Introduction
Tumor necrosis factor-α (TNF-α) is a cytokine with
complex biological activity and an autoantigen that can be used as
a therapeutic target (1,2). In addition to killing tumor cells
in vitro and in vivo, TNF-α induces inflammatory
responses, exhibits anti-viral effects, regulates the immune
system, and promotes cell proliferation and activation (3). However, when TNF-α is overexpressed,
it and other inflammatory factors can lead to a variety of
pathological conditions, such as inflammatory bowel disease,
infectious diseases and cachexia (4-6).
Therefore, neutralizing excessive TNF-α expression in the body
provides an effective tool for the treatment of such diseases
(4-6). Currently, several drugs are used
clinically to treat diseases by neutralizing excess TNF-α,
including a TNF-α human-murine chimeric antibody (infliximab), a
TNF-α soluble receptor (etanercept), a human monoclonal antibody
(aladalimumab) and a recombinant humanized anti-TNF-α fragment
antibody (certolizumab) (5).
Among these, infliximab is the most commonly used exogenous
antibody, and has been approved for the clinical treatment of
Crohn's disease. Clinical trials have demonstrated that infliximab
exhibits positive effects in ulcerative colitis (UC), such as
lowering the use of corticosteroid and reducing the colectomy rate
in acute severe UC (7,8). However, there are certain issues
involved with the aforementioned drugs, including high dosage
requirements, long-term repeated use, the ability to easily induce
hypersensitivity, and high costs, which hinder their widespread use
(9). In addition, regarding the
shortcomings of such monoclonal antibodies, the journal Nature
published an article entitled 'Can super-antibody drugs be tamed?',
which reported that the potential harm of drug-induced immune
response has become a key factor limiting their use (10).
Currently, developing immunization vaccines against
human self-proteins (i.e., autologous vaccines) has become a novel
direction for anti-molecular immunotherapy rather than the use of
monoclonal antibody drugs (11).
As autologous molecules are autoantigens with small molecular
weights and monomeric structures, often resulting in reduced
immunogenicity; they cannot induce ideal immune responses in
vivo, particularly humoral immune responses (12). Insertion of the exogenous
universal helper T-cell epitope into the proper position of a
self-antigen can help break the immune restriction and induce
neutralizing antibodies against the self-molecule (13).
The newly proposed multiple antigenic polypeptide
(MAP) design uses lysine as the core matrix, which has a low
molecular weight and weak immunogenicity; it combines with a number
of (usually 4 or 8) antigen epitopes of the same or different
monomer peptides to form a dendritic structure. This pattern mimics
the natural epitope conformation and can also activate humoral
immunity to induce high titer and affinity antibodies without
re-coupling the carrier protein (14). Based on previous investigations
associated with MAP vaccines, the authors of the current study
successfully enhanced heparinase immunogenicity using the MAP
design, and obtained high-titer and -affinity anti-heparinase
antibodies in vivo (15,16).
The biological characteristics of TNF-α are not
species-specific; indeed, the murine (m)TNF-α is highly homologous
to human TNF-α and has a similar tertiary structure (17). Its precursor has 235 amino acid
residues containing a signal peptide with 79 amino acids. In the
current study, the mTNF-α B cell epitope was predicted using
several bioinformatics techniques based on the primary structure of
mTNF-α, and associated with the interleukin-1β (IL-1β) 163-171
universal helper T-cell epitope peptide (18); then, the eight-branched MAP design
was used to synthesize a complex MAP vaccine. Next, the
immunological effects of the produced vaccine were assessed in
vitro and in vivo. Additionally, the impact of the
vaccine on disease active index (DAI), serum diamine oxidase (DAO)
levels, protein levels of colonic tissue claudin 1 and other tight
junction proteins, myeloperoxidase (MPO) activity, and epithelial
ultrastructural injuries were assessed in BALB/c mice with UC. The
aim of the present study was to provide a basis for the development
of novel TNF-α vaccines against UC.
Materials and methods
Materials
The following materials were used: mTNF-α
recombinant protein (eBioscience; Thermo Fisher Scientific, Inc.,
San Diego, CA, USA); polyclonal rabbit anti-mouse TNF-α full length
protein antibodies (cat. no. 17590-1-AP; Proteintech Tech Group,
Inc., Chicago, IL, USA); Freund's complete and incomplete adjuvants
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin (IgG) (cat.
no. BA1003; Wuhan Boster Biological Technology, Ltd., Wuhan,
China); anti-claudin 1 (cat. no. EPRR18871), -occludin (cat. no.
EPR8208), -ZO1 (cat. no. EPR19945-296; Abcam, Cambridge, UK) and
GAPDH (cat. no. 10494-1-AP; Proteintech Tech Group, Inc.)
antibodies, a western blotting chemiluminescence kit (Kangchen
Bio-tech Inc., Shanghai, China); lactate dehydrogenase (LDH) assay
kit (Jiancheng Bioengineering Institute, Nanjing, China) and
protein markers (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
were used. Mouse H22 hepatocarcinoma cells, which highly express
mTNF-α, were obtained from Huazhong University of Science and
Technology (Wuhan, China), and mouse L929 fibroblasts from American
Type Culture Collection (Manassas, VA, USA). BALB/c mice (n=48, 3-4
weeks old, 15-18 g; n=30, 4-5 weeks old; 18-20 g) were purchased
from Shanghai Sipper-BK Laboratory Animal Co. Ltd. [production
license no. SCXK (Shanghai) 2013-0016] and housed in the barrier
environment of Experimental Animal Center in Zhejiang Province [use
license no. SYXK (Zhejiang) 2014-0008]. The mice were kept in
Plexiglas® cages placed on a laminar flow ultra-clean
rack. A total of 3-4 mice were housed in each cage at a constant
temperature (25-27°C) and humidity (45-50%), under a 12-h
light/dark cycle with free access to food and sterilized water.
Water and food consumption volumes were recorded daily. The animal
studies were approved by the Ethics Committee of Zhejiang Chinese
Medical University (approval no. ZSLL-2011-95; Hangzhou, China).
Furthermore, 0.3% sodium pentobarbital was administered
intraperitoneally at a dose of 30 mg/kg to minimize perioperative
animal suffering. All animals survived till the end of the
experiments. At the end of the experiments (after 7 or 10 weeks),
all animals were anesthetized intraperitoneally with 0.3% sodium
pentobarbital (60 mg/kg), and then euthanasia was performed by
cervical dislocation.
Complex vaccine preparation
Epitope prediction
The antigenic epitopes of mouse TNF-α were predicted
using Predicting Antigenic Peptides software (http://www.mif.dfci.harvard.edu/Tool/antigenic.pl;
Harvard University, Cambridge, MA, USA), using the semi-empirical
Kolaskar and Tongaonkar methods (19), to initially determine the B cell
epitope region of mTNF-α. The 235 amino acid sequences of the
mTNF-α sequence, particularly those within the regions determined
using the Predicting Antigenic Peptides software, were then
analyzed by DNAStar 7.1 (DNASTAR, Inc., Madison, WI, USA) and the
BcePred online prediction tool (http://www.imtech.res.in/raghava/bcepred/) to evaluate
the physical and chemical properties of epitope region proteins,
including hydrophilicity, accessibility, plasticity and
antigenicity. Finally, the most likely amino acid sequence of the
predominant mTNF-α B-cell epitope was selected.
MAP synthesis
MAP synthesis was performed by Hangzhou Peptide
Biochemical Co., Ltd. on an ABI431A peptide synthesizer
(Perkin-Elmer, Inc., Waltham, MA, USA) using the eight-branched MAP
resin. The eight-branched MAP core structure was synthesized based
on the Fmoc synthesis scheme (20). The predicted predominant mTNF-α
B-cell epitope peptide and IL-1β universal helper T-cell epitope
peptide VQGEESNDK were synthesized and connected by lysine to form
a complex peptide. Then, the resulting molecule was coupled with
eight amino terminals of the eight lysine residues located at the
end of the MAP core structure. In accordance with amino acid
sequences of peptides, the amino acid was sequentially connected
one by one from the carboxy-terminus to the amino terminal end. The
collected sample was then subjected to low pressure rotary
evaporation at a constant temperature (4°C) and freeze-dried
overnight, to yield the lyophilized eight-branched TNF-α B cell
epitope-IL-1β T helper epitope complex MAP vaccine. Additionally,
an eight-branched MAP-VQGEESNDK sequence was also synthesized in
the same way, as a control, and simply consisted of eight T helper
peptide.
Immunological effects of the complex MAP
vaccine Binding affinity
The binding affinity of the complex MAP vaccine to
the mTNF-α whole protein antibody was determined using 10 mg/l of
the predicted epitope peptide, the complex MAP vaccine,
MAP-VQGEESNDK or the mTNF-α recombinant protein to coat 96-well
ELISA plates (100 µl/well) overnight at 4°C. Following
blocking with 1% bovine serum albumin (BSA; cat. no. LS000290; US
Biochemical Corp., Cleveland, OH, USA), 0.06 µg/100
µl/well of the rabbit anti-mouse full-length TNF-α antibody
was added, with 1:4,000 normal BALB/c mouse serum (Zhejiang Chinese
Medical University, Hangzhou, China) serving as a control. The
absorbance of the plates was measured at a wavelength of 490 nm
using the Imark Micoplate Absorbance Reader 13550 (Bio-Rad
Laboratories Inc.), the experiment was repeated three times.
Immunogenicity of the complex MAP
vaccine
A total of 48 male BALB/c mice (21) (3-4 weeks old, 15-18 g) were
randomly divided into four groups of 12: Epitope peptide, complex
MAP vaccine, mTNF-α recombinant protein and MAP-VQGEESNDK. The
mTNF-α recombinant group served as the control. The four peptides
were used as immunogens. The first immunization used Freund's
complete adjuvant, while boost vaccinations included Freund's
incomplete adjuvant. The initial and boost doses in the epitope
peptide, the MAP-VQGEESNDK and the complex MAP vaccine groups were
0.2 mg/mouse; 20 µg/mouse was used in the mTNF-α recombinant
protein group. Each immunogen was dissolved in 0.5 ml of PBS and
emulsified with 0.5 ml Freund's adjuvant prior to use for multiple
subcutaneous injections into the backs of the animals. Two weeks
after each vaccination, orbital blood samples were collected, and
the mice were euthanized. A total of five injections were
administered at 2-week intervals and blood was collected six times
for serum isolation. Next, antibody titers in serum were determined
using standard indirect enzyme linked immunosorbent assay (ELISA).
The 96-well ELISA plates were coated with 10 mg/l epitope peptide,
complex MAP vaccine, MAP-VQGEESNDK and mTNF-α recombinant whole
protein at 100 µl/well overnight at 4°C. After blocking with
1% BSA for 1 h at 37°C, the sections were incubated with polyclonal
rabbit anti-mouse TNF-α full length protein antibodies (dilution,
1:4,000) for 1 h at 37°C and then horseradish peroxidase-conjugated
goat anti-rabbit IgG (dilution, 1:4,000) for 1 h at 37°C to test
the immunogens; the pre-immunized serum was used as a negative
control. The ratio of the absorbance of the test sample to that of
the negative control was calculated, and a value >2.1 indicated
a positive signal; the highest dilution that yielded a positive
reaction was considered the antibody titer of the sample.
Western blot analysis
Following centrifugation, 1×109 murine
H22 cells and 1 ml of ice-cold suspension buffer was added. The
cells were then dispersed with a vortex oscillator for 5 min before
centrifugation at 1,006 × g for 5 min at 4°C. The supernatant was
discarded, and an equal volume of 2X SDS was immediately added to
the extract total protein. SDS-PAGE was performed on a 5% gel, and
a total of 35 mg protein per lane was analyzed, with the commercial
mTNF-α recombinant protein used as the positive control and the
pre-immunized mouse serum serving as a negative control. Western
blotting was performed following the manufacturer's protocol.
Immunoreactive bands were detected using chemiluminescence reagent
(Shanghai Kangchen Bio-tech Inc.).
TNF-α inhibitory effects of MAP
antiserum
Growing L929 cells were adjusted to a density of
5×107 cells/ml and 100 µl of the cells was
inoculated in each well of 96-well plates for 12 h. The antiserum
and mTNF-α in each group were diluted with 0.5 mg/l actinomycin D,
which increases the sensitivity of L929 cells to TNF-α
cytotoxicity. mTNF-α at 400 µg/l was added into each well,
which contained 10 µl of the epitope peptide antiserum, the
complex MAP vaccine antiserum, the MAP-VQGEESNDK antiserum and the
mTNF-α recombinant protein antiserum at a dilution of 1:500. In the
control wells, only the cell culture medium containing 0.5 mg/l
actinomycin D was added. After 24 h, supernatants were collected,
the absorbance was measured in triplicate at 450 nm on a microplate
reader (BioTek Instruments, Inc., Winooski, VT, USA) and the
cytotoxicity (%) of L929 cells were quantified by the LDH activity
according to the following formula: [(LDH)
E-(LDH)TS]/[(LDH)TSmax-(LDH)TS] ×100. Where, (LDH) E is the level
of experimental LDH release, (LDH)TS is the level of target
spontaneous LDH release and (LDH)TSmax is the target maximum level
of LDH release. Spontaneous LDH release was evaluated by incubating
target cells free of mTNF-α, while maximum release was assessed by
the presence of maximum mTNF-α.
Therapeutic effects of the complex MAP
vaccine in UC mice Immunization and modeling
A total of female BALB/c mice (4-5 weeks old; 18-20
g) were divided into three groups (n=10) according to body weight:
The immunization, the UC model and the blank control groups. There
was no significant difference in body weight among groups.
In the immunization group, mice were multi-point
immunized subcutaneously with the complex MAP vaccine on day 1. The
immunization dose was 0.2 mg/mouse with Freund's complete adjuvant,
followed by two booster immunizations with Freund's incomplete
adjuvant (22). The adjuvant and
antigen were fully mixed at a ratio of 1:1 and administered every 2
weeks. BALB/c mice were treated with equal volumes of PBS in the UC
model group, and the immunization scheme was the same as in the
immunization group.
The UC model was established by free access to 5%
dextran sulfate sodium (DSS) solution. After 4 weeks (corresponding
to the third immunization), mice in the immunization and UC model
groups were administered DSS for 7 days, while mice in the blank
control group drank distilled water.
DAI scoring
After drinking the DSS solution, alterations to
mouse weight and stool properties were observed daily, and fecal
occult blood was detected using the test paper method (23). A total of 3 weeks from the start
of DSS administration, data were scored as indicated in Table I. Scores of weight loss, stool
traits and fecal blood were added to determine the mean DAI score
for each mouse; thus, evaluating the disease status of the animals
(23). Meanwhile, antibody titers
in mice were detected weekly.
 | Table IMouse DAI scoring table. |
Table I
Mouse DAI scoring table.
| Score | Weight loss
(%) | Stool
traitsa | Blood stool |
|---|
| 0 | None | Normal | Normal |
| 1 | 1-5 | Loose | Occult blood
(+) |
| 2 | 5-10 | | |
| 3 | 10-15 | Diarrhea | Visible blood |
| 4 | >15 | | |
Determination of serum DAO content
Mice were intraperitoneally injected with 30 mg/kg
1% sodium pentobarbital to induce deep anesthesia. The animals were
sacrificed 3 weeks after drinking DSS. Specimens were collected
after mice had fasted for 12 h with free access to water. Eyes were
removed and orbital blood samples were collected repeatedly with a
siphon to obtain ~1 ml of blood, which was added to centrifuge
tubes containing 1% heparin. The blood samples were centrifuged at
1,006 × g for 5 min at 4°C to obtain serum. Serum DAO content was
determined using the double antibody sandwich avidin biotin
peroxidase complex (ABC)-ELISA (cat. no. ab155458; Abcam) method
(24). Optical density values
were measured at 450 nm and DAO concentrations were determined
using a standard curve.
Observation of bacterial translocation in
mesenteric lymph nodes
After blood collection, the mice were dissected.
Approximately 200 mg of mesenteric lymph nodes were extracted under
aseptic conditions and homogenized in a sterile grinder. After
centrifugation at 300 × g for 5 min at 4°C, 100 µl of the
supernatant was inoculated on a common blood agar dish for
quantitative culturing. The culture plates were incubated at 37°C
for 48 h and the mean number of colonies in the three groups were
compared.
Expression levels of tight junction
proteins
Immunoblotting was used to assess the expression
levels of tight junction proteins, including claudin 1, occludin
and ZO1, in colon tissue specimens. Approximately 0.5 cm of mouse
colon was added to the protein extraction buffer, homogenized with
a homogenizer for 30 min at 0°C and centrifuged at 21,910 × g for
15 min at 4°C. The supernatants were collected, and protein amounts
were quantified using the Lowry method.
Equal amounts of total protein (30 µg/lane)
were separated by SDS-PAGE on a 5% gel; the protein bands were
transferred onto nitrocellulose membranes. Non-specific binding was
blocked with 5% milk, which was incubated with the membranes for 2
h at 4°C. The membranes were then incubated with anti-claudin 1,
anti-occludin, anti-ZO1 antibodies (dilution, 1:1,000) and
anti-GAPDH (dilution, 1:10,000) at 4°C overnight. This was followed
by incubation with a horseradish peroxidase-conjugated secondary
antibody (dilution, 1:10,000) for 2 h at room temperature.
Detection was performed with an enhanced chemiluminescence kit.
GAPDH was used as an internal control. BandScan 5.0 software (Glyko
Inc., Novato, CA, USA) was used to analyze the gray scale values of
different bands.
Histological damage pathology
scoring
Approximately 1 cm of distal colon was fixed in 10%
formaldehyde. This was followed by conventional paraffin embedding,
slicing (4-µm-thick sections), and hematoxylin and eosin
(H&E) staining. Histological changes, including colonic mucosal
ulcers, interstitial edema, and nucleated cell infiltration were
observed using a Nikon Eclipse 400 microscope (Nikon Corporation,
Tokyo, Japan) at a magnification of ×200. The severity of colonic
tissue inflammation, depth of lesions, and crypt and epithelial
damage were evaluated by two senior pathologists. The degree of
histological damage was expressed by the histological index (HI;
Table II) (25).
 | Table IIStandard of histological index. |
Table II
Standard of histological index.
| Score | Inflammation | Depth of the
lesion | Crypt
destruction |
|---|
| 0 | None | None | None |
| 1 | Mild | Mucosal
muscularis | 1/3 basement Crypt
destruction |
| 2 | Severe | Muscular layer | 2/3 basement Crypt
destruction |
| 3 | - | Serous layer | Only complete
surface epithelium |
| 4 | - | - | All epithelial
damage |
Ultrastructure of mucous epithelium
Approximately 0.5 cm of distal ileum was fixed in
2.5% glutaraldehyde at 4°C for 6 h. Electron microscope specimens
were routinely prepared, and stained with uranium acetate and lead
citrate. All the slices were sectioned using the LKB-V type
ultramicrotome (LKB Bromma; GE Healthcare Life Sciences, Little
Chalfont, UK). The ultrastructural of mucous epithelium was
observed under the JEM-1010 type transmission electron microscope
(TEM; JEOL, Ltd., Tokyo, Japan).
Determination of MPO activity in the
colon tissue
MPO is an enzyme in neutrophil azurophilic granules,
and its activity reflects the number and activity of neutrophils.
To determine MPO activity, mouse colon tissue was accurately
weighed. Then, homogenization medium was added to the tissue based
on a tissue weight (g):volume (ml) ratio of 1:19; the resulting
solution was mixed to prepare a 5% tissue homogenate mixture.
According to the manufacturer's protocol of MPO assay kit (cat. no.
A044; Jiancheng Bioengineering Institute), the reagents were added
in order. Briefly, the prepared tissue mixture was added to 50
mmol/l phosphate buffer (pH 6.0) containing 0.5%
hexadecyltmethyl-ammonium bromide. The sample was then centrifuged
at 16,099 × g for 20 min at 4°C and the supernatant was used for
the MPO assay by utilizing 0.0005% hydrogen peroxide as a
substrate. Distilled water was used to as the blank control and
absorbance was then determined at 460 nm. Results were expressed as
units per gram weight (U/g) of wet tissue.
Statistical analysis
Statistical analysis was performed using the SPSS
17.0 software (SPSS, Inc.). Quantitative data are presented as mean
± standard deviation. Multiple samples were compared by one-way
ANOVA with the Student-Newman-Keuls post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Prediction of mTNF-α epitopes and complex
MAP vaccine synthesis
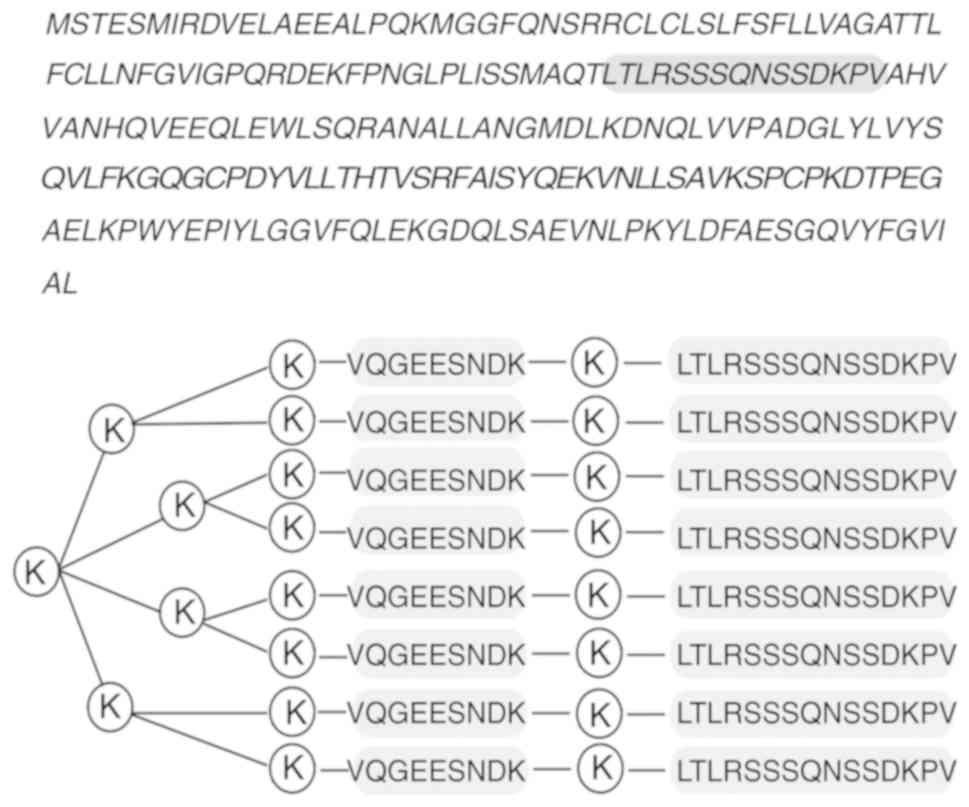
The amino acid sequence of the mTNF-α protein was
obtained from Genbank (Fig. 1).
From the results obtained with the Predicting Antigenic Peptides
software, the mouse TNF-α B-cell epitopes were located within amino
acids 10-20, 46-103, 111-125 and 146-153. The selected region of
the mTNF-α sequence was analyzed by DNAStar and Bcepred software.
The results showed that the LTLRSSSQNSSDKPV peptide in the gray
background composed of amino acids 78-92 had the best
hydrophilicity, accessibility and plasticity. Moreover, it was
located within the protein extension structure or irregular curl
structure, constituting the most likely dominant B-cell epitope.
Therefore, the TNF-α B epitope-IL-1β helper T-cell epitope complex
MAP vaccine based on the latter predicted amino acid sequence was
synthesized. Fig. 1 depicts the
structure of the complex MAP vaccine, which was purified by high
pressure liquid chromatography, to achieve a purity of ≥95%.
Binding affinity of the complex MAP
vaccine to a TNF-α whole protein antibody
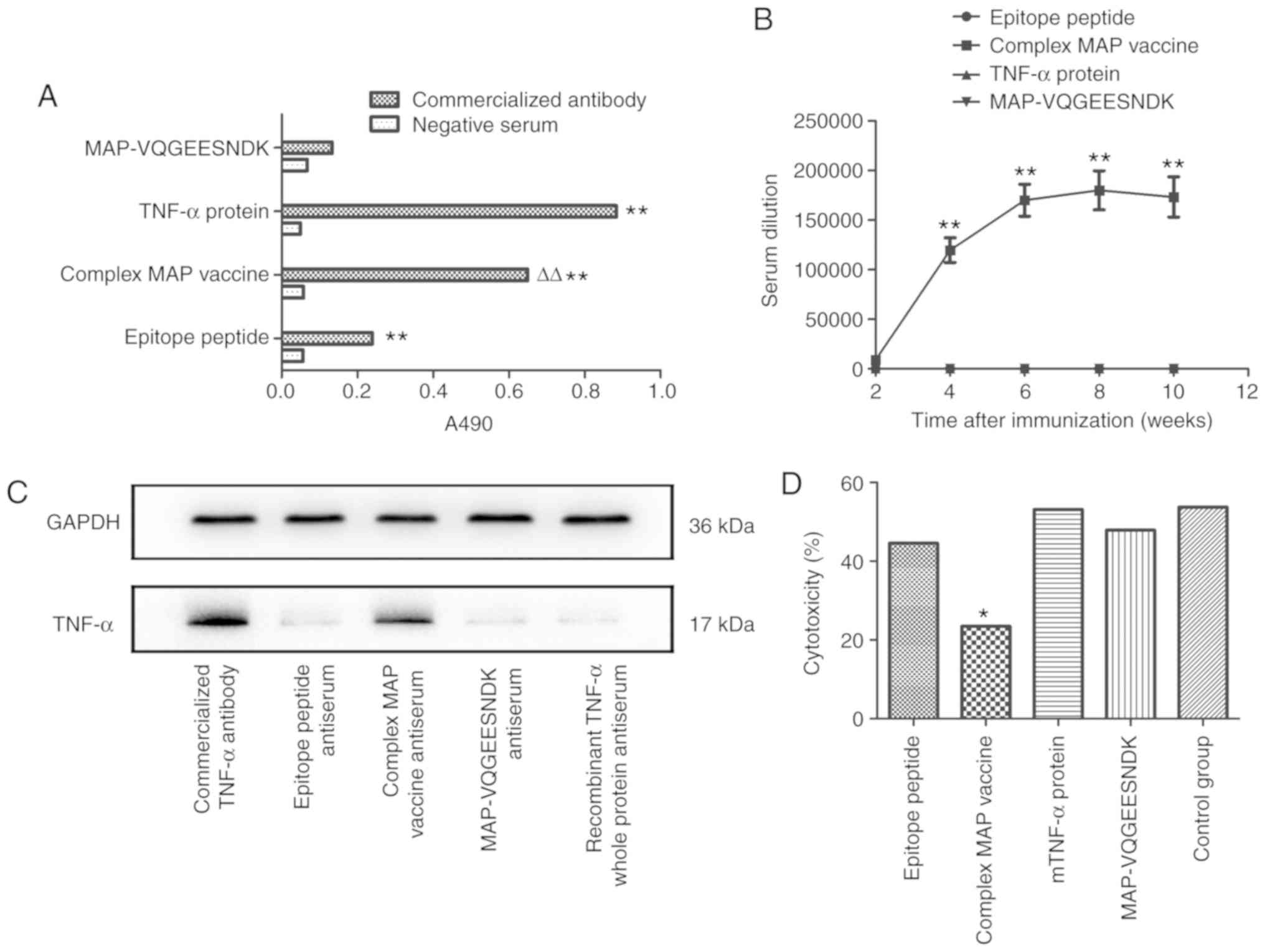
The binding affinity was detected by indirect ELISA,
and the results demonstrated that the synthesized complex MAP
vaccine had strong binding affinity with the mTNF-α whole protein
antibody in vitro. Its binding affinity with the antibody
was weaker compared with that of the mTNF-α recombinant protein,
but stronger compared with that of the epitope oligopeptide or
MAP-VQGEESNDK (P<0.01; Fig.
2A). These findings indicated that the eight-branch MAP design
and the insertion of the IL-1β helper T-cell epitope peptide
significantly enhanced the antigenicity of the predicted mTNF-α
B-cell epitope peptide LTLRSSSQNSSDKPV, and the mTNF-α B cell
epitope oligopeptide or helper T-cell epitope polypeptide,
VQGEESNDK, did not have binding affinity with the mTNF-α whole
protein antibody when compared with negative serum control.
Dynamic changes of antibody titers
Specific antibodies were detected from 2 weeks after
the first immunization with the complex MAP vaccine. Antibody
titers increased rapidly to ~1:119,000 after 4 weeks. After 6
weeks, antibody titers plateaued with a value of 1:168,000. In week
8, antibody titers reached its peak value, which declined steadily
to 1:173,000 by week 10. The epitope peptide, mTNF-α recombinant
protein and MAP-VQGEESNDK groups produced no detectable specific
antibodies (Fig. 2B), and the
IL-1β helper T-cell epitope peptide and MAP vaccine groups showed
no cross reaction. The aforementioned finding showed that a high
titer of antibody can be induced by the complex MAP vaccine.
Specificity of the antibody verified by
western blot analysis
Total protein from H22 cells was incubated with the
immunized epitope peptide, complex MAP vaccine, MAP-VQGEESNDK and
commercial mTNF-α recombinant protein antibody antiserum samples. A
rabbit anti-mouse TNF-α antibody was used as a positive control;
GAPDH was used as internal reference gene. Incubation with the
commercial mTNF-α recombinant protein antibody produced a thick
band of ~17 kDa and the band in the MAP vaccine group was also
evident. According to the specification of the commercial antibody,
17 kDa corresponds to the mTNF-α protein. The mTNF-α recombinant
protein, MAP-VQGEESNDK and epitope peptide groups did not show
overt bands in the corresponding location (Fig. 2C). The aforementioned results
suggested that the immunized antibody in the complex MAP vaccine
specifically binds to the TNF-α protein.
Effects of various antisera on TNF-α
activity
The viability of L929 cells with recombinant mTNF-α
was detected using the LDH release assay to assess the inhibitory
effects of the antisera on the biological activity of mTNF-α. The
results showed that the cytotoxicity in the epitope peptide,
complex MAP vaccine, mTNF-α, MAP-VQGEESNDK antiserum groups and the
control group were 44.59, 23.47, 53.16, 47.93 and 53.80,
respectively. This indicated that cytotoxicity measured by the LDH
activity was significantly lower in the complex MAP vaccine
antiserum group compared with in the other four groups (P<0.01)
and that TNF-α activity in the complex MAP vaccine group was
primarily inhibited by the specific antibody contained in the
antiserum. Compared with the control group, the MAP-VQGEESNDK,
epitope peptide or mTNF-α antiserum groups showed no significant
differences (Fig. 2D).
Effects of vaccination on DAI scores of
UC mouse
The DAI score of the blank control group was 0
throughout the experiment. The DAI score of the UC model group was
0 for the first 2 days of 5% DSS consumption. DAI scores gradually
increased with the duration of DSS consumption, peaking at 9.12 on
day 7. After DSS termination, the values began to decline and
dropped to 5.69 on day 21. The DAI score of the immunization group
was 0 for the first 3 days of 5% DSS consumption. It gradually
increased with the duration of DSS consumption and peaked at 6.92
on day 7; the values decreased to 1.03 on day 21 (Fig. 3). Antibody titers gradually
increased over 3 weeks. As the antibody titers increased, the
difference between the DAI scores of the immunized and UC groups
gradually increased. The results showed that DSS induced less
colonic damage in the immunization group, and repair of the colonic
damage in the immunization group was faster and more efficient
compared with the UC model group.
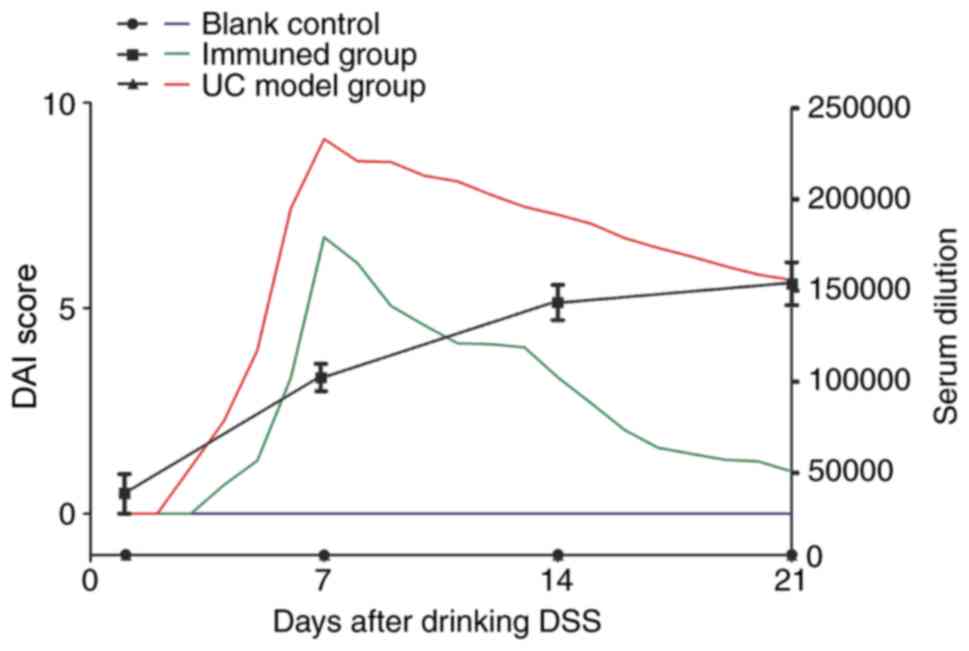 | Figure 3Mice DAI scores and serum antibody
titers after drinking DSS. From the beginning of DSS
administration, DAI scores were recorded daily and serum antibody
titers weekly for a total of 3 weeks. Throughout the whole process,
the DAI score of the blank control group was 0; No antibody titers
were detected in the blank control group and the UC model group.
The DAI score of the UC model control group was 0 the first 2 days
of 5% DSS consumption, reaching the highest value of 9.12 on day 7;
the values gradually decreased after DSS discontinuation. On day
21, scores averaged 5.69. The DAI score of the immunization group
the first 3 days was 0, reaching the highest value of 6.92 on day
7, before declining significantly to 1.03 on day 21. Antibody
titers in the immunization group was 1:4,900 on day 1. Then, the
antibody titers increased to 1:115,000 on day 7, 1:14,900 on day 14
and 1:15,100 on day 21. As the antibody titers increases, the
difference between the DAI score of the immunization group and the
DAI score of the UC model group gradually increases. UC, ulcerative
colitis; DSS, dextran sulfate sodium; DAI, disease active
index. |
Serum DAO levels in immunized mice
To assess the permeability of the intestinal mucosa
in mice, serum DAO levels in mice were measured using the double
antibody sandwich ABC-ELISA method at 7 weeks after the first
immunization. Serum DAO levels in UC model group were 3.38±0.54 and
1.56±0.37 U/ml in the immunization group, a statistically
significant difference was identified between the two groups
(P<0.05). Compared with the blank control group (1.06±0.29
U/ml), no significant difference was identified in the immunization
group (Fig. 4A). These results
indicate that the complex MAP vaccine can protect intestinal
mucosal cells in mice.
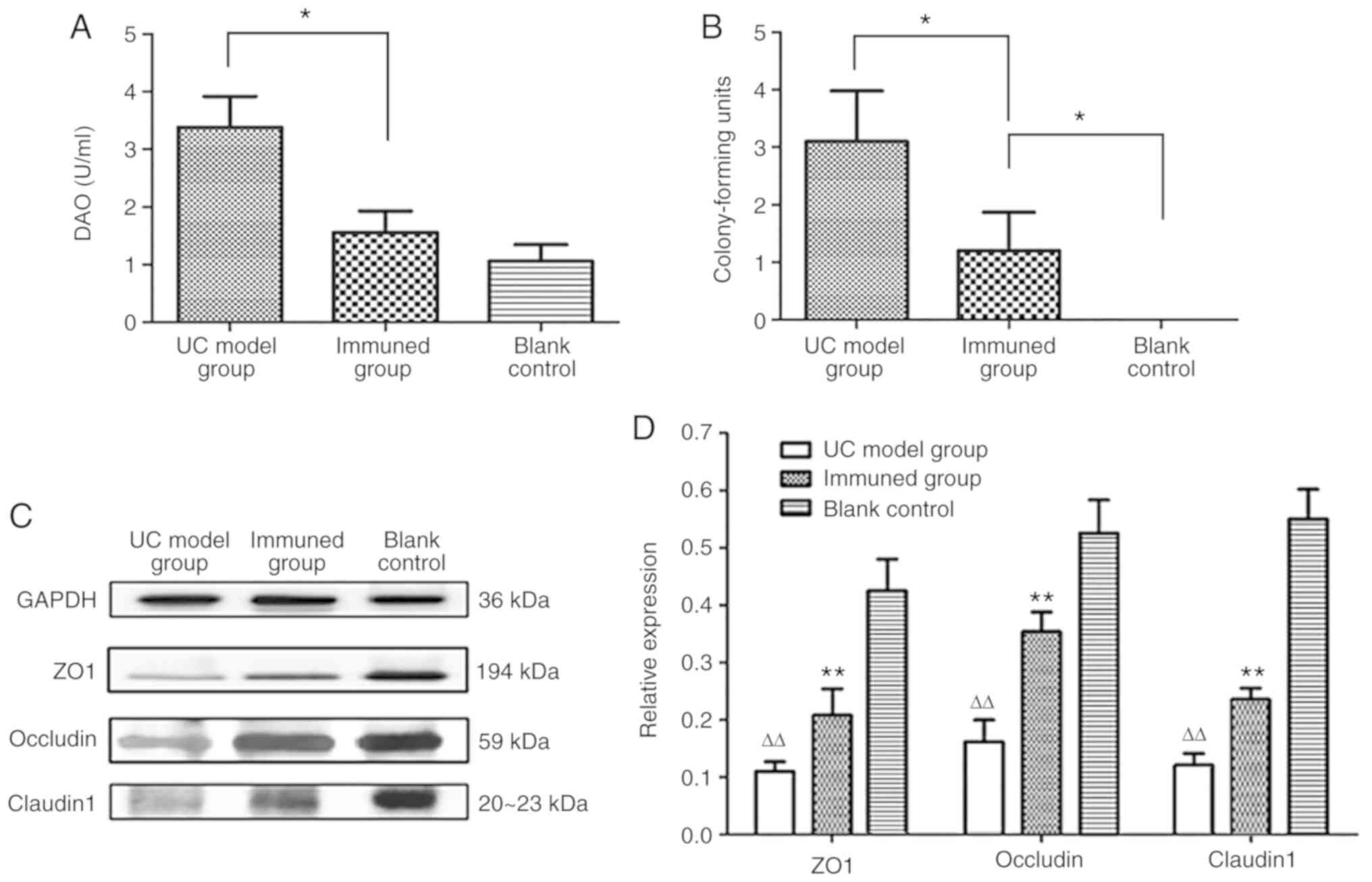 | Figure 4Assessment of intestinal mucosal
permeability. (A) The serum DAO content of each group was
determined by double antibody sandwich ABC-ELISA. The serum DAO
content was 3.38±0.54 U/Ml in the UC model group, 1.56±0.37 U/Ml in
the immunization group, and 1.06±0.29 U/Ml in the blank control
group, indicating significantly lower levels in the immunization
group compared with the UC model group, *P<0.05, vs.
UC model group. The serum DAO level in the immunization group is
slightly higher than the blank control group.
*P<0.05, vs. blank control group. (B) Mouse
colorectal lymph nodes were harvested, and bacterial colonies were
counted after culture. The blank control group had no bacterial
growth. The colony count of mesenteric lymph nodes in the model
group was 3.1±0.88, while 1.2±0.67 was obtained for the
immunization group. The accounts in the latter group was higher
than the blank control group, but significantly lower compared with
the UC model group. *P<0.05 vs. UC model group or
blank control group. (C) Western blotting was used to evaluate the
expression levels of claudin1, occludin and ZO1 in colon tissues.
With GAPDH as an internal reference, the ZO1 band was primarily
concentrated at about 194 kDa, while occludin and claudin 1 were
found ~59 kDa, and 20-23 kDa, respectively. (D) The Western
blotting results were also analyzed by the BandScan 5.0 software.
Compared with the blank control group, the expression levels of
ZO-1, occludin and claudin1 were decreased in the UC model group
(∆∆P<0.05). In the immunization group, the protein
expression was lower compared with the blank control group, but was
higher compared with the UC model group, which indicating that
immunization with the complex MAP vaccine upregulated ZO-1,
occludin and claudin1 (**P<0.05 vs. UC model group or
blank control). DAO, serum diamine oxidase; UC, ulcerative colitis;
MAP, multiple antigenic polypeptide. |
Bacterial growth in mesenteric lymph
nodes after immunization
Following blood collection in week 7, the mice were
dissected. Mesenteric lymph nodes were collected and homogenized;
the colonies were then counted following inoculation and culture.
The permeability of the intestinal mucosa was evaluated by
comparing the mean number of colonies among the three groups of
mice. The results showed that bacterial translocation did not occur
in the mesenteric lymph nodes in the blank control group. The
colony count of mesenteric lymph nodes in the UC model group was
3.1±0.88; a significantly reduced bacterial count was obtained in
the immunization group (1.2±0.67; P<0.05; Fig. 4B). The results showed that the
complex MAP vaccine can reduce intestinal bacterial translocation
to a certain extent.
Protein expression levels of claudin 1,
occludin and ZO1 in colon tissue samples after immunization
To evaluate the integrity of the mouse intestinal
mucosa, western blot analysis was used to detect the expression
levels of claudin1, occludin and ZO1 in colon tissue samples from
mice in each group. With GAPDH as an internal reference gene, the
ZO1 band was primarily concentrated at ~194 kDa, while occludin and
claudin 1 were found at ~59 kDa, and 20-23 kDa, respectively
(Fig. 4C). The results, analyzed
by the BandScan 5.0 software, showed that the expression levels of
claudin 1, occludin and ZO1 in samples from the UC model group were
significantly lower compared with those of the blank control group
(P<0.05). Compared with the blank control group, the expression
levels of claudin1, occludin and ZO1 in the immunization group were
also decreased, but significantly higher compared those in the UC
model group (P<0.05; Fig.
4D).
Histological damages and HI scores after
immunization
In the UC model group (Fig. 5A), H&E staining of murine
colon samples showed incomplete colon mucosa, multifocal ulcers
with exudation, primarily damaged epithelial cells and glands, a
large number of infiltrated inflammatory cells, evidently decreased
goblet cells, submucosal edema, lymph node aggregation, and crypt
abscesses. In the blank control group (Fig. 5B), the colonic mucosa was intact
with the glands neatly arranged. There was no evident inflammatory
cell infiltration. The colonic mucosa of the immunization group was
relatively intact, and few shallow ulcers were observed (Fig. 5C). The majority epithelial cells
and glands were intact with limited inflammatory cell infiltration;
no evident edema was observed in the submucosal layer. The mean HI
score of the UC model group was 6.75±0.62, which was significantly
higher compared with that of the blank control group. The mean HI
score of the immunization group was 2.95±0.37, which was
significantly lower compared with that of the UC model group
(Fig. 5D).
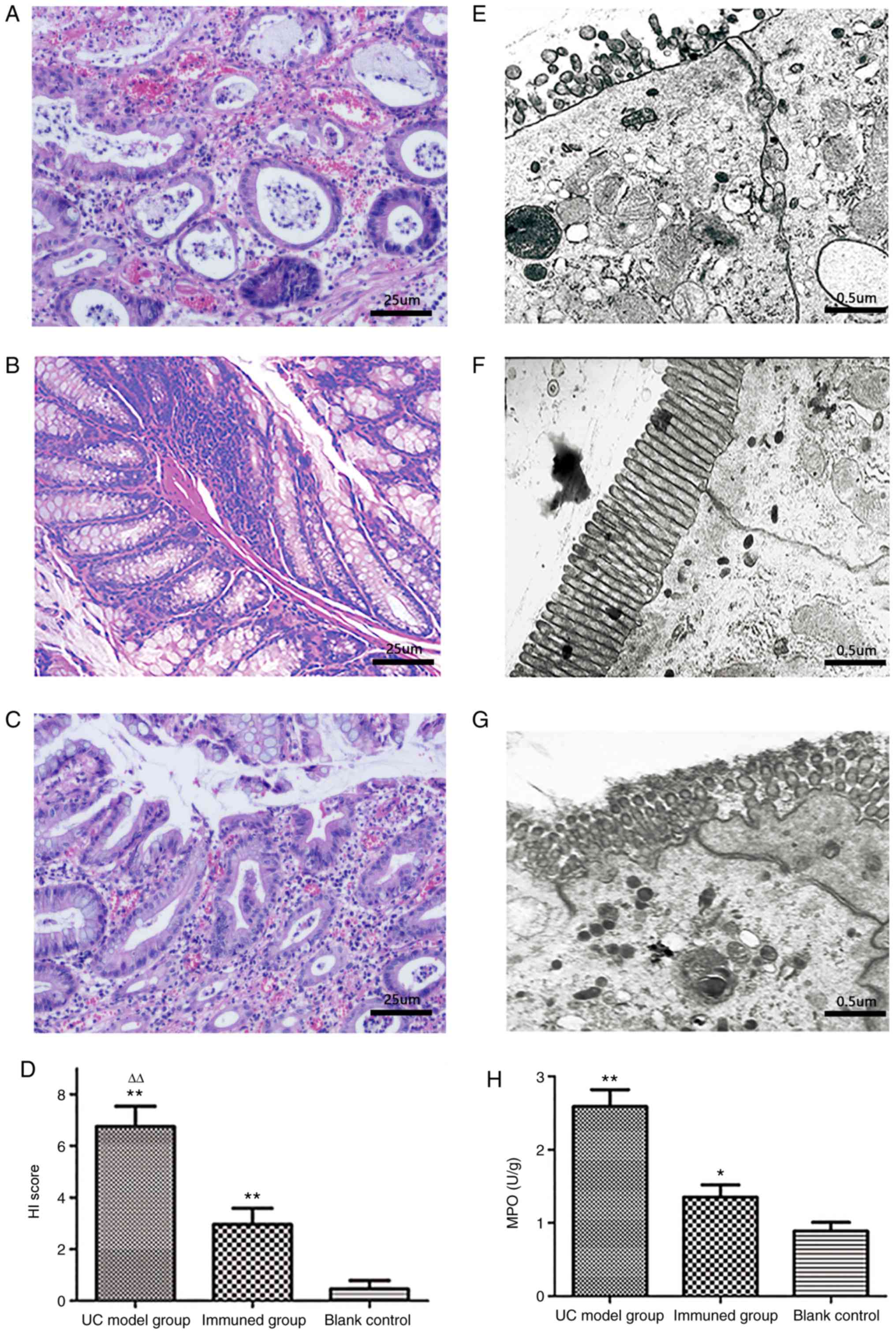 | Figure 5Evaluation of histological injury in
murine intestine. (A) The UC model group had no complete colonic
mucosa, with massive purulent exudate and mucosal hemorrhage; most
goblet cells and glands were damaged, as well as evident
inflammatory cells infiltration and crypt abscess (magnification,
×200). (B) The colonic mucosa of the blank control group was intact
with glands neatly arranged. There was no evident inflammatory cell
infiltration (magnification, ×200). (C) The colonic mucosa of the
immunization group was relatively intact with few inflammatory
cells infiltrated; goblet cells were slightly reduced
(magnification, ×200). (D) Histological damage HI scores. HI scores
in the UC model group were 6.75±0.62, significantly higher compared
with those of the blank control group. HI scores of the
immunization group were 2.95±0.37, significantly higher compared
with those of the blank control group and markedly lower compared
with those of the UC model group. **P<0.01 as vs.
blank control, ∆∆P<0.01 as vs. immunization group.
(E) Detected under TEM, evident cellular edema, atrophic and sparse
microvilli, shortened and broadened tight junction complex and
enlarged intercellular gaps were observed in the UC model group
(magnification, ×10,000). (F) In the blank control group, the
columnar mucosal epithelia were intact with microvilli neatly and
densely arranged, and the tight junction complexes were closely
connected without visible gaps (magnification, ×10,000). (G) The
ultrastructure of ileac mucous epithelia in the immunization group
were relatively normal with slight edema, and the slightly atrophic
microvilli were a bit confusedly arranged. Intercellular tight
junction complex was rather clear and intact (magnification,
×10,000). (H) MPO activity in colon tissue homogenates after
immunization. MPO activity in colonic homogenates from the UC model
group was significantly higher than that of the blank control and
immunization groups. MPO activity in the immunization group was
higher than that of the blank control group, but markedly lower
compared with that of UC model group. *P<0.05 vs.
blank control, **P<0.01 vs. immunization group or
blank control. UC, ulcerative colitis; MAP, multiple antigenic
polypeptide; TEM, transmission electron microscope; HI,
histological index; MPO, myeloperoxidase. |
Epithelial ultrastructure observed by
TEM
In the UC model group (Fig. 5E), evident mucous epithelium
injuries were detected by TEM, such as severe cellular edema,
atrophic and sparse microvilli, shortened and broadened tight
junction complex, and enlarged intercellular gaps. In the blank
control group (Fig. 5F), the
columnar mucosal epithelia were intact with microvilli neatly and
densely arranged, and the tight junction complexes were closely
connected without visible gaps. Compared with the UC model group,
the ultrastructure of ileac mucous epithelia in the immunization
group was relatively normal with slight edema in a few epithelial
cells, and the slightly atrophic microvilli were arranged a bit
disorderly (Fig. 5G).
Intercellular tight junction complexes were evident and intact.
MPO activity in colon tissue homogenates
after immunization
To evaluate the degree of colon inflammation in
mice, the authors determined MPO activity in colon tissue
homogenates from various groups. The results showed that MPO
activity in the UC model group was 2.59±0.23, which was
significantly higher compared with that in the blank control group
(0.89±0.12) and immunization group (P<0.01), indicating that
mice in the UC model group had more severe colon inflammation. MPO
activity in colonic homogenates from the immunization group was
1.35±0.17; although it was significantly higher compared with that
of the blank control group (P<0.05), this value was
significantly lower compared with that of the UC model group
(P<0.01) (Fig. 5H). These
findings indicated that the level of colon inflammation in the
immunization group was lower compared with that in the UC model
group.
Discussion
TNF-α has attracted increasing attention as a
promising autologous molecule for UC treatment (7,8,26-29). The current study aimed to induce
high antibody titers in vivo by optimizing the immunization
strategies. Using the eight-branched MAP design and inserting the
IL-1β helper T-cell epitope peptide significantly enhanced the
antigenicity of the predicted TNF-α B-cell epitope peptide
LTLRSSSQNSSDKPV. After BALB/c mouse immunization with this complex
MAP vaccine, polyclonal antisera were obtained. ELISA showed that
the eight-branched MAP could induce high TNF-α antibody titers
in vivo, which significantly increased to 119,000 after two
immunizations (4 weeks). This time point was selected for
subsequent UC experiments to assess the complex MAP vaccine in the
alleviation of UC as mice already had more anti-TNF-α antibodies.
However, the individual B cell epitope peptide, MAP-VQGEESNDK and
TNF-α protein could not induce detectable autoantibodies in
vivo, indicating that the designed complex MAP vaccine
conformation can effectively break through immune tolerance and
greatly enhance the immunogenicity of autoantigens.
As aforementioned, the complex MAP vaccine antiserum
showed a clear band at ~17 kDa, while the mTNF-α recombinant
protein, epitope peptide and MAP-VQGEESNDK antisera showed no
evident bands at the corresponding position. These results showed
that mTNF-α could not induce a specific humoral immune response
in vivo, and the eight-branched MAP design could enhance the
immunogenicity of the B-cell epitope LTLRSSSQNSSDKPV, inducing
polyclonal antibody specificity binding to TNF-α. The authors
further assessed the biological effect of the specific antibodies
using the LDH release assay, and confirmed that the complex MAP
vaccine induced specific antibodies and can significantly inhibit
the bioactivity of TNF-α.
Chey (30) applied
the TNF-α antibody infliximab to 16 patients with UC with 88% of
them demonstrating improved clinical symptoms, colonoscopy and
histology results for up to 4 months, indicating that infliximab
can alleviate the clinical symptoms of UC. In the UC mouse model,
the anti-TNF-α complex MAP vaccine significantly improved the body
weight, reduced the symptoms of bloody stool and diarrhea, and
decreased DAI scores, in agreement with the therapeutic effects of
TNF-α antibodies in passive immunotherapy of patients with UC.
Moreover, the immunization group showed a delayed increase in DAI
values and lower peak DAI values, and MPO activity in colonic
tissue in the immunization group was significantly lower compared
with the UC model group. Meanwhile, inflammatory cell infiltration
in the mouse colon tissue was reduced, the morphological structures
of mucosal epithelial cells and crypts improved, and HI scores
decreased. Taken together, these findings demonstrate that the
complex MAP vaccine had a certain preventive effect on UC incidence
and the occurrence of severe symptoms.
The increased intestinal mucosal permeability of
patients with UC is the primary indicator of intestinal mucosal
barrier function (31-34). DAO is a highly active
intracellular enzyme in the villous cell cytoplasm of the upper
layer of the mammalian intestinal mucosa. After damage and necrosis
of intestinal mucosal cells, the cells enter into the intestine,
leading to increased blood DAO activity (35). Under normal circumstances, the
intestinal barrier can effectively block intestinal parasites and
endotoxin to the intraluminal displacement (36). When a variety of factors cause
intestinal barrier damage, increased permeability, and bacterial
translocation may occur. Thus, serum DAO activity and bacterial
translocation can reflect the function of the intestinal barrier.
Studies have shown that tight junction complexes between intestinal
epithelial cells have an important role in maintaining intestinal
mucosal permeability (37-40).
The tight junction complexes primarily consist of transmembrane
proteins and cytoplasmic proteins, and contain occludin, claudin 1,
and ZO1, which reflects the permeability of the intestinal mucosa
(38-40). As demonstrated in the present
study, serum DAO levels in mice immunized with the complex MAP
vaccine were significantly lower compared with those of the UC
model group after 3 weeks of DSS consumption. Although the
bacterial colony count in lymph nodes of the immunization group was
higher compared with that of the blank control group, it was
significantly lower compared with that of the UC model group. The
expression levels of occludin, claudin1 and ZO1 in colonic tissue
homogenates of UC mice were significantly enhanced by the complex
MAP vaccine. The aforementioned findings suggested that the
newly-synthesized complex MAP vaccine can improve the permeability
of the intestinal mucosa in mice, promoting repair of the
intestinal mucosal barrier function.
In the current study, the eight-branched MAP vaccine
exhibited good antigenicity and immunogenicity, showing significant
anti-UC activity in vivo. The underlying mechanism may be
associated with the inhibition of TNF-α biological activity and
upregulation of tight junction proteins, as high titers were
produced, and polyclonal antibody specificity was determined.
However, further in-depth studies are required to determine the
optimal vaccine dose, optimize the immune strategy research,
optimize the choice of adjuvant, analyze the efficacy of the
complex MAP vaccine compared with passive immunization of
monoclonal antibodies, and to determine whether the vaccine would
induce autoimmune diseases or hypersensitivity reactions. In
conclusion, the current study provides theoretical evidence for the
further study of the synthesized complex TNF-α B cell epitope MAP
vaccine in the treatment of UC.
Acknowledgments
Not applicable.
Funding
The present study was supported by the grants of
National Natural Science Foundation of China (grant no. 81400682)
and Basic Public Welfare Research Project of Zhejiang Province
(grant no. LGF18H030009).
Availability of data and materials
The corresponding author will make available the
data generated and analyzed during this study upon reasonable
request. All materials used are included in Materials and
methods.
Authors' contributions
YS performed the experiments and contributed to
manuscript preparation. WSP and YC performed experiments and
analyzed the data. JZ designed and performed experiments; acquired,
analyzed, and interpreted the data; and prepared the manuscript.
HJW, GQR and LGC analyzed the data and contributed to manuscript
preparation. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal studies were approved by the Ethics
Committee of Zhejiang Chinese Medical University (approval no.
ZSLL-2011-95; Hangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Park JH and Brentjens RJ: Adoptive
immunotherapy for B-cell malignancies with autologous chimeric
antigen receptor modified tumor targeted T cells. Discov Med.
9:277–288. 2010.PubMed/NCBI
|
|
2
|
Castro FV, Al-Muftah M, Mulryan K, Jiang
HR, Drijfhout JW, Ali S, Rutkowski AJ, Kalaitsidou M, Gilham DE and
Stern PL: Regulation of autologous immunity to the mouse 5T4
oncofoetal antigen: Implications for immunotherapy. Cancer Immunol
Immunother. 61:1005–1018. 2012. View Article : Google Scholar
|
|
3
|
Steeland S, Libert C and Vandenbroucke RE:
A new venue of TNF targeting. Int J Mol Sci. 19:E14422018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kopylov U, Ben-Horin S, Zmora O, Eliakim R
and Katz LH: Anti-tumor necrosis factor and postoperative
complications in Crohn's disease: Systematic review and
meta-analysis. Inflamm Bowel Dis. 18:2404–2413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawalec P, Mikrut A, Wisniewska N and Pilc
A: Tumor necrosis factor-alpha antibodies (infliximab, adalimumab
and certolizumab) in Crohn's disease: Systematic review and
meta-analysis. Arch Med Sci. 9:765–779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marchioni RM and Lichtenstein GR: Tumor
necrosis factor-alpha inhibitor therapy and fetal risk: A
systematic literature review. World J Gastroenterol. 19:2591–2602.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Laharie D, Bourreille A, Branche J, Allez
M, Bouhnik Y, Filippi J, Zerbib F, Savoye G, Nachury M, Moreau J,
et al: Ciclosporin versus infliximab in patients with severe
ulcerative colitis refractory to intravenous steroids: A parallel,
open-label randomised controlled trial. Lancet. 380:1909–1915.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singh S, Heien HC, Sangaralingham LR,
Schilz SR, Kappelman MD, Shah ND and Loftus EV: Comparative
effectiveness and safety of infliximab and adalimumab in patients
with ulcerative colitis. Aliment Pharmacol Ther. 43:994–1003. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marehbian J, Arrighi HM, Hass S, Tian H
and Sandborn WJ: Adverse events associated with common therapy
regimens for moderate-to-severe Crohn's disease. Am J
Gastroenterol. 104:2524–2533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
No authors listed. Can super-antibody
drugs be tamed? Nature. 440:855–856. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rolinski J and Hus I: Breaking
immunotolerance of tumors: A new perspective for dendritic cell
therapy. J Immunotoxicol. 11:311–318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park KB, Lim BK, Ye MB, Chung SY and Nam
JH: A peptide vaccine based on a B-cell epitope on the VP1 protein
of entero-virus 70 induces a strong antibody response. Acta Virol.
56:337–342. 2012. View Article : Google Scholar
|
|
13
|
Paul S and Piontkivska H: Frequent
associations between CTL and T-Helper epitopes in HIV-1 genomes and
implications for multi-epitope vaccine designs. BMC Microbiol.
10:2122010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haro I and Gómara MJ: Design of synthetic
peptidic constructs for the vaccine development against viral
infections. Curr Protein Pept Sci. 5:425–433. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang J, Yang J, Fan D, Tao H, Wang H and
Yu T: Peptide FLNPDVLDI of heparanase is a novel HLA-A2-restricted
CTL epitope and elicits potent immunological antitumor effects in
vitro with an 8-branched design. Oncol Rep. 29:1955–1961. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J, Yang J, Cai Y, Jin N, Wang H and
Yu T: Multiple antigenic polypeptide composed of heparanase Bcell
epitopes shrinks human hepatocellular carcinoma in mice. Oncol Rep.
33:1248–1256. 2015. View Article : Google Scholar
|
|
17
|
Jia JY, Zhou HZ and Tang J: The study of
mouse TNF-α functional domain and its neutralizing antibody binding
site. Prog Biochem Biophys. 36:424–430. 2009.In Chinese.
|
|
18
|
Boraschi D and Tagliabue A: Interleukin-1
and interleukin-1 fragments as vaccine adjuvants. Methods.
19:108–113. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chakraborty S, Chakravorty R, Ahmed M,
Rahman A, Waise TM, Hassan F, Rahman M and Shamsuzzaman S: A
computational approach for identification of epitopes in dengue
virus envelope protein: A step towards designing a universal dengue
vaccine targeting endemic regions. In Silico Biol. 10:235–246.
2010.PubMed/NCBI
|
|
20
|
Yang XS, Wang HQ, Yuan QF, Xie Y, Yao ZB,
Ye XZ, Wu JM and Zhou AG: Directly synthesize Aβ_(1-15) peptide
vaccine by fmoc solid-phase peptide synthesis and study its immune
activity. J Sun Yat Sen Uni. 27:121–125. 2006.In Chinese.
|
|
21
|
Wang J, Sun N, Zhou C, Zhou X, Lu J, Wang
C and Che H: Food proteins from different allergen families
sensitize balb/c mice to family-specific immune responses. J
Immunotoxicol. 11:172–179. 2014. View Article : Google Scholar
|
|
22
|
Zhang J, Cui Y, Wu Y, Wang H and Ke J:
Prediction and identification of Bcell epitopes for tumor necrosis
factoralpha. Mol Med Rep. 16:3439–3444. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Murano M, Maemura K, Hirata I, Toshina K,
Nishikawa T, Hamamoto N, Sasaki S, Saitoh O and Katsu K:
Therapeutic effect of intracolonically administered nuclear factor
kappa B (p65) antisense oligonucleotide on mouse dextran sulphate
sodium (DSS)-induced colitis. Clin Exp Immunol. 120:51–58. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kihara N, de la Fuente SG, Fujino K,
Takahashi T, Pappas TN and Mantyh CR: Vanilloid receptor-1
containing primary sensory neurones mediate dextran sulphate sodium
induced colitis in rats. Gut. 52:713–719. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sureshbabu A, Syed MA, Boddupalli CS,
Dhodapkar MV, Homer RJ, Minoo P and Bhandari V: Conditional
overexpres-sion of TGFβ1 promotes pulmonary inflammation, apoptosis
and mortality via TGFβR2 in the developing mouse lung. Respir Res.
16:42015. View Article : Google Scholar
|
|
26
|
Thorlund K, Druyts E, Mills EJ, Fedorak RN
and Marshall JK: Adalimumab versus infliximab for the treatment of
moderate to severe ulcerative colitis in adult patients naive to
anti-TNF therapy: An indirect treatment comparison meta-analysis. J
Crohns Colitis. 8:571–581. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fausel R and Afzali A: Biologics in the
management of ulcerative colitis-comparative safety and efficacy of
TNF-alpha antagonists. Ther Clin Risk Manag. 11:63–73. 2015.
|
|
28
|
Ben-Horin S, Kopylov U and Chowers Y:
Optimizing anti-TNF treatments in inflammatory bowel disease.
Autoimmun Rev. 13:24–30. 2014. View Article : Google Scholar
|
|
29
|
de Mattos BR, Garcia MP, Nogueira JB,
Paiatto LN, Albuquerque CG, Souza CL, Fernandes LG, Tamashiro WM
and Simioni PU: Inflammatory bowel disease: An overview of immune
mechanisms and biological treatments. Mediators Inflamm.
2015:4930122015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chey WY: Infliximab for patients with
refractory ulcerative colitis. Inflamm Bowel Dis. 7(Suppl 1):
S30–S33. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Okamoto R and Watanabe M: Functional
relevance of intestinal epithelial cells in inflammatory bowel
disease. Nihon Rinsho Meneki Gakkai Kaishi. 39:522–527. 2016.
View Article : Google Scholar
|
|
32
|
Landy J, Ronde E, English N, Clark SK,
Hart AL, Knight SC, Ciclitira PJ and Al-Hassi HO: Tight junctions
in inflammatory bowel diseases and inflammatory bowel disease
associated colorectal cancer. World J Gastroenterol. 22:3117–3126.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Noth R, Stüber E, Häsler R, Nikolaus S,
Kühbacher T, Hampe J, Bewig B, Schreiber S and Arlt A: Anti-TNF-α
antibodies improve intestinal barrier function in Crohn's disease.
J Crohns Colitis. 6:464–469. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Di Sabatino A, Pender SL, Jackson CL,
Prothero JD, Gordon JN, Picariello L, Rovedatti L, Docena G,
Monteleone G, Rampton DS, et al: Functional modulation of Crohn's
disease myofibroblasts by anti-tumor necrosis factor antibodies.
Gastroenterology. 133:137–149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li JY, Lu Y and Fu XB: The significance of
changes in diamine oxidase activity in intestinal injury after
trauma. Chin Crit Care Med. 12:482–484. 2000.In Chinese.
|
|
36
|
Leaphart CL and Tepas JJ III: The gut is a
motor of organ system dysfunction. Surgery. 141:563–569. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ivanov AI, Nusrat A and Parkos CA:
Endocytosis of the apical junctional complex: Mechanisms and
possible roles in regulation of epithelial barriers. Bioessays.
27:356–365. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Furuse M, Hirase T, Itoh M, Nagafuchi A,
Yonemura S and Tsukita S: Occludin: A novel integral membrane
protein local-izing at tight junctions. J Cell Biol. 123:1777–1788.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wardill HR, Bowen JM, Al-Dasooqi N,
Sultani M, Bateman E, Stansborough R, Shirren J and Gibson RJ:
Irinotecan disrupts tight junction proteins within the gut:
Implications for chemotherapy-induced gut toxicity. Cancer Biol
Ther. 15:236–244. 2014. View Article : Google Scholar
|
|
40
|
Shang HX, Wang AQ, Bao CH, Wu HG, Chen WF,
Wu LY, Ji R, Zhao JM and Shi Y: Moxibustion combined with
acupuncture increases tight junction protein expression in Crohn's
disease patients. World J Gastroenterol. 21:4986–4996. 2015.
View Article : Google Scholar : PubMed/NCBI
|