Introduction
Myelin protein zero (MPZ) is one of the components
of myelin in Schwann cells. It comprises 21% of the protein in the
sheath of peripheral nerves, and is responsible for ensheathment
and axonal protection by linking adjacent lamellae to stabilize the
myelin assembly (1-3). There are >200 known MPZ mutations
that cause dominantly inherited peripheral neuropathies, known as
Charcot-Marie-Tooth disease type 1B (CMT1B), congenital
hypomyelinating neuropathy 2 (CHN2), or Dejerine-Sottas neuropathy
(DSN; V169fs) (4,5). CMT1B is a demyelinating neuropathy
resulting in distal muscle atrophy and DSN is a more severe form of
CMT with a childhood-onset. The symptoms of CHN2 include
respiratory difficulty, muscle weakness and incoordination,
areflexia and ataxia (6). These
mutants also cause heterogeneous neuropa-thies through diverse
pathological mechanisms due to their different gain-of-function
traits, which are not yet fully understood (7).
According to previous studies on the pathological
mechanisms of MPZ mutations, mutant MPZ proteins commonly cause
CMT1B by inducing endoplasmic reticulum (ER) stress, which is
mediated by the unfolded protein response (UPR) (8-12).
ER stress is initiated when unfolded proteins accumulate and the
production of reactive oxygen species (ROS) is induced through
oxidative protein folding. Once the concentration of unfolded
proteins exceeds the capacity of a chaperone protein, binding
immunoglobulin protein (BiP), these surplus proteins activate ER
membrane-bound proteins, such as the N-terminal ends of pancreatic
ER kinase RNA-like ER kinase (PERK), inositol-requiring enzyme 1α
(IRE1α) and activating transcription factor 6 (ATF6) (13). Activated PERK, IRE1α and ATF6
initiate the apoptotic cascade, which is initially mediated by
pro-apoptotic proteins, including C/EBP homologous protein (CHOP),
c-Jun N-terminal kinase (JNK) or microRNAs (miRNAs or miRs)
(14,15).
Amino derivatives of salicylic acids [aminosalicylic
acids (ASAs)] are safe drugs that are commonly used in clinical
practice. In particular, 4-ASA, one of the most effective
antibiotics, has been used for the treatment of tuberculosis since
1944 (16). In addition, 4- and
5-ASA can be used for the treatment of inflammatory bowel disease
owing to their anti-inflammatory properties (17). Further mechanistic studies have
indicated that 4- and 5-ASA act to scavenge free radicals that
produce inflammation (18,19).
A previous study also demonstrated that 3-, 4-, and 5-ASA reduce
the ROS concentration, thereby relieving manganese neurotoxicity in
dopaminergic human neuroblastoma cells (20).
In this study, we generated in vitro models
expressing mutant MPZ protein that caused ER stress and Schwann
cell death and investigated whether the 3 ASAs can alleviate these
pathological effects.
Materials and methods
Cell culture and transfection
Rat Schwann cells, RT4 cells (RT4-D6P2T, CRL-2768,
ATCC), were cultured in high-glucose Dulbecco's modified Eagle's
medium (DMEM; Biowest) containing 10% fetal bovine serum and 1%
penicillin-streptomycin (Biowest) at 37°C in a 5% CO2
atmosphere. The MPZ gene was amplified from the pCMV6-entry-MPZ
vector (Origene). The amplified PCR product was cloned into the
pCMV-Myc or p-EGFP(C1) vector (Clontech). Mutant genes (V169fs,
L184fs, R185fs, S226fs and R98C) were generated using the
QuikChange Site-Directed Mutagenesis kit (Stratagene). To express
wild-type MPZ and mutant MPZ genes, the RT4 cells
(2×105) seeded on 6-well culture plates were transfected
with MPZ-containing vectors [pCMV-Myc-MPZ WT/V169fs/R98C and
pEGFP(C1)-MPZ WT/V169fs], as well as their control vectors
[pCMV-Myc and pEGFP(C1)] using Lipofectamine 3000 (Invitrogen)
according to the manufacturer's instructions. Based on western blot
analysis and immunocytochemistry, the transcription efficiency was
>90%. The transfected cells were incubated at 37°C for 48 h. The
RT4 cells (2×105) were transfected with the MPZ
expression vectors and treated with the drugs (1-100 μM)
[para(4)-aminosalicylic acid
(4-ASA), sodium 4-aminosalicylic acid (s4-ASA) and 5-aminosalicylic
acid (5-ASA)] (Sigma-Aldrich), as well as their solvent (PBS)
solution as a negative control for 24 h. The direct counting of
cell numbers was performed after the collected cells were stained
with trypan blue (T8154, Sigma-Aldrich). Ten microliters of cells
were mixed with an equal volume of Trypan blue then cells were
immediately counted with a hemacytometer (Sigma-Aldrich, Z359626)
under a microscopy
Fluorescence-activated cell sorting
(FACS) for the measurement of cell death
In order to measure cell death, the RT4 cells were
seeded in 6-well culture plates at a density of 2×105
cells per well. The RT4 cells were transiently transfected with
wild-type MPZ or mutant MPZ for 24 h, then treated with the ASA
compounds (1-100 μM) for 24 h and harvested. Annexin V
fluorescein isothiocyanate (FITC) and propidium iodide (PI)
staining (BD Biosciences Pharmingen) was performed by incubating
the cells in the dark for 15 min at room temperature in binding
buffer (10 mM HEPES, 140 mM NaCl, and 2.5 mM CaCl2, at
pH 7.4) saturated with Annexin V FITC and PI. Following incubation,
the cells were washed with cold PBS, pelleted and analyzed by a
FACS VERSE analyzer (BD Biosciences). We determine the number of
live, apoptotic and necrotic cells by counting the numbers of
Annexin V-/PI- cells, Annexin
V+/PI- cells, and Annexin
V+/PI+ cells, respectively.
DCFDA assay
The control experiment involved the treatment of the
RT4 cells with Brefeldin A (Sigma-Aldrich) (5 μM) for 48 h.
For the experimental groups, the RT4 cells were transfected with
MPZ V169fs or R98C mutant vectors for 48 h with the ASA compounds
(1-100 μM) or ascorbic acid (A92902, Sigma-Aldrich). To
measure ROS levels in the control and experimental groups, the
H2DCFDA assay was performed as per the manufacturer's
recommendations (Invitrogen). Initially, the RT4 cells were
harvested and incubated with 10 μM H2DCFDA at
37°C for 40 min. After being chilled on ice, the cells were
pelleted and analyzed with a FACS VERSE analyzer (BD Biosciences).
The fluorescence intensity of H2DCFDA formed by the
reaction between H2DCFDA and intracellular ROS in
>10,000 viable cells per sample was analyzed at an excitation
wavelength of 488 nm and an emission wavelength of 525 nm using a
FACS VERSE analyzer (BD Biosciences). The experiments were repeated
at least 3 times, and the most representative histogram data are
presented.
Western blot analysis
The expression of MPZ proteins and ER stress markers
was confirmed using a standard western blot analysis. Total cells
were harvested and lysed with RIPA lysis buffer (Thermo Fisher
Scientific). The cell lysates were centrifuged at 13,000 × g for 15
min at 4°C, and the supernatants were used for quantification using
bicinchoninic acid (BCA) method. A total of 20 μg of protein
was separated and transferred onto polyvinylidene fluoride (PVDF)
membranes. The membranes were then stained with 0.1% Ponceau S
solution (Sigma-Aldrich) to ensure equal loading of the samples,
and non-specific binding was blocked with a blocking buffer (Casein
blocking buffer 10X; Sigma-Aldrich) for 1 h at room temperature.
The membranes were incubated overnight at 4°C with the following
primary antibodies: Anti-Myc-tag (1:2,000; ab9106, Abcam),
anti-binding immunoglobulin protein (BiP, #3177),
anti-C/EBP-homologous protein (CHOP, #5554), anti-cleaved caspase-3
(Asp175, #9661), anti-phospho-AKT (ser473, #9271),
anti-phospho-SAPK/JNK (Thr183/Tyr185, #9251) (all 1:1,000) (all
from Cell Signaling Technology) and anti-β-actin (A2228,
Sigma-Aldrich). Subsequently, bound antibodies were visualized with
anti-mouse (#7076) or anti-rabbit IgG HRP-linked secondary
antibodies (#7074) (all 1:2,000) (all from Cell Signaling
Technology) by incubating 1 h at room temperature and Western
blotting luminol reagent (Santa Cruz Biotechnology) in order to
detect proteins. The integrated optical densities of the
immunoreactive protein bands were measured using Image J software
(https://imagej.nih.gov).
Immunocytochemistry and confocal
imaging
For immunofluorescence staining, the RT4 cells
(2×104) were seeded on coverslips in 24-well culture
plates. After being transfected with wild-type MPZ or mutant MPZ
(pCMV-Myc-MPZ WT, V169fs, R98C and pEGFP-MPZ WT, V169fs) for 24 h,
the cells were treated with the drugs (100 μM of 4-, s4-, or
5-ASA) for 24 h. Subsequently, they were fixed in 4%
paraformaldehyde for 20 min, washed with PBS for 5 min 3 times, and
blocked with 0.1% Triton X-100 in 5% normal goat serum for 1 h at
room temperature. Fixed cells were incubated with the primary
antibodies, anti-Myc-tag (1:500; ab9106, Abcam) and anti-protein
disulfide isomerase (PDI; 1:100; #2446, Cell Signaling Technology),
overnight at 4°C. After being washed 3 times with PBS for 5 min
each time, the cells were incubated with the appropriate secondary
antibodies, including Alexa Fluor 488 goat anti-rabbit IgG
(1:1,000, A11008) and Alexa Fluor 568 goat anti-mouse IgG (1:1,000,
A11001) (Molecular Probes) for 1 h at room temperature. The cells
were finally washed and mounted in Vectashield hardset antifade
mounting medium (Vector Laboratories) with
4′,6-diamidino-2-phenylindole (DAPI) to allow for the visualization
of the nuclei. The stained sections were visualized under a laser
scanning confocal microscope (CLSM700; Carl Zeiss).
Statistical analysis
All experiments (western blot analysis, FACS,
immunocytochemistry and cell quantification) were performed at
least 3 independent times. We tested for and found normal
distributions and equal variances in our sample distributions. To
determine the statistical significance within the multiple groups
in western blot analysis and cell quantification, we used one-way
ANOVA with a post hoc Tukey's multiple comparison test to confirm
whether the F value was greater than F-critical value. For
comparisons between the groups (control vs 4-ASA treated cells
after transfection with either MPZ-V169fs or R98C vectors),
Student's t-tests were used to determine the effects of 4-ASA in
FACS analysis of Annexin V-/PI- cells,
Annexin V+/PI- cells, and Annexin
V+/PI+ cells. P<0.05 was considered
statistically significant.
Results
MPZ mutations cause Schwann cell
death
Previous studies on 2 MPZ mutants (V169fs and R98C)
indicated that these mutants induce Schwann cell death and the
elevation of ER stress in vitro and in vivo (10,21). In particular, the R98C mutant has
been reported to activate the IRE1 pathway, leading to apoptosis
in vivo (21). The V169fs
mutant has also been shown to induce ER stress and cell death by
being retained in the ER compartments of non-Schwann cells in
vitro (HeLa or 293 cell lines) (10).
To validate the induction of Schwann cell death or
ER stress by MPZ mutant proteins, we generated wild-type (WT) and 5
mutant MPZ (V169fs, L184fs, R185fs, S226fs or R98C) expression
vectors by site-directed mutagenesis. From western blot analysis
and immunocytochemistry, we confirmed the effective expression of
MPZ proteins by the transient transfection of WT and mutant MPZ
vectors into the RT4 cells with a >90% transfection efficiency
(Figs. 1, 2 and S1). In addition, we observed that the
levels of ER stress markers, such as BiP and CHOP were altered by
either MPZ-V169fs or MPZ-R98C overexpression. The CHOP expression
levels were elevated by the overexpression of MPZ-V169fs and
MPZ-R98C mutants, while the BiP level was elevated only by the
overexpression of MPZ-V169fs mutant (Fig. S1). Thus we proceeded with further
experiments using only the MPZ-V169fs and MPZ-R98C mutant.
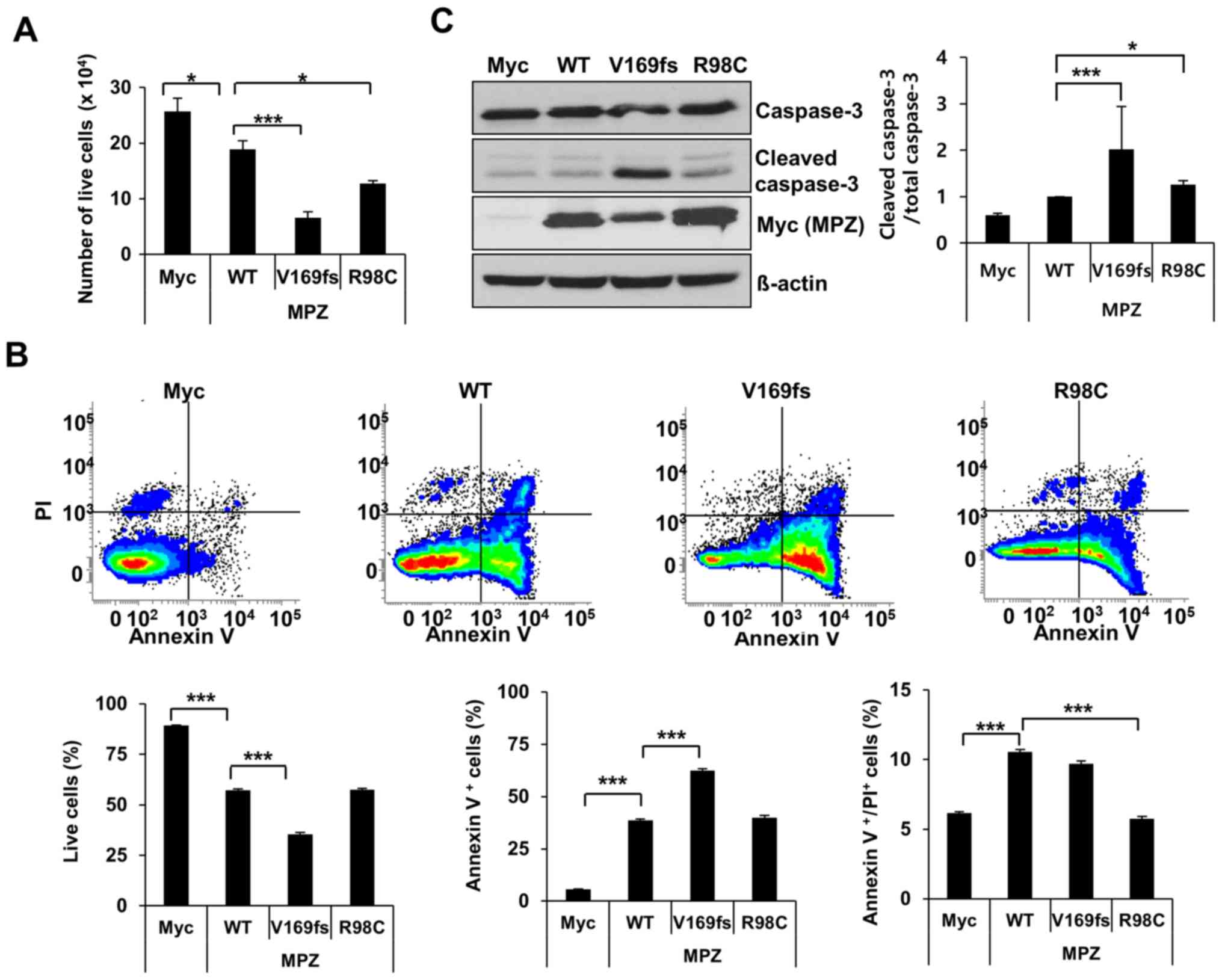
To determine whether the overexpression of two MPZ
mutants (V169fs and R98C) affects Schwann cell viability, we
transfected 2 MPZ mutant-overexpressing vectors into the RT4 cells.
Within 48 h, the detachment of transfected RT4 cells from the
bottom of the dish was observed. Manual cell counting following
Trypan blue staining indicated that the overexpression of the
V169fs or R98C mutant in the RT4 cells caused 65 or 32% significant
cell death compared to the expression of wild-type protein,
respectively (Fig. 1A). To
validate this result, the number of Annexin V- and
PI-positive RT4 cells following transfection with the MPZ mutants
were measured by FACS analysis. Expectedly, the percentage of live
RT4 cells indicated a 38% significant decrease following the
expression of the V169fs mutant compared to wild-type MPZ (Fig. 1B). This cell death was driven by
the significant induction of apoptosis. On the other hand, the
expression of the R98C mutant resulted in a 46% significant
reduction in the late apoptotic/dead cell population; however, the
number of live cells was not significantly altered. This result was
further confirmed by western blot analysis. The expression of the
V169fs mutant led to a 2-fold (P<0.05) increase in cleaved
caspase-3 levels 48 h following transfection compared to the
wild-type control (Fig. 1C).
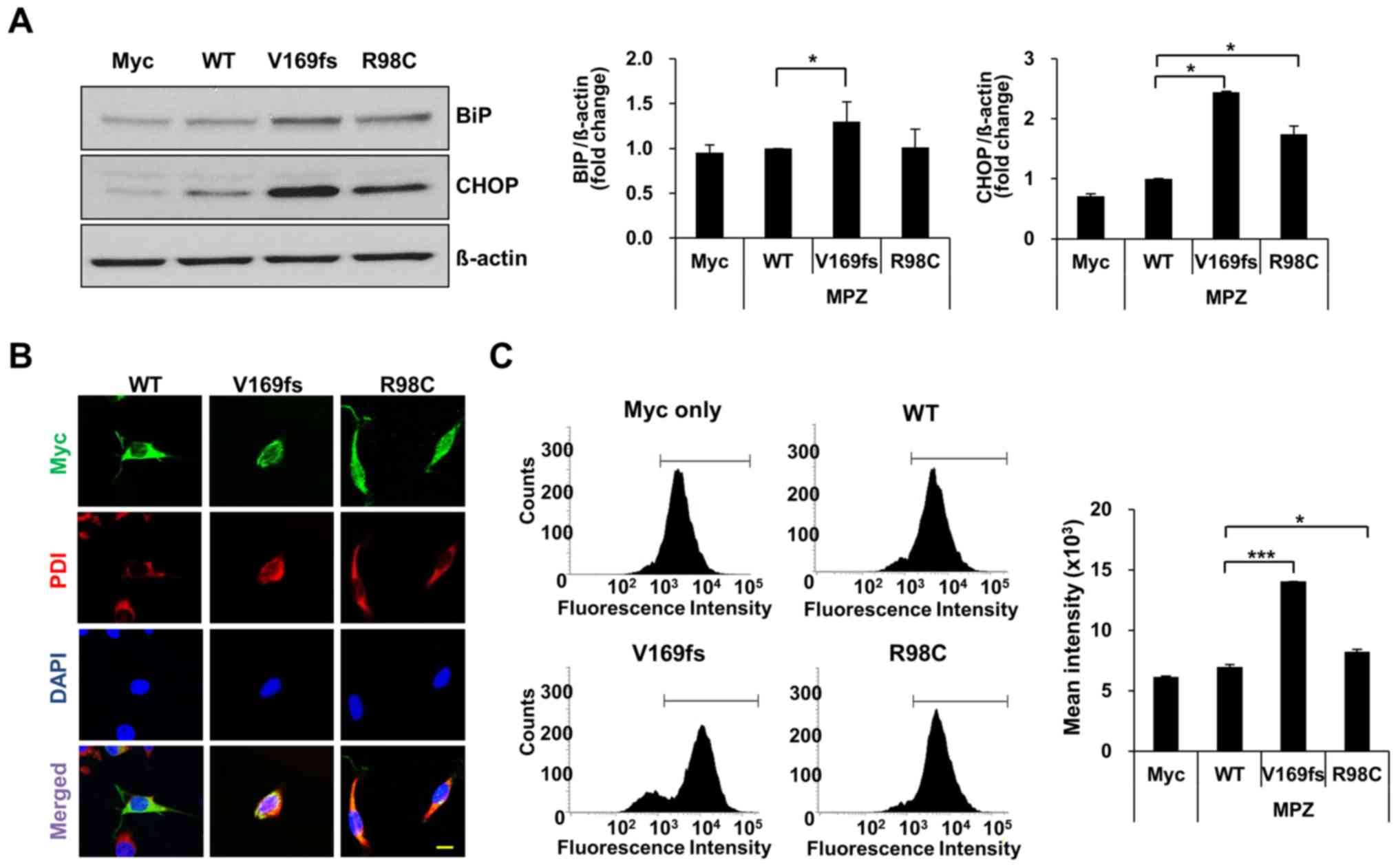
ER stress and ROS levels are elevated in
Schwann cells by the expression of MPZ mutants
As previously reported, selected MPZ mutant proteins
(S22_W28 deletion, V169fs, 550del3insG, S63del and R98C) induce ER
stress in vitro or in vivo (7,10,21-23). In this study, to determine whether
Schwann cell death is associated with ER stress in the mutant MPZ
protein (V169fs or R98C)-expressing cells, the levels of BiP and
CHOP were measured in cell lysates of transfected RT4 cells
(Fig. 2A and Fig. S1). The BiP level exhibited a
1.3-fold significant increase in the MPZ V169fs-expressing RT4
cells, whereas the overexpression of the R98C mutant did not
markedly alter the BiP levels. The level of CHOP was significantly
increased in the cells overexpressing either MPZ mutant (V169fs or
R98C) compared to the cells overexpressing the wild-type
control.
To link the elevation of ER markers to the
accumulation of mutant protein in the ER, we assessed the
localization of MPZ (wild-type/mutant) proteins in RT4 cells. We
detected MPZ with an anti-Myc antibody and marked the ER with
anti-PDI in transfected RT4 cells (Fig. 2B). Wild-type and R98C mutant
proteins were found throughout the cytosol, whereas V169fs mutant
protein co-localized with PDI.
As the retention of unfolded proteins in the ER
possibly causes an elevation of oxidative stress levels in the ER
(24,25), we also determined the extent of
induction of ROS levels using DCFDA, which detects intracellular
ROS levels through the fluorometric measurement of DCF oxidation.
Using FACS analysis, the ROS levels underwent a statistically
significant increase in the cells expressing the V169fs and R98C
mutants, respectively (Fig. 2C).
On the whole, the protein localization and ER stress levels within
the Schwann cells varied depending on the particular MPZ mutation,
yet both the V169fs and R98C mutations resulted in Schwann cell
death.
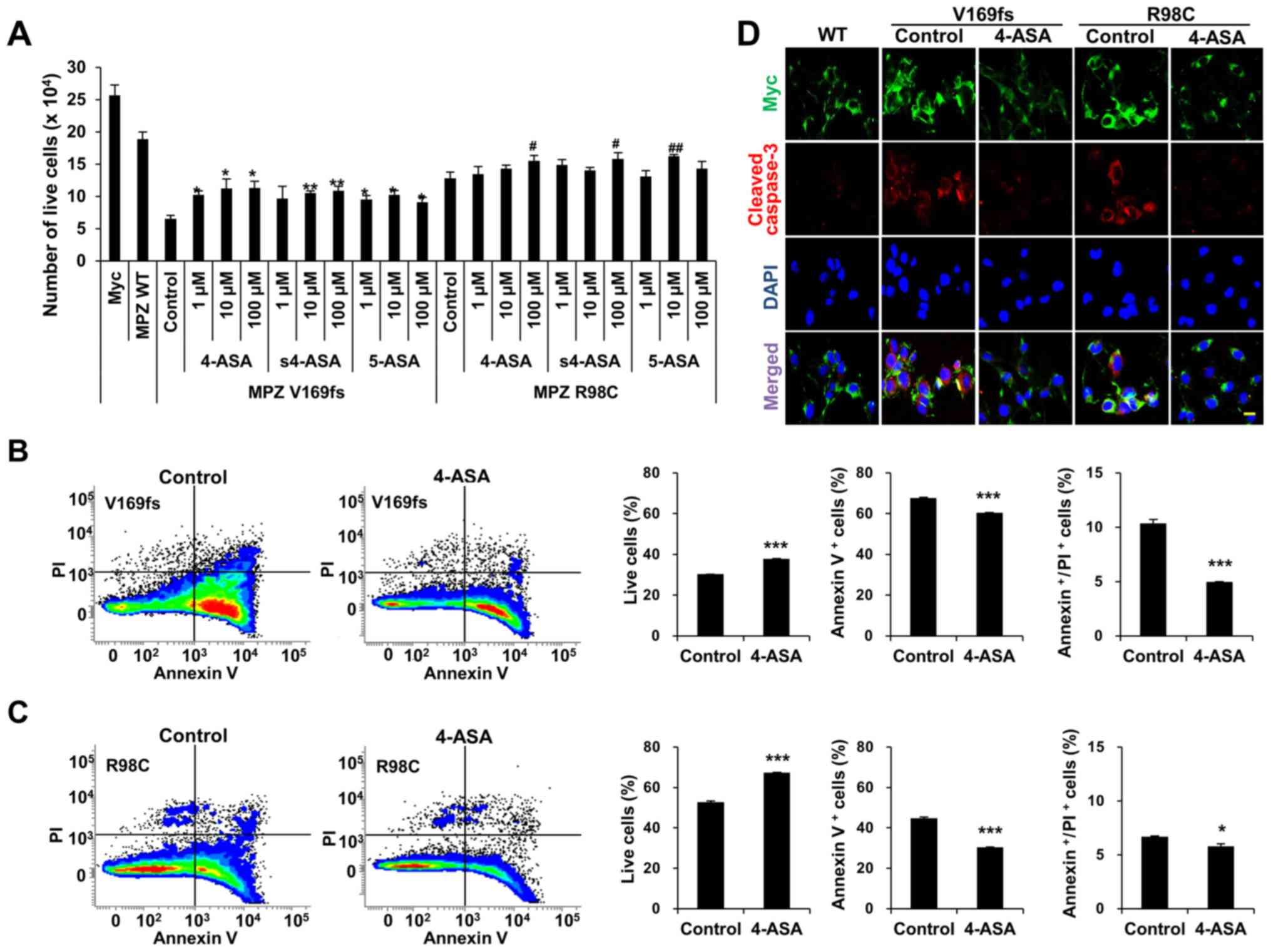
ASAs reduce the cell death induced by MPZ
mutants
To examine whether ASAs reduce Schwann cell death,
we treated the MPZ-transfected RT4 cells with 3 different
concentrations (1, 10, or 100 μM) of 4-, s4-, or 5-ASA for
24 h. This treatment resulted in the distinctively less detachment
of transfected RT4 cells. Quantitative analysis of the live cells
indicated that the number of live cells was significantly increased
following treatment with 100 μM of ASAs, except for the case
of 5-ASA treatment in the R98C MPZ mutant proteins (Fig. 3A). To verify whether the
enhancement of cell viability was dependent on the dose of ASAs, we
used one-way ANOVA with a post hoc Tukey's multiple comparison test
to compare the numbers of live cells following treatment with
various concentrations of each ASA (Fig. 3A). Although the ASA-treated group
exhibited a significant enhancement of cell viability compared to
an experimental control group, this increment was not
dose-dependent. FACS analysis of the Annexin V and PI indicated
that 4-ASA treatment significantly enhanced the number of live RT4
cells overexpressing either the V169fs (1.24-fold increase from
control group) (Fig. 3B) or R98C
mutant (1.28-fold increase from control group) (Fig. 3C). The increase in cell survival
occurred by virtue of a significant reduction in both apoptosis and
necrosis (V169fs: 10.8% reduction of apoptosis and 51.5% reduction
of necrosis; R98C: 32.4% reduction of apoptosis and 13.4% reduction
of necrosis). Treatment with s4- and 5-ASA also increased the
number of live cells via a reduction in both apoptosis and necrosis
(data not shown). The reduction of the apoptosis of the
mutant-overexpressing RT4 cells following 4-ASA treatment (100
μM) was also determined by using cleaved caspase-3 staining
(Fig. 3D). Taken together, the
results indicated that 4-, s4-, and 5-ASA significantly increased
Schwann cell viability through a significant reduction in both
apoptosis and necrosis.
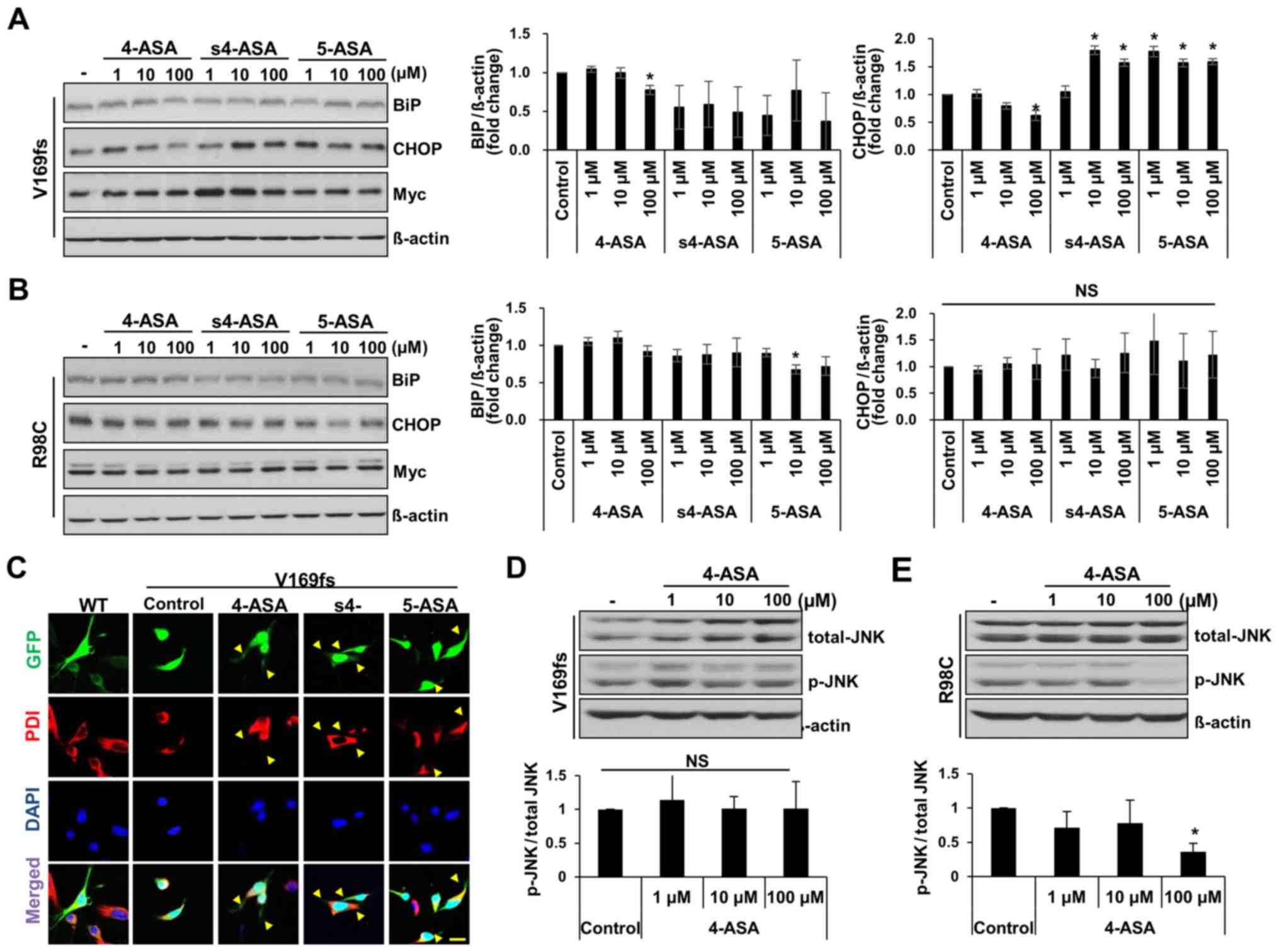
4-ASA reduces ER stress induced by MPZ
mutant proteins
4- and 5-ASA are used clinically as
anti-inflammatory drugs, particularly for patients with
inflammatory bowel disease (26).
4-ASA is recognized as an antibiotic for the treatment of
tuberculosis (27). Apart from
these known effects of the ASAs, a previous study demonstrated the
drug repositioning of ASA in reducing manganese-induced ROS
generation and cell death in human neuroblastoma cells (20). In this study, we examined whether
4-, s4-, or 5-ASA can alleviate ER stress in Schwann cells induced
by the presence of mutant MPZ proteins. We measured the level of ER
stress by detecting BiP and CHOP expression at 24 h following
treatment with the drugs. The BiP levels in the V169fs-transfected
RT4 cells were significantly decreased in response to treatment
with 100 μM 4-ASA (23% decrease from control) (Fig. 4A). However, the s4- and
5-ASA-treated cells did not exhibit a significant decrease in BiP
levels. Similarly, the CHOP level was significantly decreased only
in the 100 μM 4-ASA-treated V169fs mutant-expressing RT4
cells (37% decrease from control). On the other hand, the level of
neither ER stress marker was significantly decreased in the
R98C-transfected RT4 cells following treatment with 4-ASA in
comparison with the control, apart from the BiP level in the 5-ASA
(10 μM)-treated group (Fig.
4B). As ER stress in V169fs mutant-transfected RT4 cells
occurred due to the retention of mutant MPZ proteins within the ER
compartment, we observed the localization of MPZ V169fs protein,
which is tagged with EGFP. GFP fluorescence was observed in the
cytosol of RT4 cells following treatment with 100 μM of ASAs
(Fig. 4C). Treatment of the RT4
cells expressing V169fs mutant protein with 4-ASA resulted in a
cellular phenotype similar to that of wild-type protein-expressing
RT4 cells, in which MPZ protein was distributed throughout Schwann
cell processes (yellow arrowheads) rather being restricted to the
soma or ER compartment (PDI-positive). Treatment with s4-ASA and
5-ASA also resulted in GFP expression in Schwann cell processes,
which were shorter than those of the 4-ASA-treated RT4 cells. Taken
together, these results indicated that treatment with 100 μM
of 4-ASA ameliorated the cellular phenotypes and the ER stress
caused by MPZ V169fs mutant protein.
In R98C mutant-expressing cells, no significant
changes were observed in the BiP or CHOP levels following treatment
with any of the 3 ASAs, apart from the decrease observed in BiP
expression with 10 μM 5-ASA (Fig. 4B). This result was similar to that
of a previous study, which indicated that the ablation of CHOP did
not rescue the phenotype of R98C transgenic mice and that the R98C
mutant activates the IRE1 pathway of the UPR (21). Subsequently, in order to eludicate
the mechanism of apoptosis that results from the overexpression of
MPZ R98C protein in Schwann cells, we detected the levels of
phosphorylated JNK (p-JNK) as a marker of ER stress-induced
apoptosis (28). As expected, the
RT4 cells treated with 100 μM 4-ASA and expressing the R98C
mutant exhibited a 64% significant reduction in the p-JNK levels
(Fig. 4E), while no significant
changes were observed in the cells expressing the V169fs mutant
(Fig. 4D). Therefore, 4ASA
treatment reduced the level of UPR-mediated pro-apoptotic markers
in RT4 cells expressing the R98C MPZ mutant, while ER stress was
not affected.
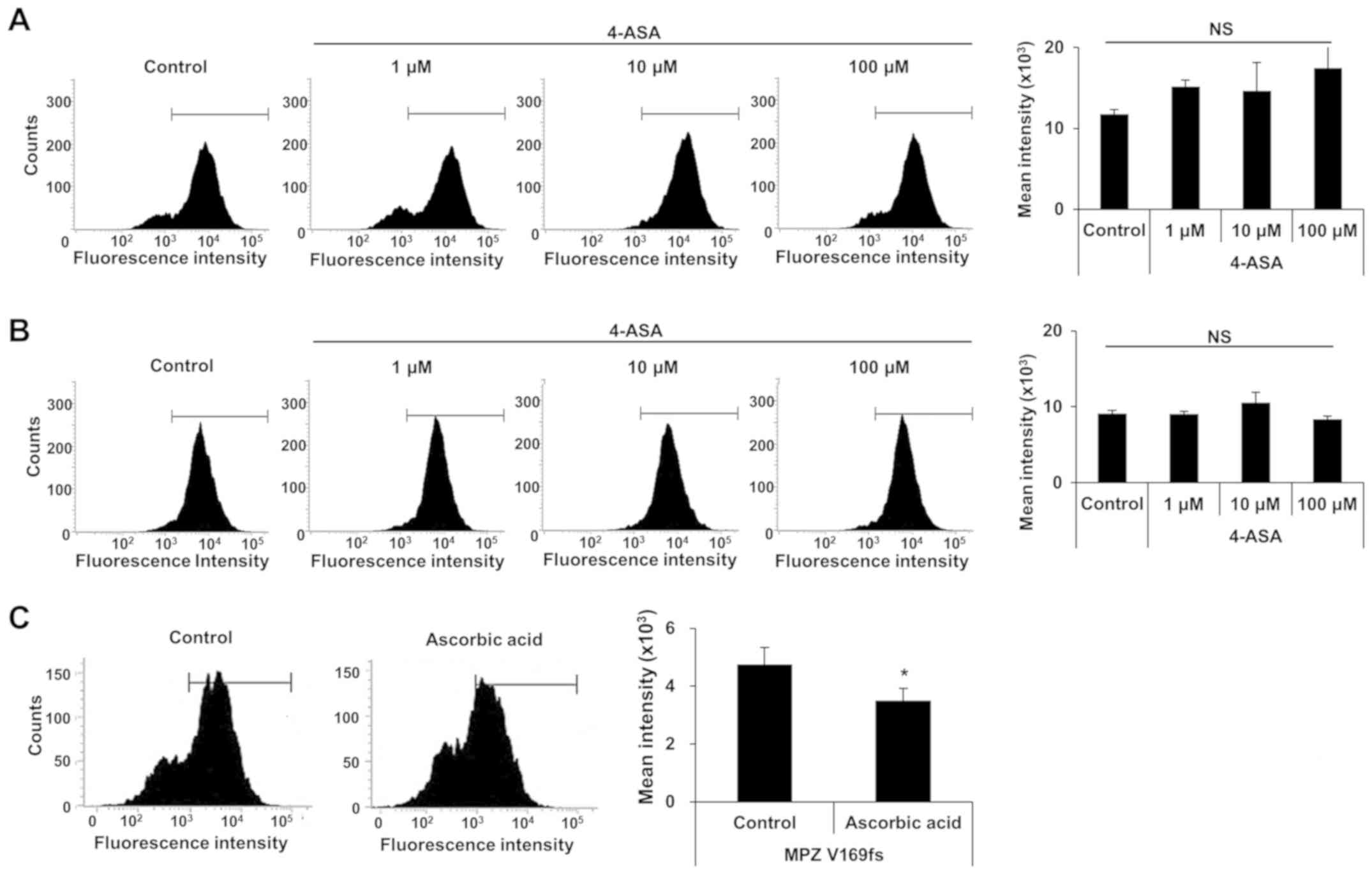
4-ASA does not affect ROS levels induced
by MPZ mutant proteins
Increased ER stress may also results in ROS
accumulation (29), which is
caused by the overexpression of mutant MPZ proteins in RT4 cells.
Thus, in this study, we examined whether 4-ASA treatment can
alleviate ROS levels. RT4 cells expressing each MPZ mutant were
treated with 3 different concentrations of 4-ASA, and the ROS level
was measured by DCFDA assay. Notably, none of the 4-ASA-treated
groups exhibited any marked changes in ROS levels (Fig. 5A and B). We also used ascorbic
acid (600 μM) to confirm the results of the DCFDA assay with
the MPZ V169fs mutant, as ascorbic acid potently inhibits ROS
production (30,31). Treatment with ascorbic acid
resulted in a 26.5% significant reduction in ROS levels (Fig. 5C). These data suggest that 4-ASA
does not affect the ROS levels, which are elevated by ER stress
caused by the expression of mutant MPZ proteins.
Discussion
Currently, there is no treatment for CMT that
affects the course of the disease. In this study, we investigated
whether amino derivatives of salicylic acids (4-, s4-, or 5-ASA)
may be effective treatments in rat Schwann cells overexpressing 2
MPZ mutations, V169fs and R98C, which cause DSN and CMT1B,
respectively (4,32). We initially generated in
vitro models over-expressing the mutant proteins in rat Schwann
cells, and found that only treatment with 100 μM 4-ASA
exhibited significant drug efficacy for those 2 MPZ mutations: i)
4-ASA treatment increased the viability of Schwann cells which
overexpressed MPZ V169fs or MPZ R8C mutant proteins; ii) 4-ASA
reduced the level of ER stress markers (BiP and CHOP) in MPZ
V169fs-expressing RT4 cells, but not in MPZ R98C-expressing cells;
iii) 4-ASA released V169fs protein retained in the ER; and iv)
4-ASA decreased the level of p-JNK, which is involved in apoptosis,
in R98C protein-expressing RT4 cells, but not in MPZ
V169fs-expressing cells.
The structure of MPZ proteins is favorable to be
retained in the ER, thus triggering the unfolded protein response
(UPR), which can be assessed by UPR markers (BiP and CHOP)
(7). Our data support previous
evidence that both the V169fs and R98C mutants similarly induced
the UPR and ER stress in vitro and in vivo,
respectively (10,21). Furthermore, the expression of the
V169fs mutant significantly elevated the levels of both ER stress
markers compared to expression of wild-type protein, whereas the
expression of the R98C mutant caused a significant increase only in
the level of CHOP. We hypothesized that the pathological mechanism
of V169fs mutant protein involves the retention of mutant proteins
in the ER causing an elevation of BiP, which consequently activates
downstream effectors (CHOP) and eventually leads to Schwann cell
apoptosis. On the other hand, the R98C mutant did not result in
this particular cellular phenotype. Based on the evidence, the 2
mutants led to protein structural changes during post-translational
modification and caused ER stress in a different manner.
Both MPZ mutants do not seem to share the apoptotic
mechanism. According to previous studies, the expression of R98C
and S63C mutant proteins did not cause the recovery of Schwann cell
apoptosis in the absence of CHOP expression in vivo,
although CHOP is important for the demyelination of MPZ mutant
nerve cells (21,23). It was also previously demonstrated
that R98C mutant protein induced Schwann cell death and UPR
activation in the ER via activation of the IRE1α/JNK pathway
instead of the PERK/CHOP pathway (21). On the other hand, there is no
evidence regarding the pathological mechanisms of action of MPZ
V169fs protein in Schwann cells, except that patients carrying this
frameshift mutation develop more severe neuropathy phenotypes than
CMT (10,33). Based on the data of this study,
the two different mutations cause different patterns of ER stress
and different mechanisms for apoptosis. Although 4-ASA treatment
(100 μM) resulted in significant reduction of apoptosis
determined by using cleaved caspase-3 staining, both the BiP and
CHOP levels were only significantly reduced in V169fs
mutant-expressing cells and p-JNK levels were only reduced in the
R98C mutant-expressing cells. Owing to the diverse function of each
MPZ mutant protein in Schwann cells, it is necessary to elucidate
the pathological mechanisms of individual mutations in order to
devise treatment strategies for patients with each pathology as
unique gain-of-function mechanisms are involved in CMT and DSN.
Recently, curcumin has been suggested as a promising
therapeutic candidate for the treatment of CMT (34), as it allows misfolded proteins to
traverse within the ER to the plasma membrane, thereby reducing
cytotoxicity (35-37). However, it must be modified for
clinical use due to its instability, low efficacy and insolubility
(33). From the view of the drug
development process, the repositioning of an established drug is a
strong alternative option. ASAs are used clinically as
anti-inflammatories and antibiotics. In this study, we evaluated
the efficacy of ASAs and found that only 4-ASA treatment was
effective in alleviating ER stress and reducing apoptosis. Although
treatment with the other ASAs (s4-ASA and 5-ASA) resulted in
similar outcomes in terms of increasing Schwann cell viability,
they did not bring about a reduction in the CHOP level, which was
elevated by expression of mutant MPZ proteins. As MPZ mutants
elevated ER stress by increasing ROS levels, we measured tje
intracellular ROS levels following treatment with 4-ASA; 4-ASA is
known to reduce the concentration of free radicals in a manner
mediated by nuclear factor-κB inhibition (18,19). Although 4-ASA reduced the
intracellular ROS levels induced by manganese neurotoxicity
(20), it seems that 4-ASA could
not suppress the MPZ mutant-driven ROS level. These data suggest
that 4-ASA may be involved in other pathways of the apoptotic cell
death that was caused by the UPR-mediated ER stress in Schwann
cells.
In conclusion, in this study, we demonstrated that
treatment with 4-ASA reduced the ER stress and SC death caused by 2
different MPZ mutants. However, the treatment efficacy was limited
and the mode of action was not clearly revealed. Thus, ASAs need to
be further developed to enhance the therapeutic efficacy for the
treatment of CMT or DSN. In addition, enhancing the in vitro
and in vivo model by the generation of point mutations at
the endogenous MPZ using CRISPR/Cas9 technique may be helpful for
the better understanding of the MPZ mutations-mediated pathogenesis
and the mode-of-action of ASAs. Thus far, there is no treatment
available that affects the course of progression of CMT. With an
eye towards identifying small molecules or drugs for CMT treatment,
4-ASA warrants further investigationr as a treatment option for CMT
or DSN.
Supplementary Materials
Funding
The present study was supported by the Korean Health
Technology R&D Project, Ministry of Health and Welfare
(HI14C3484 and HI16C0426) and by the National Research Foundation
of Korea (NRF) grants funded by the Korean government, MSIP
(NRF-2016R1A5A2007009, NRF-2017R1A2B2004699 and
NRF-2018R1A4A1024506) and MOE (2016R1D1A1B03932630).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EHC and WMM designed and performed the experiments,
interpreted the data, and wrote the manuscript. HMD and JSL
collected and analyzed data. HTP analyzed and interpreted data. BOC
and YBH generated the study concept and design, drafted the
manuscript, and provided study supervision. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Lemke G: Molecular biology of the major
myelin genes. Trends Neurosciences. 9:266–270. 1986. View Article : Google Scholar
|
|
2
|
Sutcliffe JG: The genes for myelin
revisited. Trends Genet. 4:211–213. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patzig J, Jahn O, Tenzer S, Wichert SP, de
Monasterio-Schrader P, Rosfa S, Kuharev J, Yan K, Bormuth I, Bremer
J, et al: Quantitative and integrative proteome analysis of
peripheral nerve myelin identifies novel myelin proteins and
candidate neuropathy loci. J Neurosci. 31:16369–16386. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Warner LE, Hilz MJ, Appel SH, Killian JM,
Kolodry EH, Karpati G, Carpenter S, Watters GV, Wheeler C, Witt D,
et al: Clinical phenotypes of different MPZ (P0) mutations may
include charcot-marie-tooth type 1B, dejerine-sottas, and
congenital hypomyelination. Neuron. 17:451–460. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mandich P, Mancardi GL, Varese A, Soriani
S, Di Maria E, Bellone E, Bado M, Gross L, Windebank AJ, Ajmar F
and Schenone A: Congenital hypomyelination due to myelin protein
zero Q215X mutation. Ann Neurol. 45:676–678. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murakami T, Garcia CA, Reiter LT and
Lupski JR: Charcot-marie-tooth disease and related inherited
neuropathies. Medicine (Baltimore). 75:233–250. 1996. View Article : Google Scholar
|
|
7
|
Wrabetz L, D'Antonio M, Pennuto M, Dati G,
Tinelli E, Fratta P, Previtali S, Imperiale D, Zielasek J, Toyka K,
et al: Different intracellular pathomechanisms produce diverse
myelin protein zero neuropathies in transgenic mice. J Neurosci.
26:2358–2368. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tobler AR, Notterpek L, Naef R, Taylor V,
Suter U and Shooter EM: Transport of Trembler-J mutant peripheral
myelin protein 22 is blocked in the intermediate compartment and
affects the transport of the wild-type protein by direct
interaction. J Neurosci. 19:2027–2036. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sancho S, Young P and Suter U: Regulation
of Schwann cell proliferation and apoptosis in PMP22-deficient mice
and mouse models of charcot-marie-tooth disease type 1A. Brain.
124:2177–2187. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khajavi M, Inoue K, Wiszniewski W, Ohyama
T, Snipes GJ and Lupski JR: Curcumin treatment abrogates
endoplasmic reticulum retention and aggregation-induced apoptosis
associated with neuropathy-causing myelin protein zero-truncating
mutants. Am J Hum Genet. 77:841–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Myers JK, Mobley CK and Sanders CR: The
peripheral neuropathy-linked trembler and trembler-J mutant forms
of peripheral myelin protein 22 are folding-destabilized.
Biochemistry. 47:10620–10629. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakakura M, Hadziselimovic A, Wang Z,
Schey KL and Sanders CR: Structural basis for the trembler-J
phenotype of charcot-marie-tooth disease. Structure. 19:1160–1169.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rutkowski DT and Kaufman RJ: A trip to the
ER: Coping with stress. Trends Cell Biol. 14:20–28. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Welihinda AA and Kaufman RJ: The unfolded
protein response pathway in Saccharomyces cerevisiae.
Oligomerization and trans-phosphorylation of Ire1p (Ern1p) are
required for kinase activation. J Biol Chem. 271:18181–18187. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hetz C and Mollereau B: Disturbance of
endoplasmic reticulum proteostasis in neurodegenerative diseases.
Nat Rev Neurosci. 15:233–249. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mitchison DA: Role of individual drugs in
the chemotherapy of tuberculosis. Int J Tuberc Lung Dis. 4:796–806.
2000.PubMed/NCBI
|
|
17
|
Daniel F, Seksik P, Cacheux W, Jian R and
Marteau P: Tolerance of 4-aminosalicylic acid enemas in patients
with inflammatory bowel disease and 5-aminosalicylic-induced acute
pancreatitis. Inflamm Bowel Dis. 10:258–260. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Joshi R, Kumar S, Unnikrishnan M and
Mukherjee T: Free radical scavenging reactions of sulfasalazine,
5-aminosalicylic acid and sulfapyridine: Mechanistic aspects and
antioxidant activity. Free Radic Res. 39:1163–1172. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patole J, Shingnapurkar D, Padhye S and
Ratledge C: Schiff base conjugates of p-aminosalicylic acid as
antimycobacterial agents. Bioorg Med Chem Lett. 16:1514–1517. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoon H, Lee GH, Kim DS, Kim KW, Kim HR and
Chae HJ: The effects of 3, 4 or 5 amino salicylic acids on
manganese-induced neuronal death: ER stress and mitochondrial
complexes. Toxicol In Vitro. 25:1259–1268. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saporta MA, Shy BR, Patzko A, Bai Y,
Pennuto M, Ferri C, Tinelli E, Saveri P, Kirschner D, Crowther M,
et al: MpzR98C arrests Schwann cell development in a mouse model of
early-onset charcot-marie-tooth disease type 1B. Brain.
135:2032–2047. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grandis M, Vigo T, Passalacqua M, Jain M,
Scazzola S, La Padula V, Brucal M, Benvenuto F, Nobbio L, Cadoni A,
et al: Different cellular and molecular mechanisms for early and
late-onset myelin protein zero mutations. Hum Mol Genet.
17:1877–1889. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pennuto M, Tinelli E, Malaguti M, Del
Carro U, D'Antonio M, Ron D, Quattrini A, Feltri ML and Wrabetz L:
Ablation of the UPR-mediator CHOP restores motor function and
reduces demyelination in charcot-marie-tooth 1B mice. Neuron.
57:393–405. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haynes CM, Titus EA and Cooper AA:
Degradation of misfolded proteins prevents ER-derived oxidative
stress and cell death. Mol Cell. 15:767–776. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xue X, Piao JH, Nakajima A, Sakon-Komazawa
S, Kojima Y, Mori K, Yagita H, Okumura K, Harding H and Nakano H:
Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein
response (UPR) in a reactive oxygen species (ROS)-dependent
fashion, and the UPR counteracts ROS accumulation by TNFalpha. J
Biol Chem. 280:33917–33925. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Williams C, Panaccione R, Ghosh S and
Rioux K: Optimizing clinical use of mesalazine (5-aminosalicylic
acid) in inflammatory bowel disease. Therap Adv Gastroenterol.
4:237–248. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fox W, Ellard GA and Mitchison DA: Studies
on the treatment of tuberculosis undertaken by the british medical
research council tuberculosis units 1946 1986 with relevant
subsequent publications. Int J Tuberc Lung Dis. 3(Suppl 2): pp.
S231–S279. 1999
|
|
28
|
Urano F, Wang X, Bertolotti A, Zhang Y,
Chung P, Harding HP and Ron D: Coupling of stress in the ER to
activation of JNK protein kinases by transmembrane protein kinase
IRE1. Science. 287:664–666. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao SS and Kaufman RJ: Endoplasmic
reticulum stress and oxidative stress in cell fate decision and
human disease. Antioxid Redox Signal. 21:396–413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fukumura H, Sato M, Kezuka K, Sato I, Feng
X, Okumura S, Fujita T, Yokoyama U, Eguchi H, Ishikawa Y and Saito
T: Effect of ascorbic acid on reactive oxygen species production in
chemotherapy and hyperthermia in prostate cancer cells. J Physiol
Sci. 62:251–257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Peng Y, Kwok KH, Yang PH, Ng SS, Liu J,
Wong OG, He ML, Kung HF and Lin MC: Ascorbic acid inhibits ROS
production, NF-kappa B activation and prevents ethanol-induced
growth retardation and microencephaly. Neuropharmacology.
48:426–434. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shy ME, Jáni A, Krajewski K, Grandis M,
Lewis RA, Li J, Shy RR, Balsamo J, Lilien J, Garbern JY and Kamholz
J: Phenotypic clustering in MPZ mutations. Brain. 127:371–384.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Patzko A, Bai Y, Saporta MA, Katona I, Wu
X, Vizzuso D, Feltri ML, Wang S, Dillon LM, Kamholz J, et al:
Curcumin derivatives promote schwann cell differentiation and
improve neuropathy in R98C CMT1B mice. Brain. 135:3551–3566. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Okamoto Y, Pehlivan D, Wiszniewski W, Beck
CR, Snipes GJ, Lupski JR and Khajavi M: Curcumin facilitates a
transitory cellular stress response in trembler-J mice. Hum Mol
Genet. 22:4698–4705. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Egan ME, Pearson M, Weiner SA, Rajendran
V, Rubin D, Glockner-Pagel J, Canny S, Du K, Lukacs GL and Caplan
MJ: Curcumin, a major constituent of turmeric, corrects cystic
fibrosis defects. Science. 304:600–602. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vasireddy V, Chavali VR, Joseph VT, Kadam
R, Lin JH, Jamison JA, Kompella UB, Reddy GB and Ayyagari R: Rescue
of photoreceptor degeneration by curcumin in transgenic rats with
P23H rhodopsin mutation. PLoS One. 6:e211932011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Xiao J, Zhou H, Yang S, Wu X,
Jiang C, Zhao Y, Liang D, Li X and Liang G: A novel monocarbonyl
analogue of curcumin, (1E,4E)-1,5-bis(2,3-dimethoxyphenyl)
penta-1,4-dien-3-one, induced cancer cell H460 apoptosis via
activation of endoplasmic reticulum stress signaling pathway. J Med
Chem. 54:3768–3778. 2011. View Article : Google Scholar : PubMed/NCBI
|