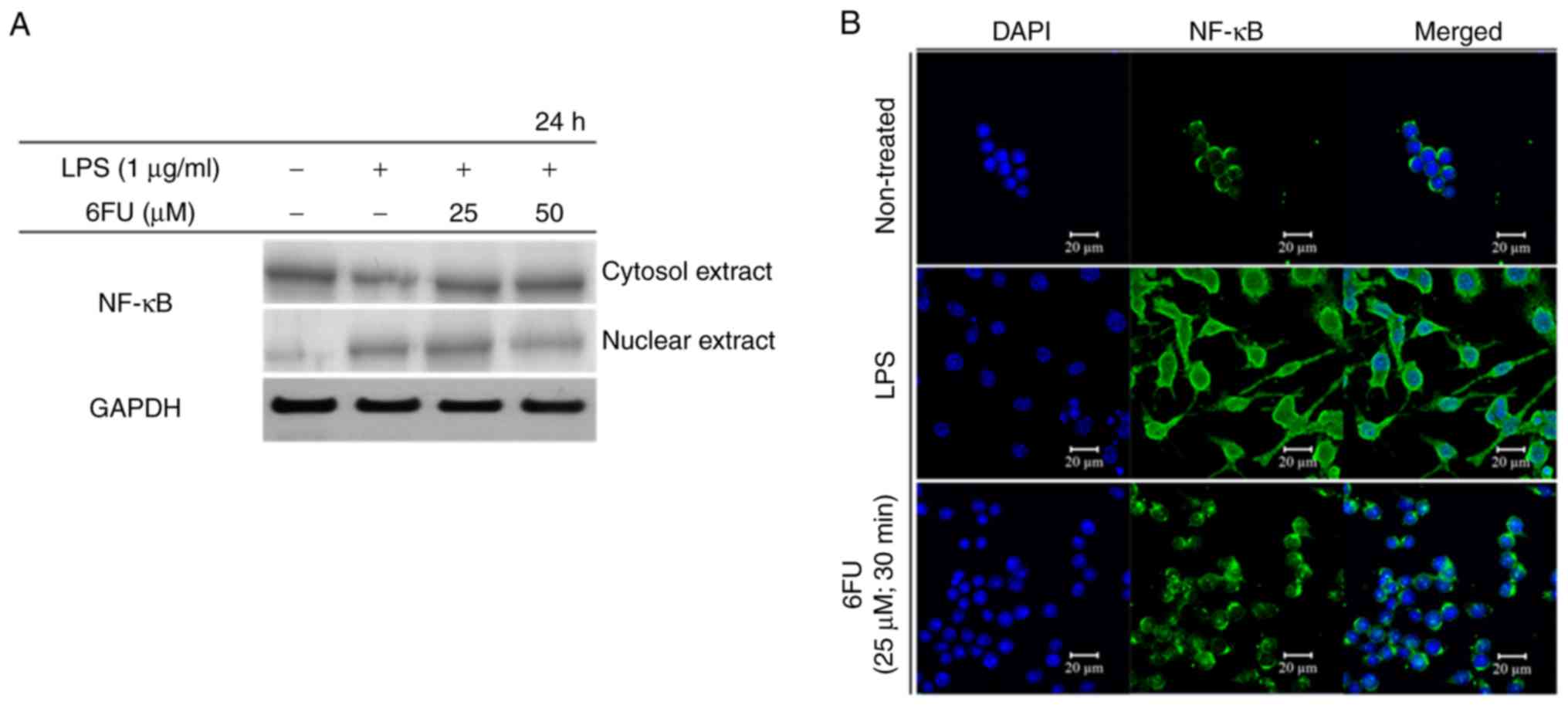
Effect of 6FU on LPS-induced activation
and translocation of NF-κB
To investigate the activity of 6FU on nuclear
translocation of NF-κB, western blot analysis and IF staining were
performed. Fig. 5A demonstrated
that 6FU decreased the concentration of NF-κB in the nucleus in
LPS-stimulated RAW 264.7 macrophages. In contrast, the expression
level of NF-κB in the cytoplasm was upregulated by 6FU.
Furthermore, 6FU inhibited nuclear translocation activity of NF-κB
in LPS-treated cells (Fig. 5B).
Therefore, 6FU decreased the expression and nuclear translocation
of NF-κB in LPS-stimulated macrophages.
Discussion
The aim of the present study was to investigate the
anti-inflammatory properties of 6FU. The cytotoxicity of 6FU on RAW
264.7 and 293 cell lines was determined. The results demonstrated
that 6FU did not exhibit any cytotoxicity on RAW 264.7 cells ≤100
µM. In 293 cells, 6FU did not demonstrate any significant
cytotoxic effect ≤50 µM. However, 100 µM 6FU in 293
cells resulted in 88.1% cell viability. These results suggested
that ≤50 µM 6FU did not demonstrate any cytotoxicity on the
murine and human cell lines, thus, ≤50 µM 6FU was used for
further investigation. It was investigated whether 6FU may regulate
production of NO in LPS-stimulated RAW 264.7 murine macrophages, as
NO is one of the principal contributors to the formation of
reactive nitrogen species and mediates the inflammatory response
(17,18). NO production was decreased by 6FU
without cytotoxic effects, compared with LPS-only-treated RAW264.7
cells.
It has been demonstrated that iNOS catalyzes the
formation and release of a large amount of NO, and COX-2 serves an
essential role in the inflammatory response as a precursor of
various biological active mediators, including PGE2 (19,20). The present results demonstrated
that 6FU markedly inhibited the protein expression level of iNOS
and COX-2 against a stimulus of inflammation in RAW 264.7 cells.
Therefore, it was suggested that 6FU has an ability to suppress
production of NO and PGE2 through downregulation of iNOS and COX-2
expression. Additionally, endotoxins, including LPS in the present
study, stimulate macrophages to express cytokines, including TNF-α,
IL-1β and IL-6, which activate inflammation-associated signaling
pathways (21,22). It was demonstrated that the mRNA
expression levels of TNF-α, IL-1β and IL-6 were significantly
decreased by 6FU compared with LPS-stimulated cells. These results
suggested that 6FU attenuated the inflammatory response by
regulating expression of iNOS, COX-2 and numerous pro-inflammatory
cytokines.
Based on these results, it was hypothesized that 6FU
may regulate the cellular signaling pathway, which is associated
with the production of NO and pro-inflammatory cytokines in
macrophages. To further investigate the mechanisms of NO and
cytokine production, the expressions of MAPK signaling proteins
were examined, which have been demonstrated to regulate various
cellular activities, cell proliferation, differentiation, migration
and the inflammatory response (6). MAPK signaling pathway proteins
consist of ERK1/2, JNK/SAPK and p38, which mediate intracellular
signaling initiated by extracellular stimuli. Among them, activated
ERK1/2 serves an essential role in the regulation of the
inflammatory response by promoting phosphorylation of its
downstream proteins (23,24). It was identified that p-ERK1/2 was
markedly decreased by treatment with 6FU; however, expression of
p-p38 and p-JNK/SAPK did not demonstrate any difference.
Furthermore, the nuclear translocation activity of p-ERK1/2 was
inhibited by 6FU in LPS-stimulated RAW 264.7 macrophages. These
results suggested that 6FU inhibits the ERK-mediated inflammatory
response by suppressing phosphorylation and translocation of
ERK1/2.
NF-κB additionally serves as one of the key
regulators of the inflammatory gene expression, which induces the
synthesis of pro-inflammatory cytokines, including iNOS and COX-2
(8). It has been investigated
that inflammatory stimuli activate NF-κB translocation from the
cytoplasm to the nucleus, and its transcriptional activity through
degradation of inhibitor of NF-κB by proteasomes (25,26). It was observed that 6FU suppressed
the translocation activity of NF-κB from the cytoplasm to the
nucleus by western blot analysis and IF staining, which
demonstrated the anti-inflammatory capacity of 6FU.
In conclusion, the present results demonstrated that
6FU downregulates the production of NO and pro-inflammatory
cytokines, including TNF-α, IL-1β and IL-6 by inhibition of the
inflammation-associated signaling pathways, including ERK1/2 and
NF-κB. Future in vivo animal studies are required to further
elucidate the detailed protein expression associated with
inflammation. In the present study, it was identified that 6FU has
potential as one of the therapeutic candidates for chronic
inflammation.
Acknowledgments
Not applicable.
Abbreviations:
|
COX-2
|
cyclooxygenase-2
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
iNOS
|
inducible nitric oxide synthase
|
|
JNK
|
c-Jun N-terminal kinase
|
|
LPS
|
lipopolysaccharide
|
|
MAPK
|
mitogen-activated protein kinase
|
|
NF-κB
|
nuclear factor κ-light-chain-enhancer
of activated B cells
|
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
JSC and GDK contributed to the design of the study
and managed the experiments. HAJ analyzed the experimental data.
C-WK conducted the nitrite assay. N-HK performed the
immunofluorescence staining. HWC conducted the cell viability
assay. S-BK and M-JK conducted the western blot analysis and
reverse transcription-polymerase chain reaction assays, and revised
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wieser V, Moschen AR and Tilg H:
Inflammation, cytokines and insulin resistance: A clinical
perspective. Arch Immunol Ther Exp (Warsz). 61:119–125. 2013.
View Article : Google Scholar
|
|
2
|
Shacter E and Weitzman SA: Chronic
inflammation and cancer. Oncology (Williston Park). 16:217–229.
2002.
|
|
3
|
Feghali CA and Wright TM: Cytokines in
acute and chronic inflammation. Front Biosci. 2:u12–d26. 1997.
View Article : Google Scholar
|
|
4
|
Lin WW and Karin M: A cytokine-mediated
link between innate immunity, inflammation, and cancer. J Clin
Invest. 117:1175–1183. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dai GF, Zhao J, Jiang ZW, Zhu LP, Xu HW,
Ma WY, Chen XR, Dong RJ, Li WY and Liu HM: Anti-inflammatory effect
of novel andrographolide derivatives through inhibition of NO and
PGE2 production. Int Immunopharmacol. 11:2144–2149. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaminska B: MAPK signalling pathways as
molecular targets for anti-inflammatory therapy-from molecular
mechanisms to therapeutic benefits. Biochim Biophys Acta.
1754:253–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koo HJ, Yoon WJ, Sohn EH, Ham YM, Jang SA,
Kwon JE, Jeong YJ, Kwak JH, Sohn E, Park SY, et al: The analgesic
and anti-inflammatory effects of Litsea japonica fruit are mediated
via suppression of NF-κB and JNK/p38 MAPK activation. Int
Immunopharmacol. 22:84–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Makarov SS: NF-kappaB as a therapeutic
target in chronic inflammation: Recent advances. Mol Med Today.
6:441–448. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang J, Zhang Y, Cao X, Fan J, Li G, Wang
Q, Diao Y, Zhao Z, Luo L and Yin Z: Lycorine inhibits
lipopolysaccharide-induced iNOS and COX-2 up-regulation in RAW264.7
cells through suppressing P38 and STATs activation and increases
the survival rate of mice after LPS challenge. Int Immunopharmacol.
12:249–256. 2012. View Article : Google Scholar
|
|
10
|
Wang D and DuBois RN: The role of COX-2 in
intestinal inflammation and colorectal cancer. Oncogene.
29:781–788. 2010. View Article : Google Scholar
|
|
11
|
Lee KC, Chang HH, Chung YH and Lee TY:
Andrographolide acts as an anti-inflammatory agent in
LPS-stimulated RAW264.7 macrophages by inhibiting STAT3-mediated
suppression of the NF-κB pathway. J Ethnopharmacol. 135:678–684.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ali MY, Seong SH, Reddy MR, Seo SY, Choi
JS and Jung HA: Kinetics and molecular docking studies of 6-formyl
umbelliferone isolated from angelica decursiva as an inhibitor of
cholinesterase and BACE1. Molecules. 22:E16042017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shan J, Fu J, Zhao Z, Kong X, Huang H, Luo
L and Yin Z: Chlorogenic acid inhibits lipopolysaccharide-induced
cyclo-oxygenase-2 expression in RAW264.7 cells through suppressing
NF-kappaB and JNK/AP-1 activation. Int Immunopharmacol.
9:1042–1048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim YS, Ahn CB and Je JY:
Anti-inflammatory action of high molecular weight mytilus edulis
hydrolysates fraction in LPS-induced RAW264.7 macrophage via NF-κB
and MAPK pathways. Food Chem. 202:9–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ham YM, Ko YJ, Song SM, Kim J, Kim KN, Yun
JH, Cho JH, Ahn G and Yoon WJ: Anti-inflammatory effect of
litsenolide B2 isolated from litsea japonica fruit via suppressing
NF-κB and MAPK pathways in LPS-induced RAW264.7 cells. J Functional
Food. 13:80–88. 2015. View Article : Google Scholar
|
|
16
|
Tang S, Shen XY, Huang HQ, Xu SW, Yu Y,
Zhou CH, Chen SR, Le K, Wang YH and Liu PQ: Cryptotanshinone
suppressed inflammatory cytokines secretion in RAW264.7 macrophages
through inhibition of the NF-κB and MAPK signaling pathways.
Inflammation. 34:111–118. 2011. View Article : Google Scholar
|
|
17
|
Kaur H and Halliwell B: Evidence for
nitric oxide-mediated oxidative damage in chronic inflammation
nitrotyrosine in serum and synovial fluid from rheumatoid patients.
FEBS Lett. 350:9–12. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nemirovskiy OV, Radabaugh M R, Aggarwal P,
Funckes-Shippy CL, Mnich SJ, Meyer DM, Sunyer T, Rodney Mathews W
and Misko TP: Plasma 3-nitrotyrosine is a biomarker in animal
models of arthritis: Pharmacological dissection of iNOS’role in
disease. Nitric Oxide. 20:150–156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng AW, Stabler TV, Bolognesi M and
Kraus VB: Selenomethionine inhibits IL-1β inducible nitric oxide
synthase (iNOS) and cyclooxygenase 2 (COX2) expression in primary
human chondrocytes. Osteoarthritis Cartilage. 19:118–125. 2011.
View Article : Google Scholar
|
|
20
|
Camacho-Barquero L, Villegas I,
Sánchez-Calvo JM, Talero E, Sánchez-Fidalgo S, Motilva V and
Alarcón de la Lastra C: Curcumin, a Curcuma longa constituent, acts
on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic
experimental colitis. Int Immunopharmacol. 7:333–342. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dumitru CD, Ceci JD, Tsatsanis C,
Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA,
Copeland NG, Kollias G and Tsichlis PN: TNF-alpha induction by LPS
is regulated posttranscriptionally via a Tpl2/ERK-dependent
pathway. Cell. 103:1071–1083. 2000. View Article : Google Scholar
|
|
22
|
Zhang WJ, Wei H, Hagen T and Frei B:
Alpha-lipoic acid attenuates LPS-induced inflammatory responses by
activating the phosphoinositide 3-kinase/Akt signaling pathway.
Proc Natl Acad Sci USA. 104:4077–4082. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Plotnikov A, Zehorai E, Procaccia S and
Seger R: The MAPK cascades: Signaling components, nuclear roles and
mechanisms of nuclear translocation. Biochim Biophys Acta.
1813:1619–1633. 2011. View Article : Google Scholar
|
|
25
|
Hseu YC, Wu FY, Wu JJ, Chen JY, Chang WH,
Lu FJ, Lai YC and Yang HL: Anti-inflammatory potential of antrodia
camphorata through inhibition of iNOS, COX-2 and cytokines via the
NF-kappaB pathway. Int Immunopharmacol. 5:1914–1925. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shie PH, Wang SY, Lay HL and Huang GJ:
4,7-Dimethoxy-5-methyl-1,3-benzodioxole from antrodia camphorata
inhibits LPS-induced inflammation via suppression of NF-κB and
induction HO-1 in RAW264.7 cells. Int Immunopharmacol. 31:186–194.
2016. View Article : Google Scholar : PubMed/NCBI
|