Introduction
Ischaemic cerebral stroke is one of the leading
causes of cerebral oedema that can lead to malignant brain oedema
and brain hernia and can potentially endanger the lives of patients
(1). The mechanisms of ischaemic
brain oedema are complex, as they involve cytotoxic oedema and
vasogenic oedema resulting from disruption of the blood-brain
barrier (BBB) (2). A previous
study found that BBB hyperpermeability allowed fluid to leak from
the intravascular space into the brain parenchyma, which caused
vasogenic cerebral oedema and increased interstitial fluid pressure
(3).
Osmotherapy is a primary treatment for cerebral
oedema. Hypertonic saline (HS), as a common osmotic dehydrating
agent, can move free water from the intracellular space into the
extracellular space through establishing an osmotic pressure
gradient, which lowers peripheral vascular resistance (4). It is well-known that administration
of 10% HS is more effective than mannitol for the treatment of
cerebral oedema (5,6). However, little is known about the
action of HS other than its osmotic effect in the treatment of
brain oedema. Zeynalov et al (7) reported that HS attenuated BBB
disruption in the presence of perivascular aquaporin 4 (AQP4) in
post-ischaemic cerebral oedema. Huang et al (8) also reported that HS downregulated
the expression of Na-K-Cl cotransporter 1 in astrocytes. This
finding suggested that HS alleviated cerebral oedema not only
through an osmotic mechanism but also through regulation of
molecular pathways.
Vascular endothelial growth factor (VEGF) is the
major regulator of microvascular permeability. It has been reported
that BBB permeability was significantly increased and the degree of
cerebral oedema decreased abruptly following treatment with VEGF
(9-11). It has also been well documented
that VEGF expression is increased in various type of cells,
including neurons, endothelial cells, astrocytes, pial cells, and
microglia/macrophages, following middle cerebral artery occlusion
(MCAO) in rats (12,13). Previous studies have focused on
astrocyte-derived VEGF (14-16), but the role of VEGF derived from
cerebral endothelial cells in the development of brain oedema
remains unclear. VEGF receptor 2 (VEGFR2), a receptor of VEGF,
serves an important role in regulating VEGF function, and it
increases vascular permeability (17,18). Previous studies have demonstrated
that VEGF-induced permeability depends on the VEGFR2-mediated
endothelial nitric oxide synthase (eNOS) pathway by downregulating
the expression of tight junction proteins (19,20). In addition, the tight junction
between endothelial cells is the key element in BBB permeability,
and disruption of tight junctions leads to BBB breakdown (21). Zonula occludens 1 (ZO1) and
occludin are important components of tight junctions, and
downregulation of these factors significantly increases the
permeability of the BBB (22).
Based on these previous findings, the present study
investigated the effect of 10% HS on cerebral oedema induced by
VEGF. Additionally, the present study investigated whether HS
regulated the expression of ZO1 and occludin via the
VEGFR2/phospholipase C γ1 (PLCγ1)/eNOS pathway. The findings are
expected to further amplify our understanding of the underlying
mechanisms and molecular roles of HS in the clinical management of
cerebral oedema.
Materials and methods
Animals and experimental groups
Male Sprague-Dawley rats (weight, 250-300 g) were
housed in groups in a pathogen-free environment (22±2°C, 55±10%
humidity and 12-h light/dark cycle) with free access to a standard
laboratory diet and water. The animals were randomly divided into
the sham group (n=30), cerebral ischaemia-reperfusion group (IR
group, n=30), cerebral ischaemia-reperfusion + normal saline group
(NS group, n=30) and cerebral ischaemia-reperfusion + 10% HS group
(HS group, n=30). Rats in the sham group were subjected to all
surgical procedures, except for transient cerebral ischaemia. Rats
in the IR group were subjected to cerebral ischemia for 2 h,
followed by reperfusion for either 12 or 24 h. Rats in the NS group
and HS group were subjected to transient cerebral ischaemia for 2
h, then treatment was administered at the beginning of reperfusion
with a continuous intravenous infusion (0.3 ml/h) of normal saline
or 10% HS, respectively, via the tail vein, until the end of the
experiment. The rats in each group were divided into two groups
according to different treatment times: A 12 h subgroup and a 24 h
subgroup. All experimental procedures involving the use of animals
were approved by the Institutional Animal Care and Use Committee,
Guangdong Province, China (approval no. GBREC2012106A). All
experiments were conducted in accordance with the National
Institute of Health Guide for the Care and Use of Laboratory
Animals.
Focal brain ischaemia-reperfusion animal
model
The rats were fasted overnight but were allowed free
access to water. Focal brain ischaemia was induced by the MCAO
method, as previously described (7). First, anaesthesia was injected
intra-muscularly with 6 mg/kg diazepam, and then anaesthesia was
continued by intraperitoneal injection with 40 mg/kg ketamine. The
right common carotid artery (CCA), internal carotid artery, and
external carotid artery were then exposed through a midline
incision in the neck. A 4-0 head-end spherical nylon suture was
inserted into the middle cerebral artery (MCA) via the CCA to
occlude the MCA for a total of 2 h. Cerebral reperfusion was
restored when the tip of the occluding suture was withdrawn to the
carotid bifurcation. The sham group rats were subjected to the same
surgical procedures but not MCAO. Neurologic examinations were
performed 2 h after the onset of occlusion. Zea-Longa test scores
were used to evaluate whether the MCAO was successful (23). The rats with neurologic deficit
scores of 1 to 3 were considered to have experienced successful
MCAO and were used for subsequent experiments.
Brain microvascular endothelial cell
culture and treatment
The brain microvascular endothelial cell (MVEC) line
bEnd.3 was obtained from American Type Culture Collection and
cultured in DMEM (Thermo Fisher Scientific, Inc.) medium
supplemented with 10% foetal bovine serum (FBS; Biological
Industries) at 37°C in a humidified incubator with 95% air and 5%
CO2. When the culture reached confluence, MVECs were
purified by removal of the non-adherent cells and subculturing of
only the adherent cells. The purified MVECs were randomly divided
into the following groups: Control group, oxygen and glucose
deprivation group (OGD group), OGD + 40 mM HS group (HS group) and
OGD + 40 mM HS + VEGF-A group (HS+VEGF-A group). The cells in the
OGD, HS and HS+VEGF-A groups were all incubated in glucose-free
medium (Thermo Fisher Scientific, Inc.) in an airtight hypoxia
chamber with 3% O2 and 5% CO2 at 37°C. After
6 h of hypoxic and glucose-free culture, the culture medium of each
group was supplemented as follows: The OGD group was cultured with
10% FBS for 24 h; the HS group was cultured with 10% FBS and 40 mM
HS for 24 h; and the HS+VEGF-A group medium was supplemented with
40 mM HS and 10 ng/ml VEGF-A simultaneously (PeproTech, Inc.) for
24 h. The cells in the control group were cultured with normal
medium containing 10% FBS, and were not subjected to hypoxia.
Evans blue (EB) staining
The permeability of the BBB was assessed by
measuring Evans blue extravasation in the ischaemic hemispheric
tissue as previously described (24,25). A solution of 2% Evans blue dye in
saline (4 ml/kg; Sigma-Aldrich; Merck KGaA) was injected
intravenously at the beginning of reperfusion. At 24 h after MCAO
and under deep anaesthesia, the rats were subjected to transcardial
perfusion with 110 ml saline to remove the intravascular Evans blue
dye. After the rats had been sacrificed by decapitation, the
hemispheres of the brain were separated along the sagittal suture.
Then, both hemispheres were weighed and kept in formamide (1 ml/100
mg) at 60°C for 24 h. The concentration of dye extracted from each
brain was determined at 620 nm using spectrophotometry. The
quantitative calculation of the dye content in the brain was based
on external standards dissolved in the same solvent.
Horseradish peroxidase (HRP) flux
assay
The permeability of the MVEC monolayer barrier was
detected by the HRP flux assay, as previously described (26). At 3, 6, 12, and 24 h after
incubation with 40 mM HS, the culture medium was replaced with DMEM
without phenol red or serum. To maintain the levels of culture
supernatant, 250 µl of medium containing 500 ng HRP
(Sigma-Aldrich; Merck KGaA) was added into the insert, and 1,250
µl of medium was added into each well. After each sample was
collected at one of the two time points, 100 µl of
peroxidase substrate (Sigma-Aldrich Merck KGaA) containing
tetramethyl benzidine and hydrogen peroxide was added to each
sample, which was then incubated for 10 min. The reaction was
terminated by adding 50 µl of 2 M sulfuric acid. The optical
density was measured at 450 nm, and the HRP transmissivity was
determined by the standard curve according to the following
equation: PHRP %=[(CHRPoxVo/CHRPixVi) ×100%], where CHRPo is the
HRP concentration in the well, CHRPi is the HRP concentration in
the insert well, Vo is the medium volume in the well, and Vi is the
medium volume in the insert.
Immunofluorescence staining
At 24 h after MCAO, frozen sections of coronal brain
were cut to 10 µm thickness at the level of the optic
chiasma and rinsed in PBS. After fixation with 4% paraformaldehyde
at room temperature for 15 min, the samples were permeabilized with
methanol, and blocked with 5% BSA in PBS for 20 min. Sections were
incubated overnight at 4°C with primary antibodies directed against
occludin [rabbit polyclonal (immunoglobulin) Ig G; 1:100; Abcam;
cat. no. ab31721), ZO1 (rabbit polyclonal IgG; 1:100; Thermo Fisher
Scientific Inc.; cat. no. 61-7300), VEGF (rabbit polyclonal IgG;
1:100; Santa Cruz Biotechnology Inc.; cat. no. sc-152), VEGF
receptor 2 (VEGFR2; rabbit polyclonal IgG; 1:100; Santa Cruz
Biotechnology Inc.; cat. no. sc-505) and vascular endothelial cell
markers von Willebrand factor (VWF; mouse monoclonal IgG; 1:100;
Santa Cruz Biotechnology Inc.; cat. no. sc-365712) and CD31 (mouse
monoclonal IgG; 1:100; Abcam; cat. no. ab119339). On the following
day, the sections were washed with PBS and incubated with the
corresponding secondary antibodies as follows: Alexa
Fluor® 488-conjugated goat anti-mouse IgG (1:100; Thermo
Fisher Scientific Inc.; cat. no. A-28175), Alexa Fluor®
555-conjugated donkey anti-rabbit IgG (1:100; Thermo Fisher
Scientific Inc.; cat. no. A-31572), Alexa Fluor®
488-conjugated donkey anti-rabbit IgG (1:100; Thermo Fisher
Scientific Inc.; cat. no. A-21206), or Alexa Fluor®
555-conjugated goat anti-mouse IgG (1:100; Thermo Fisher Scientific
Inc.; cat. no. A-21424) for 2 h at room temperature. Following
rinsing in PBS, samples were mounted with a fluorescent mounting
medium containing DAPI (cat. no. DUO82040; Sigma-Aldrich; Merck
KGaA). Colocalization was observed by a blinded observer using a
fluorescence microscope (Olympus Corporation).
For the in vitro experiments, after 6 h of
hypoxic and glucose-free culture, bEnd.3 endothelial cells were
reoxygenated and incubated with the corresponding medium, according
to different cell groups as aforementioned, for 24 h. Then the
cells were fixed with 4% paraformaldehyde for 20 min at 37°C.
Following rinsing with PBS, cells were blocked with 5% goat serum
(Jiangxi Haoran Bio-Pharma Co., Ltd.) for 30 min at 37°C and then
incubated with antibodies as described above.
For the quantification of protein expression
signals, the fluorescence intensity was calculated as follows:
Fluorescence intensity=total optical density/total fluorescence
area. Quantitative analysis was performed with Image J (v1.8.0;
National Institutes of Health).
Western blotting
Total proteins from the peri-ischaemic cerebral
cortex and bEnd.3 endothelial cells were extracted using a total
protein extraction kit (BestBio Science), according to the
manufacturer's instructions. Concentrations of the total proteins
were determined by using a bicinchoninic acid protein assay kit
(Bioworld Technology, Inc.). Protein samples were separated on
SDS-PAGE gels and electroblotted onto nitrocellulose membranes (EMD
Millipore). After blocking in TBS with 5% non-fat dry milk for 2 h
at 37°C, the membrane was incubated overnight with primary
antibodies: Occludin (rabbit polyclonal IgG; 1 µg/ml; Abcam;
cat. no. ab31721), ZO1 (rabbit polyclonal IgG; 1:1,000; Thermo
Fisher Scientific Inc.; cat. no. 61-7300), VEGF (rabbit polyclonal
IgG; 1:500; Santa Cruz Biotechnology. Inc.; cat. no. sc-152),
phosphorylated (p-) PLCγ1 (rabbit clonal IgG 1:1,000; Cell
Signalling Technology, Inc.; cat. no. 2821), eNOS (rabbit
monoclonal IgG; 1:1,000; Cell Signalling Technology, Inc.; cat. no.
32027) and β-actin (mouse monoclonal IgG; 1:1,000; Cell Signalling
Technology, Inc.; cat. no. 3700). After washing in TBST three
times, the membranes were incubated with HRP-conjugated secondary
antibody (1:1,000; Cell Signalling Technology, Inc.; cat. no. 7074
or cat. no. 7076) for 45 min at room temperature. The immunoblots
were developed using an enhanced chemiluminescence detection system
(ImageQuant LAS 500; GE Healthcare Life Sciences). The band
intensity was quantified using ImageJ 1.39u software (National
Institutes of Health).
To confirm the regulation of occludin and ZO1 by HS
through the VEGFR2/PLCγ1/eNOS pathway, bEnd.3 cells were first
stimulated with OGD for 6 h as aforementioned. Then, cells were
transferred to DMEM medium supplemented with 10% FBS and SU5416
(VEGFR2 antagonist; 10 ng/ml; Sigma-Aldrich; Merck KGaA; cat. no.
S8442), U73122 (PLCγ1 inhibitor; 1 µM; Sigma-Aldrich; Merck
KGaA; cat. no. U6756), L-NAME (eNOS inhibitor; 100 µM;
Sigma-Aldrich; Merck KGaA; cat. no. N5751) or HS (40 mM) for 24 h
at 37°C in a humidified incubator with 95% air and 5%
CO2. bEnd.3 cells, that did not undergo OGD, and were
cultured in DMEM/10% FBS, with or without 1 mM DMSO, were used as
controls.
Statistical analysis
SPSS 19.0 (IBM Corp.) was used to analyse the data.
The results are expressed as means ± standard deviation.
Differences among multiple groups were statistically analysed using
one-way ANOVA and post hoc comparisons (Bonferroni test). P<0.05
was considered to indicate a statistically significant
difference.
Results
Determination of BBB permeability
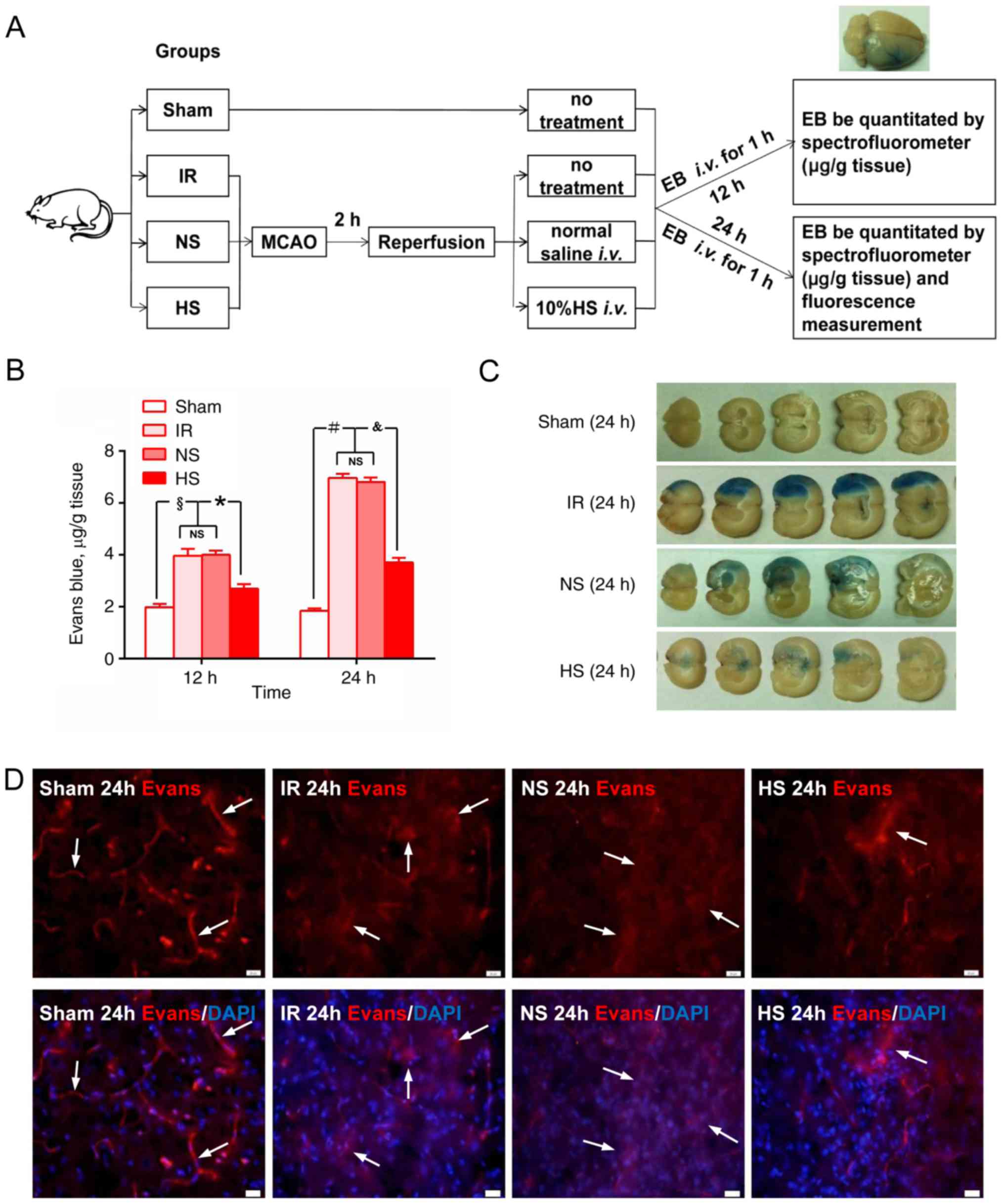
Fig. 1A shows a
schematic diagram of EB staining. The extravasation of EB was
significantly increased in samples from the IR group, NS group and
HS group at 12 and 24 h compared with those in the corresponding
sham groups (P<0.05; Fig. 1B).
However, following HS treatment, the concentration of EB in the HS
group was significantly decreased compared with that in the IR
group (P<0.05; Fig. 1B). At 24
h after MCAO, the EB staining area was obviously increased in brain
sections from the IR and NS groups, but was noticeably reduced
following 10% HS treatment in the HS group (Fig. 1C).
Using fluorescence microscopy, the contours of the
microvessels were observed to be well-delineated in the sham group.
However, in the IR and NS groups at 24 h, the vascular contours
were less evident, and increased extravasated EB was observed in
the tissue parenchyma. Following treatment with 10% HS, the
extravasation of EB was noticeably decreased (Fig. 1D).
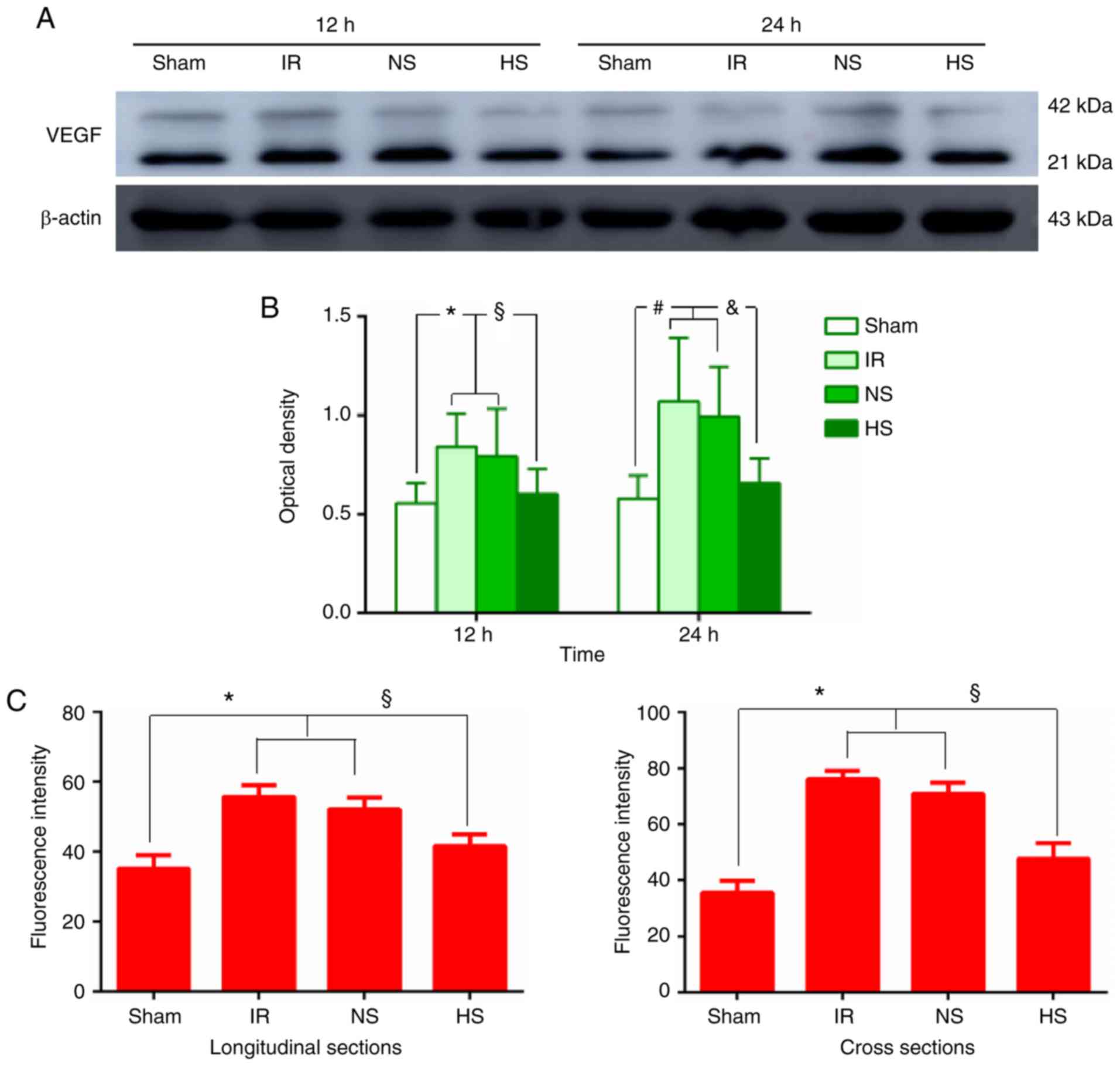
VEGF protein expression in peri-ischaemic
brain tissue
Western blot analysis revealed moderate expression
of VEGF in the sham group (Fig.
2A). At 12 and 24 h after MCAO, VEGF protein expression levels
in the IR and NS groups were significantly increased compared with
those in the sham group (Fig.
2A). However, after treatment with 10% HS, VEGF expression
levels were significantly decreased when compared with those in the
corresponding ischaemic rats (Fig.
2A). Accordingly, quantification of the western blot signals
demonstrated that the optical density of the VEGF bands in the
peri-ischaemic brain tissue increased significantly at 12 and 24 h
for the IR and NS groups compared with those in the sham group
(P<0.05; Fig. 2B). By
contrast, VEGF protein expression levels decreased significantly at
12 and 24 h in the HS group compared with those in the IR and NS
groups (P<0.05; Fig. 2B).
Immunofluorescence staining analysis revealed that
VEGF was expressed specifically in the MVECs, as confirmed by
double labelling with VWF (Fig.
2D). The staining was evident in the cross sections and in
longitudinal sections of the blood vessels (Fig. 2D). Intense VEGF immunofluorescence
was detected at 24 h in samples from the IR and NS groups compared
with the sham group (P<0.05; Fig.
2C and D). Treatment with 10% HS attenuated VEGF
immunofluorescence in samples from the HS group compared with those
from the IR and NS groups (P<0.05; Fig. 2C and D).
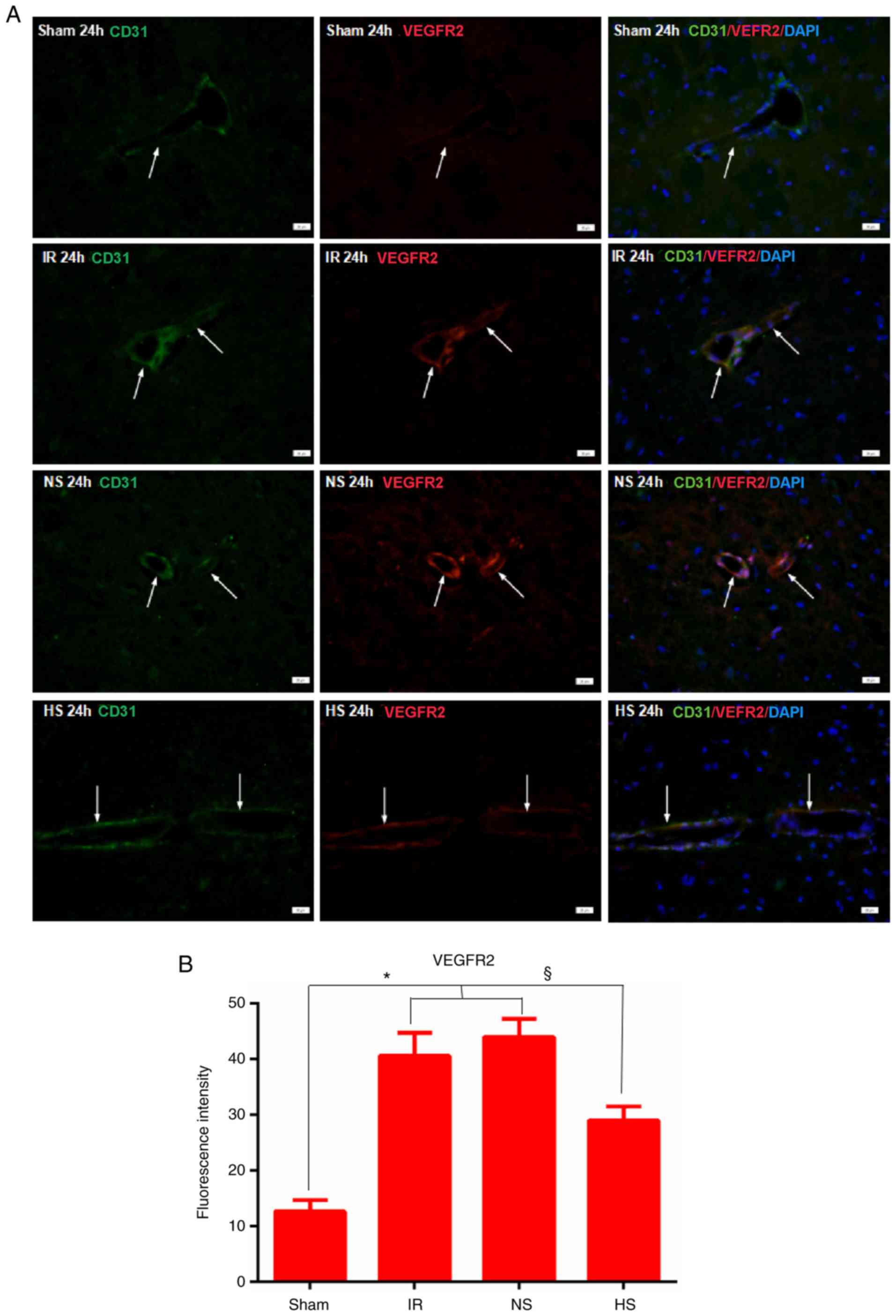
VEGFR2 protein expression in the
peri-ischaemic brain tissue
Immunofluorescence staining analysis revealed that
VEGFR2 expression was specifically detected in MVECs, as confirmed
by double labelling with CD31 (Fig.
3A). At 24 h after MCAO, very intense VEGFR2 immunoreactivity
was detected in the IR and NS groups. In the HS group, the
immunoreactivity was obviously decreased compared with that of the
IR and NS groups (Fig. 3A).
Quantification of the fluorescence intensity demonstrated that this
was significantly increased in the IR and NS groups at 24 h when
compared with that in the sham group (P<0.05; Fig. 3B). However, in the HS group,
VEGFR2 immunofluorescence was significantly decreased compared with
that in the IR and NS groups (P<0.05; Fig. 3B).
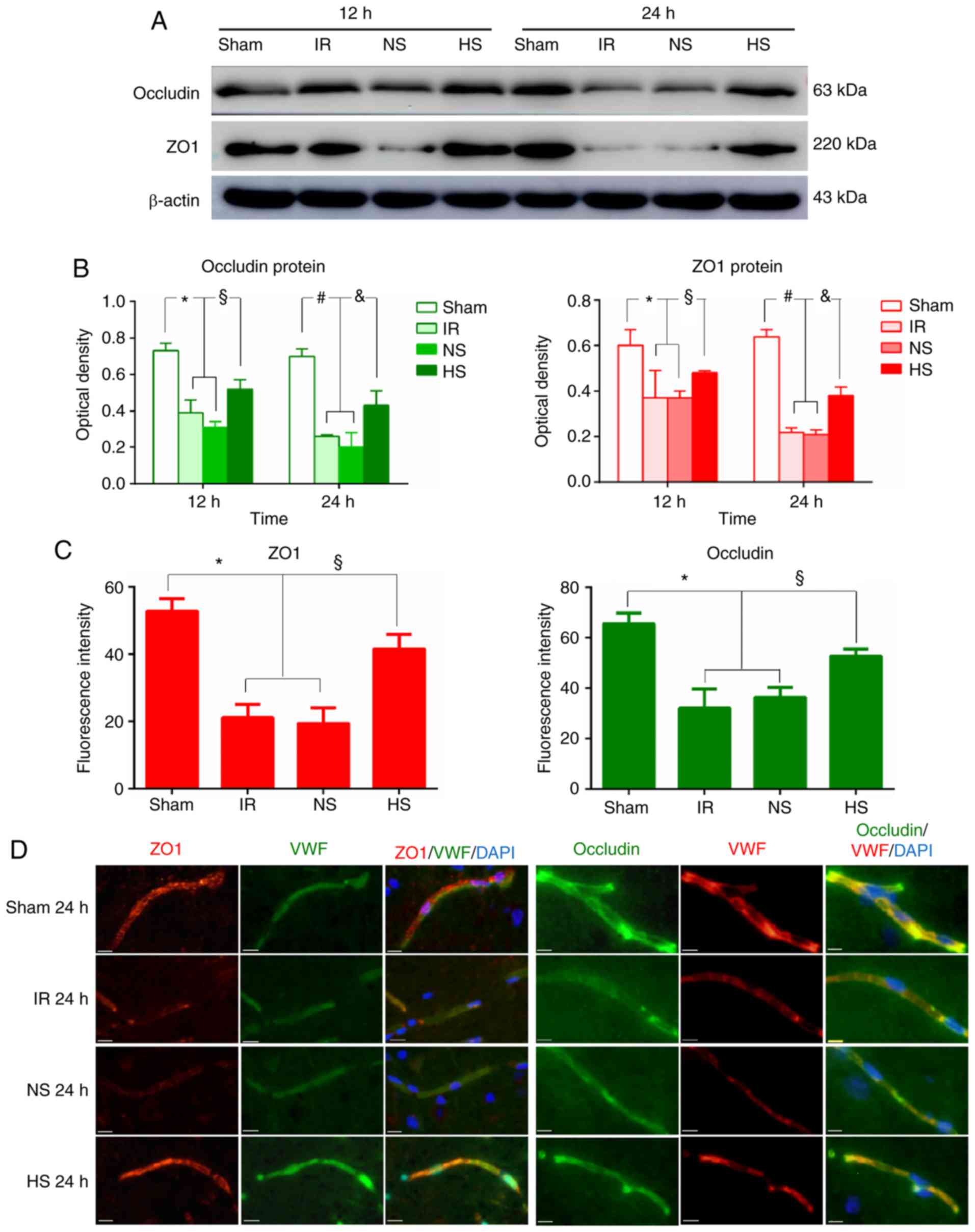
Expression of occludin and ZO1 in the
peri-ischaemic brain tissue
Western blotting analysis demonstrated that the
expression levels of occludin and ZO1 were markedly lower in the IR
and NS groups compared with that in the sham group at 12 and 24 h
after MCAO. However, after 10% HS treatment, the expression levels
of occludin and ZO1 were markedly enhanced compared with that of
the IR and the NS groups at the corresponding time points (Fig. 4A). Accordingly, quantification of
the optical densities of occludin and ZO1 revealed that their
levels were significantly decreased in the IR and NS groups
compared with the sham group at 12 and 24 h post-reperfusion
(P<0.05; Fig. 4B). Remarkably,
treatment with 10% HS markedly increased the optical density of
occludin and ZO1 compared with the IR and NS groups (P<0.05;
Fig. 4B).
To confirm the effect of focal ischaemia-reperfusion
on occludin and ZO1, immunofluorescence staining analysis was also
performed. As shown in Fig. 4D,
occludin and ZO1 expression in brain tissue was colocalized with
VWF. Very weak occludin and ZO1 immunoreactivity was detected in
the IR and NS groups compared with that in the sham group. However,
after 10% HS treatment, occludin and ZO1 immunoreactivity was
markedly enhanced. Quantification of the fluorescent signals
revealed a significant decrease in the mean fluorescence intensity
values for occludin and ZO1 in the IR and NS groups compared with
the sham group (P<0.05; Fig.
4C). Of note, a significance increase in fluorescence intensity
was observed in the HS group for both ZO1 and occludin, compared
with the IR and NS groups (P<0.05; Fig. 4C).
Determination of endothelial cell
monolayer permeability
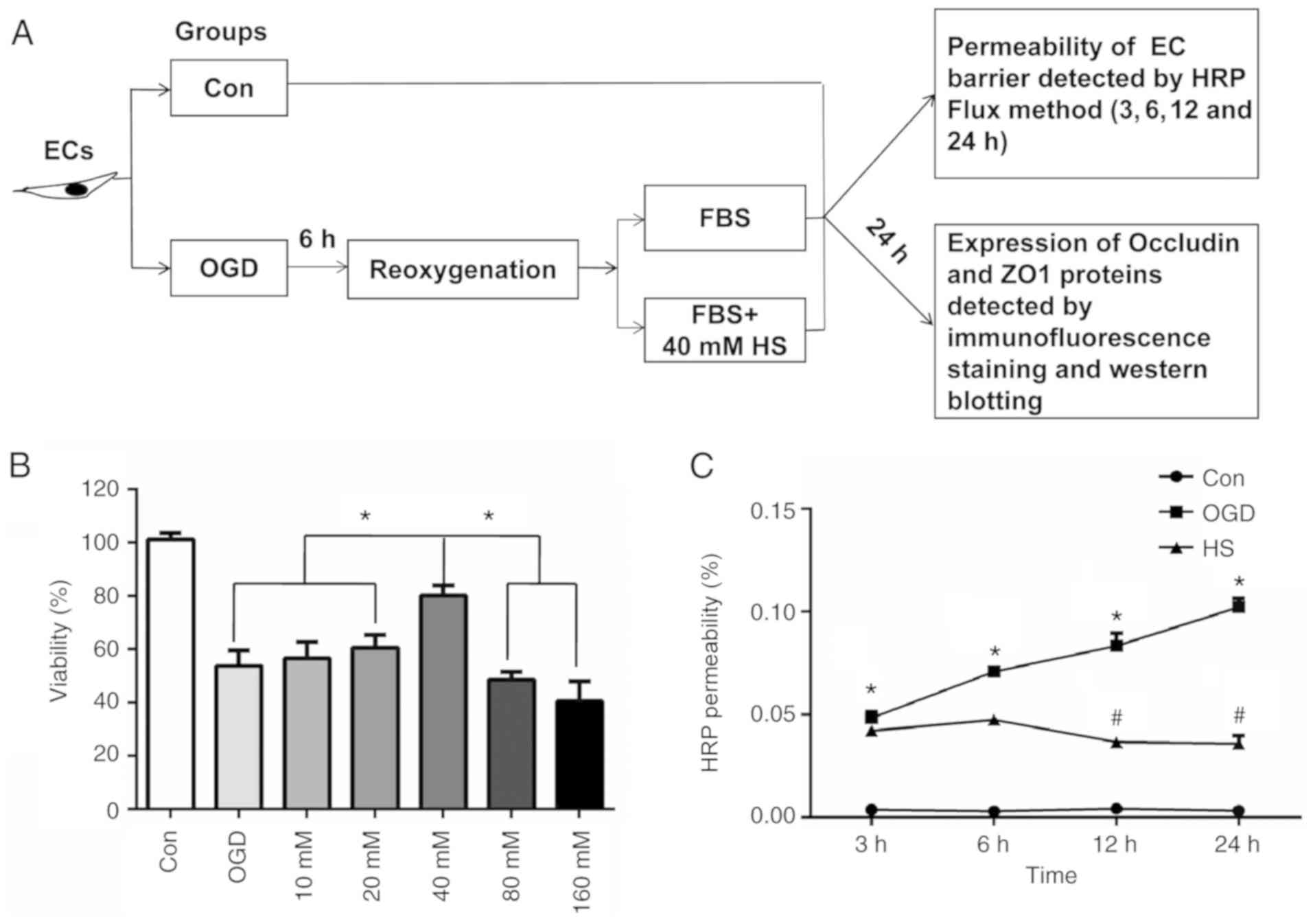
To determine the optimal concentration of HS on
bEnd.3 cells, first the effect of HS on bEnd.3 cell viability was
evaluated. The viability of the OGD-induced bEnd.3 cells increased
significantly at 24 h after treatment with 40 mM HS compared with
other concentrations of HS (10, 20, 80 and 160 mM; Fig. 5B). Therefore, the concentration of
40 mM HS was selected for the subsequent experiments in
vitro. Fig. 5A shows a
schematic diagram of the experiments conducted in vitro.
The permeability of the bEnd.3 cell monolayer was
assessed by measuring the permeability coefficient of HRP. As shown
in Fig. 5C, compared with the
control group, the HRP permeability increased significantly at 3,
6, 12, and 24 h after 6 h of OGD (P<0.05). However, when
compared with the OGD group, the HRP permeability decreased
significantly at 12 h and 24 h after treatment with 40 mM HS
(P<0.05; Fig. 5C).
HS upregulates VEGF, VEGFR2, pPLCγ1, and
eNOS protein expression in bEnd.3 endothelial cells
Immunofluorescence staining analysis demonstrated
that VEGF was expressed in the bEnd.3 MVECs, as confirmed by double
staining with CD31 (Fig. 6A).
When compared with the control group, VEGF immunofluorescence
intensity in the bEnd.3 endothelial cells was enhanced at 24 h
after OGD (P<0.05; Fig. 6A and
C), but was significantly decreased after treatment with 40 mM
HS for 24 h compared with the OGD group (P<0.05; Fig. 6A and C). Similar changes were
observed for VEGFR2 immunofluorescence expression in the bEnd.3
endothelial cells (Fig. 6B and
D).
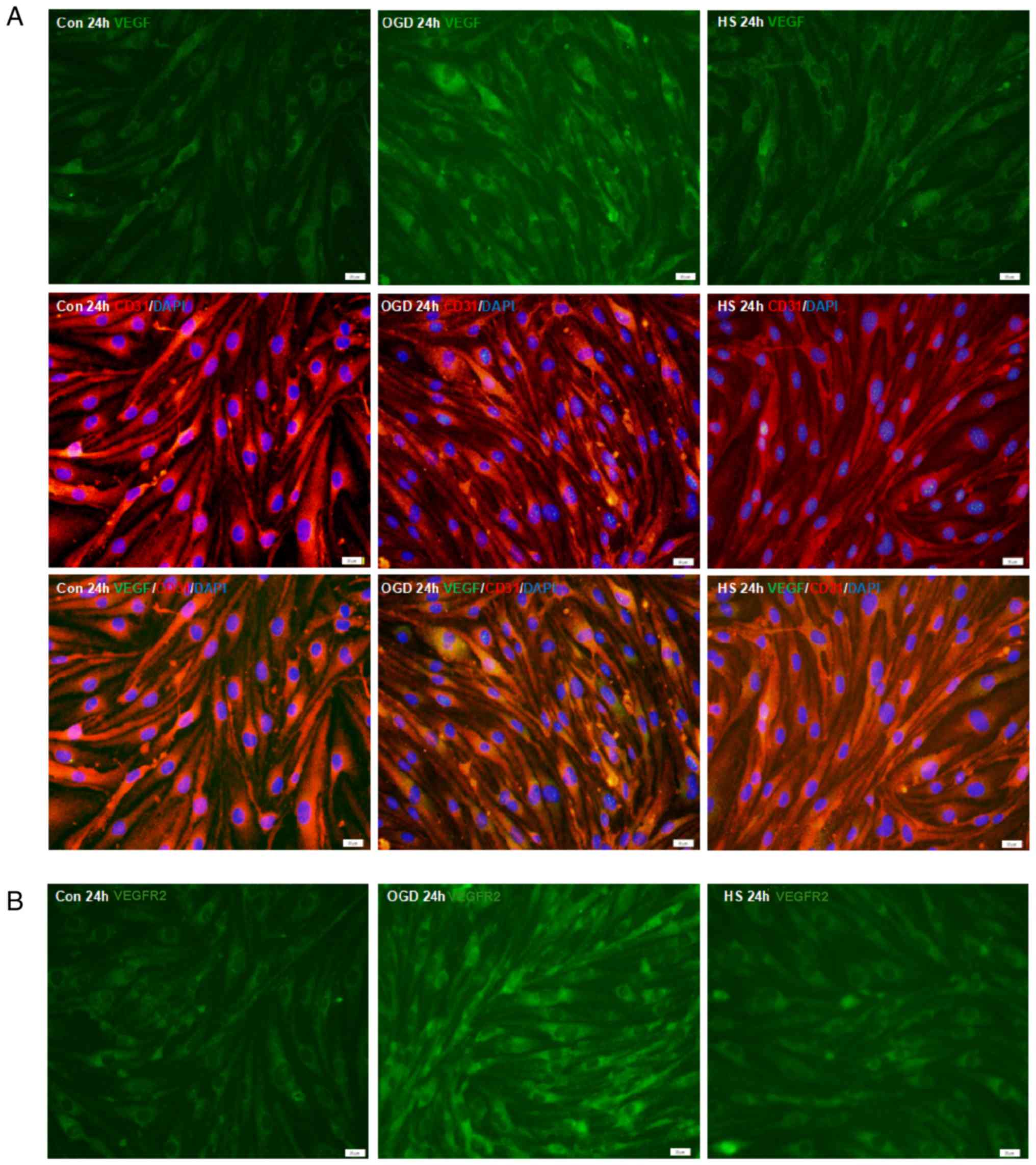 | Figure 6VEGF, VEGFR2, p-PLCγ1, PLCγ1 and eNOS
protein expression in bEnd.3 endothelial cells. (A)
Immunofluorescence staining of VEGF (green) in bEnd.3 endothelial
cells. Co-localization with the endothelial cell marker CD31 (red)
was observed. (B) Immunofluorescence staining of VEGFR2 in bEnd.3
endothelial cells. Scale bar, 20 µm. (C) Fluorescence
intensity changes of VEGF protein and (D) VEGFR2 protein in each
experimental group. §P<0.05 vs. OGD; and
*P<0.05 vs. control. (E) Representative images from
western blot analysis for the proteins VEGF (21 and 42 kDa), VEGFR2
(154 kDa), p-PLCγ1 (155 kDa), PLCγ1 (155 kDa), eNOS (140 kDa) and
β-actin (43 kDa). (F) Quantification of western blotting results in
each group. #P<0.05 vs. OGD; and
*P<0.05 vs. control. VEGF, vascular endothelial
growth factor; VEGFR2, vascular endothelial growth factor receptor
2; p-, phosphorylated; PLCγ1, phospholipase C γ1; eNOS, endothelial
nitric oxide synthase; OGD, oxygen-glucose deprivation; HS,
hypertonic saline; Con, control. |
To confirm whether the upregulated expression of
VEGF and VEGFR2 occurs through the VEGF/VEGFR2/pPLCγ1/eNOS
signalling pathway, the expression of each of these proteins was
investigated by western blotting. As shown in Fig. 6E, the expression levels of VEGF,
VEGFR2, p-PLCγ1 and eNOS were significantly increased 24 h
following OGD compared with the control (Fig. 6E and 6F). After treatment with 40 mM HS,
however, VEGF, VEGFR2, p-PLCγ1 and eNOS protein expression levels
in the HS group were significantly decreased compared with those in
the OGD group (Fig. 6E and
F).
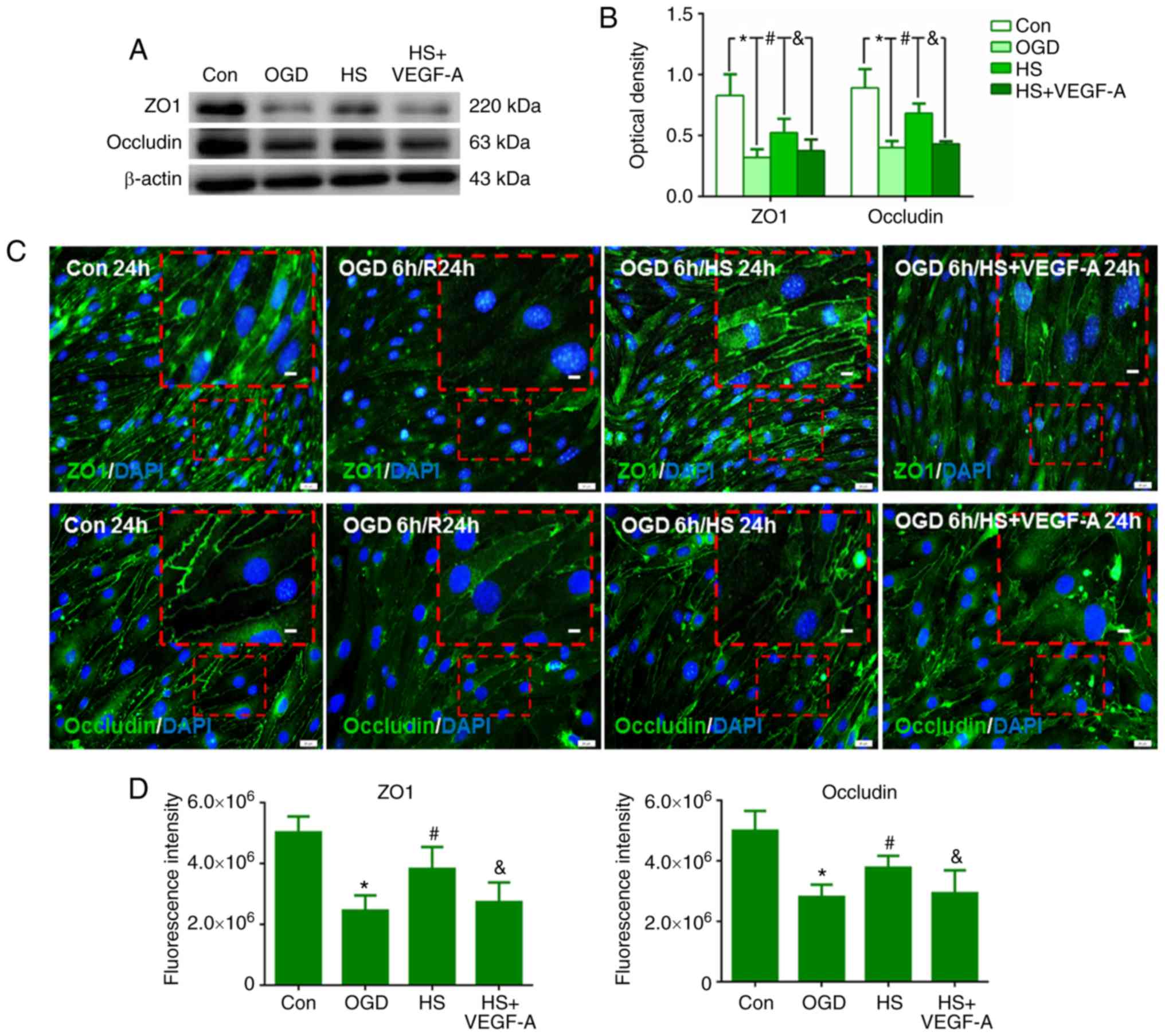
HS regulates occludin and ZO1 expression
through VEGFR2/PLCg1/eNOS in bEnd.3 endothelial cells
In the OGD group, occludin and ZO1 protein
expression levels were decreased in the bEnd.3 endothelial cells
compared with the sham group (P<0.05; Fig. 7A and B). Twenty-four hours after
treatment with 40 mM HS, however, the protein expression levels of
occludin and ZO1 in the HS group were higher than in the OGD group
(P<0.05; Fig. 7A and B).
Following a combined treatment of 10 ng/ml VEGF-A and 40 mM HS
(HS+VEGF-A group), the expression levels of both proteins were
significantly decreased at 24 h when compared with those in the HS
group (P<0.05; Fig. 7A and
B).
By immunofluorescence staining analysis of the
bEnd.3 cell monolayers, weak occludin and ZO1 immunoreactivity was
detected in the OGD group at 24 h compared with that in the control
group (P<0.05; Fig. 7C and D).
However, after treatment with 40 mM HS, occludin and ZO1
immunoreactivity was significantly increased compared with that in
the OGD group (P<0.05; Fig. 7C and
D). Following the combined treatment of 10 ng/ml VEGF-A and 40
mM HS, expression of both proteins was decreased compared with that
in the HS group (P<0.05; Fig. 7C
and D).
To further confirm the regulation of occludin and
ZO1 by HS through the VEGFR2/PLCγ1/eNOS pathway, bEnd.3 cell were
incubated with SU5416 (inhibitor of VEGFR2), U73122 (inhibitor of
PLCγ1), and L-NAME (inhibitor of eNOS). As shown in Fig. 8A and B, the protein expression
levels of occludin and ZO1 were decreased after OGD compared with
the control (P<0.05), but significantly increased following
treatment with SU5416, U73122, L-NAME and 40 mM HS (all
P<0.05).
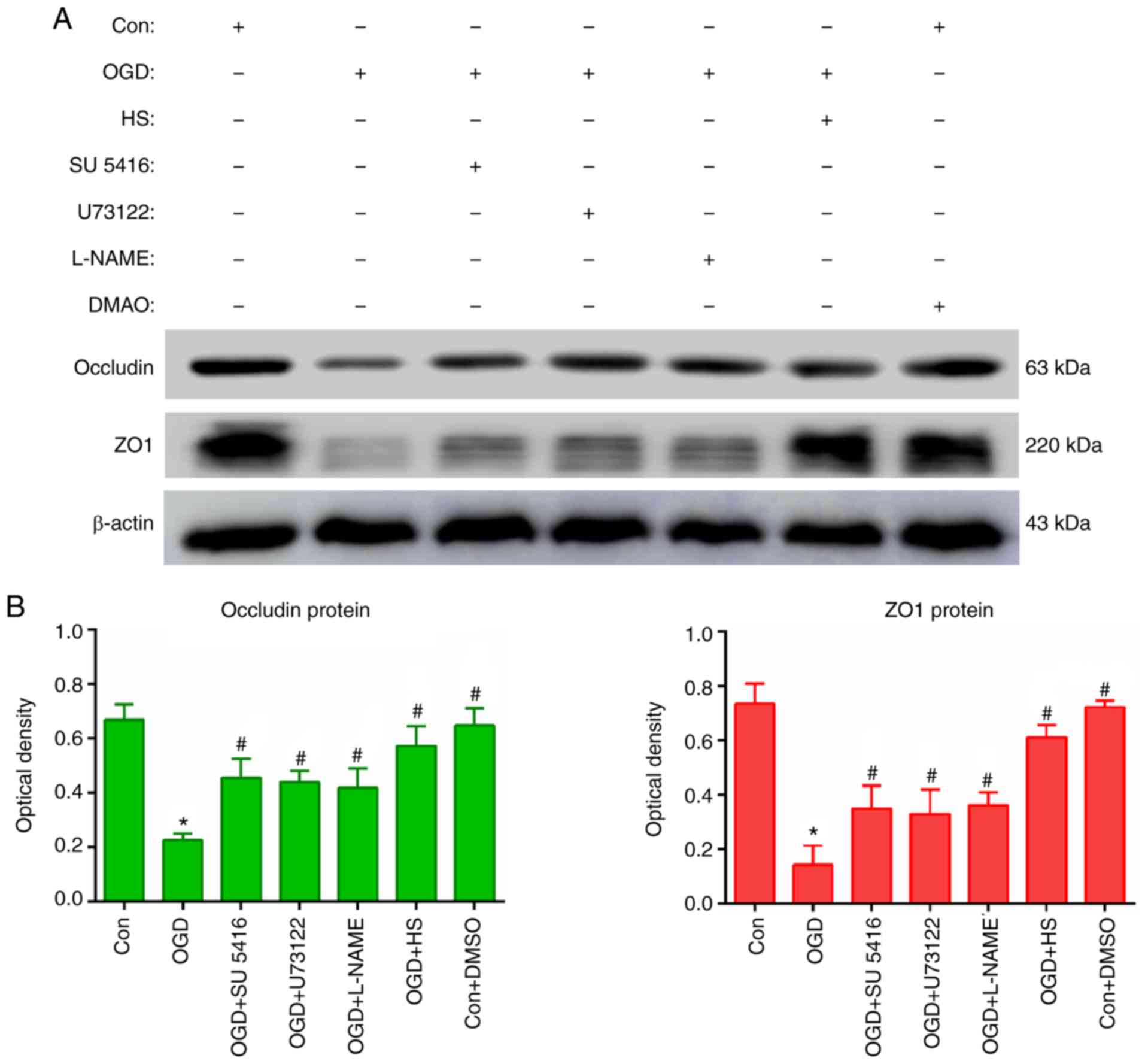 | Figure 8VEGF regulated occludin and ZO1
expression via the VEGFR2/PLCγ1/eNOS signalling pathway in bEnd.3
endothelial cells. (A) Representative images from western blot
analysis for the proteins occludin (63 kDa), ZO1 (220 kDa) and
β-actin (43 kDa), following different treatments, including OGD, 40
mM HS, SU5416 (VEGFR2 inhibitor), U73122 (PLCγ1 inhibitor) and
L-NAME (eNOS inhibitor). (B) Quantification of western blotting
results in each experimental group. *P<0.05 vs.
control; and #P<0.05 vs. OGD. VEGF, vascular
endothelial growth factor; ZO1, zonula occludens 1; VEGFR2,
vascular endothelial growth factor receptor 2; PLCγ1, phospholipase
C γ1; eNOS, endothelial nitric oxide synthase; OGD, oxygen-glucose
deprivation; HS, hypertonic saline; Con, control. |
Discussion
The BBB is a selective semipermeable membrane and
the regulated interface between the peripheral circulation and the
central nervous system (CNS) (27). The anatomical substrate of the BBB
is the cerebral microvascular endothelium, which, together with
astrocytes, pericytes, neurons, and the extracellular matrix,
constitutes a 'neurovascular unit' that is essential for the health
and function of the CNS (27).
The BBB separates the brain parenchyma from the peripheral
circulation and stabilizes the microenvironment of neurons,
protecting them from deleterious effects of certain substances in
the blood (28). The disruption
of the BBB leads to extravasation of intravascular substances into
the extracellular spaces of the brain. The present study
demonstrated that EB dye extravasation in the ischaemic hemisphere
gradually increased up to 24 h. These results are in accord with a
previous report that ischaemia-reperfusion enhanced BBB
permeability and breakdown (29).
HS is a common osmotic dehydrating agent that exerts anti-oedema
effects on an intact BBB, and the anti-oedema effect in the brain
may be associated with alleviating the disruption of the BBB
(5). As expected, the present
results demonstrated that treatment with 10% HS resulted in a
significant decrease in EB extravasation. This was further
confirmed in vitro; HRP extravasation was significantly
decreased, compared to that in the OGD group, in a bEnd.3
cell-derived cerebral MVEC monolayer barrier following incubation
with 40 mM HS for 24 h. Thus, the present study demonstrated that
10% HS ameliorated cerebral oedema through reduction of BBB
permeability and protection of BBB integrity.
VEGF is known to be an important vascular
permeability factor that can increase cerebral vascular
permeability and induce vasogenic brain oedema in multiple
diseases, such as cerebral ischaemic infarction (30), brain tumour (11), and subarachnoid haemorrhage
(9). It has been reported that
increased VEGF induces BBB leakage (31). VEGFR2, a major receptor of VEGF,
is also an important mediator of vascular permeability. It is
overexpressed in endothelial cells, astrocytes, neuronal stomata
and tissues adjacent to areas of damage by brain injury (32). A previous report suggested that
the effects of VEGF on the BBB depended on VEGFR2, and inhibition
of VEGF/VEGFR2 was beneficial for ameliorating oedema (33). In view of this, the present study
measured the expression of VEGF and VEGFR2. The results
demonstrated that the protein expression levels of VEGF and VEGFR2
were significantly upregulated after MCAO. Notably, when compared
with the IR group, the HS group exhibited lower VEGF and VEGFR2
expression levels. These changes correlated with the reduced BBB
permeability after treatment with 10% HS. These findings suggested
that HS may alleviate BBB permeability through inhibiting VEGF and
VEGFR2 expression.
It is known that endothelial cells have a
significant role in maintaining BBB permeability and integrity
(34,35), and it has been reported that VEGF
expression is also detected in vascular endothelial cells and
neurons (36). To detect whether
VEGF and VEGFR2 exhibit the same expression pattern in endothelial
cells, an in vitro hypoxia model with endothelial cells was
generated. The results were in agreement with the in vivo
experiments. Western blot analysis revealed that the protein
expression levels of VEGF and VEGFR2 were significantly increased
upon OGD, but they were markedly decreased after treatment with 40
mM HS. Immunofluorescence assays confirmed this finding,
demonstrating that HS directly suppressed the expression of VEGF
and VEGFR2 in endothelial cells.
ZO1 and occludin are important tight junction
proteins, and their downregulation significantly increases the
permeability of the BBB (22). A
previous study demonstrated that VEGF could downregulate ZO1 and
occludin expression, leading to the disruption of the BBB (37). The present study confirmed through
western blot and immunofluorescence assays that HS could also
significantly inhibit the downregulation of ZO1 and occludin.
Therefore, HS may ameliorate cerebral oedema via inhibition of
VEGF-mediated downregulation of ZO1 and occludin, which may then
protect the integrity of the BBB. It was reported that VEGF-induced
permeability depends on the VEGFR2-mediated eNOS pathway (19,20), via inhibiting the expression of
tight junction proteins. Another study also reported that the
expression levels of occludin and claudin-5 were downregulated in
microvascular endothelial cells when treated with VEGF-A; however,
they were upregulated after treatment with an anti-VEGFR2 blocking
antibody, a PLCγ1 inhibitor or eNOS inhibitor (15). In the present study, the results
demonstrated that ZO1 and occludin protein expression levels were
increased significantly after cells were incubated with SU5416,
U73122 or L-NAME. These findings suggested that the
VEGFR2/PLCγ1/eNOS signalling pathway may be involved in this
process, but further studies will be needed to fully elucidate this
mechanism.
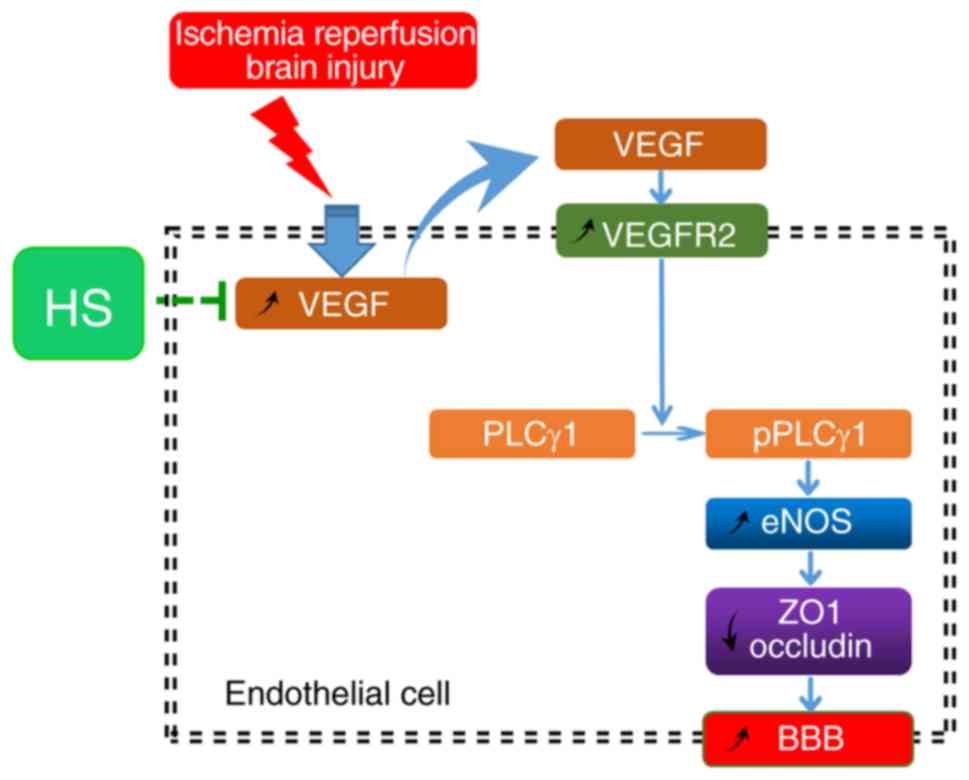
In conclusion, upregulated VEGF and VEGFR2
expression in endothelial cells induced by ischaemia-reperfusion
resulted in subsequent downregulation of ZO1 and occludin. This
downregulation of tight junction proteins may contribute to BBB
dysfunction by increasing its permeability. Finally, HS treatment
effectively alleviated this damage to the BBB, at least partly via
the VEGFR2/PLCγ1/eNOS signalling pathway (Fig. 9).
Funding
This study was supported by the Natural Science
Foundation of Guangdong province (grant nos. 2016A030311043,
2017A030313691 and 2015A030313004), the Natural Science Foundation
of Hunan province (grant no. 2017JJ2229), the National Natural
Science Foundation for Young Scientists of China (grant nos.
81701939 and 81701875) and the Science and Technology Project of
Guangdong (grant no. 2016A020215209).
Availability of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ, QW, YD and WZ conceived the study. YH, HD, SC
and BL provided materials and samples. LH, MW, YH, WJ and YD
participated in data collection and analysis. All authors read and
approved the final manuscript and consented to publish this
manuscript.
Ethics approval and consent to
participate
All experimental procedures involving the use of
animals were approved by the Institutional Animal Care and Use
Committee, Guangdong Province, China (approval no. GBREC2012106A).
All experiments were conducted in accordance with the National
Institute of Health Guide for the Care and Use of Laboratory
Animals.
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Mr Jiening Zhu and
Qiuxiong Lin for technical assistance.
References
|
1
|
Wijdicks EF, Sheth KN, Carter BS, Greer
DM, Kasner SE, Kimberly WT, Schwab S, Smith EE, Tamargo RJ,
Wintermark M, et al: Recommendations for the management of cerebral
and cerebellar infarction with swelling: A statement for healthcare
professionals from the American Heart Association/American Stroke
Association. Stroke. 45:1222–1238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gasche Y and Copin JC: Blood-brain barrier
pathophysiology and ischaemic brain oedema. Ann Fr Anesth Reanim.
22:312–319. 2003.In French. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sandoval KE and Witt KA: Blood-brain
barrier tight junction permeability and ischemic stroke. Neurobiol
Dis. 32:200–219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nout YS, Mihai G, Tovar CA, Schmalbrock P,
Bresnahan JC and Beattie MS: Hypertonic saline attenuates cord
swelling and edema in experimental spinal cord injury: A study
utilizing magnetic resonance imaging. Crit Care Med. 37:2160–2166.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zeng HK, Wang QS, Deng YY, Jiang WQ, Fang
M, Chen CB and Jiang X: A comparative study on the efficacy of 10%
hypertonic saline and equal volume of 20% mannitol in the treatment
of experimentally induced cerebral edema in adult rats. BMC
Neurosci. 11:1532010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schwarz S, Georgiadis D, Aschoff A and
Schwab S: Effects of hypertonic (10%) saline in patients with
raised intracranial pressure after stroke. Stroke. 33:136–140.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zeynalov E, Chen CH, Froehner SC, Adams
ME, Ottersen OP, Amiry-Moghaddam M and Bhardwaj A: The perivascular
pool of aquaporin-4 mediates the effect of osmotherapy in
postischemic cerebral edema. Crit Care Med. 36:2634–2640. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang LQ, Zhu GF, Deng YY, Jiang WQ, Fang
M, Chen CB, Cao W, Wen MY, Han YL and Zeng HK: Hypertonic saline
alleviates cerebral edema by inhibiting microglia-derived TNF-alpha
and IL-1beta-induced Na-K-Cl Cotransporter up-regulation. J
Neuroinflammation. 11:1022014. View Article : Google Scholar
|
|
9
|
Liu L, Fujimoto M, Kawakita F, Ichikawa N
and Suzuki H: Vascular endothelial growth factor in brain edema
formation after subarachnoid hemorrhage. Acta Neurochir Suppl.
121:173–177. 2016. View Article : Google Scholar
|
|
10
|
Bauer AT, Burgers HF, Rabie T and Marti
HH: Matrix metal-loproteinase-9 mediates hypoxia-induced vascular
leakage in the brain via tight junction rearrangement. J Cereb
Blood Flow Metab. 30:837–848. 2010. View Article : Google Scholar
|
|
11
|
Gerstner ER, Duda DG, di Tomaso E, Ryg PA,
Loeffler JS, Sorensen AG, Ivy P, Jain RK and Batchelor TT: VEGF
inhibitors in the treatment of cerebral edema in patients with
brain cancer. Nat Rev Clin Oncol. 6:229–236. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hayashi T, Abe K, Suzuki H and Itoyama Y:
Rapid induction of vascular endothelial growth factor gene
expression after transient middle cerebral artery occlusion in
rats. Stroke. 28:2039–2044. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Greenberg DA and Jin K: Vascular
endothelial growth factors (VEGFs) and stroke. Cell Mol Life Sci.
70:1753–1761. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chapouly C, Tadesse Argaw A, Horng S,
Castro K, Zhang J, Asp L, Loo H, Laitman BM, Mariani JN, Straus
Farber R, et al: Astrocytic TYMP and VEGFA drive blood-brain
barrier opening in inflammatory central nervous system lesions.
Brain. 138:1548–1567. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Argaw AT, Asp L, Zhang J, Navrazhina K,
Pham T, Mariani JN, Mahase S, Dutta DJ, Seto J, Kramer EG, et al:
Astrocyte-derived VEGF-A drives blood-brain barrier disruption in
CNS inflammatory disease. J Clin Invest. 122:2454–2468. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cobbs CS, Chen J, Greenberg DA and Graham
SH: Vascular endothelial growth factor expression in transient
focal cerebral ischemia in the rat. Neurosci Lett. 249:79–82. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Olsson AK, Dimberg A, Kreuger J and
Claesson-Welsh L: VEGF receptor signalling-in control of vascular
function. Nat Rev Mol Cell Biol. 7:359–371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shimotake J, Derugin N, Wendland M, Vexler
ZS and Ferriero DM: Vascular endothelial growth factor receptor-2
inhibition promotes cell death and limits endothelial cell
proliferation in a neonatal rodent model of stroke. Stroke.
41:343–349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bates DO and Harper SJ: Regulation of
vascular permeability by vascular endothelial growth factors.
Vascul Pharmacol. 39:225–237. 2002. View Article : Google Scholar
|
|
20
|
Fulton D, Gratton JP, McCabe TJ, Fontana
J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A and Sessa WC:
Regulation of endothelium-derived nitric oxide production by the
protein kinase Akt. Nature. 399:597–601. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luissint AC, Artus C, Glacial F,
Ganeshamoorthy K and Couraud PO: Tight junctions at the blood brain
barrier: Physiological architecture and disease-associated
dysregulation. Fluids Barriers CNS. 9:232012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiao H, Wang Z, Liu Y, Wang P and Xue Y:
Specific role of tight junction proteins claudin-5, occludin, and
ZO-1 of the blood-brain barrier in a focal cerebral ischemic
insult. J Mol Neurosci. 44:130–139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang S, Xia R, Jiang Y, Wang L and Gao F:
Vascular endothelial growth factors enhance the permeability of the
mouse blood-brain barrier. PLoS One. 9:pp. e864072014, View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uyama O, Okamura N, Yanase M, Narita M,
Kawabata K and Sugita M: Quantitative evaluation of vascular
permeability in the gerbil brain after transient ischemia using
Evans blue fluorescence. J Cereb Blood Flow Metab. 8:282–284. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang LF, Li X, Gao YB, Wang SM, Zhao L,
Dong J, Yao BW, Xu XP, Chang GM, Zhou HM, et al: Activation of
VEGF/Flk-1-ERK pathway induced blood-brain barrier injury after
microwave exposure. Mol Neurobiol. 52:478–491. 2015. View Article : Google Scholar
|
|
27
|
Hawkins BT and Davis TP: The blood-brain
barrier/neurovascular unit in health and disease. Pharmacol Rev.
57:173–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anfuso CD, Lupo G, Romeo L, Giurdanella G,
Motta C, Pascale A, Tirolo C, Marchetti B and Alberghina M:
Endothelial cell-pericyte cocultures induce PLA-2 protein
expression through activation of PKCalpha and the MAPK/ERK cascade.
J Lipid Res. 48:782–793. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee JH, Cui HS, Shin SK, Kim JM, Kim SY,
Lee JE and Koo BN: Effect of propofol post-treatment on blood-brain
barrier integrity and cerebral edema after transient cerebral
ischemia in rats. Neurochem Res. 38:2276–2286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chi OZ, Hunter C, Liu X and Weiss HR:
Effects of anti-VEGF antibody on blood-brain barrier disruption in
focal cerebral ischemia. Exp Neurol. 204:283–287. 2007. View Article : Google Scholar
|
|
31
|
Zhang ZG, Zhang L, Jiang Q, Zhang R,
Davies K, Powers C, Bruggen Nv and Chopp M: VEGF enhances
angiogenesis and promotes blood-brain barrier leakage in the
ischemic brain. J Clin Invest. 106:829–838. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lafuente JV, Argandona EG and Mitre B:
VEGFR-2 expression in brain injury: Its distribution related to
brain-blood barrier markers. J Neural Transm (Vienna). 113:487–496.
2006. View Article : Google Scholar
|
|
33
|
Vohra PK, Hoeppner LH, Sagar G, Dutta SK,
Misra S, Hubmayr RD and Mukhopadhyay D: Dopamine inhibits pulmonary
edema through the VEGF-VEGFR2 axis in a murine model of acute lung
injury. Am J Physiol Lung Cell Mol Physiol. 302:L185–L192. 2012.
View Article : Google Scholar :
|
|
34
|
Roe K, Orillo B and Verma S: West Nile
virus-induced cell adhesion molecules on human brain microvascular
endothelial cells regulate leukocyte adhesion and modulate
permeability of the in vitro blood-brain barrier model. PLoS One.
9:pp. e1025982014, View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma SC, Li Q, Peng JY, Zhouwen JL, Diao JF,
Niu JX, Wang X, Guan XD, Jia W and Jiang WG: Claudin-5 regulates
blood-brain barrier permeability by modifying brain microvascular
endothelial cell proliferation, migration, and adhesion to prevent
lung cancer metastasis. CNS Neurosci Ther. 23:947–960. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang L, Cao W, Deng Y, Zhu G, Han Y and
Zeng H: Hypertonic saline alleviates experimentally induced
cerebral oedema through suppression of vascular endothelial growth
factor and its receptor VEGFR2 expression in astrocytes. BMC
Neurosci. 17:642016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu L, Ye Z, Pan Y, Li X, Fu X, Zhang B, Li
Y, Lin W, Li X and Gao Q: Vascular endothelial growth factor
aggravates cerebral ischemia and reperfusion-induced
blood-brain-barrier disruption through regulating
LOC102640519/HOXC13/ZO-1 signaling. Exp Cell Res. 369:275–283.
2018. View Article : Google Scholar : PubMed/NCBI
|