Introduction
Due to their high prevalence, blunt chest trauma
(TxT) and hemorrhagic shock have significant effects on the
outcomes of trauma patients, causing severe modulations of the
immune system and high mortality rates (1-4).
Early mortality is mainly caused by marked blood loss, traumatic
brain injuries or non-controllable organ damage within the first
minutes and during the first hours following trauma, whereas later
mortality is mainly caused by secondary complications of the
respiratory system or, for example, the development of sepsis
during the first days to weeks of admission leading to multiple
organ failure and multiple organ dysfunction syndrome (MODS)
(1). Here, an essential factor
during the pro-inflammatory reaction of the immune system is
involved in the release of pathogen-associated molecular patterns
and/or damage-associated molecular patterns for the development of
MODS (1,5,6).
The immune system reacts to trauma with a substantial release of
pro-inflammatory cytokines, including tumor necrosis factor
(TNF)-α, interleukin (IL)-6 and IL-1β, and the expression of
intercellular adhesion molecule (ICAM)-1. Activation of the
transcription factor NF-κB serves a key role in the production,
regulation and release of these mediators (7-9).
Alcohol consumption in trauma patients constitutes
an extra variable influencing the post-traumatic immune response
with high clinical impact. Literature on the positive or negative
influence of alcohol is inconsistent. Our previous clinical and
in vivo studies found an immune-modulating influence of
alcohol, and even reduced mortality rates following isolated
hemorrhagic shock in rats (2,10,11). Phelan et al and Zambell
et al also confirmed the influence of alcohol on the immune
system, including modulated neutrophil function and cytokines TNF-α
and IL-10 in an in vivo hemorrhage model (12,13). In contrast to our results, in the
studies of Phelan et al and Zambell et al, the
changes due to alcohol consumption aggravated outcomes. In addition
to the volume or concentration of alcohol, which is known to be of
importance regarding either the positive or negative effects of
alcohol consumption (14,15), the timing of alcohol admission is
another relevant clinical question for affecting healthcare and
outcomes for trauma patients. With regard to the metabolic changes
caused by alcohol consumption, the timing of its consumption serves
a decisive role (16,17). This is partly explained by the
influence of alcohol on gluconeogenesis, as alcohol-induced
inhibition of gluconeogenesis can lead to hyperlactacidemia,
hypoglycemia and liver damage (16). However, few studies have examined
the timing of alcohol intoxication prior to trauma or the
time-dependent effects on the inflammatory response and organ
damage following trauma/hemorrhage.
Therefore, the present study investigated the
influence of either an acute ('drink and drive scenario') versus a
subacute ('evening binge drinking') alcohol-drinking scenario in an
in vivo model of TxT and hemorrhagic shock, with particular
focus on the hepatic inflammatory response and survival rates. The
study aimed to test the hypothesis that acute and subacute alcohol
intake has a beneficial influence on local inflammatory response
and liver injury in a clinically relevant double hit trauma.
Materials and methods
Animals and experimental model
The present study was approved by the Veterinary
Department of the Regional Council in Darmstadt, Germany
[Regierungspraesidium (RP) Darmstadt, Veterinaerswesen', Hessen,
Germany] and designed in accordance with the ARRIVE guidelines
(18). Animals were always
handled by group members holding a certificate of the Federation of
European Laboratory Animal Science Associations.
The experimental setting was as described previously
(17). Prior to experimental
procedures, female Lewis rats (age: 12-14 weeks, 190-240 g,
provided by Janvier Labs, France) remained in the facility for at
least 7 days for acclimation with a maximum of four animals per
cage. The animals were fasted over night before beginning the
surgical experimentation. Water was available ad libitum
during the whole housing. Animals were kept under standardized
temperature (21±2°C), relative humidity (50%), and artificial
light-dark cycles (14 h light, 10 h dark). Depending on the group
assignment, the animals either received a single dose of alcohol (5
g/kg, 30% ethanol, EtOH) or control solution (sodium chloride, NaCl
0.9%, ctrl) via oral gavage either 2 h (acute) or 12 h (subacute)
before experimentation. Previous studies using the same dose of
EtOH clearly demonstrated alcohol-dependent changes in the liver,
including decreased graft survival, disturbance of hepatic
microcirculation and increased triglyceride content (19-21). This is consistent with our
previous studies, in which fatty liver was induced by this dose and
concentration of alcohol (2,22).
Taken together, these previous data provided a valuable basis for
the use of alcohol at a dose of 5 g/kg body weight (30%) to examine
the effects of acute intoxication under inflammatory conditions
induced by hemorrhage. Prior to our previously reported studies
(2,22), preliminary experiments were
performed to establish the model of acute alcohol-induced fatty
liver using 20% EtOH, rather than 30%. Although an EtOH
concentration of 20% was initially used, a decrease in the gavage
volume was required as repeated aspirations occurred. Therefore, a
decision was made to increase the concentration to 30%.
Transaminase release and the level of hepatic fat deposition were
compared using both concentrations following H/R and a sham
procedure and resulted in no differences. The 30% concentration was
used in another previous study (23) in addition to our own previously
published study (17). For
anesthesia, isoflurane (1.2-3.0%), buprenorphine (0.05 mg/kg body
weight) and a local anesthesia to the incision sites (0.25%
Carbostesin) were applied. During anesthesia initiation, a 3.0%
isoflurane oxygen mixture was used, the vessels were cannulated
with a 2.0-2.5% isoflurane oxygen mixture depending on individual
responses to pain stimulation, the induction of trauma was
performed with a concentration of 2% isoflurane following a short
stabilization period and in the reperfusion period. At the end of
experimentation, the isoflurane concentration was reduced in a
step-by-step manner. The right femoral artery was cannulated with
polyethylene tubing to measure blood pressure, and a bilateral lung
contusion was induced, as described previously (24,25). Briefly, a Mylar polyester film
(0.190-mm, DuPont Teijin Films, Luxembourg) was fixed in a cylinder
6 cm above the chest of the animals, and a standardized pressure
wave was then applied to rupture the film and induce the lung
contusion. Subsequently, the left jugular vein and the right
carotid artery were cannulated to induce hemorrhagic shock, as
described previously (26,27).
Here, blood was withdrawn into a heparinized syringe via the
carotid artery to maintain a target mean arterial blood pressure
(MABP) of 35±3 mm Hg. For the regulation of the MABP at this level,
further withdrawal or recirculation of withdrawn blood was
performed for 60 min. The MABP was monitored using a blood pressure
analyzer (Sirecust 960, Siemens AG). Reperfusion over 30 min was
conducted with 60% of the withdrawn blood volume plus 50% Ringer's
lactate (RL) solution of the maximum removed volume via the jugular
vein. The hemorrhagic shock model was established in our laboratory
of the Department of Trauma, Hand and Reconstructive Surgery,
University Hospital Frankfurt, Goethe University, 10 years ago with
a resuscitation protocol of retransfusing 60% of the withdrawn
blood to simulate blood loss and reperfusion in traumatized
patients. As our previous studies were performed with a
resuscitation of 60% of the withdrawn blood, the present study was
also performed according to this established protocol to ensure
comparability of studies (26,27). Following removal of the catheters,
the wounds were sutured. At the end of the surgical/trauma phase,
the animals were monitored. During the entire experimentational
procedure, temperature was monitored. The animals subsequently had
free access to water and food. Postoperative analgesia was
performed via application of buprenorphine (0.05 mg/kg body weight)
twice a day.
Depending on group allocation, the animals were
sacrificed either 2 h following the end of the experiment or the
overall survival was monitored for 72 h. The sacrifice was
performed, as briefly described, by withdrawing blood via the aorta
using the same isoflurane oxygen concentration as during the
preparation period and flushing the abdominal organs with RL.
Subsequently, the left liver lobe was ligated, removed and
snapfrozen in liquid nitrogen. The remaining liver was flushed with
20 ml 10% buffered formalin solution and removed for further
handling for (immuno) histology.
During the survival evaluation, frequent and close
examination of the condition of each animal was performed. In the
occurrence of any cut-off criteria, which were defined in
accordance with the guidelines of the Ethics Committee of the RP
Darmstadt, animals were sacrificed and their mortality rate
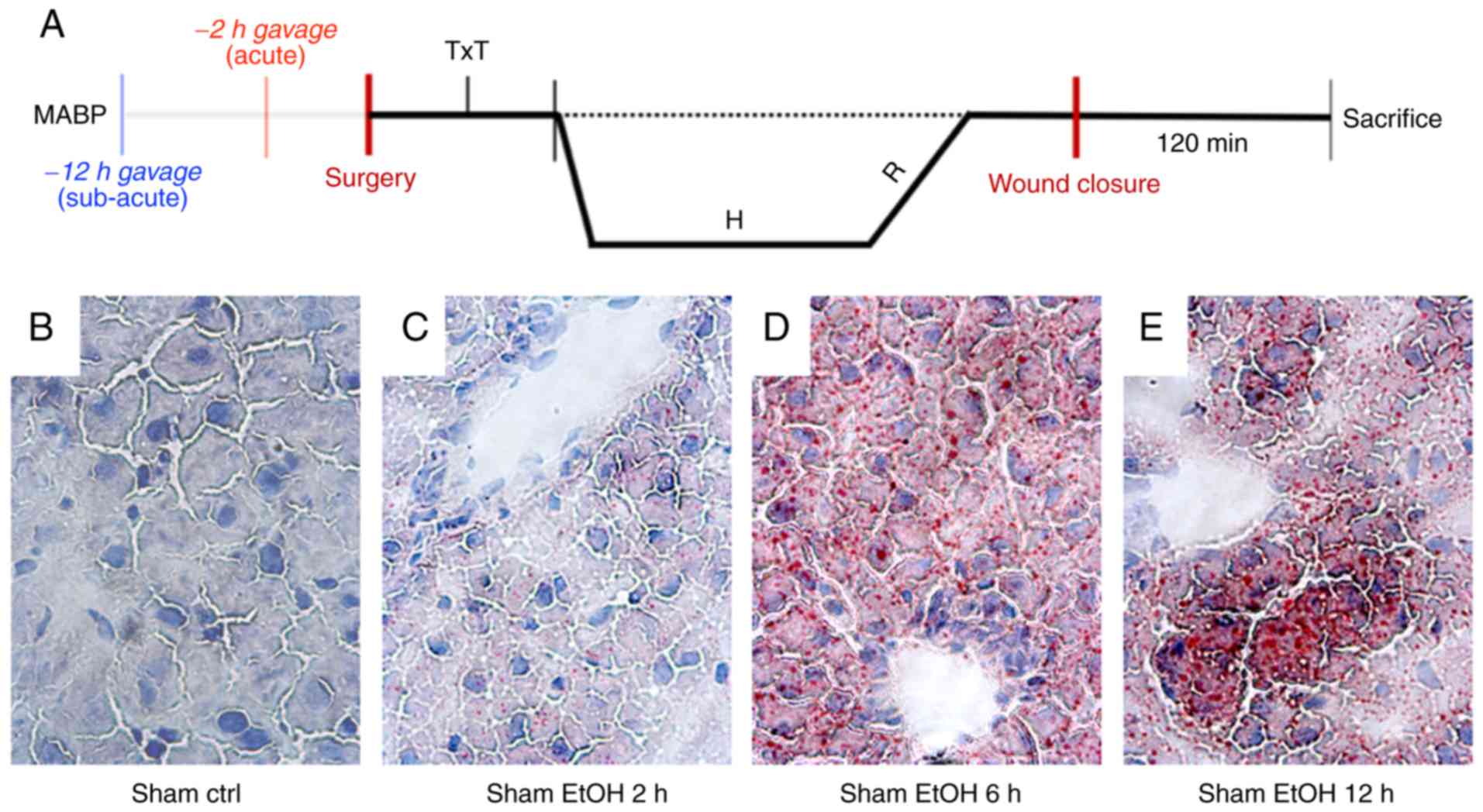
evaluated. Fig. 1A shows the
experimental overview.
Group allocation
A total of 48 animals were randomly assigned to the
subacute or acute EtOH or NaCl groups (n=6; Table I). Animals in the sham groups also
received oral gavage of EtOH or NaCl and all surgical procedures
were performed without TxT or hemorrhagic shock and resuscitation
(H/R). For survival analysis, four animals in the sham groups, and
eight animals in the TxT + H/R groups were included (Table II).
 | Table IOverview of the groups for analysis
post-sacrifice. |
Table I
Overview of the groups for analysis
post-sacrifice.
| Group | Treatment
protocol | Number of
animals |
|---|
| 1 | Sham, subacute NaCl
gavage | 6 |
| 2 | Sham, acute NaCl
gavage | 6 |
| 3 | Sham, subacute EtOH
gavage | 6 |
| 4 | Sham, acute EtOH
gavage | 6 |
| 5 | TxT + H/R, subacute
NaCl gavage | 6 |
| 6 | TxT + H/R, acute
NaCl gavage | 6 |
| 7 | TxT + H/R, subacute
EtOH gavage | 6 |
| 8 | TxT + H/R, acute
EtOH gavage | 6 |
 | Table IIOverview of the groups for survival
analysis. |
Table II
Overview of the groups for survival
analysis.
| Group | Treatment
protocol | Number of
animals |
|---|
| 9 | Sham, subacute NaCl
gavage | 4 |
| 10 | Sham, acute NaCl
gavage | 4 |
| 11 | Sham, subacute EtOH
gavage | 4 |
| 12 | Sham, acute EtOH
gavage | 4 |
| 13 | TxT + H/R, subacute
NaCl gavage | 8 |
| 14 | TxT + H/R, acute
NaCl gavage | 8 |
| 15 | TxT + H/R, subacute
EtOH gavage | 8 |
| 16 | TxT + H/R, acute
EtOH gavage | 8 |
Examination of EtOH-induced hepatic fat
accumulation
The hepatic frozen sections (3-µm) were fixed
with 10% buffered formalin. Lipids were stained at room temperature
for 50-60 min with with 2% (w/v) Oil red O working solution (0.07 g
Oil red O dissolved in 25 ml 100% methanol mixed with 10 ml 1 M
NaOH), counterstained with hematoxylin (5 g/l) for 10 min. The
images were captured by using the Zeiss Axio Observer Z1 microscope
(40X objective, Zeiss AG).
Ribonucleic acid (RNA) isolation and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) analysis
Total RNA was isolated from the snap-frozen liver
samples using the RNeasy-system (Qiagen GmbH, Hilden, Germany)
according to the manufacturer's instructions. The residual
remaining DNA were removed using the RNase-free DNase set,
according to the manufacturer's instructions (Qiagen GmbH), and the
RNA samples were stored at -80°C. The Affinity script QPCR-cDNA
synthesis kit (Stratagene; Agilent Technologies, Inc., La Jolla,
CA, USA) was used for RT-qPCR analysis using 100 ng of total RNA
for reverse transcription according to the manufacturer's
instructions. The mRNA expression levels of IL-6, IL-1β and
ICAM were determined on a Stratagene; MX3005p QPCR system
(Stratagene; Agilent Technologies, Inc.) using gene-specific
primers (rat IL-6: NM_012589, cat. no. PPR06483B;
IL-1β: NM_031512, cat. no. PPR06480B and rat ICAM:
NM_012967, cat. no. PPR42235A; all SABiosciences, SuperArray,
Frederick, MD, USA). As a reference gene, the expression of
GAPDH (rat GAPDH: NM_017008, cat. no. PPR06557B,
SABiosciences) was measured. The PCR protocol was set up with 1X
RT2 SYBR Green/Rox qPCR Master mix (SABiosciences) in a
25 µl volume according to manufacturer's instructions and as
described previously (22,28).
The relative mRNA expression of each target gene was calculated
using the comparative threshold cycle method (2−ΔΔCq
method). Briefly, the quantity of target mRNA in each sample was
normalized to GAPDH, to give ΔCq, and then to the samples
from the sham groups. The relative mRNA expression is presented as
% calculated in relation to sham following normalization to
GAPDH (29).
Quantification of cytokine protein
levels
The concentrations of the protein levels of TNF-α
and IL-1β were determined using a rat TNF-α ELISA set (Diaclone)
and rat IL-1β Tissue Culture ELISA Ready-Set-Go!®
(eBioscience; Thermo Fisher Scientific, Inc.) according to
manufacturer's instructions. ELISA was performed using the Infinite
M200 microplate reader (Tecan, Männedorf, Switzerland).
Staining of IL-10
The paraffin-embedded liver samples were sectioned
(3-µm), deparaffinized, rehydrated and stained with
anti-IL-10 antibody. Following deparaffinization, epitope recovery
was performed under a humidified atmosphere using R-Universal
epitope recovery buffer (Aptum, Kassel, Germany) for 1 h (Retriever
2010, Prestige Medical). Following washing with water and PBS,
mouse monoclonal antibody against IL-10 (Cloud-Clone-Corp.,
MAA056Ra21, 1:30) was applied as a primary antibody. Following
incubation for 1 h at room temperature, and a subsequent washing
procedure, a secondary AlexaFluor568 donkey anti-mouse antibody
(1:100, Invitrogen; Thermo Fisher Scientific, Inc., A10037) was
applied to detect specific binding. After 1 h at room temperature,
the sections were washed, and mounted using fluorescent mounting
medium containing DAPI nucleic acid stain (Vectashield HardSet
Antifade Mounting Medium with DAPI, Vector Laboratories, Ltd.,
Cambridge, UK). Fluorescence was visualized using a Zeiss inverted
fluorescence microscope AXIO Observer Z1 (Carl Zeiss AG,
Oberkochen, Germany). Representative images were captured from five
random fields. IL-10-positive cells were quantified by counting
their number in a total of five high-power fields per liver section
in a blinded manner. Data from each tissue section were pooled to
determine mean values.
Ex vivo in vitro whole blood stimulation
for the TNF-α production assay
Blood samples (50 µl) were withdrawn prior to
the onset of TxT and were diluted in 450 µl RPMI-1640
(Seromed, Berlin, Germany) in a polypropylene tube (BD Biosciences,
Franklin Lakes, NJ, USA) supplemented with 10% heat-inactivated
fetal calf serum, 100 IU/ml penicillin and 100 µg/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc.), and 20 mM
HEPES buffer (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The
samples were then stimulated with lipopolysaccharide (LPS, 10
µg/ml, E. coli 0127:B8, Sigma-Aldrich; Merck KGaA)
and incubated at 37°C and 5% CO2. After 24 h, the
samples were centrifuged for 15 min at 2,100 g at room temperature,
and the supernatant was collected and stored at −80°C. As a
reference, corresponding blood samples were incubated as described
above without adding LPS for stimulation. The concentration of
TNF-α was determined using the rat TNF-α ELISA kit (Diaclone)
according to the manufacturer's instructions.
Examination of liver injury
Plasma was stored at -80°C for later analysis of
alanine aminotransferase (ALT), aspartate amino-transferase (AST)
and lactate dehydrogenase (LDH) using the Spotchem EZ SP-4430
device (Arkray, Inc., Kyoto, Japan). The determination of
histological damage was performed by an independent veterinary
pathologist, who allocated the hematoxylin-eosin-stained liver
sections to the various experimental groups in a blinded manner.
Cell enlargement and nuclear dissolution in hepatocytes were
characterized as necrotic. The pathologist investigated the
following: Hemorrhage, necrosis, congestion, single cell
degeneration/necrosis or individualization, zonal necrosis
(perivenous), vacuolization of hepatocytes (periportal),
extramedullary hematopoiesis and granulated cytoplasm/mineralized
mitochondria.
Glucose determination
Glucose was determined prior to the onset of TxT
(baseline) using GEM Premier 4000 (Instrumentation Laboratory GmbH,
Kirchheim, Germany; BGA Set Optimedical Comfort Sampler Basic
kit).
Statistical analysis
Differences between groups were determined by
one-way analysis of variance using Kruskal-Wallis with Dunn's post
hoc test. Changes in target gene expression were analyzed by
Wilcoxon matched-pair analysis. Data are presented as the mean ±
standard error of the mean. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using GraphPad Prism 6 (GraphPad Software, Inc., San
Diego, CA, USA).
Results
Lipid deposition following EtOH
gavage
Lipid deposition was determined 2, 6 and 12 h
following EtOH or ctrl gavage by staining of lipid accumulation
with Oil red O. At 2 h post-EtOH gavage, an accumulation of lipid
droplets was present, with the most distinct occurrence after 6 h.
At 12 h post-EtOH gavage, the accumulation of lipid droplets
remained clearly visible, but regenerative fat reduction was
observed (Fig. 1B-E).
Local inflammatory changes
TxT + H/R induced a significant increase in the gene
expression of IL-6 in the liver of both ctrl groups compared with
that in the corresponding sham groups. Both EtOH trauma groups
exhibited lower gene expression of IL-6 compared with the
corresponding ctrl groups, however, only in the subacute EtOH group
was the difference significant following TxT + H/R (ctrl:
877.3±182.5 vs. EtOH: 165.1±68.2% to sham, P<0.05, Fig. 2A). The gene expression of IL-1β
was only significantly increased in the subacute ctrl group
following TxT + H/R compared with the that in the corresponding
sham group (Fig. 2B). The gene
expression pattern of ICAM-1 was comparable to that observed for
IL-6. A significant increase in the gene expression of ICAM-1 was
induced by TxT + H/R in both ctrl groups compared with that in the
corresponding sham groups (subacute ctrl: 207.6±21.7 vs. 92.7±14.8
and acute: 221.7±41.8 vs. 91.4±14.9% to sham, P<0.05, Fig. 2C). By contrast, both EtOH groups
exhibited significantly lower gene expression levels of ICAM-1
compared with that in the corresponding ctrl group following TxT +
H/R (subacute EtOH: 110.8±34.2 and acute EtOH: 101.3±32.5% to sham,
P<0.05, Fig. 2C).
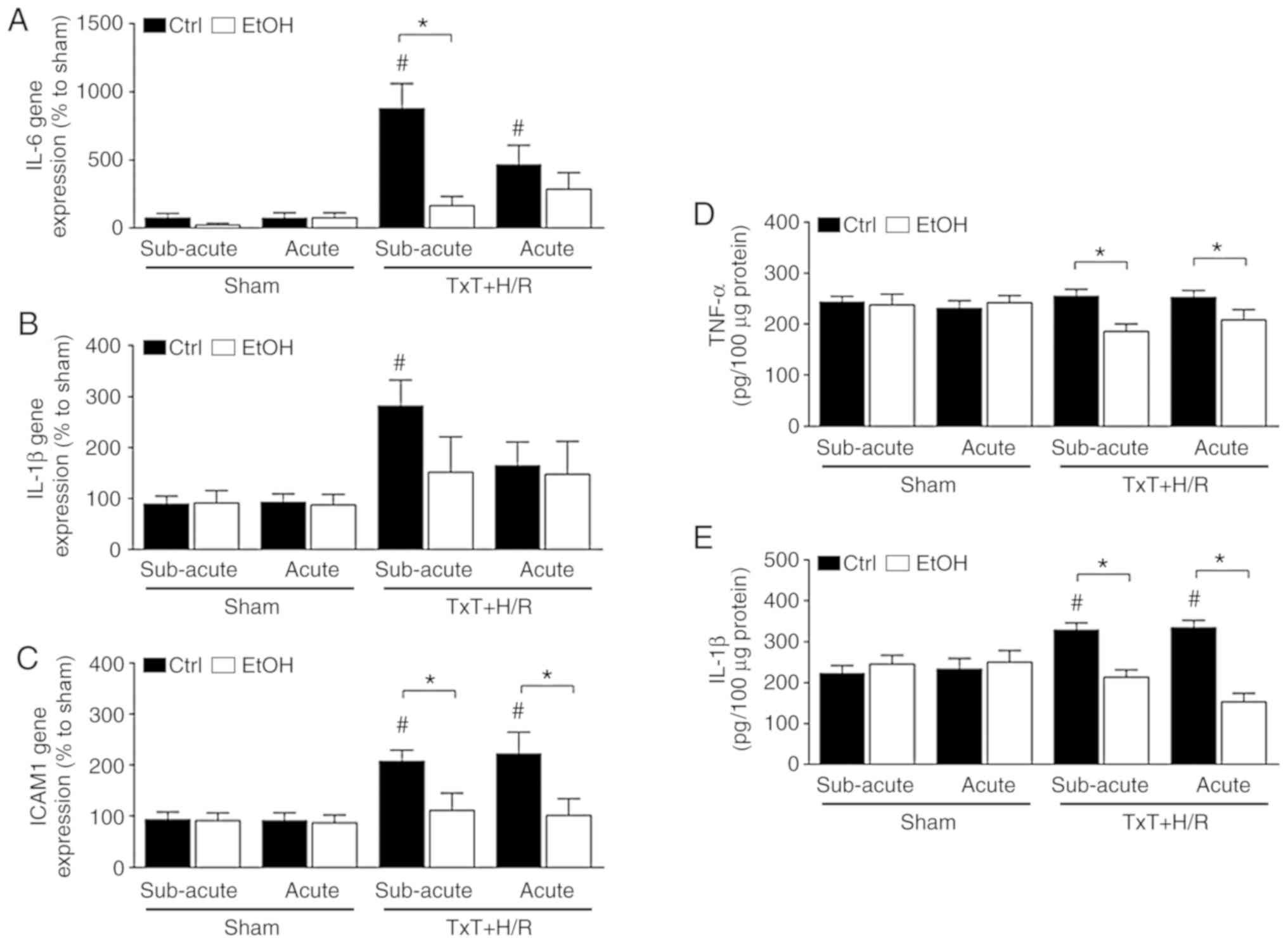 | Figure 2Inflammatory changes. Gene expression
levels of (A) IL-6, (B) IL-1β and (C) ICAM-1, and protein
expression levels of (D) TNF-α and (E) IL-1β are shown. At 12 h
(subacute) or 2 h (acute) before the experiment, female Lewis rats
received a single oral dose of EtOH or saline (ctrl), followed by
TxT + H/R. The sham group underwent all surgical procedures without
TxT + H/R. *P<0.05 between indicated groups;
#P<0.05 vs. sham group. IL, interleukin; ICAM-1,
intercellular adhesion molecule-1; TNF-α, tumor necrosis factor-α;
EtOH, alcohol; NaCl, saline; ctrl, control; TxT, blunt chest
trauma; H/R, hemorrhagic shock with resuscitation. |
In terms of the hepatic protein expression of TNF-α,
no significant changes were observed following TxT + H/R compared
with the sham. However, there were significant reductions in the
subacute and acute EtOH TxT + H/R groups compared with the
corresponding TxT + H/R ctrl groups (subacute EtOH vs. ctrl:
185.6±14.5 vs. 254.9±13.2 pg/100 µg protein and acute EtOH
vs. ctrl: 208.3±20.0 vs. 252.9±13.0 pg/100 µg protein,
P<0.05, Fig. 2D).
The hepatic protein expression of IL-1β was
significantly enhanced in both ctrl groups following TxT + H/R
exposure compared with the corresponding sham groups (subacute ctrl
vs. sham ctrl: 328.8±16.9 vs. 223.2±19.4 pg/100 µg protein;
acute ctrl vs. sham ctrl: 334.5±17.0 vs. 233.8±25.4 pg/100
µg protein, P<0.05, Fig.
2E). Both EtOH trauma groups had significantly lower protein
levels of IL-1β than the corresponding ctrl groups following TxT +
H/R exposure (P<0.05, Fig.
2E).
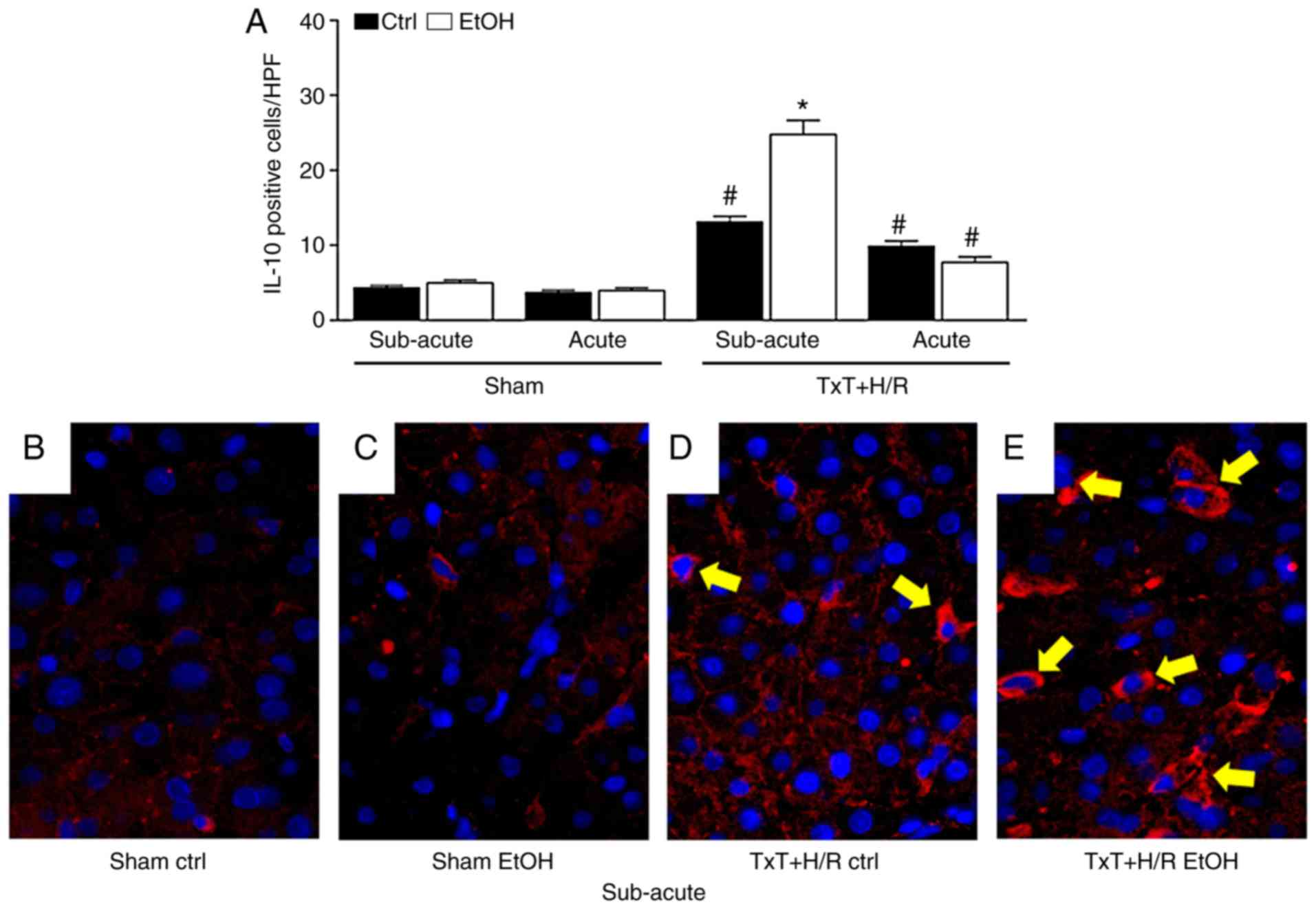
The number of positively stained cells was
comparable in all sham groups (Fig.
3A). The presence of IL-10-expressing cells in the liver was
significantly increased in all trauma groups compared with each
corresponding sham group (P<0.05, Fig. 3A). In the subacute EtOH group, a
significantly higher number of IL-10-positive cells were found
following TxT + H/R exposure compared with that in the subacute
ctrl group (24.8±1.9 vs. 13.1±0.7, respectively, P<0.05,
Fig. 3A). Representative
immunohistological images show the IL-10-positively stained cells
at 2 h post reperfusion (Fig.
3B-E).
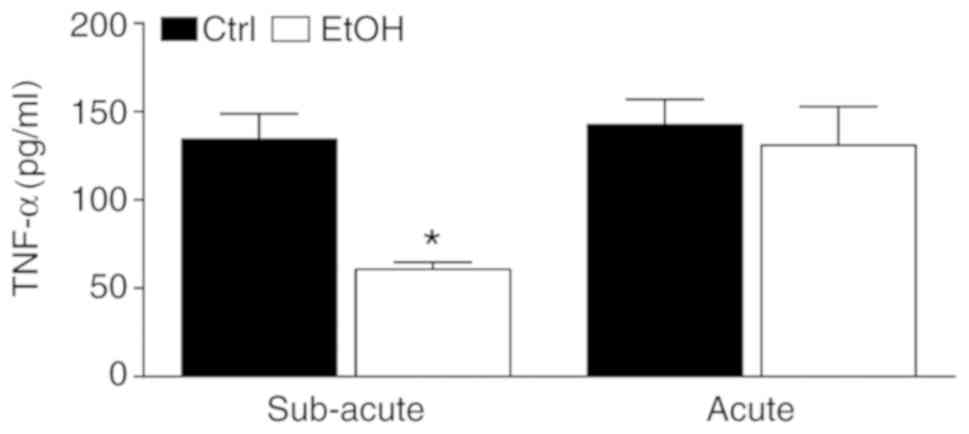
Whole-blood stimulation assay
The release of TNF-α upon stimulation with LPS
levels was determined in a whole-blood stimulation assay in order
to analyze whether there is an immunosuppression/immunotolerance
prior to the onset of trauma/hemorrhage. A significantly lower
level of TNF-α was found in the subacute EtOH group compared with
the ctrl group (60.7±4.1 vs. 134.4±14.4 pg/ml, respectively,
P<0.05, Fig. 4).
Cell and liver damage
To evaluate hepatocellular damage, AST and ALT were
determined. TxT + H/R caused a significant increase in the levels
of AST and ALT in all trauma groups compared with each
corresponding sham group (P<0.05, Fig. 5A and B). The AST and ALT levels
were further increased in the subacute EtOH trauma group compared
with the subacute ctrl trauma group, and this increase was
significant (P<0.05, Fig. 5A and
B). In terms of general cell damage, as indicated by systemic
LDH levels, TxT + H/R induced a significant increase in LDH in all
trauma groups compared with the corresponding sham groups
(P<0.05, Fig. 5C). However,
there were no significant differences among the trauma groups.
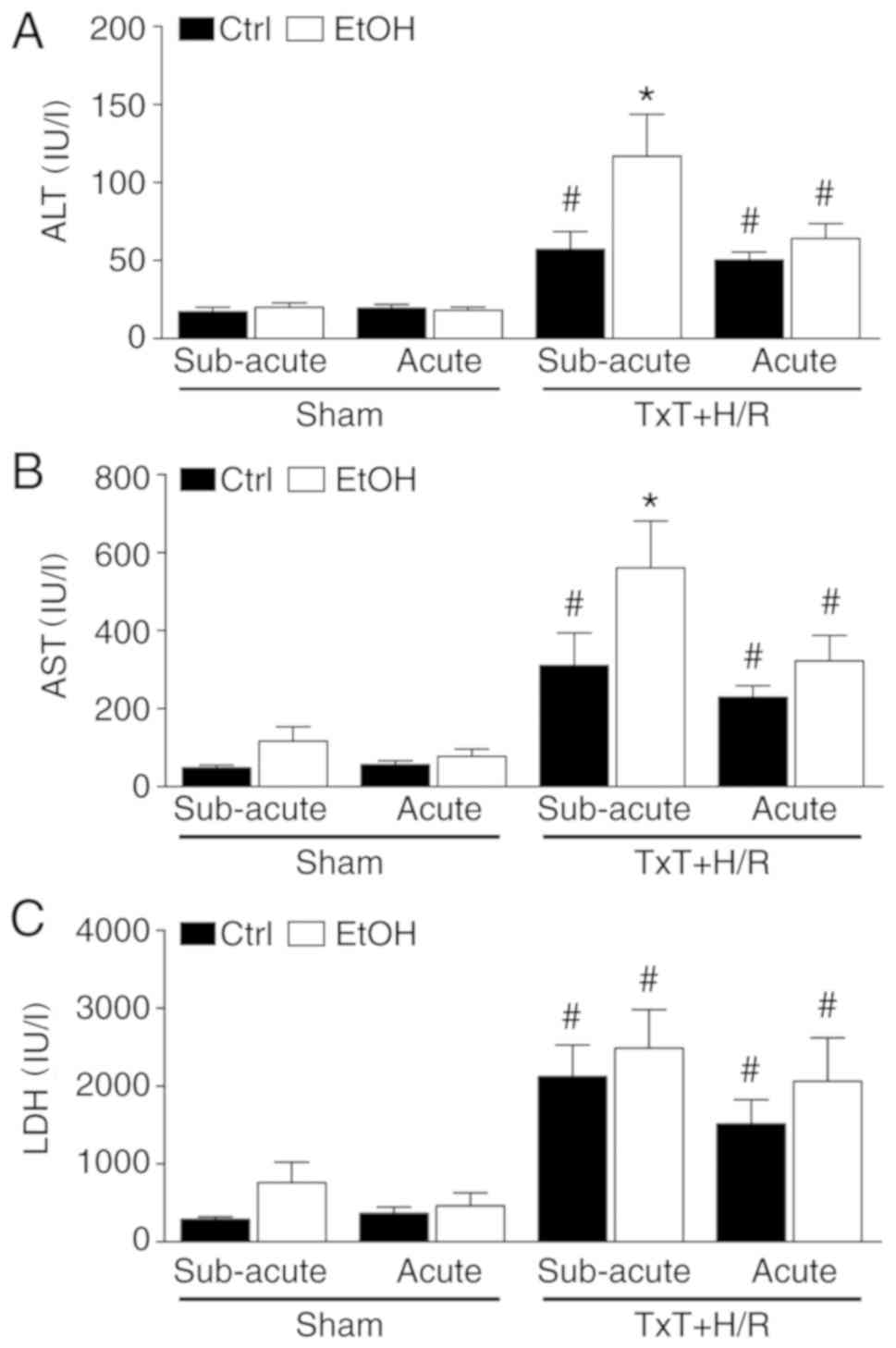 | Figure 5Cell and liver damage. (A) ALT, (B)
AST and (C) LDH levels are shown. At 12 h (subacute) or 2 h (acute)
before the experiment, female Lewis rats received a single oral
dose of EtOH or saline (ctrl), followed by TxT + H/R. The sham
group underwent all surgical procedures without TxT + H/R.
*P<0.05 vs. all groups; #P<0.05 vs.
sham group. ALT, alanine aminotransferase; AST, aspartate
aminotransferase; LDH, lactate dehydrogenase; EtOH, alcohol; ctrl,
control; TxT, blunt chest trauma; H/R, hemorrhagic shock with
resuscitation. |
Histological analysis of the liver sections from the
acute, subacute EtOH and both ctrl TxT + H/R groups revealed
significant liver damage at 2 h post-resuscitation compared with
sections from the sham groups (Fig.
6A-G). In accordance with the data from liver transaminases, in
the subacute EtOH TxT + H/R group, liver damage was significantly
higher following TxT + H/R compared with that in the subacute ctrl
group (Fig. 6A-G).
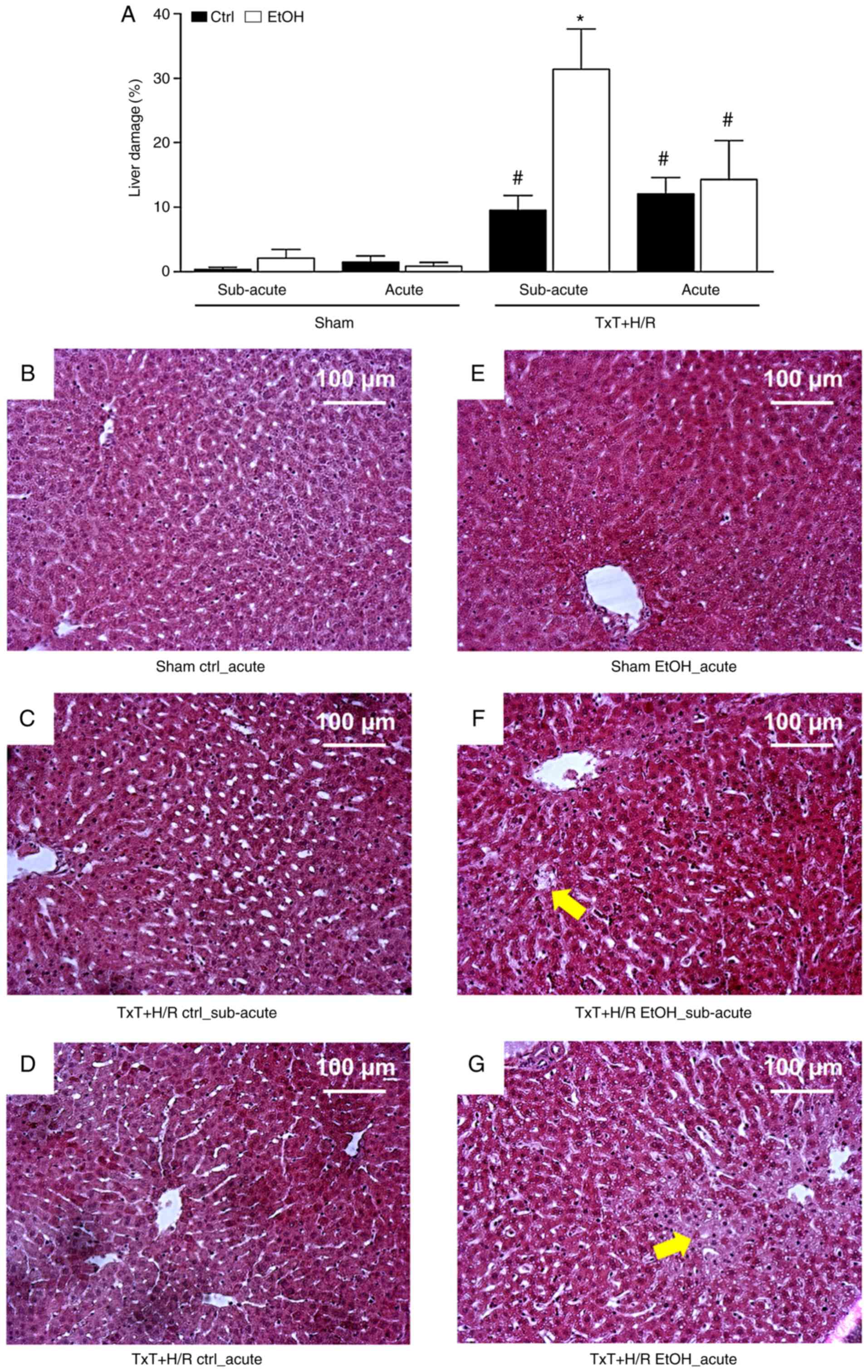 | Figure 6Liver damage. (A) Liver damage in the
treatment groups. At 12 h (subacute) or 2 h (acute) before the
experiment, female Lewis rats received a single oral dose of EtOH
or saline (ctrl), followed by TxT + H/R. The sham group underwent
all surgical procedures without TxT + H/R. *P<0.05
vs. ctrl TxT + H/R; #P<0.05 vs. sham group.
Representative liver sections (10X objective) of the (B) sham ctrl
acute, (C) sham EtOH acute, (D) TxT + H/R ctrl subacute, (E) Txt +
H/R EtOH subacute groups, (F) TxT + H/R ctrl acute and (G) TxT +
H/R EtOH acute groups are shown. Scale bar, 100 µm. Cell
enlargement and nuclear dissolution in hepatocytes as signs of
necrosis were more pronounced in the TxT + H/R groups than in the
sham groups. Arrows point to exemplary necrotic regions. EtOH,
alcohol; ctrl, control; TxT, blunt chest trauma; H/R, hemorrhagic
shock with resuscitation. |
Hypoglycemia in the subacute EtOH
group
Regarding glucose levels, a significant decrease was
observed in the subacute EtOH prior to the onset of TxT compared
with the subacute ctrl group (137.8±12.8 vs. 212.6±10.3 mg/dl,
respectively, P<0.05). There was no significant difference
between the acute EtOH group and the acute ctrl group.
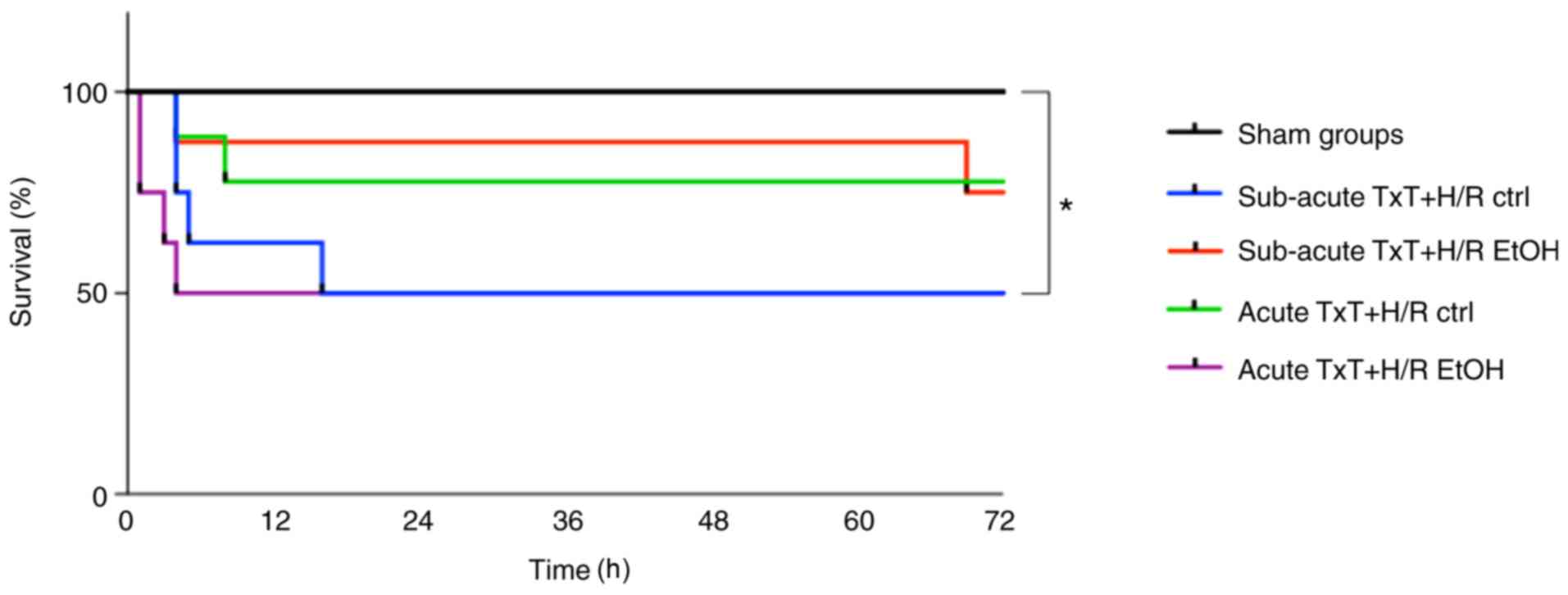
Survival analysis
Survival was assessed after 72 h. All animals in the
sham groups survived. TxT + H/R led to significantly lower survival
rates in the subacute ctrl and acute EtOH TxT + H/R groups compared
with the sham groups (P<0.05), whereas the number of animals
that died did not differ significantly among the trauma groups
(subacute TxT + H/R ctrl vs. EtOH: 4/8 (50%) vs. 2/8 (25%); acute
TxT + H/R ctrl vs. EtOH: 2/8 (25%) vs. 4/8 (50%) (Fig. 7).
Discussion
In the present study, the influence of alcohol in an
in vivo double-hit model of blunt chest trauma and
hemorrhagic shock was investigated, with particular interest in the
liver inflammatory response, organ damage and overall mortality.
Subacute and acute exposure to alcohol induced significant
anti-inflammatory changes in the local immune response to
trauma/hemorrhage. However, histological analysis of the liver
tissues revealed that no protective influence of alcohol was
evident. In addition, increased damage of the liver was present
upon subacute exposure to alcohol. Of note, increased tissue damage
was associated with an enhanced presence of IL-10-expressing cells
in the liver. These findings were paralleled by an increased
accumulation of fat in the liver and a significant hypoglycemia.
However, overall mortality rates among did not differ significantly
among groups following trauma/hemorrhage.
The liver is susceptible to the development of organ
damage following hemorrhage (2,30)
and, due to its potent immunomodulatory potential, has an important
role in the outcome of patients. The negative effects of chronic
alcohol consumption on the liver are well known, whereas the
effects of acute alcohol consumption on the liver and patient
outcomes in trauma patients constitute an interesting subject of
intensive research due to the currently controversial results. In
daily routine clinical practice, patients frequently present either
with acute alcohol intoxication, with residual alcohol levels
following evening consumption, or with chronic alcohol
intoxication. Consequently, in the present study, an experimental
setting covering the two most likely scenarios of acute alcohol
consumption, acute 'drink and drive scenario' and subacute 'evening
binge drinking' (17), was used.
In our previous study, the influence of subacute alcohol
intoxication on rats with hemorrhagic shock was examined, in which
significantly higher ALT values were found compared with those in
the control group during the time course, with a later decrease in
ALT (2). The increased liver
damage that was observed underlying subacute alcohol consumption
was in line with the results of Hu et al (31). Rats received 5 g/kg i.v. EtOH
followed by volume-controlled hemorrhagic shock, and the highest
levels of AST and ALT were found in the intoxicated hemorrhagic
shock group (31). However, in
our previous study, subacute alcohol consumption has also exhibited
hepatoprotective potential in an in vivo model of isolated
hemorrhagic shock (2). These data
are not fully in line with the results of the present study,
however, there is an important difference in the model used. In the
present study, the additional influencing factor of TxT was
included in order to mimic a more realistic clinical scenario, as
severe bleeding does not occur in isolation but rather occurs in
combination and as consequence of additional tissue trauma. In
terms of such combinatory trauma models including alcohol
intoxication, limited data exist and, to the best of our knowledge,
no studies have been performed regarding specifically blunt chest
trauma and hemorrhagic shock with previous alcohol intoxication. A
study by Desiderio examined the influence of intragastric and
intravenous EtOH application in blunt cardiac trauma in
vivo, and found significantly higher mortality rates in the
intoxicated group, which was caused by electrical-mechanical
dissociation (32,33). However, liver damage was not
addressed in their study. In the present study, significant liver
damage was detected in the NaCl and the EtOH groups. Of note, the
highest levels of ALT and AST were found in the subacute EtOH
group. This group also exhibited the most pronounced damage
histologically, which was higher than in the corresponding
reference group following trauma/hemorrhage. A trend in increased
transaminase values and liver damage was also observed in the acute
EtOH group, although the difference with the corresponding
reference group following trauma/hemorrhage was not prominent. A
possible explanation for the contradictive results regarding the
potentially hepatoprotective effects shown in previous studies and
the aggravation of liver damage as represented shown in the present
study following subacute EtOH consumption may lie in the increased
trauma severity. The present model, with the addition of TxT over
isolated hemorrhagic shock, may cause an increased vulnerability of
the total organism to a trauma/hemorrhage-induced immune response
and subsequent tissue damage. In addition, the influence of trauma
severity on the metabolism of EtOH elimination may be a possible
explanation. However, the exact relevance of trauma severity and
the timing of alcohol consumption on the difference between the
EtOH trauma groups remains to be elucidated in future
investigations. The following inflammatory changes may provide an
explanation approach.
The impact of TxT and H/R on the immune response has
been addressed previously. Seitz et al examined the
inflammatory response in a model of TxT, hemorrhagic shock or the
combination of both (34). The
highest levels of TNF-α, IL-6 and IL-10 were found in Kupffer cells
in the trauma/hemorrhage group (34). Similarly, in the present study, a
profound inflammatory reaction in the liver with elevated gene
expression levels of IL-6, IL-1β and ICAM-1 was found in TxT + H/R
control groups. By contrast, either subacute or acute exposure to
EtOH markedly attenuated pro-inflammatory gene expression following
TxT + H/R, data that is consistent with our previous report
addressing the immunosuppressive potential of acute alcohol
consumption following isolated hemorrhagic shock (2). In general, findings regarding the
immunosuppressive effect of alcohol are contradictory. Sato et
al investigated the dose-dependent influence of alcohol in an
in vivo setting of hemorrhagic shock, and found no
significant difference between the alcohol groups and the control
group regarding plasma levels of TNF-α and IL-1β (35). They identified increased mortality
in the alcohol groups, which was associated with the inhibition of
norepinephrine, epinephrine and vasopressin (35). As described above, Hu et al
observed elevated serum levels of TNF-α and IL-6 in a group of
alcohol-intoxicated animals compared to a control group following
hemorrhagic shock (31). Mathis
et al also investigated the influence of alcohol upon
hemorrhagic shock and detected an attenuation of the trauma-induced
increase in lung IL-6 and TNF-α in the alcohol group, although no
alterations in inflammatory parameters were observed in the spleen
or in lung IL-1 and IL-10 (36).
When interpreting these results, the limited comparability of the
studies due to model differences must be considered. The
experiments differ in terms of the alcohol dose, but also in the
timing and the mode of administration. Trauma severity and the
analyzed inflammatory parameters also differ. However, the
anti-inflammatory potential of alcohol consumption in the
underlying model has also been confirmed by reduced protein
expression of TNF-α and IL-1β following trauma/hemorrhage. In
accordance to these changes, the subacute EtOH group in the present
study exhibited the highest level of IL-10-positive cells in
response to trauma. The marked anti-inflammatory potential of
subacute EtOH consumption was evidently not only limited to local
liver response, but was also exerted in the reduced systemic
release of TNF-α upon LPS stimulation. In the acute setting of EtOH
consumption, the TxT + H/R-induced inflammatory response reduction
was limited to the gene expression level and did not markedly alter
IL-10-positive cells in the liver or systemic TNF-α release. These
differences between the two EtOH TxT + H/R groups may explain why
the tissue damage was differentially modulated in these groups.
Miller et al examined the inflammatory reaction and liver
damage in IL-10-knockout mice in a setting of alcoholic and
non-alcoholic steatohepatitis; and observed increased liver
inflammation, as expected (37).
These results are in line with the findings of the present study,
showing that the enhanced presence of IL-10-expressing cells is
closely associated with the reduced expression and release of
pro-inflammatory markers. Additionally, IL-10-knockout mice
exhibited less steatosis and hepatocellular damage following
alcohol exposure, an effect that is underlined by the findings of
the present study showing damage to tissue was higher with a higher
presence of IL-10-expressing cells. Furthermore, increases in IL-6
and hepatic signal transducer and activator of transcription 3
(STAT3) have been shown in IL-10-knockout mice. In double knockout
mice with additional elimination of IL-6 or STAT3, liver damage and
steatosis were re-established (37). Therefore, it was concluded that
the protective effects on the liver were associated with hepatic
IL-6/STAT3 activation, and that the inflammatory balance between
liver-protective and harmful cytokines determines the effect in a
positive or negative direction (37). Previous studies have already
documented that pro-inflammatory cytokines TNF-α and IL-6, but also
anti-inflammatory cytokines, including IL-10, are important in
promoting liver regeneration (38-43). The data obtained in the present
study does not reject the hypothesis that IL-10, as an
anti-inflammatory cytokine, may negatively regulate liver
regeneration via suppressing the pro-inflammatory response.
Therefore, IL-10 may act as a repressor of tissue regeneration,
which may have led to the observed differences among the two EtOH
groups with regard to tissue damage following TxT + H/R. The exact
role of IL-10 remains to be further elucidated.
Regarding the underlying mechanism, the activation
of NF-κB serves a key role in the production, regulation and
release of cytokines following trauma (7-9).
In a previous in vitro study, it was confirmed that the
anti-inflammatory effects of acute alcohol exposure are regulated
via the canonical NF-κB pathway (44). In addition, Mandrekar et al
confirmed the influence of alcohol on NF-κB and its subunits
(45). By contrast, in the
setting of chronic alcohol exposure, an increased activation of
NF-κB has been detected, combined with the release of
pro-inflammatory cytokines (46).
In addition, Wang et al reported the augmented activation of
NF-κB in patients with human hepatocellular carcinoma and chronic
alcohol consumption (47). It
appears that acute alcohol exposure leads to the inhibition of
NF-κB, in contrast to chronic alcohol exposure. There are two
questions that remain unanswered. The first is whether acute and
subacute exposure to alcohol potentially both reduce NF-κB, and if
this is the case, whether the exposure strategy influences NF-κB
signaling in the same way regarding the canonical or non-canonical
pathway. The answers to these questions may be key to explaining
the present data, and requires elucidation in further
investigations using specific inhibitors of the canonical and
non-canonical NF-κB pathway.
In addition to the above-mentioned anti-inflammatory
potential of EtOH, the subacute group exhibited low blood glucose
levels. Hypoglycemia can continue 24 h after alcohol consumption,
therefore, it is reasonable that this effect was observed in the
subacute group. This may be a result of the liver having to remove
EtOH from the blood, rather than managing blood glucose levels, or
by the body increasing insulin release in response to acute alcohol
consumption.
In terms of the limitations of the present study, it
is important to mention that clinical reality can only be
reproduced in a reduced form using in vivo experimentation.
Existing medication and co-existing disease have important effects
on daily routine in the treatment of trauma patients, and the
possible influence of anesthesia in the frame of the model should
be considered. Furthermore, in the present experimental setting,
sacrifice was performed 2 h following the end of experimentation
with subsequent removal of organs and blood, or survival was
assessed for 72 h. Collecting the blood at the defined time of
sacrifice grants group comparability, however, the dynamic
development of values is not represented. Furthermore, gene and
protein expression from all parameters were not consistently
measured. In addition, regeneration of the liver and the influence
of alcohol on NF-κB were not elaborated and require further
investigation. Finally, a high alcohol dose was used; it is
important in interpreting results to be aware that alcohol and its
metabolites may act dose-dependently. Therefore, a focus on ethanol
metabolism is required in future studies, particularly regarding
the extent to which elimination is influenced through trauma
severity. To mimic clinical reality in more detail, a fracture and
a traumatic brain injury may be added to the model in the future,
as clinical data show that TxT is not the leading injury in
patients intoxicated with alcohol (48-50). In addition, if applying a multiple
trauma model within a 'drink-and-drive' scenario, fracture should
be applied additionally, as applying brain injury to this model is
difficult. In the present study, two factors were crucial for
selecting an experimental setting with TxT. First, our long
standing established hemorrhagic shock model required further
expansion, and adding more than one additional injury type risks
the misinterpretation of results as it would be unclear which of
the additional injuries caused the different results compared with
previous studies. Second, due to our particular interest on
post-traumatic inflammation, additional injury with a marked
influence on the immune system was selected. Seitz et al and
Weckbach et al underlined the key role of TxT regarding the
induction of the immune response (34,51). However, the limitations described
above must be addressed in future studies.
In conclusion, the data obtained in the present
study indicate that the severity of liver damage may be dependent
on the timing of alcohol intoxication, severity of trauma and/or
the balance between pro- and anti-inflammatory responses. The data
clearly indicate the requirement for further investigations
regarding metabolic changes and the relevance of the pro- and
anti-inflammatory balance for tissue susceptibility to injury, and
a greater number of animals per group are required to evaluate
mortality.
Acknowledgments
The authors would like to thank Mrs. Katrin Jurida,
Mrs. Kerstin Kontradowitz and Mr. Alexander Schaible from the
Department of Trauma, Hand and Reconstructive Surgery, University
Hospital Frankfurt, Goethe University, for their technical
assistance.
Funding
The study was supported by grants from the Deutsche
Forschungsgemeinschaft (grant nos. DFG RE 3304/5-1 and PE
908/3-1).
Availability of data and materials
The authors can provide relevant data on
request.
Authors' contributions
NW performed the experiments, wrote the manuscript
and contributed to the organization of the project. SD and NF
contributed to the experimental procedure. KK performed the
histological analysis. MP contributed to the funding and
intellectual realization. IM contributed to the intellectual
realization and the final manuscript. BR was the head of the
project, performed the statistical analysis and contributed to the
final manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Veterinary
Department of the Regional Council in Darmstadt, Germany
(Regierungspraesidium Darmstadt, Veterinaerswesen', Hessen,
Germany) and designed in accordance with the ARRIVE guidelines
(18).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wutzler S, Lustenberger T, Relja B,
Lehnert M and Marzi I: Pathophysiology of multiple trauma:
Intensive care medicine and timing of treatment. Chirurg.
84:753–758. 2013.In German. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Relja B, Höhn C, Bormann F, Seyboth K,
Henrich D, Marzi I and Lehnert M: Acute alcohol intoxication
reduces mortality, inflammatory responses and hepatic injury after
haemorrhage and resuscitation in vivo. Br J Pharmacol.
165:1188–1199. 2012. View Article : Google Scholar :
|
|
3
|
Lefering R and Paffrath T: Reality of care
based on the data from the Trauma Registry of the German Society of
Trauma Surgery. Unfallchirurg. 115:30–32. 2012.In German.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spahn DR, Bouillon B, Cerny V, Coats TJ,
Duranteau J, Fernández-Mondéjar E, Filipescu D, Hunt BJ, Komadina
R, Nardi G, et al: Management of bleeding and coagulopathy
following major trauma: An updated European guideline. Crit Care.
17:R762013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maier M, Geiger EV, Wutzler S, Lehnert M,
Wiercinski A, Buurman WA and Marzi I: Role of lung contusions on
post-traumatic inflammatory response and organ dysfunction in
traumatized patients. Eur J Trauma Emerg Surg. 35:463–469. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huber-Lang M, Gebhard F, Schmidt CQ,
Palmer A, Denk S and Wiegner R: Complement therapeutic strategies
in trauma, hemorrhagic shock and systemic inflammation-closing
Pandora's box? Semin Immunol. 28:278–284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dong W, Cai B, Peña G, Pisarenko V, Vida
G, Doucet D, Lee M, Sharpe S, Lu Q, Xu DZ, et al: Ethyl pyruvate
prevents inflammatory responses and organ damage during
resuscitation in porcine hemorrhage. Shock. 34:205–213. 2010.
View Article : Google Scholar :
|
|
8
|
Levy RM, Mollen KP, Prince JM, Kaczorowski
DJ, Vallabhaneni R, Liu S, Tracey KJ, Lotze MT, Hackam DJ, Fink MP,
et al: Systemic inflammation and remote organ injury following
trauma require HMGB1. Am J Physiol Regul Integr Comp Physiol.
293:R1538–R1544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tak PP and Firestein GS: NF-kappaB: A key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Relja B, Menke J, Wagner N, Auner B, Voth
M, Nau C and Marzi I: Effects of positive blood alcohol
concentration on outcome and systemic interleukin-6 in major trauma
patients. Injury. 47:640–645. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wagner N, Akbarpour A, Mörs K, Voth M,
Störmann P, Auner B, Lehnert M, Marzi I and Relja B: Alcohol
intoxication reduces systemic interleukin-6 levels and leukocyte
counts after severe TBI compared with not intoxicated TBI patients.
Shock. 46:261–269. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Phelan H, Stahls P, Hunt J, Bagby GJ and
Molina PE: Impact of alcohol intoxication on hemodynamic,
metabolic, and cytokine responses to hemorrhagic shock. J Trauma.
52:675–682. 2002.PubMed/NCBI
|
|
13
|
Zambell KL, Phelan H, Vande Stouwe C,
Zhang P, Shellito JE and Molina PE: Acute alcohol intoxication
during hemorrhagic shock: Impact on host defense from infection.
Alcohol Clin Exp Res. 28:635–642. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin HL, Lin TY, Soo KM, Chen CW, Kuo LC,
Lin YK, Lee WC and Lin CL: The effect of alcohol intoxication on
mortality of blunt head injury. Biomed Res Int. 2014:6192312014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Berry C, Ley EJ, Margulies DR, Mirocha J,
Bukur M, Malinoski D and Salim A: Correlating the blood alcohol
concentration with outcome after traumatic brain injury: Too much
is not a bad thing. Am Surg. 77:1416–1419. 2011.PubMed/NCBI
|
|
16
|
Steiner JL, Crowell KT and Lang CH: Impact
of alcohol on glycemic control and insulin action. Biomolecules.
5:2223–2246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wagner N, Franz N, Dieteren S, Perl M,
Mörs K, Marzi I and Relja B: Acute alcohol binge deteriorates
metabolic and respiratory compensation capability after blunt chest
trauma followed by hemorrhagic shock-a new research model. Alcohol
Clin Exp Res. 41:1559–1567. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kilkenny C, Browne WJ, Cuthill IC, Emerson
M and Altman DG: Improving bioscience research reporting: The
ARRIVE guidelines for reporting animal research. PLoS Biol.
8:e10004122010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhong Z, Connor H, Mason RP, Qu W,
Stachlewitz RF, Gao W, Lemasters JJ and Thurman RG: Destruction of
Kupffer cells increases survival and reduces graft injury after
transplantation of fatty livers from ethanol-treated rats. Liver
Transpl Surg. 2:383–387. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ylikahri RH, Kähönen MT and Hassinen I:
Modification of metabolic effects of ethanol by fructose. Acta Med
Scand Suppl. 542:141–150. 1972.PubMed/NCBI
|
|
21
|
Enomoto N, Ikejima K, Yamashina S, Enomoto
A, Nishiura T, Nishimura T, Brenner DA, Schemmer P, Bradford BU,
Rivera CA, et al: Kupffer cell-derived prostaglandin E(2) is
involved in alcohol-induced fat accumulation in rat liver. Am J
Physiol Gastrointest Liver Physiol. 279:G100–G106. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Relja B, Wilhelm K, Wang M, Henrich D,
Marzi I and Lehnert M: Acute ethanol gavage attenuates
hemorrhage/resuscitation-induced hepatic oxidative stress in rats.
Oxid Med Cell Longev. 2012:9834272012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kozan R, Ayyildiz M, Yildirim M and Agar
E: The effect of alphatocopherol in the acute ethanol intake and
its withdrawal on penicillin-induced epilepsy. Acta Neurobiol Exp
(Wars). 69:177–188. 2009.
|
|
24
|
Liener UC, Knöferl MW, Sträter J, Barth
TF, Pauser EM, Nüssler AK, Kinzl L, Brückner UB and Gebhard F:
Induction of apoptosis following blunt chest trauma. Shock.
20:511–516. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seitz DH, Perl M, Mangold S, Neddermann A,
Braumüller ST, Zhou S, Bachem MG, Huber-Lang MS and Knöferl MW:
Pulmonary contusion induces alveolar type 2 epithelial cell
apoptosis: Role of alveolar macrophages and neutrophils. Shock.
30:537–544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lehnert M, Relja B, Sun-Young Lee V,
Schwestka B, Henrich D, Czerny C, Froh M, Borsello T and Marzi I: A
peptide inhibitor of C-jun N-terminal kinase modulates hepatic
damage and the inflammatory response after hemorrhagic shock and
resuscitation. Shock. 30:159–165. 2008.PubMed/NCBI
|
|
27
|
Relja B, Schwestka B, Lee VS, Henrich D,
Czerny C, Borsello T, Marzi I and Lehnert M: Inhibition of c-Jun
N-terminal kinase after hemorrhage but before resuscitation
mitigates hepatic damage and inflammatory response in male rats.
Shock. 32:509–516. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Relja B, Töttel E, Breig L, Henrich D,
Schneider H, Marzi I and Lehnert M: Plant polyphenols attenuate
hepatic injury after hemorrhage/resuscitation by inhibition of
apoptosis, oxidative stress, and inflammation via NF-kappaB in
rats. Eur J Nutr. 51:311–321. 2012. View Article : Google Scholar
|
|
29
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pfeifer R, Kobbe P, Darwiche SS, Billiar
TR and Pape HC: Role of hemorrhage in the induction of systemic
inflammation and remote organ damage: Analysis of combined
pseudo-fracture and hemorrhagic shock. J Orthop Res. 29:270–274.
2011. View Article : Google Scholar
|
|
31
|
Hu TM, Lee RP, Lee CJ, Subeq YM, Lin NT
and Hsu BG: Heavy ethanol intoxication increases proinflammatory
cytokines and aggravates hemorrhagic shock-induced organ damage in
rats. Mediators Inflamm. 2013:1217862013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Desiderio MA: The potentiation of the
response to blunt cardiac trauma by ethanol in dogs. J Trauma.
26:467–473. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Desiderio MA: Effects of acute, oral
ethanol on cardiovascular performance before and after experimental
blunt cardiac trauma. J Trauma. 27:267–277. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Seitz DH, Perl M, Liener UC, Tauchmann B,
Braumüller ST, Brückner UB, Gebhard F and Knöferl MW: Inflammatory
alterations in a novel combination model of blunt chest trauma and
hemorrhagic shock. J Trauma. 70:189–196. 2011. View Article : Google Scholar
|
|
35
|
Sato H, Tanaka T and Kasai K: Ethanol
consumption impairs the hemodynamic response to hemorrhagic shock
in rats. Alcohol. 47:47–52. 2013. View Article : Google Scholar
|
|
36
|
Mathis KW, Zambell K, Olubadewo JO and
Molina PE: Altered hemodynamic counter-regulation to hemorrhage by
acute moderate alcohol intoxication. Shock. 26:55–61. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miller AM, Wang H, Bertola A, Park O,
Horiguchi N, Ki SH, Yin S, Lafdil F and Gao B:
Inflammation-associated interleukin-6/signal transducer and
activator of transcription 3 activation ameliorates alcoholic and
nonalcoholic fatty liver diseases in interleukin-10-deficient mice.
Hepatology. 54:846–856. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fausto N, Campbell JS and Riehle KJ: Liver
regeneration. Hepatology. 43:S45–S53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Koniaris LG, McKillop IH, Schwartz SI and
Zimmers TA: Liver regeneration. J Am Coll Surg. 197:634–659. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang HJ, Zakhari S and Jung MK: Alcohol,
inflammation, and gut-liver-brain interactions in tissue damage and
disease development. World J Gastroenterol. 16:1304–1313. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gao B: Hepatoprotective and
anti-inflammatory cytokines in alcoholic liver disease. J
Gastroenterol Hepatol. 27(Suppl 2): S89–S93. 2012. View Article : Google Scholar
|
|
42
|
Hill DB, D'Souza NB, Lee EY, Burikhanov R,
Deaciuc IV and de Villiers WJ: A role for interleukin-10 in
alcohol-induced liver sensitization to bacterial
lipopolysaccharide. Alcohol Clin Exp Res. 26:74–82. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
El-Assal O, Hong F, Kim WH, Radaeva S and
Gao B: IL-6-deficient mice are susceptible to ethanol-induced
hepatic steatosis: IL-6 protects against ethanol-induced oxidative
stress and mitochondrial permeability transition in the liver. Cell
Mol Immunol. 1:205–211. 2004.
|
|
44
|
Mörs K, Hörauf JA, Kany S, Wagner N, Sturm
R, Woschek M, Perl M, Marzi I and Relja B: Ethanol decreases
inflammatory response in human lung epithelial cells by inhibiting
the canonical NF-κB-pathway. Cell Physiol Biochem. 43:17–30. 2017.
View Article : Google Scholar
|
|
45
|
Mandrekar P, Catalano D and Szabo G:
Inhibition of lipopoly-saccharide-mediated NFkappaB activation by
ethanol in human monocytes. Int Immunol. 11:1781–1790. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Maraslioglu M, Oppermann E, Blattner C,
Weber R, Henrich D, Jobin C, Schleucher E, Marzi I and Lehnert M:
Chronic ethanol feeding modulates inflammatory mediators,
activation of nuclear factor-κB, and responsiveness to endotoxin in
murine Kupffer cells and circulating leukocytes. Mediators Inflamm.
2014:8086952014. View Article : Google Scholar
|
|
47
|
Wang F, Yang JL, Yu KK, Xu M, Xu YZ, Chen
L, Lu YM, Fang HS, Wang XY, Hu ZQ, et al: Activation of the NF-κB
pathway as a mechanism of alcohol enhanced progression and
metastasis of human hepatocellular carcinoma. Mol Cancer.
14:102015. View Article : Google Scholar
|
|
48
|
Bradbury A: Pattern and severity of injury
sustained by pedestrians in road traffic accidents with particular
reference to the effect of alcohol. Injury. 22:132–134. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zeckey C, Dannecker S, Hildebrand F,
Mommsen P, Scherer R, Probst C, Krettek C and Frink M: Alcohol and
multiple trauma: Is there an influence on the outcome? Alcohol.
45:245–251. 2011. View Article : Google Scholar
|
|
50
|
Johnston JJE and McGovern SJ: Alcohol
related falls: An interesting pattern of injuries. Emerg Med J.
21:185–188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Weckbach S, Hohmann C, Braumueller S, Denk
S, Klohs B, Stahel PF, Gebhard F, Huber-Lang MS and Perl M:
Inflammatory and apoptotic alterations in serum and injured tissue
after experimental polytrauma in mice: Distinct early response
compared with single trauma or 'double-hit' injury. J Trauma Acute
Care Surg. 74:489–498. 2013. View Article : Google Scholar : PubMed/NCBI
|