Introduction
Musashi1 (Msi1) is an RNA-binding protein that is
expressed at high levels in the nervous system (1,2). It has been
suggested that Msi1 serves an important role in maintaining stem
cell state, differentiation and tumorigenesis (3-7). Numerous
studies have shown that Msi1 is expressed at high levels in the
nervous system and the adult intestinal epithelial stem cell
population. Msi1 is located in a small number of epithelial cells
directly above Paneth cells present in the small intestine crypts
of adult mice. This indicates that Msi1 is a marker of intestinal
epithelial stem cells (8,9). Msi1-positive cells are regarded as a
candidate for intestinal epithelial stem cells. As Msi1 protein is
localized in the cytoplasm and nucleus of cells, and Msi1-positive
cells are rarely distributed in the small intestinal crypt, it is
almost impossible to harvest living Msi1-positive cells from the
small intestine (10,11). The scarcity of Msi1-positive cells has
hindered investigation of the association between Msi1 and
intestinal epithelial stem cells.
Embryonic stem cells (ESCs) can differentiate into
various types of cells, including Msi1-positive cells (12,13). As
Msi1-positive cells are a potential candidate for intestinal
epithelial stem cells, Msi1-positive cells originated from ESCs are
expected to develop into intestinal epithelial cells. It has also
been demonstrated that Msi1 is selectively expressed in neural
progenitor cells (6,14,15). Therefore, Msi1 is considered a marker
of neuroepithelial stem cells, and Msi1-positive cells from ESCs
maintain their ability to differentiate into neuroepithelial
tissue.
Living Msi1-positive cells cannot be selected by
binding to surface antibodies due to the Msi1 protein being present
in the interior of the cell. In our previous study, a reporter gene
plasmid comprising a green fluorescent protein (GFP) sequence,
pMsi1-GFP, was constructed under the control of the Msi1 promoter
(16). Following transfection with the pMsi1-GFP vector,
Msi1-positive cells were inspected in real time and effectively
selected from a cell population derived from ESCs using flow
cytometry (FCM). The selected Msi1-positive cells were able to
differentiate into neural and intestinal epithelial-like cells
in vivo. The majority of Msi1-positive cells derived from
ESCs were prone to differentiate into neural epithelial-like cells,
and only a small number of Msi1-positive cells differentiated into
intestinal epithelial-like cells. This indicated that the selected
Msi1-positive cells were similar to neural epithelial stem cells
and not intestinal epithelial stem cells. Screening for
Msi1-positive cells that tend to differentiate into intestinal
epithelial stem cells is a prerequisite for investigating
intestinal epithelial stem cells.
During embryogenesis, intestinal epithelial tissue
develops from endoderm, and neural epithelial tissue develops from
ectoderm (17-20). Therefore, it was reasonable to presume that the
promotion of ESC differentiation into endoderm can increase the
production of intestinal epithelial tissue. Phosphatidylinositol
3-kinase (PI3K) is extensively expressed and is core in monitoring
numerous cellular processes, including cell migration, activation,
differentiation, apoptosis and angiogenesis (21-23). It has been
reported that PI3K signaling is involved in a broad array of
elementary cellular responses and serves a critical role in the
differentiation of ESCs (24-26). The suppression of PI3K signaling
promotes the differentiation of ESCs into mesendoderm and inhibits
their differentiation into ectoderm (27). Once ESCs differentiate
into mesendoderm cells, Msi1-positive cells differentiate into
intestinal epithelial stem cells, rather than neural stem cells.
Msi1-positive cells sorted from mesendoderm cells are more
favorable for the investigation of intestinal epithelial stem
cells. LY294002 (2-4-morpholinyl-8-phenlchromone), a chemical
inhibitor of PI3K, has been extensively used to examine the role of
the PI3K signaling pathway (28,29). The present study aimed tried
to determine whether suppressing PI3K signaling can promote the
differentiation of ESCs into Msi1-positive cells, which are prone
to develop into intestinal epithelial-like tissue.
Materials and methods
Reagent preparation
LY294002 (Beyotime Institute of Biotechnology,
Shanghai, China) was dissolved in dimethyl sulfoxide (DMSO) at a
concentration of 10 mg/ml and stored at −20°C. The concentrations
of LY294002 solutions finally used were 1.25, 2.5, 5, 10, 20 and 50
µmol/l.
Culture of mouse (m)ESCs and cell cycle
analysis
The ES-E14TG2a mESCs were supplied by the American
Type Culture Collection (Manassas, VA, USA). The undifferentiated
mESCs were cultivated on gelatin-coated dishes without feeder cells
in Dulbecco’s modified Eagle’s medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), supplemented with 10% fetal
calf serum (FCS; HyClone; GE Healthcare Life Sciences, Logan, UT,
USA), 10 mM HEPES (Gibco; Thermo Fisher Scientific, Inc.), 0.12%
sodium bicarbonate, 0.1 mM nonessential amino acids (HyClone), 0.1
mM 2-mercaptoethanol (2ME; Gibco; Thermo Fisher Scientific, Inc.),
100 U/ml penicillin G, 100 µg/ml streptomycin and 1,000 U/ml
leukemia inhibitory factor (LIF; Chemicon International; Thermo
Fisher Scientific, Inc.). The cells were cultured in a humidified
37°C incubator in a 5% CO2-air mixture.
The proliferation rate of mESCs was measured with
Cell Counting Kit-8 (CCK-8) (Dojindo Molecular Technologies,
Kumamoto, Japan). Briefly, the mESCs were treated with trypsin
(0.25%)/EDTA (0.02%) and 5×103 cells (100 µl
volume/well) were plated in triplicate in gelatin-coated wells of a
96-well plate in media containing 1,000 U/ml LIF. LY294002 (1.25,
2.5, 5, 10, 20 and 50 µmol/l) was mixed in triplicate wells
and cultured at 37°C for 24 h. DMSO was used as a vehicle control.
Following treatment, 10 µl of CCK-8 was added to each
microculture well, and the absorbance at 450 nm was measured with a
microplate reader following incubation for 2 h at 37°C.
Differentiation of mESCs and formation of
embryonic bodies (EBs)
To induce mESC differentiation, LIF was removed from
the mESC culture medium (EB medium). The mESCs were cultured using
the hanging-drop method (32 µl per drop) to form EBs at a
concentration of 1×106 cells/ml in EB medium with or
without LY294002. After 2 days, the harvested EBs were dissociated
with trypsin (0.25%)/EDTA (0.02%). They were inoculated at a
concentration of 1×105 cells/well in a 6-well culture
plate (Nalge Nunc International; Thermo Fisher Scientific, Inc.)
and cultured in EB medium with or without LY294002. All EB cell
cultures were maintained in a humidified chamber in a 5%
CO2-air mixture at 37°C.
Western blot analysis
Whole-cell lysates were prepared using RIPA lysis
buffer (Beyotime Institute of Biotechnology) and centrifuged
(10,000 × g) at 4°C for 15 min. Total protein in the supernatant of
the cell lysate was measured by the BCA Protein Assay kit (Beyotime
Institute of Biotechnology). The supernatants were boiled for 5 min
and were size-fractionated by sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (12% acrylamide; 40 µg protein per
sample). The proteins were transferred onto nitrocellulose filters,
following which the blots were incubated with rabbit anti-mouse
glycogen synthase kinase-3β (GSK3β; cat. no. NBP1-31353), rabbit
anti-mouse phosphorylated GSK3βS9 (cat. no. AF1590-SP;
both 1:500; Novus Biologicals, Littleton, CO, USA) and rabbit
anti-mouse β-actin (1:2,000; cat. no. 4970; Cell Signaling
Technology, Inc., Danvers, MA, USA) antibodies overnight at 4°C.
The secondary antibodies used were horseradish
peroxidase-conjugated anti-rabbit (1:4,000; cat. no. 7074; Cell
Signaling Technology, Inc.), which were incubated for 1 h at room
temperature with gentle agitation. GSK3β and GSK3βS9
were detected using an ECL chemiluminescence system (Pierce; Thermo
Fisher Scientific, Inc.). The integrated intensity for the protein
bands was determined by scanning densitometry and analyzed via
Glyko BandScan 5.0 (Glyko, Novato, CA, USA).
Construction of pMsi1-GFP and cell
transfection
A reporter gene plasmid (pMsi1-GFP) was constructed
in our previous study, and Msi1-positive cells were selected from a
cell population derived from mESCs by fluorescence-activated cell
sorting (FACS). The day before transfection, 2×105 of
the 9-day cell population derived from mESCs were counted and
seeded into 6-well plates in EB medium with or without LY294002.
For generation of a Lipofectamine-plasmid DNA complex, 150
µl of serum-free medium was mixed with 10 µl of
Lipofectamine (Invitrogen; Thermo Fisher Scientific, Inc.) and
incubated for 5 min at room temperature. Subsequently, 150
µl of DMEM was mixed with 4 µg of pMsi1-GFP, added to
Lipofectamine solution, mixed gently and incubated for 30 min at
room temperature. The transfection complexes were then added to
each well. The medium was replaced following 6 h of incubation with
EB culture medium with or without LY294002. The GFP-positive cells
were counted using FCM (Beckman Coulter, Inc., Brea, CA, USA) 24 h
following transient transfection. The cells were counterstained
with Hoechst 33342 (10 µg/ml; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at room temperature for 10 min and observed
under a fluorescence microscope (Olympus iX71; Olympus Corporation,
Tokyo, Japan).
FACS and grafting
The cells were harvested using trypsin (0.25%)/EDTA
(0.02%) 24 h following transfection. The LY-Msi1-positive cells and
EB-Msi1 positive cells were selected from 10-day cell populations
treated with and without LY294002, respectively, using FCM (488
nm), gated for a high expression level of GFP. The selected
LY/EB-Msi1 positive cells were harvested (~5.0×105 cells
per dose) whereas the unselected 10-day cell populations (treated
with or without LY294002) were injected subcutaneously into 20
non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice
(female, 4-6-week-old and 14-20 g; n=5 in each group;
Laboratory Animal Center of Sun Yat-Sen University, Guangzhou,
China). Throughout the experiment, the mice were housed in a room
with a 12/12-h light/dark cycle, at a temperature of 27°C, with 60%
relative humidity and free access to chow and water. At 2 weeks
post transplantation, the mice were sacrificed and the grafts were
removed. All experiments were performed in triplicate and repeated
separately three times. All procedures were performed in accordance
with the Animal Ethics Committee of the Second Affiliated Hospital
of Sun Yat-Sen University.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA from the cells and grafts were extracted
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
The concentration and purity of the total RNA were detected using a
UV-2450 spectrophotometer (Shimadzu Corporation, Kyoto, Japan).
According to the manufacturer’s protocol, 1 µg of total RNA
was used to perform cDNA synthesis, using a ReverTra
Ace-α-® kit (Toyobo Life Science, Osaka, Japan). qPCR
analysis was performed using a Real-time™ PCR Master Mix kit
(Toyobo Life Science) and SYBR Premix Ex Taq™ II (Takara Bio, Inc.,
Otsu, Japan) in a LightCycler 480 (Roche Diagnostics, Basel,
Switzerland). The cycling conditions were 95°C for 30 sec, followed
by 40 cycles of 95°C for 15 sec and 60°C for 30 sec. The experiment
was repeated twice and the results were averaged. Data were
analyzed using the ΔΔCt method with β-actin as the constitutive
marker (30).
The sequences of the primers used were as follows
(and reverse): Mouse Msi1, forward 5′-ATGGTGGAATGCAAGAAAGC-3′ and
reverse 5′-TAGGTGTAACCAGGGGCAAG-3′; β-actin, forward
5′-CGGCTACCACATCCAAGGAA-3′ and reverse 5′-GCTGGAATTACCGCGGCT-3′;
mouse Tubulin β III, forward 5′-CTTCGGGCAGATCTTCAGAC-3′ and reverse
5′-AGTCAACCAGCTCTGCACCT-3′; mouse Villin forward
5′-ACGGTGGTGACTGCTACCTGCT-3′ and reverse
5′-AACCACCATGCGGCCCTTGA-3′; mouse platelet-derived growth factor
receptor α (Pdgfr-α), forward 5′-AACCTTCAGCGTGGGGCCTT-3′ and
reverse 5′-ACAGTCTGGCGTGCGTCCAT-3′; mouse Leucine-rich
repeat-containing G-protein coupled receptor 5 (Lgr5), forward
5′-CACCAGCTTACCCCATGACT-3′ and reverse 5′-CTCCTGCTCTAAGGCACCAC-3′;
mouse Nestin, forward 5′-GAGAAGACAGTGAGGCAGATGAGTTA-3′ and reverse
5′-GCCTCTGTTCTCCAGCTTGCT-3.
Immunohistochemistry
Immunohistochemical analysis was performed to
measure the protein expression of Tubulin β III and Villin. The
grafts were removed from the NOD/SCID mice and fixed with 4%
paraformaldehyde overnight at 4°C, embedded in paraffin, and cut at
a thickness of 6 µm. The sections were washed for 15 min in
PBS and subjected to antigen retrieval using a microwave oven for
15 min. The sections were cooled to room temperature and incubated
for 2 h with 10% normal goat (or rabbit) serum (cat. no. kit-9710;
Ultersensitive SP kit; Fuzhou Maixin Biotech Co., Ltd., Fuzhou,
China) to reduce nonspecific binding. After a 15 min PBS wash, the
sections were incubated overnight at 4°C with rabbit anti-mouse
Tubulin β III (2.5 mg/ml in PBS; cat. no. 1967-1, Epitomics; Abcam,
Cambridge, MA, USA), or goat anti-mouse Villin (5 mg/ml in PBS;
cat. no. Sc-7672, Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
antibodies. After a 15 min PBS wash, the sections were treated with
biotinylated anti-rabbit (or goat) IgG antibody (1:200 dilution;
Ultersensitive SP kit) at 37°C for 60 min. A positive reaction was
detected using the SP method (Ultersensitive SP kit) and visualized
via a diaminobenzidine reaction (Ultersensitive SP kit). The
sections were counterstained with hematoxylin (cat. no. CTS-1099;
Maixin Biotech Co., Ltd.) at room temperature for 30 sec, prior to
being imaged under a iX71 fluorescence microscope.
Statistical analysis
Statistical analyses were performed using a
statistical software package (SAS 8 for Windows; SAS Institute,
Inc., Cary, NC, USA). Data are presented as the mean ± standard
derivation. Data were analyzed using one-way analysis of variance
and α=0.05 (P<0.05) was used to indicate a statistically
significant difference between separate groups.
Results
Effects of LY294002 in vitro: Suppression
of cell survival
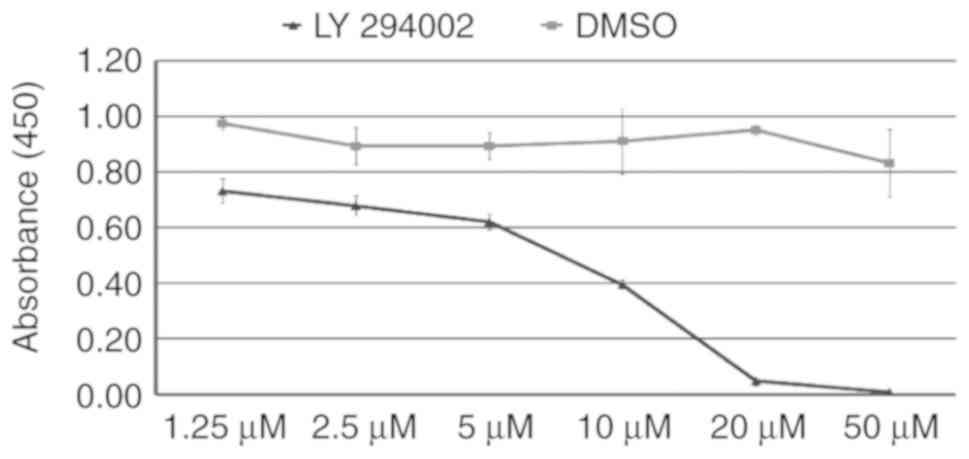
As an inhibitor of PI3K signaling, LY294002
suppresses the proliferation of mESCs (31). To determine the
appropriate concentration of LY294002 in the present study, the
mESCs were treated with LY294002 at a concentration gradient for 24
h. When the mESCs were treated with 1.25, 2.5, 5, 10, 20 and 50
µmol/l LY294002, the mean absorbance was 73.2±4.5, 68.0±3.4,
62.0±2.2, 39.5±1.7, 4.8±1.7 and 0.8±0.4%, respectively, showing a
decreasing trend. When DMSO was used as a vehicle in the cell
culture, the mean absorbance was 97.5±2.3, 89.3±6.8, 89.4±4.,
91.1±11.9, 95.2±2.0 and 83.2±1.2%, respectively. As shown in
Fig. 1, the proliferation of
mESCs was inhibited by LY294002 in a dose-dependent manner. The
inhibitory effect was significant at a concentration of >5
µmol/l. Therefore, 5 µmol/l LY294002 was subsequently
used.
Effects of LY294002 in vitro:
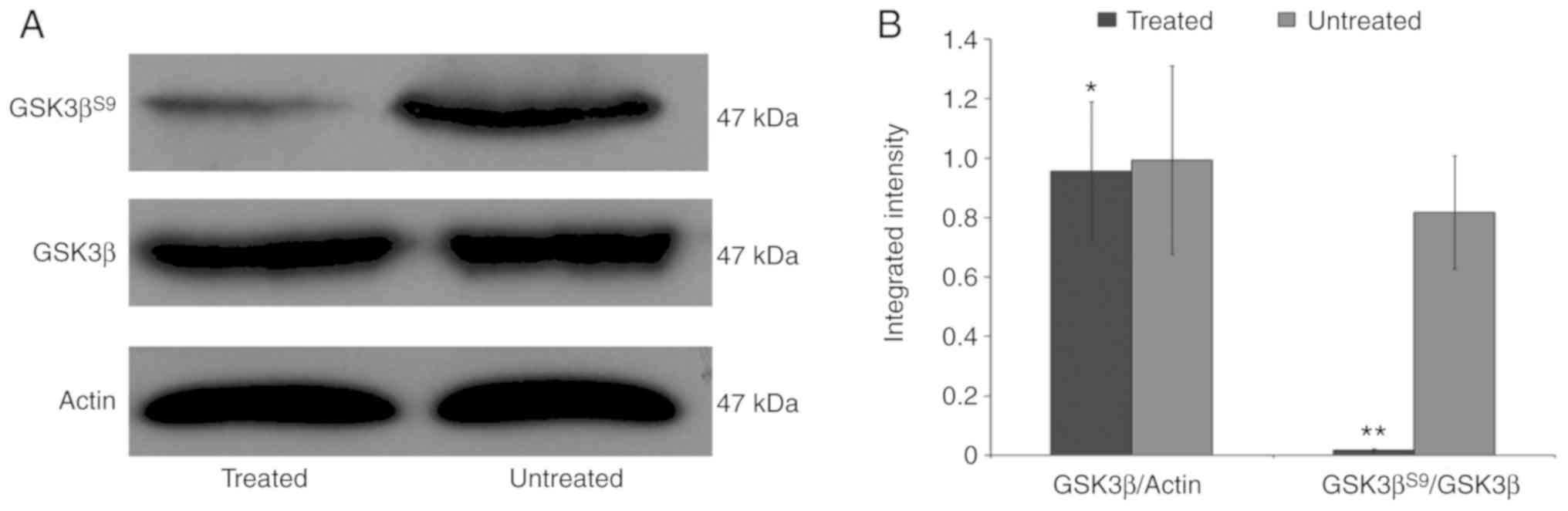
Inactivation of GSK3β in mESCs
The suppression of PI3K signaling by LY294002 was
affirmed by probing the cell lysates with phosphospecific
antibodies that discerned the activity status of downstream PI3K
signaling effectors, including GSK3β. These outcomes demonstrated
that 5 µmol/l LY294002 inhibited PI3K signaling within 72 h,
as shown by a collapse in the phosphorylation of GSK3βS9
(Fig. 2A and B). It was concluded
that 5 µmol/l LY294002 was sufficient to inhibit the
canonical PI3K signaling pathway, but had limited effect on the
proliferation of mESCs.
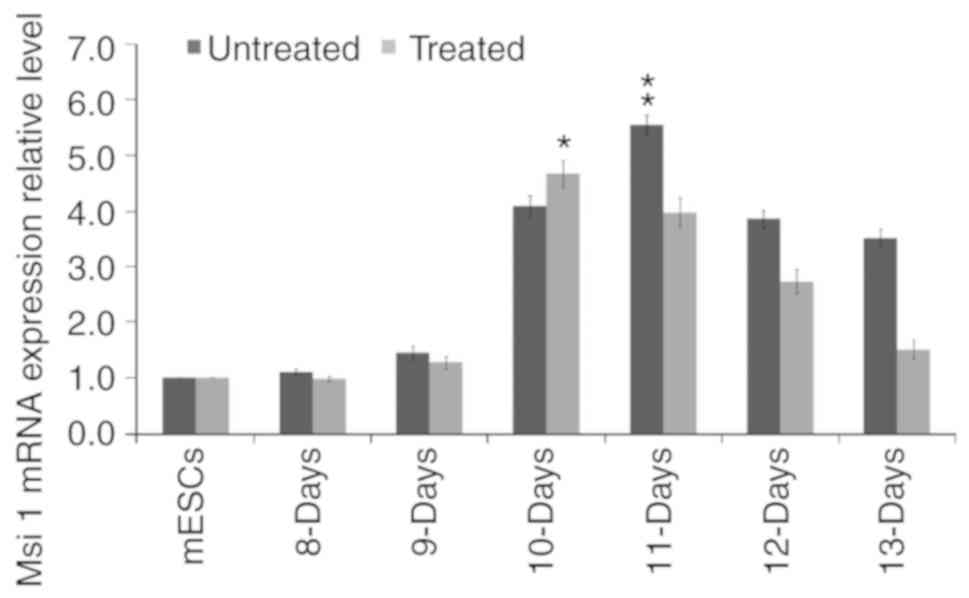
Effect of LY294002 on the mRNA expression
of Msi1 during the differentiation of mESCs
The mRNA expression levels of Msi1 were gradually
increased during the differentiation of mESCs treated with or
without 5 µmol/l LY294002. Compared with the mESCs, the mRNA
expression levels of Msi1 in the 8-13-day cell populations derived
from mESCs without LY294002 treatment were 1.103±0.060,
1.460±0.120, 4.090±0.190, 5.543±0.190, 3.867±0.159 and 3.513±0.170,
respectively. Those treated with LY94002 were 0.977±0.048,
1.2780±0.111, 4.667±0.234, 3.967±0.264, 2.730±0.210 and
1.502±0.167, respectively (Fig.
3). Taken together, the mRNA expression levels of Msi1 for cell
populations derived from mESCs without LY294002 treatment were
higher than those of the treated group at each time point, with the
exception of the 10-day cell population (P<0.05). The mRNA
expression level of Msi1 in the 10-day cell population derived from
mESCs treated with LY294002 was higher than that of the untreated
group (P<0.05).
Transient transfection of pMsi1-GFP
At 24 h post-transfection with pMsi1-GFP,
GFP-positive cells were found in the 10-day cell populations with
or without LY294002 treatment (Fig.
4A-F). The positive expression rate of GFP was estimated with
FCM. Following transfection with pMsi1-GFP, the positive expression
rates of GFP in the 10-day cell populations derived from mESCs with
or without LY294002 treatment were 16.540±2.760 and 10.040±1.980%,
respectively (Fig. 4G-I).
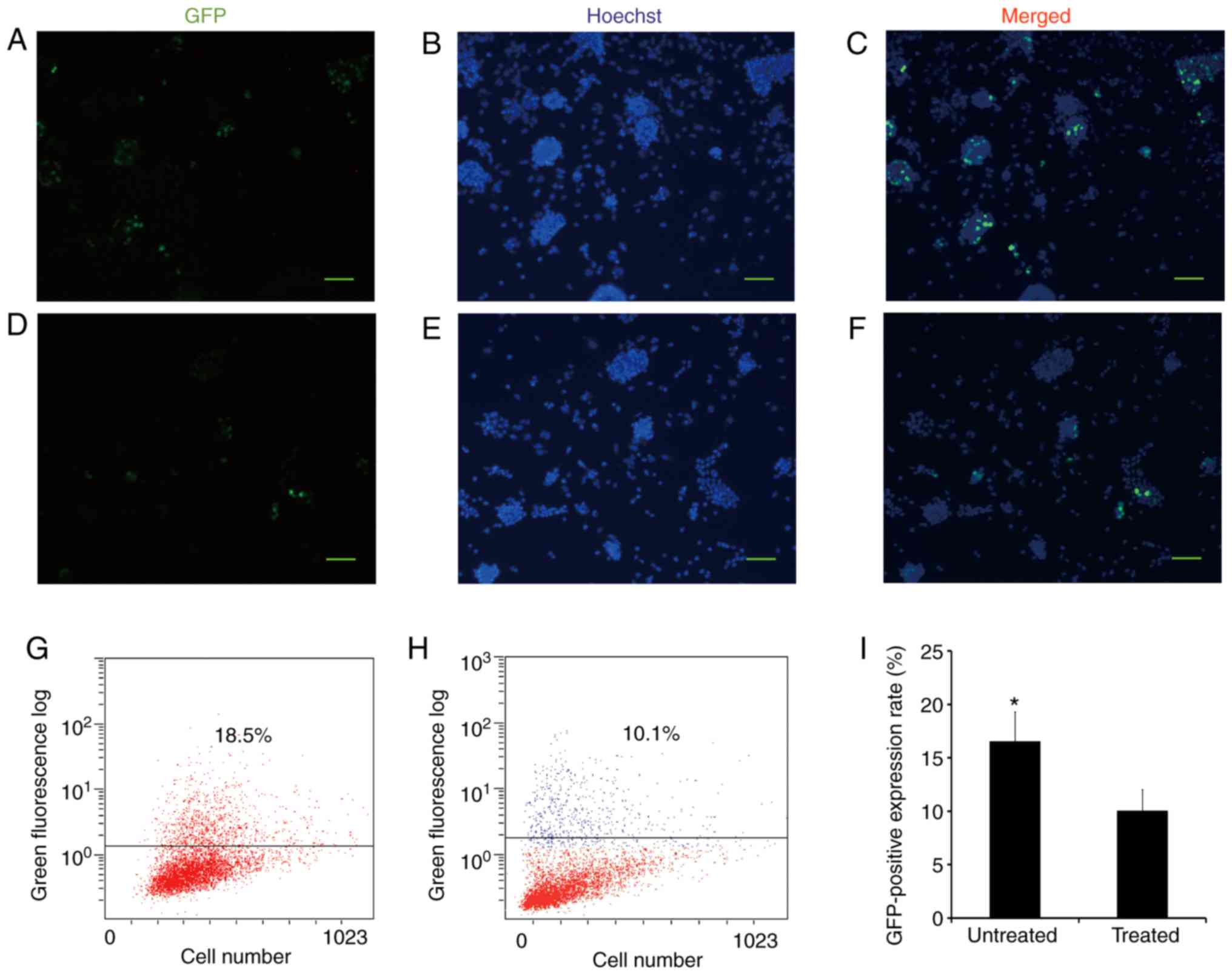 | Figure 4Transient transfection of the
pMsi1-GFP vector. pMsi1-GFP was transfected into the 9-day cell
population derived from mESCs without LY294002 treatment, as shown
in (A) GFP, (B) Hoechst and (C) merged images, and with LY294002
treatment, as shown in (D) GFP, (E) Hoechst and (F) merged images.
After 24 h, GFP-positive cells were identified in both groups under
a fluorescence microscope. Scale bar=50 µm. At 24 h
post-transfection with pMsi1-GFP, the positive expression rate of
GFP in the 10-day cell population (G) without LY294002 treatment
(G) and (H) with LY294002 treatment were calculated with flow
cytometry. (I) Positive expression rate of GFP in the 10-day cell
population without LY294002 treatment was higher than that in the
10-day cell population treated with LY294002.
*P<0.05. Msi1, Musashi 1; mESCs, mouse embryonic stem
cells; GFP, green fluorescent protein. |
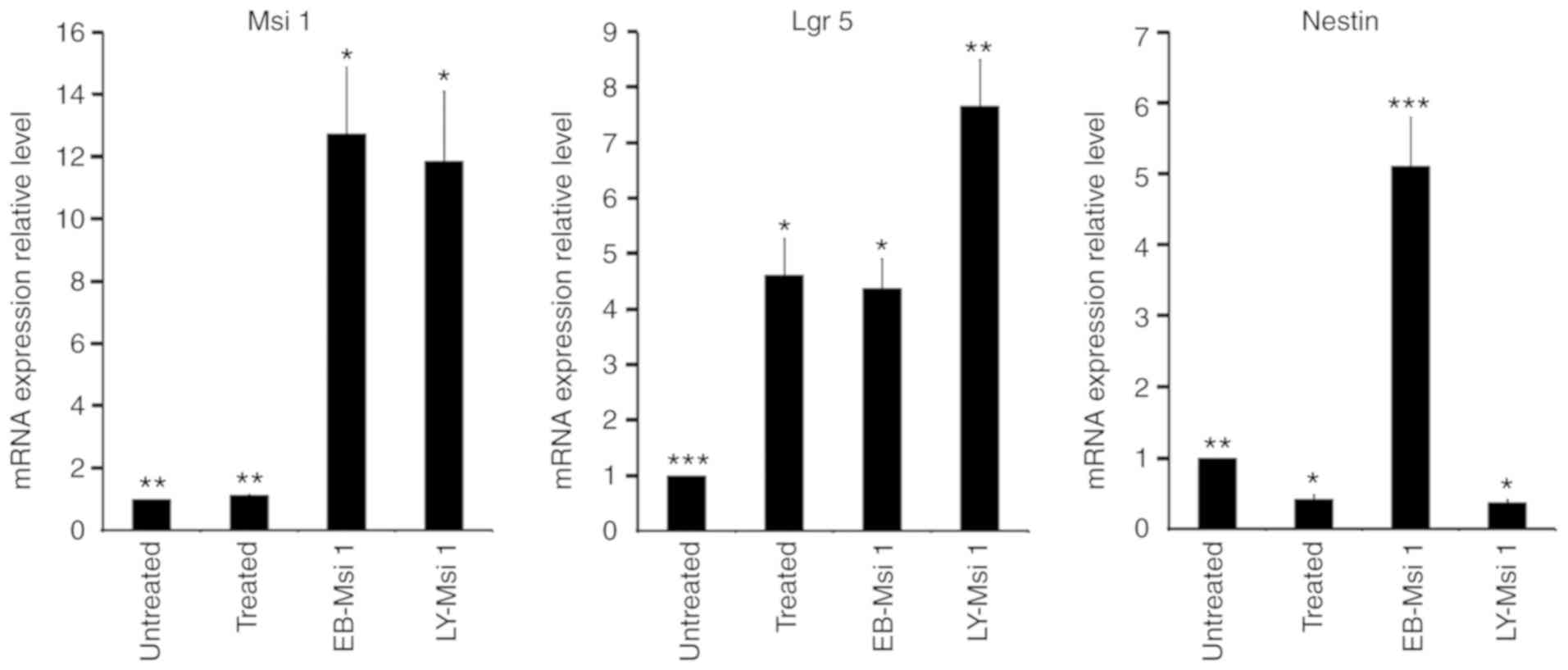
mRNA expression of markers for intestinal
and neural epithelial stem cells in selected Msi1-positive
cells
The EB-Msi1 positive cells and LY-Msi1 positive
cells were selected from the 10-day cell populations treated with
or without LY294002. Compared with the 10-day cell population
without LY294002 treatment, the mRNA expression levels of Msi1 in
the EB-Msi1 positive cells, LY-Msi1-positive cells and 10-day cell
population treated with LY294002 were 12.731±2.145, 11.864±2.231
and 1.137±0.029, respectively (Fig.
5). The mRNA expression levels of Msi1 in both Msi1-positive
cell groups were higher than those in unselected cells treated with
or without LY294002 (P<0.05). The mRNA expression level of Msi1
in EB-Msi1-positive cells was similar to that of LY-Msi1-positive
cells (P>0.05).
To determine whether Msi1-expressing cells were able
to further differentiate into neural and intestinal epithelial
cells, the mRNA expression levels of the markers for neural and
intestinal epithelial stem cells were analyzed. Lgr5 has been
reported as a marker of intestinal epithelial stem cells (32).
Compared with the 10-day cell population without LY294002, the mRNA
expression levels of Lgr5 in EB-Msi1-positive cells,
LY-Msi1-positive cells and the 10-day cell population treated with
LY294002 were 4.379±0.532, 7.665±0.844 and 4.616±0.665,
respectively (Fig. 5). The mRNA
expression level of Lgr5 in the LY-Msi1-positive cells was higher
than those in the other groups (P<0.05). The mRNA expression
levels of Lgr5 did not differ significantly between the
EB-Msi1-positive cells and the 10-day cell population treated with
LY294002 (P>0.05).
Nestin has been confirmed as a target of neural
epithelial progenitors. Compared with the 10-day cell population
without LY294002 treatment, the mRNA expression levels of Nestin in
the EB-Msi1-positive cells, LY-Msi1-positive cells and the 10-day
cell population treated with LY294002 were 5.112±0.679, 0.377±0.032
and 0.423±0.057, respectively (Fig.
5). The mRNA expression level of Nestin in the EB-Msi1-positive
cells was higher than those in the other groups (P<0.05). The
mRNA expression level of Nestin in the LY-Msi1-positive cells was
similar to that in the 10-day cell population treated with LY294002
(P>0.05), and both were significantly lower than that in the
10-day cell population without LY294002 treatment (P<0.05).
Sorting of Msi1-positive cells and
grafting assay
To observe how the Msi1-positive cells differentiate
in vivo, the present study separated and then engrafted
LY/EB-Msi1 positive cells, in addition to the unselected 10-day
cell populations treated with and without LY294002 following
pMsi1-GFP transfection, into the backs of 5-week-old NOD/SCID mice.
At 10 days post-injection, the grafts were developed. They grew
quickly, and their diameters had reached 1-2 cm at 2 weeks after
injection.
Tubulin β III has been confirmed as a marker of
mature neural epithelial cells (33-35). The mRNA expression levels
of the Tubulin β III in grafts from LY-Msi1-positive cells,
EB-Msi1-positive cells and the 10-day cell population treated with
LY294002 were 0.623±0.073, 2.08±0.318 and 0.567±0.105,
respectively, compared with the 10-day cell population without
LY294002 (Fig. 6). The mRNA
expression level of Tubulin β III in the grafts from
EB-Msi1-positive cells was higher than those in the other groups
(P<0.05). The mRNA expression levels of the Tubulin β III in the
grafts from the LY-Msi1-positive cells and the 10-day cell
population treated with LY294002 were significantly lower than
those in the grafts from EB-Msi1-positive cells and the 10-day cell
population without LY294002 (P<0.05). These results indicated
that EB-Msi1-positive cells were prone to develop into neural
epithelial-like tissue in vivo, compared with
LY-Msi1-positive cells. Therefore, LY294002 suppressed the ability
of mESCs to differentiate into neural epithelial-like tissue.
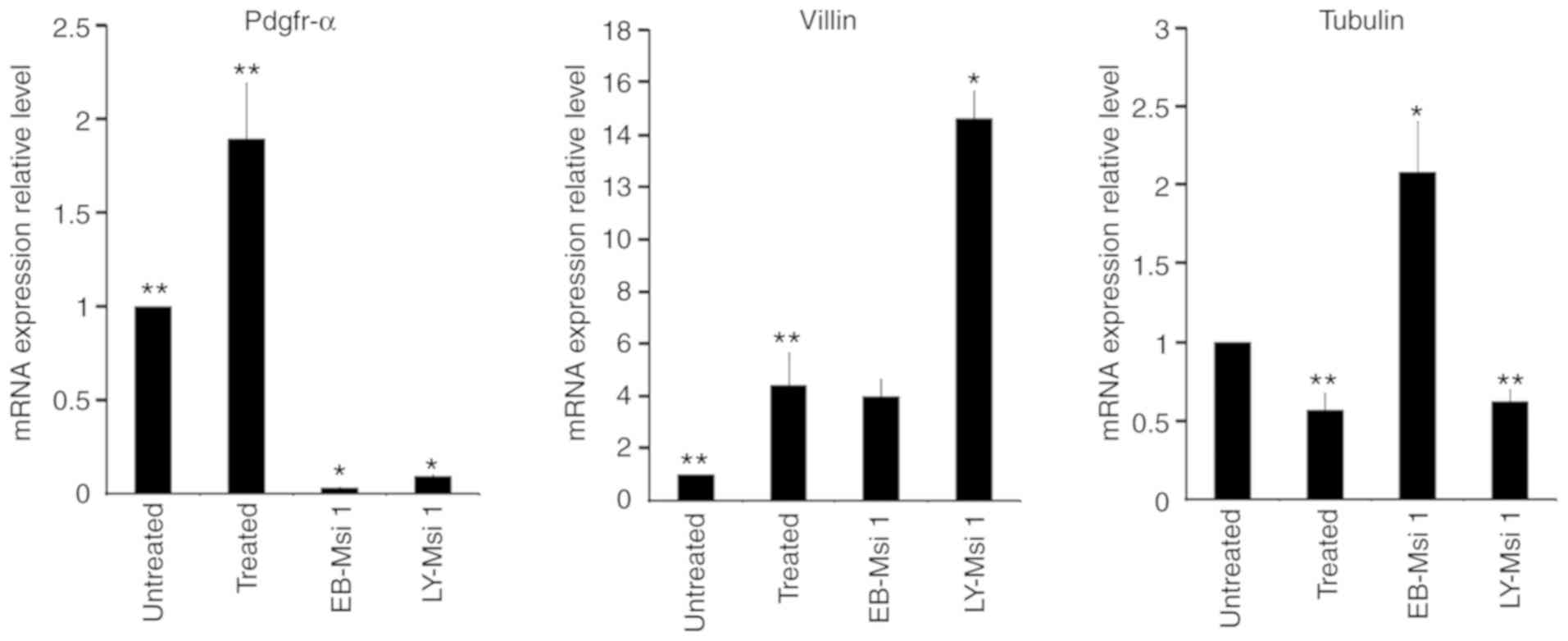
Villin, an actin bundling protein, is a dominant
structural component of the brush border of intestinal absorptive
cells (36,37). The mRNA expression levels of Villin in grafts from
the LY-Msi1-positive cells, EB-Msi1-positive cells and the 10-day
cell population treated with LY294002 were 14.612±1.053,
3.993±0.649 and 4.422±1.233, respectively, compared with that in
the 10-day cell population without LY294002 (Fig. 6). The mRNA expression level of
Villin in the grafts from LY-Msi1-positive cells was higher than
those in other groups (P<0.05). The mRNA expression levels of
the Villin in the grafts from EB-Msi1-positive cells and the 10-day
cell population treated with LY294002 were higher than that in the
grafts from the 10-day cell population without LY294002
(P<0.05). These results showed that LY294002 promoted the
differentiation of mESCs into intestinal epithelial-like cells.
LY-Msi1-positive cells were prone to develop into intestinal
epithelial-like tissue in vivo.
Pdgfr-α is a typical mesoderm marker (38-40). The
mRNA expression levels of Pdgfr-α in grafts from the
LY-Msi1-positive cells, the 10-day cell population treated with
LY294002 and EB-Msi1-positive cells were 0.094±0.005, 1.893±0.294
and 0.031±0.004-fold, respectively, compared with the 10-day cell
population without LY294002 (Fig.
6). The mRNA expression of Pdgfr-α in grafts from the 10-day
cell population treated with LY294002 was higher than in the other
groups (P<0.05). Therefore, LY294002 promoted the production of
mesoderm during the process of mESC differentiation. The mRNA
expression levels of Pdgfr-α in grafts from LY/EB-Msi1-positive
cells were significantly lower than those from the 10-day cell
populations treated with/without LY294002 (P<0.05). These
results indicated that neither EB-Msi1-positive cells nor
LY-Msi1-positive cells had the tendency to develop into mesoderm
tissue.
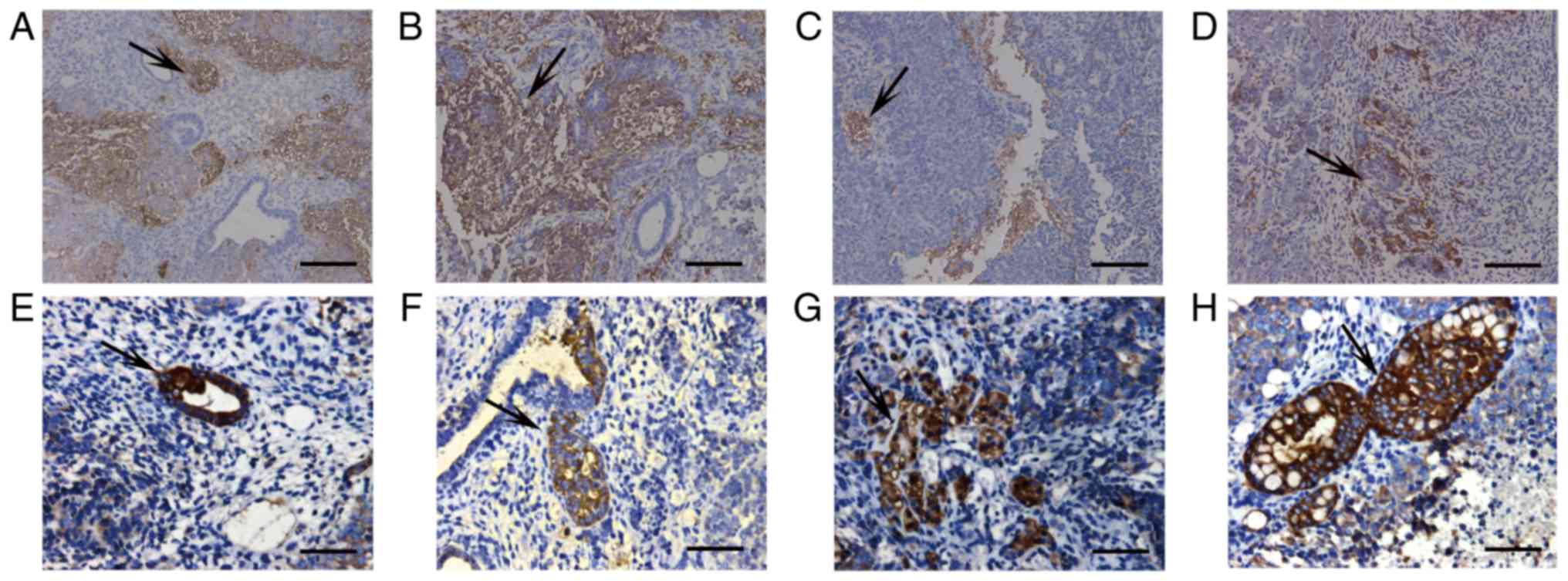
Immunohistochemical analysis of
grafts
A higher number of Tubulin β III-positive cells were
detected in the grafts from the 10-day cell population without
LY294002 and from EB-Msi1-positive cells than those from the 10-day
cell population treated with LY294002 and LY-Msi1-positive cells
(Fig. 7A-D). A high number of
Villin-positive cells were detected in the grafts from the 10-day
cell population treated with LY294002 and LY-Msi1-positive cells
than in those from the 10-day cell population without LY294002 and
EB-Msi1-positive cells (Fig.
7E-H).
Discussion
In our previous study, a pMsi1-GFP vector was
successfully constructed, which was able to identify Msi1-positive
cells, and to select Msi1-positive cells from a cell population
originating from mESCs (16). Although the selected Msi1-positive
cells had the potential to develop into neural and intestinal
epithelial-like cells, the majority tended to develop into neural
epithelial-like tissues. Only a small proportion of intestinal
epithelial-like tissues were identified in the grafts from selected
Msi1-positive cells. An additional way to harvest more intestinal
epithelial-like tissues developed from Msi1-positive cells was to
suppress the differentiation of mESCs into neutral epithelial-like
tissue.
The ectoderm gives rise to the epidermis and neural
epithelial tissue. Suppressing the differentiation of mESCs into
ectoderm can prevent neutral epithelial tissue formation. The
suppression of PI3K signaling efficiently promotes the
differentiation of mESCs into mesendoderm and then definitive
endoderm. It also prevents the differentiation of mESCs into
ectoderm (27). PI3K signaling triggers the phos-phorylation of
GSK3β on Serine9, which can be inhibited with LY294002 (41). In the
present study, LY294002 (5 µmol/l) effectively inhibited the
activity of the PI3K signaling pathway, but had limited suppressive
effect on the proliferation of mESCs. The concentration of DMSO
used did not affect cell survival, which was consistent with the
results of a study by Semba et al (42).
The PI3K signaling pathway can affect the
differentiation of mESCs (43). The inhibition of PI3K signaling
alters gene expression during the differentiation of mESCs (27).
The mRNA expression patterns of Msi1 in cells derived from mESCs
treated with and without LY294002 were similar, however, the
expression level in the treated group was lower than that in the
untreated group during the differentiation of mESCs, with the
exception of that in the 10-day cell population. The mRNA
expression of Msi1 reached a peak level in the 11-day cell
population derived from mESCs without LY294002 treatment, whereas
the mRNA expression of Msi1 reached a peak level in the 10-day cell
population derived from mESCs treated with LY294002. This result
indicated that LY294002 accelerated the differentiation of mESCs,
and shifted the peak mRNA expression of Msi1 to an earlier date.
Following transient transfection with the pMsi1-GFP vector, fewer
Msi1-positive cells were detected in the 10-day population derived
from mESCs treated with LY294002 than in the untreated group. This
result was inconsistent with the qPCR results, although the reason
remains unclear. One possibility was that LY294002 may affect the
expression of the pMsi1-GFP vector. As LY294002 suppressed the
differentiation of mESCs into ectoderm, there was a corresponding
reduction in the production of Msi1-positive cells, which are
neuronal precursor cells (6,15). Therefore, it was concluded that
LY294002 did not increase the production of Msi1-positive cells
derived from mESCs.
Although the mRNA expression of Msi1 was high in the
EB-Msi-1- and LY-Msi1-positive cells, the mRNA expression levels of
Lgr5 and Nestin were different. The mRNA expression level of Lgr5
in LY-Msi1-positive cells was higher than that in EB-Msi1-positive
cells and unselected cells derived from mESCs treated with/without
LY294002, whereas the mRNA expression level of Nestin in
EB-Msi1-positive cells was higher than that in LY-Msi1-positive
cells and unselected cells derived from mESCs treated with/without
LY294002. These results showed that LY-Msi1-positive cells appeared
to contain more intestinal epithelial stem cells, whereas
EB-Msi1-positive cells appeared to contain more neural epithelial
stem cells. As Msi1 is considered a marker of neural and intestinal
epithelial stem cells, it is reasonable to suggest that there two
subsets of Msi1-positive cells were derived from mESCs; one subset
was prone to differentiate into neural epithelial tissue, whereas
the other was prone to differentiate into intestinal epithelial
tissue (16). The suppression of PI3K signaling resulted in a
reduction of the subset that differentiated into neural epithelial
tissue and promoted the differentiation of mESCs into intestinal
epithelial tissue.
As precursor cells of neural and intestinal
epithelial cells, Msi1-positive cells lose the ability to
differentiate into certain cell types, particularly mesodermal
cells. The lower mRNA expression level of Pdgfr-α in grafts derived
from LY/EB-Msi1-positive cells provides direct evidence of
this.
As mentioned above, the LY-Msi1-positive cells and
EB-Msi1-positive cells had similar characteristics. As Msi1 is
considered a candidate marker of intestinal epithelial stem cells,
it is presumed that Villin-positive cells can be detected in the
grafts from selected Msi1-positive cells (9,37). There were also
differences in the expression levels of markers of neural and
intestinal epithelial cells. As shown in the results of the mRNA
expression analysis and immunohistochemical analysis, the
expression level of Villin was higher in grafts derived from cells
treated with LY294002 than those from cells without LY294002
treatment. In addition, the expression level of Villin was higher
in grafts derived from LY-Msi1-positive cells than in those from
EB-Msi1-positive cells. These results revealed that LY294002
promoted the differentiation of mESCs into endoderm, and increased
the production of intestinal epithelial-like cells. The
Msi1-positive cells derived from mESCs treated with LY294002 were
distinct from those without LY294002 treatment. Compared with
EB-Msi1-positive cells, more LY-Msi1-positive cells differentiated
into intestinal epithelial-like tissue. This result partially
clarified the reason why LY294002 increased the production of
intestinal epithelial-like tissue in the grafts derived from mESCs
treated with LY294002.
The marker of neural epithelial cells, Tubulin β
III, was expressed at high levels in grafts derived from
EB-Msi1-positive cells, whereas Villin, a marker of intestinal
epithelial cells, was expressed at a lower level (35). This
indicated that the majority of EB-Msi1-positive cells developed
into neural epithelial-like tissue, and only a small number of
EB-Msi1-positive cells differentiated into intestinal
epithelial-like tissue (16). Therefore, EB-Msi1-positive cells can
be regarded as ‘neural epithelial stem cells’, rather than
‘intestinal epithelial stem cells’. As LY294002 inhibited the
differentiation of mESCs into ectoderm, the expression levels of
Tubulin β III in grafts from unselected cells treated with LY294002
and LY-Msi1-positive cells were significantly lower than those from
unselected cells without LY294002 treatment and EB-Msi1-positive
cells, as shown in the results of the mRNA expression and
immunohistochemical analyses. These results suggested that LY294002
inhibited the differentiation of mESCs into neural epithelial-like
tissue (27).
In conclusion, the present study demonstrated that
LY294002 promoted the differentiation of mESCs into intestinal
epithelial-like tissue. The Msi1-positive cells selected from a
cell population derived from mESCs treated with LY294002 had more
characteristics of intestinal epithelial stem cells, and were
suitable for use as a platform for further differentiation. Due to
the low proportion of Msi1-positive cells in mesendoderm cells, it
is not possible to provide sufficient Msi1-positive cells for the
investigation of intestinal epithelial stem cells. In order to sort
out a higher number of Msi1-positive cells for intestinal
epithelial stem cell research, further investigations are required
focused on increasing the ratio of Msi1-positive cells in
mesendoderm cells (44).
Funding
The present study was supported by the Natural
Science Foundation of Guangdong Province, China (grant no.
2014A030313396) and the Youth Innovative Talents Project of the
Educational Department of Guangdong Province (grant no.
2016KQNCX028).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors’ contributions
SYL and MAT contributed equally to this work, and
conducted the experiments and drafted the manuscript. SHY
participated in the cell culturing. TY and JZC participated in the
western blot analysis. BC and PWL participated in the reverse
transcription-quantitative polymerase chain reaction studies. DMF
and FBL performed the statistical analysis and helped to draft the
manuscript. QKC participated in the design of the study and gave
the final approval of the version to be published. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All procedures were performed in accordance with the
Animal Ethics Committee of the Second Affiliated Hospital of Sun
Yat-Sen University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
Msi1
|
Musashi 1
|
|
mESCs
|
mouse embryonic stem cells
|
|
NOD/SCID
|
non-obese diabetic/severe combined
immunodeficient
|
|
GFP
|
green fluorescent protein
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
Lgr5
|
leucine-rich repeat-containing
G-protein coupled receptor
|
|
GSK3β
|
glycogen synthase kinase-3β
|
|
DMSO
|
dimethyl sulfoxide
|
|
LIF
|
leukemia inhibitory factor
|
|
CCK-8
|
Cell Counting Kit-8
|
|
Pdgf-α
|
platelet-derived growth factor
receptor α
|
|
qPCR
|
quantitative polymerase chain
reaction
|
Acknowledgments
The authors are indebted to Dr. Xiao-xue Li for
expertly reviewing of the manuscript.
References
|
1
|
Nakamura M, Okano H, Blendy JA and Montell
C: Musashi, a neural rna-binding protein required for drosophila
adult external sensory organ development. Neuron. 13:67–81. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Okano H, Imai T and Okabe M: Musashi: A
translational regulator of cell fate. J Cell Sci. 115:1355–1359.
2002.PubMed/NCBI
|
|
3
|
Sakakibara S, Imai T, Hamaguchi K, Okabe
M, Aruga J, Nakajima K, Yasutomi D, Nagata T, Kurihara Y, Uesugi S,
et al: Mouse-Musashi-1, a neural RNA-Binding protein highly
enriched in the mammalian CNS stem cell. Dev Biol. 176:230–242.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Potten CS, Booth C, Tudor GL, Booth D,
Brady G, Hurley P, Ashton G, Clarke R, Sakakibara S and Okano H:
Identification of a putative intestinal stem cell and early lineage
marker; Musashi-1. Differentiation. 71:28–41. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okano H, Kawahara H, Toriya M, Nakao K,
Shibata S and Imai T: Function of RNA-binding protein Musashi-1 in
stem cells. Exp Cell Res. 306:349–356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sakatani T, Kaneda A, Iacobuzio-Donahue
CA, Carter MG, de Boom Witzel S, Okano H, Ko MS, Ohlsson R, Longo
DL and Feinberg AP: Loss of imprinting of Igf2 alters intestinal
maturation and tumorigenesis in mice. Science. 307:1976–1978. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arumugam K, Macnicol MC and Macnicol AM:
Autoregulation of Musashi1 mRNA translation during Xenopus oocyte
maturation. Mol Reprod Dev. 79:553–563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Booth C and Potten CS: Gut instincts:
Thoughts on intestinal epithelial stem cells. J Clin Invest.
105:1493–1499. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kayahara T, Sawada M, Takaishi S, Fukui H,
Seno H, Fukuzawa H, Suzuki K, Hiai H, Kageyama R, Okano H and Chiba
T: Candidate markers for stem and early progenitor cells, Musashi-1
and Hes1, are expressed in crypt base columnar cells of mouse small
intestine. FEBS Lett. 535:131–135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukui T, Takeda H, Shu HJ, Ishihama K,
Otake S, Suzuki Y, Nishise S, Ito N, Sato T, Togashi H and Kawata
S: Investigation of Musashi-1 expressing cells in the murine model
of dextran sodium sulfate-induced colitis. Dig Dis Sci.
51:1260–1268. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kaneko J and Chiba C: Immunohistochemical
analysis of Musashi-1 expression during retinal regeneration of
adult newt. Neurosci Lett. 450:252–257. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamada T, Yoshikawa M, Takaki M, Torihashi
S, Kato Y, Nakajima Y, Ishizaka S and Tsunoda Y: In vitro
functional gut-like organ formation from mouse embryonic stem
cells. Stem Cells. 20:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takaki M, Nakayama S, Misawa H, Nakagawa T
and Kuniyasu H: In vitro formation of enteric neural network
structure in a gut-like organ differentiated from mouse embryonic
stem cells. Stem Cells. 24:1414–1422. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaneko Y, Sakakibara S, Imai T, Suzuki A,
Nakamura Y, Sawamoto K, Ogawa Y, Toyama Y, Miyata T and Okano H:
Musashi1: An evolutionally conserved marker for CNS progenitor
cells including neural stem cells. Dev Neurosci. 22:139–153. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maslov AY, Barone TA, Plunkett RJ and
Pruitt SC: Neural stem cell detection, characterization, and
age-related changes in the subventricular zone of mice. J Neurosci.
24:1726–1733. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lan SY, Yu T, Xia ZS, Yuan YH, Shi L, Lin
Y, Huang KH and Chen QK: Musashi 1-positive cells derived from
mouse embryonic stem cells can differentiate into neural and
intestinal epithelial-like cells in vivo. Cell Biol Int.
34:1171–1180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Simon TC and Gordon JI: Intestinal
epithelial cell differentiation: New insights from mice, flies and
nematodes. Curr Opin Genet Dev. 5:577–586. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hellmich HL, Kos L, Cho ES, Mahon KA and
Zimmer A: Embryonic expression of glial cell-line derived
neurotrophic factor (GDNF) suggests multiple developmental roles in
neural differentiation and epithelial-mesenchymal interactions.
Mech Dev. 54:95–105. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao N, White P and Kaestner KH:
Establishment of intestinal identity and epithelial-mesenchymal
signaling by. Dev Cell. 16:588–599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pai YJ, Abdullah NL, Mohd-Zin SW, Mohammed
RS, Rolo A, Greene ND, Abdul-Aziz NM and Copp AJ: Epithelial fusion
during neural tube morphogenesis. Birth Defects Res A Clin Mol
Teratol. 94:817–823. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng H, Fu G, Dai T and Huang H:
Migration of endothelial progenitor cells mediated by stromal
cell-derived factor-1alpha/CXCR4 via PI3K/Akt/eNOS signal
transduction pathway. J Cardiovasc Pharmacol. 50:274–280. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu J, Li M, Qu Z, Yan D, Li D and Ruan Q:
SDF-1/CXCR4-mediated migration of transplanted bone marrow stromal
cells toward areas of heart myocardial infarction through
activation of PI3K/Akt. J Cardiovasc Pharmacol. 55:496–505.
2010.PubMed/NCBI
|
|
23
|
Wang H, Yin Y, Li W, Zhao X, Yu Y, Zhu J,
Qin Z, Wang Q, Wang K, Lu W, et al: Over-expression of PDGFR-β
promotes PDGF-induced proliferation, migration, and angiogenesis of
EPCs through PI3K/Akt signaling pathway. PLoS One. 7:e305032012.
View Article : Google Scholar
|
|
24
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paling NR, Wheadon H, Bone HK and Welham
MJ: Regulation of embryonic stem cell self-renewal by
phosphoinositide 3-kinase-dependent signaling. J Biol Chem.
279:48063–48070. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Storm MP, Kumpfmueller B, Thompson B,
Kolde R, Vilo J, Hummel O, Schulz H and Welham MJ: Characterization
of the phosphoinositide 3-kinase-dependent transcriptome in murine
embryonic stem cells: Identification of novel regulators of
pluri-potency. Stem Cells. 27:764–775. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mclean AB, D’Amour KA, Jones KL,
Krishnamoorthy M, Kulik MJ, Reynolds DM, Sheppard AM, Liu H, Xu Y,
Baetge EE and Dalton S: Activin a efficiently specifies definitive
endoderm from human embryonic stem cells only when
phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells.
25:29–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Roche S, Koegl M and Courtneidge SA: The
phosphatidylinositol 3-kinase alpha is required for DNA synthesis
induced by some, but not all, growth factors. Proc Natl Acad Sci
USA. 91:9185–9189. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shivakrupa R, Bernstein A, Watring N and
Linnekin D: Phosphatidylinositol 3′-kinase is required for growth
of mast cells expressing the kit catalytic domain mutant. Cancer
Res. 63:4412–4419. 2003.PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(T)(−Delta Delta C) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
31
|
Lianguzova MS, Chuykin IA, Nordheim A and
Pospelov VA: Phosphoinositide 3-kinase inhibitor LY294002 but not
serum withdrawal suppresses proliferation of murine embryonic stem
cells. Cell Biol Int. 31:330–337. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
May R, Sureban SM, Hoang N, Riehl TE,
Lightfoot SA, Ramanujam R, Wyche JH, Anant S and Houchen CW:
Doublecortin and cam kinase-like-1 and
leucine-rich-repeat-containing G-protein-coupled receptor mark
quiescent and cycling intestinal stem cells, respectively. Stem
Cells. 27:2571–2579. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Braun H, Schäfer K and Höllt V: BetaIII
tubulin-expressing neurons reveal enhanced neurogenesis in
hippocampal and cortical structures after a contusion trauma in
rats. J Neurotrauma. 19:975–983. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guo J, Walss-Bass C and Ludueña RF: The
beta isotypes of tubulin in neuronal differentiation. Cytoskeleton
(Hoboken). 67:431–441. 2010. View Article : Google Scholar
|
|
35
|
Guo J, Qiang M and Ludueña RF: The
distribution of β-tubulin isotypes in cultured neurons from
embryonic, newborn, and adult mouse brains. Brain Res. 1420:8–18.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maunoury R, Robine S, Pringault E, Huet C,
Guénet JL, Gaillard JA and Louvard D: Villin expression in the
visceral endoderm and in the gut anlage during early mouse
embryogenesis. EMBO J. 7:3321–3329. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pinto D, Robine S, Jaisser F, EI Marjou FE
and Louvard D: Regulatory sequences of the mouse villin gene that
efficiently drive transgenic expression in immature and
differentiated epithelial cells of small and large intestines. J
Biol Chem. 274:6476–6482. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takakura N, Yoshida H, Ogura Y, Kataoka H
and Nishikawa S and Nishikawa S: PDGFR alpha expression during
mouse embryogenesis: Immunolocalization analyzed by whole-mount
immunohistostaining using the monoclonal anti-mouse PDGFR alpha
antibody APA5. J Histochem Cytochem. 45:883–893. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Karlsson L, Lindahl P, Heath JK and
Betsholtz C: Abnormal gastrointestinal development in PDGF-A and
PDGFR-(alpha) deficient mice implicates a novel mesenchymal
structure with putative instructive properties in villus
morphogenesis. Development. 127:3457–3466. 2000.PubMed/NCBI
|
|
40
|
Takebe A, Era T, Okada M, Martin Jakt L,
Kuroda Y and Nishikawa S: Microarray analysis of PDGFRα +
populations in ES cell differentiation culture identifies genes
involved in differentiation of mesoderm and mesenchyme including
ARID3b that is essential for development of embryonic mesenchymal
cells. Dev Biol. 293:25–37. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oviedo-Boyso J, Cortés-Vieyra R,
Huante-Mendoza A, Yu HB, Valdez-Alarcón JJ, Bravo-Patiño A,
Cajero-Juárez M, Finlay BB and Baizabal-Aguirre VM: The
phosphoinositide-3-kinase-Akt signaling pathway is important for
Staphylococcus aureus internalization by endothelial cells. Infect
Immun. 79:4569–4577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Semba S, Itoh N, Ito M, Harada M and
Yamakawa M: The in vitro and in vivo effects of
2-(4-morpholinyl)-8- phenyl-chromone (LY294002), a specific
inhibitor of phosphatidylinositol 3′-kinase, in human colon cancer
cells. Clin Cancer Res. 8:1957–1963. 2002.PubMed/NCBI
|
|
43
|
Xu XY, Zhang Z, Su WH, Zhang Y, Feng C,
Zhao HM, Zong ZH, Cui C and Yu BZ: Involvement of the p110 alpha
isoform of PI3K in early development of mouse embryos. Mol Reprod
Dev. 76:389–398. 2009. View Article : Google Scholar
|
|
44
|
van der Flier LG and Clevers H: Stem
cells, self-renewal, and differentiation in the intestinal
epithelium. Annu Rev Physiol. 71:241–260. 2009. View Article : Google Scholar
|