Introduction
Alzheimer’s disease (AD) is a major
neurodegenerative disease characterized by significant memory
impairment in addition to other cognitive impairments and maybe
associated with mental symptoms and behavioral abnormalities
(1-3). AD is prevalent in the elderly of
>65 years of age, with 1 newly diagnosed case every 3 sec
worldwide (4). The number of
patients with AD is estimated to increase to 135.46 million by
2050, thereby leading to serious social issues in addition to the
health, economic and social burden (5).
The accumulation of amyloid β (Aβ) and mutation of
gene polymorphisms of clusterin (CLU) can be attributed to AD
(6,7). Aβ is cleaved from the amyloid
precursor protein and a mutation in CLU causes the brain to lose
its Aβ-scavenging function. Of note, an excessive accumulation of
Aβ has been detected in the brains of patients with AD (8-10).
Accumulation of Aβ can eventually cause the cytotoxic death of
nerve cells due to oxidative stress, decreased mitochondrial
membrane potential and nuclear pyknosis (11-13). At present, modulation of
Aβ-induced neurotoxicity is an an effective therapeutic approach
for the treatment of AD; however, there are no safe and effective
therapeutics for AD at present (14-16). Therefore, the identification and
evaluation of potential protective candidates for AD treatment are
necessary.
Salidroside is a major active ingredient extracted
from Rhodiola rosea (17);
it is widely employed in traditional Chinese medicine and has been
reported to exert anti-inflammatory, anti-oxidative and
anti-autophagic effects (18-20). Based on these functions, it has
been speculated that salidroside may be able to treat AD. However,
the molecular mechanisms underlying these effects of salidroside
are currently not well understood and further research is required
to clarify them. The present study aimed to confirm the anti-AD
effects of salidroside and unravel its mechanism of action. The
toxic effects of Aβ in the PC-12 cell line were established for use
as an in vitro AD model for drug evaluation (21). The results revealed that
salidroside could effectively inhibit the toxicity and apoptosis of
PC-12 cells that was induced by Aβ. Furthermore, the protective
effect of salidroside against Aβ-induced damage in PC-12 cells was
mediated by activation of the extracellular signal regulated kinase
(ERK)1/2 and protein kinase B (AKT) signaling pathways. By
promoting cell survival and proliferation, the toxic effects of Aβ
were effectively inhibited by salidroside, thereby further
demonstrating that salidroside is a potential candidate for AD
treatment.
Materials and methods
Cell viability assay
Cell viability was evaluated using cytotoxicity
assays. Briefly, PC-12 cells were seeded into 96-well plates with
5,000 cells per well and incubated with drugs or inhibitors at the
indicated concentrations for 48 h. The salidroside was added at the
concentrations of 12.5, 25, 50, 100 or 200 µM;
Aβ1-42 was added at concentrations of 0.01 to 1
µM; while the inhibitors were added at concentrations of 5
to 20 µM. A volume of 25 µl MTT solution (5 mg/ml)
was added to each well and incubated for additional 4 h. Then,
dimethyl sulfoxide (DMSO) was added to dissolve the MTT formazan
product and the absorbance was determined at 570 nm using a
SpectraMax M5 device (Molecular Devices, LLC, Sunnyvale, CA, USA).
The relative cell viability rates of the test group were calculated
vs. the untreated controls.
Lactate dehydrogenase (LDH) assay
The quantity of LDH that is released into the
incubation medium when the cell membrane is destroyed was
determined for evaluation of Aβ-induced cytotoxicity. A total of
5×103 PC-12 cells were seeded into 96-well plates. After
incubation with 50 µM salidroside for 1 h, followed by
incubation with Aβ for additional 24 h, the activity of the LDH
that was released into the medium was determined according to the
protocol of CytoTox-ONE™ Homogeneous Membrane Integrity Assay
(Promega Corporation, Madison, WI, USA). The fluorescent intensity
was determined using a microplate reader (Multiskan™ FC; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) at an excitation
wavelength of 560 nm and an emission wavelength of 590 nm. The
percentage values of the released LDH were normalized to the
control group.
Nuclear staining
PC-002D12 cells were fixed with 3.7% formaldehyde in
PBS for 5 min at room temperature and blocked with 5% bovine serum
albumin (Sangon Biotech Co., Ltd., Shanghai, China) containing 0.1%
Triton X-100 (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 30
min at room temperature. The prepared specimens were stained with
DAPI (SouthernBiotech, Birmingham, AL, USA) at a concentration of
10 µg/ml at room temperature for 2 min and then observed
under a confocal microscope (Nikon Corporation, Tokyo, Japan).
Apoptotic cells exhibited nuclear condensation with intensely
stained nuclei, while the normal cells did not exhibit nuclear
condensation (22).
Determination of intracellular reactive
oxygen species (ROS), malondialdehyde (MDA) and superoxide
dismutase (SOD) levels
Cells were seeded in 6-well plates at a density of
5×104 cells/cm2. After 24 h of incubation,
cells were exposed to 50 µM salidroside for 1 h and to 0.3
µM Aβ for 24 h. Then, some of the cells were incubated with
5 µM fluorescent probe of H2DCF-DA for 20 min and the
fluorescence emission was visualized using a fluorescence
microscope (Nikon Corporation). The other cells were analysed using
the MDA and SOD detection kits (KeyGen Biotech Co., Ltd., Nanjing,
China).
Determination of mitochondrial membrane
potential
PC-12 cells were seeded into a black 96-well plate
with 5,000 cells per well, then incubated with 50 µM
salidroside for 1 h, followed by incubation with Aβ. Following 24
h, cells were incubated with a fluorescent probe of JC-1 (Beyotime
Institute of Biotechnology, Haimen, China) at a concentration of 5
mg/ml for 15 min at 37°C and then washed twice with PBS. The
intensity of red and green fluorescence was determined using an
Infinite M200 PRO Multimode Microplate (Tecan Group, Ltd.,
Mannedorf, Switzerland) and a fluorescence microscope (Nikon
Corporation). The mitochondrial membrane potential was calculated
using the ratio of JC-1 red/green fluorescence intensity, the color
changed from red to green when apoptosis occurred and the value was
normalized to the control group.
Caspase-3/7 activity assay
Once PC-12 cells had been incubated with salidroside
for 1 h and with Aβ for 24 h, the cells were lysed with lysis
buffer of radioimmuno-precipitation assay (RIPA; KeyGen Biotech
Co., Ltd.) and centrifuged at 12,500 x g for 5 min at 4°C.
Then, the cell lysate was incubated with 2X substrate working
solution at room temperature for 30 min in 96-well plates. The
activity of caspase-3/7 was determined using the commercially
available Caspase-Glo 3/7 Assay (Invitrogen; Thermo Fisher
Scientific, Inc.). The fluorescence intensity of each sample was
normalized to the protein concentration of the sample. All the
percentage values of caspase-3/7 activities were normalized to the
control group.
Flow cytometry assay
PC-12 cell apoptosis was evaluated with the Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) Apoptosis
Detection kit (KeyGen Biotech Co., Ltd.). Upon treatment, the
adherent and non-adherent cells were harvested. The cells were then
stained with Annexin V-FITC and PI in binding buffer for 15 min.
Flow cytometric analysis was performed on a fluorescence-activated
cell sorting flow cytometer (BD Biosciences; Franklin Lakes, NJ,
USA) and the data were analyzed with Cell Quest software (version
3.4, BD Biosciences).
Western blotting
The cells were lysed with RIPA buffer, the
concentration of proteins were determined by Bicinchoninic Acid
detection kit (KeyGen Biotech Co., Ltd.). Immunoblots were
performed with samples that contained total protein (30 µg),
which had been separated by 12% SDS-PAGE gel and then transferred
onto polyvinylidene difluoride membranes (EMD Millipore, Billerica,
MA, USA). The membranes were incubated with primary antibodies
against phosphorylated (p)-ERK1/2 (1:1,000; AF1015; Affinity
Biosciences, Cincinnati, OH, USA), total (t)-ERK (1:1,000; AF6240;
Affinity Biosciences), p-AKT (1:1,000; AF0016; Affinity
Biosciences), t-AKT (1:1,000; AF4718; Affinity Biosciences), B-cell
lymphoma (BCL)-2 (1:1,000; AF6139; Affinity Biosciences), BCL2
associated X (Bax; AF0120; 1:1,000; Affinity Biosciences) and GAPDH
(1:1,000; AF7021; Affinity Biosciences), followed by incubation
with a secondary antibody of goat anti-rabbit IgG-horseradish
peroxidase (1:5,000; sc-2004; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). Blots were developed using an enhanced
chemiluminescence detection reagent (EMD Millipore). GAPDH was used
as an internal loading control.
Statistical analysis
All experiments were repeated at least in
triplicate, all data were evaluated using SPSS v.19.0 (IBM Corp.,
Armonk, NY, USA). Data are presented as the mean ± standard
deviation. One-way analysis of variance and Bonferroni’s test were
used to compare different treatment samples. P<0.05 was
considered to indicate a statistically significant difference.
Results
Salidroside suppresses
Aβ1-42-induced cytotoxicity in PC-12 cells in a
concentration-dependent manner
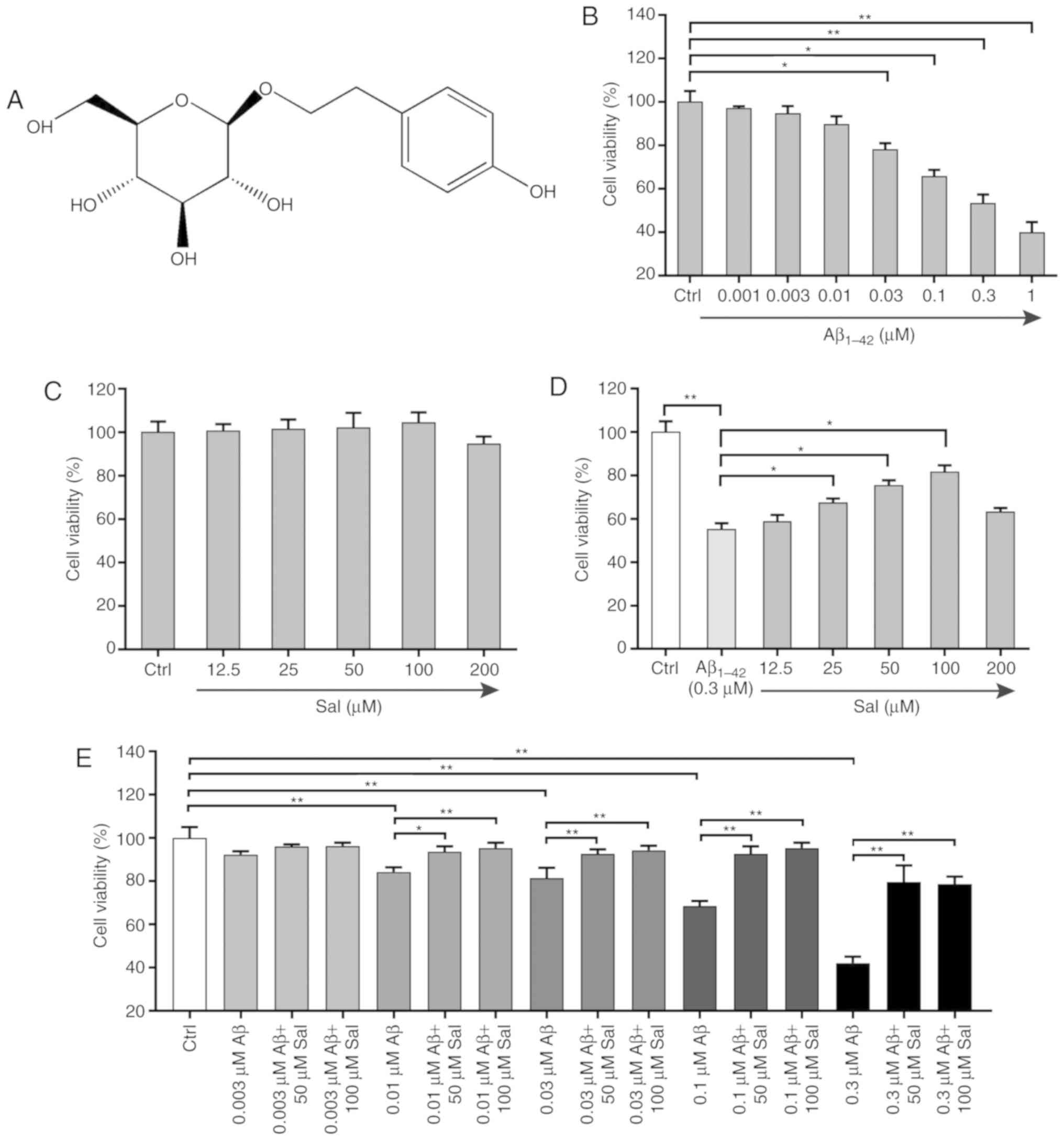
The biological activity of salidroside (Fig. 1A) was assessed by cell viability
assays. The cytotoxicity of Aβ1-42 on PC-12 cells was
first examined by MTT assay. As presented in Fig. 1B, exposure of PC-12 cells to
Aβ1-42 (0.001-1 µM) for 24 h induced a
dose-dependent decrease in cell viability, thereby demonstrating
that Aβ1-42 could induce toxicity in PC-12 cells. The
viability of PC-12 cells was only increased or slightly decreased
and the differences compared with the control group were not
significant, following treatment with salidroside (12.5-200
µM) for 24 h, thereby indicating that salidroside was not
toxic to PC-12 cells under these treatment conditions (Fig. 1C). Compared with the
Aβ1-42 group, pre-treatment with salidroside at
concentrations of 25, 50 and 100 µM significantly improved
cell viability (P<0.05; Fig.
1D). The multi-combination test results further demonstrated
that salidroside protected and rescued PC-12 cells from
Aβ1-42-induced cell death (Fig. 1E).
Salidroside suppresses
Aβ1-42-induced LDH release and apoptosis in PC-12
cells
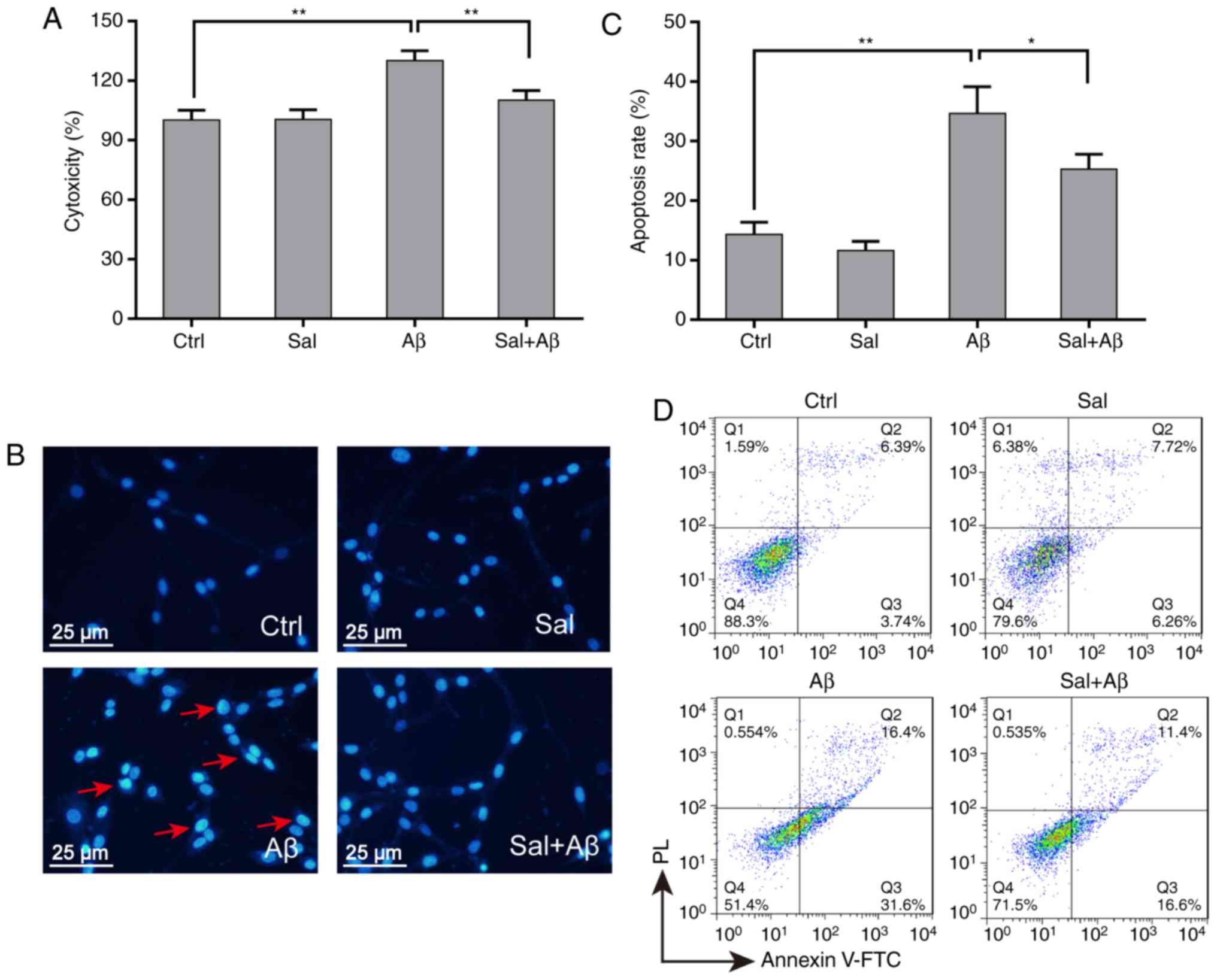
LDH assay was used for evaluation of the protective
activity of salidroside. When pre-treated with 50 µM
salidroside for 1 h, PC-12 cells exhibited a significantly reduced
Aβ1-42-induced LDH leakage (from 135-110% relative to
the control group; P<0.01; Fig.
2A). Nuclei condensation was detected in PC-12 cells upon
exposure to Aβ1-42 by DAPI staining (Fig. 2B). Pre-treatment with 50 µM
salidroside significantly inhibited Aβ1-42-induced
apoptosis compared with Aβ1-42 alone (from 35-23%;
P<0.05 Fig. 2C). To further
evaluate the influence of salidroside in response to oxidative
stress in PC-12 cells, the cells were treated with
Aβ1-42 for 24 h following being incubated with
salidroside for 1 h. Cell apoptosis rates were detected by flow
cytometry (Fig. 2D). The
apoptosis rate of PC-12 cells increased following 24 h of
Aβ1-42 treatment, while salidroside treatment could
protect PC-12 cells from Aβ1-42-induced apoptosis.
Salidroside affects Aβ1-42
induced ROS, MDA and SOD productions in PC-12 cells
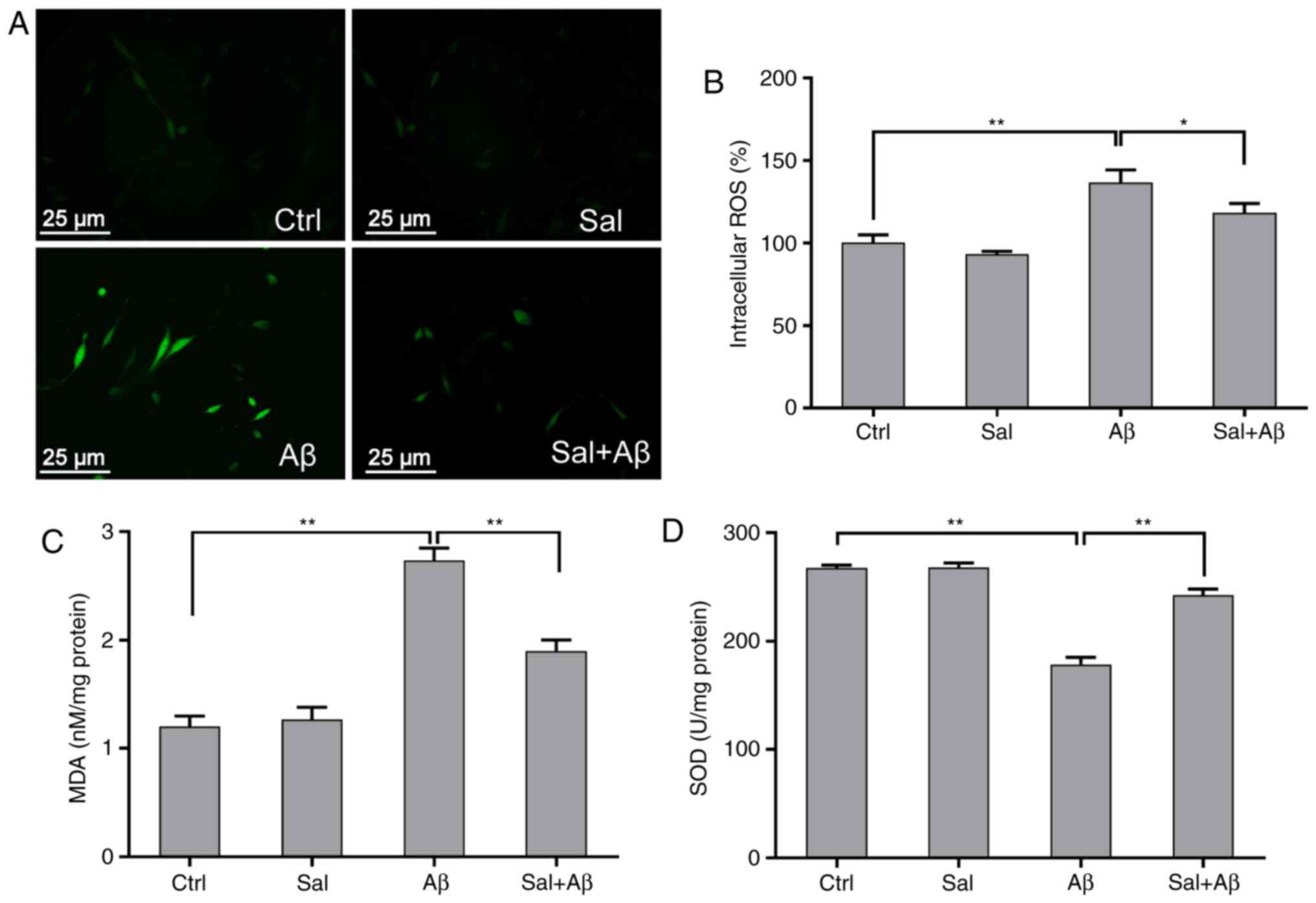
Since the toxicity of Aβ1-42 may be
affected by the generation of ROS, MDA and SOD (23), the inhibitory/promotional effect
of salidroside on ROS, MDA, and SOD production was examined. PC-12
cells were pre-treated with or without 50 µM salidroside for
1 h and then treated with Aβ1-42 for 24 h. Differences
in fluorescence intensity were observed among the different groups
(Fig. 3A). The results revealed
that Aβ1-42 could effectively induce intracellular ROS
generation, whereas salidroside could significantly inhibit the
generation of ROS induced by Aβ1-42 (from 145 to 125%
relative to control group; P<0.05; Fig. 3B). While the MDA detection results
demonstrated that the MDA levels were significantly increased due
to oxidative stress injury but significantly decreased by
salidroside pre-treatment (P<0.01; Fig. 3C). By contrast, the SOD activity
in salidroside-pretreated cells increased compared with Aβ-treated
cells (Fig. 3D).
Salidroside improves
Aβ1-42-induced alterations in the mitochondrial membrane
potential and caspase-3/7 activity
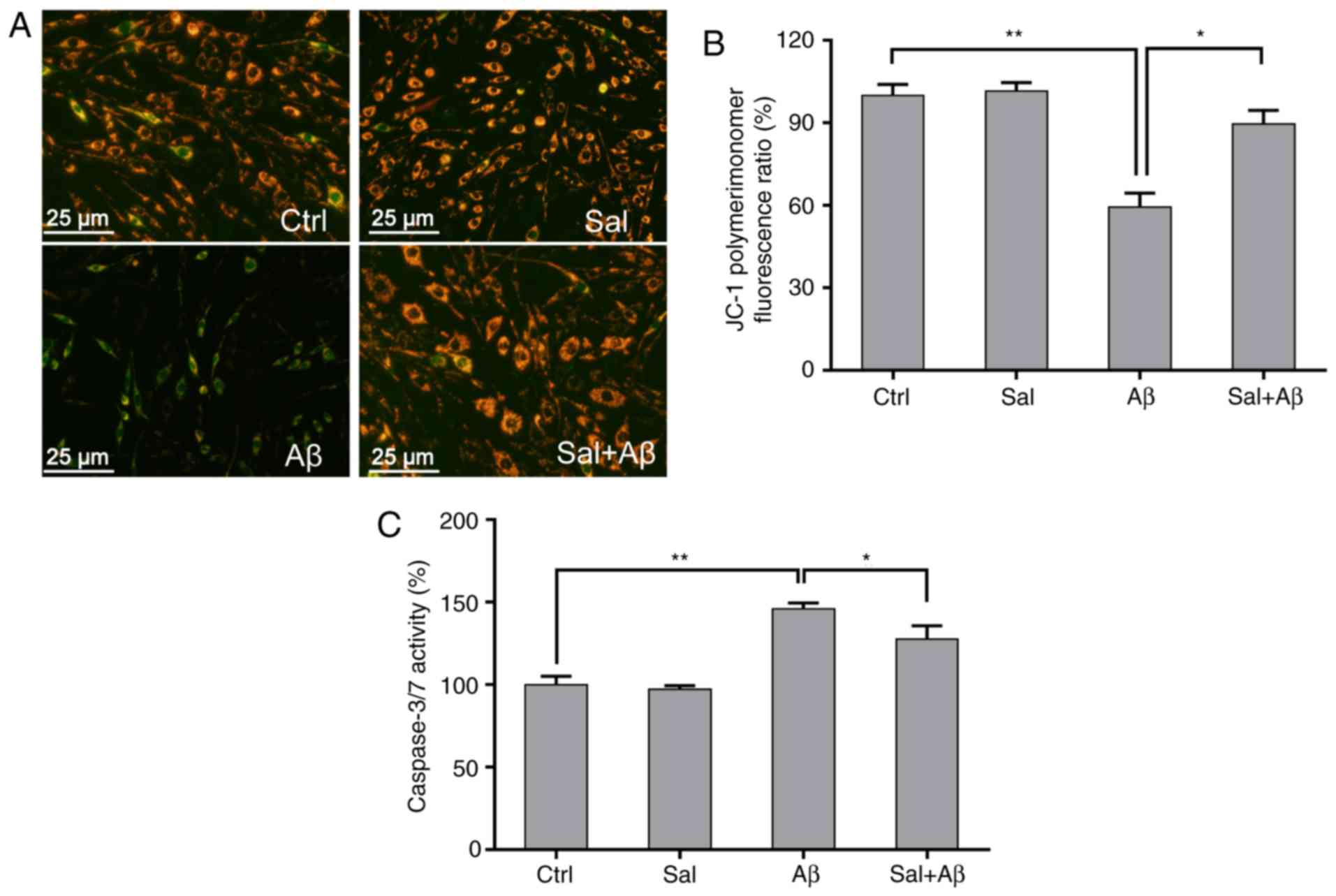
Previous studies reported that the loss of the
mitochondrial membrane potential was involved in the progression of
neuron apoptosis caused by Aβ1-42 during AD (24,25). In the present study, mitochondrial
membrane permeability was detected using the JC-1 probe in order to
evaluate the anti-apoptotic effects of salidroside. Red and green
fluorescence represented high mitochondrial membrane permeability
in viable cells and low mitochondrial membrane permeability in
apoptotic cells, respectively (Fig.
4A). When incubated with Aβ1-42 for 24 h, the
mitochondrial membrane permeability was significantly depolarized
in Aβ1-42-treated PC-12 cells compared with the control
(P<0.01), whereas pre-treatment with salidroside effectively
prevented the loss of mitochondrial membrane permeability (Fig. 4B). Treatment of PC-12 cells with
0.3 µM Aβ1-42 for 24 h triggered a significant
increase in caspase-3/7 activity compared with the control
(P<0.01), whereas pre-treatment with 50 µM salidroside
for 1 h significantly inhibited caspase-3/7 activity (P<0.05;
Fig. 4C).
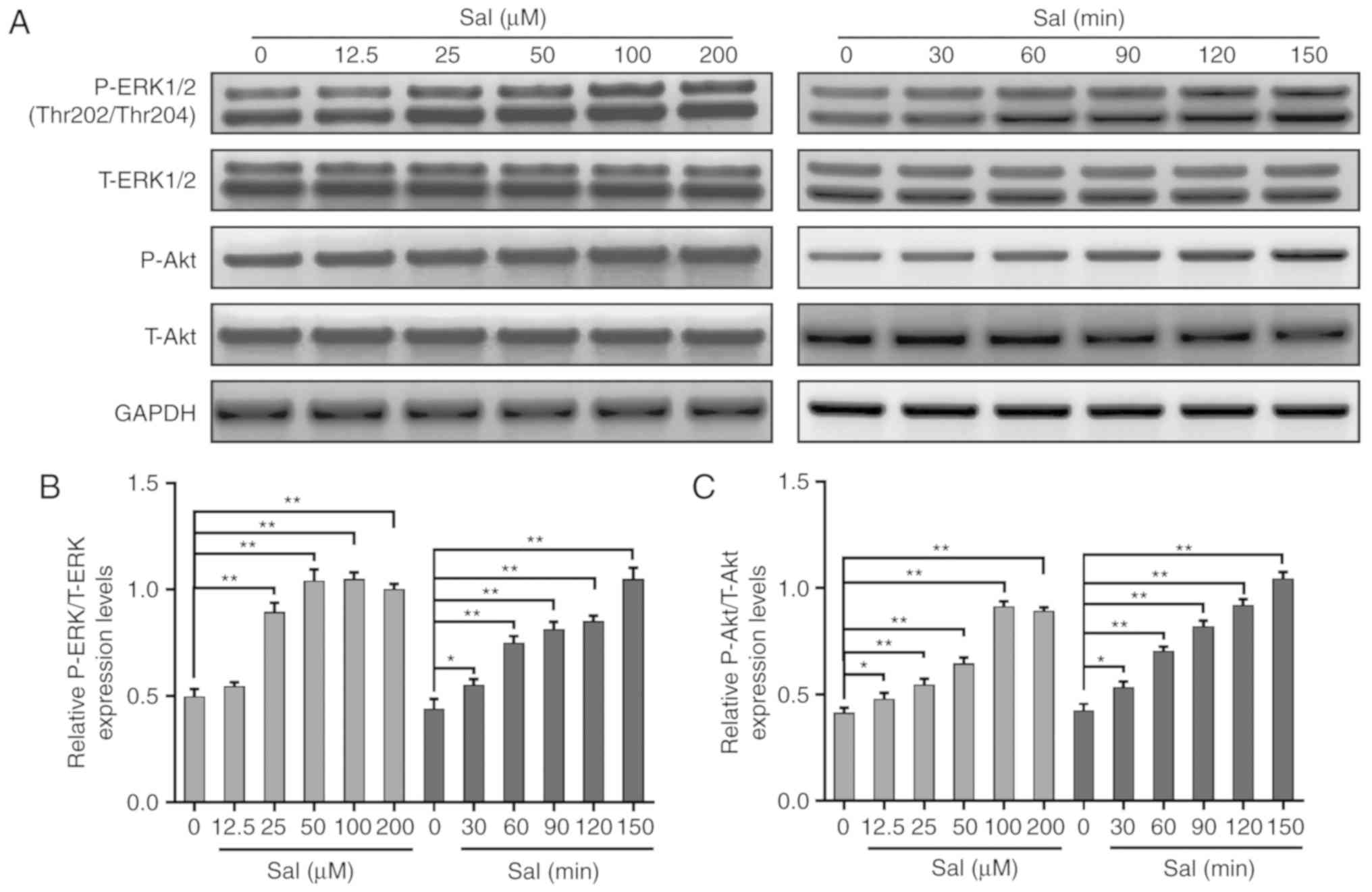
Salidroside stimulates the
phosphorylation of ERK1/2 and AKT in a time and
concentration-dependent manner in PC-12 cells
Since the ERK1/2 and AKT signaling pathways are
classical apoptosis-associated pathways, the present study
evaluated whether the anti-apoptotic effect of salidroside was
mediated by the ERK1/2 and AKT signaling pathways. The present
study examined the phosphorylated and total expression levels of
ERK1/2 and AKT in PC-12 cells treated with salidroside by western
blotting. As presented in Fig.
5A-C, the phosphorylation levels of ERK1/2 and AKT gradually
increased upon the addition of salidroside in a time and
dose-dependent manner.
Activation of the ERK1/2 and AKT
signaling pathways mediates the protective effects of salidroside
in Aβ1-42-induced PC-12 cells
To confirm the roles of the ERK1/2 and AKT signaling
pathways in the inhibitory effect of salidroside on
Aβ1-42-induced apoptosis in PC-12 cells, the specific
inhibitors of the ERK1/2 and AKT signaling pathways, PD98059 and
LY294002, respectively, were used. The two pathway inhibitors
blocked the protective effects of salidroside in cells treated with
Aβ1-42 (Fig. 6A).
PC-12 cells were pre-treated with PD98059 or LY294002 (5, 10 and 20
µM) for 30 min and then treated with 50 µM
salidroside for 1 h, and the viability of cells was determined by
MTT assay 24 h later. The protective effect of salidroside was
blocked in the presence of an all concentrations of PD98059 and
LY294002 (Fig. 6B and C). Upon
staining with DAPI, pyknosis was detected in treated PC-12 cells
(Fig. 6D). Pre-treatment with 50
µM salidroside reversed the effect of Aβ1-42 on
PC-12 cells, whereas incubation with PD98059 or LY294002 abolished
the protective effect of salidroside (Fig. 6E). The western blot results
further demonstrated that upon treatment of cells with
Aβ1-42, the incubation with PD98059 or LY294002 for 24 h
inhibited the phosphorylation of ERK1/2 and AKT, respectively.
Salidroside reversed the decrease in the phosphorylation levels of
ERK1/2 and AKT induced by Aβ1-42, whereas PD98059 and
LY294002 pre-treatment blocked the reversing effects of salidroside
(Fig. 6F). The anti Aβ-induced
apoptotic effect of salidroside may be mediated by other signaling
pathways, such as BCL-2/Bax (Fig.
S1); however, further investigation is required.
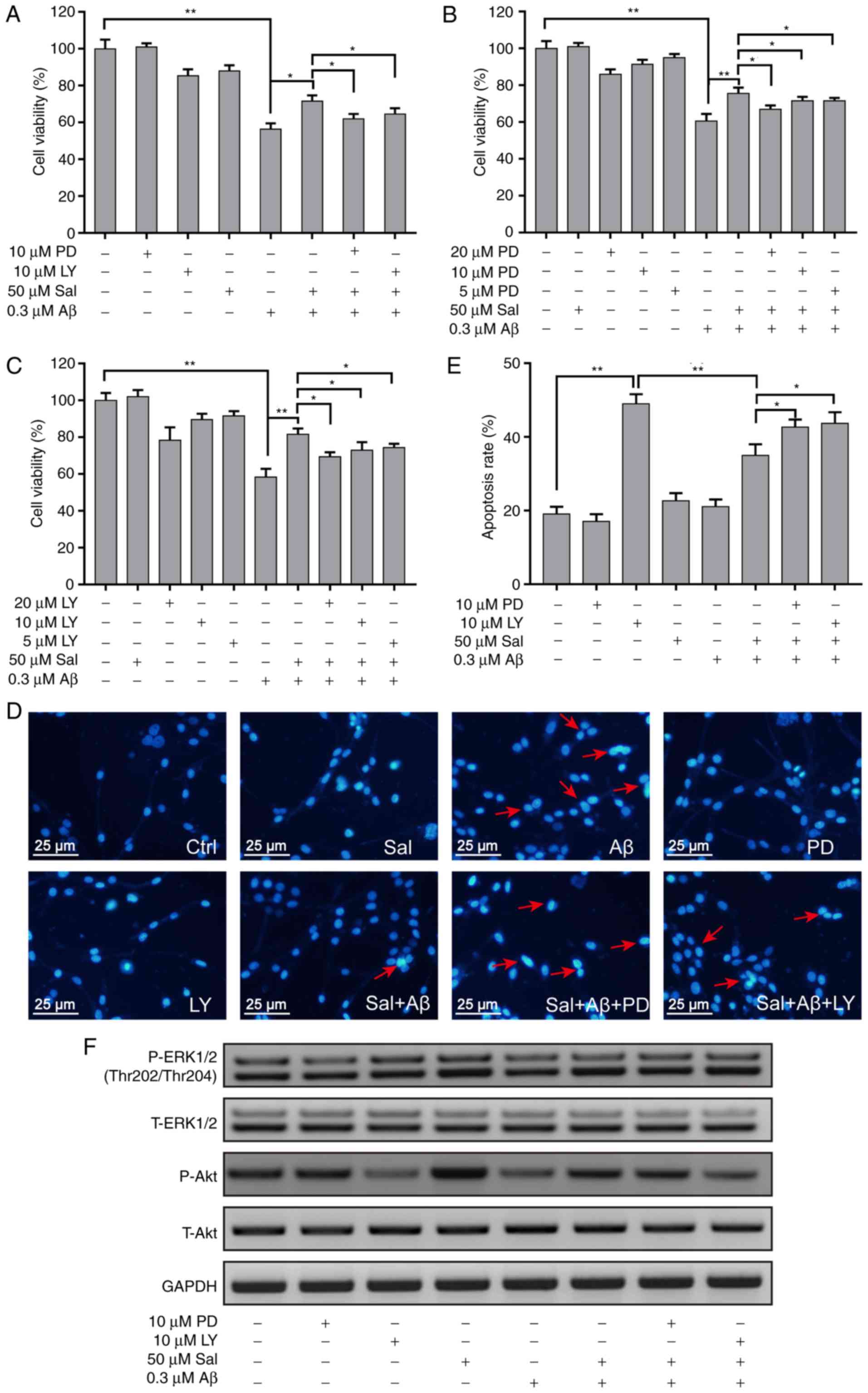 | Figure 6The ERK1/2 and AKT signaling pathways
mediate the protective effects of salidroside in PC-12 cells. PC-12
cells were exposed to 50 µM Sal for 1 h with or without
pre-treatment with (A) 10 µM PD98059 and 10 µM
LY294002 for 1 h, or (B) 5, 10 and 20 µM PD98059, or (C) 5,
10 and 20 µM LY294002, prior to being stimulated with 0.8 mM
Aβ1-42 for 24 h. Next, cell viability was determined by
MTT assay. (D) Apoptotic cells were detected by staining with
4’,6-diamidino-2-phenylindole and visualized by fluorescence
microscopy. The typical apoptotic cells were marked by arrows. (E)
The number of apoptotic nuclei with condensed chromatin was counted
from the photomicrographs and presented as a percentage of the
total number of nuclei. (F) PC-12 cells were pre-treated with the
ERK1/2 inhibitor PD98059 (10 µM) or the AKT inhibitor
LY294002 (10 µM) for 30 min and then treated with 50
µM Sal for 1 h. Subsequently, the cells were incubated with
or without 0.3 µM Aβ1-42. The expression levels
of P-ERK1/2, ERK1/2, P-AKT, AKT and GAPDH were detected by western
blotting. Data are presented as the mean ± standard deviation
(n=3). *P<0.05 and **P<0.01. ERK1/2,
extracellular signal-regulated kinases 1/2; AKT, protein kinase B;
Aβ, amyloid β1-42; P, phosphorylated; T, total;
phosphorylated; Sal, salidroside; Ctrl, control. |
Discussion
AD is a multigenetic neurodegenerative disease
caused by genetic and environmental factors (26). It is associated with a series of
physiological and pathological mechanisms, including Aβ
accumulation, abnormal phosphorylation of Tau protein, lipid
metabolism, inflammatory reaction and oxidative stress (6,10,11,27). Although hypotheses have been
proposed for its pathogenesis, no theory has been completely
validated thus far (28). The
above physiological and pathological causes eventually lead to a
neuroinflammatory reaction at the disease site, thereby resulting
in nerve cell damage or apoptosis (29). Therefore, compounds that can
effectively inhibit the nerve cell apoptosis caused by
cytotoxicity-like oxidative stress may be potential therapeutic
candidates for AD.
Salidroside has been reported to have therapeutic
effects on various diseases including cancer, pulmonary fibrosis
and cerebrovascular disease, mainly due to its pharmacological
activities, including anti-fatigue, anti-aging, anti-apoptosis,
immune regulation and free-radical scavenging (19,30,31). Based on these characteristics,
salidroside was selected in the present study to detect its effects
on AD treatment. The toxic effects of Aβ on the PC-12 cell line
were used in the present study for the in vitro screening
model. Aβ is a small peptide that consists of 42 amino acids and is
cleaved from its precursor protein. The full length and fragments
of Aβ include Aβ1-42, Aβ1-40 and
Aβ25-35, which can be used as an inducer (32). Among these fragments,
Aβ1-42 has the best induction effect of cell apoptosis
(33). Therefore,
Aβ1-42 was used in the present study to establish an
in vitro AD model and to conduct pharmacodynamic tests.
Salidroside effectively improved cell apoptosis induced by cell
pyknosis, oxidative stress and mitochondrial membrane potential
decrease in Aβ-induced PC-12 cells. Therefore, salidroside was also
most likely to exhibit activity for treating AD in vivo
systems, which needs further evaluation.
Apoptosis involves multiple signaling pathways,
including ERK1/2 and AKT (34,35). Therefore, upon confirmation of the
anti-apoptotic effect of salidroside, the effect of salidroside on
these two signaling pathways was examined. Salidroside
significantly activated the ERK1/2 and AKT signaling pathways. To
further confirm the effect exerted by the ERK1/2 and AKT signaling
pathways, the ERK1/2 inhibitor PD98059 and the AKT inhibitor
LY294002 were used (36,37). The results were consistent with
those from previous experiments.
In conclusion, salidroside effectively inhibited the
apoptosis of Aβ-induced PC-12 cells by activating the ERK1/2 and
AKT signaling pathways, thereby indicating that salidroside is a
potential candidate for the treatment of AD. The present study
provides a basis for further drug development.
Supplementary Materials
Funding
The present study was supported by the Natural
Science Foundation of China (grant no. 81771158), Science
Foundation from Health Commission of Zhejiang Province (grant no.
ZKJ-ZJ-1503, 2018278601 and 2019321345).
Availability of data and materials
The data used and analyzed in this study are
available from the corresponding author on reasonable request.
Authors’ contributions
EYY and ZLL made substantial contributions to the
design of the present study. HS, YFT, YJQ, and JPZ performed the
cell viability and apoptosis-associated experiments. YC, SSL and
MHW performed all other experiments. ZLL, YPM, and JJH analyzed
data. EYY and ZLL wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
No human trials were involved in this study.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Jarrett JT and Lansbury PT Jr: Seeding
‘one-dimensional crystallization’ of amyloid: A pathogenic
mechanism in Alzheimer’s disease and scrapie? Cell. 73:1055–1058.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Winblad B, Palmer K, Kivipelto M, Jelic V,
Fratiglioni L, Wahlund LO, Nordberg A, Bäckman L, Albert M,
Almkvist O, et al: Mild cognitive impairment-beyond controversies,
towards a consensus: Report of the international working group on
mild cognitive impairment. J Intern Med. 256:240–246. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coyle JT, Price DL and DeLong MR:
Alzheimer’s disease: A disorder of cortical cholinergic
innervation. Science. 219:1184–1190. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alzheimer’s A: 2011 Alzheimer’s disease
facts and figures. W V Med J. 107:82–83. 2011.
|
|
5
|
Colucci L, Bosco M, Fasanaro AM, Gaeta GL,
Ricci G and Amenta F: Alzheimer’s disease costs: What we know and
what we should take into account. J Alzheimers Dis. 42:1311–1324.
2014. View Article : Google Scholar
|
|
6
|
Ittner LM and Gotz J: Amyloid-β and tau -
a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci.
12:65–72. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harold D, Abraham R, Hollingworth P, Sims
R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K,
Williams A, et al: Genome-wide association study identifies
variants at CLU and PICALM associated with Alzheimer’s disease. Nat
Genet. 41:1088–1093. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bertram L and Tanzi RE: Alzheimer disease:
New light on an old CLU. Nat Rev Neurol. 6:11–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Selkoe DJ: The cell biology of
beta-amyloid precursor protein and presenilin in Alzheimer’s
disease. Trends Cell Biol. 8:447–453. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Love S and Kehoe PG: Clearance of Abeta
from the brain in Alzheimer’s disease. Foreword. Brain Pathol.
18:2392008. View Article : Google Scholar
|
|
11
|
Akwa Y, Allain H, Bentue-Ferrer D, Berr C,
Bordet R, Geerts H, Nieoullon A, Onteniente B and Vercelletto M:
Neuroprotection and neurodegenerative diseases: From biology to
clinical practice. Alzheimer Dis Assoc Disord. 19:226–239. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao J, Irwin RW, Zhao L, Nilsen J,
Hamilton RT and Brinton RD: Mitochondrial bioenergetic deficit
precedes Alzheimer’s pathology in female mouse model of Alzheimer’s
disease. Proc Natl Acad Sci USA. 106:14670–14675. 2009. View Article : Google Scholar
|
|
13
|
Zeng Z, Xu J and Zheng W: Artemisinin
protects PC12 cells against β-amyloid-induced apoptosis through
activation of the ERK1/2 signaling pathway. Redox Biol. 12:625–633.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Godyn J, Jonczyk J, Panek D and Malawska
B: Therapeutic strategies for Alzheimer’s disease in clinical
trials. Pharmacol Rep. 68:127–138. 2016. View Article : Google Scholar
|
|
15
|
Cummings JL, Morstorf T and Zhong K:
Alzheimer’s disease drug-development pipeline: Few candidates,
frequent failures. Alzheimers Res Ther. 6:372014. View Article : Google Scholar
|
|
16
|
Selkoe DJ and Hardy J: The amyloid
hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med.
8:595–608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abidov M, Crendal F, Grachev S, Seifulla R
and Ziegenfuss T: Effect of extracts from Rhodiola rosea and
Rhodiola crenulata (Crassulaceae) roots on ATP content in
mitochondria of skeletal muscles. Bull Exp Biol Med. 136:585–587.
2003. View Article : Google Scholar
|
|
18
|
Zhang J, Zhen YF, Pu-Bu-Ci-Ren, Song LG,
Kong WN, Shao TM, Li X and Chai XQ: Salidroside attenuates beta
amyloid-induced cognitive deficits via modulating oxidative stress
and inflammatory mediators in rat hippocampus. Behav Brain Res.
244:70–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Z, Li X, Simoneau AR, Jafari M and Zi
X: Rhodiola rosea extracts and salidroside decrease the growth of
bladder cancer cell lines via inhibition of the mTOR pathway and
induction of autophagy. Mol Carcinog. 51:257–267. 2012. View Article : Google Scholar
|
|
20
|
Zhang L, Yu H, Zhao X, Lin X, Tan C, Cao G
and Wang Z: Neuroprotective effects of salidroside against
beta-amyloid-induced oxidative stress in SH-SY5Y human
neuroblastoma cells. Neurochem Int. 57:547–555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu M, Waring JF, Gopalakrishnan M and Li
J: Role of GSK-3beta activation and alpha7 nAChRs in
Abeta(1–42)-induced tau phosphorylation in PC12 cells. J Neurochem.
106:1371–1377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mahbub AA, Le Maitre CL, Haywood-Small SL,
McDougall GJ, Cross NA and Jordan-Mahy N: Differential effects of
poly-phenols on proliferation and apoptosis in human myeloid and
lymphoid leukemia cell lines. Anticancer Agents Med Chem.
13:1601–1613. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar
|
|
24
|
Ly JD, Grubb DR and Lawen A: The
mitochondrial membrane potential (deltapsi(m)) in apoptosis. An
update Apoptosis. 8:115–128. 2003. View Article : Google Scholar
|
|
25
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bufill E, Bartes A, Moral A, Casadevall T,
Codinachs M, Zapater E, Carles Rovira J, Roura P, Oliva R and Blesa
R: Genetic and environmental factors that may influence in the
senile form of Alzheimer’s disease: Nested case control studies.
Neurologia. 24:108–112. 2009.In Spanish. PubMed/NCBI
|
|
27
|
Di Paolo G and Kim TW: Linking lipids to
Alzheimer’s disease: Cholesterol and beyond. Nat Rev Neurosci.
12:284–296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Butterfield DA and Boyd-Kimball D:
Oxidative stress, amyloid-β peptide, and altered key molecular
pathways in the pathogenesis and progression of Alzheimer’s
disease. J Alzheimers Dis. 62:1345–1367. 2018. View Article : Google Scholar :
|
|
29
|
Knezevic D and Mizrahi R: Molecular
imaging of neuroinflammation in Alzheimer’s disease and mild
cognitive impairment. Prog Neuropsychopharmacol Biol Psychiatry.
80:123–131. 2018. View Article : Google Scholar
|
|
30
|
Ma YG, Wang JW, Zhang YB, Wang BF, Dai ZJ,
Xie MJ and Kang HF: Salidroside improved cerebrovascular
vasodilation in streptozotocin-induced diabetic rats through
restoring the function of BKCa channel in smooth muscle cells. Cell
Tissue Res. 370:365–377. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu X, Bai Y, Liang L, Feng J and Deng F:
Salidroside improves pulmonary fibrosis by down-regulation of
cathepsin B and NF-κBp65 in rats. Zhong Nan Da Xue Xue Bao Yi Xue
Ban. 42:128–133. 2017.In Chinese. PubMed/NCBI
|
|
32
|
Richter L, Munter LM, Ness J, Hildebrand
PW, Dasari M, Unterreitmeier S, Bulic B, Beyermann M, Gust R, Reif
B, et al: Amyloid beta 42 peptide (Abeta42)-lowering compounds
directly bind to Aβ and interfere with amyloid precursor protein
(APP) transmembrane dimerization. Proc Natl Acad Sci USA.
107:14597–14602. 2010. View Article : Google Scholar
|
|
33
|
Postu PA, Noumedem JAK, Cioanca O,
Hancianu M, Mihasan M, Ciorpac M, Gorgan DL, Petre BA and Hritcu L:
Lactuca capensis reverses memory deficits in Aβ1–42-induced an
animal model of Alzheimer’s disease. J Cell Mol Med. 22:111–122.
2018. View Article : Google Scholar
|
|
34
|
Lu Z and Xu S: ERK1/2 MAP kinases in cell
survival and apoptosis. IUBMB Life. 58:621–631. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Franke TF, Hornik CP, Segev L, Shostak GA
and Sugimoto C: PI3K/Akt and apoptosis: Size matters. Oncogene.
22:8983–8998. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Imai Y, Yamagishi H, Ono Y and Ueda Y:
Versatile inhibitory effects of the flavonoid-derived PI3K/Akt
inhibitor, LY294002, on ATP-binding cassette transporters that
characterize stem cells. Clin Transl Med. 1:242012. View Article : Google Scholar
|
|
37
|
Zhu C, Qi X, Chen Y, Sun B, Dai Y and Gu
Y: PI3K/Akt and MAPK/ERK1/2 signaling pathways are involved in
IGF-1-induced VEGF-C upregulation in breast cancer. J Cancer Res
Clin Oncol. 137:1587–1594. 2011. View Article : Google Scholar : PubMed/NCBI
|