Introduction
Lung cancer is one of the leading causes of
cancer-associated mortality in China and globally (1). Lung cancer can be classified into
non-small cell lung cancer (NSCLC), accounting for ~80-85% of
cases, and small cell lung cancer (SCLC), accounting for ~10-15% of
cases (2-4). At present, the standard treatment
strategy for lung cancer includes surgical resection, chemotherapy
and radiation therapy (5).
Furthermore, chemotherapy remains one of the commonly used
therapeutic regimens for advanced lung cancer (6). Gemcitabine (GEM) as a first-line
therapeutic drug has been used to treat lung cancer, but GEM
resistance poses a major limitation to the efficacy of GEM
chemotherapy (7). Therefore, it
is important to develop novel agents and therapeutic strategies to
overcome resistance.
Numerous recent studies focused on natural products,
which may be sources of novel natural antitumor agents (8-10).
Furthermore, a number of antitumor drugs, including paclitaxel,
docetaxel and vinorelbine, have been developed from natural
products and are successfully used to treat cancer (11). It has been reported that certain
natural products exert anticancer effects through a number of
mechanisms of action, including the inhibition of
phosphoinositide-3 kinase (PI3K)/Akt, induction of endoplasmic
reticulum (ER) stress and the generation of reactive oxygen species
(ROS) in various cancer types, including colorectal, lung and
prostate cancer (12-14). Additionally, Wang et al
(15) reported that licoricidin
enhances GEM-induced cytotoxicity by suppressing the Akt pathways
in osteosarcoma cells. Cheng et al (16) reported that resveratrol enhances
the sensitivity of pancreatic cancer cells to GEM via the
accumulation of ROS.
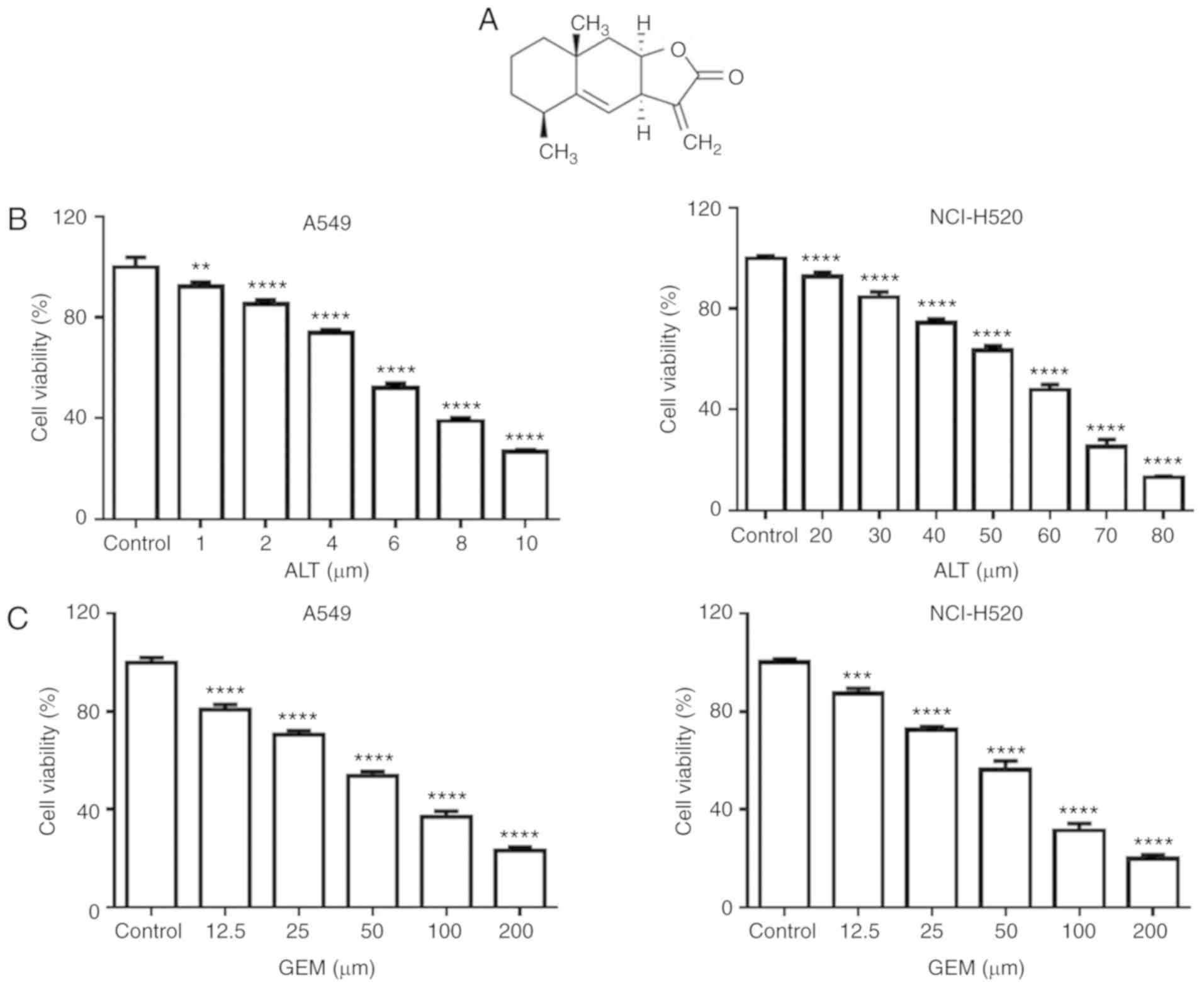
Alantolactone (ALT), a sesquiterpene lactone
compound isolated from Inula helenium (Fig. 1A), has been reported to exert
anticancer effects against various types of cancer. ALT was
demonstrated to promote apoptosis in colorectal cancer cells via
ROS overproduction (17). ALT may
induce apoptosis of human cervical cancer cells via ROS generation
(18). In MDA-MB-231 breast
cancer cells, ALT induces apoptosis via the ROS-mediated
mitochondrion-dependent pathway (19). Furthermore, ALT may trigger
apoptosis and induce cell cycle arrest in the
G1/G0 phase in SK-MES-1 lung squamous cancer
cells (20). Additionally, Maryam
et al (21) reported that
ALT enhanced the chemosensitivity of A549 cells to doxorubicin via
ROS-mediated inhibition of signal transducer and activator of
transcription 3 activation.
In the present study, it was first examined whether
ALT may enhance the sensitivity of human lung cancer cells to GEM
and then the underlying mechanisms were investigated. The results
provide evidence that ALT in combination with GEM may be a
promising strategy for treating lung cancer.
Materials and methods
Reagents and antibodies
ALT and N-acetyl-L-cysteine (NAC; a ROS scavenger)
were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum
(FBS) were from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Anti-CCAAT-enhancer-binding protein homologous protein (CHOP)
monoclonal antibody (cat. no. 5554), anti-phosphorylated (p)
eukaryotic initiation factor 2α [p-eIF2α (Ser51); cat. no. 9721],
anti-eIF2α (cat. no. 9722), anti-cyclin A2 (cat. no. 4656),
anti-p-Akt (cat. no. 4058), anti-Akt (cat. no. 9272),
anti-p-glycogen synthase kinase 3β [p-GSK3β (Ser9); cat. no. 5558],
anti-GSK3β (cat. no. 12456), anti-β-actin (cat. no. 4967) and
anti-caspase-3 (cat. no. 9662) were purchased from Cell Signal
Technology, Inc. (Danvers, MA, USA). Anti-p21 (cat. no. 195720) was
purchased from R&D Systems, Inc. (Minneapolis, MN, USA).
Horseradish peroxidase (HRP)-conjugated anti-mouse IgG (cat. no.
7076) and HRP-conjugated anti-rabbit IgG (cat. no. 7074) were
purchased from Cell Signal Technology Inc.. Tunicamycin (TM; an ER
stress agonist) was purchased from Merck KGaA. LY294002 (a PI3K
inhibitor) was purchased from MedChem Express (Monmouth Junction,
NJ, USA).
Cell culture
The A549 and NCI-H520 human lung carcinoma cells
lines were provided by the Cell Bank of the Shanghai Institute of
Biochemistry and Cell Biology (Shanghai, China). The cells were
cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin and
100 µg/ml streptomycin at 37°C in an atmosphere containing
5% CO2.
Cell viability assay
The A549 and NCI-H520 cells were seeded in 96-well
plates at a density of 1.5×104 cells/well and incubated
for 24 h at 37°C. Cell viability was detected by using a Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan), following the manufacturer's protocol. In brief,
the A549 cells were treated with 0, 1, 2, 4, 6, 8 and 10 µM
ALT or 0, 12.5, 25, 50, 100, and 200 µM GEM for 24 h at
37°C. The NCI-H520 cells were treated with 0, 20, 30, 40, 50, 60,
70 and 80 µM ALT or 0, 12.5, 25, 50, 100 and 200 µM
GEM for 24 h at 37°C. Furthermore, the A549 cells were treated with
or without 4 µM ALT and 25 µM GEM for 24 h at 37°C.
The NCI-H520 cells were treated with or without 40 µM ALT
and 25 µM GEM for 24 h at 37°C. In addition, LY294002 (4
µm) was added 1 h prior to certain treatments, and then the
A549 and NCI-H520 cells were treated with or without GEM (25
µm) for 24 h at 37°C. A total of 10 µl CCK-8 solution
was added to each well during the last 4 h of incubation at 37°C.
The absorbance at 450 nm was detected using an ELISA reader (Tecan
Group Ltd., Mannedorf, Switzerland). The experiments were performed
in triplicate. Additionally, morphological changes in A549 and
NCI-H520 cells treated with different concentrations of ALT and GEM
for 24 h were monitored using an inverted light microscope
(magnification, ×200).
Cell cycle analysis
The A549 and NCI-H520 cells were seeded in 6-well
plates at a density of 2.5×105 cells/well for 24 h at
37°C. The cell cycle distribution was analyzed with a Cell Cycle
Detection kit (cat. no. KGA512; Nanjing KeyGen Biotech Co., Ltd.,
Nanjing, China) and a FACScalibur flow cytometer (BD Biosciences,
San Jose, CA, USA), according to the manufacturer's protocols. In
brief, the A549 cells were treated with or without 4 µM ALT
and 25 µM GEM for 24 h at 37°C. The NCI-H520 cells were
treated with or without 40 µM ALT and 25 µM GEM for
24 h at 37°C. Cells were trypsinized and single-cell suspensions
were fixed with 75% ethanol overnight at 4°C. Propidium iodide (PI)
was used to stain the DNA in the samples for 15 min at 25°C, and
flow cytometry was used to detect cell cycle distribution. The
results were analyzed with ModFit LT 3.0 (Verity Software House,
Inc., Topsham, ME, USA). The experiments were performed in
triplicate.
Assay of cell apoptosis
The A549 and NCI-H520 cells were seeded in 6-well
plates at a density of 2.5×105 cells/well for 24 h at
37°C. Cell apoptosis was assessed using an Annexin V-fluorescein
isothiocyanate (FITC) Apoptosis Detection kit (cat. no. KGA104;
Nanjing KeyGen Biotech Co., Ltd.), according to the manufacturer's
protocol. In brief, the A549 and NCI-H520 cells were pre-treated
with NAC (8 mM) for 2 h at 37°C. The A549 cells were then treated
with or without 4 µM ALT and 25 µM GEM for 24 h at
37°C. The NCI-H520 cells were treated with or without 40 µM
ALT and 25 µM GEM for 24 h at 37°C. Annexin V-FITC and PI
were used to stain the cells for 15 min at 37°C in dark room. The
apoptotic cells were then detected with a FACScalibur flow
cytometer and analyzed by FlowJo 7.6 software (FlowJo LLC, Ashland,
OR, USA). The experiments were performed in triplicate.
Measurement of ROS generation
Intracellular ROS production was measured with a ROS
assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China) using flow cytom-etry, according to the manufacturer's
protocol. In brief, the A549 cells were treated with or without 4
µM ALT and 25 µM GEM for 12 h at 37°C. The NCI-H520
cells were treated with or without 40 µM ALT and 25
µM GEM for 12 h at 37°C. The cells were then incubated with
10 µM 2′,7′-dichlorodihy-drofluorescein diacetate for 15 min
at 37°C in the dark. The cells were analyzed using a FACScalibur
flow cytometer. The results were analyzed with FlowJo 7.6 software
and the experiments were performed in triplicate.
Western blot analysis
The A549 and NCI-H520 cells were seeded in 35-mm
cell culture dishes at a density of 2.5×105 cells/well
for 24 h at 37°C. The A549 cells were treated with or without 4
µM ALT and 25 µM GEM for 12 h at 37°C. The NCI-H520
cells were treated with or without 40 µM ALT and 25
µM GEM for 12 h at 37°C. Furthermore, the A549 cells were
treated with ALT (4 µm) and NCI-H520 cells were treated with
ALT (40 µm) for 3, 6 or 12 h. In addition, the A549 and
NCI-H520 cells were treated with LY294002 (4 µm) for 12 h.
In a separate experiment, the A549 and NCI-H520 cells were
pre-treated with NAC (8 mM) for 2 h at 37°C, followed by treatment
with or without 4 µM ALT and 25 µM GEM for 12 h at
37°C for the A549 cells, and treatment with or without 40 µM
ALT and 25 µM GEM for 12 h at 37°C for the NCI-H520
cells.
Following the different treatments, the A549 and
NCI-H520 cells were harvested and lysed in RIPA buffer (Beyotime
Institute of Biotechnology, Haimen, China) with protease inhibitors
(Sigma Aldrich, St. Louis, MO, USA) on ice for 30 min and then
centrifuged at 12,000 × g for 10 min at 4°C, as described
previously (22). The proteins
were then quantified with a Bicinchoninic Acid Protein assay kit
(Thermo Fisher Scientific, Inc.). Equal amounts of protein samples
(40 µg protein/well) were separated by 10-15% SDS-PAGE,
according to molecular weight of protein. Akt and p-Akt were
separated by 10% SDS-PAGE. GSK3β, p-GSK3β, eIF2α, p-eIF2α, cyclin
A2 and β-actin were separated by 12% SDS-PAGE. CHOP, p21 and
caspase-3 were separated by 15% SDS-PAGE. Subsequently, the protein
samples were transferred onto nitrocellulose membranes (Pall Life
Sciences, Port Washington, NY, USA). The membranes were blocked
with 5% non-fat milk for 2 h at 25°C, followed by incubation with
anti-CHOP (1:2,000 dilution), anti-p-eIF2α (Ser51) (1:3,000
dilution), anti-eIF2α (1:4,000 dilution), anti-cyclin A2 (1:1,000
dilution), anti-p-Akt (1:2,000 dilution), anti-Akt (1:3,000
dilution), anti-p-GSK3β (Ser9) (1:5,000 dilution), anti-GSK3β
(1:5,000 dilution), anti-β-actin (1:6,000 dilution), anti-caspase-3
(1:1,000 dilution) and anti-p21 (1:1,000 dilution) antibodies
overnight at 4°C. The membranes were incubated with a
HRP-conjugated secondary antibody (1:20,000 dilution) at room
temperature for 2 h. The reaction was visualized using SuperSignal
West Pico chemiluminescent Substrate (Pierce; Thermo Fisher
Scientific, Inc.), followed by exposure to Kodak X-ray film (Kodak,
Rochester, NY, USA).
Statistical analysis
Values are expressed as the mean ± standard
deviation. Data were analyzed using GraphPad Prism 6.0 (GraphPad
Software Inc., La Jolla, CA, USA). Differences between groups were
determined by one-way analysis of variance, followed by Dunnett's
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
ALT enhances GEM-induced cytotoxicity in
A549 and NCI-H520 cells
To assess whether ALT synergizes with GEM to inhibit
cell proliferation, A549 and NCI-H520 cells were first treated with
increasing doses of ALT and GEM for 24 h, and the cell viability
was assessed with a CCK-8 assay. As depicted in Fig. 1B and C, ALT or GEM significantly
decreased the growth of A549 and NCI-H520 cells in a dose-dependent
manner. It was notable that A549 cells [half maximal inhibitory
concentration (IC50) =6.63±1.10 µM] were more
sensitive to ALT, compared with NCI-H520 cells (IC50
=59.91±1.16 µM), while the sensitivity of A549 cells
(IC50 =8.39±1.02 µM) and NCI-H520
(IC50 =58.76±1.06 µM) to GEM was similar. Based
on the results, 4 µM ALT and 25 µM GEM were used in
A549 cells for the subsequent experiments. NCI-H520 cells were
treated with 40 µM ALT and 25 µM GEM for the
subsequent experiments. The cell viability of A549 and NCI-H520
cells was investigated with a CCK-8 assay. Furthermore, the results
indicated that the viability of A549 and NCI-H520 cells was
significantly decreased by combined treatment, compared with ALT or
GEM treatment alone (Fig. 1D).
Morphological observation also indicated that combined treatment
notably decreased the percentage of surviving cells, compared with
those subjected to ALT or GEM treatment alone (Fig. 1E). These results indicated that
ALT significantly enhances the synergism of GEM by inhibiting the
cell proliferation of A549 and NCI-H520 cells.
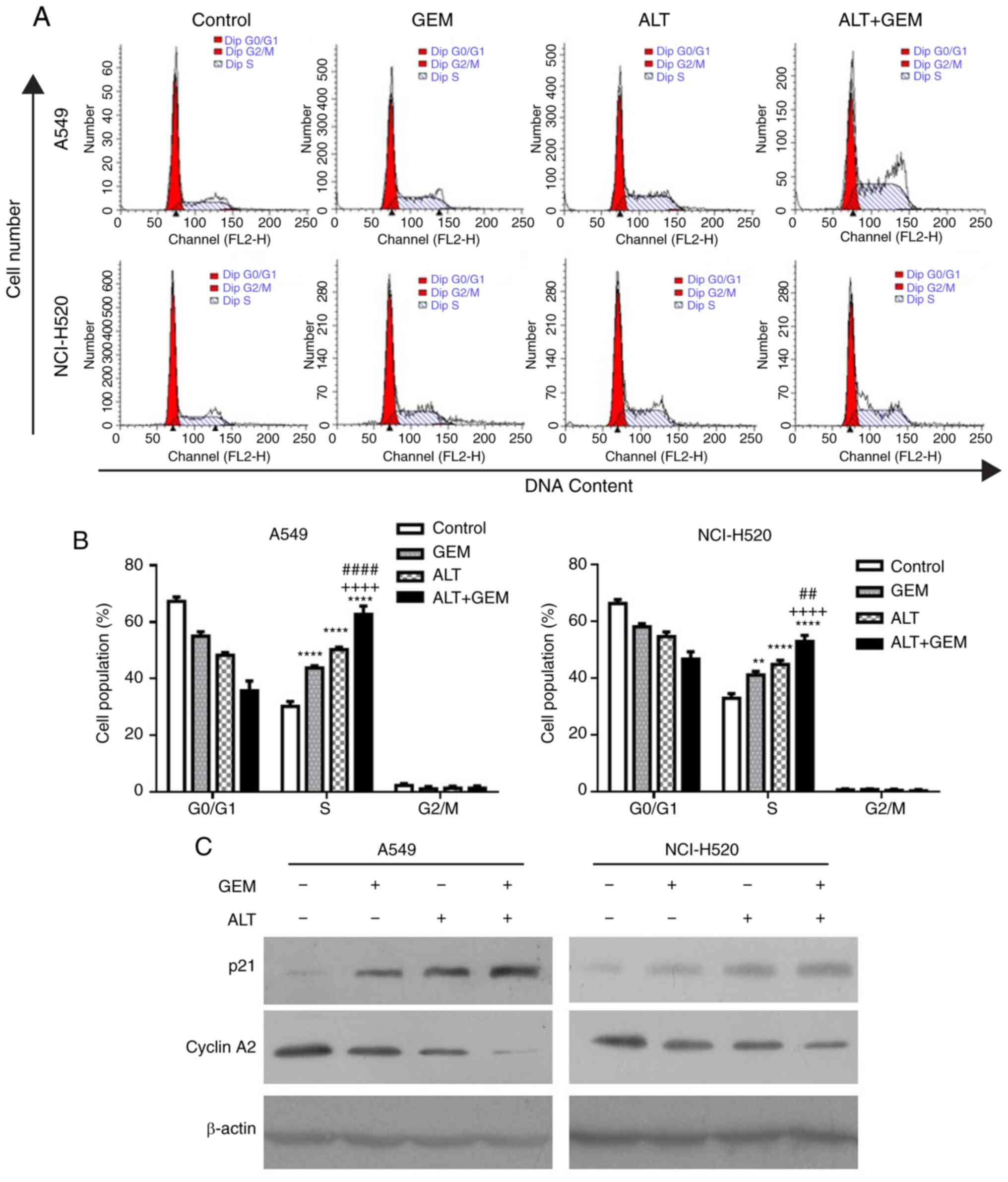
ALT enhances GEM-induced cell cycle
arrest in A549 and NCI-H520 cells
Numerous studies demonstrated that cell cycle arrest
induced by anticancer drugs has an important role in cell growth
inhibition (23-25). To verify whether cell cycle arrest
is involved in the GEM sensitization effect, the impact of ALT and
GEM on the cell cycle distribution was determined in A549 and
NCI-H520 cells with flow cytometry. The cell cycle assay
demonstrated that ALT, GEM and their co-treatment significantly
induced S-phase arrest, compared with the control group (Fig. 2A and B). The combination treatment
with ALT and GEM significantly increased the S-phase arrest,
compared with that caused by ALT or GEM treatment alone (Fig. 2A and B). Furthermore, western blot
analysis was performed to examine the expression levels of
S-phase-associated proteins in A549 and NCI-H520 cells. As depicted
in Fig. 2C, ALT, GEM and
co-treatment with ALT and GEM caused a notable upregulation of the
expression levels of p21 but a downregulation of cyclin A2,
compared with that in the control group. Compared with that in the
mono-treatment groups, the levels of cyclin A2 in the co-treatment
group were decreased, while the levels of p21 were increased in
A549 and NCI-H520 cells. These results indicated that S-phase
arrest contributes to the synergistic effect induced by ALT on the
growth inhibition properties of GEM in A549 and NCI-H520 cells.
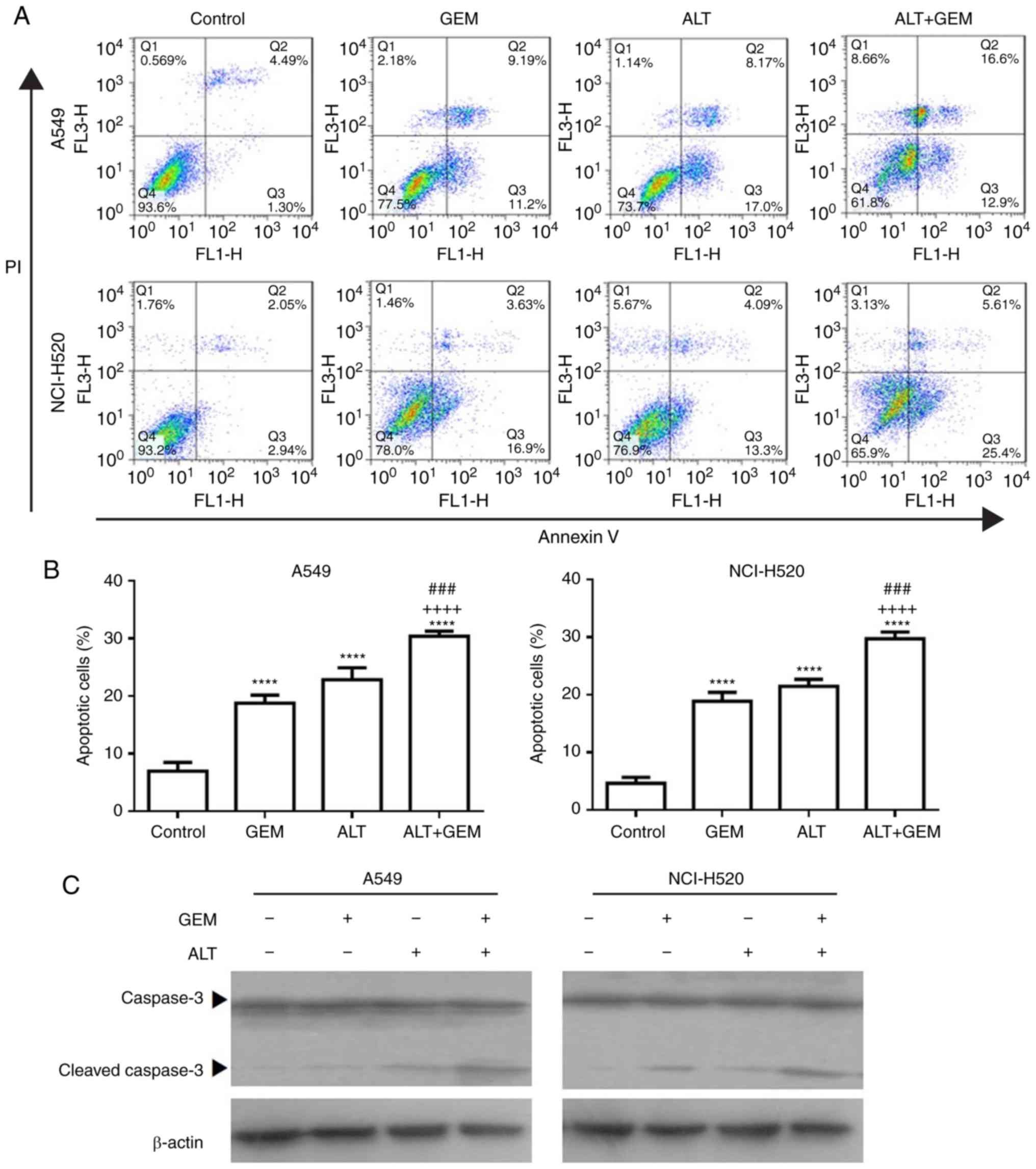
ALT enhances GEM-induced apoptosis in
A549 and NCI-H520 cells
To further investigate the synergistic effect
induced by ALT on the growth inhibition by GEM, the apoptosis of
A549 and NCI-H520 cells treated with ALT and GEM was examined with
flow cytometry. As depicted in Fig.
3A and B, ALT, GEM and their combination significantly
increased the apoptotic rate of A549 and NCI-H520 cells. Compared
with that in the mono-treatment groups, the apoptotic rate was
significantly increased in the combined group (Fig. 3A and B). The levels of the
apoptosis-associated protein caspase-3 in A549 and NCI-H520 cells
were also examined with western blot analysis. It was observed that
ALT, GEM and their combination notably increased the expression
levels of activation of caspase-3. Compared with those in the
mono-treatment groups, the levels of activation of caspase-3 were
notably increased in the combined treatment group (Fig. 3C). These results demonstrated that
ALT significantly enhances the sensitivity to GEM by inducing cell
apoptosis in A549 and NCI-H520 cells.
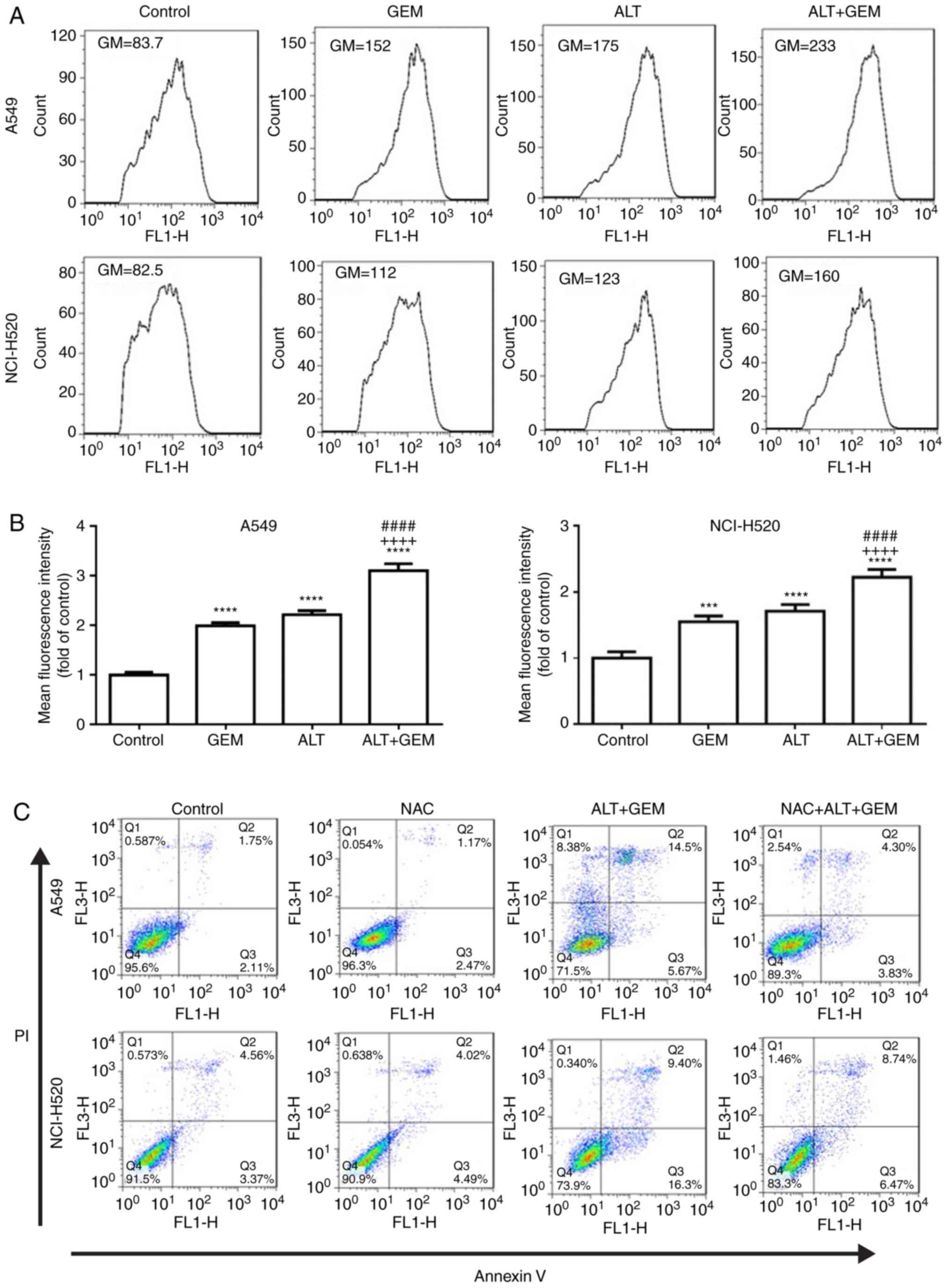
ALT sensitizes GEM-mediated cell
apoptosis via ROS production in A549 and NCI-H520 cells
Previous studies indicated that ROS has a critical
role in anticancer drug-mediated apoptosis (26,27). Therefore, the effects of ALT and
GEM on ROS production in A549 and NCI-H520 cells were assessed by
flow cytometry. As depicted in Fig.
4A and B, ALT, GEM and their combination significantly
increased ROS accumulation in A549 and NCI-H520 cells. Compared
with the mono-treatments, the co-treatment significantly enhanced
ROS accumulation. To further investigate whether ROS generation
contributes to ALT- and GEM-mediated apoptosis, A549 and NCI-H520
cells were pre-treated with NAC (ROS scavenger) prior to treating
the cells with ALT and GEM, and apoptosis was then detected by flow
cytometry. The results indicated that pre-treatment with NAC
significantly decreased ALT- and GEM-induced apoptosis in A549 and
NCI-H520 cells (Fig. 4C and D).
Additionally, western blot analysis revealed that pre-treatment
with NAC notably attenuated the increases in the levels of
activation of caspase-3 induced by ALT and GEM treatment in A549
and NCI-H520 cells (Fig. 4E).
Thus, these results demonstrated that ALT enhances GEM-mediated
apoptosis via increasing the intracellular ROS production in A549
and NCI-H520 cells.
ALT sensitizes A549 and NCI-H520 cells to
GEM-mediated cell apoptosis via induction of ROS-mediated ER
stress
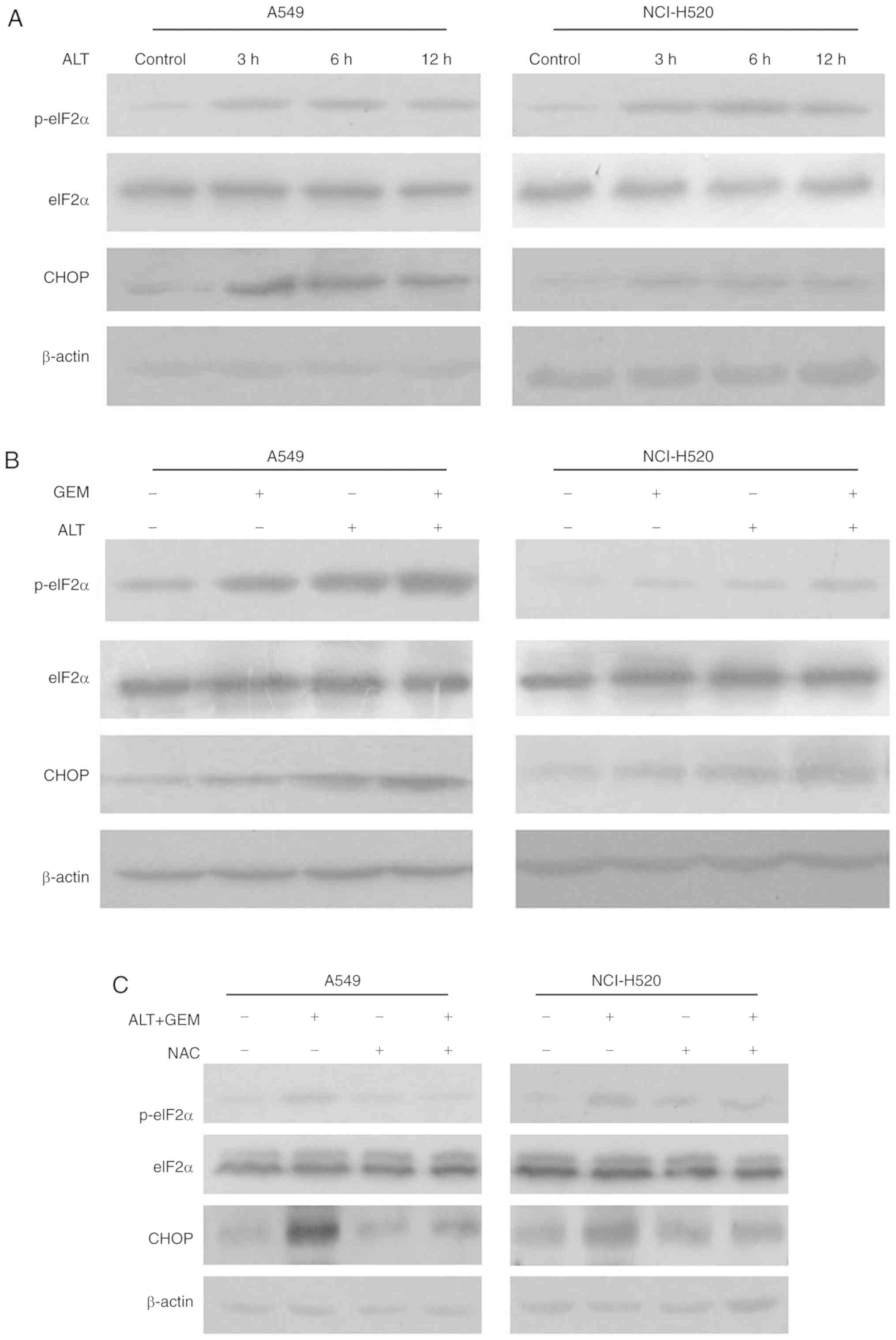
It has been reported that ROS generation induces
cell apoptosis through activating ER stress pathways in a variety
of cancer cell types, including prostate, bladder and lung cancer
(28-30). To confirm whether ER stress is
involved in ROS-mediated apoptosis induced by the test drugs, the
effect of ALT and GEM on hallmarks of ER-associated apoptosis,
including eIF2α phosphorylation and CHOP expression, was determined
in A549 and NCI-H520 cells with western blot analysis. As indicated
in Fig. 5A, the levels of eIF2α
phosphorylation and CHOP expression were notably unregulated by ALT
in A549 and NCI-H520 cells. Combination treatment with ALT and GEM
notably increased the phosphorylation of eIF2α and CHOP expression,
compared with that caused by each drug alone (Fig. 5B). Furthermore, A549 and NCI-H520
cells were treated with ALT and GEM in the presence of NAC and the
phosphorylation of eIF2α and the expression of CHOP were then
measured. It was revealed that NAC attenuated the phosphorylation
of eIF2α and the expression of CHOP induced by ALT and GEM
(Fig. 5C). To further confirm
whether ER stress is involved in GEM-mediated growth inhibition,
the effects of TM, an ER stress agonist, on GEM-induced growth
inhibition of A549 and NCI-H520 cells were assessed. As depicted in
Fig. 5D, combination treatment
with TM and GEM significantly decreased the cell viability compared
with GEM alone. Collectively, these results revealed that ALT
increases GEM-mediated apoptosis via ROS-mediated ER stress
activation.
ALT sensitizes GEM-mediated cell
apoptosis via ROS-mediated inhibition of the Akt/GSK3β pathway in
A549 and NCI-H520 cells
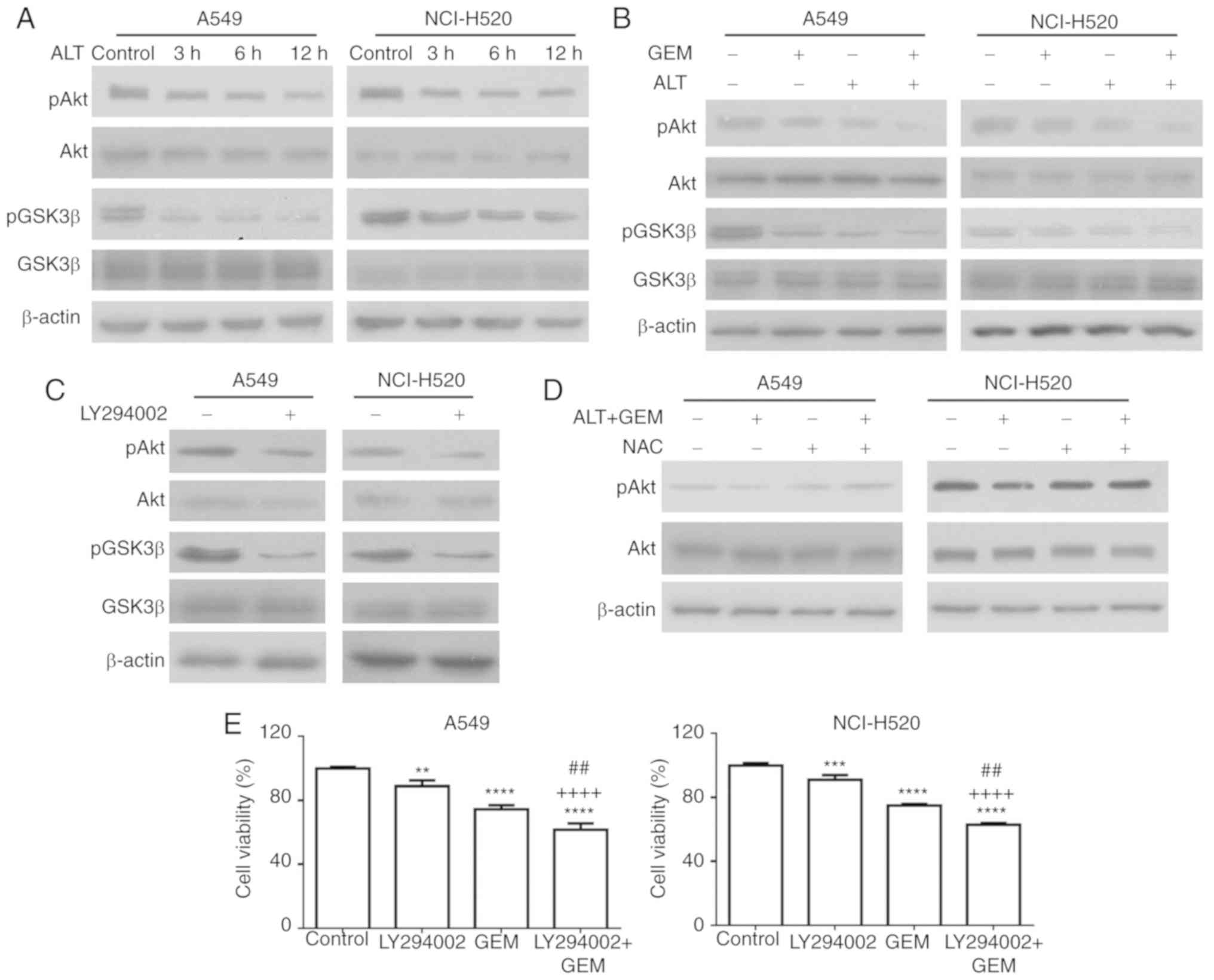
The Akt pathway is an important pathway associated
with ROS-mediated apoptosis in various cancer cell types, including
bladder, lung and pancreatic cancer (31-34). To determine whether the Akt
pathway is involved in ROS-mediated apoptosis, the effects of ALT
and GEM on the levels of the expression of Akt, GSK3β, p-Akt and
p-GSK3β in A549 and NCI-H520 cells were determined by western blot
analysis. As depicted in Fig. 6A,
the levels of p-Akt and p-GSK3β (Ser 9) were notably decreased by
ALT, but the levels of total Akt and GSK3β were not notably
affected. Furthermore, combination treatment notably inhibited the
levels of p-Akt and p-GSK3β, compared with the mono-treatment
groups (Fig. 6B). In another
experiment, A549 and NCI-H520 cells were treated with LY294002, a
PI3K inhibitor, and the levels of p-GSK3β and p-Akt were detected
with western blot analysis. As depicted in Fig. 6C, LY294002 notably decreased the
levels of p-GSK3β and p-Akt in A549 and NCI-H520 cells. To further
determine the role of the Akt pathway in ROS-mediated cell
apoptosis, A549 and NCI-H520 cells were pre-treated with NAC
followed by the test drugs, and the effect on the levels of p-Akt
was determined. As depicted in Fig.
6D, pre-treatment with NAC reduced the effect of co-treatment
with ALT and GEM on p-Akt. The effect of pre-treatment with
LY294002 on cell growth inhibition induced by GEM was then
assessed. As depicted in Fig. 6E,
LY294002 pre-treatment significantly increased the GEM-induced
inhibition of A549 and NCI-H520 cell growth. Collectively, these
results indicated that ALT increases GEM-mediated apoptosis via
ROS-mediated activation of the Akt/GSK3β pathway.
Discussion
GEM, as a first-line chemotherapeutic drug, is
frequently used for the treatment of a number of cancer types,
including lung cancer (35,36). However, GEM resistance is common
in lung cancer treatment and critically limits the outcome
(37,38). Thus, novel strategies for the
effective treatment of lung cancer are urgently required. Previous
studies demonstrated that ALT has anticancer activity against
various human cancer cell types, including colorectal, cervical and
breast cancer (17-19). Additionally, it has been reported
that ALT enhanced the chemosensitivity of cancer cells (21,39). In the present study, it was
investigated whether ALT and GEM have a synergistic effect on lung
cancer. It was observed that ALT significantly inhibits the
proliferation of A549 and NCI-H520 cells, and induces
G0/G1 arrest and cell apoptosis in these cell
lines. It was also demonstrated that ALT enhances the antitumor
effect of GEM on A549 and NCI-H520 cells via ROS-mediated ER stress
and the Akt/GSK3β pathway. In the future, the upstream mechanisms
of the effects observed in the present study should be further
investigated.
Previous studies indicated that induction of cell
cycle arrest is an efficient strategy in anticancer therapy
(23,40,41). GEM has been reported to inhibit
cell proliferation and induce S-phase arrest in lung cancer cells
(42-44). It has also been reported that ALT
induces G2/M-phase arrest and G1/G0-phase
arrest in MDA-MB-231 breast cancer cells (19) and lung squamous cancer SK-MES-1
cells (20), respectively. In the
present study, ALT or GEM induced S-phase arrest in A549 and
NCI-H520 cells. Compared with ALT or GEM alone, the combination
significantly increased the accumulation of A549 and NCI-H520 cells
in the S phase. Additionally, cell cycle-associated protein
analysis revealed that ALT alone or GEM alone caused an
upregulation in the expression of cyclin-dependent kinase inhibitor
p21 and a downregulation of cyclin A2. Furthermore, compared with
ALT or GEM alone, their combination caused a notable upregulation
in the expression of p21 and downregulation of cyclin A2. These
results indicated that ALT enhanced the anti-proliferative effect
of GEM in A549 and NCI-H520 cells via p21 and cyclin A2-mediated
S-phase arrest.
Apoptosis is an important cellular process and
numerous anticancer drugs, including paclitaxel, doxorubicin,
carboplatin and curcumin, prevent tumor progression via inducing
cell apoptosis (45-47). It is well known that ALT and GEM
induce apoptosis in lung cancer (21,48). The present results demonstrated
that ALT and GEM induce apoptosis in A549 and NCI-H520 cells. The
combination of the two drugs significantly increased the rate of
apoptosis of A549 and NCI-H520 cells, compared with that achieved
with each drug alone. Cell apoptosis-associated protein analysis
revealed that ALT or GEM may induce cell apoptosis by activation of
caspase-3 in A549 and NCI-H520 cells. Cell apoptosis-associated
protein analysis also revealed that the drug combination notably
increases the level of activation of caspase-3. These results
indicated that ALT enhances GEM-induced cell apoptosis by
activation of caspase-3 in A549 and NCI-H520 cells.
High levels of ROS have been documented to induce
apoptosis in various cancer types, including bladder, lung and
cervical cancer, which have a notable role in cell apoptosis
induced by anticancer drugs (29,34,49). Previous research demonstrated that
ALT induces cell apoptosis in various cancer types, including
colorectal, cervical and breast cancer, via increasing the
generation of ROS (17-19). Maryam et al (21) demonstrated that ALT enhances the
chemosensitivity of A549 lung adenocarcinoma cells to doxorubicin
via ROS generation. Cheng et al (16) also reported that resveratrol
enhances the sensitivity of pancreatic cancer cells to GEM via
inducing the accumulation of ROS. In the present study, an increase
in ROS generation was observed in ALT- or GEM-treated A549 and
NCI-H520 cells. Compared with ALT or GEM alone, their combination
significantly increased the accumulation of ROS in A549 and
NCI-H520 cells. Furthermore, the apoptosis of A549 and NCI-H520
cells treated by ALT and GEM combined was attenuated by NAC.
Additionally, ALT- and GEM-mediated upregulation of activation of
caspase-3 in A549 and NCI-H520 cells was also decreased by
pre-treatment with NAC. Overall, the present results demonstrated
that ALT enhanced GEM-induced cell apoptosis via increasing the
accumulation of ROS in A549 and NCI-H520 cells.
The ER as a central cellular organelle is well known
to regulate multiple cellular functions, including protein folding,
protein maturation, ER quality control and the maintenance of
cellular homeostasis (50,51).
The accumulation of misfolded proteins in the ER may disrupt ER
function, cause ER stress and induce cell apoptosis (52). ER stress has become a novel target
for potential anticancer drugs (53). It has also been demonstrated that
increased ROS generation induced by anticancer drugs triggers ER
stress-mediated apoptosis in various cancer types, including
bladder, prostate and cervical cancer (29,54,55). Maryam et al (21) reported that ALT enhances the
chemosensitivity of A549 lung adenocarcinoma cells to doxorubicin
via the ROS-mediated ER stress apoptosis pathway. Consistent with
this, the present study indicated that ALT caused a notable
deregulation of ER stress-associated proteins, including increases
in eIF2α phosphorylation and CHOP expression in A549 and NCI-H520
cells. Combination treatment with ALT and GEM notably increased the
phosphorylation of eIF2α and CHOP expression, compared with that
obtained with each drug alone. Furthermore, inhibition of ROS
generation by NAC abrogated the ALT- and GEM-induced ER stress
activation in A549 and NCI-H520 cells. Additionally, combination
treatment with TM significantly enhanced the effect of GEM to
decrease the viability of lung cancer cells. Collectively, these
results indicated that ALT enhances GEM-mediated apoptosis via the
ROS-mediated, ER stress-induced apoptosis pathway.
The Akt pathway is involved in regulating cell
survival and death (56).
Therefore, inhibition of the Akt signaling pathway has been
considered an effective approach for the treatment of human cancer
types, including prostate and gastric cancer (57,58). It has been reported that the
inhibition of Akt induced cancer cell apoptosis via inhibition of
various downstream targets, including inhibition of the
phosphorylation of GSK3β at Ser9 (59,60). Furthermore, evidence indicated
that increased ROS generation induced by anticancer drugs triggered
cell apoptosis via inhibition of the Akt signaling pathway
(31,61). Li et al (62) reported that phenoxodiol enhances
the antitumor activity of GEM against gallbladder cancer through
suppressing the Akt pathway. Furthermore, Tuya et al
(63) reported that trichosanthin
enhances the antitumor effect of GEM via inhibition of the Akt
pathway in NSCLC. In the present study, it was confirmed that ALT
reduces the levels of p-Akt and p-GSK3β in A549 and NCI-H520 cells.
Combination treatment of ALT and GEM notably reduced the levels of
p-Akt and p-GSK3β, compared with that in the mono-treatment groups.
Furthermore, pre-treatment of NAC efficiently abrogated the
combination treatment-induced reduction in the levels of p-Akt and
p-GSK3β. Additionally, LY294002 decreased the levels of p-GSK3β and
increased GEM-induced cell growth inhibition in A549 and NCI-H520
cells. These results indicated that ALT enhances GEM-mediated
apoptosis via ROS-mediated inhibition of the Akt/GSK3β pathway.
In conclusion, the present study demonstrated that
ALT enhances the antitumor efficacy of GEM via the ROS-mediated
inhibition of the Akt/GSK3β pathway and activation of the ER stress
pathway in A549 and NCI-H520 cells. These results indicated that
the combination of ALT and GEM may provide a potential clinical
strategy for lung cancer treatment. Furthermore, these results will
be further validated in in vivo experiments.
Funding
This study was supported by grants from the
Scientific Research Program Funded by Shaanxi Provincial Education
Department (program no. 16JK1761), the National Key Research and
Development Program (grant. no. 2016YFC0905001) and the National
Natural Science Foundation of China (grant. no. 81471710).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JiqW and JiaW conducted the experiments and analyzed
the data. JiqW made substantial contributions to the design of the
present study and prepared the manuscript. YZ, XL, JizW, BL and YL
performed the western blotting and analyzed the data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feldman MW and Roughgarden J: A
population's stationary distribution and chance of extinction in a
stochastic environment with remarks on the theory of species
packing. Theor Popul Biol. 7:197–207. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carvajal-Hausdorf D, Altan M, Velcheti V,
Gettinger SN, Herbst RS, Rimm DL and Schalper KA: Expression and
clinical significance of PD-L1, B7-H3, B7-H4 and TILs in human
small cell lung Cancer (SCLC). J Immunother Cancer. 7:652019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Torre LA, Siegel RL and Jemal A: Lung
cancer statistics. Adv Exp Med Biol. 893:1–19. 2016. View Article : Google Scholar
|
|
6
|
Lee SH: Chemotherapy for lung cancer in
the era of personalized medicine. Tuberc Respir Dis (Seoul). Dec
20–2018, Epub ahead of print.
|
|
7
|
Jia Y and Xie J: Promising molecular
mechanisms responsible for gemcitabine resistance in cancer. Genes
Dis. 2:299–306. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qin JJ, Li X, Hunt C, Wang W, Wang H and
Zhang R: Natural products targeting the p53-MDM-2 pathway and
mutant p53: Recent advances and implications in cancer medicine.
Genes Dis. 5:204–219. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Albuquerque KRS, Pacheco NM, Del Rosario
Loyo Casao T, de Melo FCSA, Novaes RD and Goncalves RV:
Applicability of plant extracts in preclinical studies of melanoma:
A systematic review. Mediators Inflamm. 2018:67979242018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim C and Kim B: Anti-cancer natural
products and their bioactive compounds inducing ER stress-mediated
apoptosis: A review. Nutrients. 10:E10212018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Demain AL and Vaishnav P: Natural products
for cancer chemotherapy. Microb Biotechnol. 4:687–699. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meng LQ, Wang Y, Luo YH, Piao XJ, Liu C,
Wang Y, Zhang Y, Wang JR, Wang H, Xu WT, et al: Quinalizarin
induces apoptosis through reactive oxygen species (ROS)-mediated
mitogen-activated protein kinase (MAPK) and signal transducer and
activator of transcription 3 (STAT3) signaling pathways in
colorectal cancer cells. Med Sci Monit. 24:3710–3719. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu F, Gao S, Yang Y, Zhao X, Fan Y, Ma W,
Yang D, Yang A and Yu Y: Antitumor activity of curcumin by
modulation of apoptosis and autophagy in human lung cancer A549
cells through inhibiting PI3K/Akt/mTOR pathway. Oncol Rep.
39:1523–1531. 2018.PubMed/NCBI
|
|
14
|
Huang H, Xie H, Pan Y, Zheng K, Xia Y and
Chen W: Plumbagin triggers ER stress-mediated apoptosis in prostate
cancer cells via induction of ROS. Cell Physiol Biochem.
45:267–280. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Wang S, Liu J, Lu Y and Li D:
Licoricidin enhances gemcitabine-induced cytotoxicity in
osteosarcoma cells by suppressing the Akt and NF-kappaB signal
pathways. Chem Biol Interact. 290:44–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng L, Yan B, Chen K, Jiang Z, Zhou C,
Cao J, Qian W, Li J, Sun L, Ma J, et al: Resveratrol-induced
downregulation of NAF-1 enhances the sensitivity of pancreatic
cancer cells to gemcitabine via the ROS/Nrf2 signaling pathways.
Oxid Med Cell Longev. 2018:94820182018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ding Y, Wang H, Niu J, Luo M, Gou Y, Miao
L, Zou Z and Cheng Y: Induction of ROS overload by alantolactone
prompts oxidative DNA damage and apoptosis in colorectal cancer
cells. Int J Mol Sci. 17:5582016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang Y, Xu H and Wang J: Alantolactone
induces apoptosis of human cervical cancer cells via reactive
oxygen species generation, glutathione depletion and inhibition of
the Bcl-2/Bax signaling pathway. Oncol Lett. 11:4203–4207. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cui L, Bu W, Song J, Feng L, Xu T, Liu D,
Ding W, Wang J, Li C, Ma B, et al: Apoptosis induction by
alantolactone in breast cancer MDA-MB-231 cells through reactive
oxygen species-mediated mitochondrion-dependent pathway. Arch Pharm
Res. 41:299–313. 2018. View Article : Google Scholar
|
|
20
|
Zhao P, Pan Z, Luo Y, Zhang L, Li X, Zhang
G, Zhang Y, Cui R, Sun M and Zhang X: Alantolactone induces
apoptosis and cell cycle arrest on lung squamous cancer SK-MES-1
cells. J Biochem Mol Toxicol. 29:199–206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maryam A, Mehmood T, Zhang H, Li Y, Khan M
and Ma T: Alantolactone induces apoptosis, promotes STAT3
glutathionylation and enhances chemosensitivity of A549 lung
adenocarcinoma cells to doxorubicin via oxidative stress. Sci Rep.
7:62422017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Wang J, Hui B, Sun W, Li B, Shi
F, Che S, Chai L and Song L: Pristimerin enhances the effect of
cisplatin by inhibiting the miR23a/Akt/GSK3beta signaling pathway
and suppressing autophagy in lung cancer cells. Int J Mol Med.
43:1382–1394. 2019.PubMed/NCBI
|
|
23
|
Razak NA, Abu N, Ho WY, Zamberi NR, Tan
SW, Alitheen NB, Long K and Yeap SK: Cytotoxicity of eupatorin in
MCF-7 and MDA-MB-231 human breast cancer cells via cell cycle
arrest, anti-angiogenesis and induction of apoptosis. Sci Rep.
9:15142019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang X, Zhao L, Zhang T, Xi J, Liu S, Ren
L, Zheng Y and Zhang H: Protosappanin B promotes apoptosis and
causes G1 cell cycle arrest in human bladder cancer cells. Sci Rep.
9:10482019. View Article : Google Scholar :
|
|
25
|
Geng YD, Zhang L, Wang GY, Feng XJ, Chen
ZL, Jiang L and Shen AZ: Xanthatin mediates G2/M cell cycle arrest,
autophagy and apoptosis via ROS/XIAP signaling in human colon
cancer cells. Nat Prod Res. 1–5. 2018.Epub ahead of print.
View Article : Google Scholar
|
|
26
|
Zhou GZ, Li AF, Sun YH and Sun GC: A novel
synthetic curcumin derivative MHMM-41 induces ROS-mediated
apoptosis and migration blocking of human lung cancer cells A549.
Biomed Pharmacother. 103:391–398. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma G, Luo W, Lu J, Ma DL, Leung CH, Wang Y
and Chen X: Cucurbitacin E induces caspase-dependent apoptosis and
protective autophagy mediated by ROS in lung cancer cells. Chem
Biol Interact. 253:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen W, Li P, Liu Y, Yang Y, Ye X, Zhang F
and Huang H: Isoalantolactone induces apoptosis through
ROS-mediated ER stress and inhibition of STAT3 in prostate cancer
cells. J Exp Clin Cancer Res. 37:3092018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu Y, Tong Y, Ying J, Lei Z, Wan L, Zhu X,
Ye F, Mao P, Wu X, Pan R, et al: Chrysin induces cell growth
arrest, apoptosis, and ER stress and inhibits the activation of
STAT3 through the generation of ROS in bladder cancer cells. Oncol
Lett. 15:9117–9125. 2018.PubMed/NCBI
|
|
30
|
Ge G, Yan Y and Cai H: Ginsenoside Rh2
inhibited proliferation by inducing ROS mediated ER stress
dependent apoptosis in lung cancer cells. Biol Pharm Bull.
40:2117–2124. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ji L, Zhong B, Jiang X, Mao F, Liu G, Song
B, Wang CY, Jiao Y, Wang JP, Xu ZB, et al: Actein induces autophagy
and apoptosis in human bladder cancer by potentiating ROS/JNK and
inhibiting AKT pathways. Oncotarget. 8:112498–112515. 2017.
View Article : Google Scholar
|
|
32
|
Ahn KI, Choi EO, Kwon DH, HwangBo H, Kim
MY, Kim HJ, Ji SY, Hong SH, Jeong JW, Park C, et al: Induction of
apoptosis by ethanol extract of Citrus unshiu Markovich peel in
human bladder cancer T24 cells through ROS-mediated inactivation of
the PI3K/Akt pathway. Biosci Trends. 11:565–573. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lai ZQ, Ip SP, Liao HJ, Lu Z, Xie JH, Su
ZR, Chen YL, Xian YF, Leung PS and Lin ZX: Brucein D, a naturally
occurring tetracyclic triterpene quassinoid, induces apoptosis in
pancreatic cancer through ROS-associated PI3K/Akt signaling
pathway. Front Pharmacol. 8:9362017. View Article : Google Scholar
|
|
34
|
Guo CL, Wang LJ, Zhao Y, Liu H, Li XQ,
Jiang B, Luo J, Guo SJ, Wu N and Shi DY: A novel bromophenol
derivative BOS-102 induces cell cycle arrest and Apoptosis in human
A549 lung cancer cells via ROS-Mediated PI3K/Akt and the MAPK
signaling pathway. Mar Drugs. 16:E432018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Amrutkar M and Gladhaug IP: Pancreatic
cancer chemoresistance to gemcitabine. Cancers (Basel). 9:E1572017.
View Article : Google Scholar
|
|
36
|
Li Y, Wang LR, Chen J, Lou Y and Zhang GB:
First-line gemcitabine plus cisplatin in nonsmall cell lung cancer
patients. Dis Markers. 2014:9604582014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu B and Tao ZZ: Piceatannol enhances the
antitumor efficacy of gemcitabine in human A549 non-small cell lung
cancer cells. Oncol Res. 22:213–217. 2014. View Article : Google Scholar
|
|
38
|
Gebregiworgis T, Bhinderwala F, Purohit V,
Chaika NV, Singh PK and Powers R: Insights into gemcitabine
resistance and the potential for therapeutic monitoring.
Metabolomics. 14:1562018. View Article : Google Scholar
|
|
39
|
Yao Y, Xia D, Bian Y, Sun Y, Zhu F, Pan B,
Niu M, Zhao K, Wu Q, Qiao J, et al: Alantolactone induces G-1 phase
arrest and apoptosis of multiple myeloma cells and overcomes
bortezomib resistance. Apoptosis. 20:1122–1133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Y, Ji P, Liu J, Broaddus RR, Xue F
and Zhang W: Centrosome-associated regulators of the G(2)/M
checkpoint as targets for cancer therapy. Mol Cancer. 8:82009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu Y, Kang X, Niu G, He S, Zhang T, Bai
Y, Li Y, Hao H, Chen C, Shou Z and Li B: Shikonin induces apoptosis
and prosurvival autophagy in human melanoma A375 cells via
ROS-mediated ER stress and p38 pathways. Artif Cells Nanomed
Biotechnol. 47:626–635. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li J, Pan YY and Zhang Y: Synergistic
interaction between sorafenib and gemcitabine in EGFR-TKI-sensitive
and EGFR-TKI-resistant human lung cancer cell lines. Oncol Lett.
5:440–446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li J, Wang S, Su ZF and Yuan Y:
Synergistic effects of sorafenib in combination with gemcitabine or
pemetrexed in lung cancer cell lines with K-ras mutations. Contemp
Oncol (Pozn). 20:33–38. 2016.
|
|
44
|
Tang Y, Wang Y and Teng X:
Sequence-dependent effect of gemcitabine and cisplatin on A549
non-small-cell lung cancer cells. Mol Med Rep. 8:221–226. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang YF, Zhu DJ, Chen XW, Chen QK, Luo
ZT, Liu CC, Wang GX, Zhang WJ and Liao NZ: Curcumin enhances the
effects of irinotecan on colorectal cancer cells through the
generation of reactive oxygen species and activation of the
endoplasmic reticulum stress pathway. Oncotarget. 8:40264–40275.
2017.PubMed/NCBI
|
|
46
|
Broecker-Preuss M, Becher-Boveleth N,
Muller S and Mann K: The BH3 mimetic drug ABT-737 induces apoptosis
and acts synergistically with chemotherapeutic drugs in thyroid
carcinoma cells. Cancer Cell Int. 16:272016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gregoraszczuk EL, Rak-Mardyła A, Ryś J,
Jakubowicz J and Urbański K: Effect of chemotherapeutic drugs on
Caspase-3 activity, as a key biomarker for Apoptosis in ovarian
tumor cell cultured as monolayer. A Pilot Study Iran J Pharm Res.
14:1153–1161. 2015.
|
|
48
|
Teng JP, Yang ZY, Zhu YM, Ni D, Zhu ZJ and
Li XQ: Gemcitabine and cisplatin for treatment of lung cancer in
vitro and vivo. Eur Rev Med Pharmacol Sci. 22:3819–3825.
2018.PubMed/NCBI
|
|
49
|
Seervi M, Rani A, Sharma AK and Santhosh
Kumar TR: ROS mediated ER stress induces Bax-Bak dependent and
independent apoptosis in response to Thioridazine. Biomed
Pharmacother. 106:200–209. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yadav RK, Chae SW, Kim HR and Chae HJ:
Endoplasmic reticulum stress and cancer. J Cancer Prev. 19:75–88.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chipurupalli S, Kannan E, Tergaonkar V,
D'Andrea R and Robinson N: Hypoxia induced ER stress response as an
adaptive mechanism in cancer. Int J Mol Sci. 20:E7492019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang M and Kaufman RJ: The impact of the
endoplasmic reticulum protein-folding environment on cancer
development. Nat Rev Cancer. 14:581–597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Logothetis C, Aparicio A and Thompson TC:
ER stress in prostate cancer: A therapeutically exploitable
vulnerability? Sci Transl Med. 10:pp. eaat39752018, View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wu S, Yang Y, Li F, Huang L, Han Z, Wang
G, Yu H and Li H: Chelerythrine induced cell death through
ROS-dependent ER stress in human prostate cancer cells. Onco
Targets Ther. 11:2593–2601. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lin CL, Lee CH, Chen CM, Cheng CW, Chen
PN, Ying TH and Hsieh YH: Protodioscin induces Apoptosis through
ROS-Mediated endoplasmic reticulum stress via the JNK/p38
activation pathways in human cervical cancer cells. Cell Physiol
Biochem. 46:322–334. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Madhunapantula SV, Mosca PJ and Robertson
GP: The Akt signaling pathway: An emerging therapeutic target in
malignant melanoma. Cancer Biol Ther. 12:1032–1049. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hong SW, Shin JS, Moon JH, Kim YS, Lee J,
Choi EK, Ha SH, Lee DH, Chung HN, Kim JE, et al: NVP-BEZ235, a dual
PI3K/mTOR inhibitor, induces cell death through alternate routes in
prostate cancer cells depending on the PTEN genotype. Apoptosis.
19:895–904. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu M, Li CM, Chen ZF, Ji R, Guo QH, Li Q,
Zhang HL and Zhou YN: Celecoxib regulates apoptosis and autophagy
via the PI3K/Akt signaling pathway in SGC-7901 gastric cancer
cells. Int J Mol Med. 33:1451–1458. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xue M, Ji X, Xue C, Liang H, Ge Y, He X
and Zhang L, Bian K and Zhang L: Caspase-dependent and
caspase-independent induction of apoptosis in breast cancer by
fucoidan via the PI3K/AKT/GSK3β pathway in vivo and in vitro.
Biomed Pharmacother. 94:898–908. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yang Q, Wen L, Meng Z and Chen Y: Blockage
of endoplasmic reticulum stress attenuates nilotinib-induced
cardiotoxicity by inhibition of the Akt-GSK3β-Nox4 signaling. Eur J
Pharmacol. 822:85–94. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Song X, Wang Z, Liang H, Zhang W, Ye Y, Li
H, Hu Y, Zhang Y, Weng H, Lu J, et al: Dioscin induces gallbladder
cancer Apoptosis by inhibiting ROS-Mediated PI3K/AKT signalling.
Int J Biol Sci. 13:782–793. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li Y, Huang X, Huang Z and Feng J:
Phenoxodiol enhances the antitumor activity of gemcitabine in
gallbladder cancer through suppressing Akt/mTOR pathway. Cell
Biochem Biophys. 70:1337–1342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Tuya N, Wang Y, Tong L, Gao W, Yu R and
Xue L: Trichosanthin enhances the antitumor effect of gemcitabine
in non-small cell lung cancer via inhibition of the PI3K/AKT
pathway. Exp Ther Med. 14:5767–5772. 2017.PubMed/NCBI
|