Introduction
Pharyngolaryngeal cancer is one of the common
malignant tumors that occurs in the head and neck, and its
development is a highly complex progress during which plenty of
mechanisms, for example, oncogene activation, tumor suppressor gene
inactivation and abnormal expression of apoptosis-associated genes,
are involved (1,2). Although comprehensive treatment for
pharyngolaryngeal cancer has been developed recently, the overall
survival rate has not yet improved to an acceptable level (3,4).
Investigating the molecular mechanism of laryngeal cancer and
searching for an effective therapeutic molecular target remains to
be done (3).
MicroRNA (miRNA/miR) has been proved to be able to
inhibit the synthesis of specific proteins by binding to specific
3′-untranslated region (UTR) of the target mRNAs, while they
participate in the regulation of cell growth, proliferation,
differentiation and apoptosis (5,6).
Under the circumstance of mutation, deletion or abnormal
expression, miRNAs can serve roles similar to oncogenes or tumor
suppressor genes, participating in the process of proliferation,
differentiation and apoptosis of tumor cells (7,8).
Early in 2008, Chang et al (9) screened the differential expression
profiles of miRNAs in pharyngolaryngeal cancer and normal tissues
using gene chip techniques, and demonstrated that the levels of 13
miRNAs in pharyngolaryngeal cancer tissues were significantly
different compared with in normal tissues. Furthermore, a previous
study has revealed that miRNA was strongly associated with the
development of laryngeal carcinoma (10). In addition, a comprehensive study
revealed that smoking-specific miRNAs are altered in head and neck
squamous cell carcinoma as well (11). Therefore, investigating the
association between miRNA and pharyngolaryngeal cancer is remains
necessary as it would be able to provide a comprehensive
understanding of the mechanism of the development of
pharyngolaryngeal cancer.
Furthermore, previous studies have demonstrated that
miRNAs are a potential tumor biomarkers that could interact with a
variety of signaling pathways, including mitogen-activated protein
kinase (MAPK) and Wnt/Frizzled, therefore participating in the
occurrence and development of tumors (12,13). MAPKs serve an important role in
multiple biological processes including cell proliferation and
differentiation. MAPK kinase kinase 9 (MAP3K9), alternatively known
as mixed-lineage kinases1, has been classified as an oncoprotein
(14). It has been reported that
miRNAs could inhibit the proliferation, migration, invasion and EMT
process of cancer cells by regulating MAP3K9 (15,16).
In this study the aim was to investigate whether
miR-490-5p, the miRNA reported previously to act as a critical
modulator in tumors (17), could
affect the hallmarks of pharyngolaryngeal cancer including
proliferation, migration, invasion and the epithelial-mesenchymal
transition (EMT) process. The underlying mechanism of the
modulation of miR-490-5p on these effects was also
investigated.
Materials and methods
Tissue samples
Tumor and tumor-adjacent tissues samples of 45
patients with laryngeal carcinoma admitted to the People's Hospital
of Xinjiang Uygur Autonomous Region (Xinjiang, China) from February
2009 to October 2013 were selected. These patients including 41
males and 4 females, the age of these patients ranged from 42-76
years old, with an average of 59.6±7.9 years. The data of overall
survival (OS) rates of 45 cases were also collected from the
hospital. The cut-off line of low and high expression of miR-490-5p
were determined according to the median of its expression in these
cases. The present study was approved by the Ethics Committee of
the People's Hospital of Xinjiang Uygur Autonomous Region, and an
informed consent was obtained from each patient.
Bioinformatics analysis
To investigate the target gene of miR-490-5p, the
biological information online analysis software of Targetscan
(http://www.targetscan.org/vert_72/),
miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/php/index.php) and
miRDB (http://mirdb.org/miRDB/) were used.
Cell culturing, transfection and
grouping
NP69, BICR 18, FaDu, HNE-3 and Detroit 562 cell
lines were purchased from the Type Culture Collection of the
Chinese Academy of Sciences. HaCaT cell lines (cat. no. 300493)
were purchased from CLS Cell Lines Service GmbH. NP69 cell lines
acted as the normal laryngeal cells, while HaCaT cell lines acted
as normal tissue cells. The HNE-3, FaDu, NP69 cell lines were
cultured in RPMI-1640 medium (Thermo Fisher Scientific, Inc.),
whereas Detroit 562, BICR 18 and HaCaT cell lines were cultured in
Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific,
Inc.). The medium was supplemented with 10% fetal bovine serum
(FBS; Thermo Fisher Scientific, Inc.). Cells were cultured in an
incubator filled with 5% CO2 at 37°C and subcultured
every 2-3 days. The overexpression plasmid of miR-490-5p (mimics,
5′-CCAUGGAUACUCCCCAGGCGGGU-3′) and MAP3K9 as well as their
corresponding negative control (NC) plasmids were synthesized by
Shanghai Genepharma Company and loaded into the pcDNA 3.1 plasmids.
The transfection of all plasmids (50 nM) was conducted using
Lipofectamine™ 2000 Transfection Reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) following the manufacturer's protocol. Following
transfection for 24 h, the efficiency of transfection was detected
using reverse transcription-quantitative polymerase chain reaction
(RT-qPCR). For observing the effect of upregulated miR-490-5p on
cell viability and predicting the target of miR-490-5p, BICR 18 and
FaDu cells were treated with medium alone, negative control (NC)
plasmid of miR-490-5p or the overexpression plasmid of miR-490-5p
(mimics) and grouped respectively as control, NC and miR-490-5p
mimics groups. The expression vector of MAP3K9 cDNA was transfected
into BICR 18 and FaDu cells to achieve the effect of MAP3K9
overexpression. For determining the effectiveness of the
overexpression of MAP3K9 in cells, BICR 18 and FaDu cells were
treated with medium alone, NC plasmid of MAP3K9 or overexpression
plasmid of MAP3K9-cDNA, which were respectively grouped as control,
NC and MAP3K9 groups. For the investigation of the role of MAP3K9
in the regulation of miR-490-5p on cell viability, migration,
invasion, EMT and colony formation, BICR 18 and FaDu cells were
treated with medium alone, NC plasmid of miR-490-5p, overexpression
plasmid of miR-490-5p (mimics), overexpression plasmid of
miR-490-5p (mimics) and MAP3K9 as well as the overexpression
plasmid of MAP3K9, which were grouped as control, NC, mimics,
mimics + MAP3K9 and MAP3K9 group, respectively.
RT-qPCR analysis
According to the manufacturer's protocol, total RNA
of cells was extracted by TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.) and cDNAs were synthesized by iScript™ cDNA
Synthesis kit (Bio-Rad Laboratories, Inc.). The RT reaction was
performed at 42°C for 30 min, followed by reverse transcriptase
inactivation at 85°C for 15 sec. qPCR was performed using Fast
Start Universal SYBR-Green Master kit (Roche Diagnostics) and the
gene-specific primers. The reactions were run in 96-well plates
using the following formula and program: 2X SYBR-Green master mix
10 μl, cDNA template 1 μl, forward primer (10
μM) 1 μl, reverse primer (10 μM) 1 μl,
ddH2O 7 μl, 2 min of hot start at 95°C 40 cycles
for 15 sec at 95°C for 60 sec at 60°C and for 2 min at 10°C. The
data were analyzed by the 2-ΔΔCq method (18) with normalization to the control.
Primers used are listed in Table
I.
 | Table IThe sequences of primers. |
Table I
The sequences of primers.
| Primer name | Direction | Sequence
(5′-3′) |
|---|
| GAPDH | F |
GAGTCAACGGATTTGGTCGT |
| GAPDH | R |
CATGGGTGGAATCATATTGGA |
| E-cadherin | F |
TACACTGCCCAGGAGCCAGA |
| E-cadherin | R |
TGGCACCAGTGTCCGGATTA |
| Vimentin | F |
AGGCAAAGCAGGAGTCCACTGA |
| Vimentin | R |
ATCTGGCGTTCCAGGGACTCAT |
| MMP-9 | F |
CTCTGGAGGTTCGACGTGAA |
| MMP-9 | R |
TCAACTCACTCCGGGAACTC |
| TIMP-2 | F |
CCAGAAGAAGAGCCTGAACCA |
| TIMP-2 | R |
GTCCATCCAGAGGCACTCATC |
| miR-490-5p-RT | |
CTCAACTGGTGTCGTGGAGTC |
| |
GGCAATTCAGTTGAGUCCACCCA |
| miR-490-5p | F |
CATGGATCTCCAGGTGG |
| miR-490-5p | R |
GAACATGTCTGCGTATCTC |
| TNKS2 | F |
GTGAATGCCCAAGACAAAGGAGG |
| TNKS2 | R |
GGTGTGAAAGCCCATTTGTCCG |
| MAP3K9 | F |
AGGGTTCACCAGCCTTATGGAG |
| MAP3K9 | R |
GGTGAATGCTGTAGGCGACTCT |
| NUFIP2 | F |
AGCACCAGGAAACGCCGAAGAA |
| NUFIP2 | R |
CTTGCTGGTTGCCATTGAGGAC |
| CLCC1 | F |
TTCAGACTGGCAACAAGAGCCC |
| CLCC1 | R |
TTCCACCTGTCTCCTTGGGCTT |
| ABCC2 | F |
GCCAACTTGTGGCTGTGATAGG |
| ABCC2 | R |
ATCCAGGACTGCTGTGGGACAT |
The assessment of cell viability by Cell
Counting Kit-8 (CCK-8)
The assessment of cell viability was conducted using
CCK-8 (HY-K0301; MedChemExpress). Cells in each group described
above were seeded in a 96-well plate at a density of
104-105 cells/well in 100 μl of
culture medium and incubated in an incubator with CO2 at
37°C for 24 and 48 h. 10 μl of CCK-8 solution was added to
each well of the plate, which was further incubated for another 1 h
in the incubator. The absorbance of each sample was measured at 450
nm using a microplate reader (Multiskan; Thermo Fisher Scientific,
Inc.) after the plate had been agitated on an orbital shaker for 1
min.
Luciferase assay
Cells were seeded in a 24-well plate at a cell
density 6×104 cells/well. The point mutation of MAP3K9
in BICR 18 and FaDu cells was created by the Quick-Change
Site-Directed Mutagenesis kit (Stratagene; Agilent Technologies,
Inc.). Cells carrying wild or mutant type of MAP3K9 were grouped
and treated as aforementioned. miR-490-5p was co-transfected with
control vectors pRL-TK (Promega Corporation) coding for
Renilla luciferase. Transfection was conducted using
Lipofectamine™ 2000. Following transfection for 24 h, the
luciferase activity was determined by GloMax 20/20 Luminometer
(Promega Corporation). DNA sequences were confirmed by sequencing.
Dual-Glo luciferase assay kit (Promega Corporation) was used to
detect the luciferase activity after the cells had been gathered
and lysed 48 h. Values of the firefly luciferase were normalized to
Renilla.
The extraction of total protein
Cells were lysed in 1 ml of radioimmunoprecipitation
assay buffer (cat. no. 89900; Thermo Fisher Scientific, Inc.) and
centrifuged at 4°C 16,000 × g for 30 min, and the supernatant of
cells was then gathered. The concentration of total protein was
determined by the Pierce™ BCA Protein Assay kit (Thermo Fisher
Scientific, Inc.). According to the protocol given by the
manufacturer, 20 μl of standard proteins was diluted and 25
μl of samples were added into a 96-well plate. A total of
200 μl of the Working Regent was added to each well and well
mixed for 30 sec. Plate was placed in the dark at 37°C for 30 min.
The OD at 562 nm was read on a microplate reader (Multiskan; Thermo
Fisher Scientific, Inc.).
Western blotting
Protein samples (20 μg/lane) were separated
on 10% SDS-PAGE for 60 min at 80 V and transferred to a
polyvinylidene difluoride membrane for 60 min at 80 V. The membrane
was washed once in PBS with 0.2% Tween 20 (PBST) and then blocked
with 5% non-fat milk for 1 h at room temperature (RT). The membrane
was further probed with anti-MAP3K9 antibody (cat. no. ab228752),
anti-β actin antibody (cat. no. ab115777), anti-matrix
metalloproteinase (MMP)-9 antibody (cat. no. ab76003), anti-tissue
inhibitor of metalloproteinase 1 (TIMP2) antibody (cat. no.
ab180630), anti-epithelial (E)-Cadherin antibody (cat.no. ab40772),
anti-vimentin antibody (cat. no. ab92547; all 1:1,000; Abcam) in
non-fat milk and incubated overnight at 4°C. The membranes were
then washed 3 times in PBST for 5 min and horseradish
peroxidase-conjugated anti-rabbit immunoglobulin G secondary
antibodies (cat. no. ab205718; Abcam) were added at 1:2,000
dilution in non-fat milk for 1 h at RT. The membranes were then
exposed to Pierce™ enhanced chemiluminescence (ECL) plus western
blotting substrate (Thermo Fisher Scientific, Inc.) for 1 min in
the dark. The resulting bands were scanned and the images were
captured in the ChemiDoc MP (Bio-Rad Laboratories Inc.) and the
gray level of the bands was analyzed by the ImageJ software
(version 1.46; National Institutes of Health).
Wound healing test
Cells were seeded in the wells of a 6-well plate at
a density of 5×104 cells/well until 90% confluence was
reached. Each confluent cell monolayer was scratched by a sterile
plastic tip in order to produce a straight gap. The debris and the
edge of the gap were washed with PBS. The cells were cultured in
complete medium for 24 h and the relative wound distance was
analyzed based on the images under the inverted light microscope
(Olympus IX71; Olympus Corporation) at the same observation site.
The distance between the gaps was set as the mean of the distance
between upper, middle and lower edges. The distance in the images
was measured by ImageJ software.
The assessment of cell invasion rate by
Transwell
A total of 3×104 cells with 100 μl
serum-free medium were planted in the top chamber of a Transwell
apparatus (8-μm) with a Matrigel-coated membrane (BD
Biosciences) for Transwell invasion assay. The bottom chamber was
filled with medium supplimented with 10% FBS. Following incubation
of the Transwell for 12 h, the cells that invaded into the surface
of the bottom chamber were fixed with 4% paraformaldehyde for 15
min at room temperature, stained with 0.5% crystal violet for 20
min at room temperature and counted under an Olympus IX71
microscope. The images of the bottom of each camber in the same
site were imaged.
Plate colony formation assay
Cells were collected and seeded into the wells of a
6-well plate at a density of 200 cells/dish for 48 h. A total of
700 μg/ml of G418 (Abcam) was used to detect the positive
cell clones for 3 weeks till the visible cell colony was visible.
Medium was replaced every 3 days. Next, cell colony formations were
gently washed with PBS and fixed with methanol for 15 min at room
temperature. Colonies were stained with 0.5% crystal violet for 20
min at room temperature. The stain was then carefully washed off
with running water and dried. Each colony in the wells was imaged
and counted under an Olympus IX71 microscope.
Statistical analysis
All the data analysis was performed by GraphPad
Prism v7.0 software (Graphpad Software, Inc.) and presented as mean
± standard deviation. The variantions between different groups were
analyzed by one-way analysis of variance followed by Turkey's
multiple comparison. The comparison of the OS rates was analyzed by
log-rank test. P<0.05 was considered to indicate a statistically
significant difference.
Results
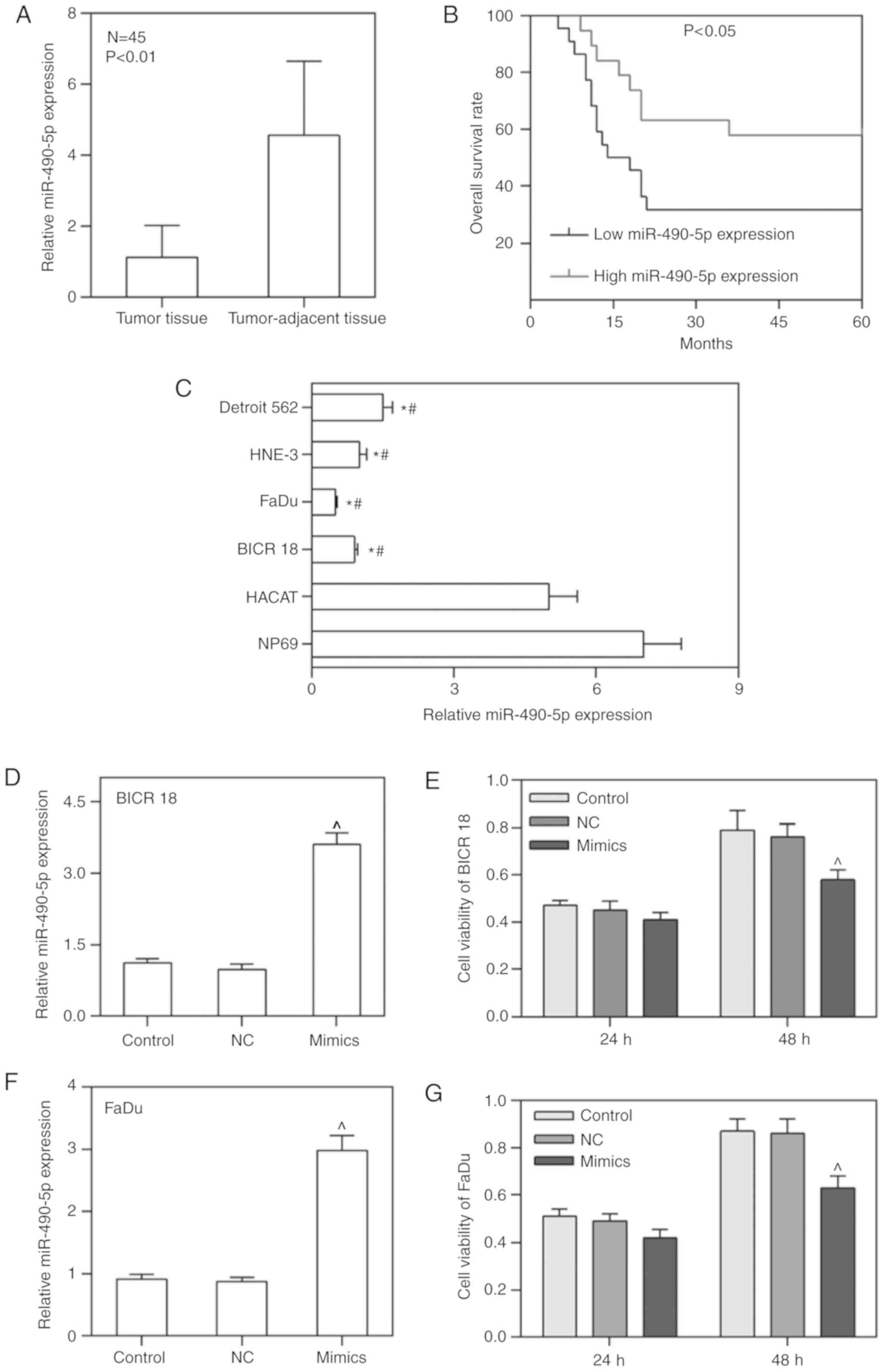
miR-490-5p expression is depressed in
primary pharyngolaryngeal cancer tissues and cell lines, leading to
an unfavorable prognosis
To confirm that the expression of miR-490-5p was
downregulated in primary pharyngolaryngeal cancer tissues and cell
lines as well as its effect on the prognosis, the mRNA level of
miR-490-5p in tumor tissues, tumor-adjacent tissues, NP69, HaCaT,
BICR 18, FaDu, HNE-3 and Detroit 562 cell lines was measured. As
expected, the expression of miR-490-5p in tumor tissues was
significantly decreased compared with in tumor-adjacent tissues
(P<0.01; Fig. 1A). Similarly,
the expression of miR-490-5p in BICR 18, FaDu, HNE-3 and Detroit
562 cells were decreased compared with HaCaT and NP69, particularly
in BICR 18 and FaDu cells (P<0.05; Fig. 1C). Therefore, BICR 18 and FaDu
cells were selected for later experiments. Furthermore, patients
with low miR-490-5p expression demonstrated a significantly
decreased OS rate compared with high miR-490-5p expression
(P<0.05; Fig. 1B). The data
from the present study suggested that the downregulation of
miR-490-5p in pharyngolaryngeal cancer was a common condition,
leading to an unfavorable prognosis.
miR-490-5p overexpression decreases the
cell viability of pharyngolaryngeal cancer cells
In order to determine whether the upregulation of
miR-490-5p could affect the cell viability of pharyngolaryngeal
cancer cells, the miR-490-5p overexpression plasmid (mimics) was
transfected into BICR 18 and FaDu cells, and the cell viabilities
of BICR 18 and FaDu cells 24 and 48 h following culturing were
measured. miR-490-5p was successfully overexpressed in BICR 18 and
FaDu cells. The cell viabilities in BICR 18 and FaDu cells were
significantly decreased in the mimic group compared with the NC
groups following 48 h of culturing (P<0.05; Fig. 1D-G). The results demonstrated that
the upregulation of miR-490-5p was able to lower the proliferation
of pharyngolaryngeal cancer cells.
miR-490-5p could target MAP3K9 in BICR 18
and FaDu cell lines
To understand how the upregulation of miR-490-5p led
to decreased cell viability of BICR 18 and FaDu cells, the targets
of miR-490-5p were predicted using targetscan, miRTarBase and miRDB
databases and verified using a luciferase assay. The results of the
Venn diagram demonstrated that TNKS2, MAP3K9, NUFIP2, CLCC1 and
ABCC2 were target genes for miR-490-5p in results from targetscan,
miRTarBase and miRDB databases (Fig.
2A). It was suggested that these five genes may be the target
for miR-490-5p. Furthermore, the relative mRNA expression of MAP3K9
in miR-490-5p mimics group was significantly decreased compared
with in NC groups in the BICR 18 and FaDu cells, while the other
four genes exhibited no significant difference between the
miR-490-5p mimics group and NC group (P<0.05; Fig. 2B and C). Moreover, the relative
luciferase activities in the miR-490-5p mimics groups were
significantly decreased compared with NC groups when the sequence
of MAP3K9-3′-UTR was wild type, however, no significance difference
was exhibited between NC and miR-490-5p mimics groups when the
sequence of MAP3K9-3′-UTR was mutated in both BICR 18 and FaDu
cells (P<0.05; Fig. 2D-F).
Therefore, it was proved that MAP3K9 was the target gene for
miR-490-5p. The expression of MAP3K9 in tumor tissues was
significantly increased compared with in tumor-adjacent tissues
(P<0.01; Fig. 2H), and the
expression of MAP3K9 in tumor tissues and tumor-adjacent tissues
was opposite to that of miR-490-5p (P<0.01; Fig. 2G and H).
 | Figure 2Prediction of the target of
miR-490-5p. (A) Venn diagram exhibiting the most possible targets
of miR-490-5p (TNKS2, MAP3K9, NUFIP2, CLCC1 and ABCC2) predicting
from targetscan, miRTarBase and miRDB databases. (B) Relative mRNA
expressions of TNKS2, MAP3K9, NUFIP2, CLCC1 and ABCC2 in control,
NC and miR-490-5p mimics groups of BICR 18 cells. (C) Relative mRNA
expressions of TNKS2, MAP3K9, NUFIP2, CLCC1 and ABCC2 in control,
NC and miR-490-5p mimics groups of FaDu cells. (D) The possible
complementary sequences of MAP3K9 3′-UTR and miR-490-5p. (E)
Relative luciferase activities in control, NC, mimics groups of
BICR 18 cells when the sequences of MAP3K9-3′-UTR was wild or
mutant type. (F) Relative luciferase activities in control, NC,
mimics groups of FaDu cells when the sequences of MAP3K9-3′-UTR was
wild or mutant type. (G) Relative miR-490-5p expression in
laryngeal tumor and tumor-adjacent tissues. (H) Relative MAP3K9
expression in laryngeal tumor and tumor-adjacent tissues. Bars
indicated the mean ± standard deviation. *P<0.05 vs.
NC groups. miR, microRNA; NC, negative control; UTR, untranslated
region; MAPK, mitogen activated protein kinase. |
MAP3K9 overexpression partially reverses
the inhibitory effect of the miR-490-5p mimic on cell
viability
In order to confirm that MAP3K9 was involved in the
regulation of miR-490-5p on the cell proliferation, the expression
of MAP3K9 was overexpressed in BICR 18 and FaDu cells, and further
observed whether it could affect the cell viability in
miR-490-5p-upregulated BICR 18 and FaDu cells. As demonstrated in
the results of the present study, MAP3K9 was significantly
overexpressed in BICR 18 and FaDu cells (P<0.05; Fig. 3A-D). Furthermore, the cell
viabilities in miR-490-5p mimics + MAP3K9 groups were increased
compared with the miR-490-5p mimics groups in both BICR 18 and FaDu
cells, the cell viabilities in miR-490-5p mimics groups were
decreased compared with in the NC groups in both BICR 18 and FaDu
cells, while the cell viabilities in MAP3K9 groups were increased
compared with in NC groups (P<0.05; Fig. 3E and F). Therefore, it indicated
that miR-490-5p could target and inhibit the expression of MAP3K9
and lead to the downregulation of proliferation of
pharyngolaryngeal cancer cells.
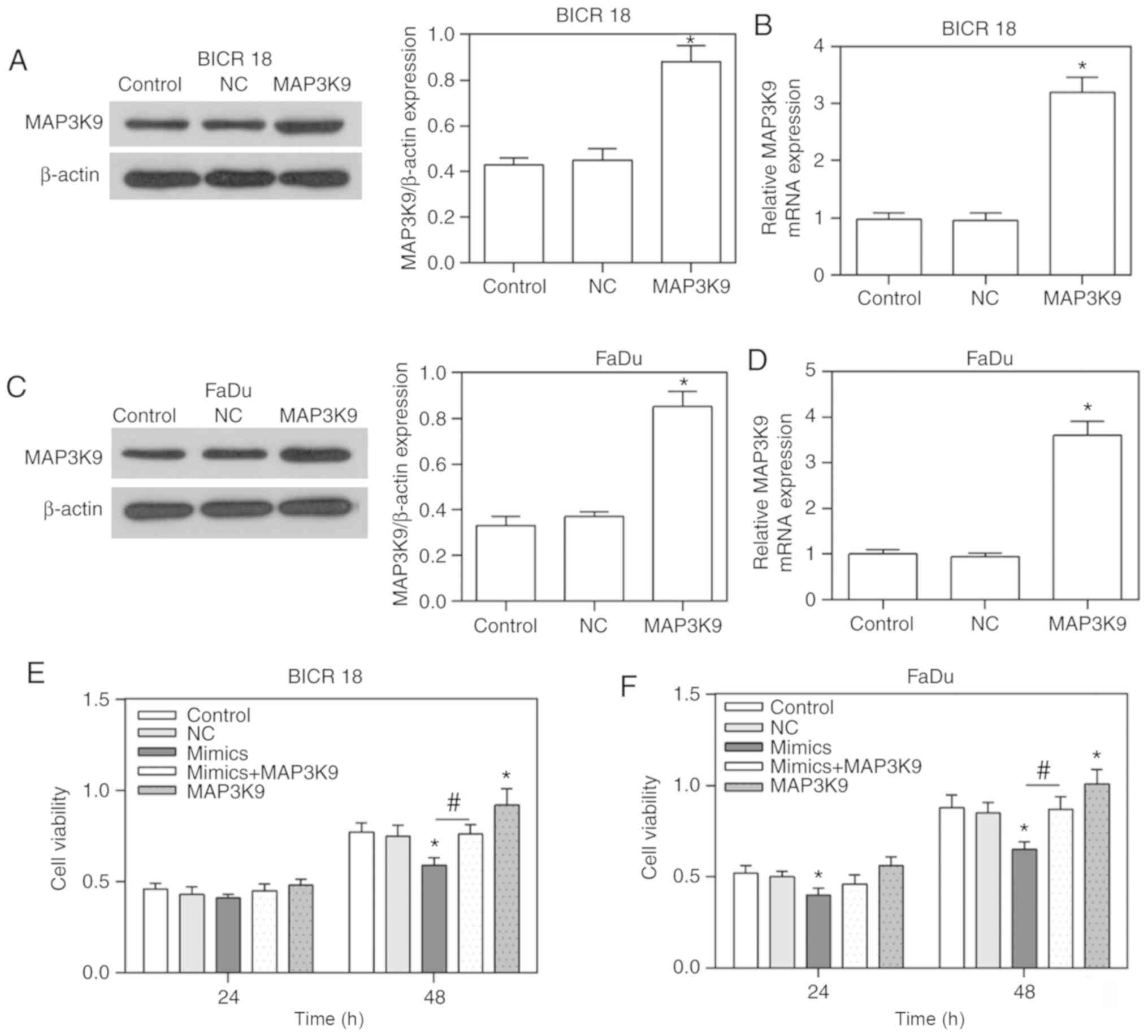 | Figure 3Overexpression of MAP3K9 in BICR 18
and FaDu cells as well as the alteration of cell viability in
control, NC, miR-490-5p mimics, miR-490-5p mimics + MAP3K9 and
MAP3K9 groups in BICR 18 and FaDu cells. (A) The protein expression
of MAP3K9 in control, NC and MAP3K9 groups of BICR 18 cells. (B)
Relative MAP3K9 mRNA expression in control, NC and MAP3K9 groups of
BICR 18 cells. (C) The protein expression of MAP3K9 in control, NC
and MAP3K9 groups of FaDu cells. (D) Relative MAP3K9 mRNA
expression in control, NC and MAP3K9 groups of FaDu cells. (E) The
cell viabilities in each group of BICR 18 cells following 24 and 48
h of culturing since the transfection. (F) The cell viabilities in
each group of FaDu cells following 24 and 48 h of culturing since
the transfection. Bars indicate the mean ± standard deviation.
*P<0.05 vs. NC groups; #P<0.05 vs.
miR-490-5p mimics groups. miR, microRNA; NC, negative control;
MAPK, mitogen activated protein kinase. |
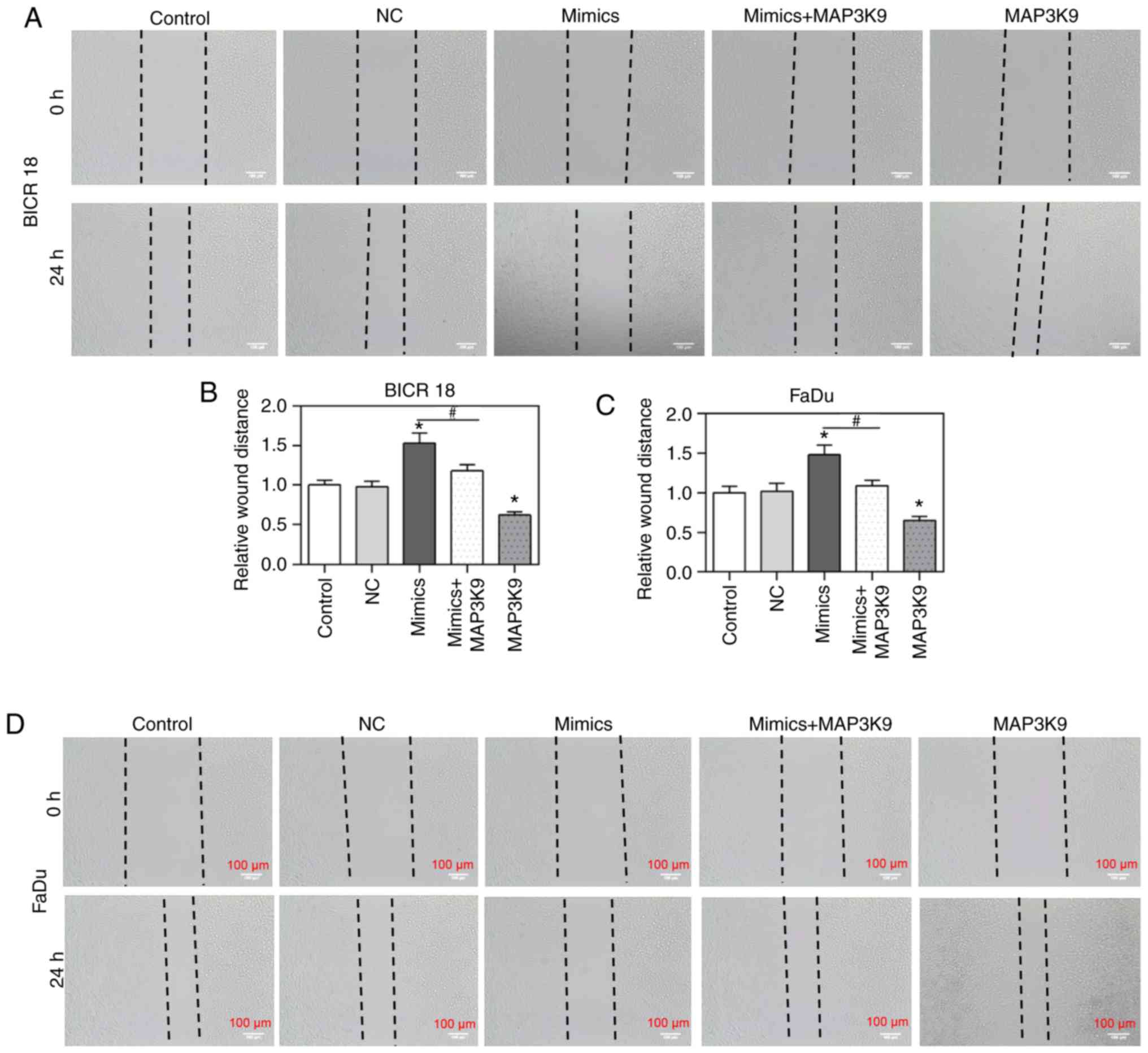
MAP3K9 overexpression partially reverses
the inhibitory effect of miR-490-5p mimic on cell migration
To confirm that MAP3K9 was involved in the
regulation of miR-490-5p on the cell migration, the alteration of
cell migration rate was measured by wound healing assay in BICR 18
and FaDu cells. The relative wound distances in miR-490-5p mimics
groups were identified to be increased compared with in the NC
groups but decreased in MAP3K9 groups compared with in NC groups in
BICR 18 and FaDu cells (P<0.05; Fig. 4). Significantly, the cell
viability in miR-490-5p mimics + MAP3K9 groups was decreased
compared with in the miR-490-5p mimics groups in BICR 18 and FaDu
cells (P<0.05; Fig. 4). The
results of the present study suggested that miR-490-5p could target
and inhibit the expression of MAP3K9, further leading to the
downregulation of the migration of pharyngolaryngeal cancer
cells.
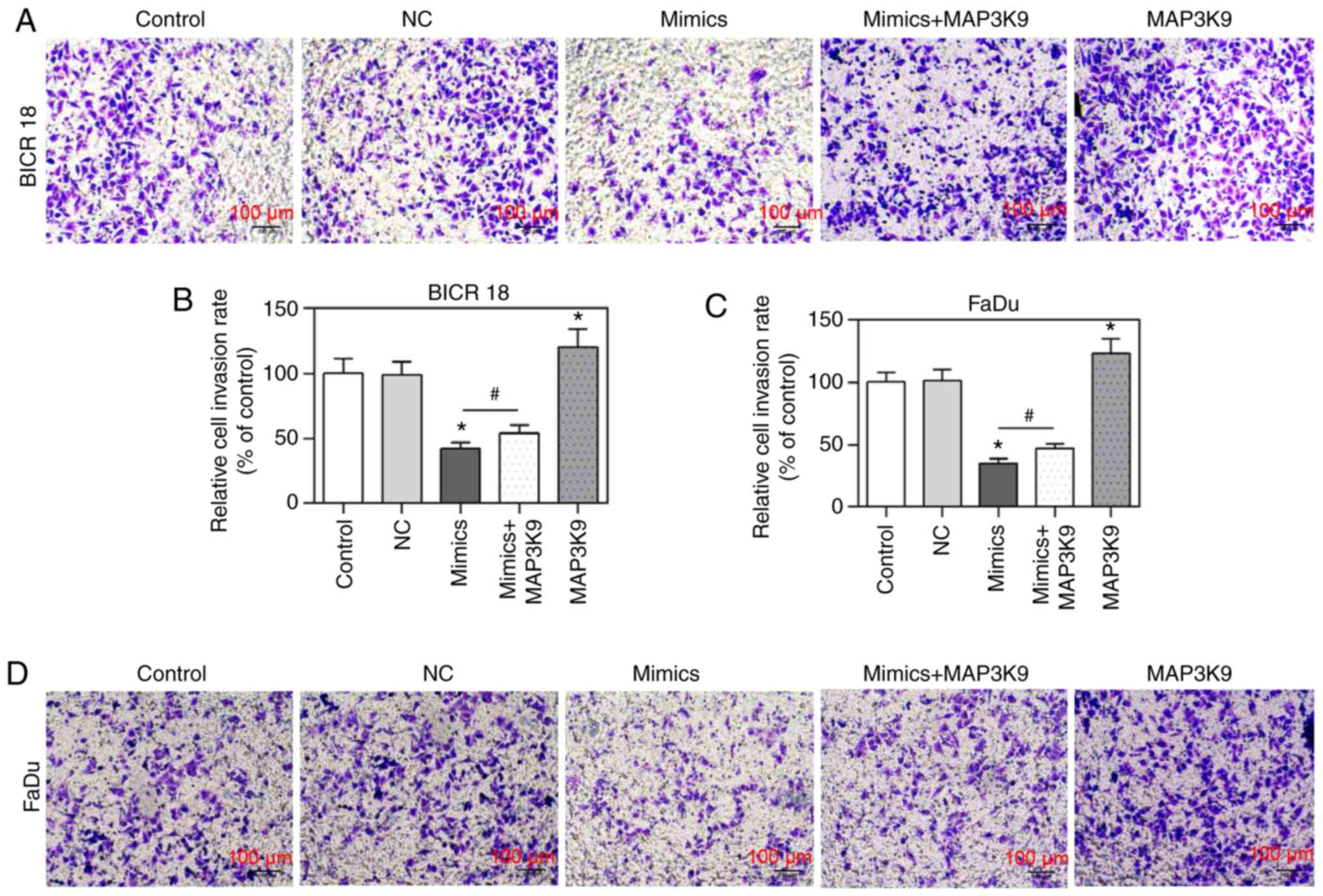
MAP3K9 overexpression partially reverses
the inhibitory effect of miR-490-5p mimic on cell invasion
To confirm that MAP3K9 was involved in the
regulation of miR-490-5p on cell invasion, the change of cell
invasion rate was measured by Transwell invasion assay in BICR 18
and FaDu cells. In the present study, the relative cell invasion
rates (of control) in miR-490-5p mimics group were observed to be
significantly decreased compared with in the NC groups in BICR 18
and FaDu cells and the relative cell invasion rates (of control) in
MAP3K9 group was significantly increased compared with in NC groups
in BICR 18 and FaDu cells (P<0.05; Fig. 5). Moreover, the relative cell
invasion rates (of control) in miR-490-5p mimics + MAP3K9 groups
were significantly increased compared with in the miR-490-5p mimics
groups in BICR 18 and FaDu cells (P<0.05; Fig. 5). It suggested that miR-490-5p
could target and inhibit the expression of MAP3K9 and further lead
to the downregulation of the invasion of pharyngolaryngeal cancer
cells.
MAP3K9 overexpression partially reverses
the inhibitory effect of miR-490-5p mimic on EMT process
To confirm that MAP3K9 was involved in the
regulation of miR-490-5p on the migration, invasion and EMT
process, the expression of MMP-9, TIMP-2, E-cadherin and vimentin
was measured in BICR 18 and FaDu cells. It was demonstrated that
the expression of MMP-9 and vimentin were significantly decreased,
while the expression of TIMP-2 and E-cadherin were significantly
increased in the miR-490-5p mimics group (P<0.05; Fig. 6). Compared with BICR 18 and FaDu
cells in the NC groups, the expression of MMP-9 and vimentin were
significantly increased, while the expression of TIMP-2 and
E-cadherin were significantly decreased in MAP3K9 groups, compared
with BICR 18 and FaDu cells in the NC groups (P<0.05; Fig. 6). In addition, the expression of
MMP-9 and vimentin in miR-490-5p mimics + MAP3K9 groups were
significantly increased compared with in the miR-490-5p mimics
groups, while the expression of TIMP-2 and E-cadherin in miR-490-5p
mimics + MAP3K9 groups were significantly decreased in BICR 18 and
FaDu cells (P<0.05; Fig. 6) in
the miR-490-5p mimics groups. The results of mRNA and protein
expression levels were consistent, apart from the relative protein
level of TIMP-2 in FaDu cells. It indicated that miR-490-5p could
target and inhibit the expression of MAP3K9 and lead to the further
downregulation of migration, invasion and EMT process of
pharyngolaryngeal cancer cells.
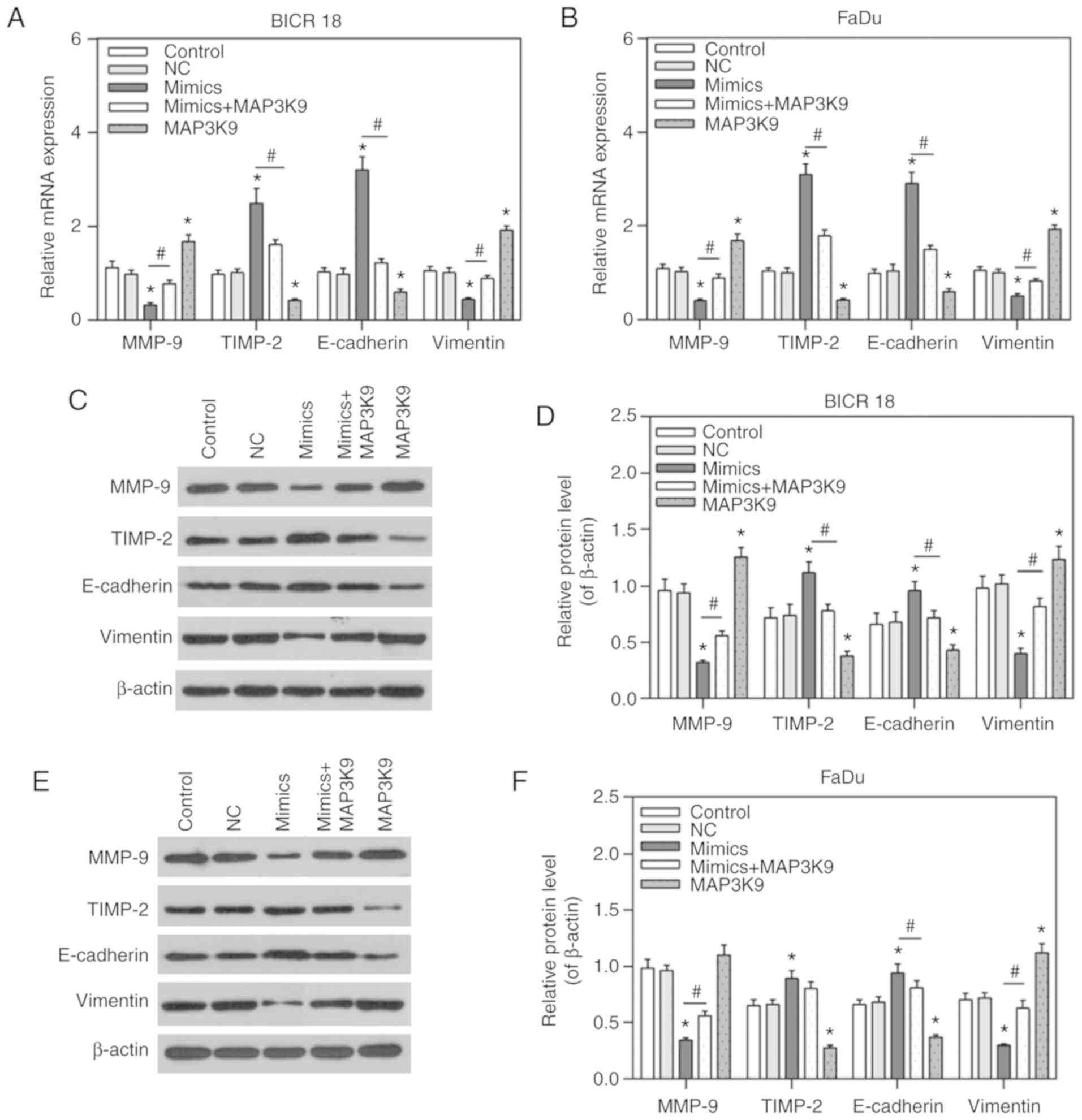 | Figure 6Expression of MMP-9, TIMP-2,
E-cadherin and vimentin in the control, NC, miR-490-5p mimics,
miR-490-5p mimics + MAP3K9 and MAP3K9 groups of BICR 18 and FaDu
cells. (A) Relative mRNA expressions of MMP-9, TIMP-2, E-cadherin
and vimentin in each group of BICR 18 cells. (B) Relative mRNA
expressions of MMP-9, TIMP-2, E-cadherin and vimentin in each group
of FaDu cells. (C) The original outcomes of western blot of the
expressions of MMP-9, TIMP-2, E-cadherin and vimentin in each group
of BICR 18 cells. (D) Relative protein levels of MMP-9, TIMP-2,
E-cadherin and vimentin (of β-actin) in each group of BICR 18 cells
(E) The original outcomes of western blotting of the expression of
MMP-9, TIMP-2, E-cadherin and vimentin in each group of FaDu cells.
(F) Relative protein levels of MMP-9, TIMP-2, E-cadherin and
vimentin (of β-actin) in each group of FaDu cells. Bars indicates
the mean ± standard deviation. *P<0.05 vs. NC groups;
#P<0.05 vs. miR-490-5p mimics groups. miR, microRNA;
NC, negative control; MAPK, mitogen activated protein kinase; E,
epithelial; TIMP-2, tissue inhibitor of metalloproteinase; MMP,
matrix metalloproteinase. |
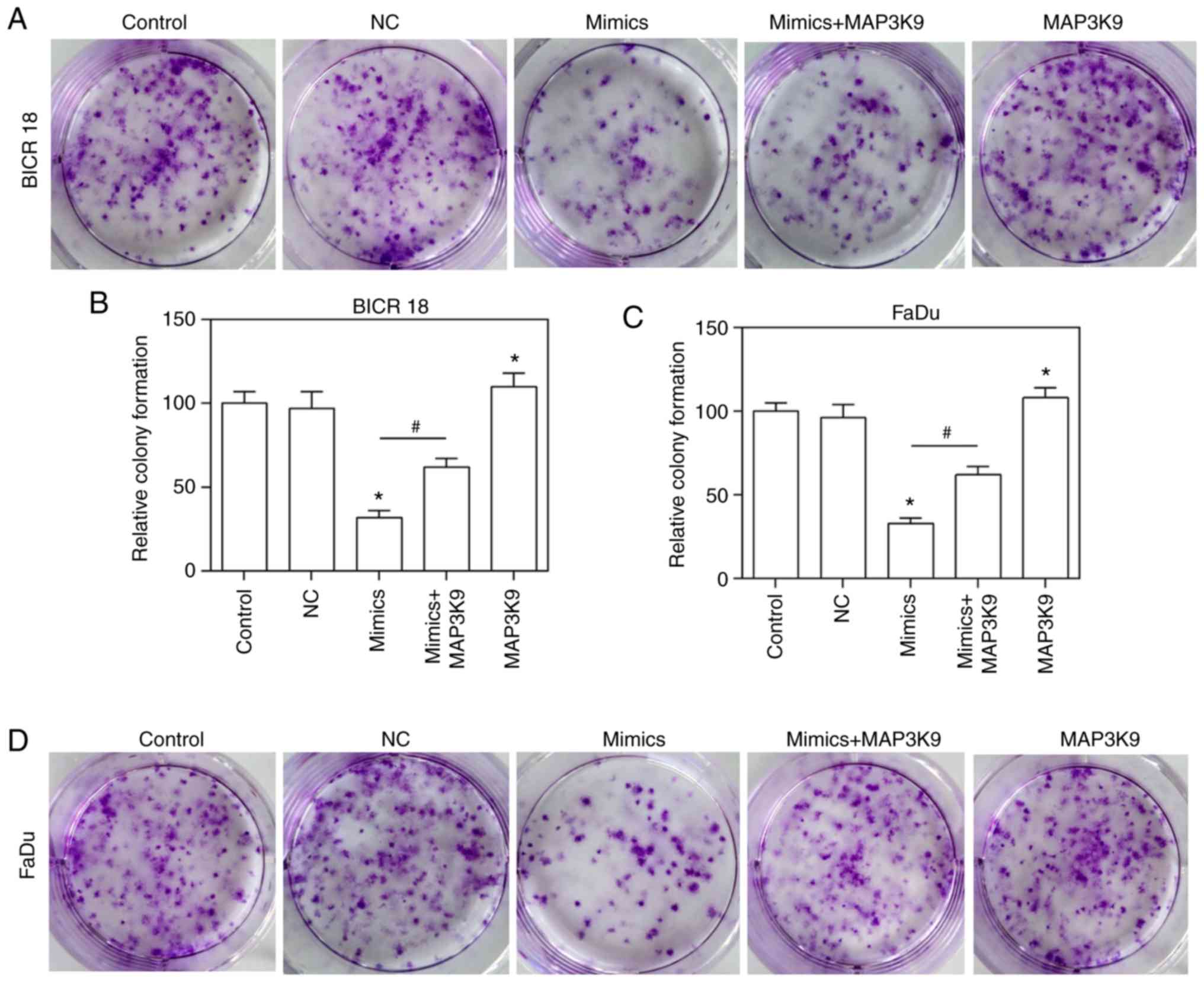
MAP3K9 overexpression partially reverses
the inhibitory effect of the miR-490-5p mimic on the cloning of
ability of pharyngolaryngeal cancer cells
To confirm that MAP3K9 was involved in the
regulation of miR-490-5p on the ability of growth, the growth
ability of BICR 18 and FaDu cells was measured by plate clone
formation assay. The relative colony formation in the miR-490-5p
mimics and MAP3K9 groups was significantly decreased and increased,
respectively compared with in NC groups in BICR 18 and FaDu cells
(P<0.05; Fig. 7). Furthermore,
the relative colony formation in miR-490-5p mimics + MAP3K9 groups
was significantly increased compared with the miR-490-5p mimics
groups in BICR 18 and FaDu cells (Fig. 7; P<0.05). It suggested that
miR-490-5p could target and inhibit the expression of MAP3K9 and
lead to the downregulation of the ability of cloning of
pharyngolaryngeal cancer cells.
Discussion
In the present study, it was identified that
miR-490-5p was downregulated in laryngeal tumor tissue,
tumor-adjacent tissue and tumor cell lines, and that patients who
demonstrated a low expression level of miR-490-5p exhibited a poor
prognosis during the development of pharyngolaryngeal cancer.
Furthermore, it was demonstrated that miR-490-5p could precisely
target MAP3K9, which served a critical role in the modulation of
miR-490-5p on the proliferation, migration, invasion, EMT and
colony formation of BICR 18 and FaDu cells.
In the present study, it was identified that the
expression of miR-490-5p was decreased in laryngeal tumor tissues
and cell lines, leading to an unfavorable prognosis. According to
the study of Xu et al (17) decreased expression of miR-490-5p
was detected in hepatocellular carcinoma (HCC) tissues and cells.
Furthermore, Fang et al (19) reported that the expression level
of miR-490-5p was connected with survival rate of HCC patients.
Therefore, it was hypothesized that miR-490-5p seemed to be
downregulated in most pharyngolaryngeal cancer tissues and cell
lines, leading to a poor prognosis of patients. miR-490-5p
expression was also detected in NP69, HaCaT, BICR 18, FaDu, HNE-3
and Detroit 562 cells, and the result demonstrated that miR-490-5p
expression in pharyngolaryngeal cancer cells was decreased,
particularly in BICR 18 and FaDu cells, which further confirmed
that the expression of miR-490-5p was downregulated in
pharyngolaryngeal cancer cells. In addition, laryngeal carcinoma is
one of the most common head and neck neoplasms, which is mainly
represented by squamous cell carcinoma was predominant, BICR 18 and
FaDu cells are squamous cells (20,21). Therefore, BICR 18 and FaDu cells
were selected for later experiments.
Furthermore, it was identified that miR-490-5p could
decrease the cell viabilities of BICR 18 and FaDu cells. Lan et
al (22) revealed that the
overexpression of miR-490-5p inhibited the proliferation of T24
cells (bladder cancer cells). Therefore, it was hypothesized that
the overexpression of miR-490-5p could eventually decrease the
proliferation of pharyngolaryngeal cancer cells.
The underlying mechanism of the effect of miR-490-5p
on the proliferation of pharyngolaryngeal cancer cells attracted
the authors' attention. Targetscan, miRTarBase and miRDB databases
are widely used tools for predicting the targets of microRNAs. A
total of five candidates were screened out as the targets for
miR-490-5p and it was confirmed that MAP3K9 was the most accurate
target for miR-490-5p by luciferase assay. To date, to the best of
our knowledge, no study has demonstrated that MAP3K9 was the target
of miR-490-5p. Therefore, it was the first time that MAP3K9 was
proved to be the target for miR-490-5p in BICR 18 and FaDu cell
lines.
Through elevating the expression of MAP3K9 in BICR
18 and FaDu cells, which overexpressed miR-490-5p (mimic), it was
demonstrated that miR-490-5p may regulate the proliferation of
these two cell lines via targeting MAP3K9. In the present study, it
was identified that the overexpression of miR-490-5p could decrease
cell viability or colony formation by the downregulation of MAP3K9
in cells. MAP3K9 is the upstream kinase in the MAPK signaling
pathway, belonging to the MAPK kinase kinase (MAPKK) family
(23,24). MAP3K9 is composed of 1,104 amino
acids, receiving various stimulatory signals from receptors of cell
membranes (23,24). Its phosphorylation can activate
the downstream MAPKK and further activate MAPK, which ultimately
activate the whole MAPK signaling pathway, which is the classic
pathway that regulates plenty of cellular functions including cell
proliferation, differentiation, migration and apoptosis (23,24). Nie et al (15) reported that miR-148b increased
proliferation in human renal cancer cells by targeting MAP3K9. Xia
et al (16) also revealed
that MAP3K9 directly targets miR-7, which increased the
proliferation of pancreatic cancer cells. This evidence indirectly
supported the hypothesis that MAP3K9 participated in the regulation
of miR-490-5p on the proliferation of pharyngolaryngeal cancer
cells.
Migration and invasion represent the metastatic
ability of cancer which is the most important hallmark of tumors
(25). It was identified that the
overexpressed miR-490-5p could decrease cell migration and invasion
by inhibiting MAP3K9. Chen et al (26) reported that miR-490-5p inhibited
the migration and invasion of HCC. Furthermore, the participation
of MAP3K9 in regulating the migration and invasion of PC cells had
been demonstrated (16).
Therefore, miR-490-5p may regulate the migration and invasion of
pharyngolaryngeal cancer cells by targeting MAP3K9.
MMP-9 belongs to the MMP family and degrades type IV
collagen effectively, therefore destroying the integrity of the
basal membrane, which is associated with infiltration and
metastasis of tumors (27,28).
MMP-9 is one of the invasion and migration marker proteins, and a
number of studies have demonstrated that the upregulation of MMP-9
could enhance the migration and invasion of a variety of cancer
cells including gastric cancer, esophageal squamous cell cancer and
oral cancer (29-31). E-cadherin not only inhibits the
communication between tumor cells, but also the movement and
invasion of tumors (32). The
downregulation of E-cadherin could significantly lower the risk of
EMT of tumor cells (32,33). vimentin is one of the cytoskeletal
proteins and is expressed mainly in cells derived from mesoderm,
which is considered to be a key factor in EMT (34,35). TIMP-2 is a type of endogenous
enzyme that inhibits the degradation of the extracellular matrix
and maintains the stability of the environment within the tissues,
therefore resisting the initiation of the EMT process (36,37). Notably, it was identified that
miR-490-5p overexpression could suppress MMP-9, vimentin expression
and increase E-cadherin, TIMP-2 expression in the BICR 18 and FaDu
cells by downregulating MAP3K9 expression. This suggests that
miR-490-5p could inhibit the migration, invasion and EMT process of
pharyngolaryngeal cancer cells by targeting MAP3K9. Chen et
al (38) revealed that
miR-490-5p may be a tumor suppressor and was involved in clear cell
renal cell carcinomas metastasis. Luo et al (39) demonstrated that the overexpression
of miR-148a inhibited cutaneous squamous cell carcinoma cell
metastasis by downregulating MAP3K9. Though this study was
identified to directly support the present study's findings, it was
hypothesized that miR-490-5p may regulate the EMT progress of
pharyngolaryngeal cancer cells by targeting MAP3K9.
In the present study, the MAP3K9 expression cDNA
vector was transfected into cells to achieve the effect of MAP3K9
overexpression, the cDNA contains the complete UTR region and
miR-490-5p can bind to the target site of MAP3K9 to rescue the
phenotype of miR-490-5p. It was identified that overexpression of
miR-490-5p inhibited the proliferation, migration, invasion and EMT
process of BICR 18 and FaDu cells by targeting MAP3K9, however, the
effect of silencing miR-490-5p on the proliferation, migration,
invasion and EMT process of laryngeal cancer cells remains unknown,
and needs to be further investigated. In addition, to verify
whether miR-490-5p/MAP3K9 serves a role in the apoptosis,
migration, invasion and EMT of laryngeal cancer cells via
participating in specific signaling pathways, also needs to be
discussed in the future. This study has certain limitations, in the
experiment to detect the expression of miR-490-5p in tumor and
tumor-adjacent tissues, the results would have been more reliable
if the experiment of the in-situ hybridization had been conducted.
Furthermore, with reference to the research methods of other
studies (40-42), the effect of cell proliferation on
migration and invasion was not excluded when detecting the effect
of miR-490-5p on cell migration and invasion.
In conclusion, miR-490-5p could target MAP3K9 and
further modulate the proliferation, migration, invasion and EMT of
pharyngolaryngeal cancer cells. The present study provides a novel
entry point to the treatment of pharyngolaryngeal cancer.
Funding
No funding received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
Substantial contributions to conception and design:
AA. Data acquisition, data analysis and interpretation: XC, ML and
YT. Drafting the article or critically revising it for important
intellectual content: XC and ML. Final approval of the version to
be published: All authors, Agreement to be accountable for all
aspects of the work in ensuring that questions associated with the
accuracy or integrity of the work are appropriately investigated
and resolved: AA and XC.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. The present study was approved by the Ethics Committee
of the People's Hospital of Xinjiang Uygur Autonomous Region, and
an informed consent was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Acknowledgments
Not applicable.
References
|
1
|
de Miguel-Luken MJ, Chaves-Conde M, de
Miguel-Luken V, Muñoz-Galván S, López-Guerra JL, Mateos JC, Pachón
J, Chinchón D, Suarez V and Carnero A: MAP17 (PDZKIP1) as a novel
prognostic biomarker for laryngeal cancer. Oncotarget.
6:12625–12636. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Butler A, Rigby MH, Scott J, Trites J,
Hart R and Taylor SM: A retrospective review in the management of
T3 laryngeal squamous cell carcinoma: An expanding indication for
transoral laser microsurgery. J Otolaryngol Head Neck Surg.
45:342016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Q, Liu Y, Hu G, Wang R, Zhao Y and
Zhang M: The survival rate and larynx preservation in elderly
cancer patients who received surgical operation: A retrospective
cohort study. Int J Surg. 36:342–346. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Henriques de Figueiredo B and Grégoire V:
How to minimize morbidity in radiotherapy of pharyngolaryngeal
tumors? Curr Opin Otolaryngol Head Neck Surg. 24:163–169. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cui N, Hao G, Zhao Z, Wang F, Cao J and
Yang A: MicroRNA-224 regulates self-renewal of mouse spermatogonial
stem cells via targeting DMRT1. J Cell Mol Med. 20:1503–1512. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meza-Sosa KF, Valle-Garcia D, Pedraza-Alva
G and Perez-Martinez L: Role of microRNAs in central nervous system
development and pathology. J Neurosci Res. 90:1–12. 2012.
View Article : Google Scholar
|
|
7
|
Leng R, Zha L and Tang L: miR-718
represses VEGF and inhibits ovarian cancer cell progression. FEBS
Lett. 588:2078–2086. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tutar Y: miRNA and cancer; computational
and experimental approaches. Curr Pharm Biotechnol. 15:4292014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang SS, Jiang WW, Smith I, Poeta LM,
Begum S, Glazer C, Shan S, Westra W, Sidransky D and Califano JA:
MicroRNA alterations in head and neck squamous cell carcinoma. Int
J Cancer. 123:2791–2797. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin C, Zhang Y and Li J: Upregulation of
miR-196a promotes cell proliferation by downregulating
p27kip1 in laryngeal cancer. Biol Res. 49:402016.
View Article : Google Scholar
|
|
11
|
Krishnan AR, Zheng H, Kwok JG, Qu Y, Zou
AE, Korrapati A, Li PX, Califano JA, Hovell MF, Wang-Rodriguez J
and Ongkeko WM: A comprehensive study of smoking-specific microRNA
alterations in head and neck squamous cell carcinoma. Oral Oncol.
72:56–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhan YH, Liu J, Qu XJ, Hou KZ, Wang KF,
Liu YP and Wu B: β-Elemene induces apoptosis in human renal-cell
carcinoma 7860 cells through inhibition of MAPK/ERK and
PI3K/Akt/mTOR signalling pathways. Asian Pac J Cancer Prev.
13:2739–2744. 2012. View Article : Google Scholar
|
|
13
|
Du HF, Ou LP, Song XD, Fan YR, Yang X, Tan
B, Quan Z, Luo CL and Wu XH: Nuclear factor-κB signaling pathway is
involved in phospholipase Cε-regulated proliferation in human renal
cell carcinoma cells. Mol Cell Biochem. 389:265–275. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hannes S, Abhari BA and Fulda S: Smac
mimetic triggers necroptosis in pancreatic carcinoma cells when
caspase activation is blocked. Cancer Lett. 380:31–38. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nie F, Liu T, Zhong L, Yang X, Liu Y, Xia
H, Liu X, Wang X, Liu Z, Zhou L, et al: MicroRNA-148b enhances
proliferation and apoptosis in human renal cancer cells via
directly targeting MAP3K9. Mol Med Rep. 13:83–90. 2016. View Article : Google Scholar :
|
|
16
|
Xia J, Cao T, Ma C, Shi Y, Sun Y, Wang ZP
and Ma J: miR-7 suppresses tumor progression by directly targeting
MAP3K9 in pancreatic cancer. Mol Ther Nucleic Acids. 13:121–132.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu B, Xu T, Liu H, Min Q, Wang S and Song
Q: miR-490-5p suppresses cell proliferation and invasion by
targeting BUB1 in hepatocellular carcinoma cells. Pharmacology.
100:269–282. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using rea time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Fang ZQ, Li MC, Zhang YQ and Liu XG:
miR-490-5p inhibits the metastasis of hepatocellular carcinoma by
down-regulating E2F2 and ECT2. J Cell Biochem. 119:8317–8324. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu W, Feng L, Li P, Wang Y, Du Y, Chen X,
Wu S, Zhao G and Lou W: Effects of HPV-16 infection on
hypopharyngeal squamous cell carcinoma and FaDu cells. Oncol Rep.
35:99–106. 2016. View Article : Google Scholar
|
|
21
|
Li H, Wawrose JS, Gooding WE, Garraway LA,
Lui VW, Peyser ND and Grandis JR: Genomic analysis of head and neck
squamous cell carcinoma cell lines and human tumors: A rational
approach to preclinical model selection. Mol Cancer Res.
12:571–582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lan G, Yang L, Xie X, Peng L and Wang Y:
MicroRNA-490-5p is a novel tumor suppressor targeting c-FOS in
human bladder cancer. Arch Med Sci. 11:561–569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Durkin JT, Holskin BP, Kopec KK, Reed MS,
Spais CM, Steffy BM, Gessner G, Angeles TS, Pohl J, Ator MA and
Meyer SL: Phosphoregulation of mixed-lineage kinase 1 activity by
multiple phosphorylation in the activation loop. Biochemistry.
43:16348–16355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Slattery ML, Hines LH, Lundgreen A,
Baumgartner KB, Wolff RK, Stern MC and John EM: Diet and lifestyle
factors interact with MAPK genes to influence survival: The Breast
Cancer Health Disparities Study. Cancer Causes Control.
25:1211–1225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen W, Ye L, Wen D and Chen F: miR-490-5p
inhibits hepatocellular carcinoma cell proliferation, migration and
invasion by directly regulating ROBO1. Pathol Oncol Res. 25:1–9.
2019. View Article : Google Scholar
|
|
27
|
Yang Z, Li K, Liang Q, Zheng G, Zhang S,
Lao X, Liang Y and Liao G: Elevated hydrostatic pressure promotes
ameloblastoma cell invasion through upregulation of MMP-2 and MMP-9
expression via Wnt/β-catenin signalling. J Oral Pathol Med.
47:836–846. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X, Tao Y and Li X: Expression of
MMP-9/TIMP-2 in nasal polyps and its functional implications. Int J
Clin Exp Pathol. 8:14556–14561. 2015.
|
|
29
|
Chang X, Xu X, Xue X, Ma J, Li Z, Deng P,
Chen J, Zhang S, Zhi Y and Dai D: NDRG1 controls gastric cancer
migration and invasion through regulating MMP-9. Pathol Oncol Res.
22:789–796. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang L, Song X, Zhu J, Li M, Ji Y, Wu F,
Chen Y, Cui X, Hu J, Wang L, et al: Tumor suppressor microRNA-34a
inhibits cell migration and invasion by targeting
MMP-2/MMP-9/FNDC3B in esophageal squamous cell carcinoma. Int J
Oncol. 51:378–388. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hsu LS, Huang RH, Lai HW, Hsu HT, Sung WW,
Hsieh MJ, Wu CY, Lin YM, Chen MK, Lo YS and Chen CJ: KLF6 inhibited
oral cancer migration and invasion via downregulation of
mesenchymal markers and inhibition of MMP-9 activities. Int J Med
Sci. 14:530–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yazdani J, Ghavimi MA, Jabbari Hagh E and
Ahmadpour F: The role of E-cadherin as a prognostic biomarker in
head and neck squamous carcinoma: A systematic review and
meta-analysis. Mol Diagn Ther. 22:523–535. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tiwari N, Gheldof A, Tatari M and
Christofori G: EMT as the ultimate survival mechanism of cancer
cells. Semin Cancer Biol. 22:194–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu S, Du Y, Beckford J and Alachkar H:
Upregulation of the EMT marker vimentin is associated with poor
clinical outcome in acute myeloid leukemia. J Transl Med.
16:1702018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu CY, Lin HH, Tang MJ and Wang YK:
Vimentin contributes to epithelial-mesenchymal transition cancer
cell mechanics by mediating cytoskeletal organization and focal
adhesion maturation. Oncotarget. 6:15966–15983. 2015.PubMed/NCBI
|
|
36
|
Łukaszewicz-Zając M, Mroczko B,
Guzińska-Ustymowicz K, Pryczynicz A, Gryko M, Kemona A, Kędra B and
Szmitkowski M: Matrix metalloproteinase 2 (MMP-2) and their tissue
inhibitor 2 (TIMP-2) in gastric cancer patients. Adv Med Sci.
58:235–243. 2013. View Article : Google Scholar
|
|
37
|
Urbaniak-Kujda D, Kapelko-Slowik K, Prajs
I, Dybko J, Wolowiec D, Biernat M, Slowik M and Kuliczkowski K:
Increased expression of metalloproteinase-2 and -9 (MMP-2, MMP-9),
tissue inhibitor of metalloproteinase-1 and -2 (TIMP-1, TIMP-2),
and EMMPRIN (CD147) in multiple myeloma. Hematology. 21:26–33.
2016. View Article : Google Scholar
|
|
38
|
Chen K, Zeng J, Tang K, Xiao H, Hu J,
Huang C, Yao W, Yu G, Xiao W, Guan W, et al: miR-490-5p suppresses
tumour growth in renal cell carcinoma through targeting PIK3CA.
Biol Cell. 108:41–50. 2016. View Article : Google Scholar
|
|
39
|
Luo Q, Li W, Zhao T, Tian X, Liu Y and
Zhang X: Role of miR-148a in cutaneous squamous cell carcinoma by
repression of MAPK pathway. Arch Biochem Biophys. 583:47–54. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mao D, Qiao L, Lu H and Feng Y: B-cell
translocation gene 3 overexpression inhibits proliferation and
invasion of colorectal cancer SW480 cells via Wnt/β-catenin
signaling pathway. Neoplasma. 63:705–716. 2016. View Article : Google Scholar
|
|
41
|
Jia S, Qu T, Wang X, Feng M, Yang Y, Feng
X, Ma R, Li W, Hu Y, Feng Y, et al: KIAA1199 promotes migration and
invasion by Wnt/β-catenin pathway and MMPs mediated EMT progression
and serves as a poor prognosis marker in gastric cancer. PLoS One.
12:e01750582017. View Article : Google Scholar
|
|
42
|
Yang D, Du G, Xu A, Xi X and Li D:
Expression of miR-149-3p inhibits proliferation, migration, and
invasion of bladder cancer by targeting S100A4. Am J Cancer Res.
7:2209–2219. 2017.PubMed/NCBI
|