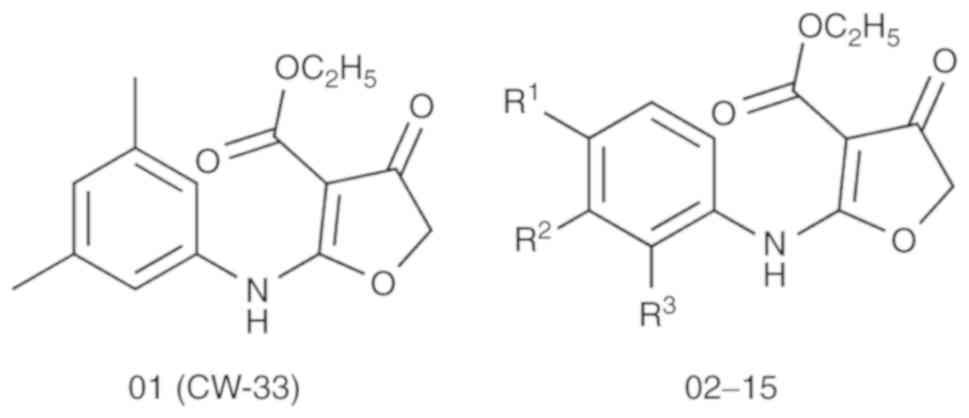
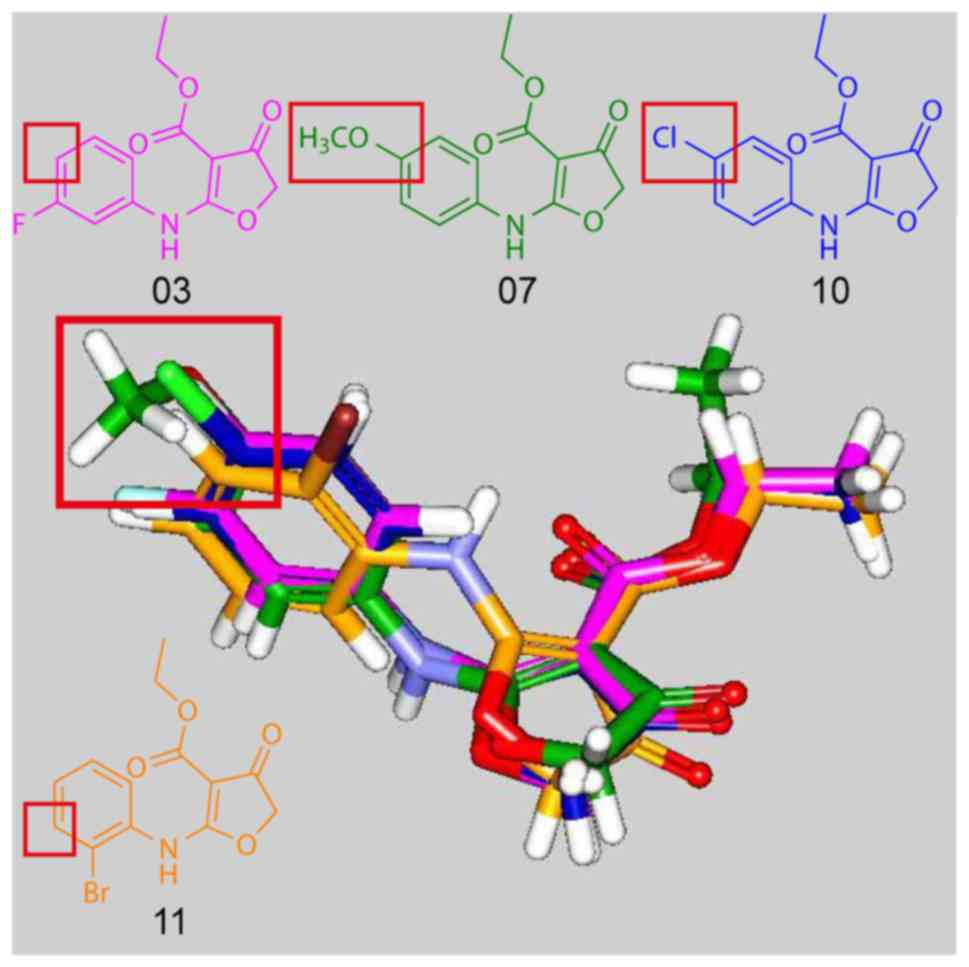
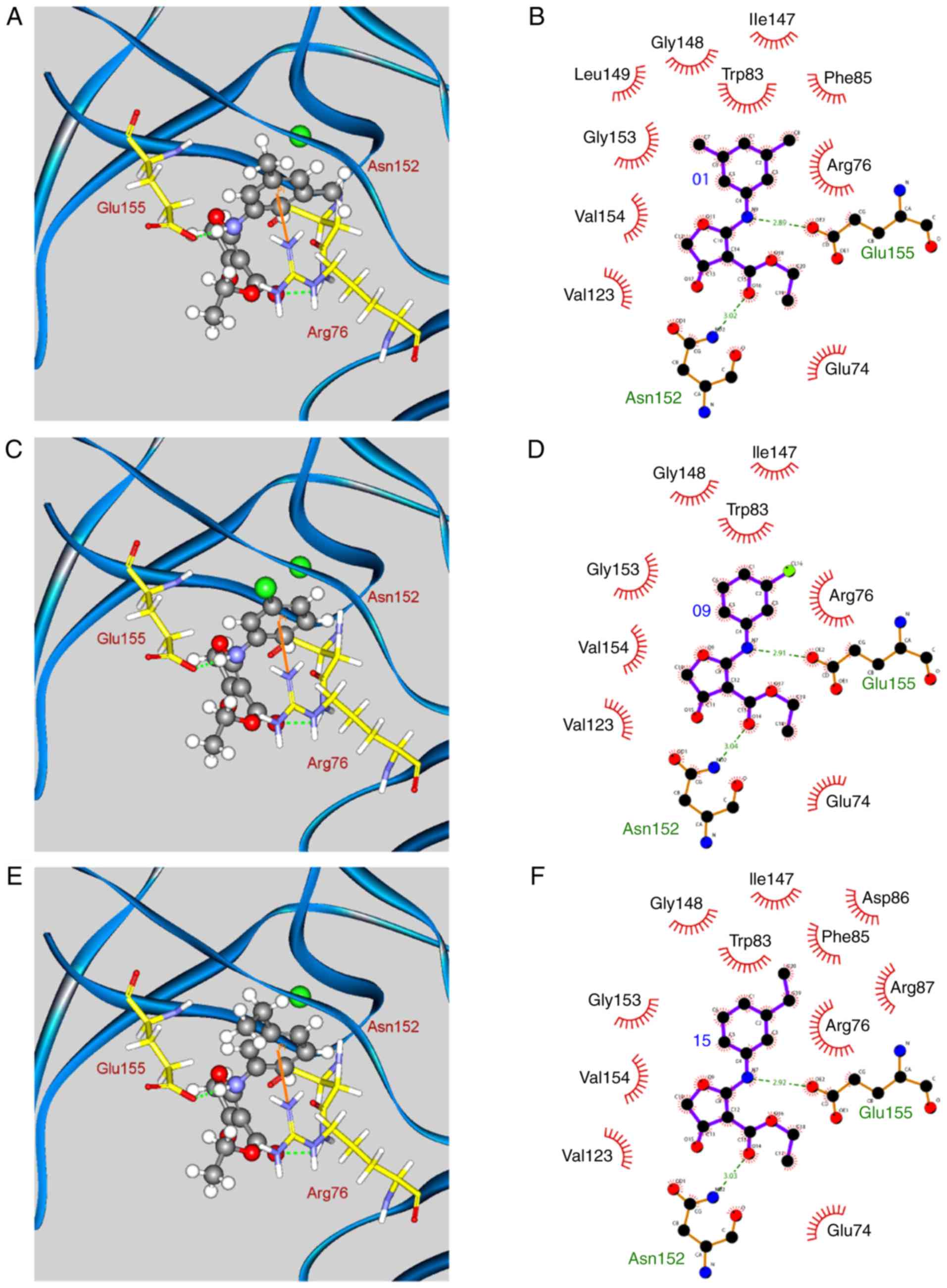
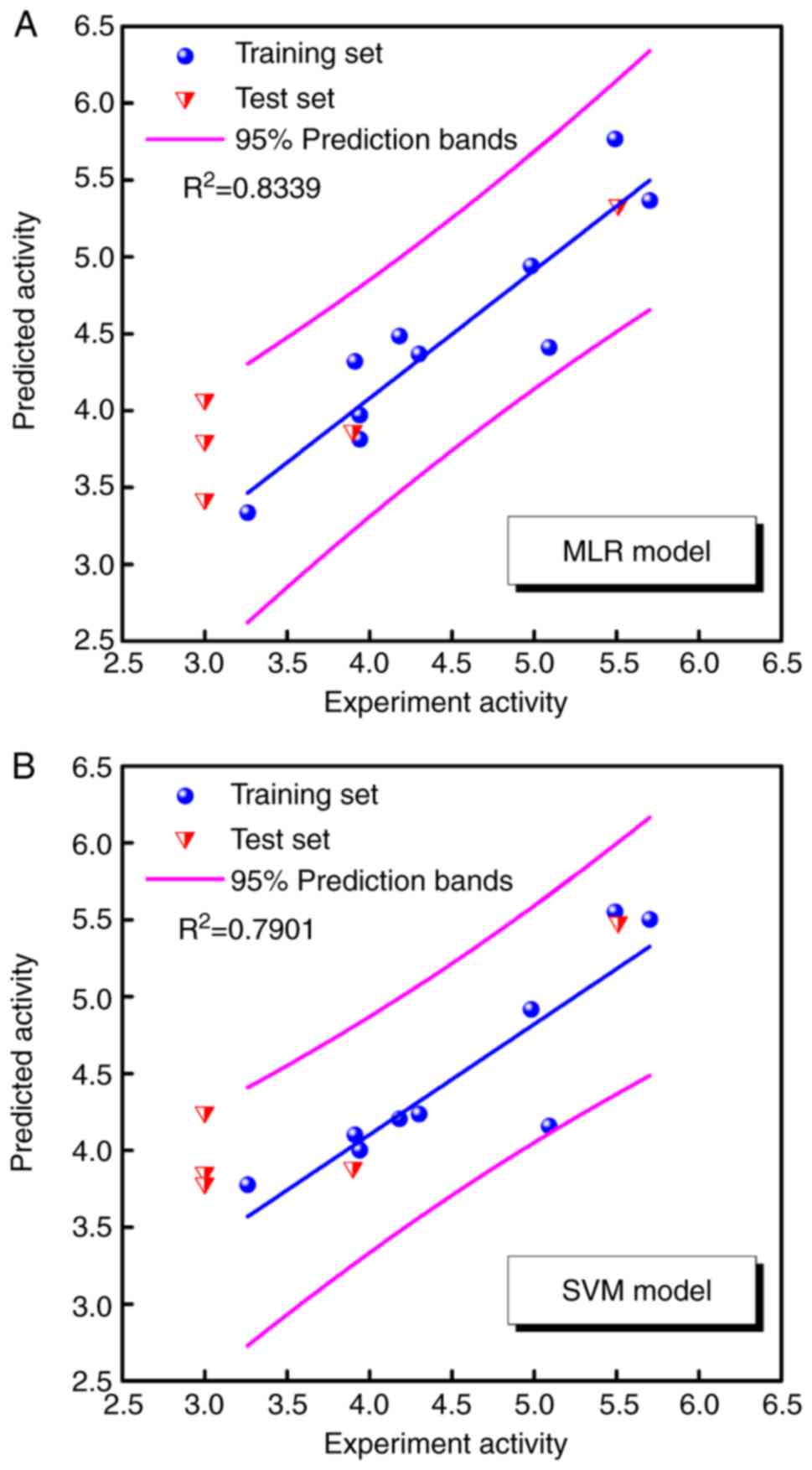
|
1
|
Unni SK, Růžek D, Chhatbar C, Mishra R,
Johri MK and Singh SK: Japanese encephalitis virus: From genome to
infectome. Microbes Infect. 13:312–321. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Simon LV and Kruse B: Encephalitis,
Japanese. StatPearls. StatPearls Publishing; Treasure Island, FL:
2017
|
|
3
|
van den Hurk AF, Ritchie SA and Mackenzie
JS: Ecology and geographical expansion of Japanese encephalitis
virus. Annu Rev Entomol. 54:17–35. 2009. View Article : Google Scholar
|
|
4
|
Karunaratne SH and Hemingway J:
Insecticide resistance spectra and resistance mechanisms in
populations of Japanese encephalitis vector mosquitoes, Culex
tritaeniorhynchus and Cx. gelidus, in Sri Lanka. Med Vet Entomol.
14:430–436. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thenmozhi V, Rajendran R, Ayanar K,
Manavalan R and Tyagi BK: Long-term study of Japanese encephalitis
virus infection in Anopheles subpictus in Cuddalore district, Tamil
Nadu, South India. Trop Med Int Health. 11:288–293. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumar K, Arshad SS, Selvarajah GT, Abu J,
Toung OP, Abba Y, Bande F, Yasmin AR, Sharma R, Ong BL, et al:
Prevalence and risk factors of Japanese encephalitis virus (JEV) in
livestock and companion animal in high-risk areas in Malaysia. Trop
Anim Health Prod. 50:741–752. 2018. View Article : Google Scholar :
|
|
7
|
Solomon T, Ni H, Beasley DW, Ekkelenkamp
M, Cardosa MJ and Barrett AD: Origin and evolution of Japanese
encephalitis virus in southeast Asia. J Virol. 77:3091–3098. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schiøler KL, Samuel M and Wai KL: Vaccines
for preventing Japanese encephalitis. Cochrane Database Syst Rev.
CD0042632007.PubMed/NCBI
|
|
9
|
Turtle L and Driver C: Risk assessment for
Japanese encephalitis vaccination. Hum Vaccin Immunother.
14:213–217. 2018. View Article : Google Scholar :
|
|
10
|
Jelinek T, Burchard GD, Dieckmann S,
Bühler S, Paulke-Korinek M, Nothdurft HD, Reisinger E, Ahmed K,
Bosse D, Meyer S, et al: Short-term immunogenicity and safety of an
accelerated pre-exposure prophylaxis regimen with Japanese
encephalitis vaccine in combination with a rabies vaccine: A phase
III, multicenter, observer-blind study. J Travel Med. 22:225–231.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh A, Mitra M, Sampath G, Venugopal P,
Rao JV, Krishnamurthy B, Gupta MK, Sri Krishna S, Sudhakar B, Rao
NB, et al: A Japanese encephalitis vaccine from India induces
durable and cross-protective immunity against temporally and
spatially wide-ranging global field strains. J Infect Dis.
212:715–725. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vu TD, Nguyen QD, Tran HTA, Bosch-Castells
V, Zocchetti C and Houillon G: Immunogenicity and safety of a
single dose of a live attenuated Japanese encephalitis chimeric
virus vaccine in Vietnam: A single-arm, single-center study. Int J
Infect Dis. 66:137–142. 2018. View Article : Google Scholar
|
|
13
|
Fan YC, Lin JW, Liao SY, Chen JM, Chen YY,
Chiu HC, Shih CC, Chen CM, Chang RY, King CC, et al: Virulence of
Japanese encephalitis virus genotypes I and III, Taiwan. Emerg
Infect Dis. 23:1883–1886. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Connor B and Bunn WB: The changing
epidemiology of Japanese encephalitis and New data: The
implications for new recommendations for Japanese encephalitis
vaccine. Trop Dis Travel Med Vaccines. 3:142017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ginsburg AS, Meghani A, Halstead SB and
Yaich M: Use of the live attenuated Japanese Encephalitis vaccine
SA 14-14-2 in children: A review of safety and tolerability
studies. Hum Vaccin Immunother. 13:2222–2231. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Solomon T, Dung NM, Kneen R, Gainsborough
M, Vaughn DW and Khanh VT: Japanese encephalitis. J Neurol
Neurosurg Psychiatry. 68:405–415. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hegde NR and Gore MM: Japanese
encephalitis vaccines: Immunogenicity, protective efficacy,
effectiveness, and impact on the burden of disease. Hum Vaccin
Immunother. 13:1–18. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang SH, Lien JC, Chen CJ, Liu YC, Wang
CY, Ping CF, Lin YF, Huang AC and Lin CW: Antiviral activity of a
novel compound CW-33 against Japanese encephalitis virus through
inhibiting intracellular calcium overload. Int J Mol Sci.
17:E13862016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang CY, Huang AC, Hour MJ, Huang SH, Kung
SH, Chen CH, Chen IC, Chang YS, Lien JC and Lin CW: Antiviral
potential of a novel compound CW-33 against enterovirus A71 via
inhibition of viral 2A protease. Viruses. 7:3155–3171. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lien JC, Wang CY, Lai HC, Lu CY, Lin YF,
Gao GY, Chen KC, Huang AC, Huang SH and Lin CW: Structure analysis
and antiviral activity of CW-33 analogues against Japanese
encephalitis virus. Sci Rep. 8:165952018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takegami T, Simamura E, Hirai K and Koyama
J: Inhibitory effect of furanonaphthoquinone derivatives on the
replication of Japanese encephalitis virus. Antiviral Res.
37:37–45. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bera AK, Kuhn RJ and Smith JL: Functional
characterization of cis and trans activity of the Flavivirus
NS2B-NS3 protease. J Biol Chem. 282:12883–12892. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weinert T, Olieric V, Waltersperger S,
Panepucci E, Chen L, Zhang H, Zhou D, Rose J, Ebihara A, Kuramitsu
S, et al: Fast native-SAD phasing for routine macromolecular
structure determination. Nat Methods. 12:131–133. 2015. View Article : Google Scholar
|
|
24
|
Brooks BR, Bruccoleri RE, Olafson BD,
States DJ, Swaminathan S and Karplus M: CHARMM: A program for
macromolecular energy minimization and dynamics calculations. J
Comput Chem. 4:187–217. 1983. View Article : Google Scholar
|
|
25
|
Venkatachalam CM, Jiang X, Oldfield T and
Waldman M: LigandFit: A novel method for the shape-directed rapid
docking of ligands to protein active sites. J Mol Graph Model.
21:289–307. 2003. View Article : Google Scholar
|
|
26
|
Gehlhaar DK, Verkhivker GM, Rejto PA,
Sherman CJ, Fogel DB, Fogel LJ and Freer ST: Molecular recognition
of the inhibitor Ag- 1343 by Hiv-1 protease: Conformationally
flexible docking by evolutionary programming. Chem Biol. 2:317–324.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gehlhaar DK, Bouzida D and Rejto Paul A:
Reduced dimensionality in ligand-protein structure prediction:
Covalent inhibitors of serine proteases and design of site-directed
combinatorial libraries. Rational Drug Design American Chemical
Society. 292–311. 1999. View Article : Google Scholar
|
|
28
|
Muegge I and Martin YC: A general and fast
scoring function for protein- ligand interactions: A simplified
potential approach. J Med Chem. 42:791–804. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Laskowski RA and Swindells MB: LigPlot+:
Multiple ligand-protein interaction diagrams for drug discovery. J
Chem Inf Model. 51:2778–2786. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fan RE, Chen PH and Lin CJ: Working set
selection using second order information for training support
vector machines. J Mach Learn Res. 6:1889–1918. 2005.
|
|
31
|
Rogers D and Hopfinger AJ: Application of
genetic function approximation to quantitative structure-activity
relationships and quantitative structure-property relationships. J
Chem Inf Comput Sci. 34:8541994. View Article : Google Scholar
|
|
32
|
Li H, Sutter J and Hoffman R: HypoGen: An
automated system for generating 3D predictive pharmacophore models.
Pharmacophore Perception, Development and Use in Drug Design. Guner
O: International University Line; La Jolla: 2000
|
|
33
|
Cramer RD, Patterson DE and Bunce JD:
Comparative molecular field analysis (CoMFA). 1. Effect of shape on
binding of steroids to carrier proteins. J Am Chem Soc.
110:5959–5967. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Klebe G, Abraham U and Mietzner T:
Molecular similarity indices in a comparative analysis (CoMSIA) of
drug molecules to correlate and predict their biological activity.
J Med Chem. 37:4130–4146. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang CC, Hsieh YC, Lee SJ, Wu SH, Liao CL,
Tsao CH, Chao YS, Chern JH, Wu CP and Yueh A: Novel dengue virus-
specific NS2B/NS3 protease inhibitor, BP2109, discovered by a
high-throughput screening assay. Antimicrob Agents Chemother.
55:229–238. 2011. View Article : Google Scholar
|
|
36
|
Seniya C, Mishra H, Yadav A, Sagar N,
Chaturvedi B, Uchadia K and Wadhwa G: Antiviral potential of
4-hydroxypanduratin A, secondary metabolite of Fingerroot,
Boesenbergia pandurata (Schult.), towards Japanese Encephalitis
virus NS2B/NS3 protease. Bioinformation. 9:54–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hall LH, Mohney B and Kier LB: The
electrotopological state: Structure information at the atomic level
for molecular graphs. J Chem Inf Comput Sci. 31:76–82. 1991.
View Article : Google Scholar
|
|
38
|
Hall LH and Kier LB: The E-state as the
basis for molecular structure space definition and structure
similarity. J Chem Inf Comput Sci. 40:784–791. 2000. View Article : Google Scholar : PubMed/NCBI
|