Introduction
Osteosarcoma (OS), the most frequent malignant bone
tumour, often occurs in children, accounting for 2.4% of all
malignancies and ~20% of all primary bone tumours in paediatric
patients (1,2). Despite the rapid advances in
treatment and diagnosis, the survival of OS patients remains poor,
and its recurrence rate is as high as 30-40% due to its highly
aggressive and metastatic properties (3,4).
The current standard chemotherapy methods only provide 65-70%
long-term disease-free survival for OS patients without metastasis,
and 70% OS patients with recurrence die within 5 years (5,6).
Thus, it is of high urgency to uncover the mechanisms of OS
tumourigenesis, particularly its invasion and migration.
MicroRNAs (miRNAs) are small endogenous RNAs that
are involved in various steps of tumourigenesis, including in cell
proliferation, apoptosis, migration and invasion (7,8).
miR-29a, which is considered as a tumour suppressor, serves an
important role in the development of several types of cancer, such
as lung (9), prostate (10) and gastric cancer (11). A previous study has also reported
that miR-29a is downregulated in OS (12); however, the involvement and
mechanisms for miR-29a in the development of OS have rarely been
reported (12). The
hyper-methylation of certain tumour suppressor gene promoters plays
a crucial role in cancer progression, and the interaction between
methylation of anti-oncogenes and miRNAs has been demonstrated in
several types of cancer (13,14). Recently, it was reported that
overexpression of DNA methyltransferase 3B (DNMT3B) was correlated
to the downregulation of miR-29a in juvenile myelomonocytic
leukaemia (15). However, to the
best of our knowledge, no study has investigated the association
between DNMT3B and miR-29a in OS.
The suppressor of cytokine signalling (SOCS) family
consists of eight members, namely SOCS1-7, as well as the
cytokine-inducible SH2-containing protein (16). SOCS1 can act as both tumour
suppressor and oncogene in cancer development (17), and the methylation of SOCS1
promoter regions by methyltransferases is reportedly involved in
the tumourigenesis of several cancer types. The methylation of
SOCS1 promotes the growth and proliferation of acute myeloid
leukaemia cells through the JAK2/STAT signalling pathway (18). In addition, SOCS1 has been
demonstrated to suppress the metastasis of melanoma cells (19) and human prostate cancer cells
(20). However, SOCS1 can also
act as an oncogene, and silencing SOCS1 in monocytes enhanced the
tumour-killing activity of macrophages (21). A previous study demonstrated that
SOCS1 was involved in the development of OS (22). However, deeper insights on the
function of SOCS1 in OS are still needed, and no study has focussed
on the association between the miR-29a/DNMT3B axis and SOCS1, or
its influence on the apoptosis, invasion and migration of OS
cells.
The SOCS1/nuclear factor (NF)-κB pathway serves a
vital role in inflammatory processes (23,24). SOCS1 regulates the
epithelial-mesenchymal transition (EMT) and bone metastasis of
prostate cancer via the NF-κB signalling pathway (25). SOCS1, the key factor in our
research, is an upstream protein of NF-κB (26), while several studies have reported
that the NF-κB signalling pathway is associated with tumour
invasion and migration. It was also demonstrated that NF-κB
promoted EMT, migration and invasion in pancreatic carcinoma cells
(27). Furthermore, a previous
review demonstrated that activation of the TNF-α/NF-κB pathway may
contribute to tumor development and promote cell migration and
invasion by activating Snail and epithelial-mesenchymal
transformation (28). Therefore,
we hypothesized that miR-29a may modulate tumourigenesis in OS by
regulating the SOCS1/NF-κB signalling pathway by directly targeting
DNMT3B.
In the present study, it was observed that miR-29a
promoted apoptosis, and inhibited invasion, migration and EMT in OS
cells via the SOCS1/NF-κB signalling pathway by targeting DNMT3B.
To the best of our knowledge, this is the first study examining the
association between miR-29a, DNMT3B and SOCS1 in OS tumourigenesis.
The current study may provide a better understanding of the
miR-29a/DNMT3B/SOCS1 axis in the development of OS and provide
novel therapeutic targets for OS treatment.
Materials and methods
Tissue samples
A total of 30 paired OS tissues and adjacent
non-tumour tissue samples were used in the present study, which
were obtained from patients who underwent radical resection at the
Second Xiangya Hospital, Central South University (Changsha,
China). Samples were frozen immediately after surgical resection
and stored in liquid nitrogen until required for further assay.
Histological analysis was conducted to confirm the pathology of all
tissues (29). Written informed
consent was obtained from all patients, and the study was approved
by the Ethics Committee of the Second Xiangya Hospital, Central
South University. The clinicopathological features of all OS
patients are listed in Table
I.
 | Table IAssociation of miR-29a, DNMT3B and
SOCS1 expression with the clinicopathological characteristics of
osteosarcoma patients. |
Table I
Association of miR-29a, DNMT3B and
SOCS1 expression with the clinicopathological characteristics of
osteosarcoma patients.
| Clinical
parameters | Cases (n) | miR-29a expression
| P-value | DNMT3B expression
| P-value | SOCS1 expression
| P-value |
|---|
| High | Low | High | Low | High | Low |
|---|
| Age (years) | | | | | | | | | | |
| <18 years | 20 | 11 | 9 | 0.6999 | 8 | 12 | 0.2451 | 9 | 11 | 0.6999 |
| ≥18 years | 10 | 4 | 6 | | 7 | 3 | | 6 | 4 | |
| Sex | | | | | | | | | | |
| Male | 17 | 6 | 11 | 0.1394 | 10 | 7 | 0.4621 | 12 | 5 | 0.0253 |
| Female | 13 | 9 | 4 | | 5 | 8 | | 3 | 10 | |
| Tumor size
(cm) | | | | | | | | | | |
| <5 cm | 12 | 7 | 5 | 0.7104 | 4 | 8 | 0.2635 | 3 | 9 | 0.0604 |
| ≥5 cm | 18 | 8 | 10 | | 11 | 7 | | 12 | 6 | |
| TNM stage | | | | | | | | | | |
| I | 14 | 12 | 2 | 0.0007 | 4 | 10 | 0.0656 | 11 | 3 | 0.0092 |
| II+III | 16 | 3 | 13 | | 11 | 5 | | 4 | 12 | |
| Distant
metastasis | | | | | | | | | | |
| Yes | 16 | 4 | 12 | 0.0092 | 10 | 6 | 0.2723 | 14 | 2 | <0.0001 |
| No | 14 | 11 | 3 | | 5 | 9 | | 1 | 13 | |
Cell culture and transfection
The OS cell lines U2OS, MG-63 and Saos-2, as well as
the normal hFOB 1.19 osteoblast cells, were all purchased from ATCC
(Manassas, VA, USA). Briefly, cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 10% foetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) and 100 µg/ml
penicillin-streptomycin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) at 37°C and 5% CO2. When the OS cells reached
70-80% confluence, they were transfected with miR-29a mimics,
miR-29a inhibitor, negative control (NC) mimics, NC inhibitor (all
10 nM; GeneChem Corp., Shanghai, China), SOCS1 siRNA (siSOCS1) or
NC siRNA (siNC) for 48 h using the Lipo6000 transfection reagent
(Beyotime Institute of Biotechnology, Haimen, China) according to
the manufacturer's protocol. After 48 h, the transfection rate was
determined using RT-qPCR. To inhibit the level of methylation,
5-aza-2′-deoxycytidine (5-Aza; 10 µM; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) was added to the cells. Briefly, U2OS or
MG63 cells were treated with 10 µM 5-Aza after cells reached
70-80% confluence or cells were treated with 10 µM 5-Aza
after transfection of miR-29a mimics for 24 h. Cells not treated
with 5-Aza were used as controls. The cells were further cultured
for 24 h for further experiments.
293T cells (American Type Culture Collection) were
also cultured in DMEM (Thermo Fisher Scientific, Inc.) containing
10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 100
µg/ml penicillin-streptomycin at 37°C and 5%
CO2.
Dual-luciferase reporter assay
The predicted binding region of DNMT3B and miR-29a
was obtained using Targetscan 7.2 (http://www.targetscan.org). To confirm whether DNMT3B
was a direct target of miR-29a, a dual-luciferase reporter assay
was conducted. Briefly, the DNMT3B 3′-untranslated region with the
predicted miR-29a binding site (WT) or mutant binding site (MUT)
was amplified, and then sub-cloned into the pGL4.10 luciferase
reporter vector. 293T cells were then co-transfected with the
miR-29a mimics or NC mimics using the Lipo6000 reagent (Beyotime
Institute of Biotechnology) according to the manufacturer's
protocol. After 48 h of transfection, a luciferase reporter assay
was performed using a Bright-Glo™ Luciferase Assay System (Promega
Corporation, Madison, WI, USA). The luciferase activity was
normalised to the value of the Renilla luciferase
activity.
Apoptosis assay
To study the effect of miR-29a on apoptosis, cells
were stained with an Annexin V/propidium iodide (PI) double
staining kit (BD Biosciences, Franklin Lakes, NJ, USA) according to
the manufacturer's protocol. Briefly, the cells were seeded at
density of 3×105 in 24-well plates, and were collected
after 48 h of transfection, washed twice with cold PBS and
resuspended in 1X binding buffer. Cells were stained with 5
µl Annexin V-FITC for 15 min and then with 5 µl PI
for 10 min in the dark at room temperature. Apoptosis was measured
by flow cytometry (BD Biosciences).
Wound healing assay
Cell migration was determined by performing a
scratch-wound healing assay. Briefly, cells at density of
1×106 /well were seeded into 6-well plates for 24 h.
Cell layers were scratched using a 100-µl pipette tip to
form a wound-like gap. The cells were then maintained in DMEM with
10% FBS, and images were captured at 0 and 24 h. ImageJ software
(National Institutes of Health, Bethesda, MD, USA) was used to
analyse the wound width. The migration distance of cells was
measured according to the following formula: Migration rate
(%)=(W0h-W24h)/W0h ×100%.
Transwell assay
A Transwell assay was used to determine the cell
invasion ability. Briefly, 2×105 cells were plated in
the top chamber of 24-well Transwell inserts with a Matrigel-coated
membrane (BD Biosciences) in serum-free medium. As a
chemoattractant, DMEM with 20% FBS was added to the bottom
compartment. Cells were cultured for 24 h at 37°C, and the
non-invading cells on the upper surface were removed with a cotton
swab. The invaded cells on the lower surface were fixed with 4%
paraformaldehyde and stained with 0.1% crystal violet. Invading
cells were finally counted under an inverted microscope (Zeiss,
Oberkochen, Germany).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
assay
The expression levels of miR-29a, DNMT3B and SOCS1
were measured using RT-qPCR. Briefly, the extraction of total RNA
was conducted using the TRIzol reagent (Tiangen Biotech, Beijing,
China). For extraction of miRNA, the mirVana miRNA isolation kit
(Ambion; Thermo Fisher Scientific, Inc.) was used. The RNA
concentration was determined using a NanoDrop ND-1000
spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific,
Inc.). Subsequently, a Prime-Script™ One Step RT-qPCR kit (Takara
Biotechnology Co., Ltd., Dalian, China) and a TaqMan MicroRNA
Reverse Transcription kit (Applied Biosystems Life Technologies)
were used to convert RNA to cDNA for mRNA and miRNA, respectively.
Subsequently, qPCR analysis was performed in an Applied Biosystems
7500 Real Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) using SYBR Green PCR Master Mix (Solarbio Science
& Technology Co., Ltd., Beijing, China) using the following
conditions: Initial activation step at 95°C for 5 sec, 35 cycles of
denaturation at 94°C for 15 sec, annealing at 55°C for 25 sec, and
extension at 70°C 30 sec. The primers used in qPCR were as follows:
miR-29a forward, 5′-CGCGGATCCTGGATTTAGTAAGATTTGGGC-3′, and reverse,
5′-CCGGAATTCACATGCAATTCAGGTCAGTG-3′; DNMT3B forward,
5′-GCCCATTCGATCTGGTGATT-3′, and reverse,
5′-GGCGGTAGAACTCAAAGAAGAG-3′; SOCS1 forward,
5′-TGGTTGTAGCAGCTTGTGTCTGG-3′, and reverse,
5′-CCTGGTTTGTGCAAAGATACTGGG-3′; U6 snRNA forward,
5′-ATTGGAACGATACAGAGAAGATT-3′, and reverse
5′-GGAACGCTTCACGAATTTG-3′; GAPDH forward,
5′-CCACAGTCCATGCCATCAC-3′, and reverse 5′-GCTTCACCACCTTCTTGATG-3′.
GAPDH and U6 small nuclear RNA (U6 snRNA) were used as internal
references for mRNA and miRNA, respectively. The relative
expression level was calculated by the 2−ΔΔCq method
(30).
Methylation-specific PCR (MSP) assay
For analysis of the methylation level of SOCS1, the
MSP method was used. Briefly, DNA was isolated from the cell lines
using a QIAamp Fast DNA Tissue kit (Qiagen, Valencia, CA, USA).
Subsequent to transforming unmethylated cytosine residues to uracil
using an EpiTect MSP kit (Qiagen), the bisulphite-treated DNA was
amplified using the following MSP primers: SOCS1 (methylated
samples) forward, 5′-TTCGCGTGTATTTTTAGGTCGGTC-3′, and reverse,
5′-CGACACAACTCCTACAACGACCG-3′; SOCS1 (unmethylated samples)
forward, 5′-TTATGAGTATTTGTGTGTATTTTTAGGTTGGTT-3′, and reverse,
5′-CACTAACAACACAACTCCTACAACAACCA-3′. The PCR reaction and
quantification methods were conducted as aforementioned.
Western blot assay
Western blotting was used to test the protein levels
of DNMT3B and SOCS1, as well as the levels of proteins associated
with EMT, apoptosis and the NF-κB signalling pathway. Briefly,
total proteins were extracted from the cells or tissues using RIPA
lysis buffer (Cell Signaling Technology, Inc., Danvers, MA, USA)
containing a protease inhibitor cocktail (Roche, Mannheim,
Germany). Nuclear proteins were extracted using a nucleoprotein kit
(Merck KGaA, West Point, PA, USA) according to the manufacturer's
protocol. Next, the protein concentration was determined using a
BCA Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc.).
Samples were then separated by 10% SDS-PAGE, transferred to PVDF
membranes, blocked by 5% non-fat milk at room temperature for 1 h
and incubated at 4°C overnight with a corresponding primary
antibody: DNMT3B (ab79822; 1:1,000), SOCS1 (ab9870; 1:2,000),
fibronectin (ab2413; 1:1,000), vimentin (ab8978; 1:1,000),
N-cadherin (ab18203; 1:500), E-cadherin (ab15148; 1:500), matrix
metalloproteinase (MMP)-2 (ab37150; 1:2,000), MMP-9 (ab73734;
1:2,000), p65 (ab16502; 1:1,000), IκBα (ab32518; 1:1,000), p-IκB-α
(ab133462; 1:10,000), cleaved caspase-3 (ab13847; 1:500),
pro-caspase-3 (ab32499; 1:10,000), cleaved poly(ADP-ribose)
polymerase (PARP; ab4830; 1:1,000), PARP (ab74290; 1:1,000), B-cell
lymphoma-2 (Bcl-2; ab196495; 1:500), Bcl-2-associated X protein
(Bax; ab53154; 1:500) and GAPDH (ab8245; 1:2,000). Samples were
then incubated with horseradish peroxidase-conjugated anti-rabbit
immunoglobulin G secondary antibody (ab6721; 1:1,000) at room
temperature for 45 min. All primary and secondary antibodies were
obtained from Abcam (Cambridge, UK). The signal was developed using
an ECL system (Beyotime Institute of Biotechnology) according to
the manufacturer's protocol. The protein levels were quantified
using Quantity One software (Bio-Rad Laboratories, Inc., Hercules,
CA, USA), and GAPDH was used as an internal reference.
Statistical analysis
All experiments were repeated at least three times,
and one representative result is presented. Data were analysed with
Prism software, version 6.0 (GraphPad Software, San Diego, CA,
USA). The data are expressed as the mean ± standard deviation.
Comparison between two groups in Fig.
1 was performed using paired Student's t test, while comparison
between two groups in other figures using unpaired Student's t
test. Comparison among three or more groups was conducted using
one-way analysis of variance, followed by Tukey's post-hoc test.
The correlations between miR-29a/DNMT3B/SOCS1 expression level and
the clinicopathological characteristics of patients with OS were
assessed by the χ2 test. A P-value of <0.05 was
considered to denote that the difference was statistically
significant.
Results
miR-29a and SOCS1 are downregulated, and
DNMT3B is upregulated in OS tissues
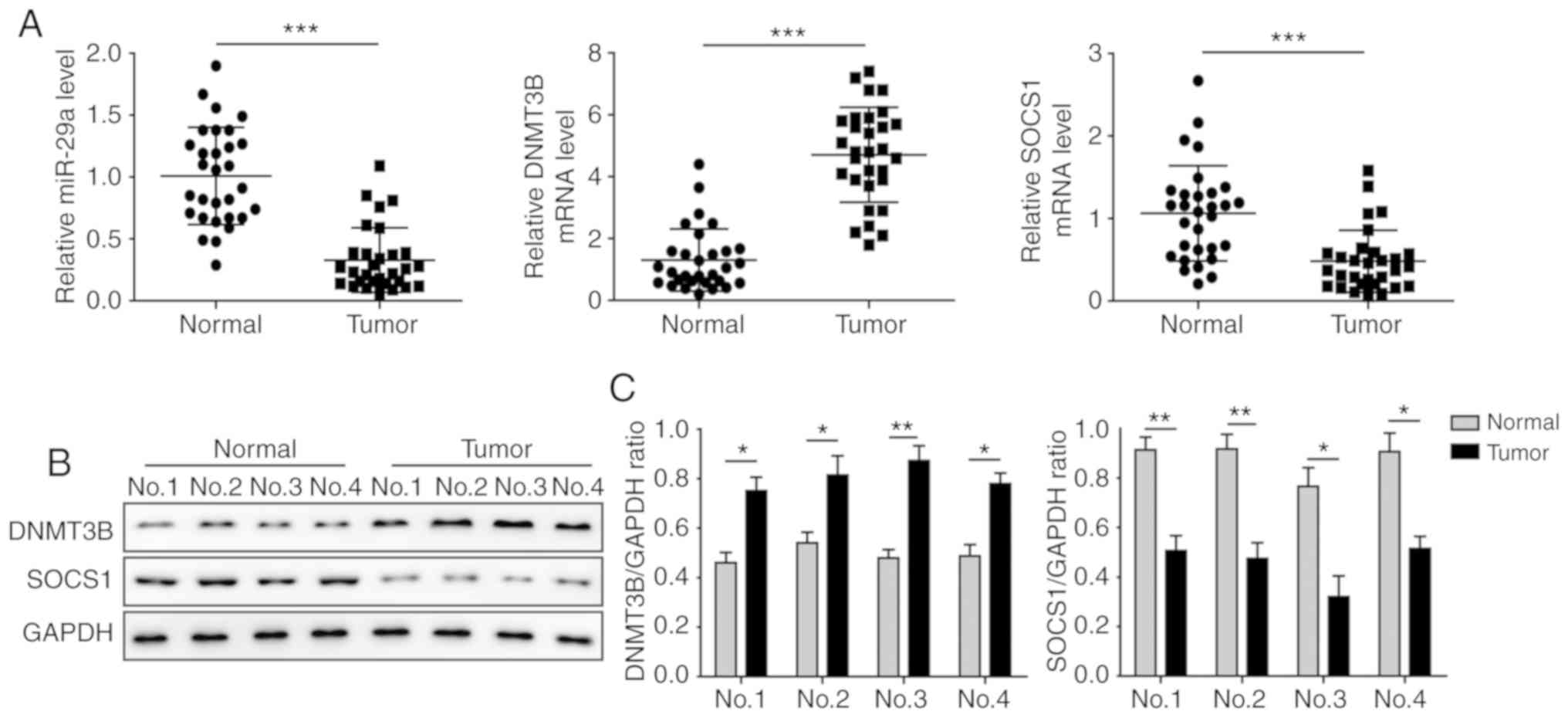
The levels of miR-29a, DNMT3B and SOCS1 in OS and
adjacent normal tissues were determined by RT-qPCR and western blot
analysis. The results demonstrated that, in OS tissues, miR-29a and
SOCS1 were significantly downregulated, and DNMT3B was
significantly upregulated compared with the adjacent non-tumour
tissues (Fig. 1A). The protein
level of SOCS1 was also markedly decreased in tumour tissues, while
DNMT3B was significantly increased (Fig. 1B and C). As shown in Table I, miR-29a and SOCS1 expression
were found to be associated with an advanced clinical stage
(P<0.05) and distant metastasis (P<0.05). All these results
suggested that miR-29a and SOCS1 may be tumour biomarkers of OS
progression.
miR-29a and SOCS1 are downregulated, and
DNMT3B is upregulated in OS cells
To further investigate the mechanisms of the
miR-29a/DNMT3B/SOCS1 axis in OS, the levels of miR-29a, DNMT3B and
SOCS1 were determined in OS cells. As shown in Fig. 2A, in the three OS cell lines
investigated, miR-29a and SOCS1 were significantly downregulated,
while DNMT3B was significantly upregulated compared with the normal
hFOB 1.19 cells, which is consistent with the results in tissue
samples. In addition, the MSP assay revealed that the methylation
level of SOCS1 was significantly increased in OS cells in
comparison with that in hFOB 1.19 cells (Fig. 2B). Furthermore, protein level
changes of DNMT3B and SOCS1 were similar to the alterations at the
mRNA level: The expression of SOCS1 was significantly inhibited,
while that of DNMT3B was significantly induced in OS cells
(Fig. 2C and D). The migration
assay revealed that migration distances in all OS cell lines were
significantly higher compared with those in hFOB 1.19 cells,
suggesting that OS cells exhibit strong migration ability. Among
the three OS cells, U2OS and MG-63 cells exhibited stronger
migration abilities in comparison with the Saos-2 cells (Fig. 2E and F). Considering their
migration ability and expression of miR-29a, DNMT3B and SOCS1, the
U2OS and MG-63 cell lines were selected for use in subsequent
experiments.
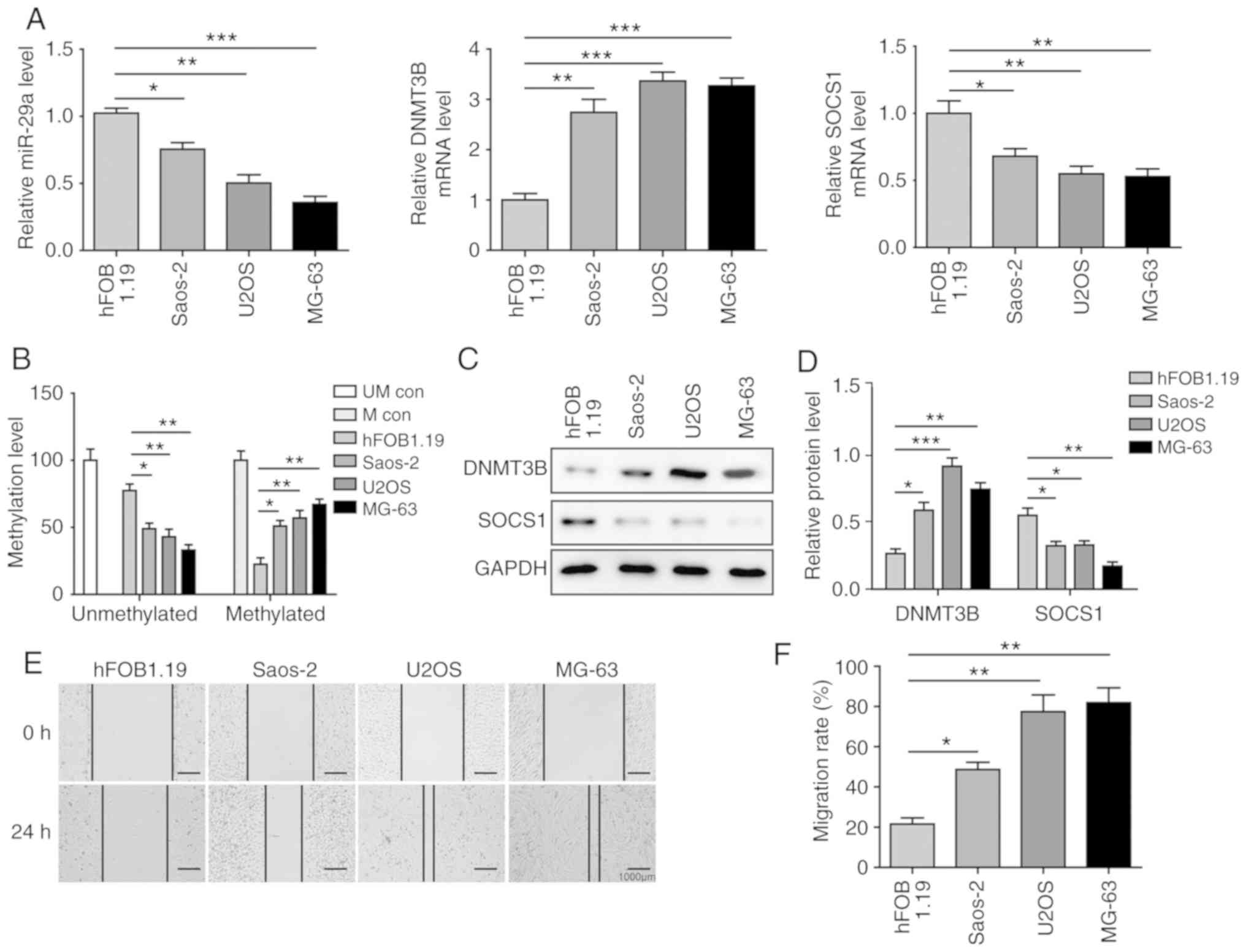 | Figure 2miR-29a and SOCS1 are downregulated,
and DNMT3B is upregulated in OS cells. (A) Expression levels of
miR-29a, DNMT3B and SOCS1 in different OS cell lines and normal
hFOB1.19 cells, assessed by reverse transcription-quantitative PCR.
(B) Methylation level of SOCS1 in different OS cell lines and
normal hFOB1.19 cells, examined by methylation-specific PCR. (C)
Western blots and (D) quantified protein levels of DNMT3B and SOCS1
in three OS cell lines and normal hFOB1.19 cells. (E)
Representative wound healing assay images (magnification, ×40) and
(F) migration distance of different OS cell lines. Data are
expressed as the mean ± standard deviation. Comparison among three
or more groups was conducted using one-way analysis of variance
followed by Tukey's post hoc test. *P<0.05,
**P<0.01 and ***P<0.001. miR-29a,
microRNA-29a; SOCS1, suppressor of cytokine signalling 1; DNMT3B,
DNA methyltransferase 3B; OS, osteosarcoma; PCR, polymerase chain
reaction; UM con, unmethylated control; M con, methylated
control. |
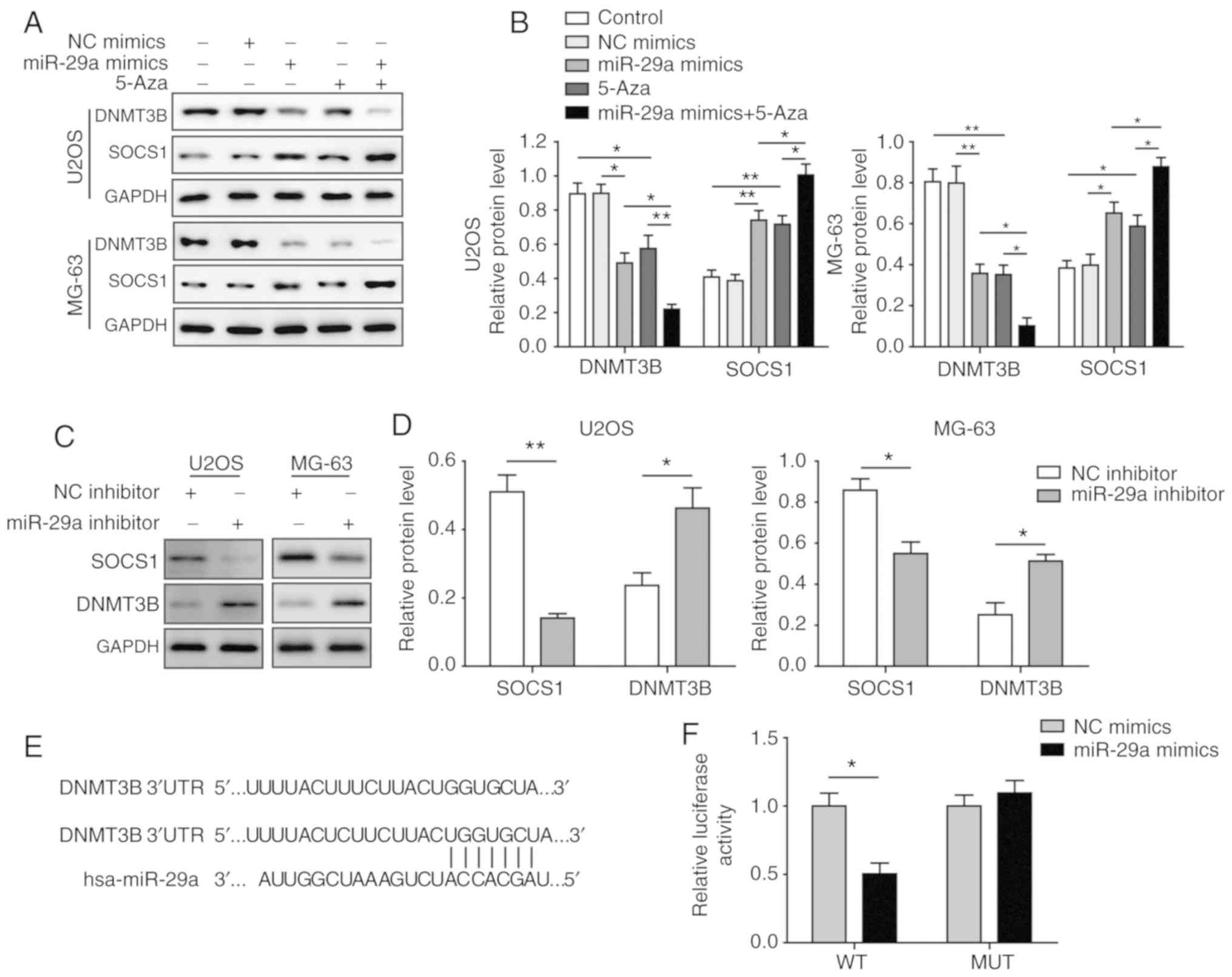
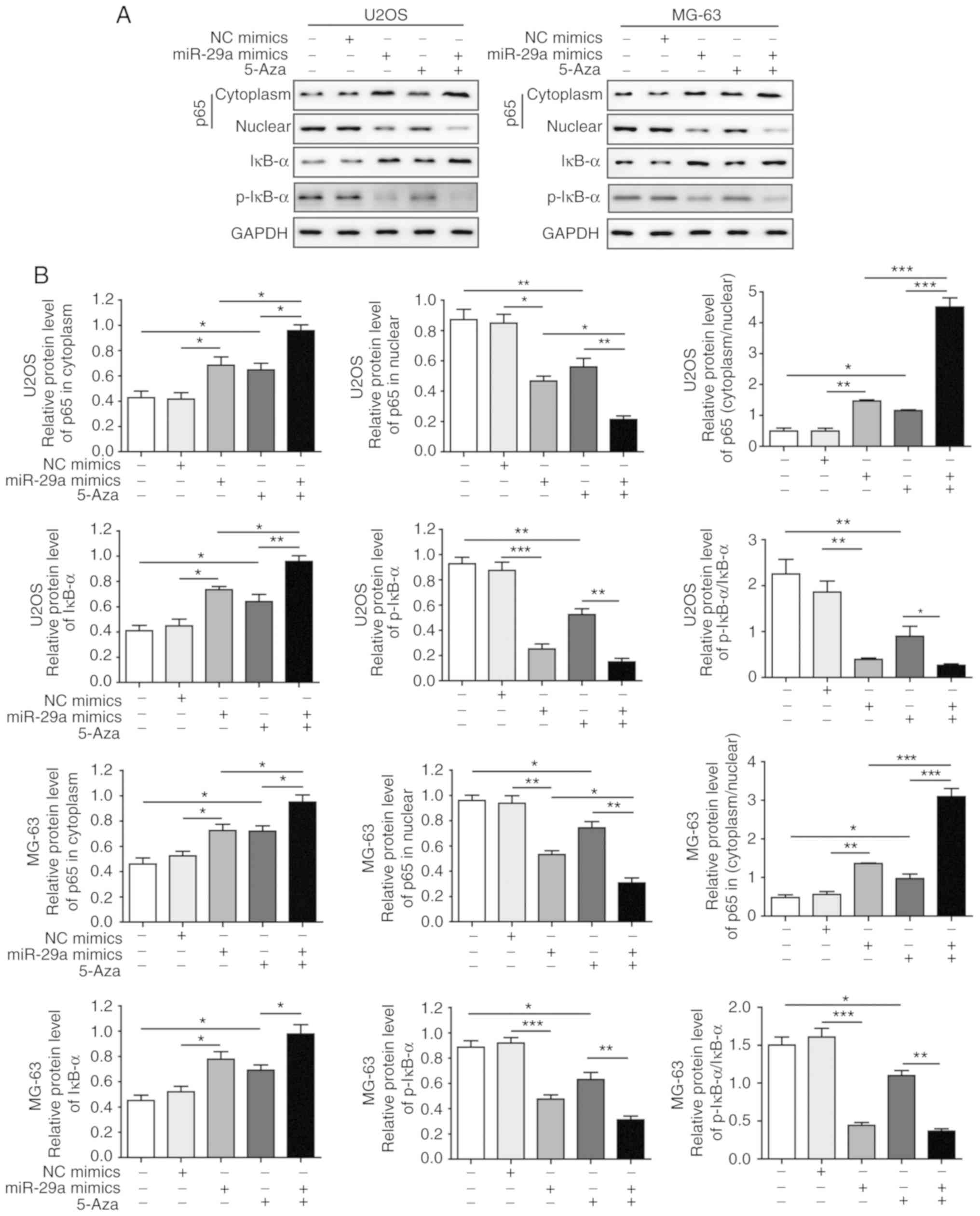
miR-29a overexpression downregulates the
methylation of SOCS1 and upregulates SOCS1 expression through
negatively targeting DNMT3B
The miR-29a mimics and the methyltransferase
inhibitor 5-Aza were used to treat U2OS and MG-63 cells. The
results demonstrated that miR-29a was significantly upregulated in
the two cell lines following transfection with the miR-29a mimics
(Fig. 3A), while co-treatment
with 5-Aza did not markedly affect miR-29a expression. Furthermore,
the overexpression of miR-29a, as well as the treatment with 5-Aza,
significantly downregulated DNMT3B and upregulated SOCS1 levels,
while the combination treatment with miR-29a mimics and 5-Aza
further enhanced these effects (Fig.
3B and C). By contrast, when cells were transfected with
miR-29a inhibitor, the opposite results were observed. Upon
inhibition of miR-29a, the expression levels of miR-29a and SOCS1
were significantly downregulated, while DNMT3B was markedly
elevated (Fig. 3D). In the
methylation analysis of SOCS1, it was observed that the methylation
level was significantly inhibited when cells were transfected with
miR-29a mimics or treated with 5-Aza, and miR-29a mimics synergised
with 5-Aza to significantly exacerbate this effect (Fig. 3E). Alteration of the protein
levels of DNMT3B and SOCS1 was consistent with the mRNA results
(Fig. 4A-D).
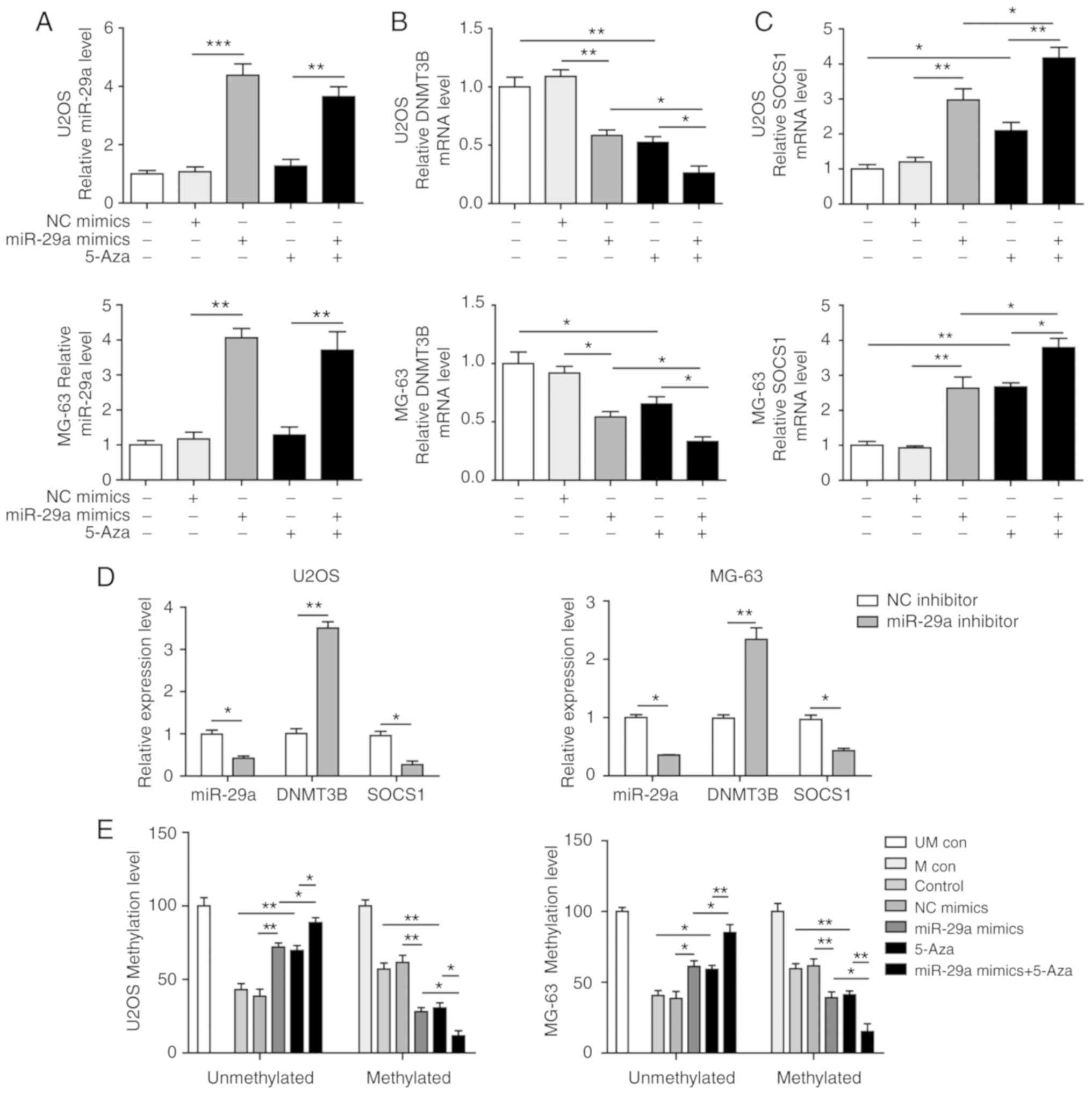 | Figure 3miR-29a downregulates the methylation
of SOCS1 and upregulates SOCS1 expression in U2OS and MG-63 cells.
(A) miR-29a, (B) DNMT3B and (C) SOCS1 expression levels in cells
transfected with miR-29a mimics and/or treated with 5-Aza. (D)
Expression of miR-29a, DNMT3B and SOCS1 in cells transfected with
miR-29a inhibitor or NC, as measured by reverse
transcription-quantitative polymerase chain reaction. (E)
Methylation level of SOCS1 in cells transfected with miR-29a mimics
and/or treated with 5-Aza. Comparison between two groups was
performed using unpaired Student's t-test, while comparison among
three or more groups was conducted using one-way analysis of
variance followed by Tukey's post hoc test. Data are expressed as
the mean ± standard deviation. *P<0.05,
**P<0.01 and ***P<0.001. miR-29a,
microRNA-29a; SOCS1, suppressor of cytokine signalling 1; DNMT3B,
DNA methyltransferase 3B; 5-Aza, 5-aza-2′-deoxycytidine; NC,
negative control; UM con, unmethylated control; M con, methylated
control. |
To further investigate the aforementioned changes, a
dual-luciferase reporter assay was conducted to determine whether
DNMT3B was a direct target of miR-29a. The binding sites between
miR-29a and DNMT3B are indicated in Fig. 4E. In U2OS and MG-63 cells, the
relative luciferase activity in the DNMT3B-WT group was
significantly downregulated by transfection with miR-29a mimics as
compared with cells trans-fected with miR-29a NC. By contrast, no
significant change was observed in the DNMT3B-MUT group upon
transfection with miR-29a mimics or NC (Fig. 4F), indicating that DNMT3B is a
direct target of miR-29a. Taken together, these results suggested
that miR-29a upregulated SOCS1 by directly targeting DNMT3B, which
inhibited the methylation level of SOCS1.
miR-29a promotes apoptosis of U2OS and
MG-63 cells
Apoptosis was assayed to further investigate the
effects of miR-29a on U2OS and MG-63 cells. The results
demonstrated that the apoptosis rate was significantly promoted
when the two cell lines were transfected with miR-29a mimics or
treated with 5-Aza (Fig. 5A and
B). The highest apoptosis rate was observed in cells that were
simultaneously transfected with miR-29a mimics and treated with
5-Aza. When transfected with miR-29a inhibitor, the cell apoptosis
was slightly suppressed, although no significant difference was
observed (Fig. 5C and D).
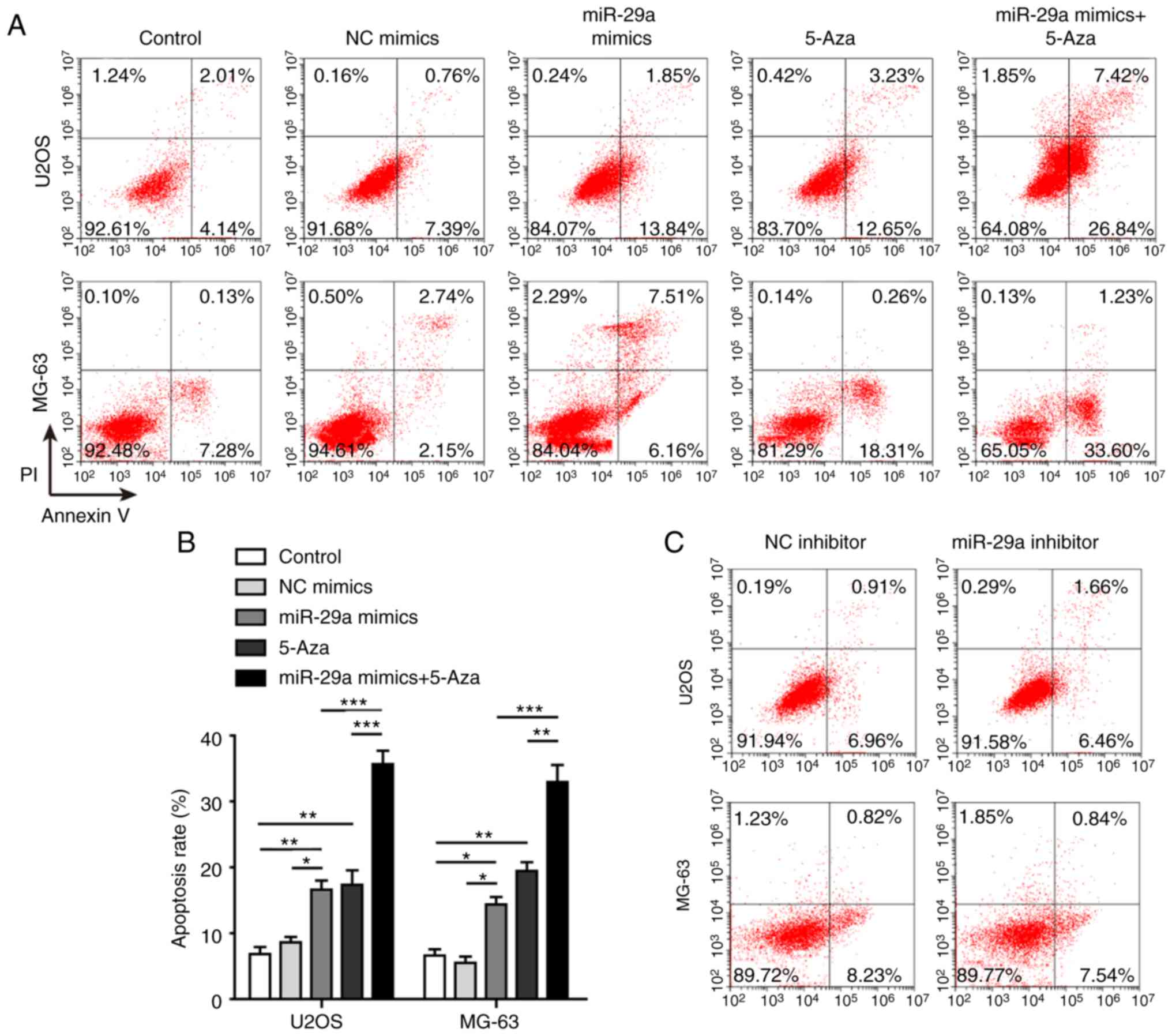 | Figure 5miR-29a promotes apoptosis of U2OS
and MG-63 cells. (A) Flow cytometry results and (B) quantified
apoptosis rate of cells transfected with miR-29a mimics and/or
treated with 5-Aza. (C) Flow cytometry results and (D) quantified
apoptosis rate of cells transfected with miR-29a inhibitor or NC.
(E) Western blots and (F) quantified protein levels of cleaved
caspase-3, uncleaved caspase-3, cleaved PARP, uncleaved PARP, Bax
and Bcl-2. Data are expressed as the mean ± standard deviation.
Comparison between two groups was performed using unpaired
Student's t-test, while comparison among three or more groups was
conducted using one-way analysis of variance followed by Tukey's
post hoc test. *P<0.05, **P<0.01 and
***P<0.001. miR-29a, microRNA-29a; NC, negative
control; PARP, poly(ADP-ribose) polymerase; Bcl-2, B-cell
lymphoma-2; Bax, Bcl-2-associated X protein; N.S.,
non-significant. |
Furthermore, the levels of several
apoptosis-associated proteins, including uncleaved caspase-3,
cleaved caspase-3, uncleaved PARP, cleaved PARP, Bax and Bcl-2,
were measured. In the two cell lines, overexpression of miR-29a
significantly increased the expression levels of cleaved caspase-3,
cleaved PARP and Bax, whereas it markedly decreased the levels of
uncleaved caspase-3, uncleaved PARP and Bcl-2. Co-treatment with
5-Aza further promoted these effects on apoptosis-associated
proteins, the ratio of cleaved/uncleaved caspase-3 and
cleaved/uncleaved PARP were significantly enhanced when cells were
transfected with miR-29a mimics and 5-Aza (Fig. 5E and F).
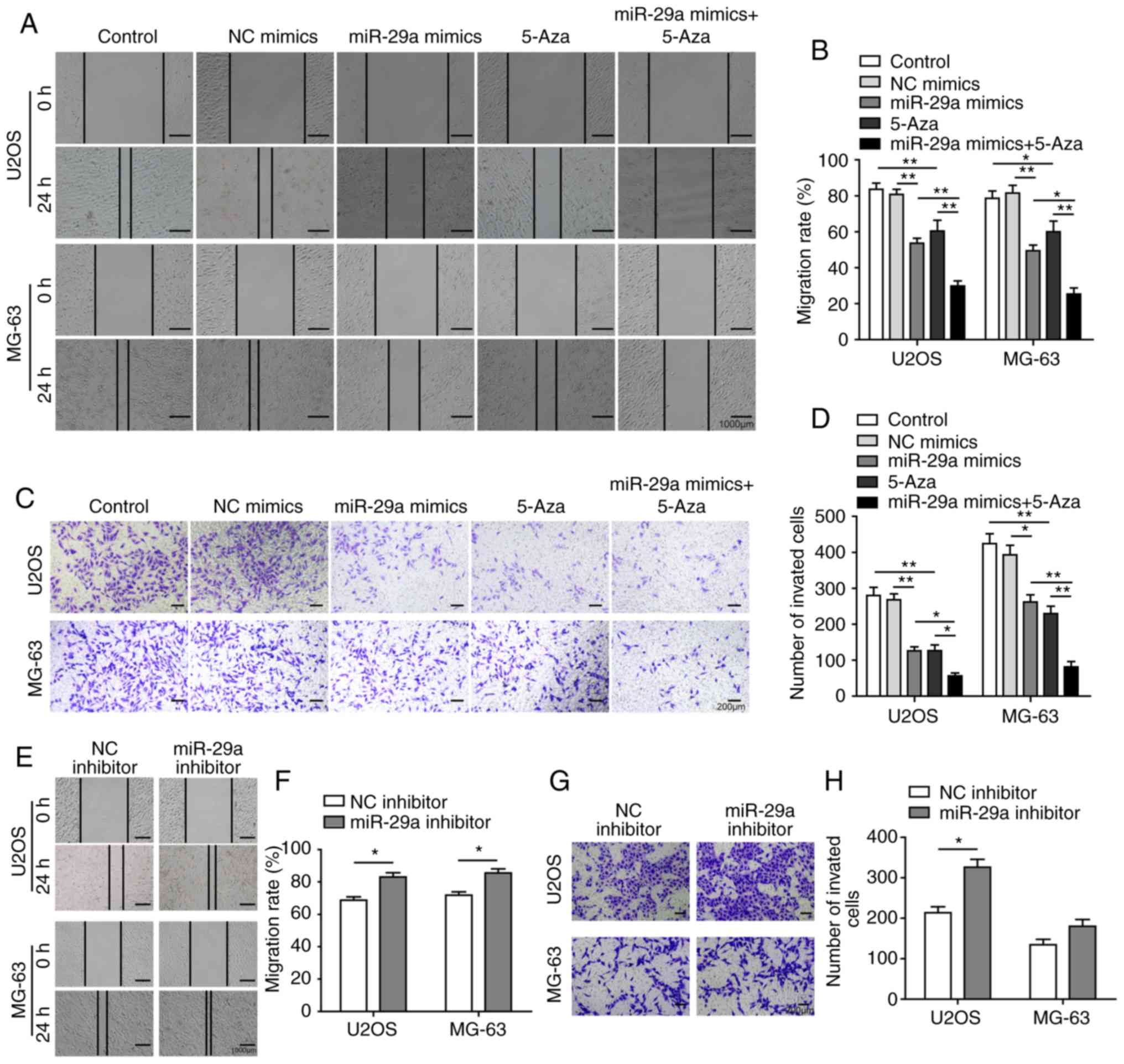
miR-29a inhibits cell invasion, migration
and EMT process of U2OS and MG-63 cells
The effects of miR-29a on the cell invasion,
migration and EMT process were subsequently investigated. The
invasion and migration abilities were significantly inhibited by
transfection with miR-29a mimics or treatment with 5-Aza, while the
inhibitory effects were further enhanced in cells treated with the
combination of miR-29a mimics and 5-Aza (Fig. 6A-D). However, when transfected
with the miR-29a inhibitor, the invasion and migration abilities of
the cells were evidently promoted (Fig. 6E-H). All these results indicated
that miR-29a was able to promote apoptosis, as well as inhibit cell
invasion and migration, in U2OS and MG-63 cells.
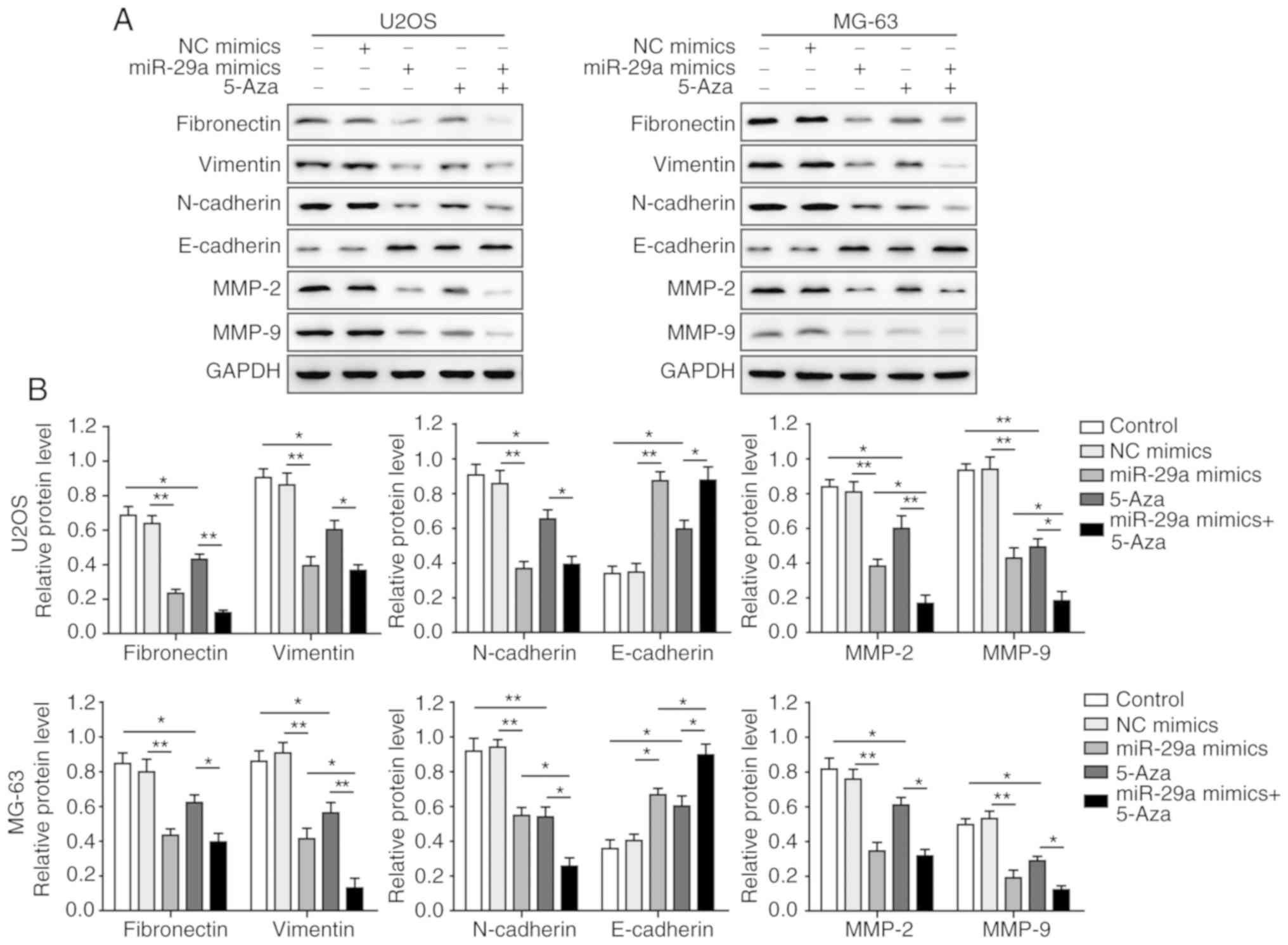
Furthermore, as shown in Fig. 7A and B, the levels of fibronectin,
vimentin, N-cadherin, MMP-2 and MMP-9 were significantly inhibited
in U2OS and MG-63 cells by overex-pression of miR-29a or treatment
with 5-Aza, as compared with the control group. However, the level
of E-cadherin was markedly increased in the miR-29a mimics or 5-Aza
groups. Combination treatment with miR-29a mimics and 5-Aza further
promoted the aforementioned effects, suggesting that miR-29a
inhibited the EMT process of U2OS and MG-63 cells.
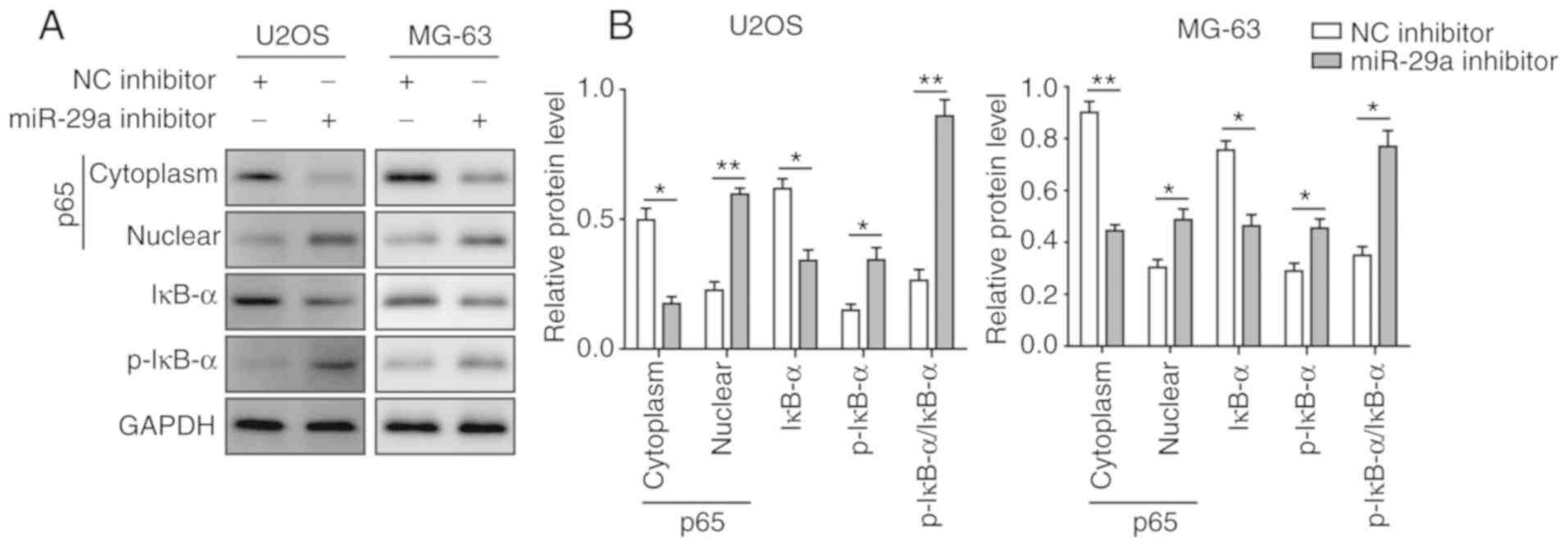
miR-29a inhibits NF-κB signalling pathway
to suppress OS tumourigenesis
Considering that SOCS1 can affect NF-κB signalling
and thus influence the invasion and migration of cancer cells, the
current study subsequently investigated the effects of miR-29a and
5-Aza on NF-κB signalling. The results revealed that the expression
of cytoplasmic p65 was significantly elevated and that of nuclear
p65 was significantly decreased when cells were transfected with
miR-29a mimics or treated with 5-Aza, indicating that the nuclear
translocation of p65 was significantly inhibited by miR-29a and
5-Aza. In addition, the expression of IκB-α was significantly
enhanced, while p-IκB-α expression and the ratio of p-IκB-α/IκB-α
were significantly decreased when cells were transfected with
miR-29a mimics or treated with 5-Aza. All these effects were most
significant under the combination of miR-29a mimics and 5-Aza
(Fig. 8A and B). However, when
cells were transfected with miR-29a inhibitor, the opposite results
were observed. Upon the suppression of miR-29a, the expression of
cytoplasmic p65 was significantly decreased, while nuclear p65 was
significantly elevated. Furthermore, the expression of IκB-α was
significantly decreased, while the expression of p-IκB-α and the
ratio of p-IκB-α/IκB-α were markedly enhanced (Fig. 9A and B). These results
collectively suggested that miR-29a inhibited the activation of
NF-κB signalling, which modulated OS tumourigenesis.
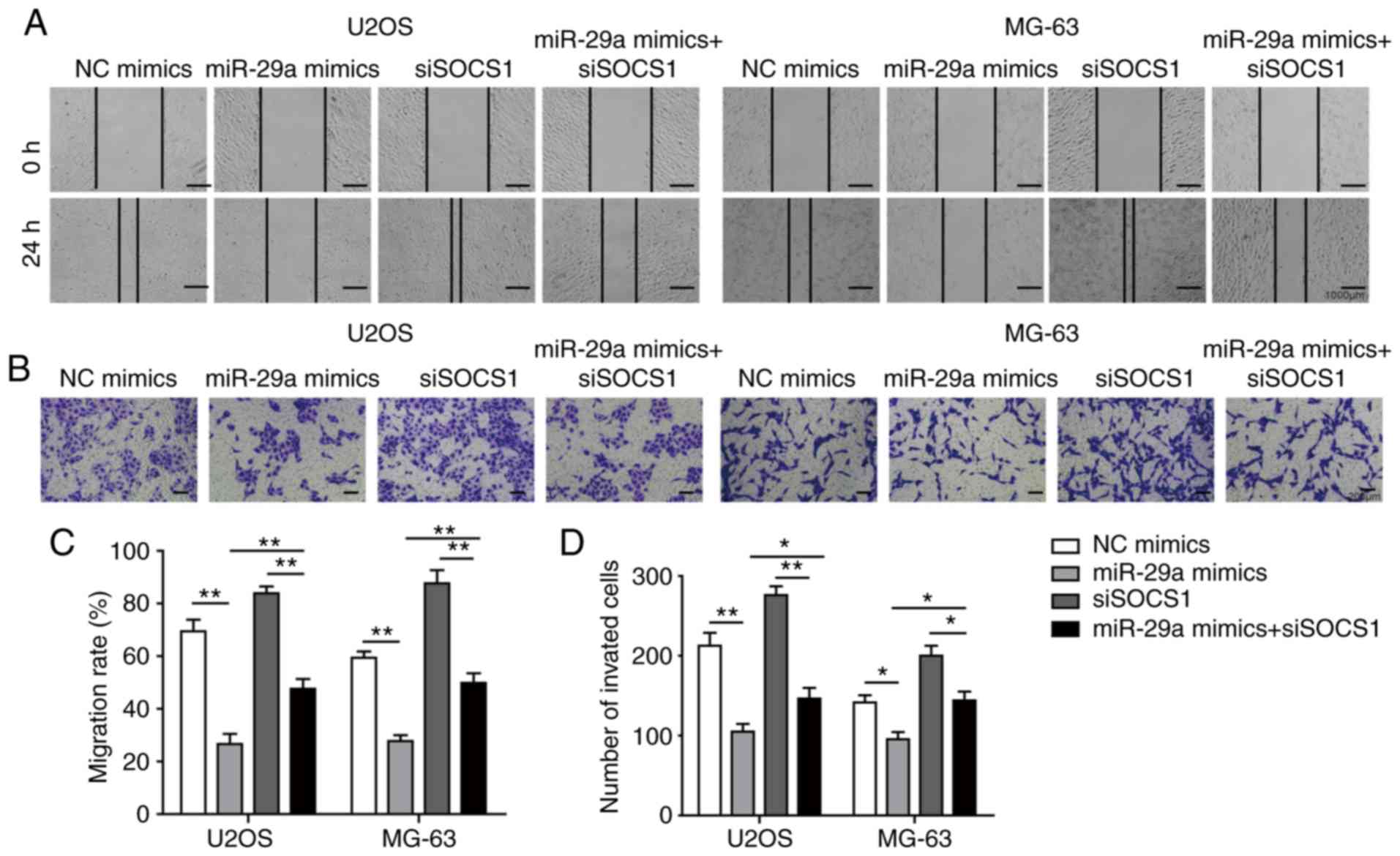
miR-29a suppresses the invasion and
migration of OS cells through the SOCS1/NF-κB signalling
pathway
Finally, cells were co-transfected with siSOCS1 and
miR-29a mimics to examine the effects on cell apoptosis, invasion
and migration. The results revealed that, when co-transfected with
siSOCS1 and miR-29a mimics, the miR-29a-induced increase in SOCS1
expression was significantly decreased by siSOCS1 (Fig. 10A-C). The cell apoptosis rate
exhibited no significant difference in cells transfected with
siSOCS1 compared with the control group. However, the apoptosis
rate was increased by miR-29a mimics, whereas this was markedly
decreased when co-transfected with siSOCS1 and miR-29a mimics
(Fig. 10D and E), suggesting
that transfection with siSOCS1 reversed the effects of miR-29a
treatment. Furthermore, the detection of apoptosis-associated
proteins demonstrated that miR-29a mimics significantly enhanced
the expression levels of cleaved caspase-3, cleaved PARP and Bax,
while they markedly decreased the levels of uncleaved caspase-3,
uncleaved PARP and Bcl-2; however, co-transfection with siSOCS1
reversed these effects (Fig. 10F and
G). Additionally, the miR-29a mimics-induced ratio of
cleaved/uncleaved caspase-3 and cleaved/uncleaved PARP were
markedly reversed by the knockdown of SOCS1 as compared with the
miR-29a mimics alone (Fig. 10F and
G). Similarly, the inhibition of cell invasion and migration
abilities of miR-29a mimics were reversed by co-transfection with
siSOCS1 (Fig. 11A-D). All these
results further confirmed that miR-29a suppressed the invasion and
migration of OS cells through the SOCS1/NF-κB signalling
pathway.
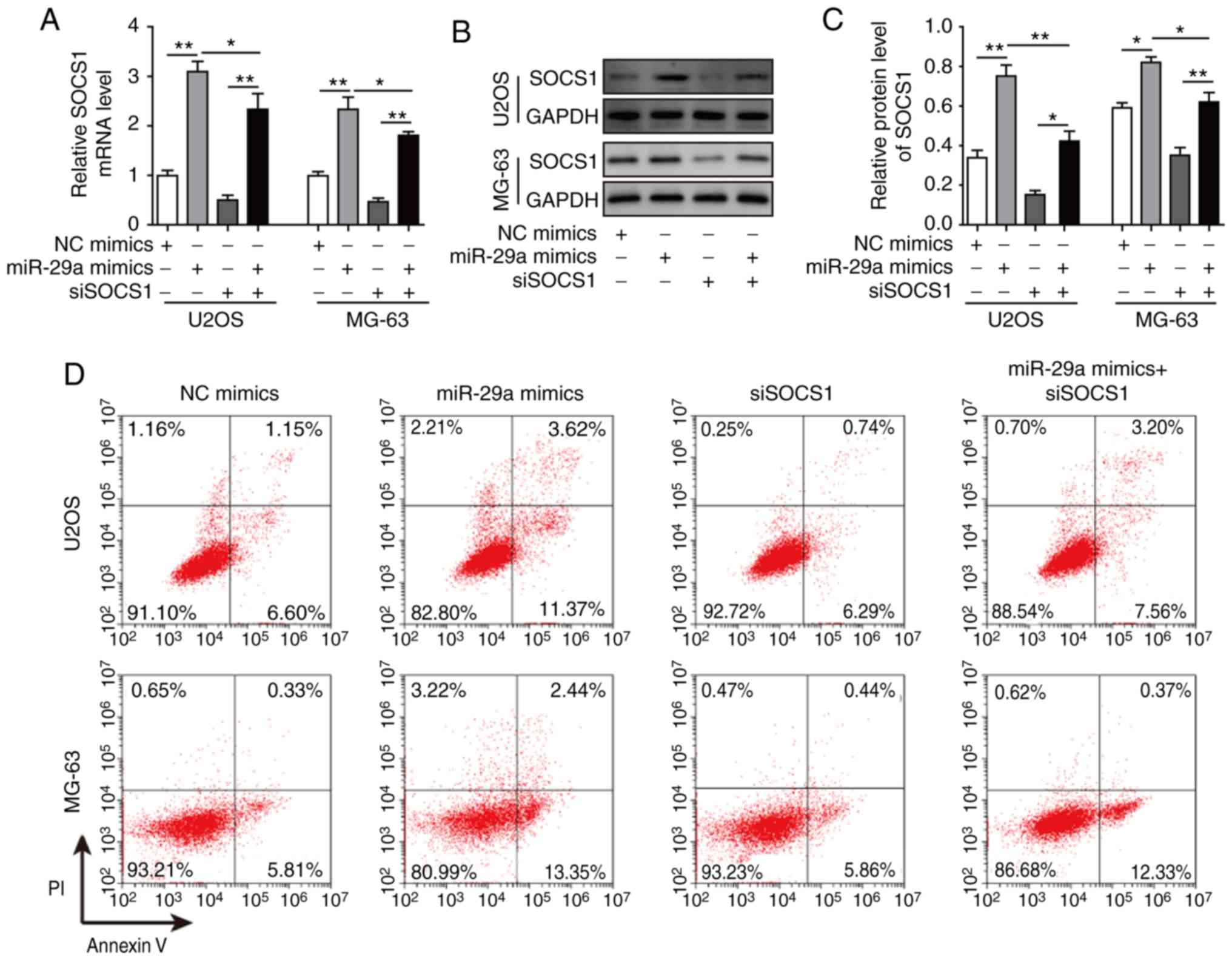 | Figure 10miR-29a promotes cell apoptosis of
U2OS and MG-63 osteosarcoma cells through the SOCS1/NF-κB
signalling pathway. (A) mRNA expression of SOCS1 in cells
transfected with miR-29a mimics and/or siSOCS1, assessed by reverse
transcription-quantitative polymerase chain reaction. (B) Western
blots and (C) quantified protein level of SOCS1 in cells
transfected with miR-29a mimics and/or siSOCS1. (D) Flow cytometry
results and (E) quantified apoptosis rate of cells transfected with
miR-29a mimics and/or siSOCS1. (F) Western blots and (G) quantified
protein levels of cleaved caspase-3, uncleaved caspase-3, cleaved
PARP, uncleaved PARP, Bax and Bcl-2 in cells transfected with
miR-29a mimics and/or siSOCS1. Data are expressed as the mean ±
standard deviation. Comparison among three or more groups was
conducted using one-way analysis of variance followed by Tukey's
post hoc test. *P<0.05, **P<0.01 and
***P<0.001. miR-29a, microRNA-29a; SOCS1, suppressor
of cytokine signalling 1; siSOCS1, SOCS1 siRNA; NC, negative
control; PARP, poly (ADP-ribose) polymerase; Bcl-2, B-cell
lymphoma-2; Bax, Bcl-2-associated X protein. |
Discussion
Despite developments in therapeutic methods and
diagnosis, the survival rate of OS patients remains poor due to the
high aggressiveness and metastasis properties of this tumour.
miR-29a, which is considered to be a tumour suppressor, has been
reported to be abnormally expressed in OS (12). However, to date, no study has
focussed on the roles of the miR-29a/DNMT3B/SOCS1 axis in the
invasion and migration of OS. In the present study, it was
demonstrated for the first time that miR-29a promoted apoptosis,
and inhibited invasion, migration and EMT in OS cells by directly
targeting DNMT3B and then suppressing the methylation level of
SOCS1. In addition, these effects were found to be associated with
inhibition of the NF-κB signalling pathway.
The clinical significance of miR-29a, DNMT3B and
SOCS1 in cancer has been reported in several studies. It has been
reported that miR-29a was significantly upregulated in tissue
samples of colorectal cancer patients and may be used as a
promising diagnostic biomarker (31). Zhang et al (32) revealed that miR-29a and miR-29b
were downregulated in OS tissues. Furthermore, Liu et al
(15) reported that
overexpression of DNMT3B was correlated to the downregulation of
miR-29a in juvenile myelomonocytic leukaemia patients. Lv et
al (33) further demonstrated
that SOCS1 expression was significantly lower in breast cancer
tissues, and was correlated with lymph node metastasis and clinical
staging. In the present study, it was also observed that miR-29a
and SOCS1 were downregulated, and DNMT3B was upregulated in OS
tissues and cells. In addition, miR-29a and SOCS1 expression levels
were associated with advanced clinical stage and distant
metastasis.
The role of miR-29a as a tumour suppressor, and the
effects of SOCS1 and DNMT3B on tumour development have been
demonstrated in several previous studies. For instance, it has been
reported that miR-29a suppresses cell proliferation and migration
by downregulating IGF1R in hepatocellular carcinoma (34). In addition, the downregulation of
SOCS1 promotes cell growth and tumourigenesis in gastric cancer
(35). Another study indicated
that mahanine induced the demethylation of the RASSF1A promoter in
prostate cancer cells by downregulating DNMT1 and DNMT3B (36).
Several related studies have focussed on the
association of miR-29a with SOCS1 or DNMT3B. Chen et al
(37) reported that miR-29a was
able to promote metastasis of hepato-cellular carcinoma through the
ten-eleven translocation (TET)-SOCS1-MMP-9 signalling axis. DNMT3B
was also revealed to be a target of miR-29a in neuroblastoma
(38). Recently, Fu et al
(39) demonstrated that the
upregulation of DNMT3A/3B could enhance the methylation level of
SOCS1-CpG islands. However, no study has reported whether miR-29a
influences the expression of DNMT3B or affects the methylation of
SOCS1 in OS cells. In the present study, using a dual-luciferase
reporter assay, it was observed that miR-29a directly targets
DNMT3B. It was also demonstrated for the first time that miR-29a
promoted the apoptosis, and inhibited the invasion, migration and
EMT of OS cells by directly targeting DNMT3B and then
downregulating the methylation level of SOCS1.
The regulatory effect of SOCS1 on the EMT and NF-κB
signalling is well demonstrated. SOCS1 regulates the EMT and
metastasis of prostate cancer (20). In the present study, it was
demonstrated that the inhibition of NF-κB signalling was also
involved in the aforementioned process. Gebeshuber et al
(40) reported that miR-29a
suppressed EMT and metastasis in lung cancer. The study by Kogure
et al (41) further
demonstrated that miR-29a was associated with epigenetic regulation
of transforming growth factor β-induced EMT in hepatocellular
carcinoma. Furthermore, miR-29a was reported to regulate the
lipopolysaccharide-induced inflammatory responses through the
Akt1/NF-κB pathway (42). In the
current research, the results revealed that miR-29a inhibited the
NF-κB signalling pathway in OS, and the inhibition effect of
miR-29a on cell invasion, migration and EMT in OS cells was
reversed by inhibition of SOCS1, indicating that the effects of
this miRNA may be through the inhibition of SOCS1/NF-κB
signalling.
In conclusion, this study investigated the roles of
the miR-29a/DNMT3B/SOCS1 axis on the invasion and migration of OS
cells. The results revealed that miR-29a promoted the apoptosis and
inhibited the metastasis of OS cells via inhibition of the
SOCS1/NF-κB signalling pathway by directly targeting DNMT3B. The
current study may provide deeper insights into the role of the
miR-29a/DNMT3B/SOCS1 axis in the development of OS, as well as
provide novel therapeutic targets for OS treatment.
Acknowledgments
The authors would like to express their sincere
gratitude to the reviewers for their constructive comments.
Funding
No funding was received.
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
JDN conceived the study. HLG, YT, XZM, DS, DY and
JDN collected the data. HLG, YT and JDN analysed the data. HLG, YT,
XZM, DYS, DY and JDN performed the experiments. DS and JDN provided
the resources and supervised the study. HLG wrote the original
draft. DYS and JDN reviewed and edited the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Second Xiangya Hospital, Central South University. Written
informed consent was obtained from all patients prior to
participation.
Patient consent for publication
Written informed consent for publication was
obtained from all patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment-where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar
|
|
3
|
Broadhead ML, Clark JC, Myers DE, Dass CR
and Choong PF: The molecular pathogenesis of osteosarcoma: A
review. Sarcoma. 2011:9592482011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kansara M, Teng MW, Smyth MJ and Thomas
DM: Translational biology of osteosarcoma. Nat Rev Cancer.
14:722–735. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Di Fiore R, Drago-Ferrante R, Pentimalli
F, Di Marzo D, Forte IM, D'Anneo A, Carlisi D, De Blasio A,
Giuliano M, Tesoriere G, et al: MicroRNA-29b-1 impairs in vitro
cell proliferation, self-renewal and chemoresistance of human
osteosarcoma 3AB-OS cancer stem cells. Int J Oncol. 45:2013–2023.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Durfee RA, Mohammed M and Luu HH: Review
of osteosarcoma and current management. Rheumatol Ther. 3:221–243.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie B, Ding Q, Han H and Wu D: miRCancer:
A microRNA-cancer association database constructed by text mining
on literature. Bioinformatics. 29:638–644. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dinh TK, Fendler W, Chałubińska-Fendler J,
Acharya SS, O'Leary C, Deraska PV, D'Andrea AD, Chowdhury D and
Kozono D: Circulating miR-29a and miR-150 correlate with delivered
dose during thoracic radiation therapy for non-small cell lung
cancer. Radiat Oncol. 11:612016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pasqualini L, Bu H, Puhr M, Narisu N,
Rainer J, Schlick B, Schäfer G, Angelova M, Trajanoski Z, Börno ST,
et al: miR-22 and miR-29a are members of the androgen receptor
cistrome modulating LAMC1 and Mcl-1 in prostate cancer. Mol
Endocrinol. 29:1037–1054. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang H, Bai M, Deng T, Liu R, Wang X, Qu
Y, Duan J, Zhang L, Ning T, Ge S, et al: Cell-derived microvesicles
mediate the delivery of miR-29a/c to suppress angiogenesis in
gastric carcinoma. Cancer Lett. 375:331–339. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sampson VB, Yoo S, Kumar A, Vetter NS and
Kolb EA: MicroRNAs and potential targets in osteosarcoma: Review.
Front Pediatr. 3:692015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He DX, Gu XT, Li YR, Jiang L, Jin J and Ma
X: Methylation- regulated miR-149 modulates chemoresistance by
targeting GlcNAc N-deacetylase/N-sulfotransferase-1 in human breast
cancer. FEBS J. 281:4718–4730. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Pan Q, Wan X, Mao Y, Lu W, Xie X and
Cheng X: Methylation-associated Has-miR-9 deregulation in
paclitaxel- resistant epithelial ovarian carcinoma. BMC Cancer.
15:5092015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu YL, Lensing SY, Yan Y, Webster C and
Emanuel PD: Overexpression Of DNMT3a and DNMT3b is related to
down-regulation of Mir-29a in juvenile myelomonocytic leukemia
(JMML). Blood. 122:48902013.
|
|
16
|
Babon JJ and Nicola NA: The biology and
mechanism of action of suppressor of cytokine signaling 3. Growth
Factors. 30:207–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Beaurivage C, Champagne A, Tobelaim WS,
Pomerleau V, Menendez A and Saucier C: SOCS1 in cancer: An oncogene
and a tumor suppressor. Cytokine. 82:87–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang XH, Yang L, Liu XJ, Zhan Y, Pan YX,
Wang XZ and Luo JM: Association between methylation of tumor
suppressor gene SOCS1 and acute myeloid leukemia. Oncol Rep.
40:1008–1016. 2018.PubMed/NCBI
|
|
19
|
Huang FJ, Steeg P, Price J, Sawaya R and
Huang S: Suppressor of cytokine signaling-1 (SOCS-1) negatively
regulates angiogenesis, invasion, and brain metastasis of melanoma
cells. Cancer Res. 68:2008. View Article : Google Scholar
|
|
20
|
Shao N, Ma G, Zhang J and Zhu W:
miR-221-5p enhances cell proliferation and metastasis through
post-transcriptional regulation of SOCS1 in human prostate cancer.
BMC Urol. 18:142018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hashimoto M, Ayada T, Kinjyo I, Hiwatashi
K, Yoshida H, Okada Y, Kobayashi T and Yoshimura A: Silencing of
SOCS1 in macrophages suppresses tumor development by enhancing
antitumor inflammation. Cancer Sci. 100:730–736. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gaspericampani A, Pancotti F and Roncuzzi
L: Abstract 4396: Caveolin-1 as an oncopromoter in solidtumors: A
role mediated by STAT3 in vitro. Cancer Res. 73(8 Suppl): S4396.
2014.
|
|
23
|
Zhang H, Zhao Z, Pang X, Yang J, Yu H,
Zhang Y, Zhou H and Zhao J: Genistein protects against
Ox-LDL-induced inflammation through microRNA-155/SOCS1-mediated
repression of NF-ĸB signaling pathway in HUVECs. Inflammation.
40:1450–1459. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee YJ, Han JY, Byun J, Park HJ, Park EM,
Chong YH, Cho MS and Kang JL: Inhibiting Mer receptor tyrosine
kinase suppresses STAT1, SOCS1/3, and NF-κB activation and enhances
inflammatory responses in lipopolysaccharide-induced acute lung
injury. J Leukoc Biol. 91:921–932. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ren D, Yang Q, Dai Y, Guo W, Du H, Song L
and Peng X: Oncogenic miR-210-3p promotes prostate cancer cell EMT
and bone metastasis via NF-κB signaling pathway. Mol Cancer.
16:1172017. View Article : Google Scholar
|
|
26
|
Liu X, Li J, Peng X, Lv B, Wang P, Zhao X
and Yu B: Geraniin inhibits LPS-induced THP-1 macrophages switching
to M1 phenotype via SOCS1/NF-κB pathway. Inflammation.
39:1421–1433. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maier HJ, Schmidt-Strassburger U, Huber
MA, Wiedemann EM, Beug H and Wirth T: NF-kappaB promotes
epithelial-mesenchymal transition, migration and invasion of
pancreatic carcinoma cells. Cancer Lett. 295:214–228. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu Y and Zhou BP:
TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and
invasion. Br J Cancer. 102:639–644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Meister P, Konrad E, Lob G, Janka G, Keyl
W and Stürz H: Osteosarcoma: Histological evaluation and grading.
Arch Orthop Trauma Surg. 94:91–98. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
31
|
Brunet Vega A, Pericay C, Moya I, Ferrer
A, Dotor E, Pisa A, Casalots A, Serra-Aracil X, Oliva JC, Ruiz A
and Saigí E: microRNA expression profile in stage III colorectal
cancer: Circulating miR-18a and miR-29a as promising biomarkers.
Oncol Rep. 30:320–326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang W, Qian JX, Yi HL, Yang ZD, Wang CF,
Chen JY, Wei XZ, Fu Q and Ma H: The microRNA-29 plays a central
role in osteosarcoma pathogenesis and progression. Mol Biol (Mosk).
46:622–627. 2012. View Article : Google Scholar
|
|
33
|
Lv Y, Song G and Li P: Correlation of
SOCS-1 gene with onset and prognosis of breast cancer. Oncol Lett.
16:383–387. 2018.PubMed/NCBI
|
|
34
|
Wang X, Liu S, Cao L, Zhang T, Yue D, Wang
L, Ping Y, He Q, Zhang C, Wang M, et al: miR-29a-3p suppresses cell
proliferation and migration by downregulating IGF1R in
hepatocellular carcinoma. Oncotarget. 8:86592–86603.
2017.PubMed/NCBI
|
|
35
|
Qin S, Ai F, Ji WF, Rao W, Zhang HC and
Yao WJ: miR-19a promotes cell growth and tumorigenesis through
targeting SOCS1 in gastric cancer. Asian Pac J Cancer Prev.
14:835–840. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Agarwal S, Amin KS, Jagadeesh S, Baishay
G, Rao PG, Barua NC, Bhattacharya S and Banerjee PP: Mahanine
restores RASSF1A expression by down-regulating DNMT1 and DNMT3B in
prostate cancer cells. Mol Cancer. 12:992013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen Q, Dan Y, Zhang Y, Yu L, Li XD, Zhou
ZJ, Zhou SL, Gao DM, Hu J, Jin C, et al: MicroRNA-29a induces loss
of 5-hydroxymethylcytosine and promotes metastasis of
hepatocellular carcinoma through a TET-SOCS1-MMP9 signaling axis.
Cell Death Dis. 8:e29062017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Maugeri M, Barbagallo D, Barbagallo C,
Banelli B, Di Mauro S, Purrello F, Magro G, Ragusa M, Di Pietro C,
Romani M and Purrello M: Altered expression of miRNAs and
methylation of their promoters are correlated in neuroblastoma.
Oncotarget. 7:83330–83341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fu X, Song X, Li Y, Tan D and Liu G:
Hepatitis B virus X protein upregulates DNA methyltransferase 3A/3B
and enhances SOCS-1CpG island methylation. Mol Med Rep. 13:301–308.
2016. View Article : Google Scholar
|
|
40
|
Gebeshuber CA, Zatloukal K and Martinez J:
miR-29a suppresses tristetraprolin, which is a regulator of
epithelial polarity and metastasis. EMBO Rep. 10:400–405. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kogure T, Kondo Y, Kakazu E, Ninomiya M,
Kimura O and Shimosegawa T: Involvement of miRNA-29a in epigenetic
regulation of transforming growth factor-β-induced
epithelial-mesenchymal transition in hepatocellular carcinoma.
Hepatol Res. 44:907–919. 2014. View Article : Google Scholar
|
|
42
|
Tang B, Li X, Ren Y, Wang J, Xu D, Hang Y,
Zhou T, Li F and Wang L: MicroRNA-29a regulates lipopolysaccharide
(LPS)-induced inflammatory responses in murine macrophages through
the Akt1/NF-κB pathway. Exp Cell Res. 360:74–80. 2017. View Article : Google Scholar : PubMed/NCBI
|