Introduction
Sepsis is a life-threatening clinical syndrome, and
is characterized by systemic inflammatory reactions that occur due
to infection (1). It is a serious
complication of severe trauma, burns and surgery, and is also a
major cause of infections and multiple organ dysfunction syndrome
(2-4). Sepsis arises as a result of the
complex interactions of pro- and anti-inflammatory processes caused
by factors, such as Gram-negative and Gram-positive bacteria; the
competition between the host immune response and pathogens
determines patient survival (5).
In the United States of America, the morbidity from severe sepsis
has been reported to be ~300 cases per 100,000 residents, with a
25% fatality rate (6). The
mortality of patients is the highest in those with respiratory
tract infections, particularly pneumonia (6). At present, the administration of
immunostimulatory therapy to patients at the appropriate stage
represents a major advancement in sepsis treatment.
The majority of patients who are critically ill are
deficient in vitamin D [serum 1,25-dihydroxyvitamin D3 (VD)
concentrations <20-30 ng/ml] (7). Patients with sepsis in emergency
departments and intensive care units generally exhibit lower levels
of serum VD compared with healthy controls (8,9). A
previous study revealed a correlation between VD deficiency and
high mortality in sepsis patients (10). Besides regulating
calcium-phosphate homeostasis, VD affects innate and adaptive
immunity and their interplay; VD may induce immune imbalance,
potentially affecting the pathogenesis of certain infections
(11,12). Upregulated expression of
biomarkers associated with sepsis-induced systemic inflammation are
important indicators of VD deficiency (9); however, few studies have
investigated the specific mechanisms by which VD modulates the
inflammatory response in patients with sepsis.
Toll-like receptor (TLR) family members (TLR1-TLR10)
serve a critical role in mediating immune responses against
microbial infections via the recognition of microbial components,
including bacterial lipopolysaccharide, lipoproteins, peptidoglycan
and single-stranded and double-stranded viral RNAs (13,14). Induction of TLR signaling pathways
arises from the conserved cytoplasmic Toll/interleukin (IL)-1
receptor (TIR) domains of vertebrate TLRs (15). The myeloid differentiation primary
response gene 88 (MyD88) contains the TIR domain at the C-terminal.
MyD88 was identified as an important adapter for all TLRs,
recruiting IL-1 receptor-associated kinase to TLRs, activating
downstream effector proteins to stimulate the secretion of
inflammatory cytokines (16,17). Two other TIR domain-containing
molecules, TIR domain-containing Adapter Protein (TIRAP) and
TIR-domain-containing adapter-inducing interferon-β (TRIF), have
been identified to be involved in the TLR-signaling pathways
(18). Specifically, TIRAP
participates in the TLR2- and TLR4-MyD88-dependent signaling
pathway, whereas the adapter TRIF has a critical role in TLR3- and
TLR4-mediated MyD88-dependent signaling (19,20).
Monocytes serve a critical role in mediating
anti-bacterial responses (21),
and an abnormal inflammatory response of monocytes could result in
severe pathologies, such as sepsis (22). In sepsis, monocytes recognize
Gram-negative bacteria (endotoxins) and induce a dysfunctional
immune- inflammatory response via TLR4 (23,24), in which MyD88- and TRIF-dependent
pathways are involved (25). The
expression of pro-inflammatory cytokine genes, including IL-6,
C-X-C motif ligand (CXCL)1, chemokine ligand (CCL)3, and tumor
necrosis factor (TNF) via activation of nuclear factor-κB (26,27) can be triggered via the
MyD88-dependent pathway (28). On
the contrary, the expression of type I IFNs (IFN-α and IFN-β) and
IFN-inducible chemokines, such as CXCL10, CCL5 and CCL2 (19) can be induced by the TRIF-dependent
pathway. TRIF also regulates the differentiation of monocytes to
dendritic cells and their mobilization to the lymph nodes (29). A previous study reported that VD
regulates the TLR4 pathway in colon cancer, induced by inflammatory
stimuli (30). Sadeghi et
al (31) demonstrated a
dose-dependent decrease in TLR2 and TLR4 mRNA and protein
expression in VD-treated human monocytes. We hypothesized a strong
association between VD and TLR signaling, particularly via TLR4, in
sepsis.
In the present study, cell viability, inflammatory
cytokine production, and the expression of TLR4-signaling
components were determined in a lipopolysaccharide (LPS)-induced
sepsis model using human newborn and adult monocytes. We further
investigated whether VD has an effect on the sepsis-induced
inflammation response through the TLR4 signaling pathway. Our
findings may improve understanding of the action of VD in
anti-inflammatory responses in bacterial pathogen infections.
Materials and methods
Monocytes isolation from newborns and
healthy adults
Peripheral blood was collected from 20 healthy
newborns (12 male and 8 female) and 20 healthy adult volunteers (10
male and 10 female, 46.7±21.3 years old) from March 2017 to January
2018 according to a protocol approved by the Ethics Committee of
the Ruian People's Hospital. Prior to peripheral blood collection,
written informed consent was provided by the pregnant females and
volunteers. None of the subjects had a history of intra-amniotic
infection or any other infection. Blood collected from left arm
underwent anti-coagulation by the addition of heparin (10 U/ml).
Monocytes in the form of peripheral blood mononuclear cells (PBMCs)
were extracted from peripheral blood, using Ficoll Hypaque (GE
Healthcare) density gradient centrifugation according to the
manufacturer's protocols. The isolated cells were cultured in
RPMI-1640 medium (HyClone; GE Healthcare Life Sciences)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin (Beyotime
Institute of Biotechnology). Subsequently, the cells were
maintained in 24-well plates at 37°C.
Establishment of a sepsis cell model
LPS from E. coli, Serotype R 1515 (Re;
liquid) was purchased from Alexis Biochemicals and used at a
concentration of 10 ng/ml. For the induction of the sepsis cell
model, PBMCs (5×106 cells/ml) were seeded onto 6-well
plates and stimulated without or with LPS of different
concentrations (10, 20, 40 and 80 ng/ml) at indicated time points
(24, 48 and 72 h), 37°C, and were classified into four groups:
Newborn control (NC), newborn sepsis (NS), adult control (AC) and
adult sepsis (AS). PBS was used as a control. The cell viability
assay on diverse of concentrations of LPS was performed to
determine the cytotoxic effects.
Treatment of monocytes with VD
VD (Nature's Bounty) was dissolved and diluted in
RPMI-1640 medium to final concentrations of 10−7,
10−9 and 10−11 M. Then, the monocytes were
pre-treated with or without VD (10−7, 10−9 or
10−11 M) for 4 h, stimulated with or without LPS (10
ng/ml) for 48 h at 37°C, and incubated in a humidified 5%
CO2. environment at 37°C.
Suppressing TLR4 expression in
monocytes
Monocytes from the four groups (NC, NS, AC and AS)
were pre-incubated with 1 µg/ml anti-TLR4 (ab22048) or IgG2b
(ab91366, both Abcam) for 1 h and stimulated with VD
(10−9 M) for 48 h at 37°C to give a total of 16 groups,
which were as follows: NC + IgG, NC + TLR4, NS + IgG, NS + TLR4, AC
+ IgG, AC + TLR4, AS + IgG, AS + TLR4, NC + IgG + VD, NC + TLR4 +
VD, NS + IgG + VD, NS + TLR4 + VD, AC + IgG + VD, AC + TLR4 + VD,
AS + IgG + VD and AS + TLR4 + VD. Comparisons were performed
between the groups with TLR4 and without TLR4.
Plasmid transfection
Small interfering RNA (si)MyD88 and siTRIF, and a
negative control (siCon) were chemically synthesized by Shanghai
GenePharma Co., Ltd. (Shanghai, China). The sequences were as
follows: siNC, 5′-GCG ACG AUC UGC CUA AGA UTT-3′; siMyD88, 5′-GCC
UGU CUC UGU UCU UGA ATT-3′ and siTRIF, 5′-GCC AGC AAC UUG GAA AUC
ATT-3′. To determine the function of MyD88 and TRIF in
vitro, monocytes from the four groups (NC, NS, AC, and AS) were
transfected with 10 nM siMyD88, siTRIF and siCon using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions and
stimulated with VD (10−9 M) for 48 h at 37°C. For MyD88
knockdown, the monocytes were further classified into 16 groups as
follows: NC + siCon, NC + siMyD88, NS + siCon, NS + siMyD88, AC +
siCon, AC + siMyD88, AS + siCon, AS + siMyD88, NC + siCon + VD, NC
+ siMyD88 + VD, NS + siCon + VD, NS + siMyD88 + VD, AC + siCon +
VD, AC + siMyD88 + VD, AS + siCon + VD and AS + siMyD88 + VD.
Correspondingly, a total of 16 groups, including NC + siCon, NC +
siTRIF, NS + siCon, NS + siTRIF, AC + siCon, AC + siTRIF, AS +
siCon, AS + siTRIF, NC + siCon + VD, NC + siTRIF + VD, NS + siCon +
VD, NS + siTRIF + VD, AC + siCon + VD, AC + siTRIF + VD, AS + siCon
+ VD and AS + siTRIF + VD were used to investigate the function of
TRIF in the sepsis cell model. Comparisons were performed between
the groups transfected with siCon and siMyD88 or siTRIF.
Cell Counting Kit-8 (CCK-8) assay
Three independent experiments were performed to
assess the cytotoxic effects of LPS on monocytes from the AC and NC
groups using a CCK-8 assay (Dojindo Molecular Technologies, Inc.)
according to the manufacturer's protocols. Briefly, the monocytes
were seeded onto a 96-well plate at a density of 3,000 cells per
well and incubated for 24 h at 37°C. The monocytes were then
treated as indicated. After incubation with 24, 48 and 72 h, the
CCK-8 reagent (10 µl) was added to each well and 4 h later,
each sample was measured at 450 nm using a BioTek microplate
reader.
Trypan blue assay
The viability of the different monocyte groups was
determined by trypan blue staining. Briefly, the monocytes were
seeded onto each well of the 24-well culture dish. When they
reached 60-70% confluence, trypsin and an equal volume of trypan
blue (0.2%) were added. The cell viability was observed after
incubation for 1-2 min at room temperature with trypan blue using
light microscopy. There are four 1 × 1 mm squares of one chamber
and the average number of cells per square (all hemocytometers
consist of two chambers, each is divided into nine 1 mm2
squares) is determined. For an accurate determination, cells in 4
corner squares plus the central big square (20-50 cells/square)
were analyzed. If the cell density is higher than 200 cells/square,
cell suspension should be further diluted.
Cytokine analysis
To confirm effective cell stimulation by the various
treatments, the levels of key pro-inflammatory cytokines, TNF-α and
IL-6, and the anti-inflammatory cytokine IL-10, were determined in
the supernatants from monocytes using ELISA. Briefly, the levels of
IL-6, IL-10, and TNF-α in the cell culture supernatants collected
after 24, 48 and 72 h incubation were determined using commercial
ELISA kits (cat. nos. GR106328-2, GR106292-2 and GR106397-2,
respectively, Genorise Scientific Inc.) according to the
manufacturer's instructions.
Reverse transcription-quantitative PCR
(RT-qPCR)
Monocytes were collected, and cellular RNAs from the
different cell groups were extracted using TRIzol®
reagent (Thermo Fisher Scientific, Inc.) and then
reverse-transcribed into cDNA using PrimeScript™ RT reagent Kit
with gDNA Eraser (Takara Bio, Inc.) according to manufacturer's
instructions. qPCR was performed using an ABI 7300 RT-PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). After
determining RNA concentration, in vitro RT was performed in
a 20 µl with a system including 4 µl 25 mM
MgCl2, 2 µl Reverse Transcription 10X Buffer, 2
µl 10 mM dNTP Mixture, 0.5 µl Ribonuclease Inhibitor,
15 U AMV Reverse Transcriptase, 0.5 µg random Primers, 1
µg total RNA and Nuclease-Free Water to a final volume of 20
µl under 42°C for 15 min and 85°C denaturation (Promega
Corporation). qPCR was then performed using SYBR Premix Ex Taq GC
kit (Takara Bio, Inc.) with 7.5 µl 2X premix, 10 mM forward
and reverse primers and dH2O to a final volume of 15
µl under the following conditions: 94°C denaturation for 30
sec, followed by 40 cycles each containing 94°C denaturation for 5
sec, and 60°C annealing for 30 sec, final extension of 72°C for 2
min with a LightCycler 480 instrument (Roche Diagnostics). The
primer sequences were used as follows: VDR, forward 5′-CCC TTG ACC
TCT TCC CGC TGGT T-3′, reverse, 5′-TCA CTG ACG CGG TAC TTG TAG TCT
TGG TTG-3′; TLR4, forward 5′-AGA CCT GTC CCT GAA CCC TAT-3′,
reverse, 5′-CGA TGG ACT TCT AAA CCA GCC A-3′; MyD88, forward 5′-GGC
TGC TCT CAA CAT GCG A-3′, reverse, 5′-CTG TGT CCG CAC GTT CAA
GA-3′; TRIF, forward 5′-CCT GGA ATC ATC ATC GGA ACA G-3′, reverse
5′-TGA GTG GTC TAT GGC GT C CT-3′; and GAPDH, forward 5′-TGT TCG
TCA TGG GTG TGA AC-3′, reverse, 5′-ATG GCA TGG ACT GTG GTC AT-3′.
All the samples were analyzed in triplicates, and the
2−ΔΔCq method was used to calculate the gene expression
levels (32).
Western blot analysis
For western blot analysis, protein (30-50 µg)
extracts were obtained by lysing the monocytes in a buffer as
previously reported (33). After
protein quantitation using a BCA protein assay, protein extracts
were separated by 10% SDS-PAGE and transferred onto a
polyvinylidene difluoride membrane. The membrane was blocked with
10% skim milk at room temperature for 1 h, probed with primary
antibodies against TLR4 (sc-293072, 1:1,000), MyD88 (sc-74532,
1:1,000), and TRIF (sc-514384, 1:1,000) from Santa Cruz
Biotechnology, Inc., and GAPDH (cat. no. 14C10, 1:1,000, Cell
Signaling Technology, Inc.) overnight at 4°C. Then, the membrane
was incubated with a horseradish peroxidase-conjugated secondary
antibody (PI31430, 1:10,000; Pierce; Thermo Fisher Scientific,
Inc.) at room temperature for 1 h. The signals were visualized
using enhanced chemiluminescence detection (GE Healthcare). Band
densities were quantified with ImageJ 1.43r (National Institutes of
Health).
Statistical analysis
Statistical analysis was conducted using GraphPad
Prism software version 6 (GraphPad Software, Inc.). The results are
expressed as the mean ± standard deviation of at least three
independent experiments. The differences between groups were
assessed using one-way analysis for variance followed by a Tukey's
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Establishment and evaluation of the
sepsis cell model
To construct a sepsis cell model, the monocytes from
newborns and healthy adults were stimulated with LPS or control
(PBS) for 24, 48 or 72 h. After treatment, no significant
difference in the expression of VD receptor (VDR) mRNA expression
was detected among the groups (Fig.
1A). Subsequently, different concentrations of LPS 10, 20, 40
and 80 ng/ml were tested. The cell viability assay indicated that
40 and 80 ng/ml LPS exhibited significant cytotoxic effects in the
NS and AS groups compared with the respective controls (Fig. 1B). We thus used LPS (10 ng/ml) to
induce a sepsis cell model. The viability of monocytes from the NC,
NS, AC and AS groups was determined at 24, 48 or 72 h after
treatment by a CCK-8 assay and trypan blue assay, respectively. As
presented in Fig. 1C, the CCK-8
assay indicated that the viability of monocytes was significantly
decreased in the NS and AS groups after 72 h of treatment compared
with the corresponding controls. In the trypan blue assay, monocyte
viability was significantly decreased in the NS and AS groups
compared with the corresponding controls (Fig. 1D and E).
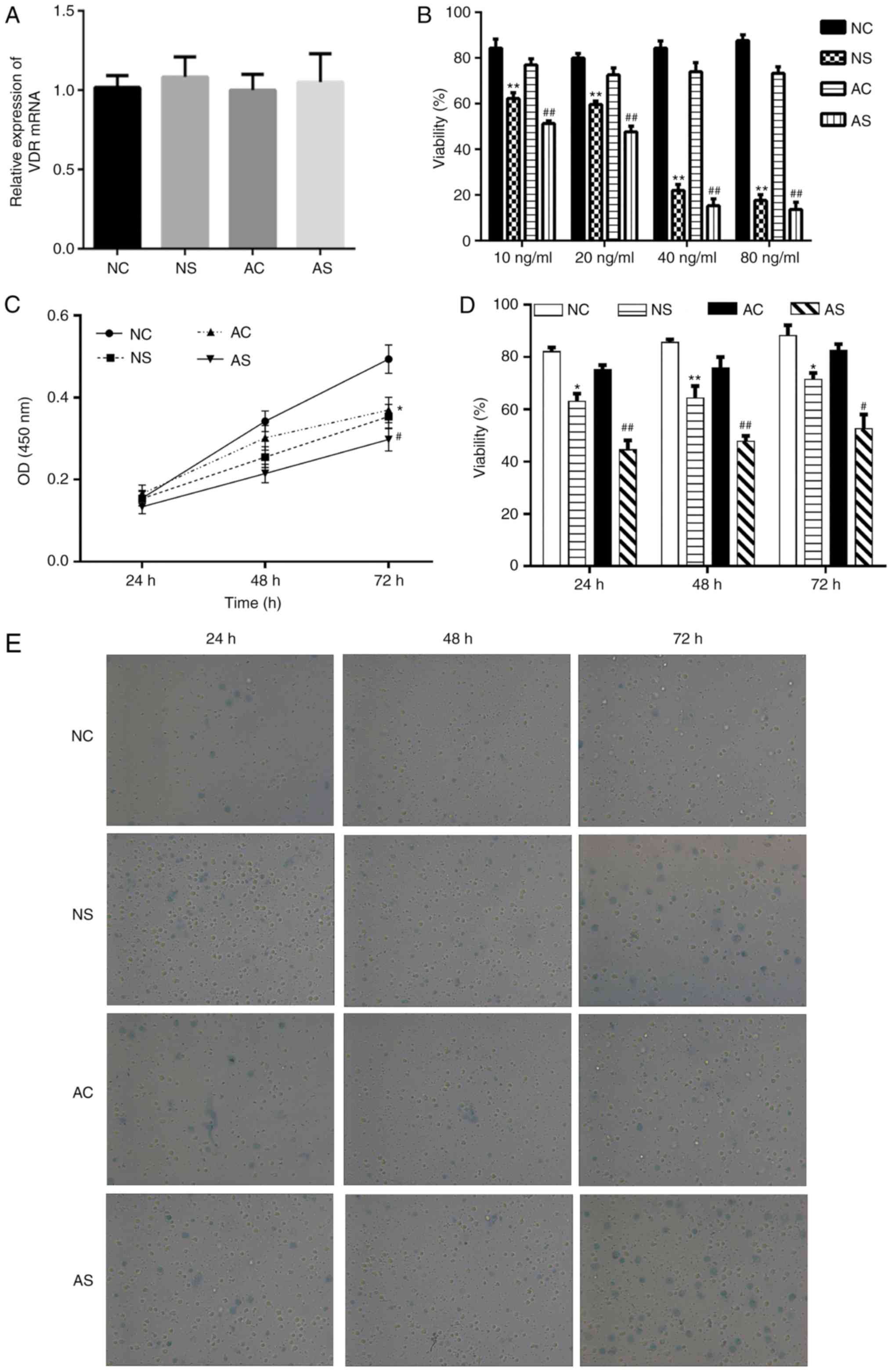 | Figure 1Effects of LPS on cell viability in
monocytes from newborns and healthy adults. (A) The expression of
VDR after treatment with LPS. (B) Percentage of trypan blue
staining-negative cells in total cells (viability %) upon treatment
with different concentrations of LPS (10, 20, 40 and 80 ng/ml). (C)
Monocytes of newborns and healthy adults were stimulated with or
without LPS (10 ng/ml) at 24, 48 and 72 h, respectively. OD at 450
nm revealed the cell viability as determined by a Cell Counting
Kit-8. (D and E) Percentage of trypan blue staining negative cells
in total cells (viability %) and representative images
(magnification, ×200). *P<0.05,
**P<0.01, NS vs. NC; #P<0.05,
##P<0.01, AS vs. AC. AC, adult control; AS, adult
sepsis; NC: Newborn control; NS, newborn sepsis; LPS,
lipopolysaccharide; OD, optical density; VDR, vitamin D
receptor. |
Subsequently, the effects of LPS on the release of
cytokines were determined. As shown in Fig. 2A and E, IL-6 and TNF-α production
in monocytes from newborns and healthy adults with sepsis was
significantly increased with LPS treatment compared with the
respective controls. Anti-inflammatory cytokine IL-10 expression
was increased with LPS treatment for 48 h in the NS and AS groups
compared with the controls; a significantly higher IL-10 level was
observed in the NS group compared with NC group at 72 h. However,
no significant changes were observed after 72 h of LPS stimulation
in monocytes from healthy adults. We also investigated whether LPS
stimulation regulated TLR signaling. As shown in Fig. 2B, the expression levels of TLR4,
MyD88 and TRIF mRNA in monocytes from the NS and AS groups were
significantly increased when compared with the NC or AC groups.
These results were further confirmed by western blotting (Fig. 2C and D). Therefore, LPS
stimulation for 48 h was used in subsequent experiments.
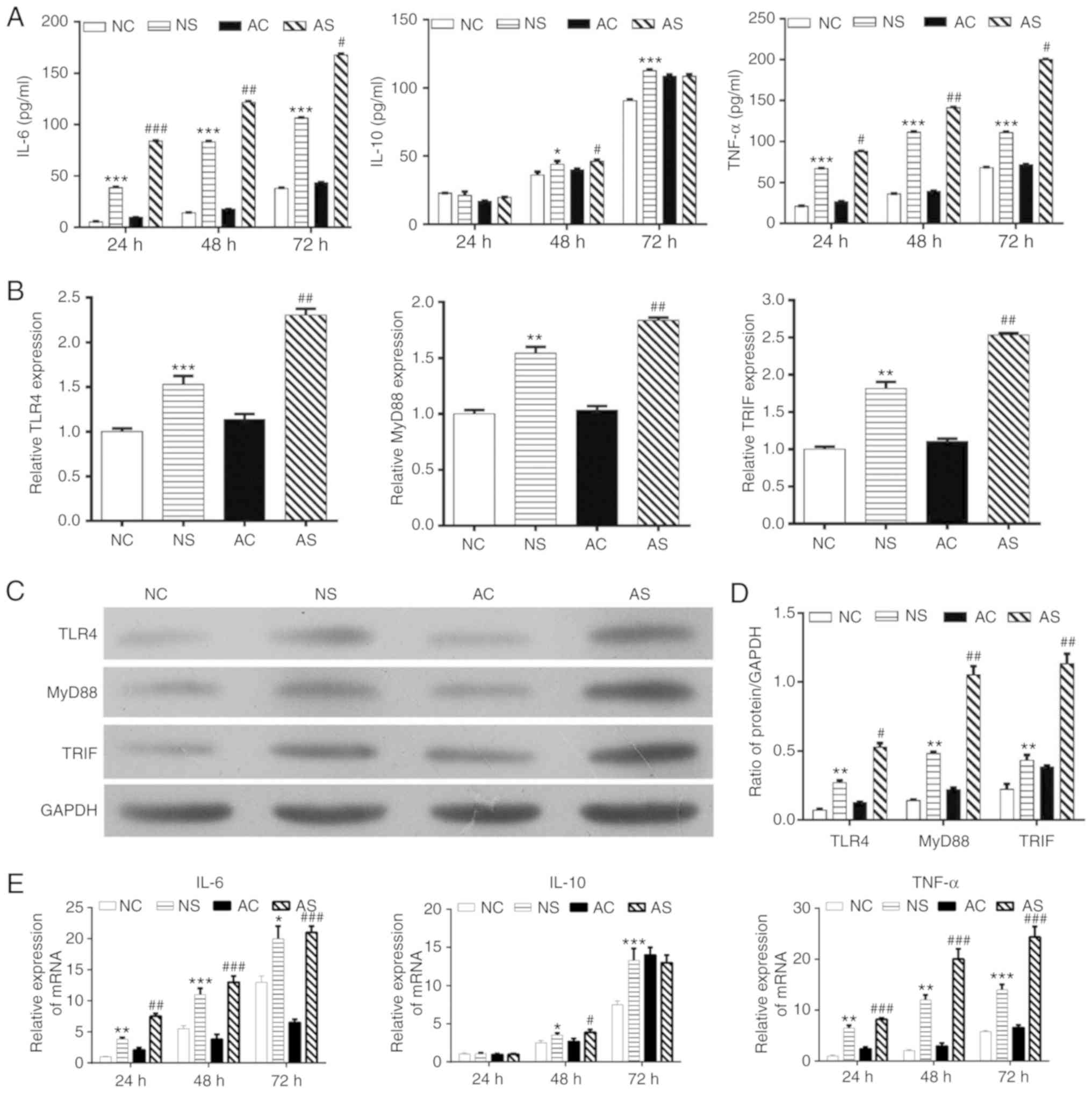 | Figure 2Effects of LPS stimulation on
inflammation-related cytokines. (A) Levels of TNF-α, IL-6 and IL-10
in the culture supernatants from monocytes in the NC, NS, AC, and
AS groups at 24, 48 and 72 h by ELISA. (B) mRNA expression of TLR4,
MyD88 and TRIF evaluated by RT-qPCR. (C) Representative blot of
TLR4, MyD88 and TRIF from western blotting. (D) Densitometry
analysis of TLR4, MyD88 and TRIF expression; monocytes of newborns
and healthy adults were stimulated with or without LPS for 48 h.
(E) mRNA expression levels of IL-6, IL-10 and TNF-α in the culture
supernatants from monocytes in the NC, NS, AC and AS groups at 24,
48 and 72 h by RT-qPCR. *P<0.05,
**P<0.01, ***P<0.001, NS vs. NC;
#P<0.05, ##P<0.01,
###P<0.001, AS vs. AC. AC, adult control; AS, adult
sepsis; NC, newborn control; NS, newborn sepsis; IL, interleukin;
LPS, lipopolysaccharide; MyD88, myeloid differentiation primary
response gene 88; RT-qPCR, reverse transcription-quantitative PCR;
TLR4, Toll-like receptor 4; TNF-α, tumor necrosis factor-α; TRIF,
Toll-IL-1 resistance-domain-containing adapter-inducing
interferon-β. |
VD attenuates LPS-induced
pro-inflammatory cytokines release in monocytes
VD has been reported to have anti-inflammatory and
immune regulatory functions (34,35). We investigated whether VD could
attenuate LPS-induced cytokine release and TLR4 signaling in
monocytes from newborns and healthy adults. Monocytes were treated
with VD (10−7, 10−9 or 10−11 M)
and LPS for 48 h. As presented in Fig. 3A and C, the expression of IL-6,
IL-10 and TNF-α decreased in the NC, NS, AC and AS groups in a VD
concentration-dependent manner. RT-qPCR indicated that the
expression of TLR4, MyD88 and TRIF decreased following VD treatment
in the four monocyte groups (Fig.
3B).
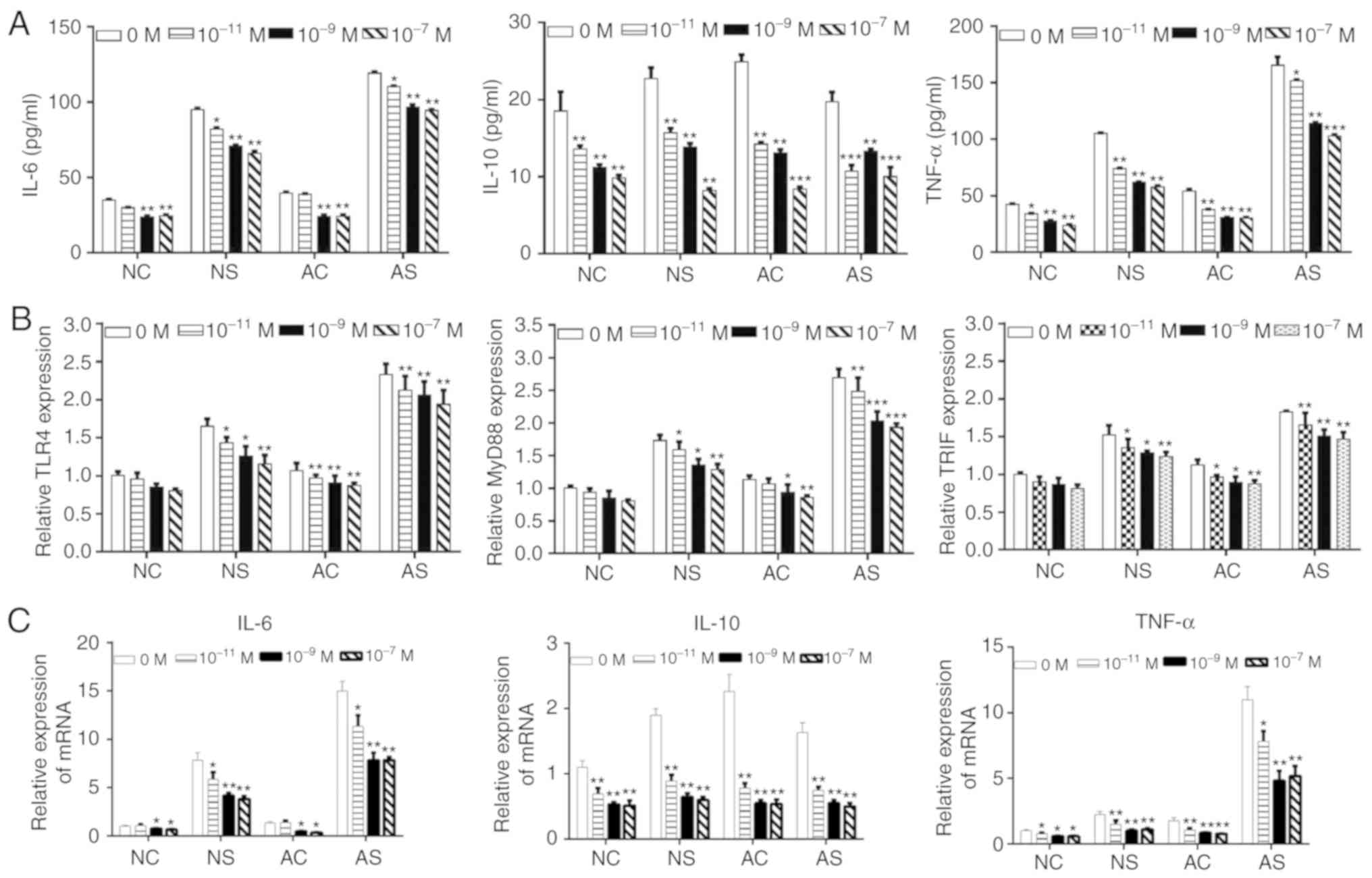 | Figure 3Effects of VD on IL-6, IL-10 and
TNF-α release, and TLR signaling in lipopolysaccharide-stimulated
monocytes from newborns and healthy adults. (A) Levels of IL-6,
IL-10 and TNF-α in the NC, NS, AC and AS groups after treatment
with VD at concentrations of 10−7, 10−9 and
10−11 M by ELISA. (B) TLR4, MyD88 and TRIF mRNA
expression in the NC, NS, AC and AS groups after treatment with VD
at concentrations of 10−7, 10−9 and
10−11 M. (C) mRNA expression levels of IL-6, IL-10 and
TNF-α in the NC, NS, AC and AS groups after treatment with VD at
concentrations of 10−7, 10−9 and
10−11 M by reverse transcription-quantitative PCR.
*P<0.05, **P<0.01,
***P<0.001 vs. 0 M VD. AC, adult control; AS, adult
sepsis; NC, newborn control; NS, newborn sepsis; VD, 1,25-dihydroxy
vitamin D3; IL, interleukin; MyD88, myeloid differentiation primary
response gene 88; TLR4, Toll-like receptor 4; TNF-α, tumor necrosis
factor-α; TRIF, Toll-IL-1 resistance-domain-containing
adapter-inducing interferon-β. |
Anti-inflammatory effects of VD in
LPS-stimulated monocytes is mediated by TLR4 signaling
To further investigate whether the anti-inflammatory
effects of VD in monocytes occur in a TLR4-dependent manner,
anti-TLR4 antibody was used to inhibit TLR4 signaling. An IgG
antibody was used as isotype control. In the NC, NS, AC and AS
groups, the cells were treated with IgG or anti-TLR4, IgG + VD or
anti-TLR4 + VD. The levels of IL-6, IL-10 and TNF-α were evaluated
by ELISA. As shown in Fig. 4A and
C, blockade of TLR4 significantly downregulated the levels of
IL-6 and TNF-α but not IL-10. Furthermore, inhibition of TLR4
significantly decreased the levels of MyD88 and TRIF mRNA compared
with the IgG group, as detected by RT-qPCR (Fig. 4B).
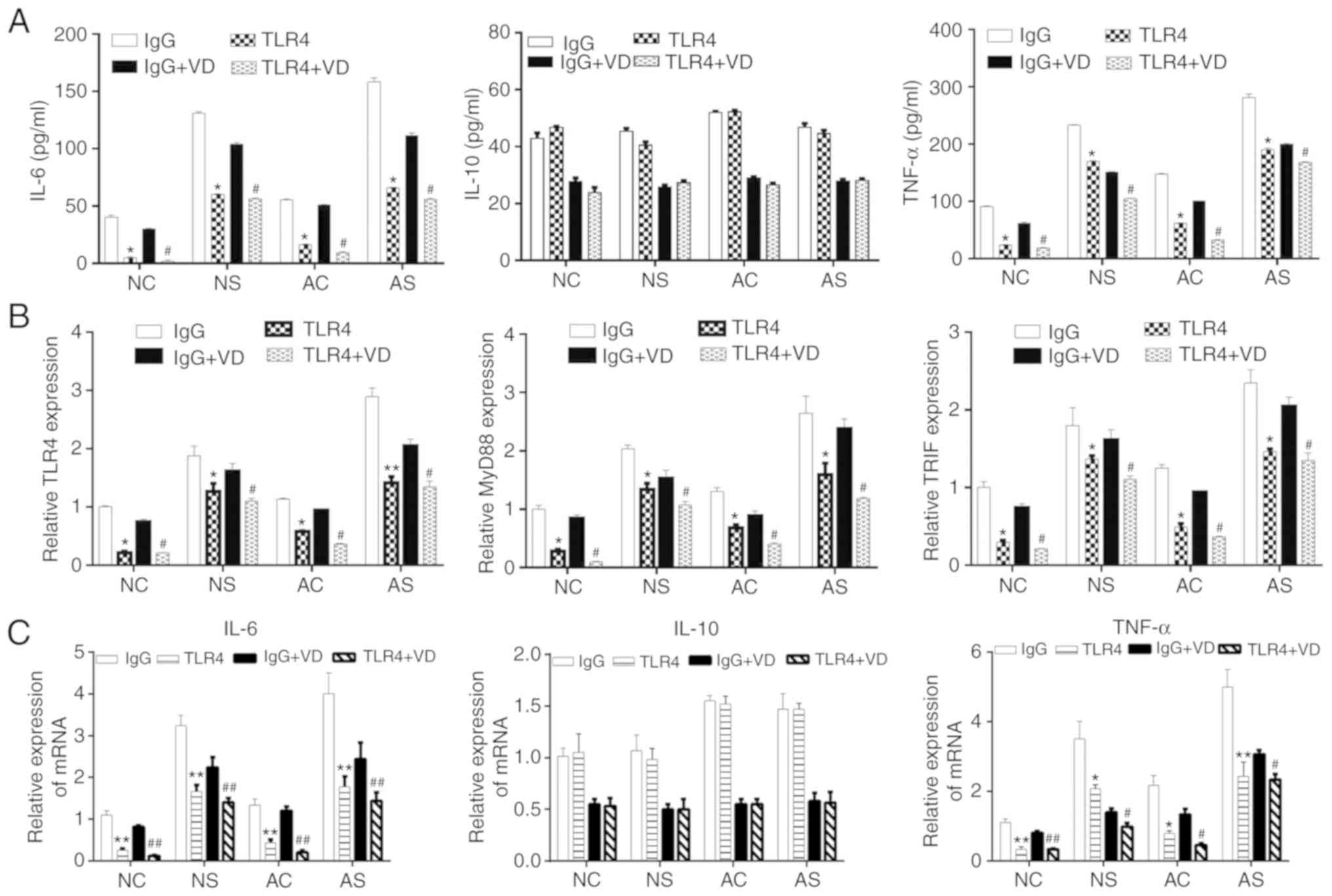 | Figure 4Effects of blocking TLR4 on the
protein levels of TNF-α, IL-6, IL-10, and mRNA levels of TLR4,
MyD88 and TRIF in monocytes stimulated with lipopolysaccharide. (A)
Levels of IL-6, IL-10 and TNF-α in the NC, NS, AC, and AS groups.
The release of inflammation-related cytokines TNF-α, IL-6, and
IL-10 was measured in the culture supernatants from monocytes in
four groups (NC, NS, AC and AS) after treatment with VD at
concentration of 10−9 M by ELISA. (B) mRNA levels of
TLR4, MyD88 and TRIF in the four groups (NC, NS, AC and AS) after
treatment with VD at concentration of 10−9. (C) Levels
of IL-6, IL-10 and TNF-α mRNA in the culture supernatants from
monocytes in the four groups (NC, NS, AC and AS) after treatment
with VD at a concentration of 10−9 M by reverse
transcription-quantitative PCR. *P<0.05,
**P<0.01, IgG vs. TLR4; #P<0.05,
##P<0.01, IgG + VD vs. TLR4 + VD. AC, adult control;
AS, adult sepsis; NC, newborn control; NS, newborn sepsis; VD,
1,25-dihydroxy vitamin D3; IL, interleukin; MyD88, myeloid
differentiation primary response gene 88; TLR4, Toll-like receptor
4; TNF-α, tumor necrosis factor-α; TRIF, Toll-IL-1
resistance-domain-containing adapter-inducing interferon-β. |
We also used si-RNA targeting MyD88 (siMyD88) and
TRIF (siTRIF) to further confirm our findings (Fig. S1). As shown in Figs. 5A and C, and 6A, downregulation of MyD88 or TRIF
significantly suppressed IL-6 and TNF-α production in the siMyD88
or siTRIF, and siMyD88 + VD or siTRIF + VD groups compared with the
siCon and siCon + VD groups, respectively. On the contrary, no
significant difference was observed in IL-10 levels in these
groups. siMyD88 and siTRIF treatment significantly decreased the
expression of TLR4, MyD88 and TRIF (Figs. 5B and 6B) in monocytes from siMyD88 or siTRIF,
and siMyD88 + VD or siTRIF + VD groups compared with the siCon and
siCon + VD groups, respectively.
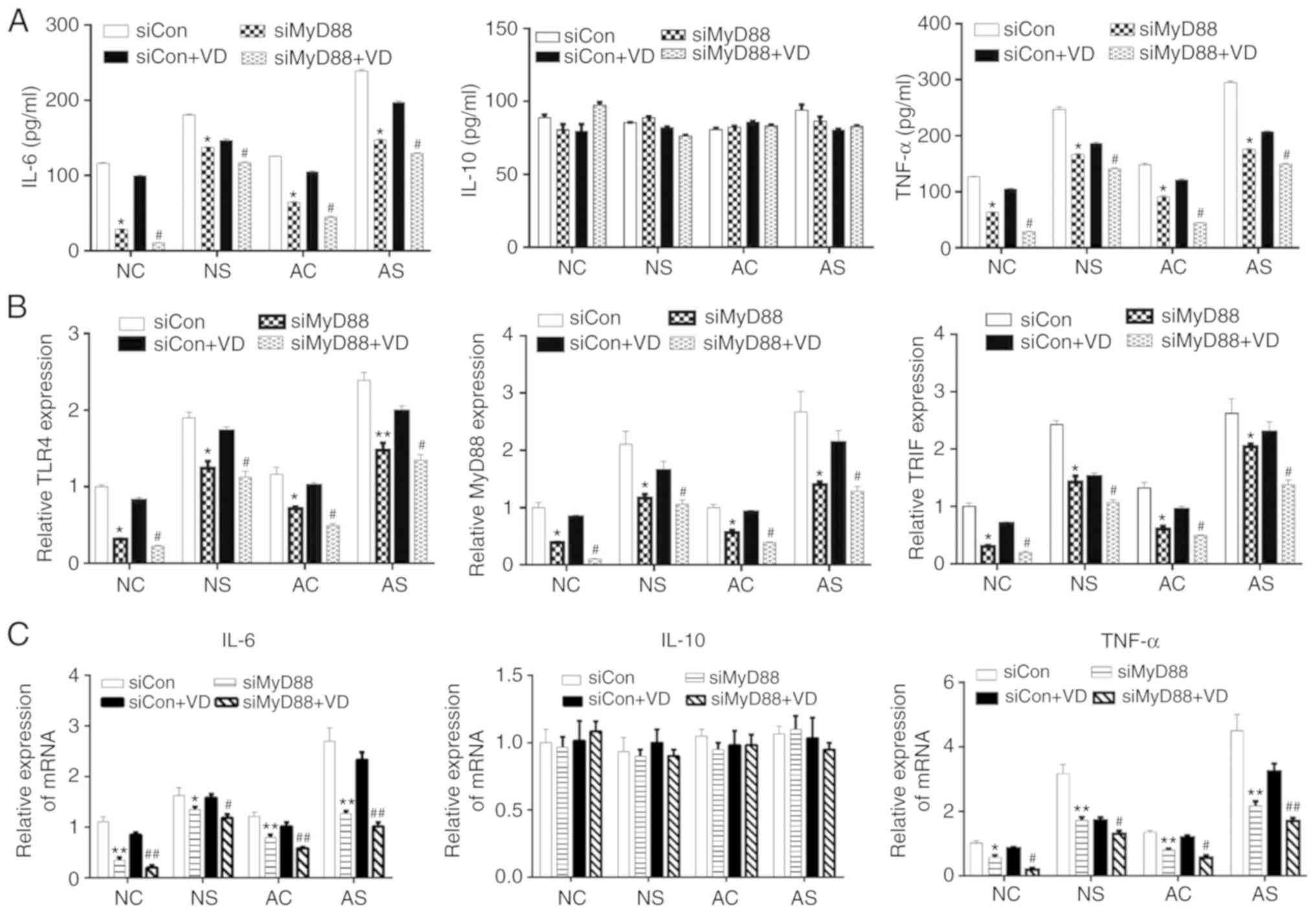 | Figure 5Effects of MyD88 downregulation on
the expression of protein levels. TNF-α, IL-6, IL-10, and mRNA
levels of TLR4, TLR4 and TRIF. (A) The release of IL-6, IL-10 and
TNF-α was measured by ELISA. (B) mRNA expression levels of TLR4,
MyD88 and TRIF. (C) Levels of IL-6, IL-10 and TNF-α mRNA detected
by reverse transcription-quantitative PCR. *P<0.05,
**P<0.01, siCon vs. siMyD88; #P<0.05,
##P<0.01, siCon + VD vs. siMyD88 + VD. AC, adult
control; AS, adult sepsis; NC, newborn control; NS, newborn sepsis;
VD, 1,25-dihydroxy vitamin D3; Con, control; IL, interleukin;
MyD88, myeloid differentiation primary response gene 88; si, small
interfering RNA; TLR4, Toll-like receptor 4; TNF-α, tumor necrosis
factor-α; TRIF, Toll-IL-1 resistance-domain-containing
adapter-inducing interferon-β. |
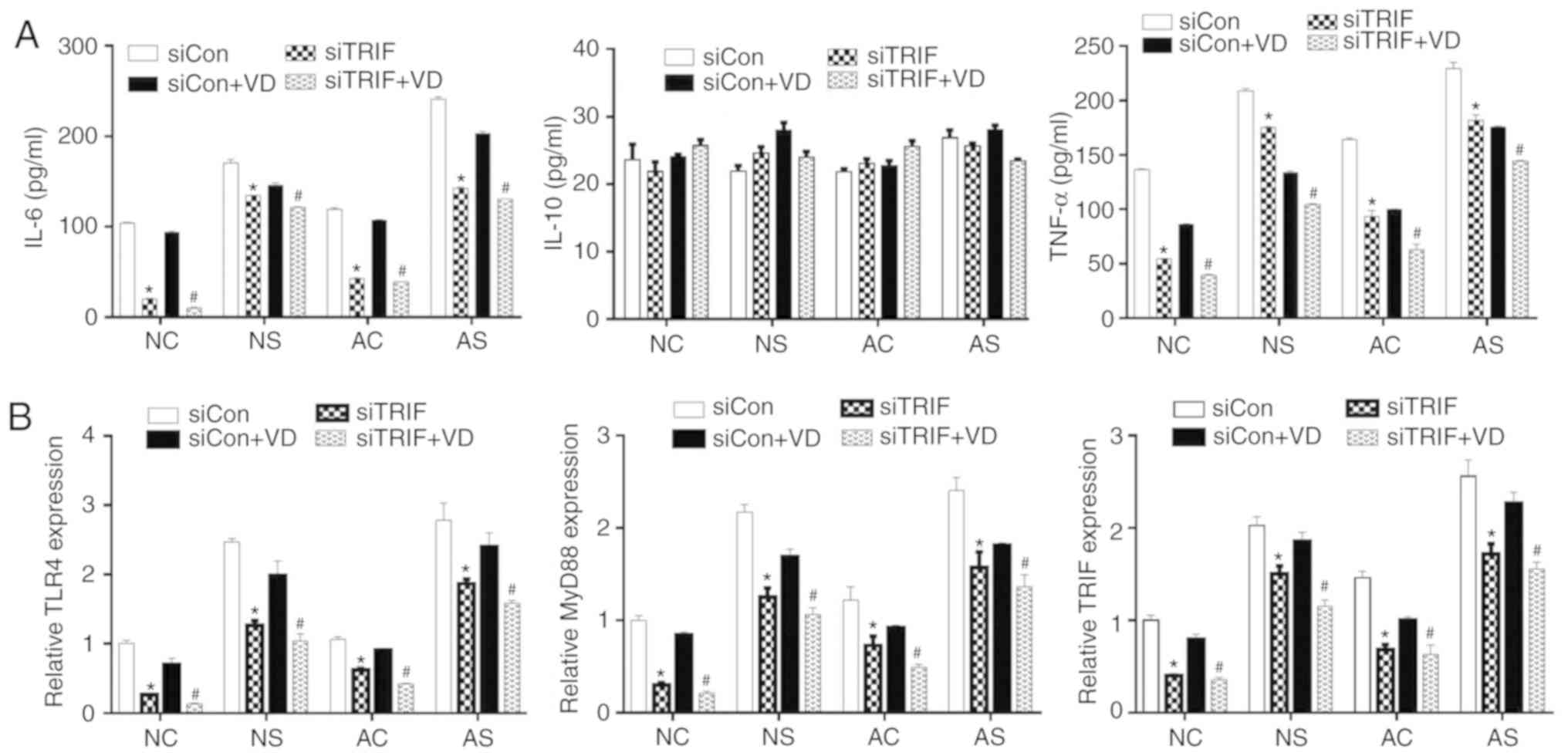 | Figure 6Effects of TRIF downregulation on the
expression of TNF-α, IL-6, IL-10, TLR4 and MyD88. (A) The release
of IL-6, IL-10 and TNF-α was measured by ELISA. (B) mRNA expression
levels of TLR4, MyD88 and TRIF as determined by reverse
transcription-quantitative PCR. *P<0.05, siCon vs.
siTRIF; #P<0.05, siCon + VD vs. siTRIF + VD. AC,
adult control; AS, adult sepsis; NC, newborn control; NS, newborn
sepsis; VD, 1,25-dihydroxy vitamin D3; Con, control; IL,
interleukin; MyD88, myeloid differentiation primary response gene
88; si, small interfering RNA; TLR4, Toll-like receptor 4; TNF-α,
tumor necrosis factor-α; TRIF, Toll-IL-1
resistance-domain-containing adapter-inducing interferon-β. |
Discussion
The results of the present study revealed that LPS
stimulation significantly attenuated cell viability, increased the
production of pro-inflammatory cytokines (IL-6 and TNF-α), and
promoted the expression of TLR4, MyD88 and TRIF. VD was determined
to inhibit pro-inflammatory responses in monocytes induced by LPS
stimulation involving the TLR4/MyD88-dependent and TRIF-dependent
signaling pathways. Deficiency of TLR4, MyD88 or TRIF impaired the
expression of the remaining two, which was accompanied with low
levels of IL-6 and TNF-α. Thus, a feedback loop may occur, whereby
the secretion of these pro-inflammatory cytokines is regulated.
Cytokines are endogenous inflammatory proteins and
function as mediators in the pathology and physiology of systemic
inflammatory response syndrome (SIRS) caused by sepsis (36). Pro-inflammatory cytokines, such as
TNF-α, IL-6 and IL-β are essential for initiating the inflammatory
process in host defense against infection, whereas overproduction
of these cytokines contributes to multiple organ failure and
mortality due to septic shock (37,38). Chawla et al (39) reported that a high concentration
of IL-6 acts as a risk factor for acute kidney injury in patients
with severe sepsis. TNF-α appears to be a key cytokine in the
pathogenesis of septic shock (40). In addition, anti-inflammatory
factors, including transforming growth factor-β, IL-10 and
prostaglandin E2 may be formed to alleviate persistent inflammation
(41). Monocytes serve a critical
role in the development of SIRS caused by sepsis (21). Activated monocytes in sepsis
induce a dysfunctional immune-inflammatory response in which the
production of anti-inflammatory cytokines, IL-6 and TNF-α are
released (23,24). In the present study, significantly
elevated expression levels of the pro-inflammatory cytokines IL-6
and TNF-α were observed; however, the levels of the
anti-inflammatory cytokine IL-10 were unaffected in monocytes
treated with LPS. As the immune system of newborns is yet to
develop further, we isolated monocytes from both newborns and
healthy adults. Although a similar trend was observed compared
between the treatment group and control group, the levels of
inflammatory factors in newborns were generally lower than those in
healthy adults (42). These
findings supported the fact that monocytes serve an important role
in the development of sepsis.
Emerging evidence from clinical studies has revealed
a high prevalence of VD deficiency or insufficiency among patients
with severe infections, such as sepsis (9). A previous study has proposed that VD
had a modulatory effect on the inflammatory response in animal
models of sepsis (43). In the
present study, VD reduced the expression of pro-inflammatory
factors (IL-6 and TNF-α) in a dose-dependent manner, suggesting
that VD exerted an inhibitory effect on inflammatory reactions;
thus, VD may reduce sepsis-induced organ dysfunction and
mortality.
MyD88 was identified as a necessary component for
the activation of all TLR-mediated innate immunity processes, and
TRIF is involved in the TLR4-mediated MyD88-independent signaling
pathway (44). To investigate the
molecular mechanism underlying the suppression of inflammatory
cytokine secretion by VD, we inhibited TLR4 and its downstream
molecules. In this study, it was observed that blocking TLR4 in
newborn and adult monocytes hindered IL-6 and TNF-α production, and
inhibited the expression of MyD88 and TRIF. VD treatment resulted
in a dose-dependent decrease in TLR4 expression and its downstream
molecules, MyD88 and TRIF. Evidence has shown that TLR-4 mRNA was
downregulated after treatment with anti-TLR4 antibody (45). Anti-TLR4 monoclonal antibody
attenuated lung injury caused by mechanical ventilation,
inflammation and edema in rats by inhibiting the TLR4/MyD88
signaling pathway (46).
Consistently, we observed that TLR4 was also decreased after
treatment with the anti-TLR4 antibody, suggesting that the
inhibition of TLR4 interferes with the cascades involving the
TLR4/MyD88 signaling pathway. To the best of our knowledge, our
study is the first to demonstrate for the first time that VD
induced the suppression of IL-6 and TNF-α in sepsis, which is
likely mediated by the inhibition of the TLR4-MyD88-dependent and
TRIF-dependent pathways. Of note, VD did not significantly affect
the production of IL-10 in LPS-stimulated human monocytes.
Feedback mechanisms are necessary to sustain
effective immunity, inflammation and tissue homeostasis (47). Hegyi et al (48) demonstrated a disturbance of AMP in
psoriasis mediated by a pro-inflammatory feedback loop was induced
by the VD analog, calcipotriol. White (49) confirmed that the VD-signaling
pathway can be induced though TLR2 or TLR4 signaling via elevated
VDR and VS 1-α-hydroxylase expression; a positive feedback loop is
formed with the involvement of VD, TLR2 and CD14. Interestingly,
downregulation of MyD88 suppressed the pro-inflammatory response,
and reduced the levels of TLR4 and TRIF. A similar pattern of
results was observed in the cells with TRIF silencing. Thus, we
proposed the hypothesis that the effects of VD on TLR4, MyD88 and
TRIF expression in monocytes constituted a feedback loop in sepsis,
which may be associated with the biosynthesis of pro-inflammatory
cytokines. Our preliminary data suggest the role of VD in the
attenuation of inflammation induced by LPS. However, the effects of
VD in the treatment of sepsis should be further evaluated within
in vivo models, such as LPS induction and cecal ligation
models.
In summary, the present study reported that VD
decreased the release of the pro-inflammatory cytokines IL-6 and
TNF-α by inhibiting the TLR4/MyD88 and TLR4/TRIF signaling pathways
in LPS-stimulated monocytes. Our findings suggest that VD may be
considered as a potential therapeutic agent in the treatment of
sepsis, given its reported anti-inflammatory properties.
Supplementary Data
Acknowledgments
Not applicable.
Funding
This study was supported by Wenzhou Science &
Technology Bureau Project (grant. nos. Y20150128 and Y20180236),
Wenzhou Health Bureau Project (grant. no. 2015B37), and Ruian
Science & Technology Bureau Project (grant. no. MS2017006).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
GZ and ZL conceived and designed the experiments.
GZ, NW, YH, and MP performed the experiments. GZ analyzed the data.
GZ and ZL wrote the paper. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The research protocols were approved by the Ethics
committee of Ruian People's Hospital. Written informed consent was
provided by patients and/the patient's family.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rivers E, Nguyen B, Havstad S, Ressler J,
Muzzin A, Knoblich B, Peterson E and Tomlanovich M; Early
Goal-Directed Therapy Collaborative Group: Early goal-directed
therapy in the treatment of severe sepsis and septic shock. N Engl
J Med. 345:1368–1377. 2001. View Article : Google Scholar
|
|
2
|
Waydhas C, Nast-Kolb D, Jochum M, Trupka
A, Lenk S, Fritz H, Duswald KH and Schweiberer L: Inflammatory
mediators, infection, sepsis, and multiple organ failure after
severe trauma. Arch Surg. 127:460–467. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barber RC, Chang LY, Arnoldo BD, Purdue
GF, Hunt JL, Horton JW and Aragaki CC: Innate immunity SNPs are
associated with risk for severe sepsis after burn injury. Clin Med
Res. 4:250–255. 2006. View Article : Google Scholar
|
|
4
|
Aird WC: The role of the endothelium in
severe sepsis and multiple organ dysfunction syndrome. Blood.
101:3765–3777. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bar-Or D, Carrick MM, Mains CW, Rael LT,
Slone D and Brody EN: Sepsis, oxidative stress, and hypoxia: Are
there clues to better treatment? Redox Rep. 20:193–197. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaukonen KM, Bailey M, Suzuki S, Pilcher D
and Bellomo R: Mortality related to severe sepsis and septic shock
among critically ill patients in Australia and New Zealand,
2000-2012. JAMA. 311:1308–1316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holick MF: Vitamin D deficiency. N Engl J
Med. 357:266–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ginde AA, Camargo CA Jr and Shapiro NI:
Vitamin D insufficiency and sepsis severity in emergency department
patients with suspected infection. Acad Emerg Med. 18:551–554.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jeng L, Yamshchikov AV, Judd SE, Blumberg
HM, Martin GS, Ziegler TR and Tangpricha V: Alterations in vitamin
D status and anti-microbial peptide levels in patients in the
intensive care unit with sepsis. J Transl Med. 7:282009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cecchi A, Bonizzoli M, Douar S, Mangini M,
Paladini S, Gazzini B, Degl'Innocenti S, Linden M, Zagli G and
Peris A: Vitamin D deficiency in septic patients at ICU admission
is not a mortality predictor. Minerva Anestesiol. 77:1184–1189.
2011.PubMed/NCBI
|
|
11
|
Moller S, Laigaard F, Olgaard K and
Hemmingsen C: Effect of 1,25-dihydroxy-vitamin D3 in experimental
sepsis. Int J Med Sci. 4:190–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hewison M, Zehnder D, Chakraverty R and
Adams JS: Vitamin D and barrier function: A novel role for
extra-renal 1 alpha-hydroxylase. Mol Cell Endocrinol. 215:31–38.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsumoto M, Kikkawa S, Kohase M, Miyake K
and Seya T: Establishment of a monoclonal antibody against human
Toll-like receptor 3 that blocks double-stranded RNA-mediated
signaling. Biochem Biophys Res Commun. 293:1364–1369. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawasaki T and Kawai T: Toll-like receptor
signaling pathways. Front Immunol. 5:4612014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akira S and Takeda K: Toll-like receptor
signalling. Nat Rev Immunol. 4:499–511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rathinam VA, Appledorn DM, Hoag KA,
Amalfitano A and Mansfield LS: Campylobacter jejuni-induced
activation of dendritic cells involves cooperative signaling
through Toll-like receptor 4 (TLR4)-MyD88 and TLR4-TRIF axes.
Infect Immun. 77:2499–2507. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Medzhitov R, Preston-Hurlburt P, Kopp E,
Stadlen A, Chen C, Ghosh S and Janeway CA Jr: MyD88 is an adaptor
protein in the hToll/IL-1 receptor family signaling pathways. Mol
Cell. 2:253–258. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamamoto M and Akira S: TIR
domain-containing adaptors regulate TLR-mediated signaling
pathways. Nihon Rinsho. 62:2197–2203. 2004.In Japanese. PubMed/NCBI
|
|
19
|
Yamamoto M, Sato S, Hemmi H, Hoshino K,
Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K and
Akira S: Role of adaptor TRIF in the MyD88-independent toll-like
receptor signaling pathway. Science. 301:640–643. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Horng T, Barton GM and Medzhitov R: TIRAP:
An adapter molecule in the Toll signaling pathway. Nat Immunol.
2:835–841. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shalova IN, Kajiji T, Lim JY, Gómez-Piña
V, Fernández-Ruíz I, Arnalich F, Iau PT, López-Collazo E, Wong SC
and Biswas SK: CD16 regulates TRIF-dependent TLR4 response in human
monocytes and their subsets. J Immunol. 188:3584–3593. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Castanheira FVES, de Lima KA, Cebinelli
GCM, Sônego F, Kanashiro A, Colon DF, Borges V, Czaikoski PG, Mota
JM, Cunha TM, et al: CCR5-Positive inflammatory monocytes are
crucial for control of sepsis. Shock Dec. 7:2018.Epub ahead of
print.
|
|
23
|
Medzhitov R, Preston-Hurlburt P and
Janeway CA Jr: A human homologue of the Drosophila Toll protein
signals activation of adaptive immunity. Nature. 388:394–397. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Akira S: Innate immunity to pathogens:
Diversity in receptors for microbial recognition. Immunol Rev.
227:5–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rosadini CV, Zanoni I, Odendall C, Green
ER, Paczosa MK, Philip NH, Brodsky IE, Mecsas J and Kagan JC: A
single bacterial immune evasion strategy dismantles both MyD88 and
TRIF signaling pathways downstream of TLR4. Cell Host Microbe.
18:682–693. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bjorkbacka H, Fitzgerald KA, Huet F, Li X,
Gregory JA, Lee MA, Ordija CM, Dowley NE, Golenbock DT and Freeman
MW: The induction of macrophage gene expression by LPS
predominantly utilizes Myd88-independent signaling cascades.
Physiol Genomics. 19:319–330. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kawai T, Adachi O, Ogawa T, Takeda K and
Akira S: Unresponsiveness of MyD88-deficient mice to endotoxin.
Immunity. 11:115–122. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Diomede F, Zingariello M, Cavalcanti MFXB,
Merciaro I, Pizzicannella J, De Isla N, Caputi S, Ballerini P and
Trubiani O: MyD88/ERK/NFkB pathways and pro-inflammatory cytokines
release in periodontal ligament stem cells stimulated by
Porphyromonas gingivalis. Eur J Histochem. 61:27912017.
|
|
29
|
Cheong C, Matos I, Choi JH, Dandamudi DB,
Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G,
et al: Microbial stimulation fully differentiates monocytes to
DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell.
143:416–429. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Murillo G, Nagpal V, Tiwari N, Benya RV
and Mehta RG: Actions of vitamin D are mediated by the TLR4 pathway
in inflammation-induced colon cancer. J Steroid Biochem Mol Biol.
121:403–407. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sadeghi K, Wessner B, Laggner U, Ploder M,
Tamandl D, Friedl J, Zügel U, Steinmeyer A, Pollak A, Roth E, et
al: Vitamin D3 down-regulates monocyte TLR expression and triggers
hypo-responsiveness to pathogen-associated molecular patterns. Eur
J Immunol. 36:361–370. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Giannoni E, Guignard L, Knaup Reymond M,
Perreau M, Roth-Kleiner M, Calandra T and Roger T: Estradiol and
progesterone strongly inhibit the innate immune response of
mononuclear cells in newborns. Infect Immun. 79:2690–2698. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Manion M, Hullsiek KH, Wilson EMP, Rhame
F, Kojic E, Gibson D, Hammer J, Patel P, Brooks JT, Baker JV, et
al: Vitamin D deficiency is associated with IL-6 levels and
monocyte activation in HIV-infected persons. PLoS One.
12:e01755172017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wei R and Christakos S: Mechanisms
underlying the regulation of innate and adaptive immunity by
vitamin D. Nutrients. 7:8251–8260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gogos CA, Drosou E, Bassaris HP and
Skoutelis A: Pro- versus anti-inflammatory cytokine profile in
patients with severe sepsis: A marker for prognosis and future
therapeutic options. J Infect Dis. 181:176–180. 2000. View Article : Google Scholar
|
|
37
|
Pinsky MR, Vincent JL, Deviere J, Alegre
M, Kahn RJ and Dupont E: Serum cytokine levels in human septic
shock Relation to multiple-system organ failure and mortality.
Chest. 103:565–575. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Parrillo JE, Parker MM, Natanson C,
Suffredini AF, Danner RL, Cunnion RE and Ognibene FP: Septic shock
in humans. Advances in the understanding of pathogenesis,
cardiovascular dysfunction, and therapy. Ann Intern Med.
113:227–242. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chawla LS, Seneff MG, Nelson DR, Williams
M, Levy H, Kimmel PL and Macias WL: Elevated plasma concentrations
of IL-6 and elevated APACHE II score predict acute kidney injury in
patients with severe sepsis. Clin J Am Soc Nephrol. 2:22–30. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mira JP, Cariou A, Grall F, Delclaux C,
Losser MR, Heshmati F, Cheval C, Monchi M, Teboul JL, Riché F, et
al: Association of TNF2, a TNF-alpha promoter polymorphism, with
septic shock susceptibility and mortality: A multicenter study.
JAMA. 282:561–568. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Haveman JW, Muller Kobold AC, Tervaert JW,
van den Berg AP, Tulleken JE, Kallenberg CG and The TH: The central
role of monocytes in the pathogenesis of sepsis: Consequences for
immunomonitoring and treatment. Neth J Med. 55:132–141. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li YP, Yu SL, Huang ZJ, Huang J, Pan J,
Feng X, Zhang XG, Wang JH and Wang J: An impaired inflammatory
cytokine response to gram-negative LPS in human neonates is
associated with the defective TLR-mediated signaling pathway. J
Clin Immunol. 35:218–226. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Watkins RR, Yamshchikov AV, Lemonovich TL
and Salata RA: The role of vitamin D deficiency in sepsis and
potential therapeutic implications. J Infect. 63:321–326. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Takeda K and Akira S: TLR signaling
pathways. Semin Immunol. 16:3–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou S, Wang G and Zhang W: Effect of
TLR4/MyD88 signaling pathway on sepsis-associated acute respiratory
distress syndrome in rats, via regulation of macrophage activation
and inflammatory response. Exp Ther Med. 15:3376–3384.
2018.PubMed/NCBI
|
|
46
|
Huang C, Pan L, Lin F, Dai H and Fu R:
Monoclonal antibody against Toll-like receptor 4 attenuates
ventilator-induced lung injury in rats by inhibiting MyD88- and
NF-kappaB-dependent signaling. International Int J Mol Med.
39:693–700. 2017. View Article : Google Scholar
|
|
47
|
Chen Y, Liu W, Sun T, Huang Y, Wang Y, Deb
DK, Yoon D, Kong J, Thadhani R and Li YC: 1,25-Dihydroxyvitamin D
promotes negative feedback regulation of TLR signaling via
targeting microRNA-155-SOCS1 in macrophages. J Immunol.
190:3687–3695. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hegyi Z, Zwicker S, Bureik D, Peric M,
Koglin S, Batycka-Baran A, Prinz JC, Ruzicka T, Schauber J and Wolf
R: Vitamin D analog calcipotriol suppresses the Th17
cytokine-induced proinflammatory S100 'alarmins' psoriasin (S100A7)
and koebnerisin (S100A15) in psoriasis. J Invest Dermatol.
132:1416–1424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
White JH: Vitamin D signaling, infectious
diseases, and regulation of innate immunity. Infect Immun.
76:3837–3843. 2008. View Article : Google Scholar : PubMed/NCBI
|




















