Introduction
Mesenchymal stem cell (MSC)-based therapies for
obesity, acute myocardial infarction (MI) and graft-versus-host
disease (GvHD) have been proven to be highly efficient (1–3),
emerging as promising approaches to regenerative medicine.
Extensive evidence suggests that transplantation of MSCs is
characterized by hypoimmunogenicity (4) and multipotent differentiation
potential, and is in accordance with ethical guidelines. However,
the poor retention and survival of the transplanted MSCs limit
their therapeutic efficacy in clinical trials. Previous studies
have verified that hypoxic preconditioning (HPC) promotes the
survival of MSCs after transplantation (5,6).
Furthermore, leptin has been confirmed to play a vital role in
HPC-elicited resistance of MSCs to apoptosis (7).
Energy production by glycolysis is crucial for cell
fate, particularly in the absence of oxygen. The interesting aspect
of MSCs is that they preferentially use glycolysis in an
undifferentiated or anti-apoptotic state, whereas they rely on
mitochondrial oxidative phosphorylation (OXPHOS) during their
proliferative phase (6). Leptin,
a peptidase secreted by adipocytes, is closely associated with the
peripheral signaling pathway of glucose homeostasis (8). A recent study demonstrated that
leptin increased mitochondrial fusion through the glycogen synthase
kinase (GSK)3/OMA1/optic atrophy (OPA)1 signaling pathway to
prevent apoptosis of MSCs in a hostile microenvironment (9).
Mitochondria are highly dynamic organelles that can
move and change their morphology through fission and fusion.
Sustainable dynamic homeostasis of fusion and fission is
responsible for maintaining mitochondrial respiration, morphology
and content. OPA1 is a mitochondrial inner membrane protein that
has at least five isoforms, which mediate fusion and fission in the
inner mitochondrial membrane; the long OPA1 isoforms (L-OPA1) are
associated with fusion, whereas the short OPA1 isoforms (S-OPA1)
are associated with fission (10–12). Emerging evidence indicates the
potential of regulating mitochondrial dynamics in metabolic
alterations (13). In
hypothalamic pro-opiomelanocortin neurons, mitochondrial
fragmentation suppresses the response to glucose in a fed state
(14). In activated effector T
cells, mitochondrial fusion increases glycolysis to promote
cellular immunotherapy against cancer cells (15). However, there is no research
directly indicating whether OPA1-induced stimulation of glycolysis
is involved in the rescue of MSCs following leptin treatment. The
aim of the present study was to determine whether leptin targeting
OPA1 increases glycolysis in MSCs to improve their survival rate in
response to hypoxia, and to investigate the underlying
mechanism.
Materials and methods
Cell isolation, culture and hypoxic
injury
Human bone marrow was collected from patients
undergoing hip replacement surgery after obtaining their informed
consent. The study protocol was approved by the Human Ethics
Committee of Guizhou Provincial People's Hospital (Guiyang, China).
MSCs were isolated and cultured as previously reported (9).
MSCs were cultured with human recombinant leptin (50
ng/ml; R&D Systems, Inc.) in Dulbecco's modified Eagle's medium
(DMEM; Corning, Inc.) with 10% fetal bovine serum (FBS; Thermo
Fisher Scientific, Inc.) under normoxic conditions for 24 h, and
were then exposed to DMEM without glucose or FBS in a hypoxic
chamber (0.5% O2/5% CO2; BioSpherix, Ltd.)
for an additional 24 h.
Cell siRNA transfection
A siRNA silencing the expression of human OPA1
(siOPA1) and a scramble siRNA (NC) were purchased from GenePharma.
siRNA transfection was coordinated with Lipofectamine RNAi MAX
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Transfection of 50 nM siRNA and the
specific sequences of OPA1 (sense, CCA UGU GGC CCU AUU UAA ATT;
antisense, UUU AAA UAG GGC CAC AUG GTT) were conducted as described
previously (9).
Annexin V/PI staining
The harvested cells (2×105 cells per
sample) were incubated with a mixture of Annexin V and PI for 30
min according to the manufacturer's instructions (Dojindo Molecular
Technologies, Inc.). Apoptotic cells were quantified by flow
cytometry (BD Biosciences).
Oxygen consumption rate (OCR)
The oxygen consumption of the intact cells was
measured as described previously (16,17). Briefly, the harvested cells
(1×106 cells) were resuspended with 1 ml MiR05
[containing 0.5 mM EGTA, 3 mM MgCl2•6H2O, 60
mM potassium lactobionate, 20 mM taurine, 10 mM
KH2PO4, 20 mM HEPES, 110 mM sucrose and 1 g/l
fatty acid-free bovine serum albumin (BSA) at pH 7.1], and were
then transferred into the experimental chamber. Subsequently, using
Oroboros Oxygraph-2k, the OCR was analyzed by titrating various
substrates/inhibitors of the respiratory chain in a stepwise manner
at 30°C (Oroboros Instruments GmbH).
Extracellular acidification rate
(ECAR)
ECAR was measured by FLUOstar Omega (BMG LABTECH
GmbH) according to the manufacturer's instructions. Briefly, MSCs
were plated in a 96-well plate (1×104 cells/well)
overnight. The next day, after replacing with fresh culture media,
10 μl reconstituted pH-Xtra reagent was added into each well
(a well with 10 μl fresh culture media without cells was
used as blank control). Subsequently, 100 μl mineral oil was
promptly added into each well, and fluorescence intensity was
measured by a fluorescence plate reader (380 nm excitation and 615
nm emission).
Western blot analysis
Western blotting was conducted as described
previously (7). Proteins were
extracted from MSCs using RIPA solution (Beyotime Institute of
Biotechnology) with protease inhibitor cocktail (Roche
Diagnostics). Protein concentrations were quantified using BCA
Protein Assay Kit (Thermo Fisher Scientific, Inc.) and separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis (8-10%),
followed by transfer to polyvinylidene difluoride membranes (EMD
Millipore). After blocking with 5% BSA for 1 h at room temperature,
the proteins of interest were incubated with primary antibodies
overnight at 4°C: Cleaved caspase-3 (1:500, Cell Signaling
Technology, Inc.); voltage-dependent anion channel (VDAC) (1:500,
Santa Cruz Biotechnology, Inc.); poly-OPA1 (1:1,000, BD
Biosciences); glucose transporter (GLUT)4 (1:500, Santa Cruz
Biotechnology, Inc.); GLUT1 (1:1,000, Abcam); sodium-glucose
symporter (SGLT)1 (1:1,000, Abcam); β-actin (1:3,000, KangChen
Biotech. Co., Ltd.). On the following day, the membranes were
incubated with anti-rabbit and anti-mouse secondary antibody
(1:5,000, Abcam) for 1 h at room temperature, and then detected
using the Gel Doc EZ Imaging System (Bio-Rad Laboratories, Inc.)
with an ECL kit (Merck KGaA), and analyzed using Image Lab software
(Bio-Rad Laboratories, Inc.).
Reverse transcription–quantitative
polymerase chain reaction (RT–qPCR) analysis
The gene expression levels were measured by
extracting total RNA using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's
instructions. RT-qPCR was performed as previously described
(18). Briefly, the RNA
concentration was measured by a NanoDrop spectrophotometer (Thermo
Fisher Scientific, Inc.). Total RNA (2 μg) was
reverse–transcribed to cDNA using a Reverse Transcription kit
(Takara). RT-qPCR was performed using SYBR Green PCR Master Mix
(Takara). The cycling conditions were as follows: 95°C for 10 min,
followed by 40 cycles of 15 sec at 95°C and 30 sec at 60°C. mRNA
levels were normalized to β-actin and determined using the
2−ΔΔCq method. The gene primers are listed in Table I.
 | Table IHuman primers for quantitative
polymerase chain reaction analysis. |
Table I
Human primers for quantitative
polymerase chain reaction analysis.
| Primer sequences
5′-3′
|
|---|
| Gene | Forward | Reverse |
|---|
| GAPDH |
TCAACGTGTGGTCATCTCCG |
TGGTCATCAACCCTTCCACG |
| HK–1 |
ATGGTGGATGGTGAGCAAGG C |
AGGAACCCTCGTTTGGTGA |
| LDH–α |
GTTCGGAGGACCCAGCAATTA |
GGCTCCTACAGCAAGGACAC |
| PDH–α |
GAGTTTGTGTGGGTTCCTCCA |
GTGGGTCGCTGGAGTAGATG |
| cpt–1α |
ATGACGGCTATGGTGTGTCG |
TTCCAGCCCAGCACATGAAC |
| GLUT1 |
ACAACCAGACATGGGTCCAC |
AGGACTTGCCCAGTTTCGAG |
| GLUT3 |
CGAGACCCAGAGATGCTGTAA |
CTGTGTCCCCATCGCTGTAAT |
| GLUT4 |
GGCTCTACCCCGATGGTTTC |
GTGTCCCTGGTGAAGAGTGC |
| GLUT5 |
GACCAGGTTCCTGAAAGGGT |
AGATACACCCCTTCCTTCATGC |
| GLUT9 |
GCACCAGGCACTGGAATTAAAC |
TCCTCGGTCCTTTTTACTGAGC |
| SGLT1 |
TCCTGCTTGCTATTTTCTGGA |
ATAATCGTGGGACAGTTGCTG |
| β–actin |
CTCGCCTTTGCCGATCC |
GAATCCTTCTGACCCATGCC |
Transmission electron microscopy
(TEM)
Mitochondrial ultrastructure was examined by TEM.
Briefly, the cells were fixed with 2.5% glutaraldehyde for at least
4 h. After washing with phosphate–buffered saline, the cells were
post–fixed with 1% OsO4 for 1-2 h. Then, following
dehydration by the ethanol gradient method, the cells were
infiltrated by acetone overnight. Subsequently, the specimens were
embedded in Spurr resin, heated at 70°C for 9 h and sectioned with
Leica EM UC7 Ultramicrotome (Leica Microsystems GmbH). Uranyl
acetate and alkaline lead citrate were used to stain the sections.
Images were captured randomly using Hitachi H-7650 TEM (Hitachi,
Ltd.).
Periodic acid–Schiff (PAS) staining and
glucose oxidase method
Glycogen content was measured by PAS staining
according to the manufacturer's instructions (SenBeiJia
Biotechnology, Co.). Briefly, the harvested cells were fixed by PAS
fixing liquid for 10 min. After washing and drying, the cells were
incubated in periodic acid solution at room temperature for 15 min
and stained by Schiff reagent for 30 min in the dark. The images
were captured randomly at a magnification of ×200. Glucose content
was detected using the glucose oxidase method according to the
manufacturer's instructions (Applygen Technologies, Inc), and
absorbance was measured at 570 nm by a microplate reader (Bio-Rad
Laboratories, Inc.).
ATP measurement
Cellular ATP content was analyzed by a
luciferin/luciferase-based kit according to the manufacturer's
instructions (Beyotime Institute of Biotechnology).
Lactate measurement
The lactate content was measured using a lactate
assay kit (Jiancheng Biotechnology) according to the manufacturer's
instructions.
Sotagliflozin (LX4211) treatment
assay
MSCs were incubated with leptin (50 ng/ml) under
normal culture conditions for 24 h. Subsequently, these MSCs were
cultured with leptin in the presence or absence of 36 nM
sotagliflozin (LX4211; Selleck Chemicals), an inhibitor of SGLT1,
and subjected to hypoxia and glucose + serum deprivation for an
additional 24 h.
MI mouse model and MSC
transplantation
A MI mouse model was induced by ligation of the left
anterior descending coronary artery in 10-12-week-old male C57BL/6J
mice (20-25 g), as previously described (7). MSCs (1.5×105 cells in 20
μl DMEM per mouse) were transplanted to the border zone of
the infarct.
Sirius Red staining
The mice were euthanized, and the hearts were
harvested and embedded in paraffin on day 28 post–MI. The tissue
samples were cut into 3-μm sections and stained with Sirius
Red (Beijing Solarbio Science & Technology Co., Ltd.), as
described previously (19).
Infarct areas were analyzed according to the sum of the endocardial
and epicardial length of the infarct zone in proportion to the
total length of the endocardial and epicardial left ventricle using
Image-Pro-Plus software (Media Cybernetics, Inc.).
Echocardiography
Echocardiography was performed on day 28 post-MI, as
described previously (20).
Cardiac morphology and function were analyzed via a Vevo 2100
system (VisualSonics, Inc.). Left ventricular ejection fraction was
measured for at least three cardiac cycle intervals.
Statistical analysis
All experiments were performed at least three times.
All data are presented as mean ± standard error of the mean.
Student's t–test was used to determine significance when comparing
two sets of data. One-way analysis of variance followed by Tukey's
post hoc test was used for comparison of more than three sets of
data. All data were analyzed using SPSS 17.0 (SPSS Inc.). The
statistics were presented by GraphPad Prism 5 (GraphPad Software,
Inc.). P<0.05 was considered to indicate statistically
significant differences.
Results
Leptin enhances the survival of MSCs by
preventing apoptosis in vitro
In order to examine the protective effect of leptin
on MSCs in response to the microenvironment after transplantation,
MSCs were incubated with recombinant leptin (MSCs-Lep) for 24 h
under normal culture conditions, and then exposed to hypoxia and
glucose + serum deprivation for an additional 24 h in vitro.
The solvent of leptin, Tris buffer, was used as control
(MSCs-Con).
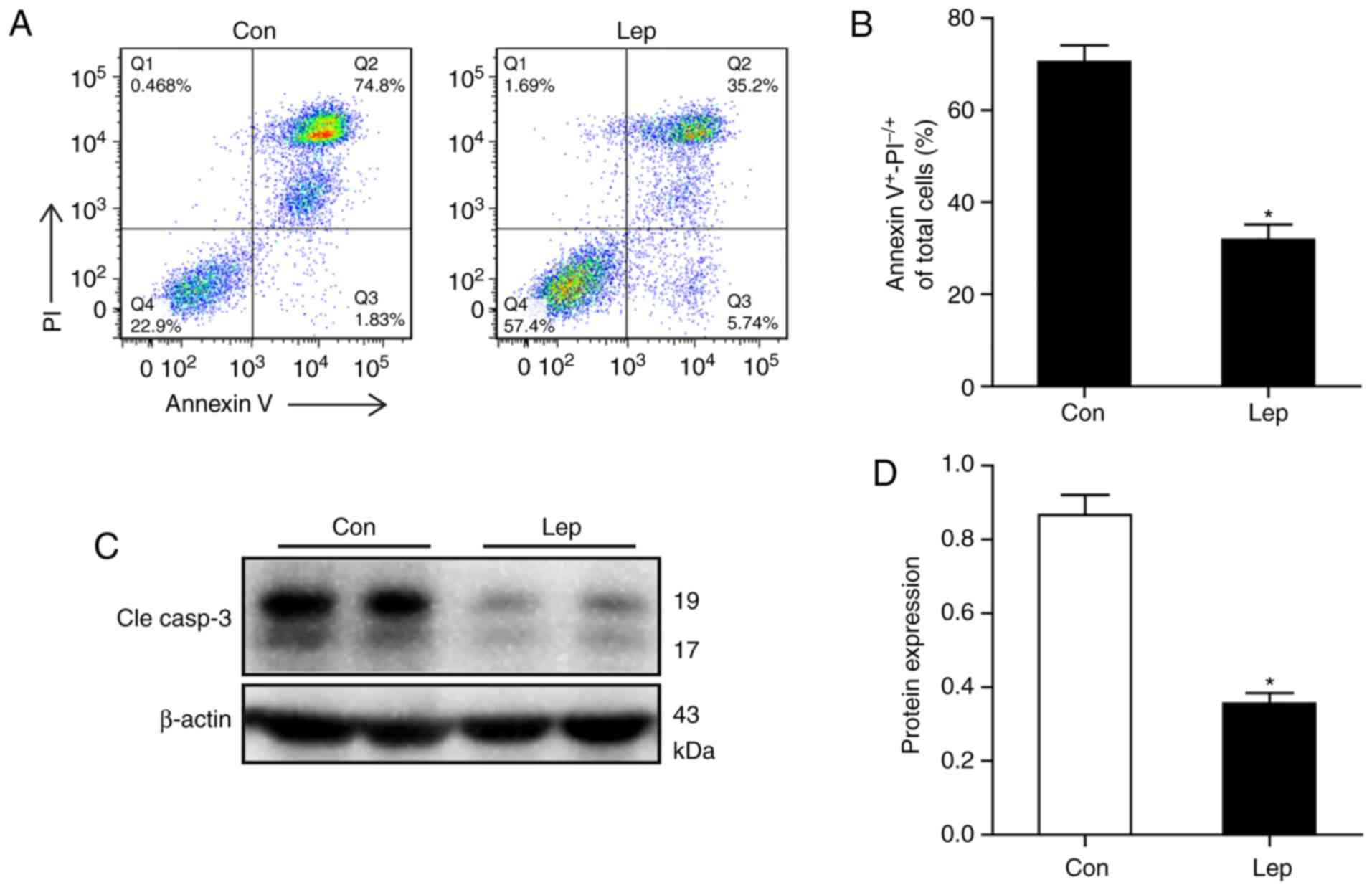
To distinguish the apoptotic cells affected by
hypoxia, the percentage of Annexin V+ and
PI−/+ staining was calculated by flow cytometry.
Decreased apoptotic ratio was detected in MSCs-Lep, while a higher
percentage of Annexin V+ and PI−/+ staining
was detected in MSCs-Con (Fig. 1A and
B). Similarly, lower expression of cleaved caspase-3 was
observed in MSCs-Lep compared with MSCs-Con (Fig. 1C and D). These data suggest that
leptin exerted a cytoprotective effect on MSCs against hypoxic
insults.
Increased mitochondrial fusion is induced
by leptin
Mitochondrial morphology is closely associated with
cell fate; an abundance of pro-apoptotic factors is stored in
mitochondrial cristae, and these pro-apoptotic proteins are
released from the mitochondria into the cytoplasm to activate the
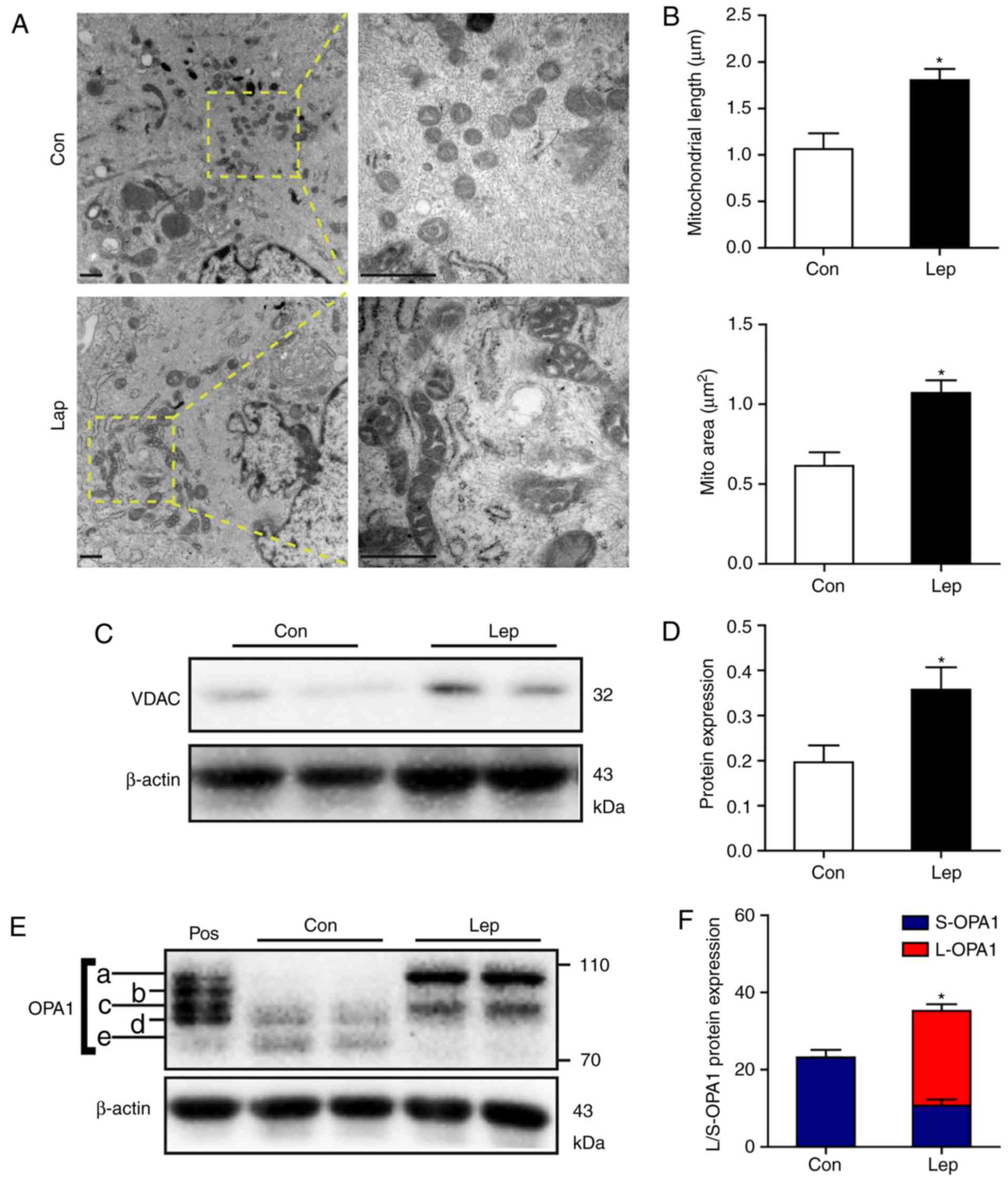
apoptosis signaling pathway (21-23). First, mitochondrial morphology was
evaluated by TEM. Elongated tubular mitochondria were prominent in
MSCs-Lep under hypoxic stress, while the mitochondria observed in
MSCs-Con were fewer and sparsely distributed (Fig. 2A). In contrast to MSCs-Con,
increased mitochondrial length and area were observed in MSCs-Lep
(Fig. 2B). Moreover, the
increased protein level of VDAC indicated that the mitochondrial
content was higher in MSCs-Lep compared with MSCs-Con (Fig. 2C and D).
The different isoforms of OPA1 modulate
mitochondrial inner membrane dynamics, among which L-OPA1 (isoforms
a-b, ~85-100 kDa) are implicated in fusion, whereas S-OPA1
(isoforms c-e, ~75-85 kDa) are associated with fission (12,24). It was previously reported that
leptin enhanced the accumulation of L-OPA1 via OMA1 ubiquitination,
thereby inducing mitochondrial fusion, to prevent apoptosis of MSCs
(9). Similarly, in the present
study, significant accumulation of L-OPA1 was detected in MSCs-Lep,
with higher protein expression of S-OPA1 in MSCs-Con (Fig. 2E and F), indicating that leptin
promotes mitochondrial fusion through accumulation of L-OPA1.
Leptin promotes glycolysis in response to
hypoxic stress
Glucose uptake and glycolysis are crucial for MSC
survival, particularly in hypoxia (6). Despite the fact that leptin was
found to prevent MSC apoptosis, whether glucose metabolic
alterations were involved in the mechanism underlying
leptin-induced MSC survival remained largely unclear. In order to
determine the changes in glycolysis of MSCs, the glycogen assay was
performed, which revealed that leptin induced an increase in the
glycogen content of MSCs-Lep compared with MSCs-Con, as determined
by PAS staining (Fig. 3A).
Furthermore, cellular glucose was examined using the glucose
oxidase method. In contrast to MSCs-Con, increased glucose uptake,
elicited by leptin treatment, was found at the time point of
hypoxic insults (Fig. 3B).
Moreover, several essential genes linked to glucose metabolism were
analyzed, including glyceraldehyde-3-phosphate dehydrogenase
(GAPDH), hexo-kinase (HK)-1, lactate dehydrogenase (LDH)-α, and
pyruvate dehydrogenase (PDH)-α, as well as key genes of fatty acid
oxidation, such as carnitine palmitoyltransferase (cpt)-1α
(25). In contrast to MSCs-Con,
increased gene expression of GAPDH, HK-1, LDH-α and PDH-α, and
decreased gene expression of cpt-1α, were observed in MSCs-Lep
(Fig. 3C). Subsequently, the
increased lactate content of the supernatant was assessed in
MSCs-Lep compared with MSCs-Con (Fig.
3D) and was found to be decreased in MSCs-Lep (Fig. 3D). Furthermore, the level of
adenosine triphosphate (ATP) production was higher in MSCs-Lep
compared with that in MSCs-Con (Fig.
3E), and increased ECAR was measured in MSCs-Lep compared with
MSCs-Con (Fig. 3F), indicating
that leptin activated glycolysis to produce energy. However, a
markedly lower OCR was observed in MSCs-Lep compared with MSCs-Con
(Fig. 3G), demonstrating that
mitochondrial OXPHOS was insufficient following leptin
administration. Therefore, increased glycolysis of MSCs was
observed in association with improved survival following leptin
treatment under hypoxic conditions.
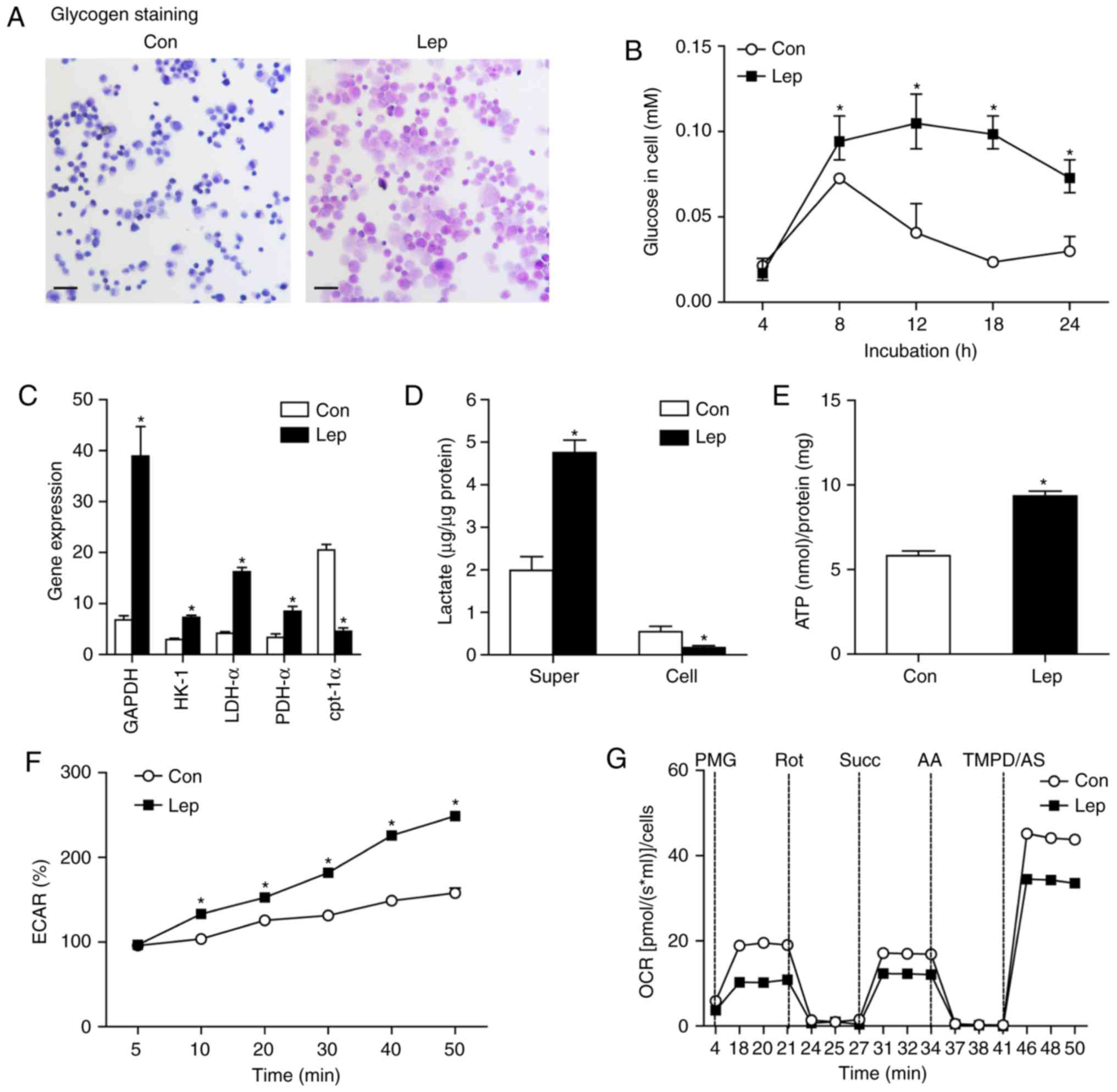 | Figure 3Glycolysis was induced by leptin
treatment. (A) Following leptin treatment, MSCs were exposed to
hypoxia and glucose + serum deprivation for 24 h. Representative
images of glycogen staining were revealed in MSCs-Con and MSCs-Lep
by the Periodic acid-Schiff (PAS) method. Scale bar: 50 μm.
(B) Glucose content was determined using the glucose oxidase method
when MSCs were exposed to glucose + serum deprivation during the
time course of hypoxia. (C) The gene expression of GAPDH, HK-1,
LDH-α, PDH-α and cpt-1α was detected by RT-qPCR and normalized to
β-actin. (D) Lactate content of supernatant or cellular was
measured in MSCs-Con and MSCs-Lep, respectively. (E) Cellular ATP
levels were measured by luciferin/luciferase-based assay, and the
data were calibrated by protein content. (F) Following exposure to
hypoxia and glucose + serum deprivation for 24 h, the ECAR of MSCs
was measured at selected time points and is shown as percentage
relative to untreated control MSCs (1×104
cells/96-well). (G) Mitochondrial oxygen consumption rate (OCR) was
determined in MSCs-Con and MSCs-Lep using an Oroboros instrument.
The data were analyzed after sequential injection of a mixture of 5
mM pyruvate + 0.5 mM malate + 10 mM glutamate (PMG), 0.1 μM
rotenone (Rot), 10 mM succinate (Succ), 2.5 μM antimycin A
(AA) and a mixture of 0.5 mM TMPD +2 mM ascorbate (TMPD/AS), and
calculated as the mean of each time. Data are presented as mean ±
standard error of the mean; n=3 (*P<0.05). MSCs,
mesenchymal stem cells; HK, hexokinase; LDH, lactate dehydrogenase;
PDH, pyruvate dehydrogenase; cpt, carnitine palmitoyltransferase;
RT–qPCR, reverse transcription–quantitative polymerase chain
reaction; ECAR, extracellular acidification rate. |
OPA1 is involved in leptin–induced
glycolysis
Improved glycolysis regulated by leptin has been
confirmed based on the increased insulin sensitivity and reduction
of hepatic gluconeogenesis (8,26).
In addition, insulin regulates glucose uptake through the
Akt-mTOR-NFκB-OPA1 signaling pathway in cardiomyocytes (27). These results prompted us to
investigate whether the anti-apoptotic effect of leptin was
mediated via OPA1-induced glycolysis. OPA1 gene expression was
silenced by small interfering RNA (siOPA1) treatment. The siOPA1
transfection efficiency was verified by western blotting (Fig. S1). In the siOPA1 group, no
significant effect of leptin on glycogen staining (Fig. 4A) or the glucose content (Fig. 4B) was observed. The increase in
the gene expression levels of GAPDH, HK-1, LDH-α and PDH-α
following leptin treatment was blunted by OPA1 silencing, and there
was no significant difference in cpt–1α gene expression between the
siOPA1 + Con and siOPA1 + Lep groups (Fig. 4C). Similarly, we also observed
that the increased lactate levels in the supernatant and ECAR in
MSCs-Lep were blunted by siOPA1 treatment, whereas the reduced
cellular lactate in MSCs-Lep started to accumulate again (Fig. 4D and F). Following siOPA1
transfection, ATP production was not altered in the siOPA1 + Con or
siOPA1 + Lep groups, while a slight reduction in ATP was detected
when comparing the NC + Con and siOPA1 + Con groups (Fig. 4E). These results indicate that
improvement of leptin-induced glycolysis is primarily dependent on
OPA1. Following siOPA1 transfection, the levels of OCR did not
change in the siOPA1 + Con and siOPA1 + Lep groups, while they were
decreased compared with the NC + Con group (Fig. 4G), which was in agreement with an
earlier study reporting that OPA1 can regulate mitochondrial
metabolism (28).
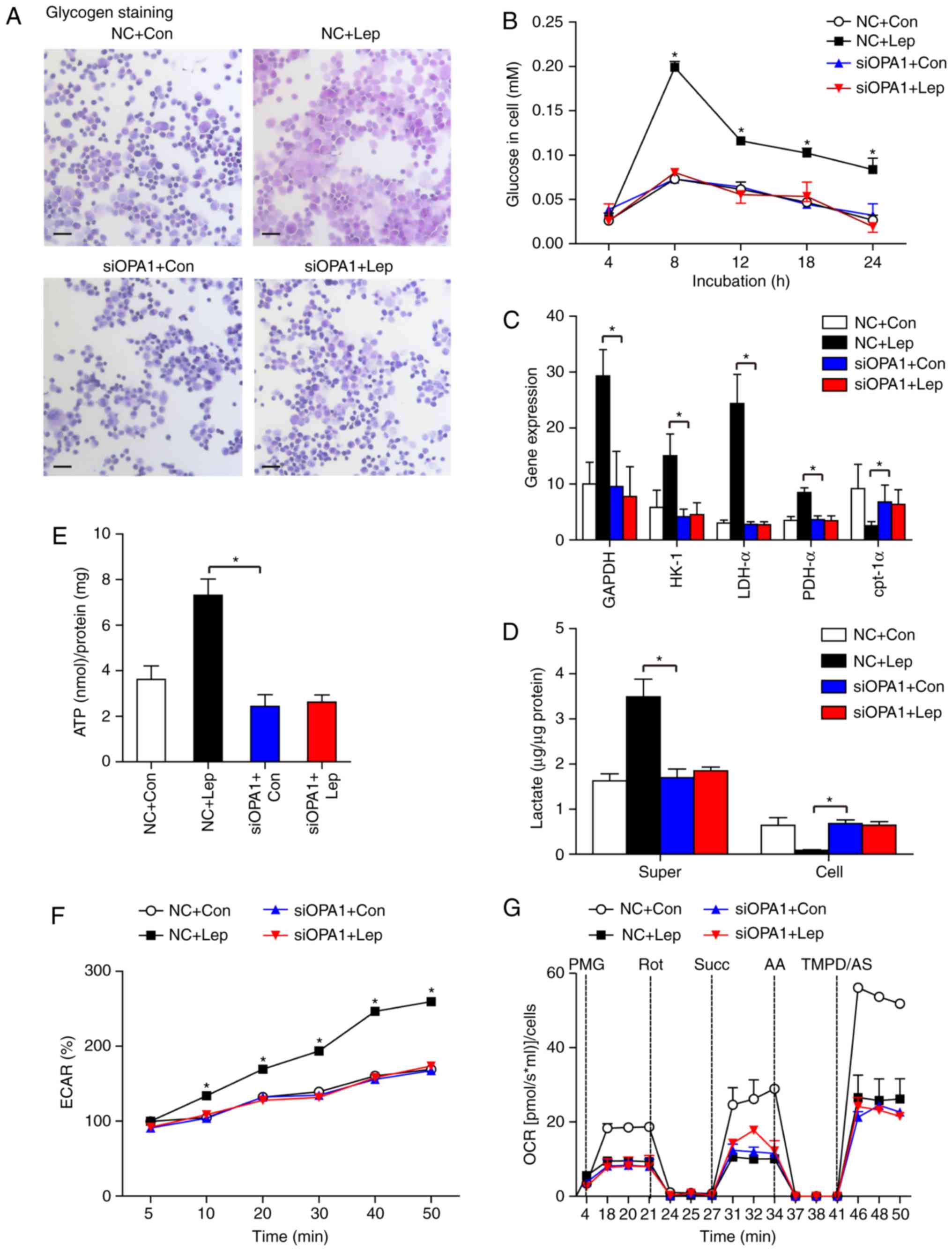 | Figure 4Leptin-induced glycolysis was
required for OPA1. (A) Representative images of glycogen staining
in MSCs transfected with siOPA1 in the presence or absence of
leptin treatment, following exposure to hypoxia and glucose + serum
deprivation for 24 h. A scramble siRNA (NC) was used as gene
silencing control. Scale bar: 50 μm. (B) The glucose content
was analyzed in siOPA1/NC-transfected MSCs, with or without leptin
treatment. (C) The mRNA level of GAPDH, HK-1, LDH-α, PDH-α and
cpt-1α was assessed in the NC and siOPA1 groups, with or without
leptin treatment, following exposure to glucose + serum deprivation
during the time course of hypoxia. (D) Supernatant or cellular
lactate content and (E) cellular ATP levels were measured in the NC
and siOPA1 groups in the presence or absence of leptin treatment.
(F) Cellular ECAR was measured at selected time points in NC + Con,
NC + Lep, siOPA1 + Con and siOPA1 + Lep cells, relative to
untreated control cells. (G) The OCR of the abovementioned cells
was quantified using an Oroboros instrument. Each in vitro
experiment was performed three times. Data are presented as mean ±
standard error of the mean; n=3 (*P<0.05). OPA1,
optic atrophy 1; MSCs, mesenchymal stem cells; HK, hexokinase; LDH,
lactate dehydrogenase; PDH, pyruvate dehydrogenase; cpt, carnitine
palmitoyltransferase; OCR, oxygen consumption rate; ECAR,
extracellular acidification rate. |
Leptin increases the expression level of
glucose trans–porters
Glucose uptake is essential for cell growth,
metabolism and proliferation. Glucose diffusion across the cell
membrane is accomplished by glucose transporters, including
Na+-independent facilitated diffusion glucose
transporters (GLUTs), sodium-glucose symporters 1 (SGLT1) and
SWEETs; GLUTs and SGLT1 are responsible for glucose transport in
mammals, whereas SWEETs is mainly found in plants (29). To explore how leptin regulates
glucose transport, several major glucose transporters were
investigated to identify the obviously increased gene expression of
GLUT1, GLUT4 and SGLT1 (Fig. 5A).
GLUT1 acts as 'housekeeping' gene, as it is ubiquitous in all
tissues, whereas GLUT4 is principally found in adipocytes and
muscle (30). Consequently, the
protein levels of GLUT1, GLUT4 and SGLT1 were measured; we observed
activation of SGLT1 at the protein level in MSCs–Lep compared with
MSCs–Con, while no significant impact on the expression levels of
GLUT1 and GLUT4 was noted following leptin treatment (Fig. 5B and C). Subsequently, to confirm
that leptin modulates glycolysis through the SGLT1 protein,
leptin–treated MSCs were administered sotagliflozin (LX4211), which
is as an inhibitor of SGLT1 (31). We observed that the increase in
glucose content mediated by leptin was reversed after LX4211
administration (Fig. 5D and E).
Moreover, the upregulated gene expression of GAPDH, HK-1, LDH-α and
PDH-α by leptin treatment was blunted by LX4211 administration, in
contrast with the unchanged gene expression of cpt-1α in the Con +
LX4211 and Lep + LX4211 groups (Fig.
5F). Additionally, the increased lactate content of the
supernatant and the increased ECAR level mediated by leptin were
blocked by LX4211 administration, while the reduction in cellular
lactate accumulation following leptin treatment was hindered by
LX4211 administration (Fig. 5G and
H), suggesting that leptin modulates glycolysis in MSCs
primarily through SGLT1 activation. However, a slightly higher
lactate content of the supernatant and ECAR level were observed in
the Lep + LX4211 group compared with the Con + LX4211 group
(Fig. 5G and H), indicating that
there may be another SGLT1-independent pathway involved in
leptin-mediated glycolysis, at least in part.
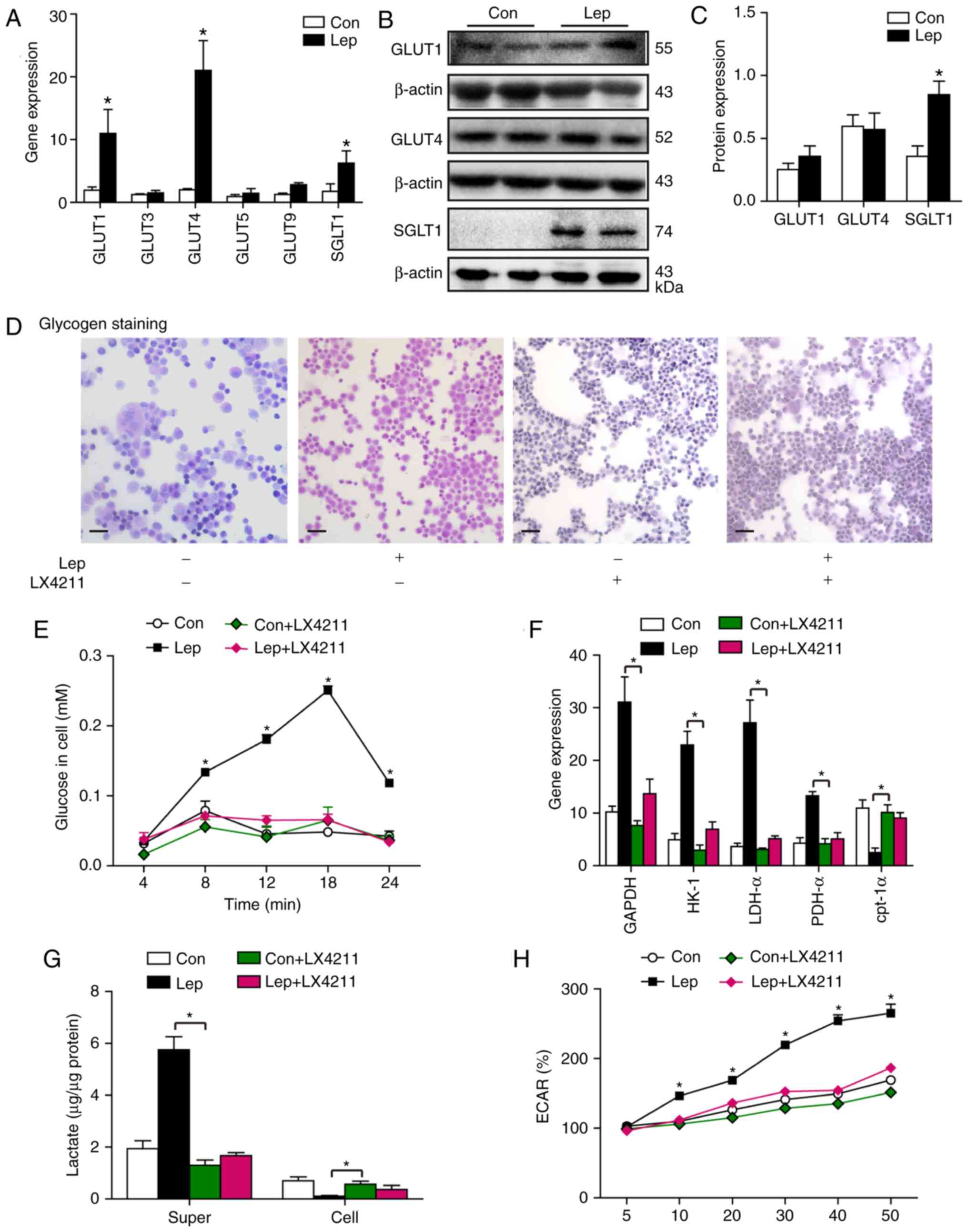 | Figure 5Leptin induced the expression of
glucose transporter SGLT1. (A) Gene expression of pivotal glucose
transporters, such as the GLUT family and SGLT1 after leptin
administration. (B and C) The protein expression levels of GLUT1,
GLUT4 and SGLT1 were assessed by western blotting and quantified
using densitometry. (D and E) After incubation with leptin, the
MSCs were treated with leptin in the presence or absence of 36 nM
LX4211 (an inhibitor of SGLT1), with exposure to hypoxia and
glucose + serum deprivation for an additional 24 h. Glycogen
staining and glucose content were evaluated in the Con, Lep, Con +
LX4211 and Lep + LX4211 groups. Scale bar, 50 μm. (F) The
mRNA expression levels of GAPDH, HK-1, LDH-α, PDH-α and cpt-1α
under LX4211 treatment were analyzed in the abovementioned cells.
(G) The supernatant or cellular lactate content and (H) ECAR (%)
were measured in the abovementioned cells. Individual experiments
were repeated three times. Data are presented as mean ± standard
error of the mean; n=3 (*P<0.05). SGLT1,
sodium-glucose symporter 1; GLUT, glucose transporters; MSCs,
mesenchymal stem cells; HK, hexokinase; LDH, lactate dehydrogenase;
PDH, pyruvate dehydrogenase; cpt, carnitine palmitoyltransferase;
ECAR, extracellular acidification rate. |
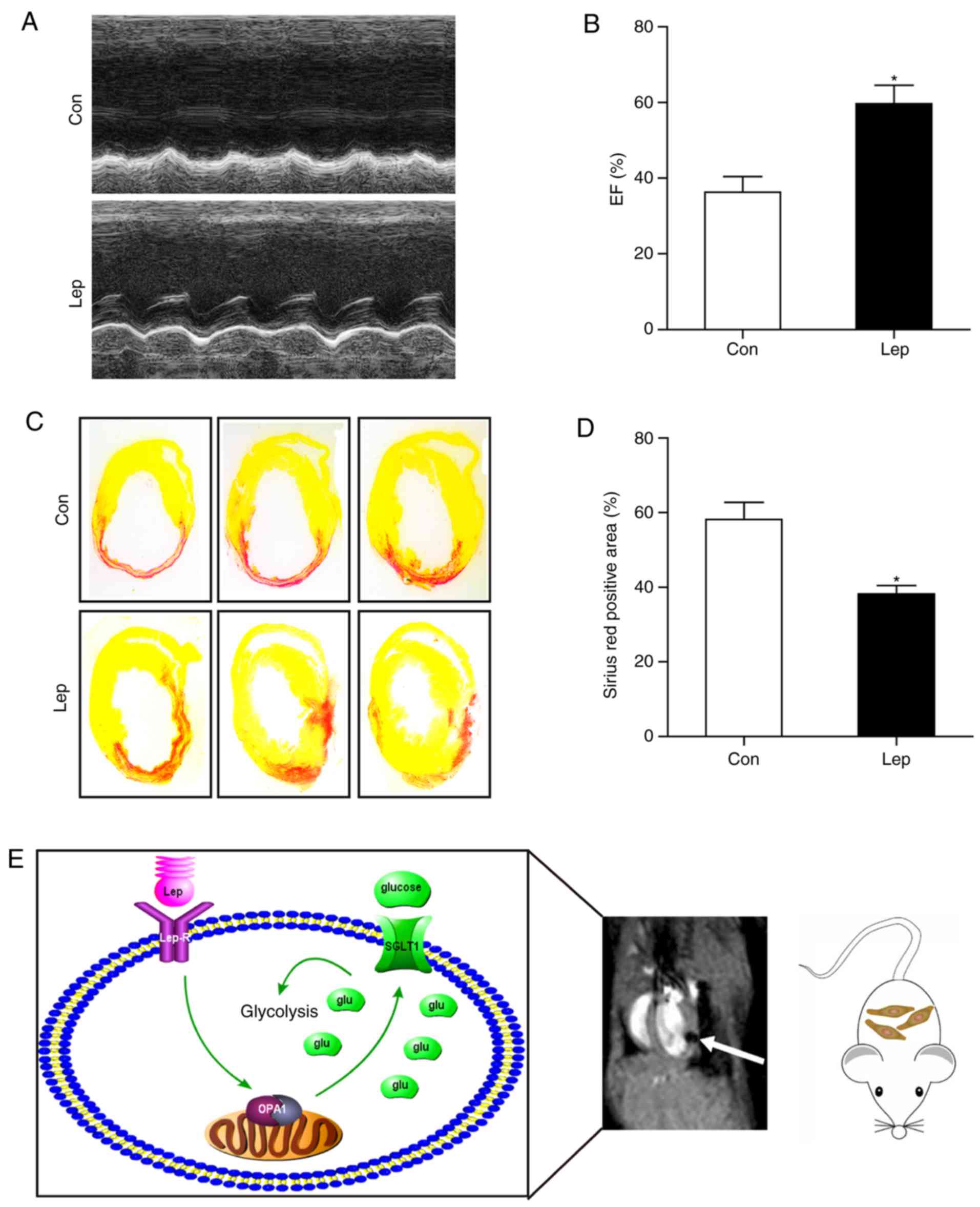
Survival of transplanted MSCs is improved
by leptin treatment in vivo
To evaluate the leptin-mediated MSC survival in
vivo, MSCs were transplanted into the border zone of the
infarcted area in an MI mouse model. On the 28th day after MI,
cardiac function was analyzed by two-dimensional echo-cardiography
examination, and the infarct size was measured by Sirius Red
staining. Notably, in contrast to MSCs-Con, improved cardiac
function (Fig. 6A and B) and
decreased infarct size (Fig. 6C and
D) were observed in MSCs-Lep. Taken together, these data
suggest that leptin endowed MSCs with apoptotic resistance through
a switch to glycolysis, mediated at least in part via SGLT1, which
was highly dependent on OPA1 (Fig.
6E).
Discussion
A previous study demonstrated the role of leptin in
apoptotic resistance of MSCs, and demonstrated that leptin
stimulated the GSK3/OMA1/OPA1 signaling pathway to promote
mitochondrial fusion and protect mitochondrial integrity (9). The present study focused on a new
aspect of cell metabolism. Leptin was found to improve glucose
metabolism in order to provide energy for MSCs under hypoxic
conditions, and the leptin-regulated glycolysis was dependent on
the mitochondrial fusion protein OPA1. It was revealed that
modulation of glycolysis by leptin was dependent on OPA1 primarily
through activation of SGLT1. These data prompt further
investigation of the association of the mitochondrial protein OPA1
with the glucose transporter SGLT1, in order to provide a
pharmacological strategy of MSCs therapy for MI injury.
Additionally, accumulating evidence indicates that
HPC promotes the survival of MSCs (32,33). The main characteristic of the MSC
microenvironment is a low-oxygen atmosphere, in which 5-10%
O2 may promote MSC proliferation (6), although differentiation of MSCs can
be stimulated at 2% O2 (34). Hypoxia-induced glycogen storage
promotes the effectiveness of MSC therapy (35). Accompanied by increased lactate
production rates, increased survival of MSCs may be observed in
hypoxia (5). The enhanced
survival of MSCs is abolished in the presence of 2DG (an inhibitor
of hexokinase) (36). These
findings indicate that glucose metabolism is crucial for MSC
survival under hypoxic conditions. Consistent with previous
studies, our results demonstrated that increased glycolysis was
associated with improved MSC survival. Although long-term
engraftment of transplanted MSCs has proven that only few
transplanted cells survived at day 28 post-MI in a previous study
(32), the therapeutic effects of
MSCs are considered to be primarily due to paracrine mechanisms
according to the related literature (37-39).
Leptin is a major adipostatic hormone involved in
energy balance through neuroendocrine mechanisms (26). With the increasing incidence of
insulin resistance and type 2 diabetes mellitus, the role of leptin
in the regulation of glucose homeo-stasis has attracted the
attention of scholars (40).
Leptin improves insulin resistance and inhibits hepatic
gluconeo-genesis (8,26). Leptin administration was shown to
increase glucose infusion rate via the PI3K signaling pathway in a
neuronal network (41). Moreover,
leptin can increase insulin and glucagon gene expression and β-cell
mass to regulate glucose homeostasis (42).
Regarding cellular energy production, mitochondria
produce ATP through OXPHOS, whereas another function of
mitochondria is associated with cell apoptosis (12). A sustainable mitochondrial dynamic
homeostasis of fusion and fission is required for cellular health,
while excessive mitochondrial fragmentation or mitophagy predispose
to cell death (23). The present
study demonstrated a reduction in OCR and ATP following siOPA1
treatment, with or without leptin. As a mitochondrial inner
membrane protein, OPA1, plays a key role in mitochondrial
respiratory efficiency and cristae remodeling (43). Additionally, in agreement with an
earlier hypothesis, OPA1 regulates mitochondrial metabolism
(28). In the present study,
although we failed to demonstrate a restoration of mitochondrial
function by leptin treatment, it may be hypothesized that the role
of mitochondrial fusion in leptin-improved MSC survival was through
inhibiting the release of proapoptotic proteins from the
mitochondria to the cytoplasm, a phenomenon referred to as
stress-induced mitochondrial hyperfusion (SIMH) (44). In addition, the decreased
expression of cpt-1α can inhibit fatty acids from entering the
mitochondria and reduce lipogenesis, thereby stimulating glucose
metabolism (45). However, cpt-1α
reduction may inhibit glucose oxidation without affecting
glycolysis, as previously suggested (46). Taken together, these results
combined with the results on the accumulation of lactate in the
supernatant and the elevated levels of ECAR, indicate that the
increased ATP production by leptin treatment may be primarily
attributed to cellular glycolysis due to insufficient oxygen supply
under hypoxic conditions. Intriguingly, a higher lactate content of
the supernatant and reduced cellular lactate were detected in
leptin-treated MSCs, which may be associated with MCT4 upregulation
to extrude intracellular lactate and promote MSC survival in
hypoxia (38).
Glucose transporters are necessary for glycolysis;
however, the number of studies investigating transporter regulating
networks and their activity is limited. It has also been reported
that members of the GLUT family may be phosphorylated at various
residues (e.g., C-terminal domain) (29,30). We analyzed the mRNA levels of
several major transporters, and found high expression of GLUT1,
GLUT4 and SGLT1. GLUT1 acts as a 'housekeeping' gene and plays a
major role in the brain, whereas GLUT4 is principally found in
muscles and SGLT1 is known to be an active sodium-coupled glucose
transporter in intestinal cells (30). Despite the high expression of
GLUT1 and GLUT4 mRNA, higher protein levels were not detected. As
previously reported (47-49), although leptin did not affect the
protein levels, it activated the translocation of GLUT1 and GLUT4.
Additionally, following treatment with an inhibitor of SGLT1
(LX4211), there was a mild increase in glycolysis mediated by
leptin; thus, the translocation of GLUT1 and GLUT4 may be involved
in leptin-modulated glycolysis. Further research on phosphorylated
transporters and glucose regulatory networks is required.
In conclusion, leptin appears to play a key role in
promoting MSC glycolysis in order to resist apoptosis in hypoxia.
OPA1, which is associated with mitochondrial fusion, was found to
be involved in leptin-modulated glycolysis, primarily through SGLT1
activation. Thus, the findings of the present study may provide a
new pharmacological strategy involving leptin-regulated glucose
metabolism via the OPA1 signaling pathway in MSC-based therapy for
MI.
Supplementary Data
Funding
The present study was supported by the Clinical
Research Center Project of the Department of Science and Technology
of Guizhou Province [grant no. (2017) 5405] and the National
Clinical Key Specialty Construction Project of China [grant no.
(2013) 544].
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
LY: Conception and design, financial support,
accountability for all aspects of the work; FY: Experiments, data
analysis, figure assembly and writing of the manuscript; BL and MH:
Experimental testing and data analysis; YY, XL and YZ: Manuscript
revision, data analysis; HL, LZ and ST: Supervision of experimental
testing; YW and LW: MI mouse model establishment; YP: Cardiac
function analysis by echo-cardiography. All authors approved the
final version of the manuscript submitted for publication.
Ethics approval and consent to
participate
The experimental protocols were approved by the
Human Ethics Committee of Guizhou Provincial People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
HPC
|
hypoxic preconditioning
|
|
OPA1
|
optic atrophy 1
|
|
VDAC
|
voltage-dependent anion channel
|
|
TEM
|
transmission electron microscopy
|
|
OXPHOS
|
oxidative phosphorylation
|
|
OCR
|
oxygen consumption rate
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
HK-1
|
hexokinase-1
|
|
LDH-α
|
lactate dehydrogenase-α
|
|
PDH-α
|
pyruvate dehydrogenase-α
|
|
cpt-1α
|
carnitine palmitoyltransferase-1α
|
|
GLUTs
|
glucose transporters
|
|
SGLT1
|
sodium-glucose symporter 1
|
|
EF
|
ejection fraction
|
Acknowledgments
Not applicable.
References
|
1
|
Lee CW, Hsiao WT and Lee OK: Mesenchymal
stromal cell-based therapies reduce obesity and metabolic syndromes
induced by a high-fat diet. Transl Res. 182:61–74. e82017.
View Article : Google Scholar
|
|
2
|
Bottcher M, Hofmann AD, Bruns H, Haibach
M, Loschinski R, Saul D, Mackensen A, Le Blanc K, Jitschin R and
Mougiakakos D: Mesenchymal stromal cells disrupt mTOr-signaling and
aerobic glycolysis during T-cell activation. Stem Cells.
34:516–521. 2016. View Article : Google Scholar
|
|
3
|
Hare JM, Traverse JH, Henry TD, Dib N,
Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE,
Gammon RS, et al: A randomized, double-blind, placebo-controlled,
doseescalation study of intravenous adult human mesenchymal stem
cells (prochymal) after acute myocardial infarction. J Am Coll
Cardiol. 54:2277–2286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang XP, Sun Z, Miyagi Y, McDonald
Kinkaid H, Zhang L, Weisel RD and Li RK: Differentiation of
allogeneic mesenchymal stem cells induces immunogenicity and limits
their long-term benefits for myocardial repair. Circulation.
122:2419–2429. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Meester C, Timmermans AD, Balteau M,
Ginion A, Roelants V, Noppe G, Porporato PE, Sonveaux P, Viollet B,
Sakamoto K, et al: Role of AMP-activated protein kinase in
regulating hypoxic survival and proliferation of mesenchymal stem
cells. Cardiovasc Res. 101:20–29. 2014. View Article : Google Scholar
|
|
6
|
Buravkova LB, Andreeva ER, Gogvadze V and
Zhivotovsky B: Mesenchymal stem cells and hypoxia: Where are we?
Mitochondrion. 19:105–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu X, Wu R, Jiang Z, Wang L, Chen P, Zhang
L, Yang L, Wu Y, Chen H, Chen H, et al: Leptin signaling is
required for augmented therapeutic properties of mesenchymal stem
cells conferred by hypoxia preconditioning. Stem Cells.
32:2702–2713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kelesidis T, Kelesidis I, Chou S and
Mantzoros CS: Narrative review: The role of leptin in human
physiology: Emerging clinical applications. Ann Intern Med.
152:93–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang F, Wu R, Jiang Z, Chen J, Nan J, Su
S, Zhang N, Wang C, Zhao J, Ni C, et al: Leptin increases
mitochondrial OPA1 via GSK3-mediated OMA1 ubiquitination to enhance
therapeutic effects of mesenchymal stem cell transplantation. Cell
Death Dis. 9:5562018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pernas L and Scorrano L: Mito-morphosis:
Mitochondrial fusion, fission, and cristae remodeling as key
mediators of cellular function. Annu Rev Physiol. 78:505–531. 2016.
View Article : Google Scholar
|
|
11
|
Head B, Griparic L, Amiri M, Gandre-Babbe
S and van der Bliek AM: Inducible proteolytic inactivation of OPA1
mediated by the OMA1 protease in mammalian cells. J Cell Biol.
187:959–966. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Quiros PM, Langer T and Lopez-Otin C: New
roles for mitochondrial proteases in health, ageing and disease.
Nat Rev Mol Cell Biol. 16:345–359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wai T and Langer T: Mitochondrial dynamics
and metabolic regulation. Trends Endocrinol Metab. 27:105–117.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Santoro A, Campolo M, Liu C, Sesaki H,
Meli R, Liu ZW, Kim JD and Diano S: DRP1 suppresses leptin and
glucose sensing of POMC neurons. Cell Metab. 25:647–660. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Buck MD, O'Sullivan D, Klein Geltink RI,
Curtis JD, Chang CH, Sanin DE, Qiu J, Kretz O, Braas D, van der
Windt GJ, et al: Mitochondrial dynamics controls T cell fate
through metabolic programming. Cell. 166:63–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nan J, Hu H, Sun Y, Zhu L, Wang Y, Zhong
Z, Zhao J, Zhang N, Ye J, Wang Y, et al: TNFR2 stimulation promotes
mitochondrial fusion via stat3- and NF-kB-dependent activation of
OPA1 expression. Circ Res. 121:392–410. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuznetsov AV, Veksler V, Gellerich FN,
Saks V, Margreiter R and Kunz WS: Analysis of mitochondrial
function in situ in permeabilized muscle fibers, tissues and cells.
Nat Protoc. 3:965–976. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang N, Ye F, Zhu W, Hu D, Xiao C, Nan J,
Su S, Wang Y, Liu M, Gao K, et al: Cardiac ankyrin repeat protein
attenuates cardiomyocyte apoptosis by upregulation of Bcl-2
expression. Biochim Biophys Acta. 1863:3040–3049. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang C, Chen H, Zhu W, Xu Y, Liu M, Zhu L,
Yang F, Zhang L, Liu X, Zhong Z, et al: Nicotine accelerates
atherosclerosis in apolipoprotein E–deficient mice by activating α7
nicotinic acetylcholine receptor on mast cells. Arterioscler Thromb
Vasc Biol. 37:53–65. 2017. View Article : Google Scholar
|
|
20
|
Liu X, Hu D, Zeng Z, Zhu W, Zhang N, Yu H,
Chen H, Wang K, Wang Y, Wang L, et al: SRT1720 promotes survival of
aged human mesenchymal stem cells via FAIM: A pharmacological
strategy to improve stem cell-based therapy for rat myocardial
infarction. Cell Death Dis. 8:e27312017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lahera V, de Las Heras N, Lopez-Farre A,
Manucha W and Ferder L: Role of mitochondrial dysfunction in
hypertension and obesity. Curr Hypertens Rep. 19:112017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Giuliano M, Lauricella M, Calvaruso G,
Carabillò M, Emanuele S, Vento R and Tesoriere G: The apoptotic
effects and synergistic interaction of sodium butyrate and MG132 in
human retinoblastoma Y79 cells. Cancer Res. 59:5586–5595.
1999.PubMed/NCBI
|
|
23
|
Chan DC: Fusion and fission: Interlinked
processes critical for mitochondrial health. Annu Rev Genet.
46:265–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Quirós PM, Ramsay AJ, Sala D,
Fernández-Vizarra E, Rodríguez F, Peinado JR, Fernández-García MS,
Vega JA, Enríquez JA, Zorzano A and López-Otín C: Loss of
mitochondrial protease OMA1 alters processing of the GTPase OPA1
and causes obesity and defective thermogenesis in mice. EMBO J.
31:2117–2133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Wong K, Giles A, Jiang J, Lee JW,
Adams AC, Kharitonenkov A, Yang Q, Gao B, Guarente L and Zang M:
Hepatic SIRT1 attenuates hepatic steatosis and controls energy
balance in mice by inducing fibroblast growth factor 21.
Gastroenterology. 146:539–549e7. 2014. View Article : Google Scholar :
|
|
26
|
Flier JS, Harris M and Hollenberg AN:
Leptin, nutrition, and the thyroid: The why, the wherefore, and the
wiring. J Clin Invest. 105:859–861. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Parra V, Verdejo HE, Iglewski M, Del Campo
A, Troncoso R, Jones D, Zhu Y, Kuzmicic J, Pennanen C,
Lopez-Crisosto C, et al: Insulin stimulates mitochondrial fusion
and function in cardiomyocytes via the Akt-mTOR-NFκB-Opa-1
signaling pathway. Diabetes. 63:75–88. 2014. View Article : Google Scholar
|
|
28
|
Patten DA, Wong J, Khacho M, Soubannier V,
Mailloux RJ, Pilon-Larose K, MacLaurin JG, Park DS, McBride HM,
Trinkle-Mulcahy L, et al: OPA1-dependent cristae modulation is
essential for cellular adaptation to metabolic demand. EMBO J.
33:2676–2691. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen LQ, Cheung LS, Feng L, Tanner W and
Frommer WB: Transport of sugars. Annu Rev Biochem. 84:865–894.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deng D, Sun P, Yan C, Ke M, Jiang X, Xiong
L, Ren W, Hirata K, Yamamoto M, Fan S and Yan N: Molecular basis of
ligand recognition and transport by glucose transporters. Nature.
526:391–396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zambrowicz B, Freiman J, Brown PM, Frazier
KS, Turnage A, Bronner J, Ruff D, Shadoan M, Banks P, Mseeh F, et
al: LX4211, a dual SGLT1/SGLT2 inhibitor, improved glycemic control
in patients with type 2 diabetes in a randomized,
placebo-controlled trial. Clin Pharmacol Ther. 92:158–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu X, Xu Y, Zhong Z, Wu Y, Zhao J, Wang Y,
Cheng H, Kong M, Zhang F, Chen Q, et al: A large-scale
investigation of hypoxia-preconditioned allogeneic mesenchymal stem
cells for myocardial repair in nonhuman primates: Paracrine
activity without remuscularization. Circ Res. 118:970–983. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lavrentieva A, Majore I, Kasper C and Hass
R: Effects of hypoxic culture conditions on umbilical cord-derived
human mesenchymal stem cells. Cell Commun Signal. 8:182010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Volkmer E, Kallukalam BC, Maertz J, Otto
S, Drosse I, Polzer H, Bocker W, Stengele M, Docheva D, Mutschler W
and Schieker M: Hypoxic preconditioning of human mesenchymal stem
cells overcomes hypoxia-induced inhibition of osteogenic
differentiation. Tissue Eng Part A. 16:153–164. 2010. View Article : Google Scholar
|
|
35
|
Zhu H, Sun A, Zou Y and Ge J: Inducible
metabolic adaptation promotes mesenchymal stem cell therapy for
ischemia: A hypoxia-induced and glycogen-based energy prestorage
strategy. Arterioscler Thromb Vasc Biol. 34:870–876. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mylotte LA, Duffy AM, Murphy M, O'Brien T,
Samali A, Barry F and Szegezdi E: Metabolic flexibility permits
mesenchymal stem cell survival in an ischemic environment. Stem
Cells. 26:1325–1336. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hsiao ST, Asgari A, Lokmic Z, Sinclair R,
Dusting GJ, Lim SY and Dilley RJ: Comparative analysis of paracrine
factor expression in human adult mesenchymal stem cells derived
from bone marrow, adipose, and dermal tissue. Stem Cells Dev.
21:2189–2203. 2012. View Article : Google Scholar :
|
|
38
|
Saraswati S, Guo Y, Atkinson J and Young
PP: Prolonged hypoxia induces monocarboxylate transporter-4
expression in mesenchymal stem cells resulting in a secretome that
is deleterious to cardiovascular repair. Stem Cells. 33:1333–1344.
2015. View Article : Google Scholar :
|
|
39
|
Tachibana A, Santoso MR, Mahmoudi M,
Shukla P, Wang L, Bennett M, Goldstone AB, Wang M, Fukushi M, Ebert
AD, et al: Paracrine effects of the pluripotent stem cell-derived
cardiac myocytes salvage the injured myocardium. Circ Res.
121:e22–e36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Morton GJ and Schwartz MW: Leptin and the
central nervous system control of glucose metabolism. Physiol Rev.
91:389–411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rasmussen BA, Breen DM, Duca FA, Côté CD,
Zadeh-Tahmasebi M, Filippi BM and Lam TK: Jejunal leptin-PI3K
signaling lowers glucose production. Cell Metab. 19:155–161. 2014.
View Article : Google Scholar
|
|
42
|
Michel M, Page-McCaw PS, Chen W and Cone
RD: Leptin signaling regulates glucose homeostasis, but not
adipostasis, in the zebrafish. Proc Natl Acad Sci USA.
113:3084–3089. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Varanita T, Soriano ME, Romanello V,
Zaglia T, Quintana-Cabrera R, Semenzato M, Menabò R, Costa V,
Civiletto G, Pesce P, et al: The opa1-dependent mitochondrial
cristae remodeling pathway controls atrophic, apoptotic, and
ischemic tissue damage. Cell Metab. 21:834–844. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tondera D, Grandemange S, Jourdain A,
Karbowski M, Mattenberger Y, Herzig S, Da Cruz S, Clerc P, Raschke
I, Merkwirth C, et al: SLP-2 is required for stress-induced
mitochondrial hyperfusion. EMBO J. 28:1589–1600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bertero E and Maack C: Metabolic
remodelling in heart failure. Nat Rev Cardiol. 15:457–470. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gamble J and Lopaschuk GD: Glycolysis and
glucose oxidation during reperfusion of ischemic hearts from
diabetic rats. Biochim Biophys Acta. 1225:191–199. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Benomar Y, Naour N, Aubourg A, Bailleux V,
Gertler A, Djiane J, Guerre-Millo M and Taouis M: Insulin and
leptin induce Glut4 plasma membrane translocation and glucose
uptake in a human neuronal cell line by a phosphatidylinositol
3-kinase-dependent mechanism. Endocrinology. 147:2550–2556. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Berti L, Kellerer M, Capp E and Häring HU:
Leptin stimulates glucose transport and glycogen synthesis in C2C12
myotubes: Evidence for a PI3-kinase mediated effect. Diabetologia.
40:606–609. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Berti L and Gammeltoft S: Leptin
stimulates glucose uptake in C2C12 muscle cells by activation of
ERK2. Mol Cell Endocrinol. 157:121–130. 1999. View Article : Google Scholar
|