Introduction
Striatal dopamine insufficiency is a major
contributor to the motor symptoms of PD (1,2).
The striatum, which is the main component of the basal ganglia, is
a mass-like structure formed by a cluster of cells. In rodents,
90-95% of striatal neurons are projection neurons, which are
involved in performing striatal functions and are simultaneously
regulated by striatal inter-neurons (3). Importantly, striatal projection
neurons receive both excitatory inputs from the cerebral cortex and
thalamus and inhibitory inputs from the substantia nigra pars
compacta (SNc). Then, the striatal neurons provide feedback to the
stri-atum and cerebral cortex after relaying through the substantia
nigra and thalamus, which exert regulatory functions on the
striatum (4-7). Neurotransmitter inputs target
striatal neurons (especially projection neurons), and coordination
of the excitatory and inhibitory inputs maintains the structural
integrity and functional stability of the striatal neurons
(8). Striatal dopamine depletion
leads to an imbalance among the cortex, thalamus and SNc; thus,
dopamine depletion induces a relative increase in corticostriatal
glutamatergic inputs, which is considered the main mechanism
underlying striatal neuronal damage (9,10).
If the excitatory input to the cortical striatum is reduced after
striatal dopamine deprivation, the balance between excitability and
inhibitory input on striatal neurons can be restored. A partial
decortication approach was used in the present study to decrease
corticostriatal glutamatergic inputs(Fig. S1).
The pathological mechanisms underlying striatal
neuron injury after dopamine depletion induced by
6-hydroxydopa-mine (6OHDA) remain unclear. When the dopaminergic
input from the midbrain is removed, a result is a relative increase
in cortical excitatory input to the striatal neurons, which is also
considered a possible cause. The loss of dopaminergic neurons in
the substantia nigra results in striatal dopamine neurotransmitter
depletion. Striatal dopamine depletion induces an imbalance between
excitatory and inhibitory afferents to the striatum and leads to
morphological changes and complex physiological changes in the
striatum (11,12). A previous study confirmed that
6OHDA-induced PD rats exhibited behavioral disorders associated
with striatal function, such as muscular tension, learning, memory
and cognitive deficits (13). The
present study examined the effect of decortication on dopamine
depletion-induced behavioral disorders. Changes have also
previously been identified in the morphology and protein and gene
expression levels of striatal neurons in PD rat models (14,15). One study showed that the neuronal
damage manifests as the loss of dendritic spines (16). Although other reports have
described apoptotic changes in striatal neurons (17-19), the potential mechanisms remain
unclear. To determine whether the changes in striatal neurons were
associated with the relative increase in glutamatergic inputs,
dopamine depletion, glutamate depletion and dopamine + glutamate
depletion models were used to investigate histopathological and
protein changes in striatal neurons (Fig. S1).
In light of the aforementioned findings, the present
study aimed to confirm the lesion mechanism of striatal neurons and
the regulatory effects of cortical glutamatergic inputs in PD
pathological processes, which is of importance for further
understanding of the pathological mechanisms of PD.
Materials and methods
Experimental animals
Male 8-week-old Sprague-Dawley (SD) rats weighing
200-250 g were used for the present study. All rats were obtained
from the Center for Experimental Animals of Sun Yat-sen University.
All rat care and procedures involved were approved by the Animal
Care and Use Committee of Sun Yat-sen University (no. SYXK
GUANGDONG 2011-0029). Animal welfare and experimental procedures
were carried out strictly in accordance with the Guide for the Care
and Use of Laboratory Animals (Guide for Ethical Review of Animal
Welfare of China, 2018) (20).
The SD rats were individually housed in an air-conditioned room
(temperature 22±0.5°C, relative humidity, 40-70%) under a 12 h
light-dark cycle, with water and food available ad
libitum.
SD rats (n=96) were randomly assigned to the control
group (n=24), 6OHDA group (n=24), ibotenic acid (IA) group (n=24)
and 6OHDA+IA group (n=24). In each group, six animals were used for
Golgi-Cox staining, six for immunohistochemistry and electron
microscopy (EM) detection, and six for western blotting; the
remaining animals were used for reverse transcription-quantitative
(RT-q)PCR.
For clarity and conciseness, the data and
statistical analyses presented in the text include only the
control, 6OHDA, IA and 6OHDA+IA groups unless otherwise
indicated.
Treatment of animals
6OHDA is a neurotransmitter analog that is used to
induce nigrostriatal dopamine depletion (21). IA is a neurotoxic isoxazole that
is used to induce corticostriatal glutamate depletion. The rats
were deeply anesthetized with sodium pentobarbital [50 mg/kg,
intraperitoneal (i.p.); cat. no. P3761; Sigma-Aldrich; Merck KGaA]
and fixed on a Kopf stereotaxic frame (Stoelting Co.). Burr holes
were made in the skull over the primary motor cortex (M1) and the
right medial forebrain bundle (MFB). The rats in the 6OHDA group
were injected with 8 µl 6OHDA (2 µg/µl; 6OHDA
dissolved in 0.9% saline containing 0.01% ascorbic acid as an
antioxidant; cat. no. H116; Sigma-Aldrich; Merck KGaA) in the right
MFB [anterior-posterior (AP), -3.6; medial-lateral (ML), −0.19; and
dorsal ventral (DV), -8.2] using a 10 µl syringe (Hamilton
Company). The PD rat models used in the present study were
described in a previous report (22). The rats in the IA group were
injected with 1 µl of 45 nM IA (CAS no. 2552-55-8;
Sigma-Aldrich; Merck KGaA) in the primary motor cortex (AP, −1.7;
ML, −2.2; and DV, −1.7). The rats in the 6OHDA+IA group were
injected with both 6OHDA and IA on the right side using the same
methods (16,23). The rats in the vehicle control
group were injected with solvent at the same volume in the same
injection location. The rats in the 6OHDA vehicle control group
were injected with 8 µl solvent (0.9% saline containing
0.01% ascorbic acid) in the right MFB. The rats in the IA vehicle
control group were injected with 1 µl solvent (0.9% saline)
in the primary motor cortex. The rats in the 6OHDA+IA vehicle
control group were injected with both 6OHDA solvent and IA solvent
on the right side using the same methods.
During the 3 weeks following 6OHDA lesions, rats
were subcutaneously injected with apomorphine (APO; cat. no.
2073/50; Tocris Bioscience) at a dose of 0.25 mg/kg, and the number
of 360° contralateral rotations within 30 min were counted. Only
rats with a significant number of contra-lateral rotations (>7
cycles/minute or >210 total cycles) were included. The effect of
APO on motor asymmetry and rotation to the uninjured side (the left
in the present study) in a 30 min period were recorded by two
examiners who were blinded to animal states. The rotation behavior
test method was described in detail in a previous study (14). All rats were sacrificed at 28 days
after surgery and further examined. All brains were quickly removed
and used for Golgi-Cox staining, immunohistochemistry, EM
detection, western blotting and RT-qPCR. Moreover,
immunohistochemical staining for tyrosine hydroxylase was performed
to ensure the success of the model after slicing. Only the data and
tissue from the rats with TH-positive fibers (<5%) in the right
striatum were used in the subsequent analyses. The extent of
TH-positive fibers after 6OHDA lesion development was shown in a
previous study (13). The
APO-induced rotation test and immunohistochemical staining of TH
were designed to ensure that successful dopamine depletion-induced
PD models were used for further examination (data not shown) and
have been described previously (13,14). A total of 4 weeks after
development of the IA lesions, the focal cortical lesions were
examined using a light microscope (LM).
Behavioral tests
Grip strength test
During the 3 weeks following the 6OHDA lesions, the
grip strength of each rat was evaluated by recording the time spent
hanging on a steel wire that was 2 mm in diameter and 35 mm in
length, and suspended 50 cm above the horizontal surface of the
ground (24). The test was
performed three times/day over a 5 day period. All animals
underwent these test (n=24/group). The observers were blinded to
the rat treatment conditions.
Morris water maze task
During the 3 weeks following the 6OHDA lesions, the
rats were trained twice a day for 5 days. All animals underwent
these tests (n=24/group). A probe test was conducted on the last
day of the Morris water maze task (25). During the training process, the
target platform (diameter, 10 cm) was located in a fixed position.
In each session, the animals were released from four designated
starting points (north, east, south and west) and allowed to swim
until they reached the target platform or for 2 min. Once the rats
reached the platform or failed to find the platform within 2 min,
they remained on the platform for 30 sec. In each session, the
latency to reach the platform within 2 min was recorded using the
TopScan™ 2.0 behavior analysis system (CleverSys,
Inc.).
Golgi staining
The methods for Golgi staining were previously
described in detail (26). At 4
weeks following the 6OHDA lesions, all the rats were deeply
anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and
perfused with 0.9% saline. The brains were harvested and stained
using the FD Rapid GolgiStain™ kit in accordance with the
manufacturer's instructions (cat. no. PK 401; FD Neuro
Technologies.). The brains were first stored in the dark for 14
days in Golgi-Cox solution and then submerged in 30% sucrose for 3
days. The brains were sectioned coronally at a thickness of 50
µm using a vibratome (Leica VT1200S; Leica Microsystems
GmbH). Sections were collected and the stain was developed with
ammonium hydroxide for 30 min. Sections were immersed in deionized
water for another 30 min and then washed with water, dehydrated,
cleared and mounted using a resin. Dendrites and dendritic spines
were photographed using a Leica DM 2500B microscope equipped with a
×40 objective. The Neuron J v1.4.1 software (National Institutes of
Health) was used to measure the total dendrite lengths and spine
numbers. The dendrite and dendritic spine morphologies were
examined in the striatal neurons and the data were analyzed using
Sholl analysis (27). The third
dendrite order of the striatal neurons from the dorsal-lateral
striatum of each group was quantitatively analyzed with regard to
the spine numbers. In the present study, the experimental area of
interest was the dorsal-lateral zone of the striatum, which is the
sensorimotor area of the striatum. The location of this region in
the striatum was described in an earlier study (15). The dendritic spine densities were
measured on dendritic segments 10 µm in length. The
densities of dendritic spine were determined on striatal neurons
from the dorsal-lateral striatum. In Golgi-stained slices, the
total dendritic length was calculated by summing the lengths of
each branch segment within a dendrite and then summing the total
lengths of each of the dendrites for each neuron. The spine numbers
from 10 µm of the dendrite were counted as spine density
(28).
Immunohistochemistry
During the 4 weeks following 6OHDA lesions, rats
(n=3/group) were anesthetized with 0.4% pentobarbital sodium at 50
mg/kg and perfused transcardially with PBS (500 ml; 0.1 M) followed
by 500 ml of 4% paraformaldehyde (in 0.1 M phosphate buffer, pH
7.4). The brain tissue samples were then removed and immersed in 4%
paraformaldehyde overnight at 4°C. The brain tissue was cut into 30
µm sections with a vibratome. The sections were pretreated
with 0.3% H2O2 and 0.1% Triton-X 100 for 30
min at room temperature. BSA (3%; Sigma-Aldrich; Merck KGaA; cat.
no. B2064) was used to block non-specific binding sites at room
temperature for 30 min. The sections were incubated at 4°C for 24 h
with the following primary antibodies: Mouse anti-TH (1:1,000; EMD
Millipore; cat. no. MAB318) diluted with 0.1 M PBS (pH 7.4)
containing 0.5% BSA and 0.3% Triton X-100. The sections were rinsed
and incubated in anti-mouse IgG (1:200; Sigma-Aldrich; Merck KGaA;
cat. no. M4280) diluted with the aforementioned buffer for 3 h at
room temperature, followed by incubation in homologous PAP complex
(1:200; Sigma-Aldrich; Merck KGaA; cat. no. P1291) at room
temperature for 2 h. The peroxidase reaction was performed using
3,3′-diaminobenzidine (0.05% in 0.1 M PBS, pH 7.4; Sigma-Aldrich;
Merck KGaA; cat. no. 11718096001) at room temperature for 1-2 min.
Sections were mounted onto gelatin-coated slides, dehydrated,
permeabilized with xylene and covered with neutral balsam.
To perform conventional double-label
immunofluorescence, sections were incubated overnight at 4°C with
primary antibody. The primary antibodies were mouse anti-NeuN
(1:800; EMD Millipore; cat. no. 2884594) and rabbit anti-caspase-3
(1:500; Cell Signaling Technology, Inc.; cat. no. 9662) diluted
with 0.1 M PBS, pH 7.4, containing 0.5% BSA and 0.3% Triton X-100.
The sections were subsequently incubated with anti-mouse
fluorescent IgG (1:200; Alexa Fluor 480; Molecular Probes; cat. no.
A11029; Thermo Fisher Scientific, Inc.) and anti-rabbit fluorescent
IgG (1:200; Alexa Fluor 594; Molecular Probes; Thermo Fisher
Scientific, Inc.; cat. no. A32740) for 3 h at room temperature. The
section containing the striatum was observed by confocal microscopy
(Nikon C2; Nikon Corporation; magnification ×20). The number of
positive cells was counted in five randomly selected squares
(100×100 µm) in the dorsal-lateral striatum.
EM
During the 4 weeks following the 6OHDA lesions, rats
(n=3/group) used for EM were perfused in the aforementioned manner
(as per the immunohistochemistry analysis), but 0.6% glutaraldehyde
was added to the fixative. All brains were quickly removed and
immersed in 4% paraformaldehyde + 15% saturated picric acid in 0.1
M PB overnight at 4°C, then sectioned at 50 µm by vibratome.
Five sections were used in the EM analysis for each animal. The
methods of EM used were previously described in detail (26). The processed slices were rinsed in
sodium cacodylate buffer (0.1 M, pH 7.2), then postfixed with 2%
osmium tetroxide (cat. no. 18456; PELCO; Ted Pella, Inc.) for 1 h,
dehydrated in a graded series of ethyl alcohols, impregnated with
1% uranyl acetate in 100% alcohol, and flat-embedded in Spurr's
resin (cat. no. 18010; PELCO Eponate 12™ kit; Ted Pella, Inc.). All
sections were examined with an LM. The dorsal-lateral striatal
areas were cut from the slide and glued to the top of a resin
block. The slices were mounted on mesh grids and stained with 0.4%
lead citrate and 4.0% uranyl acetate using an LKB ultramicrotome
(cat. no. EM UC6; Leica Microsystems, GmbH). The EM Tecnai G2
Spirit Twin (FEI; Thermo Fisher Scientific, Inc.) was used to
examine the tissue sections. The number of dendritic spines was
counted in the area of the dorsal-lateral striatum using the
captured images. For the EM data, the analysis and quantification
were carried out on digital EM images. Five nonoverlapping squares
with a size of 100 µm2 in each image were
selected. The number of spines/100 µm2 was
counted as the spine density. For each animal, the analysis was
based on 30 EM images (29).
Western blotting
During the 4 weeks following the 6OHDA lesions, rats
(n=6/group) were sacrificed by decapitation after deep anesthesia
with sodium pentobarbital (50 mg/kg, i.p.), and the striatum of
each rat was extracted and homogenized. The striatal tissue was
extracted and lysed with RIPA buffer (Beyotime Institute of
Biotechnology). Protein was quantified using a bicinchoninic acid
kit (Beyotime Institute of Biotechnology). A total of 30 µg
protein sample was separated by SDS-PAGE (10%) and transferred onto
a PVDF membrane (cat. no. IPVH00010; EMD Millipore). After the
transfer, the membranes were blocked with 5% dried skim milk in
Tris-buffered saline with Tween-20 at room temperature for 1.5 h
and then incubated and shaken overnight at 4°C with the following
primary antibodies: Rabbit anti-caspase-3 (1:5,000; Cell Signaling
Technology, Inc.; cat. no. 9662), rabbit anti-cleaved caspase-3
(1:2,500; Cell Signaling Technology, Inc.; cat. no. 9661) and
rabbit anti-β-actin (1:2,000; EMD Millipore; cat. no. ABT1485). The
membranes were incubated with horseradish peroxidase
(HRP)-conjugated goat-anti-rabbit IgG antibody (1:5,000; cat. no.
AP307P; EMD Millipore) for 2 h at room temperature. The
immunoreactive bands were visualized with chemiluminescent HRP
substrate (cat. no. WBKLs0500; EMD Millipore). The protein bands
were visualized using a Bio-Rad GelDoc XR+ system (Bio-Rad
Laboratories, Inc.) and quantified using ImageJ v1.8.0 software
(National Institutes of Health).
RT-qPCR
During the 4 weeks following the 6OHDA lesions, rats
(n=6/group) were sacrificed by decapitation after deep anesthesia
with sodium pentobarbital (50 mg/kg, i.p.), and the striatum of
each rat was extracted from the brain. According to the
manufacturer's instructions, total RNA was isolated using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). cDNA was synthesized using the SuperScript VILO cDNA
Synthesis kit (cat. no. 11754250, Invitrogen; Thermo Fisher
Scientific, Inc.). The samples were kept at 42°C for 60 min on the
PCR instrument, after which they were kept at 70°C for 5 min to
inactivate the reverse transcriptase. qPCR was performed with
SYBR-Green Master Mix (ABI; Thermo Fisher Scientific, Inc.) on an
ABI PRISM 7000 Sequence Detection (Applied Biosystems; Thermo
Fisher Scientific, Inc.) under the following conditions: 50°C for 5
min, 95°C for 10 min, followed by 45 cycles at 95°C for 30 sec and
60°C for 30 sec. Relative gene expression was analyzed using the
2−ΔΔCq method (30).
The primers were as follows: Caspase-3 forward, 5′-GGA CCT GTG
GACCTG AAA AA-3′; Caspase-3 reverse, 5′-GCA TGC CAT ATC ATC GTC
AG-3′; β-actin forward, 5′-GAA CCC TAA GGC C AA C-3′; and β-actin
reverse, 5′-TGT CAC GCA CGA TT T CC-3′. The cycling conditions were
previously described (22). The
melting curves were analyzed using the 7500 system SDS software
v.2.0.6 (Applied Biosystems™; Thermo Fisher Scientific, Inc.; cat.
no. 4377354).
Statistical analysis
The investigators were blinded when analyzing the
morphological data. IBM SPSS Statistics v22.0 software (IBM Corp.)
was used for all statistical analyses. Data are expressed as the
mean ± SD. Least significant difference post hoc tests were used to
examine the statistical significance among the four groups.
Behavioral indicators were examined by one-way ANOVA followed by
least significant difference post hoc tests. Comparisons among
groups were examined by two-way analysis of variance, and P<0.05
was considered to indicate a statistically significant
difference.
Results
Decortication alleviates the behavioral
dysfunction induced by dopamine depletion in the PD rat model
As reported in a previous study, muscular tension is
markedly upregulated in rats treated with 6OHDA to eliminate
dopaminergic neurons (13). The
grip strength test showed that the hanging time was significantly
increased in the 6OHDA group (22.17±1.47 sec) compared with the
control group (11.67±2.16 sec) and the IA group (14.67±2.66 sec;
P<0.05). Notably, the hanging time was significantly decreased
in the 6OHDA+IA group (12.83±1.72 sec; P<0.05; Table I) compared to the 6OHDA group, and
slightly decreased compared to the IA group.
 | Table IMeasurements from and comparisons
between the behavioral tests. |
Table I
Measurements from and comparisons
between the behavioral tests.
| Test items | Group
|
|---|
| Control | 6OHDA | IA | 6OHDA+IA |
|---|
| Hang time, sec | 11.67±2.16 | 22.17±1.47a | 14.67±2.66a,b | 12.83±1.72b |
| Latency, sec | 40.0±16.91 | 92.56±17.18a | 42.61±32.09 | 45.50±17.86b |
Cognitive deficits are common clinical symptoms of
PD that seriously affect the quality of life of patients. Thus, the
water maze task was used to investigate the cognitive deficits in
the experimental rat model. The probe trial of the Morris water
maze task showed that the latency of the 6OHDA group (92.56±17.18
sec) was significantly longer than that of the control group
(40.0±16.91 sec; P<0.05), whereas the latency of the 6OHDA+IA
group (45.50±17.86 sec) was significantly shorter than that of the
6OHDA group (92.56±17.18 sec; P<0.05; Table I).
Motor dysfunction caused by upregulated muscle
tension, and cognitive deficits, are the main clinical symptoms of
PD. These results indicated that decortication alleviated the
behavioral dysfunction induced by dopamine depletion in the PD rat
model.
Decortication offsets dendrite lesions
and spinal loss of striatal neurons in the PD rat model
Dendrites and dendritic spines are the main
structures of striatal neurons. Therefore, Golgi staining was
employed to investigate the morphological characteristics of the
striatal neurons (27,31). LM analysis showed that the
striatal neuronal dendrites were short, sparse and even broken in
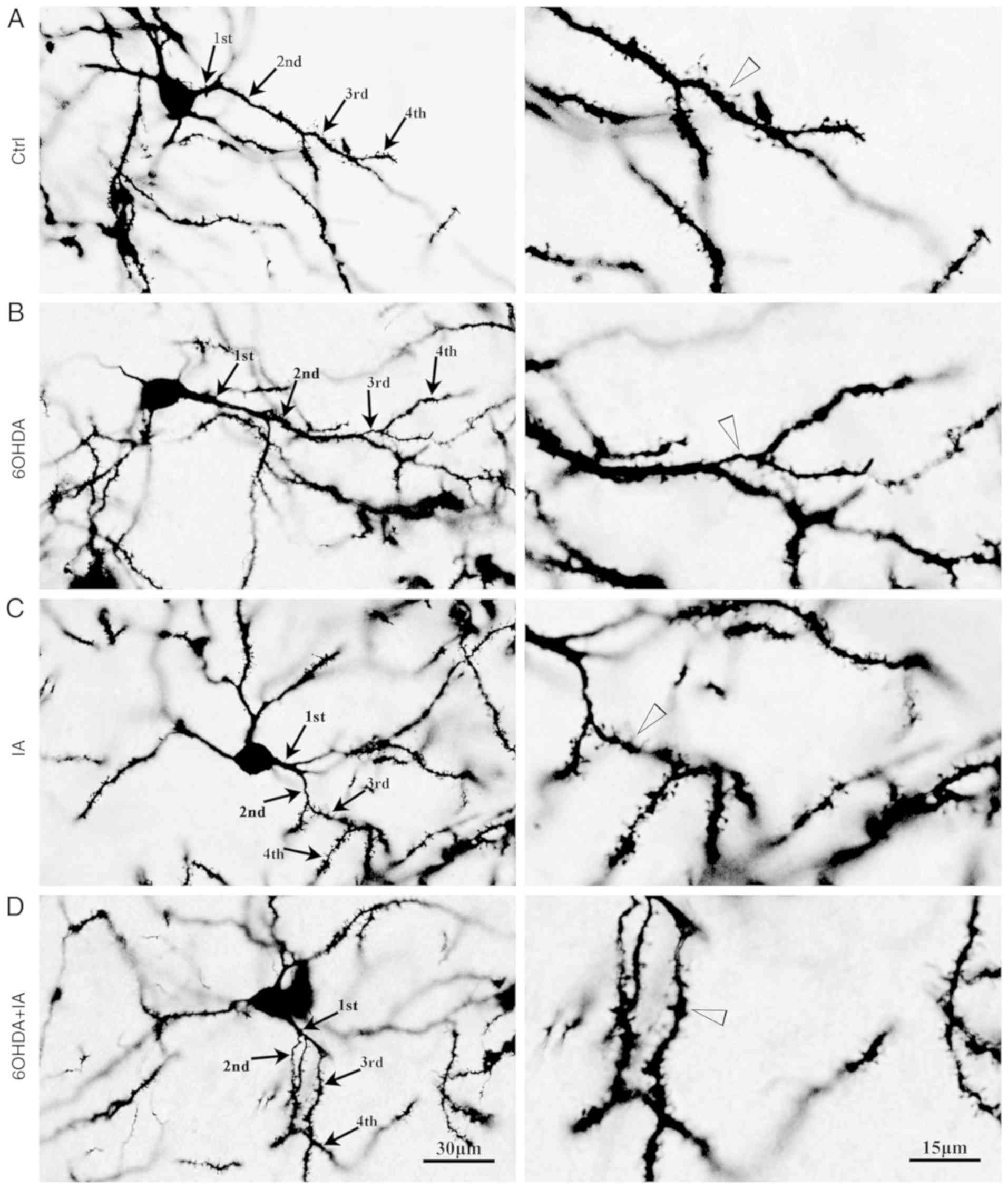
the 6OHDA group (Fig. 1). The
statistical data showed that the total dendritic length of single
neurons in the 6OHDA group (454.1±33.69 µm) was
significantly decreased compared to that in the control group
(615.6±42.44 µm; P<0.05; Fig. 2A), whereas the total dendritic
length in the 6OHDA+IA group (586.4±50.72 µm) was
significantly increased compared to that in the 6OHDA group
(P<0.05; Fig. 2A).
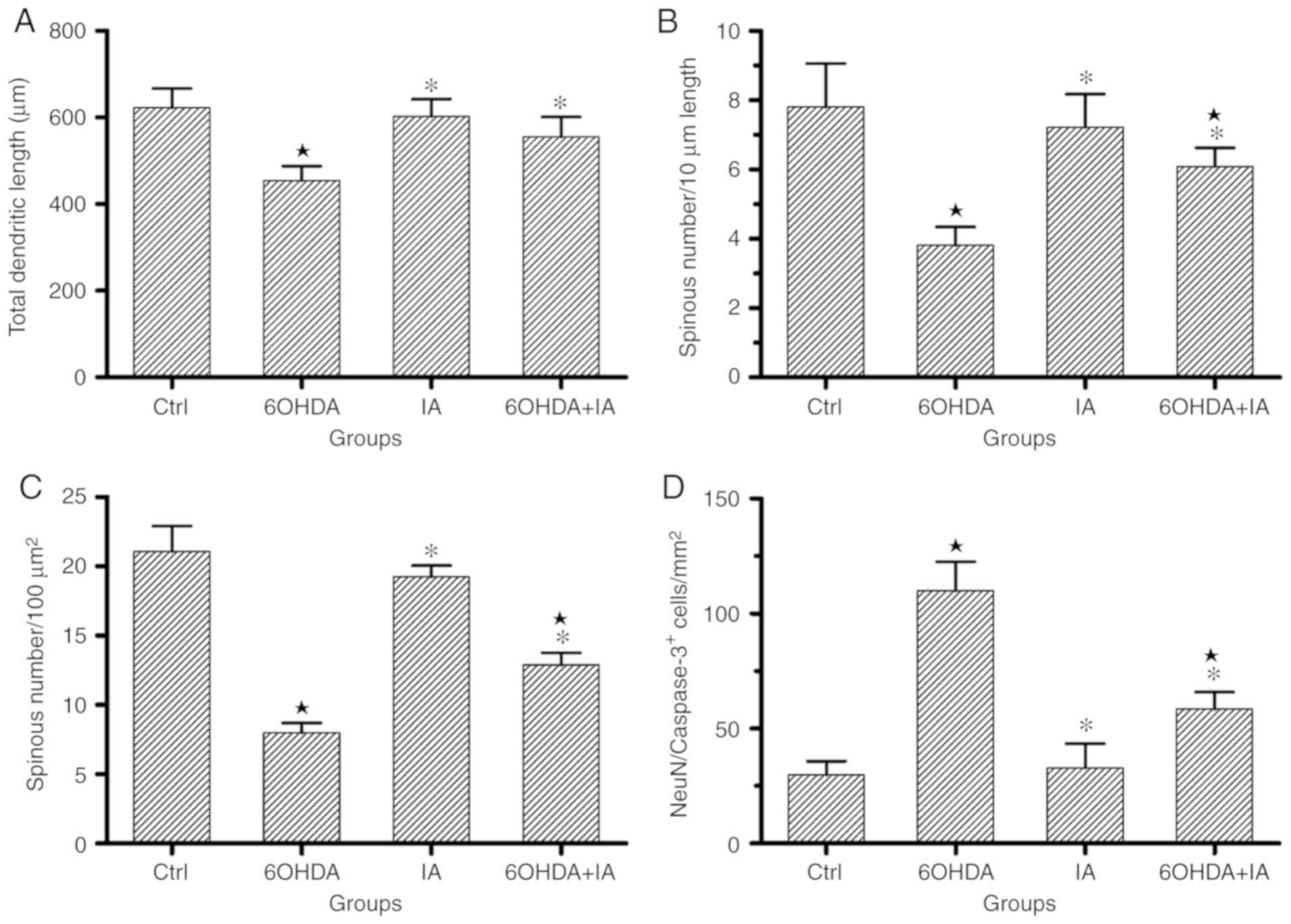 | Figure 2Statistical processing and
comparisons of the experimental data. (A) Comparison of the total
dendritic length of single neuron. An increased total dendritic
length was observed in the 6OHDA + IA group compared with that in
the 6OHDA group. (B) Comparison of the dendritic spine densities
(spines/10 µm) as evaluated by Golgi staining, showing a
severe loss of dendritic spines in the 6OHDA group. However, an
increased density of dendritic spines was observed in the 6OHDA +
IA rats compared to the density in the 6OHDA rats. (C) Comparison
of the dendritic spine densities (number/100 µm2)
in the electron microscopy experiment, consistent with the Golgi
staining experiment results. The statistical analysis showed a
significant reduction in the dendritic spine density in the 6OHDA
group, but an increase in the density in the 6OHDA + IA group. (D)
Comparison of double-labeling immunofluorescence for
NeuN/caspase-3, which shows a significant increase in the number of
NeuN/caspase-3 double-labeled neurons in the 6OHDA group compared
with the numbers in the control and 6OHDA + IA groups. Data are
presented as the mean ± SD (n=6/group), two-way ANOVA.
*P<0.05 vs. respective Ctrl group; *P<0.05 vs.
respective 6OHDA group. NeuN, neuron-specific protein; Ctrl,
control; 6OHDA, 6-hydroxydopamine; IA, ibotenic acid. |
The dendritic spines of the striatal neurons could
be clearly observed in the brain slices using Golgi staining.
Therefore, Golgi staining was also used to determine the dendritic
spine density in the striatal neurons. The dendritic spine density
was significantly downregulated in the 6OHDA group (3.8±0.53)
compared with the density in the control group (7.80±1.26;
P<0.05; Figs. 1 and 2B), whereas the dendritic spine density
was upregulated in the 6OHDA+IA group (6.09±0.54) compared with the
density in the 6OHDA group (P<0.05; Figs. 1 and 2B).
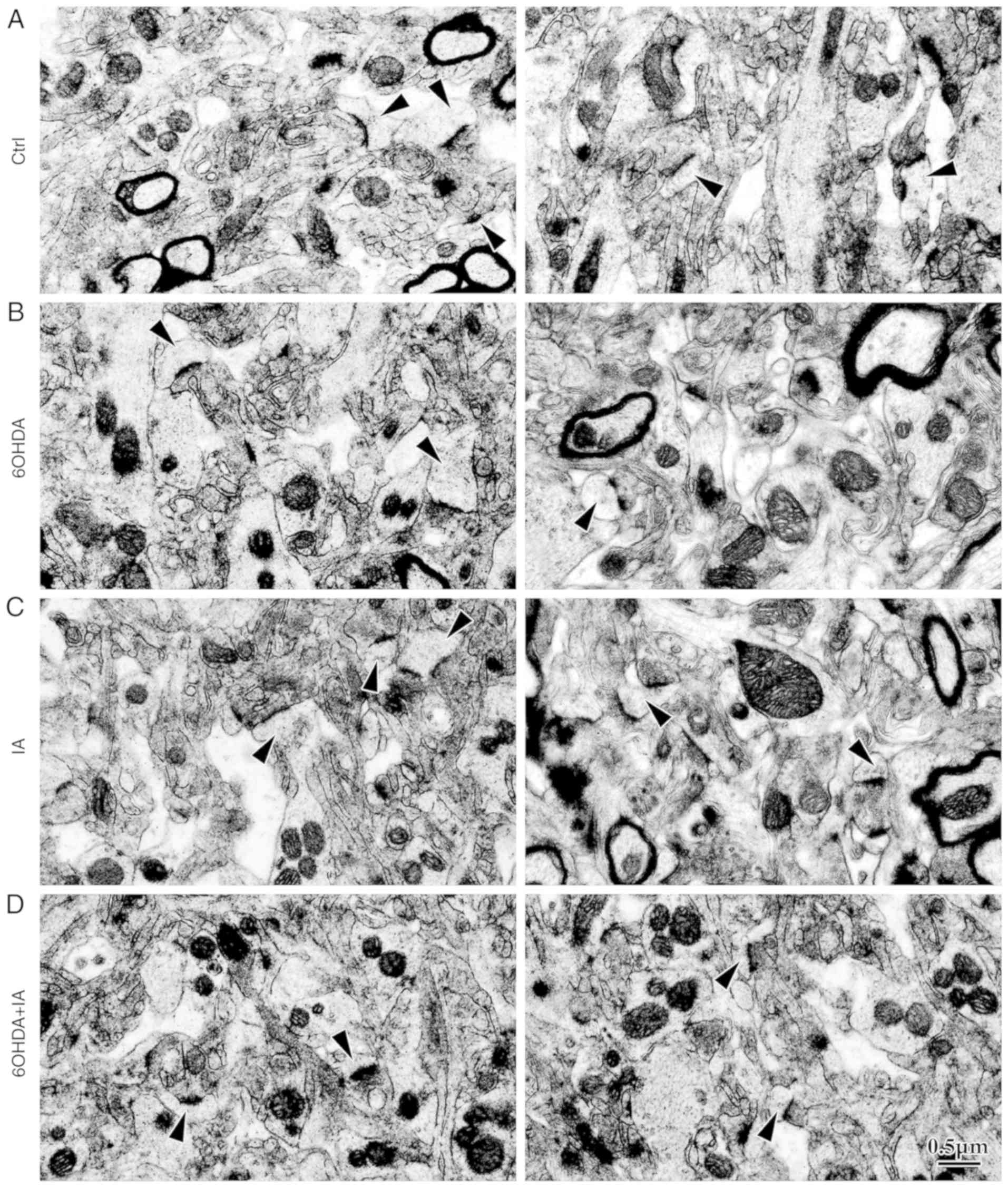
To investigate excitatory synaptic inputs on the
dendritic spines, the microstructures of the striatal neurons were
observed by EM. The dendritic spine density in the 6OHDA group
(7.10±0.70) was significantly decreased compared to that in the
control (21.07±1.83) and IA (19.24±0.82; P<0.05; Figs. 3 and 2C) groups. However, the dendritic spine
density was significantly increased in the 6OHDA+IA group
(12.89±0.8727) compared to that in the 6OHDA group (P<0.05;
Figs. 3 and 2C). Similarly, no difference was
observed between the control and IA (P>0.05; Figs. 3 and 2C) groups. These results suggested that
decortication rescued the morphological alterations in the striatal
neurons in the 6OHDA-treated rats.
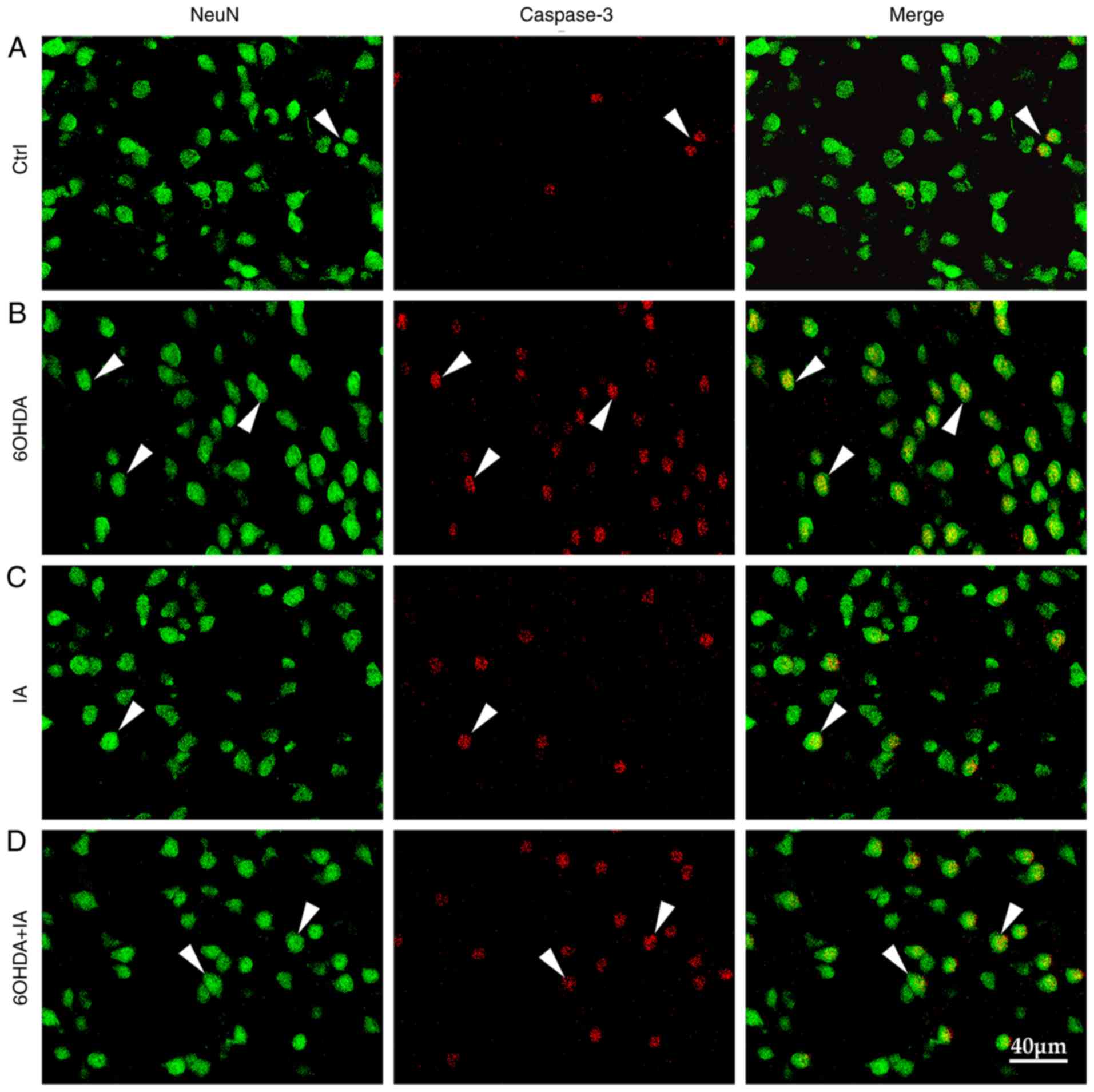
Cortical regulation effects dopamine
depletion-induced striatal neuron lesions
Apoptosis induced by dopamine deficiency contributes
to the decrease in striatal neurons in PD. Thus, NeuN and caspase-3
expression was investigated in the experimental rat model. NeuN is
a specific marker of neurons, whereas caspase-3 is a specific
marker of apoptosis. Immunofluorescence analysis showed that the
quantity of caspase-3-positive neurons was significantly
upregulated in the 6OHDA group (110.00±12.62) compared to the
quantity in the control group (29.79±6.06; P<0.05; Figs. 4 and 2D) and in the IA group (32.84±10.61;
P<0.05; Figs. 4 and 2D). Notably, the quantity of
caspase-3-positive neurons was downregulated in the 6OHDA + IA
group (58.42±7.40) compared to the quantity in the 6OHDA group
(P<0.05; Figs. 4 and 2D).
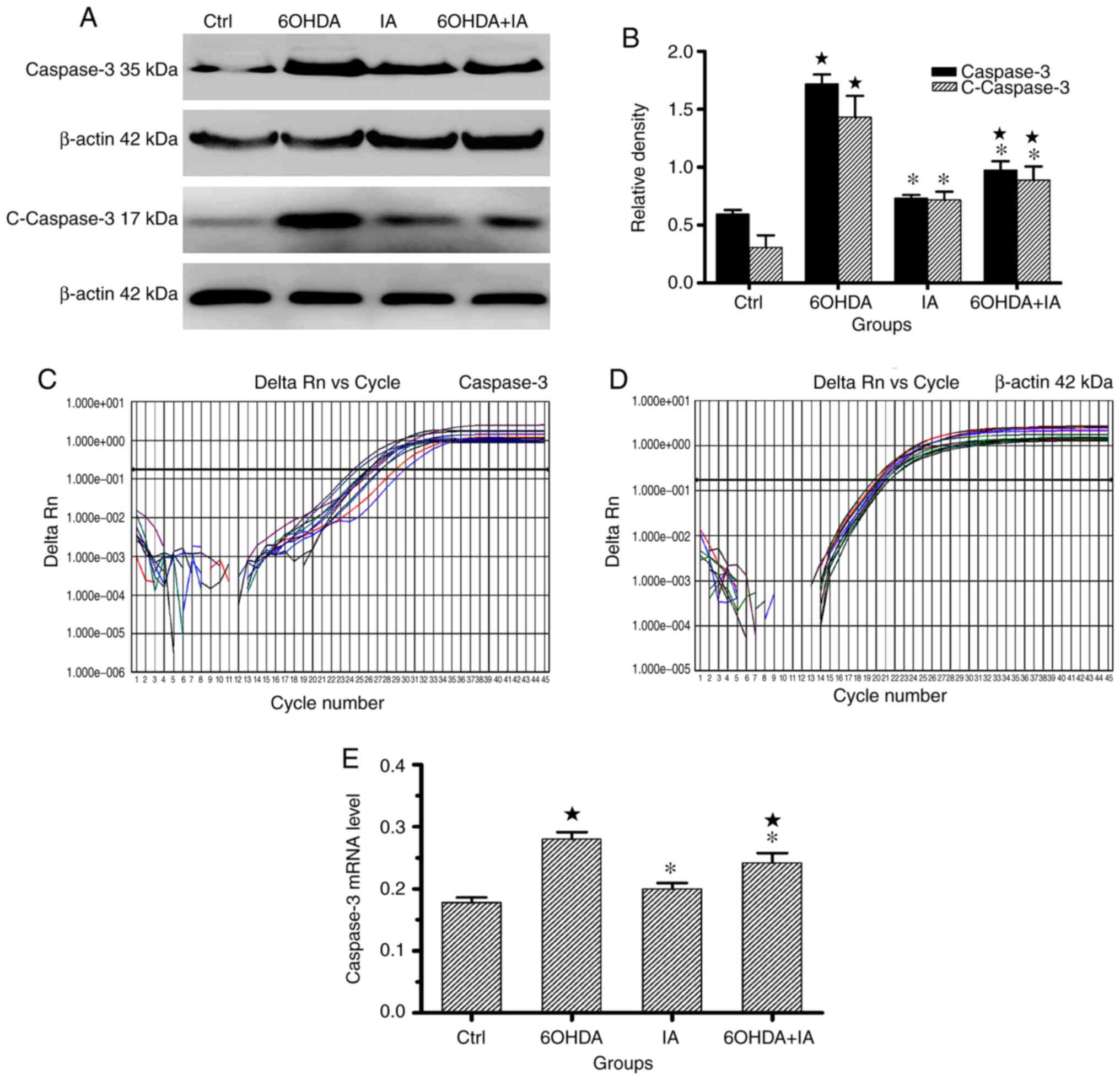
Western blotting and RT-qPCR analyses indicated that
total caspase-3 expression was significantly upregulated in the
6OHDA group (1.80±0.20 and 0.28±0.03, respectively) compared to the
control group (0.59±0.08 and 0.18±0.02, respectively; P<0.05;
Fig. 5) and the IA group
(0.73±0.03 and 0.24±0.04, respectively; P<0.05; Fig. 5). Similarly, total caspase-3
expression levels were decreased in the 6OHDA+IA group (0.75±0.07
and 0.20±0.02) compared to the expression levels in the 6OHDA group
(P<0.05; Fig. 5). Western
blotting indicated that cleaved caspase-3 expression was
significantly upregulated in the 6OHDA group (1.430±0.19) compared
to the control group (0.31±0.11; P<0.05; Fig. 5). Cleaved caspase-3 expression
levels were decreased in the 6OHDA+IA group (0.89±0.12) compared to
the expression levels in the 6OHDA group (P<0.05; Fig. 5). These results demonstrated that
decortication offset the downregulated apoptosis in the
6OHDA-treated rats.
In summary, these results suggested that
decortication alleviated the abnormal morphology of striatal
neurons and motor dysfunction in the 6OHDA-treated PD rat
model.
Discussion
The results of the present study showed that partial
decortication ameliorates the loss of dendritic spines on striatal
neurons in the dopamine depletion-induced PD rat model. Researchers
who study dendritic spines accept synaptic input frequency and the
connection points provided by dendrites and spines as the basis of
the structural integrity of the striatal synapses and the stability
of the neural circuits, and their normal function is to maintain
striatal function, while they are associated with neurodegenerative
diseases (32). The striatum,
which is a critical component of the basal ganglia, mainly consists
of projection neurons and interneurons. In total, ~90% of all
striatal neurons in rodents are striatal projection neurons.
Interneurons make up ~10% of striatal neurons (33). The projection neurons comprise
direct and indirect pathway neurons. Direct and indirect pathway
neurons have no significant distinctions in their morphology,
quantity and distribution, and both secrete the inhibitory
neurotransmitter γ-aminobutyric acid (GABA). However, the
projection areas of the direct and indirect pathway neurons are
different (34). The direct
pathway neurons project to the substantia nigra pars reticulata
(SNr), whereas indirect pathway neurons project to the SNr after
relaying into the external segment of the globus pallidus and the
subthalamic nucleus. As a crucial part of the motor center, the
striatum plays an important role in muscular tension and fine motor
behavior. The striatum also has a vital role in learning, memory
and cognition (35-37). The function of the striatum relies
on the projection neurons in the direct and indirect pathways.
After transport to the SNr through the direct and indirect pathways
and relaying in the thalamus, nerve impulses from striatal
projection neurons regulate motor cortex activities. In addition,
the activity of the striatal projection neurons is strictly
regulated by interneurons and neurons from the cortex, thalamus and
midbrain (38,39). Normally, the glutamatergic
excitatory inputs from the cortex and thalamus and the dopaminergic
inputs from the SNc coordinate with each other to maintain the
functional stabilization and structural integrity of the striatal
neurons (8).
It is generally known that the striatum is involved
in various neurodegenerative diseases, such as PD and Huntington's
disease (HD). Striatal neurons exhibit individual vulnerability in
brain injury (39-41). For example, the projection neurons
are vulnerable to ischemic damage, while the interneurons display
resistance and even hyperplasia in middle cerebral artery occlusion
models. The striatal projection neurons exhibit severe damage in
HD, evidenced by the rupture of dendrites and dendritic spine loss
(42). The fundamental cause of
PD is the loss and dysfunction of dopaminergic neurons in the
substantia nigra. Therefore, the question remains as to how
dopamine deficiency in PD affects striatal neurons. Gerfen et
al (38) found that the
activity of striatal dopamine receptor D2 neurons was
upregulated in PD, with severe damage and loss of dendritic spines.
However, striatal dopamine receptor D1 neurons are not
seriously affected. In the present study the quantity of
caspase-3-positive neurons was increased in the 6OHDA-treated rats.
In addition, western blotting and RT-qPCR further demonstrated that
the expression of caspase-3 was upregulated at both the protein and
mRNA levels. These results suggested that striatal neuron apoptosis
did occur after dopamine was deleted. Dendritic spines that receive
synaptic input are highly sensitive to injury. For example,
striatal dendritic spines undergo severe loss in cerebral ischemia
and HD (43,44). It was further demonstrated that
the total dendritic length and dendritic density of the striatal
projection neurons was decreased in the PD rats treated with
6OHDA.
Striatal projection neurons receive glutamatergic
inputs from the cortex and thalamus, as well as dopaminergic inputs
from the SNc. The balance of these synaptic inputs is critical to
maintain the function and integrity of the striatum (45). The disruption of this balance
between excitatory and inhibitory synaptic inputs (16), as well as that among DA, GABA and
acetylcholine synaptic inputs (46), is the primary cause of striatal
neuronal damage (47,48). To confirm this hypothesis, the
present study evaluated the striatal dendritic regression in the PD
rats treated with 6OHDA after decortication. Garcia et al
(16) presented evidence of the
glutamate depletion in the striatum following decortication. A
noteworthy finding was that the decortication ameliorated neuronal
dendritic lesions and dendritic spine loss. Moreover, the
decortication also alleviated motor dysfunction in the PD rats.
Other researchers have shown that dopamine depletion decreases grip
strength (49); however previous
studies (24,50), as well as the present study,
reached the opposite conclusion (13). This inconsistency may be due to
the different measurement methods and experimental animals. Other
researchers have measured the grip strength of the forelimbs using
a digital grip force meter; the animals are positioned to grab the
grid with their forelimbs and are gently pulled to record the grip
strength. The present study tested the duration and tension of grip
strength, whereas other studies have tested the power of grip
strength. On the other hand, the present results indicated that
decortication alleviated the cognitive deficits induced by dopamine
depletion. The striatal complexes in rodents can be roughly divided
into the dorsolateral region (participating in sensorimotor
circuits) and the ventromedial region (participating in associative
circuits). Different striatal subregions receive inputs from
distinct cortical areas and thus control different physiological
functions, including the processes of learning and memory. The
dorsolateral region analyzed by the present study receives inputs
from the motor cortex (51). The
striatum also receives input from other brain regions. A few areas
of M1 were damaged. This may be the reason why there was no
statistical difference between the IA group and the control group
in the water maze test. However, the body weight of the animals was
not assessed as part of the experiment and that is a limitation of
the current work, and as such may guide future experiments. These
results indicated that the presynaptic glutamatergic inputs offset
the striatal neuronal lesions in 6OHDA-treated PD rats
In summary, the present study demonstrated that the
relative excess of cortical excitatory inputs is important for
striatal neuronal lesions in a PD rat model following treatment
with 6OHDA, which shed new light on the pathology and treatment of
PD.
Supplementary Data
Acknowledgments
The authors would like to thank Ms. Wanjun Guan
(Core Lab Plat for Medical, Zhongshan School of Medicine, Sun
Yat-sen University, China) for providing support for instruments
and equipment, and Dr Ming Zhuang (The Frist Affiliated Hospital,
Sun Yat-sen University) for providing advice on writing.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81471288) and by the
National Key R&D Program of China (grant no.
2017YFA0104704).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL and YZ designed the experiments. YZ, BL, XZ and
JW performed all the experiments and drafted the manuscript. BL,
XZ, JW and SC carried out data acquisition. SC, ZC, TC and ZH
performed the data analysis. TC, ZH and WL participated in the
revision of the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All rat care and procedures involved were approved
by the Animal Care and Use Committee of Sun Yat-sen University (no.
SYXK GUANGDONG 2011-0029).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hornykiewicz O: Dopamine
(3-hydroxytyramine) and brain function. Pharmacol Rev. 18:925–964.
1966.PubMed/NCBI
|
|
2
|
Gibb WR and Lees AJ: Anatomy,
pigmentation, ventral and dorsal subpopulations of the substantia
nigra, and differential cell death in parkinson's disease. J Neurol
Neurosurg Psychiatry. 54:388–396. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Villalba RM and Smith Y: Differential
striatal spine pathology in Parkinson's disease and cocaine
addiction: A key role of dopamine. Neuroscience. 251:2–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Penney JB and Young AB: Speculations on
the functional anatomy of basal ganglia disorders. Annu Rev
Neurosci. 6:73–94. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
DeLong MR: Primate models of movement
disorders of basal ganglia origin. Trends Neurosci. 13:281–285.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Albin RL, Young AB and Penney JB: The
functional anatomy of disorders of the basal ganglia. Trends
Neurosci. 18:63–64. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
DeLong MR and Wichmann T: Basal ganglia
circuits as targets for neuromodulation in parkinson disease. JAMA
Neurol. 72:1354–1360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kreitzer AC and Malenka RC: Striatal
plasticity and basal ganglia circuit function. Neuron. 60:543–54.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kreitzer AC: Physiology and pharmacology
of striatal neurons. Annu Rev Neurosci. 32:127–147. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parker JG, Marshall JD, Ahanonu B, Wu YW,
Kim TH, Grewe BF, Zhang Y, Li JZ, Ding JB, Ehlers MD and Schnitzer
MJ: Diametric neural ensemble dynamics in parkinsonian and
dyskinetic states. Nature. 557:177–182. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jimenez-Shahed J: A review of current and
novel levodopa formulations for the treatment of parkinson's
disease. Ther Deliv. 7:179–191. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiao D: Acupuncture for parkinson's
disease: A review of clinical, animal, and functional magnetic
resonance imaging studies. J Tradit Chin Med. 35:709–717. 2015.
View Article : Google Scholar
|
|
13
|
Ma Y, Zhan M, OuYang L, Li Y, Chen S, Wu
J, Chen J, Luo C and Lei W: The effects of unilateral 6-OHDA lesion
in medial forebrain bundle on the motor, cognitive dysfunctions and
vulnerability of different striatal interneuron types in rats.
Behav Brain Res. 1:37–45. 2014. View Article : Google Scholar
|
|
14
|
Jia Y, Mo SJ, Feng QQ, Zhan ML, OuYang LS,
Chen JC, Ma YX, Wu JJ and Lei WL: EPO-dependent activation of
PI3K/Akt/FoxO3a signalling mediates neuroprotection in in vitro and
in vivo models of parkinson's disease. J Mol Neurosci. 53:117–124.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma Y, Feng Q, Ouyang L, Mu S, Liu B, Li Y,
Chen S and Lei W: Morphological diversity of GABAergic and
cholinergic inter-neurons in the striatal dorsolateral and
ventromedial regions of rats. Cell Mol Neurobiol. 34:351–359. 2014.
View Article : Google Scholar
|
|
16
|
Garcia BG, Neely MD and Deutch AY:
Cortical regulation of striatal medium spiny neuron dendritic
remodeling in parkinsonism: Modulation of glutamate release
reverses dopamine depletion-induced dendritic spine loss. Cereb
Cortex. 20:2423–2432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mitchell IJ, Lawson S, Moser B, Laidlaw
SM, Cooper AJ, Walkinshaw G and Waters CM: Glutamate-induced
apoptosis results in a loss of striatal neurons in the parkinsonian
rat. Neuroscience. 63:1–5. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hernandez-Baltazar D, Mendoza-Garrido ME
and Martinez- Fong D: Activation of GSK-3β and caspase-3 occurs in
nigral dopamine neurons during the development of apoptosis
activated by a striatal injection of 6-hydroxydopamine. PLoS One.
8:e709512013. View Article : Google Scholar
|
|
19
|
Shah M, Rajagopalan S, Xu L, Voshavar C,
Shurubor Y, Beal F, Andersen JK and Dutta AK: The high-affinity
D2/D3 agonist D512 protects PC12 cells from 6-OHDA-induced
apoptotic cell death and rescues dopaminergic neurons in the MPTP
mouse model of parkinson's disease. J Neurochem. 131:74–85. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
General administration of quality
supervision, inspection and quarantine of the Republic People's of
China: Laboratory animal - Guideline for Ethical Review of Animal
Welfare (GB/T 35892-2018). General Administration of Press and
Publication of the People's Republic of China; Beijing: 2018,
http://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=9BA619057D5C13103622A10FF4BA5D14.
|
|
21
|
Deumens R, Blokland A and Prickaerts J:
Modeling parkinson's disease in rats: An evaluation of 6-OHDA
lesions of the nigrostriatal pathway. Exp Neurol. 175:303–317.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng X, Wu J, Zhu Y, Chen S, Chen Z, Chen
T, Huang Z, Wei J, Li Y and Lei W: A Comparative study for
striatal-direct and-indirect pathway neurons to DA
depletion-induced lesion in a PD rat model. Neurochem Int.
118:14–22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Wu JJ, Ouyang S, Jia LS, Liu Y, Mu
BB, Ma SH, Wang YX, Wei WP, Li JYYL, et al: Cortical regulation of
striatal projection neurons and interneurons in a parkinson's
disease rat model. Neural Regen Res. 11:1969–1975. 2016. View Article : Google Scholar
|
|
24
|
Shear DA, Dong J, Gundy CD, Haik-Creguer
KL and Dunbar GL: Comparison of intrastriatal injections of
quinolinic acid and 3-nitropropionic acid for use in animal models
of Huntington's disease. Prog Neuropsychopharmacol Biol Psychiatry.
22:1217–1240. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vorhees CV and Williams MT: Morris water
maze: Procedures for assessing spatial and related forms of
learning and memory. Nat Protoc. 1:848–858. 2006. View Article : Google Scholar
|
|
26
|
Mu S, Lin E, Liu B, Ma Y, OuYang L, Li Y,
Chen S, Zhang J and Lei W: Melatonin reduces projection neuronal
injury induced by 3-nitropropionic acid in the rat striatum.
Neurodegener Dis. 14:139–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Garcia-Segura LM and Perez-Marquez J: A
new mathematical function to evaluate neuronal morphology using the
Sholl analysis. J Neurosci Methods. 226:103–109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Graveland GA, Williams RS and DiFiglia M:
A golgi study of the human neostriatum: Neurons and afferent
fibers. J Comp Neurol. 234:317–333. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deng YP, Wong T, Bricker-Anthony C, Deng B
and Reiner A: Loss of corticostriatal and thalamostriatal synaptic
terminals precedes striatal projection neuron pathology in
heterozygous Q140 Huntington's disease mice. Neurobiol Dis.
60:89–107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
31
|
Wearne SL, Rodriguez A, Ehlenberger DB,
Rocher AB, Henderson SC and Hof PR: New techniques for imaging,
digiti-zation and analysis of three-dimensional neural morphology
on multiple scales. Neuroscience. 136:661–680. 2005. View Article : Google Scholar
|
|
32
|
Lammel S, Lim BK and Malenka RC: Reward
and aversion in a heterogeneous midbrain dopamine system.
Neuropharmacology. 76(Pt B): 351–359. 2014. View Article : Google Scholar
|
|
33
|
Kawaguchi Y, Wilson CJ, Augood SJ and
Emson PC: Striatal interneurones: Chemical, physiological and
morphological characterization. Trends Neurosci. 18:527–535. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gerfen CR and Surmeier DJ: Modulation of
striatal projection systems by dopamine. Annu Rev Neurosci.
34:441–466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mowery TM, Penikis KB, Young SK, Ferrer
CE, Kotak VC and Sanes DH: The sensory striatum is permanently
impaired by transient developmental deprivation. Cell Rep.
19:2462–2468. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhai S, Tanimura A, Graves SM, Shen W and
Surmeier DJ: Striatal synapses, circuits, and Parkinson's disease.
Curr Opin Neurobiol. 48:9–16. 2018. View Article : Google Scholar
|
|
37
|
Radl D, Chiacchiaretta M, Lewis RG,
Brami-Cherrier K, Arcuri L and Borrelli E: Differential regulation
of striatal motor behavior and related cellular responses by
dopamine D2L and D2S isoforms. Proc Natl Acad Sci USA. 115:198–203.
2018. View Article : Google Scholar
|
|
38
|
Gerfen CR: Indirect-pathway neurons lose
their spines in parkinson disease. Nat Neurosci. 9:157–158. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Parker PR, Lalive AL and Kreitzer AC:
Pathway-specific remodeling of thalamostriatal synapses in
parkinsonian mice. Neuron. 89:734–740. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yoshioka H, Niizuma K, Katsu M, Sakata H,
Okami N and Chan PH: Consistent injury to medium spiny neurons and
white matter in the mouse striatum after prolonged transient global
cerebral ischemia. J Neurotrauma. 28:649–660. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
de Oliveira PA, Ben J, Matheus FC,
Schwarzbold ML, Moreira ELG, Rial D, Walz R and Prediger RD:
Moderate traumatic brain injury increases the vulnerability to
neurotoxicity induced by systemic administration of
6-hydroxydopamine in mice. Brain Res. 1663:78–86. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Beal MF, Ferrante RJ, Swartz KJ and Kowall
NW: Chronic quinolinic acid lesions in rats closely resemble
huntington's disease. J Neurosci. 11:1649–1659. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Graham RK, Pouladi MA, Joshi P, Lu G, Deng
Y, Wu NP, Figueroa BE, Metzler M, André VM, Slow EJ, et al:
Differential susceptibility to excitotoxic stress in YAC128 mouse
models of huntington disease between initiation and progression of
disease. J Neurosci. 29:2193–2204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chakraborty J, Nthenge-Ngumbau DN, Rajamma
U and Mohanakumar KP: Melatonin protects against behavioural
dysfunctions and dendritic spine damage in 3-nitropropionic
acid-induced rat model of huntington's disease. Behav Brain Res.
264:91–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sardi S, Vardi R, Sheinin A, Goldental A
and Kanter I: New types of experiments reveal that a neuron
functions as multiple independent threshold units. Sci Rep.
7:180362017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shen W, Tian X, Day M, Ulrich S, Tkatch T,
Nathanson NM and Surmeier DJ: Cholinergic modulation of Kir2
channels selectively elevates dendritic excitability in
striatopallidal neurons. Nat Neurosci. 10:1458–1466. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Romashkova JA and Makarov SS: NF-kappaB is
a target of AKT in anti-apoptotic PDGF signalling. Nature.
401:86–90. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ruiz-Calvo A, Maroto IB, Bajo-Grañeras R,
Chiarlone A, Gaudioso Á, Ferrero JJ, Resel E, Sánchez-Prieto J,
Rodríguez-Navarro JA, Marsicano G, et al: Pathway-specific control
of striatal neuron vulnerability by corticostriatal cannabinoid CB1
receptors. Cereb Cortex. 28:307–322. 2018. View Article : Google Scholar
|
|
49
|
Singh S and Kumar P: Neuroprotective
potential of curcumin in combination with piperine against
6-hydroxy dopamine induced motor deficit and neurochemical
alterations in rats. Inflammopharmacology. 25:69–79. 2017.
View Article : Google Scholar
|
|
50
|
Leung TC, Lui CN, Chen LW, Yung WH, Chan
YS and Yung KK: Ceftriaxone ameliorates motor deficits and protects
dopaminergic neurons in 6-hydroxydopamine-lesioned rats. ACS Chem
Neurosci. 3:22–30. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Packard MG and Knowlton BJ: Learning and
memory functions of the basal ganglia. Annu Rev Neurosci.
25:563–593. 2002. View Article : Google Scholar : PubMed/NCBI
|