Introduction
Leukemia is a malignant disease characterized by the
abnormal growth of hematopoietic stem cells. According to the
American Cancer Society and the National Cancer Institute in 2016,
~468,000 patients were diagnosed with leukemia in the United States
(1). As one of the most common
blood tumors in adult leukemia, acute myeloid leukemia (AML), which
is characterized by abnormal proliferation and the accumulation of
a large number of unusual hematopoietic stem cells in bone marrow,
peripheral blood and even in other tissues, could result in the
destruction of the hematopoietic system, and the morbidity and
mortality of the disease has exhibited an annual increase (1,2).
At present, cell-dependent therapy, hepatocyte transplantation,
targeted therapy, chemotherapy and radiotherapy have been applied
for the treatment of leukemia under different circumstances
(3-6). In regard to induction therapy for
AML, the main chemotherapy regimen has been the combination of
anthracyclines and cytarabine for the past three decades (7). Although a recent multicenter
clinical phase III trial revealed that the complete remission rate
of patients with AML could be as high as 79%, the overall survival
and relapse-free survival were only 20 and 15 months, respectively
(8). Therefore, relapse of drug
resistance is still a leading cause of mortality in patients with
AML, and it is also a major issue in the attempt to maintain longer
survival times after initial remission (9).
The etiology of AML is very complex, and current
research has indicated that chromosomal abnormalities and
reproducible genetic abnormalities were the main mechanisms of
morbidity in patients with AML (10-12). High mobility group A2 (HMGA2), a
member of the high mobility group protein superfamily, is widely
accepted as a new oncogene (13,14). HNGA2 has the physiological
functions of inducing gene transcription, integrating retrovirus
into chromosomes, inducing transformation and promoting the
activation of cancer cells; it also plays an important role in
maintaining stem cell differentiation potential and self-renewal
ability (15-17). Based on the high expression of
HMGA2 at the embryonic stage, the association between HMGA2 and
stem cells was studied. We previously reported that the expression
of HMGA2 was high in embryonic stem cells and its expression
gradually decreased with age (18). Meyer et al (19) suggested that the level of HMGA2
was increased in the CML-accelerated and CML-blastic phases, when
compared with that in the CML-chronic phase. Furthermore, the
expression of HMGA2 was negatively correlated to let-7b (19,20). In addition, HMGA2 could accelerate
the G2/M phase of cell cycle transformation or induce
epithelial-mesenchymal transition to promote tumorigenesis,
invasion and metastasis (16,21).
However, the role of HMGA2 in AML and the underlying
mechanism are still unclear. Several signaling pathways have been
reported to be important in the progression of leukemia including
the Wnt/β-catenin, PI3K/Akt/mTOR, NF-κB and Janus kinase/STAT
signaling pathways (22-25). The aim of the present study was to
investigate the Wnt/β-catenin signaling pathway in regulation of
HMGA2 in AML cells.
Materials and methods
Cell culture
The human myeloid leukemia cell lines, NB4, HL60,
KG1, U937, Kasumi-1, THP-1 and K562 were purchased from American
Type Culture Collection. All cells were cultured at 37°C in 5%
CO2 atmosphere in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) containing with 10% fetal bovine serum (FBS;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and
streptomycin (North China Pharmaceutical Co., Ltd.).
NB4 and HL60 cells were selected to conduct the
following experiments. Both cell lines were treated with 10
µg/ml dauno-rubicin (DNR; Shenzhen Main Luck Pharmaceuticals
Inc.) for 24 h at 37°C to evaluate the cell sensitivity to DNR. In
addition, NB4 and HL60 cells were treated with 10 µM XAV939
(MedChemExpress USA), or 10 µM XAV939 + 10 µg/ml DNR
and 20 mM LiCl (Sigma Aldrich; Merck KGaA), or 20 mM LiCl + 10
µg/ml DNR for 24 h at 37°C to perform mechanism-related
experiments.
Cell transfection
HL60 and NB4 cells, with or without drug treatments,
were respectively seeded in 6-well plates (1.0×105) for
24 h at 37°C before transfection. Silencing HMGA2 [small
interfering RNA (siRNA/si-) HMGA2; forward,
5′-AGAUUGAGAUUGAAAGUGCCU-3′ and reverse,
5′-GCACUUUCAAUCUCAAUCUCU-3′], overexpressing HMGA2 (HMGA2) and
negative control (NC) plasmids (5 µg/well of each plasmid)
were synthetized by Invitrogen (Thermo Fisher Scientific, Inc.).
Lipofectamine 2000™ (Invitrogen; Thermo Fisher Scientific, Inc.)
was applied to determine transient transfection according to
manufacturer's protocol. siHMGA2, siNC, HMGA2 or NC and
Lipofectamine 2000™ were respectively added to Opti-Minimum
Essential Medium (MEM; Gibco; Thermo Fisher Scientific, Inc.)
medium. The Lipofectamine/siRNA or Lipofectamine/overexpressing RNA
mixtures were cultured at 20°C for 10 min and then Opti-MEM
RPMI-1640 medium was added. After 6 h of culture, the media was
changed back to RPMI-1640 medium containing 10% FBS. After 24 h
culture, cells were used in the subsequent experiments.
Cell viability
Cell viability was determined using a 3-(4,
5-dimethylthiazol-2-yl)-2, 5 diphenyltetrazolium bromide (MTT;
Beyotime Institute of Biotechnology) assay. After transfection or
treatment with drugs for 24, 48 or 72 h, 5×103 cells per
well were seeded into 96-well plates and cultured at 37°C with 5%
CO2. Subsequently, 10 µl MTT was added into each
well containing culture medium for a further 1 h. Then, 100
µl DMSO was added to dissolve crystals once the media was
removed. The optical density values were detected using a
Microplate Reader (Thermo Fisher Scientific, Inc.) at 490 nm.
Wound scratch and Transwell assays
Cells (5×104) were seeded in 12-well
plates and incubated at 37°C for 24 h. A sterile pipette tip (10
µl) was used to draw a wound in the center of the plate. The
plates were gently washed 3 times with PBS. Then, the cells were
cultured in serum-free medium for 0 or 48 h. The scratch area was
measured using ImageJ software version 1.8.0 (National Institutes
of Health).
The invasion activity of cells was detected using a
24-well transwell chamber coated with Matrigel (Corning, Inc.).
After 24 h of transfection treatment, the cells were resuspended in
serum-free medium and 1×105 cells were added into the
coated upper chamber. RPMI-1640 medium containing 10% FBS was added
to the lower chamber and the cells were incubated for 48 h at 37°C
in an environment with a 5% CO2. The cells were fixed
with 4% formaldehyde for 20 min at 25°C and stained with 1% crystal
violet for a further 15 min at 37°C. The number of invading cells
was counted at ×200 magnification using a light microscope.
Flow cytometry
Transfected cells (5×105) were digested
with 0.25% trypsin and centrifugated at 1,000 × g for 5 min at
37°C. The apoptosis assay was performed using Annexin V-FITC. The
cells (5×105 cells/well) were washed twice using washing
buffer, and the suspension was cultured with the Annexin V-FITC and
propidium iodide apoptosis kit [cat. no. 70-AP101-60; MultiSciences
(Lianke) Biotech Co., Ltd.] in the dark at 25°C for 20 min
according to the manufacturer's instructions. Binding buffer was
subsequently added to each well. A flow cytometer was used to
detect samples within 1 h and BD CellQuest™ Pro Software version
1.2 was used for analysis (BD Biosciences).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA in cultured cells or cells treated with
drugs or plasmids was extracted with TRIzol regent (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. The Superscript II first-strand cDNA synthesis System
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to perform
RT. RT-qPCR was carried out using the SYBR Fast qPCR Mix
(Invitrogen; Thermo Fisher Scientific, Inc.) for HMGA2, X-linked
inhibitor of apoptosis (XIAP), Bcl-2 and Bax. GAPDH was used as an
internal control. The thermocy-cling conditions of qPCR were as
follows: For HMGA2 and GAPDH, 95°C for 3 min, 95°C for 1 min
followed by 30 cycles of 60°C for 30 sec and 72°C for 30 sec; for
XIAP, 95°C for 3 min, 95°C for 30 sec followed by 35 cycles of 58°C
for 30 sec and 72°C for 30 sec; and for Bcl-2 and Bax, 95°C for 5
min, 95°C for 10 sec followed by 40 cycles of 60°C for 34 sec.
Primers were purchased commercially (Invitrogen; Thermo Fisher
Scientific, Inc.) and the sequences are listed in Table I. The expression levels of the
above genes were determined using the 2−ΔΔCq method
(26).
 | Table IPrimers used in reverse
transcription-quantitative PCR. |
Table I
Primers used in reverse
transcription-quantitative PCR.
| Gene | Primer | Sequence
(5′-3′) |
|---|
| HMGA2 | Forward |
AGTCCCTCTAAAGCAGCTCAAAAG |
| Reverse |
GCCATTTCCTAGGTCTGCCTC |
| XIAP | Forward |
ATGACTTTTAACAGTTTTGAAGG |
| Reverse |
GCTCGTGCCAGTGTTGATGCTG |
| Bcl-2 | Forward |
GGATTGTGGCCTTCTTTGAG |
| Reverse |
TACCCAGCCTCCGTTATCCT |
| Bax | Forward |
CCGATTCATCTACCCTGCTG |
| Reverse |
TGAGCAATTCCAGAGGCAGT |
| GAPDH | Forward |
AGCCACATCGCTCAGACAC |
| Reverse |
GCCCAATACGACCAAATCC |
Western blot analysis
RIPA lysis buffer (Thermo Fisher Scientific, Inc.)
was used to extract total protein from the cultured cells.
Subsequently, protein concentration was determined using an
Enhanced BCA Protein Assay kit (Beyotime Institute of
Biotechnology). The proteins (20 µg/lane) were subjected to
12% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF)
membranes (EMD Millipore). Then 5% milk PBS with 0.1% Triton X-100
was applied to block the membranes at room temperature for 2 h,
which were then incubated with the following: Anti-HMGA2 antibody
(cat. no. ab97276; 1:2,000; Abcam), anti-XIAP antibody (cat. no.
ab21278; 1:1,000; Abcam), anti-Bcl-2 antibody (cat. no. ab32124;
1:1,000; Abcam), anti-cleaved caspase-3 antibody (cat. no. ab2302;
1:1,000; Abcam), anti-Wnt antibody (cat. no. ab28472; 1:1,000;
Abcam), anti-non-phospho (Np)-β-catenin antibody (cat. no. 8814;
1:1,000; Cell Signaling Technology, Inc.) and anti-GAPDH antibody
(cat. no. ab9485; 1:2,500; Abcam) overnight at 4°C. The membranes
were then incubated with the appropriate horseradish
peroxidase-conjugated secondary antibody (1:2,000; cat. no.
SA00001-2; ProteinTech Group, Inc.) at 4°C for 1 h after washing
with PBST (containing 0.05% Tween-20) three times. Protein bands
were detected with ECL (Thermo Fisher Scientific, Inc.) and
visualized using Quantity One software version 4.6.2 (Bio-Rad
Laboratories, Inc.).
Statistical analysis
Statistical analysis was detected by GraphPad Prism
version 6.0 software (GraphPad Software, Inc.). All data were
presented as the mean ± standard deviation from three independent
experiments. Differences were analyzed using one-way analysis of
variance following Tukey's post hoc test for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression and transfection efficiency of
HMGA2 in AML cells
The 7 AML cell lines, including NB4, HL60, KG1,
U937, Kasumi-1, THP-1 and K562, were analyzed to determine the
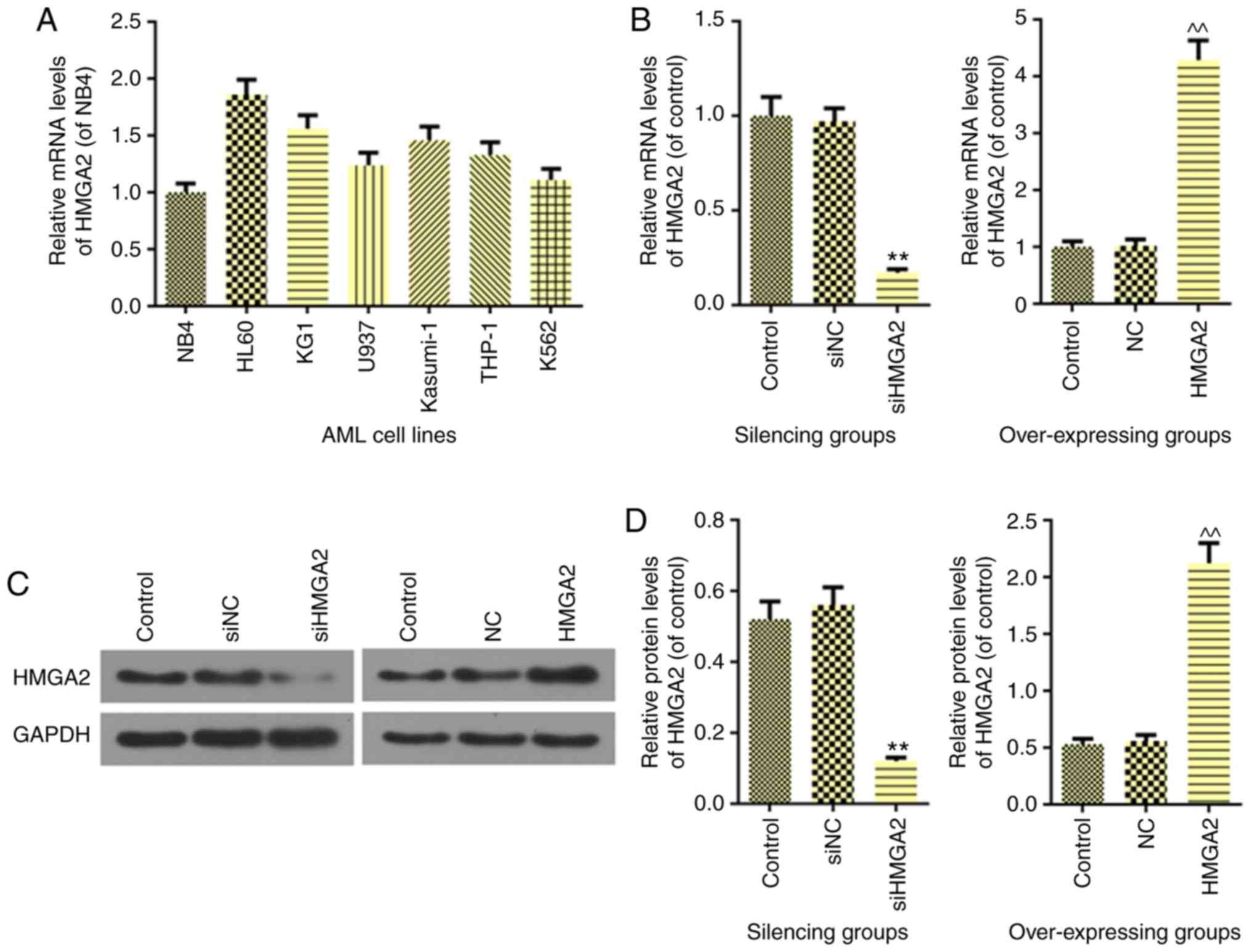
expression of HMGA2. As shown in Fig.
1A, the HL60, KG1, U937, Kasumi-1, THP-1 and K562 cell lines
had higher expressions of HMGA2 than NB4 cells. Therefore, NB4,
which had a relatively low expression of HMGA2, was selected for
the HMGA2 overexpression experiments, and HL60 was selected for the
siHMGA2 transfection experiments. RT-qPCR (Fig. 1B) and western blot analysis
(Fig. 1C and D) revealed a
decreased expression of HMGA2 (P<0.01) in siHMGA2 HL60 cells and
an elevated expression of HMGA2 (P<0.01) in NB4 cells, thereby
indicating successful transfection.
HMGA2 regulates the proliferation,
apoptosis, migration and invasion of AML HL60 and NB4 cells
As aforementioned, the present study selected two
AML cell lines, which either possessed a high or low expression of
HMGA2, and were subsequently transfected with either silencing or
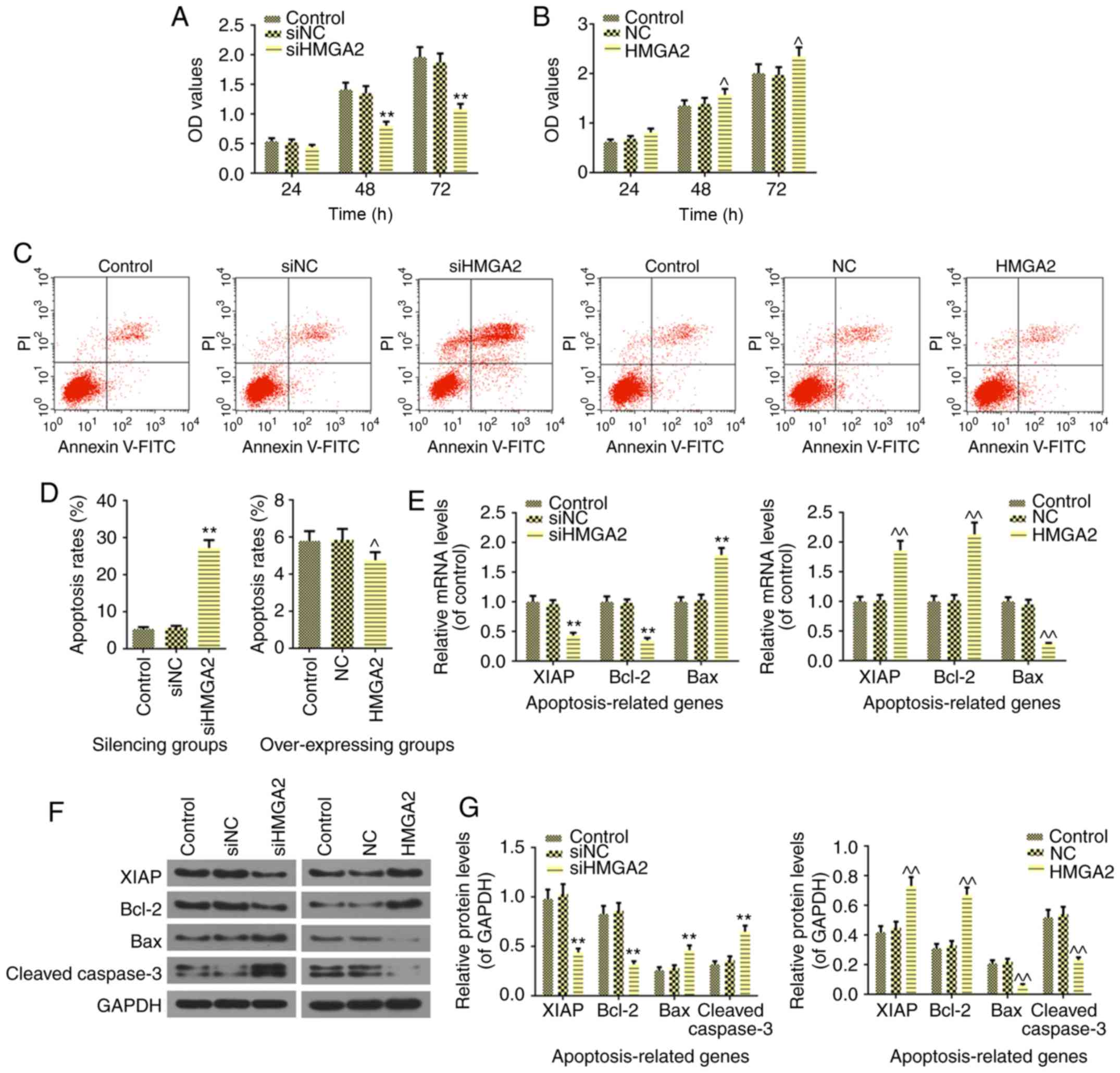
overexpression HMGA2 plasmids. The results revealed that silencing
HMGA2 decreased HL60 cell viability in 24 h; however, no
significant difference in comparison to control was identified.
However, siHMGA2 significantly inhibited cell proliferation from 48
h, compared with the control (P<0.01; Fig. 2A). In addition, in NB4 cell,
overexpressing HMGA2 promoted cell viability starting from 48 h
(P<0.05; Fig. 2B). In regard
to cell apoptosis, the Annexin-V-FITC assay revealed that silencing
HMGA2 significantly induced cell apoptosis (P<0.01) and
overexpression of HMGA2 produced the opposite result (P<0.05;
Fig. 2C and D). These results
were in agreement with the expression levels of the
apoptosis-related genes in AML cells. Silencing HMGA2 significantly
suppressed the mRNA and protein expressions of XIAP (P<0.01) and
Bcl-2 (P<0.01), and significantly increased Bax and cleaved
caspase-3 the mRNA and protein levels (P<0.01; Fig. 2E-G). In addition, significantly
increased expression levels of XIAP and Bcl-2, and significantly
reduced expression levels of Bax and cleaved caspase-3 were found
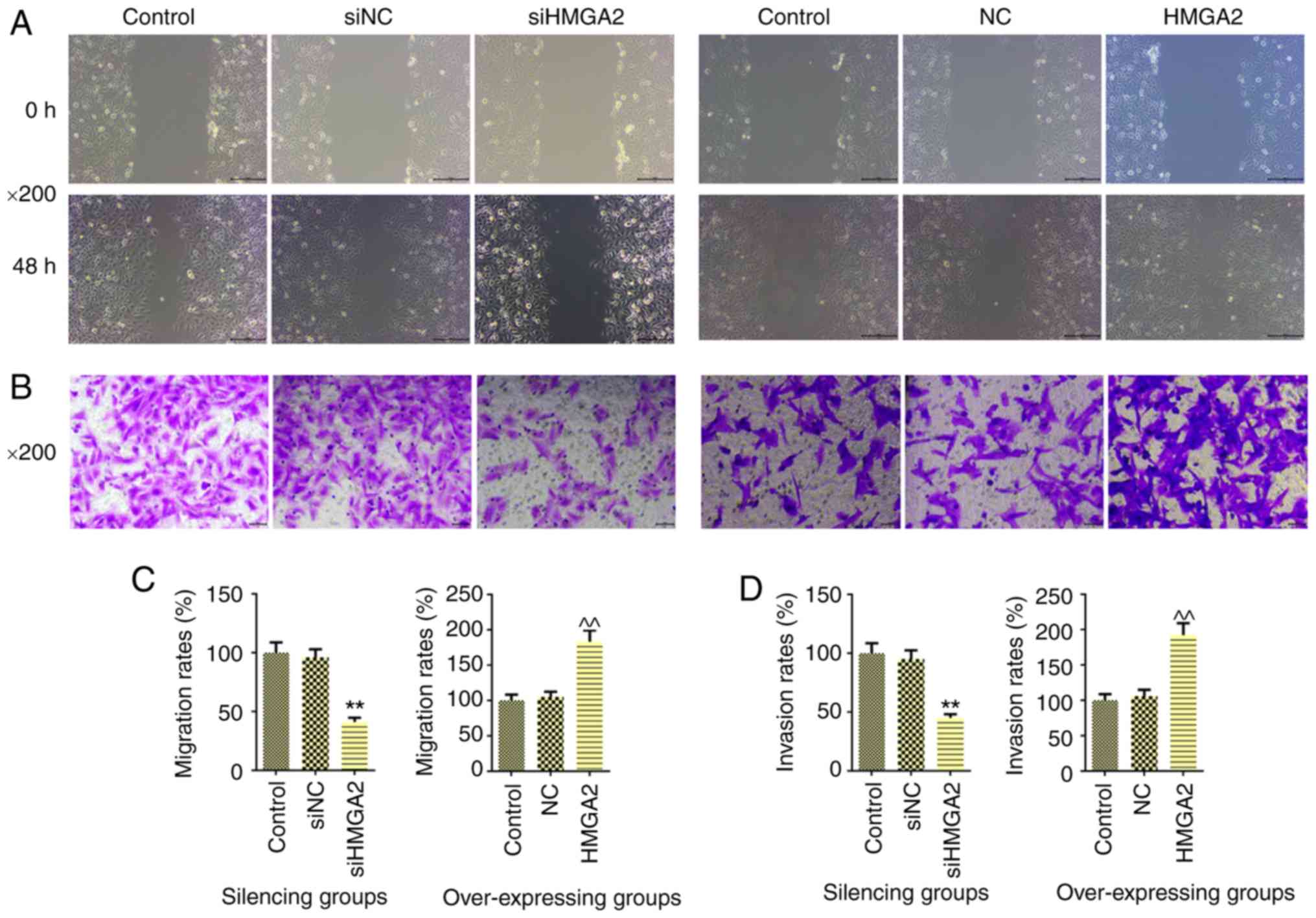
in the NB4 cells overexpressing HMGA2 (P<0.01; Fig. 2E-G). Subsequently, the effect of
HMGA2 on cell migration (Fig. 3A)
and invasion (Fig. 3B) was
investigated. As excepted, silencing HMGA2 significantly inhibited
cell migration (P<0.01; Fig.
3C) and invasion (P<0.01; Fig.
3D). By contrast, overexpression of HMGA2 contributed to the
promotion of cell migration and invasion (P<0.01).
Effect of HMGA2 on cell sensitivity to
DNR in AML HL60 and NB4 cells
DNR in combination with Cytarabine is widely
accepted as a classical AML induction mitigation scheme (27). In addition, Idarubicin has been
approved by the US Food and Drug Administration for the combination
chemotherapy for AML since 1990 (28). DNR has a certain inhibitory effect
on AML, which was confirmed in the present study as presented in
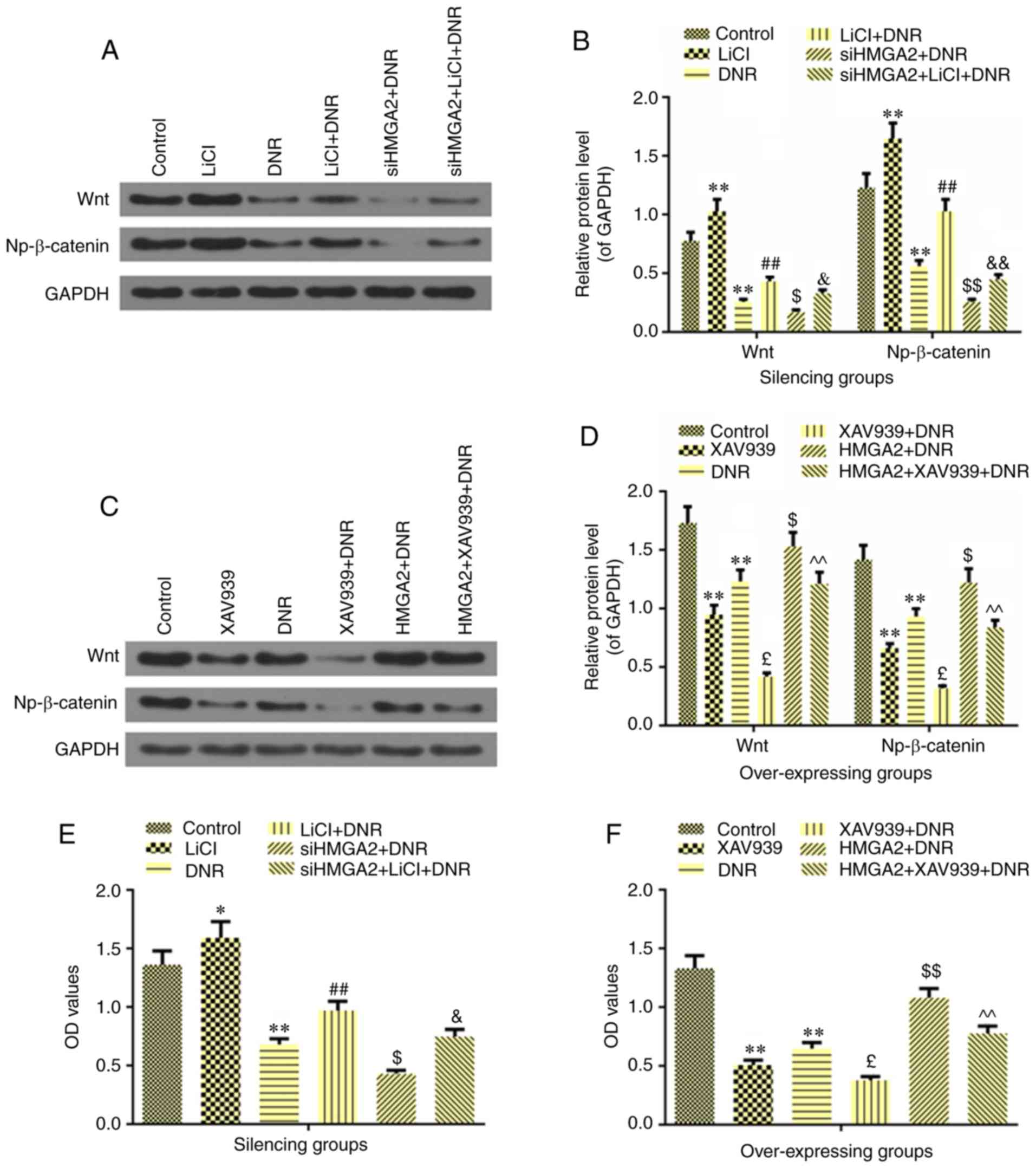
Fig. 4A (P<0.01). In addition,
silencing HMGA2 contributed to the inhibitory effect on cell
viability induced by DNR (P<0.05; Fig. 4A). Nevertheless, overexpression of
HMGA2 could significantly reverse the DNR-induced reduction of cell
proliferation (P<0.01; Fig.
4A). Furthermore, the results demonstrated that the mRNA and
protein levels of HMGA2 were not altered when comparing the NC and
NC + DNR groups, indicating that DNR did not affect HMGA2
expression (P>0.05). Compared with siNC + DNR, siHMGA2 in
combination with DNR significantly downregulated the expression of
HMGA2 both at the mRNA and protein levels (P<0.01; Fig. 4B-D). However, in NB4 cells, the
effect of DNR or HMGA2 overexpression + DNR on the expression of
HMGA2 was opposite to that observed in HL60 cell (P<0.01;
Fig. 4B-D).
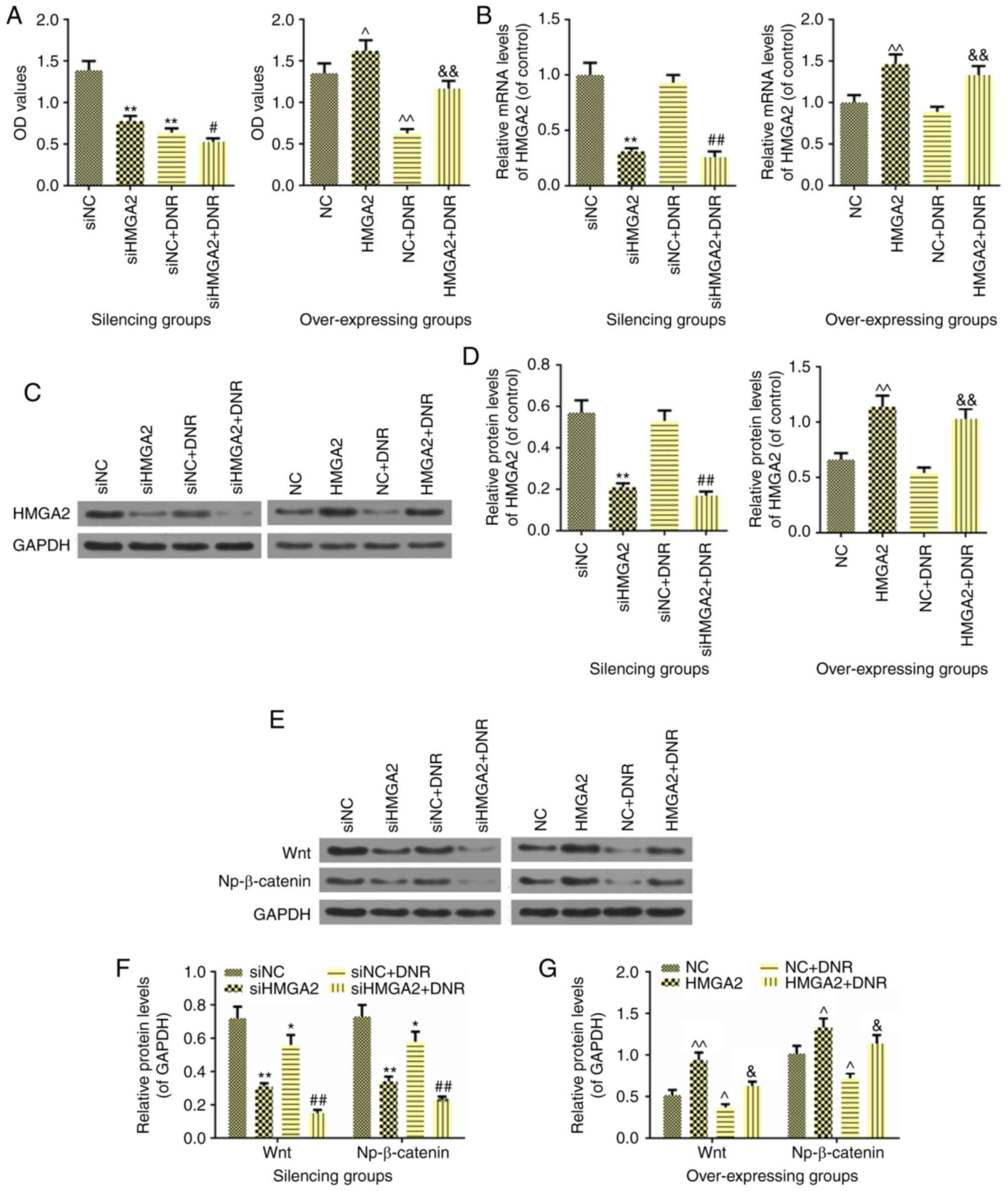 | Figure 4Effects of HMGA2 on cell sensitivity
to DNR and the expressions of Wnt and Np-β-catenin in AML HL60 and
NB4 cells. (A) The effect of HMGA2 on cell sensitivity to DNR as
determined by MTT assay. (B) The mRNA level of HMGA2 was detected
by reverse transcription-quantitative PCR to observe the effect of
silencing or overexpressing HMGA2 on DNR in AML HL60 and NB4 cells.
(C and D) The protein level of HMGA2 was detected by western
blotting to observe the effect of silencing or overexpressing HMGA2
on DNR in AML HL60 and NB4 cells. (E) The effects of HMGA2 on the
expressions of Wnt and Np-β-catenin, which were inhibited by DNR,
were detected by western blot analysis in AML HL60 and NB4 cells.
(F) Relative protein levels of Wnt and Np-β-catenin, which were
regulated by silencing HMGA2 in HL60 cell. (G) Relative protein
levels of Wnt and Np-β-catenin, which were regulated by
overexpressing HMGA2 in NB4 cell. GAPDH was used as an internal
control. Data were presented as the mean ± standard deviation from
three independent experiments. *P<0.05 and
**P<0.01 vs. of HL60 cell siNC; ^P<0.05
and ^^P<0.01 vs. NB4 cell NC; #P<0.05
and ##P<0.01 vs. siNC+DNR; &P<0.05
and &&P<0.01 vs. NC+DNR. DNR, daunorubicin;
AML, acute myelocytic leukemia; HMGA2, high-mobility group AT-hook
2 overexpression group; si-, small interfering RNA; NC, negative
control; Np-β-catenin, non-phospho-β-catenin. |
HMGA2 affects the DNR-induced inhibitory
effect of Wnt/β-catenin signaling in AML HL60 and NB4 cells
An increasing body of evidence has indicated that
abnormal activation of Wnt/β-catenin signaling contributes to the
progression of tumors (29-31). Therefore, the present study
investigated whether HMGA2 regulated this signaling pathway.
Western blot analysis revealed that DNR could inhibit the protein
expressions of Wnt and Np-β-catenin (P<0.05; Fig. 4E-G), but its inhibitory effect was
not more significant than siHMGA2 treatment (P<0.01). Notably,
when siHMGA2 was combined with DNR, the Wnt and Np-β-catenin
protein levels were significantly reduced (P<0.01), indicating
that AML cells treated with this combination had suppressed
Wnt/β-catenin signaling. On the other hand, overexpression of HMGA2
not only increased the protein expressions of Wnt (P<0.01) and
Np-β-catenin (P<0.05), but also attenuated the inhibitory
effects of DNR on the Wnt and Np-β-catenin levels (P<0.05;
Fig. 4E and G). For the
experiments involving the agonist and antagonist of Wnt/β-catenin
signaling, LiCl and XAV939 were employed, respectively. As
expected, the agonist LiCl significantly promoted the expressions
of Wnt and Np-β-catenin (P<0.01; Fig. 5A and B), and the antagonist XAV939
significantly inhibited Wnt/β-catenin signaling activation
(P<0.01; Fig. 5C and D). The
results further revealed that DNR partially suppressed the
promotional effects of LiCl on the expressions of Wnt and
Np-β-catenin (P<0.01; Fig. 5A and
B). In addition, siHMGA2 could further enhance the inhibitory
effect of DNR, which was partially reversed by LiCl (Wnt,
P<0.05; Np-β-catenin, P<0.01). As shown in Fig. 5C and D, XAV939 in combination with
DNR had the strongest inhibitory effect on Wnt and Np-β-catenin
expressions, when compared with treatment alone (P<0.01).
Overexpression of HMGA2 could significantly upregulate the Wnt and
Np-β-catenin protein levels in DNR treated cells, compared with DNR
only treatment (P<0.05) and the effect of HMGA2 overexpression
in turn was partially reversed by the combination of XAV939 and DNR
(P<0.01; Fig. 5C and D). In
addition, LiCl could induce cell proliferation (P<0.05; Fig. 5E) as expected and XAV939
significantly inhibited cell viability (P<0.01; Fig. 5F). DNR could also significantly
reverse the changes in the cell viability of the cells treated with
LiCl (P<0.01) or XAV939 (P<0.05). Furthermore, silencing
HMGA2 suppressed the increase in cell viability in DNR-treated HL60
cells, which was partially reversed by LiCl (P<0.05). Similarly,
overexpressing HMGA2 increased cell viability in DNR-induced NB4
cells, and this effect was partially reversed by XAV939 treatment
(P<0.01).
Discussion
The present study investigated the HMGA2 levels in
several AML cell lines, amongst which the NB4 cell line had
relatively reduced expression of HMGA2, and HL60 cells had the
greatest expression of HMGA2. Subsequently, these two cell lines
were selected to be used in the following experiments. To the best
of our knowledge, previous studies have only reported that HMGA2
has a high expression in a large number of malignant tumors
including thyroid, ovarian, prostate, gallbladder and bladder
cancers, and gastric adenocarcinoma and esophageal squamous cell
carcinoma (32-35). In addition, it has been reported
that elevated HMGA2 levels were detected in AML (36-39) and Nyquist et al (39) demonstrated that t(12;13)(q14;q31)
led to HMGA2 upregulation in AML. Through transfection with siHMGA2
in HL60 cells and overexpression HMGA2 in NB4 cells, the present
study revealed that silencing HMGA2 could inhibit cell
proliferation, migration and invasion as well as induce cell
apoptosis. The present in vitro experiments were in
agreement with the results obtained by Tan et al (38) who reported that reduced expression
of HMGA2 in AML cells also suppressed cell proliferation. In
addition, a marked reduction in XIAP and Bcl-2 expression levels
and upregulation of Bax and cleaved caspase-3 levels occurred in
following siHMGA2 transfection in HL60 cells. It has been well
established that XIAP is the most potent endogenous caspase
inhibitor in the IAP family, which is the only endogenous protein
capable of acting on both the initiation and effect of caspases
(40,41). If XIAP is activated, the junction
region of its baculoviral IAP repeat 1 (BIR1) and BIR2 domains can
bind to the active sites of the effectual caspase-3,7 to
competitively inhibit the activity of caspase-3,7 (42). Saraei et al (43) also suggested that XIAP could be
putative in resensitizing tumor necrosis factor-related
apoptosis-inducing ligand in leukemia.
The present study is, to the best of our knowledge,
not the first to determine the levels of HMGA2 in AML cells, but is
the first to study the effect of it on DNR in regard to AML cell
sensitivity. DNR, as an anthracycline-based chemotherapy drug, is
also a cycle nonspecific agent with strong anti-tumor properties.
Currently, almost all first-line standard regimens contain DNR
(44). Quiney et al
(45) reported that there were
some patients with DNR resistance in the clinic. The present
results revealed that silencing HMGA2 could enhance the inhibition
of AML cells by DNR (10 µg/ml), while overexpressing HMGA2
presented the opposite result in comparison with that produced by
silencing HMGA2. Previous studies have demonstrated that targeting
HMGA2 could regulate chemoresistance in several types of cancers,
such as colorectal cancer in which HMGA2 could increase the
chemoresistance to 5-fluorouracil by activating disheveled segment
polarity protein 2/Wnt signaling (46).
The molecular mechanism of the role of HMGA2 in the
genesis and development of AML is not clearly defined. A previous
study has suggested that HMGA2 could promote the growth of AML
cells by regulating the Akt signaling (38). Tan et al (47) subsequently demonstrated that
silencing HMGA2 induced the terminal differentiation of myeloid
leukemia primary blasts and cell lines. Ohshima et al
(48) suggested that HMGA2 and
the let-7 family were negatively regulated and were correlated with
the invasiveness of gastric cancer. This negative regulatory
effects contributed to tumorigenesis via the regulation of some
molecular signaling pathways such as the growth factor signaling
pathway and Ras signaling pathway (48). Watanabe et al (49) believed that the upregulation of
HMGA2 expression activated the Ras signaling pathway, leading to
the development of pancreatic cancer. The Wnt signaling pathway not
only played a key role in regulating embryonic development, but
also its abnormal activation was closely associated with the
progression of tumors (50-53). Therefore, the present study
ultimately indicated that the Wnt/β-catenin pathway was the
underlying mechanism. β-catenin cannot be degraded in the presence
of Wnt signaling. Thus, a large number of free β-catenins
accumulate in the cytoplasm and enter the nucleus in order to bind
to the transcription factor T cytokine/lymphocyte enhancer, which
initiates a series of downstream target molecules such as c-myc and
cyclin D1 expression, thereby participating in cell proliferation
and apoptosis (54). The present
study revealed that silencing HMGA2 markedly inhibited Wnt and
Np-β-catenin (active) protein levels of Wnt signaling and enhanced
protein sensitivities to DNR; moreover, the activity of the Wnt
signaling agonist LiCl was partially reversed in a previous study
(55). To the best of our
knowledge, the present study is the first to investigate the effect
of HMGA2 on the regulation of Wnt/β-catenin in AML cells. However,
in gastric cancer, Zha et al (56) had already confirmed that HMGA2 was
conducive to EMT by activating Wnt/β-catenin signaling. Similarly,
Wend et al (57) suggested
that the Wnt10B/β-catenin signaling was closely associated with
HMGA2 and promoted metastatic triple-negative breast cancer cell
proliferation.
In conclusion, the present study demonstrated that
HMGA2 played important roles in driving AML progression in HL60 and
NB4 cells, potentially through the activation of the Wnt/β-catenin
signaling pathway. In addition, it was revealed that HMGA2 could
regulate AML cell sensitivity to DNR. As such HMGA2 may be a
promising molecular marker for AML diagnosis.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SY made substantial contributions to the conception
and design of the study. YG, QH, GW, SC, MZ and YW acquired,
analyzed and interpreted the data. SY and YG drafted the article
and revised it critically for important intellectual content. All
authors gave final approval of the version to be published. All
authors agree to be held accountable for all aspects of the work in
ensuring that questions related to the accuracy or integrity of the
work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Döhner H, Weisdorf DJ and Bloomfield CD:
Acute myeloid leukemia. N Engl J Med. 373:1136–1152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee SC and Abdel-Wahab O: Therapeutic
targeting of splicing in cancer. Nat Med. 22:976–986. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buchner M and Müschen M: Targeting the
B-cell receptor signaling pathway in B lymphoid malignancies. Curr
Opin Hematol. 21:341–349. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Piemontese S, Ciceri F, Labopin M,
Bacigalupo A, Huang H, Santarone S, Gorin NC, Koc Y, Wu D, Beelen
D, et al: A survey on unmanipulated haploidentical hematopoietic
stem cell transplantation in adults with acute leukemia. Leukemia.
29:1069–1075. 2015. View Article : Google Scholar
|
|
6
|
Herbaux C, Genet P, Bouabdallah K, Pignon
JM, Debarri H, Guidez S, Betrian S, Leleu X, Facon T, Morschhauser
F, et al: Bendamustine is effective in T-cell prolymphocytic
leukaemia. Br J Haematol. 168:916–919. 2015. View Article : Google Scholar
|
|
7
|
Döhner H, Estey EH, Amadori S, Appelbaum
FR, Büchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson
RA, et al: Diagnosis and management of acute myeloid leukemia in
adults: Recommendations from an international expert panel, on
behalf of the European LeukemiaNet. Blood. 115:453–474. 2010.
View Article : Google Scholar
|
|
8
|
Walter RB, Othus M, Burnett AK, Löwenberg
B, Kantarjian HM, Ossenkoppele GJ, Hills RK, Ravandi F, Pabst T,
Evans A, et al: Resistance prediction in AML: Analysis of 4601
patients from MRC/NCRI, HOVON/SAKK, SWOG and MD Anderson cancer
center. Leukemia. 29:312–320. 2015. View Article : Google Scholar
|
|
9
|
Ramos NR, Mo CC, Karp JE and Hourigan CS:
Current approaches in the treatment of relapsed and refractory
acute myeloid leukemia. J Clin Med. 4:665–695. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mrózek K, Heerema NA and Bloomfield CD:
Cytogenetics in acute leukemia. Blood Rev. 18:115–136. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rowley JD: Chromosomal translocations:
Revisited yet again. Blood. 112:2183–2189. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin J, Yu M, Hu C, Ye L, Xie L, Jin J,
Chen F and Tong H: Pesticide exposure as a risk factor for
myelodysplastic syndromes: A meta-analysis based on 1,942 cases and
5,359 controls. PLoS One. 9:e1108502014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu J, Liu Z, Shao C, Gong Y, Hernando E,
Lee P, Narita M, Muller W, Liu J and Wei JJ: HMGA2
overexpression-induced ovarian surface epithelial transformation is
mediated through regulation of EMT genes. Cancer Res. 71:349–359.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Young AR and Narita M: Oncogenic HMGA2:
Short or small? Genes Dev. 21:1005–1009. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fedele M and Fusco A: HMGA and cancer.
Biochim Biophys Acta. 1799:48–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morishita A, Zaidi MR, Mitoro A,
Sankarasharma D, Szabolcs M, Okada Y, D'Armiento J and Chada K:
HMGA2 is a driver of tumor metastasis. Cancer Res. 73:4289–4299.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Natarajan S, Hombach-Klonisch S, Dröge P
and Klonisch T: HMGA2 inhibits apoptosis through interaction with
ATR-CHK1 signaling complex in human cancer cells. Neoplasia.
15:263–280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li O, Li J and Dröge P: DNA architectural
factor and proto-oncogene HMGA2 regulates key developmental genes
in pluripotent human embryonic stem cells. FEBS Lett.
581:3533–3537. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meyer B, Krisponeit D, Junghanss C, Murua
Escobar H and Bullerdiek J: Quantitative expression analysis in
peripheral blood of patients with chronic myeloid leukaemia:
Correlation between HMGA2 expression and white blood cell count.
Leuk Lymphoma. 48:2008–2013. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei J, Li H, Wang S, Li T, Fan J, Liang X,
Li J, Han Q, Zhu L, Fan L and Zhao RC: let-7 enhances osteogenesis
and bone formation while repressing adipogenesis of human
stromal/mesenchymal stem cells by regulating HMGA2. Stem Cells Dev.
23:1452–1463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fusco A and Fedele M: Roles of HMGA
proteins in cancer. Nat Rev Cancer. 7:899–910. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma S, Yang LL, Niu T, Cheng C, Zhong L,
Zheng MW, Xiong Y, Li LL, Xiang R, Chen LJ, et al: SKLB-677, an
FLT3 and Wnt/β-catenin signaling inhibitor, displays potent
activity in models of FLT3-driven AML. Sci Rep. 5:156462015.
View Article : Google Scholar
|
|
23
|
Sokolowski KM, Koprowski S, Kunnimalaiyaan
S, Balamurugan M, Gamblin TC and Kunnimalaiyaan M: Potential
molecular targeted therapeutics: Role of PI3-K/Akt/mTOR inhibition
in cancer. Anticancer Agents Med Chem. 16:29–37. 2016. View Article : Google Scholar
|
|
24
|
Li J, Volk A, Zhang J, Cannova J, Dai S,
Hao C, Hu C, Sun J, Xu Y, Wei W, et al: Sensitizing leukemia stem
cells to NF-κB inhibitor treatment in vivo by inactivation of both
TNF and IL-1 signaling. Oncotarget. 8:8420–8435. 2017.PubMed/NCBI
|
|
25
|
Cook AM, Li L, Ho Y, Lin A, Li L, Stein A,
Forman S, Perrotti D, Jove R and Bhatia R: Role of altered growth
factor receptor-mediated JAK2 signaling in growth and maintenance
of human acute myeloid leukemia stem cells. Blood. 123:2826–2837.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Lin TL and Levy MY: Acute myeloid
leukemia: Focus on novel therapeutic strategies. Clin Med Insights
Oncol. 6:205–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Masaoka T, Ogawa M, Yamada K, Kimura K and
Ohashi Y: A phase II comparative study of idarubicin plus
cytarabine versus daunorubicin plus cytarabine in adult acute
myeloid leukemia. Semin Hematol. 33(Suppl 3): S12–S17. 1996.
|
|
29
|
Shi S, Chen X, Liu H, Yu K, Bao Y, Chai J,
Gao H and Zou L: LGR5 acts as a target of miR-340 5p in the
suppression of cell progression and drug resistance in breast
cancer via Wnt/β-catenin pathway. Gene. 683:47–53. 2019. View Article : Google Scholar
|
|
30
|
Wang J, Mook RA Jr, Ren XR, Zhang Q, Jing
G, Lu M, Spasojevic I, Lyerly HK, Hsu D and Chen W: Identification
of DK419, a potent inhibitor of Wnt/β-catenin signaling and
colorectal cancer growth. Bioorg Med Chem. 26:5435–5442. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu P, Chen B, Gu Y and Liu Q: PNMA1,
regulated by miR-33a 5p promotes proliferation and EMT in
hepatocellular carcinoma by activating the Wnt/β-catenin pathway.
Biomed Pharmacother. 108:492–499. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jin L, Lloyd RV, Henry MR, Erickson LA,
Sebo TJ, Rumilla KM and Zhang J: The diagnostic utility of
combination of HMGA2 and IMP3 qRT-PCR testing in thyroid neoplasms.
Appl Immunohistochem Mol Morphol. 23:36–43. 2015. View Article : Google Scholar
|
|
33
|
Zhu C, Li J, Cheng G, Zhou H, Tao L, Cai
H, Li P, Cao Q, Ju X, Meng X, et al: miR-154 inhibits EMT by
targeting HMGA2 in prostate cancer cells. Mol Cell Biochem.
379:69–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ding X, Wang Y, Ma X, Guo H, Yan X, Chi Q,
Li J, Hou Y and Wang C: Expression of HMGA2 in bladder cancer and
its association with epithelial-to-mesenchymal transition. Cell
Prolif. 47:146–151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kong D, Su G, Zha L, Zhang H, Xiang J, Xu
W, Tang Y and Wang Z: Coexpression of HMGA2 and Oct4 predicts an
unfavorable prognosis in human gastric cancer. Med Oncol.
31:1302014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pierantoni GM, Santulli B, Caliendo I,
Pentimalli F, Chiappetta G, Zanesi N, Santoro M, Bulrich F and
Fusco A: HMGA2 locus rearrangement in a case of acute lymphoblastic
leukemia. Int J Oncol. 23:363–367. 2003.PubMed/NCBI
|
|
37
|
Marquis M, Beaubois C, Lavallée VP,
Abrahamowicz M, Danieli C, Lemieux S, Ahmad I, Wei A, Ting SB,
Fleming S, et al: High expression of HMGA2 independently predicts
poor clinical outcomes in acute myeloid leukemia. Blood Cancer J.
8:682018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tan L, Wei X, Zheng L, Zeng J, Liu H, Yang
S and Tan H: Amplified HMGA2 promotes cell growth by regulating Akt
pathway in AML. J Cancer Res Clin Oncol. 142:389–399. 2016.
View Article : Google Scholar
|
|
39
|
Nyquist KB, Panagopoulos I, Thorsen J,
Roberto R, Wik HS, Tierens A, Heim S and Micci F: t(12;13)(q14;q31)
leading to HMGA2 upregulation in acute myeloid leukaemia. Br J
Haematol. 157:769–771. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fulda S and Vucic D: Targeting IAP
proteins for therapeutic intervention in cancer. Nat Rev Drug
Discov. 11:109–124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dean EJ, Ranson M, Blackhall F and Dive C:
X-linked inhibitor of apoptosis protein as a therapeutic target.
Expert Opin Ther Targets. 11:1459–1471. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mastrangelo E, Vachette P, Cossu F,
Malvezzi F, Bolognesi M and Milani M: The activator of apoptosis
Smac-DIABLO acts as a tetramer in solution. Biophys J. 108:714–723.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Saraei R, Soleimani M, Movassaghpour
Akbari AA, Farshdousti Hagh M, Hassanzadeh A and Solali S: The role
of XIAP in resistance to TNF-related apoptosis-inducing ligand
(TRAIL) in Leukemia. Biomed Pharmacother. 107:1010–1019. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
O'Donnell MR, Abboud CN, Altman J,
Appelbaum FR, Arber DA, Attar E, Borate U, Coutre SE, Damon LE,
Goorha S, et al: NCCN Clinical Practice Guidelines Acute myeloid
leukemia. J Natl Compr Canc Netw. 10:984–1021. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Quiney C, Billard C, Faussat AM,
Salanoubat C and Kolb JP: Hyperforin inhibits P-gp and BCRP
activities in chronic lymphocytic leukaemia cells and myeloid
cells. Leuk Lymphoma. 48:1587–1599. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu X, Wang Y, Deng H, Liu C, Wu J and Lai
M: HMGA2 enhances 5-fluorouracil chemoresistance in colorectal
cancer via the Dvl2/Wnt pathway. Oncotarget. 9:9963–9974. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tan L, Xu H, Chen G, Wei X, Yu B, Ye J, Xu
L and Tan H: Silencing of HMGA2 reverses retardance of cell
differentiation in human myeloid leukaemia. Br J Cancer.
118:405–415. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ohshima K, Inoue K, Fujiwara A, Hatakeyama
K, Kanto K, Watanabe Y, Muramatsu K, Fukuda Y, Ogura S, Yamaguchi K
and Mochizuki T: Let-7 microRNA family is selectively secreted into
the extracellular environment via exosomes in a metastatic gastric
cancer cell line. PLoS One. 5:e132472010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Watanabe S, Ueda Y, Akaboshi S, Hino Y,
Sekita Y and Nakao M: HMGA2 maintains oncogenic RAS-induced
epithelial-mesenchymal transition in human pancreatic cancer cells.
Am J Pathol. 174:854–868. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lin Y, Meng F, Lu Z, Chen K, Tao Y, Ouyang
Y and Cao X: Knockdown of PKM2 suppresses tumor progression in
human cervical cancer by modulating epithelial-mesenchymal
transition via Wnt/β-catenin signaling. Cancer Manag Res.
10:4191–4202. 2018. View Article : Google Scholar :
|
|
51
|
Yang F, Xiao Z and Zhang S: Knockdown of
miR-194 5p inhibits cell proliferation, migration and invasion in
breast cancer by regulating the Wnt/β-catenin signaling pathway.
Int J Mol Med. 42:3355–3363. 2018.PubMed/NCBI
|
|
52
|
Zhao C, Qiao C, Zong L and Chen Y: Long
non-coding RNA-CCAT2 promotes the occurrence of non-small cell lung
cancer by regulating the Wnt/β-catenin signaling pathway. Oncol
Lett. 16:4600–4606. 2018.PubMed/NCBI
|
|
53
|
Liu L, Jiang H, Zhao J and Wen H: MiRNA-16
inhibited oral squamous carcinoma tumor growth in vitro and in vivo
via suppressing Wnt/β-catenin signaling pathway. Onco Targets Ther.
11:5111–5119. 2018. View Article : Google Scholar :
|
|
54
|
Kumar R, Kotapalli V, Naz A, Gowrishankar
S, Rao S, Pollack JR and Bashyam MD: XPNPEP3 is a novel
transcriptional target of canonical Wnt/β-catenin signaling. Genes
Chromosomes Cancer. 57:304–310. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Qiu F, Shin Y, Chen D, Cheng R, Chen Q,
Zhou K, Larrick JW, Mendelson AR and Ma JX: Anti-angiogenic effect
of a humanized antibody blocking the Wnt/β-catenin signaling
pathway. Microvasc Res. 119:29–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zha L, Zhang J, Tang W, Zhang N, He M, Guo
Y and Wang Z: HMGA2 elicits EMT by activating the Wnt/β-catenin
pathway in gastric cancer. Dig Dis Sci. 58:724–733. 2013.
View Article : Google Scholar
|
|
57
|
Wend P, Runke S, Wend K, Anchondo B,
Yesayan M, Jardon M, Hardie N, Loddenkemper C, Ulasov I, Lesniak
MS, et al: WNT10B/β-catenin signalling induces HMGA2 and
proliferation in metastatic triple-negative breast cancer. EMBO Mol
Med. 5:264–279. 2013. View Article : Google Scholar : PubMed/NCBI
|