Introduction
An 'itch' was defined as 'an unpleasant sensation
that elicits the desire or reflex to scratch' by Samuel
Hafenreffer, almost 1,350 years ago (1). It is still difficult to treat
pruritus, or chronic itchy skin, as its pathophysiological
mechanisms remain elusive (2-5).
Similar to many other diseases, itch manifests in two forms. Acute
itch is relieved easily by scratching, while chronic itch, which is
further categorized according to dermatological, systemic,
neuropathic and psychogenic subtypes based on clinical relevance,
remains a challenge to cure (6).
Although Sun and Chen indicated gastrin-releasing peptide receptor
(GRPR) as the first dedicated molecule that mediates pruritus, the
itch pathogenesis remains unclear (7).
According to a number of studies, pruritus and pain
are two fundamental sensory perceptions that share close
associations in neural pathways (8-10).
Although opioids, which affect the body by activating opioid
receptors, such as the μ-opioid receptor (MOP), δ-opioid
receptor (DOP), κ-opioid receptor (KOP) and nociceptin/orphanin FQ
peptide receptor (NOP), are integral transmitters in pain pathways,
it has been reported that they play important roles in the
development and maintenance of chronic itch (11). The effect of opioid receptors on
itch appears to be subtype-dependent. For example, activated MOP
results in pruritus, whereas activated KOP suppresses itching. A
previous study by Ahmadi et al revealed that endogenous
opioid and gene expression levels of MOP were unaltered in brain
areas of cholestasis-induced analgesia and pruritus in rats
(12). Serotonin or
5-hydroxytryptamine (5-HT), which has been identified as a potent
inducer of itching, is synthesized by enterochromaffin cells in the
gastrointestinal tract and serotoninergic neurons in the central
nervous system, although only a small percentage is synthesized by
the latter (13-16). Depending on sequence homology and
interrelated second messenger systems, serotonin receptors are
classified into 7 groups (5-HT1-7) with 15 subtypes. 5-HT3
receptors are ligand-gated non-selective cation channels, while the
other 5-HT receptors are G-protein coupled receptors (17,18). As selective serotonin reuptake
inhibitors have been increasingly used in the treatment of pruritus
in the clinical setting, the anti-pruritic role of centrally
released serotonin has been highlighted by numerous studies
(19). The importance 5-HT in
acute and chronic itch has been increasingly reported; however, the
specific subtypes of 5-HT receptors that are involved in
serotonergic itch signal transduction have not yet been fully
investigated due to the complexity of the serotonergic system.
Although the exact subtypes of 5-HT receptors involved in different
mouse models of chronic itch have yet to be elucidated, these
studies reflect the integral role of serotonergic signaling in
chronic itch. Microglia and astrocytes have also been reported to
promote chronic pain by producing related mediators, such as
proinflammatory cytokines, growth factors, and chemokines that
activate and sensitize nociceptive neurons in the spinal cord
(20-23). Moreover, Shiratori-Hayashi et
al indicated that astrocytes in the spinal cord played a
dominate role in chronic itch by activating the signal transducer
and activator of transcription 3 (STAT3) (24). Despite the prominent manifestation
of the roles of glial cells in the genesis and maintenance of
chronic pain, their effects on itching remain unclear.
Chemokines, which are generally of low molecular
weight ranging from 7 to 15 kDa, have been classified into 4
subfamilies based on the number and location of cysteine residues
at the n-terminus named CC, CXC, XC and CX3C (25). As chemoattractants, their main
functions are to control the migration and residence of all immune
cells in various immune responses (26). Although chemokines are well-known
regulators of peripheral immune cell trafficking, several
chemokines have been proven to be involved in chronic pain in the
spinal cord (27,28). In the central nervous system,
chemokines have been reported to function in neuronal development,
synaptic transmission and disease-associated neuroinflammation
through a G-protein coupled receptor under both physiological and
pathological conditions (29-31).
Chronic itch, which can result from immune
dysfunction, is a common component of a number of inflammatory skin
diseases, such as dry skin, atopic dermatitis, contact dermatitis
and allergic contact dermatitis. The application of
acetone/ether/water (AEW) weakens the water-retaining capacity of
skin by breaking the cutaneous barrier function, leading to dry
skin and severe pruritus (32).
The dry skin model recapitulates the pathophysiological
characteristics of chronic itch that are observed in human
dermatitis, such as senile xerosis, atopic dermatitis, seasonal
xerosis in winter, renal failure and cholestasis (33,34). Diphenylcyclopropenone (DCP) is an
immunotherapy agent for alopecia areata that typically induces
eczematous skin, contact dermatitis and severe pruritic in
patients. Treatment with DCP in both mice and rats often results in
increased and persistent scratching behaviors (35,36).
It is well-known that inflammatory mediators in the
peripheral and central nervous systems can lead to pain and itch
hypersensitivity by activating or sensitizing nociceptive and
pruriceptive neurons directly (37). Given the remarkable function of
related mediators in chronic itch, the investigation of whether the
expression of these mediators is dysregulated in chronic itch
conditions is integral. In this study, we investigated the gene
expression of several related molecules and receptors that may
represent novel candidate itch transducers in two types of chronic
itch models. We report that the levels of multiple mediators, such
as several 5-HT receptors and chemokines in the spinal cord were
altered and may be significant candidates for investigating the
transmission and development of chronic itch further.
Materials and methods
Animals and animal care
A total of 64 adult male C57BL/6J mice aged 8-10
weeks (weighing 20-25 g) were raised in the Animal Centre of Tongji
Hospital (license no. 42000600018076). They were maintained in a
vivarium (temperature, 22-24°C; humidity, 50-60%) with sufficient
food and water and a 12 h light/12 h dark cycle. All experiments
were performed under protocols approved by the Institutional Animal
Care and Use Committee of Tongji Hospital, Huazhong University of
Science and Technology (IRB ID:TJ-A0803).
Experimental design
It has been reported by numbers of researchers that
AEW-induced dry skin itch and DCP-induced contact dermatitis can be
successful established in C57BL/6J mice and that the pruritic
behavior is stable (38-40). In addition, the present study was
built upon our previous study (16). Therefore, we selected C57BL/6J
mice for use in this study. Three sets of experiments were
performed as follows: i) Experiment A: Mice were randomly assigned
to 2 groups, namely the water group (n=10) and the AEW group
(n=10). Pruritic behavior was measured. ii) Experiment B: Mice were
divided into the acetone group (n=10) and the DCP group (n=10).
Scratching behaviors were video-recorded. iii) Experiment C: Mice
were randomly assigned to 3 groups, namely the naïve control group
(n=8), the AEW group (n=8) and the DCP group (n=8). After behavior
testing was completed, C5-C8 tissue was prepared for reverse
transcription-quantitative PCR (RT-qPCR).
AEW-induced model of chronic itch
Mice were shaved at the back of neck and randomly
assigned to the AEW group (n=10) and the control group (n=10). The
neck skin of the mice in the AEW group was painted with acetone and
diethyl ether for 15 sec (1:1) followed by water for 30 sec twice
per day (9:00 and 16:00) for 8 days consecutively as previously
described (32). The mice in the
control group were painted with water for 45 sec. Spontaneous
scratching was recorded on the day before applying AEW and on day
9, following the final AEW treatment.
DCP-induced chronic itch model
Mice were shaved at the back of neck and assigned to
the DCP group (n=10) and control group (n=10). The neck skin of the
mice in the DCP group was painted with 0.1 ml 1% DCP (Shanghai
Aladdin Biochem Technology Co., Ltd.) dissolved in acetone on day 1
and day 7 under conventional conditions (35). The neck skin of the mice in the
control group was painted with 0.1 ml acetone. According to our
preliminary experiment results, scratching behaviors were
video-recorded on the day before the DCP application and day 10,
following the final DCP treatment.
Behavioral test
From 3 days before the test, the mice were put in a
plastic chamber (9×9×13 cm) for 30 min each day to acclimatize to
the testing environment. Each time, the mice were allowed to
acclimatize for 30 min. The measuring cases were placed on a
transparent glass, which was elevated by a metal floor; spontaneous
itch behavior was video-recorded below the glass in the absence of
any observer for 1 h. As previously reported, a scratching bout is
defined as lifting a hind paw towards the shaved region and
returning the hind paw back to the floor or mouth for licking
(36). The behavior videotapes
were assessed in a blinded manner.
Tissue collection
After the behavioral testing was completed, the mice
were placed in a 1 liter Plexiglass anesthetic induction chamber.
The induction chamber was filled with 4% isoflurane in 100% oxygen.
In order to examine the loss of righting reflex, the chamber was
tipped over. After losing their righting reflex, the mice were laid
in abdominal position on a heating pad and maintained with 2%
isoflurane in 100% oxygen via nose cone. After achieving surgical
anesthetic depth, the treated skins at the back of neck were cut
and collected. Subsequently, the mice were euthanized by rapid
decapitation. The mouse heads were removed, and the spinal columns
were opened to expose the cervical spinal cord. The cervical spinal
cord was cut into 2 segments. The lower cervical segment of each
spinal cord (C5-8) was collected in a RNase-free cryogenic vial and
kept at −80°C for subsequent analysis after freezing in liquid
nitrogen.
RT-qPCR
Total RNA was extracted from the lower cervical
spinal cords (C5-8) using the RNAiso Plus kit (Takara) according to
the manufacturer's instructions (41). RNA purity and concentration were
determined using Nano Drop Lite (Thermo Fisher Scientific). High
quality samples containing 1 μg of total RNA were reverse
transcribed to synthesize cDNA using the Prime Script RT Reagent
kit (Takara). qPCR was performed on a LightCycler system (StepOne,
Applied Biosystems) using SYBR Premix Ex Taq II (Takara). The total
reaction volume was 20 μl, and reaction conditions were
established according to manufacturer's protocol as follows: 1
cycle of denaturation at 95°C for 3 min, followed by 40 cycles of
denaturation at 95°C for 30 sec, annealing at 60°C for 20 sec, and
extension at 72°C for 10 sec. Each reaction was concluded with a
melting curve analysis to test the amplicon specificity by heating
from 55 to 95°C in 0.5°C increments/30 sec, with fluorescence
recorded at each increment. The data was quantified using a
comparative CT method with the formula for relative fold
change=2−ΔΔcq as previously described and compared with
the housekeeping gene average (42). The experiments were performed in
triplicate. The primers sequences used are listed Table I.
 | Table IPrimer sequences used in RT-qPCR. |
Table I
Primer sequences used in RT-qPCR.
| Gene | Forward (5′ to
3′) | Reverse (5′ to
3′) |
|---|
| Oprm1 |
GCCCTCTATTCTATCGTGT |
TAGTGGCTAAGGCATCTGC |
| Oprk1 |
TCTTCGTCTTTGCCTTTGT |
GATTTCGGTCCTTCTCTCG |
| Oprd1 |
CTGCGTGTTCCTCTTTGCC |
GCTGCGGTCCTTCTCCTTG |
| Oprl1 |
CACAAGTGGAGGATGAAGA |
ATGAAGGAAAAAAGGAAGA |
| Htr1a |
TACTCCACTTTCGGCGCTTT |
GGCTGACCATTCAGGCTCTT |
| Htr1b |
TACACGGTCTACTCCACGGT |
CGGTCTTGTTGGGTGTCTGT |
| Htr1d |
GCTCTGGACAGATACTGGGC |
AGTCGGACATCTCCTCGTGA |
| Htr1f |
CAACAGTTGAGCCTGCCACA |
GCCAGCCCAGACAGAGTGAG |
| Htr2a |
AACCCCATTCACCATAGCCG |
CCGAAGACTGGGATTGGCAT |
| Htr2b |
TGGATGGGTCTCACAGGGAT |
AAAGGGGCACCACATAAGCA |
| Htr2c |
GCATAGCCGGTTCAATTCGC |
TTGCTTTCGTCCCTCAGTCC |
| Htr3a |
GACCATCTTCATTGTGCGGC |
AGTGGTTTCCCATGGCTGAG |
| Htr3b |
CCAGTTCCGGTCCATCAACA |
CACGGCAAGGTAGATTCGGA |
| Htr4 |
GATGACCCCTCTACGCATCG |
CCAGCCTTGCATTATGGGGA |
| Htr5a |
TCTTCCTGTGGTTGGGCTATT |
CCCTTGGCAGATGGATCTTGT |
| Htr5b |
CTCCTATGCTGTCTTCTCCACC |
CCACGAGTCTCCGCTTGTC |
| Htr6 |
GCATAGCTCAGGCCGTATGT |
TCCCGCATGAAGAGGGGATA |
| Htr7 |
TTCTGCAACGTCTTCATCG |
ATTCTGCCTCACGGGGTA |
| Nppb |
GGCCTCACAAAAGAACACCC |
CAGGCAGAGTCAGAAACTGGA |
| Npr1 |
GGCTGTGAAACGTGTGAACC |
GTCGGTACAAGCTCCCACAA |
| Grp |
TGGGCTGTGGGACACTTAAT |
GCTTCTAGGAGGTCCAGCAAA |
| Grpr |
GGGAAGACGGGAAATGCTGT |
ATGTTGGTTCTGTCCCAGCC |
| Gfap |
TTGCTGGAGGGCGAAGAAAA |
TGGTGAGCCTGTATTGGGAC |
| Iba1 |
GCTTTTGGACTGCTGAAGGC |
GTTTGGACGGCAGATCCTCA |
| Map2 |
ACCTTCCTCCATCCTCCCTC |
TCCTGCTCTGCGAATTGGTT |
| Ccl1 |
TGCTGCTTGAACACCTTGAA |
TTAGTTGAGGCGCAGCTTTCT |
| Ccl2 |
CCTGCTGCTACTCATTCACCA |
ATTCCTTCTTGGGGTCAGCA |
| Ccl3 |
CCCAGCCAGGTGTCATTTTC |
CAGGCATTCAGTTCCAGGTCA |
| Ccl4 |
CACCATGAAGCTCTGCGTGTC |
GCAGGAAGTGGGAGGGTCAG |
| Ccl5 |
GTGCCCACGTCAAGGAGTAT |
TTCTCTGGGTTGGCACACAC |
| Ccl6 |
TATCCTTGTGGCTGTCCTTGG |
TACATGGGATCTGTGTGGCA |
| Ccl7 |
GATCTCTGCCACGCTTCTGT |
ATAGCCTCCTCGACCCACTT |
| Ccl8 |
TCTACGCAGTGCTTCTTTGC |
GCAGGTGACTGGAGCCTTAT |
| Ccl9 |
GCCCAGATCACACATGCAAC |
AGGACAGGCAGCAATCTGAA |
| Ccl11 |
AGAGCTCCACAGCGCTTCTA |
GGAAGTTGGGATGGAGCCTG |
| Ccl12 |
CAGTCCTCAGGTATTGGCTGG |
GGACACTGGCTGCTTGTGAT |
| Ccl19 |
TTCACGCCACAGGAGGACA |
TTCCGCATCATTAGCACCC |
| Ccl20 |
CCAGGCAGAAGCAAGCAACTAC |
CGGCCATCTGTCTTGTGAAAC |
| Ccl21 |
TGGACCCAAGGCAGTGATG |
CGGGATGGGACAGCCTAAAC |
| Ccl22 |
CTTGCTGTGGCAATTCAGACC |
GAGGGTGACGGATGTAGTCC |
| Ccl24 |
TGAACTCTGAGCTGTGCCTGAC |
TCTTATGGCCCTTCTTGGTGA |
| Ccl25 |
AGTGGAAGCTGCAACCTACG |
GCACTCCTCACGCTTGTACT |
| Ccl27 |
TTTCCTTGGCTGCGAATGTG |
CTGCTTGGGAGTGGCTGTCT |
| Ccl28 |
GCTGTGTGTGTGGCTTTTCAA |
GGCTCTCATCCACTGCTTCA |
| Cxcl1 |
ACTTCCAGACTCGCCCAA |
TTCCGACCTCCTGATAAACA |
| Cxcl2 |
CCCTAAAGAAACCTCGTGCC |
TCAGAAATCGGGTGCCAG |
| Cxcl3 |
CCTACCAAGGGTTGATTTTGAGAC |
AGTGGCTATGACTTCTGTCTGGGT |
| Cxcl4 |
GTGAGATTGGGTGAAGGGAT |
TGAGGGTAAACGGGGAGAA |
| Cxcl5 |
CTCAGTCATAGCCGCAACG |
GGGATAAGGGATGTGGGTAG |
| Cxcl7 |
TGTGCTGATGTGGAAGTGATG |
TGACGATTCTCTTGACGCC |
| Cxcl9 |
CCTGGCAACAGAGAGTGACA |
GCTTACCCAGGTCAGTGTCTAT |
| Cxcl10 |
GAGGTGCCTTCTTAGGTCATAC |
GGAATAGACTCTGCTTTCACTTTG |
| Cxcl11 |
GAAGGTCACAGCCATAGCCC |
TTGTCGCAGCCGTTACTCG |
| Cxcl12 |
ACATTTGGGGATGCTCAGTA |
CTAAGCCCTGGAAATCACAA |
| Cxcl13 |
ATCGGATTCAAGTTACGCC |
GTTTGGTTCAGTTGGATTGC |
| Cxcl14 |
CAAAAGGCTTGCTAGGGATT |
CACTTGATGAAGCGTTTGGT |
| Cxcl15 |
CTGTGGCTCTGGACAAGTATG |
GAAATCAACTGTCTCCTCTGCTAA |
| Cxcl16 |
TTTGGAATGAGGAAAGGTAGG |
GCAAAGGGCATTGTAAGGT |
| Cxcl17 |
TCATCTCCCTTGAGCCCCT |
TGCTTGTGTAGACTTGGTAGGC |
| Xcl1 |
CCAAATGGGTGAAAGCAGCG |
TCAGGGTTATCGCTGTGCTG |
| Cx3cl1 |
CTCACGAATCCCAGTGGCTT |
TTTCTCCTTCGGGTCAGCAC |
| Ccr1 |
CACTCACCGTACCTGTAGCC |
GCAAATATCAGACGCACGGC |
| Ccr2 |
GCCATCATAAAGGAGCCATACC |
ATGCCGTGGATGAACTGAGG |
| Ccr3 |
ATGGAGTAAAGTAGTCGCAGTGG |
GCCATTCTACTTGTCTCTGGT |
| Ccr4 |
CATCTCGGATTTGCTGTTCGT |
TGCCGCTGTAGAAGCCCAC |
| Ccr5 |
CCTAGCCAGAGGAGGTGAGACA |
GCAGGGTGCTGACATACCATAA |
| Ccr6 |
GCATTTCCTGGGACTTGCTT |
AGGTACTCCTGCCTCAGTGGTT |
| Ccr7 |
TGTGATTTCTACAGCCCCCAG |
AAAATGACAAGGAGAGCCACCA |
| Ccr8 |
GAAACCTCAGAAGAAAGGCTCG |
GCGGTGAAGAAATCAGGGTAG |
| Ccr9 |
GGAGGCTGGTCTGCATTATCTT |
AAGGCTTGTGAGTTCTGTGGG |
| Ccr10 |
GGACCAAGCCCACAGAGCA |
AGGGAGACACTGGGTTGGAAG |
| Cxcr1 |
CAATGGCCGAGGCTGAATA |
GAAGGGACACCAGTGCATAAAA |
| Cxcr2 |
TAGGTGTCCCACAGGTGAAAAG |
AGAATAGAGGGCATGCCAGAG |
| Cxcr3 |
CCCAACCACAAGTGCCAAAG |
TTCCAGAAGAAAGGCAAAGTCC |
| Cxcr4 |
ACGGCTGTAGAGCGAGTGTTG |
TGGGCAGGAAGATCCTATTGA |
| Cxcr5 |
GGACATGGGCTCCATCACATAC |
TCCCATCATACCCAGGAGGAA |
| Cxcr6 |
TGGCTCTTCAACAATTCCAGTG |
ACCAGGGAGTTTCCTAGCAGTC |
| Cxcr7 |
GAGGACACCCCACAAATCACT |
TGCACATCCATGGTCTTGAGG |
| Xcr1 |
CATGGGTTCTTGGCCTCAG |
GTGTGAGGTTGTAGGGAGCC |
| Cx3cr1 |
GTCACCATTAGTCTGGGCGT |
CCCAGACACTCGTTGTCCTT |
| Gapdh |
GACAAAATGGTGAAGGTCGGT |
GAGGTCAATGAAGGGGTCG |
Histological analysis
The treated skin was collected to perform a
histological examination. After the skin samples were fixed with 4%
paraformaldehyde overnight and embedded with paraffin, the tissues
were sectioned at 14 μm using a cryostat and stained with
hematoxylin and eosin (H&E), as previously described (32). The stained sections were observed
and captured using a bright light microscope (Olympus). The
thickness of the epidermal was determined by measuring the distance
between the junction of dermal-epidermal and surface of the
epidermal. Epidermal thickness was quantified by Image J software
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All quantification data are expressed as the means ±
SEM, and error bars represented SEM. Behavioral tests were
performed as well as the Student's t-test, measurements of
epidermal thickness, and RT-qPCR analysis with one-way ANOVA and
Dunn's post hoc test, both of which were performed using GraphPad
Prism software (GraphPad Software, Inc.). P<0.05 was considered
to indicate a statistically significant difference.
Results
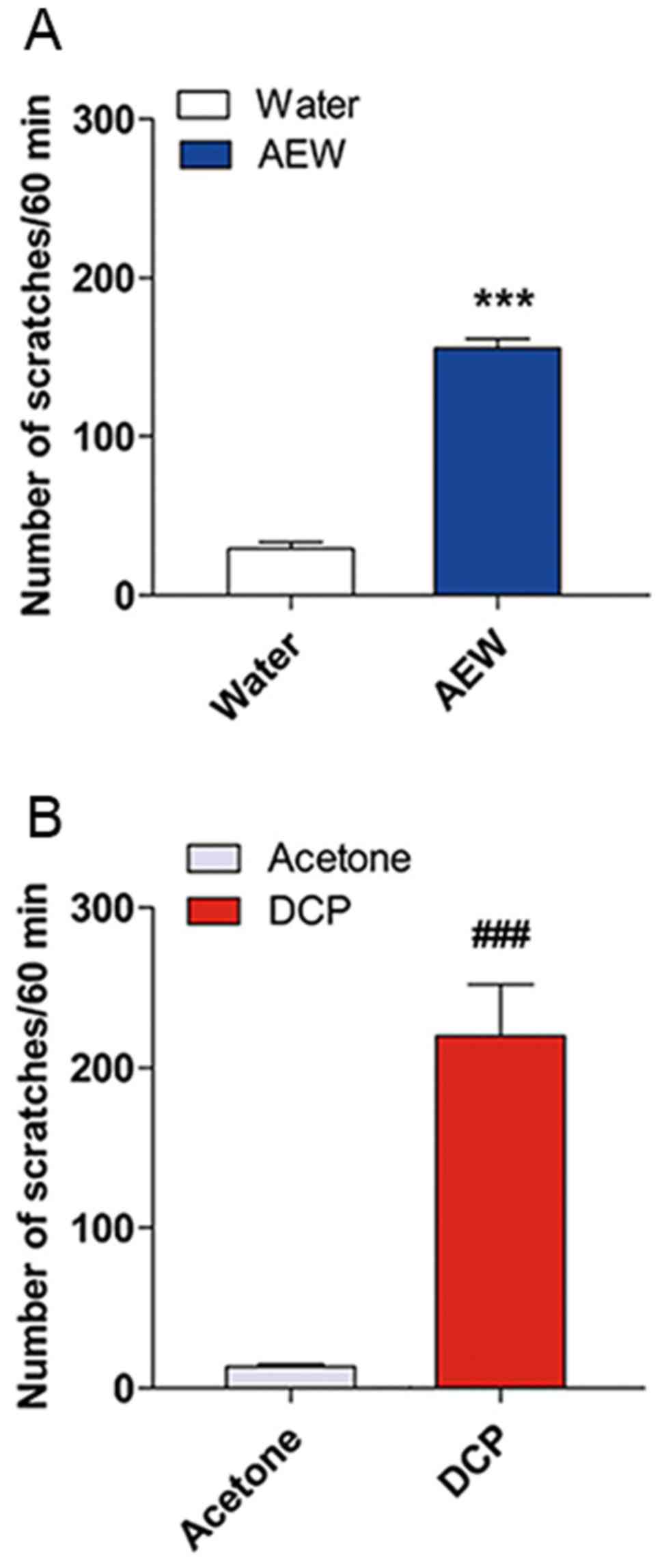
AEW- and DCP-induced scratching behaviors
in mice
The number of spontaneous scratching bouts (SSB)
within 1 h between the model group and control group were assessed
in a blinded manner. As shown in Fig.
1, the AEW-treated mice exhibited a significantly greater
number of SSBs (SSB=155.5±6.009, n=8, P<0.001) at day 9 compared
with the water-treated mice (SSB=29.38±3.923, n=8). The number of
SSBs in the DCP-treated mice (SSB=219.9±32.31, n=7) exhibited an
obvious difference between the acetone-treated mice at day 10
(SSB=13.29±1.599, n=7, P<0.001).
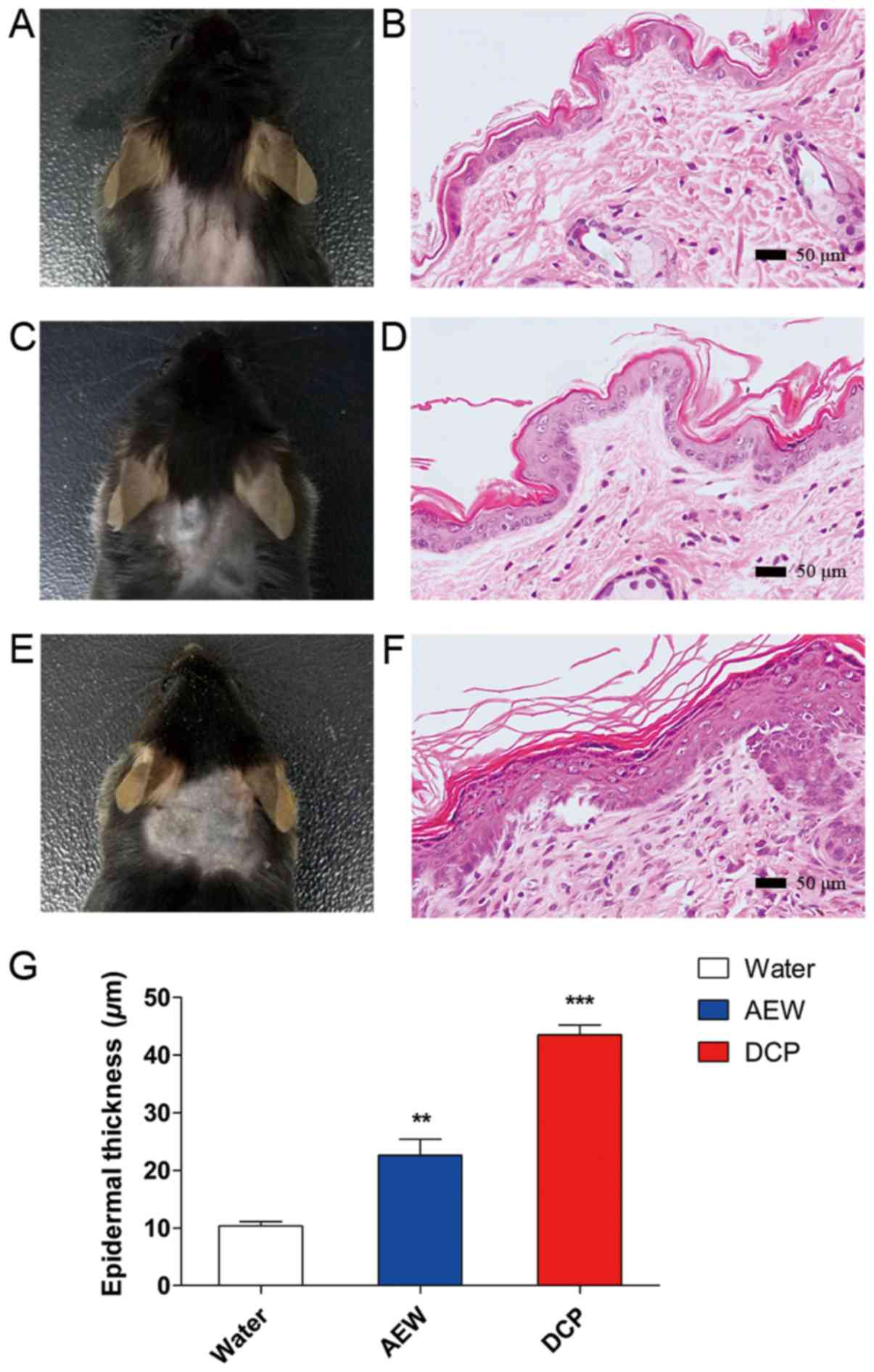
Histological observations of the
skin
H&E staining of skins of mice from the naïve,
AEW and DCP groups is shown in Fig. 2
(B, D and F respectively). The skins of the naïve mice were
smooth and soft (Fig. 2A). The
skins of the mice in the AEW group were rough and sclerotic
(Fig. 2C). The skins of the
DCP-treated mice manifested congestion and sclerosis, accompanied
with scratches and incrustation (Fig.
2E). There was apparent epidermal hyperplasia in the AEW and
DCP groups compared with the naïve control group, as shown in
Fig. 2G (AEW vs. naïve,
P<0.05; DCP vs. naïve, P<0.001).
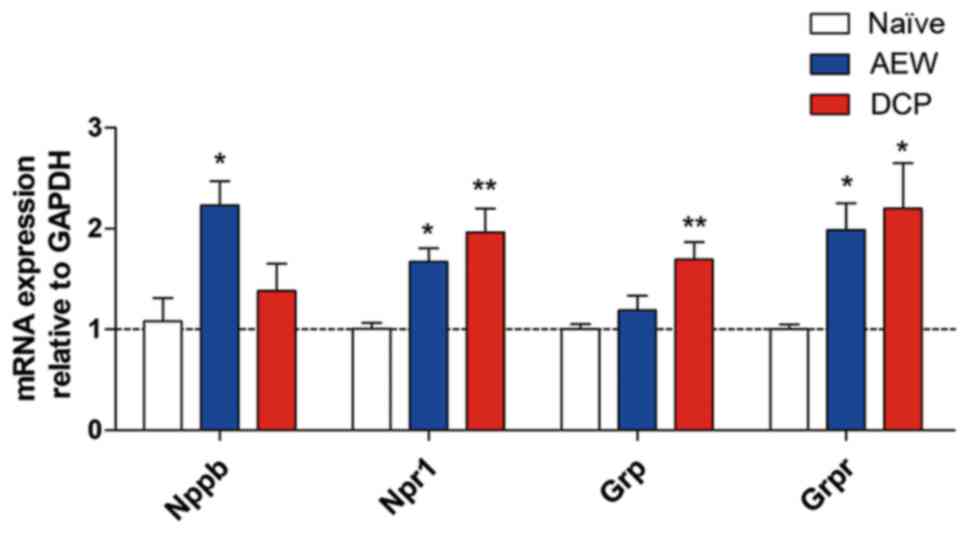
mRNA expression of representative itch
markers in AEW group and DCP group
The mRNA expression of natriuretic peptide B (Nppb)
was upregulated in the AEW group, and that of gastrin releasing
peptide (Grp) was upregulated in the DCP group. Additionally, the
mRNA expression levels of their receptors, Npr1 and Grpr, were
increased in both chronic itch groups (Fig. 3).
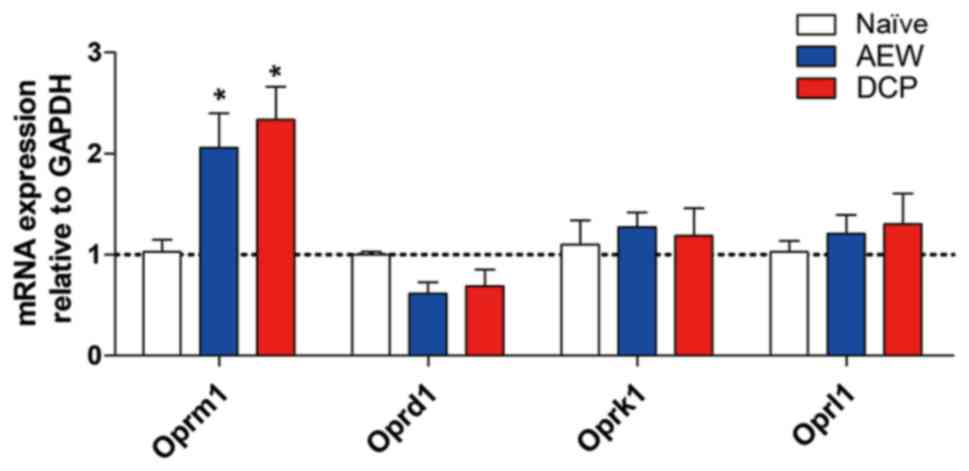
Oprm1 gene expression is increased in the
AEW group and DCP group
The fold change of opioid receptor mu 1 (Oprm1) in
the lower cervical spinal cord (C5-8) was increased in both the AEW
and DCP groups compared with the naïve mice (P<0.05). The gene
expression of other opioid receptors, such as opioid receptor kappa
1 (Oprk1), opioid receptor delta 1 (Oprd1) and opioid related
nociceptin receptor 1 (Oprl1) exhibited no significant difference
in the AEW or DCP groups compared with the naïve mice (Fig. 4).
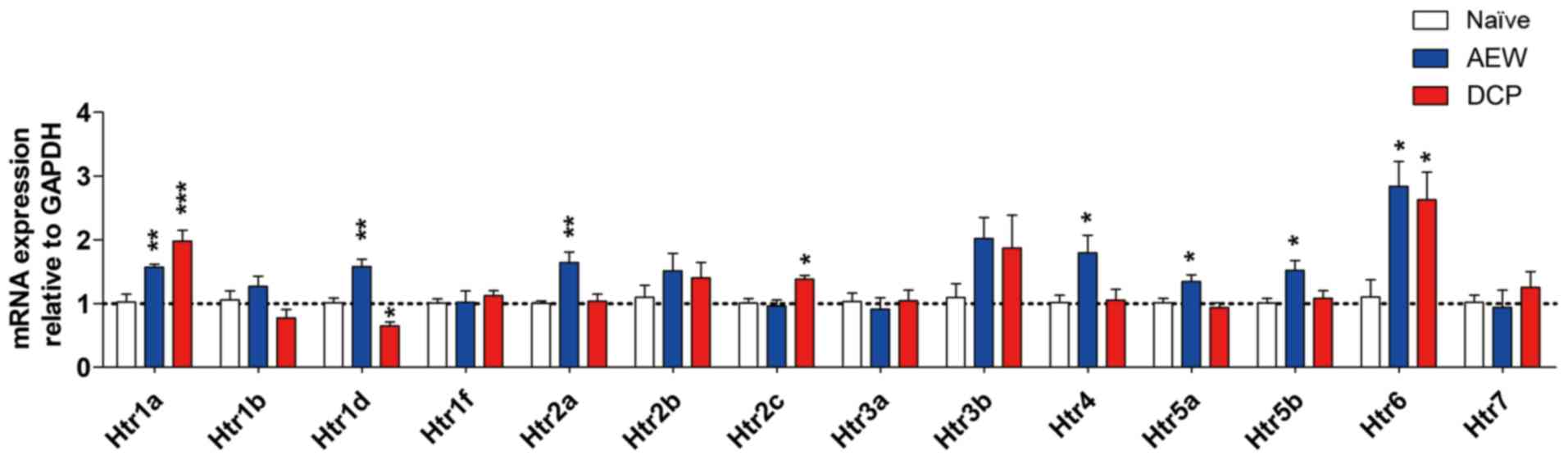
Differential expression profiles of
5-hydroxytryptamine receptors (Htrs) in the AEW and DCP groups
The fold changes of all Htrs in the two models of
different types of chronic itch compared with the naïve control
mice are presented in Fig. 5.
There was a significant increase in the expression levels of Htr1a
(P<0.01), Htr1d (P<0.01), Htr2a (P<0.01), Htr4
(P<0.05), Htr5a (P<0.05), Htr5b (P<0.05) and Htr6
(P<0.05) in the AEW group, whereas there was a significant
increase in the expression levels of Htr1a (P<0.001), Htr2c
(P<0.05) and Htr6 (P<0.05) in the DCP group. The Htr1a and
Htr6 expression levels were increased in both groups. Only Htr1d
expression was significantly decreased in the DCP group (P<0.05;
Fig. 5).
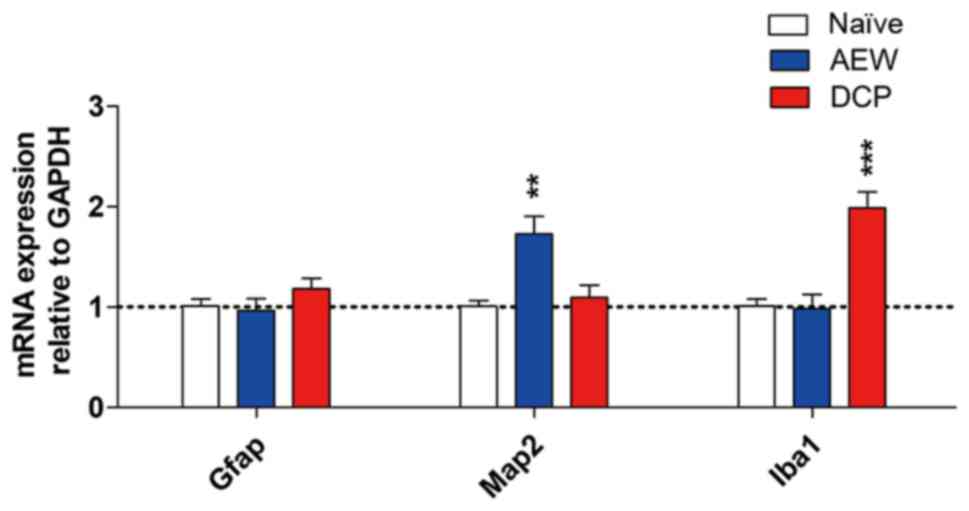
mRNA expression of neuronal and
microglial markers in the AEW and DCP groups
In order to determine whether neurons and glia are
activated in the spinal cords of mice with chronic itch, the mRNA
expression levels of neuronal marker microtubule-associated protein
2 (Map2), astrocytic marker glial fibrillary acidic protein (Gfap)
and the expression of the microglial marker ionized calcium binding
adaptor molecule 1 (Iba1) were evaluated by RT-qPCR. The fold
change of Map2 exhibited elevated an upregulation in the AEW group
(P<0.01). The expression of Iba1 (P<0.001) was significantly
upregulated in the DCP group compared with the naïve mice. The mRNA
expression of Gfap did not exhibit an obvious change in either
model of chronic itch (Fig.
6).
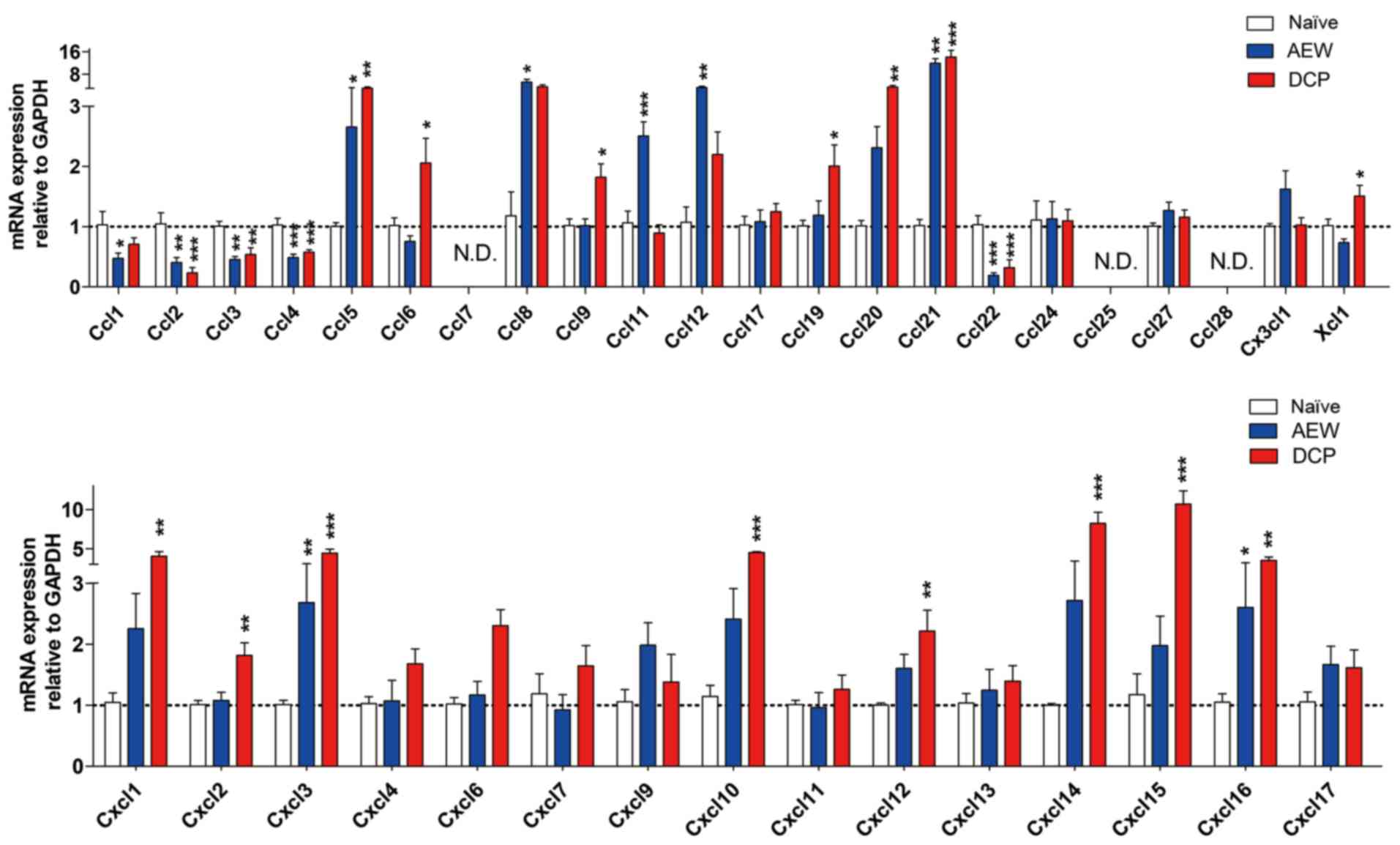
Expression profile of chemokine ligands
between AEW group and DCP group
In both models of chronic itch, the mRNA expression
levels of C-C motif chemokine ligand (Ccl)2, Ccl3, Ccl4 and Ccl22
were downregulated, whereas those of Ccl5, Ccl8, Ccl21, Cxcl3 and
Cxcl16 were upregulated in the lower cervical spinal cord. We did
not detect any gene expression of Ccl7, Ccl25 and Ccl28 within 40
cycles. Additionally, the mRNA expression of Ccl1 was decreased,
whereas the expression levels of Ccl11 and Ccl12 were increased in
the AEW group. In addition, in the DCP group, the mRNA expression
levels of Ccl6, Ccl19, Ccl20, Xcl1, Cxcl1, Cxcl2, Cxcl10, Cxcl12,
Cxcl14 and Cxcl15 were upregulated (Fig. 7).
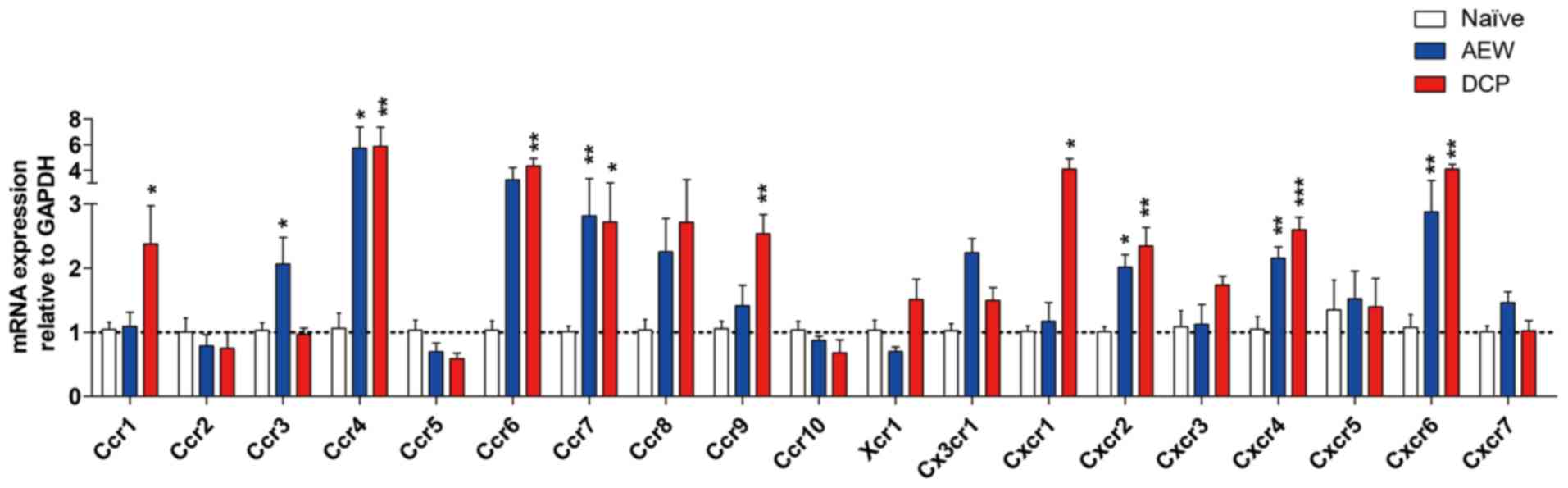
Expression profiles of chemokine
receptors in two models of different types of chronic itch induced
by AEW and DCP
The gene expression levels of Ccr4, Ccr7, Cxcr2,
Cxcr4 and Cxcr6 were increased in both chronic itch groups. The
fold change of Ccr3 in the AEW group, and that of Ccr1, Ccr6, Ccr9
and Cxcr1 in the DCP group was significantly increased (Fig. 8). The detailed association of
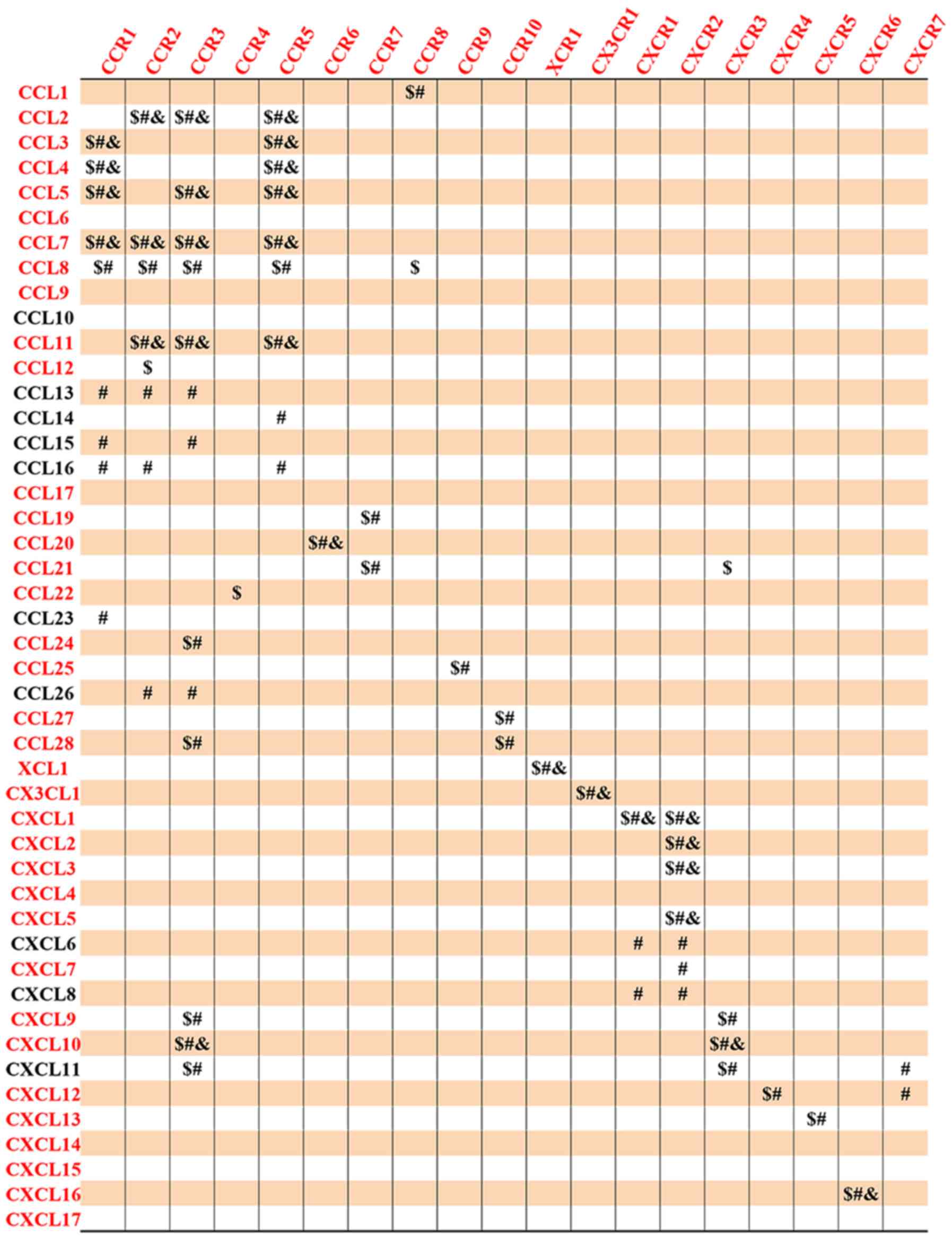
chemokine ligands and chemokine receptors is shown in Fig. 9.
Discussion
Itch and pain closely share mechanisms in neural
pathways and are mediated by similar neuronal cell types and
molecules. The current knowledge on itch has arisen from the
investigation of pain (1,8,9).
Thus, valuable information on pain mediators aids our understanding
of the underlying mechanisms of itch. Chronic itch is induced by
peripheral neuropathy, nerve irritation, central hypersensitivity,
or immune dysfunction, which can lead to itch hypersensitivity by
releasing inflammatory mediators such as proinflammatory cytokines,
growth factors and chemokines to activate or sensitize peripheral
and central nervous systems (37). Therefore, we aimed to screen and
discover critical and specific molecules or mediators in the spinal
cord during chronic itch conditions.
Acting as a local irritant, DCP induces local
sensitization and immune response (36). In contrast to DCP-induced contact
dermatitis, AEW induces dry skin (32,35). Skin dryness is one of the most
common symptoms of chronic itch conditions; for example, atopic
dermatitis, uremic pruritus and cholestatic pruritus (43). B-type natriuretic peptide (BNP)
and natriuretic peptide receptor A (NPRA), which are encoded by the
Nppb and Npr1 genes, have been defined as itch-selective
neuropeptides and receptors (44-46). The GRP/GRPR system has been
reported to include the first-known itch-specific molecules
(7). There has been controversy
surrounding the expression of GRP in peripheral nerves (45,47). However, GRP and its receptor,
GRPR, are highly expressed in the spinal cord. GRP and GRPR
expression levels are increased in the skin and spinal cord of
primates exhibiting chronic itch symptoms (48). In this study, after the models of
chronic itch were established, we examined the mRNA levels of most
representative itch markers, such as Nppb, Npr1, Grp and Grpr in
the spinal cords of AEW- and DCP-treated mice. We found that the
mRNA expression levels of Nppb and Npr1 were increased in the mice
with AEW-induced itch. In addition, our results revealed that the
mRNA expression levels of Grp and Grpr were increased in the spinal
cords of mice with DCP-induced chronic itch. A previous study by
Miyamoto et al demonstrated that inflammatory cells did not
infiltrate the dermis of dry-skin samples from AEW-treated mice,
suggesting that skin inflammation involves different mechanisms in
contact dermatitis compared with dry-skin pruritus (32). Therefore, the differential
expression in two types of chronic itch model suggests that the
NPPB/NPR1 system plays a major role in chronic dry skin-associated
pruritus, while the GRP/GRPR system is more related to chronic
pruritus caused by contact dermatitis. Taken together, we
identified herein that the application of AEW and DCP produces
profound scratching behaviors and chronic itch in mice, as also
previously described (32,36,49).
In this study, most genes that we screened which
appeared to be associated with itch behavior have been verified.
Previously, the intrathecal application or microinjection of the
agonist of KOP, DOP and NOP did not elicit obvious scratching in
monkeys (50-52). However, MOP is required for
intrathecal morphine-induced itch, and MOP antagonist blocks
intrathecal morphine-induced itch (11). Miyamoto et al demonstrated
that the subcutaneous injection of naloxone and naltrexone, which
are antagonists of MOP, suppressed spontaneous scratching in
AEW-treated mice, suggesting that MOP may be involved in the
development of AEW-induced itch condition (32). Likewise, the results of this study
demonstrated that Oprm1 expression increased in both types of
models of chronic itch. Combining previous findings and our
results, it can be inferred that Oprm1 plays an important role in
itch mechanisms and other opioid receptor subtypes may not be
involved in AEW- and DCP-induced chronic itch. According to
pharmacological studies, the 5-HT1 and 5-HT2 receptors are involved
in itch perception (53,54). Consistent with these findings, our
results further confirmed that the gene expression levels of Htr1a,
Htr1d, Htr2a and Htr2c were upregulated in the AEW- or DCP-induced
chronic itch condition. In addition, a study conducted by Tian
et al identified that the mRNA expression levels of Htr1d,
Htr2a, Htr2c, Htr5a, Htr5b and Htr6 were increased in the
trigeminal ganglia and dorsal root ganglia of rats exhibiting
cholestatic itch (55).
Furthermore, the study by Morita et al demonstrated that
5-HT7 was a key mediator of acute serotonergic itch and chronic
atopic dermatitis itch (56).
However, a recent study confirmed the role of Htr2a, but not Htr7,
in allergic contact dermatitis itch and of Htr7, but not Htr2a, in
dry skin-associated chronic itch (57). Although these two studies both
used knockout mice to verify the roles of 5-HT receptors, residual
pruritus behaviors in animals lacking Htr7 or Htr2a have still been
observed, suggesting that other 5-HT receptors or molecules are
required for the development of chronic itch models. In this study,
the increased mRNA level of Htr2a in the spinal cord was
preliminarily screened. Whether Htr2a is involved in dry skin itch
requires further verification. Although the exact subtypes of 5-HT
receptors involved in different mouse models of chronic itch have
yet to be elucidated, these studies and our results highlight the
crucial roles of serotonergic signaling in chronic itch pathology.
In brief, we found that the gene expression levels of Htr1a, Htr6
and Oprm1 were upregulated in both models of chronic itch.
In order to determine whether neurons and glia were
activated in the spinal cord of mice with chronic itch, we examined
neuronal and microglial marker expression between the AEW and DCP
groups. Previously, Liu et al employed pharmacological and
transgenic approaches to verify that AEW could induce the
activation of astrocytes in the spinal cord of mice. Interestingly,
they also found that spinal astrogliosis was affected by scratching
behavior, which was abrogated when mice were prevented from
scratching pruritus skin by Elizabethan Collars (49). This novel finding was consistent
with that in the study by Wilson et al, in which the extent
of keratinocyte hyperplasia in the skin of AEW mice was found to be
scratch-dependent (58). By
contrast, in this study, we found that the expression level of
Map2, which is a neuronal marker, was much higher than that of Gfap
in the spinal cords of AEW-induced itch mice. A recent study
highlighted the importance of Map2 in controlling the axonal entry
of cargo vesicles and regulating their distribution along the
distal axon. It suggested that Map2 controls axonal cargo transport
and drives synaptic and secretory vesicle accumulation in the
periphery (59). Therefore, the
increase in the expression of Map2 in the AEW group may be
attributed to the increased secretory vesicle in neurons. In
addition, Map2 has been reported to play an important role in
neuronal morphogenesis and to affect microtubule density and the
length of dendrites (60). The
increased expression of Map2 may enhance the sprouting and synaptic
plasticity of dendrites, resulting in neural hypersensitivity.
Therefore, in long-term skin dryness, pathological changes of the
central nervous system, such as neuropathic itch may occur and lead
to Map2 upregulation, which enhances the sprouting and synaptic
plasticity of dendrites. Another explanation is that the expression
of Map2 is not restricted to neurons. For instance, it has been
reported before that reactive astrocytes may express
neuron-specific enolase and Map2 (61,62). We also found the expression level
of Iba1, which is a microglial marker, was prominently increased in
the spinal cords of mice with DCP-induced chronic itch. It has been
established that the mediation of gene expression varies over time
(63-66). The main difference between the two
studies is that Liu et al examined expression levels in the
cervical dorsal horn 5 days after AEW treatment while we examined
the cervical spinal cord 9 after treatment. In long-term skin
dryness, pathological changes of the central nervous system, such
as neuropathic itch may occur and can lead to the upregulation of
Map2, which enhances the sprouting and synaptic plasticity of
dendrites.
Over the past decade, a dermatology study
demonstrated the expression patterns of chemokines in the skin of a
chronic proliferative dermatitis mutant mouse model, which was a
useful way of investigating the role of chemokines in eosinophil
accumulation in chronic inflammation (67). Chemokine ligands and receptors,
which participate in the induction and maintenance of inflammation,
have been studied in several diseases models as inflammatory
mediators. Although chemokines and their receptors have been
implicated in the pathophysiology of chronic pain, they have been
largely unexplored in chronic itch conditions. Among the chemokines
examined in this study, the mRNA expression levels of Ccl2, Ccl3,
Ccl4 and Ccl22 were downregulated in the two mouse models of
chronic itch. Another study observed the conspicuously decreased
serum concentration of CCL2, CCL3, CCL4 and CCL5 by an
enzyme-linked immunosorbent assay in the ragweed-allergic patients
out of the pollen season. It seemed that the allergic subjects
protected against the initiation of an allergic inflammatory
reaction by maintaining a low physiologic concentration of
chemokines (68). Thus, the
decrease in the levels of these chemokines may be a mechanism
through which tissues can be protected from damage by continuously
recruiting cascaded immune mediators to the lesion sites.
Consistent with our findings of the upregulation of Ccl21 and Iba1
in DCP-induced itch, Biber et al identified that neuronal
Ccl21 upregulated the expression of ionotropic purinoceptors P2X4
in spinal cord microglia (69).
The release of Ccl21, which is a potent microglial activator, was
markedly increased after neural hyperexcitability or injury
(70). In our study, the mRNA
levels of Ccl21 were dramatically upregulated in two different
chronic itch models, suggesting that the activation of microglia
cells may be triggered by Ccl21. Therefore, Ccl21 may be a
promising drug target in chronic itch intervention. A recent study
by Jing et al investigated whether spinal cord chemokines
could contribute to the development of chronic itch. They reported
that Cxcr3-deficiency mice showed reduced scratching in chronic
itch models induced by AEW, DCP and 2,4-dinitro-1-fluorobenzene
(DNFB). Moreover, their results revealed the mRNA and protein
expression levels of CXCR3 and CXCL10 in the spinal cord 7 days
after AEW treatment increased significantly, suggesting that spinal
cord chemokines may be involved in the alteration of skin induced
by itch mediators (71). We found
that the mRNA level of Cxcl10 was similarly up-regulated in
DCP-induced contact dermatitis in our results. However, we did not
detect significant alteration of Cxcl10 or Cxcr3 in AEW-induced
dry-skin itch. Their results showed that SSB in the group of
wild-type mice treated with AEW at day 7 reached over 350 within 1
h. Our data demonstrated that SSB reached 155.5 by day 9. As
mentioned, scratching behaviors can affect glial activation of
spinal cord in AEW mice (49,58). This difference may be the result
from methodological differences in model construction. The
up-regulation of CXCL12-CXCR4 chemokine ligand-receptor systems has
been reported in the thalamus of diabetic monkeys accompanied with
neuroinflammation (23). The
results of this study demonstrated that the mRNA levels of both
Cxcl12 and Cxcr4 were upregulated in the DCP-treated mice. In
addition, our results revealed that some chemokines along with
their receptors were upregulated in two types of chronic itch mice,
Ccl21-Ccr7, Cxcl3-Cxcr2 and Cxcl16-Cxcr6, suggesting that these
ligand-receptor systems may play an important role in chronic itch
development. However, the actual role of these signaling pathways
must be verified in further research. In summary, it seems that
some of these related genes in the spinal cord may be involved in
the mediation of skin inflammation in chronic itch conditions. In
particular, the activation of glial cells is vital for the
development of chronic itch, suggesting that maintaining a balance
of chemokines in the spinal cord may provide a new direction for
therapeutic development. The details of the association between
chemokine ligands and chemokine receptors are shown in Fig. 9.
This study documents the profiles of several itch
mediators in two types of chronic itch. Moreover, we observed that
the levels of several chemokine ligands and their receptors were
simultaneously upregulated in both dry-skin and contact dermatitis
chronic itch. Nevertheless, there are some limitations to our
research. The exact role of the differentially expressed genes in
chronic itch warrants further investigation in future studies. The
changes in mRNA levels may not always reflect the changes in
protein levels, since many factors influence the expression of
proteins. For instance, the processes regulated protein expression
between transcription and translation could be affected at many
different stages and many different ways. In addition, following
translation, protein expression can also be regulated
post-translational modifications. However, the transcription level
data can suggest whether the protein is present or not and roughly
what at level to expect to find the protein. For example, a highly
abundant protein will usually have a highly expressed mRNA. The
transcription data is useful for identifying potential candidates
for follow-up work at the protein level. The goal of this study was
to draw out the gene expression patterns of itch-related mediators
in chronic itch model. To a certain extent, it was a way to narrow
the range and select the candidate mediators for further
investigating mechanisms of chronic itch. Western blot analysis may
be use in any future study by our group to confirm the results of
the present study. In conclusion, the data of this study identify
several molecular determinants of itch in the spinal cord and
provides a useful baseline in gene level for functional
experimentation or crosstalk between these related mediators. Our
research may thus contribute to future research on endogenous
triggers of chronic itch signaling, which may provide significant
breakthroughs for the treatment of obstinate pruritus.
Funding
This study was supported by grants from National
Natural Science Foundation of P.R. China (Nos. 81670240, 81271766
and 81674057), the National Natural Science Foundation of Hubei
Province (Nos. 2016CFB625 and 2016CFB324) and the Special Fund of
Fundamental Scientific Research Business Expense for Higher School
of Central Government (2012 TS060 to HBX).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
BWL performed the RT-qPCR experiments, analyzed the
data and wrote the manuscript. ZXL and ZGH established the itch
models and collected tissues, QW and CL performed the histology
experiments. XWZ, HY and HBX contributed to the study concept and
design, and supervised the project. All authors have read and
approved the manuscript.
Ethics approval and consent to
participate
This study was performed following the approval of
the Institutional Ethical Committee of Tongji Hospital, Tongji
Medical College, Huazhong University of Science and Technology (no.
TJ-A20150803).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Ikoma A, Steinhoff M, Stander S,
Yosipovitch G and Schmelz M: The neurobiology of itch. Nat Rev
Neurosci. 7:535–547. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
He ZG, Liu BW, Li ZX, Liu C, Liu C and
Xiang HB: Altered expression profiling of spinal genes modulated by
compound 48/80 in a mouse itch model. J Anesth Perioper Med.
4:220–224. 2017. View Article : Google Scholar
|
|
3
|
Chen M, Li ZX, Wang Q and Xiang HB:
Altered expression of differential genes in thoracic spinal cord
involved in experimental cholestatic itch mouse model. Curr Med
Sci. 38:679–683. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ding DF, Li RC, Xiong QJ, Zhou L and Xiang
HB: Pulsed radiofrequency to the great occipital nerve for the
treatment of intractable postherpetic itch: A case report. Int J
Clin Exp Med. 7:3497–3500. 2014.PubMed/NCBI
|
|
5
|
Liu BW, Li ZX, He ZG, Liu C, Xiong J and
Xiang HB: Altered expression of target genes of spinal cord in
different itch models compared with capsaicin assessed by RT-qPCR
validation. Oncotarget. 8:74423–74433. 2017.PubMed/NCBI
|
|
6
|
Bernhard JD: Itch and pruritus: What are
they, and how should itches be classified? Dermatol Ther.
18:288–291. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun YG and Chen ZF: A gastrin-releasing
peptide receptor mediates the itch sensation in the spinal cord.
Nature. 448:700–703. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ikoma A, Rukwied R, Stander S, Steinhoff
M, Miyachi Y and Schmelz M: Neurophysiology of pruritus:
Interaction of itch and pain. Arch Dermatol. 139:1475–1478. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moser HR and Giesler GJ Jr: Itch and
analgesia resulting from intrathecal application of morphine:
Contrasting effects on different populations of trigeminothalamic
tract neurons. J Neurosci. 33:6093–6101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akiyama T and Carstens E: Neural
processing of itch. Neuroscience. 250:697–714. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ko MC: Neuraxial opioid-induced itch and
its pharmacological antagonism. Handb Exp Pharmacol. 226:315–335.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ahmadi S, Karami Z, Mohammadian A,
Khosrobakhsh F and Rostamzadeh J: Cholestasis induced
antinociception and decreased gene expression of MOR1 in rat brain.
Neuroscience. 284:78–86. 2015. View Article : Google Scholar
|
|
13
|
Liu C, Liu TT, He ZG, Shu B and Xiang HB:
Inhibition of itch-related responses by selectively ablated
serotonergic signals at the rostral ventromedial medulla in mice.
Int J Clin Exp Pathol. 7:8917–8921. 2014.
|
|
14
|
Li HJ, Johnston B, Aiello D, Caffrey DR,
Giel-Moloney M, Rindi G and Leiter AB: Distinct cellular origins
for serotonin-expressing and enterochromaffin-like cells in the
gastric corpus. Gastroenterology. 146:754–764. 2014. View Article : Google Scholar :
|
|
15
|
Hoon MA: Molecular dissection of itch.
Curr Opin Neurobiol. 34:61–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu T, He Z, Tian X, Kamal GM, Li Z, Liu
Z, Liu H, Xu F, Wang J and Xiang H: Specific patterns of spinal
metabolites underlying alpha-Me-5-HT-evoked pruritus compared with
histamine and capsaicin assessed by proton nuclear magnetic
resonance spectroscopy. Biochim Biophys Acta Mol Basis Dis.
1863:1222–1230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Berger M, Gray JA and Roth BL: The
expanded biology of serotonin. Annu Rev Med. 60:355–366. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Beattie DT and Smith JA: Serotonin
pharmacology in the gastrointestinal tract: A review. Naunyn
Schmiedebergs Arch Pharmacol. 377:181–203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Steinhoff M, Cevikbas F, Ikoma A and
Berger TG: Pruritus: Management algorithms and experimental
therapies. Semin Cutan Med Surg. 30:127–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ji RR, Xu ZZ and Gao YJ: Emerging targets
in neuroinflammation-driven chronic pain. Nat Rev Drug Discov.
13:533–548. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kawasaki Y, Zhang L, Cheng JK and Ji RR:
Cytokine mechanisms of central sensitization: Distinct and
overlapping role of interleukin-1beta, interleukin-6, and tumor
necrosis factor-alpha in regulating synaptic and neuronal activity
in the superficial spinal cord. J Neurosci. 28:5189–5194. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsuda M, Shigemoto-Mogami Y, Koizumi S,
Mizokoshi A, Kohsaka S, Salter MW and Inoue K: P2X4 receptors
induced in spinal microglia gate tactile allodynia after nerve
injury. Nature. 424:778–783. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kiguchi N, Ding H, Peters CM, Kock ND,
Kishioka S, Cline JM, Wagner JD and Ko MC: Altered expression of
glial markers, chemokines, and opioid receptors in the spinal cord
of type 2 diabetic monkeys. Biochim Biophys Acta Mol Basis Dis.
1863:274–283. 2017. View Article : Google Scholar
|
|
24
|
Shiratori-Hayashi M, Koga K, Tozaki-Saitoh
H, Kohro Y, Toyonaga H, Yamaguchi C, Hasegawa A, Nakahara T,
Hachisuka J, Akira S, et al: STAT3-dependent reactive astrogliosis
in the spinal dorsal horn underlies chronic itch. Nat Med.
21:927–931. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zlotnik A and Yoshie O: The chemokine
superfamily revisited. Immunity. 36:705–716. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Melik-Parsadaniantz S and Rostene W:
Chemokines and neuromodulation. J Neuroimmunol. 198:62–68. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
White FA, Jung H and Miller RJ: Chemokines
and the pathophysiology of neuropathic pain. Proc Natl Acad Sci
USA. 104:20151–20158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao YJ and Ji RR: Chemokines,
neuronal-glial interactions, and central processing of neuropathic
pain. Pharmacol Ther. 126:56–68. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Charo IF and Ransohoff RM: The many roles
of chemokines and chemokine receptors in inflammation. N Engl J
Med. 354:610–621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Asensio VC and Campbell IL: Chemokines in
the CNS: Plurifunctional mediators in diverse states. Trends
Neurosci. 22:504–512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Savarin-Vuaillat C and Ransohoff RM:
Chemokines and chemokine receptors in neurological disease: Raise,
retain, or reduce? Neurotherapeutics. 4:590–601. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miyamoto T, Nojima H, Shinkado T,
Nakahashi T and Kuraishi Y: Itch-associated response induced by
experimental dry skin in mice. Jpn J Pharmacol. 88:285–292. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thaipisuttikul Y: Pruritic skin diseases
in the elderly. J Dermatol. 25:153–157. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Di Nardo A, Wertz P, Giannetti A and
Seidenari S: Ceramide and cholesterol composition of the skin of
patients with atopic dermatitis. Acta Derm Venereol. 78:27–30.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY
and Chen ZF: Cellular basis of itch sensation. Science.
325:1531–1534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang TT, Shen FY, Ma LQ, Wen W, Wang B,
Peng YZ, Wang ZR and Zhao X: Potentiation of synaptic transmission
in Rat anterior cingulate cortex by chronic itch. Mol Brain.
9:732016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ji RR: Neuroimmune interactions in itch:
Do chronic itch, chronic pain, and chronic cough share similar
mechanisms? Pulm Pharmacol Ther. 35:81–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Valtcheva MV, Samineni VK, Golden JP,
Gereau RW IV and Davidson S: Enhanced nonpeptidergic intraepidermal
fiber density and an expanded subset of chloroquine-responsive
trigeminal neurons in a mouse model of dry skin itch. J Pain.
16:346–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Geng X, Shi H, Ye F, Du H, Qian L, Gu L,
Wu G, Zhu C, Yang Y, Wang C, et al: Matrine inhibits itching by
lowering the activity of calcium channel. Sci Rep. 8:113282018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Y, Liu J, Li M, Dai S, Liang J and Ji
W: The effect of kinin B1 receptor on chronic itching
sensitization. Mol Pain. 11:702015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xu Y, Zhang XH and Pang YZ: Association of
tyrosinase (TYR) and tyrosinase-related protein 1 (TYRP1) with
melanic plumage color in korean quails (Coturnix coturnix).
Asian-Australas J Anim Sci. 26:1518–1522. 2013. View Article : Google Scholar
|
|
42
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nature Protoc.
3:1101–1108. 2008. View Article : Google Scholar
|
|
43
|
Han L and Dong X: Itch mechanisms and
circuits. Annu Rev Biophys. 43:331–355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shimizu Y, Sonoda A, Nogi C, Ogushi Y,
Kanda R, Yamaguchi S, Nohara N, Aoki T, Yamada K, Nakata J, et al:
B-type (brain) natriuretic peptide and pruritus in hemodialysis
patients. Int J Nephrol Renovasc Dis. 7:329–335. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mishra SK and Hoon MA: The cells and
circuitry for itch responses in mice. Science. 340:968–971. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kiguchi N, Sukhtankar DD, Ding H, Tanaka
K, Kishioka S, Peters CM and Ko MC: Spinal functions of B-type
natriuretic peptide, gastrin-releasing peptide, and their cognate
receptors for regulating itch in mice. J Pharmacol Exp Ther.
356:596–603. 2016. View Article : Google Scholar :
|
|
47
|
Liu XY, Wan L, Huo FQ, Barry DM, Li H,
Zhao ZQ and Chen ZF: B-type natriuretic peptide is neither
itch-specific nor functions upstream of the GRP-GRPR signaling
pathway. Mol Pain. 10:42014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nattkemper LA, Zhao ZQ, Nichols AJ, Papoiu
ADP, Shively CA, Chen ZF and Yosipovitch G: Overexpression of the
gastrin-releasing peptide in cutaneous nerve fibers and its
receptor in the spinal cord in primates with chronic itch. J Invest
Dermatol. 133:2489–2492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu T, Han Q, Chen G, Huang Y, Zhao LX,
Berta T, Gao YJ and Ji RR: Toll-like receptor 4 contributes to
chronic itch, alloknesis, and spinal astrocyte activation in male
mice. Pain. 157:806–817. 2016. View Article : Google Scholar :
|
|
50
|
Thomas DA, Williams GM, Iwata K, Kenshalo
DR Jr and Dubner R: Effects of central administration of opioids on
facial scratching in monkeys. Brain Res. 585:315–317. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ko MC, Lee H, Harrison C, Clark MJ, Song
HF, Naughton NN, Woods JH and Traynor JR: Studies of micro-,
kappa-, and delta-opioid receptor density and G protein activation
in the cortex and thalamus of monkeys. J Pharmacol Exp Ther.
306:179–186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ko MC, Wei H, Woods JH and Kennedy RT:
Effects of intrathecally administered nociceptin/orphanin FQ in
monkeys: Behavioral and mass spectrometric studies. J Pharmacol Exp
Ther. 318:1257–1264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ishiuji Y, Coghill RC, Patel TS, Dawn A,
Fountain J, Oshiro Y and Yosipovitch G: Repetitive scratching and
noxious heat do not inhibit histamine-induced itch in atopic
dermatitis. Br J Dermatol. 158:78–83. 2008.
|
|
54
|
Kim DK, Kim HJ, Kim H, Koh JY, Kim KM, Noh
MS, Kim JJ and Lee CH: Involvement of serotonin receptors 5-HT1 and
5-HT2 in 12(S)-HPETE-induced scratching in mice. Eur J Pharmacol.
579:390–394. 2008. View Article : Google Scholar
|
|
55
|
Tian B, Wang XL, Huang Y, Chen LH, Cheng
RX, Zhou FM, Guo R, Li JC and Liu T: Peripheral and spinal 5-HT
receptors participate in cholestatic itch and antinociception
induced by bile duct ligation in rats. Sci Rep. 6:362862016.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Morita T, McClain SP, Batia LM, Pellegrino
M, Wilson SR, Kienzler MA, Lyman K, Olsen AS, Wong JF, Stucky CL,
et al: HTR7 Mediates serotonergic acute and chronic itch. Neuron.
87:124–138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Luo J, Feng J, Yu G, Yang P, Mack MR, Du
J, Yu W, Qian A, Zhang Y, Liu S, et al: Transient receptor
potential vanilloid 4-expressing macrophages and keratinocytes
contribute differentially to allergic and nonallergic chronic itch.
J Allergy Clin Immunol. 141:608–619. 2018. View Article : Google Scholar
|
|
58
|
Wilson SR, Nelson AM, Batia L, Morita T,
Estandian D, Owens DM, Lumpkin EA and Bautista DM: The ion channel
TRPA1 is required for chronic itch. J Neurosci. 33:9283–9294. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ribeiro LF and de Wit J: Neuronal
polarity: MAP2 shifts secretory vesicles into high gear for
long-haul transport down the axon. Neuron. 94:223–225. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mohan R and John A: Microtubule-associated
proteins as direct crosslinkers of actin filaments and
microtubules. IUBMB Life. 67:395–403. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Geisert EE Jr, Johnson HG and Binder LI:
Expression of microtubule-associated protein 2 by reactive
astrocytes. Proc Natl Acad Sci USA. 87:3967–3971. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ridet JL, Malhotra SK, Privat A and Gage
FH: Reactive astrocytes: Cellular and molecular cues to biological
function. Trends Neurosci. 20:570–577. 1997. View Article : Google Scholar
|
|
63
|
Harrison BJ, Venkat G, Hutson T, Rau KK,
Bunge MB, Mendell LM, Gage FH, Johnson RD, Hill C and Rouchka EC:
Transcriptional changes in sensory ganglia associated with primary
afferent axon collateral sprouting in spared dermatome model. Genom
Data. 6:249–252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Knowlton WM, Palkar R, Lippoldt EK, McCoy
DD, Baluch F, Chen J and McKemy DD: A sensory-labeled line for
cold: TRPM8-expressing sensory neurons define the cellular basis
for cold, cold pain, and cooling-mediated analgesia. J Neurosci.
33:2837–2848. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yang W, Rudick CN, Hoxha E, Allsop SA,
Dimitrakoff JD and Klumpp DJ: Ca(2+)/calmodulin-dependent protein
kinase II is associated with pelvic pain of neurogenic cystitis. Am
J Physiol Renal Physiol. 303:F350–F356. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ke C, Gao F, Tian X, Li C, Shi D, He W and
Tian Y: Slit2/Robo1 mediation of synaptic plasticity contributes to
bone cancer pain. Mol Neurobiol. 54:295–307. 2017. View Article : Google Scholar
|
|
67
|
Renninger ML, Seymour R, Lillard JW Jr,
Sundberg JP and HogenEsch H: Increased expression of chemokines in
the skin of chronic proliferative dermatitis mutant mice. Exp
Dermatol. 14:906–913. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kostova Z, Batsalova T, Moten D, Teneva I
and Dzhambazov B: Ragweed-allergic subjects have decreased serum
levels of chemokines CCL2, CCL3, CCL4 and CCL5 out of the pollen
season. Cent-Eur J Immunol. 40:442–446. 2015. View Article : Google Scholar
|
|
69
|
Biber K, Tsuda M, Tozaki-Saitoh H,
Tsukamoto K, Toyomitsu E, Masuda T, Boddeke H and Inoue K: Neuronal
CCL21 up-regulates microglia P2X4 expression and initiates
neuropathic pain development. EMBO J. 30:1864–1873. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hulsebosch CE, Hains BC, Crown ED and
Carlton SM: Mechanisms of chronic central neuropathic pain after
spinal cord injury. Brain Res Rev. 60:202–213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jing PB, Cao DL, Li SS, Zhu M, Bai XQ, Wu
XB and Gao YJ: Chemokine receptor CXCR3 in the spinal cord
contributes to chronic itch in mice. Neurosci Bull. 34:54–63. 2017.
View Article : Google Scholar : PubMed/NCBI
|