Introduction
Inflammatory cells, such as macrophages and
microglia, are activated and accumulate in heart and brain tissues
following ischemic onset, causing inflammatory injury (1). Acute liver failure is a
life-threatening disease with a high mortality rate worldwide and a
substantial impact on public health (2). This disease is characterized by
hepatic dysfunction, irregular liver biochemical values and
coagulopathy. It is associated with rapidly enlightened multiple
organ failure, which can have devastating consequences. The current
methods for diagnosing acute liver failure are underdeveloped;
liver transplantation is the most common treatment strategy
(3). Therefore, there is an unmet
medical need to develop novel therapeutic strategies for acute
liver failure. Acute liver failure is caused by
inflammation-mediated hepatocellular injury, which strictly
resembles the innate immune response induced by exposure to
lipopolysaccharide (LPS). The LPS-induced acute liver injury model
is a well-established animal model that may precisely mimic
clinical indicators in humans (4). LPS induces Kupffer cell activation
via the Toll-like receptor 4 signaling pathway, activates nuclear
factor (NF)-κB, and recruits the release of inflammatory cytokines,
including interleukin (IL)-1β, IL-6 and tumor necrosis factor
(TNF)-α (5). As the same
inflammatory mediators appear to be associated with the
pathogenesis of acute liver injury, their inhibition is essential
for developing therapeutic strategies, which can be evaluated in
animal models of LPS-induced liver failure.
The mitogen-activated protein kinase (MAPK)
signaling pathway is reported to be involved in proinflammatory
responses in LPS-induced macrophages (6). Three major MAPK families have been
recognized, including extracellular signal-related kinase (ERK),
p38 and c-Jun N-terminal kinase (JNK). ERK stimulation is
associated with the LPS-induced production of TNF-α in macrophages
(7). In addition, the triggering
of p38 is involved in the production of inflammatory stimulators
for leucocyte recruitment and activation, and p38 controls the
expression of several inflammation-related genes, including TNF-α
and IL-6 (8,9). Furthermore, JNK is induced by
bacterial endotoxins, inflammatory cytokines, hypoxia and
ultraviolet radiation (10).
Therefore, suppression of the MAPK pathway may reveal the
anti-inflammatory activities of drugs. NF-κB is a transcription
factor that has a significant contribution in inflammation
(11), and NF-κB is composed of
p65 and p50 subunits. In normal unstimulated cells, NF-κB is
present in a stable form in the cytosol as the inhibitor of NF-κB
(IκB)-NF-κB complex. Following LPS stimulation, IκB is activated by
the degradation and phosphorylation of IκB via IκB kinase (IKK).
This phosphorylation results in the detachment of IκB from the
IκB-NF-κB complex, thereby enabling NF-κB to translocate to the
nucleus and triggering the transcription of proinflammatory genes,
including inducible nitric oxide synthase (iNOS), TNF-α and IL-6
(12). By contrast, the Akt
pathway regulates cellular activation, inflammatory responses and
apoptosis (13). A previous study
established that the Akt pathway executes a decelerating mechanism
to control proinflammatory mediators in LPS-induced microglia by
inhibiting the JNK and p38 MAPK pathways (14). Therefore, an inhibitor of
NF-κB/Akt may be active as an anti-inflammatory agent.
Metal complexes are useful resources for drug design
due to their potential mechanisms of action. A large group of
metal-based drugs have been designed for their various oxidation
states and overall coordination geometries (15,16). The application of metal complexes
in medicine has recently been stimulated due to the clinical
success of anticancer cisplatin and other platinum (II) compounds.
Furthermore, the high structural diversity of metal complexes is an
attractive platform for designing drugs for other conditions,
including neurodegeneration, microbial and parasitic infections and
inflammation (17). In our
previous study, a substantial number of ruthenium metal compounds
were identified as being effective antiplatelet agents for the
prevention and treatment of thrombotic diseases (18,19). Therefore, it is clear that metal
complexes have the potential to offer an alternative to
anti-inflammatory organic drugs. The present study aimed to
investigate the ability of a novel ruthenium-based metal complex,
TQ-5, on inhibiting the LPS-induced expression of iNOS and
subsequent production of TNF-α, nitric oxide (NO) and IL-1β in RAW
264.7 macrophage cells and in mouse liver injury models. In
addition, the study aimed to clarify the underlying protective
mechanisms of TQ-5 in a mouse liver injury model by investigating
the involvement of NF-κB, MAPK and Akt signaling molecules.
Materials and methods
Chemicals and reagents
Fetal bovine serum (FBS), Dulbecco's modified
Eagle's medium (DMEM), L-glutamine penicillin/streptomycin, and
anti-α-tubulin (cat. no. MS-581-P1) monoclonal antibodies (mAbs)
were purchased from Invitrogen (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). LPS (Escherichia coli 0127:B8),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich
(St. Louis, MO, USA). Anti-iNOS (cat. no. sc-650) polyclonal
antibody (pAb) was purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Anti-TNF-α (cat. no. 3707), anti-JNK (cat. no.
9252), anti-phospho-c-JNK (Thr183/Tyr185; cat. no. 9251),
anti-phospho-p44/p42 ERK (Thr202/Tyr204; cat. no. 9101),
anti-phospho-p38 MAPK (Thr180/Tyr182; cat. no. 9211),
anti-phospho-Akt (cat. no. 9271) pAbs, anti-phospho-p65 (Ser536;
cat. no. 3033), anti-p65 (cat. no. 4764), anti-IκBα (cat. no.
4812), anti-ERK (cat. no. 9107), anti-Akt (cat. no. 2920), and
anti-p38 MAPK (cat. no. 9217) mAbs were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Anti-IL-1β (cat. no.
5128) pAb was purchased from BioVision, Inc. (Milpitas, CA, USA).
Horseradish peroxidase (HRP)-conjugated donkey anti-rabbit
immunoglobulin G (IgG; cat. no. RPN4301) and sheep anti-mouse IgG
(cat. no. RPN4201) were purchased from Amersham; GE Healthcare Life
Sciences (Chalfont, UK). Western blotting detection reagent for
enhanced chemiluminescence (ECL) and Hybond™-P polyvinylidene
difluoride (PVDF) blotting membranes were purchased from GE
Healthcare Life Sciences.
TQ-5 synthesis and RAW 264.7 cell
cultivation
The ruthenium metal complex TQ-5 and its ligand (L)
were synthesized according to the method described in our previous
study (18). The RAW 264.7 cells
were obtained from ATCC (cat. no. TIB-71) and cultured in DMEM
supplemented with 10% FBS and 100 U/ml penicillin G and 100 mg/ml
streptomycin at 37°C in a humidified atmosphere of 5%
CO2/95% air (20).
Cell viability assay
The RAW 264.7 cells (2×105 cells per
well) were seeded into 24-well culture plates with DMEM containing
10% FBS for 24 h. The cells were treated with various
concentrations of TQ-5 (10, 20 and 40 µM) or solvent control (0.1%
DMSO) for 20 min and then stimulated with LPS (1 µg/ml) or left
unstimulated for 24 h at 37°C. Cell viability was measured using an
MTT assay (20). The cell
viability index was calculated as follows: (absorbance of treated
cells/absorbance of control cells) ×100%. The absorbance of samples
was determined at 570 nm using an MRX absorbance reader (Dynex
Technologies, Chantilly, VA, USA).
Determination of NO production
To determine NO production, the content of
nitrite/nitrate, as stable oxidative end products of NO, was
measured as previously described (20) with minor modifications. The RAW
264.7 cells were seeded into 6-cm dishes (8×105) with
DMEM containing 10% FBS for 24 h. The cells were treated with TQ-5
(10-40 μM) or solvent control (0.1% DMSO) for 20 min and
then stimulated with LPS (1 μg/ml) or left unstimulated for
24 h. These conditioned supernatants were collected and mixed with
equal volumes of Griess reagent. The absorbance of samples was
determined at 550 nm by using an MRX absorbance reader. The
concentrations of nitrite/nitrate were calculated using a standard
curve through linear regression of absorbance measurements of
standard solutions (sodium nitrite dissolved in the same culture
medium).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the RAW 264.7 cells
using the NucleoSpin® RNA kit (Macherey-Nagel, Düren,
Germany). RT-qPCR analysis was performed using Fast
SYBR®-Green Master mix (Thermo Fisher Scientific, Inc.),
following the manufacturer's instructions, to determine the
expression of target genes, and the results were normalized using
the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Amplification was performed using a StepOne Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The cycling
conditions were as follows: Hot-start activation at 95°C for 20 sec
followed by 40 cycles of denaturation at 95°C for 3 sec and
annealing/extension at 60°C for 30 sec. The following primers were
used in the present study: TNF-α, forward
5′-TCTTCTGTCTACTGAACTTCGG-3′ and reverse
5′-AAGATGATCRGAGTGTGAGGG-3′; IL-1β, forward
5′-AACCTGCTGGTGTGTGACGTTC-3′ and reverse 5′-CAGCACGAGGCTTTTTTGTTG
T-3′; iNOS, forward 5′-CGAAACGCTTCACTTCCAA-3′ and reverse
5′-TGAGCCTATATTGCTGTGGCT-3′; and GAPDH, forward
5′-GAACATCATCCCTGCATCCA-3′ and reverse 5′-GCCAGTGAGCTTCCCGTTC-3′.
Densitometry quantification was performed using the comparative CT
method (2-ΔΔCq) (21).
Samples were normalized by GAPDH.
Animals
A total of 26 male C57BL/6 mice (22-25 g; 8 weeks
old) were obtained from BioLasco Taiwan Co., Ltd. (Taipei, Taiwan).
The mice were kept in cages at a temperature of 22±4°C and a
relative humidity of 50±20% under a 12 h light-dark cycle.
Experimental mice received a standard pellet diet and water ad
libitum. All animal experiments and care procedures conformed
to the Guide for the Care and Use of Laboratory Animals
(LAC-2016-0395) and were approved by the Institutional Animal Care
and Use Committee of Taipei Medical University (Taipei,
Taiwan).
LPS-induced acute liver inflammation in
mice
The mice were divided into the following four
groups: i) Control, ii) LPS (2.5 mg/kg), iii) TQ-5 (2 mg/kg) + LPS
(2.5 mg/kg), and iv) TQ-5 (4 mg/kg) + LPS (2.5 mg/kg). The mice
were initially pretreated intraperitoneally with TQ-5 or 0.1% DMSO,
and 2 h following the administration of TQ-5, LPS was injected
intraperitoneally. The mice were sacrificed following 6 h of LPS
stimulation, and liver tissues were quickly removed and stored at
-80°C until analysis.
Assessment of hepatic function
The mice were sacrificed following 6 h of LPS (2.5
mg/kg) stimulation. Subsequently, blood was collected and serum was
separated by centrifugation at 500 × g for 10 min at room
temperature. The serum alanine aminotransferase (ALT) and aspartate
aminotransferase (AST) levels were determined to assess liver
function using the Vet-Test® chemistry analyzer (IDEXX,
Westbrook, ME, USA). Enzyme activities are expressed as
international units per liter.
Western blotting
Western blot analysis was performed in cells and
liver tissue homogenates by following a previously described method
(20). In brief, the RAW 264.7
cells (8×105 cells/dish) were seeded onto 6-cm dishes
with DMEM containing 10% FBS for 24 h. The cells were pretreated
with TQ-5 or 0.1% DMSO for 20 min and then stimulated with LPS (1
μg/ml) or left unstimulated according to the experimental
design. Subsequently, the proteins from the cells and liver tissues
were extracted using lysis buffer (containing 50 mM HEPES, 5 mM
EDTA, 50 mM NaCl and 1% Triton X-100). The extracted protein
samples (50 μg) were applied for 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, and the separated
proteins were then electrophoretically transferred onto PVDF
membranes (0.45-μm). The membranes were blocked with 5%
skimmed milk in Tris-buffered saline in Tween-20 (TBST) buffer (10
mM Tris-base, 100 mM NaCl and 0.01% Tween-20) for 30 min at room
temperature and then recognized with various primary antibodies
(anti-iNOS, anti-TNF-α, anti-JNK, anti-phospho-c-JNK,
anti-phospho-p 4 4/p 42 ERK, anti-phospho-p38 MAPK,
anti-phospho-Akt, anti-phospho-p65, anti-p65, anti-IκBα, anti-ERK,
anti-Akt, anti-p38 MAPK, anti-IL-1β or anti-α-tubulin; all, 1:1,000
in TBST) for 2 h at 4°C prior to incubation with secondary antibody
(HRP-conjugated anti-mouse IgG or anti-rabbit IgG) for 1 h at room
temperature. The ECL system was used to detect the immunoreactive
bands. Densitometry of the protein bands was performed using
Biolight Windows Application, V2000.01 (Bio-Profil, VilberLourmat,
France).
Statistical analysis
All results are expressed as the mean ± standard
error of the mean and are accompanied by the number of observations
(n). Multiple group comparisons were assessed using one-way
analysis of variance followed by analysis using the Newman-Keuls
method. P<0.05 was considered to indicate a statistically
significant difference. Statistical analyses were performed using
SAS (version 9.2; SAS Institute, Inc., Cary, NC, USA).
Results
Impact of TQ-5 on the viability and
morphology of RAW 264.7 Cells
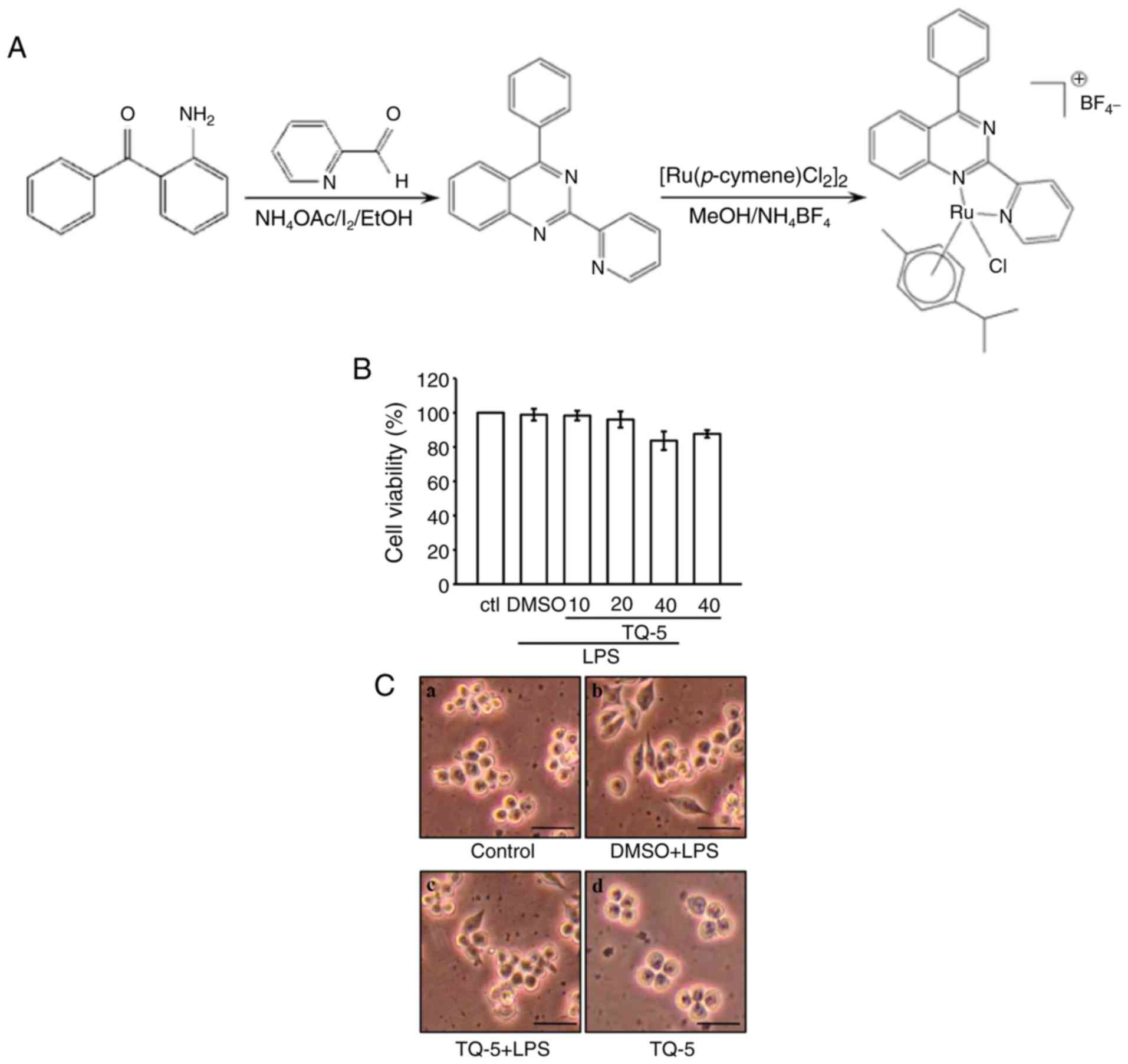
The toxicity of TQ-5 (Fig. 1A) was first examined in RAW 264.7
cells using the MTT assay. At concentrations of 10, 20 and 40
μM, TQ-5 alone or in the presence of LPS did not induce
cytotoxicity in RAW 264.7 cells, as shown in Fig. 1B. This observation was further
confirmed by examining the cell morphology, and the results
revealed that normal macrophage cells exhibited a round morphology
(Fig. 1C-a); by contrast, the
LPS-stimulated cells exhibited an uneven morphology with
pseudopodia formation and cell spreading (Fig. 1C-b). This modification was
condensed by TQ-5 pretreatment, as shown in Fig. 1C-c. Additionally, TQ-5 at 40
μM did not prominently affect the normal round morphology of
the LPS-stimulated cells, which exhibited a morphology identical to
that of the unstimulated cells (Fig.
1C-d). This finding indicates that the effects of TQ-5 on RAW
264.7 cells are flexible and not cytotoxic.
Akt, but not MAPK, pathways are involved
in mediating the effects of TQ-5 on reducing LPS-induced
inflammation in RAW 264.7 macrophages
Previous studies have demonstrated that the MAPK and
Akt signaling pathways are associated with LPS-induced inflammation
in macrophages (22,23). Therefore, the potential
involvement of these pathways in the TQ-5-mediated alleviation of
LPS-induced inflammatory events was examined in the present study.
The results indicated that LPS treatment significantly promoted the
phosphorylation of JNK, p38 MAPK and ERK, in addition to Akt
(Fig. 2A-D). However, the
LPS-induced phosphorylation of MAPK was not completely abrogated by
pretreatment with TQ-5; this metal complex inhibited the
phosphorylation of Akt in a concentration-dependent manner.
Therefore, TQ-5 suppressed the inflammatory responses by
inactivating only Akt signaling pathways and not MAPK signaling
pathways in LPS-stimulated RAW 264.7 macrophages.
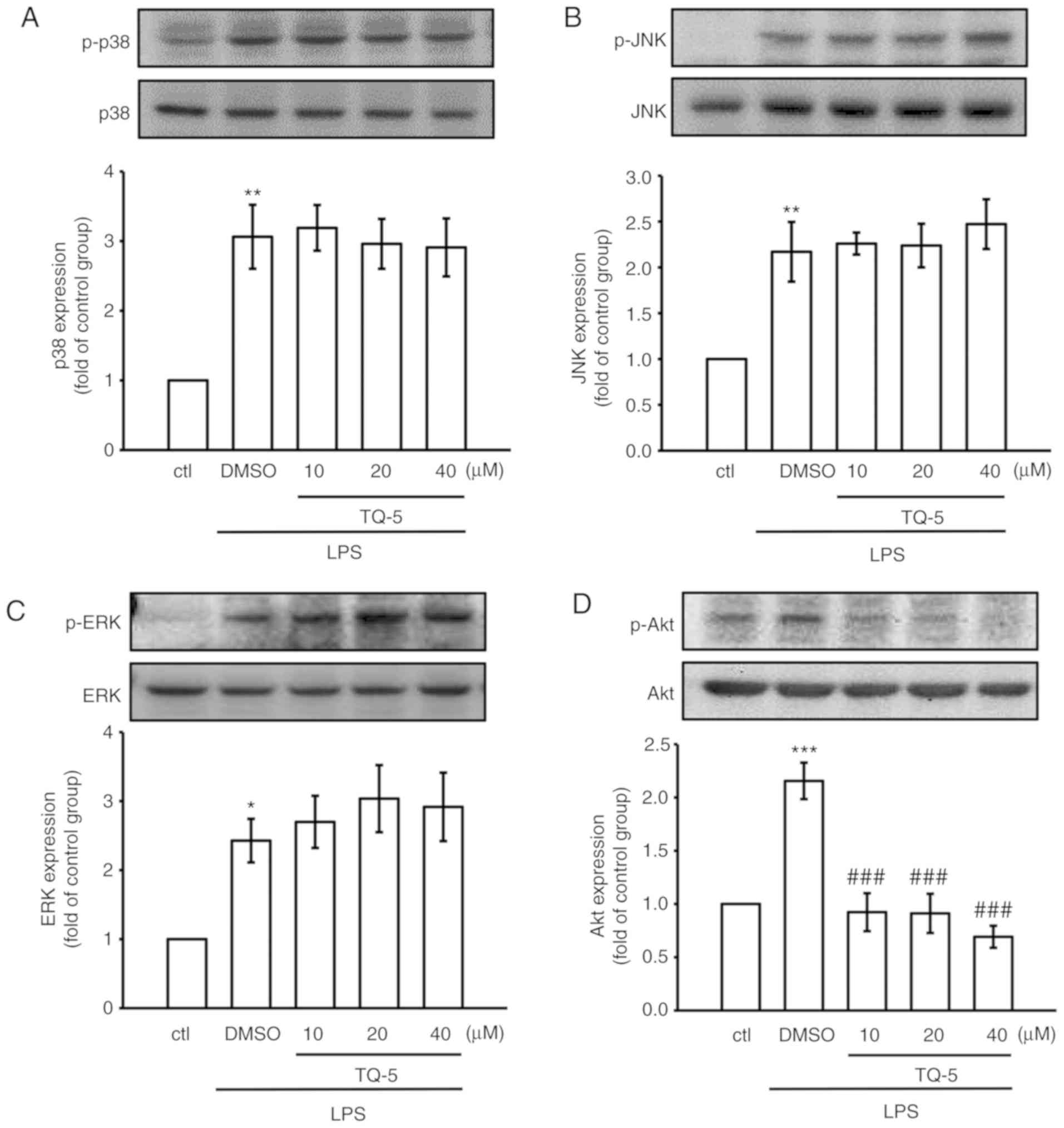 | Figure 2Effect of TQ-5 on LPS-induced MAPK
and Akt signaling pathways in RAW cells. RAW cells were treated
with 0.1% DMSO or various concentrations of TQ-5 (10, 20 and 40
μM) for 20 min, followed by LPS (1 μg/ml) for 30 min.
The expression of phosphorylated (A) p38 MAPK, (B) JNK, (C) ERK and
(D) Akt were detected by immunoblotting. Data are expressed as the
mean ± SEM (n=4). *P<0.05, **P<0.01 and
***P<0.001 compared with the ctrl group;
###P<0.001 compared with the LPS group. LPS,
lipopolysaccharide; MAPK, mitogen-activated protein kinase; JNK,
c-Jun N-terminal kinase; ERK, extracellular signal-regulated
kinase; p-, phosphorylated; DMSO, dimethyl sulfoxide; ctl,
control. |
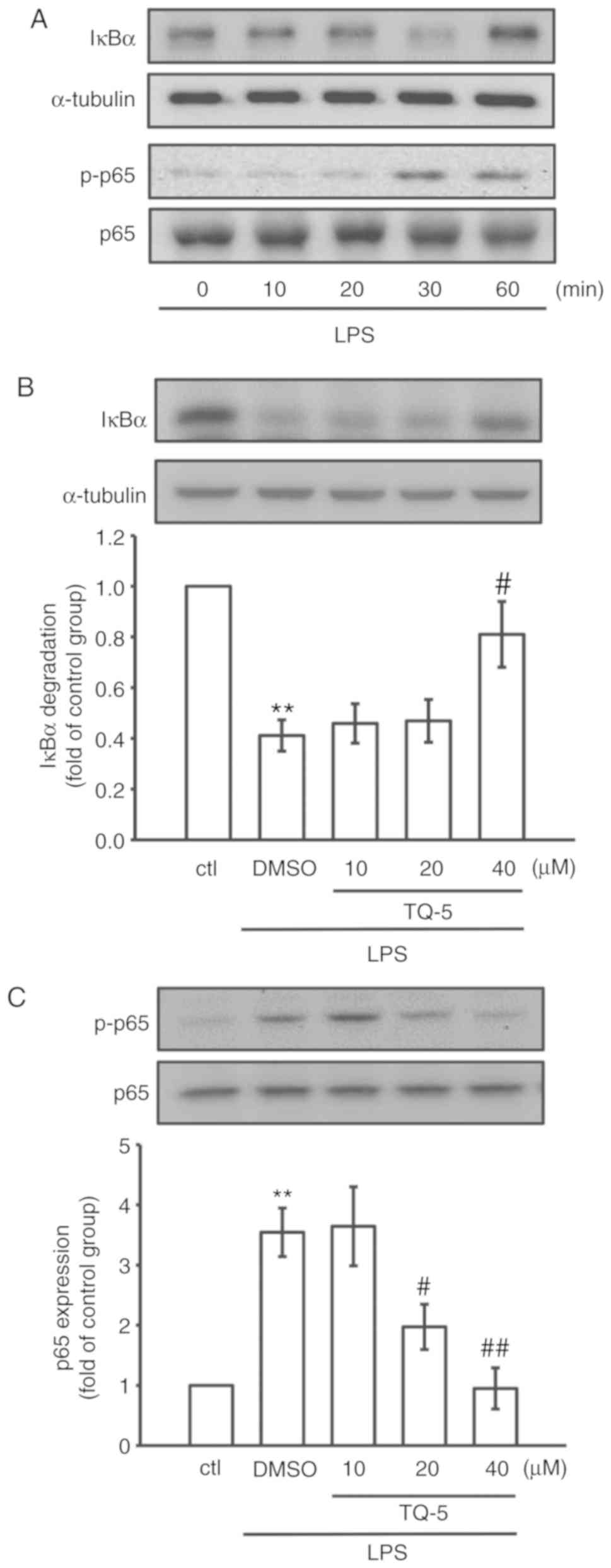
TQ-5 normalizes the LPS-induced
activation of NF-κB in RAW Cells
NF-κB is considered a prerequisite for the
transcription of genes associated with inflammatory processes
(22); therefore, the ability of
TQ-5 to inhibit the activation of NF-κB was investigated in the
present study. As shown in Fig.
3A, LPS evidently promoted the phosphorylation of NF-κB p65
with a concurrent degradation of IκBα after 30 min of exposure;
therefore, this time point was selected for subsequent experiments.
TQ-5 (40 μM) pretreatment markedly restored IκBα degradation
(Fig. 3B) and effectively reduced
the phosphorylation of NF-κBp65 (Fig.
3C) in the LPS-stimulated cells. These observations suggest
that TQ-5 can act as a negative regulator of LPS-stimulated NF-κB
activation in RAW 264.7 cells.
TQ-5 inhibits LPS-induced proinflammatory
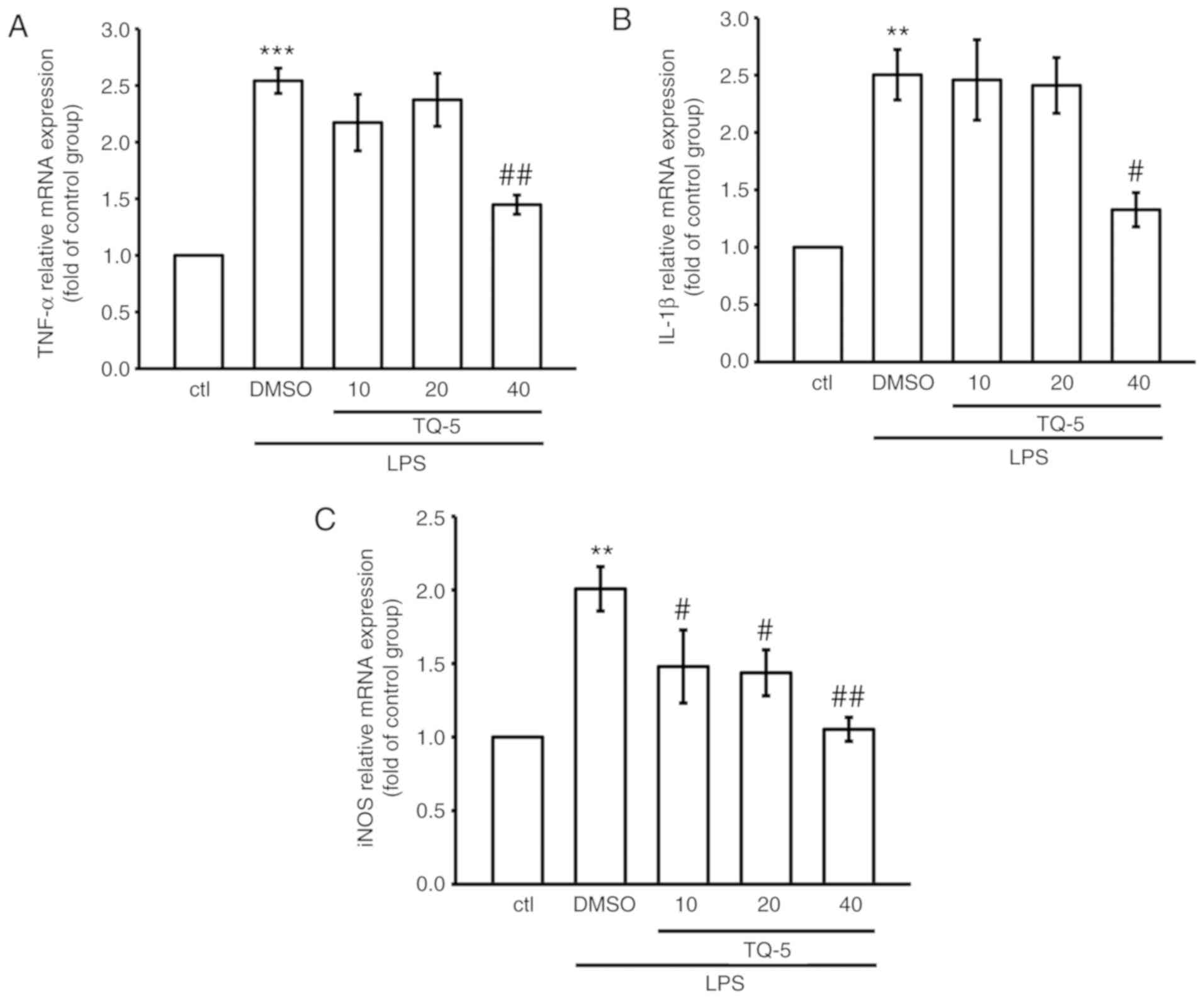
cytokines and mediators in RAW macrophages
To observe the effects of TQ-5 on the LPS-induced
production of typical proinflammatory cytokines (TNF-α and IL-1β)
and mediators (NO and iNOS) in RAW 264.7 cells, the cells were
pretreated with various concentrations of TQ-5 (10, 20 and 40
μM) for 20 min. Subsequently, the cells were stimulated for
24 h with 1 μg/ml LPS. The proinflammatory cytokine (TNF-α
and IL-1β) and mediator (iNOS) levels in the cellular supernatants
were assessed using immunoblotting, and NO was examined using the
Griess reagent. As indicated in Fig.
4A-D, the stimulation of RAW 264.7 cells with LPS alone
significantly increased the expression levels of TNF-α, IL-1β and
iNOS and the production of NO; however, these elevations were
significantly reduced by TQ-5 at a maximum concentration of 40
μM. In addition, TQ-5 (40 μM) suppressed the
LPS-stimulated mRNA expression of TNF-α, IL-1β and iNOS (Fig. 5A-C). These results suggest that
TQ-5 inhibits the production of NO via the downregulation of iNOS
and that the regulation of cytokine production serves a role in the
TQ-5-mediated inhibition of inflammatory events in RAW cells.
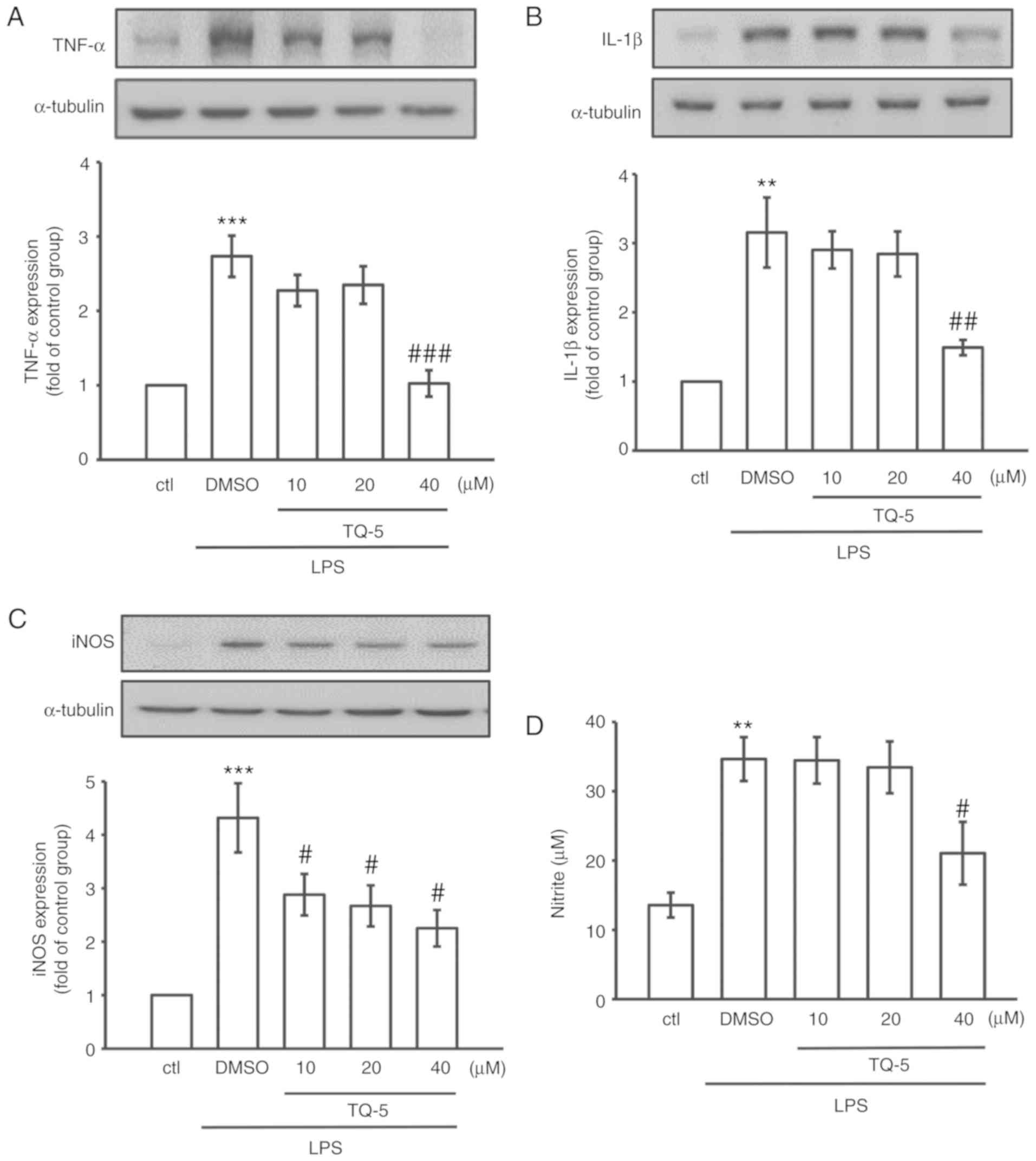 | Figure 4TQ-5 attenuates the LPS-induced
expression of TNF-α, IL-1β and iNOS and production of NO in RAW
cells. Cells were pretreated with TQ-5 (10, 20 and 40 μM)
for 20 min and then stimulated by LPS (1 μg/ml) for 24 h.
The protein levels of (A) TNF-α, (B) IL-1β and (C) iNOS were
evaluated. (D) Cells were pretreated with TQ-5 (10, 20 and 40
μM) for 20 min and then stimulated by LPS (1 μg/ml)
for 24 h. NO was measured using Griess reagent (1% sulfanilamide
and 0.1% naphthalenediamine dissolved in 2.5% phosphoric acid).
Data are presented as the mean ± SEM (n=4); **P<0.01
and ***P<0.001 compared with the ctl group;
#P<0.05, ##P<0.01 and
###P<0.001 compared with the LPS group. LPS,
lipopolysaccharide; TNF-α, tumor necrosis factor-α; IL-1β,
interleukin-1β; iNOS, inducible nitric oxide synthase; NO, nitric
oxide; DMSO, dimethyl sulfoxide; ctl, control. |
TQ-5 attenuates acute liver injury in
LPS-challenged mice
LPS-induced acute liver injury in mice is an
extensively used model for investigating the mechanism of
hepatoprotective and anti-inflammatory agents (24). To further establish the in
vitro results of the effects of TQ-5 in inflammatory lesions,
LPS was used to develop liver injury mouse models. Serum ALT and
AST levels are vital indicators of liver dysfunction. Therefore,
the levels of these enzymes were observed following LPS stimulation
in the absence or presence of TQ-5. The body weight and survival
rate was observed in mice of the treatment groups, as shown in
Table SI. As shown in Fig. 6A and B, the intraperitoneal
injection of 2.5 mg/kg LPS significantly increased serum ALT and
AST levels, whereas TQ-5 treatment reduced these levels. LPS
stimulated the expression of TNF-α, IL-1β, iNOS and p65 in the
mouse liver (Fig. 6C-F).
Consistent with the in vitro interpretations, downregulated
phosphorylation of p65 and reduced expression levels of TNF-α,
IL-1β and iNOS were confirmed as TQ-5-mediated in vivo
protective effects. These results suggested that TQ-5 provided
protection against liver injury by inhibiting inflammatory
processes.
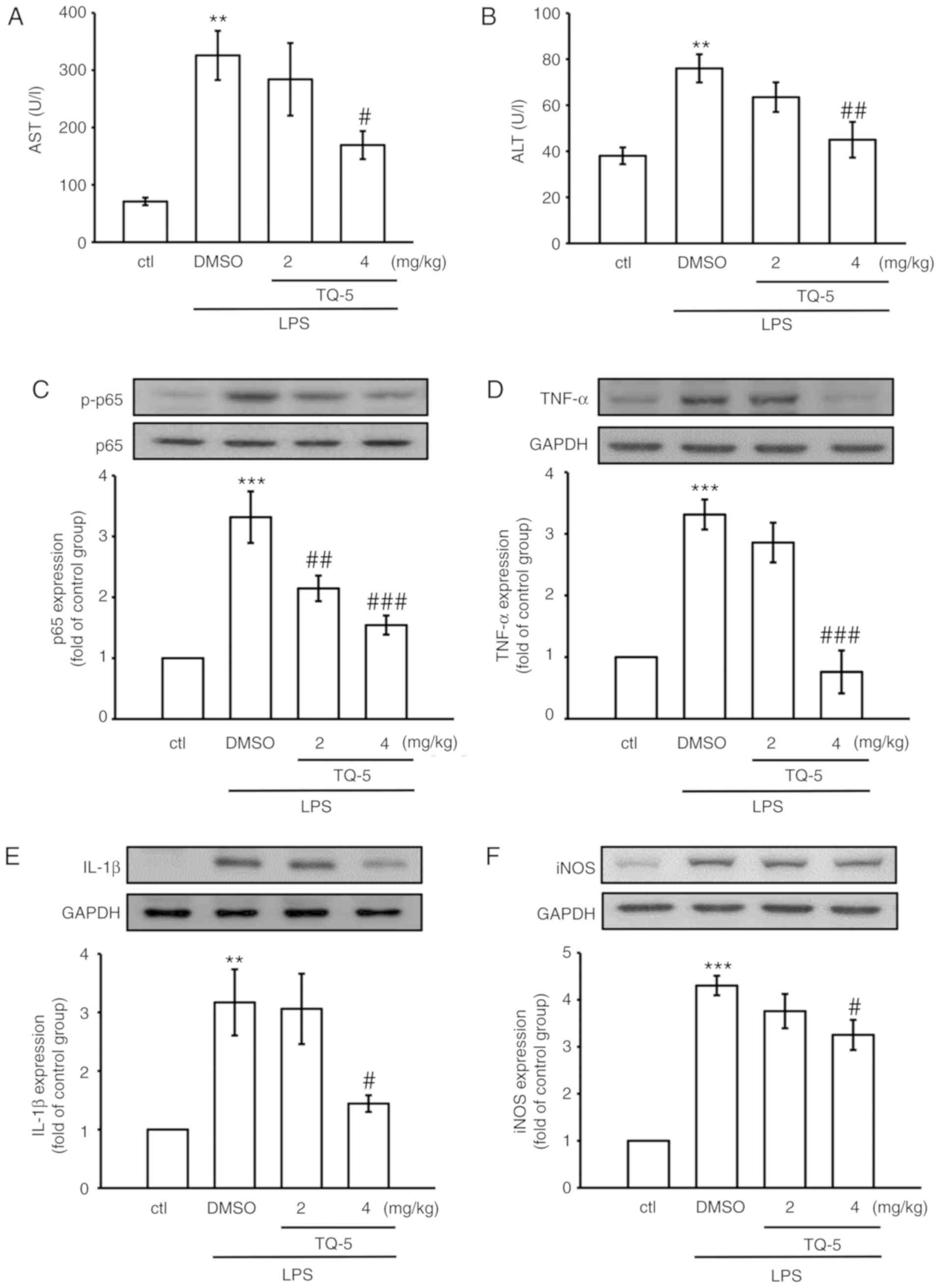 | Figure 6TQ-5 protects against LPS-induced
liver injury in mice via inhibiting the expression of p-p65, TNF-α,
IL-1β and iNOS. Mice were administered with an intraperitoneal
injection of TQ-5 (2 and 4 mg/kg) 2 h prior to administration of
LPS (2.5 mg/kg). After 6 h of LPS challenge, blood and liver
tissues were collected to evaluate serum (A) AST and (B) ALT and
the protein expression of (C) p-p65, (D) TNF-α, (E) IL-1β and (F)
iNOS. Data are presented as the mean ± SEM (n=6);
**P<0.01 and ***P<0.001 compared with
the ctl group; #P<0.05, ##P<0.01 and
###P<0.001 compared with the LPS group. LPS,
lipopolysaccharide; AST, aspartate aminotransferase; ALT, alanine
aminotransferase; TNF-α, tumor necrosis factor-α; IL-1β,
interleukin-1β; iNOS, inducible nitric oxide synthase; p-,
phosphorylated; DMSO, dimethyl sulfoxide; ctl, control. |
Discussion
The results of the present study demonstrated that
TQ-5, a newly synthesized ruthenium metal complex, exhibited active
anti-inflammatory property via impeding the LPS-induced
inflammatory mediators (NO and iNOS), proinflammatory cytokines
(TNF-α and IL-1β), Akt, phosphorylation of NF-κBp65 and degradation
of IκBα in RAW 264.7 macrophages. In addition, this novel metal
complex protected against liver injury in mice through suppressing
the phosphorylation of p65 and consequently inhibited the
expression of TNF-α, IL-1β and iNOS. These in vivo results
are consistent with in vitro data and suggested that TQ-5
protected against liver injury by inhibiting the inflammatory
processes induced by LPS. These data demonstrated that TQ-5
exhibited potent anti-inflammatory activity by mediating inhibition
of the Akt/NF-κB signaling pathways; therefore, this metal complex
may serve as a promising lead for the development of
anti-inflammatory agents to treat acute liver failure. The RAW
264.7 cell line is one of the most commonly used cell lines for
investigating inflammatory reactions as LPS can stimulate these
cells and elicit the production of inflammatory mediators,
including TNF-α, IL-6 and iNOS (25). In addition, the RAW264.7 cell line
is exclusively competent for determining infection-related
proinflammatory mediators (26).
Initially, MTT assays were performed to measure the viability of
RAW 264.7 cells and confirm that any anti-inflammatory effects of
TQ-5 were not ascribed to decreasing RAW 264.7 cell viability
(Fig. 1B). Based on these
results, the concentrations of TQ-5 showing no cytotoxicity were
selected for subsequent experiments.
LPS triggered macrophages and subsequently produced
proinflammatory mediators, including NO and iNOS, in addition to
numerous cytokines, including TNF-α and IL-6 (27). Capillarisin, a natural flavonoid,
reportedly inhibits the expression of iNOS in LPS-induced RAW 264.7
macrophages (28). Other studies
have demonstrated that eight 2-phenylnaphtha-lenes (PNAP-1-8)
inhibited the increment of iNOS expression and NO production in
LPS-induced RAW 264.7 cells and suggested that the development of
inflammation involves TNF-α (29). Hämäläinen et al (30) reported that kaempferol, a natural
flavonol, inhibits the LPS-induced production of NO and expression
of iNOS in activated macrophages. More appropriately, studies have
reported that rhodium (III) complex inhibits the production of NO
(31), and gold (I) complex
expressively reduces the production of TNF-α and IL-1β in
LPS-activated macrophages (32).
Similarly, the results of the present study showed that ruthenium
complex TQ-5 reversed the LPS-induced elevation of TNF-α, IL-1β, NO
and iNOS in RAW 264.7 cells. Therefore, these findings suggest that
TQ-5 can inhibit inflammatory events by reducing the expression of
iNOS, TNF-α and IL-1β and production of NO.
The NF-κB family serves vital roles in inflammation,
immunity and survival. Upon LPS stimulation, IKK phosphorylates
IκBα, leading to the nuclear translocation of NF-κB (33). The suppression of NF-κB activation
by the flavonoid compounds capillarisin and genistein reportedly
inhibits proinflammatory mediators iNOS, COX-II, TNF-α and IL-6 in
LPS-stimulated RAW264.7 cells (34). In addition, one study demonstrated
that pretreatment with phenylnaphthalenes PNAP-6 and PNAP-8 caused
higher cytosolic levels of IκBα and NF-κBp65 in LPS-stimulated
cells, thereby suppressing the translocation of NF-κBp65 to the
nucleus (29). The copper complex
(Cu2+) was reported to inhibit the activation of NF-κB
by preventing IκBα degradation in Jurkat T cells (35). Zinc and copper complexes have also
been found to inhibit the activation of NF-κB in LPS-stimulated RAW
264.7 cells (36). In the present
study, ruthenium complex TQ-5 inhibited the degradation of IκBα and
phosphorylation of NF-κBp65 in LPS-induced RAW 264.7 macrophages.
This finding suggested that TQ-5 inhibited LPS-induced
proinflammatory mediators by regulating NF-κB signaling
cascades.
In addition to NF-κB, MAPKs are induced by numerous
extracellular stimuli, resulting in the downstream phosphorylation
of crucial signaling molecules associated with inflammation
(37). The MAPK family serves an
essential role in LPS-induced inflammatory cytokine production in
several cell types (38). In
cells activated by LPS, the phosphorylation of MAPK is involved in
activating the transcription factors of NF-κB and subsequently
induces cytokine production (39). Another report describes that
proinflammatory mediators are controlled by the downregulation of
MAPK and NF-κB in LPS-treated RAW 264.7 cells (40). By contrast, the present study
showed that pretreatment of RAW 264.7 cells with TQ-5 did not
reduce the LPS-induced phosphorylation of p38 MAPK, JNK or ERK.
However, the LPS-stimulated phosphorylation of Akt was
concentration-dependently inhibited by TQ-5 in RAW 264.7 cells.
Consistent with this result, a previous study demonstrated that
myricetin, a natural flavonol, exhibits anti-inflammatory effects
through inhibition of the Akt signaling pathway in LPS-induced RAW
cells (41). This indicates that
Akt pathways, but not MAPK-dependent pathways, are involved in the
anti-inflammatory effect of TQ-5 on LPS-induced RAW cells.
The mouse LPS-induced acute liver injury model is
one of the most frequently used research models. In the
inflammatory event, LPS stimulates Kupffer cells to release TNF-α,
IL-1β and IL-6; activates the NF-κB pathway; and successively
increases the production of iNOS. These cellular signaling pathways
serves vital roles in acute liver injury and inflammation (42,43). LPS also stimulates TNF-α, IL-6,
and IL-1β in a model of ischemia-reperfusion liver injury (44,45). Therefore, it is hypothesized that
the interference of LPS-induced inflammatory reactions may be
advantageous for attenuating inflammation-associated liver
disorders. In the present study, serum ALT and AST levels were
measured to examine the effect of TQ-5 on liver injury. ALT is a
specific marker for hepatic parenchymal injury, whereas AST is a
nonspecific marker for hepatic injury. The results showed that TQ-5
ameliorated liver damage, as evidenced by the reduced levels of
serum ALT and AST. The expression of hepatic NF-κB, TNF-α, IL-1β
and iNOS were assessed to further identify the mechanism underlying
the anti-inflammatory and hepatoprotective effects of TQ-5. TQ-5
significantly inhibited the LPS-induced hepatic expression of p-p65
and consequently reduced the expression of TNF-α, IL-1β and iNOS in
the mouse liver. Together, these results suggest that TQ-5 impedes
LPS-induced liver damage through the suppression of NF-κB,
proinflammatory cytokines and mediators.
In conclusion, the anti-inflammatory and
hepatoprotective effects of a newly synthesized novel ruthenium
complex, TQ-5, were evaluated using LPS-stimulated RAW 264.7 cells
and a mouse liver injury model, respectively. TQ-5 suppressed the
LPS-induced production of NO, TNF-α, IL-1β and iNOS by inhibiting
signaling molecules Akt and NF-κB in RAW 264.7 cells without
inducing cytotoxicity. This novel metal complex exhibited defensive
effects against LPS-induced liver injury in vivo. These
findings suggest that TQ-5 may be a potential drug candidate for
the development of anti-inflammatory agents to treat acute liver
failure.
Supplementary Data
Funding
This study was supported by grants from the Ministry
of Science and Technology of Taiwan (grant nos. MOST
107-2314-B-195-002 and MOST 107-2221-E-195-001), the University
Grants Commission, India (grant nos. MRP-MAJOR-CHEM-2013-5144;
69/2014 F. No. 10-11/12UGC), Cathay General Hospital (grant no.
CGH-MR-A10715) and MacKay Memorial Hospital (grant no.
MMH108-17).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TJ, KHL and YCL designed the study and wrote the
manuscript. MV contributed to the chemical synthesis and analysis.
SMH, CHH, CWH and CCC performed the experiments and analyzed the
data. All authors contributed clarifications and guidance on the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments and care procedures conformed
to the Guide for the Care and Use of Laboratory Animals
(LAC-2016-0395) and were approved by the Institutional
Animal Care and Use Committee of Taipei Medical
University (Taipei, Taiwan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Denker SP, Ji S, Dingman A, Lee SY,
Derugin N, Wendland MF and Vexler ZS: Macrophages are comprised of
resident brain microglia not infiltrating peripheral monocytes
acutely after neonatal stroke. J Neurochem. 100:893–904. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kimbell B and Murray SA: What is the
patient experience in advanced liver disease? A scoping review of
the literature. BMJ Support Palliat Care. 5:471–480. 2015.
View Article : Google Scholar
|
|
3
|
Newland CD: Acute liver failure. Pediatr
Ann. 45:e433–e438. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuroda N, Inoue K, Ikeda T, Hara Y, Wake K
and Sato T: Apoptotic response through a high mobility box 1
protein-dependent mechanism in LPS/GalN-induced mouse liver failure
and glycyrrhizin-mediated inhibition. PLoS One. 9:e928842014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moratalla A, Caparrós E, Juanola O,
Portune K, Puig-Kröger A, Estrada-Capetillo L, Bellot P,
Gómez-Hurtado I, Piñero P, Zapater P, et al: Bifidobacterium
pseudocatenulatum CECT7765 induces an M2 anti-inflammatory
transition in macrophages from patients with cirrhosis. J Hepatol.
64:135–145. 2016. View Article : Google Scholar
|
|
6
|
Jeong YH, Oh YC, Cho WK, Shin H, Lee KY
and Ma JY: Anti-inflammatory effects of Viola yedoensis and the
application of cell extraction methods for investigating bioactive
constituents in macrophages. BMC Complement Altern Med. 16:1802016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dumitru CD, Ceci JD, Tsatsanis C,
Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA,
Copeland NG, Kollias G and Tsichlis PN: TNF-alpha induction by LPS
is regulated posttranscriptionally via a Tpl2/ERK-dependent
pathway. Cell. 103:1071–1083. 2000. View Article : Google Scholar
|
|
8
|
Ho FM, Lai CC, Huang LJ, Kuo TC, Chao CM
and Lin WW: The anti-inflammatory carbazole, LCY-2-CHO, inhibits
lipopolysaccharide-induced inflammatory mediator expression through
inhibition of the p38 mitogen-activated protein kinase signaling
pathway in macrophages. Br J Pharmacol. 141:1037–1047. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klintman D, Li X, Santen S, Schramm R,
Jeppsson B and Thorlacius H: p38 mitogen-activated protein
kinase-dependent chemokine production, leukocyte recruitment, and
hepatocellular apoptosis in endotoxemic liver injury. Ann Surg.
242:830–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bennett BL, Sasaki DT, Murray BW, O'Leary
EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Peirce S, Satoh Y, et
al: SP600125, an anthrapyrazolone inhibitor of Jun N-terminal
kinase. Proc Natl Acad Sci USA. 98:13681–13686. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
DiDonato JA, Mercurio F and Karin M:
Phosphorylation of I kappa B alpha precedes but is not sufficient
for its dissociation from NF-kappa B. Mol Cell Biol. 15:1302–1311.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tak PP and Firestein GS: NF-kappaB: A key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guha M and Mackman N: The
phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide
activation of signaling pathways and expression of inflammatory
mediators in human monocytic cells. J Biol Chem. 277:32124–32132.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Basri AM, Lord RM, Allison SJ,
Rodríguez-Bárzano A, Lucas SJ, Janeway FD, Shepherd HJ, Pask CM,
Philips RM and McGowan PC: Bis-picolinamide Ruthenium (III)
dihalide complexes: Dichloride-to-diiodide exchange generates
single trans isomers with high potency and cancer cell selectivity.
Chemistry. 23:6341–6356. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saeed HK, Saeed IQ, Buurma NJ and Thomas
JA: The structure of linkers affects the DNA binding properties of
tethered dinuclear Ruthenium (II) metallo-intercalators. Chemistry.
23:5467–5477. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Faller P and Hureau C: A bioinorganic view
of Alzheimer's disease: When misplaced metal ions (re)direct the
electrons to the wrong target. Chemistry. 18:15910–15920. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hsia CH, Velusamy M, Sheu JR, Khamrang T,
Jayakumar T, Lu WJ, Lin KH and Chang CC: A novel ruthenium
(II)-derived organometallic compound, TQ-6, potently inhibits
platelet aggregation: Ex vivo and in vivo studies. Sci Rep.
7:95562017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jayakumar T, Hsu CY, Khamrang T, Hsia CH,
Hsia CW, Manubolu M and Sheu JR: Possible molecular targets of
novel ruthenium complexes in antiplatelet therapy. Int J Mol Sci.
19:E18182018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang BP, Lin CH, Chen HM, Lin JT, Cheng
YF and Kao SH: AMPK activation inhibits expression of
proinflammatory mediators through downregulation of PI3K/p38 MAPK
and NF-κB signaling in murine macrophages. DNA Cell Biol.
34:133–141. 2015. View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Jung JS, Choi MJ, Lee YY, Moon BI, Park JS
and Kim HS: Suppression of lipopolysaccharide-induced
neuroinflammation by morin via MAPK, PI3K/Akt, and PKA/HO-1
signaling pathway modulation. J Agric Food Chem. 65:373–382. 2017.
View Article : Google Scholar
|
|
23
|
Jing Y, Ai Q, Lin L, Dai J, Jia M, Zhou D,
Che Q, Wan J, Jiang R and Zhang L: Protective effects of garcinol
in mice with lipopolysaccharide/D-galactosamine-induced apoptotic
liver injury. Int Immunopharmacol. 19:373–380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fan GW, Zhang Y, Jiang X, Zhu Y, Wang B,
Su L, Cao W, Zhang H and Gao X: Anti-inflammatory activity of
baicalein in LPS-stimulated RAW264.7 macrophages via estrogen
receptor and NF-κB-dependent pathways. Inflammation. 36:1584–1591.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hartley JW, Evans LH, Green KY, Naghashfar
Z, Macias AR, Zerfas PM and Ward JM: Expression of infectious
murine leukemia viruses by RAW264.7 cells, a potential complication
for studies with a widely used mouse macrophage cell line.
Retrovirology. 5:12008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peng XX, Zhang SH, Wang XL, Ye TJ, Li H,
Yan XF, Wei L, Wu ZP, Hu J, Zou CP, et al: Panax Notoginseng flower
saponins (PNFS) inhibit LPS-stimulated NO overproduction and iNOS
gene overexpression via the suppression of TLR4-mediated
MAPK/NF-kappa B signaling pathways in RAW264.7 macrophages. Chin
Med. 10:152015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan MH, Chiou YS, Tsai ML and Ho CT:
Anti-inflammatory activity of traditional Chinese medicinal herbs.
J Tradit Complement Med. 1:8–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han S, Lee JH, Kim C, Nam D, Chung WS, Lee
SG and Ahn KS, Cho SK, Cho M and Ahn KS: Capillarisin inhibits
iNOS, COX-2 expression, and proinflammatory cytokines in
LPS-induced RAW 264.7 macrophages via the suppression of ERK, JNK,
and NF-κB activation. Immunopharmacol Immunotoxicol. 35:34–42.
2013. View Article : Google Scholar
|
|
29
|
Chang CF, Liao KC and Chen CH:
2-Phenylnaphthalene derivatives inhibit lipopolysaccharide-induced
pro-inflammatory mediators by downregulating of MAPK/NF-κB pathways
in RAW 264.7 macrophage cells. PLoS One. 12:e01689452017.
View Article : Google Scholar
|
|
30
|
Hämäläinen M, Nieminen R, Vuorela P,
Heinonen M and Moilanen E: Anti-inflammatory effects of flavonoids:
Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and
NF-kappaB activations, whereas flavone, isorhamnetin, naringenin,
and pelargonidin inhibit only NF-kappaB activation along with their
inhibitory effect on iNOS expression and NO production in activated
macrophages. Mediators Inflamm. 2007:456732007. View Article : Google Scholar
|
|
31
|
Liu LJ, Lin S, Chan DS, Vong CT, Hoi PM,
Wong CY, Ma DL and Leung CH: A rhodium (III) complex inhibits
LPS-induced nitric oxide production and angiogenic activity in
cellulo. J Inorg Biochem. 140:23–28. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Leung CH, Lin S, Zhong HJ and Ma DL: Metal
complexes as potential modulators of inflammatory and autoimmune
responses. Chem Sci. 6:871–884. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chang CF, Chau YP, Kung HN and Lu KS: The
lipopolysaccharide-induced pro-inflammatory response in RAW264.7
cells is attenuated by an unsaturated fatty acid-bovine serum
albumin complex and enhanced by a saturated fatty acid-bovine serum
albumin complex. Inflamm Res. 61:151–160. 2012. View Article : Google Scholar
|
|
34
|
Ji G, Zhang Y, Yang Q, Cheng S, Hao J,
Zhao X and Jiang Z: Genistein suppresses LPS-induced inflammatory
response through inhibiting NF-κB following AMP kinase activation
in RAW 264.7 macrophages. PLoS One. 7:e531012012. View Article : Google Scholar
|
|
35
|
Satake H, Suzuki K, Aoki T, Otsuka M,
Sugiura Y, Yamamoto T and Inoue J: Cupric ion blocks NF-kappa B
activation through inhibiting the signal-induced phosphorylation of
I kappa B alpha. Biochem Biophys Res Commun. 216:568–573. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jeon KI, Jeong JY and Jue DM:
Thiol-reactive metal compounds inhibit NF-kappa B activation by
blocking I kappa B kinase. J Immunol. 164:5981–5989. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kaminska B: MAPK signalling pathways as
molecular targets for anti-inflammatory therapy-from molecular
mechanisms to therapeutic benefits. Biochim Biophys Acta.
1754:253–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zong Y, Sun L, Liu B, Deng YS, Zhan D,
Chen YL, He Y, Liu J, Zhang ZJ, Sun J and Lu D: Resveratrol
inhibits LPS-induced MAPKs activation via activation of the
phosphatidylinositol 3-kinase pathway in murine RAW 264.7
macrophage cells. PLoS One. 7:e441072012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Z, Jiang W, Zhang Z, Qian M and Du B:
Nitidine chloride inhibits LPS-induced inflammatory cytokines
production via MAPK and NF-kappaB pathway in RAW 264.7 cells. J
Ethnopharmacol. 144:145–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Intayoung P, Limtrakul P and Yodkeeree S:
Antiinflammatory activities of crebanine by inhibition of NF κB and
AP-1 activation through suppressing MAPKs and Akt signaling in
LPS-induced RAW264.7 macrophages. Biol Pharm Bull. 39:54–61. 2016.
View Article : Google Scholar
|
|
41
|
Hou W, Hu S, Su Z, Wang Q, Meng G, Guo T,
Zhang J and Ga P: Myricetin attenuates LPS-induced inflammation in
RAW 264.7 macrophages and mouse models. Future Med Chem.
10:2253–2264. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Karin M: Nuclear factor-kappa B in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen F, Castranova V, Shi X and Demers LM:
New insights into the role of nuclear factor-kappaB, a ubiquitous
transcription factor in the initiation of diseases. Clin Chem.
45:7–17. 1999.PubMed/NCBI
|
|
44
|
Colletti LM and Green M: Lung and liver
injury following hepatic ischemia/reperfusion in the rat is
increased by exogenous lipopolysaccharide which also increases
hepatic TNF production in vivo and in vitro. Shock. 16:312–319.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sheu JR, Chen ZC, Hsu MJ, Wang SH, Jung
KW, Wu WF, Pan SH, Teng RD, Yang CH and Hsieh CY: CME-1, a novel
polysaccharide, suppresses iNOS expression in
lipopolysaccharide-stimulated macrophages through
ceramide-initiated protein phosphatase 2A activation. J Cell Mol
Med. 22:999–1013. 2018.
|