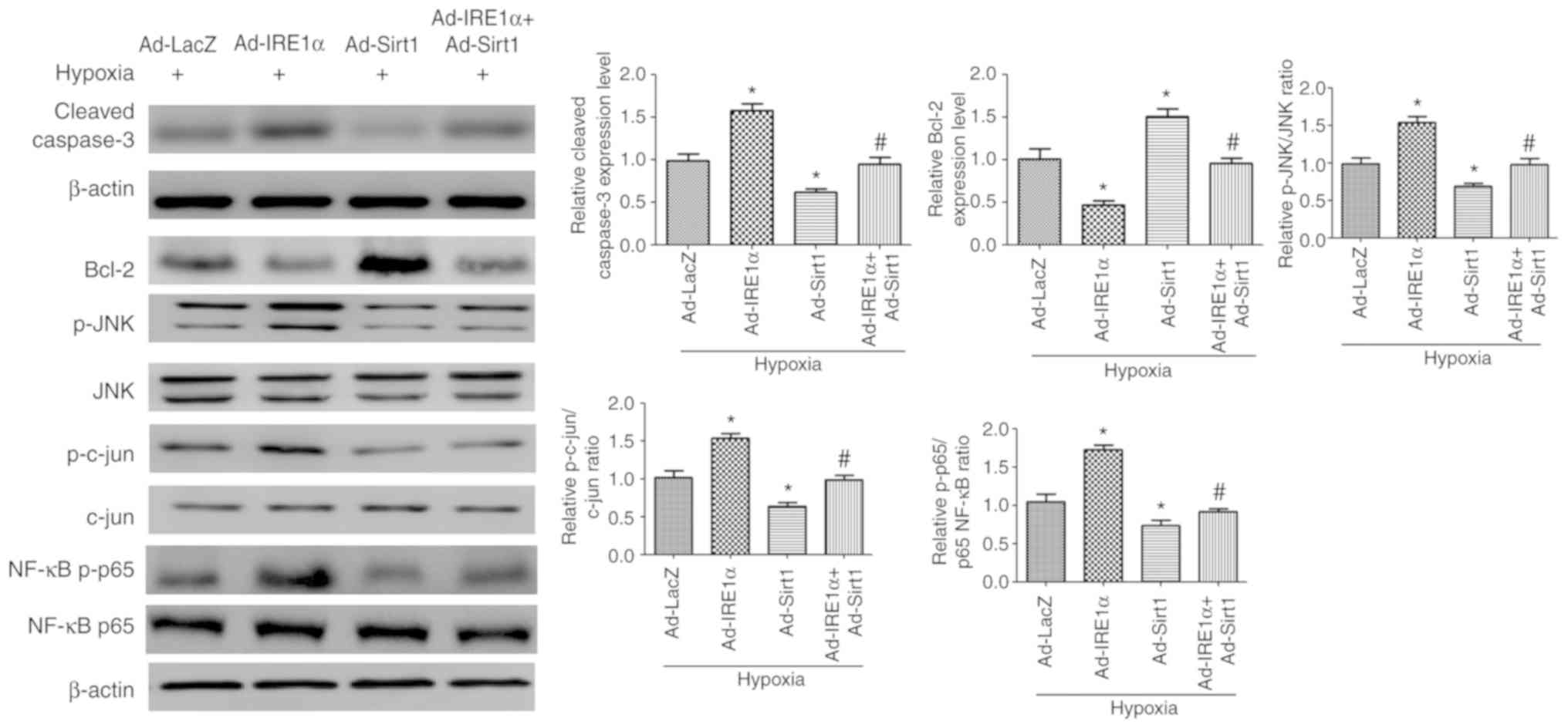
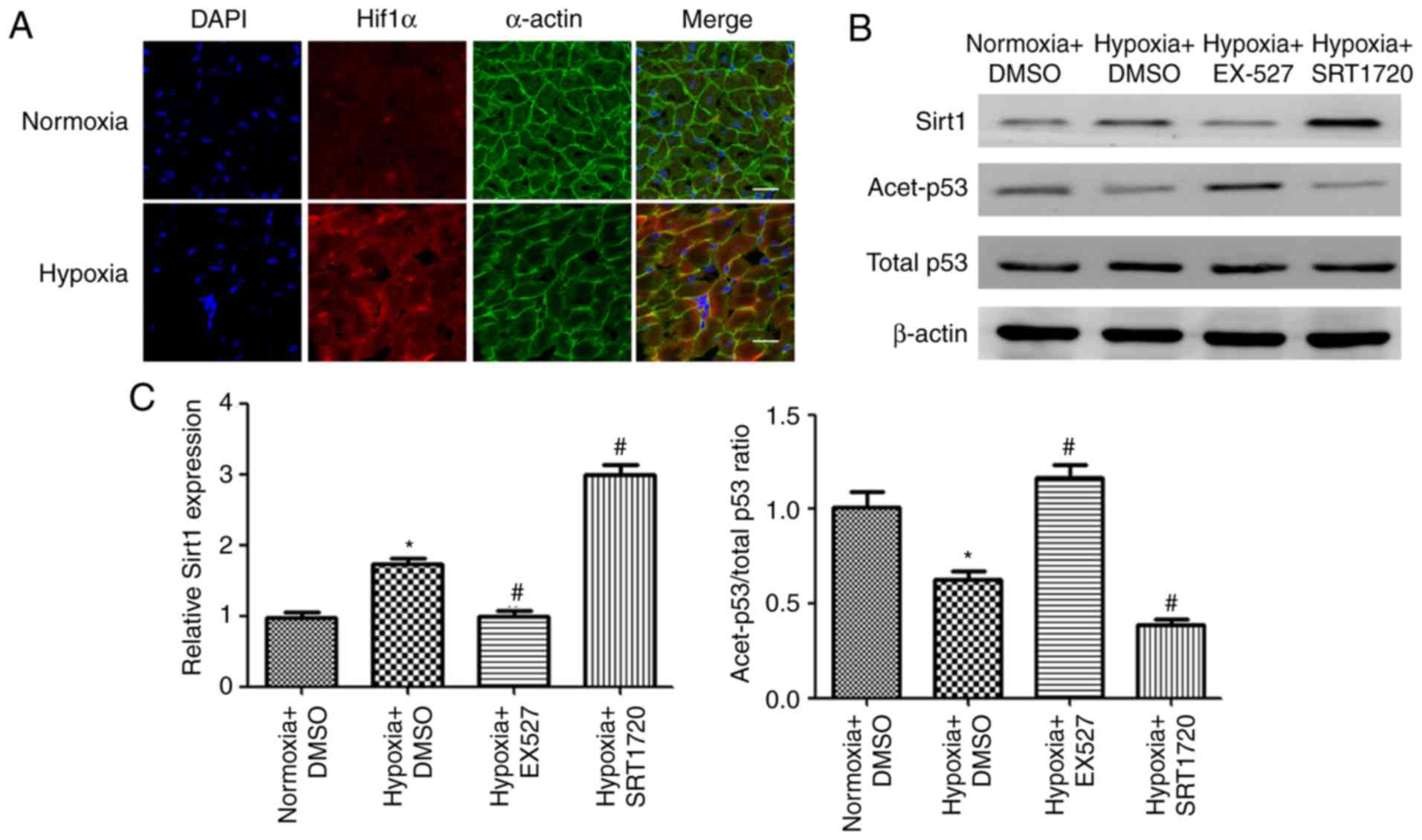
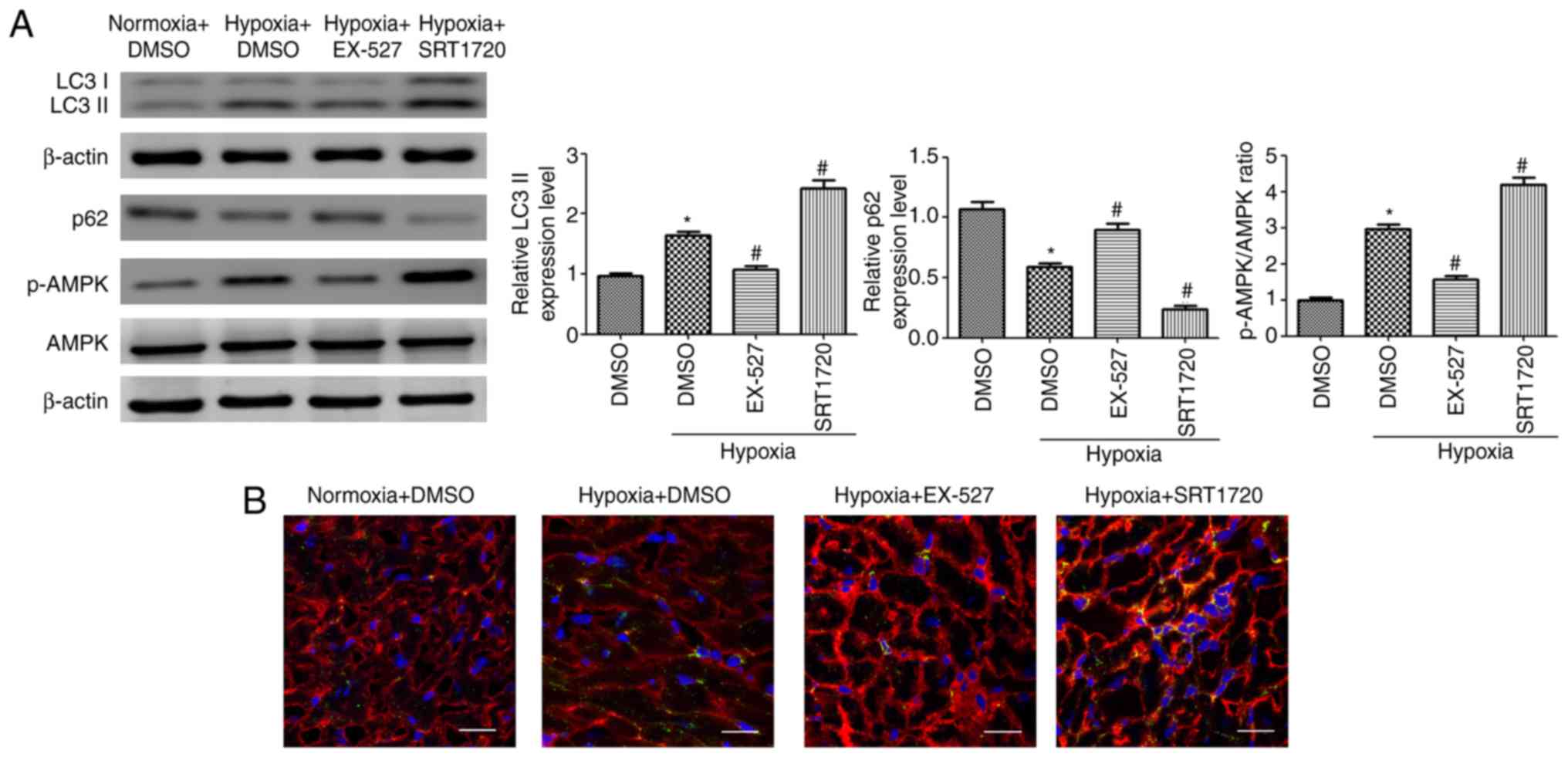
|
1
|
Anastasiou D and Krek W: SIRT1: Linking
adaptive cellular responses to aging-associated changes in
organismal physiology. Physiology (Bethesda). 21:404–410. 2006.
|
|
2
|
Liu L, Liu C, Zhang Q, Shen J, Zhang H,
Shan J, Duan G, Guo D, Chen X, Cheng J, et al: SIRT1-mediated
transcriptional regulation of SOX2 is important for self-renewal of
liver cancer stem cells. Hepatology. 64:814–827. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hariharan N, Maejima Y, Nakae J, Paik J,
Depinho RA and Sadoshima J: Deacetylation of FoxO by Sirt1 plays an
essential role in mediating starvation-induced autophagy in cardiac
myocytes. Cir Res. 107:1470–1482. 2010. View Article : Google Scholar
|
|
4
|
Liu B, Ghosh S, Yang X, Zheng H, Liu X,
Wang Z, Jin G, Zheng B, Kennedy BK, Suh Y, et al: Resveratrol
rescues SIRT1-dependent adult stem cell decline and alleviates
progeroid features in laminopathy-based progeria. Cell Metab.
16:738–750. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang Y, Duan W, Li Y, Jin Z, Yan J, Yu S
and Yi D: Novel role of silent information regulator 1 in
myocardial ischemia. Circulation. 128:2232–2240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alcendor RR, Gao S, Zhai P, Zablocki D,
Holle E, Yu X, Tian B, Wagner T, Vatner SF and Sadoshima J: Sirt1
regulates aging and resistance to oxidative stress in the heart.
Circ Res. 100:1512–1521. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ding C, Zou Q, Wang F, Wu H, Wang W, Li H
and Huang B: HGF and BFGF secretion by human adipose-derived stem
cells improves ovarian function during natural aging via activation
of the SIRT1/FOXO1 signaling pathway. Cell Physiol Biochem.
45:1316–1332. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsu CP, Zhai P, Yamamoto T, Maejima Y,
Matsushima S, Hariharan N, Shao D, Takagi H, Oka S and Sadoshima J:
Silent information regulator 1 protects the heart from
ischemia/reperfusion. Circulation. 122:2170–2182. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He S, Liu P, Jian Z, Li J, Zhu Y, Feng Z
and Xiao Y: miR-138 protects cardiomyocytes from hypoxia-induced
apoptosis via MLK3/JNK/c-jun pathway. Biochem Biophys Res Commun.
441:763–769. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lim JH, Lee YM, Chun YS, Chen J, Kim JE
and Park JW: Sirtuin 1 modulates cellular responses to hypoxia by
deacetylating hypoxia-inducible factor 1alpha. Mol Cell.
38:864–878. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dioum EM, Chen R, Alexander MS, Zhang Q,
Hogg RT, Gerard RD and Garcia JA: Regulation of hypoxia-inducible
factor 2alpha signaling by the stress-responsive deacetylase
sirtuin 1. Science. 324:1289–1293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rabinowitz JD and White E: Autophagy and
metabolism. Science. 330:1344–1348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakai A, Yamaguchi O, Takeda T, Higuchi Y,
Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, et
al: The role of autophagy in cardiomyocytes in the basal state and
in response to hemodynamic stress. Nat Med. 13:619–624. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu L, Jin X, Hu CF, Li R, Zhou Z and Shen
CX: Exosomes derived from mesenchymal stem cells rescue myocardial
ischaemia/reperfusion injury by inducing cardiomyocyte autophagy
Via AMPK and Akt pathways. Cell Physiol Biochem. 43:52–68. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang H, Liu B, Li T, Zhu Y, Luo G, Jiang
Y, Tang F, Jian Z and Xiao Y: AMPK activation serves a critical
role in mitochondria quality control via modulating mitophagy in
the heart under chronic hypoxia. Int J Mol Med. 41:69–76. 2018.
|
|
16
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shore GC, Papa FR and Oakes SA: Signaling
cell death from the endoplasmic reticulum stress response. Curr
Opin Cell Biol. 23:143–149. 2011. View Article : Google Scholar :
|
|
18
|
Zhao W, Han F and Shi Y: IRE1alpha pathway
of endoplasmic reticulum stress induces neuronal apoptosis in the
locus coeruleus of rats under single prolonged stress. Prog
Neuropsychopharmacol Biol Psychiatry. 69:11–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brozzi F, Nardelli TR, Lopes M, Millard I,
Barthson J, Igoillo-Esteve M, Grieco FA, Villate O, Oliveira JM,
Casimir M, et al: Cytokines induce endoplasmic reticulum stress in
human, rat and mouse beta cells via different mechanisms.
Diabetologia. 58:2307–2316. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brozzi F, Gerlo S, Grieco FA, Juusola M,
Balhuizen A, Lievens S, Gysemans C, Bugliani M, Mathieu C,
Marchetti P, et al: Ubiquitin D regulates IRE1α/c-Jun N-terminal
Kinase (JNK) protein-dependent apoptosis in pancreatic beta cells.
J Biol Chem. 291:12040–12056. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jain K, Suryakumar G, Ganju L and Singh
SB: Amelioration of ER stress by 4-phenylbutyric acid reduces
chronic hypoxia induced cardiac damage and improves hypoxic
tolerance through upregulation of HIF-1α. Vascul Pharmacol.
83:36–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu B, Zhang HG, Zhu Y, Jiang YH, Luo GP,
Tang FQ, Jian Z and Xiao YB: Cardiac resident macrophages are
involved in hypoxiainduced postnatal cardiomyocyte proliferation.
Mol Med Rep. 15:3541–3548. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng W, Lu YB, Liang ST, Zhang QJ, Xu J,
She ZG, Zhang ZQ, Yang RF, Mao BB, Xu Z, et al: SIRT1 mediates the
protective function of Nkx2.5 during stress in cardiomyocytes.
Basic Res Cardiol. 108:3642013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sommer RJ, Hijazi ZM and Rhodes JF:
Pathophysiology of congenital heart disease in the adult: Part III:
Complex congenital heart disease. Circulation. 117:1340–1350. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu L, Wang Q, Zhang L, Fang Z, Zhao F, Lv
Z, Gu Z, Zhang J, Wang J, Zen K, et al: Hypoxia induces PGC-1alpha
expression and mitochondrial biogenesis in the myocardium of TOF
patients. Cell Res. 20:676–687. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu Y, Feng Z, Jian Z and Xiao Y: Long
noncoding RNA TUG1 promotes cardiac fibroblast transformation to
myofibroblasts via miR29c in chronic hypoxia. Mol Med Rep.
18:3451–3460. 2018.PubMed/NCBI
|
|
27
|
Yan J, Duan J, Wu X, Guo C, Yin Y, Zhu Y,
Hu T, Wei G, Wen A and Xi M: Total saponins from Aralia taibaiensis
protect against myocardial ischemia/reperfusion injury through AMPK
pathway. Int J Mol Med. 36:1538–1546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gu Y, Gao L, Chen Y, Xu Z, Yu K, Zhang D,
Zhang G and Zhang X: Sanggenon C protects against cardiomyocyte
hypoxia injury by increasing autophagy. Mol Med Rep. 16:8130–8136.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu MH, Lin XL, Guo DM, Zhang Y, Yuan C,
Tan TP, Chen YD, Wu SJ, Ye ZF and He J: Resveratrol protects
cardiomyocytes from doxorubicin-induced apoptosis through the
AMPK/P53 pathway. Mol Med Rep. 13:1281–1286. 2016. View Article : Google Scholar
|
|
30
|
Hamid T, Gu Y, Ortines RV, Bhattacharya C,
Wang G, Xuan YT and Prabhu SD: Divergent tumor necrosis factor
receptor-related remodeling responses in heart failure: Role of
nuclear factor-kappaB and inflammatory activation. Circulation.
119:1386–1397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Milne JC, Lambert PD, Schenk S, Carney DP,
Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, et al: Small
molecule activators of SIRT1 as therapeutics for the treatment of
type 2 diabetes. Nature. 450:712–716. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vachharajani VT, Liu T, Brown CM, Wang X,
Buechler NL, Wells JD, Yoza BK and McCall CE: SIRT1 inhibition
during the hypoinflammatory phenotype of sepsis enhances immunity
and improves outcome. J Leukoc Biol. 96:785–796. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song S, Tan J, Miao Y, Li M and Zhang Q:
Crosstalk of autophagy and apoptosis: Involvement of the dual role
of autophagy under ER stress. J Cell Physiol. 232:2977–2984. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thorburn A: Apoptosis and autophagy:
Regulatory connections between two supposedly different processes.
Apoptosis. 13:1–9. 2008. View Article : Google Scholar :
|