Introduction
Osteoarthritis (OA), which is characterized by
progressive joint dysfunction and cartilage degradation, is the
most prevalent joint disease and is considered as one of the major
health problems, particularly for middle-aged and elderly people
(1-3). Although the pathophysiology of OA
remains poorly understood, an inflammatory component, which is
marked by symptoms including joint pain and stiffness, is involved
in OA development and progression (4,5).
IL-1β, which is significantly increased in
chondrocytes as well as synovial cells of patients with OA, is
known to serve a pivotal role in the pathogenesis of OA (6). Numerous studies have demonstrated
that IL-1β can not only increase chondrocyte production of
inflammatory mediators, including inducible nitric oxide (NO)
synthase (iNOS), cyclooxygenase-2 (COX-2) and prostaglandin E2
(PGE2), but also enhance chondrocyte expression of cartilage
catabolic enzymes, including matrix metalloproteinases (MMPs) and a
disintegrin and metalloproteinase with thrombospondin motifs
(ADAMTS) (7-9). IL-1β is considered to be one of the
most promising targets for treating OA and IL-1β stimulation is
used as a conventional in vitro model to study OA (10-14).
Peimine is the main compound extracted from
Bulbus Fritillariae (BF). BF is a traditional Chinese
medicine that has been used as an antitussive and antiasthma drug
for >2,000 years due to its high therapeutic effects, low
toxicity and few side effects (15). Peimine has been reported to have
antioxidant, anti-inflammatory and pain suppressing effects
(16-19). However, to the best of our
knowledge, the effects of Peimine on OA have not been studied.
Therefore, the aim of the present study was to investigate the
potential beneficial effects and the underlying mechanism of
Peimine on OA using both in vivo and in vitro
models.
Materials and methods
Medium and reagents
Peimine was purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany; cat. no. SMB00446), dissolved in DMSO and
stored at −20°C until use. Anisomycin (cat. no. SC-3524) and
U-46619 (cat. no. SC-201242) were gained from Santa Cruz
Biotechnology, Inc., (Dallas, TX, USA). IL-1β was obtained from
R&D Systems, Inc. (Minneapolis, MN, USA; cat. no. 401-ML),
dissolved in 0.1% bovine serum albumin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and stored at −80°C prior to use. A PGE2 ELISA
kit was purchased from R&D Systems, Inc., (cat. no. KGE004B),
the Nitric Oxide Assay kit was acquired from Abcam (Cambridge, UK;
cat. no. ab65328) and the Cell Counting Kit-8 (CCK)-8 kit was
purchased from Sigma-Aldrich (Merck KGaA; cat. no. 96992). The
Prime Script reverse transcription (RT) reagent kit and SYBR for
the RT-quantitative polymerase chain reaction (PCR) experiments
were obtained from Takara Bio, Inc. (Otsu, Japan). Primary
antibodies of iNOS (cat. no. 13120), COX-2 (cat. no. 4842), P38
(cat. no. 9212), phosphorylated (P)-P38 (Thr180/Tyr192; cat. no.
4511), ERK (cat. no. 4695), P-ERK (Thr202/Tyr204; cat. no. 4370),
JNK (cat. no. 9252) and P-JNK (Thr183/Tyr185; cat. no. 4668) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Primary antibodies of MMP-3 (cat. no. 1908-1) and MMP-13 (cat. no.
1923-1) were acquired from Epitomics, Inc., (Abcam), the primary
antibody for ADAMTS-5 (cat. no. PA5-14350) was purchased from
Thermo Fisher Scientific, Inc., (Waltham, MA, USA) and the primary
antibody of GAPDH was obtained from Wuhan Boster Biological
Technology, Ltd., (Wuhan, China; cat. no. BM3876). Horseradish
peroxidase-conjugated secondary antibodies, including anti-rabbit
immunoglobulin (Ig)G (cat. no. W4011) and anti-mouse IgG (cat. no.
W4021), were acquired from Promega Corporation (Madison, WI, USA).
The basic medium consisted of low glucose Dulbecco's modified
Eagles medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.)
containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 2 mM L-glutamine, 50 U/ml penicillin and 0.05
mg/ml streptomycin.
Primary chondrocyte isolation and
culture
Primary chondrocytes were isolated from the femoral
heads, femoral condyles and the tibial plateau of 4-day-old
C57BL/6J mice as described previously (20). Briefly, the cartilage was minced
into 1-mm3 pieces and digested in 3 mg/ml collagenase D
solution for 45 min at 37°C twice. Subsequently, the cartilage
pieces were retrieved and digested in 0.5 mg/ml collagenase D
solution overnight. The digestion solution was then retrieved and
centrifuged at 400 × g at room temperature for 5 min to obtain the
chondrocytes. The isolated chondrocytes were placed on a treated
plate and cultured in the basic medium at 37°C with 5%
CO2. The second passage was used for the
experiments.
Cell viability assay
The chondrocytes were seeded in 96-well plates at
the same density (1×104 cells/well) and cultured in the
basic medium for 24 h. Subsequently, the cells were treated with
the vehicle (DMSO) or different concentrations of Peimine (5, 10
and 20 μg/ml) with or without IL-1β (10 ng/ml) for a further
24 h. The CCK-8 was used to quantify cell viability according to
the manufacturer's protocol.
NO and PGE2 measurement
The chondrocytes were seeded in 6-well plates at the
same density (3×105 cells/ml) and cultured in the basic
medium for 24 h. Subsequently, the cells were treated with the
vehicle (DMSO) or different doses of Peimine (5, 10 and 20
μg/ml) with or without IL-1β (10 ng/ml) for a further 24 h.
The culture medium supernatant from each sample was harvested and
kept for further analysis. The fresh culture medium was used as a
blank control. The NO concentration was measured using the Nitric
Oxide Assay kit and the PGE2 concentration was measured using the
PGE2 ELISA kit according to the respective manufacturer's protocol.
All assays were performed in duplicate.
Western blot analysis
The western blot analysis was performed as
previously described (21). The
chondrocytes were seeded in 6-well plates at a density of
3×105 cells/ml and cultured for 24 h in the basic
medium. Subsequently, the cells were treated with the vehicle
(DMSO) or different doses of Peimine (5, 10 and 20 μg/ml)
with or without IL-1β (10 ng/ml). The total protein from the
chondrocytes was collected using radioimmunoprecipitation assay
lysis buffer acquired from Sigma-Aldrich (Merck KGaA). A
bicinchoninic acid protein assay kit obtained from Thermo Fisher
Scientific, Inc., (cat. no. 23225) was used to measure the
concentrations of the proteins. A total of 40 μg protein
from each sample was loaded on 10% SDS-polyacrylamide gels and then
transferred to polyvinylidene difluoride membranes. After blocking
in 5% non-fat milk for 1 h at room temperature, the membranes were
incubated with the aforementioned primary antibodies at 4°C
overnight (GAPDH primary antibody was diluted 1:400, all the other
primary antibodies were diluted 1:1,000). The membranes were washed
twice and then incubated with the corresponding secondary
antibodies for 1 h (diluted 1:4,000). Subsequently, the ECL reagent
from Millipore (Billerica, MA, USA; cat no. 345818-100ML) was
applied to the membranes and the immunoreactive proteins were
detected using premium autoradiography films (Denville Scientific,
Holliston, MA, USA; cat. no. E3218). Finally, the grey value of the
bands was quantified using ImageJ2 software (National Institute of
Health, Bethesda, MD, USA) (22).
RNA isolation and RT-qPCR
The chondrocytes were placed in 6-well plates at the
same density (3×105 cells/ml) and cultured in the basic
medium for 24 h. Subsequently, the cells were treated with the
vehicle (DMSO) or different concentrations of Peimine (5, 10 and 20
μg/ml) with or without IL-1β (10 ng/ml) for the following 24
h. Total RNA from each well was isolated using a RNAeasy Mini kit
from Qiagen, Inc., (Valencia, CA, USA) according to the
manufacturer's protocol. cDNA was synthesized using the Prime
Script RT reagent kit. qPCR was performed using SYBR and the
thermocycling condition was as follows: 95°C for 10 min, followed
by 40 cycles at 95°C for 5 sec, 60°C for 30 sec and 72°C for 30
sec, and a final extension step at 72°C for 3 min. The relative
mRNA expression levels were calculated using the 2−ΔΔCq
method (23). GAPDH was used as
the housekeeping gene to normalize the expression of the target
genes. The primer sequences were as follows: GAPDH forward
5′-TGCACCACCAACTGCTTAG-3′ and reverse 5′-GGATGCAGGGATGATGTTC-3′
(24); MMP-1 forward
5′-AACTACATTTAGGGGAGAGGTGT-3′, and reverse
5′-GCAGCGTCAAGTTTAACTGGAA-3′ (25); MMP-3 forward
5′-TCCTGATGTTGGTGGCTTCAG-3′, and reverse
5′-TGTCTTGGCAAATCCGGTGTA-3′ (26); MMP-9 forward
5′-ACCACATCGAACTTCGA-3′, and reverse 5′-CGACCATACAGATACTG-3′
(27); MMP-13 forward
5′-TGATGGACCTTCTGGTCTTCTGG-3′, and reverse
5′-CATCCACATGGTTGGGAAGTTCT-3′ (27); ADAMDTS-4 forward
5′-CATCCGAAACCCTGTCAACTTG-3′, and reverse
5′-GCCCATCATCTTCCACAATAGC-3′ (27); ADAMDTS-5 forward
5′-GCCATTGTAATAACCCTGCACC-3′, and reverse
5′-TCAGTCCCATCCGTAACCTTTG-3′ (27).
Establishment of the murine OA model
The animal experiment was designed and conducted in
accordance with the Guide for the Care and Use of Laboratory
Animals by the US National Institutes of Health, and was approved
by the Ethics Committee on Animal Experimentation of Tongji Medical
College, Huazhong University of Science and Technology (Wuhan,
China). The mice were housed under standard laboratory conditions
(20-25°C; humidity, 40-70%; 16/8-h light/dark cycle), and provided
with food and water ad libitum. A total of 15, 12-week-old
C57BL/6 male mice were purchased from the Experimental Animal
Center of Tongji Medical College and were divided randomly into the
following three groups (n=5): Sham/Vehicle group (sham operation +
vehicle treatment), OA/Vehicle group (OA operation + vehicle
treatment) and OA/Peimine group (OA operation + Peimine treatment).
The right knee of each mouse was used for the experiments.
Intraperitoneal injections of xylazine (10 mg/kg) and ketamine (100
mg/kg) were performed to anesthetize mice. For the mice in the
OA/Vehicle and OA/Peimine groups, OA was induced using the medial
meniscal tear (MMT) model, in which the medial collateral ligament
and medial meniscus of the knee were identified and excised, and
the other structures were preserved. In the Sham/Vehicle group, the
mice underwent sham operations; the joint cavity was opened without
transection of the medial meniscus ligament and medial meniscus.
Following surgery, the mice in the OA/Peimine group were
administered Peimine (20 mg/kg) 5 days a week for 8 weeks. The
Peimine was first dissolved in 20% ethanol to produce the 5 mg/ml
working solution and then the solution was administered to mice by
oral gavage at a dose of 4 ml/kg. For the mice in the Sham/Vehicle
and OA/Vehicle groups, a vehicle solution (20% ethanol in distilled
water) was administered by oral gavage at a dose of 4 ml/kg for 5
days a week. After a total period of 8 weeks, all mice were
sacrificed with an overdose injection of pentobarbital (180 mg/kg)
and the right knee joints of each mouse were collected for further
evaluation.
Histological analysis
The right knee joints from the mice were dissected
and fixed in 4% paraformaldehyde overnight at 4°. Subsequently, the
samples were decalcified in 10% EDTA at 4°C for 4 weeks and
embedded in paraffin. The specimens were cut into 5-μm-thick
sections along the sagittal plane. The tissue sections were stained
with Safranin O/Fast Green at room temperature for 45 min using
Safranin O/Fast Green staining kit (ScienCell Research Laboratories
lnc., Carlsbad, CA, USA; cat. no. 8348) according to the
manufacturer's protocol. The severity of OA-associated alterations
were assessed under a microscope using the Osteoarthritis Research
Society International (OARSI) histopathology scoring system
(28). Two pathologists (K. Chen
and W. Huang) independently reviewed the severity of OA and their
results were then compared. In cases of a discrepancy between the
two reviewers, a third investigator (P. Cheng) was consulted until
a mutual consensus was reached.
Statistical analysis
All experiments were repeated at least three times.
All data are expressed as the mean ± standard deviation.
Differences between groups were analyzed by one-way analysis of
variance with the Tukey-Kramer honest significant difference test
using SPSS software, version 16.0 (SPSS, Inc., Chicago, IL, USA).
The unpaired t-test was used for comparisons between two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of Peimine on chondrocyte
viability
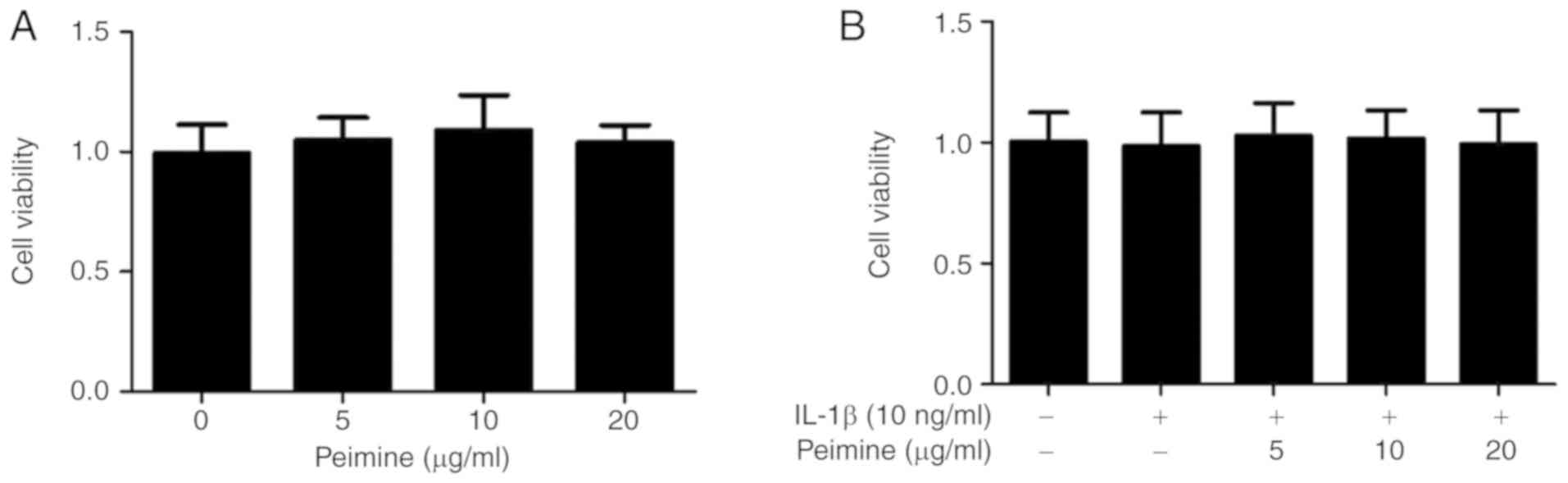
The potential cytotoxicity of Peimine on mouse
chondrocytes was tested using a CCK-8 kit. The chondrocytes were
treated with a vehicle or different doses of Peimine (5, 10 and 20
μg/ml) in the presence or absence of IL-1β for 24 h. As
presented in Fig. 1A and B, the
different doses of Peimine exhibited no cytotoxicity on the mouse
chondrocytes. Therefore, these concentrations of Peimine (5, 10 and
20 μg/ml) were used in the subsequent experiments (Fig. 1).
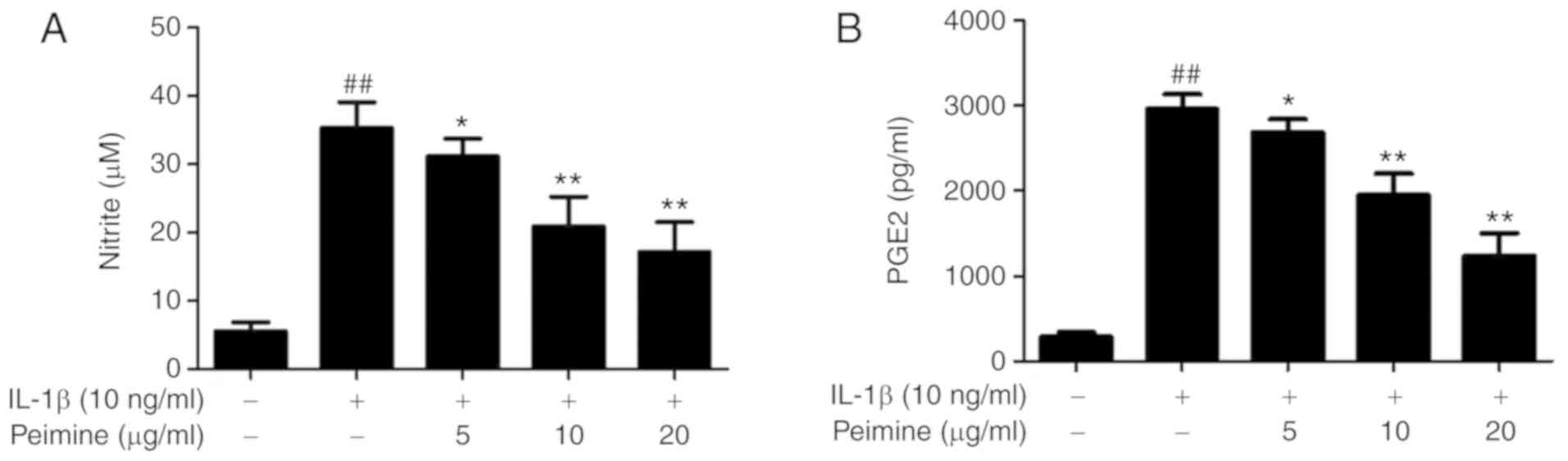
Effect of Peimine on IL-1β-induced NO and
PGE2 production in chondrocytes
It has been reported that IL-1β can induce
production of inflammatory mediators such as NO and PGE2 in
chondrocytes (29,30). In order to evaluate the effect of
Peimine on IL-1β-induced NO and PGE2 production, mouse chondrocytes
were stimulated with IL-1β (10 ng/ml) and treated with different
doses of Peimine (0, 5, 10 and 20 μg/ml) for 24 h. The NO
concentration was measured using a Nitric Oxide Assay kit and the
PGE2 concentration was measured using a PGE2 ELISA kit. As
expected, the concentrations of NO and PGE2 were significantly
elevated following treatment with IL-1β compared with the control
group (P<0.01; Fig. 2).
Furthermore, treatment with Peimine significantly reduced
IL-1β-induced NO and PGE2 production in a dose-dependent manner
(P<0.05; Fig. 2).
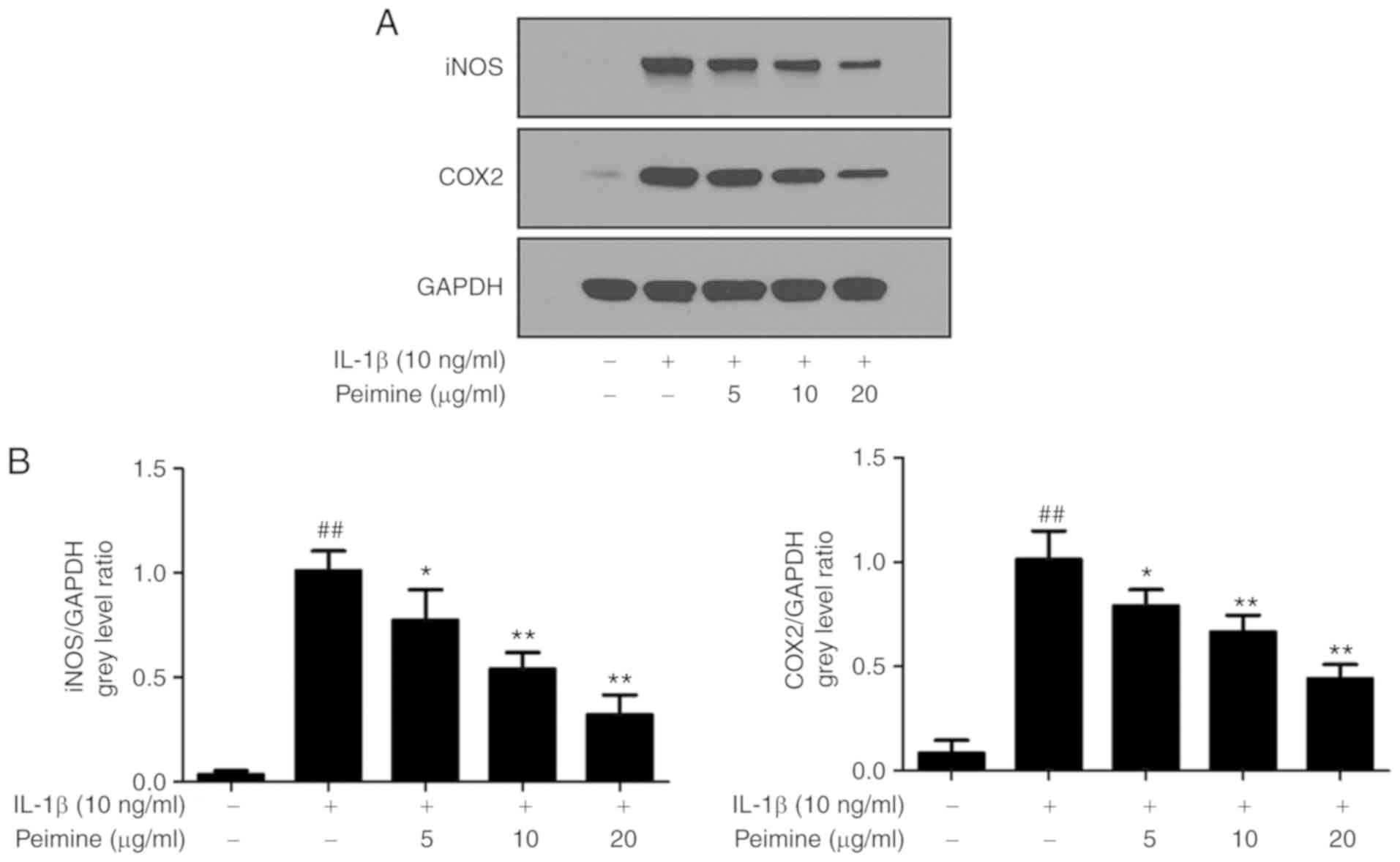
Effect of Peimine on IL-1β-induced
protein expression of iNOS and COX-2 in chondrocytes
The effect of Peimine on IL-1β-induced protein
expression of inflammatory mediators, including iNOS and COX-2, was
evaluated using western blot analysis. As expected, following
stimulation with IL-1β (10 ng/ml, 24 h), the protein expression
levels of iNOS and COX-2 were significantly increased compared with
the control (P<0.01; Fig. 3).
However, when the chondrocytes were treated with Peimine together
with IL-1β, the protein expression of iNOS and COX-2 was
significantly inhibited in a dose-dependent manner (P<0.05),
indicating that Peimine prohibits IL-1β-induced protein expression
of inflammatory mediators, namely iNOS and COX-2 (Fig. 3).
Effect of Peimine on IL-1β-induced
expression of MMP-1, MMP-3, MMP-9, MMP-13, ADAMTS-4 and ADAMTS-5 in
chondrocytes
A number of studies have demonstrated that IL-1β
stimulation can induce the expression of MMP-1, MMP-3, MMP-9,
MMP-13, ADAMTS-4 and ADAMTS-5, which are major cartilage
degradation enzymes in OA (4,5).
To investigate whether Peimine can inhibit the IL-1β-induced
overexpres-sion of these enzymes, mouse chondrocytes were treated
with different doses of Peimine in the presence or absence of IL-1β
for 24 h. According to the RT-quantitative PCR results, the mRNA
overexpression of MMP-1, MMP-3, MMP-9, MMP-13, ADAMTS-4 and
ADAMTS-5 induced by IL-1β was inhibited by Peimine in a
dose-dependent manner (Fig.
4).
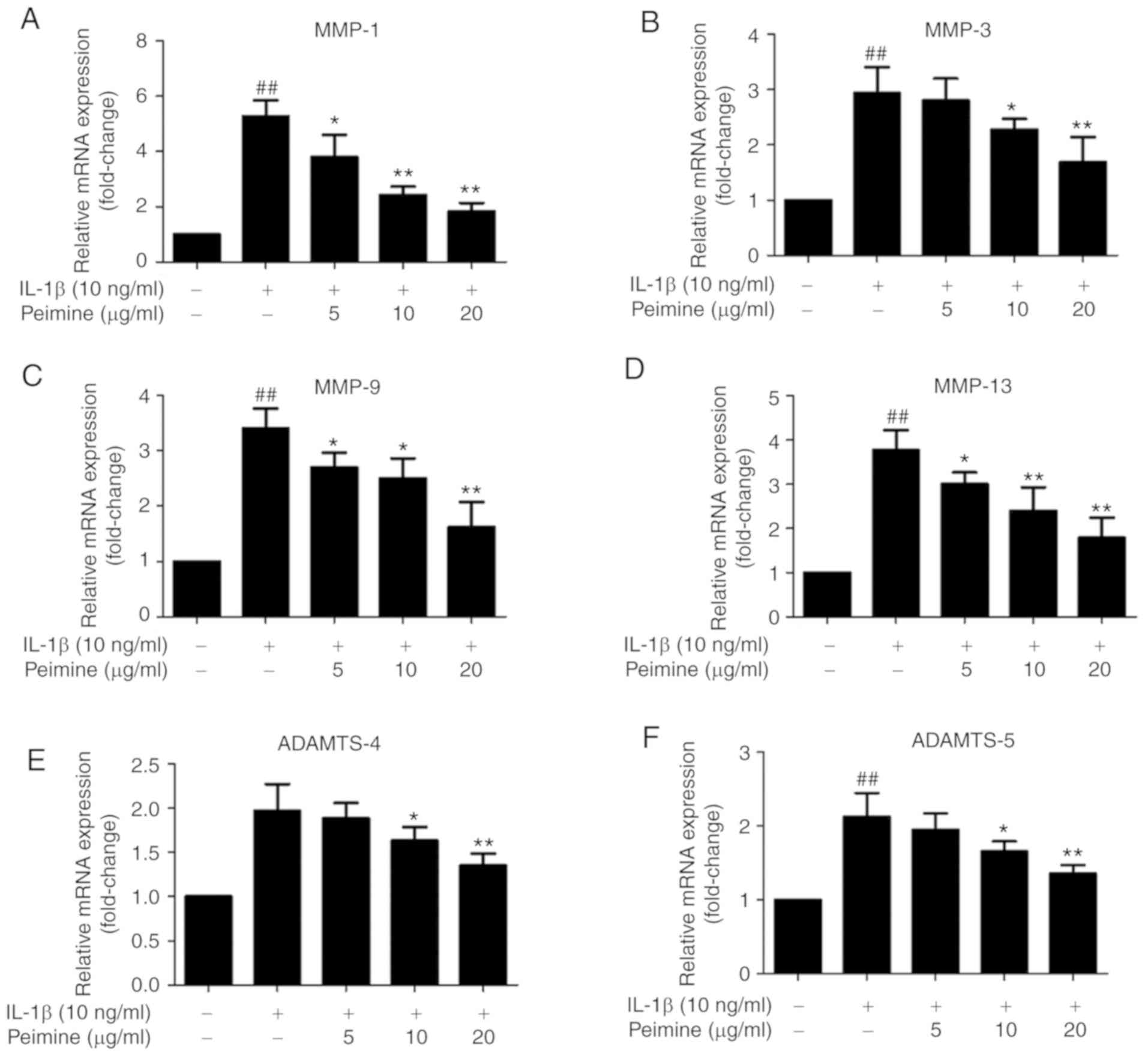 | Figure 4Effect of Peimine on IL-1β-induced
expression of MMP-1, MMP-3, MMP-9, MMP-13, ADAMTS-4 and ADAMTS-5 in
chondrocytes. Chondrocytes were treated with Peimine (5, 10, 20
ug/ml) or vehicle in the absence or presence of IL-1β (10 ng/ml)
for 24 h. Total RNA from each group was isolated. mRNA expressions
of (A) MMP-1, (B) MMP-3, (C) MMP-9, (D) MMP-13, (E) ADAMTS-4 and
(F) ADAMTS-5 in chondrocytes was determined using reverse
transcription-polymerase chain reaction. The experiments were
repeated 3 times separately. #P<0.05 and
##P<0.01 vs. the control group; *P<0.05
and **P<0.01 vs. IL-1β group. MMP, matrix
metalloproteinase; IL, interleukin; ADAMTS, a disintegrin and
metalloproteinase with thrombospondin motifs. |
Effect of Peimine on IL-1β-induced MAPK
activation in chondrocytes
Given the result that Peimine can inhibit
IL-1β-induced inflammation in mouse chondrocytes, the underlying
mechanism of this inhibitory effect was subsequently investigated.
MAPK signaling pathways, which serve a significant role in
inflammation, are considered as promising therapeutic targets for
inflammatory diseases and OA (31,32). Furthermore, it has been reported
that Peimine can inhibit MAPK signaling pathways in
lipopolysaccharide (LPS)-induced macrophages (15). Therefore, the effects of Peimine
on IL-1β-induced MAPK activation in chondrocytes were evaluated.
Cells were treated with different concentrations of Peimine (0, 5,
10 and 20 μg/ml) in the presence or absence of IL-1β (10
ng/ml) for 24 h, and then the total protein was isolated and
western blot analysis was performed. As expected, IL-1β stimulation
significantly increased the phosphorylation of ERK, JNK and p38
(P<0.01; Fig. 5). Peimine
treatment inhibited this phosphorylation in a dose-dependent manner
(Fig. 5).
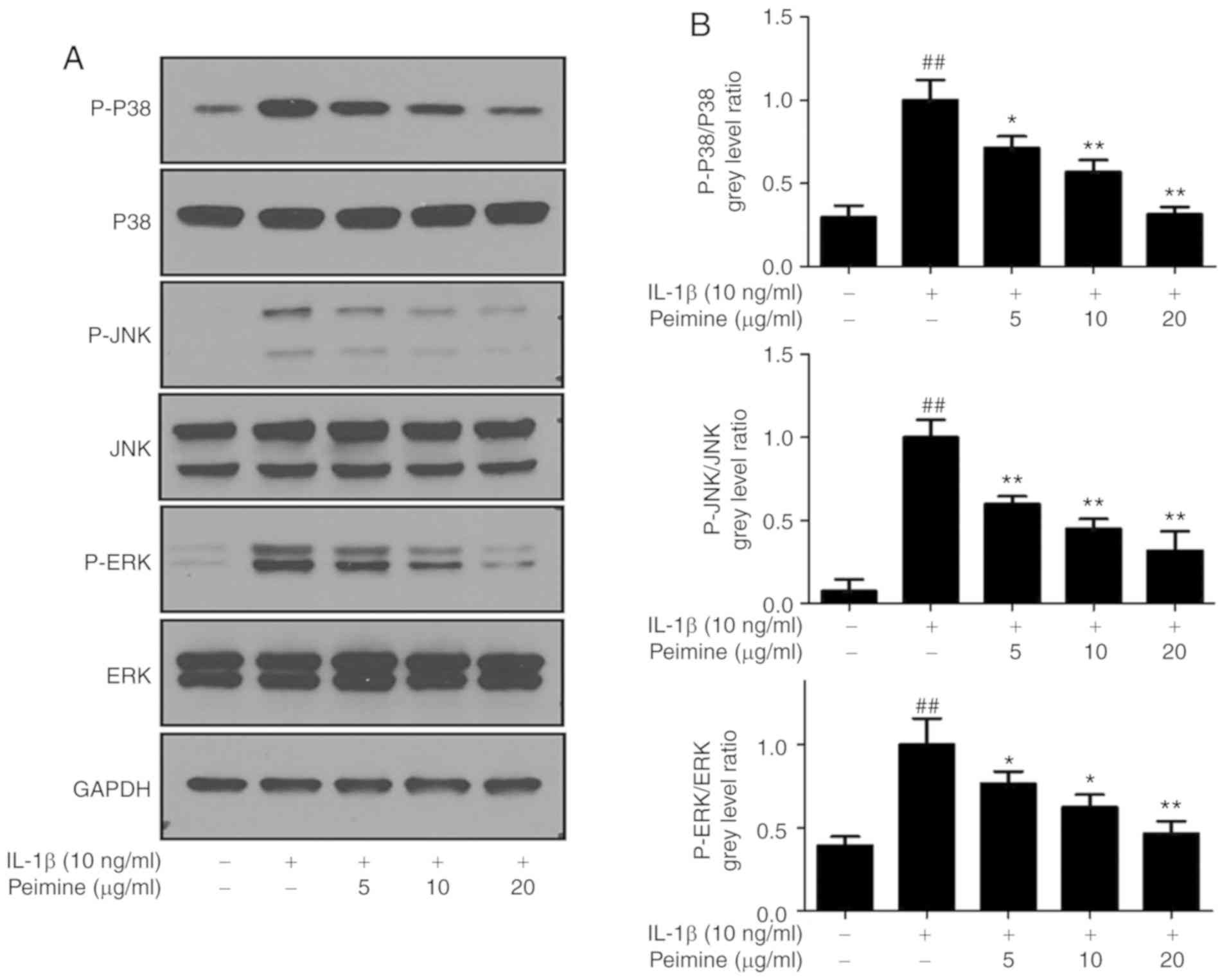 | Figure 5Effect of Peimine on
mitogen-activated protein kinase signaling. Chondrocytes were
treated with Peimine (5, 10, 20 μg/ml) or vehicle in the
absence or presence of IL-1β (10 ng/ml) for 24 h. Total proteins
from chondrocytes in each group were isolated, protein expression
levels were determined using western blot analysis. (A)
Representative images of western blotting for P-P38, P38, P-JNK,
JNK, P-ERK, ERK and GAPDH. (B) Relative protein expression was
quantified using Image-J software. The experiments were repeated 3
times separately. ##P<0.01 vs. the control group;
*P<0.05 and **P<0.01 vs. the IL-1β
group. IL, interleukin; JNK, Janus kinase; P-ERK, phosphorylated
extracellular signal regulated kinase. |
In addition, anisomycin, a p38 and JNK activator, or
U-46619, a p38 and ERK activator were used together with Peimine
(20 μg/ml) on IL-1β-treated chondrocytes. As expected, the
phosphorylation levels of p38 and JNK increased in the Anisomycin +
Peimine group compared with the Peimine group, and the
phosphorylation levels of p38 and ERK increased in the U-46619 +
Peimine group compared with the Peimine group (Fig. 6A and B). Furthermore, the protein
levels of MMP-3, MMP-13, ADAMTS-5, iNOS and COX-2 signifi-cantly
increased following the activation of MAPK signaling compared with
the Peimine group (P<0.05; Fig. 6C
and D). These results indicate that the protective effect of
Peimine on IL-1β-treated chondrocytes was attenuated following
activation of MAPK signaling. Therefore, the data indicate that
Peimine inhibits IL-1β-induced inflammation in mouse chondrocytes
via inhibition of MAPK signaling pathways.
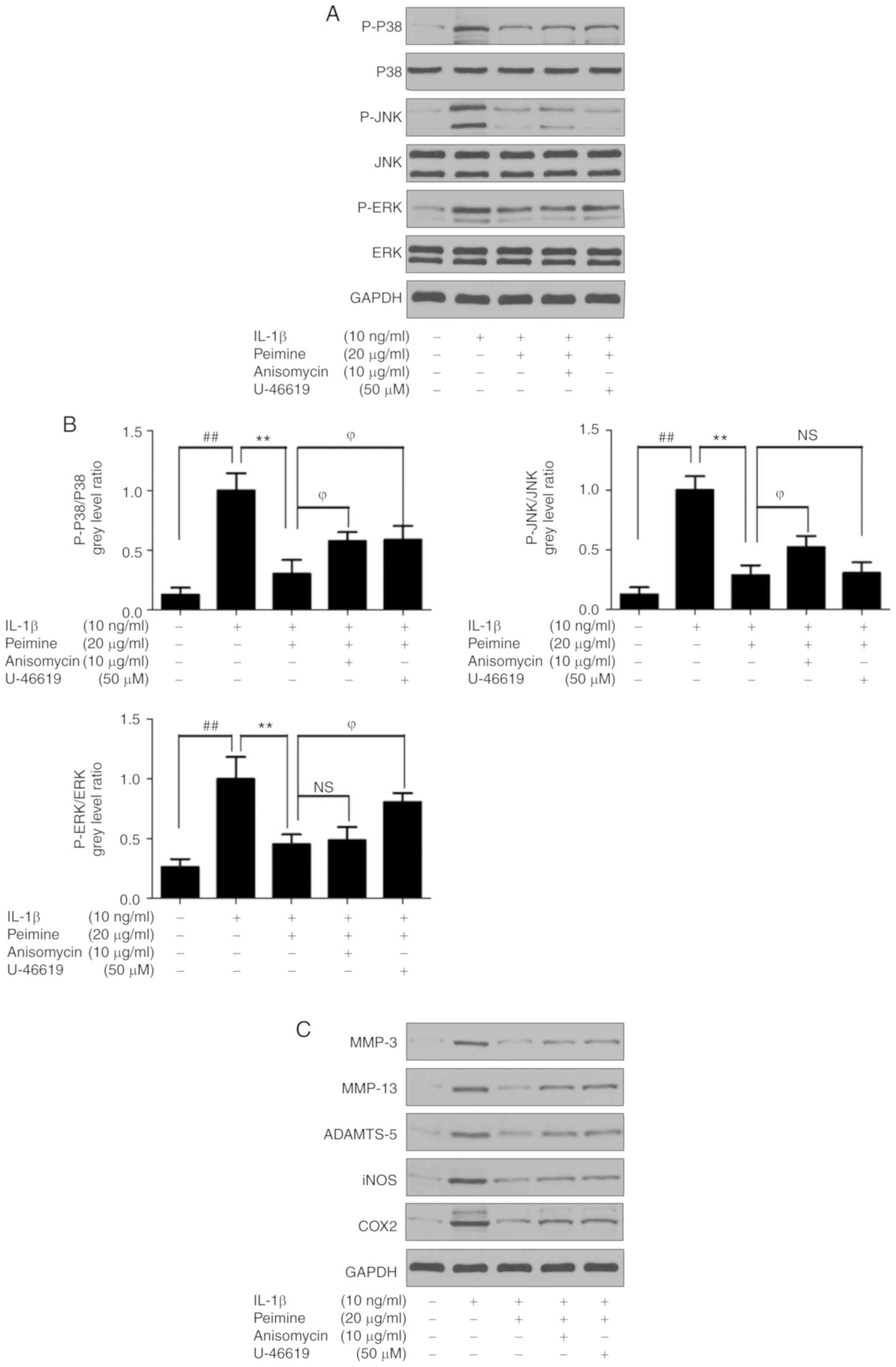 | Figure 6Effect of Peimine on IL-1β treated
chondrocytes is alleviated following the activation of
mitogen-activated protein kinase signaling. Chondrocytes were
treated with Peimine (20 μg/ml) or vehicle in the absence or
presence of IL-1β (10 ng/ml) for 24 h. In 'Peimine + Anisomycin'
group and 'Peimine + U-46619′, 10 μg/ml Anisomycin or 50
μM was treated together with Peimine (20 μg/ml) and
IL-1β (10 ng/ml) for 24 h. Total proteins from chondrocytes in each
group were isolated, protein expression levels were determined
using western blotting. (A) Representative images of western
blotting for P-P38, P38, P-JNK, JNK, P-ERK, ERK and GAPDH. (B)
Relative protein expression was quantified using Image-J software.
(C) Representative images of western blotting for COX2, iNOS,
MMP-3, MMP-13, ADAMTS-5 and GAPDH. (D) Relative protein expression
was quantified using Image-J software. ##P<0.01 vs.
the control group; **P<0.01 vs. the IL-1β group;
ϕP<0.05 vs. 'IL-1β+ Peimine' group. IL, interleukin;
JNK, Janus kinase; P-ERK, phosphorylated extracellular signal
regulated kinase; MMP, matrix metalloproteinase; COX-2,
cyclo-oxygenase-2; iNOS, inducible nitric oxide synthase; ADAMSTS,
a disintegrin and metalloproteinase with thrombospondin motifs; NS,
not significant. |
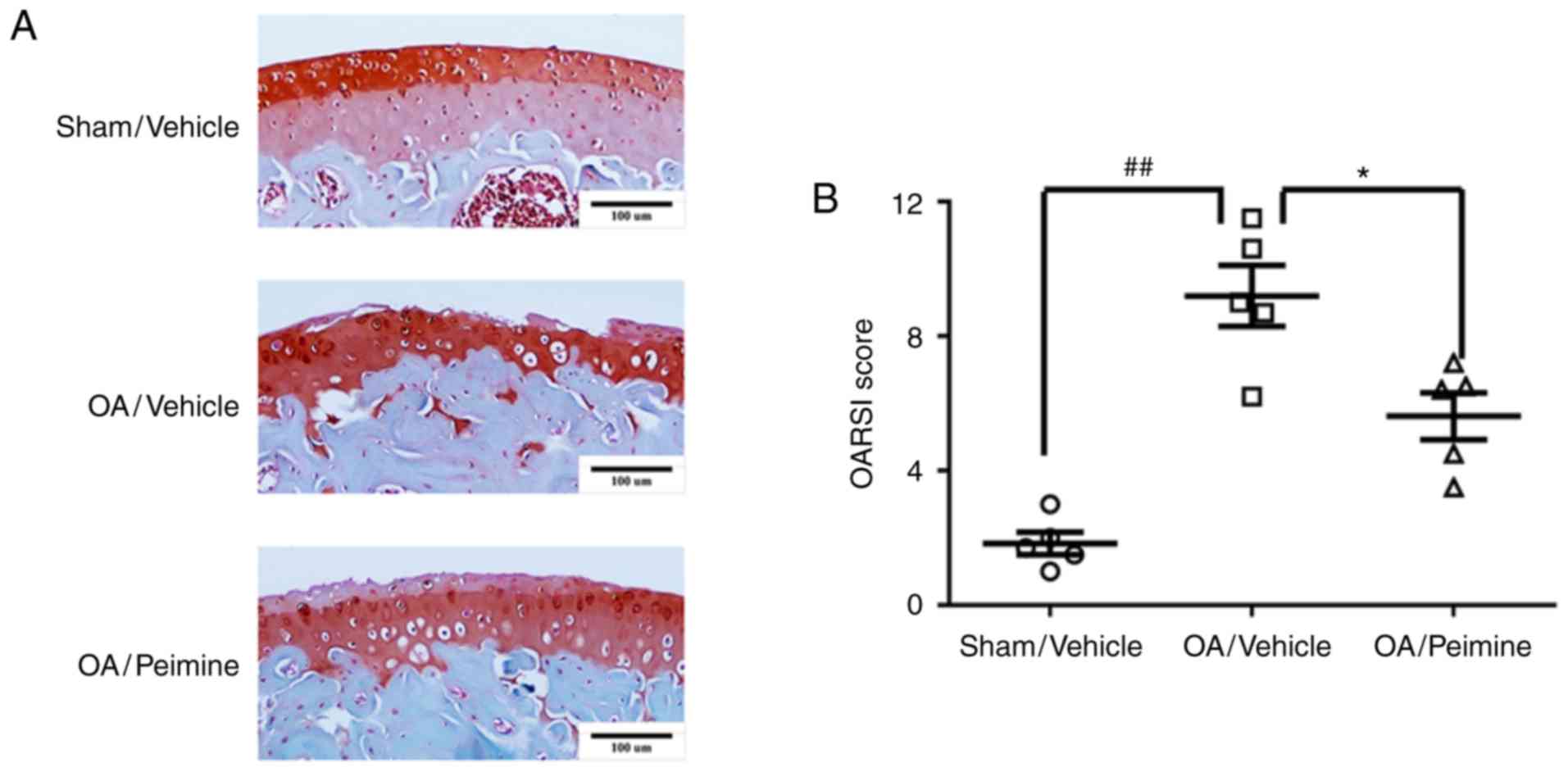
Effect of Peimine on cartilage
degradation in an OA model
A mouse OA model was established to evaluate whether
Peimine has a protective effect on OA in vivo. The mice were
randomly divided into the following three groups: Sham/Vehicle
group (sham operation + vehicle treatment), OA/Vehicle group (OA
operation + vehicle treatment) and OA/Peimine group (OA operation +
Peimine treatment). Following surgery, the vehicle or Peimine (20
mg/kg) was administered to the mice by oral gavage 5 days a week
for a total period of 8 weeks. Histological analysis was performed
using Safranin O/Fast Green staining and the severity of
OA-associated changes was assessed using the OARSI scoring system.
As presented in Fig. 7A, the
joint surface was intact and almost no cartilage destruction was
observed in the mice of the Sham/Vehicle group. As expected, the
joint surface was discontinued and severe cartilage destruction was
observed in the mice of the OA/Vehicle group. However, the
morphology of the joint surface in the OA/Peimine group was much
better than that of the OA/Vehicle group. Furthermore, the OARSI
scores were significantly increased in the OA/Peimine group
compared with the OA/Vehicle group, indicating that Peimine exerts
protective effects against the development of OA (P<0.05;
Fig. 7B).
Discussion
OA, which is characterized by progressive articular
cartilage destruction, is the most common type of joint disease
(33). It is estimated that 25%
of the adult population in the US will be affected by OA by the
year 2020 and OA will become one of the major health problems
(34,35). However, there are no effective
interventions to restore degraded cartilage or decelerate disease
progression. Therefore, there is an urgent requirement to identify
novel drugs to treat OA efficiently.
An accumulating number of studies have reported that
compounds extracted from plants have the potential to protect
against OA (7,36,37). Peimine, the main compound
extracted from BF, has been demonstrated to have antioxidant,
anti-inflammatory and pain suppressing effects (16-19). In the present study, the effects
of Peimine on IL-1β-induced inflammation were evaluated in mouse
chondrocytes in vitro and the potential beneficial effects
of Peimine on OA were also investigated in a mouse model in
vivo.
The results of the present study revealed the
following observations that are, to the best of our knowledge,
novel: i) Peimine reduces IL-1β-induced production of the
inflammatory mediators iNOS, COX-2 and PGE2 in mouse chondrocytes;
ii) Peimine suppresses IL-1β-induced chondrocyte expression of the
cartilage catabolic enzymes MMP-1, MMP-3, MMP-9, MMP-13, ADAMTS-4
and ADAMTS-5 in mice; iii) Peimine inhibits the MAPK signaling
pathway in a dose-dependent manner in mouse chondrocytes; iv)
Peimine has the potential to slow the progression of OA in a mouse
model of OA.
Inflammatory cytokines including IL-1β and tumor
necrosis factor-α serve a pivotal role in the pathogenesis of OA
(5). The levels of IL-1β are
significantly increased in articular chondrocytes from patients
with OA (38). The increased
levels of IL-1β stimulate chondrocytes to produce more inflammatory
mediators, including iNOS, NO, COX-2 and PGE2 (39,40). IL-1β also enhances the degradation
of the extracellular matrix via increased chondrocyte expression of
cartilage catabolic enzymes, including MMPs and ADAMTS (41,42). As a result of its important role
in the progression of OA, IL-1β stimulation is used as a
conventional in vitro model to study OA and was used in the
present study.
In the present study, mouse chondrocytes were
stimulated with IL-1β (10 ng/ml) for 24 h. The data demonstrated
that the concentrations of NO and PGE2 were upregulated and the
protein expression levels of iNOS and COX-2 were elevated in the
chondrocyte culture medium supernatant. These results are in
accordance with a previous study, suggesting that IL-1β stimulates
the OA microenvironment in vitro (37). iNOS, a member of the NO synthase
family of enzymes, is used to synthesized NO (39). Another inflammatory mediator,
PGE2, is the predominant product of COX-2 (43). It has been established that PGE2
and COX-2 serve key roles in the pathogenesis of OA, including
inhibition of matrix synthesis and promotion of cartilage
degradation (39). Targeting of
PGE2 and COX-2 is considered a promising approach for the
therapeutic intervention of OA (44). The data of the present study
demonstrate that Peimine treatment alleviates IL-1β-induced
expression of iNOS, NO, COX-2 and PGE2, indicating that Peimine
inhibits IL-1β-induced inflammation in mouse chondrocytes.
The progressive cartilage destruction is the most
characteristic change in OA (45). IL-1β stimulates chondrocytes to
produce proteolytic enzymes, including aggrecanases and MMPs, which
contribute to the destruction of the cartilage matrix (46). Among the various
chondrocyte-secreted enzymes, MMP-13 and ADAMTS-5 are considered as
the pivotal factors that accelerate the cartilage destruction in OA
(47). Other enzymes, including
MMP-1, MMP-3, MMP-9 and ADAMTS-4, also participate in the
progression of OA (48). In the
present study, IL-1β stimulation (10 ng/ml, 24 h) increased the
mRNA expression levels of MMP-1, MMP-3, MMP-9, MMP-13, ADAMTS-4 and
ADAMTS-5 in the mouse chondrocytes compared with the control group,
which is in accordance with previous studies (7,36,37). Furthermore, Peimine treatment
inhibited the elevated mRNA expression levels in a dose-dependent
manner. These data suggest that Peimine ameliorates the increased
expression levels of catabolic enzymes caused by IL-1β and protects
cartilage from IL-1β-induced destruction.
MAPK signaling pathways are involved in multiple
cellular activities, including cell survival, proliferation and
inflammation (49). Accumulating
evidence has suggested that MAPK signaling serves a significant
role in the progression of OA (4). Furthermore, a previous study
reported that Peimine can inhibit MAPK signaling pathways in
LPS-induced macrophages (15). In
order to elucidate the anti-inflammatory mechanisms of Peimine in
chondrocytes, MAPK signaling pathway was evaluated in the present
study. Following stimulation with IL-1β for 24 h, MAPK signaling
pathway in the mouse chondrocyte cells was activated, as revealed
by the significantly increased phosphorylation levels of p38, ERK
and JNK. However, the Peimine treatment reduced the expression
levels of P-p38, P-ERK and P-JNK in a dose-dependent manner. These
data suggest that Peimine inhibits IL-1β-induced inflammation in
mouse chondrocytes via inhibition of the MAPK signaling pathway.
However, this is just one of the associated signaling pathways and
there is a high possibility that a number of other mechanisms are
involved in the anti-inflammation effects of Peimine on mouse
chondrocytes.
To investigate the effects of Peimine on OA in
vivo, a mouse OA model based on MMT was established in the
present study. After the surgery and indicated treatments, the
severity of OA-associated changes was assessed using the OARSI
scoring system. The mice in the OA/Vehicle group (MMT operation +
vehicle treatment) exhibited increased OARSI scores compared with
the Sham/Vehicle group (sham operation + vehicle treatment). This
result suggests that the mouse OA model was established
successfully. The OARSI scores of the mice in the OA/Peimine group
(OA operation + Peimine treatment) were significantly reduced
compared with the OA/Vehicle group, indicating that Peimine exerts
protective effects against the development of OA.
It is important to note the limitations within the
present study that represent the future directions for the present
study. First, the data suggest that the MAPK signaling pathway is
involved in the anti-inflammation effects of Peimine on mouse
chondrocytes. However, there is a high possibility that a number of
other mechanisms are also involved. Second, the in vivo and
in vitro experimental models are both based on mice, and it
remains to be verified whether Peimine exhibits the same effects on
humans. Third, Peimine is the main compound extracted from BF.
Although BF has been used in Chinese traditional medicine for
>2,000 years due to its low toxicity and few side effects, it
remains unclear whether Peimine has side effects when used for a
long period of time to treat OA.
In conclusion, the results of the present study
report the anti-inflammatory effects of Peimine in OA for the first
time to the best of our knowledge. Peimine inhibits IL-1β-induced
iNOS and COX-2 production and expression of MMPs and ADAMTs in a
dose-dependent manner via blockage of IL-1β-induced MAPK signaling
activation. Furthermore, Peimine has protective effects against the
development of OA in a mouse model of OA. These results indicate
that Peimine is a promising therapeutic agent for OA.
Abbreviations:
|
OA
|
osteoarthritis
|
|
IL-1β
|
interleukin-1β
|
|
NO
|
nitric oxide
|
|
iNOS
|
inducible nitric oxide synthase
|
|
COX-2
|
cyclooxygenase-2
|
|
MMP
|
matrix metalloproteinase
|
|
ADAMTS
|
a disintegrin and metalloproteinase
with thrombospondin motifs
|
|
MAPK
|
mitogen-activated protein kinase
|
|
BF
|
Bulbus Fritillariae
|
|
MMT
|
medial meniscal tear
|
Funding
The present study was supported by research grants
from the National Natural Science Foundation of China (grant nos.
81672168 and 81472082, awarded to AC), the Natural Science
Foundation of Hubei Province of China (grant no. 2018CFB714,
awarded to WZ), and the Hubei Province Health and Family Planning
Scientific Research Project (grant no. WJ2019Q028, awarded to
PC).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PC and AC designed the experiments. KC, ZL, WH, SL,
ZW, XJ, CZ, FG, WZ and HL performed the experiments. ZW, XJ, KC and
ZL performed the measurements and analysis. FG, KC and PC drafted
the manuscript. WZ and ZL revised the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee on
Animal Experimentation of Tongji Medical College, Huazhong
University of Science and Technology (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Chen D, Shen J, Zhao W, Wang T, Han L,
Hamilton JL and Im HJ: Osteoarthritis: Toward a comprehensive
understanding of pathological mechanism. Bone Res. 5:160442017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buckwalter JA, Saltzman C and Brown T: The
impact of osteoarthritis: Implications for research. Clin Orthop
Relat Res. (Suppl 427): S6–S15. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bitton R: The economic burden of
osteoarthritis. Am J Manag Care. 15(Suppl 8): S230–S235.
2009.PubMed/NCBI
|
|
4
|
Goldring MB and Otero M: Inflammation in
osteoarthritis. Curr Opin Rheumatol. 23:471–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu-Bryan R and Terkeltaub R: Emerging
regulators of the inflammatory process in osteoarthritis. Nat Rev
Rheumatol. 11:35–44. 2015. View Article : Google Scholar :
|
|
6
|
Daheshia M and Yao JQ: The interleukin 1β
pathway in the pathogenesis of osteoarthritis. J Rheumatol.
35:2306–2312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park C, Jeong JW, Lee DS, Yim MJ, Lee JM,
Han MH, Kim S, Kim HS, Kim GY, Park EK, et al: Sargassum
serratifolium extract attenuates interleukin-1β-induced oxidative
stress and inflammatory response in chondrocytes by suppressing the
activation of NF-κB, p38 MAPK, and PI3K/Akt. Int J Mol Sci.
19:E23082018. View Article : Google Scholar
|
|
8
|
Ding QH, Cheng Y, Chen WP, Zhong HM and
Wang XH: Celastrol, an inhibitor of heat shock protein 90beta
potently suppresses the expression of matrix metalloproteinases,
inducible nitric oxide synthase and cyclooxygenase-2 in primary
human osteoarthritic chondrocytes. Eur J Pharmacol. 708:1–7. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roman-Blas JA and Jimenez SA: NF-kappaB as
a potential therapeutic target in osteoarthritis and rheumatoid
arthritis. Osteoarthritis Cartilage. 14:839–848. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen WP, Hu ZN, Jin LB and Wu LD:
Licochalcone A inhibits MMPs and ADAMTSs via the NF-κB and
Wnt/β-catenin signaling pathways in rat chondrocytes. Cell Physiol
Biochem. 43:937–944. 2017. View Article : Google Scholar
|
|
11
|
Largo R, Alvarez-Soria MA, Díez-Ortego I,
Calvo E, Sánchez-Pernaute O, Egido J and Herrero-Beaumont G:
Glucosamine inhibits IL-1beta-induced NFkappaB activation in human
osteoarthritic chondrocytes. Osteoarthritis Cartilage. 11:290–298.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Frisbie DD, Ghivizzani SC, Robbins PD,
Evans CH and McIlwraith CW: Treatment of experimental equine
osteoarthritis by in vivo delivery of the equine interleukin-1
receptor antagonist gene. Gene Ther. 9:12–20. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clements KM, Price JS, Chambers MG, Visco
DM, Poole AR and Mason RM: Gene deletion of either
interleukin-1beta, interleukin-1beta-converting enzyme, inducible
nitric oxide synthase, or stromelysin 1 accelerates the development
of knee osteoarthritis in mice after surgical transection of the
medial collateral ligament and partial medial meniscectomy.
Arthritis Rheum. 48:3452–3463. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chevalier X, Giraudeau B, Conrozier T,
Marliere J, Kiefer P and Goupille P: Safety study of intraarticular
injection of interleukin 1 receptor antagonist in patients with
painful knee osteoarthritis: A multicenter study. J Rheumatol.
32:1317–1323. 2005.PubMed/NCBI
|
|
15
|
Yi PF, Wu YC, Dong HB, Guo Y, Wei Q, Zhang
C, Song Z, Qin QQ, Lv S, Wu SC and Fu BD: Peimine impairs
pro-inflammatory cytokine secretion through the inhibition of the
activation of NF-κB and MAPK in LPS-induced RAW264.7 macrophages.
Immunopharmacol Immunotoxicol. 35:567–572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu J, Zhao W, Pan L, Zhang A, Chen Q, Xu
K, Lu H and Chen Y: Peimine, a main active ingredient of
Fritillaria, exhibits anti-inflammatory and pain suppression
properties at the cellular level. Fitoterapia. 111:1–6. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cho IH, Lee MJ, Kim JH, Han NY, Shin KW,
Sohn Y and Jung HS: Fritillaria ussuriensis extract inhibits the
production of inflammatory cytokine and MAPKs in mast cells. Biosci
Biotechnol Biochem. 75:1440–1445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park JH, Lee B, Kim HK, Kim EY, Kim JH,
Min JH, Kim S, Sohn Y and Jung HS: Peimine inhibits the production
of proinflammatory cytokines through regulation of the
phosphorylation of NF-kappaB and MAPKs in HMC-1 cells. Pharmacogn
Mag. 13(Suppl 2): S359–S364. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu K, Mo C, Xiao H, Jiang Y, Ye B and Wang
S: Imperialine and verticinone from bulbs of Fritillaria wabuensis
inhibit pro-inflammatory mediators in LPS-stimulated RAW 264.7
macrophages. Planta Med. 81:821–829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gosset M, Berenbaum F, Thirion S and
Jacques C: Primary culture and phenotyping of murine chondrocytes.
Nat Protoc. 3:1253–1260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen K, Lv ZT, Cheng P, Zhu WT, Liang S,
Yang Q, Parkman VA, Zhou CH, Jing XZ, Liu H, et al: Boldine
ameliorates estrogen deficiency-induced bone loss via inhibiting
bone resorption. Front Pharmacol. 9:10462018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schindelin J, Rueden CT, Hiner MC and
Eliceiri KW: The ImageJ ecosystem: An open platform for biomedical
image analysis. Mol Reprod Dev. 82:518–529. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Shohet RV, Kisanuki YY, Zhao XS, Siddiquee
Z, Franco F and Yanagisawa M: Mice with cardiomyocyte-specific
disruption of the endothelin-1 gene are resistant to hyperthyroid
cardiac hypertrophy. Proc Natl Acad Sci USA. 101:2088–2093. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mu X, Urso ML, Murray K, Fu F and Li Y:
Relaxin regulates MMP expression and promotes satellite cell
mobilization during muscle healing in both young and aged mice. Am
J Pathol. 177:2399–2410. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li S, Zhang Y, Sun Y, Cao W and Cui L:
Exposure to fermentation supernatant of Staphylococcus aureus
accelerated dedifferentiation of chondrocytes and production of
antimicrobial peptides. J Orthop Res. 36:443–451. 2018.
|
|
27
|
Kim JH, Lee G, Won Y, Lee M, Kwak JS, Chun
CH and Chun JS: Matrix cross-linking-mediated mechanotransduction
promotes posttraumatic osteoarthritis. Proc Natl Acad Sci USA.
112:9424–9429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pritzker KP, Gay S, Jimenez SA, Ostergaard
K, Pelletier JP, Revell PA, Salter D and van den Berg WB:
Osteoarthritis cartilage histopathology: Grading and staging.
Osteoarthritis Cartilage. 14:13–29. 2006. View Article : Google Scholar
|
|
29
|
Chowdhury TT, Bader DL and Lee DA: Dynamic
compression inhibits the synthesis of nitric oxide and PGE(2) by
IL-1beta-stimulated chondrocytes cultured in agarose constructs.
Biochem Biophys Res Commun. 285:1168–1174. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang G, Im HJ and Wang JH: Repetitive
mechanical stretching modulates IL-1beta induced COX-2, MMP-1
expression, and PGE2 production in human patellar tendon
fibroblasts. Gene. 363:166–172. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaminska B: MAPK signalling pathways as
molecular targets for anti-inflammatory therapy-from molecular
mechanisms to therapeutic benefits. Biochim Biophys Acta.
1754:253–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Loeser RF, Erickson EA and Long DL:
Mitogen-activated protein kinases as therapeutic targets in
osteoarthritis. Curr Opin Rheumatol. 20:581–586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brittberg M, Gomoll AH, Canseco JA, Far J,
Lind M and Hui J: Cartilage repair in the degenerative ageing knee.
Acta Orthop. 87:26–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Helmick CG, Felson DT, Lawrence RC,
Gabriel S, Hirsch R, Kwoh CK, Liang MH, Kremers HM, Mayes MD,
Merkel PA, et al: Estimates of the prevalence of arthritis and
other rheumatic conditions in the United States. Part I Arthritis
Rheum. 58:15–25. 2008. View Article : Google Scholar
|
|
35
|
Lawrence RC, Felson DT, Helmick CG, Arnold
LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG,
et al: Estimates of the prevalence of arthritis and other rheumatic
conditions in the United States. Part II Arthritis Rheum. 58:26–35.
2008. View Article : Google Scholar
|
|
36
|
Huang X, Pan Q, Mao Z, Zhang R, Ma X, Xi Y
and You H: Sinapic acid inhibits the IL-1β-induced inflammation via
MAPK down-regulation in rat chondrocytes. Inflammation. 41:562–568.
2018. View Article : Google Scholar
|
|
37
|
Pan T, Shi X, Chen H, Chen R, Wu D, Lin Z,
Zhang J and Pan J: Geniposide suppresses interleukin-1β-induced
inflammation and apoptosis in rat chondrocytes via the
PI3K/Akt/NF-κB signaling pathway. Inflammation. 41:390–399. 2018.
View Article : Google Scholar
|
|
38
|
Pelletier JP, McCollum R, Cloutier JM and
Martel-Pelletier J: Synthesis of metalloproteases and interleukin 6
(IL-6) in human osteoarthritic synovial membrane is an IL-1
mediated process. J Rheumatol Suppl. 43:109–114. 1995.PubMed/NCBI
|
|
39
|
Amin AR, Dave M, Attur M and Abramson SB:
COX-2, NO, and cartilage damage and repair. Curr Rheumatol Rep.
2:447–453. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Abramson SB, Attur M, Amin AR and Clancy
R: Nitric oxide and inflammatory mediators in the perpetuation of
osteoarthritis. Curr Rheumatol Rep. 3:535–541. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fan Z, Bau B, Yang H, Soeder S and Aigner
T: Freshly isolated osteoarthritic chondrocytes are catabolically
more active than normal chondrocytes, but less responsive to
catabolic stimulation with interleukin-1beta. Arthritis Rheum.
52:136–143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Elliott S, Hays E, Mayor M, Sporn M and
Vincenti M: The triterpenoid CDDO inhibits expression of matrix
metalloproteinase-1, matrix metalloproteinase-13 and Bcl-3 in
primary human chon-drocytes. Arthritis Res Ther. 5:R285–R291. 2003.
View Article : Google Scholar
|
|
43
|
Arasapam G, Scherer M, Cool JC, Foster BK
and Xian CJ: Roles of COX-2 and iNOS in the bony repair of the
injured growth plate cartilage. J Cell Biochem. 99:450–461. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nah SS, Choi IY, Lee CK, Oh JS, Kim YG,
Moon HB and Yoo B: Effects of advanced glycation end products on
the expression of COX-2, PGE2 and NO in human osteoarthritic
chondrocytes. Rheumatology (Oxford). 47:425–431. 2008. View Article : Google Scholar
|
|
45
|
Loeser RF: Molecular mechanisms of
cartilage destruction: Mechanics, inflammatory mediators, and aging
collide. Arthritis Rheum. 54:1357–1360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ishiguro N, Kojima T and Poole AR:
Mechanism of cartilage destruction in osteoarthritis. Nagoya J Med
Sci. 65:73–84. 2002.
|
|
47
|
van den Berg WB: Osteoarthritis year 2010
in review: Pathomechanisms. Osteoarthritis Cartilage. 19:338–341.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Huang K and Wu LD: Aggrecanase and
aggrecan degradation in osteoarthritis: A review. J Int Med Res.
36:1149–1160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|